Significance Statement
Sex differences in the predisposition to CKD development or progression are well known. However, the underlying mechanisms involved remain unclear. The authors found markedly greater renal EGF receptor (EGFR) expression levels in adult wild-type male versus female mice, and a similar sex difference in EGFR expression in normal adult human kidneys. In Dsk5 mutant mice with constitutive EGFR activation, males developed significant spontaneous glomerular and tubulointerstitial injury, whereas females were relatively spared. In female mice, oophorectomy did not affect renal EGFR expression, but testosterone increased it; in male mice, castration decreased renal EGFR expression. These findings indicate that differential expression in renal EGFR expression plays a role in sex differences in susceptibility to progressive kidney injury, one that may be mediated in part by testosterone.
Keywords: DSK5, tubulointerstitial fibrosis, glomerulosclerosis, EGFR, testosterone
Visual Abstract
Abstract
Background
Sex differences mediating predisposition to kidney injury are well known, with evidence indicating lower CKD incidence rates and slower decline in renal function in nondiabetic CKD for premenopausal women compared with men. However, signaling pathways involved have not been elucidated to date. The EGF receptor (EGFR) is widely expressed in the kidney in glomeruli and tubules, and persistent and dysregulated EGFR activation mediates progressive renal injury.
Methods
To investigate the sex differences in response to renal injury, we examined EGFR expression in mice, in human kidney tissue, and in cultured cell lines.
Results
In wild type mice, renal mRNA and protein EGFR levels were comparable in males and females at postnatal day 7 but were significantly lower in age-matched adult females than in adult males. Similar gender differences in renal EGFR expression were detected in normal adult human kidneys. In Dsk5 mutant mice with a gain-of-function allele that increases basal EGFR kinase activity, males had progressive glomerulopathy, albuminuria, loss of podocytes, and tubulointerstitial fibrosis, but female Dsk5 mice had minimal kidney injury. Oophorectomy had no effect on renal EGFR levels in female Dsk5 mice, while castration protected against the kidney injury in male Dsk5 mice, in association with a reduction in EGFR expression to levels seen in females. Conversely, testosterone increased EGFR expression and renal injury in female Dsk5 mice. Testosterone directly stimulated EGFR expression in cultured kidney cells.
Conclusions
These studies indicate that differential renal EGFR expression plays a role in the sex differences in susceptibility to progressive kidney injury that may be mediated at least in part by testosterone.
Until recently, most experimental studies of kidney disease have not taken the sex of experimental animals into account, and many, if not most studies have utilized predominantly one sex or another, with the use of males far outnumbering that of females. However, there is increasing awareness of differences in injury patterns and responses between sexes, and the National Institutes of Health has now mandated explicitly that consideration of potential sex differences be included in funded research. Although few formal comparisons of sex differences in progressive experimental renal injury have been performed, epidemiologic evidence indicates that premenopausal women have a lower incidence of hypertension, less microvascular disease in diabetes, decreased prevalence of CKD, and slower decline in renal function with nondiabetic CKD.1–4 A recent analysis of a large and diverse cohort of patients with CKD emphasized that women had a lower risk of progression to ESRD and death, even when adjusting for multiple covariants.5
The EGF receptor (EGFR) is the prototypical receptor of the family of ErbB receptors (ErbBs), which consists of four transmembrane receptors belonging to the receptor tyrosine kinase superfamily and includes EGFRs ErbB1/HER1, ErbB2/Neu/HER2, ErbB3/HER3, and ErbB4/HER4.6,7 EGFR is widely expressed in different cell types of the mammalian kidney, including glomerulus, proximal tubule, and cortical and medullary collecting duct.8–11 Receptor activation leads to phosphorylation on specific tyrosine residues within the cytoplasmic tail that serve as docking sites for a variety of signaling molecules activating intracellular pathways, including the MAP kinase, JAK/STAT, src kinase, and PI3K pathways, which regulate cell proliferation, differentiation, and apoptosis.6,7,12
Studies by us and others have implicated EGFR activation in glomerular and tubulointerstitial injury in experimental models of GN, diabetic nephropathy, unilateral ureteral obstruction, and progressive tubulointerstitial fibrosis.13–20 Angiotensin II and TGF-β, two major pathways involved in renal fibrosis, can transactivate EGFR,21,22 whereas EGFR activation also leads to increased renal TGF-β expression.21,22 There have been previous reports that in other organs, sex differences can modulate expression of proteins in the EGFR axis, including expression of the EGFR ligand, EGF,23 and EGFR.24 In this study, we investigated sex differences in the development and progression of chronic renal injury in heterozygous Dsk5 mutant mice, which contain a mutation within the EGFR kinase domain that stabilizes the activation loop, producing a gain-of-function allele that increases basal receptor tyrosine kinase activity without other intervention.
Methods
Animal Studies
All animal experiments were performed in accordance with the guidelines of the Institutional Animal Care and Use Committee of Vanderbilt University Medical Center. For tissue acquisition, the animals were anesthetized with pentobarbital (70 mg/kg, administered intraperitoneally) and given heparin (1000 U/kg, administered intraperitoneally) to minimize coagulation. One kidney was removed for immunoblotting and quantitative PCR, and the other kidney was perfused with FPAS (3.7% formaldehyde, 10 mmol/l sodium-m-periodate, 40 mmol/l phosphate buffer, and 1% acetic acid) through cannulation of the aortic trunk using the left ventricle.25 The fixed kidney was dehydrated through a graded series of ethanol, embedded in paraffin, sectioned at 4-µm thickness, and mounted on glass slides.
The previously described hHB-EGF+/Tg mice26 were bred onto a C57BL/6 background, a relatively fibrosis-resistant mouse strain. Male homozygous hHB-EGFTg/Tg mice develop spontaneous fibrosis.14 Female and male hHB-EGFTg/Tg mice were genotyped using PCR and used for experiments at 15 weeks of age.
Castration and Oophorectomy and Testosterone Supplementation
Prepubertal (21 day old) wild-type female or male mice were ovariectomized or castrated, respectively. These mice were euthanized after 6 weeks for renal EGFR expression analysis. For male Dsk5 mice aged 21 days old, castration was carried out, and BP and albuminuria were monitored throughout the experimental period (up to 6 months old). Seven weeks of age female wild-type or Dsk5 mice were administrated testosterone dissolved in cotton seed oil at a dose of 10 mg/kg per day via intramuscular injection daily for 8 weeks. In another subset, female Dsk5 mice were given testosterone with or without an EGFR tyrosine kinase inhibitor, erlotinib, at a dose of 80 mg/kg per day via gastric gavage for 8 weeks, as described previously.15,16
BP Measurements
BP was measured in awake mice with a tail-cuff microphonic manometer.27 In brief, mice were trained for three consecutive days at room temperature (Monday to Wednesday) before systolic BP (SBP) was recorded in the following 2 days (Thursday and Friday) using a tail-cuff monitor (BP-2000 BP Analysis System; Visitech Systems). SBPs recorded on two consecutive days were averaged and used as the SBP from one mouse.
