Abstract
The strain Bifidobacterium animalis 01, isolated from centenarians, showed promising antioxidant potential in our previous studies. In this study, the genome information on strain 01 and the important antioxidant components are presented. The complete genome comprises a single circular chromosome (1,931,632 bp; 60.49% G + C content) with 1569 coding DNA sequences, 52 tRNA, and 9 rRNA operons. Based on phylogenomic analyses, strain 01 was designated as B. animalis subsp. lactis 01. The genomic analysis reveals that at least eight protein-coding genes are antioxidant-related genes. The conditions for simulating the oxidative stress have been determined. The results of quantitative reverse transcription PCR further demonstrated that the genes encoding the thioredoxin system (ahpC, ahpF, bcp, trxB, trxA, nrdH, and msrAB) and non-enzyme factors of the divalent cation transporter gene (mntH) were upregulated under the H2O2 challenge, indicating that the eight genes were effective components of the antioxidant system. The results of this study could benefit for understanding the antioxidant mechanism of B. animalis 01 and future utilization of it as a potential antioxidant agent.
Electronic supplementary material
The online version of this article (10.1007/s13205-019-1890-6) contains supplementary material, which is available to authorized users.
Keywords: Bifidobacterium animalis subsp. lactis 01, Complete genome sequence, Antioxidant activity, H2O2 challenge
Introduction
Reactive oxygen species (ROS) are produced by aerobic respiration and immune defense of organisms (Lü et al. 2010). The excessive amount of ROS can result in cellular damage, which promotes chronic diseases, such as cardiovascular diseases, diabetes, and cancer (Stephens et al. 2009; Sosa et al. 2013). The consumption of antioxidant supplements has been proposed to alleviate ROS and presumed beneficial for human health (Lobo et al. 2010). It has been shown that probiotic strains present significant antioxidant abilities (Mishra et al. 2015; Amaretti et al. 2013). In addition, certain probiotics can act as antioxidants to maintain intestinal redox balance in the gut by adhering to the intestinal lumen and colonizing the intestine (Tang et al. 2018).
A number of recent studies have reported that Bifidobacterium spp. showed great antioxidative activities (Mishra et al. 2015). The antioxidant capacity of B. animalis subsp. lactis INL1 was evaluated by 2,2ʹ-azino-bis (3-ethylbenzothiazoline-6-sulphonic acid) and ·OH technique. The different fractions, such as culture supernatant and lysate supernatant, exhibited high radical scavenging activity with both radicals (Loyeau et al. 2018). In B. longum, silent information regulator 2 could positively regulate the activity of its antioxidant enzymes (Guo et al. 2017). B. bifidum ATCC 29521 could decrease the intracellular level of ROS, and its incubated extracts also showed antioxidative activity by chelating metal ions (Wang et al. 2016). B. subsp. lactis DSMZ 23032 showed an antioxidative capacity with total antioxidant activity, trolox equivalent antioxidant capacity, and total glutathione values (Amaretti et al. 2013). Therefore, these species are potential candidates of natural antioxidant bioresource to promote the human health.
B. animalis 01 was isolated from feces of healthy centenarian volunteers in Guangxi, China. B. animalis 01 itself and its protein extracted have been observed to possess antioxidant activity both in vitro and in vivo (Zhang et al. 2009; Shen et al. 2010, 2011). However, the antioxidant mechanism of this strain is still largely unknown. In this study, to further investigate its antioxidant mechanism, the complete genome information of strain 01 and its integral components of antioxidant defense system are presented. The results of this study lay the theoretical foundation for the future application of strain 01 in the prevention of oxidative stress-related disorders.
Methods
Genome sequencing, assembly, and annotation
The genomic DNA of strain 01 was extracted using the QIAGEN DNA Extraction Kit according to the manufacturer’s instruction (Qiagen, CA, USA). Genome sequencing was performed by the Illumina Hiseq 2000 platform (2 × 100 bp). After sequencing, the short reads were assembled by SOAPdenovo v2.04 (http://soap.genomics.org.cn) (Luo et al. 2012).
After gap closing by SOAP GapCloser, a draft genome with 23 scaffolds was achieved. Gaps between scaffolds were closed by polymerase chain reaction (PCR) and Sanger sequencing. Genome annotation was applied by RAST (Overbeek et al. 2008). COG and Pfam (http://pfam.xfam.org/) were used to predict functional genes. The genes related to antioxidant activities were identified from the genome of B. animalis 01 using BLAST from NCBI (http://blast.ncbi.nlm.nih.gov/Blast.cgi).
