Abstract
The long-term survival of any multicellular species depends on the success of its germline in producing high-quality gametes and maximizing survival of the offspring. Studies in Drosophila melanogaster have led our growing understanding of how germline stem cell (GSC) lineages maintain their function and adjust their behavior according to varying environmental and/or physiological conditions. This review compares and contrasts the local regulation of GSCs by their specialized microenvironments, or niches; discusses how diet and diet-dependent factors, mating, and microorganisms modulate GSCs and their developing progeny; and briefly describes the tie between physiology and development during the larval phase of the germline cycle. Finally, it concludes with broad comparisons with other organisms and some future directions for further investigation.
Keywords: FlyBook, Drosophila, oogenesis, ovary, spermatogenesis, testis, germline stem cells, adipocytes, physiology, diet, microorganisms
THE germline is key for the perpetuation of all species of multicellular organisms. The development of gametes from germ cell precursors is therefore heavily guarded and optimized for the efficient production of high-quality sperm and eggs with maximal chance of survival for the resulting offspring. In Drosophila melanogaster, germline stem cells (GSCs) are established during development and function throughout adulthood to generate gametes. In adults, GSCs reside in specialized microenvironments, or niches, that produce local signals required for GSC self-renewal and normal activity levels (Greenspan et al. 2015; Laws and Drummond-Barbosa 2017). In addition to local niche signals, however, GSCs, along with their developing progeny, also sense and respond to a wealth of circulating factors that vary according to diet, metabolic status, and other environmental and/or physiological inputs. The complex integration of a multitude of local and systemic factors results in the finely tuned control of the GSC lineage in the context of a whole organism. This review compares and contrasts the regulation of male and female adult GSC lineages at the local and systemic levels to ensure the balance between self-renewal and differentiation, and the modulation of survival, proliferation, and growth according to changing environmental and physiological conditions.
Control of Adult Male and Female Germline Stem Cells by Their Niches
GSCs established during development continue to be maintained in adult males and females to support the production of sperm and eggs, respectively, thanks to their residence in specialized microenvironments, or niches (Greenspan et al. 2015; Laws and Drummond-Barbosa 2017). In fact, the first experimental demonstrations of adult tissue stem cell niches were carried out in Drosophila in the early 2000s (Xie and Spradling 1998, 2000; Kiger et al. 2001; Tulina and Matunis 2001). Since then, the field has seen an explosion in the number of factors shown to be required for proper regulation of stem cells at the local level.
Asymmetric stem cell divisions
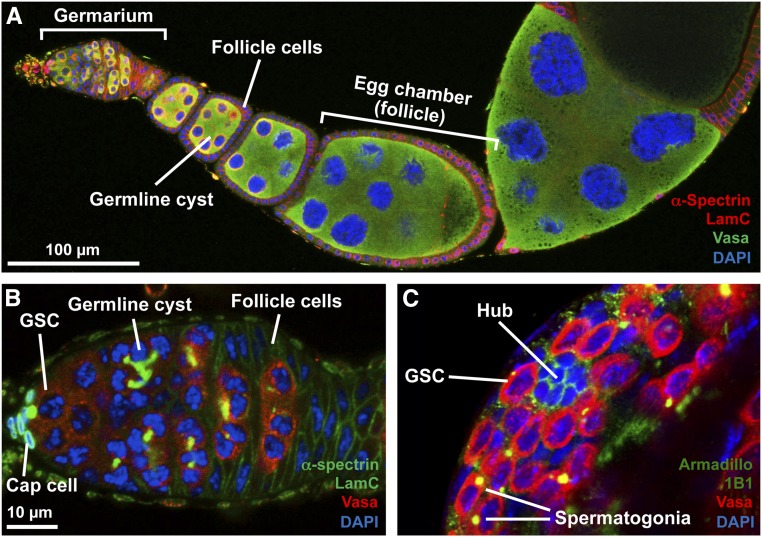
The Drosophila male and female GSC niches share multiple anatomic similarities but differ in significant ways (Figure 1). Each Drosophila ovary is composed of 16–20 ovarioles, and each ovariole contains a stem cell niche with two-to-three GSCs, such that females carry around three-to-five dozen GSCs per ovary. Each testis, in contrast, has a single niche housing a total of 6–12 GSCs (Greenspan et al. 2015; Laws and Drummond-Barbosa 2017). The female GSC niche is composed of postmitotic somatic cells, including terminal filament cells, four-to-eight cap cells that physically adhere to GSCs, and a subset of escort cells. In males, GSCs physically associate with 10–15 somatic hub cells, which also house a second stem cell population, the somatic cyst stem cells (CySCs). Each GSC division yields a self-renewed GSC, and another daughter cell destined for differentiation: a female cystoblast or a male gonialblast. The cystoblast and gonialblast undergo four rounds of mitotic divisions with incomplete cytokinesis to generate 16-cell cysts interconnected by cytoplasmic bridges. In females, early dividing germ cells remain closely associated with long escort cell processes, and the newly formed 16-cell cyst is subsequently enveloped by follicle cells to form an egg chamber (or follicle) that develops through 14 stages of oogenesis. Only one of the female cyst cells gives rise to an oocyte, whereas the remaining cells become supporting nurse cells. In males, two postmitotic somatic cyst cells derived from CySCs envelop the gonialblast and remain associated with the resulting germline cyst. In each male cyst, all 16 germ cells (referred to as spermatogonia) undergo meiosis to form 64 spermatids that further develop into sperm (Greenspan et al. 2015; Laws and Drummond-Barbosa 2017).
Figure 1.
Drosophila GSC lineages. (A) Confocal image of an ovariole showing the anterior germarium followed by developing egg chambers (or follicles). Each egg chamber is composed of a 16-cell germline cyst surrounded by a monolayer of follicle cells. (B) Image of germarium showing GSCs juxtaposed to cap cells. GSCs give rise to cystoblasts that divide to give rise to 2-, 4-, 8-, and 16-cell germline cysts. Follicle cells surround each 16-cell germline cyst to form an egg chamber that buds off the germarium. (C) Anterior tip of a testis showing the hub surrounded by GSCs. GSCs give rise to gonialblasts that divide to form germline cysts collectively called spermatogonia. α-Spectrin [red in (A); green in (B)] labels fusomes and follicle cell membranes; LamC [red in (A); green in (B)] labels cap cell nuclear envelopes; Armadillo [green in (C)] labels hub cells; 1B1 [green in (C)] labels fusomes; Vasa [green in (A) and red in (B and C)] labels germ cells; and DAPI (blue) labels nuclei. (B and C) are shown at the same magnification. GSC, germline stem cell.
GSCs divide asymmetrically with respect to the position of the resulting daughters relative to the niche in both sexes. The self-renewed GSC remains in the niche, whereas the cystoblast or gonialblast is distally displaced, such that it falls outside of the local influence of the niche (Greenspan et al. 2015). A major role of secreted molecules produced by the niche is to create a signaling microenvironment that represses the differentiation of GSCs, but niche signals also contribute to proliferation control and stem cell protection, as discussed below. In addition to serving as a source of diffusible extracellular signals, the GSC niche serves as a physical anchor through adhesion molecules to ensure the long-term retention of GSCs within this unique signaling milieu (Gilboa and Lehmann 2004; Greenspan et al. 2015). Gap junctions physically connect the niche to GSCs and mediate additional communication through unknown factors (Gilboa and Lehmann 2004). Thus, the niche contributes to the asymmetric outcome of GSC divisions through diverse mechanisms.
In addition to asymmetry imposed by the external environment, GSC divisions also exhibit intrinsic asymmetry. As GSCs divide in both sexes, a specialized organelle, the fusome, goes through stereotypical morphological changes that are oriented relative to the position of the niche and correlate with progression through the cell cycle, as has been extensively described in females (de Cuevas and Spradling 1998; Hsu et al. 2008; Ables and Drummond-Barbosa 2010). The cell cycle in GSCs is also distinct in that G2 is exceptionally long, and Cyclin E expression and activity persist through G2 and M phases in females, unlike its more typical G1/S expression pattern in dividing cysts (Hsu et al. 2008; Ables and Drummond-Barbosa 2013). Asymmetric inheritance of centrosomes during GSC division has been well documented in males: the mother centrosome localizes near the hub, whereas the daughter centrosome moves to the opposite side, foreshadowing spindle orientation perpendicular to the niche (Pereira and Yamashita 2011). In contrast to an initial report that females do not require centrioles or centrosomes for asymmetric GSC divisions (Stevens et al. 2007), more recent studies suggest that activation of the small GTPase Rac at the GSC–cap cell interface regulates centrosome position and spindle orientation (Lu et al. 2012), and that the daughter centrosome is preferentially inherited by the female GSC (Salzmann et al. 2014). Additional studies provide intriguing evidence that sister chromatids of sex chromosomes and a subset of histones are also asymmetrically segregated during male GSC division (Tran et al. 2012; Yadlapalli and Yamashita 2013; Xie et al. 2015). Differences in the levels of ribosome biosynthesis and protein translation in GSCs vs. their early daughters are required for their respective self-renewal vs. differentiation fates (Fichelson et al. 2009; Slaidina and Lehmann 2014; Zhang et al. 2014). How these various asymmetries relate mechanistically to one another and to the niche remain largely unknown.
The niche signaling environment
GSCs are physically retained in the niche via E-cadherin-mediated adherens junctions to ensure they are continuously exposed to the unique combination of signals provided by the niche. E-cadherin accumulates at the GSC–niche junction in both sexes (Song et al. 2002; Wang et al. 2006), and direct evidence based on genetic mosaic analysis in females indicates that E-cadherin is required for the maintenance of GSCs (Song et al. 2002). E-cadherin has also been proposed to mediate cell competition favoring occupancy of the niche by GSCs instead of “accidentally differentiated” cells (Jin et al. 2008). E-cadherin expression and localization is regulated by multiple factors, including the GTPase Rab11 (Bogard et al. 2007), the translational initiation factor eIF4A (Shen et al. 2009), and Lisencephaly-1 (Chen et al. 2010). A similar requirement for E-cadherin is likely the case in male GSCs, as genes required for E-cadherin expression, maturation, or localization also promote GSC maintenance (Wang et al. 2006; Srinivasan et al. 2012; Lim et al. 2015).
Multiple key developmental pathways are used reiteratively in niches to promote GSC self-renewal in males and females, including bone morphogenetic protein (BMP) and Janus kinase (JAK)/signal transducer and activator of transcription (STAT) signaling components (Greenspan et al. 2015). As described below, these signaling pathways can repress differentiation through more direct mechanisms or by ensuring the retention of GSCs in the niche, and their specific roles differ in male vs. female niches.
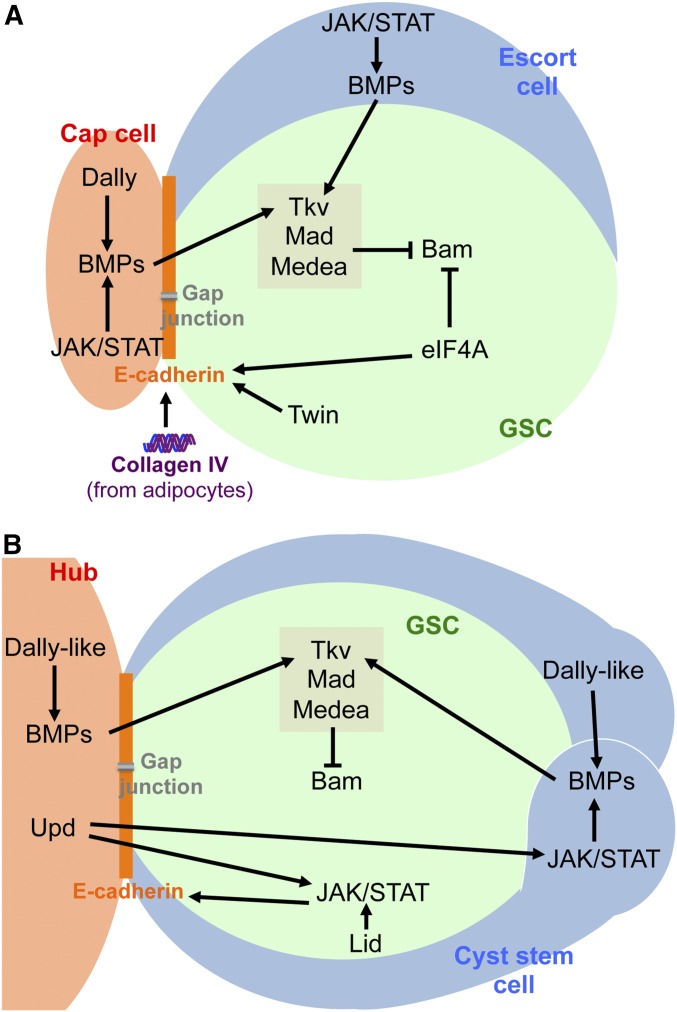
The discovery that a BMP signal produced by the niche directly controls female GSCs marked a key milestone toward the experimental definition of stem cell niches and of their signaling roles (Figure 2A). Using a combination of overexpression and genetic mosaic analysis, Xie and Spradling (1998) demonstrated that the BMP signal Decapentaplegic (Dpp), produced from a localized source, acts directly on GSCs to promote their maintenance and proliferation (Xie and Spradling 1998). Subsequent work showed that, upon reception of the Dpp signals by GSCs, activation of Mad and Medea leads to their direct binding to a silencer element, and repression of bag of marbles (bam) (Chen and McKearin 2003a,b; Song et al. 2004), which encodes a key differentiation factor (McKearin and Ohlstein 1995; Ohlstein and McKearin 1997). As in female GSCs, BMP signaling is also required for the maintenance of male GSCs through Bam repression (Shivdasani and Ingham 2003; Kawase et al. 2004; Schulz et al. 2004) (Figure 2B). In parallel to BMP signaling, the translational initiation factor eIF4A directly inactivates Bam and maintains E-cadherin expression, further reinforcing GSC maintenance in females (Shen et al. 2009). The Drosophila CCR4 homolog Twin (part of a deadenylase complex) acts in female GSCs to enhance E-cadherin expression and also in their early progeny to promote Bam-induced differentiation (Fu et al. 2015). Not surprisingly, many mechanisms for precise spatial regulation of BMP expression/signaling have evolved to ensure that the BMP pathway is robustly active in GSCs but actively repressed in its differentiating daughters (Chen et al. 2011; Ayyub et al. 2015; Dolezal et al. 2015; Inaba et al. 2015; Luo et al. 2015, 2017; Morawa et al. 2015; Newton et al. 2015; Mottier-Pavie et al. 2016).
