Abstract
A well-known side effect of statin therapy is myopathy. We report a case of statin induced necrotizing autoimmune myopathy, a rare variant of statin-induced myopathy. A 64-year-old gentleman on atorvastatin presented with muscle weakness. Initial laboratory results showed elevated liver function tests, a creatine phosphokinase (CPK) of 8200 IU/L, and positive urine myoglobin. Despite discontinuing atorvastatin, his CPK remained persistently elevated. Muscle biopsy was consistent with necrotizing myopathy. Anti-HMG CoA reductase antibody was strongly positive. Steroids followed by intravenous immunoglobulin were given. The patient’s muscle weakness, CPK, and liver functions gradually improved, and he was eventually discharged on oral steroids. Statin induced necrotizing autoimmune myopathy should be considered when discontinuing statin does not lead to muscle recovery and improvement in CPK. Diagnosis is confirmed by positive anti-HMG-CoA reductase autoantibody.
Keywords: Autoimmune myopathy, Myopathy, Statins
1. Learning objective
Statin-induced necrotizing autoimmune myopathy is a rare side effect of statin therapy characterized by muscle weakness and marked elevation of CPK. Diagnosis requires a high index of suspicion, especially with persistent elevation of CPK despite statin discontinuation. Diagnosis is confirmed by positive anti-HMG-CoA reductase autoantibody.
2. Introduction
Statins are among the most widely prescribed medications. They play a major role in reducing the incidence of cardiovascular diseases in susceptible patients. A well-known side effect of statin therapy is myopathy. We report a case of statin-induced necrotizing autoimmune myopathy (SINAM), a rare variant of statin-induced myopathy, that was successfully treated with steroids and immunosuppressive therapy.
3. Case report
A 64-year-old gentleman presented with muscle weakness for 4 months. His medical history was significant for hypertension, coronary artery disease, diabetes mellitus, and hyperlipidemia. Family history was noncontributory. His medications included aspirin, metoprolol, lisinopril, and insulin—in addition to atorvastatin, which he has been taking for 20 years. On initial evaluation, he was vitally stable. Results of his physical examination were significant for weakness in all extremities. Laboratory results showed elevated aspartate aminotransferase, 265 IU/L (reference range 10–40 IU/L); alanine aminotransferase, 398 IU/L (reference range 10–40 IU/L); creatine phosphokinase (CPK), 8200 IU/L (reference range 55–170 IU/L); creatinine, 1.3 (reference range 0.6–1.4 mg/dL); and positive urine myoglobin.
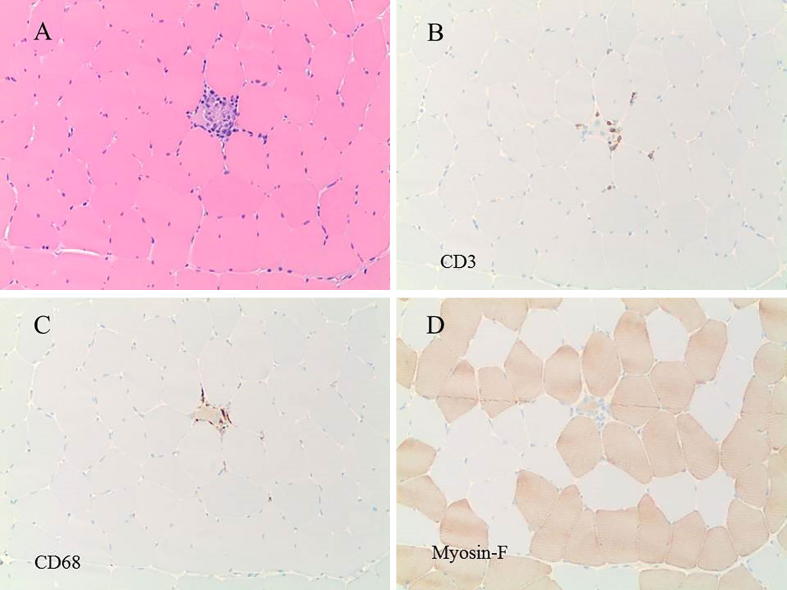
Despite discontinuing atorvastatin, his CPK remained persistently elevated. Tests for antinuclear antibody, anti-Jo 1 antibody, anti-double-stranded DNA antibody, anti-scl-70 antibody, and paraneoplastic autoantibodies were all negative. Computed tomography of the chest, abdomen, and pelvis was unremarkable, showing no evidence of malignancy. Electromyography (EMG) showed myopathy with fibrillations, suggestive of an inflammatory myopathy. The left thigh muscle was biopsied to determine the underlying myopathy. The biopsy showed focal necrotic fibers without significant endomysial inflammation (Fig. 1a). Immunohistochemistry reactions revealed rare T lymphocytes (positive for CD3; Fig. 1b), and macrophages (positive for CD68; Fig. 1c) near the necrotic fibers in the biopsy. Immunohistochemistry reaction for fast myosin showed a normal mosaic pattern of positive (type 2) and negative (type 1) fibers (Fig. 1d). These findings of a necrotizing myopathy without significant inflammation are indicative of a toxic–metabolic, or humoral mediated autoimmune injury, rather than a cell-mediated inflammatory myopathy (e.g., polymyositis or dermatomyositis). Test for anti-HMG CoA reductase antibody, ordered prior to the muscle biopsy, eventually was reported as strongly positive.
