Abstract
Introduction:
Preterm birth (PTB) and in-utero inflammation are recognized risk factors of neurodevelopmental disabilities (NDDs); however, their combined role in NDDs is unknown. We examined the independent and joint association of PTB and placental histological findings with the childhood risk of NDDs (overall and by subgroups including autism spectrum disorder (ASD) and ADHD).
Methods:
We analyzed data from the Boston Birth Cohort, where mother-infant pairs were enrolled at birth and followed from birth onwards. Birth outcomes, placental pathology and NDDs were obtained from electronic medical records. Placental pathology was categorized using a standardized classification system proposed by the Amsterdam Placental Workshop Group.
Results:
PTB (all, including spontaneous, medically indicated) was an independent risk factor for NDDs. Placental histological chorioamnionitis (CA) and PTB additively increased the odds of NDDs (aOR: 2.16, 95% CI: 1.37, 3.39), as well as ADHD (aOR: 2.75, 95% CI: 1.55, 4.90), other developmental disabilities (aOR: 1.96, 95% CI: 1.18, 3.25) and possibly ASD (aOR: 2.31, 95% CI: 0.99, 5.39). The above associations were more pronounced in spontaneous than medically indicated PTB. PTB alone in the absence of CA only had a moderate association with ASD and ADHD. Placental maternal vascular malperfusion alone or in combination with PTB was not associated with the risk of NDDs.
Discussion:
Our study provided new insights on PTB and NDDs by further considering preterm subtypes and placental histology. We revealed that children of spontaneous PTB along with histological CA were at the highest risk for a spectrum of NDDs.
Keywords: Preterm, chorioamnionitis, maternal vascular malperfusion, autism, ADHD, neurodevelopmental disabilities
Introduction
Neurodevelopmental disabilities (NDDs) are a group of conditions, characterized by limitations in several developmental domains, which have an onset in the early developmental period [1, 2]. Autism Spectrum Disorder (ASD) and Attention Deficit Hyperactivity Disorder (ADHD) are the most common neurodevelopmental conditions with a prevalence of ~1.5% and 5 –10%, respectively [3] [2, 4]. There are important commonalities between these conditions, with studies showing higher rates of co-morbidity among children with ASD and ADHD [5]. Similarly, young children with ASD often share features with children with NDDs such as global developmental delay and language delay [6]. To date, few studies have examined whether there are common early life antecedents underlying the co-morbidities. This report will focus on two early life factors: preterm birth (PTB) and placental histological findings.
PTB (defined as <37 weeks of gestation) is a known risk factor for a range of NDDs [7–9]. PTB represents a heterogeneous entity with different underlying pathophysiologies [10]; however, preterm sub-types (such as spontaneous vs. medically indicated PTB) have not been frequently considered in epidemiological studies that assess NDD outcomes. PTB is also associated with placental pathology [11] [12, 13]. Specifically, histological chorioamnionitis (CA), a condition of intrauterine infection/inflammation and maternal vascular malperfusion (MVM) are the 2 most common pathological placental conditions associated with PTB [14–19]. While the relationship between CA, MVM and some of the early morbidities has been extensively studied, there is a dearth of cohort studies that have assessed the long-term associations of these placental pathologies with NDDs [20] [12, 21]. This is not surprising given the challenges of studying incident NDDs, which does not manifest immediately after birth, but only long after the placenta is discarded [22, 23]. There are important gaps in this field and it is unclear whether placental pathological findings in combination with PTB influence neurodevelopment later in life [20].
By analyzing data from a large prospective birth cohort, we sought to address aforementioned gaps by examining the individual and joint effects of PTB and placental histological findings (specifically CA, MVM) on the risk of NDDs, defined as the presence of ASD, ADHD or other developmental disabilities (DD) in childhood. We further examined whether the associations differ by preterm subtypes (spontaneous vs. medically indicated) and by specific neurodevelopmental conditions, such as ASD, ADHD and other DD. Dysfunction of the placenta exposes the fetus to an unfavorable intrauterine environment, which may potentially facilitate preterm delivery and damage the developing brain [14]. Given the critical role the placenta plays in fetal growth and development, exploration of its underlying pathology along with PTB may possibly hold important clues about the pathogenesis of NDDs.
Methods
Participation and data collection procedure
The Boston Birth Cohort (BBC) is a prospective cohort study that was initiated to investigate the environmental and genetic determinants of preterm delivery. Exclusion criteria for initial enrollment were multiple-gestation pregnancies, chromosomal abnormalities, major birth defects and preterm deliveries as a result of maternal trauma. Between 1998 and 2015, mothers who delivered at the Boston Medical Center were invited to participate in the study. After obtaining informed consent, mothers were interviewed 24–72 hours after delivery and a standardized questionnaire was used to collect demographic data, medical, reproductive history and substance abuse [24, 25]. A standardized abstraction form was used to extract data from medical records review, including prenatal and intrapartum clinical care, pregnancy complications, birth outcomes, ultrasonographic findings, laboratory test results and placental pathology reports [24]. A sub-set of the originally enrolled children who continued to receive pediatric care at the BMC were included in this study, and they were followed-up until 2018. The Institutional Review Boards (IRB) of Boston University Medical Center and Johns Hopkins Bloomberg School of Public Health approved both the baseline and follow-up studies.
Exposure
Placentas were obtained by the labor and delivery nurse at the time of delivery and were sent to the BMC’s perinatal pathologist to be processed and reviewed. During the course of the BBC study, a new pathologist took over the examination of the placenta. Prior to this transition, for training purposes, a sub-set of placental pathology slides (n=298) was randomly selected and independently reviewed by two placenta pathologists, who then compared the readings and reached consensus on the review of pathology findings [24].
In accordance with the College of American Pathologists guidelines [26], the perinatal pathologists examined all placentas when clinically indicated. Perinatal pathologists had no knowledge of subsequent neurodevelopmental outcomes. Fresh placentas were fixed with 10% neutral buffered formalin for at least 24 to 48 hours. Placental disc was serially sectioned every 2–3 cm and dissection of placental plate was performed for diagnostic interpretation. A rolled section of membranes and umbilical cord in cassette 1, and three transmural/full thickness sections of placental plate, including fetal and maternal surfaces, in cassettes 2–4, were routinely sampled. Grossly identified lesions were further sampled. Pathological placental lesions were diagnosed based on commonly used, recommended criteria [24]. For the purposes of this project, a placental pathologist recoded the placental diagnosis into predominantly eight categories, in line with the classification proposed by Redline [27, 28]. A second perinatal pathologist further confirmed the coding. Broadly, the categories were: CA; MVM; marginal (venous) abruption; umbilical cord obstruction; fetal vascular malperfusion; villous stromal-vascular abnormalities; and a miscellaneous group (Supplemental Table 1).
As described earlier [29–31], gestational age at birth was characterized based on first day of last menstrual period data and early ultrasound data. Children with gestational age ≥ 37 completed weeks of gestation were categorized as full-term and those <37 weeks were categorized as preterm. Preterm children were further categorized into spontaneous and medically indicated preterm based on maternal prenatal and perinatal medical records [32].
