Abstract
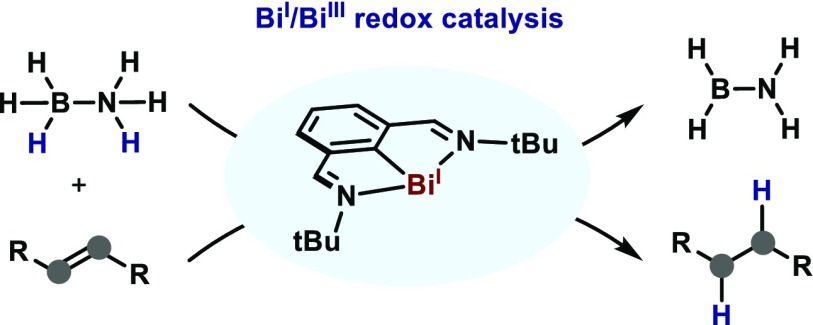
A catalytic transfer-hydrogenation utilizing a well-defined Bi(I) complex as catalyst and ammonia-borane as transfer agent has been developed. This transformation represents a unique example of low-valent pnictogen catalysis cycling between oxidation states I and III, and proved useful for the hydrogenation of azoarenes and the partial reduction of nitroarenes. Interestingly, the bismuthinidene catalyst performs well in the presence of low-valent transition-metal sensitive functional groups and presents orthogonal reactivity compared to analogous phosphorus-based catalysis. Mechanistic investigations suggest the intermediacy of an elusive bismuthine species, which is proposed to be responsible for the hydrogenation and the formation of hydrogen.
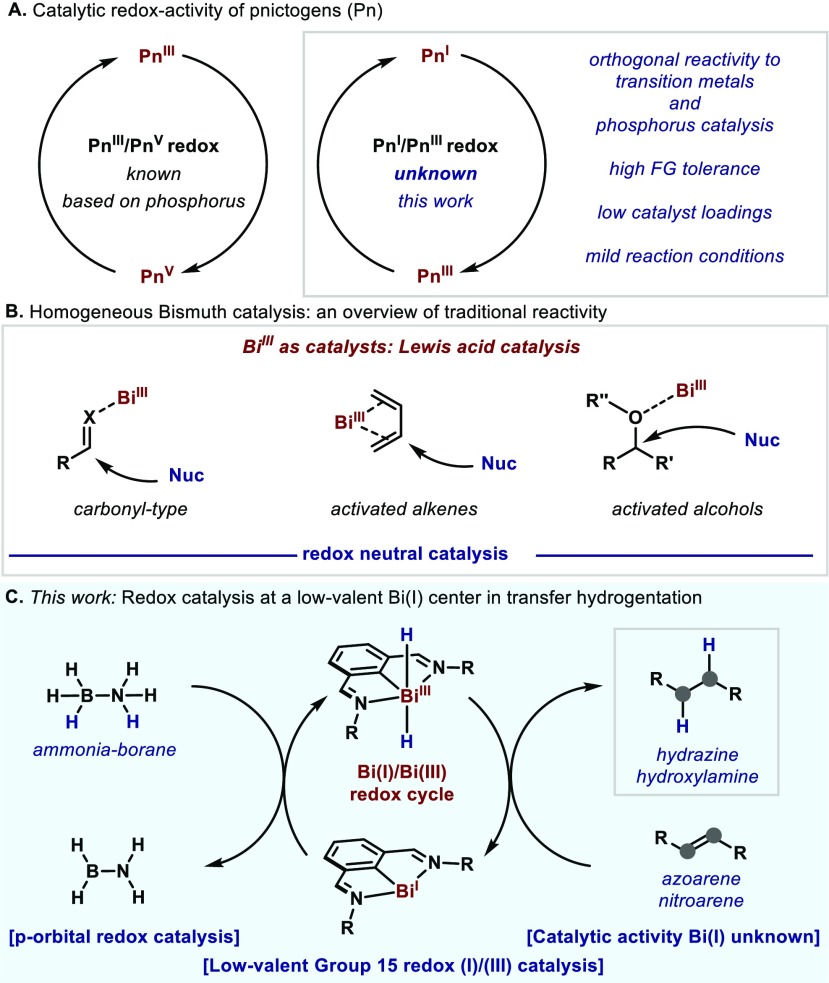
For over half a century, the use of noble metal catalysis has revolutionized the way chemists assemble molecules via the construction of new bonds.1 The enormous impact of these metals at industrial level has led to their exploitation, becoming less abundant and hence more expensive. In recent years, an increasing attention has been placed in unlocking the potential of more abundant first-row transition metals thus becoming powerful sustainable alternatives.2 In parallel, approaches that depart from transition metals have also gained momentum; for example the use of alkali and alkaline,3 main group,4 and the use of Frustrated Lewis Pairs,5 have become promising alternatives to transition metals (TM) thus emulating their behavior and reactivity. However, despite the wealth of literature in these areas, the quest for conferring TM-like catalytic properties to main group elements is still in its infancy.6 In this regard, the ability of pnictogens to maneuver between distinct oxidation states represents a promising approach. Recently, Radosevich reported a variety of P(III)/P(V) redox platforms, which show catalytic activity toward a variety of transformations (Figure 1A).7 This reactivity is based on highly strained P(III) compounds, where the lone pair becomes easily oxidizable and thus more prone to nucleophilic attacks and formal oxidative additions.8 Such a groundbreaking approach opened the door to the possibility of performing catalytic redox processes beyond the TM block. With the aim of investigating unconventional catalytic redox processes of nontransition metals, we have recently started a program which focuses on the exploitation of the redox abilities of bismuth (Bi) to be applied in organic synthesis. Bi represents the last stable element in the periodic table,9 with properties at the interface of metalloids and main group.10 Importantly, Bi has been considered nontoxic and largely more abundant than commonly employed TM such as Pd, Rh or Ir,11 thus highlighting its potential toward developing truly sustainable catalytic strategies. Yet, the use of bismuth in organic synthesis has been largely dominated