Short abstract
Objective
Hypertension is a risk factor for development of white matter hyperintensities (WMHs). However, the relationship between hypertension and WMHs remains obscure. We sought to clarify this relationship using clinical data from different regions of China.
Methods
We analyzed the data of 333 patients with WMHs in this study. All included patients underwent conventional magnetic resonance imaging (MRI) examination. A primary diagnosis of WMHs was made according to MRI findings. The volume burden of WMHs was investigated using the Fazekas scale, which is widely used to rate the degree of WMHs. We conducted retrospective clinical analysis of the data in this study.
Results
Our findings showed that WMHs in patients with hypertension were associated with diabetes, cardiovascular diseases, history of cerebral infarct, and plasma glucose and triglyceride levels. Fazekas scale scores for WMHs increased with increased blood pressure values in patients with hypertension.
Conclusion
This analysis indicates that hypertension is an independent contributor to the prevalence and severity of WMHs.
Keywords: Prevalence, relevance, risk factor, white matter hyperintensities, hypertension, magnetic resonance imaging
Introduction
White matter hyperintensities (WMHs) refer to increased hyperintensity signals detected on T2-weighted magnetic resonance imaging (T2-MRI), fluid-attenuated inversion recovery (FLAIR), and proton density MRI sequences. WMHs are often considered white matter changes or white matter lesions as they present with hypointensities on computed tomography in areas of cerebral white matter. A variety of imaging and clinical studies have shown that WMHs are closely linked with subtle functional impairment, cognitive impairment, dementia, and a higher prevalence of neuropsychiatric disorders such as major depressive disorder, bipolar disorder, and schizophrenia.1–3 A close association has been identified between WMHs and dementia and neurodegenerative diseases, including Alzheimer disease and Parkinson disease.4–6
WMHs have a detrimental role in the onset and development of cognitive impairment and dementia.7 The presence of WMHs prior to mild cognitive impairment implies an increased risk for dementia.8 Growing evidence has shown that the presence of WMHs is correlated with high-risk factors of cerebrovascular diseases including age,9 hypertension,10 diabetes mellitus,11 hyperhomocysteinemia,12 hyperlipidemia,13 hypersensitive C-reactive protein,14 smoking, and other unhealthy lifestyle factors.15,16 Age is most closely related to the onset and development of WMHs, which are commonly observed in the brain of elderly individuals and can also be found in middle-aged individuals.17 The second strongest risk factor for WMHs is hypertension. WMHs appear to mediate the association of hypertension with concurrent cognitive impairment and the mechanisms of ischemia, micro-hemorrhages, cerebral small vessel damage, dysfunction of the blood–brain barrier, and/or deformation of the myelin sheath.18 Research results strongly support a linear relationship between blood pressure values and the volume of WMHs. Recent multivariable regression analyses have shown a significant relationship between WMH volume and hypertension, diabetes, smoking, and education levels.19 However, the relationship between the volume of WMHs and hypertension remains obscure. Therefore, it is essential to clarify this relationship using additional clinical data. In the current study, we investigated clinical data of WMHs and the relationship between hypertension and WMHs, in an analysis of the association between blood pressure values in patients with hypertension and the volume of WMHs.
Methods
Study population and design
This retrospective cohort analysis was performed in Renmin Hospital, Hubei University of Medicine in Shiyan, Hubei Province, China (serving approximately 80,000 inpatients and 1,000,000 outpatients per year), and the Examination Center of Magnetic Resonance Imaging of the Fourth Affiliated Hospital of Harbin Medical University (serving approximately 100,000 inpatients and 1,500,000 outpatients per year). WMHs were diagnosed using MRI examination and evidence of WMHs on T2-MRI. Controls were patients with no findings of WMHs on T2-MRI. Each diagnosis was extracted from hospital medical records. This retrospective analysis of clinical data was approved by our institutional medical ethics committee. The analysis was executed in accordance with the relevant guidelines and regulations. The requirement for informed consent was waived owing to the retrospective nature of this analysis.
