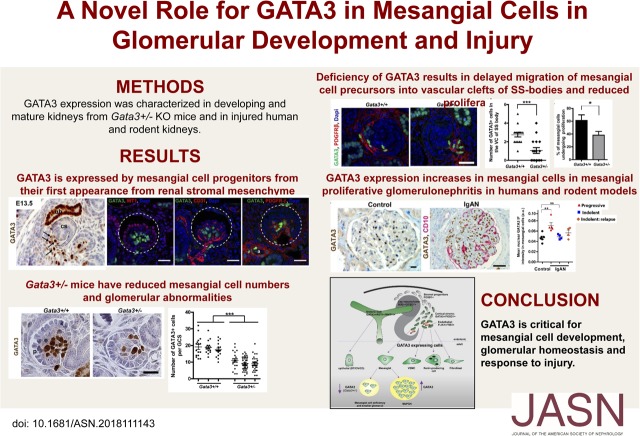
Significance Statement
Mesangial cells play a crucial role in maintaining glomerular homeostasis and injuries to these cells often result in progression to CKD like IgA and diabetic nephropathies. However, the transcription factors involved in mesangial cell development and function are largely unknown. The authors describe the role transcription factor GATA3 plays in mesangial cells in embryonic kidneys and healthy and injured adult glomeruli. Mice with haploinsufficiency of GATA3 have too few MC precursor cells and glomerular abnormalities. GATA3 expression increases in mesangial cells in mesangial proliferative GN in humans and rodent models suggesting GATA3 is important for glomerular homeostasis and response to injury. GATA3 also may be a useful a nuclear marker of human mesangial cells.
Keywords: GATA3, IgA nephropathy, kidney development, transcription factors
Visual Abstract
Abstract
Background
GATA3 is a dual-zinc finger transcription factor that regulates gene expression in many developing tissues. In the kidney, GATA3 is essential for ureteric bud branching, and mice without it fail to develop kidneys. In humans, autosomal dominant GATA3 mutations can cause renal aplasia as part of the hypoparathyroidism, renal dysplasia, deafness (HDR) syndrome that includes mesangioproliferative GN. This suggests that GATA3 may have a previously unrecognized role in glomerular development or injury.
Methods
To determine GATA3’s role in glomerular development or injury, we assessed GATA3 expression in developing and mature kidneys from Gata3 heterozygous (+/−) knockout mice, as well as injured human and rodent kidneys.
Results
We show that GATA3 is expressed by FOXD1 lineage stromal progenitor cells, and a subset of these cells mature into mesangial cells (MCs) that continue to express GATA3 in adult kidneys. In mice, we uncover that GATA3 is essential for normal glomerular development, and mice with haploinsufficiency of Gata3 have too few MC precursors and glomerular abnormalities. Expression of GATA3 is maintained in MCs of adult kidneys and is markedly increased in rodent models of mesangioproliferative GN and in IgA nephropathy, suggesting that GATA3 plays a critical role in the maintenance of glomerular homeostasis.
Conclusions
These results provide new insights on the role GATA3 plays in MC development and response to injury. It also shows that GATA3 may be a novel and robust nuclear marker for identifying MCs in tissue sections.
GATA binding protein 3 (GATA3) is a member of an evolutionary conserved family of six dual zinc-finger transcription factors (GATA1–6) with essential roles in cell lineage commitment and differentiation. GATA3 binds and remodels inaccessible chromatin,1 which is essential for cellular reprogramming during embryonic development and in response to injury. GATA3 is crucial for the normal development of many fetal tissues, including breast, parathyroid glands, kidney, and inner ear.2−7 In adults, GATA3 is required for maintenance of cell differentiation; for example, in mammary luminal epithelial cells and in parathyroid chief cells.2,3 GATA3 is also the master regulator of leukocyte differentiation into Th2 lymphocytes and group 2 innate lymphoid cells (ILC2), both of which modulate inflammation and promote tissue repair.8–10
In the kidney, GATA3 is essential for formation of the nephric duct and ureteric buds that give rise to branching of the ureteric tree and subsequently the collecting ducts.4,5 Deletion of Gata3 in mice leads to failed kidney development,6 whereas specific inactivation in the nephric duct causes ectopic ureteric budding and a spectrum of urogenital malformations.4 Mutations of GATA3 in humans cause syndromic autosomal dominant hypoparathyroidism, sensorineural deafness, and renal dysplasia (HDR).11−13 HDR often results in progressive kidney disease caused by congenital renal aplasia, hypoplasia, or dysplasia (41%); vesicoureteral reflux (16%); and cysts or pelvicalyceal deformities (11%).14 More recently, HDR has been associated with nephrotic syndrome and isolated cases of GN,15,16 whereas Gata3 hypomorphic mutant mice rescued with a yeast artificial chromosome Gata3 transgene, whose expression was limited to renal tubules and not glomeruli, have been shown to suffer glomerular mesangial cell (MC) defects.17 In genome-wide transcriptome studies, GATA3 has been identified as a highly enriched transcript in MCs,18,19 but the functional significance of GATA3 expression in MCs in health and disease has not been explored. Moreover, GN in HDR could reflect the effect of GATA3 deficiency on immunity because overexpression in T cells protects against lupus nephritis in BXSB/MpJ-Yaa mice,20 and on ILC2s, which are reported to be central to renal repair.8 Alternatively it could be evidence of a novel role for GATA3 in glomerular development or homeostasis; this study was designed to determine whether this is the case.
We show that GATA3 is expressed by FOXD1+ stromal cells, and that a subset, which mature into MCs, continues to express GATA3 in adult kidneys. In mice, we uncover an essential role for GATA3 in glomerular development and document marked changes in mesangial GATA3 expression in GN (both rodent models and IgA nephropathy [IgAN]). The results provide novel insights into MC development and responses to injury, identifying GATA3 as a new and robust marker for MCs in tissue sections.
Methods
Animals
Studies with congenic Gata3+/taulacZ (Gata3+/−) mice21 on an FVB/N background were approved by the University of Oxford Ethical Review Committee and were licensed under the Animal (Scientific Procedures) Act 1986, issued by the UK Government Home Office Department. Kidneys were dissected and fixed overnight in 4% paraformaldehyde at 4°C. Embryos were collected with the day of the vaginal plug designated as embryonic day (E) 0.5 and staged by morphologic criteria, which included somite number and eye and limb morphology.
Mesangial Proliferative Anti-Thy1.1 Nephritis Model
Anti-Thy1.1 nephritis was induced in male Wistar rats (Charles River Laboratories, Wilmington, MA) by injection of specific (OX7) antibody as described.22
Nephrotoxic Nephritis Mouse Model
Nephrotoxic serum nephritis was induced in 8- to 10-week-old male C57BL/6 wild-type mice by intraperitoneal injection of 2.5 mg of nephrotoxic sheep serum per gram of mouse body weight, as described.23 Controls were injected intraperitoneally with an equal amount of nonspecific sheep IgG.
Human Kidney Biopsy Samples
The use of patient material was approved by the Ethics Committee of Medical University of Vienna (ethical approval no. EK 1673/2013) before commencement of the study, and patients provided informed written consent in agreement with the Declaration of Helsinki. Human kidney biopsy samples either from time-zero renal allograft biopsy samples (n=12) or those that were diagnosed with steroid-responsive minimal change disease (n=5), membranous nephropathy (MGN) (n=7), lupus nephritis class IV (LN) (n=13), or IgAN (n=25) (Supplemental Tables 1–3) were processed for preparation of 2-μm sections for immunohistologic evaluation.
MC Culture
Primary MC cultures were established from glomeruli isolated from kidney cortex tissue (sampled from patients undergoing nephrectomy because of renal cell carcinoma) by the differential sieving method described before.24,25 MCs outgrowths were grown in RPMI 1640 medium containing 10% FCS, 1% penicillin/streptomycin (Gibco), and ITS (Sigma-Aldrich). Cells were harvested on days 7, 21, 29, and 43 after seeding glomeruli for preparation of RNA and protein lysates. Conditionally immortalized human glomerular MCs were kindly provided by Professor Moin Saleem (University of Bristol) and grown as described.25 Cells were growth-arrested in serum-free medium for 24 hours before addition of PDGF-BB (5–60 ng/ml), TGF-β (2 ng/ml), TNF-α (10 ng/ml), IL4 (25 ng/ml), and INF-γ (20 ng/ml). After 24–48 hours cells were harvested for preparation of RNA or protein lysates.
Quantitative RT-PCR
Total RNA was extracted from frozen kidney tissue and primary mesangial or conditionally immortalized MCs with RNeasy mini kit (Qiagen), quantified by NanoDrop spectrophotometer and used to prepare cDNA with SuperScript III reverse transcription (Invitrogen). Quantitative PCR was performed using KAPA SYBR FAST master mix (Kapa Biosystems) and specific primers (Supplemental Table 4). Samples were analyzed on a qTOWER3 thermal cycler (Analytik Jena), and the relative level of GATA3 expression was normalized to the level of GADPH expression using the 2−ΔΔCT method.
