Abstract
Metformin is the most widely prescribed drug for type 2 diabetes. Chemically, metformin is a hydrophilic base that functions as an organic cation, suggesting that it may have the capacity to inhibit the tubular reabsorption of peptide radiotracers. The purpose of this study was to investigate whether metformin could reduce renal uptake of peptidyl radiotracers and serve as a radioprotective agent for peptide receptor radionuclide therapy (PRRT).
Methods:
We used two radiolabeled peptides: a 68Ga-labeled cyclic (TNYL-RAW) peptide (68Ga-NOTA-c(TNYL-RAW) (NOTA: 1,4,7 triazacyclononane-1,4,7-trisacetic acid) targeting EphB4 receptors and an 111In- or 64Cu-labeled octreotide (111In/64Cu-DOTA-octreotide) (DOTA: 1,4,7,10 triazacyclododecane-1,4,7,10-tetraacetic acid) targeting somatostatin receptors. Each radiotracer was injected intravenously into normal Swiss mice or tumor-bearing nude mice in the presence or absence of metformin administered intravenously or orally. Micro–positron emission tomography or micro–single-photon emission computed tomography images were acquired at different times after radiotracer injection, and biodistribution studies were performed at the end of the imaging session. To assess the radioprotective effect of metformin on the kidneys, normal Swiss mice received two doses of 111In-DOTA-octreotide in the presence or absence of metformin, and renal function was analyzed via blood chemistry and histology.
Results:
Intravenous injection of metformin with 68Ga-NOTA-c(TNYL-RAW) or 111In-DOTA-octreotide reduced the renal uptake of the radiotracer by 60% and 35%, respectively, compared to uptake without metformin. These reductions were accompanied by greater uptake in the tumors for both radiolabeled peptides. Moreover, the renal uptake of 111In-DOTA-octreotide was significantly reduced when metformin was administered via oral gavage. Significantly more radioactivity was recovered in the urine collected over a period of 24 h after intravenous injection of 64Cu-DOTA-octreotide in mice that received oral metformin than in mice that received vehicle. Finally, co-administration of 111In-DOTA-octreotide with metformin mitigated radio-nephrotoxicity.
Conclusion:
Metformin inhibits kidney uptake of peptidyl radiotracers, protecting the kidney from nephrotoxicity. Further studies are needed to elucidate the mechanisms of these finding and to optimize mitigation of radiation-induced damage to kidney in PRRT.
Keywords: EphB4 receptors, peptide, copper-64, positron emission tomography, nephrotoxicity, radiotherapy
Graphic Abstract

Introduction
Radioisotopes used in peptide nuclear imaging or peptide receptor radionuclide therapy (PRRT) must have low uptake in normal organs and minimal side effects in non-target organs, including the kidneys. Low renal uptake of these radiotracers can improve imaging quality by reducing background radioactivity. Most radiotracers are cleared from the body via the renal-urinary route. Owning to relatively high kidney reabsorption and retention of radiotracers, nephrotoxicity is often the dose-limiting toxic effect of PRRT1.
Several approaches have been used to reduce the risk of radiotoxicity to the kidneys, such as developing highly specific peptides or monoclonal antibodies with low kidney uptake. Alternatively, some positively charged amino acids such as D- or L-lysine reduce the renal uptake of peptide-based radiotracers by inhibiting their tubular reabsorption2–5. For example, co-administration of a combination of lysine and arginine with 111In-DTPA-octreotide (DTPA: diethylenetriaminepentaacetic acid) led to reduction of the radiation dose to the kidneys by about 35–50%, allowing higher treatment doses and thus higher radiation doses to the tumor5–6. A similar reduction of renal uptake of 111In-DOTA-Tyr3-octreotate (DOTA: 1,4,7,10 triazacyclododecane-1,4,7,10-tetraacetic acid) has been demonstrated with amifostine, which could be converted to a positively charged compound, 2-((aminopropyl)amino)ethanethiol, in normal tissues7. Intraperitoneal injection of L-lysine immediately before intravenous injection of 213Bi-DOTA-octreotate reduced renal uptake of the radiotracer, prolonging survival of tumor-bearing mice receiving 213Bi-DOTA-octreotate–mediated alpha particle therapy8. A 4-h continuous intravenous infusion of L-lysine/L-arginine starting 30 min prior to 177Lu-DOTA-TATE administration is recommended to prevent renal toxicity and has become standard clinical practice in neuroendocrine tumors9. The receptors involved in reducing kidney uptake of radiotracers by L-lysine/L-arginine have not yet been characterized completely, but megalin and cubilin are thought to be involved1, 10.
Metformin, which is very widely used as a first-line treatment for type 2 diabetes11, works by preventing the liver from converting fats and amino acids into glucose. Metformin also activates adenosine monophosphate–activated protein kinase, an enzyme that helps cells respond more effectively to insulin12. The antitumor activity of metformin has attracted considerable attention in recent years for its potential in cancer prevention13–15. On a molecular level, metformin has a strong basic biguanide group that may have the capacity to inhibit the tubular reabsorption of glomerularly filtered peptides4. We hypothesized that metformin can considerably reduce kidney uptake of peptidyl radiotracers, resulting in a relatively high tumor-to-kidney uptake ratio that is favorable for diagnostic and therapeutic applications. In this study, we investigated the role of metformin in the biodistribution of two peptide radiotracers: a 68Ga-labeled peptide targeting EphB4 receptors and an 111In/64Cu-labeled octreotide targeting somatostatin receptors.
Experimental Methods
Materials
1,1-Dimethylbiguanide hydrochloride (metformin) was purchased from Sigma-Aldrich (St. Louis, MO). S-2-(4-Isothiocyanatobenzyl)-1,4,7-triazacyclononane-1,4,7-triacetic acid (p-SCN-Bn-NOTA) was obtained from Macrocyclics (Plano, TX). DOTA-(Tyr³)-octreotide was purchased from Bachem (Torrance, CA). Amino acid derivatives were purchased from Novabiochem (San Diego, CA) or Chem-Impex International (Wood Dale, IL). All other chemical reagents and solvents were purchased from Sigma-Aldrich and were used as received unless otherwise specified. 68GaCl3 was obtained from elution using a 68Ge/68Ga generator (Eckert & Ziegler, Berlin, Germany). 111InCl3 was obtained from Triad Isotopes (Houston, TX). 64CuCl2 was supplied by the Cyclotron Radiochemistry Facility at The University of Texas MD Anderson Cancer Center (Houston, TX).