Measurements of Blood Glucose and Urinary Albumin Excretion
Fasting blood glucose was evaluated with a B-glucose analyzer (HemoCue, Lake Forest, CA) on saphenous vein samples from conscious mice at 2:00 pm after fasting for 6 hours, initiated at 8:00 am. Urinary albumin and creatinine excretion were determined using Albuwell M kits (Exocell, Philadelphia, PA). Albuminuria was expressed as urinary albumin concentration versus creatinine concentration ratio (µg/mg).
Cell Culture
Human renal cortex proximal tubule epithelial cell lines RPTEC/TERT1 (ATCCCRL-4031) were purchased from ATCC and cultured in DMEM-F-12 supplemented with hTERT RPTEC growth kit. The cells were starved for 24 hours with 1% FBS, followed by testosterone treatment at different concentrations for 24 or 48 hours. Immortalized mouse podocyte cells were cultured as previously described.28 Briefly, cells were maintained at 33°C in RPMI 1640 medium containing 100 U/ml IFNγ and 10% FBS, and induced to differentiate by shifting them to 37°C and culturing in RPMI 1640 medium containing 10% FBS without IFNγ, for 10 days. The differentiated podocytes were made quiescent in RPMI 1640 medium containing 5.5 mM of glucose and 1% FBS for 24 hours followed by testosterone treatment. Immortalized mesangial cells were obtained from the Immortomouse as previously described.29 Immortalized mesangial cells were propagated at 33°C in the presence of 100 IU/ml IFNγ. For experiments, cells were cultured at 37°C without IFNγ for a minimum of 3 days before use.
Chromatin Immunoprecipitation Assay
An Imprint ChIP Kit was used as per the manufacturer’s instructions (Sigma-Aldrich). Briefly, RPTEC/TERT1 were starved for 24 hours with 5% charcoal-stripped FBS (A3382101; Gibco), followed by 100 nM testosterone stimulation for 6 hours. Two million cells were gently crosslinked (1% formalin, 10 minutes), sheared to approximately 1000–200 bp DNA fragments, then subjected to immunoprecipitation with antibodies against androgen receptor (AR; catalog no. 39781, 5 μg per sample; Active Motif), or POLR2A (MA1–46093, 1 µg per sample; Thermo Fisher) or IgG (1 μg per sample) as a negative control. The chromatin-associated DNAs were purified and measured by PCR using primers spanning AR potential open chromatin regions in EGFR upstream of the transcription start site. Potential AR binding regions were predicted by a meme online tool (http://meme-suitep.org/) and Encode public database. The primer sequences for EGFR were ACCCATTCTTCTCTGTTCCCTG (forward) and AGCCTTCATAGTACGGCTTGT (reverse).
Antibodies
Rat anti-mouse F4/80 (MCA497R, a marker of macrophages), CD68 (MCA1957), CD3 (MCA1477, a marker of T lymphocytes), CD4 (MCA2961), CD8a (MCA2694), and Ly-6G (Gr-1, MCA2387, a marker of neutrophils) were purchased from AbD Serotec (now Bio-Rad); goat anti–phospho-EGFR (Tyr 1173, SC-12351) and anti-human connective tissue growth factor (CTGF; SC-14939), rabbit anti–phospho-EGFR (Tyr845, SC-23420-R) and anti–IL-6 (SC-12912T) were purchased from Santa Cruz Biotechnology; rabbit anti-Wilms Tumor Protein (WT1, a marker of podocytes; ab89901) and TNF-α (ab6671) were from Abcam; goat anti-EGFR (E1282) and mouse anti–α-smooth muscle actin (α-SMA; a marker of myofibroblasts, A5228) were from Sigma (St. Louis, MO); rabbit anti-collagen I (600–401–103–01) was purchased from Rockland Immunochemicals; rat anti–KIM-1 (a marker of kidney epithelial injury, MAB1817–100) and mouse anti–4-hydroxynonenal (a marker of oxidative stress, no. 198960) were from R&D Systems; and rabbit anti–p-ERK (4370S) was from Cell Signaling Technology.
Real-Time PCR
Total tissue RNAs from kidneys, livers, or aortae were isolated using TRIzol reagent (Invitrogen). SuperScript III First-Strand Synthesis System kit (Invitrogen) was used to synthesize cDNA from equal amounts of total RNA from each sample. Quantitative real-time PCR was performed using TaqMan real-time PCR (7900HT; Applied Biosystems). The Master Mix and all gene probes were also purchased from Applied Biosystems. The probes used in the experiments included mouse S18 (Mm02601778), collagen I (col1a1, Mm00801666), collagen III (col3a1, Mm01254476), collagen IV (col4a1, Mm01210125), α-SMA (acta2, Mm01546133), fibronectin 1 (fn1, Mm01256744), CTGF (Mm01192933), TGF-β (Mm00441726), KIM-1 (TIM-1, Mm00506686), NGAL (Mm01324470), F4/80 (Emr1, Mm00802529), CD3 (cd3d, Mm00442746), IL-6 (Mm00446190), TNF-α (Mm99999068), IL-1α (Mm00439621), IL-1β (Mm00434228), CCL2 (MCP-1, Mm00441242), GM-CSF (csf2, Mm01290062), IL-23 (Mm00518984), IRF5 (Mm00496477), EGFR (Mm00433023), HB-EGF (Mm00439307), amphiregulin (Mm00437583), TGF-α (Mm00446232), Epiregulin (Mm00514794), and EPGN (Mm00504344).
Immunoblotting Analysis
Whole kidney tissue was homogenized using lysis buffer (10 mmol/l Tris–HCl [pH 7.4], 50 mmol/l NaCl, 2 mmol/l EGTA, 2 mmol/l EDTA, 0.5% nonidet P-40, 0.1% SDS, 100 μmol/l Na3VO4, 100 mmol/l NaF, 0.5% sodium deoxycholate, 10 mmol/l sodium pyrophosphate, 1 mmol/l PMSF, 10 μg/ml aprotinin, and 10 μg/ml leupeptin) and centrifuged at 15,000×g for 20 minutes at 4°C. The cultured cells were lysed with the same lysis buffer. The BCA protein assay kit (Thermo Scientific, Waltham, MA) was used to measure the protein concentration of each sample. Western analysis was performed as previously described.16
Human Kidneys
Noncancerous kidney tissue from ten adult patients (five male and five female) undergoing nephrectomy for renal carcinoma was utilized for analysis. Patient characteristics and renal histologic analysis are included in Table 1. Samples were deidentified, and studies were approved by our institutional review board.
Table 1.
Characterization of patient samples utilized for renal EGFR analysis
| Gender | Age, yr | Serum Creatinine | BUN | Creatinine Clearance | Global Glomerular Sclerosis (%) | Segmental Glomerular Sclerosis (%) | Mesangial Expansion (0–3 Scale) | Interstitial Fibrosis, % | Arteriole Hyalinosis (0–3 Scale) |
|---|---|---|---|---|---|---|---|---|---|
| Male (n=5) | 56.9±4.60 | 1.0±0.09 | 17.3±1.80 | 80.5±8.71 | 6.6±4.12 | 0.4±0.45 | 0±0 | 5.0±0 | 1.0±1.0 |
| Female (n=5) | 57.1±4.61 | 0.9±0.09 | 17.2±3.60 | 77.9±9.96 | 17.0±3.19 | 0.7±0.72 | 1.3±0.25 | 7.5±2.5 | 0.5±0.5 |
Data are presented as mean±SD.