H2O2 treatment of B. animalis subsp. lactis 01
As the most stable ROS, H2O2, which can diffuse through cells and form other active ROS, is generated from nearly all sources of oxidative cycle. Thus, H2O2 was added to the cultured strain 01 to induce the oxidative stress. Strain 01 was anaerobically cultured in De Man, Rogosa, and Sharpe (MRS) broth (AoBoxing, Beijing, China) at 37 °C until an OD600 of ~ 1.1, after which H2O2 was supplemented in a series (0.5, 1.0, 1.5, 2.0, and 2.5 mM), with the control containing no H2O2. The samples were collected at 0, 30, and 60 min after H2O2 addition and immediately diluted and plated on MRS with 0.05% cysteine agar. The plates were incubated anaerobically at 37 °C for 48 h before enumeration. The replicates were prepared in duplicate.
Determination of intracellular ROS
The production of intracellular ROS was measured using flow cytometry with 2′,7′-dichloro-uorescein diacetate (DCFH-DA) staining, as described previously (Li et al. 2017). After treatments with 1.5 mM H2O2 for 0, 30, and 60 min, cells were washed twice with phosphate buffer saline (PBS) (pH 7.2), and then, DCFH-DA (10 μM) was added to the cells for 30 min at 37 °C. The cells were washed with PBS to remove extracellular DCFH-DA and resuspended in PBS. The intracellular ROS levels were measured by FACSCalibur flow cytometry (BD Biosciences, USA) at an excitation wavelength of 485 nm and an emission wavelength of 530 nm.
Real-time quantitative PCR (RT-qPCR) analysis
B. animalis subsp. lactis 01 was cultured as described above, after which H2O2 was supplemented at the selected concentration for 0 (T0), 5 (T1), 30 (T2), or 60 min (T3). The total RNAs of different samples were isolated using the Trizol reagent (Invitrogen, United States) following the manufacturer’s instruction. To completely eliminate the DNA, the RNA samples were treated with 50 µg/mL RNase-free DNaseI (Takara, Japan). The quality of isolated RNAs was evaluated by gel electrophoresis. The RNA samples were reverse transcribed into single-stranded cDNA using PrimeScript first-strand cDNA Synthesis Kit (Takara, Japan). RT-qPCR was performed using 7500 Fast Real-Time PCR system (Applied Biosystems) with SYBR FAST qPCR Kit (Kapa Biosystems, USA). The primers sequences for RT-qPCR are listed in Table S1. The qPCR data were analyzed by the 2−ΔΔCT method (Livak and Schmittgen 2001). And the 16S rRNA gene was used as an internal reference. Table S1 lists the gene-specific primer sequences.
Availability of data and materials
The complete genome sequence of B. animalis subsp. lactis 01 was deposited at GenBank under the accession number CP035497. This strain has been deposited in China Center of Industrial Culture Collection under the accession number CICC no. 24193. The community metadata standards the “Minimal Information about any (X) Sequence” (MixS), which is shown in Table 1.
Table 1.