Figure 2.
Regulation of GSCs by their local microenvironments. (A) Diagram of a female GSC in the niche microenvironment represented by a cap cell and an escort cell. (B) Diagram of a male GSC attached to the hub. GSCs are attached to cap cells (A) or the hub (B) via E-cadherin-mediated adhesion, and receive local signals from the niche that control their fate. See text for additional details. BMP, bone morphogenetic protein; GSC, germline stem cell; JAK, Janus kinase; STAT, signal transducer and activator of transcription.
The first niche signal identified in males was Unpaired, which is expressed specifically in the hub, and activates JAK and the downstream transcriptional factor Stat92E, in both GSC and CySCs to promote their maintenance (Kiger et al. 2001; Tulina and Matunis 2001). JAK/STAT activation in adult male GSCs promotes their adhesion to the hub (Leatherman and Dinardo 2010) and protects them from death (Hasan et al. 2015), and proper expression of Stat92E in male GSCs requires the H3K4me3-specific histone demethylase encoded by lid (Tarayrah et al. 2015). In contrast, female GSCs do not respond directly to JAK/STAT; instead, JAK/STAT acts in cap and escort cells to regulate Dpp expression (Greenspan et al. 2015). In CySCs, JAK/STAT signaling promotes self-renewal; incidentally, experiments involving the expansion of CySCs by overactivation of the JAK/STAT pathway showed that CySCs, in addition to the hub, serve as a source of BMP signals to promote self-renewal of GSCs (Leatherman and Dinardo 2010). In addition to local extracellular signals, communication through gap junctions between GSCs and neighboring somatic cells also occurs in both males and females (Tazuke et al. 2002; Smendziuk et al. 2015), although the nature of signals traveling through these junctions remains unknown.
Extracellular matrix components and heparan sulfate proteoglycans (HSPGs) also influence adult GSCs, at least in part through modulating niche signaling. In females, the glypican Division abnormally delayed (Dally), a major cell surface HSPG, is highly expressed in cap cells, where it is required for full BMP signaling and GSC maintenance (Guo and Wang 2009). In males, a similar role in regulating GSC number is fulfilled by the other Drosophila glypican, Dally-like, which also controls centrosome positioning during asymmetric division (Hayashi et al. 2009; Levings et al. 2016). Adipocyte-derived collagen IV acts via β-integrin/FAK signaling to control E-cadherin levels at the GSC–cap cell junction (Weaver and Drummond-Barbosa 2018). Integrins, which can serve as collagen IV receptors (Chen et al. 2013), function in males both during development (to anchor the hub in its proper position) and adulthood (to maintain hub integrity) (Tanentzapf et al. 2007; Lee et al. 2016). For example, stimulation of βPS integrin signaling by Shriveled, a conserved ligand secreted by somatic cells and GSCs, maintains hub architecture and modulates E-cadherin levels, and thereby GSC numbers (Lee et al. 2016). Future studies should address the roles that various extracellular matrix proteins play in maintaining the physical integrity and signaling environment in adult male and female GSC niches.
Transition of GSC daughters to differentiation
As GSC daughters develop from cystoblasts/gonialblasts to 16-cell cysts, they require intimate contact with somatic cells for proper differentiation (Greenspan et al. 2015; Laws and Drummond-Barbosa 2017). In females, stationary escort cells with dynamic long processes help propel the early differentiating germline, whereas in males, each gonialblast remains encased by the same pair of somatic cyst cells through its differentiation into a 16-cell cyst. In both cases, these closely interacting somatic cells create environments that foster differentiation, in stark contrast to GSC niches.
In females, many mechanisms operate to ensure that BMP signaling is off in early differentiating germ cells (Chen et al. 2011). Dpp expression is repressed in escort cells to restrict it to cap cells, and its diffusion within the adult niche is also controlled by glypicans and possibly collagen IV (Wang et al. 2008; Chen et al. 2011; Van De Bor et al. 2015). Wnt signaling in escort cells contributes to germ cell differentiation by limiting Dpp signaling to the GSC niche (Hamada-Kawaguchi et al. 2014; Luo et al. 2015; Wang et al. 2015; Mottier-Pavie et al. 2016). High levels of the BMP type I receptor Tkv in anterior escort cells, induced by Wnt ligands produced by cap cells, have been proposed to function as a Dpp sink to further restrict its diffusion (Luo et al. 2015). A complex between the E3 ubiquitin ligase Smurf and the serine/threonine kinase Fused ensures that Tkv is degraded in cystoblasts, whereas the translational repressor TRIM-NHL-domain protein Brain tumor (Brat) (in combination with Pumilio) downregulates protein expression of the downstream transcriptional regulators Mad, Medea, and schnurri in cystoblasts (Chen et al. 2011; Harris et al. 2011; Newton et al. 2015). Additional mechanisms involving regulation of escort cell processes, the epidermal growth factor (EGF) receptor pathway, Hedgehog (Hh) signaling, and the Hippo pathway effector Yorkie also contribute to repression of BMP signaling in early female differentiating germ cells (Xie 2013; Liu et al. 2015; Lu et al. 2015; Mottier-Pavie et al. 2016; Huang et al. 2017).
The repression of BMP expression in somatic cells outside of the female GSC niche is multifaceted, underscoring the critical role it plays in the decision between self-renewal and differentiation of GSCs. Several epigenetic regulators, including the histone lysine-specific demethylase Lsd1 and Polycomb repressive complex 1 (PRC1) (antagonizing the Trithorax group Brahma), contribute to the prevention of ectopic dpp (and glass bottom boat, gbb, which encodes another BMP signal) expression in escort cells (Eliazer et al. 2011; Li et al. 2016). Wnt signaling in escort cells also contributes to low dpp mRNA levels (while BMP signaling represses Wnt signaling in anterior escort cells) (Mottier-Pavie et al. 2016), although it remains unclear how Wnt signaling interacts with epigenetic regulators of dpp. Hh and Yorkie signaling in escort cells act in parallel to suppress gbb and dpp mRNA expression, respectively (Liu et al. 2015; Lu et al. 2015; Huang et al. 2017), although a separate study concluded that Yorkie regulates escort cell function and germ cell differentiation independently of dpp (Li et al. 2015). Knockdown of cul-2 (which encodes a component of Cullin-2 E3 ligase) was also shown to result in ectopic Dpp expression and expansion of GSC-like cells (Ayyub et al. 2015), indicating the existence of post-transcriptional mechanisms of Dpp repression in escort cells as well.
Relatively less is known about the role of somatic cyst cells in the differentiation of early germ cells in the testis. In addition to Bam expression in germ cells, EGF receptor signaling within somatic cyst cells is required for appropriate differentiation of the underlying germline (Greenspan et al. 2015), although the downstream signals involved are unknown. The epigenetic regulator Enhancer of Polycomb acts in cyst cells and genetically interacts with the EGF pathway for proper differentiation of cyst cells themselves and of the germline (Feng et al. 2017). Heterotypic gap junctions formed by somatic Inx2 and germline Zpg/Inx4 mediate further cyst cell–germ cell communication that is essential for differentiation (Smendziuk et al. 2015). Proper male germline differentiation also requires the gradual formation of a permeability barrier mediated by septate junctions in cyst cells (Fairchild et al. 2015). Many other factors contributing to the control of GSC self-renewal and differentiation of their daughters remain to be studied, as suggested by recent RNAi screens in both males and females (Yan et al. 2014; Liu et al. 2016; Fairchild et al. 2017).
Intracellular circuitry balancing GSC self-renewal and daughter differentiation
The sharp switch from self-renewal to differentiation mode in the GSC daughter distal to the niche is reinforced by a complex intracellular circuitry involving multiple post-transcriptional regulators that have been intensely studied in females (Moschall et al. 2017). GSC self-renewal requires the translational repressors Pumilio and Nanos (Lin and Spradling 1997; Wang and Lin 2004) in addition to Dpp signaling, whereas Bam drives cystoblast differentiation (see above). Brat was identified as a major differentiation factor that is actively repressed in GSCs by Pumilio/Nanos, and later functions with Pumilio in cystoblasts to translationally repress specific targets (see above) to turn off signaling downstream of Dpp (Harris et al. 2011). Bam (in a complex with the RNA-binding protein Bgcn) represses nanos mRNA translation (Li et al. 2009). Notably, repression of nanos mRNA translation by Bam is female-specific and requires the RNAi-binding protein Sex lethal (Sxl) (Chau et al. 2009), a major post-transcriptional regulator that controls female-specific development (Moschall et al. 2017). Specifically, Sxl (in a complex with Bam, Bgcn, and the TRIM-NHL-domain protein Mei-P26) binds the 3′-UTR of nanos mRNA to rapidly downregulate Nanos expression in cystoblasts and early germline cysts (Chau et al. 2012; Li et al. 2013), thus reinforcing the transition to differentiation.
Small noncoding RNAs also regulate the balance between GSC maintenance and daughter differentiation. For example, microRNAs (miRNAs) are required for GSC maintenance, based on the analysis of genetic mosaics for genes encoding the RNAse Dicer-1 and its cofactor Loquacious (both involved in miRNA biogenesis) (Jin and Xie 2007; Park et al. 2007). Interestingly, Mei-P26 has multiple interactions with the miRNA pathway in regulating the early GSC lineage. In GSCs, Mei-P26 associates with miRNA pathway components to repress translation of target mRNAs, and loss of mei-P26 causes premature translation of the BMP antagonist Brat, thereby impairing BMP signaling and leading to premature Bam expression (Li et al. 2012). In differentiating GSC daughters, the Argonaute family protein Ago1 (which is guided by miRNAs to bind the 3′-UTR of target RNAs) interacts with Brat and Mei-P26, which now antagonizes the miRNA pathway to promote differentiation of early cysts (Neumüller et al. 2008). Mei-P26 is also strongly expressed in 16-cell cysts, and is required at this later stage for differentiation of nurse cells and oocytes (Neumüller et al. 2008). Finally, piRNAs also have complex roles in transcriptional and post-transcriptional gene regulation, and in the repression of transposable elements, a topic that has been extensively reviewed elsewhere (Juliano et al. 2011; Gleason et al. 2018; Rojas-Ríos and Simonelig 2018).
Dedifferentiation of early differentiating germ cells
Remarkably, despite all of the mechanisms ensuring proper differentiation, dedifferentiation of early germ cells can occur in both males and females under certain conditions, presumably as a strategy to replenish GSCs (Greenspan et al. 2015). Upon extensive GSC loss due to forced differentiation induced by genetic manipulations, early germline cysts can break down and repopulate the niche as functional GSCs in testes and ovaries (Brawley and Matunis 2004; Kai and Spradling 2004; Sheng et al. 2009). Subsequent studies relying on lineage tracing using Bam promoter-induced labeling systems indicated that dedifferentiation might occur physiologically in aging males and in a small number of germ cells in early stages of differentiation in females (Cheng et al. 2008; Liu et al. 2015). Interestingly, a recent study using a similar lineage-tracing approach showed that spermatogonial dedifferentiation mediated by JNK signaling is required to maintain a functional GSC pool during chronic stress, including starvation and frequent mating (Herrera and Bach 2018). However, the molecular mechanisms underlying dedifferentiation remain largely unknown.
Adult GSC Responses to Physiological and Environmental Factors
GSCs and their niches exist within complex organisms composed of many tissues and organs that are exposed to nutrients, metabolites, hormones, and other factors shared through their circulatory system. These circulating factors change in response to nutrient availability, the metabolic state of different organs, age, interactions with microorganisms, and other inputs, serving as a powerful communication means to coordinate reproduction with stresses and opportunities experienced by the organism. Although it has been known for a long time that oogenesis is profoundly affected by diet, the discovery that female GSCs and their early differentiating progeny are specifically regulated by diet occurred within the past two decades (Drummond-Barbosa and Spradling 2001). Since then, the field has witnessed intense research on diet-dependent mechanisms regulating the GSC lineage in both males and female (Laws and Drummond-Barbosa 2017). There is also growing evidence that other physiological factors can also impact GSCs and their niche (Ameku and Niwa 2016; Sullivan 2016; Ables and Drummond-Barbosa 2017; Laws and Drummond-Barbosa 2017).
Diet and diet-dependent signaling
Oogenesis demands high levels of energy and resources; therefore, many mechanisms have evolved to tightly couple its multiple steps to the availability of nutrients. Females produce 60-fold more eggs on a yeast-rich compared to a yeast-free diet, and entry into vitellogenesis and retention of mature oocytes (i.e., inhibition of ovulation) are major diet-regulated steps (Drummond-Barbosa and Spradling 2001). Using a lineage-tracing system, Drummond-Barbosa and Spradling (2001) showed for the first time that previtellogenic stages, including GSCs, are also highly responsive to nutrient availability (Drummond-Barbosa and Spradling 2001). GSCs proliferate two-to-three times faster on rich relative to poor diets, in coordination with their early progeny (Drummond-Barbosa and Spradling 2001). The cell cycle of GSCs on a rich diet has a relatively long G2 phase and a very short G1 phase, and both of these phases are proportionately lengthened in response to a poor diet (Hsu et al. 2008). In addition, a poor diet increases the rate of early germline cyst apoptotic/autophagic death in germarium region 2, where 16-cell germline cysts are not yet fully surrounded by follicle cells (Drummond-Barbosa and Spradling 2001; Hou et al. 2008). Follicles also grow at different rates, with coordinated changes in follicle cell proliferation and growth of the underlying germline cyst prior to vitellogenesis (Drummond-Barbosa and Spradling 2001). In addition to growing more slowly on a poor diet, germline cysts within previtellogenic follicles cortically reorganize their microtubule cytoskeleton and accumulate enlarged ribonucleoprotein-containing P bodies (Shimada et al. 2011), a subset of which are closely associated with U bodies (Buckingham and Liu 2011). This multifaceted ovarian response to diet is fast and largely reversible within hours (Drummond-Barbosa and Spradling 2001; Shimada et al. 2011). In females maintained on a poor diet for weeks (instead of days), the rates of GSC loss are significantly higher than those of females on a rich diet (Hsu and Drummond-Barbosa 2009), further adding to the wide range of oogenesis processes affected by diet.
