Short abstract
Objective
We aimed to explore potential microRNAs (miRNAs) and target genes related to atrial fibrillation (AF).
Methods
Data for microarrays GSE70887 and GSE68475, both of which include AF and control groups, were downloaded from the Gene Expression Omnibus database. Differentially expressed miRNAs between AF and control groups were identified within each microarray, and the intersection of these two sets was obtained. These miRNAs were mapped to target genes in the miRNet database. Functional annotation and enrichment analysis of these target genes was performed in the DAVID database. The protein-protein interaction (PPI) network from the STRING database and the miRNA-target-gene network were merged into a PPI-miRNA network using Cytoscape software. Modules of this network containing miRNAs were detected and further analyzed.
Results
Ten differentially expressed miRNAs and 1520 target genes were identified. Three PPI-miRNA modules were constructed, which contained miR-424, miR-15a, miR-542-3p, and miR-421 as well as their target genes, CDK1, CDK6, and CCND3.
Conclusion
The identified miRNAs and genes may be related to the pathogenesis of AF. Thus, they may be potential biomarkers for diagnosis and targets for treatment of AF.
Keywords: MicroRNAs, atrial fibrillation, computational biology, microarray analysis, cyclin D kinase, cyclin D3
Introduction
Atrial fibrillation (AF) is one of the most common types of cardiac arrhythmia.1 It is an important risk factor for consequences such as stroke and atrial appendage thrombus formation,1,2 which may increase the risk of mortality in patients with AF.3,4 Therefore, revealing the mechanism of pathogenesis of AF is important and could indicate pathways to prevent and treat AF.
MicroRNAs (miRNAs) are small, non-coding RNA molecules involved in RNA silencing and posttranscriptional regulation of gene expression.5,6 Recent studies have revealed important roles for miRNAs in various physiological and pathological cardiac processes, such as the regulation of cardiac excitability and formation of arrhythmia,7,8 cardiac hypertrophy,9 myocardial infarction,9,10 and diabetic cardiomyopathy.11 An increasing number of tissue and circulating miRNAs have been identified as biomarkers of AF, involved in the development,12 progression,12–14 and complications15 of the disease.
Microarray analysis has become a powerful tool in the characterization of many pathophysiological processes. In this study, we used publicly available miRNA microarray data to perform de novo analyses. We focused on complementary bioinformatics to explore the data more deeply and aimed to construct an updated prediction of new miRNAs and genes as biomarkers of chronic AF to supplement currently available data.
Materials and methods
The data used in this study were obtained from open access databases on the internet and did not require ethical permission or patient consent for use.
Microarray data and differentially expressed miRNAs
We searched the Gene Expression Omnibus (GEO) database (http://www.ncbi.nlm.nih.gov/geo/), using key words atrial fibrillation, microRNA, and miRNA, and selected microarrays with data of good cross-comparability. Microarray data for GSE70887 and GSE68475 were selected and downloaded; the corresponding platforms were GPL19546 (Agilent-021827 Human miRNA Microarray; Agilent Technologies, Santa Clara, CA, USA) and GPL15018 (Agilent-031181 Unrestricted_Human_miRNA_V16.0_Microarray 030840; Agilent Technologies), respectively. GSE70887 included four atrial tissue samples from patients with chronic AF (AF group) and four from patients without AF (control group). GSE68475 included 10 atrial tissue samples from patients with persistent or permanent (≥6 months) AF (AF group) and 11 atrial tissue samples from patients with normal sinus rhythm (control group).
Differentially expressed miRNAs between the AF group and control group were screened within each microarray using the online tool GEO2R in the GEO database. GEO2R is an R-programming-based language for analysis of gene expression datasets by t-test or analysis of variance (ANOVA). It can help identify differentially expressed miRNAs between two groups of samples under the same conditions.16 Differentially expressed miRNAs were selected by using cut-off values of P < 0.05 and |log2FC| > 0.5, where FC = fold change. After these two sets of differentially expressed miRNAs were selected, we obtained their intersection for the following analyses.