Immunohistochemistry Staining and Quantitative Image Analysis
Immunostaining was carried out as previously described.16 For phosphoprotein staining, antigen retrieval was performed by boiling section in citric acid buffer (pH 6.0; 100 mmol/l) three times, for 5 minutes each. For mouse primary antibodies, the M.O.M. kit was used to reduce endogenous mouse Ig staining. For phospho-EGFR and WT1 fluorescence staining, after antigen retrieval the sections were blocked for 1 hour with PBS containing 10% normal donkey serum, then phospho-EGFR or WT1 antibody diluted in PBS containing 1% normal donkey serum, 1% BSA, 0.3% Triton X-100, and 0.001% sodium azide were added for 16 hours. After washing with PBS, fluorescence-labeled second antibody was added. Staining on the slides was imaged with a Nikon TE300 fluorescence microscope and a spot-cam digital camera (Diagnostic Instruments, Sterling Heights, MI). On the basis of the distinctive density and color of immunostaining in video images, the number, size, and position of stained cells were quantified using the BIOQUANT true-color windows system (R&M Biometrics) as previously described.16 Six representative fields from each animal were quantified, and their average was used as data from one animal sample.
Masson Trichrome, Sirius Red, and Periodic Acid–Schiff Staining
These stains were performed according to the protocol provided by the manufacturer (Sigma).
Statistical Analyses
All values are presented as means±SEM. Fisher exact test, ANOVA, and Bonferroni t tests were used for statistical analysis.
Results
Wild-Type Mice Exhibit Sex Differences in Renal EGFR Activation
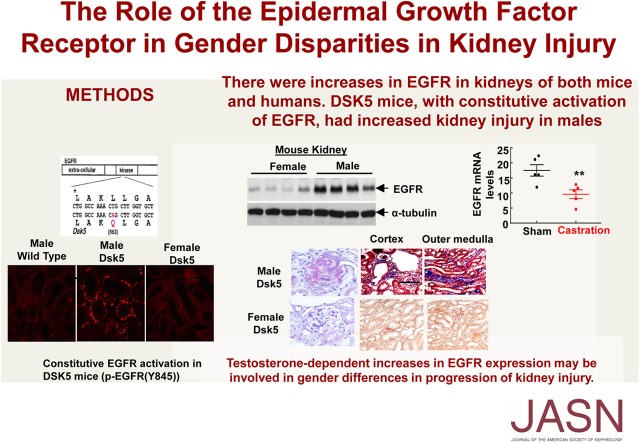
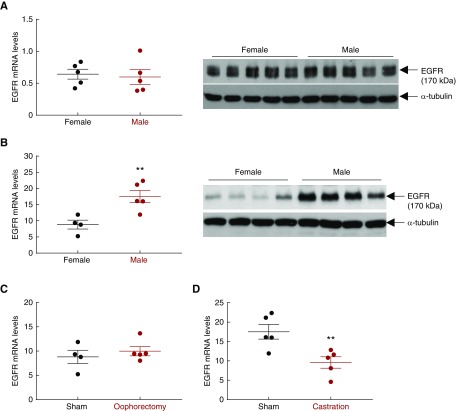
To investigate the potential role of EGFR expression and activation underlying sex differences in kidney injury, we first compared EGFR expression in kidneys from prepubertal age-matched wild-type male mice and female mice at age 7 days versus a postpubertal state at age 3 months.30 As indicated in Figure 1A, both renal EGFR mRNA and protein levels were comparable between wild-type female and male mice at age 7 days. In contrast, both renal EGFR mRNA and protein levels were significantly higher in wild-type male than female mice at age 3 months (Figure 1B). Prepubertal oophorectomy (removal of both ovaries at age 21 days) in wild-type female mice did not significantly increase renal EGFR mRNA expression (Figure 1C). In contrast, prepubertal castration of wild-type males decreased renal EGFR levels to that seen in the wild-type females (Figure 1, C and D).
Figure 1.
Mouse kidney EGFR expression is androgen-dependent. (A) Renal EGFR mRNA and protein levels were comparable between wild-type male and female mice at prepubertal 7 days of age. n=5. (B) Renal EGFR mRNA and protein levels were significantly higher in wild-type male than female mice at postpubertal 3 months of age (**P<0.01; n=4 in female group and n=5 in male group). (C) Oophorectomy (bilateral removal of ovaries) of wild-type female mice at age 21 days had no effect on renal EGFR expression 6 weeks later. n=4 in sham group and n=5 in ovariectomized group. (D) Castration of wild-type male mice at age 21 days led to decreased renal EGFR expression 6 weeks later (**P<0.01; n=5 in each group).
Dsk5 Mice Exhibit Sex Differences in Renal EGFR Activation
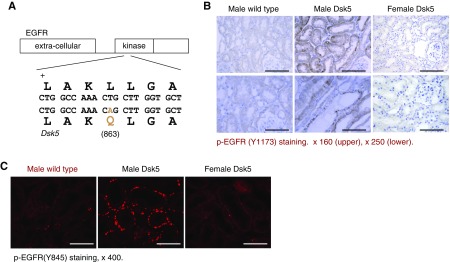
Dsk5 mice have a gain-of-function mutation for EGFR tyrosine kinase activity due to a leucine to glutamine substitution in the EGFR tyrosine kinase domain (Figure 3A) that results in constitutively active receptor activation in the absence of ligand stimulation.31 No previous study has examined the renal phenotype of these mice. Immunohistochemical staining showed increased phospho-EGFR (Y1173) staining in male Dsk5 mice, but not in female Dsk5 mice (Figure 3B), and immunofluorescence staining with phospho-EGFR (Y845) was also increased in male mouse kidney (Figure 3C), confirming EGFR tyrosine kinase activity was constitutively activated in male Dsk5 mouse kidneys.
Figure 3.
Confirmation of basal activation of renal EGFR in Dsk5 mice. (A) Dsk5 mice have a Leu863Gln mutation within the kinase domain that stabilizes the receptor activation loop, producing a gain-of-function mutation of basal EGFR kinase activity. (B) Immunohistochemical staining determined increased renal basal EGFR activation in male but not female Dsk5 mice at 15 weeks of age as indicated by increased phospho-EGFR (Y1173) staining in male Dsk5 mice. Original magnification, ×160 for upper panel (scale bar, 62.5 6 µm) and ×250 (scale bar, 40 µm) for lower panel. (C) Immunofluorescence staining with another phospho-EGFR (Y845) antibody also confirmed increases in renal EGFR activation in male Dsk5 mice, although only minimal renal EGFR activation was found in age-matched male wild-type mice and female Dsk5 mice. Original magnification, ×400 (scale bar, 25 µm).