Spermatogenesis is also coupled to dietary inputs at early spermatogonial stages, but thus far there is little evidence of regulation of later stages, in agreement with the relatively lower demands imposed by gametogenesis in males compared to females. In males maintained on a yeast-free diet for prolonged periods of time (15–20 days), GSC numbers decrease (McLeod et al. 2010). Another study showed that the number of GSCs is reduced from around eight to six GSCs within 3–6 days of yeast deprivation, after which it remains stable for at least 2 weeks (Yang and Yamashita 2015). Rates of GSC proliferation also decrease in response to a yeast-free diet, although the timing of this response is controversial. Yang and Yamashita (2015) reported that GSC proliferation transiently slows down within the first 2 days on a poor diet, but then becomes comparable to GSC division rates on a rich diet for ≥ 18 days subsequently (Yang and Yamashita 2015), while McLeod et al. (2010) found reduced proliferation rates at 20 days on a yeast-free diet (McLeod et al. 2010). Two- and four-cell spermatogonia also show increased rates of death in starved males although, unlike in females (Drummond-Barbosa and Spradling 2001; Hou et al. 2008), these dying germline cysts are negative for apoptotic markers (Yang and Yamashita 2015). Instead, apoptotic somatic cyst cells trigger the death of underlying germ cells through a process involving spichthyin, which encodes a protein associated with lysosomes during phagocytosis of spermatogonia by cyst cells (Yang and Yamashita 2015; Chiang et al. 2017). Blocking of this death process leads to continued GSC loss beyond 6 days on a yeast-free diet and to impaired germline recovery upon switching to a yeast-rich diet (Yang and Yamashita 2015; Chiang et al. 2017). Interestingly, reducing (instead of eliminating) dietary yeast leads to distinct responses in males. Lower levels of dietary yeast and sugar cause centrosome misorientation in GSCs, which in turn leads to a slowdown of their proliferation (Roth et al. 2012). In addition, Mair et al. (2010) reported that although GSC numbers are similar in well-fed males vs. those on low yeast at 10 and 20 days, GSCs are maintained better under low-yeast conditions at 30 days (Mair et al. 2010).
Multiple diet-dependent signaling pathways and extensive interorgan communication regulate these dietary responses (Figure 3). As described above, male and female GSCs, and their early progeny, respond to diet in comparable (although not identical) ways. These responses also involve similar signaling pathways; however, the precise mechanisms of regulation of male and female GSCs, and early descendants, by diet-dependent pathways appear quite distinct.
Figure 3.
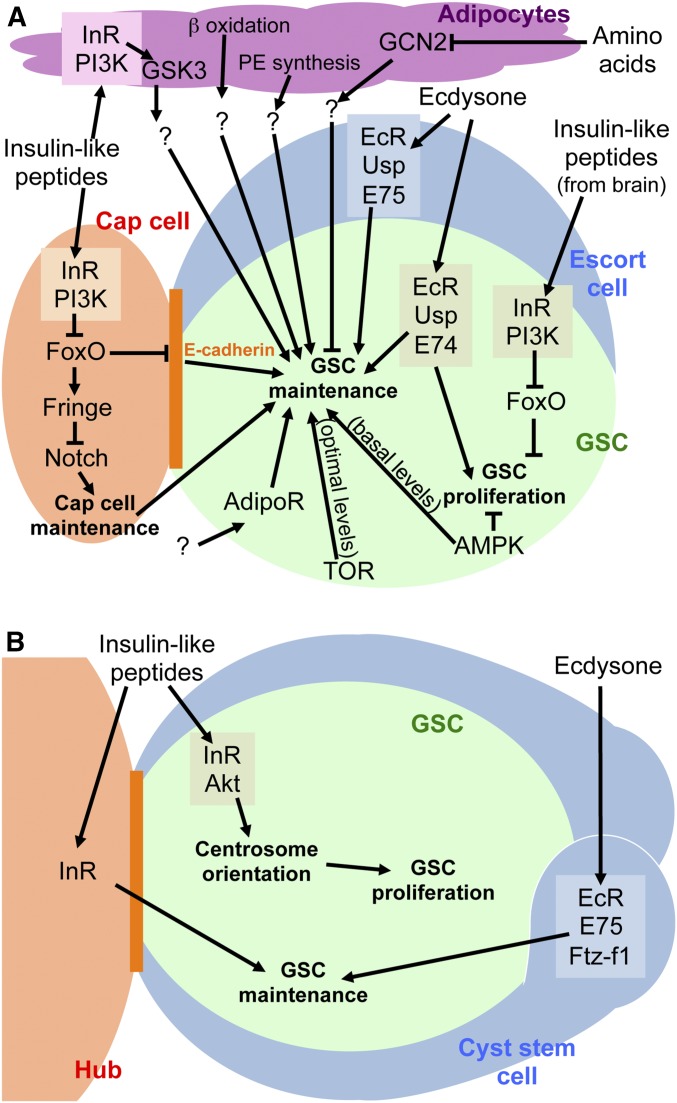
Systemic regulation of GSCs. (A) Diagram illustrating that female GSCs are regulated by interorgan communication involving the brain and adipocytes through signals that act on the niche, GSCs, or through intermediate organs. (B) Diagram of male GSC next to hub illustrating the known roles of insulin-like peptides and ecdysone in controlling GSC maintenance and proliferation. See text for additional details. EcR, ecdysone receptor; GSC, germline stem cell; InR, insulin receptor; PI3K, phosphoinositide 3 kinase; TOR, target of rapamycin; PE, phosphatidylethanolamine.
Insulin, target of rapamycin, and AMP-dependent kinase signaling:
Insulin, Target of Rapamycin (TOR), and AMP-dependent kinase (AMPK) signaling represent a core set of interweaved diet-dependent pathways that are used reiteratively throughout development and adulthood to control downstream responses in a context-dependent manner (Riera et al. 2016; Laws and Drummond-Barbosa 2017). Activation of the insulin receptor, a receptor tyrosine kinase, by insulin-like peptides results in phosphorylation of insulin receptor substrate, encoded by chico. Downstream activation of phosphoinositide 3 kinase (PI3K) leads to conversion of phosphatidylinositol (4,5)-bisphosphate to phosphatidylinositol (3,4,5)-trisphosphate at the cell membrane, and recruitment of the serine/threonine kinase Akt (also known as protein kinase B), which phosphorylates multiple downstream substrates, including the transcription factor Forkhead box, subgroup O (FoxO) and Tuberous Sclerosis Complex 2 (TSC2; also known as Tuberin). FoxO phosphorylation leads to its retention in the cytoplasm, preventing activation of transcription of its downstream targets. TSC2, together with TSC1 (also known as Hamartin), negatively regulates the nutrient sensor TOR by acting as a GTPase-activating protein for the small GTPase Rheb, a positive regulator of the TOR serine/threonine kinase (Saxton and Sabatini 2017). Activation of Rheb activity downstream of the insulin pathway or other growth factor signaling is integrated with amino acid availability inputs through regulation of TOR subcellular localization, resulting in TOR activation and phosphorylation of its downstream effectors (Wolfson and Sabatini 2017). The cellular energy sensor AMPK is activated under a high AMP-to-ATP ratio (i.e., low energy availability) and also in response to the upstream tumor suppressor Lkb1. AMPK has many downstream targets, including TSC2, which is activated by AMPK to inhibit TOR signaling (Lin and Hardie 2018; Khan et al. 2019). Not surprisingly, these highly conserved pathways mediate diverse responses of GSC lineages to dietary inputs (Laws and Drummond-Barbosa 2017).
Diet-dependent pathways directly control GSC proliferation. In females, cell-specific ablation and genetic mosaic analysis showed that insulin-like peptides produced in brain neurosecretory cells directly stimulate the insulin receptor on GSCs to promote their proliferation, primarily at the level of the G2 phase of the cell cycle through PI3K inhibition of FoxO (LaFever and Drummond-Barbosa 2005; Hsu et al. 2008). Live-imaging culture experiments showed that addition of exogenous insulin promotes G2/M progression in GSCs (Morris and Spradling 2011), consistent with in vivo results. TOR signaling within female GSCs also stimulates progression through G2 largely independently of insulin signaling (LaFever et al. 2010). In contrast to the positive role of insulin and TOR signaling, AMPK function is required for the inhibition of GSC proliferation on a poor diet (Laws and Drummond-Barbosa 2016). Although the roles of TOR and AMPK signaling in male GSCs have not been examined, insulin signaling was also shown to promote the proliferation of male GSCs at G2/M (Ueishi et al. 2009). Subsequent studies using expression of dominant-negative and constitutively active transgenes in the germline showed that insulin signaling contributes to proper centrosome orientation through Akt on a rich diet, and that increased misorientation on a poor diet slows down male GSC proliferation downstream of the centrosome orientation checkpoint (Roth et al. 2012).
GSC maintenance also requires insulin signaling but, at least in females, the mechanisms are very distinct from those regulating GSC proliferation. The insulin receptor is not required in female GSCs to promote their maintenance; instead, insulin-like peptides act through PI3K/FoxO on the niche to control cap cell number and E-cadherin levels at the GSC–cap cell junction (Hsu and Drummond-Barbosa 2009). Insulin signaling acts intrinsically in cap cells to promote their maintenance by inhibiting FoxO and allowing Notch activation (Hsu and Drummond-Barbosa 2009, 2011), which had been previously been shown to control cap cell number in adult females (Song et al. 2007). Under low insulin signaling, FoxO directly activates excessive transcription of the glycosyltransferase Fringe, leading to inhibition of Notch signaling in cap cells (Yang et al. 2013). Intriguingly, a recent study reported that diet-induced cap cell loss is reversible, possibly through the recruitment of escort cells (Bonfini et al. 2015); however, this study did not include lineage-tracing experiments. In males, GSC maintenance appears to require insulin signaling intrinsically in GSCs, and the GSC loss induced by 20 days of starvation can be avoided by expression of transgenes that constitutively activate insulin signaling either in germ cells or in the hub, suggesting an additional contribution through the niche (McLeod et al. 2010). In stark contrast to the lack of requirement for the insulin receptor in the germline for female GSC maintenance (Hsu and Drummond-Barbosa 2009), optimal levels of TOR signaling are intrinsically required for GSC maintenance because genetic mosaics with mutations either in Tor (reducing TOR activity) or Tsc1/2 (increasing TOR activity) show GSC loss (LaFever et al. 2010; Sun et al. 2010). In fact, female GSC loss is much more severe in response to increased TOR activity (LaFever et al. 2010; Sun et al. 2010) due to decreased BMP signaling and differentiation of GSCs, which can be rescued by the TOR inhibitor rapamycin (Sun et al. 2010). Basal AMPK activity also appears to play a role in female GSC maintenance that is independent of nutrient stress because AMPK mutant GSCs are lost faster relative to controls on a rich diet (Laws and Drummond-Barbosa 2016), when AMPK activity levels are expected to be low (Lin and Hardie 2018).
Insulin, TOR, and AMPK signaling also control the descendants of GSCs at multiple points during their development, further underscoring their versatility in tying diet to reproduction. In females, TOR promotes the survival of early dividing cysts in the germarium (LaFever et al. 2010), in contrast to insulin signaling, which has no intrinsic role in early cyst survival (LaFever and Drummond-Barbosa 2005). Active inhibition of TOR signaling by GATOR1 [which is involved in amino acid control of TOR (Bar-Peled and Sabatini 2014)] on a poor diet is required to protect previtellogenic follicles against apoptosis (Wei and Lilly 2014), while on a rich diet GATOR1 is antagonized by GATOR2 to allow oocyte growth (Wei et al. 2014). Insulin and TOR signaling work together to stimulate follicle growth and progression through vitellogenesis, by acting in both the germline and follicle cells (LaFever and Drummond-Barbosa 2005; Hsu et al. 2008; LaFever et al. 2010; Pritchett and McCall 2012; Wei et al. 2014, 2016; Cai et al. 2016), and spermatocyte growth also requires insulin signaling (Ueishi et al. 2009). AMPK is required in the germline for the degeneration of vitellogenic follicles on a poor diet; however, AMPK does not function in the germline to control follicle growth, acting solely in follicle cells to repress growth of the underlying germline (Laws and Drummond-Barbosa 2016). Insulin signaling also acts on follicle cells of previtellogenic follicles to indirectly mediate the effects on P body accumulation and microtubule rearrangements induced by diet (Shimada et al. 2011; Burn et al. 2015). Finally, decreased Akt activity during late oogenesis leads to stimulation of GSK3 to induce mitochondrial quiescence and glycogen accumulation in mature eggs (Sieber et al. 2016), although in this case Akt is presumably regulated at the developmental instead of dietary level.
Ecdysone signaling:
The Drosophila steroid hormone ecdysone has many well-characterized roles in the control of developmental processes, metabolism, and reproduction (Ables and Drummond-Barbosa 2017). Ecdysone binds to a heterodimeric receptor encoded by ecdysone receptor (EcR) and ultraspiracle (usp), leading to subsequent activation of its many transcriptional targets, including the transcriptional factors E74, E75, Broad, E78, and Ftz-f1 (Ables and Drummond-Barbosa 2017). In adult females, the major source of ecdysone is vitellogenic follicles in the ovary, and ecdysone production is stimulated in response to nutrients and insulin signaling (Schwartz et al. 1985; Tu et al. 2002), presumably indirectly as a result of effects on vitellogenesis progression. Ecdysone production is at least 20-fold lower in males (Schwartz et al. 1985), and it is unclear if or how its levels are regulated in this context. Nevertheless, there is evidence for ecdysone regulation of both female and male GSCs and their progeny.