The community metadata standards the “Minimal Information about any (X) Sequence” (MixS) of Bifidobacterium animalis subsp. lactis 01
| Investigation | |||
| Investigation type | investigation_type | BA | |
| Project name | project_name | PRJNA516982 Bifidobacterium animalis strain:01 | |
| Environment | |||
| Collection date | collection_date | Missing (before 2000) | |
| Geographic location (latitude and longitude) | lat_lon | ||
| Geographic location (country and/or sea, region) | geo_loc_name | China: Guangxi | |
| Environment (biome) | biome | Homo sapiens | |
| Environment (feature) | feature | Gut | |
| Environment (material) | material | Feces | |
| MIMS/MIENS extension | |||
| Environmental package | env_package | Feces | |
| Depth | depth | Meter | 1 |
| Elevation | elev | Meter | 0 |
| Nucleic acid sequence source | |||
| Number of replicons | num_replicons | 3 | |
| Reference for biomaterial | ref_biomaterial | 10.1016/j.anaerobe.2010.06.006; 10.1007/s00284-010-9827-7; 10.1016/j.foodchem.2008.12.006 | |
| Observed biotic relationship | biotic_relationship | Free-living and particle-associated | |
| Trophic level | trophic_level | Heterotroph | |
| Relationship to oxygen | rel_to_oxygen | Anaerobes | |
| Isolation and growth condition | isol_growth_condt | B. animalis subsp. lactis 01 grows optimally at a range of 37 °C, a pH of 6.5; culture media: MRS | |
| Sequencing | |||
| Sequencing method | sequencing_meth | Illumina | |
| Assembly | assembly | Assembler: SOAPdenovo v2.04 | |
| Finishing strategy | finishing_strategy | Statuts: finished; | |
| Relevant electronic resources | url | http://www.ncbi.nlm.nih.gov/genomeprj/CP035497 | |
Results and discussion
General features
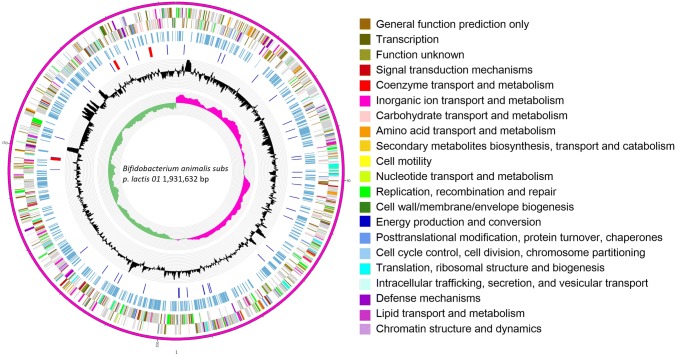
The complete genome of B. animalis 01 contains a circular chromosome of 1,931,632 bp and no plasmid, with G + C contents of 60.49%. The chromosome contains 1569 protein-coding genes, 52 tRNA, and 9 rRNA genes (Fig. 1). The identified genes were classified into 21 functional categories according to Clusters of Orthologous Groups (COG) of protein designation (Tatusov et al. 2003) (Table S2). Strain 01 was known as B. animalis 01 in our previous studies (Zhang et al. 2009; Shen et al. 2010, 2011). However, on the basis of the comparative phylogenomic analysis of B. animalis genomes, strain 01 should be now reassigned to denote B. animalis subsp. lactis 01 (Fig. S1).
Fig. 1.
Circular genome map of Bifidobacterium animalis subsp. lactis 01. Circles are shown from the outside to inner. Ring 1, Genome sequences. Ring 2 and 3, COG annotated coding sequences. Ring 4, KEGG enzymes. Ring 5, RNA genes. Ring 6, GC content. Ring 7, GC skew. Very short features were enlarged to enhance visibility. Clustered genes, such as several rRNA genes, may appear as one line due to space limitations. The image was created by software Circos
Identification of gene-coding antioxidant system
According to the annotation of genome, eight antioxidant-related genes of strain 01 were identified (Table 2), included alkyl hydroperoxide reductase subunits C and F (ahpC and ahpF), bacterioferritin comigratory proteins (bcp), thioredoxin reductase (trxB), thioredoxin (trxA), glutaredoxin-like proteins (nrdH), peptide methionine sulfoxide reductase (msrAB), and divalent metal cation transporter (mntH). Among these genes, ahpC, bcp, ahpF, trxB, trxA, nrdH, and msrAB are the basic components of the thioredoxin (Trx) system, which is a functional antioxidant system in protecting cells from oxidative damage (Lu and Holmgren 2014). MntH actively acquires a high-affinity manganese. The involvement of manganese is critical for defensing against ROS (Huang et al. 2017).
Table 2.