MiRNA-target-gene mapping
All differentially expressed miRNAs were uploaded to the miRNet database (http://www.mirnet.ca)17 to map their corresponding target genes. The miRNA-target-gene network was constructed based on this mapping.
Functional enrichment analysis
The target genes were then annotated and enriched in the Database for Annotation, Visualization, and Integrated Discovery (DAVID; https://david-d.ncifcrf.gov/).18,19 Then, gene ontology: biological process (GO-BP) terms and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways were obtained. P-values < 0.05 were considered statistically significant.
Prediction of protein-protein interaction
We then applied Search Tool for the Retrieval of Interacting Genes (STRING, http://string-db.org/)20 to construct a protein-protein interaction (PPI) network based on the target genes detected above. Interactions of proteins in the STRING database were screened by using a confidence score criterion; PPI pairs with a confidence score >0.9 were considered significant in this analysis.
PPI-miRNA network construction and module clustering analysis
The PPI network and miRNA-target-gene network were merged into a whole PPI-miRNA network by using the Cytoscape software (https://cytoscape.org). This network was calculated by using the Cytoscape plugin “MCODE” to explore potential functional modules. Degree cutoff value was set to 2 and the node score cutoff to 0.2 as the criteria in this process. Modules containing miRNAs were selected.
Results
Identification of differentially expressed miRNAs and miRNA-target-gene mapping
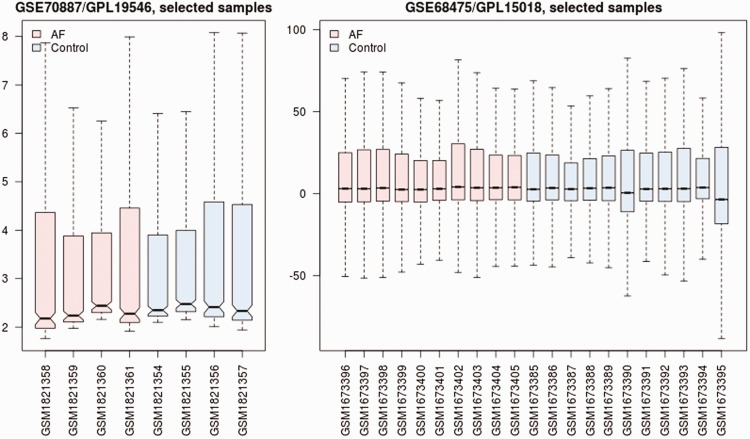
Data cross-compatibility was assessed (Figure 1) and 10 significant differentially expressed miRNAs were identified (Table 1). To explore the possible targets of the 10 miRNAs identified above, miRNA-target gene mapping was implemented in miRNet. A total of 1520 genes and 2235 miRNA-target-gene pairs were identified.
Figure 1.
Cross-comparability assessment of microarray data of GSE70887 and GSE68475 (http://www.ncbi.nlm.nih.gov/geo/). GSE70887 included four atrial tissue samples from patients with chronic atrial fibrillation (AF) and four from patients without AF (control). GSE68475 included 10 atrial tissue samples from patients with persistent or permanent (≥6 months) AF (AF) and 11 from patients with normal sinus rhythm (control).
Table 1.
A list of differentially expressed miRNAs.
| miRNA | P-value | Log2FC |
|---|---|---|
| hsa-miR-503 | 0.000628 | 0.828181 |
| hsa-miR-542-3p | 0.00223 | 0.607736 |
| hsa-miR-208b | 0.004467 | 1.729022 |
| hsa-miR-421 | 0.016152 | 0.501949 |
| hsa-miR-22* | 0.022647 | 0.675245 |
| hsa-miR-126* | 0.023377 | 0.595347 |
| hsa-miR-424 | 0.031095 | 0.604423 |
| hsa-miR-224 | 0.035734 | 0.665672 |
| hsa-miR-1285 | 0.043129 | −0.504033 |
| hsa-miR-15a | 0.047701 | 0.530578 |
The miR* nomenclature indicates a miRNA that originates from the same hairpin structure of the corresponding predominant miR (without *) and is thought to be complementary to the predominant miR. FC (fold change) values are expressed as the comparison of AF versus controls.