Data collection
We randomly selected patients with WMHs and obtained the MRI data of all patients. Included patients underwent conventional MRI examination, including T1- and T2-weighted gradient-echo imaging (GRE), T2-weighted FLAIR, or diffusion-weighted MRI (DWI) sequences. The following patients were excluded: (1) ethnic origin other than Han Chinese; (2) WMHs owing to brain injury; (3) WMHs owing to brain infectious diseases; (4) WMHs owing to brain immunological diseases; (5) WMHs owing to brain congenital diseases; and (4) WMHs owing to other abnormal aging processes in the brain. The clinical and demographic characteristics of all patients were obtained from the MRI Examination Centre and Hospital Medical Records Information Management System, including age, sex, risk factors, initial vital signs, symptoms, laboratory findings, and clinical outcomes.
We investigated the volume burden of WMHs using the Fazekas scale, which is widely used to rate the degree of WMHs.20,21 WMHs are divided into periventricular hyperintensities (PVH) and deep white-matter hyperintensities (DWMH). PVH and DWMH were considered separately and rated from 0–3 on a 4-point scale (Table 1). Each case in this study had a score of at least 1 for either PVH or DWMH. The hypertension grades used in this study were as follows (in mmHg): grade 1: 140–159 (systolic blood pressure, SBP) and 90–99 (diastolic blood pressure, DBP), grade 2: 160–179 (SBP) and 100–109 (DBP), and grade 3: above 180 (SBP) and above 110 (DBP).
Table 1.
The Fazekas scale.
| Fazekas scale score | PVH | DWMH |
|---|---|---|
| 0 | Absent | Absent |
| 1 | Caps or pencil-thin lining around ventricles | Punctate foci |
| 2 | Smooth halo around ventricles (6–10 mm regular margins) | Beginning confluence |
| 3 | Irregular halo (>10 mm) | Large confluent areas |
PVH: periventricular hyperintensities, DWMH: deep white matter hyperintensities.
Statistical analysis
Statistical analysis was performed using GraphPad Prism 5 (GraphPad Software, San Diego, CA, USA) and Microsoft Excel spreadsheets (Microsoft Corporation, Redmond, WA, USA). For parameters with normal distributions and homogeneous variances, significant differences between the mean values in two groups were verified using a Student t-test. A chi-squared test was used to analyze the associations between two different variables. A P-value of less than 0.05 was taken to indicate statistical significance.
Results
Spatial distribution features of WMHs
We analyzed the data of 333 patients (aged 20 to 86 years) with WMHs in this study. Features of the spatial distribution of WMHs are described in Table 2. The most common location of WMHs in this study was the frontal lobe, based on the MRI findings. This was followed by, in descending order, the basal ganglia, parietal lobe, semiovale center, pons, temporal lobe, thalamus, occipital lobe, corona radiate, brainstem, opisthencephalon, and insular lobe.
Table 2.
Spatial distribution features of white matter hyperintensities.
| Imaging features | Cases (n=333) | Percentage (%) |
|---|---|---|
| Frontal lobe | 188 | 56.46 |
| Basal ganglia | 146 | 43.84 |
| Parietal lobe | 125 | 37.54 |
| Semiovale center | 41 | 12.31 |
| Pons | 31 | 9.31 |
| Temporal lobe | 24 | 7.21 |
| Thalamus | 22 | 6.61 |
| Occipital lobe | 11 | 3.30 |
| Corona radiata | 10 | 3.00 |
| Brainstem | 5 | 1.50 |
| Opisthencephalon | 4 | 1.20 |
| Insular lobe | 2 | 0.60 |
Evaluation of WMH variables
Among the 333 patients with WMHs, 169 had hypertension and 164 did not have hypertension. Table 3 demonstrates that WMHs in patients with hypertension were associated with the following factors: diabetes, cardiovascular diseases, history of cerebral infarct, and plasma glucose and triglyceride levels.