Definitions for ASD, ADHD, other DD and neurotypical children
Information regarding child’s neurodevelopmental outcomes was documented in the electronic medical records (EMR). Based on EMR ICD codes, children that were ever diagnosed with autism (299.00), Asperger syndrome (299.80), and/or pervasive developmental disorder not otherwise specified (299.90) constituted the ASD cases. Children ever diagnosed with ADHD (314.0–314.9) constituted the ADHD cases. Children ever diagnosed with language delay, coordination disorders, or learning disorders (315.0–315.5) constituted the other DD cases. Neurotypical children were those that were never diagnosed with ASD, ADHD, other DD, intellectual disabilities, oppositional defiant disorder, conduct disorder or congenital anomalies.
Covariates
Covariates were selected a priori based on the existing literature, including our own work in the BBC [30, 33–35]. Covariates that were used for adjustments included maternal age at delivery, smoking during pregnancy (ever smoked 3 months before pregnancy/during pregnancy vs. not smoked before pregnancy/during pregnancy), parity (not including the index pregnancy), maternal education (high school or less vs. some college or more), race/ethnicity (black, white, Hispanic and Other) and child’s sex (female vs. male). Missingness in covariates was minimal and handled by assigning them to the largest categories. Since gestational age is in the causal pathway between the placental pathology and NDDs, it was not adjusted for, but rather assessed in the additive model.
Statistical Analysis
The outcomes in this study were ASD, ADHD and other DD, that were assessed separately and together as NDDs. The exposure was PTB and placental pathology, specifically CA and MVM. Exposures were considered as dichotomous variables. The characteristics of the study sample for neurotypical and NDDs (i.e. children with ASD, ADHD and other DD) were calculated using chi-square tests for categorical variables and ANOVA for continuous variables. Logistic regression models were applied to assess the crude and adjusted associations between exposures and outcomes. For PTB and placental pathology, we performed an independent analysis, followed by an analysis to assess the joint effects. All results are presented as odds ratio. Throughout, we used 2-sided statistical tests with a significance level of 0.05. Data were analyzed using STATA version 13.0 (StataCorp, College Station, TX).
Results
This study included 363 neurotypical children and 77 ASD, 215 ADHD and 376 other DD children (Figure 1). The demographic and clinical characteristics of mothers and children are presented in Table 1 and have been documented earlier in this cohort [25, 31, 36, 37]. We found that the risk factors such as higher maternal BMI, diabetes mellitus, maternal smoking during pregnancy, male sex, PTB and low birth weight were more frequent in children with NDDs (Table 1). There were no significant differences in the incidence of placental pathology between neurotypical children and those with NDDs (Table 1).
Figure 1.
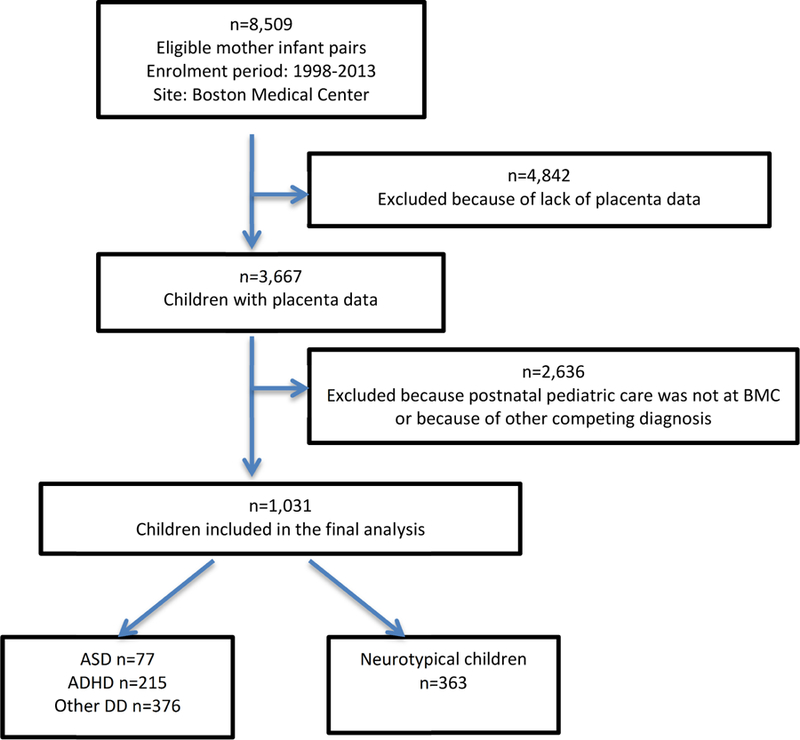
Flowchart of initial enrolment and postnatal follow-up of the Boston Birth Cohort and the Sample Included in the analysis
Table 1:
Maternal and child characteristics of neurotypical children and those with neurodevelopmental disabilities (NDDs), including ASD, ADHD, and other DD*
| Overall (n=1031) | Neurotypical (n=363) | Neurodevelopmental disabilities (n=668) | P value | |
|---|---|---|---|---|
| Maternal characteristics | ||||
| Age at birth (yrs), mean (SD) | 29.0 (6.6) | 28.6 (6.6) | 29.2 (6.5) | 0.14 |
| Parity (%) | 0.77 | |||
| 0 | 465 (45.1) | 166 (45.7) | 299 (44.8) | |
| 1 or more | 566 (54.9) | 197 (54.3) | 369 (55.2) | |
| Mother's education (%) | 0.64 | |||
| High School or less | 647 (62.8) | 221 (60.9) | 426 (63.8) | |
| Some college or more | 375 (36.4) | 139 (38.3) | 236 (35.3) | |
| Missing | 9 (0.9) | 3 (0.8) | 6 (0.9) | |
| Maternal BMI (%) | 0.01 | |||
| Underweight (<18.5) + Normal Weight (≥18.5–<25) | 432 (41.9) | 173 (47.7) | 259 (38.8) | |
| Overweight (≥25–<30) | 281 (27.3) | 95 (26.2) | 186 (27.8) | |
| Obesity (≥30) | 318 (30.8) | 95 (26.2) | 223 (33.4) | |
| Diabetes mellitus (%) | 0.008 | |||
| No | 878 (85.2) | 324 (89.3) | 554 (82.9) | |
| Gestational diabetes | 89 (8.6) | 27 (7.4) | 62 (9.3) | |
| Pre-gestational diabetes | 64 (6.2) | 12 (3.3) | 52 (7.8) | |
| Smoking during & 3 months prior to pregnancy (%) | 0.003 | |||
| No | 803 (77.9) | 300 (82.6) | 503 (75.3) | |
| Yes | 224 (21.7) | 60 (16.5) | 164 (24.6) | |
| Missing | 4 (0.4) | 3 (0.8) | 1 (0.2) | |
| Child’s characteristics | ||||
| Sex (%) | <0.001 | |||
| Male | 535 (51.9) | 144 (39.7) | 391 (58.5) | |
| Female | 496 (48.1) | 219 (60.3) | 277 (41.5) | |
| Race-ethnicity (%) | 0.53 | |||
| Black | 688 (66.7) | 232 (63.9) | 456 (68.3) | |
| White | 75 (7.3) | 29 (8.0) | 46 (6.9) | |
| Hispanic | 206 (20.0) | 77 (21.2) | 129 (19.3) | |
| Other | 62 (6.0) | 25 (6.9) | 37 (5.5) | |
| Gestational age (%) | <0.001 | |||
| Term | 511 (49.6) | 211 (58.1) | 300 (44.9) | |
| Preterm (<37 weeks) | 520 (50.4) | 152 (41.9) | 368 (55.1) | |
| Birth weight (g) | 2460.4 (923.7) | 2673.7 (822.8) | 2344.5 (955.0) | <0.001 |
| Major placental pathology categories based on the Amsterdam recommendation | ||||
| Intrauterine infection/inflammation | 0.14 | |||
| No | 767 (74.4) | 280 (77.1) | 487 (72.9) | |
| Yes | 264 (25.6) | 83 (22.9) | 181 (27.1) | |
| Chronic villitis | 0.88 | |||
| No | 1016 (98.6) | 358 (98.6) | 658 (98.5) | |
| Yes | 15 (1.5) | 5 (1.4) | 10 (1.5) | |
| Maternal vascular malperfusion | 0.97 | |||
| No | 627 (60.8) | 221 (60.9) | 406 (60.8) | |
| Yes | 404 (39.2) | 142 (39.1) | 262 (39.2) | |
| Marginal (venous) abruption | 0.41 | |||
| No | 993 (96.3) | 352 (97.0) | 641 (96.0) | |
| Yes | 38 (3.7) | 11 (3.0) | 27 (4.0) | |
| Umbilical cord (UC) obstruction | 0.41 | |||
| No | 1022 (99.1) | 361 (99.5) | 661 (99.0) | |
| Yes | 9 (0.9) | 2 (0.6) | 7 (1.1) | |
| Fetal vascular malperfusion | 0.25 | |||
| No | 1013 (98.3) | 359 (98.9) | 654 (97.9) | |
| Yes | 18 (1.8) | 4 (1.1) | 14 (2.1) | |
| Villous stromal-vascular abnormalities | 0.11 | |||
| No | 1012 (98.2) | 353 (97.3) | 659 (98.7) | |
| Yes | 19 (1.8) | 10 (2.8) | 9 (1.4) | |
| Miscellaneous findings | 0.22 | |||
| No | 294 (28.5) | 95 (26.2) | 199 (29.8) | |
| Yes | 737 (71.5) | 268 (73.8) | 469 (70.2) | |
ASD – Autism Spectrum Disorder; ADHD – Attention-Deficit/Hyperactivity Disorder; DD – Developmental disabilities.