Functional enrichment analysis
The 1520 genes identified were uploaded to DAVID for functional enrichment analysis. Numerous GO-BP terms and KEGG pathways were enriched, and those with the smallest P-values are listed in Tables 2 and 3. Specifically, biological processes related to cell growth and apoptosis were highly enriched.
Table 2.
| GO ID | Term | Gene counts | P-value |
|---|---|---|---|
| GO:0046907 | Intracellular transport | 103 | 2.75E-10 |
| GO:0007049 | Cell cycle | 114 | 1.42E-09 |
| GO:0045449 | Regulation of transcription | 294 | 2.22E-09 |
| GO:0045184 | Establishment of protein localization | 109 | 2.51E-08 |
| GO:0043067 | Regulation of programmed cell death | 113 | 3.66E-08 |
| GO:0010941 | Regulation of cell death | 113 | 4.47E-08 |
| GO:0015031 | Protein transport | 107 | 5.61E-08 |
| GO:0010608 | Posttranscriptional regulation of gene expression | 43 | 7.31E-08 |
| GO:0042981 | Regulation of apoptosis | 111 | 7.51E-08 |
| GO:0043069 | Negative regulation of programmed cell death | 61 | 1.12E-07 |
GO, gene ontology.
Table 3.
| KEGG Pathway | Gene counts | P-value |
|---|---|---|
| hsa05200: Pathways in cancer | 76 | 1.00E-14 |
| hsa05215: Prostate cancer | 29 | 2.20E-09 |
| hsa05210: Colorectal cancer | 28 | 2.52E-09 |
| hsa05220: Chronic myeloid leukemia | 23 | 4.79E-07 |
| hsa05212: Pancreatic cancer | 22 | 9.72E-07 |
| hsa04115: p53 signaling pathway | 21 | 1.53E-06 |
| hsa05213: Endometrial cancer | 18 | 1.85E-06 |
| hsa05221: Acute myeloid leukemia | 19 | 2.17E-06 |
| hsa05218: Melanoma | 20 | 1.29E-05 |
| hsa04722: Neurotrophin signaling pathway | 28 | 1.44E-05 |
KEGG, Kyoto Encyclopedia of Genes and Genomes.
PPI pairs and PPI-miRNA network
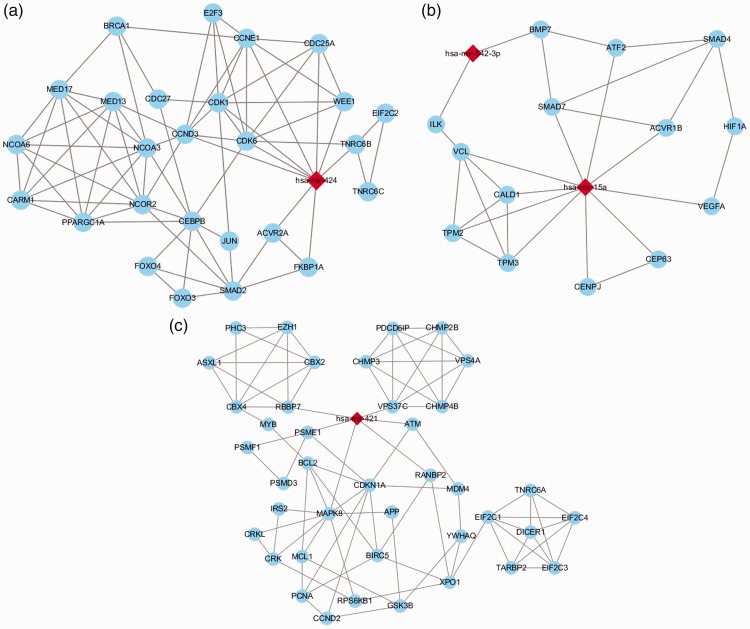
The genes targeted by the differentially expressed miRNAs were imported to the STRING database, and 2543 PPI pairs were obtained. Using the Cytoscape software, we merged the PPI network and miRNA-target-gene network into a whole PPI-miRNA network. Module clustering analysis of this network was then performed. Three significant modules containing miRNAs (miR-424, miR-421, miR-15a, and miR-542-3p) were obtained (Figure 2). Genes linking to miR-424 with the highest node scores (CDK1, CDK6, and CCND3) in module A (Figure 2a) were involved in biological processes related to cell growth and apoptosis, as mentioned above.