Table 3.
Variables of patients with white matter hyperintensities.
| Variables | All (n = 333) | Without hypertension (n = 164) | With hypertension (n = 169) | P-value |
|---|---|---|---|---|
| Age (y), mean ± SD | 63.00 ± 11.85 | 62.00 ± 12.23 | 63.96 ± 11.42 | 0.670 |
| Men, n (%) | 159 (47.75) | 80 (48.78) | 79 (46.75) | 0.710 |
| Smoking, n (%) | 75 (22.52) | 34 (20.73) | 41 (24.26) | 0.441 |
| Drinking, n (%) | 110 (33.03) | 58 (35.37) | 52 (30.77) | 0.373 |
| Hyperlipidemia, n (%) | 14 (4.20) | 9 (5.49) | 5 (2.96) | 0.25 |
| Diabetes mellitus, n (%) | 48 (14.41) | 12 (7.32) | 36 (21.30) | <0.05 |
| Cardiovascular diseases, n (%) | 88 (26.43) | 25 (15.24) | 63 (37.28) | <0.05 |
| History of cerebral infarct, n (%) | 70 (21.02) | 22 (13.41) | 48 (28.40) | <0.05 |
| SBP (mmHg), mean ± SD | 147.77 ± 30.83 | 123.00 ± 9.87 | 177.88 ± 19.79 | <0.05 |
| DBP (mmHg), mean ± SD | 87.53 ± 15.91 | 76.46 ± 8.49 | 101.18 ± 11.77 | <0.05 |
| Pulse pressure (mmHg), mean ± SD | 64.10 ± 20.47 | 47.62 ± 9.29 | 76.92 ± 17.39 | <0.05 |
| Fasting plasma glucose (mmol/L), mean ± SD | 5.82 ± 2.05 | 5.57 ± 1.69 | 6.05 ± 2.32 | <0.05 |
| LDL-C (mmol/L), mean ± SD | 2.45 ± 0.80 | 2.45 ± 0.72 | 2.45 ± 0.87 | 0.960 |
| HDL-C (mmol/L), mean ± SD | 1.30 ± 0.38 | 1.34 ± 0.37 | 1.27 ± 0.31 | 0.118 |
| Total cholesterol (mmol/L), mean ± SD | 4.11 ± 1.07 | 4.11 ± 0.89 | 4.11 ± 1.21 | 0.963 |
| Triglyceride (mmol/L), mean ± SD | 1.46 ± 1.08 | 1.38 ± 0.87 | 4.09 ± 0.23 | <0.05 |
SBP: systolic blood pressure, DBP: diastolic blood pressure, LDL-C: low-density lipoprotein cholesterol, HDL-C: high-density lipoprotein cholesterol.
Increased WMH volume with increased blood pressure values in patients with hypertension
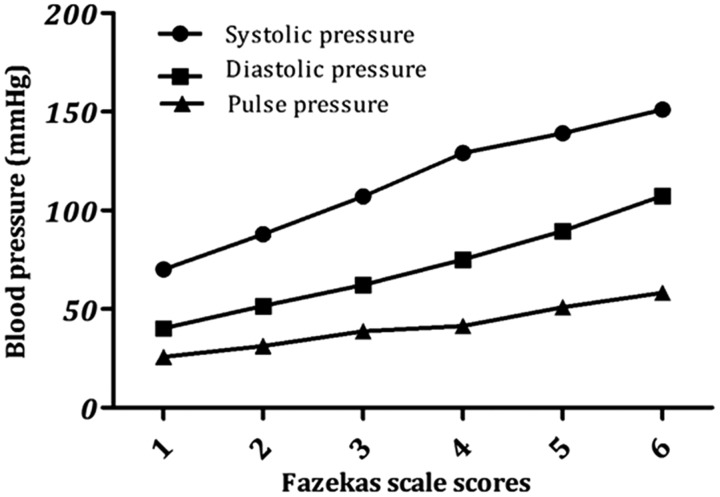
Table 4 presents the distribution of Fazekas scale scores. Figure 1 shows that scores on the Fazekas scale increased with blood pressure values in patients with hypertension, including systolic, diastolic, and pulse pressure (P < 0.0001). The same results were found using a chi-squared test (Tables 5 and 6). The data in the tables show that Fazekas scale scores were significantly different in each hypertension grade, indicating that the volume of WMHs is related to blood pressure in patients with hypertension.