Microarray Analysis
Human kidney biopsy specimens for Affymetrix microarray expression data (HG-U133A Array) were obtained within the scope of the European Renal cDNA Bank Kröner-Fresenius Biopsy Bank.26,27 Diagnostic renal biopsy specimens were received from patients after an informed written consent, approved by the local ethics committees. After renal biopsy, the tissue was transferred to RNase inhibitor and microdissected into glomerular and tubulointerstitial compartments. Total RNA was isolated from microdissected glomeruli, reverse transcribed, and linearly amplified as described.28 Microarray expression data presented in this study was obtained from individual patients with IgAN (n=27). Pretransplantation kidney biopsy specimens from living donors (n=6) were used as controls. Fragmentation, hybridization, staining, and imaging were performed with the Affymetrix HG-U 133A system (Affymetrix, Santa Clara, CA), according to the manufacturer’s instructions. CEL file normalization was performed with the Robust Multichip Average method using RMAExpress (version 1.0.5) and the human Entrez-Gene custom CDF annotation from Brain Array, version 18 (http://brainarray.mbni.med.umich.edu/Brainarray/default.asp). To identify differentially expressed genes, the significance analysis of microarrays) method was applied using TiGR (MeV, version 4.8.1).29 A q-value <5% was considered to be statistically significant.
Western Blot Analysis
Total protein was extracted in lysis buffer (50 mM Tris, 150 mM NaCl, 0.1% SDS, 0.5% sodium deoxycholate, 1% Triton ×100, 1 mM PMSF, 1% protease inhibitor cocktail) and separated by 10% SDS-PAGE, followed by transfer onto polyvinylidene difluoride membranes (Immobilon-FL). Membranes were blocked in Odyssey buffer (LI-COR) for 1 hour at room temperature, followed by incubation with primary antibodies against GATA3 and α-tubulin (Supplemental Table 5) diluted in Odyssey buffer overnight at 4°C. After washing, membranes were incubated with secondary antibodies targeting rabbit or mouse IgG (IRDye 680RD and IRDye 800CW conjugates; LI-COR). Detection was performed using LI-COR Odyssey infrared scanner and quantification performed using LI-COR Image Studio Lite.
Histology, Immunohistochemistry, and Immunofluorescence
Fixed mouse kidneys or embryos were processed for embedding in paraffin and cut into 2–6 μm sections for hematoxylin and eosin staining, periodic acid–Schiff staining, or for immunostaining. Deparaffinized kidney sections were rehydrated in graded alcohols (100%, 96%, 70%, and 50%), and antigen retrieval was performed in citrate buffer in the autoclave at 120°C for 20 minutes. For immunohistochemistry (IHC) signal detection, endogenous peroxidase activity was quenched by incubation in 3% (vol/vol) H2O2 for 10 minutes. Where biotinylated secondary antibodies were used, sections were incubated with avidin block for 10 minutes. Sections were blocked in 10% goat serum for 30 minutes at room temperature and incubated overnight at 4°C with primary antibodies (Supplemental Table 5). For IHC detection, the immobilized antibodies were detected by UltraVision-LP HRP Polymer detection system specific for anti-mouse and anti-rabbit IgG (ThermoFisher Scientific), or with biotinylated secondary antibodies and AB reagents (Vector Laboratories) according to manufacturer’s instructions. DAB (Vector Laboratories) and hematoxylin were used as the chromogen and the nuclear counterstain, respectively. In negative controls, primary antibodies were omitted. For immunoflourescence (IF) detection the following secondary antibodies were used: Alexa Fluor 488 goat anti-mouse IgG1, Alexa Fluor 488 goat anti-mouse IgG (H+L), Alexa Fluor 546 goat anti-mouse IgG2A, Alexa Fluor 546 goat anti-rabbit IgG, Alexa Fluor 546 goat anti-rat IgG, Alexa Fluor 647 goat anti-rabbit IgG, Alexa Fluor 594 donkey anti-goat IgG (ThermoFisher Scientific). Dapi was used to visualize the nuclei.
Quantitative Image Analysis
Immunostained tissue slides were visualized and digitized using Zeiss AxioCam 512 color camera attached to a Zeiss Imager M2 microscope; by a confocal laser scanning microscope (LSM700; Carl Zeiss); or by digital slide scanner (3DHISTECH Pannoramic 250 Flash equipped with Adimec Q-12A-180Fc camera). Images were analyzed with the ZEN2012 software (Carl Zeiss) and quantification was performed with ImageJ (National Institutes of Health) and CellProfiler software.30 Cell Profiler pipelines are available upon request. Briefly, glomerular cross section (GCS) areas were outlined and cells were identified by nuclear Dapi staining. To determine the percentage of positively stained cells, IF intensity thresholds for each stain (nuclear or cytoplasmic) were determined either automatically using an inbuilt classifier (Cell Profiler Analyst) or by plotting mean intensity values per cell on a scatter plot (example shown in Supplemental Material). The number of positively stained cells were expressed as percentage of total Dapi+ cells per GCS area in more than ten glomeruli per animal/genotype. Measurements of immunohistochemically stained areas were expressed as a percentage of total GCS areas (μm2) in more than ten glomeruli per animal/genotype.
Statistical Analyses
All values are expressed as means±SD. Statistical significance (defined as P<0.05) was evaluated using t tests, Mann–Whitney U tests, or ANOVA for multiple group comparisons. Statistical analysis was performed using GraphPad Prism 6.0 (GraphPad Software Inc., San Diego, CA).
Results
Characterization of GATA3 Expression in Healthy Adult Kidneys
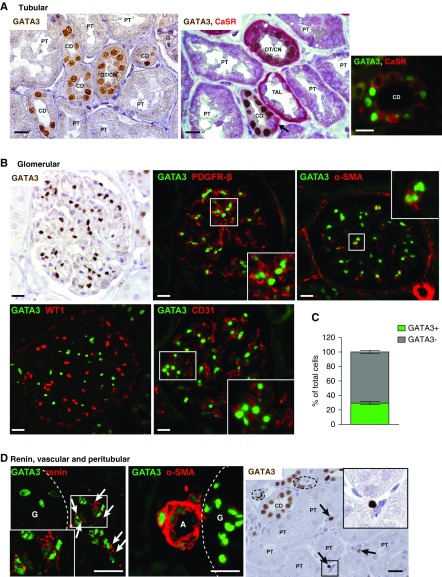
GATA3 has an established role in kidney development, but whether GATA3 is expressed in mature kidneys, or involved in homeostasis as in other tissues, is unknown.2,3,9 Using renal allograft biopsy specimens from healthy adults to analyze GATA3 expression showed strong expression in collecting duct (CD) epithelium (Figure 1A) (originating from GATA3-expressing ureteric bud progenitors) and in distal and connecting tubules. In CDs, GATA3 was found in principal but not intercalated cells, as demonstrated by the absence of coexpression with calcium sensing receptor (CaSR) (Figure 1A). By contrast, GATA3 was uniformly undetectable in proximal tubular epithelium arising from progenitors in the mesenchymal cap, as do podocytes and glomerular parietal epithelial cells. Thus, renal epithelial expression of GATA3 is maintained in cells that arise from ureteric bud progenitors, suggesting a function in homeostasis.
Figure 1.
Expression of GATA3 in healthy adult kidneys. (A) IHC for GATA3 (brown) in time-zero renal allograft biopsy samples from healthy adults demonstrated strong nuclear expression of GATA3 in a subset of tubular epithelial cells. CaSR (red) was used as a nephron segment marker53 in double staining, which showed that GATA3 was absent from proximal tubules (PT) and thick ascending limbs (TAL), but was expressed in distal/connecting nephron segments (DT/CN) and in principal cells of the CDs where it was absent from intercalated cells (arrow). IF staining for GATA3 (green) and CaSR (red) confirmed the cell specific expression of GATA3 in principal cells of the CD, which do not express CaSR. Scale bars, 20 μm. (B) Glomerular GATA3 expression was detected by IHC (brown) and by IF (green) where it was confined to MCs costaining with MC markers PDGFR-β and α-SMA (red). GATA3 did no colocalize with WT-1 or CD31, markers of epithelial (podocyte and parietal) and endothelial cells, respectively. Scale bars, 20 μm. (C) Quantification of the number of GATA3-expressing cells in GCS areas (n≥10) from healthy human kidneys (n=6). (D) GATA3 colocalized with the aspartyl-protease renin (red) in juxtaglomerular cells (arrows) and with α-SMA (red) in smooth muscle cells of the afferent arterioles (A). Peritubular expression of GATA3 was detected by IHC in a subset of interstitial fibroblasts (arrows). Arterioles indicated by dashed lines. Scale bars, 20 μm. Daltonized versions of Immunofluorescence pictures are provided in Supplemental Appendix 1. G, glomerulus.
There was strong nuclear GATA3 expression in MCs identified by colocalization with PDGF receptor β (PDGFR-β) and α-smooth muscle actin (α-SMA). GATA3 did not colocalize with Wilms tumor protein (WT-1) or CD31, demonstrating it was not expressed by podocytes, parietal epithelial cells, or endothelial cells (Figure 1B). In healthy human kidneys, 28.42%±2.18% (mean±SD) of the cells per GCS expressed GATA3, consistent with the expected proportion of MCs in the glomerular tuft (Figure 1C). GATA3 expression and its colocalization with cell-specific markers was conserved between human, mouse, and rat kidneys (Supplemental Figure 1).
GATA3 was detected in renin-expressing cells of the juxtaglomerular apparatus, revealing a novel role of GATA3 in renal endocrine cells. GATA3 was also expressed in renal smooth muscle cells (VSMCs) colocalized with α-SMA, and in a subset of peritubular fibroblasts (Figure 1D). MCs, renin-secreting cells, VSMCs, and some renal fibroblasts have recently been reported to share a common renal stromal progenitor,31−34 and so it is striking that they all express GATA3 in the adult kidney. This led us to analyze embryonic mouse and human kidneys to investigate GATA3 expression in renal stromal cells during development.