Radiolabeling
68Ga labeling of NOTA-c(TNYL-RAW).
The method for synthesis and the characterization of NOTA-cyclic(Lys-Thr-Asn-Tyr-Leu-Phe-Ser-Pro-Asn-Gly-Pro-ILe-Ala-Arg-Ala-Trp-Asp) [NOTA-c(TNYL-RAW)], including its binding affinity to EphB4 receptors, are presented in the Supplementary Information section. For labeling with the positron emitter 68Ga, 68GaCl3 in 5 mL of 0.1 N HCl (370 MBq, 10 mCi) was obtained by elution using the 68Ge/68Ga generator. Ultrapure concentrated HCl (12 M) was added to the 68GaCl3 solution to a final concentration of 5.5 M. The solution was injected through a preconditioned separation cartridge (Chromafix 30-PS-HCO3; Macherey-Nagel, Bethlehem, PA). The cartridge column was dried using argon, and 68GaCl3 was eluted by 0.2 mL de-ionized water and directly added to a solution of NOTA-c(TNYL-RAW) (10 μg) in 0.3 mL of 0.3 M NaOAc (pH 4.5). The reaction mixture was then kept at 60°C for 30 min. The 68Ga-labeled peptide was further purified, if necessary, by the Agilent HPLC 1100 system (Agilent Technologies, Santa Clara, CA) using a C-18 column (Vydac, 4.6 × 250 mm, 10 μm) eluted with a linear gradient of 10%–90% acetonitrile in a 0.1% aqueous trifluoroacetic acid (TFA) solution over 35 min at a flow rate of 1.0 mL/min. 68Ga-NOTA-c(TNYL-RAW) (retention time, tR = 13.6 min) was collected in 1- to 2-mL fractions. The solvent was then removed in a rotavapor, reconstituted in saline solution, and passed through a 0.22-μm filter for immediate use in the animal experiments.
The stability of 68Ga-NOTA-c(TNYL-RAW) was tested in an aqueous solution containing 20% mouse plasma. Purified 68Ga-NOTA-c(TNYL-RAW) (100 μCi) in 1 mL of phosphate-buffered saline solution (PBS; pH 7.4) containing 20% mouse plasma was incubated at 37°C. Aliquots of the incubation mixtures were removed at 0.5, 1, 2, and 4 h and analyzed to monitor changes in radiotracer peak activity using the Agilent 1100 HPLC system equipped with a C-18 column (Vydac, 4.6 × 250 mm, 10 μm). The column was eluted as already described. The stability of the radiotracer was expressed as a percentage of intact radiolabeled peptide of the total activity. Triplicate measurements were obtained.
111In labeling of DOTA-[Tyr3]-octreotide.
To a solution of DOTA-[Tyr3]-octreotide in 0.2 M ammonium acetate buffer (pH 5.5; 50 μL, 0.1 mg/mL) was added an aliquot of 185 MBq (5 mCi) of 111InCl3 (80–160 μL solution in 0.05 M HCl). The reaction mixture was incubated at 60°C for 30 min. The radiochemical purity, defined as the ratio of counts in the product compared to the total counts on the plate, was evaluated by instant thin-layer chromatography-silica gel–impregnated strip (ITCL-SG, Agilent Technologies). The ITLC-SG strip was developed with 0.1 M citric acid in saline solution. The strips were scanned using an AR-2000 radio imaging scanner (Eckert & Ziegler). 111In-DOTA-[Tyr3]-octreotide remained at the original spot, while free 111In moved to the solvent front. The radiolabeled compound had radiochemical purity of >98% and was used without further purification.
64Cu labeling of DOTA-[Tyr3]-octreotide.
To a solution of DOTA-[Tyr3]-octreotide in 0.1 M sodium acetate buffer (pH 5.0; 100 μL, 0.1 mg/mL) was added an aliquot of 74 MBq (2 mCi) of 64CuCl2 (5 μL solution in 0.01 M HCl). The reaction mixture was incubated at 80°C for 30 min. The radiochemical purity was checked by ITLC-SG strip as described above. The radiolabeled compound had radiochemical purity of >98% and was used without further purification.
Cell lines and culture
A375SM human melanoma cells, which express EphB4, and MDA-MB-231 human breast cancer cells, which express relatively low levels of EphB4, were obtained from ATCC (Manassas, VA) and were grown in Dulbecco modified Eagle/F12 medium supplemented with 10% fetal bovine serum, 100 IU/mL penicillin, and 100 μg/mL streptomycin (Invitrogen, Carlsbad, CA) at 37°C in a humidified atmosphere with 5% CO2. HT1080 human fibrosarcoma cells (ATCC) stably expressing human somatostatin receptor type 2 (SSTR2) were obtained as reported elsewhere16–17 and were grown in Dulbecco modified Eagle medium containing 1× glutamine, penicillin, streptomycin, and 10% fetal bovine serum.
Animal models
All animal studies were performed under the guidelines and approval of the Institutional Animal Care and Use Committee at MD Anderson Cancer Center. Female athymic nude mice (4–6 weeks old) were obtained from Charles River Laboratories (Wilmington, MA). EphB4+ A375SM or SSTR2+ HT1080 tumor cells were harvested by trypsinization. After centrifugation of the cell suspensions at 1000 rpm for 5 min, the culture medium was aspirated, and 0.1 mL of A375SM or HT1080 tumor cells in culture medium were injected into each flank subcutaneously (1×106 cells/mouse). When tumors were about 1 cm in diameter, micro–positron emission tomography/computed tomography (μPET/CT) or micro–single-photon emission computed tomography/computed tomography (μSPECT/CT) imaging and biodistribution analysis were performed. Immediately after each experiment was completed, all animals were killed humanely by CO2 inhalation.
μPET/CT and biodistribution of 68Ga-NOTA-c(TNYL-RAW) in mice bearing A375SM tumors after intravenous co-injection with metformin
Female nude mice bearing A375SM tumors (n=3,4) were imaged with μPET/CT at 1 h and 4 h after intravenous injection of 68Ga-NOTA-c(TNYL-RAW) at a dose of 7.4 MBq (200 μCi in 100 μL of saline solution) with or without co-injection of metformin (1.2 mg/mouse, 48 mg/kg). Tumor-bearing mice were anesthetized with isoflurane (2% in O2) and placed in a prone position. Images were acquired over a period of 15 min using an Inveon PET/CT system (Siemens Preclinical Solutions, Knoxville, TN). The spatial resolution of the μPET system is approximately 1.4 mm. The CT imaging parameters were as follows: x-ray voltage, 80 kVp; anode current, 500 mA; exposure time of each of the 360 rotational steps, 300–350 ms. Images were reconstructed using the two-dimensional ordered-subset expectation maximization algorithm provided by the manufacturer. PET and CT image fusion and image analysis were performed using the Inveon Research Workplace software (Siemens Preclinical Solutions). At the end of the imaging session (4 h post-injection), the animals were euthanized, and their organs were removed and weighed, and their radioactivities were counted using a Packard Cobra gamma counter (PerkinElmer Inc., Waltham, MA). The percentage of injected dose per gram of tissue (%ID/g) was calculated. Values are expressed as mean ± standard error of mean (SEM).