Ecdysone signaling influences GSC maintenance in males and females through different mechanisms. Genetic mosaic analyses showed that ecdysone signaling is intrinsically required in female GSCs for their proliferation and maintenance via E74 (with a smaller contribution from Broad), but independently of E75 or of the coactivator Taiman (Ables and Drummond-Barbosa 2010). Ecdysone signaling enhances the response of GSCs to BMP signals, and ecdysone pathway components show a strong genetic interaction with BMP pathway components and the chromatin-remodeling factors encoded by iswi and nurf301 (Ables and Drummond-Barbosa 2010). Noncell-autonomous mechanisms are likely at play, given that knockdown of EcR, usp, or E75 in escort cells also leads to increased loss of GSCs (Morris and Spradling 2012). Similarly, expression of a dominant-negative form of EcR, E75, or ftz-f1 (but not broad) in the somatic cyst stem cell lineage in males also leads to GSC loss, which is rescued by ecdysone feeding (Li et al. 2014). A potential germline requirement for ecdysone signaling for GSC maintenance was not tested in this study; however, the nonautonomous role involves a genetic interaction between EcR and Nurf301 (Li et al. 2014), resembling the case in females. A recent genetic mosaic screen identified additional ecdysone targets controlling female GSCs and their early progeny (Ables et al. 2016).
Ecdysone is indirectly required through somatic cells for early germline development in females and males. Overexpression of dominant-negative or wild-type EcR, or knockdown of EcR or taiman in escort cells, leads to germaria filled with undifferentiated precystoblasts that fail to form cysts, suggesting that ecdysone signaling is required in escort cells for early germline differentiation (König et al. 2011). Another study showed that knockdown of EcR, usp, or E75 in escort cells disrupts 16-cell cyst production; this later effect on differentiation might be due to differences in the strength of RNAi manipulation (Morris and Spradling 2012). In this same study, knockdown of Usp, EcR, or E75 in the somatic lineage in males did not perturb early germline development (Morris and Spradling 2012). In contrast, temperature-sensitive EcR mutants showed increased death of differentiating germ cells in testes, and this phenotype was rescued by EcR expression in the somatic lineage (Li et al. 2014). A third study concluded that ecdysone antagonizes EGF signaling to regulate germline cyst differentiation in males because reduction of EcR signaling in somatic cyst cells rescues germline differentiation defects caused by mutation of the EGF receptor ligand Spitz (Qian et al. 2014). Not surprisingly, these studies show that differences in the strengths of genetic manipulations or in specific experimental strategies might lead to distinct and/or apparently contradictory conclusions.
Ecdysone signaling also controls later developing follicles. For example, E78 genetically interacts with EcR to control follicle development past stage 4/5 (Ables et al. 2015). EcR and E75 are required in the germline for progression of vitellogenic stages (Buszczak et al. 1999). Ecdysone also promotes lipid uptake in stage 10 oocytes by inducing the transcription factor sterol regulatory element-binding protein (SREBP) and the lipoprotein receptor LpR2 in oocytes, and acts systemically to stimulate female feeding (Sieber and Spradling 2015). Finally, a developmentally controlled increase in ecdysone activity in mature follicle cells is required for their response to neuronal ovulatory stimuli (Knapp and Sun 2017).
Unknown adipocyte factors:
Different tissues and organs undergo extensive crosstalk through systemic factors to modulate a large variety of processes such that multicellular organisms can function as a whole. The regulation of germline lineages in adult female and male Drosophila is no exception, as the brain–germline connection through insulin-like peptides discussed above illustrates. Recent studies have shown that, in addition to the role of adipocytes as a source of collagen IV for the female GSC niche (Weaver and Drummond-Barbosa 2018), as-yet-unspecified adipocyte factors also contribute to the regulation of the female GSC lineage. For example, insulin-like peptides act on adipocytes via GSK-3β and independently of FoxO to control female GSC numbers through unknown systemic factors (Armstrong and Drummond-Barbosa 2018). Unidentified signals downstream of adipocyte insulin signaling also affect early germline cyst survival and vitellogenesis (Armstrong and Drummond-Barbosa 2018). Amino acid sensing in adipocytes controls GSC number through the conserved GCN2 kinase and ovulation through TOR (Armstrong et al. 2014); however, the relevant secreted/exported factors downstream of GCN2 and TOR signaling in adipocytes remain undiscovered. The Drosophila homolog of adiponectin receptor, which in mammals responds to adipocyte-derived adiponectin (Fang and Judd 2018), is required within GSCs for their maintenance, and its overexpression partially reverses normal GSC loss with age (Laws et al. 2015), although the identity and source of the Drosophila ligand for this receptor are unknown. An isobaric tags for relative and absolute quantification proteomic analysis identified multiple metabolic pathways that are regulated in adipocytes within 12 hr of dietary changes, and knockdown of regulatory enzymes in some of these pathways exclusively in adult adipocytes was shown to have specific effects in the female GSC lineage (Matsuoka et al. 2017). For example, enzymes involved in pyruvate/acetyl-CoA synthesis promote early cyst survival, whereas fatty acid oxidation and phosphatidylethanolamine synthesis helps maintain normal GSC numbers (Matsuoka et al. 2017). It is still unclear how these pathways influence oogenesis (e.g., through exported signaling metabolites or more indirect mechanisms) or to what degree regulation of these pathways by diet (as opposed to other potential stimuli) contributes to the dietary response of oogenesis. The potential role of adipocytes in controlling the male GSC lineage remains to be investigated. It will also be important to investigate the communication of GSC lineages with additional organs besides adipocytes and the brain, including muscles and intestine.
Mating
During mating, males transfer sperm, pheromones, and proteins, including accessory gland proteins, to females (Billeter and Wolfner 2018). These transferred factors act on the reproductive tract and other tissues to affect copulation duration, female appetite and food preference, ovulation, and remating (Billeter and Wolfner 2018). For example, the best-studied accessory gland protein, sex peptide, reaches the female hemolymph and stimulates its G protein-coupled receptor in the nervous system to induce various downstream responses (Ferveur 2010; Carmel et al. 2016). The role of mating specifically on GSCs and their descendants is just beginning to be explored. A recent study showed that mating temporarily increases GSC number and stimulates GSC proliferation (Ameku and Niwa 2016). These effects are mediated in part through male sex peptide acting on female neurons to increase ovarian ecdysone production (Ameku and Niwa 2016). In addition, sex peptide stimulates the release of neuropeptide F from enteroendocrine cells of the midgut, which in turn modulates BMP signaling in GSCs via a functional interaction with ecdysone (Ameku et al. 2018). These findings are consistent with the known role of ecdysone in the control of GSC proliferation and maintenance (Ables and Drummond-Barbosa 2010; Morris and Spradling 2012), and with the observation that genome-wide responses to mating include a network of genes that interact with EcR (Gerrard et al. 2013). Mating or sex peptide injection also stimulates vitellogenesis in later oocytes (Soller et al. 1997), and mating increases the titers of juvenile hormone (Sugime et al. 2017), a sesquiterpenoid produced by the corpora allata and known to stimulate vitellogenesis (Gruntenko and Rauschenbach 2018).
The effects of mating on the male germline, potentially as a result of the loss of sperm/seminal fluid to females, or of the physical or pheromonic experience, are much less well studied. Nevertheless, some studies suggest that this question is worth exploring. For example, studies using fluorescently labeled sperm showed that males adjust ejaculate sizes according to perceived female quality and risk of sperm competition, such that more sperm are delivered to large, young, or mated females (Lupold et al. 2011). Also, reproduction earlier in life reduces male fertility during aging owing to lower sperm counts (Partridge and Prowse 1997), although the cellular and molecular mechanisms involved or more short-term effects remain unclear.
Microorganisms
Microorganisms can have a profound impact on reproduction and whole-body physiology. As the examples below illustrate, the widespread intracellular bacteria Wolbacchia pipientis and the gut microbiome can affect the germline through largely unknown mechanisms.
The endosymbiont Wolbachia:
Wolbachia affects early oogenesis in multiple Drosophila species, in many cases exhibiting stem cell niche tropism. D. mauritiana infected with a Wolbachia strain shows bacterial accumulation in the GSC niche. These infected females display a twofold increase in GSC proliferation and a twofold decrease in early germline cyst death, laying approximately four times as many eggs as uninfected controls (Fast et al. 2011). Infected males also show an enrichment of Wolbachia in the GSC niche and higher GSC division rates, albeit less pronounced than in females (Fast et al. 2011). Another study showed that although GSC niche tropism is not observed in D. melanogaster in particular, 6 of 11 additional species of Drosophila have Wolbachia infection of the female GSC niche (Toomey et al. 2013). In addition, somatic follicle cell niche tropism of Wolbachia is widespread among Drosophila species, and appears to serve as a mechanism to increase the bacterial density in the germline and thereby maximize vertical transmission (Toomey et al. 2013). Interestingly, niche tropism is much less common and more evolutionarily divergent in males than in females, does not affect male reproduction, and appears to be mechanistically distinct from GSC niche enrichment in females (Toomey and Frydman 2014). A virulent Wolbachia strain was also shown to affect survival of early germline cysts in females. D. melanogaster w1118 females infected with the virulent Wolbachia strain wMelPop, but not Canton-S females infected with the widely occurring strain wMel, show higher levels of apoptosis in region 2 of the germarium relative to tetracycline-treated (uninfected) counterparts (Zhukova and Kiseleva 2012).
The molecular mechanisms through which Wolbachia influences different aspects of germline development remain largely unknown. The effects of Wolbachia are similar to those of physiological factors such as diet, suggesting that Wolbachia infection might influence signaling pathways that normally control oogenesis. In support of this possibility, Wolbachia infection suppresses phenotypes caused by overexpression of a dominant-negative insulin receptor transgene, namely dwarfism, reduced fecundity, and lifespan extension (Ikeya et al. 2009). Analysis of ovarian proteomes from D. melanogaster and D. simulans infected with endogenous and variant Wolbachia strains revealed context-specific proteomic responses to Wolbachia, suggesting that changes in protein abundance are involved in Wolbachia-Drosophila interactions during oogenesis (Christensen et al. 2016).
A series of studies focused on the interaction of Wolbachia with genetic mutations in Drosophila have provided significant mechanistic insight into how Wolbachia affects early oogenesis. In D. melanogaster, Wolbachia injection suppresses the sterility resulting from blocked differentiation and the overproliferation of germ cells caused by mutations in Sxl (Starr and Cline 2002). The presence of Wolbachia also increases the fertility of bam hypomorphic mutants, and long-term interactions between different Drosophila species and Wolbachia appear to drive the rapid evolution of bam, preferentially affecting its function in females (and presumably the decision between female GSC self-renewal and daughter differentiation) (Flores et al. 2015). A recent study showed that the ability of Wolbachia to suppress Sxl and bam appears to involve a common mechanism, as Wolbachia infection suppresses GSC loss and bam upregulation in Sxl mutants (Ote et al. 2016). A functional screen identified TomO as a Wolbachia protein secreted into the cytoplasm of the female germline. TomO associates with nanos mRNA, disrupting its interaction with the translational inhibitor Cup, and leading to elevated levels of Nanos protein and suppression of GSC loss in Sxl mutants (Ote et al. 2016). Additional genetics-based studies using Drosophila—and eventually also Wolbachia—mutants will continue to shed light on the mechanistic aspects of the coevolution of Wolbachia and the Drosophila germline.
The gut microbiome:
The population of microbes in the Drosophila midgut is shaped by diet, changes with aging, and modulates host metabolism, physiology, and behavior (Lewis et al. 2014; Bonfini et al. 2016; Wong et al. 2016, 2017). Therefore, it is conceivable that the gut microbiome indirectly affects GSCs and their descendants. In fact, a recent report showed that removal of gut bacteria from Drosophila represses oogenesis. Specifically, elimination of extracellular bacteria using egg dechorionation and sterilization led to reduced numbers of developing follicles and laid eggs. These oogenesis phenotypes were fully rescued by recolonization with Acetobacter through the host Aldehyde dehydrogenase (Aldh) gene, although specific oogenesis processes were not analyzed (Elgart et al. 2016). How the microbiome affects germ cell biology in either males or female remains largely unknown.
Other factors
Many additional factors influence the behavior of Drosophila GSCs and their progeny. For example, iron homeostasis influences female GSCs (Matsuoka et al. 2017), copper is required for male fertility (Steiger et al. 2010), and histone biotinylation represses retrotransposons in the germline (Chew et al. 2008), although the roles of micronutrients in GSC lineages remain largely unknown. DNA damage induced by genetic manipulation or radiation leads to loss of GSCs and death of early germline cysts (Panagopoulos et al. 2007; Hasan et al. 2015; Ma et al. 2016). Sleep alterations appear to influence the dynamics of male GSC proliferation (Tulina et al. 2014). Notably, adult Drosophila can also enter a state of reproductive dormancy (diapause) triggered by low temperatures (12°) and short photoperiods, and characterized by the complete absence of developing vitellogenic follicles (Saunders et al. 1989). Insulin signaling was recently shown to regulate reproductive diapause (Schiesari et al. 2016); however, the cellular and molecular mechanisms underlying diapause remain largely unknown. It is not surprising that seemingly endless external and physiological factors modulate GSC lineages; after all, they reflect the environmental pressures that have shaped germline function for optimal transmission of genetic information during evolution.
Safeguarding the Full Germline Cycle: Tying Physiology and Development
The tight control of adult GSCs and their progeny ensures the optimal production of gametes according to available resources and other physiological constraints and demands. However, once a fertilized egg develops into a newly hatched and actively eating larva, it too will encounter varying conditions that shape the growth and development of its various organs and cell types, including the germline itself. Thus, successful long-term survival of a species requires tight regulation of early germline development according to environmental and physiological factors as well. As summarized below, similar signaling pathways are employed in adults and during the development of GSCs, although the mechanistic details vary.
Primordial germ cells (PGCs) and somatic gonadal precursors form the embryonic gonad. In females, these initial ∼12 PGCs reenter mitosis at the first instar larval stage and increase in numbers to ∼100 by the late third instar, in a process that requires extensive soma–germline interactions and multiple signals, including EGF, Wingless, Hh, activin, and Dpp/Gbb (Sato et al. 2010; Gilboa 2015). In contrast to the case in adult females, where adipocyte-derived collagen IV promotes GSC maintenance via E-cadherin and independently of BMP signals (Weaver and Drummond-Barbosa 2018), collagen IV produced by larval hemocytes inhibits BMP signaling during female niche development to restrict the number of established GSCs (Van De Bor et al. 2015).