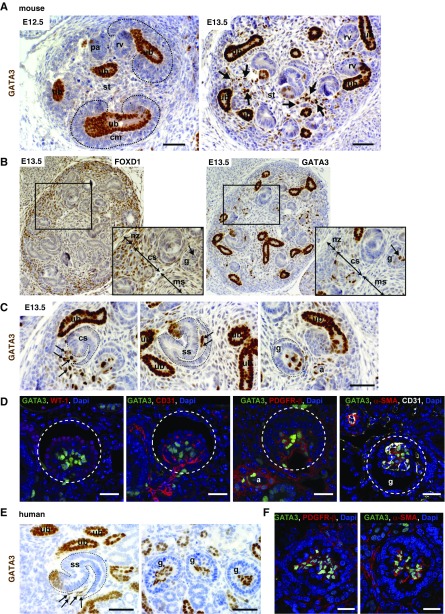
GATA3 Is Expressed in Embryonic Renal Stromal Mesenchyme and Is a Marker of Mesangial Progenitor Cells
GATA3 spatio-temporal expression in renal stromal mesenchyme and its cell derivatives was examined in mouse embryos at various time points. At E12.5, strong GATA3 expression was limited to the ureteric buds and tips; it was very weakly expressed in interstitial stromal cells (Figure 2A), but absent from cap mesenchyme that provides progenitor cells for the nephron. By E13.5, GATA3 was strongly expressed in a subset of spindle-shaped mesenchymal cells occupying spaces between ureteric bud branches and induced nephrons interior to the nephrogenic zone (Figure 2, A and B). These stromal cells are characterized by expression of forkhead-box D1 (FOXD1) transcription factor from E10.5,34,35 and a subset of them expressed GATA3 from E13.5 (Figure 2B). GATA3-expressing cells were observed at the entrance of the vascular cleft of the comma-shaped body, within the cleft of the s-shaped (SS) body, and in the cap-stage glomeruli (Figure 2C). GATA3 colocalized with PDGFR-β and α-SMA in the core of the capillary tuft, but not with WT-1 or CD31 (Figure 2D). This identifies GATA3 as an early marker for MC precursors. Interestingly, FOXD1 expression was no longer detected in these MC precursors, suggesting downregulated expression during differentiation from stromal progenitors (Figure 2B). There was no colocalization between GATA3 and CD31 either in glomeruli or arterioles (Figure 2D), demonstrating its expression in the renal vasculature was confined to VSMCs.
Figure 2.
Expression of GATA3 in developing mouse and human kidneys. (A) ICH for GATA3 (brown) in wild-type mouse embryos at E12.5 and E13.5. GATA3 expression is strong in ureteric bud (ub) cell nuclei, but absent from cap mesenchyme (cm). Arrows indicate increased expression of GATA3 in stromal cells (st) in E13.5 kidneys. Scale bars, 50 μm. (B) IHC for FOXD1 (left panel) and GATA3 (right panel) in E13.5 mouse kidney serial sections. Expression of FOXD1 and GATA3 overlaps in a subset of stromal cells in the capsular stoma (cs). FOXD1 expression is absent from MCs where GATA3 is strongly expressed (arrow). Higher magnification images of the boxed regions are shown. (C) At E13.5, GATA3-expressing stromal cells (arrows) can be seen migrating into the vascular cleft of the comma-shaped (cs) and SS bodies and reside within the core of the glomerulus (g). Smooth muscle cells of the developing arterioles (a) also express GATA3. Scale bars, 50 μm. (D) Co-IF with anti-GATA3 (green) and anti–WT-1 (podocyte marker), anti-CD31 (endothelial marker), anti–PDGFR-β, and anti–α-SMA (mesangial markers) antibodies in mouse E13.5 kidneys showing localization of GATA3 in MCs of the developing glomeruli (g, outlined) and in smooth muscle cells of arterioles (a). Scale bars, 20 μm. (E) IHC for GATA3 (brown) in 23-week human fetal kidney demonstrating GATA3 expression in renal stromal cells (arrows) located within the vascular cleft of the SS bodies and the core of the glomeruli (g). Scale bars, 50 μm. (F) Co-IF in 23-week human fetal kidney with anti-GATA3 (green) and anti–PDGFR-β (red, left panel) and anti–α-SMA (red, right panel) antibodies demonstrating GATA3 expression in MCs of glomeruli. Scale bars, 20 μm. ms, medullary stroma; nz, nephrogenic zone.
A similar pattern of GATA3 expression was observed in 23-week human fetal kidneys. GATA3-expressing cells were detected in the vascular cleft of the SS body and within capillary loop-stage glomeruli where GATA3 colocalized with α-SMA and PDGFR-β, demonstrating its expression by MC precursors in the core of the renal corpuscle (Figure 2, E and F, Supplemental Figure 2). GATA3 was also expressed in developing renal VSMCs where it colocalized with α-SMA, PDGFR-β, and renin, which is expressed throughout the developing renal vasculature (Supplemental Figure 2).31 GATA3 expression preceded renin expression in renal VSMCs in both human and mouse embryos (data not shown).
Unexpectedly, expression of GATA3 was also observed in the distal SS body (Figure 2, C and E, Supplemental Figure 3), revealing that GATA3 is also a marker of distal-tubule precursors; its persistent expression in the distal and connecting nephron segments in the mature kidney (Figure 1) suggests that GATA3 is required for differentiation and maturation of these distal-tubule epithelial cell precursors.
GATA3 Expression Is Downregulated in MCs in vitro
GATA3 was uniformly highly expressed in human MCs during development and in mature adult glomeruli, as well as in rodents. GATA3 expression (quantitative RT-PCR and Western blot) was similarly high in glomeruli freshly isolated from human kidneys, but was rapidly downregulated in primary cultures of MCs derived from them: reduced in the early (7-day) outgrowths and negligible after 21 days (Supplemental Figure 4A). These unexpected results were supported by the absence of GATA3 in a conditionally immortalized human MC line,25 both in their proliferative, “permissive” temperature (33°C), and after growth arrest and differentiation at 37°C (Supplemental Figure 4B). GATA3 expression, assessed by protein or mRNA, could not be rescued by addition of cytokines, including PDGF-BB (activates MC proliferation36), TNF-α, TGF-β, IL4 (potent stimulus of T cell GATA3), or INF-γ (Supplemental Figure 4B). The loss of GATA3 from primary MC cultures precludes further analysis of GATA3 functions in vitro, and raises more general issues about using cultured MC to study MC-specific cellular pathways.
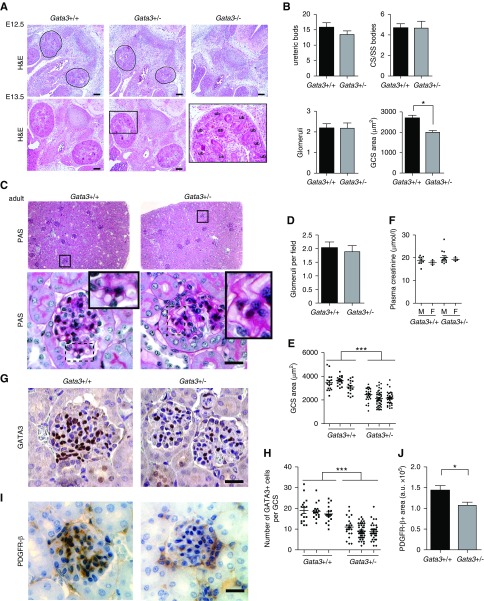
MC Defects in Gata3-Deficient Mice
Homozygous Gata3 knockout (Gata3−/−) mouse embryos had gross developmental abnormalities with absent metanephric kidneys at E12.5 and died shortly thereafter (Figure 3A). In contrast, kidneys of Gata3+/− mice at E12.5 and E13.5 were of similar size and morphology to those of age-matched wild-type littermates with similar numbers of ureteric buds, comma-shaped/SS bodies and developing glomeruli, and subsequently developed into mature kidneys (Figure 3, A–C). However, analysis of GCS areas in both developing and mature kidneys revealed a significant reduction in Gata3+/− mice compared with wild-type littermates (Figure 3, B–D). Periodic acid–Schiff staining showed that Gata3+/− mice had weaker glomerular basement membrane staining and a higher frequency of glomerular capillary loop dilation (Figure 3C). Although plasma parameters for kidney function, including creatinine, urea, albumin, and total protein concentrations, were not significantly different between 6-week-old Gata3+/− mice and age- and sex-matched wild-type littermates, a subset of male Gata3+/− mice exhibited elevated plasma creatinine concentrations (Figure 3E, Supplemental Table 6). Immunohistochemical analysis of glomerular GATA3 expression and quantification of GATA3+ cells revealed that Gata3+/− mice had fewer MCs (Figure 3, F and G), which was confirmed by a reduction in PDGFR-β staining (Figure 3, H and I).
Figure 3.