Figure 2.
Three protein-protein interaction (PPI)-miRNA modules were constructed using Cytoscape software. Modules A, B, and C involved miR-424, both miR-542-3p and miR-15a, and miR-421, respectively. Red diamonds represent up-regulated miRNAs, and blue ellipses represent target genes.
Discussion
AF is the leading cause of stroke and atrial appendage thrombus formation worldwide and is a major medical problem.1,2 In the present study, we used publicly available miRNA microarray data and novel bioinformatic approaches to predict the potential key miRNAs and genes associated with the pathogenesis of AF.
In our functional enrichment analysis, biological processes related to cell growth and apoptosis were highly enriched. Moreover, in the PPI-miRNA network, we showed that genes CDK1, CDK6, and CCND3, linked to miR-424, were involved in these biological processes, suggesting their role in the pathogenesis of AF.
The role of abnormal cell growth and apoptosis in AF and other heart diseases has been studied previously. Expression of certain genes associated with cell growth and the cell cycle has been found to be altered in patients with AF, especially in fibroblasts of the heart.21,22 AF is associated with structural remodeling of the atria, the hallmark of which is development and progression of atrial fibrosis, in which growth of fibroblasts is stimulated.23 In contrast, dedifferentiation of atrial myocytes has been found in AF, and this process is mediated by atrial fibroblast activation.24
CDK1 (Cyclin-dependent kinase 1) is a highly conserved protein that functions as a serine/threonine kinase and is a key player in cell cycle regulation.25 In an animal study, isoproterenol-induced cardiac fibrosis was shown to be associated with increased levels of CDK1 in fibroblasts exclusively in the adult mouse heart; thus, targeting CDK1 in the diseased heart may inhibit fibrosis and confer cardioprotection.26 Another study showed that estrogen loss is associated with cardiac fibrosis and diastolic dysfunction. GPR30 (G protein-coupled estrogen receptor) is expressed in rat cardiac fibroblasts, and activation of GPR30 limits proliferation of these cells by suppressing cell cycle proteins including CDK1.27
CDK6 is a member of the family of serine/threonine kinases and a key regulator during the G1/S cell cycle transition.28 Aberrant CDK6 expression has been reported in various cancer types,29–32 suggesting its involvement in aberrant cell cycle and uncontrolled cell growth. CDK6 has also been reported to be involved in certain heart diseases. For example, inflammation is associated with various cardiovascular diseases, of which C-reactive protein is a marker. C-reactive protein treatment of cardiac myocytes results in a significant reduction in the levels of CDK6 in a concentration-dependent manner.33 In turn, depression of CDK6 contributes to the effect of attenuation of miR-1 on provoking cardiomyocyte hypertrophy.34
CCND3, the gene encoding cyclin D3, is a regulator of progression through the G1 phase of the cell cycle. It is an oncogene in various B-cell lymphoma subtypes.35,36 Rho-associated coiled-coil kinases play a role in cardiac cell physiology and are also expressed in the developing heart, the blockade of which decreases expression of cell cycle proteins, including cyclin D3, in cardiomyocytes.37
MiR-424 plays a role in cell cycle regulation.38,39 It is reported to be involved in the differentiation of cardiac myocytes, and altered expression of miR-424 is associated with certain heart diseases, such as heart failure40 and tetralogy of Fallot.41 In our PPI-miRNA module A (Figure 2a), CDK1, CDK6, and CCND3 were co-regulated by miR-424. Hence, miR-424 may be a central regulator in the pathogenesis of AF.
Other miRNAs, such as miR-15a, miR-542-3p, and miR-421 (Figure 2b and 2c), were shown by our results to be possible key regulators in AF, although there was little evidence of direct associations with AF.