Dsk5 Mice Exhibit Sex Differences in Renal Injury
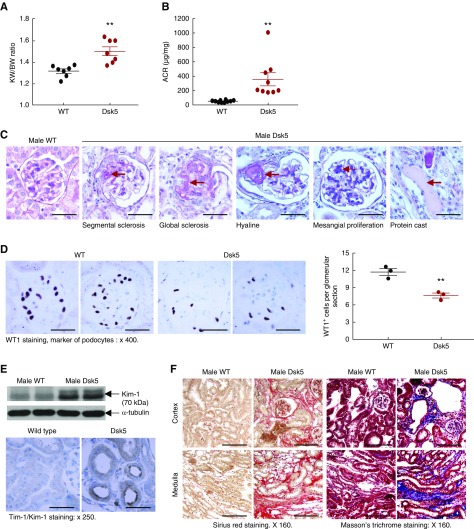
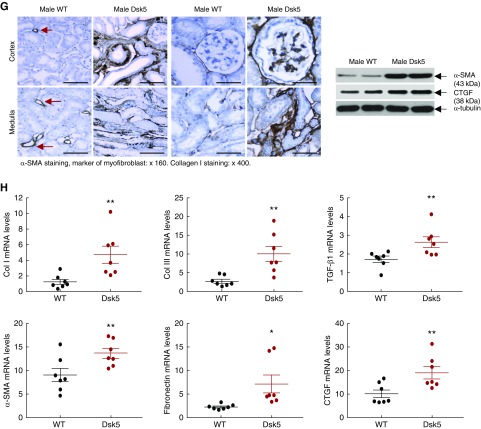
Fasting blood glucose was comparable between male wild-type and Dsk5 mice (mg/dl: 116.8±3.2 versus 115.7±3.0 of wild type; P>0.05; n=9 in each group). Although body weights in male Dsk5 and wild-type mice were comparable, kidney weights were significantly greater in male Dsk mice (Figure 4A), consistent with a previous report that EGFR activation is involved in kidney growth.32 Kidneys from 15-week-old male Dsk5 mice exhibited significant glomerular injury, as evidenced by increased albuminuria (Figure 4B), mesangial proliferation, hyaline, segmental sclerosis, and global sclerosis, as well as tubular protein casts (Figure 4C). There was a significant decrease in WT1 immunostaining, an index of differentiated podocytes (Figure 4D). Male Dsk5 mouse kidneys also had tubular damage, as indicated by increased expression of the tubule injury marker KIM-1 (Figure 4E), and significant tubulointerstitial fibrosis as indicated by increased Masson trichrome and Sirius Red staining (Figure 3F). Renal protein and mRNA levels of profibrotic and fibrotic components, including TGF-β1, CTGF, α-SMA, fibronectin, collagen I, and collagen III, were all increased in male Dsk5 mice (Figure 4, G and H). In addition, there was increased infiltration of macrophages (increased CD68 and F4/80 expression levels and F4/80-positive cells), T cells and neutrophils (increased CD3, CD4, CD8α, and Gr-1), increased proinflammatory cytokine expression (TNFα, IL-6, IL-1α, and MCP-1), and increased oxidative stress (4- hydroxynonenal staining, a marker of oxidative stress) (Supplemental Figures 1 and 2).
Figure 4.
Male Dsk5 mice developed spontaneous kidney injury. Male wild-type and Dsk5 mice were euthanized at 15 weeks of age. (A) Male Dsk5 mice had kidney hypertrophy as indicated by increased kidney weight versus body weight (KW/BW) ratios (**P<0.01; n=7). (B) Male Dsk5 mice had increased albuminuria as indicated by increased urinary albumin-to-creatinine ratio (ACR) at 15 weeks of age (**P<0.01; n=10 in wild-type group and n=9 in Dsk5 group). (C) Period acid–Schiff staining showed that kidneys of male Dsk5 mice exhibited segmental and global glomerular sclerosis, mesangial expansion, hyaline, and protein casts. Original magnification, ×400 (scale bar, 25 µm). (D) Male Dsk5 mice had loss of podocytes as indicated by two representative photomicrographs from wild-type and Dsk5 mice (left panel) and quantitatively decreased WT1-positive cells (right panel; **P<0.01; n=3). Original magnification, ×400 (scale bar, 25 µm). (E) Male Dsk5 mice had epithelial cell injury as indicated by increased renal expression levels of KIM-1, a marker of proximal tubule epithelial injury. Original magnification, ×250 (scale bar, 40 µm). (F) Male Dsk5 mice exhibited tubulointerstitial fibrosis as indicated by Sirius Red and Masson trichrome staining. Original magnification, ×160 (scale bar, 62.5 µm). (G) Immunostaining and immunoblotting determined that male Dsk5 mice had increased renal expression levels of α-SMA, a marker of myofibroblasts, CTGF, and collagen I. Original magnification, ×160 for α-SMA (scale bar, 62.5 µm) and ×400 for collagen I (scale bar, 25 µm). Arrows indicate blood vessels. (H) Kidneys of male Dsk5 mice had increased mRNA levels of profibrotic and fibrotic components, including α-SMA, CTGF, TGF-β, collagen I and III (Col I and Col III), and fibronectin. *P<0.05; **P<0.01. n=7 in each group.
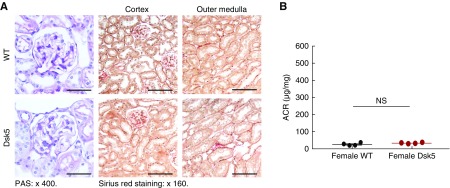
A striking finding was that although male Dsk5 mice developed glomerular injury and tubulointerstitial fibrosis at 15 weeks of age as described above, female Dsk5 mice had only minimal glomerular injury, interstitial fibrosis, and albuminuria even at 30 weeks of age (Figure 2). Renal EGFR activation was markedly lower in female Dsk5 mice than in male Dsk5 mice (Figure 3C).
Figure 2.
Female Dsk5 mice had minimal kidney injury at 30 weeks of age. Female wild-type and Dsk5 mice were euthanized at 30 weeks of age. (A) Female Dsk5 mice had minimal glomerular injury and tubulointerstitial fibrosis. Original magnification, ×400 for periodic acid–Schiff (PAS) staining (scale bar, 25 µm), ×160 for Sirius Red staining (scale bar, 62.5 µm). (B) Albuminuria was comparable between female wild-type and Dsk5 mice. n=4 in each group.