Characterization of embryonic and mature kidneys from Gata3 deficient mice. (A) Hematoxylin and eosin (H&S) stained sections of mouse embryonic kidneys at E12.5 and E13.5 show that Gata3+/− mice have kidneys of similar size and morphology to those of age-matched wild-type littermates (Gata3+/+), whereas Gata3−/− mice have gross developmental abnormalities and absent metanephric kidneys at E12.5. Corresponding higher magnification image of boxed region (left panel). Scale bars, 100 μm. (B) Morphologic characterization of embryonic kidneys from E13.5 Gata3+/− (n=4) and wild-type (n=3) embryos was performed by quantifying the number of ureteric bud tips, comma-shaped (CS) and S-shaped (ss) bodies, cap-stage glomeruli in >15 4-μm sections passing through the midline plane per kidney; GCS areas were measured in at least ten cap-stage glomeruli per embryo (*P<0.05; unpaired t test). (C) Kidney sections from adult mice stained with periodic acid–Schiff (PAS) show that Gata3+/− mice developed mature kidneys with similar numbers of glomeruli. Higher magnification images reveal capillary loop dilation and thickened glomerular basement membrane in Gata3+/− mice. (D) Glomeruli were counted in >50 fields (500×500 μm) of digitized images of whole kidney sections (n=4 per kidney) from age-matched Gata3+/− (n=3) and wild-type (n=3) adult mice. (E) Quantification of GCS areas (n>20 per kidney) in Gata3+/− (n=3) and wild-type (n=3) adult mice (***P<0.001). (F) Plasma creatinine measurements in 6-week old male (M) and female (F) Gata3+/+ (n=12) and Gata3+/− (n=16) mice. (G) IHC for GATA3 (brown) in adult kidneys from wild-type and Gata3+/− mice show reduced number of GATA3+ MCs in Gata3+/− mice. (H) Quantification of the number of GATA3+ cells within glomeruli (n>20 per animal) of wild-type (n=3) and Gata3+/− (n=3) mice (***P<0.001). (I) Confirmation of reduced MC number using an independent mesangial marker PDGFR-β by IHC (brown) in adult kidneys from wild-type and Gata3+/− mice. (J) Quantification of the area occupied by PDGFR-β stain within glomeruli (n>10 per animal) of wild-type (n=3) and Gata3+/− (n=3) mice (*P<0.05). Scale bars, 20 μm.
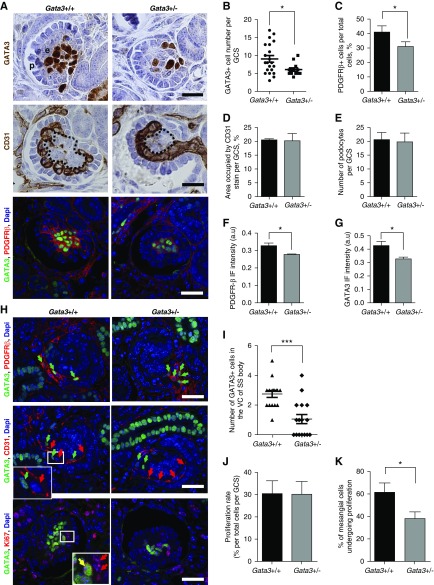
We next analyzed the effect of GATA3 haploinsufficiency on glomerular maturation. By E15.5, developing glomeruli are organized into discrete cellular compartments, and analysis of GATA3 and PDGFR-β expression showed that Gata3+/− mice had significantly fewer MC precursors than wild-type littermates and smaller total GCS areas (Figure 4, A–C). Despite normal CD31+ endothelial and WT-1+ podocyte cell numbers, the mesangial cores were significantly smaller in the Gata3+/− mice and capillaries were poorly organized (Figure 4, A, D, and E). Quantification of the PDGFR-β IF signal intensity indicated that expression of PDGFR-β was attenuated in MCs of Gata3+/− compared with wild-type mice, as was the GATA3 IF signal, confirming mesangial GATA3 haploinsufficiency, albeit by approximately 30% reduction of the wild-type levels. Although PDGFR-β expression preceded GATA3 expression in stromal mesenchyme (Supplemental Figure 5), these observations suggest that GATA3 regulates PDGFR-β expression in MCs in maturing glomeruli.
Figure 4.
Gata3+/− mice have MC defects. (A) IHC for GATA3 (brown) in developing glomeruli of E15.5 wild-type and Gata3+/− embryos reveals a reduction in the number of MCs in Gata3+/− mice (top panel). IHC for the endothelial marker CD31 (brown) shows abnormalities in capillary loop organization and smaller mesangium cores (outlined) in glomeruli of Gata3+/− mice (middle panel). Co-IF with anti-GATA3 (green) and anti–PDGFR-β (red) antibodies (lower panel) in cap-stage glomeruli. Scale bars, 20 μm. (B) The number of GATA3-expressing cells within glomeruli (n≥12 per genotype) of Gata3+/− (n=4) embryos are significantly reduced compared with wild-type (n=3) (*P<0.05). (C) Quantification of the number of PDGFR-β–expressing cells within glomeruli (n≥15 per genotype) of wild-type (n=3) and Gata3+/− (n=4) embryos confirms reduced number of MCs in Gata3+/− mice (*P<0.05). (D) Areas occupied by CD31 stain within glomeruli (n≥20 per genotype) of wild-type (n=3) and Gata3+/− (n=4) embryos are similar, showing that endothelial cell numbers are not affected in Gata3+/− mice. (E) Quantification of the number of podocytes within glomeruli (n≥30 per genotype) of wild-type (n=3) and Gata3+/− (n=4) embryos shows that podocyte numbers in Gata3+/− mice are similar to wild-type. (F) The mean cytoplasmic intensity of the PDGFR-β IF signal is significantly reduced in glomeruli (n≥15 per genotype) of Gata3+/− (n=4) compared with wild-type (n=3) embryos. *P<0.05. (G) The mean nuclear intensity of the GATA3 IF signal is significantly reduced in MCs of Gata3+/− mice (*P<0.05). (H) In wild-type kidneys cells expressing high levels of GATA3 (green) and PDGFR-β (red) migrate from the stroma into the vascular cleft of the SS body (arrows, top panel); these cells are closely associated with CD31+ endothelial progenitors (red arrows, middle panel). In Gata3+/− littermates fewer GATA3+PDGFR+ cells can be seen within the vascular cleft; instead they group around the entrance to the cleft and are dissociated from CD31+ cells. Co-IF for GATA3 and Ki67 (bottom panel) in cap-stage glomeruli shows that fewer GATA3+ MCs are proliferating (i.e., GATA3+Ki67+) in Gata3+/− kidneys, but the number of Ki67+ endothelial or podocyte cells are unaffected. (I) Quantification of the number of GATA3+ cells in the vascular cleft (VC; n≥15 per genotype) of the SS bodies in Gata3+/+ (n=3) and Gata3+/− (n=4) kidneys (***P<0.001). (J) The percentage of Ki67+ cells per total cells within GCS areas (n≥15 per genotype) are similar between wild-type (n=3) and Gata3+/− (n=4) kidneys. (K) A smaller proportion of GATA3+ cells in cap-stage glomeruli are proliferating (i.e., GATA3+Ki67+, yellow arrow) in Gata3+/− kidneys (*P<0.05).
Next we determined the mechanisms to explain reduced MC number in Gata3+/− mice by investigating recruitment of MC precursors into the developing glomeruli and MC proliferation rates in maturing glomeruli. Quantification of GATA3+ PDGFR-β+ cells in the vascular cleft of SS bodies revealed that fewer MC precursors migrated into the cleft in Gata3+/− mice compared with age-matched wild-type littermates (Figure 4, H and I, Supplemental Figure 6). Moreover, in wild-type embryos, GATA3+ MC precursors closely followed CD31+ endothelial progenitors, whereas this association was disrupted in Gata3+/− mice and GATA3+ MC progenitors were found to lag behind migrating endothelial cells and group together around the entrance to the cleft, presumably as a result of attenuated PDGFR-β expression (Figure 4H, Supplemental Figure 6). Analysis of proliferation rates within the cap-stage maturing glomeruli using the Ki67 proliferation marker demonstrated that although total glomerular proliferation rates were unaffected in Gata3+/− mice, reflecting normal endothelial cell proliferation, the proportion of GATA3+ cells that costained for Ki67 was significantly reduced revealing perturbed mesangial progenitor cell proliferation rates in Gata3+/− mice (Figure 4, H, J, and K).
Thus, Gata3+/− mice have impaired MC progenitor migration and proliferation during development resulting in a paucity of MCs in adult kidneys. These findings identify a nonredundant role for GATA3 in normal glomerular development; its persistent expression in mature MCs suggests a potential contribution to homeostasis and the response to injury. We tested this by analyzing GATA3 expression in rodent GN models and in human renal biopsy samples.
GATA3 Expression Is Increased in MCs in Thy.1.1 Nephritis in Rats and Nephrotoxic Nephritis in Mice
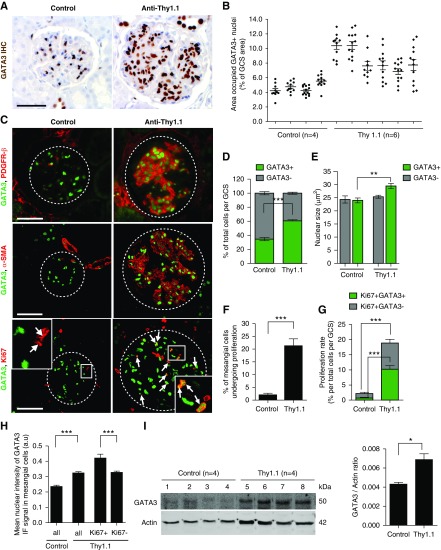
Glomerular GATA3 expression was quantified in rats with Thy1.1 nephritis. On day 8 post disease induction (maximal MC activation and proliferation), there was a striking increase in the number of GATA3-expressing nuclei in glomeruli compared with control rats, and also of the proportion of total GATA3-positive GCS area, although the extent varied between glomeruli (Figure 5, A and B). The nuclear staining was localized to cells that expressed PDGFR-β and α-SMA, confirming they were MCs (Figure 5C). There were significantly more GATA3+ MCs in the nephritic rats than in controls (mean±SD: 60.6%±3.3% and 34.5%±5.2%, respectively; P<0.001) (Figure 5D). Moreover, the nuclei of the GATA3+ MCs in nephritic rats were significantly enlarged, indicating cellular hypertrophy (Figure 5E). MC proliferation assessed by GATA3 and Ki67 double-staining was rare in healthy rats: less than a quarter of glomeruli contained a single double-positive cell, and only 1.55%±3.06% of GATA3+ MCs expressed Ki67 (Figure 5, C and F). Proliferating MCs accounted for 0.55%±1.04% of total cells per GCS (Figure 5G) and a quarter of total proliferating cells per GCS, most of which were endothelial cells (Supplemental Figure 7). GATA3+Ki67+ MCs were much more common in Thy1.1 nephritis, accounting for 10.94%±4.24% of total cells per GCS area, 20.89%±8.33% of total GATA3+ MCs, and 54.78%±15.92% of the total glomerular proliferating cells (Figure 5, C, F, and G). Again, endothelial cells accounted for most of the remainder (Supplemental Figure 7). Interestingly, 18.01%±10.17% of MCs of Thy1.1 rats had GATA3 IF intensity above the highest control GATA3 IF signal (Figure 5H, Supplemental Figure 8), indicating more GATA3 protein. Significantly more GATA3HIGH MCs were proliferating (Ki67+) than the GATA3NORMAL cells (49.90%±17.58% compared with 24.14%±6.99%, respectively; P<0.05) and the mean GATA3 IF intensity was greater in proliferating (GATA3+Ki67+) compared with nonproliferating Thy1.1 MCs (Figure 5H). Immunoblotting of glomerular protein lysates revealed that GATA3 abundance was almost two-fold higher in glomeruli from Thy1.1 compared with control rats (Figure 5I). Thus, GATA3 expression is upregulated in proliferating MCs in injured glomeruli.