Circulating miR-15a has been found to be up-regulated in diffuse myocardial fibrosis.42 In addition, two genes (SMAD7 and VEGFA) directly linked to miR-15a in our module B (Figure 2b) are reported to be involved in the pathogenesis of AF. Degradation of Smad7 protein (possibly mediated by miR-21) may decrease the inhibitory feedback regulation of transforming growth factor (TGF)-β1/Smad signaling and serves as a key mechanism of AF-induced atrial fibrosis.43,44 In contrast, miR-15a has been shown to down-regulate the level of Smad7 in other diseases.45,46 Hence, miR-15a may play a role in AF-induced atrial fibrosis via the Smad7 pathway.
VEGFA is a member of the platelet-derived growth factor (PDGF)/vascular endothelial growth factor (VEGF) family. It induces proliferation and migration of vascular endothelial cells and is essential for both physiological and pathological angiogenesis. It has been reported that the plasma level of VEGF is higher in patients with AF and that the interaction among this angiogenic marker, platelets, and tissue factor (TF) may alter endothelial integrity, thereby contributing to the prothrombotic state in AF.47,48 In contrast, miR-15a level has been reported to be positively correlated with VEGFA level49,50 in other pathologic conditions. Thus, an elevated miR-15a level may predispose AF patients to thromboembolic events via the VEGFA pathway, and early changes in miR-15a level could be a new predictor of prognosis.
Finally, miR-542-3p and miR-421 have been shown to be related to certain cardiovascular diseases. MiR-542-3p displays antiangiogenic characteristics in endothelial cells.51 In a genetic mouse model of diabetes, miR-542-3p is down-regulated in the heart with diabetic cardiomyopathy.52 MiR-542-3p is significantly down-regulated in ischemic stroke and may be an important regulator in its pathological processes and development.53,54 Transcription factor E2F1-dependent miR-421 regulates mitochondrial fragmentation and myocardial infarction by targeting Pink1.55 Moreover, ACE2 (angiotensin converting enzyme 2) plays critical roles in several pathologies, including cardiovascular disease. Moreover, miR-421 seems to have the ability to down-regulate ACE2, which reveals a novel potential therapeutic target for cardiovascular diseases.56
In summary, our results suggest that miRNAs including miR-424, miR-421, miR-15a, and miR-542-3p as well as genes including CDK1, CDK6, and CCND3 may have potential as new biomarkers for AF. These miRNAs and genes may be involved in the pathogenesis of AF via cell cycle or apoptotic pathways and may be potential therapeutic targets for AF. However, this study has some limitations. The microarray data included only a small number of patients. The results are not yet supported by confirmatory laboratory experiments. Hence, further studies are needed to verify the clinical applications of these findings.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This work was funded by grants from the National Natural Science Foundation of China (grant no. 81601711) and the Natural Scientific Foundation of Guangdong Province (grant no. 2016A030313255) to Zhongxing Wang. The funding sources had no involvement in study design; collection, analysis, and interpretation of data; writing the report; or the decision to submit the article for publication.