Androgen Contributes to Sex Differences in Renal Injury in Dsk5 Mice
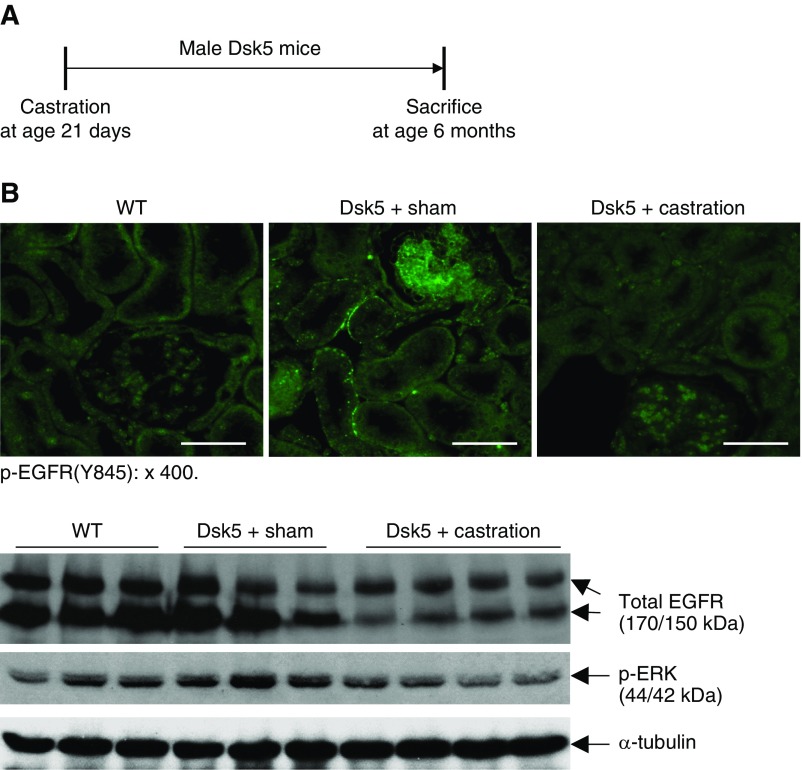
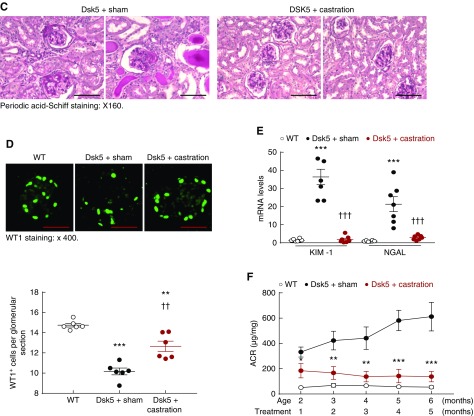
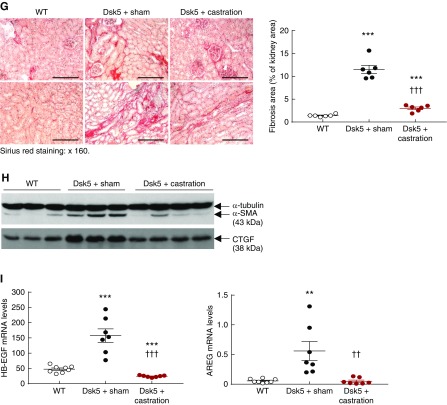
To investigate whether renal injury in male Dsk5 mice was related to androgen-mediated increases in EGFR expression, we determined the effect of prepubertal castration on development of functional and structural renal abnormalities (Figure 5A). Castration led to decreases in renal EGFR activation, total renal EGFR levels, and activation of ERK, a known downstream signaling molecule (Figure 5B). Kidney hypertrophy seen in male Dsk5 mice was also diminished by castration (Supplemental Figure 3). Histologically, kidneys from castrated Dsk5 mice exhibited only mesangial expansion (Figure 5C) and had attenuated loss of podocytes and tubular injury as indicated by significant decreases in the expression of both KIM-1 and NGAL, two markers of tubular injury (Figure 5, D and E). Progressive increases in albuminuria were also prevented by castration (Figure 5F). There was markedly decreased tubulointerstitial fibrosis seen in castrated male Dsk5 mice, as indicated by Masson trichrome staining (Supplemental Figure 4), Sirius Red staining (Figure 5G) and decreased profibrotic and fibrotic components, including TGF-β, CTGF, α-SMA, fibronectin, collagen I, collagen III, and collagen IV (Figure 5H, Supplemental Figure 5), as well as decreased renal immune cell infiltration and expression levels of proinflammatory cytokines/chemokines (Supplemental Figure 6). At 6 months of age, sham male Dsk5 mice developed hypertension, in association with cardiac hypertrophy, and these parameters were attenuated by castration (Supplemental Figure 7).
Figure 5.
Castration led to decreases in renal EGFR expression and injury in male Dsk5 mice. (A) Male Dsk5 mice were castrated at 21 days of age and euthanized at 6 months of age. (B) Castration led to decreases in renal EGFR activation as indicated by phospho-EGFR (Y845) immunofluorescence staining, total renal EGFR expression, and p-ERK levels, a downstream signaling of EGFR activation. Original magnification, ×400 (scale bar, 20 µm). (C) Glomerular sclerosis, tubular dilation, and protein casts were apparent in sham male Dsk5 mice, whereas only mesangial expansion was seen in castrated male Dsk5 mice. Original magnification, ×160 (scale bar, 62.5 µm). (D) Loss of WT1 staining, a marker of podocytes, seen in male Dsk5 mice was significantly attenuated but was not completely prevented by castration (**P<0.01; ***P<0.001 versus wild-type group; ††P<0.01 versus sham Dsk5 group; n=6 in each group). Original magnification, ×400 (scale bar, 25 µm). (E) Tubular injury seen in male Dsk5 mice was dramatically attenuated by castration as indicated by significant reduction of renal expression levels of KIM-1 and NGAL, two markers of tubular injury (***P<0.001 versus wild-type group; †††P<0.001 versus sham Dsk5 group; n=8 in wild-type group and n=7 in other groups). (F) Castration prevented progressive albuminuria seen in male Dsk5 mice (*P<0.05; **P<0.01; ***P<0.001 versus sham Dsk5 mice; n=8 in wild-type group and n=7 in other groups). (G) Castration significantly reduced tubulointerstitial fibrosis in male Dsk5 mice as indicated by Sirius Red staining (***P<0.001 versus wild-type group; †††P<0.001 versus sham Dsk5 group; n=6). Original magnification, ×160 (scale bar, 80 µm). (H) Castration decreased renal expression levels of α-SMA and CTGF in male Dsk5 mice. (I) Male Dsk5 mice had significant increases in renal expression levels of HB-EGF and amphiregulin (AREG), two ligands for EGFR, which were significantly attenuated by castration (**P<0.01, ***P<0.001 versus wild-type group; ††P<0.01, †††P<0.001 versus sham Dsk5 group; n=8 in wild-type group and n=7 in other groups).
In cultured cells, EGFR activation can lead to increases in the expression of its ligands.33 In male Dsk5 kidneys, renal mRNA levels of multiple EGFR ligands, including HB-EGF, amphiregulin (Figure 5I), TGF-α, epigen, and epiregulin (Supplemental Figure 8), were all higher in male Dsk5 mice than wild-type mice, and castration led to decreases in all these ligands in Dsk5 mice.