Figure 1.
(a) H&E-stained section of the muscle biopsy, showing a necrotic fiber with some inflammatory cells. (b) Immunohistochemistry reaction for CD3 revealed a few T lymphocytes around the necrotic fiber. (c) Immunohistochemistry reaction for CD68 revealed macrophages in the necrotic fiber. (d) Immunohistochemistry reaction for fast myosin showed a normal pattern of positive (type 2) and negative (type 1) fibers. H&E = hematoxylin and eosin.
A diagnosis of statin-induced necrotizing autoimmune myopathy (SINAM) was thus confirmed, and the patient was started on aggressive intravenous (IV) hydration, IV dexamethasone 50 mg every 6 hours for 3 days, followed by oral prednisone 70 mg (1 mg/kg/d). When IV fluids were slowed down and steroid therapy was complete, CPK began to rise again. He was started on IV immunoglobulins (IVIG) infusion 2 mg/kg/d for 3 days. His CPK improved and stabilized at about 1000; meanwhile, his creatinine was within normal range throughout the hospital stay. He was discharged home on prednisone 70 mg daily. However, his CPK started increasing again within 1 month. Methotrexate was started, and prednisone was tapered to 15 mg daily. His CPK normalized and his weakness improved dramatically within several weeks.
4. Discussion
Statins are notorious for causing toxic myopathy. An early study in 2008 [1] reported a 20% incidence of mild musculoskeletal problems in patients on statins. However, a more recent study in 2014 reported that myalgia occurred in approximately equal numbers in patients treated with statins versus placebo [2]. In rare cases, statins can cause more serious muscle damage such as rhabdomyolysis, with a reported incidence of three to seven per 100,000 patients on statin therapy per year [3]. In the majority of cases, the patients recovered spontaneously after the statins have been discontinued.
SINAM is an uncommon side effect of statin therapy and is thought to occur in two to three of every 100,000 patients on statins. Its pathophysiology remains unknown, but is thought to occur secondary to statin-induced autoimmunity against HMG-CoA reductase, the rate-limiting enzyme involved in cholesterol synthesis, in genetically susceptible individuals. Patients classically present with symmetrical proximal muscle weakness and pain, including muscle cramps, difficulty in climbing stairs, difficulty rising from a chair, and generalized weakness [4]. The onset is highly variable [5], ranging from weeks to years after initiation of statin therapy, as with our patient who was on a statin for 20 years without any reported side effects prior to this presentation.
Diagnosis of SINAM requires a high index of suspicion, especially with persistent elevation of CPK and nonimprovement in symptoms, despite discontinuing statin therapy. CPK is typically elevated, reaching >2000 IU/L in 90% of cases. EMG shows small-amplitude motor-unit potentials with increased spontaneous activity. Muscle edema may be present on magnetic resonance imaging [4]. Muscle biopsy typically reveals muscle cell necrosis, regeneration and cellular infiltrates composed mainly of macrophages, with small numbers of T lymphocytes (CD3+, CD4+, and CD8+), and CD123+ plasmacytoid dendritic cells [6]. Diagnosis is confirmed by testing for anti-HMG CoA reductase autoantibody. In antibody-negative patients, alternative diagnoses should be considered [4]. In rare cases, anti-HMG-CoA reductase autoantibodies may be positive in patients with autoimmune myopathy who have never been prescribed statin therapy [7].