μSPECT/CT and biodistribution of 111In-DOTA-[Tyr3]-octreotide in mice with SSTR2-expressing HT1080 tumors after intravenous co-injection with metformin
For the μSPECT/CT study, female nude mice bearing HT1080 tumors expressing SSTR2 (n=4/group) were injected through the tail vein with 7.4 MBq (200 μCi) of 111In DOTA-[Tyr3]-octreotide per mouse with or without co-injection of 1.2 mg of metformin (48 mg/kg). μSPECT images were acquired using the ALBIRA SPECT-CT imaging system18 with a pinhole collimator at a field of view of 80 mm for 15 min, followed by standard CT scanning. Images were reconstructed using three-dimensional maximum likelihood and ordered-subset expectation maximization algorithms, whereas the CT images were obtained using a three dimension–based, filtered back projection algorithm. SPECT and CT image fusion and image analysis were performed using PMOD Base Functionality software (PMOD Technologies LLC, Zürich, Switzerland). The animals were euthanized 24 h after radiotracer injection. The organs of interest were removed and weighed, and their radioactivities were counted. The %ID/g values are expressed as mean ± SEM.
Biodistribution and urinary excretion of radiolabeled DOTA-[Tyr3]-octreotide after oral administration of metformin
To determine whether oral administration of metformin enhances renal clearance of radiolabeled octreotide, healthy female Swiss mice were administered metformin orally (formulated in 0.5% [weight/volume] methylcellulose in PBS, 200 mg/mL) at a daily dose of 600 mg/kg for 3 days (n=4). Age-matched control mice were administered vehicle (0.5% methylcellulose in PBS) via oral gavage daily for 3 days (n=5). Each mouse in each group was then injected intravenously with 111In-DOTA-[Tyr3]-octreotide at a dose of 7.4 MBq/mouse (200 μCi/mouse, 100 μL). Twenty-four hours later, mice were euthanized; major organs were collected and weighed, and counted for radioactivity.
In a separate study, excretion of radiotracer into the urine via the renal system was assessed. Groups of normal female Swiss mice were administered vehicle (0.5% methylcellulose in PBS) or metformin in 0.5% methylcellulose in PBS at a dose of 300 mg/kg via oral gavage daily for 7 days. Mice in each group then received an intravenous injection of 64Cu-DOTA-[Tyr3]-octreotide at a dose of 0.74 MBq/mouse (20 μCi/mouse, 100 μL). The mice were placed in metabolic cages, and urine from each mouse was collected over a period of 24 h for radioactivity counting. The counts are expressed as percentage of total injected dose.
Radiotoxicity to kidney after intravenous injection of 111In-DOTA-[Tyr3]-octreotide
Eighteen normal female Swiss mice (6 weeks old) were randomly divided into three groups (n=6/group): untreated controls, those receiving 111In-DOTA-[Tyr3]-octreotide alone, and those receiving metformin and 111In-DOTA-[Tyr3]-octreotide. In the combined treatment group, metformin (dissolved in 0.5% methylcellulose in PBS, 200 mg/mL) was given via oral gavage at a daily dose of 600 mg/kg/dose for 3 days, and 111In-DOTA-[Tyr3]-octreotide was injected intravenously immediately after the last metformin dose at a dose of 22.2 MBq/mouse (600 μCi/mouse). A second dose (also 22.2 MBq/mouse) of 111In-DOTA-[Tyr3]-octreotide was given 3 days later. Three weeks after the last radiotracer injection, retro-orbital blood was collected and serum levels of blood urea nitrogen (BUN) and creatinine (SCr) were quantified by an automatic biochemistry analyzer (Beckman Coulter, Inc., Brea, CA). The mice then were euthanized, and their kidneys were collected and fixed with 10% buffered formalin. Paraffin-embedded tissue blocks were processed for hematoxylin and eosin (H&E) staining and histologic examination. The H&E-stained slides were quantified according to a reported method19 by counting the percentage of tubules that displayed cell necrosis, loss of brush border, cast formation, or tubule dilatation as follows: 0 = none, 1 ≤10%, 2 = 11–25%, 3 = 26–45%, 4 = 46–75%, and 5 ≥75%. A total of 10 fields (×200) were reviewed for each slide.
Statistical analysis
Biodistribution and toxicity study results between metformin-treated groups and vehicle-treated groups or untreated control groups were compared by using the Student t-test. A p-value <0.05 was considered statistically significant.
RESULTS
Chemistry and radiochemistry
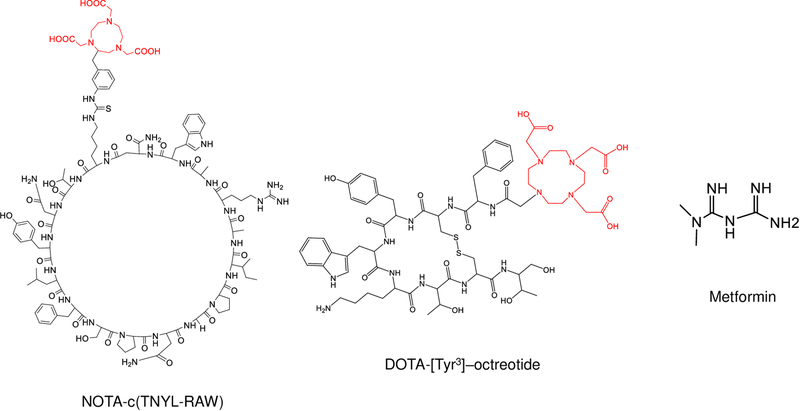
The structures of NOTA-c(TNYL-RAW), DOTA-[Tyr3]-octreotide, and metformin are shown in Figure 1. NOTA-c(TNYL-RAW) was synthesized by standard Fmoc chemistry. The purity and identity of the peptides were confirmed by high-resolution liquid chromatography–mass spectrometry. Figure S1 shows representative sensorgrams obtained from surface plasmon resonance analyses of c(TNYL-RAW) and a scrambled peptide, with fitted curves obtained using a global 1:1 mass transfer model. The binding kinetics and binding affinity of c(TNYL-RAW) to EphB4 receptors were as follows: Kon = 1.09 × 106 [M−1S−1], Koff = 4.82 × 10−3 [S−1], and KD = 4.41 ×10−9 [M].