Table 4.
Distribution of Fazekas scale scores.
| Fazekas scale score | Cases (n=333) | Proportion (%) |
|---|---|---|
| 1 | 98 | 29.43 |
| 2 | 81 | 24.32 |
| 3 | 60 | 18.02 |
| 4 | 39 | 11.71 |
| 5 | 30 | 9.01 |
| 6 | 25 | 7.51 |
Figure 1.
Fazekas scale scores for white matter hyperintensities (WMHs) increase with blood pressure values.
Table 5.
Relationship between Fazekas scale score and hypertension grade.
| Hypertension grade | n |
Fazekas scale score |
|||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | ||
| 1 | 92 | 77 | 9 | 3 | 2 | 1 | 1 |
| 2 | 117 | 35 | 28 | 26 | 16 | 8 | 4 |
| 3 | 124 | 14 | 20 | 31 | 33 | 16 | 10 |
| Chi-squared | 134.7 | ||||||
| P-value | <0.0001 | ||||||
Table 6.
Comparison of different hypertension grades according to Fazekas scale score.
| Hypertension grade | n |
Fazekas scale score |
|||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | ||
| 1 | 92 | 77 | 9 | 3 | 2 | 1 | 1 |
| 2 | 117 | 35 | 28 | 26 | 16 | 8 | 4 |
| Chi-squared | 59.92 | ||||||
| P-value | <0.0001 | ||||||
| 2 | 117 | 35 | 28 | 26 | 16 | 8 | 4 |
| 3 | 124 | 14 | 20 | 31 | 33 | 16 | 10 |
| Chi-squared | 21.72 | ||||||
| P-value | 0.0006 | ||||||
| 1 | 92 | 77 | 9 | 3 | 2 | 1 | 1 |
| 3 | 124 | 14 | 20 | 31 | 33 | 16 | 10 |
| Chi-squared | 116.9 | ||||||
| P-value | <0.0001 | ||||||
Discussion
WMHs refer to a hyperintense signal on T2-MRI, FLAIR, or proton density MRI sequences in cerebral white matter. There are numerous existing studies suggesting that the occurrence and development of WMHs are associated with many risk factors, such as age, hypertension, obesity, diabetes, hyperhomocysteinemia cardiovascular diseases, and unhealthy lifestyle.16,22,23 The findings of the present clinical retrospective analysis suggested that the severity of WMHs, according to volume burden measured using the Fazekas scale, increases with increased blood pressure values in patients with hypertension.
WMHs were previously known as leukoaraiosis.24,25 Leukoaraiosis is derived from “araiosis” or rarefaction of white matter. However, the current ability to identify or assign weights to specific causes and pathologies was previously lacking. There are many knowledge gaps in the understanding of this radiological finding, although some authors have used the label “ischemic leukoaraiosis”. Hypertension enhances brain WMH changes in which demyelination and axonal degeneration predominate, in addition to changes in neurotransmitter systems, corticosteroids, the microvascular environment, and calcium homeostasis in the late stages of age-related diseases.7,26 In particular, early changes in interstitial fluid and water content in patients with hypertension have been investigated,27,28 conditions that may finally lead to demyelination, axonal damage, and even atrophy.