Percentages may not add up due to rounding
Maternal and child characteristics of term and preterm children are presented in Supplemental Table 1 and placental diagnostic categories and frequency of placenta pathology findings stratified by term and PTBs are presented in Supplemental Tables 2 and 3. Compared to children born at term, preterm children had increased odds of NDDs in both unadjusted and adjusted models (aOR: 1.62, 95% CI: 1.24, 2.11) (Table 2). When stratified further, the increased odds were noted for both spontaneous PTB (aOR: 1.69, 95% CI: 1.24, 2.31) and medically indicated PTB (aOR: 1.51, 95% CI: 1.06, 2.15). However, exposure to CA alone was not associated with increased odds of NDDs (aOR: 1.27, 95% CI: 0.93, 1.72).
Table 2.
Individual and joint association of preterm birth and histological chorioamnionitis (CA) with child’s risk of neurodevelopmental disabilities (NDDs), including ASD, ADHD, and other DD*
| Total n | NDD n | Unadjusted | Adjusted** | |
|---|---|---|---|---|
| Individual associations | ||||
| Term | 511 | 300 | 1.0 (Ref) | |
| Preterm | 520 | 368 | 1.70 (1.32, 2.20) | 1.62 (1.24, 2.11) |
| Spontaneous | 316 | 227 | 1.79 (1.33, 2.43) | 1.69 (1.24, 2.31) |
| Medically indicated | 204 | 141 | 1.57 (1.11, 2.22) | 1.51 (1.06, 2.15) |
| No CA | 767 | 487 | 1.0 (Ref) | |
| CA | 264 | 181 | 1.25 (0.93, 1.69) | 1.27 (0.93, 1.72) |
| Combined association of preterm with CA | ||||
| Term, no CA | 383 | 222 | 1.0 (Ref) | |
| Term, CA | 128 | 78 | 1.13 (0.75, 1.70) | 1.16 (0.76, 1.77) |
| Preterm, no CA | 384 | 265 | 1.61 (1.20, 2.17) | 1.55 (1.14, 2.10) |
| Preterm, CA | 136 | 103 | 2.26 (1.46, 3.52) | 2.16 (1.37, 3.39) |
| Combined association of spontaneous preterm with CA | ||||
| Term, no CA | 383 | 222 | 1.0 (Ref) | |
| Term, CA | 128 | 78 | 1.13 (0.75, 1.70) | 1.12 (0.73, 1.72) |
| Spontaneous preterm, no CA | 199 | 137 | 1.60 (1.12, 2.30) | 1.47 (1.01, 2.14) |
| Spontaneous preterm, CA | 117 | 90 | 2.42 (1.50, 3.89) | 2.38 (1.46, 3.88) |
| Combined association of medically indicated preterm with CA | ||||
| Term, no CA | 383 | 222 | 1.0 (Ref) | |
| Term, CA | 128 | 78 | 1.13 (0.75, 1.70) | 1.17 (0.77, 1.80) |
| Medically indicated preterm, no CA | 185 | 128 | 1.63 (1.12, 2.36) | 1.59 (1.08, 2.33) |
| Medically indicated preterm, CA | 19 | 13 | 1.57 (0.58, 4.22) | 1.38 (0.49, 3.87) |
ASD – Autism Spectrum Disorder; ADHD – Attention-Deficit/Hyperactivity Disorder; DD – Developmental disabilities.
Adjusted for maternal smoking, age, education, parity, race and child’s sex
Test of interactions were performed for all the above analyses, but none reached statistical significance (p<0.05).
We assessed the combined association between PTB and CA on the odds of NDDs. PTB increased the odds of NDDs, both in the absence (aOR: 1.55, 95% CI: 1.14, 2.10) and presence of CA (aOR: 2.16, 95% CI: 1.37, 3.39), when compared to term babies without CA exposure. Similarly, for spontaneous PTB babies, the odds of NDDs increased among those without (aOR: 1.47, 95% CI: 1.01, 2.14) or with exposure CA (aOR: 2.38, 95% CI: 1.46, 3.88). Among medically indicated preterm babies, increased odds of NDDs were noted only in those not exposed to CA (aOR: 1.59, 95% CI: 1.08, 2.33) (Table 2). There was no statistically significant interaction between PTB (including spontaneous, medically indicated), CA and odds of NDDs (data not shown).
Next, we looked at the individual and joint associations of PTB and placental pathology with specific outcomes – ASD, ADHD and other DD (Table 3). Compared to term children, those born preterm were at increased odds of ASD (aOR: 2.06, 95% CI: 1.22, 3.49) and other DD (aOR: 1.68, 95% CI: 1.25, 2.27). While spontaneous PTB was also associated with greater odds of ASD (aOR: 2.10, 95% CI: 1.15, 3.85), increased odds of other DD was observed in both spontaneous (aOR: 1.75, 95% CI: 1.24, 2.48) and medically indicated preterm babies (aOR: 1.57, 95% CI: 1.05, 2.33). CA was associated with increased odds of ADHD in both unadjusted and adjusted models (aOR: 1.74, 95% CI: 1.15, 2.62).