by stoichiometric reactions based on Bi(V) or Bi(III) species, and catalytic strategies primarily focused on the soft Lewis-acid properties of Bi(III) salts (Figure 1B).12 In addition, methods beyond the classic reactivity of Bi salts have recently attracted increasing attention.13 In contrast to the wealth of methods using high-valent Bi species,14 attention to its low-valent counterparts has been scarce. Low-valent Bi(I) compounds are known in the literature,15 yet seldom monomeric Bi species have been isolated.16 Generally, the formation of Bi(I) compounds is achieved through a highly unstable Bi(III) dihydride, which rapidly extrudes H2 upon ligand coupling.16,17 Inspired by these results, herein we present a transfer hydrogenation of azoarenes and nitro compounds with ammonia-borane (AB) catalyzed by a well-defined and stable Bi(I) complex. Preliminary mechanistic investigations point out at a catalytic platform involving extremely reactive Bi(III) hydride intermediates (Figure 1C).18 To the best of our knowledge, this is the first example of a catalytic redox cycle in the pnictogens group maneuvering in a Pn(I)/Pn(III) redox cycle.
Figure 1.
(A) Catalytic redox-activity of pnictogens. (B) Typical reactivity of Bi(III) in catalysis. (C) Bi(I)/Bi(III) redox catalysis.
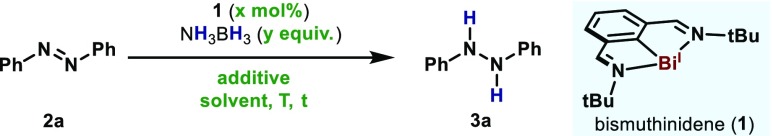
Initially, we attempted the transfer hydrogenation using a bismuthinidene complex (1)16b as catalyst in THF at 50 °C (Table 1, entry 1). To our delight, 2a was completely converted to 3a using 1.0 equiv of AB as reducing agent. When the catalyst loading and the reaction time were reduced, lower yields were obtained (entry 2), but the addition of 2.0 equiv of AB using 1 mol % of 1 resulted in good yields of 3a (entry 3). Yields were substantially diminished at lower temperatures (entry 4), but the reaction performed well in halogenated solvents (entry 5). Such reactivity is in stark contrast to the high reactivity of low-valent TM, which react with halogenated compounds leading to catalyst deactivation or decomposition. The use of other polar solvents (entries 6 and 7) was not beneficial, but noticeably, the addition of 1.0 equiv of H2O improved the yield and reduced reaction times (entry 8). Furthermore, the reaction did not proceed in absence of catalyst (entry 9) or AB complex (entry 10).
Table 1. Optimization of the Bi(I)-Catalyzed Transfer Hydrogenation.
| entry | X | Y | solvent | T (°C) | t (h) | 3a, yield (%)a |
|---|---|---|---|---|---|---|
| 1 | 4 | 1 | THF | 50 | 24 | 99 |
| 2 | 1 | 1 | THF | 50 | 16 | 57 |
| 3 | 1 | 2 | THF | 50 | 16 | 86 |
| 4 | 1 | 2 | THF | 35 | 16 | 53 |
| 5 | 1 | 2 | DCE | 50 | 16 | 76 |
| 6 | 1 | 2 | 1,4-dioxane | 50 | 16 | 87 |
| 7 | 1 | 2 | acetone | 50 | 16 | 45 |
| 8b | 1 | 1 | THF | 35 | 3 | 99 (99)c |
| 9b | – | 1 | THF | 35 | 16 | traces |
| 10b | 1 | – | THF | 35 | 16 | traces |
Yield calculated by 1H NMR using 1,3,5-trimethoxybenzene as internal standard.
With 1.0 equiv of H2O.
Isolated yield.