There is strong evidence that hypertension is an independent risk factor for the occurrence and development of WMHs.16,29 Subtle hemodynamic abnormalities and reduced blood flow appear to occur in cerebral white matter during the early stages of hypertension.30 Studies have shown that a higher WMH burden in individuals with hypertension is associated with lower perfusion in areas with WMHs.31,32 Some findings suggest that microstructural white matter alterations develop over the course of hypertension and may persist, despite adequate treatment.33,34 Our investigation further confirmed that hypertension is an independent contributor to the prevalence and progression of WMHs in Chinese patients. These clinical results revealed that hypertension, together with other vascular risk factors, promotes the occurrence of WMHs. It is important to note that these results are generally consistent with the research data on the relationship between hypertension and WMHs.35 Taken together, these results suggest that the prevention and management of hypertension may be vital to preserving brain health in aging, as well as slowing down the progression of WMHs.33
WMHs may be regarded as an inflammatory microvascular encephalopathy that is associated with major degenerative factors including hypertension, diabetes, ischemia, stroke, dementia, and loss of cognitive function.36,37 The presence of WMHs also doubles the risk of dementia and triples the risk of stroke, highlighting the public health importance of the research findings.7,37 WMHs are linked with vascular risk factors, including smoking, diabetes, and higher levels of homocysteine. The clinical status of individuals with different risk factors could explain much of the heterogeneity in WMHs and clinical syndromes.37 The SPRINT MIND study reported that intensive lowering of blood pressure could prevent mild cognitive impairment, in concert with slower progression of WMHs.38 Therefore, it is important to lower risk factors in general, for the prevention and management of WMHs at population level, in addition to intensive lowering of blood pressure in appropriate groups.
Overall, the findings of this investigation supported that hypertension is an independent contributor to the prevalence and severity of WMHs. The present analysis indicates that the prevention and management of hypertension will be of great importance in preserving brain health during aging and in slowing the progression of WMHs. Lowering hypertension as well as other risk factors will generally yield significant benefits in the prevention and management of WMHs at population level. However, longitudinal studies, such as a prospective multicenter randomized controlled trial, are needed to confirm these findings. Because this study was not a randomized controlled trial, there may be bias present in participant selection and stratification. This was a retrospective analysis with a small sample size; therefore, the results cannot be extrapolated to all patients with hypertension. Further studies using longitudinal data are needed to confirm the temporal sequence of events and the present conclusions to clarify the exact association between WMH volume and blood pressure values in patients with hypertension.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This work was supported by grants from the Natural Science Foundation of Hubei Province (2015CFB260), the Hubei Province Health and Family Planning Scientific Research Project (WJ2015MB219), and the Shiyan Natural Science of Renmin Hospital, Hubei University of Medicine, to Dr Zhiyou Cai.