Figure 5.
Increased GATA3 expression in the Thy1.1 mesangial proliferative nephritis model. (A) IHC for GATA3 (brown) in kidney sections of rats with Thy1.1 mesangial proliferative nephritis (day 8 post antibody administration) and control rats. Scale bar, 50 µm. (B) Quantification of the area occupied by GATA3 stain, corrected for GCS area (expressed as a % of GCS) (each dot represents a single glomerulus; mean±SEM shown). (C) Colocalization of GATA3 (green) with PDGFR-β (red, top panel) and α-SMA (red, middle panel) in glomerular MCs and with Ki67 (red, bottom panel) in proliferating MCs (arrows). (D) Quantitative analysis of digitized images of sections stained by IF revealed an increase in the number of GATA3-expressing nuclei per total cell number (Dapi stained) per GCS area in Thy1.1 rat kidneys compared with control kidneys (***P<0.001; n=3 rats per group, 10–15 glomeruli per rat). (E) Measurement of the nuclear area (Dapi stained) showed that GATA3-expressing cells in Thy1.1 rat glomeruli had enlarged nuclei compared with GATA3-expressing cells in control rats or GATA3-negative cells, indicating MC hypertrophy in Th1.1 nephritis (**P<0.01; n=3 rats per group, 10–15 glomeruli per rat). (F) Quantification of the proportion of MCs (GATA3+) undergoing proliferation (Ki67+GATA3+) per GSC area (***P<0.001; n=3 rats per group, 10–15 glomeruli per rat). (G) Proliferation rates assessed by Ki67 and GATA3 coimmunostaining. (***P<0.001; n=3 rats per group, 10–15 glomeruli per rat). (H) The mean nuclear intensity of the GATA3 IF signal significantly increased in nuclei of MCs (GATA3+) in Thy1.1 rats compared with controls (***P<0.001), indicating that GATA3 protein expression is upregulated in MCs in mesangial proliferative nephritis. Proliferating MCs (GATA3+, Ki67+) had a higher GATA3 IF signal intensity compared with nonproliferating MCs (GATA3+, Ki67−) (***P<0.001; n=3 rats per group, 10–15 glomeruli per rat). (I) Western blot analysis of glomerular lysates from Thy1.1 nephritic and control rats reveals profound upregulation of GATA3 protein (50 kDa). GATA3 protein levels normalized to total actin are shown (*P<0.05).
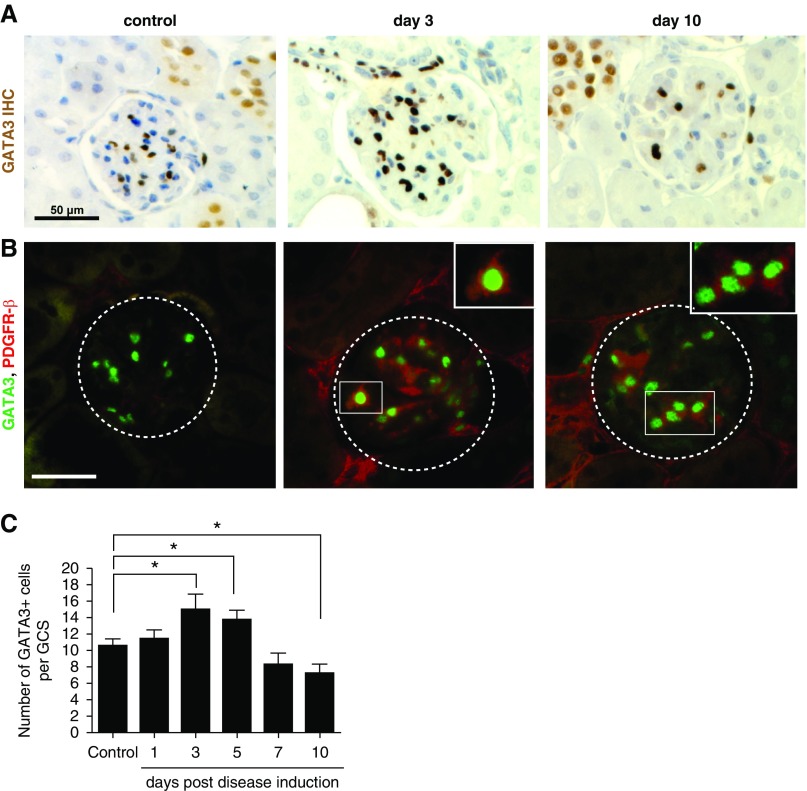
To confirm that changes in GATA3 expression were not unique to glomeruli with extensive mesangiolysis, we repeated the analyses in a nephrotoxic nephritis mouse model. On days 3 and 5 after anti-GBM antibody injection, glomeruli from nephritic mice had significantly more GATA3-expressing cells. Staining intensity was significantly greater, and the GATA3-expressing nuclei were enlarged (Figure 6). Later the number of GATA3-expressing MCs decreased, although some persisted even in severely scarred glomeruli.
Figure 6.
Glomerular expression of GATA3 in mice induced with nephrotoxic nephritis. (A) IHC for GATA3 (brown) in kidney sections of mice that were administered nephrotoxic antibodies to induce nephritis by days 3 and 10; and in corresponding control mice. Scale bar, 50 µm. (B) Co-IF with anti-GATA3 (green) and anti–PDGFR-β (red) antibodies demonstrating an increase in GATA3 IF signal in MCs coexpressing PDGFR-β at day 3 post disease induction (boxed region). Scale bar, 50 μm. (C) Quantification of the number of GATA3-expressing nuclei within a GCS area (n≥20) reveals an increase in GATA3+ MC number on the third and fifth day post disease induction, and a reduction in the number of GATA3+ cells by day 10. *P<0.05.
GATA3 as a MC Marker in Human Mesangial Proliferative Disease
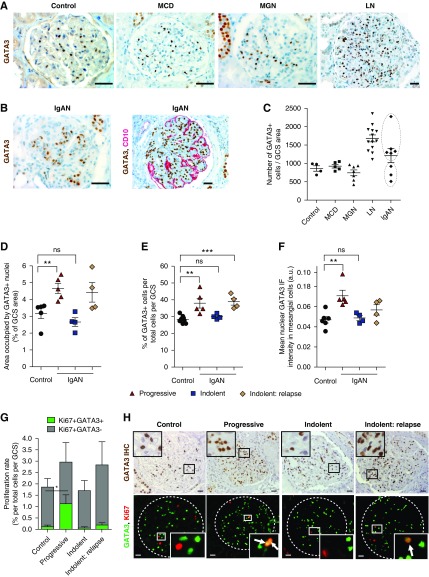
We next examined GATA3 expression in kidney biopsy specimens from patients with four different glomerular diseases with and without mesangial proliferation: systemic LN,37 IgAN, MGN, and steroid-responsive minimal change disease. There was no difference in the number of glomerular GATA3-expressing nuclei in biopsy specimens from time-zero renal allograft controls, MGN, or minimal change disease (Figure 7A). By contrast there were significantly more GATA3-expressing nuclei in all lupus nephritis biopsy specimens and a subset of IgAN cases (Figure 7B). There were two IgAN groups segregated by the degree of glomerular GATA3 expression: those with increased mesangial GATA3+ cell number and those with normal expression (Figure 7C). To verify the bimodal distribution, we analyzed renal biopsy specimens from a second group of nine patients with IgAN who had a second biopsy and in whom we could correlate the results with clinical data. Glomerular GATA3 expression increased in five patients and was normal in four, whether assessed by area occupied by GATA3+ nuclei-to-total GCS ratio, corrected GATA3+ cell number, or the mean nuclear GATA3 IF intensity (Figure 7, A–C). IgAN GATA3HIGH expressers also had significantly more MC proliferation (Ki67+) (GATA3 HIGH: 0.44%±0.62%; GATA3NORMAL: 0.12%±0.16%; P<0.05) (Figure 7, G and H). These GATA3HIGH patients with IgAN had sustained heavy proteinuria either at the time of the biopsy or developed it within 6 months and three had increased serum creatinine concentrations (progressive group, Supplemental Table 3). Of note, in this progressive group we also observed extraglomerular GATA3+ cell expansion often localized to the juxtaglomerular region, and in several cases, expansion of GATA3+ cells in the periglomerular fibrotic regions, associated both with ostensibly normal as well as highly sclerotic glomeruli where GATA3 expression persisted in the few remaining MCs (Supplemental Figure 9A). The GATA3NORMAL patients, however, had a normal serum creatinine both when biopsied and 6 months later (indolent group). However, all four had clinical relapses between 10 and 21 years later with increases in serum creatinine that provoked another renal biopsy (indolent: relapse). All four had increased GATA3+ MC number, MC proliferation, and GATA3 expression, demonstrating a correlation with progressive disease (Figure 7, D–H). Grouping the patients according to their Oxford Mesangial and Endocapillary hypercellularity, Segmental glomerulosclerosis, Tubular atrophy score of mesangial hypercellularity (M, zero, or one) showed a significant difference in GATA3 expression demonstrating the utility of GATA3 as a MC marker when evaluating MC expansion and proliferation (Supplemental Figure 9).