Diet-dependent signaling pathways also modulate PGCs, thereby shaping the number of niches formed during late larval stages in females (Gilboa 2015). Insulin and TOR signaling cell-autonomously stimulate the proliferation of PGCs, and also control the number, size, and behavior of somatic cells in the developing ovary (Gancz and Gilboa 2013). In addition, insulin, but not TOR, signaling in somatic cells can indirectly regulate PGC number and differentiation. Overexpression of the insulin receptor in somatic cells leads to precocious PGC differentiation and, conversely, reduced insulin signaling decreases the fraction of differentiated PGCs in late larval stages (Gancz and Gilboa 2013). Thus, under low nutrient conditions, delayed differentiation buys additional time for slowly proliferating PGCs to increase their numbers.
Systemic regulation by ecdysone acting on PGCs and somatic niche precursors is a major factor controlling the timing of the switch from PGC proliferation to differentiation in females (Gilboa 2015). During early larval stages, EcR and its coreceptor Usp repress ecdysone targets and niche differentiation. However, starting in midlarval third instar, ecdysone stimulates EcR signaling and downstream expression of Br-Z1, one of four related transcription factors encoded by the broad complex locus (BR-C), to promote gradual differentiation of niches. As late larvae leave their food source in search of a site for pupation, the first wave of bam expression and PGC differentiation occurs in response to an ecdysone pulse (Gancz et al. 2011). A subset of PGCs attached to the newly formed niches and exposed to BMP signals are protected from differentiation and become adult GSCs (Gilboa 2015).
The transcription factor Broad represents a point of conversion for other factors controlling GSC niche formation. Although activin, a TGFβ superfamily ligand, promotes proliferation of somatic precursors earlier in ovarian development, activin is also required in somatic cells during late third-instar larvae for proper protein levels of Br-Z1 in response to ecdysone, thereby promoting niche and PGC differentiation (Lengil et al. 2015). In addition, the chromatin-binding protein Combgap tightly regulates long-range chromatin contacts between EcR-bound regions within the BR-C and EcR-mediated transcription from the BR-C locus, thereby influencing ovarian growth and GSC niche development (Hitrik et al. 2016).
Perspectives
The connection between the germline and an organism’s physiological status is universal. For example, in adult Caenorhabditis elegans worms, starvation leads to the arrest of proliferating germ cells in G2 and inhibition of meiotic entry within a few hours; these effects are reversed upon refeeding (Seidel and Kimble 2015). Long-term complete removal of food leads to reduced egg laying and subsequent maternal death due to internal hatching of progeny; animals that survive undergo adult reproductive diapause, where only ∼35 germ cells survive, resist starvation, and are able to repopulate the germline upon refeeding (Angelo and Van Gilst 2009). In contrast to the case in Drosophila, however, insulin and ribosomal protein S6 kinase control larval, but not adult, germ cell proliferation (Pinkston et al. 2006; Michaelson et al. 2010; Korta et al. 2012), suggesting distinct mechanisms regulating adult germ cell division rates in Drosophila vs. C. elegans. In mice, TOR signaling is required within germ cells for the formation and maintenance of self-renewing spermatogonia (Serra et al. 2019) and for follicle development and oocyte quality (Guo et al. 2018). In humans, diet influences fertility in males and females (Panth et al. 2018), although human studies are typically correlative.
Extensive interorgan communication controls fertility in other organisms as well. In C. elegans, diet- and pheromone-regulated expression of TGFβ in chemosensory neurons controls niche function and germ cell entry into meiosis/differentiation (Dalfó et al. 2012; Pekar et al. 2017). In mammals, the hypothalamic–pituitary–gonadal axis, involving multiple hormones, is essential for ovarian and testicular function (Kanda 2018). This axis in turn is responsive to circadian, stress, and metabolic alterations (Carmo-Silva and Cavadas 2017; Evans and Anderson 2018). High-fat diet-induced obesity reduces male fertility in mammalian models (Crean and Senior 2019), although many of the mechanisms at play remain unclear. Not surprisingly, obesity also increases the risk of infertility in both men and women (Fan et al. 2018; Panth et al. 2018; Reynolds and Gordon 2018; Silvestris et al. 2018).
The essential role of GSCs (or earlier germ cell precursors in women) in maintaining the germline—which in turn ensures species survival—underscores the urgency of expanding our knowledge of the complex regulation of the GSC lineage. The Drosophila system remains a top choice to address many questions that remain, such as: how do GSCs maintain the quality of their genome, epigenome, and key organelles; how do interactions with the microbiome—including bacteria, fungi, and viruses—affect the GSC lineage; and what additional interorgan communication mechanisms ensure faithful coupling of germline function to the organism’s external and physiological environments? Thus far, studies have revealed just the tip of the iceberg representing the complexity of germ cell regulation, which likely involves nearly every organ through multiple systemic factors acting through a wide range of mechanisms responsive to a diverse set of inputs.
Acknowledgments
I thank Lesley Weaver for the ovariole and germarium images; Maggie de Cuevas and Erika Matunis for the testis image; and members of the Drummond-Barbosa laboratory and thoughtful anonymous reviewers for valuable comments on the manuscript. D.D.-B. is funded by National Institutes of Health grants R01 GM069875 and R01 GM125121.
Footnotes
Communicating editor: A. Spradling
Literature Cited
- Ables E. T., and Drummond-Barbosa D., 2010. The steroid hormone ecdysone functions with intrinsic chromatin remodeling factors to control female germline stem cells in Drosophila. Cell Stem Cell 7: 581–592. 10.1016/j.stem.2010.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ables E. T., and Drummond-Barbosa D., 2013. Cyclin E controls Drosophila female germline stem cell maintenance independently of its role in proliferation by modulating responsiveness to niche signals. Development 140: 530–540. 10.1242/dev.088583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ables E. T., and Drummond-Barbosa D., 2017. Steroid hormones and the physiological regulation of tissue-resident stem cells: lessons from the Drosophila ovary. Curr. Stem Cell Rep. 3: 9–18. 10.1007/s40778-017-0070-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ables E. T., Bois K. E., Garcia C. A., and Drummond-Barbosa D., 2015. Ecdysone response gene E78 controls ovarian germline stem cell niche formation and follicle survival in Drosophila. Dev. Biol. 400: 33–42. 10.1016/j.ydbio.2015.01.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ables E. T., Hwang G. H., Finger D. S., Hinnant T. D., and Drummond-Barbosa D., 2016. A genetic mosaic screen reveals ecdysone-responsive genes regulating Drosophila oogenesis. G3 (Bethesda) 6: 2629–2642. 10.1534/g3.116.028951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ameku T., and Niwa R., 2016. Mating-induced increase in germline stem cells via the neuroendocrine system in female Drosophila. PLoS Genet. 12: e1006123 10.1371/journal.pgen.1006123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ameku T., Yoshinari Y., Texada M. J., Kondo S., Amezawa K. et al. , 2018. Midgut-derived neuropeptide F controls germline stem cell proliferation in a mating-dependent manner. PLoS Biol. 16: e2005004 10.1371/journal.pbio.2005004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angelo G., and Van Gilst M. R., 2009. Starvation protects germline stem cells and extends reproductive longevity in C. elegans. Science 326: 954–958. 10.1126/science.1178343 [DOI] [PubMed] [Google Scholar]
- Armstrong A. R., and Drummond-Barbosa D., 2018. Insulin signaling acts in adult adipocytes via GSK-3beta and independently of FOXO to control Drosophila female germline stem cell numbers. Dev. Biol. 440: 31–39. 10.1016/j.ydbio.2018.04.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong A. R., Laws K. M., and Drummond-Barbosa D., 2014. Adipocyte amino acid sensing controls adult germline stem cell number via the amino acid response pathway and independently of Target of Rapamycin signaling in Drosophila. Development 141: 4479–4488. 10.1242/dev.116467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayyub C., Banerjee K. K., and Joti P., 2015. Reduction of Cullin-2 in somatic cells disrupts differentiation of germline stem cells in the Drosophila ovary. Dev. Biol. 405: 269–279. 10.1016/j.ydbio.2015.07.019 [DOI] [PubMed] [Google Scholar]
- Bar-Peled L., and Sabatini D. M., 2014. Regulation of mTORC1 by amino acids. Trends Cell Biol. 24: 400–406. 10.1016/j.tcb.2014.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billeter J. C., and Wolfner M. F., 2018. Chemical cues that guide female reproduction in Drosophila melanogaster. J. Chem. Ecol. 44: 750–769. 10.1007/s10886-018-0947-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogard N., Lan L., Xu J., and Cohen R. S., 2007. Rab11 maintains connections between germline stem cells and niche cells in the Drosophila ovary. Development 134: 3413–3418. 10.1242/dev.008466 [DOI] [PubMed] [Google Scholar]
- Bonfini A., Wilkin M. B., and Baron M., 2015. Reversible regulation of stem cell niche size associated with dietary control of Notch signalling. BMC Dev. Biol. 15: 8 10.1186/s12861-015-0059-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonfini A., Liu X., and Buchon N., 2016. From pathogens to microbiota: how Drosophila intestinal stem cells react to gut microbes. Dev. Comp. Immunol. 64: 22–38. 10.1016/j.dci.2016.02.008 [DOI] [PubMed] [Google Scholar]
- Brawley C., and Matunis E., 2004. Regeneration of male germline stem cells by spermatogonial dedifferentiation in vivo. Science 304: 1331–1334. 10.1126/science.1097676 [DOI] [PubMed] [Google Scholar]
- Buckingham M., and Liu J. L., 2011. U bodies respond to nutrient stress in Drosophila. Exp. Cell Res. 317: 2835–2844. 10.1016/j.yexcr.2011.09.001 [DOI] [PubMed] [Google Scholar]
- Burn K. M., Shimada Y., Ayers K., Vemuganti S., Lu F. et al. , 2015. Somatic insulin signaling regulates a germline starvation response in Drosophila egg chambers. Dev. Biol. 398: 206–217. 10.1016/j.ydbio.2014.11.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buszczak M., Freeman M. R., Carlson J. R., Bender M., Cooley L. et al. , 1999. Ecdysone response genes govern egg chamber development during mid-oogenesis in Drosophila. Development 126: 4581–4589. [DOI] [PubMed] [Google Scholar]
- Cai W., Wei Y., Jarnik M., Reich J., and Lilly M. A., 2016. The GATOR2 component Wdr24 regulates TORC1 activity and lysosome function. PLoS Genet. 12: e1006036 10.1371/journal.pgen.1006036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmel I., Tram U., and Heifetz Y., 2016. Mating induces developmental changes in the insect female reproductive tract. Curr. Opin. Insect Sci. 13: 106–113. 10.1016/j.cois.2016.03.002 [DOI] [PubMed] [Google Scholar]
- Carmo-Silva S., and Cavadas C., 2017. Hypothalamic dysfunction in obesity and metabolic disorders. Adv. Neurobiol. 19: 73–116. 10.1007/978-3-319-63260-5_4 [DOI] [PubMed] [Google Scholar]
- Chau J., Kulnane L. S., and Salz H. K., 2009. Sex-lethal facilitates the transition from germline stem cell to committed daughter cell in the Drosophila ovary. Genetics 182: 121–132. 10.1534/genetics.109.100693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chau J., Kulnane L. S., and Salz H. K., 2012. Sex-lethal enables germline stem cell differentiation by down-regulating Nanos protein levels during Drosophila oogenesis. Proc. Natl. Acad. Sci. USA 109: 9465–9470. 10.1073/pnas.1120473109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D., and McKearin D., 2003a Dpp signaling silences bam transcription directly to establish asymmetric divisions of germline stem cells. Curr. Biol. 13: 1786–1791. 10.1016/j.cub.2003.09.033 [DOI] [PubMed] [Google Scholar]
- Chen D., and McKearin D. M., 2003b A discrete transcriptional silencer in the bam gene determines asymmetric division of the Drosophila germline stem cell. Development 130: 1159–1170. 10.1242/dev.00325 [DOI] [PubMed] [Google Scholar]
- Chen S., Kaneko S., Ma X., Chen X., Ip Y. T. et al. , 2010. Lissencephaly-1 controls germline stem cell self-renewal through modulating bone morphogenetic protein signaling and niche adhesion. Proc. Natl. Acad. Sci. USA 107: 19939–19944. 10.1073/pnas.1008606107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S., Wang S., and Xie T., 2011. Restricting self-renewal signals within the stem cell niche: multiple levels of control. Curr. Opin. Genet. Dev. 21: 684–689. 10.1016/j.gde.2011.07.008 [DOI] [PubMed] [Google Scholar]