References
- 1.Chai YL, Hilal S, Chong JP, et al. Growth differentiation factor-15 and white matter hyperintensities in cognitive impairment and dementia. Medicine (Baltimore) 2016; 95: e4566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Prins ND, Scheltens P. White matter hyperintensities, cognitive impairment and dementia: an update. Nat Rev Neurol 2015; 11: 157–165. [DOI] [PubMed] [Google Scholar]
- 3.Jorgensen KN, Nerland S, Norbom LB, et al. Increased MRI-based cortical grey/white-matter contrast in sensory and motor regions in schizophrenia and bipolar disorder. Psychol Med 2016; 46: 1971–1985. [DOI] [PubMed] [Google Scholar]
- 4.Koncz R, Sachdev PS. Are the brain's vascular and Alzheimer pathologies additive or interactive? Curr Opin Psychiatry 2018; 31: 147–152. [DOI] [PubMed] [Google Scholar]
- 5.Ogama N, Sakurai T, Saji N, et al. Frontal white matter hyperintensity is associated with verbal aggressiveness in elderly women with Alzheimer disease and amnestic mild cognitive impairment. Dement Geriatr Cogn Dis Extra 2018; 8: 138–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dadar M, Zeighami Y, Yau Y, et al. White matter hyperintensities are linked to future cognitive decline in de novo Parkinson's disease patients. Neuroimage Clin 2018; 20: 892–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hase Y, Horsburgh K, Ihara M, et al. White matter degeneration in vascular and other ageing-related dementias. J Neurochem 2018; 144: 617–633. [DOI] [PubMed] [Google Scholar]
- 8.Joki H, Higashiyama Y, Nakae Y, et al. White matter hyperintensities on MRI in dementia with Lewy bodies, Parkinson's disease with dementia, and Alzheimer's disease. J Neurol Sci 2018; 385: 99–104. [DOI] [PubMed] [Google Scholar]
- 9.Zhuang FJ, Chen Y, He WB, et al. Prevalence of white matter hyperintensities increases with age. Neural Regen Res 2018; 13: 2141–2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zerna C, Yu AYX, Modi J, et al. Association of white matter hyperintensities with short-term outcomes in patients with minor cerebrovascular events. Stroke 2018; 49: 919–923. [DOI] [PubMed] [Google Scholar]
- 11.van Bloemendaal L, Ijzerman RG, Ten Kulve JS, et al. Alterations in white matter volume and integrity in obesity and type 2 diabetes. Metab Brain Dis 2016; 31: 621–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wong A, Mok V, Fan YH, et al. Hyperhomocysteinemia is associated with volumetric white matter change in patients with small vessel disease. J Neurol 2006; 253: 441–447. [DOI] [PubMed] [Google Scholar]
- 13.Jimenez-Conde J, Biffi A, Rahman R, et al. Hyperlipidemia and reduced white matter hyperintensity volume in patients with ischemic stroke. Stroke 2010; 41: 437–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu YC. C-reactive protein and cerebral white matter lesions. Clin Neurol Neurosurg 2012; 114: 99–100. [DOI] [PubMed] [Google Scholar]
- 15.Avci AY, Lakadamyali H, Arikan S, et al. High sensitivity C-reactive protein and cerebral white matter hyperintensities on magnetic resonance imaging in migraine patients. J Headache Pain 2015; 16: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Basile AM, Pantoni L, Pracucci G, et al. Age, hypertension, and lacunar stroke are the major determinants of the severity of age-related white matter changes. The LADIS (Leukoaraiosis and Disability in the Elderly) Study. Cerebrovasc Dis 2006; 21: 315–322. [DOI] [PubMed] [Google Scholar]
- 17.Reed T, Kirkwood SC, DeCarli C, et al. Relationship of family history scores for stroke and hypertension to quantitative measures of white-matter hyperintensities and stroke volume in elderly males. Neuroepidemiology 2000; 19: 76–86. [DOI] [PubMed] [Google Scholar]
- 18.Hajjar I, Quach L, Yang F, et al. Hypertension, white matter hyperintensities, and concurrent impairments in mobility, cognition, and mood: the Cardiovascular Health Study. Circulation 2011; 123: 858–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Habes M, Erus G, Toledo JB, et al. White matter hyperintensities and imaging patterns of brain ageing in the general population. Brain 2016; 139: 1164–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang X, Ding L, Yang L, et al. Relationship between white matter hyperintensities penumbra and cavity formation. Med Sci Monit 2016; 22: 41–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sudo FK, Alves CE, Alves GS, et al. White matter hyperintensities, executive function and global cognitive performance in vascular mild cognitive impairment. Arq Neuropsiquiatr 2013; 71: 431–436. [DOI] [PubMed] [Google Scholar]