References
- 1.Della Bella P, Pasquale V. Faculty of 1000 evaluation for Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation Internet. F1000 - Post-publication peer review of the biomedical literature. Available from: 10.3410/f.718304413.793492095. [DOI]
- 2.Kannel WB, Wolf PA, Benjamin EJ, et al. Prevalence, incidence, prognosis, and predisposing conditions for atrial fibrillation: population-based estimates. Am J Cardiol 1998; 82: 2N–9N. [DOI] [PubMed] [Google Scholar]
- 3.Andersson T, Magnuson A, Bryngelsson IL, et al. All-cause mortality in 272,186 patients hospitalized with incident atrial fibrillation 1995-2008: a Swedish nationwide long-term case-control study. Eur Heart J 2013; 34: 1061–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benjamin EJ, Wolf PA, D’Agostino RB, et al. Impact of atrial fibrillation on the risk of death: the Framingham heart study. Circulation 1998; 98: 946–952. [DOI] [PubMed] [Google Scholar]
- 5.Ambros V. The functions of animal microRNAs. Nature 2004; 431: 350–355. [DOI] [PubMed] [Google Scholar]
- 6.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 2004; 116: 281–297. [DOI] [PubMed] [Google Scholar]
- 7.Luo X, Pan Z, Shan H, et al. MicroRNA-26 governs profibrillatory inward-rectifier potassium current changes in atrial fibrillation. J Clin Invest 2013; 123: 1939–1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McManus DD, Freedman JE. MicroRNAs in platelet function and cardiovascular disease. Nat Rev Cardiol 2015; 12: 711–717. [DOI] [PubMed] [Google Scholar]
- 9.Carè A, Catalucci D, Felicetti F, et al. MicroRNA-133 controls cardiac hypertrophy. Nat Med 2007; 13: 613–618. [DOI] [PubMed] [Google Scholar]
- 10.Yang B, Lin H, Xiao J, et al. The muscle-specific microRNA miR-1 regulates cardiac arrhythmogenic potential by targeting GJA1 and KCNJ2. Nat Med 2007; 13: 486–491. [DOI] [PubMed] [Google Scholar]
- 11.Feng B, Chen S, George B, et al. miR133a regulates cardiomyocyte hypertrophy in diabetes. Diabetes Metab Res Rev 2010; 26: 40–49. [DOI] [PubMed] [Google Scholar]
- 12.Galenko O, Jacobs V, Knight S, et al. The role of microRNAs in the development, regulation, and treatment of atrial fibrillation. J Interv Card Electrophysiol 2019. 10.1007/s10840-018-0495-z. [DOI] [PubMed] [Google Scholar]
- 13.van den Berg NWE, Kawasaki M, Berger WR, et al. MicroRNAs in atrial fibrillation: from expression signatures to functional implications. Cardiovasc Drugs Ther 2017; 31: 345–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang Z, Chen XJ, Qian C, et al. Signal transducer and activator of transcription 3/MicroRNA-21 feedback loop contributes to atrial fibrillation by promoting atrial fibrosis in a rat sterile pericarditis model. Circ Arrhythm Electrophysiol 2016; 9: pii: e003396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hsieh CS, Huang PS, Chang SN, et al. Genome-wide copy number variation association study of atrial fibrillation related thromboembolic stroke. J Clin Med 2019; 8: pii: E332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smyth GK. limma: linear models for microarray data Statistics for Biology and Health. pp. 397–420. [Google Scholar]
- 17.Fan Y, Siklenka K, Arora SK, et al. miRNet - dissecting miRNA-target interactions and functional associations through network-based visual analysis. Nucleic Acids Res 2016; 44: W135–W141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang DW, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc 2009; 4: 44–57. [DOI] [PubMed] [Google Scholar]
- 19.Huang DW, Sherman BT, Lempicki RA. Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res 2009; 37: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Szklarczyk D, Franceschini A, Wyder S, et al. STRING v10: protein-protein interaction networks, integrated over the tree of life. Nucleic Acids Res 2015; 43: D447–D452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Qi XY, Huang H, Ordog B, et al. Fibroblast inward-rectifier potassium current upregulation in profibrillatory atrial remodeling. Circ Res 2015; 116: 836–845. [DOI] [PubMed] [Google Scholar]