Figure 7.
Glomerular expression of GATA3 in patient renal biopsy samples. (A) IHC for GATA3 (brown) in sections of kidney biopsy samples from unaffected individuals (control) and patients with minimal change disease (MCD), membranous GN (MGN), and systemic lupus nephritis (LN). Scale bars, 50 µm. (B) IHC for GATA3 in sections of kidney biopsy samples from patients with IgA nephritis (IgAN) (brown, right panel), and dual ICH for GATA3 and CD10, a podocyte marker (red, left panel). Scale bars, 50 µm. (C) Numbers of GATA3+ cells were quantified in glomeruli of normal (n=4), MCD (n=5), MGN (n=7), LN (n=13), and IgAN (n=7) kidney biopsy samples, corrected for GCS area (in mm2; average GCS area in control is 0.027±0.007 mm2, mean±SD) and expressed as a ratio (GATA3+ cell number divided by GCS area). Each dot represents a mean of at least eight GCS areas per patient and mean±SEM per disease group is shown. Two populations were evident in the IgAN group (outlined). (D–F) Kidney biopsy samples from a second cohort of patients with IgAN with aggressive (n=5) or indolent (n=4; paired biopsy samples taken at first diagnosis and at relapse) disease were analyzed for GATA3 expression and time-zero renal allograft biopsy samples were used as controls. Areas occupied by GATA3 IHC stain, expressed as a percentage GCS area, were markedly increased in the aggressive IgAN group (P<0.01), but not in indolent IgAN group (D). Similarly, analysis of sections stained by IF revealed an increase in the number of GATA3-expressing nuclei per total cell number (Dapi stained) per GCS area in the aggressive IgAN group, but not in the indolent group, which only showed an increase in the repeat biopsy after relapse (E). The mean nuclear intensity of the GATA3 IF signal significantly increased in nuclei of MCs (GATA3+) in the aggressive IgAN group (F). Each dot represents a mean of at least eight GCS areas per patient and mean±SEM per group is shown. For each measurement, differences between means were considered statistically significant at *P<0.05; **P<0.01; ***P<0.001. (G) Assessment of the total (Ki67+) and mesangial (Ki67+, GATA3+) proliferation rates (expressed as a percentage of total cells per GCS area) revealed that patients with aggressive IgAN (n=5) had increased MC proliferation rates compared with controls (0.44%±0.62% versus 0.12%±0.16%; *P<0.05). A least eight GCS areas per patient were analyzed and mean±SEM per group is shown. (H) Representative sections of kidney biopsy samples from patients with IgAN stained by IHC for GATA3 (brown, top panel), and by IF for GATA3 and Ki67 showing localization of GATA3 in MCs and proliferating MCs (arrows), respectively. Scale bars, 20 μm. ns, not significant.
Recent transcriptomic analysis of differentially expressed genes in IgAN glomeruli identified GATA3 as an upregulated gene.38 We interrogated the cDNA expression data from microdissected glomeruli (European Renal cDNA Bank) and revealed a 2.1-fold increase (q<0.001) in GATA3 expression in IgAN (n=27) compared with living donors (n=6), providing independent confirmation that GATA3 expression is upregulated in IgAN.
Discussion
MCs are descendants of FOXD1-expressing stromal progenitor cells in the developing kidney,31 but the transcription factors responsible for this specification are largely unknown. Here, we identify GATA3 as one such transcription factor in developing kidneys by demonstrating its expression in FOXD1-stromal cells, that its first expression is coincident with stromal cell differentiation, and that GATA3 deficiency in Gata3+/− mice reduces the frequency of mesangial precursors and their proliferation in embryonic mouse kidneys, resulting in small glomeruli containing fewer MCs in adult mice. Furthermore, expression of GATA3 in MCs of mature kidneys increases in mesangial proliferative GN in humans and rodent models, suggesting a homeostatic importance after injury, and thus a potential therapeutic target. In addition, GATA3 has practical utility as a simple, robust way to quantify MCs in GN.
Kidney organogenesis involves three major progenitor populations: the well characterized RET1+/WNT11+ ureteric bud, SIX2+/CITED1+ cap mesenchymal cells, and less well described FOXD1+ stromal mesenchymal cells.34,35,39 FOXD1 expression occurs from E10.5 in stromal cells, precursors of VSMCs, MCs, renin-producing cells, pericytes, and interstitial fibroblasts,35,40 including myofibroblasts in CKD.41 Deletion of Foxd1 causes severe kidney malformations, including reduced ureteric bud branching and decreased nephron numbers; aberrant vessel-patterning; and fewer mesangial and renin cells.42,43 Identification of GATA3 in two of the major progenitor populations (ureteric bud and stroma) and continued GATA3 expression by all descendant cell populations in mature kidneys (Figure 8), highlights its importance to renal development and initiation and maintenance of cell phenotype, explaining the wide spectrum of kidney abnormalities associated with GATA3 deficiency in patients with HDR. Renal expression of GATA3 is controlled by enhancer elements that are uniquely active in the two progenitor populations, and although a recent study showed that GATA3 expression can be directed to ureteric bud-derived renal tubules,17 the location of the stromal/mesangial-specific GATA3 regulatory elements are yet to be identified. Absence of CD malformation in Gata3+/− mice supports previous reports17,44 that threshold levels for GATA3 activity are cell-specific, and that MCs require a high level of GATA3 activity such that a 30% reduction in protein expression, as we see in Gata3+/− MCs (Figure 4G), is enough to result in their aberrant development, whereas ureteric bud–derived tubules are unaffected by this level of GATA3 deficiency. Indeed, recent single-cell RNA-sequencing analysis on isolated MCs identified GATA3 as the third most enriched transcript expressed by every single MC.19
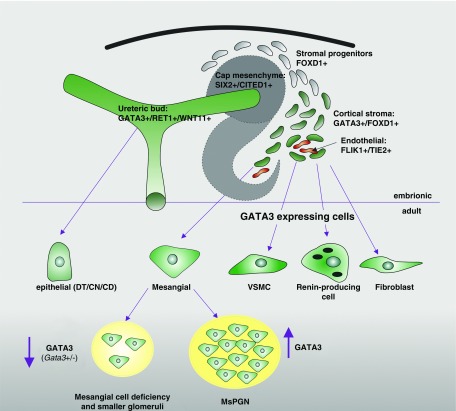
Figure 8.
Schematic diagram showing location of GATA3 expression (green) in the embryonic and adult kidney. GATA3 is expressed by two major renal progenitor populations, ureteric bud and stromal cells, and continues to be expressed by all cell types that originate from them, i.e., epithelial cells of the CDs/DN/CN and stromal cell descendants (mesangial, VSMCs, renin-producing cells, and fibroblasts). Deficiency in GATA3 results in a paucity of MCs and smaller glomeruli, and increased GATA3 expression is associated with mesangial proliferative GN.
The paucity of MCs in Gata3+/− mice is reminiscent of parathyroid defects reported in these mice with smaller parathyroid-primordia containing fewer CaSR-expressing cells, and failure to initiate proliferation to enlarge the parathyroid mass in response to a low calcium/vitamin D diet.3 Our current findings demonstrate that MC progenitors from Gata3+/− mice have reduced proliferations rates and that GATA3 expression is upregulated in proliferating MCs in experimental mesangial proliferative GN and in biopsy specimens of patients with IgAN, suggesting that transcriptional GATA3 activity is required for proliferation in response to signals promoting MC activation in injured glomeruli. In breast and neuroblastoma tumor cells GATA3 promotes cell proliferation by facilitating G1/S transition through transcriptional regulation of the CCND1 gene.45,46 PDGF-B, TGF-β, and IL-6 signaling pathways have pivotal roles in MC proliferation.36,47,48 Whether GATA3 is involved in these pathways remains to be elucidated. Our findings that fewer MC precursors enter the vascular cleft of the SS body are reminiscent of the MC defects reported in mice with stromal-mesenchyme specific deletion of RBP-J, a downstream effector of Notch signaling.32,49 RPB-J has been shown to directly regulate GATA3 expression in T-helper 2 cells,50,51 which indicates that the Notch-RBP-J-GATA3 regulatory axis may be involved in specification and differentiation of MCs from FOXD1+ stromal mesenchyme.