Antioxidant-related genes in the B. animalis subsp. lactis 01 genome
| COG category | Proposed function | Gene name | Locus tag(s) | Coordinatesc | Protein size |
|---|---|---|---|---|---|
| Oa | Alkyl hydroperoxide reductase subunit C | ahpC | ET527_04440 | 994497..995060(+) | 187aa |
| O | Alkyl hydroperoxide reductase protein F | ahpF | ET527_04445 | 995079..996851(+) | 590aa |
| O | bacterioferritin comigratory proteins | bcp | ET527_04555 | 1021161..1021646(+) | 161aa |
| O | Thioredoxin reductase | trxB | ET527_08195 | 1923658..1924620(+) | 320aa |
| O | Thioredoxin | trxA | ET527_06980 | 1609487..1609882(+) | 131aa |
| O | Glutaredoxin-like protein | nrdH | ET527_01755 | 409364..409627(−) | 87aa |
| O | Peptide methionine sulfoxide reductase msrA/msrB | msrAB | ET527_05320 | 1193250..1194242(−) | 330aa |
| Pb | Divalent metal cation transporter mntH | mntH | ET527_07110 | 1640760..1642118(−) | 452aa |
aPost-translational modification, protein turnover, chaperones
bInorganic ion transport and metabolism
cGenes on forward strand (+); genes on reverse strand (−)
Resistance to H2O2
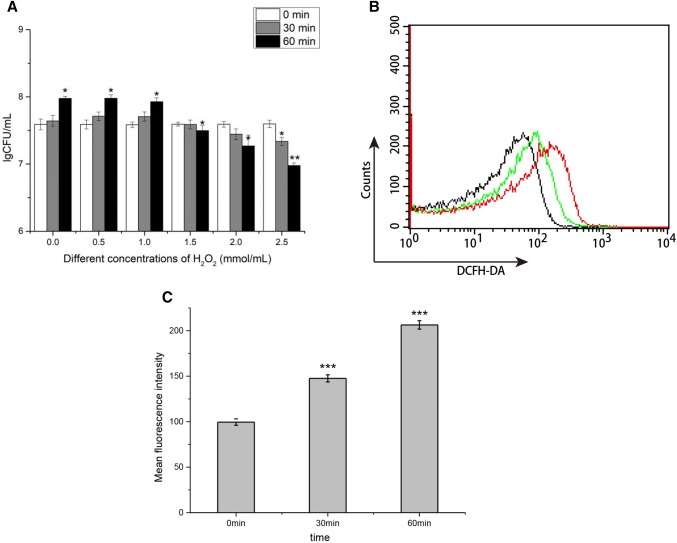
As shown in Fig. 2a, the datas of lgCFU were used to select appropriate H2O2 concentrations for oxidative stress induction and H2O2 challenge. The increased exogenous levels of H2O2 resulted in growth defect of strain 01. The strain 01 could survive well during the 1.0 mM of H2O2 challenge, indicating that these levels of H2O2 caused no metabolic disturbances which could impact the normal growth. The growth was stopped at a critical level of 1.5 mM of H2O2, which indicated a non-lethal stress. Significant cell death after the 60 min treatment with 2.0 mM of H2O2 was found. From Fig. 2b, c, we could also find the increase of intracellular ROS concentration in strain 01 treated by 1.5 mM of H2O2 with time variation. Therefore, 1.5 mM of H2O2 was selected for the oxidative stress induction and H2O2 challenge.
Fig. 2.
Effect of H2O2 on survival and production of ROS in B. animalis subsp. lactis 01. a Survival of early exponential phase B. animalis subsp. lactis 01 over 30 min and 60 min in MRS broth medium with H2O2 concentration of 0, 0.5, 1.0, 1.5, 2.0, and 2.5 mM. b Representative histograms of DCFH-DA fluorescence. Black line represented control B. animalis subsp. lactis 01 cells without H2O2 treatment (0 min). Green line represented B. animalis subsp. lactis 01 cells treated with 1.5 mM H2O2 (30 min), while red line represented cells treated with 1.5 mM H2O2 (60 min). c The percentage of mean fluorescence intensity relative to control cells. Data presented are the mean ± SD (n = 3). Error bars represent standard deviations. Statistical significance was calculated using Holm–Sidak Student’s t test (*p < 0.05, **p < 0.01, and ***p < 0.001)
Evaluation of the expression profiles of the antioxidant-related genes during H2O2 stress
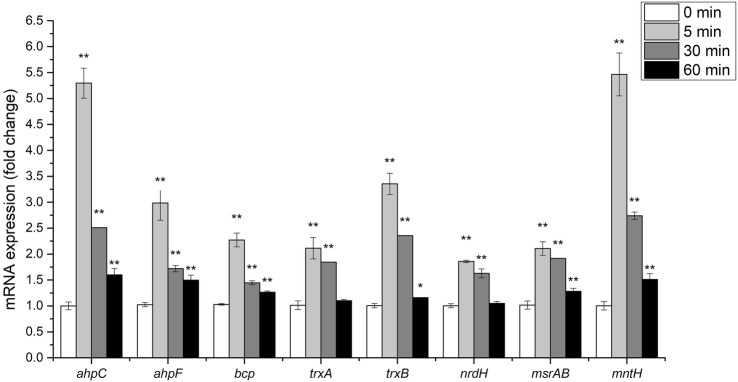
As shown in Fig. 3, the transcription rates of eight antioxidant-related genes in strain 01 were elevated by 1.5 mM of H2O2. The gene expressions at T1 were higher than those at T2 and T3, indicating that strain 01 exhibited antioxidant activity with high values by scavenging H2O2 within minutes. The ahpC showed 5.3-fold upregulated expression, and the expression of ahpF increased by 3.35-fold after 5 min of oxidative stress exposure (T1). The trend of other relative gene expressions (T1) in the thioredoxin system was as follows: trxB > bcp > trxA > msrAB > nrdH. The expression of mntH was upregulated by 5.46-fold after 5 min of oxidative stress exposure.