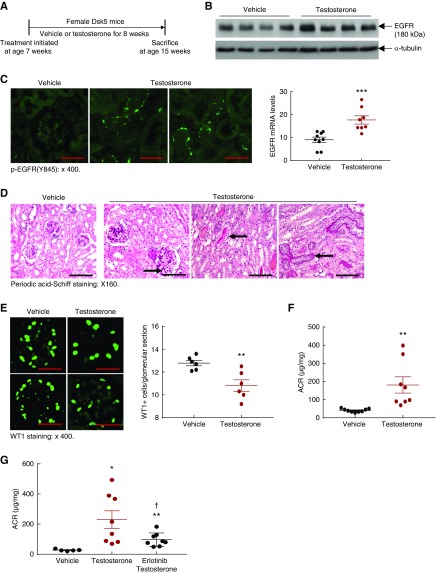
To investigate whether the minimal renal injury in female Dsk5 mice was secondary to a relative lack of androgen, we treated female Dsk5 mice with vehicle or testosterone (10 mg/kg per day, daily, administered intramuscularly) for 8 weeks, beginning at 7 weeks of age (Figure 6A). Testosterone supplementation led to increases in renal EGFR mRNA and protein levels as well as EGFR activation (Figure 6, B and C). Testosterone supplementation also led to mesangial expansion, tubular atrophy, and protein casts in association with loss of podocytes and increased albuminuria (Figure 6, D–F). To investigate further whether increased EGFR tyrosine kidney activity contributed to testosterone-mediated kidney injury in female Dsk5 mice, we treated age-matched female Dsk5 mice with testosterone with or without erlotinib, an inhibitor of EGFR tyrosine kinase activity.8,10 As indicated in Figure 6G, testosterone-induced albuminuria was significantly but not completely prevented by treatment with erlotinib.
Figure 6.
Testosterone supplementation led to increases in renal EGFR expression and injury in female Dsk5 mice. (A) Seven-week-old female Dsk5 mice were given testosterone at a dose of 10 mg/kg per day for 8 weeks and were euthanized at 15 weeks of age. (B and C) Testosterone administration led to increases in renal EGFR activation, mRNA, and protein levels (***P<0.001 versus vehicle; n=9 in vehicle group and n=8 in testosterone group). Original magnification, ×400 (scale bar, 25 µm). (D) Testosterone treatment led to mesangial expansion, protein casts, and tubular epithelial cell atrophy in female Dsk5 mice (arrows). Original magnification, ×160 (scale bar, 50 µm). (E) Testosterone administration caused loss WT1 staining, a marker of podocytes, in female Dsk5 mice (**P<0.01; n=6). Original magnification, ×400 (scale bar, 25 µm). (F) Testosterone supplementation led to increased albuminuria in female Dsk5 mice (**P<0.01; n=9 in vehicle group and n=8 in testosterone group). (G) Testosterone-induced albuminuria in female Dsk5 mice was prevented by erlotinib, an inhibitor of EGFR tyrosine kinase activity (*P<0.05, **P<0.01 versus vehicle group; †P<0.05 versus testosterone group; n=5 in vehicle group and n=8 in testosterone and testosterone plus erlotinib groups).
Androgen Increases EGFR Protein Levels in Cultured Renal Cell Lines
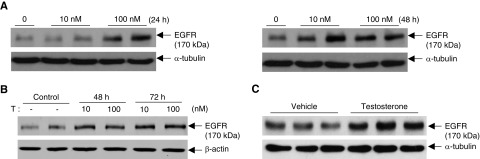
Testosterone administration to human renal cortical proximal tubule epithelial cells (RPTEC/TERT1) increased EGFR expression in a concentration-dependent manner at 24 and 48 hours (Figure 7A, Supplemental Figure 9). In addition, testosterone also stimulated EGFR expression in cultured mouse podocytes (Figure 7B) and cultured mouse mesangial cells (Figure 7C).
Figure 7.
Testosterone stimulated EGFR expression in cultured cells. (A) Human renal cortex proximal tubule epithelial cells were treated with testosterone for 24 and 48 hours. Testosterone stimulated EGFR expression at both time points. (B) Testosterone at 10 or 100 nM stimulated EGFR expression in differentiated podocytes at 48 and 72 hours. (C) Testosterone at 10 nM stimulated EGFR expression in differentiated mesangial cells at 48 hours.
As indicated in Supplemental Figure 10A, testosterone treatment led to AR translocation to nuclei of RPTEC/TERT1. To investigate the potential mechanism regarding androgen regulation of EGFR expression in the kidney, chromatin immunoprecipitation PCR was performed. The potential AR binding regions in the promoter or in the vicinity of EGFR gene were predicted as described in Methods and illustrated in Supplemental Figure 11. We determined that testosterone administration increased promoter binding of RNA polymerase II, indicative of transcriptional activation (Supplemental Figure 10B). AR was also found to be able to directly bind to the promoter of the EGFR gene after testosterone treatment (Supplemental Figure 10B).
Human Kidneys Show Sex Differences in EGFR Expression
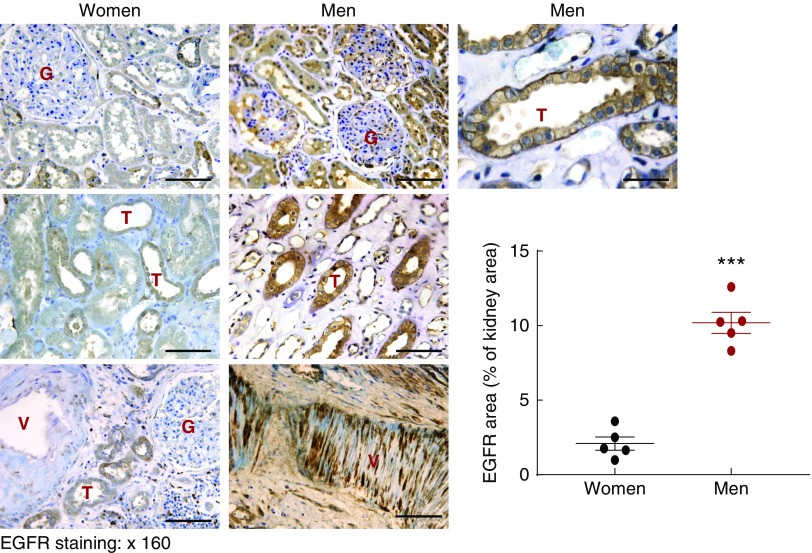
To determine whether the sexual dimorphism of EGFR expression was also seen in human kidney, we investigated EGFR expression levels in tissue samples of relatively normal adult human kidneys. As indicated in Figure 8, EGFR expression in tubular epithelial cells, glomeruli, and vascular smooth muscle cells was markedly higher in male versus female kidneys (***P<0.001; n=5 in each group).
Figure 8.
Renal EGFR expression levels were higher in man than in women. Relatively normal human kidney sections from nephrectomy were stained with EGFR antibody. EGFR immunostaining was found in tubular epithelial cells (T), glomerulus (G), and arterials (V), and renal EGFR expression was higher in men and in women (***P<0.001; n=5 in each group). Original magnification, ×160 (scale bar, 50 µm).
Discussion
It is recognized that there is a sexual dimorphism in the propensity for development of chronic renal injury in experimental animals. In addition, premenopausal women have decreased rates of the incidence and progression of CKD.34 In experimental animals, the propensity for development of kidney injury in males has been shown to be due to detrimental effects of testosterone rather than any protective effect of estrogen.35–37 Furthermore, bodybuilders who abuse anabolic steroids have been found to be at increased risk for development of focal glomerulosclerosis and tubulointerstitial disease.38 The sex differences seen in kidney disease are undoubtedly multifactorial; however, our studies suggest that sex hormone–dependent differences in EGFR expression may play a significant role.