Table 3:
Individual and joint association of preterm birth and histological chorioamnionitis (CA) with child’s risk of ASD, ADHD, and other DD*, using a common reference group of neurotypical children
| Total n | ASD n | Unadjusted | Adjusted | Total n | ADHD n | Unadjusted | Adjusted | Total n | DD n | Unadjusted | Adjusted** | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Individual associations | ||||||||||||
| Term | 241 | 30 | 1.0 (Ref) | 316 | 105 | 1.0 (Ref) | 376 | 165 | 1.0 (Ref) | |||
| Preterm | 199 | 47 | 2.17 (1.31, 3.60) | 2.06 (1.22, 3.49) | 262 | 110 | 1.45 (1.04, 2.04) | 1.34 (0.93, 1.92) | 363 | 211 | 1.78 (1.33, 2.38) | 1.68 (1.25, 2.27) |
| Spontaneous | 118 | 29 | 2.29 (1.30, 4.04) | 2.10 (1.15, 3.85) | 158 | 69 | 1.56 (1.05, 2.31) | 1.48 (0.97, 2.26) | 218 | 129 | 1.85 (1.32, 2.60) | 1.75 (1.24, 2.48) |
| Medically Indicated | 81 | 18 | 2.01 (1.05, 3.84) | 1.89 (0.96, 3.70) | 104 | 41 | 1.31 (0.83, 2.07) | 1.15 (0.70, 1.91) | 145 | 82 | 1.66 (1.13, 2.45) | 1.57 (1.05, 2.33) |
| No CA | 340 | 60 | 1.0 (Ref) | 428 | 148 | 1.0 (Ref) | 559 | 279 | 1.0 (Ref) | |||
| CA | 100 | 17 | 0.96 (0.53, 1.73) | 1.03 (0.56, 1.92) | 150 | 67 | 1.53 (1.05, 2.23) | 1.74 (1.15, 2.62) | 180 | 97 | 1.17 (0.84, 1.64) | 1.17 (0.83, 1.65) |
| Combined association of preterm with CA | ||||||||||||
| Term, no CA | 185 | 24 | 1.0 (Ref) | 239 | 78 | 1.0 (Ref) | 281 | 120 | 1.0 (Ref) | |||
| Term, CA | 56 | 6 | 0.81 (0.31, 2.08) | 0.86 (0.32, 2.29) | 77 | 27 | 1.11 (0.65, 1.91) | 1.14 (0.64, 2.03) | 95 | 45 | 1.21 (0.76, 1.93) | 1.25 (0.77, 2.01) |
| Preterm, no CA | 155 | 36 | 2.03 (1.15, 3.58) | 1.92 (1.06, 3.46) | 189 | 70 | 1.21 (0.81, 1.81) | 1.04 (0.68, 1.60) | 278 | 159 | 1.79 (1.28, 2.51) | 1.73 (1.23, 2.43) |
| Preterm, CA | 44 | 11 | 2.24 (1.00, 5.01) | 2.31 (0.99, 5.39) | 73 | 40 | 2.50 (1.47, 4.27) | 2.75 (1.55, 4.90) | 85 | 52 | 2.11 (1.29, 3.47) | 1.96 (1.18, 3.25) |
| Combined association of spontaneous preterm with CA | ||||||||||||
| Term, no CA | 185 | 24 | 1.0 (Ref) | 239 | 78 | 1.0 (Ref) | 281 | 120 | 1.0 (Ref) | |||
| Term, CA | 56 | 6 | 0.81 (0.31, 2.08) | 0.85 (0.32, 2.26) | 77 | 27 | 1.11 (0.65, 1.91) | 1.11 (0.62, 1.98) | 95 | 45 | 1.21 (0.76, 1.93) | 1.20 (0.74, 1.94) |
| Spon. preterm, no CA | 81 | 19 | 2.06 (1.05, 4.01) | 1.82 (0.89, 3.70) | 99 | 37 | 1.23 (0.76, 2.01) | 1.04 (0.62, 1.77) | 143 | 81 | 1.75 (1.17, 2.63) | 1.64 (1.08, 2.49) |
| Spon. preterm, CA | 37 | 10 | 2.48 (1.07, 5.78) | 2.59 (1.04, 6.46) | 59 | 32 | 2.45 (1.37, 4.37) | 2.80 (1.49, 5.24) | 75 | 48 | 2.39 (1.41, 4.04) | 2.29 (1.33, 3.92) |
| Combined association of medically indicated preterm with CA | ||||||||||||
| Term, no CA | 185 | 24 | 1.0 (Ref) | 239 | 78 | 1.0 (Ref) | 281 | 120 | 1.0 (Ref) | |||
| Term, CA | 56 | 6 | 0.81 (0.31, 2.08) | 0.86 (0.33, 2.29) | 77 | 27 | 1.11 (0.65, 1.91) | 1.13 (0.63, 2.04) | 95 | 45 | 1.21 (0.76, 1.93) | 1.27 (0.78, 2.05) |
| Med. Preterm, no CA | 74 | 17 | 2.00 (1.00, 3.99) | 1.90 (0.93, 3.89) | 90 | 33 | 1.20 (0.72, 1.98) | 1.03 (0.59, 1.79) | 135 | 78 | 1.84 (1.21, 2.78) | 1.76 (1.15, 2.70) |
| Med. Preterm, CA | 7 | 1 | 1.12 (0.13, 9.69) | 1.08 (0.11, 10.21) | 14 | 8 | 2.75 (0.92, 8.21) | 2.93 (0.88, 9.74) | 10 | 4 | 0.89 (0.25, 3.24) | 0.75 (0.19, 2.88) |
ASD – Autism Spectrum Disorder; ADHD – Attention-Deficit/Hyperactivity Disorder; DD – Developmental disabilities; Med. Preterm – Medically indicated preterm; Spon. Preterm – Spontaneous preterm
Adjusted for maternal smoking, age, education, parity, race and child’s sex
Test of interactions were performed for all the above analyses, but none reached statistical significance (p<0.05)
Compared to term children that were not exposed to CA, increased odds of ADHD (aOR: 2.75, 95% CI: 1.55, 4.90) and other DD (aOR: 1.96, 95% CI: 1.18, 3.25) was observed among preterm babies with CA exposure. This association also approached significance for ASD (aOR: 2.31, 95% CI: 0.99, 5.39). When restricting the analysis to those that had spontaneous PTB with CA exposure, the associations became slightly stronger for ASD (aOR: 2.59, 95% CI: 1.04, 6.46), ADHD (aOR: 2.80, 95% CI: 1.49, 5.24) and other DD (aOR: 2.29, 95% CI: 1.33, 3.92). The association between medically indicated PTB with CA approached significance with ADHD (aOR: 2.93, 95% CI: 0.88, 9.74), but not with ASD or other DD (Table 3).
Exposure to MVM was not associated with increased odds of NDDs (aOR: 1.00, 95% CI: 0.76, 1.31) (Table 4). When joint effects of PTB and MVM was considered, PTB without (aOR: 1.95, 95% CI: 1.38, 2.74) or with MVM (aOR: 1.51, 95% CI: 1.04, 2.20) exposure was associated with increased odds of NDDs. Even in the absence of MVM and CA, PTB was still associated with increased odds of NDDs (aOR: 1.58, 95% CI: 1.08, 2.32). When PTB was further stratified, increased odds of NDDs were observed for both spontaneous PTB without MVM exposure (aOR: 2.01, 95% CI: 1.37, 2.94) and medically indicated PTB without MVM exposure (aOR: 1.79, 95% CI: 1.05, 3.05). There was no statistically significant interaction between PTB (including spontaneous, medically indicated), MVM and odds of NDDs (data not shown).