Fig. 3.
Effects of hydrogen peroxide on expressions of antioxidant-related genes in B. animalis subsp. lactis 01. The bar chart showed the relative mRNA levels of eight tested genes of cells in the presence of 1.5 mM H2O2 at the early exponential phase. Data presented are the mean ± SD (n = 3). Error bars represent standard deviations. Statistical significance was calculated using Holm–Sidak Student’s t test (*p < 0.05 and **p < 0.01)
AhpC is a typical peroxiredoxin which is directly regenerated by AhpF through electron transfer. The ahpC and ahpF complex, which is widely distributed in prokaryotes, is known to efficiently detoxify H2O2 (Poole et al. 2000; Seaver and Imlay 2001). The ahpC-overexpressing B. longum subsp. longum NCC2705 showed increased resistance to the endogenous H2O2 versus the control strain (Zuo et al. 2014). Other studies have shown the upregulation of ahpC in B. longum subsp. longum BBMN68 and B. animalis subsp. lactis BL-04 under oxidative challenge (Oberg et al. 2013; Xiao et al. 2011). In Salmonella typhimurium, ahpC is classified as a highly catalytic 2-Cys Prx, featuring a catalytic rate of more than 107/M/s (Parsonage et al. 2008). These evidences indicate that the ensemble of ahpC and ahpF is one of the most important antioxidant proteins in strain 01.
The thioredoxin system plays a crucial role in defensing against ROS for anaerobes (Jean et al. 2004; Mishra and Imlay 2013). Besides the AhpC and AhpF complex, other antioxidant proteins may also play important roles. Bcp, a member of peroxiredoxin, exhibits hydroperoxide peroxidase activities (Jeong et al. 2000). Poorly defined in Bifidobacterium sp., bcp was characterized with antioxidant functions from Candidatus liberibacter asiaticus and Thermococcus kodakaraensis KOD1 (Singh et al. 2017; Lee et al. 2015). The methionine residues are easily oxidized by ROS. Methionine sulfoxide reductase AB (msrAB) can catalyze the methionine sulfoxides back to methionine, and the cyclic interconversion of methionine can lead to the removal of ROS (Lee et al. 2009). Strain 01 showed significant upregulation of trxB and trxA, along with nrdH. The three genes were predicted for regenerating bcp and oxidizing msrAB by electron donation in response to the oxidative stress in strain 01.
Manganese has been proven beneficial for defensing against oxidative stress, acting as a co-factor of antioxidant enzymes and non-proteinaceous manganese antioxidants (Wang et al. 2014). The acquisition of manganese is crucial for cell manganese homeostasis. MntH, a high-affinity transporter, is highly selective for manganese (Kehres and Maguire 2003). The ability to obtain manganese plays an important role in catalase-void bacteria (Turner et al. 2015). To effectively cope with oxidative stress, strain 01 enhanced manganese import through the increased expression levels of mntH.