Figure 1. Structures of NOTA-c(TNYL-RAW), DOTA-[Tyr3]-octreotide, and metformin.
Radiometal chelators are highlighted in red.
The radiolabeling efficiency of 68Ga-NOTA-c(TNYL-RAW) was greater than 90%, and the radiolabeling efficiency of 111In/64Cu-DOTA-[Tyr3]-octreotide was greater than 98%. The radiochemical purity of 68Ga-NOTA-c(TNYL-RAW), defined as the ratio of the main product peak to all peaks, was determined by high-performance liquid chromatography (HPLC) to be >98% after purification (Fig. S2). The specific activity levels of the 68Ga-NOTA-c(TNYL-RAW) and 111In-DOTA-[Tyr3]-octreotide used in the in vitro and in vivo experiments were typically 74–92 MBq/nmol (2.0–2.5 mCi/nmol) and 37–55 MBq/nmol (1–1.5 mCi/nmol), respectively, at the end of synthesis. 68Ga-NOTA-c(TNYL-RAW) was stable in PBS containing 20% mouse plasma for up to 4 h at 37°C (Fig. S2).
Metformin reduced renal uptake of 68Ga-NOTA-c(TNYL-RAW) after intravenous co-administration
We used the A375SM human melanoma cell line to establish tumors in mice for a μPET/CT imaging study of 68Ga-c(TNYL-RAW). Overexpression of EphB4 in A375SM cells was confirmed by Western blot analysis (Fig. S3).
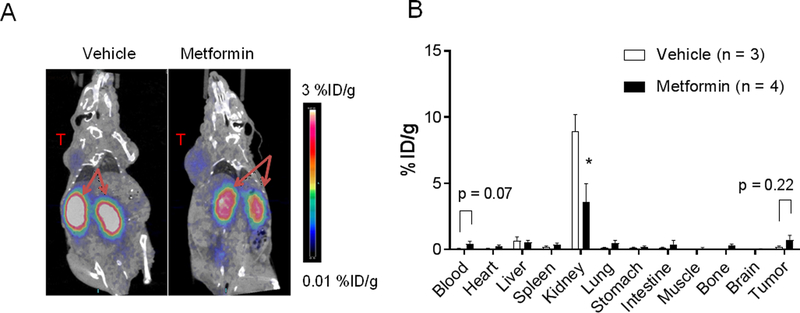
When the radiotracer was co-injected intravenously with metformin, μPET/CT images showed drastic reduction in kidney uptake of 68Ga-NOTA-c(TNYL-RAW) 1 h after intravenous injection compared with mice that received radiotracer only; this reduction was accompanied by enhanced delineation of the radiotracer in the tumor (Fig. 2A). Biodistribution data obtained 4 h after injection showed that co-injection of metformin with the 68Ga-NOTA-c(TNYL-RAW) reduced uptake of the radiotracer in the kidney by 59.5% (8.92±2.23 vs.3.61±2.75; p<0.05). Tumor uptake was also increased by metformin co-injection, from 0.22±0.05 %ID/g to 0.74±0.33 %ID/g (Fig. 2B), accompanied by increases in tumor-to-kidney uptake ratio from 0.03±0.01 %ID/g to 0.46±0.72 %ID/g, p=0.35). The tumor-to-blood uptake ratio was decreased by metformin co-injection from 3.18±0.36 %ID/g to 1.52±0.25 %ID/g, p<0.01). No difference was found in liver uptake of the radiotracer.
Figure 2. Metformin reduces renal uptake of 68Ga-NOTA-c(TNYL-RAW).
(A) Representative coronal μPET/CT images of EphB4+ A375SM tumor–bearing mice acquired 1 h after intravenous injection of 68Ga-NOTA-c(TNYL-RAW). Arrows, kidney; T, tumor. (B) Biodistribution of 68Ga-NOTA-c(TNYL-RAW) 4 h after radiotracer injection. Metformin (48 mg/kg) or vehicle was co-injected with radiotracer. Data are presented as mean ± SEM. *p<0.05.
Metformin reduced renal uptake of 111In-DOTA-[Tyr3]-octreotide after intravenous co-administration
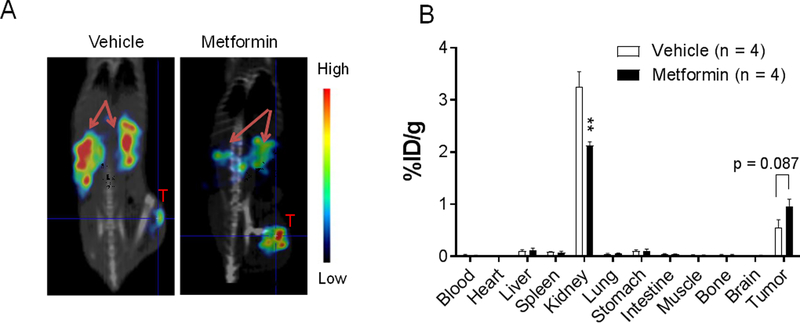
To further confirm that metformin can suppress renal uptake of peptide radiotracers, we acquired μSPECT/CT images of mice administered 111In-DOTA-[Tyr3]-octreotide with or without intravenous co-injection of metformin. When 111In-DOTA-[Tyr3]-octreotide was co-injected with metformin, renal retention of the radiotracer was clearly reduced in the μSPECT/CT images 4 h after injection, and this reduction was accompanied by increased tumor uptake of the radiotracer (Fig. 3A). Biodistribution data obtained at 24 h after injection showed that metformin co-injection significantly reduced uptake of 111In-DOTA-[Tyr3]-octreotide in the kidney (3.26±0.56 %ID/g vs. 2.12±0.15 %ID/g; p<0.01) and significantly increased the tumor-to-kidney uptake ratio (0.17±0.05 %ID/g vs. 0.45±0.06 %ID/g, p=0.01). The tumor-to-blood ration also increased from 38.2±34.7 %ID/g to 74.7±49.3 %ID/g (p=0.27) by metformin co-injection. Differences in radiotracer uptake in all the other major organs and in the tumor (p=0.08) were not statistically significant (Fig. 3B).