There is increasing evidence that continuous dysregulated EGFR activation is related to renal injury and fibrogenesis.19,21,39,40 In models of tubulointerstitial fibrosis and diabetic nephropathy, administration of EGFR tyrosine kinase inhibitors or genetic deletion of EGFR markedly reduced kidney injury.15,16,41 Constitutively activated EGFR in male Dsk5 mice led to progressive glomerulosclerosis and tubulointerstitial fibrosis, further confirming a detrimental role for aberrant and persistent increases in EGFR expression and activation seen in a variety of experimental models of progressive renal injury, as well as in human CKD.
Renal EGFR mRNA and protein levels were comparable between male and female mice at postnatal day 7, but their levels are significantly higher in adult male than female mice. Castration markedly decreased renal EGFR expression and injury in male Dsk5 mice. Oophorectomy did not increase renal EGFR expression in wild-type mice. Although female Dsk5 mice were almost completely spared from progressive renal injury, testosterone increased renal EGFR expression and renal injury. There have not been previous studies demonstrating a role for testosterone-mediated EGFR expression in the kidney, but it has been reported that testosterone can increase EGFR expression in the liver.42 We confirmed that EGFR mRNA levels were higher in livers of adult male mice but were comparable in aortae (Supplemental Figure 12A).
The ligands for EGFR (EGF, TGF-α, HB-EGF, amphiregulin, betacellulin, epiregulin) are produced as membrane-bound prohormones that are cleaved to their active, soluble form by proteolysis, predominantly by members of the ADAM family, especially TACE/ADAM17. Pharmacologic or genetic inhibition of TACE/ADAM17 has been shown to reduce renal tubulointerstitial fibrosis induced by angiotensin II infusion and unilateral ureteral obstruction.13,43 We have also observed increased susceptibility for male mice to develop renal injury in a model of tubulointerstitial fibrosis resulting from the selective renal tubule overexpression of the EGFR ligand HB-EGF (Supplemental Figure 13).14 Interestingly, we found that the mRNA levels of EGFR ligands, including amphiregulin, HB-EGF, and betacellulin, were also higher in adult wild-type male than female mice (Supplemental Figure 12B), suggesting that hormonal upregulation of EGFR ligands may also contribute to renal injury, either directly or as secondary to EGFR activation.32
In summary, these studies demonstrate that constitutive EGFR activation promotes glomerular and tubulointerstitial injury in male mice, but not in female mice. This sex difference in susceptibility for renal injury is mediated at least in part by androgen-dependent EGFR expression, and suggest that differences in EGFR expression between sexes may be a factor in differences in incidence and rates of progression of CKD.
Disclosures
None.
Funding
These studies were supported by National Institutes of Health grants DK51265, DK95785, and DK62794 (to Dr. Harris and Dr. Zhang); DK103067 and the Vanderbilt O’Brien Center (P30DK114809) (to Dr. Harris, Dr. Pozzi, Dr. Fogo, and Dr. Zhang); Department of Veterans Affairs merit award 00507969 (to Dr. Harris); American Diabetes Association grant 1-18-IBS-267 (to Dr. Chen); and the Vanderbilt Center for Kidney Disease.
Supplementary Material
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2018121244/-/DCSupplemental.
Supplemental Figure 1. Kidneys of male Dsk5 mice had increased immune cell infiltration and proinflammatory factors.
Supplemental Figure 2. Kidneys of male Dsk5 mice had increased neutrophil infiltration and oxidative stress.
Supplemental Figure 3. Castration attenuated kidney hypertrophy in male Dsk5 mice.
Supplemental Figure 4. Castration attenuated kidney tubulointerstitial fibrosis in male Dsk5 mice.
Supplemental Figure 5. Castration reduced renal fibrosis in male Dsk5 mice.
Supplemental Figure 6. Castration decreased renal immune cell infiltration and proinflammatory cytokine/chemokine levels in male Dsk5 mice.
Supplemental Figure 7. Castration attenuated heart hypertrophy and prevented hypertension in male Dsk5 mice.
Supplemental Figure 8. Castration decreased renal expression levels of EGFR ligands in male Dsk5 mice.
Supplemental Figure 9. Testosterone stimulated EGFR expression in cultured cells.
Supplemental Figure 10. ChIP analysis of AR interaction with EGFR promoter.
Supplemental Figure 11. Analysis of potential AR binding sites in EGFR promoter.
Supplemental Figure 12. Expression of EGFR in liver and aorta.
Supplemental Figure 13. Female hHB-EGFTg/Tg mice had less renal tubulointerstitial fibrosis than males.
References
- 1.Neugarten J, Acharya A, Silbiger SR: Effect of gender on the progression of nondiabetic renal disease: A meta-analysis. J Am Soc Nephrol 11: 319–329, 2000 [DOI] [PubMed] [Google Scholar]
- 2.Yu M, Ryu DR, Kim SJ, Choi KB, Kang DH: Clinical implication of metabolic syndrome on chronic kidney disease depends on gender and menopausal status: Results from the Korean National Health and Nutrition Examination survey. Nephrol Dial Transplant 25: 469–477, 2010 [DOI] [PubMed] [Google Scholar]
- 3.Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, et al. : Heart disease and stroke statistics-2017 update: A report from the American Heart Association. Circulation 135: e146–e603, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maric-Bilkan C: Sex differences in micro- and macro-vascular complications of diabetes mellitus. Clin Sci (Lond) 131: 833–846, 2017 [DOI] [PubMed] [Google Scholar]
- 5.Ricardo AC, Yang W, Sha D, Appel LJ, Chen J, Krousel-Wood M, et al.: Sex-related disparities in CKD progression. J Am Soc Nephrol 30: 137–146, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schlessinger J: Ligand-induced, receptor-mediated dimerization and activation of EGF receptor. Cell 110: 669–672, 2002 [DOI] [PubMed] [Google Scholar]
- 7.Hynes NE, Lane HA: ERBB receptors and cancer: The complexity of targeted inhibitors. Nat Rev Cancer 5: 341–354, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Harris RC: Response of rat inner medullary collecting duct to epidermal growth factor. Am J Physiol 256: F1117–F1124, 1989 [DOI] [PubMed] [Google Scholar]
- 9.Harris RC, Hoover RL, Jacobson HR, Badr KF: Evidence for glomerular actions of epidermal growth factor in the rat. J Clin Invest 82: 1028–1039, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Breyer MD, Redha R, Breyer JA: Segmental distribution of epidermal growth factor binding sites in rabbit nephron. Am J Physiol 259: F553–F558, 1990 [DOI] [PubMed] [Google Scholar]
- 11.Chen JC, Chen JK, Nagai K, Plieth D, Tan M, Lee TC, et al. : EGFR signaling promotes TGFβ-dependent renal fibrosis. J Am Soc Nephrol 23: 215–224, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yarden Y, Sliwkowski MX: Untangling the ErbB signalling network. Nat Rev Mol Cell Biol 2: 127–137, 2001 [DOI] [PubMed] [Google Scholar]
- 13.Lautrette A, Li S, Alili R, Sunnarborg SW, Burtin M, Lee DC, et al.: Angiotensin II and EGF receptor cross-talk in chronic kidney diseases: a new therapeutic approach. Nat Med 11: 867–874, 2005 [DOI] [PubMed] [Google Scholar]