Using GATA3 as a nuclear mesangial marker allowed us to precisely evaluate MC expansion and mesangial proliferation rates in mesangial proliferative GN, and demonstrate interglomerular variation. Inconsistencies in reporting mesangial cellularity are common when applying the Mesangial and Endocapillary hypercellularity, Segmental glomerulosclerosis, Tubular atrophy score in IgAN biopsy specimens.52 Using GATA3 as a nuclear marker specific for MCs may be of great utility in quantification of mesangial hypercellularity and evaluation of disease progression. Indeed, expansion of GATA3-expressing nuclei was more prominent in biopsy samples from patients with aggressive IgAN and higher serum creatinine. Mesangial proliferation in these biopsy specimens was elevated compared with biopsy samples from patients with indolent IgAN and lower serum creatinine. Moreover, quantifying GATA3 expression showed, in agreement with the Thy1.1 model, that GATA3 expression increased in MCs from biopsy specimens with higher mesangial proliferation. This suggests that aberrant GATA3 expression is associated with activation of MCs and abnormal proliferation and extracellular matrix deposition. Transcriptomic-profiling of MCs isolated from stromal-specific Gata3-deficient mice with induced experimental mesangial injury will identify the genes and pathways regulated by this integral MC transcription factor.
In summary, we identified a novel, nonredundant role for GATA3 in MCs from their first appearance in embryonic kidney to mature glomeruli in health and disease. Furthermore, GATA3 expression increases in MCs in mesangial proliferative GN and haploinsufficiency of GATA3 results in MC defects, suggesting its importance for glomerular homeostasis and response to injury. Moreover, GATA3 is a new, clinically applicable nuclear marker of MCs, superior to current markers.
Disclosures
Dr. Aigner reports personal fees from Sanofi, non-financial support from Chiesi, outside the submitted work. Prof. Thakker reports grants from Medical Research Council, grants from Wellcome Trust, grants from National Institute for Health Research, grants from EU Horizon 2020 Marie Curie Actions Initial Training Networks, grants from Kidney Research UK, grants from National Institute for Health Research, Translational Research Collaboration, grants from Marshall Smith Syndrome Foundation, during the conduct of the study; grants from NPS/ Shire Pharmaceuticals, grants from Novartis Pharma AG, grants from GlaxoSmithKline, personal fees from Ipsen, personal fees from AstraZeneca, outside the submitted work. All of the remaining authors have nothing to disclose.
Funding
Dr. Grigorieva was funded by a Seventh Framework Programme of the European Union Marie Curie IEF Fellowship (grant number 302739 [2011]). This project has received funding from the European Union Horizon 2020 research and innovation programme under grant agreement 668036 (RELENT). The European Renal cDNA Bank Kröner-Fresenius Biopsy Bank is supported by the Else Kröner-Fresenius Foundation.
Supplementary Material
Acknowledgments
We thank Dr. Enikoe Kallay for kindly providing the CaSR antibody, Anton Jäger for assistance with the graphical abstract, and Dr. Robert Steadman for his valuable comments on the manuscript. We also thank all participating centers of the European Renal cDNA Bank, Kröner–Fresenius Biopsy Bank (ERCB-KFB), and their patients for their cooperation. Dr. Grigorieva, Prof. Rees, and Prof. Kain designed the study. Prof. Thakker, Prof. Ostendorf, Prof. Floege, and Dr. Panzer made substantial contributions to acquisition of data and revised the article critically for important intellectual content. Dr. Grigorieva, Dr. Grigorieva, Ms. Schachner, and Ms. Neudert carried out experiments. Dr. Grigorieva, Dr. Oszwald, and Dr. Lindenmeyer analyzed the data. Dr. Grigorieva made the figures. Dr. Grigorieva, Prof. Rees, and Prof. Kain drafted and revised the paper, all authors approved the final version of the manuscript.
The opinions expressed in this publication are those of the authors and do not purport to reflect the official position or views of the EC, its agencies, or officers.
For a list of active members at the time of the study, see Shved et al.54
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “Mesangial Cells: The Tuft Guys of Glomerular Development,” on pages 1551–1553.
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2018111143/-/DCSupplemental.
Supplemental Figure 1. GATA3 expression in the kidney is conserved between human, rat, and mouse.
Supplemental Figure 2. Expression of GATA3 in the human fetal kidney.
Supplemental Figure 3. GATA3 is a marker of distal tubule precursor cells.
Supplemental Figure 4. GATA3 expression is downregulated in primary MC cultures.
Supplemental Figure 5. Expression of PDGFR-β precedes GATA3 expression in stromal mesenchyme.
Supplemental Figure 6. Impaired recruitment of GATA3+ MC precursors into the developing glomeruli in Gata3+/− mice.
Supplemental Figure 7. Proliferating endothelial cells in the Thy1.1 mesangial proliferative nephritis model.
Supplemental Figure 8. Quantification of GATA3 IF intensity.
Supplemental Figure 9. Extraglomerular and crescentic GATA3 expression in renal biopsy samples.
Supplemental Table 1. Patient clinical parameters.
Supplemental Table 2. Clinical parameters of patients in the Lupus nephritis cohort.
Supplemental Table 3. Clinical parameters of patients in the IgAN cohort.
Supplemental Table 4. Primer sequences.
Supplemental Table 5. Primary antibodies.
Supplemental Table 6. Gata3+/− mouse kidney function parameters.
Supplemental Appendix 1. Daltonized versions of Figures 1, 2, and 4–7, and Supplemental Figures 2, 7, and 9.
References
- 1.Takaku M, Grimm SA, Shimbo T, Perera L, Menafra R, Stunnenberg HG, et al.: GATA3-dependent cellular reprogramming requires activation-domain dependent recruitment of a chromatin remodeler. Genome Biol 17: 36, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kouros-Mehr H, Slorach EM, Sternlicht MD, Werb Z: GATA-3 maintains the differentiation of the luminal cell fate in the mammary gland. Cell 127: 1041–1055, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grigorieva IV, Mirczuk S, Gaynor KU, Nesbit MA, Grigorieva EF, Wei Q, et al.: Gata3-deficient mice develop parathyroid abnormalities due to dysregulation of the parathyroid-specific transcription factor Gcm2. J Clin Invest 120: 2144–2155, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grote D, Boualia SK, Souabni A, Merkel C, Chi X, Costantini F, et al.: Gata3 acts downstream of beta-catenin signaling to prevent ectopic metanephric kidney induction. PLoS Genet 4: e1000316, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grote D, Souabni A, Busslinger M, Bouchard M: Pax 2/8-regulated Gata 3 expression is necessary for morphogenesis and guidance of the nephric duct in the developing kidney. Development 133: 53–61, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Lim KC, Lakshmanan G, Crawford SE, Gu Y, Grosveld F, Engel JD: Gata3 loss leads to embryonic lethality due to noradrenaline deficiency of the sympathetic nervous system. Nat Genet 25: 209–212, 2000 [DOI] [PubMed] [Google Scholar]
- 7.van der Wees J, van Looij MA, de Ruiter MM, Elias H, van der Burg H, Liem SS, et al.: Hearing loss following Gata3 haploinsufficiency is caused by cochlear disorder. Neurobiol Dis 16: 169–178, 2004 [DOI] [PubMed] [Google Scholar]
- 8.Riedel JH, Becker M, Kopp K, Düster M, Brix SR, Meyer-Schwesinger C, et al.: IL-33-mediated expansion of type 2 innate lymphoid cells protects from progressive glomerulosclerosis. J Am Soc Nephrol 28: 2068–2080, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hosoya T, Maillard I, Engel JD: From the cradle to the grave: Activities of GATA-3 throughout T-cell development and differentiation. Immunol Rev 238: 110–125, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mjösberg J, Bernink J, Golebski K, Karrich JJ, Peters CP, Blom B, et al.: The transcription factor GATA3 is essential for the function of human type 2 innate lymphoid cells. Immunity 37: 649–659, 2012 [DOI] [PubMed] [Google Scholar]
- 11.Van Esch H, Groenen P, Nesbit MA, Schuffenhauer S, Lichtner P, Vanderlinden G, et al.: GATA3 haplo-insufficiency causes human HDR syndrome. Nature 406: 419–422, 2000 [DOI] [PubMed] [Google Scholar]
- 12.Ali A, Christie PT, Grigorieva IV, Harding B, Van Esch H, Ahmed SF, et al.: Functional characterization of GATA3 mutations causing the hypoparathyroidism-deafness-renal (HDR) dysplasia syndrome: Insight into mechanisms of DNA binding by the GATA3 transcription factor. Hum Mol Genet 16: 265–275, 2007 [DOI] [PubMed] [Google Scholar]
- 13.Gaynor KU, Grigorieva IV, Nesbit MA, Cranston T, Gomes T, Gortner L, et al.: A missense GATA3 mutation, Thr272Ile, causes the hypoparathyroidism, deafness, and renal dysplasia syndrome. J Clin Endocrinol Metab 94: 3897–3904, 2009 [DOI] [PubMed] [Google Scholar]
- 14.Belge H, Dahan K, Cambier JF, Benoit V, Morelle J, Bloch J, et al.: Clinical and mutational spectrum of hypoparathyroidism, deafness and renal dysplasia syndrome. Nephrol Dial Transplant 32: 830–837, 2017 [DOI] [PubMed] [Google Scholar]
- 15.Chenouard A, Isidor B, Allain-Launay E, Moreau A, Le Bideau M, Roussey G: Renal phenotypic variability in HDR syndrome: Glomerular nephropathy as a novel finding. Eur J Pediatr 172: 107–110, 2013 [DOI] [PubMed] [Google Scholar]