Figure 3. Metformin reduces renal uptake of 111In-DOTA-[Tyr3]-octreotide.
(A) Representative coronal μSPECT/CT images of HT1080 tumor–bearing mice acquired 4 h after intravenous injection of the radiotracer. Arrows, kidney; T, tumor. (B) Biodistribution of 111In-DOTA-[Tyr3] 24 h after radiotracer injection. Metformin (48 mg/kg) or vehicle was co-injected with radiotracer. Data are presented as mean ± SEM. **p<0.01.
Metformin reduced renal uptake of radiolabeled 111In-DOTA-[Tyr3]-octreotide after oral administration
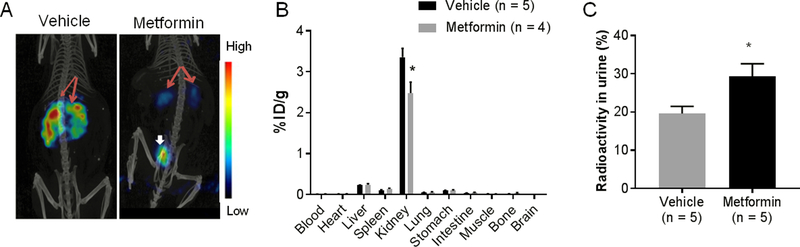
Because metformin is administered orally in standard patient care, we determined whether orally administered metformin could suppress renal retention of peptide radiotracers and increase excretion into the urine as intravenously administered metformin did. Figure 4A shows representative μSPECT/CT images of normal Swiss mice acquired 4 h after intravenous injection of 111In-DOTA-[Tyr3]-octreotide. The mice pretreated with 3 daily metformin doses (via oral gavage at a cumulative dose of 1.8 g/kg) showed significantly reduced kidney uptake of the radiotracer and increased clearance into the bladder compared with mice that received radiotracer only. These observations were confirmed by biodistribution data derived by the cut-and-count method (Fig. 4B).
Figure 4. Orally administered metformin reduces renal uptake while increasing urine excretion of radiolabeled octreotide.
(A) Representative coronal μSPECT/CT images of normal female Swiss mice acquired 4 h after intravenous injection of 111In-DOTA-[Tyr3]-octreotide. Red arrows, kidney; white arrow, bladder. (B) Biodistribution of 111In-DOTA-[Tyr3]-octreotide 24 h after intravenous injection. Metformin was administered via oral gavage at a daily dose of 600 mg/kg for 3 days immediately before radiotracer injection. (C) Percentage of total injected radioactivity recovered from the urine over a 24-h period after intravenous injection of 64Cu-DOTA-[Tyr3-octreotide. Metformin was administered via oral gavage at a daily dose of 300 mg/kg for 7 days immediately before radiotracer injection. In both experiments, control mice received oral administration of vehicle (0.5% weight/volume methylcellulose) only. Data are presented as mean ± SEM. *p<0.05.
Metformin increased urinary excretion of 64Cu-DOTA-[Tyr3]-octreotide
Mice that had received 7 daily metformin doses (via oral gavage at a cumulative dose of 2.1 g/kg) had significantly greater radioactivity in their urine collected over 24 h than mice pretreated with vehicle only (Fig. 4C). The radioactivity recovered in the urine increased by 49.5%, from 19.6±1.9 to 29.3±3.3 percent of total injected dose (p<0.05).
Metformin reduced radiotoxicity of 111In-DOTA-[Tyr3]-octreotide to kidneys
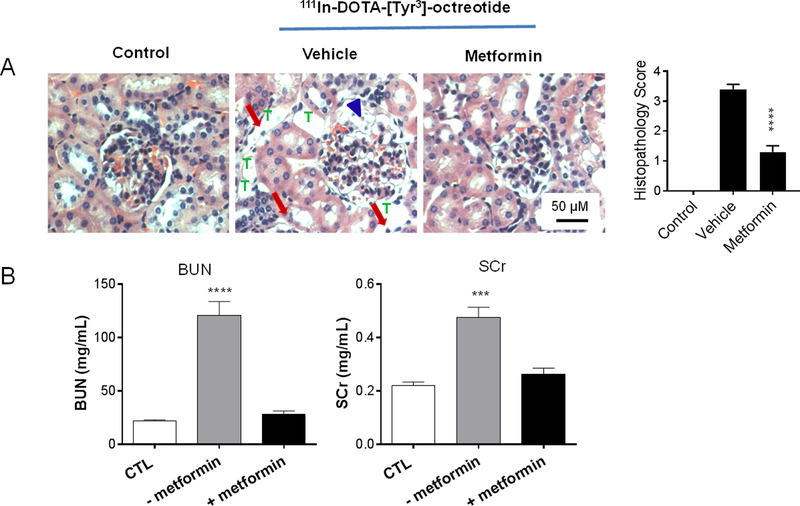
To determine whether metformin protects kidneys from radiotoxicity caused by peptide radiotracers, we analyzed kidney function after two intravenous injections of 111In-DOTA-[Tyr3]-octreotide at a dose of 22.2 MBq/mouse (600 μCi/mouse/dose, 3-day interval) with or without metformin pretreatment for 3 days. As shown in Figure 5A, metformin reduced radiation-induced necrotic damage to kidney glomerular cells and tubular cells. Histologic scores in the outer medulla were 0 in non-treated controls, 3.4±0.16 in animals treated with vehicle and radiotracer, and 1.3±0.21 in animals treated with metformin and radiotracer (p<0.0001 vs. vehicle + radiotracer, n=10) (Fig. 5A). Kidney function as measured by changes in BUN and SCr levels showed significant nephrotoxicity in mice pretreated with vehicle solution by oral gavage followed by 111In-DOTA-[Tyr3]-octreotide. In contrast, mice pretreated with metformin by oral gavage prior to 111In-DOTA-[Tyr3]-octreotide injections had baseline levels of BUN and SCr. Thus, metformin effectively mitigated the impact of 111In-DOTA-[Tyr3]-octreotide on kidney function (Fig. 5B).
Figure 5. The protective effect of metformin on kidney structure and function in mice treated with 111In-DOTA-[Tyr3]-octreotide.