- Chen S., Lewallen M., and Xie T., 2013. Adhesion in the stem cell niche: biological roles and regulation. Development 140: 255–265. 10.1242/dev.083139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng J., Turkel N., Hemati N., Fuller M. T., Hunt A. J. et al. , 2008. Centrosome misorientation reduces stem cell division during ageing. Nature 456: 599–604. 10.1038/nature07386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chew Y. C., West J. T., Kratzer S. J., Ilvarsonn A. M., Eissenberg J. C. et al. , 2008. Biotinylation of histones represses transposable elements in human and mouse cells and cell lines and in Drosophila melanogaster. J. Nutr. 138: 2316–2322. 10.3945/jn.108.098673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang A. C., Yang H., and Yamashita Y. M., 2017. spict, a cyst cell-specific gene, regulates starvation-induced spermatogonial cell death in the Drosophila testis. Sci. Rep. 7: 40245 10.1038/srep40245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen S., Perez Dulzaides R., Hedrick V. E., Momtaz A. J., Nakayasu E. S. et al. , 2016. Wolbachia endosymbionts modify Drosophila ovary protein levels in a context-dependent manner. Appl. Environ. Microbiol. 82: 5354–5363. 10.1128/AEM.01255-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crean A. J., and Senior A. M., 2019. High-fat diets reduce male reproductive success in animal models: a systematic review and meta-analysis. Obes. Rev. 20: 921–933. 10.1111/obr.12827 [DOI] [PubMed] [Google Scholar]
- Dalfó D., Michaelson D., and Hubbard E. J., 2012. Sensory regulation of the C. elegans germline through TGF-β-dependent signaling in the niche. Curr. Biol. 22: 712–719. 10.1016/j.cub.2012.02.064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Cuevas M., and Spradling A. C., 1998. Morphogenesis of the Drosophila fusome and its implications for oocyte specification. Development 125: 2781–2789. [DOI] [PubMed] [Google Scholar]
- Dolezal D., Liu Z., Zhou Q., and Pignoni F., 2015. Fly LMBR1/LIMR-type protein Lilipod promotes germ-line stem cell self-renewal by enhancing BMP signaling. Proc. Natl. Acad. Sci. USA 112: 13928–13933. 10.1073/pnas.1509856112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond-Barbosa D., and Spradling A. C., 2001. Stem cells and their progeny respond to nutritional changes during Drosophila oogenesis. Dev. Biol. 231: 265–278. 10.1006/dbio.2000.0135 [DOI] [PubMed] [Google Scholar]
- Elgart M., Stern S., Salton O., Gnainsky Y., Heifetz Y. et al. , 2016. Impact of gut microbiota on the fly’s germ line. Nat. Commun. 7: 11280 10.1038/ncomms11280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliazer S., Shalaby N. A., and Buszczak M., 2011. Loss of lysine-specific demethylase 1 nonautonomously causes stem cell tumors in the Drosophila ovary. Proc. Natl. Acad. Sci. USA 108: 7064–7069. 10.1073/pnas.1015874108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans M. C., and Anderson G. M., 2018. Integration of circadian and metabolic control of reproductive function. Endocrinology 159: 3661–3673. 10.1210/en.2018-00691 [DOI] [PubMed] [Google Scholar]
- Fairchild M. J., Smendziuk C. M., and Tanentzapf G., 2015. A somatic permeability barrier around the germline is essential for Drosophila spermatogenesis. Development 142: 268–281. 10.1242/dev.114967 [DOI] [PubMed] [Google Scholar]
- Fairchild M. J., Islam F., and Tanentzapf G., 2017. Identification of genetic networks that act in the somatic cells of the testis to mediate the developmental program of spermatogenesis. PLoS Genet. 13: e1007026 10.1371/journal.pgen.1007026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan W., Xu Y., Liu Y., Zhang Z., Lu L. et al. , 2018. Obesity or overweight, a chronic inflammatory status in male reproductive system, leads to mice and human subfertility. Front. Physiol. 8: 1117 10.3389/fphys.2017.01117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang H., and Judd R. L., 2018. Adiponectin regulation and function. Compr. Physiol. 8: 1031–1063. 10.1002/cphy.c170046 [DOI] [PubMed] [Google Scholar]
- Fast E. M., Toomey M. E., Panaram K., Desjardins D., Kolaczyk E. D. et al. , 2011. Wolbachia enhance Drosophila stem cell proliferation and target the germline stem cell niche. Science 334: 990–992. 10.1126/science.1209609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng L., Shi Z., and Chen X., 2017. Enhancer of polycomb coordinates multiple signaling pathways to promote both cyst and germline stem cell differentiation in the Drosophila adult testis. PLoS Genet. 13: e1006571 10.1371/journal.pgen.1006571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferveur J. F., 2010. Drosophila female courtship and mating behaviors: sensory signals, genes, neural structures and evolution. Curr. Opin. Neurobiol. 20: 764–769. 10.1016/j.conb.2010.09.007 [DOI] [PubMed] [Google Scholar]
- Fichelson P., Moch C., Ivanovitch K., Martin C., Sidor C. M. et al. , 2009. Live-imaging of single stem cells within their niche reveals that a U3snoRNP component segregates asymmetrically and is required for self-renewal in Drosophila. Nat. Cell Biol. 11: 685–693. 10.1038/ncb1874 [DOI] [PubMed] [Google Scholar]
- Flores H. A., Bubnell J. E., Aquadro C. F., and Barbash D. A., 2015. The Drosophila bag of marbles gene interacts genetically with Wolbachia and shows female-specific effects of divergence. PLoS Genet. 11: e1005453 10.1371/journal.pgen.1005453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Z., Geng C., Wang H., Yang Z., Weng C. et al. , 2015. Twin promotes the maintenance and differentiation of germline stem cell lineage through modulation of multiple pathways. Cell Rep. 13: 1366–1379. 10.1016/j.celrep.2015.10.017 [DOI] [PubMed] [Google Scholar]
- Gancz D., and Gilboa L., 2013. Insulin and Target of rapamycin signaling orchestrate the development of ovarian niche-stem cell units in Drosophila. Development 140: 4145–4154. 10.1242/dev.093773 [DOI] [PubMed] [Google Scholar]
- Gancz D., Lengil T., and Gilboa L., 2011. Coordinated regulation of niche and stem cell precursors by hormonal signaling. PLoS Biol. 9: e1001202 10.1371/journal.pbio.1001202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerrard D. T., Fricke C., Edward D. A., Edwards D. R., and Chapman T., 2013. Genome-wide responses of female fruit flies subjected to divergent mating regimes. PLoS One 8: e68136 10.1371/journal.pone.0068136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilboa L., 2015. Organizing stem cell units in the Drosophila ovary. Curr. Opin. Genet. Dev. 32: 31–36. 10.1016/j.gde.2015.01.005 [DOI] [PubMed] [Google Scholar]
- Gilboa L., and Lehmann R., 2004. How different is Venus from Mars? The genetics of germ-line stem cells in Drosophila females and males. Development 131: 4895–4905. 10.1242/dev.01373 [DOI] [PubMed] [Google Scholar]
- Gleason R. J., Anand A., Kai T., and Chen X., 2018. Protecting and diversifying the germline. Genetics 208: 435–471. 10.1534/genetics.117.300208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenspan L. J., de Cuevas M., and Matunis E., 2015. Genetics of gonadal stem cell renewal. Annu. Rev. Cell Dev. Biol. 31: 291–315. 10.1146/annurev-cellbio-100913-013344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruntenko N. E., and Rauschenbach I. Y., 2018. The role of insulin signalling in the endocrine stress response in Drosophila melanogaster: a mini-review. Gen. Comp. Endocrinol. 258: 134–139. 10.1016/j.ygcen.2017.05.019 [DOI] [PubMed] [Google Scholar]
- Guo J., Zhang T., Guo Y., Sun T., Li H. et al. , 2018. Oocyte stage-specific effects of MTOR determine granulosa cell fate and oocyte quality in mice. Proc. Natl. Acad. Sci. USA 115: E5326–E5333. 10.1073/pnas.1800352115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Z., and Wang Z., 2009. The glypican Dally is required in the niche for the maintenance of germline stem cells and short-range BMP signaling in the Drosophila ovary. Development 136: 3627–3635. 10.1242/dev.036939 [DOI] [PubMed] [Google Scholar]
- Hamada-Kawaguchi N., Nore B. F., Kuwada Y., Smith C. I., and Yamamoto D., 2014. Btk29A promotes Wnt4 signaling in the niche to terminate germ cell proliferation in Drosophila. Science 343: 294–297. 10.1126/science.1244512 [DOI] [PubMed] [Google Scholar]
- Harris R. E., Pargett M., Sutcliffe C., Umulis D., and Ashe H. L., 2011. Brat promotes stem cell differentiation via control of a bistable switch that restricts BMP signaling. Dev. Cell 20: 72–83. 10.1016/j.devcel.2010.11.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasan S., Hetie P., and Matunis E. L., 2015. Niche signaling promotes stem cell survival in the Drosophila testis via the JAK-STAT target DIAP1. Dev. Biol. 404: 27–39. 10.1016/j.ydbio.2015.04.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi Y., Kobayashi S., and Nakato H., 2009. Drosophila glypicans regulate the germline stem cell niche. J. Cell Biol. 187: 473–480. 10.1083/jcb.200904118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera S. C., and Bach E. A., 2018. JNK signaling triggers spermatogonial dedifferentiation during chronic stress to maintain the germline stem cell pool in the Drosophila testis. Elife 7: e36095. 10.7554/eLife.36095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitrik A., Popliker M., Gancz D., Mukamel Z., Lifshitz A. et al. , 2016. Combgap promotes ovarian niche development and chromatin association of EcR-binding regions in BR-C. PLoS Genet. 12: e1006330 10.1371/journal.pgen.1006330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou Y. C., Chittaranjan S., Barbosa S. G., McCall K., and Gorski S. M., 2008. Effector caspase Dcp-1 and IAP protein Bruce regulate starvation-induced autophagy during Drosophila melanogaster oogenesis. J. Cell Biol. 182: 1127–1139. 10.1083/jcb.200712091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu H. J., and Drummond-Barbosa D., 2009. Insulin levels control female germline stem cell maintenance via the niche in Drosophila. Proc. Natl. Acad. Sci. USA 106: 1117–1121. 10.1073/pnas.0809144106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu H. J., and Drummond-Barbosa D., 2011. Insulin signals control the competence of the Drosophila female germline stem cell niche to respond to Notch ligands. Dev. Biol. 350: 290–300. 10.1016/j.ydbio.2010.11.032 [DOI] [PubMed] [Google Scholar]
- Hsu H. J., LaFever L., and Drummond-Barbosa D., 2008. Diet controls normal and tumorous germline stem cells via insulin-dependent and -independent mechanisms in Drosophila. Dev. Biol. 313: 700–712. 10.1016/j.ydbio.2007.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J., Reilein A., and Kalderon D., 2017. Yorkie and Hedgehog independently restrict BMP production in Escort cells to permit germline differentiation in the Drosophila ovary. Development 144: 2584–2594. 10.1242/dev.147702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeya T., Broughton S., Alic N., Grandison R., and Partridge L., 2009. The endosymbiont Wolbachia increases insulin/IGF-like signalling in Drosophila. Proc. Biol. Sci. 276: 3799–3807. 10.1098/rspb.2009.0778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inaba M., Buszczak M., and Yamashita Y. M., 2015. Nanotubes mediate niche-stem-cell signalling in the Drosophila testis. Nature 523: 329–332. 10.1038/nature14602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Z., and Xie T., 2007. Dcr-1 maintains Drosophila ovarian stem cells. Curr. Biol. 17: 539–544. 10.1016/j.cub.2007.01.050 [DOI] [PubMed] [Google Scholar]
- Jin Z., Kirilly D., Weng C., Kawase E., Song X. et al. , 2008. Differentiation-defective stem cells outcompete normal stem cells for niche occupancy in the Drosophila ovary. Cell Stem Cell 2: 39–49. 10.1016/j.stem.2007.10.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juliano C., Wang J., and Lin H., 2011. Uniting germline and stem cells: the function of Piwi proteins and the piRNA pathway in diverse organisms. Annu. Rev. Genet. 45: 447–469. 10.1146/annurev-genet-110410-132541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kai T., and Spradling A., 2004. Differentiating germ cells can revert into functional stem cells in Drosophila melanogaster ovaries. Nature 428: 564–569. 10.1038/nature02436 [DOI] [PubMed] [Google Scholar]
- Kanda S., 2018. Evolution of the regulatory mechanisms for the hypothalamic-pituitary-gonadal axis in vertebrates-hypothesis from a comparative view. Gen. Comp. Endocrinol. DOI: 10.1016/j.ygcen.2018.11.014. 10.1016/j.ygcen.2018.11.014 [DOI] [PubMed] [Google Scholar]
- Kawase E., Wong M. D., Ding B. C., and Xie T., 2004. Gbb/Bmp signaling is essential for maintaining germline stem cells and for repressing bam transcription in the Drosophila testis. Development 131: 1365–1375. 10.1242/dev.01025 [DOI] [PubMed] [Google Scholar]
- Khan A. H., Zou Z., Xiang Y., Chen S., and Tian X. L., 2019. Conserved signaling pathways genetically associated with longevity across the species. Biochim Biophys Acta Mol Basis Dis 1865: 1745–1755. 10.1016/j.bbadis.2018.09.001 [DOI] [PubMed] [Google Scholar]
- Kiger A. A., Jones D. L., Schulz C., Rogers M. B., and Fuller M. T., 2001. Stem cell self-renewal specified by JAK-STAT activation in response to a support cell cue. Science 294: 2542–2545. 10.1126/science.1066707 [DOI] [PubMed] [Google Scholar]
- Knapp E., and Sun J., 2017. Steroid signaling in mature follicles is important for Drosophila ovulation. Proc. Natl. Acad. Sci. USA 114: 699–704. 10.1073/pnas.1614383114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- König A., Yatsenko A. S., Weiss M., and Shcherbata H. R., 2011. Ecdysteroids affect Drosophila ovarian stem cell niche formation and early germline differentiation. EMBO J. 30: 1549–1562. 10.1038/emboj.2011.73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korta D. Z., Tuck S., and Hubbard E. J., 2012. S6K links cell fate, cell cycle and nutrient response in C. elegans germline stem/progenitor cells. Development 139: 859–870. 10.1242/dev.074047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaFever L., and Drummond-Barbosa D., 2005. Direct control of germline stem cell division and cyst growth by neural insulin in Drosophila. Science 309: 1071–1073. 10.1126/science.1111410 [DOI] [PubMed] [Google Scholar]