Conclusions
Unsurprisingly, anaerobic bacteria would exhibit antioxidant activity. The antioxidant activity is important for the survival of gut microbiota in GIT filled with ROS. Strain 01 lacks the most common antioxidant enzymes, such as superoxide dismutase, catalase, and glutathione peroxidase. The antioxidant capacities of strain 01 may be associated with the thioredoxin system and manganese. Our study provides the transcriptional landscape of strain 01 under H2O2 challenge, and the results are highly meaningful for understanding the molecular mechanisms of ROS resistance in Bifidobacterium sp.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Fig. S1. Neighbor-joining phylogenetic tree constructed from the complete genomes of B. animalis strains (JPEG 81 kb)
Abbreviations
- ROS
Reactive oxygen species
- PCR
Polymerase chain reaction
- RT-qPCR
Real-time quantitative PCR
- COG
Clusters of orthologous groups
- CFU
Colony-forming units
- CDS
Coding DNA sequences
- RAST
Rapid annotation using subsystem technology
- GIT
Gastrointestinal tract
- CICC
China Center of Industrial Culture Collection
Author contributions
JLZ and PLL designed the experiments. JLZ and SBW performed the experiments. JLZ, ZZ, SBW, and YXQ analyzed the results and wrote the manuscript. All authors read and approved the final manuscript.
Funding
This project was funded by the National Natural Science Foundation of China (31671831 and 31471707).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no competing interests.
References
- Amaretti A, Di Nunzio M, Pompei A, Raimondi S, Rossi M, Bordoni A. Antioxidant properties of potentially probiotic bacteria: in vitro and in vivo activities. Appl Microbiol Biotechnol. 2013;97:809–817. doi: 10.1007/s00253-012-4241-7. [DOI] [PubMed] [Google Scholar]
- Guo Q, Li S, Xie Y, et al. The NAD-dependent deacetylase, Bifidobacterium longum, Sir2 in response to oxidative stress by deacetylating FOXO3a and sigH (σ H) in, Bifidobacterium longum, and HEK293T cells respectively. Free Radical Biol Med. 2017;108:929–939. doi: 10.1016/j.freeradbiomed.2017.05.012. [DOI] [PubMed] [Google Scholar]
- Huang X, Shin JH, Pinochet-Barros A, Su TT, Helmann JD. Bacillus subtilis MntR coordinates the transcriptional regulation of manganese uptake and efflux systems. Mol Microbiol. 2017;103:253–268. doi: 10.1111/mmi.13554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jean D, Briolat V, Reysset G. Oxidative stress response in Clostridium perfringens. Microbiology. 2004;150:1649–1659. doi: 10.1099/mic.0.27017-0. [DOI] [PubMed] [Google Scholar]
- Jeong W, Cha MK, Kim IH. Thioredoxin-dependent hydroperoxide peroxidase activity of bacterioferritin comigratory protein (BCP) as a new member of the thiol-specific antioxidant protein (TSA)/alkyl hydroperoxide peroxidase C (AhpC) family. J Biol Chem. 2000;275(4):2924–2930. doi: 10.1074/jbc.275.4.2924. [DOI] [PubMed] [Google Scholar]
- Kehres DG, Maguire ME. Emerging themes in manganese transport, biochemistry and pathogenesis in bacteria. FEMS Microbiol Rev. 2003;27:263–290. doi: 10.1016/s0168-6445(03)00052-4. [DOI] [PubMed] [Google Scholar]
- Lee BC, Dikiy A, Kim HY, Gladyshev VN. Functions and evolution of selenoprotein methionine sulfoxide reductases. Biochim Biophys Acta. 2009;1790(11):1471–1477. doi: 10.1016/j.bbagen.2009.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Chung JM, Yun HJ, et al. New insight into multifunctional role of peroxiredoxin family protein: determination of DNA protection properties of bacterioferritin comigratory protein under hyperthermal and oxidative stresses. Biochem Biophys Res Commun. 2015;469:1028–1033. doi: 10.1016/j.bbrc.2015.12.099. [DOI] [PubMed] [Google Scholar]