(A) Representative photomicrographs of hematoxylin and eosin–stained sections of kidneys from treated (vehicle, metformin) and untreated (control) mice. Arrowheads: glomerular cell necrosis; arrows, tubular cell necrosis; T, tubular vacuoles. (B) Renal function parameters after radiotracer injection in the presence and absence of metformin. Radiotracer was injected intravenously twice 3 days apart at 600 μCi/mouse/dose. Metformin was administered with oral gavage at a daily dose of 600 mg/kg for 3 days immediately before the first radiotracer injection (cumulative dose, 1.8g/kg). Data are presented as mean ± SEM (n=6). BUN, blood urea nitrogen; SCr, serum creatinine; CTL, no-treatment control. ****p<0.0001; ***p<0.001 compared to both no-treatment and metformin groups.
DISCUSSION
Animal and clinical studies have shown that co-administration of lysine or arginine can significantly reduce renal uptake of radiolabeled peptides.3, 5, 20–22 For example, the renal uptake of radiolabeled octreotide was reduced by up to 40% with oral or intravenous administration of lysine.20 Renal uptake of [111In-DTPA]octreotide was inhibited by a combination of 25 g of lysine and 25 g of arginine reduced in patients by 33%.5 We found that metformin also effectively reduced renal accumulation of radiolabeled peptides whether it was co-injected intravenously with the radiolabeled peptides or was administered by oral gavage as a pretreatment before radiotracer injection. Moreover, metformin administered through oral gavage protected the mouse kidneys from radiation-induced nephrotoxicity.
Retention of therapeutic radiotracers in the kidneys at a relatively high radiation dose can lead to kidney failure, and PRRT in particular can lead to dose-limiting nephrotoxicity20. A high radioactivity background in the kidneys also limits the detection of tumor near a kidney23. It is known that radiolabeled peptides containing hydrophilic amino acids are excreted primarily through the kidneys. This process involves glomerular filtration, tubular reabsorption via pinocytosis and/or receptor-mediated endocytosis, and subsequent lysosomal degradation. Both the basement membrane of the glomerulus and the proximal tubular cell surface are negatively charged, and thus positively charged peptides can bind to the negatively charged basement membrane via electrostatic interaction and be retained there10. Positively charged low-molecular-weight species such as lysine and arginine are used to interfere in the interaction of peptides with proximal tubular cells and to reduce renal uptake and retention of peptide radiotracers2–3. A combination of 25 g L-lysine and 25 g L-arginine in 1 L volume as a standard 4-h infusion protocol for kidney protection during PRRT has been found to be safe in patients. However, vomiting and nausea occur in about 15% and 30%, respectively, of patients treated with this kidney protection regimen24.
As the most frequently prescribed medication for type 2 diabetes25, metformin is taken by more than 120 million people worldwide every year. Metformin also has anticancer and cancer-preventive effects26–27. Since metformin is a structural analogue of the amino acid arginine, we hypothesized that metformin may act as a competitor in the charge-dependent renal absorption of radiolabeled peptides. Our hypothesis was supported by μPET/CT and biodistribution data showing that renal uptake of 68Ga-NOTA-c(TNYL-RAW), a positively charged cyclic peptide containing an arginine moiety, was significantly reduced when the peptide was co-injected with metformin in mice engrafted with human A375SM tumors. On average, co-injection with metformin reduced renal uptake of 68Ga-NOTA-c(TNYL-RAW) by about 60%. We also found that the reduced renal uptake of 68Ga-NOTA-c(TNYL-RAW) with co-injection of metformin resulted in a concomitant increase of the radiotracer’s uptake in the tumor. We extended our findings to the clinically used radiotracer 111In-DOTA-[Tyr3]-octreotide. Again, co-administration of metformin through intravenous injection significantly reduced the renal uptake of the radiotracer in mice, by about 35% at 24 h after intravenous injection, and improved visualization of SSTR2+ HT1080 tumors by μSPECT/CT. Because metformin is administered orally by patients, we further investigated the effects of orally administered metformin on renal clearance of 111In-DOTA-[Tyr3]-octreotide. Our data confirm that metformin administered by oral gavage could effectively reduce renal uptake of 111In-DOTA-[Tyr3]-octreotide, by about 26%.
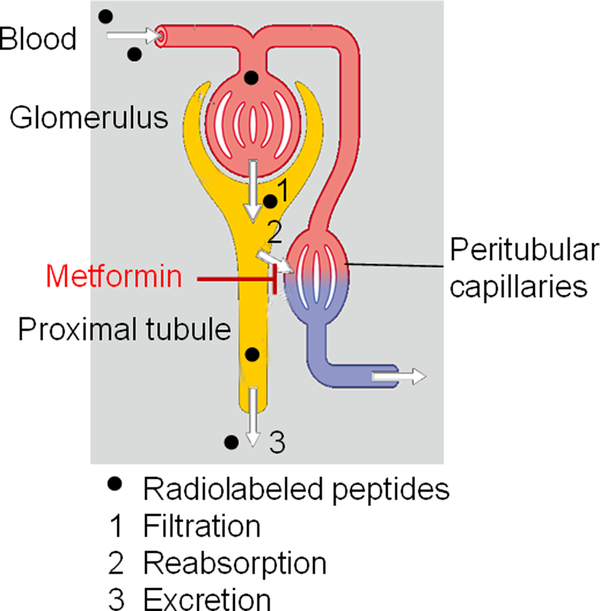
Although the mechanism for this metformin action is not clear, involvement of the megalin receptor system is likely, as the megalin system was shown to be essential for kidney uptake of 111In-labeled octreotide28. Octreotide analogues are actively reabsorbed in the proximal tubular cells by receptor-mediated endocytosis, in part through megalin21, 28. Metformin reduced kidney uptake of 111In-DOTA-[Tyr3]-octreotide to the same level as lysine did (~35% reduction)6. These data are in accord with the role of cationic amino acids such as lysine or arginine in saturating binding sites on megalin protein toward positively charged peptides. Thus, metformin may interact with the tubular cell surface through electrostatic repulsion to block reabsorption and retention of radiolabeled peptides in the proximal tubular cells, as schematically depicted in Figure 6. In support of this mechanism, we observed significantly increased urinary excretion of 64Cu-DOTA-[Tyr3]-octreotide when metformin was administered via oral gavage before radiotracer injection. Because multiple mechanisms are involved in glomerular filtration and tubular reabsorption of peptides, further studies are needed to clarify the role of metformin in reduction of renal uptake of radiolabeled peptides and to determine whether metformin’s effect on renal uptake of radiotracers can be extended to a broader spectrum of peptides of different radioisotope, charge, and size.