- 14.Overstreet JM, Wang Y, Wang X, Niu A, Gewin LS, Yao B, et al.: Selective activation of epidermal growth factor receptor in renal proximal tubule induces tubulointerstitial fibrosis. FASEB J 31: 4407–4421, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li Z, Li Y, Overstreet JM, Chung S, Niu A, Fan X, et al. : Inhibition of epidermal growth factor receptor activation is associated with improved diabetic nephropathy and insulin resistance in type 2 diabetes. Diabetes 67: 1847–1857, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang MZ, Wang Y, Paueksakon P, Harris RC: Epidermal growth factor receptor inhibition slows progression of diabetic nephropathy in association with a decrease in endoplasmic reticulum stress and an increase in autophagy. Diabetes 63: 2063–2072, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zeng F, Kloepfer LA, Finney C, Diedrich A, Harris RC: Specific endothelial heparin-binding EGF-like growth factor deletion ameliorates renal injury induced by chronic angiotensin II infusion. Am J Physiol Renal Physiol 311: F695–F707, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen J, Harris RC: Interaction of the EGF receptor and the hippo pathway in the diabetic kidney. J Am Soc Nephrol 27: 1689–1700, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu N, Guo JK, Pang M, Tolbert E, Ponnusamy M, Gong R, et al.: Genetic or pharmacologic blockade of EGFR inhibits renal fibrosis. J Am Soc Nephrol 23: 854–867, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen X, Abair TD, Ibanez MR, Su Y, Frey MR, Dise RS, et al.: Integrin alpha1beta1 controls reactive oxygen species synthesis by negatively regulating epidermal growth factor receptor-mediated Rac activation. Mol Cell Biol 27: 3313–3326, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen J, Chen JK, Harris RC: Angiotensin II Induces epithelial-to-mesenchymal transition in renal epithelial cells through reactive oxygen species/Src/Caveolin-mediated activation of an EGFR-ERK signaling pathway. Mol Cell Biol 35: 981–991, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen J, Chen JK, Harris RC. EGF receptor deletion in podocytes attenuates diabetic nephropathy. J Am Soc Nephrol 26: 1115–1125, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hiramatsu M, Kashimata M, Takayama F, Minami N: Developmental changes in and hormonal modulation of epidermal growth factor concentration in the rat submandibular gland. J Endocrinol 140: 357–363, 1994 [DOI] [PubMed] [Google Scholar]
- 24.Wang L, Xiao J, Gu W, Chen H: Sex difference of Egfr expression and molecular pathway in the liver: Impact on drug design and cancer treatments? J Cancer 7: 671–680, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang MZ, Hao CM, Breyer MD, Harris RC, McKanna JA: Mineralocorticoid regulation of cyclooxygenase-2 expression in rat renal medulla. Am J Physiol Renal Physiol 283: F509–F516, 2002 [DOI] [PubMed] [Google Scholar]
- 26.Zhang MZ, Yao B, Yang S, Jang L, Wang S, Fan X, et al. : CSF-1 signaling mediates recovery from acute kidney injury. J Clin Invest 122: 4519–4532, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yao B, Harris RC, Zhang MZ. Intrarenal dopamine attenuates deoxycorticosterone acetate/high salt-induced blood pressure elevation in part through activation of a medullary cyclooxygenase 2 pathway. Hypertension 54: 1077–1083, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mundel P, Reiser J, Zúñiga Mejía Borja A, Pavenstädt H, Davidson GR, Kriz W, et al.: Rearrangements of the cytoskeleton and cell contacts induce process formation during differentiation of conditionally immortalized mouse podocyte cell lines. Exp Cell Res 236: 248–258, 1997 [DOI] [PubMed] [Google Scholar]
- 29.Borza CM, Pozzi A. The role of cell-extracellular matrix interactions in glomerular injury. Exp Cell Res 318: 1001–1010, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jean-Faucher C, Berger M, de Turckheim M, Veyssiere G, Jean C: Developmental patterns of plasma and testicular testosterone in mice from birth to adulthood. Acta Endocrinol (Copenh) 89: 780–788, 1978 [DOI] [PubMed] [Google Scholar]
- 31.Fitch KR, McGowan KA, van Raamsdonk CD, Fuchs H, Lee D, Puech A, et al. : Genetics of dark skin in mice. Genes Dev 17: 214–228, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen JK, Nagai K, Chen J, Plieth D, Hino M, Xu J, et al. : Phosphatidylinositol 3-kinase signaling determines kidney size. J Clin Invest 125: 2429–2444, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barnard JA, Graves-Deal R, Pittelkow MR, DuBois R, Cook P, Ramsey GW, et al.: Auto- and cross-induction within the mammalian epidermal growth factor-related peptide family. J Biol Chem 269: 22817–22822, 1994 [PubMed] [Google Scholar]
- 34.Silbiger SR, Neugarten J: The impact of gender on the progression of chronic renal disease. Am J Kidney Dis 25: 515–533, 1995 [DOI] [PubMed] [Google Scholar]
- 35.Doublier S, Lupia E, Catanuto P, Periera-Simon S, Xia X, Korach K, et al. : Testosterone and 17β-estradiol have opposite effects on podocyte apoptosis that precedes glomerulosclerosis in female estrogen receptor knockout mice. Kidney Int 79: 404–413, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baylis C: Age-dependent glomerular damage in the rat. Dissociation between glomerular injury and both glomerular hypertension and hypertrophy. Male gender as a primary risk factor. J Clin Invest 94: 1823–1839, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hewitson TD, Boon WC, Simpson ER, Smith ER, Samuel CS: Estrogens do not protect, but androgens exacerbate, collagen accumulation in the female mouse kidney after ureteric obstruction. Life Sci 158: 130–136, 2016 [DOI] [PubMed] [Google Scholar]
- 38.Herlitz LC, Markowitz GS, Farris AB, Schwimmer JA, Stokes MB, Kunis C, et al. : Development of focal segmental glomerulosclerosis after anabolic steroid abuse. J Am Soc Nephrol 21: 163–172, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tang J, Liu N, Tolbert E, Ponnusamy M, Ma L, Gong R, et al.: Sustained activation of EGFR triggers renal fibrogenesis after acute kidney injury. Am J Pathol 183: 160–172, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li YY, Lam SK, Mak JC, Zheng CY, Ho JC: Erlotinib-induced autophagy in epidermal growth factor receptor mutated non-small cell lung cancer. Lung Cancer 81: 354–361, 2013 [DOI] [PubMed] [Google Scholar]
- 41.Advani A, Wiggins KJ, Cox AJ, Zhang Y, Gilbert RE, Kelly DJ: Inhibition of the epidermal growth factor receptor preserves podocytes and attenuates albuminuria in experimental diabetic nephropathy. Nephrology (Carlton) 16: 573–581, 2011 [DOI] [PubMed] [Google Scholar]
- 42.Noguchi S, Ohba Y, Oka T: Pretranslational enhancement of epidermal growth factor receptor by direct effect of testosterone in mouse liver. Endocrinology 128: 2141–2148, 1991 [DOI] [PubMed] [Google Scholar]
- 43.Kefaloyianni E, Muthu ML, Kaeppler J, Sun X, Sabbisetti V, Chalaris A, et al. : ADAM17 substrate release in proximal tubule drives kidney fibrosis. JCI Insight 1, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.