- Li Z, Tan J, Shao L, et al. Selenium-mediated protection in reversing the sensitivity of bacterium to the bactericidal antibiotics. J Trace Elem Med Biol. 2017;41:23–31. doi: 10.1016/j.jtemb.2017.02.007. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lobo V, Patil A, Phatak A, Chandra N. Free radicals, antioxidants and functional foods: impact on human health. Pharmacogn Rev. 2010;4:118–126. doi: 10.4103/0973-7847.70902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loyeau P, Spotti M, Braber NV, Rossi Y, Montenegro M, Vinderola G, Carrara C. Microencapsulation of Bifidobacterium animalis subsp. lactis INL1 using whey proteins and dextrans conjugates as wall materials. Food Hydrocoll. 2018;85:129–135. doi: 10.1016/j.foodhyd.2018.06.051. [DOI] [Google Scholar]
- Lu J, Holmgren A. The thioredoxin antioxidant system. Free Radical Biol Med. 2014;66:75–87. doi: 10.1016/j.freeradbiomed.2013.07.036. [DOI] [PubMed] [Google Scholar]
- Lü J, Lin PH, Yao Q, Chen C. Chemical and molecular mechanisms of antioxidants: experimental approaches and model systems. J Cell Mol Med. 2010;14:840–860. doi: 10.1111/j.1582-4934.2009.00897.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo R, Liu B, Xie Y, Li Z, HuangW Yuan J, He G, Chen Y, Pan Q, Liu Y. SOAPdenovo2: an empirically improved memory-efficient short-read de novo assembler. Gigascience. 2012;1:18. doi: 10.1186/2047-217x-1-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra S, Imlay JA. An anaerobic bacterium, Bacteroides thetaiotaomicron, uses a consortium of enzymes to scavenge hydrogen peroxide. Mol Microbiol. 2013;90:1356–1371. doi: 10.1111/mmi.12438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra V, Shah C, Mokashe N, et al. Probiotics as potential antioxidants: a systematic review. J Agric Food Chem. 2015;63(14):3615–3626. doi: 10.1021/jf506326t. [DOI] [PubMed] [Google Scholar]
- Oberg TS, Ward RE, Steele JL, Broadbent JR. Genetic and physiological responses of Bifidobacterium animalis subsp. lactis to hydrogen peroxide stress. J Bacteriol. 2013;195:3743–3751. doi: 10.1128/jb.00279-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overbeek RA, Osterman AL, Robert O, Olsen GJ, Folker M, Michael K, Glass EM, Svetlana G, Kevin F, Edwards RA. The RAST server: rapid annotations using subsystems technology. BMC Genom. 2008;9:75. doi: 10.1186/1471-2164-9-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsonage D, Karplus PA, Poole LB. Substrate specificity and redox potential of AhpC, a bacterial peroxiredoxin. Proc Natl Acad Sci USA. 2008;105:8209–8214. doi: 10.1073/pnas.0708308105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole LB, Higuchi M, Shimada M, Calzi ML, Kamio Y. Streptococcus mutans H2O2-forming NADH oxidase is an alkyl hydroperoxide reductase protein. Free Radical Biol Med. 2000;28:108–120. doi: 10.1016/s0891-5849(99)00218-x. [DOI] [PubMed] [Google Scholar]
- Seaver LC, Imlay JA. Alkyl hydroperoxide reductase is the primary scavenger of endogenous hydrogen peroxide in Escherichia coli. J Bacteriol. 2001;183:7173–7181. doi: 10.1128/jb.183.24.7173-7181.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Q, Zhang B, Xu R, Wang Y, Ding X, Li P. Antioxidant activity in vitro of the selenium-contained protein from the Se-enriched Bifidobacterium animalis 01. Anaerobe. 2010;16:380–386. doi: 10.1016/j.anaerobe.2010.06.006. [DOI] [PubMed] [Google Scholar]
- Shen Q, Shang N, Li P. In vitro and in vivo antioxidant activity of Bifidobacterium animalis 01 isolated from centenarians. Curr Microbiol. 2011;62:1097–1103. doi: 10.1007/s00284-010-9827-7. [DOI] [PubMed] [Google Scholar]
- Singh A, Kumar N, Tomar PPS, et al. Characterization of a bacterioferritin comigratory protein family 1-Cys peroxiredoxin from Candidatus Liberibacter asiaticus. Protoplasma. 2017;254(4):1–17. doi: 10.1007/s00709-016-1062-z. [DOI] [PubMed] [Google Scholar]
- Sosa V, Moliné T, Somoza R, et al. Oxidative stress and cancer: an overview. Ageing Res Rev. 2013;12:376–390. doi: 10.1016/j.arr.2012.10.004. [DOI] [PubMed] [Google Scholar]
- Stephens JW, Khanolkar MP, Bain SC. The biological relevance and measurement of plasma markers of oxidative stress in diabetes and cardiovascular disease. Atherosclerosis. 2009;202:321–329. doi: 10.1016/j.atherosclerosis.2008.06.006. [DOI] [PubMed] [Google Scholar]