Figure 6.
Proposed mechanism of metformin’s action in reducing renal uptake of radiolabeled peptides.
We demonstrated the protective effects of metformin against radiation-induced kidney damage in the setting of intravenous 111In-DOTA-[Tyr3]-octreotide and oral administration of metformin, the route used by patients. Our results agree with those of Melis et al.1, who studied dosimetry and nephrotoxicity of several 111In-labeled peptides, including 111In-labeled octreotide. These researchers observed that, at a single injected dose of 40–50 MBq (1.0–1.35 mCi), 111In-labeled peptides impaired kidney function, as shown by increased BUN and SCr levels. In our studies, mice received two injections of 111In-DOTA-[Tyr3]-octreotide for a total dose of 44 MBq per mouse. We found that 111In-DOTA-[Tyr3]-octreotide caused both structural and functional kidney damage in mice 3 weeks after radiotracer injection. Administration of metformin prevented such damage and reduced BUN and SCr levels to the baseline. Histopathologic evaluation revealed that histologic damage in both glomeruli and tubuli was reversed in mice given metformin. Thus, reduction in radiotracer uptake in the kidneys protected the kidneys from radiotoxicity caused by the high dose of 111In-DOTA-[Tyr3]-octreotide. This may have significant implications for patients already on metformin who undergo PRRT. Additional benefits of metformin may include improved imaging properties at reduced radiotracer dose or reduced acquisition time. Oral administration of metformin is a standard clinical approach, much less expensive, better patient compliance, and with less adverse effect potential than the 4-h intravenous infusion of L-lysine/L-arginine solution, the current standard clinical protocol used to prevent renal toxicity in PRRT for neuroendocrine tumors.
In the clinic, metformin is administered orally at a daily dose of up to 2.0 gram for type 2 diabetes. Rare cases of lactic acidosis have been reported in patients receiving metformin hydrochloride tablets (approximately 0.03 cases/1000 patient-years). When metformin is implicated as the cause of lactic acidosis, metformin plasma levels of greater than 5 μg/mL are generally found (https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=da99b8c4-f85e-409f-93a8-0e4758d74552). Therefore, in planning future clinical studies combination metformin and PRRT, the renal function should be assessed and metformin dose titrated so that its plasma levels are kept below 5 μg/mL.
In conclusion, we found that the widely used FDA-approved drug metformin inhibits renal uptake of radiolabeled peptides and thus can be used in this setting to mitigate radiation-induced damage to kidney tissue. Given that metformin is not metabolized and is well tolerated by most patients29, and that many patients with neuroendocrine tumors have co-existing type 2 diabetes treated with metformin, it is of great clinical interest to further optimize and validate the metformin renal toxicity protection protocol for neuroendocrine tumor patients receiving PRRT.
Supplementary Material
Acknowledgments
We thank Kathryn L. Hale of the Department of Scientific Publications, MD Anderson Cancer Center, for editing the manuscript. This work was supported in part by the John S. Dunn Foundation. This research was conducted at the Center for Advanced Biomedical Imaging, MD Anderson Cancer center, in part with equipment support from GE Healthcare. The Research Animal Support Facility, Research Cyclotron Facility, and Small Animal Imaging Facility are supported by a Cancer Center Support Grant from the National Institutes of Health (P30CA016672).
Footnotes
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website. Supplementary tables and figures are provided.
The authors declare no conflicts of interest.
References
- (1).Melis M; Vegt E; Konijnenberg MW; de Visser M; Bijster M; Vermeij M; Krenning EP; Boerman OC; de Jong M, Nephrotoxicity in Mice after Repeated Imaging Using 111In-Labeled Peptides. J. Nucl. Med 2010, 51, 973–977. [DOI] [PubMed] [Google Scholar]
- (2).Kobayashi H; Yoo TM; Kim IS; Kim M-K; Le N; Webber KO; Pastan I; Paik CH; Eckelman WC; Carrasquillo JA, L-Lysine Effectively Blocks Renal Uptake of 125I- or 99mTc-Labeled Anti-Tac Disulfide-Stabilized Fv Fragment. Cancer Res. 1996, 56, 3788–3795. [PubMed] [Google Scholar]
- (3).Bernard BF; Krenning EP; Breeman WAP; Rolleman EJ; Bakker WH; Visser TJ; Mäcke H; de Jong M, D-Lysine Reduction of Indium-111 Octreotide and Yttrium-90 Octreotide Renal Uptake. J. Nucl. Med 1997, 38, 1929–1933. [PubMed] [Google Scholar]
- (4).Behr TM; Becker WS; Sharkey RM; Juweid ME; Dunn RM; Bair HJ; Wolf FG; Goldenberg DM, Reduction of Renal Uptake of Monoclonal Antibody Fragments by Amino Acid Infusion. J. Nucl. Med 1996, 37, 829–833. [PubMed] [Google Scholar]
- (5).Rolleman E; Valkema R; de Jong M; Kooij P; Krenning E, Safe and Effective Inhibition of Renal Uptake of Radiolabelled Octreotide by a Combination of Lysine and Arginine. Eur. J. Nucl. Med. Mol. Imaging 2003, 30, 9–15. [DOI] [PubMed] [Google Scholar]
- (6).Gotthardt M; van Eerd-Vismale J; Oyen WJ; de Jong M; Zhang H; Rolleman E; Maecke HR; Behe M; Boerman O, Indication for Different Mechanisms of Kidney Uptake of Radiolabeled Peptides. J. Nucl. Med 2007, 48, 596–601. [DOI] [PubMed] [Google Scholar]
- (7).Melis M; Valkema R; Krenning EP; de Jong M, Reduction of Renal Uptake of Radiolabeled Octreotate by Amifostine Coadministration. J. Nucl. Med 2012, 53, 749–753. [DOI] [PubMed] [Google Scholar]