Table 4:
Individual and joint association of preterm birth and histological maternal vascular malperfusion (MVM) with child’s risk of neurodevelopmental disabilities (NDDs), including ASD, ADHD, and other DD*
| Total n | NDD n | Unadjusted | Adjusted** | |
|---|---|---|---|---|
| No MVM | 627 | 406 | 1.0 (Ref) | |
| MVM | 404 | 262 | 1.00 (0.77, 1.30) | 1.00 (0.76, 1.31) |
| Combined association of preterm with MVM | ||||
| Term, no MVM | 317 | 180 | 1.0 (Ref) | |
| Term, MVM | 194 | 120 | 1.23 (0.86, 1.78) | 1.23 (0.85, 1.80) |
| Preterm, no MVM | 310 | 226 | 2.05 (1.47, 2.86) | 1.95 (1.38, 2.74) |
| Preterm, MVM | 210 | 142 | 1.59 (1.10, 2.29) | 1.51 (1.04, 2.20) |
| Preterm, no MVM, no CA | 203 | 140 | 1.69 (1.17, 2.45) | 1.58 (1.08, 2.32) |
| Preterm, MVM, and CA | 29 | 17 | 1.08 (0.50, 2.33) | 0.90 (0.40, 2.01) |
| Combined association of spontaneous preterm with MVM | ||||
| Term, no MVM | 317 | 180 | 1.0 (Ref) | |
| Term, MVM | 194 | 120 | 1.23 (0.86, 1.78) | 1.24 (0.85, 1.82) |
| Spontaneous preterm, no MVM | 225 | 166 | 2.14 (1.48, 3.10) | 2.01 (1.37, 2.94) |
| Spontaneous preterm, MVM | 91 | 61 | 1.55 (0.95, 2.53) | 1.50 (0.91, 2.50) |
| Combined association of medically indicated preterm with MVM | ||||
| Term, no MVM | 317 | 180 | 1.0 (Ref) | |
| Term, MVM | 194 | 120 | 1.23 (0.86, 1.78) | 1.24 (0.85, 1.81) |
| Medically indicated preterm, no MVM | 85 | 60 | 1.83 (1.09, 3.06) | 1.79 (1.05, 3.05) |
| Medically indicated preterm, MVM | 119 | 81 | 1.62 (1.04, 2.53) | 1.53 (0.97, 2.42) |
ASD – Autism Spectrum Disorder; ADHD – Attention-Deficit/Hyperactivity Disorder; DD – Developmental disabilities. CA - Chorioamnionitis
Adjusted for maternal smoking, age, education, parity, race and child’s sex
Test of interactions were performed for all the above analyses, but none reached statistical significance (p<0.05)
Discussion
To our knowledge, this is the first prospective birth cohort study to simultaneously assess placental pathology and PTB and its subtypes, in relation to incident NDDs and its specific conditions: ASD, ADHD and other DD. Consistent with the literature, our results showed that PTB, especially spontaneous PTB, have an increased odds of NDDs, and specifically ASD and other DD [8, 38, 39]. Although CA, by itself, was not associated with greater odds of NDDs, we showed that children born preterm with CA exposure had increased odds of NDDs, as well as ADHD, other DD and possibly ASD. We further teased out the differences in NDD risk between overall, spontaneous and medically indicated preterm and provided empirical evidence to support the hypothesis that PTB, especially spontaneous PTB, possibly triggered by infection/inflammation in the presence of CA increases the risk of adverse neurodevelopmental outcomes [40].
It is well known that ASD, ADHD and other DD share a number of commonalities such as early onset, male preponderance, cognitive impairments and delays or deviance in the development of brain structure/function [41]. Because of these shared disturbances, it is theorized that the spectrum of NDDs may share certain underlying pathology, rather than being etiologically discrete entities [1, 42]. Recent studies on biological risk factors support the co-occurrence, with factors like PTB, maternal autoimmune disease and maternal infections being associated with many of the neurodevelopmental disorders [5, 25]. Our study lends support to the theory that certain prenatal risk factors, along with placental pathology, serve as a common thread across the spectrum of NDDs thereby help explain some of the co-morbidities we observe in children with NDDs.
When considered in the context of existing studies, our findings on ASD are consistent with Straughen et al., the only other study that assessed histological placental inflammation in the context of ASD [43]. However, Straughen et al. did not specially study the effects of placental pathology in children born preterm, which is an important risk factor, as noted in our study. Regarding other DD, the results were also consistent with previous studies, including a meta-analysis that showed an association between CA exposure and other DD such as speech delay and hearing loss [19, 21, 44]. The increased odds of NDDs observed with PTB, both in the absence and presence of CA, suggests PTB could increase the risk independent of CA and impact neurodevelopment through multiple pathways resulting in significant brain injury or more subtle impairments in brain development [45].
The underlying mechanism for increased odds of NDDs among preterm babies with CA is complex and not fully understood. It is possible that CA triggers a fetal inflammatory response with release of proinflammatory cytokines that could directly impact the immature brain and increase susceptibility to neurodevelopmental disorders, including ASD and ADHD [46–49]. Evidence suggests that CA, as well as inflammatory cytokines can result in white matter brain lesions [50–52]. Independent studies have shown that white matter alterations are associated with attention deficit [53–55] and ASD [56, 57]. Furthermore, a recent study showed that abnormalities in white matter are seen in both ASD and ADHD, suggesting a common underlying neural mechanism for these two frequently co-occurring conditions [58]. Another possible hypothesis is that CA, PTB and NDD are common consequences of the aberrant developmental biology, in which case, CA and PTB may be early indicators of the adverse NDD outcome [59].
An additional finding from this study is the lack of association between term babies exposed to CA and odds of NDDs, as well as individual conditions such as ASD, ADHD and other DD. Literature suggests that, for term babies, CA may be a heterogeneous condition with greater likelihood of localized inflammation and a maternal, rather than fetal origin [60, 61]. Thus, the etiology and pathogenesis may differ [60, 61], with placental inflammation of fetal origin more predictive of brain injury than placental inflammation of maternal origin [19]. This is further supported by studies on other neurodevelopmental conditions, which have shown the incidence of cerebral palsy is low among those that are born at term or closer to term [60].
Overall, MVM was not associated with NDDs. While our findings did not support the role of MVM in ASD (data not shown), a recent study showed that there might be variations in chorionic surface vascular network between placentas associated with high-risk ASD pregnancies and those from general population [62].
Our study has several strengths. First, this is one of the few prospective birth cohort studies that have concurrently assessed PTB (its subtypes), placental pathology and long-term neurodevelopmental outcomes. Most studies of placenta and neurodevelopmental outcomes are case reports or case studies, because prospective studies are difficult to conduct, as neurodevelopmental conditions don’t manifest immediately after birth [22, 23]. Second, our investigation was conducted in a larger birth cohort, whereas most previous studies had much smaller sample sizes. Third, our study’s internal validity was strengthened by verification of placental examinations and diagnostic coding done by a perinatal pathologist [24], using a standardized classification system that will enable comparison of this study’s findings with future studies [24].