- 22.Ohki R, Yamamoto K, Ueno S, et al. Gene expression profiling of human atrial myocardium with atrial fibrillation by DNA microarray analysis. Int J Cardiol 2005; 102: 233–238. [DOI] [PubMed] [Google Scholar]
- 23.Dzeshka MS, Lip GYH, Snezhitskiy V, et al. Cardiac fibrosis in patients with atrial fibrillation: mechanisms and clinical implications. J Am Coll Cardiol 2015; 66: 943–959. [DOI] [PubMed] [Google Scholar]
- 24.Rücker-Martin C, Pecker F, Godreau D, et al. Dedifferentiation of atrial myocytes during atrial fibrillation: role of fibroblast proliferation in vitro. Cardiovasc Res 2002; 55: 38–52. [DOI] [PubMed] [Google Scholar]
- 25.Young PG. The cell cycle: principles of control. Primers in biology. By David O Morgan. London (United Kingdom): New Science Press; distributed by Sinauer Associates, Sunderland (Massachusetts). $49.95 (paper). xxvii 297 p; ill.; index. ISBN: 0-87893-508-8. 2007. Q Rev Biol 2008; 83: 113–113. [Google Scholar]
- 26.Gaspard GJ, MacLean J, Rioux D, et al. A novel β-adrenergic response element regulates both basal and agonist-induced expression of cyclin-dependent kinase 1 gene in cardiac fibroblasts. Am J Physiol Cell Physiol 2014; 306: C540–C550. [DOI] [PubMed] [Google Scholar]
- 27.Wang H, Zhao Z, Lin M, et al. Activation of GPR30 inhibits cardiac fibroblast proliferation. Mol Cell Biochem 2015; 405: 135–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Malumbres M, Marcos M, Mariano B. Mammalian cyclin-dependent kinases. Trends Biochem Sci 2005; 30: 630–641. [DOI] [PubMed] [Google Scholar]
- 29.Lee KH, Lotterman C, Karikari C, et al. Epigenetic silencing of MicroRNA miR-107 regulates cyclin-dependent kinase 6 expression in pancreatic cancer. Pancreatology 2009; 9: 293–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chilosi M, Doglioni C, Yan Z, et al. Differential expression of cyclin-dependent kinase 6 in cortical thymocytes and T-cell lymphoblastic lymphoma/leukemia. Am J Pathol 1998; 152: 209–217. [PMC free article] [PubMed] [Google Scholar]
- 31.Costello JF, Plass C, Arap W, et al. Cyclin-dependent kinase 6 (CDK6) amplification in human gliomas identified using two-dimensional separation of genomic DNA. Cancer Res 1997; 57: 1250–1254. [PubMed] [Google Scholar]
- 32.Mendrzyk F, Radlwimmer B, Joos S, et al. Genomic and protein expression profiling identifies CDK6 as novel independent prognostic marker in medulloblastoma. J Clin Oncol 2005; 23: 8853–8862. [DOI] [PubMed] [Google Scholar]
- 33.Choi JW, Lee KH, Kim SH, et al. C-reactive protein induces p53-mediated cell cycle arrest in H9c2 cardiac myocytes. Biochem Biophys Res Commun 2011; 410: 525–530. [DOI] [PubMed] [Google Scholar]
- 34.Yuan W, Tang C, Zhu W, et al. CDK6 mediates the effect of attenuation of miR-1 on provoking cardiomyocyte hypertrophy. Mol Cell Biochem 2016; 412: 289–296. [DOI] [PubMed] [Google Scholar]
- 35.Sonoki T. Cyclin D3 is a target gene of t(6; 14)(p21.1; q32.3) of mature B-cell malignancies. Blood 2001; 98: 2837–2844. [DOI] [PubMed] [Google Scholar]
- 36.Kasugai Y, Tagawa H, Kameoka Y, et al. Identification of CCND3 and BYSL as candidate targets for the 6p21 amplification in diffuse large B-cell lymphoma. Clin Cancer Res 2005; 11: 8265–8272. [DOI] [PubMed] [Google Scholar]
- 37.Zhao Z, Rivkees SA. Rho-associated kinases play an essential role in cardiac morphogenesis and cardiomyocyte proliferation. Dev Dyn 2003; 226: 24–32. [DOI] [PubMed] [Google Scholar]