- 16.Kamezaki M, Kusaba T, Adachi T, Yamashita N, Nakata M, Ota N, et al.: Unusual proliferative glomerulonephritis in a patient diagnosed to have hypoparathyroidism, sensorineural deafness, and renal dysplasia (HDR) syndrome with a novel mutation in the GATA3 gene. Intern Med 56: 1393–1397, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moriguchi T, Yu L, Otsuki A, Ainoya K, Lim KC, Yamamoto M, et al.: Gata3 hypomorphic mutant mice rescued with a yeast artificial chromosome transgene suffer a glomerular mesangial cell defect. Mol Cell Biol 36: 2272–2281, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brunskill EW, Potter SS: Changes in the gene expression programs of renal mesangial cells during diabetic nephropathy. BMC Nephrol 13: 70, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lu Y, Ye Y, Yang Q, Shi S: Single-cell RNA-sequence analysis of mouse glomerular mesangial cells uncovers mesangial cell essential genes. Kidney Int 92: 504–513, 2017 [DOI] [PubMed] [Google Scholar]
- 20.Yoh K, Shibuya K, Morito N, Nakano T, Ishizaki K, Shimohata H, et al.: Transgenic overexpression of GATA-3 in T lymphocytes improves autoimmune glomerulonephritis in mice with a BXSB/MpJ-Yaa genetic background. J Am Soc Nephrol 14: 2494–2502, 2003 [DOI] [PubMed] [Google Scholar]
- 21.van Doorninck JH, van Der Wees J, Karis A, Goedknegt E, Engel JD, Coesmans M, et al.: GATA-3 is involved in the development of serotonergic neurons in the caudal raphe nuclei. J Neurosci 19: RC12, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Floege J, Ostendorf T, Janssen U, Burg M, Radeke HH, Vargeese C, et al.: Novel approach to specific growth factor inhibition in vivo: Antagonism of platelet-derived growth factor in glomerulonephritis by aptamers. Am J Pathol 154: 169–179, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Panzer U, Steinmetz OM, Paust HJ, Meyer-Schwesinger C, Peters A, Turner JE, et al.: Chemokine receptor CXCR3 mediates T cell recruitment and tissue injury in nephrotoxic nephritis in mice. J Am Soc Nephrol 18: 2071–2084, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Kreisberg JI, Karnovsky MJ: Glomerular cells in culture. Kidney Int 23: 439–447, 1983 [DOI] [PubMed] [Google Scholar]
- 25.Sarrab RM, Lennon R, Ni L, Wherlock MD, Welsh GI, Saleem MA: Establishment of conditionally immortalized human glomerular mesangial cells in culture, with unique migratory properties. Am J Physiol Renal Physiol 301: F1131–F1138, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cohen CD, Frach K, Schlöndorff D, Kretzler M: Quantitative gene expression analysis in renal biopsies: A novel protocol for a high-throughput multicenter application. Kidney Int 61: 133–140, 2002 [DOI] [PubMed] [Google Scholar]
- 27.Martini S, Nair V, Keller BJ, Eichinger F, Hawkins JJ, Randolph A, et al.: European Renal cDNA Bank; C-PROBE Cohort; CKDGen Consortium : Integrative biology identifies shared transcriptional networks in CKD. J Am Soc Nephrol 25: 2559–2572, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cohen CD, Klingenhoff A, Boucherot A, Nitsche A, Henger A, Brunner B, et al.: Comparative promoter analysis allows de novo identification of specialized cell junction-associated proteins. Proc Natl Acad Sci U S A 103: 5682–5687, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tusher VG, Tibshirani R, Chu G: Significance analysis of microarrays applied to the ionizing radiation response. Proc Natl Acad Sci U S A 98: 5116–5121, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jones TR, Kang IH, Wheeler DB, Lindquist RA, Papallo A, Sabatini DM, et al.: CellProfiler Analyst: Data exploration and analysis software for complex image-based screens. BMC Bioinformatics 9: 482, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sequeira Lopez ML, Gomez RA: Development of the renal arterioles. J Am Soc Nephrol 22: 2156–2165, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lin EE, Sequeira-Lopez ML, Gomez RA: RBP-J in FOXD1+ renal stromal progenitors is crucial for the proper development and assembly of the kidney vasculature and glomerular mesangial cells. Am J Physiol Renal Physiol 306: F249–F258, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sequeira-Lopez ML, Lin EE, Li M, Hu Y, Sigmund CD, Gomez RA: The earliest metanephric arteriolar progenitors and their role in kidney vascular development. Am J Physiol Regul Integr Comp Physiol 308: R138–R149, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kobayashi A, Mugford JW, Krautzberger AM, Naiman N, Liao J, McMahon AP: Identification of a multipotent self-renewing stromal progenitor population during mammalian kidney organogenesis. Stem Cell Reports 3: 650–662, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hatini V, Huh SO, Herzlinger D, Soares VC, Lai E: Essential role of stromal mesenchyme in kidney morphogenesis revealed by targeted disruption of Winged Helix transcription factor BF-2. Genes Dev 10: 1467–1478, 1996 [DOI] [PubMed] [Google Scholar]
- 36.Floege J, van Roeyen C, Boor P, Ostendorf T: The role of PDGF-D in mesangioproliferative glomerulonephritis. Contrib Nephrol 157: 153–158, 2007 [DOI] [PubMed] [Google Scholar]
- 37.Weening JJ, D’Agati VD, Schwartz MM, Seshan SV, Alpers CE, Appel GB, et al.: The classification of glomerulonephritis in systemic lupus erythematosus revisited. J Am Soc Nephrol 15: 241–250, 2004 [DOI] [PubMed] [Google Scholar]
- 38.Liu P, Lassén E, Nair V, Berthier CC, Suguro M, Sihlbom C, et al.: Transcriptomic and proteomic profiling provides insight into mesangial cell function in IgA nephropathy. J Am Soc Nephrol 28: 2961–2972, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Little MH, McMahon AP: Mammalian kidney development: Principles, progress, and projections. Cold Spring Harb Perspect Biol 4, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li W, Hartwig S, Rosenblum ND: Developmental origins and functions of stromal cells in the normal and diseased mammalian kidney. Dev Dyn 243: 853–863, 2014 [DOI] [PubMed] [Google Scholar]
- 41.Humphreys BD, Lin SL, Kobayashi A, Hudson TE, Nowlin BT, Bonventre JV, et al.: Fate tracing reveals the pericyte and not epithelial origin of myofibroblasts in kidney fibrosis. Am J Pathol 176: 85–97, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Levinson RS, Batourina E, Choi C, Vorontchikhina M, Kitajewski J, Mendelsohn CL: Foxd1-dependent signals control cellularity in the renal capsule, a structure required for normal renal development. Development 132: 529–539, 2005 [DOI] [PubMed] [Google Scholar]
- 43.Hum S, Rymer C, Schaefer C, Bushnell D, Sims-Lucas S: Ablation of the renal stroma defines its critical role in nephron progenitor and vasculature patterning. PLoS One 9: e88400, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hasegawa SL, Moriguchi T, Rao A, Kuroha T, Engel JD, Lim KC: Dosage-dependent rescue of definitive nephrogenesis by a distant Gata3 enhancer. Dev Biol 301: 568–577, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shan L, Li X, Liu L, Ding X, Wang Q, Zheng Y, et al.: GATA3 cooperates with PARP1 to regulate CCND1 transcription through modulating histone H1 incorporation. Oncogene 33: 3205–3216, 2014 [DOI] [PubMed] [Google Scholar]
- 46.Molenaar JJ, Ebus ME, Koster J, Santo E, Geerts D, Versteeg R, et al.: Cyclin D1 is a direct transcriptional target of GATA3 in neuroblastoma tumor cells. Oncogene 29: 2739–2745, 2010 [DOI] [PubMed] [Google Scholar]
- 47.Schlöndorff D, Banas B: The mesangial cell revisited: No cell is an island. J Am Soc Nephrol 20: 1179–1187, 2009 [DOI] [PubMed] [Google Scholar]
- 48.Eitner F, Westerhuis R, Burg M, Weinhold B, Gröne HJ, Ostendorf T, et al.: Role of interleukin-6 in mediating mesangial cell proliferation and matrix production in vivo. Kidney Int 51: 69–78, 1997 [DOI] [PubMed] [Google Scholar]
- 49.Boyle SC, Liu Z, Kopan R: Notch signaling is required for the formation of mesangial cells from a stromal mesenchyme precursor during kidney development. Development 141: 346–354, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Amsen D, Antov A, Jankovic D, Sher A, Radtke F, Souabni A, et al.: Direct regulation of Gata3 expression determines the T helper differentiation potential of Notch. Immunity 27: 89–99, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fang TC, Yashiro-Ohtani Y, Del Bianco C, Knoblock DM, Blacklow SC, Pear WS: Notch directly regulates Gata3 expression during T helper 2 cell differentiation. Immunity 27: 100–110, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Trimarchi H, Barratt J, Cattran DC, Cook HT, Coppo R, Haas M, et al.: IgAN Classification Working Group of the International IgA Nephropathy Network and the Renal Pathology Society; Conference Participants : Oxford classification of IgA nephropathy 2016: An update from the IgA nephropathy classification working group. Kidney Int 91: 1014–1021, 2017 [DOI] [PubMed] [Google Scholar]
- 53.Graca JA, Schepelmann M, Brennan SC, Reens J, Chang W, Yan P, et al.: Comparative expression of the extracellular calcium-sensing receptor in the mouse, rat, and human kidney. Am J Physiol Renal Physiol 310: F518–F533, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shved N, Warsow G, Eichinger F, Hoogewijs D, Brandt S, Wild P, et al. : Transcriptome-based network analysis reveals renal cell type-specific dysregulation of hypoxia-associated transcripts. Sci Rep. 7: 8576, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.