Our study has notable limitations. First, the outcome definitions were based on EMR data and thus, could have been subjected to outcome misclassification. However, this may not be differential, considering the prospective study design. Second, although well-known risk factors of NDDs were adjusted for, there is a possibility of residual confounding. Third, in this birth cohort, placental examination was performed per prenatal and perinatal clinical indications such as preterm, small for gestational age and asphyxia. On one hand, we had an enriched sample with high-risk children; on the other hand, we cannot completely rule out the possibility of selection bias. However, this bias would not have influenced future NDD outcomes given our prospective study design. Also, this study analyzed a sub-set of children who continued to receive pediatric care at the BMC, which could also be a concern for selection bias. However, the associations between well-established factors such as gender of the child and maternal age with NDD were replicated in our study sample, assuaging some of the concerns. Fourth, an absence of definitive immunohistochemical or molecular diagnostic testing for specific placental lesions precludes validation. Fifth, like other studies that employ placental histopathology, sampling limitations in our study could not be completely avoided. In particular, certain lesions (e.g. decidual vasculopathy and infarction) might have been localized and difficult to appreciate in gross examination. However, this limitation was assuaged by having all placental examinations and tissue sampling performed by a perinatal pathologist, grossly examining placental disc every 2–3 cm, and obtaining transmural sections of the placenta along with sections of grossly identified lesions [24]. Sixth, despite showing a joint effect of PTB and CA on NDDs, it was not possible to conduct a mediation analysis since in utero temporal ordering of PTB and CA was unclear, as both of these were measured at birth. Seventh, it was challenging to tease out PTB and antenatal steroid effects on NDD because by clinical indication, very preterm babies were most likely to receive antenatal steroids and also at greatest risk of NDD. Eighth, other DD may comprise of heterogeneous group of conditions and their diagnosis might have been varied based on age of assessment. However, additional stratified analysis in this study based on the length of follow-up did not alter the association between PTB and other DD (data not shown). Finally, our population included urban low-income minority population, who were at high risk for PTBs, and thus, caution should be exercised when generalizing the study findings to populations with different characteristics.
In summary, using a standardized placental pathology classification system, this hypothesis generating study showed that PTB, especially spontaneous PTB, along with histological CA increased the odds of NDDs, including ADHD, other DD and possibly ASD. Further, our findings support the emerging theory that PTB along with histological CA may represent a common prenatal risk factor for NDDs and thus could potentially help explain the co-morbidities we observe in children with NDDs. Our data suggest that placental pathology findings may be valuable, along with types of PTB for identifying newborns at high risk of developing NDDs and could potentially shed light on pathogenic processes underlying NDDs [63]. We hope that our findings will stimulate future clinical and mechanistic studies, which may provide further insight and inform clinical risk assessment and prevention strategies for reducing long-term neurodevelopmental sequela among high-risk children [12, 23].
Supplementary Material
Highlights.
PTB and histological chorioamnionitis (CA) additively increase the risk of NDDs.
Spontaneous PTB with histological CA are associated with highest risk of NDDs.
Maternal vascular malperfusion does not increase the risk of NDDs.
Acknowledgement
The authors would like to thank the study participants and the field staff without whom this study would not have been possible.
Role of funding source:
This study is supported in part by the Health Resources and Services Administration (HRSA) of the U.S. Department of Health and Human Services (HHS) under grant number R40MC27443, Autism Field-initiated Innovative Research Studies Program; and grant number UJ2MC31074, Autism Single Investigator Innovation Program. This information or content and conclusions are those of the author and should not be construed as the official position or policy of, nor should any endorsements be inferred by HRSA, HHS or the U.S. Government.
The funding agencies had no involvement in the collection, analysis, or interpretation of data; in the writing of the report; or in the decision to submit the article for publication
The Boston Birth Cohort (the parent study) was supported in part by the March of Dimes PERI grants (20-FY02-56, #21-FY07-605); and the National Institutes of Health (NIH) grants (R21ES011666, R01HD041702, R21HD066471, U01AI090727, R21AI079872, R01HD086013, 2R01HD041702).
Abbreviations
- ASD
Autism Spectrum Disorder
- ADHD
Attention Deficit Hyperactivity Disorder
- CA
Chorioamnionitis
- DD
Developmental Disabilities
- EMR
Electronic medical records
- MVM
Maternal vascular malperfusion
- NDD
Neurodevelopmental disabilities
- PTB
Preterm birth
Footnotes
Declarations of interest: none
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Shevell M, Global developmental delay and mental retardation or intellectual disability: conceptualization, evaluation, and etiology, Pediatr Clin North Am 55(5) (2008) 1071–84, xi. [DOI] [PubMed] [Google Scholar]
- [2].Ramtekkar UP, DSM-5 Changes in Attention Deficit Hyperactivity Disorder and Autism Spectrum Disorder: Implications for Comorbid Sleep Issues, Children (Basel) 4(8) (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Lyall K, Croen L, Daniels J, Fallin MD, Ladd-Acosta C, Lee BK, Park BY, Snyder NW, Schendel D, Volk H, Windham GC, Newschaffer C, The Changing Epidemiology of Autism Spectrum Disorders, Annu Rev Public Health 38 (2017) 81–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Buss C, Entringer S, Davis EP, Hobel CJ, Swanson JM, Wadhwa PD, Sandman CA, Impaired executive function mediates the association between maternal pre-pregnancy body mass index and child ADHD symptoms, PLoS One 7(6) (2012) e37758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Craig F, Lamanna AL, Margari F, Matera E, Simone M, Margari L, Overlap Between Autism Spectrum Disorders and Attention Deficit Hyperactivity Disorder: Searching for Distinctive/Common Clinical Features, Autism Res 8(3) (2015) 328–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Ventola P, Kleinman J, Pandey J, Wilson L, Esser E, Boorstein H, Dumont-Mathieu T, Marshia G, Barton M, Hodgson S, Green J, Volkmar F, Chawarska K, Babitz T, Robins D, Fein D, Differentiating between autism spectrum disorders and other developmental disabilities in children who failed a screening instrument for ASD, J Autism Dev Disord 37(3) (2007) 425–36. [DOI] [PubMed] [Google Scholar]