- 38.Finnerty JR, Wang WX, Hébert SS, et al. The miR-15/107 group of microRNA genes: evolutionary biology, cellular functions, and roles in human diseases. J Mol Biol 2010; 402: 491–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kozomara A, Griffiths-Jones S. miRBase: integrating microRNA annotation and deep-sequencing data. Nucleic Acids Res 2011; 39: D152–D157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Marques FZ, Vizi D, Khammy O, et al. The transcardiac gradient of cardio-microRNAs in the failing heart. Eur J Heart Fail 2016; 18: 1000–1008. [DOI] [PubMed] [Google Scholar]
- 41.Zhang J, Chang JJ, Xu F, et al. MicroRNA deregulation in right ventricular outflow tract myocardium in nonsyndromic tetralogy of Fallot. Can J Cardiol 2013; 29: 1695–1703. [DOI] [PubMed] [Google Scholar]
- 42.Fang L, Ellims AH, Moore XL, et al. Circulating microRNAs as biomarkers for diffuse myocardial fibrosis in patients with hypertrophic cardiomyopathy. J Transl Med 2015; 13: 314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.He X, Gao X, Peng L, et al. Atrial fibrillation induces myocardial fibrosis through angiotensin II type 1 receptor-specific Arkadia-mediated downregulation of Smad7. Circ Res 2011; 108: 164–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.He X, Zhang K, Gao X, et al. Rapid atrial pacing induces myocardial fibrosis by down-regulating Smad7 via microRNA-21 in rabbit. Heart Vessels 2016; 31: 1696–1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu N, Jiao T, Huang Y, et al. Hepatitis B virus regulates apoptosis and tumorigenesis through the microRNA-15a-Smad7-transforming growth factor beta pathway. J Virol 2015; 89: 2739–2749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang Y. The inhibition of microRNA-15a suppresses hepatitis B virus-associated liver cancer cell growth through the Smad/TGF-β pathway. Oncol Rep 2017; 37: 3520–3526. [DOI] [PubMed] [Google Scholar]
- 47.Choudhury A, Freestone B, Patel J, et al. Relationship of soluble CD40 ligand to vascular endothelial growth factor, angiopoietins, and tissue factor in atrial fibrillation: a link among platelet activation, angiogenesis, and thrombosis? Chest 2007; 132: 1913–1919. [DOI] [PubMed] [Google Scholar]
- 48.Freestone B, Chong AY, Lim HS, et al. Angiogenic factors in atrial fibrillation: a possible role in thrombogenesis? Ann Med 2005; 37: 365–372. [DOI] [PubMed] [Google Scholar]
- 49.Shang J, He Q, Chen Y, et al. miR-15a-5p suppresses inflammation and fibrosis of peritoneal mesothelial cells induced by peritoneal dialysis via targeting VEGFA. J Cell Physiol 2019; 234: 9746–9755. [DOI] [PubMed] [Google Scholar]
- 50.Nakano T, Inoue Y, Shimojo N, et al. Lower levels of hsa-mir-15a, which decreases VEGFA, in the CD4+ T cells of pediatric patients with asthma. J Allergy Clin Immunol 2013; 132: 1224–1227. [DOI] [PubMed] [Google Scholar]
- 51.Luo SX, Su-Xia L, Bei-Bei C, et al. Decreased expression of miR-542-3p exerts growth inhibitory functions in esophageal cancer. J Cancer Res Ther 2015; 11: 24. [DOI] [PubMed] [Google Scholar]
- 52.Chavali V, Tyagi SC, Mishra PK. Differential expression of dicer, miRNAs, and inflammatory markers in diabetic Ins2+/− Akita hearts. Cell Biochem Biophys 2014; 68: 25–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.He T, Qi F, Jia L, et al. MicroRNA-542-3p inhibits tumour angiogenesis by targeting angiopoietin-2. J Pathol 2014; 232: 499–508. [DOI] [PubMed] [Google Scholar]
- 54.He W, Chen S, Chen X, et al. Bioinformatic analysis of potential microRNAs in ischemic stroke. J Stroke Cerebrovasc Dis 2016; 25: 1753–1759. [DOI] [PubMed] [Google Scholar]
- 55.Wang K, Zhou LY, Wang JX, et al. E2F1-dependent miR-421 regulates mitochondrial fragmentation and myocardial infarction by targeting Pink1. Nat Commun 2015; 6: 7619. [DOI] [PubMed] [Google Scholar]
- 56.Lambert DW, Lambert LA, Clarke NE, et al. Angiotensin-converting enzyme 2 is subject to post-transcriptional regulation by miR-421. Clin Sci 2014; 127: 243–249. [DOI] [PubMed] [Google Scholar]