Treatment centers on discontinuing statins indefinitely and initiating immunosuppressive therapy. Immunosuppressive therapy is essential, as only a few cases have reported improvement with discontinuation of statins alone [8]. Unfortunately, there are no randomized clinical trials to guide management. However, experts suggest starting with prednisone at a dose of 1 mg per kilogram of bodyweight [4]. Other immunosuppressants, such as methotrexate, azathioprine, or mycophenolate mofetil, are used as adjunctive therapy if myopathy does not improve or continues to progress. Nonresponders and more severe cases require IVIG or rituximab therapy. IVIG can also be used as monotherapy, and may even be considered as first-line therapy for diabetic patients [9]. Long-term treatment plans should be tailored for each patient. Immunosuppressive therapy is tapered when patients recover their full strength. Prognosis is usually good, although some patients may experience relapses and may require long-term treatment [10].
5. Conclusion
SINAM is a rare side effect of statin therapy characterized by symmetric proximal muscle weakness and marked elevation of CPK. Diagnosis requires a high index of suspicion, especially with persistent elevation of CPK despite statin discontinuation. Diagnosis is confirmed by positive anti-HMG-CoA reductase autoantibody. Treatment entails steroids, immunosuppressive therapy and discontinuation of statins indefinitely. Prognosis is usually good, although some patients may experience relapses and require long-term therapy.
Acknowledgments
Acknowledgment
The case was presented at the annual meeting of the American College of Physicians in 2018.
Conflict of interest
The authors declare that there is no conflict of interest.
Disclosure: Authors have nothing to disclose with regard to commercial support.
Footnotes
Peer review under responsibility of King Saud University.
References
- 1.Josan K., Majumdar S.R., McAlister F.A. The efficacy and safety of intensive statin therapy: a meta-analysis of randomized trials. Can Med Assoc J. 2008;178:576–584. doi: 10.1503/cmaj.070675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ganga H.V., Slim H.B., Thompson P.D. A systematic review of statin-induced muscle problems in clinical trials. Am Heart J. 2014;168:6–15. doi: 10.1016/j.ahj.2014.03.019. [DOI] [PubMed] [Google Scholar]
- 3.Law M., Rudnicka A.R. Statin safety: a systematic review. Am J Cardiol. 2006;97:52c–60c. doi: 10.1016/j.amjcard.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 4.Mammen A.L. Statin-associated autoimmune myopathy. New Engl J Med. 2016;374:664–669. doi: 10.1056/NEJMra1515161. [DOI] [PubMed] [Google Scholar]
- 5.Klein M., Mann H., Plestilova L., Zamecnik J., Betteridge Z., McHugh N. Increasing incidence of immune-mediated necrotizing myopathy: single-centre experience. Rheumatology (Oxford, England) 2015;54:2010–2014. doi: 10.1093/rheumatology/kev229. [DOI] [PubMed] [Google Scholar]
- 6.Chung T., Christopher-Stine L., Paik J.J., Corse A., Mammen A.L. The composition of cellular infiltrates in anti-HMG-CoA reductase-associated myopathy. Muscle Nerve. 2015;52:189–195. doi: 10.1002/mus.24642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Christopher-Stine L., Casciola-Rosen L.A., Hong G., Chung T., Corse A.M., Mammen A.L. A novel autoantibody recognizing 200-kd and 100-kd proteins is associated with an immune-mediated necrotizing myopathy. Arthritis Rheum. 2010;62:2757–2766. doi: 10.1002/art.27572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Allenbach Y., Drouot L., Rigolet A., Charuel J.L., Jouen F., Romero N.B. Anti-HMGCR autoantibodies in European patients with autoimmune necrotizing myopathies: inconstant exposure to statin. Medicine. 2014;93:150–157. doi: 10.1097/MD.0000000000000028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mammen A.L., Tiniakou E. Intravenous immune globulin for statin-triggered autoimmune myopathy. New Engl J Med. 2015;373:1680–1682. doi: 10.1056/NEJMc1506163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grable-Esposito P., Katzberg H.D., Greenberg S.A., Srinivasan J., Katz J., Amato A.A. Immune-mediated necrotizing myopathy associated with statins. Muscle Nerve. 2010;41:185–190. doi: 10.1002/mus.21486. [DOI] [PubMed] [Google Scholar]