- [7].Aarnoudse-Moens CS, Weisglas-Kuperus N, van Goudoever JB, Oosterlaan J, Meta-analysis of neurobehavioral outcomes in very preterm and/or very low birth weight children, Pediatrics 124(2) (2009) 717–28. [DOI] [PubMed] [Google Scholar]
- [8].Limperopoulos C, Bassan H, Sullivan NR, Soul JS, Robertson RL Jr., Moore M, Ringer SA, Volpe JJ, du Plessis AJ, Positive screening for autism in ex-preterm infants: prevalence and risk factors, Pediatrics 121(4) (2008) 758–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Wood NS, Marlow N, Costeloe K, Gibson AT, Wilkinson AR, Neurologic and developmental disability after extremely preterm birth. EPICure Study Group, N Engl J Med 343(6) (2000) 378–84. [DOI] [PubMed] [Google Scholar]
- [10].Villar J, Papageorghiou AT, Knight HE, Gravett MG, Iams J, Waller SA, Kramer M, Culhane JF, Barros FC, Conde-Agudelo A, Bhutta ZA, Goldenberg RL, The preterm birth syndrome: a prototype phenotypic classification, Am J Obstet Gynecol 206(2) (2012) 119–23. [DOI] [PubMed] [Google Scholar]
- [11].Galinsky R, Polglase GR, Hooper SB, Black MJ, Moss TJ, The consequences of chorioamnionitis: preterm birth and effects on development, J Pregnancy 2013 (2013) 412831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Hodyl NA, Aboustate N, Bianco-Miotto T, Roberts CT, Clifton VL, Stark MJ, Child neurodevelopmental outcomes following preterm and term birth: What can the placenta tell us?, Placenta 57 (2017) 79–86. [DOI] [PubMed] [Google Scholar]
- [13].Roescher AM, Hitzert MM, Timmer A, Verhagen EA, Erwich JJ, Bos AF, Placental pathology is associated with illness severity in preterm infants in the first twenty-four hours after birth, Early Hum Dev 87(4) (2011) 315–9. [DOI] [PubMed] [Google Scholar]
- [14].van Vliet EO, de Kieviet JF, van der Voorn JP, Been JV, Oosterlaan J, van Elburg RM, Placental pathology and long-term neurodevelopment of very preterm infants, Am J Obstet Gynecol 206(6) (2012) 489 e1–7. [DOI] [PubMed] [Google Scholar]
- [15].Peng CC, Chang JH, Lin HY, Cheng PJ, Su BH, Intrauterine inflammation, infection, or both (Triple I): A new concept for chorioamnionitis, Pediatr Neonatol 59(3) (2018) 231–237. [DOI] [PubMed] [Google Scholar]
- [16].Redline RW, Placental pathology: a systematic approach with clinical correlations, Placenta 29 Suppl A (2008) S86–91. [DOI] [PubMed] [Google Scholar]
- [17].Lee J, Kim JS, Park JW, Park CW, Park JS, Jun JK, Yoon BH, Chronic chorioamnionitis is the most common placental lesion in late preterm birth, Placenta 34(8) (2013) 681–9. [DOI] [PubMed] [Google Scholar]
- [18].Scifres CM, Parks WT, Feghali M, Caritis SN, Catov JM, Placental maternal vascular malperfusion and adverse pregnancy outcomes in gestational diabetes mellitus, Placenta 49 (2017) 10–15. [DOI] [PubMed] [Google Scholar]
- [19].Rovira N, Alarcon A, Iriondo M, Ibanez M, Poo P, Cusi V, Agut T, Pertierra A, Krauel X, Impact of histological chorioamnionitis, funisitis and clinical chorioamnionitis on neurodevelopmental outcome of preterm infants, Early Hum Dev 87(4) (2011) 253–7. [DOI] [PubMed] [Google Scholar]
- [20].Soucy-Giguere L, Gasse C, Giguere Y, Demers S, Bujold E, Boutin A, Intra-amniotic inflammation and child neurodevelopment: a systematic review protocol, Syst Rev 7(1) (2018) 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Pappas A, Kendrick DE, Shankaran S, Stoll BJ, Bell EF, Laptook AR, Walsh MC, Das A, Hale EC, Newman NS, Higgins RD, Eunice H Kennedy Shriver National Institute of Child, N. Human Development Neonatal Research, Chorioamnionitis and early childhood outcomes among extremely low-gestational-age neonates, JAMA Pediatr 168(2) (2014) 137–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Nelson KB, Blair E, The placenta and neurologic and psychiatric outcomes in the child: study design matters, Placenta 32(9) (2011) 623–625. [DOI] [PubMed] [Google Scholar]
- [23].Roescher AM, Timmer A, Erwich JJ, Bos AF, Placental pathology, perinatal death, neonatal outcome, and neurological development: a systematic review, PLoS One 9(2) (2014) e89419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Bustamante Helfrich B, Chilukuri N, He H, Cerda SR, Hong X, Wang G, Pearson C, Burd I, Wang X, Maternal vascular malperfusion of the placental bed associated with hypertensive disorders in the Boston Birth Cohort, Placenta 52 (2017) 106–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Brucato M, Ladd-Acosta C, Li M, Caruso D, Hong X, Kaczaniuk J, Stuart EA, Fallin MD, Wang X, Prenatal exposure to fever is associated with autism spectrum disorder in the boston birth cohort, Autism Res 10(11) (2017) 1878–1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Langston C, Kaplan C, Macpherson T, Manci E, Peevy K, Clark B, Murtagh C, Cox S, Glenn G, Practice guideline for examination of the placenta: developed by the Placental Pathology Practice Guideline Development Task Force of the College of American Pathologists, Arch Pathol Lab Med 121(5) (1997) 449–76. [PubMed] [Google Scholar]
- [27].Redline RW, The clinical implications of placental diagnoses, Semin Perinatol 39(1) (2015) 2–8. [DOI] [PubMed] [Google Scholar]
- [28].Redline RW, Classification of placental lesions, Am J Obstet Gynecol 213(4 Suppl) (2015) S21–8. [DOI] [PubMed] [Google Scholar]
- [29].Wang X, Zuckerman B, Pearson C, Kaufman G, Chen C, Wang G, Niu T, Wise PH, Bauchner H, Xu X, Maternal cigarette smoking, metabolic gene polymorphism, and infant birth weight, Jama 287(2) (2002) 195–202. [DOI] [PubMed] [Google Scholar]
- [30].Wang G, Divall S, Radovick S, Paige D, Ning Y, Chen Z, Ji Y, Hong X, Walker SO, Caruso D, Pearson C, Wang MC, Zuckerman B, Cheng TL, Wang X, Preterm birth and random plasma insulin levels at birth and in early childhood, Jama 311(6) (2014) 587–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Raghavan R, Fallin MD, Hong X, Wang G, Ji Y, Stuart EA, Paige D, Wang X, Cord and Early Childhood Plasma Adiponectin Levels and Autism Risk: A Prospective Birth Cohort Study, J Autism Dev Disord (2018). [DOI] [PMC free article] [PubMed]
- [32].Olapeju B, Saifuddin A, Wang G, Ji Y, Hong X, Raghavan R, Summers A, Keiser A, Ji H, Zuckerman B, Yarrington C, Hao L, Surkan PJ, Cheng TL, Wang X, Maternal postpartum plasma folate status and preterm birth in a high-risk US population, Public Health Nutr (2018) 1–11. [DOI] [PMC free article] [PubMed]
- [33].Wang G, Hu FB, Mistry KB, Zhang C, Ren F, Huo Y, Paige D, Bartell T, Hong X, Caruso D, Ji Z, Chen Z, Ji Y, Pearson C, Ji H, Zuckerman B, Cheng TL, Wang X, Association Between Maternal Prepregnancy Body Mass Index and Plasma Folate Concentrations With Child Metabolic Health, JAMA pediatrics 170(8) (2016) e160845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Raghavan R, Riley AW, Volk H, Caruso D, Hironaka L, Sices L, Hong X, Wang G, Ji Y, Brucato M, Wahl A, Stivers T, Pearson C, Zuckerman B, Stuart EA, Landa R, Fallin MD, Wang X, Maternal Multivitamin Intake, Plasma Folate and Vitamin B12 Levels and Autism Spectrum Disorder Risk in Offspring, Paediatric and perinatal epidemiology (2017). [DOI] [PMC free article] [PubMed]