- 22.Muscari A, Faccioli L, Ghinelli M, et al. Hypertension and other determinants of white matter lesions in stroke patients. J Clin Hypertens (Greenwich) 2016; 18: 907–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.King KS, Peshock RM, Rossetti HC, et al. Effect of normal aging versus hypertension, abnormal body mass index, and diabetes mellitus on white matter hyperintensity volume. Stroke 2014; 45: 255–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ropele S, Seewann A, Gouw AA, et al. Quantitation of brain tissue changes associated with white matter hyperintensities by diffusion-weighted and magnetization transfer imaging: the LADIS (Leukoaraiosis and Disability in the Elderly) study. J Magn Reson Imaging 2009; 29: 268–274. [DOI] [PubMed] [Google Scholar]
- 25.Gouw AA, van der Flier WM, Fazekas F, et al. Progression of white matter hyperintensities and incidence of new lacunes over a 3-year period: the Leukoaraiosis and Disability study. Stroke 2008; 39: 1414–1420. [DOI] [PubMed] [Google Scholar]
- 26.Raz N, Rodrigue KM. Differential aging of the brain: patterns, cognitive correlates and modifiers. Neurosci Biobehav Rev 2006; 30: 730–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Francis F, Ballerini L, Wardlaw JM. Perivascular spaces and their associations with risk factors, clinical disorders and neuroimaging features: a systematic review and meta-analysis. Int J Stroke 2019; 1747493019830321. [DOI] [PubMed] [Google Scholar]
- 28.Shams S, Martola J, Charidimou A, et al. Topography and determinants of Magnetic Resonance Imaging (MRI)-visible perivascular spaces in a large memory clinic cohort. J Am Heart Assoc 2017; 6: pii: e006279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fennema-Notestine C, McEvoy LK, Notestine R, et al. White matter disease in midlife is heritable, related to hypertension, and shares some genetic influence with systolic blood pressure. Neuroimage Clin 2016; 12: 737–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang T, Li Y, Guo X, et al. Reduced perfusion in normal-appearing white matter in mild to moderate hypertension as revealed by 3D pseudocontinuous arterial spin labeling. J Magn Reson Imaging 2016; 43: 635–643. [DOI] [PubMed] [Google Scholar]
- 31.Pavlovic AM, Pekmezovic T, Trajkovic JZ, et al. Increased risk of cognitive impairment and more severe brain lesions in hypertensive compared to non-hypertensive patients with cerebral small vessel disease. J Clin Hypertens (Greenwich) 2018; 20: 1260–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ni R, Chu L, Xu D, et al. Risk factors of cerebral microbleeds in young and middle-aged patients with hypertension. Neurol Res 2018; 40: 413–418. [DOI] [PubMed] [Google Scholar]
- 33.McEvoy LK, Fennema-Notestine C, Eyler LT, et al. Hypertension-related alterations in white matter microstructure detectable in middle age. Hypertension 2015; 66: 317–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yau PL, Hempel R, Tirsi A, et al. Cerebral white matter and retinal arterial health in hypertension and type 2 diabetes mellitus. Int J Hypertens 2013; 2013: 329602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.de Leeuw FE, Richard F, de Groot JC, et al. Interaction between hypertension, apoE, and cerebral white matter lesions. Stroke 2004; 35: 1057–1060. [DOI] [PubMed] [Google Scholar]
- 36.Ryu WS, Woo SH, Schellingerhout D, et al. Grading and interpretation of white matter hyperintensities using statistical maps. Stroke 2014; 45: 3567–3575. [DOI] [PubMed] [Google Scholar]
- 37.Wardlaw JM, Valdes Hernandez MC, Munoz-Maniega S. What are white matter hyperintensities made of? Relevance to vascular cognitive impairment. J Am Heart Assoc 2015; 4: 001140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kjeldsen SE, Narkiewicz K, Burnier M, et al. Intensive blood pressure lowering prevents mild cognitive impairment and possible dementia and slows development of white matter lesions in brain: the SPRINT Memory and Cognition IN Decreased Hypertension (SPRINT MIND) study. Blood Press 2018; 27: 247–248. [DOI] [PubMed] [Google Scholar]