- LaFever L., Feoktistov A., Hsu H. J., and Drummond-Barbosa D., 2010. Specific roles of Target of rapamycin in the control of stem cells and their progeny in the Drosophila ovary. Development 137: 2117–2126. 10.1242/dev.050351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laws K. M., and Drummond-Barbosa D., 2016. AMP-activated protein kinase has diet-dependent and -independent roles in Drosophila oogenesis. Dev. Biol. 420: 90–99. 10.1016/j.ydbio.2016.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laws K. M., and Drummond-Barbosa D., 2017. Control of germline stem cell lineages by diet and physiology. Results Probl. Cell Differ. 59: 67–99. 10.1007/978-3-319-44820-6_3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laws K. M., Sampson L. L., and Drummond-Barbosa D., 2015. Insulin-independent role of adiponectin receptor signaling in Drosophila germline stem cell maintenance. Dev. Biol. 399: 226–236. 10.1016/j.ydbio.2014.12.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leatherman J. L., and Dinardo S., 2010. Germline self-renewal requires cyst stem cells and stat regulates niche adhesion in Drosophila testes. Nat. Cell Biol. 12: 806–811. 10.1038/ncb2086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. Y., Chen J. Y., Shaw J. L., and Chang K. T., 2016. Maintenance of stem cell niche integrity by a novel activator of integrin signaling. PLoS Genet. 12: e1006043 10.1371/journal.pgen.1006043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lengil T., Gancz D., and Gilboa L., 2015. Activin signaling balances proliferation and differentiation of ovarian niche precursors and enables adjustment of niche numbers. Development 142: 883–892. 10.1242/dev.113902 [DOI] [PubMed] [Google Scholar]
- Levings D. C., Arashiro T., and Nakato H., 2016. Heparan sulfate regulates the number and centrosome positioning of Drosophila male germline stem cells. Mol. Biol. Cell 27: 888–896. 10.1091/mbc.E15-07-0528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis Z., Heys C., Prescott M., and Lize A., 2014. You are what you eat. Gut Microbes 5: 541–543. 10.4161/gmic.29153 [DOI] [PubMed] [Google Scholar]
- Li C., Kan L., Chen Y., Zheng X., Li W. et al. , 2015. Ci antagonizes Hippo signaling in the somatic cells of the ovary to drive germline stem cell differentiation. Cell Res. 25: 1152–1170. 10.1038/cr.2015.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Yang F., Chen H., Deng B., and Xi R., 2016. Control of germline stem cell differentiation by Polycomb and Trithorax group genes in the niche microenvironment. Development 143: 3449–3458. 10.1242/dev.137638 [DOI] [PubMed] [Google Scholar]
- Li Y., Minor N. T., Park J. K., McKearin D. M., and Maines J. Z., 2009. Bam and Bgcn antagonize Nanos-dependent germ-line stem cell maintenance. Proc. Natl. Acad. Sci. USA 106: 9304–9309. 10.1073/pnas.0901452106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Maines J. Z., Tastan O. Y., McKearin D. M., and Buszczak M., 2012. Mei-P26 regulates the maintenance of ovarian germline stem cells by promoting BMP signaling. Development 139: 1547–1556. 10.1242/dev.077412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Zhang Q., Carreira-Rosario A., Maines J. Z., McKearin D. M. et al. , 2013. Mei-p26 cooperates with Bam, Bgcn and Sxl to promote early germline development in the Drosophila ovary. PLoS One 8: e58301 10.1371/journal.pone.0058301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Ma Q., Cherry C. M., and Matunis E. L., 2014. Steroid signaling promotes stem cell maintenance in the Drosophila testis. Dev. Biol. 394: 129–141. 10.1016/j.ydbio.2014.07.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim C., Gandhi S., Biniossek M. L., Feng L., Schilling O. et al. , 2015. An aminopeptidase in the Drosophila testicular niche acts in germline stem cell maintenance and spermatogonial dedifferentiation. Cell Rep. 13: 315–325. 10.1016/j.celrep.2015.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin H., and Spradling A. C., 1997. A novel group of pumilio mutations affects the asymmetric division of germline stem cells in the Drosophila ovary. Development 124: 2463–2476. [DOI] [PubMed] [Google Scholar]
- Lin S. C., and Hardie D. G., 2018. AMPK: sensing glucose as well as cellular energy status. Cell Metab. 27: 299–313. 10.1016/j.cmet.2017.10.009 [DOI] [PubMed] [Google Scholar]
- Liu Y., Ge Q., Chan B., Liu H., Singh S. R. et al. , 2016. Whole-animal genome-wide RNAi screen identifies networks regulating male germline stem cells in Drosophila. Nat. Commun. 7: 12149 10.1038/ncomms12149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z., Zhong G., Chai P. C., Luo L., Liu S. et al. , 2015. Coordinated niche-associated signals promote germline homeostasis in the Drosophila ovary. J. Cell Biol. 211: 469–484. 10.1083/jcb.201503033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu T., Wang S., Gao Y., Mao Y., Yang Z. et al. , 2015. COP9-Hedgehog axis regulates the function of the germline stem cell progeny differentiation niche in the Drosophila ovary. Development 142: 4242–4252. 10.1242/dev.124768 [DOI] [PubMed] [Google Scholar]
- Lu W., Casanueva M. O., Mahowald A. P., Kato M., Lauterbach D. et al. , 2012. Niche-associated activation of rac promotes the asymmetric division of Drosophila female germline stem cells. PLoS Biol. 10: e1001357 10.1371/journal.pbio.1001357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo L., Wang H., Fan C., Liu S., and Cai Y., 2015. Wnt ligands regulate Tkv expression to constrain Dpp activity in the Drosophila ovarian stem cell niche. J. Cell Biol. 209: 595–608. 10.1083/jcb.201409142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo L., Siah C. K., and Cai Y., 2017. Engrailed acts with Nejire to control decapentaplegic expression in the Drosophila ovarian stem cell niche. Development 144: 3224–3231. 10.1242/dev.145474 [DOI] [PubMed] [Google Scholar]
- Lupold S., Manier M. K., Ala-Honkola O., Belote J. M., and Pitnick S., 2011. Male Drosophila melanogaster adjust ejaculate size based on female mating status, fecundity, and age. Behav. Ecol. 22: 184–191. 10.1093/beheco/arq193 [DOI] [Google Scholar]
- Ma X., Han Y., Song X., Do T., Yang Z. et al. , 2016. DNA damage-induced Lok/CHK2 activation compromises germline stem cell self-renewal and lineage differentiation. Development 143: 4312–4323. 10.1242/dev.141069 [DOI] [PubMed] [Google Scholar]
- Mair W., McLeod C. J., Wang L., and Jones D. L., 2010. Dietary restriction enhances germline stem cell maintenance. Aging Cell 9: 916–918. 10.1111/j.1474-9726.2010.00602.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuoka S., Armstrong A. R., Sampson L. L., Laws K. M., and Drummond-Barbosa D., 2017. Adipocyte metabolic pathways regulated by diet control the female germline stem cell lineage in Drosophila melanogaster. Genetics 206: 953–971. 10.1534/genetics.117.201921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKearin D., and Ohlstein B., 1995. A role for the Drosophila bag-of-marbles protein in the differentiation of cystoblasts from germline stem cells. Development 121: 2937–2947. [DOI] [PubMed] [Google Scholar]
- McLeod C. J., Wang L., Wong C., and Jones D. L., 2010. Stem cell dynamics in response to nutrient availability. Curr. Biol. 20: 2100–2105. 10.1016/j.cub.2010.10.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaelson D., Korta D. Z., Capua Y., and Hubbard E. J., 2010. Insulin signaling promotes germline proliferation in C. elegans. Development 137: 671–680. 10.1242/dev.042523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morawa K. S., Schneider M., and Klein T., 2015. Lgd regulates the activity of the BMP/Dpp signalling pathway during Drosophila oogenesis. Development 142: 1325–1335. 10.1242/dev.112961 [DOI] [PubMed] [Google Scholar]
- Morris L. X., and Spradling A. C., 2011. Long-term live imaging provides new insight into stem cell regulation and germline-soma coordination in the Drosophila ovary. Development 138: 2207–2215. 10.1242/dev.065508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris L. X., and Spradling A. C., 2012. Steroid signaling within Drosophila ovarian epithelial cells sex-specifically modulates early germ cell development and meiotic entry. PLoS One 7: e46109 10.1371/journal.pone.0046109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moschall R., Gaik M., and Medenbach J., 2017. Promiscuity in post-transcriptional control of gene expression: Drosophila sex-lethal and its regulatory partnerships. FEBS Lett. 591: 1471–1488. 10.1002/1873-3468.12652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mottier-Pavie V. I., Palacios V., Eliazer S., Scoggin S., and Buszczak M., 2016. The Wnt pathway limits BMP signaling outside of the germline stem cell niche in Drosophila ovaries. Dev. Biol. 417: 50–62. 10.1016/j.ydbio.2016.06.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumüller R. A., Betschinger J., Fischer A., Bushati N., Poernbacher I. et al. , 2008. Mei-P26 regulates microRNAs and cell growth in the Drosophila ovarian stem cell lineage. Nature 454: 241–245. 10.1038/nature07014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton F. G., Harris R. E., Sutcliffe C., and Ashe H. L., 2015. Coordinate post-transcriptional repression of Dpp-dependent transcription factors attenuates signal range during development. Development 142: 3362–3373. 10.1242/dev.123273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlstein B., and McKearin D., 1997. Ectopic expression of the Drosophila Bam protein eliminates oogenic germline stem cells. Development 124: 3651–3662. [DOI] [PubMed] [Google Scholar]
- Ote M., Ueyama M., and Yamamoto D., 2016. Wolbachia protein TomO targets nanos mRNA and restores germ stem cells in Drosophila sex-lethal mutants. Curr. Biol. 26: 2223–2232. 10.1016/j.cub.2016.06.054 [DOI] [PubMed] [Google Scholar]
- Panagopoulos D. J., Chavdoula E. D., Nezis I. P., and Margaritis L. H., 2007. Cell death induced by GSM 900-MHz and DCS 1800-MHz mobile telephony radiation. Mutat. Res. 626: 69–78. 10.1016/j.mrgentox.2006.08.008 [DOI] [PubMed] [Google Scholar]
- Panth N., Gavarkovs A., Tamez M., and Mattei J., 2018. The influence of diet on fertility and the implications for public health nutrition in the United States. Front. Public Health 6: 211 10.3389/fpubh.2018.00211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J. K., Liu X., Strauss T. J., McKearin D. M., and Liu Q., 2007. The miRNA pathway intrinsically controls self-renewal of Drosophila germline stem cells. Curr. Biol. 17: 533–538. 10.1016/j.cub.2007.01.060 [DOI] [PubMed] [Google Scholar]
- Partridge L., and Prowse N., 1997. The effects of reproduction on longevity and fertility in male Drosophila melanogaster. J. Insect Physiol. 43: 501–512. 10.1016/S0022-1910(97)00014-0 [DOI] [PubMed] [Google Scholar]
- Pekar O., Ow M. C., Hui K. Y., Noyes M. B., Hall S. E. et al. , 2017. Linking the environment, DAF-7/TGFbeta signaling and LAG-2/DSL ligand expression in the germline stem cell niche. Development 144: 2896–2906. 10.1242/dev.147660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira G., and Yamashita Y. M., 2011. Fly meets yeast: checking the correct orientation of cell division. Trends Cell Biol. 21: 526–533. 10.1016/j.tcb.2011.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinkston J. M., Garigan D., Hansen M., and Kenyon C., 2006. Mutations that increase the life span of C. elegans inhibit tumor growth. Science 313: 971–975. 10.1126/science.1121908 [DOI] [PubMed] [Google Scholar]
- Pritchett T. L., and McCall K., 2012. Role of the insulin/Tor signaling network in starvation-induced programmed cell death in Drosophila oogenesis. Cell Death Differ. 19: 1069–1079. 10.1038/cdd.2011.200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian Y., Dominado N., Zoller R., Ng C., Kudyba K. et al. , 2014. Ecdysone signaling opposes epidermal growth factor signaling in regulating cyst differentiation in the male gonad of Drosophila melanogaster. Dev. Biol. 394: 217–227. 10.1016/j.ydbio.2014.08.019 [DOI] [PubMed] [Google Scholar]
- Reynolds R., and Gordon A., 2018. Obesity, fertility and pregnancy: can we intervene to improve outcomes? J. Endocrinol. DOI: 10.1530/JOE-18-0199. 10.1530/JOE-18-0199 [DOI] [PubMed] [Google Scholar]
- Riera C. E., Merkwirth C., De Magalhaes Filho C. D., and Dillin A., 2016. Signaling networks determining life span. Annu. Rev. Biochem. 85: 35–64. 10.1146/annurev-biochem-060815-014451 [DOI] [PubMed] [Google Scholar]
- Rojas-Ríos P., and Simonelig M., 2018. piRNAs and PIWI proteins: regulators of gene expression in development and stem cells. Development 145: dev161786. 10.1242/dev.161786 [DOI] [PubMed] [Google Scholar]
- Roth T. M., Chiang C. Y., Inaba M., Yuan H., Salzmann V. et al. , 2012. Centrosome misorientation mediates slowing of the cell cycle under limited nutrient conditions in Drosophila male germline stem cells. Mol. Biol. Cell 23: 1524–1532. 10.1091/mbc.e11-12-0999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salzmann V., Chen C., Chiang C. Y., Tiyaboonchai A., Mayer M. et al. , 2014. Centrosome-dependent asymmetric inheritance of the midbody ring in Drosophila germline stem cell division. Mol. Biol. Cell 25: 267–275. 10.1091/mbc.e13-09-0541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T., Ogata J., and Niki Y., 2010. BMP and Hh signaling affects primordial germ cell division in Drosophila. Zool. Sci. 27: 804–810. 10.2108/zsj.27.804 [DOI] [PubMed] [Google Scholar]
- Saunders D. S., Henrich V. C., and Gilbert L. I., 1989. Induction of diapause in Drosophila melanogaster: photoperiodic regulation and the impact of arrhythmic clock mutations on time measurement. Proc. Natl. Acad. Sci. USA 86: 3748–3752. 10.1073/pnas.86.10.3748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxton R. A., and Sabatini D. M., 2017. mTOR signaling in growth, metabolism, and disease. Cell 169: 361–371. 10.1016/j.cell.2017.03.035 [DOI] [PubMed] [Google Scholar]