- [35].Li M, Fallin MD, Riley A, Landa R, Walker SO, Silverstein M, Caruso D, Pearson C, Kiang S, Dahm JL, Hong X, Wang G, Wang MC, Zuckerman B, Wang X, The Association of Maternal Obesity and Diabetes With Autism and Other Developmental Disabilities, Pediatrics 137(2) (2016) 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Li M, Fallin MD, Riley A, Landa R, Walker SO, Silverstein M, Caruso D, Pearson C, Kiang S, Dahm JL, Hong X, Wang G, Wang MC, Zuckerman B, Wang X, The Association of Maternal Obesity and Diabetes With Autism and Other Developmental Disabilities, Pediatrics 137(2) (2016) e20152206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Raghavan R, Zuckerman B, Hong X, Wang G, Ji Y, Paige D, DiBari J, Zhang C, Fallin MD, Wang X, Fetal and Infancy Growth Pattern, Cord and Early Childhood Plasma Leptin, and Development of Autism Spectrum Disorder in the Boston Birth Cohort, Autism Res (2018). [DOI] [PMC free article] [PubMed]
- [38].Arpino C, Compagnone E, Montanaro ML, Cacciatore D, De Luca A, Cerulli A, Di Girolamo S, Curatolo P, Preterm birth and neurodevelopmental outcome: a review, Childs Nerv Syst 26(9) (2010) 1139–49. [DOI] [PubMed] [Google Scholar]
- [39].Darcy-Mahoney A, Minter B, Higgins M, Guo Y, Williams B, Head Zauche LM, Birth K, Probability of an Autism Diagnosis by Gestational Age(), Newborn Infant Nurs Rev 16(4) (2016) 322–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Gagliardi L, Rusconi F, Da Fre M, Mello G, Carnielli V, Di Lallo D, Macagno F, Miniaci S, Corchia C, Cuttini M, Pregnancy disorders leading to very preterm birth influence neonatal outcomes: results of the population-based ACTION cohort study, Pediatr Res 73(6) (2013) 794–801. [DOI] [PubMed] [Google Scholar]
- [41].Visser JC, Rommelse NN, Greven CU, Buitelaar JK, Autism spectrum disorder and attention-deficit/hyperactivity disorder in early childhood: A review of unique and shared characteristics and developmental antecedents, Neurosci Biobehav Rev 65 (2016) 229–63. [DOI] [PubMed] [Google Scholar]
- [42].Owen MJ, O’Donovan MC, Schizophrenia and the neurodevelopmental continuum:evidence from genomics, World Psychiatry 16(3) (2017) 227–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Straughen JK, Misra DP, Divine G, Shah R, Perez G, VanHorn S, Onbreyt V, Dygulska B, Schmitt R, Lederman S, Narula P, Salafia CM, The association between placental histopathology and autism spectrum disorder, Placenta 57 (2017) 183–188. [DOI] [PubMed] [Google Scholar]
- [44].Suppiej A, Franzoi M, Vedovato S, Marucco A, Chiarelli S, Zanardo V, Neurodevelopmental outcome in preterm histological chorioamnionitis, Early Hum Dev 85(3) (2009) 187–9. [DOI] [PubMed] [Google Scholar]
- [45].Duerden EG, Taylor MJ, Miller SP, Brain development in infants born preterm: looking beyond injury, Semin Pediatr Neurol 20(2) (2013) 65–74. [DOI] [PubMed] [Google Scholar]
- [46].Redline RW, Severe fetal placental vascular lesions in term infants with neurologic impairment, Am J Obstet Gynecol 192(2) (2005) 452–7. [DOI] [PubMed] [Google Scholar]
- [47].Chau V, McFadden DE, Poskitt KJ, Miller SP, Chorioamnionitis in the pathogenesis of brain injury in preterm infants, Clin Perinatol 41(1) (2014) 83–103. [DOI] [PubMed] [Google Scholar]
- [48].Huleihel M, Golan H, Hallak M, Intrauterine infection/inflammation during pregnancy and offspring brain damages: possible mechanisms involved, Reprod Biol Endocrinol 2 (2004) 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Burd I, Balakrishnan B, Kannan S, Models of fetal brain injury, intrauterine inflammation, and preterm birth, Am J Reprod Immunol 67(4) (2012) 287–94. [DOI] [PubMed] [Google Scholar]
- [50].Wu YW, Colford JM Jr., Chorioamnionitis as a risk factor for cerebral palsy: A meta-analysis, JAMA 284(11) (2000) 1417–24. [DOI] [PubMed] [Google Scholar]
- [51].Dammann O, Kuban KC, Leviton A, Perinatal infection, fetal inflammatory response, white matter damage, and cognitive limitations in children born preterm, Ment Retard Dev Disabil Res Rev 8(1) (2002) 46–50. [DOI] [PubMed] [Google Scholar]
- [52].Malaeb S, Dammann O, Fetal inflammatory response and brain injury in the preterm newborn, J Child Neurol 24(9) (2009) 1119–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].O’Shea TM, Downey LC, Kuban KK, Extreme prematurity and attention deficit: epidemiology and prevention, Front Hum Neurosci 7 (2013) 578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Nagel BJ, Bathula D, Herting M, Schmitt C, Kroenke CD, Fair D, Nigg JT, Altered white matter microstructure in children with attention-deficit/hyperactivity disorder, J Am Acad Child Adolesc Psychiatry 50(3) (2011) 283–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Silk TJ, Vance A, Rinehart N, Bradshaw JL, Cunnington R, White-matter abnormalities in attention deficit hyperactivity disorder: a diffusion tensor imaging study, Hum Brain Mapp 30(9) (2009) 2757–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Yamagata B, Itahashi T, Nakamura M, Mimura M, Hashimoto RI, Kato N, Aoki Y, White matter endophenotypes and correlates for the clinical diagnosis of autism spectrum disorder, Soc Cogn Affect Neurosci 13(7) (2018) 765–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Ameis SH, Catani M, Altered white matter connectivity as a neural substrate for social impairment in Autism Spectrum Disorder, Cortex 62 (2015) 158–81. [DOI] [PubMed] [Google Scholar]
- [58].Aoki Y, Yoncheva YN, Chen B, Nath T, Sharp D, Lazar M, Velasco P, Milham MP, Di Martino A, Association of White Matter Structure With Autism Spectrum Disorder and Attention-Deficit/Hyperactivity Disorder, JAMA Psychiatry 74(11) (2017) 1120–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Behnia F, Parets SE, Kechichian T, Yin H, Dutta EH, Saade GR, Smith AK, Menon R, Fetal DNA methylation of autism spectrum disorders candidate genes: association with spontaneous preterm birth, Am J Obstet Gynecol 212(4) (2015) 533 e1–9. [DOI] [PubMed] [Google Scholar]
- [60].Conti N, Torricelli M, Voltolini C, Vannuccini S, Clifton VL, Bloise E, Petraglia F, Term histologic chorioamnionitis: a heterogeneous condition, Eur J Obstet Gynecol Reprod Biol 188 (2015) 34–8. [DOI] [PubMed] [Google Scholar]
- [61].Torricelli M, Voltolini C, Toti P, Vellucci FL, Conti N, Cannoni A, Moncini I, Occhini R, Severi FM, Petraglia F, Histologic chorioamnionitis: different histologic features at different gestational ages, J Matern Fetal Neonatal Med 27(9) (2014) 910–3. [DOI] [PubMed] [Google Scholar]
- [62].Chang JM, Zeng H, Han R, Chang YM, Shah R, Salafia CM, Newschaffer C, Miller RK, Katzman P, Moye J, Fallin M, Walker CK, Croen L, Autism risk classification using placental chorionic surface vascular network features, BMC Med Inform Decis Mak 17(1) (2017) 162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Redline RW, O’Riordan MA, Placental lesions associated with cerebral palsy and neurologic impairment following term birth, Arch Pathol Lab Med 124(12) (2000) 1785–91. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