- Tang W, Li C, He Z, et al. Probiotic properties and cellular antioxidant activity of Lactobacillus plantarum MA2 isolated from Tibetan kefir grains. Probiotics Antimicrob Proteins. 2018;10:523–533. doi: 10.1007/s12602-017-9349-8. [DOI] [PubMed] [Google Scholar]
- Tatusov RL, Fedorova ND, Jackson JD, et al. The COG database: an updated version includes eukaryotes. BMC Bioinform. 2003;4:41. doi: 10.1186/1471-2105-4-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner AG, Cheryl-lynn YO, Gillen CM, et al. Manganese homeostasis in group A Streptococcus is critical for resistance to oxidative stress and virulence. MBio. 2015;6(2):e00278-15. doi: 10.1128/mbio.00278-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Tong H, Dong X. PerR-regulated manganese ion uptake contributes to oxidative stress defense in an oral streptococcus. Appl Environ Microb. 2014;80:2351–2359. doi: 10.1128/aem.00064-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang BG, Xu HB, Xu F, et al. Efficacy of oral Bifidobacterium bifidum ATCC 29521 on microflora and antioxidant in mice. Can J Microbiol. 2016;62:249–262. doi: 10.1139/cjm-2015-0685. [DOI] [PubMed] [Google Scholar]
- Xiao M, Xu P, Zhao J, et al. Oxidative stress-related responses of Bifidobacterium longum subsp. longum BBMN68 at the proteomic level after exposure to oxygen. Microbiology. 2011;157:1573–1588. doi: 10.1099/mic.0.044297-0. [DOI] [PubMed] [Google Scholar]
- Zhang B, Zhou K, Zhang J, et al. Accumulation and species distribution of selenium in Se-enriched bacterial cells of the Bifidobacterium animalis 01. Food Chem. 2009;115(2):727–734. doi: 10.1016/j.foodchem.2008.12.006. [DOI] [Google Scholar]
- Zuo F, Yu R, Khaskheli GB, Ma H, et al. Homologous overexpression of alkyl hydroperoxide reductase subunit C (ahpC) protects Bifidobacterium longum strain NCC2705 from oxidative stress. Res Microbiol. 2014;165:581–589. doi: 10.1016/j.resmic.2014.05.040. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Neighbor-joining phylogenetic tree constructed from the complete genomes of B. animalis strains (JPEG 81 kb)
Data Availability Statement
The complete genome sequence of B. animalis subsp. lactis 01 was deposited at GenBank under the accession number CP035497. This strain has been deposited in China Center of Industrial Culture Collection under the accession number CICC no. 24193. The community metadata standards the “Minimal Information about any (X) Sequence” (MixS), which is shown in Table 1.
Table 1.
The community metadata standards the “Minimal Information about any (X) Sequence” (MixS) of Bifidobacterium animalis subsp. lactis 01
| Investigation | |||
| Investigation type | investigation_type | BA | |
| Project name | project_name | PRJNA516982 Bifidobacterium animalis strain:01 | |
| Environment | |||
| Collection date | collection_date | Missing (before 2000) | |
| Geographic location (latitude and longitude) | lat_lon | ||
| Geographic location (country and/or sea, region) | geo_loc_name | China: Guangxi | |
| Environment (biome) | biome | Homo sapiens | |
| Environment (feature) | feature | Gut | |
| Environment (material) | material | Feces | |
| MIMS/MIENS extension | |||
| Environmental package | env_package | Feces | |
| Depth | depth | Meter | 1 |
| Elevation | elev | Meter | 0 |
| Nucleic acid sequence source | |||
| Number of replicons | num_replicons | 3 | |
| Reference for biomaterial | ref_biomaterial | 10.1016/j.anaerobe.2010.06.006; 10.1007/s00284-010-9827-7; 10.1016/j.foodchem.2008.12.006 | |
| Observed biotic relationship | biotic_relationship | Free-living and particle-associated | |
| Trophic level | trophic_level | Heterotroph | |
| Relationship to oxygen | rel_to_oxygen | Anaerobes | |
| Isolation and growth condition | isol_growth_condt | B. animalis subsp. lactis 01 grows optimally at a range of 37 °C, a pH of 6.5; culture media: MRS | |
| Sequencing | |||
| Sequencing method | sequencing_meth | Illumina | |
| Assembly | assembly | Assembler: SOAPdenovo v2.04 | |
| Finishing strategy | finishing_strategy | Statuts: finished; | |
| Relevant electronic resources | url | http://www.ncbi.nlm.nih.gov/genomeprj/CP035497 | |