- Schiesari L., Andreatta G., Kyriacou C. P., O’Connor M. B., and Costa R., 2016. The insulin-like proteins dILPs-2/5 determine diapause inducibility in Drosophila. PLoS One 11: e0163680 10.1371/journal.pone.0163680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz C., Kiger A. A., Tazuke S. I., Yamashita Y. M., Pantalena-Filho L. C. et al. , 2004. A misexpression screen reveals effects of bag-of-marbles and TGF beta class signaling on the Drosophila male germ-line stem cell lineage. Genetics 167: 707–723. 10.1534/genetics.103.023184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz M. B., Kelly T. J., Imberski R. B., and Rubenstein E. C., 1985. The effects of nutrition and methoprene treatment on ovarian ecdysteroid synthesis in Drosophila melanogaster. J. Insect Physiol. 31: 947–957. 10.1016/0022-1910(85)90029-0 [DOI] [Google Scholar]
- Seidel H. S., and Kimble J., 2015. Cell-cycle quiescence maintains Caenorhabditis elegans germline stem cells independent of GLP-1/Notch. Elife 4: e10832. 10.7554/eLife.10832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serra N., Velte E. K., Niedenberger B. A., Kirsanov O., and Geyer C. B., 2019. The mTORC1 component RPTOR is required for maintenance of the foundational spermatogonial stem cell pool in mice†. Biol. Reprod. 100: 429–439. 10.1093/biolre/ioy198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen R., Weng C., Yu J., and Xie T., 2009. eIF4A controls germline stem cell self-renewal by directly inhibiting BAM function in the Drosophila ovary. Proc. Natl. Acad. Sci. USA 106: 11623–11628. 10.1073/pnas.0903325106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng X. R., Brawley C. M., and Matunis E. L., 2009. Dedifferentiating spermatogonia outcompete somatic stem cells for niche occupancy in the Drosophila testis. Cell Stem Cell 5: 191–203. 10.1016/j.stem.2009.05.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada Y., Burn K. M., Niwa R., and Cooley L., 2011. Reversible response of protein localization and microtubule organization to nutrient stress during Drosophila early oogenesis. Dev. Biol. 355: 250–262. 10.1016/j.ydbio.2011.04.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shivdasani A. A., and Ingham P. W., 2003. Regulation of stem cell maintenance and transit amplifying cell proliferation by tgf-beta signaling in Drosophila spermatogenesis. Curr. Biol. 13: 2065–2072. 10.1016/j.cub.2003.10.063 [DOI] [PubMed] [Google Scholar]
- Sieber M. H., and Spradling A. C., 2015. Steroid signaling establishes a female metabolic state and regulates SREBP to control oocyte lipid accumulation. Curr. Biol. 25: 993–1004. 10.1016/j.cub.2015.02.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieber M. H., Thomsen M. B., and Spradling A. C., 2016. Electron transport chain remodeling by GSK3 during oogenesis connects nutrient state to reproduction. Cell 164: 420–432. 10.1016/j.cell.2015.12.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvestris E., de Pergola G., Rosania R., and Loverro G., 2018. Obesity as disruptor of the female fertility. Reprod. Biol. Endocrinol. 16: 22 10.1186/s12958-018-0336-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slaidina M., and Lehmann R., 2014. Translational control in germline stem cell development. J. Cell Biol. 207: 13–21. 10.1083/jcb.201407102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smendziuk C. M., Messenberg A., Vogl A. W., and Tanentzapf G., 2015. Bi-directional gap junction-mediated soma-germline communication is essential for spermatogenesis. Development 142: 2598–2609. 10.1242/dev.123448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soller M., Bownes M., and Kubli E., 1997. Mating and sex peptide stimulate the accumulation of yolk in oocytes of Drosophila melanogaster. Eur. J. Biochem. 243: 732–738. 10.1111/j.1432-1033.1997.00732.x [DOI] [PubMed] [Google Scholar]
- Song X., Zhu C. H., Doan C., and Xie T., 2002. Germline stem cells anchored by adherens junctions in the Drosophila ovary niches. Science 296: 1855–1857. 10.1126/science.1069871 [DOI] [PubMed] [Google Scholar]
- Song X., Wong M. D., Kawase E., Xi R., Ding B. C. et al. , 2004. Bmp signals from niche cells directly repress transcription of a differentiation-promoting gene, bag of marbles, in germline stem cells in the Drosophila ovary. Development 131: 1353–1364. 10.1242/dev.01026 [DOI] [PubMed] [Google Scholar]
- Song X., Call G. B., Kirilly D., and Xie T., 2007. Notch signaling controls germline stem cell niche formation in the Drosophila ovary. Development 134: 1071–1080. 10.1242/dev.003392 [DOI] [PubMed] [Google Scholar]
- Srinivasan S., Mahowald A. P., and Fuller M. T., 2012. The receptor tyrosine phosphatase Lar regulates adhesion between Drosophila male germline stem cells and the niche. Development 139: 1381–1390. 10.1242/dev.070052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starr D. J., and Cline T. W., 2002. A host parasite interaction rescues Drosophila oogenesis defects. Nature 418: 76–79. 10.1038/nature00843 [DOI] [PubMed] [Google Scholar]
- Steiger D., Fetchko M., Vardanyan A., Atanesyan L., Steiner K. et al. , 2010. The Drosophila copper transporter Ctr1C functions in male fertility. J. Biol. Chem. 285: 17089–17097. 10.1074/jbc.M109.090282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens N. R., Raposo A. A., Basto R., St Johnston D., and Raff J. W., 2007. From stem cell to embryo without centrioles. Curr. Biol. 17: 1498–1503. 10.1016/j.cub.2007.07.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugime Y., Watanabe D., Yasuno Y., Shinada T., Miura T. et al. , 2017. Upregulation of juvenile hormone titers in female Drosophila melanogaster through mating experiences and host food occupied by eggs and larvae. Zool. Sci. 34: 52–57. 10.2108/zs160150 [DOI] [PubMed] [Google Scholar]
- Sullivan W., 2016. Endosymbiosis: the remarkable healing powers of Wolbachia. Curr. Biol. 26: R797–R799. 10.1016/j.cub.2016.07.045 [DOI] [PubMed] [Google Scholar]
- Sun P., Quan Z., Zhang B., Wu T., and Xi R., 2010. TSC1/2 tumour suppressor complex maintains Drosophila germline stem cells by preventing differentiation. Development 137: 2461–2469. 10.1242/dev.051466 [DOI] [PubMed] [Google Scholar]
- Tanentzapf G., Devenport D., Godt D., and Brown N. H., 2007. Integrin-dependent anchoring of a stem-cell niche. Nat. Cell Biol. 9: 1413–1418. 10.1038/ncb1660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarayrah L., Li Y., Gan Q., and Chen X., 2015. Epigenetic regulator Lid maintains germline stem cells through regulating JAK-STAT signaling pathway activity. Biol. Open 4: 1518–1527. 10.1242/bio.013961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tazuke S. I., Schulz C., Gilboa L., Fogarty M., Mahowald A. P. et al. , 2002. A germline-specific gap junction protein required for survival of differentiating early germ cells. Development 129: 2529–2539. [DOI] [PubMed] [Google Scholar]
- Toomey M. E., and Frydman H. M., 2014. Extreme divergence of Wolbachia tropism for the stem-cell-niche in the Drosophila testis. PLoS Pathog. 10: e1004577 10.1371/journal.ppat.1004577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toomey M. E., Panaram K., Fast E. M., Beatty C., and Frydman H. M., 2013. Evolutionarily conserved Wolbachia-encoded factors control pattern of stem-cell niche tropism in Drosophila ovaries and favor infection. Proc. Natl. Acad. Sci. USA 110: 10788–10793. 10.1073/pnas.1301524110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran V., Lim C., Xie J., and Chen X., 2012. Asymmetric division of Drosophila male germline stem cell shows asymmetric histone distribution. Science 338: 679–682. 10.1126/science.1226028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu M. P., Yin C. M., and Tatar M., 2002. Impaired ovarian ecdysone synthesis of Drosophila melanogaster insulin receptor mutants. Aging Cell 1: 158–160. 10.1046/j.1474-9728.2002.00016.x [DOI] [PubMed] [Google Scholar]
- Tulina N., and Matunis E., 2001. Control of stem cell self-renewal in Drosophila spermatogenesis by JAK-STAT signaling. Science 294: 2546–2549. 10.1126/science.1066700 [DOI] [PubMed] [Google Scholar]
- Tulina N. M., Chen W. F., Chen J. H., Sowcik M., and Sehgal A., 2014. Day-night cycles and the sleep-promoting factor, Sleepless, affect stem cell activity in the Drosophila testis. Proc. Natl. Acad. Sci. USA 111: 3026–3031. 10.1073/pnas.1316552111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueishi S., Shimizu H., and H. Inoue Y., 2009. Male germline stem cell division and spermatocyte growth require insulin signaling in Drosophila. Cell Struct. Funct. 34: 61–69. 10.1247/csf.08042 [DOI] [PubMed] [Google Scholar]
- Van De Bor V., Zimniak G., Papone L., Cerezo D., Malbouyres M. et al. , 2015. Companion blood cells control ovarian stem cell niche microenvironment and homeostasis. Cell Rep. 13: 546–560. 10.1016/j.celrep.2015.09.008 [DOI] [PubMed] [Google Scholar]
- Wang H., Singh S. R., Zheng Z., Oh S. W., Chen X. et al. , 2006. Rap-GEF signaling controls stem cell anchoring to their niche through regulating DE-cadherin-mediated cell adhesion in the Drosophila testis. Dev. Cell 10: 117–126. 10.1016/j.devcel.2005.11.004 [DOI] [PubMed] [Google Scholar]
- Wang S., Gao Y., Song X., Ma X., Zhu X. et al. , 2015. Wnt signaling-mediated redox regulation maintains the germ line stem cell differentiation niche. Elife 4: e08174 10.7554/eLife.08174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Harris R. E., Bayston L. J., and Ashe H. L., 2008. Type IV collagens regulate BMP signalling in Drosophila. Nature 455: 72–77. 10.1038/nature07214 [DOI] [PubMed] [Google Scholar]
- Wang Z., and Lin H., 2004. Nanos maintains germline stem cell self-renewal by preventing differentiation. Science 303: 2016–2019. 10.1126/science.1093983 [DOI] [PubMed] [Google Scholar]
- Weaver L. N., and Drummond-Barbosa D., 2018. Maintenance of proper germline stem cell number requires adipocyte collagen in adult Drosophila females. Genetics 209: 1155–1166. 10.1534/genetics.118.301137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Y., and Lilly M. A., 2014. The TORC1 inhibitors Nprl2 and Nprl3 mediate an adaptive response to amino-acid starvation in Drosophila. Cell Death Differ. 21: 1460–1468. 10.1038/cdd.2014.63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Y., Reveal B., Reich J., Laursen W. J., Senger S. et al. , 2014. TORC1 regulators Iml1/GATOR1 and GATOR2 control meiotic entry and oocyte development in Drosophila. Proc. Natl. Acad. Sci. USA 111: E5670–E5677. 10.1073/pnas.1419156112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Y., Reveal B., Cai W., and Lilly M. A., 2016. The GATOR1 complex regulates metabolic homeostasis and the response to nutrient stress in Drosophila melanogaster. G3 (Bethesda) 6: 3859–3867. 10.1534/g3.116.035337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfson R. L., and Sabatini D. M., 2017. The dawn of the age of amino acid sensors for the mTORC1 pathway. Cell Metab. 26: 301–309. 10.1016/j.cmet.2017.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong A. C., Vanhove A. S., and Watnick P. I., 2016. The interplay between intestinal bacteria and host metabolism in health and disease: lessons from Drosophila melanogaster. Dis. Model. Mech. 9: 271–281. 10.1242/dmm.023408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong A. C., Wang Q. P., Morimoto J., Senior A. M., Lihoreau M. et al. , 2017. Gut microbiota modifies olfactory-guided microbial preferences and foraging decisions in Drosophila. Curr. Biol. 27: 2397–2404.e4. 10.1016/j.cub.2017.07.022 [DOI] [PubMed] [Google Scholar]
- Xie J., Wooten M., Tran V., Chen B. C., Pozmanter C. et al. , 2015. Histone H3 threonine phosphorylation regulates asymmetric histone inheritance in the Drosophila male germline. Cell 163: 920–933. 10.1016/j.cell.2015.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie T., 2013. Control of germline stem cell self-renewal and differentiation in the Drosophila ovary: concerted actions of niche signals and intrinsic factors. Wiley Interdiscip. Rev. Dev. Biol. 2: 261–273. 10.1002/wdev.60 [DOI] [PubMed] [Google Scholar]
- Xie T., and Spradling A. C., 1998. Decapentaplegic is essential for the maintenance and division of germline stem cells in the Drosophila ovary. Cell 94: 251–260. 10.1016/S0092-8674(00)81424-5 [DOI] [PubMed] [Google Scholar]
- Xie T., and Spradling A. C., 2000. A niche maintaining germ line stem cells in the Drosophila ovary. Science 290: 328–330. 10.1126/science.290.5490.328 [DOI] [PubMed] [Google Scholar]
- Yadlapalli S., and Yamashita Y. M., 2013. Chromosome-specific nonrandom sister chromatid segregation during stem-cell division. Nature 498: 251–254. 10.1038/nature12106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan D., Neumuller R. A., Buckner M., Ayers K., Li H. et al. , 2014. A regulatory network of Drosophila germline stem cell self-renewal. Dev. Cell 28: 459–473. 10.1016/j.devcel.2014.01.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H., and Yamashita Y. M., 2015. The regulated elimination of transit-amplifying cells preserves tissue homeostasis during protein starvation in Drosophila testis. Development 142: 1756–1766. 10.1242/dev.122663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S. A., Wang W. D., Chen C. T., Tseng C. Y., Chen Y. N. et al. , 2013. FOXO/Fringe is necessary for maintenance of the germline stem cell niche in response to insulin insufficiency. Dev. Biol. 382: 124–135. 10.1016/j.ydbio.2013.07.018 [DOI] [PubMed] [Google Scholar]
- Zhang Q., Shalaby N. A., and Buszczak M., 2014. Changes in rRNA transcription influence proliferation and cell fate within a stem cell lineage. Science 343: 298–301. 10.1126/science.1246384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhukova M. V., and Kiseleva E., 2012. The virulent Wolbachia strain wMelPop increases the frequency of apoptosis in the female germline cells of Drosophila melanogaster. BMC Microbiol. 12: S15 10.1186/1471-2180-12-S1-S15 [DOI] [PMC free article] [PubMed] [Google Scholar]