- (8).Chan HS; Konijnenberg MW; Daniels T; Nysus M; Makvandi M; de Blois E; Breeman WA; Atcher RW; de Jong M; Norenberg JP, Improved Safety and Efficacy of (213)Bi-Dotatate-Targeted Alpha Therapy of Somatostatin Receptor-Expressing Neuroendocrine Tumors in Mice Pre-Treated with L-Lysine. EJNMMI Res. 2016, 6, 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Strosberg J; El-Haddad G; Wolin E; Hendifar A; Yao J; Chasen B; Mittra E; Kunz PL; Kulke MH; Jacene H; Bushnell D; O’Dorisio TM; Baum RP; Kulkarni HR; Caplin M; Lebtahi R; Hobday T; Delpassand E; Van Cutsem E; Benson A; Srirajaskanthan R; Pavel M; Mora J; Berlin J; Grande E; Reed N; Seregni E; Oberg K; Lopera Sierra M; Santoro P; Thevenet T; Erion JL; Ruszniewski P; Kwekkeboom D; Krenning E; Investigators N-T, Phase 3 Trial of (177)Lu-DOTATATE for Midgut Neuroendocrine Tumors. N. Engl. J. Med 2017, 376, 125–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Behr TM; Goldenberg DM; Becker W, Reducing the Renal Uptake of Radiolabeled Antibody Fragments and Peptides for Diagnosis and Therapy: Present Status, Future Prospects and Limitations. Eur. J. Nucl. Med 1998, 25, 201–212. [DOI] [PubMed] [Google Scholar]
- (11).Knowler WC; Barrett-Connor E; Fowler SE; Hamman RF; Lachin JM; Walker EA; Nathan DM; Diabetes Prevention Program Research, G., Reduction in the Incidence of Type 2 Diabetes with Lifestyle Intervention or Metformin. N. Engl. J. Med 2002, 346, 393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Coughlan KA; Valentine RJ; Ruderman NB; Saha AK, Ampk Activation: A Therapeutic Target for Type 2 Diabetes? Diabetes Metab. Syndr. Obes 2014, 7, 241–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Yu X; Mao W; Zhai Y; Tong C; Liu M; Ma L; Yu X; Li S, Anti-Tumor Activity of Metformin: From Metabolic and Epigenetic Perspectives. Oncotarget 2017, 8, 5619–5628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Dowling RJ; Goodwin PJ; Stambolic V, Understanding the Benefit of Metformin Use in Cancer Treatment. BMC Med. 2011, 9, 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Gong J; Kelekar G; Shen J; Shen J; Kaur S; Mita M, The Expanding Role of Metformin in Cancer: An Update on Antitumor Mechanisms and Clinical Development. Target. Oncol 2016, 11, 447–467. [DOI] [PubMed] [Google Scholar]
- (16).Kundra V; Mannting F; Jones AG; Kassis AI, Noninvasive Monitoring of Somatostatin Receptor Type 2 Chimeric Gene Transfer. J. Nucl. Med 2002, 43, 406–412. [PubMed] [Google Scholar]
- (17).Yang D; Han L; Kundra V, Exogenous Gene Expression in Tumors: Noninvasive Quantification with Functional and Anatomic Imaging in a Mouse Model. Radiology 2005, 235, 950–958. [DOI] [PubMed] [Google Scholar]
- (18).Sánchez F; Orero A; Soriano A; Correcher C; Conde P; González A; Hernández L; Moliner L; Rodríguez-Alvarez MJ; Vidal LF; Benlloch JM; Chapman SE; Leevy WM, Albira: A Small Animal PET/SPECT/CT Imaging System. Med. Phys 2013, 40, 051906. [DOI] [PubMed] [Google Scholar]
- (19).Melnikov VY; Ecder T; Fantuzzi G; Siegmund B; Lucia MS; Dinarello CA; Schrier RW; Edelstein CL, Impaired IL-18 Processing Protects Caspase-1-Deficient Mice from Ischemic Acute Renal Failure. J. Clin. Invest 2001, 107, 1145–1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Verwijnen SM; Krenning EP; Valkema R; Huijmans JGM; de Jong M, Oral Versus Intravenous Administration of Lysine: Equal Effectiveness in Reduction of Renal Uptake of [111In-DTPA]Octreotide. J. Nucl. Med 2005, 46, 2057–2060. [PubMed] [Google Scholar]
- (21).Trejtnar F; Novy Z; Petrik M; Laznickova A; Melicharova L; Vankova M; Laznicek M, In Vitro Comparison of Renal Handling and Uptake of Two Somatostatin Receptor-Specific Peptides Labeled with Indium-111. Ann. Nucl. Med 2008, 22, 859–867. [DOI] [PubMed] [Google Scholar]
- (22).Flook AM; Yang J; Miao Y, Evaluation of New Tc-99m-Labeled Arg-X-Asp-Conjugated Α-Melanocyte Stimulating Hormone Peptides for Melanoma Imaging. Molecular Pharmaceutics 2013, 10, 3417–3424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Müller C; Schibli R; Krenning EP; de Jong M, Pemetrexed Improves Tumor Selectivity of 111In-DTPA-Folate in Mice with Folate Receptor–Positive Ovarian Cancer. J. Nucl. Med 2008, 49, 623–629. [DOI] [PubMed] [Google Scholar]
- (24).Kwekkeboom DJ; Teunissen JJ; Bakker WH; Kooij PP; de Herder WW; Feelders RA; van Eijck CH; Esser JP; Kam BL; Krenning EP, Radiolabeled Somatostatin Analog [177Lu-Dota0,Tyr3]Octreotate in Patients with Endocrine Gastroenteropancreatic Tumors. J. Clin. Oncol 2005, 23, 2754–2762. [DOI] [PubMed] [Google Scholar]
- (25).Bednar F; Simeone DM, Metformin and Cancer Stem Cells: Old Drug, New Targets. Cancer Prev. Res 2012, 5, 351–354. [DOI] [PubMed] [Google Scholar]
- (26).Martin-Castillo B; Vazquez-Martin A; Oliveras-Ferraros C; Menendez JA, Metformin and Cancer: Doses, Mechanisms and the Dandelion and Hormetic Phenomena. Cell Cycle 2010, 9, 1057–1064. [DOI] [PubMed] [Google Scholar]
- (27).Hirsch HA; Iliopoulos D; Tsichlis PN; Struhl K, Metformin Selectively Targets Cancer Stem Cells, and Acts Together with Chemotherapy to Block Tumor Growth and Prolong Remission. Cancer Res. 2009, 69, 7507–7511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).de Jong M; Barone R; Krenning E; Bernard B; Melis M; Visser T; Gekle M; Willnow TE; Walrand S; Jamar F; Pauwels S, Megalin Is Essential for Renal Proximal Tubule Reabsorption of (111)in-Dtpa-Octreotide. J. Nucl. Med 2005, 46, 1696–1700. [PubMed] [Google Scholar]
- (29).Gong L; Goswami S; Giacomini KM; Altman RB; Klein TE, Metformin Pathways: Pharmacokinetics and Pharmacodynamics. Pharmacogenet. Genomics 2012, 22, 820–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.