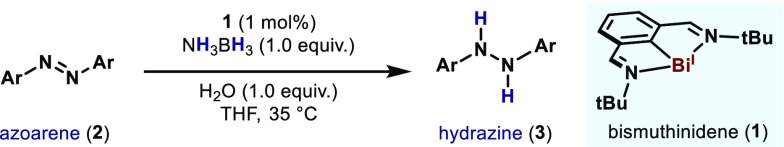
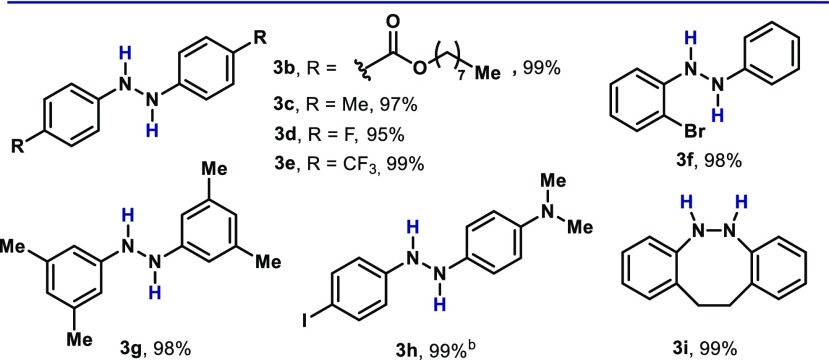
With these optimal reaction conditions in hand, we explored the influence of different substituents in the azoarene (2). As shown in Table 2, the protocol boded well with substrates bearing ester groups, without reduction of the carbonyl moiety (3b). Electron-rich azoarenes can also be reduced (3c), as well as substrates containing electron-withdrawing functionalities, such as fluoride (3d) and trifluoromethyl (3e). The presence of a bromide group at the ortho position of the reactive functionality did not inhibit the reactivity (3f). Azoarenes bearing substituents at the meta position also reacted smoothly under the optimized conditions (3g). Interestingly, unsymmetrical azoarenes in a push–pull electronic situation were also tolerated (3h). It is noteworthy that the presence of the iodide group did not affect the reaction outcome, further indicating the stability of 1 toward oxidative additions to labile bonds. Although cyclic compounds can also be completely reduced (3i), aromatic azoarenes has proven.19
Table 2. Scope of the Transfer Hydrogenation of Azoarenesa.
Isolated yields.
Yield calculated by 1H NMR using 1,3,5-trimethoxybenzene as internal standard.
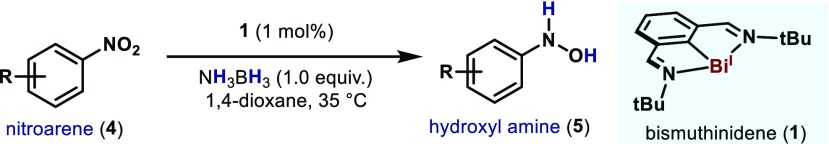
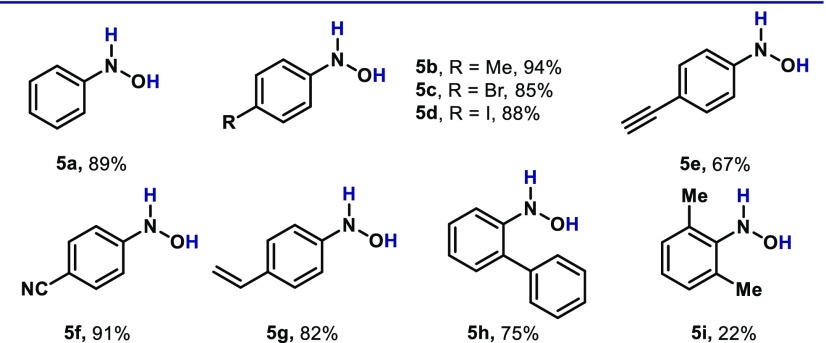
At this point, we decided to expand the protocol to other unsaturated functionalities such as nitroarenes (4, Table 3). Contrarily to the majority of TM-catalyzed reductions, this protocol is highly selective toward the formation of N-arylhydroxylamines.20 With a slight modification from the optimized protocol,21 a variety of electronically distinct nitroarenes could be reduced in excellent yields. For example, simple nitrobenzene was reduced to N-phenylhydroxylamine (5a) in 89% yield. Electron-rich nitroarenes are also amenable to this reactivity (5b), as well as substrates containing carbon–halogen bonds such as bromide (5c) and iodide (5d). Compounds bearing unsaturated functionalities such as alkyne (5e), nitrile (5f) and alkene (5g) were also obtained in excellent yields. Interestingly, when 2-phenylnitrobenzene (4h) was subjected to the reaction conditions, excellent yields were obtained of the corresponding N-hydroxylamine (5h). This result is complementary to P(III) catalysis, with which intramolecular Cadogan-type reactions en route to carbazole have been observed.7i Finally, sterically congested nitroarenes delivered the corresponding N-hydroxylamines (5i) albeit in lower yields.
Table 3. Scope of the Transfer Hydrogenation of Nitroarenesa.
Isolated yields.
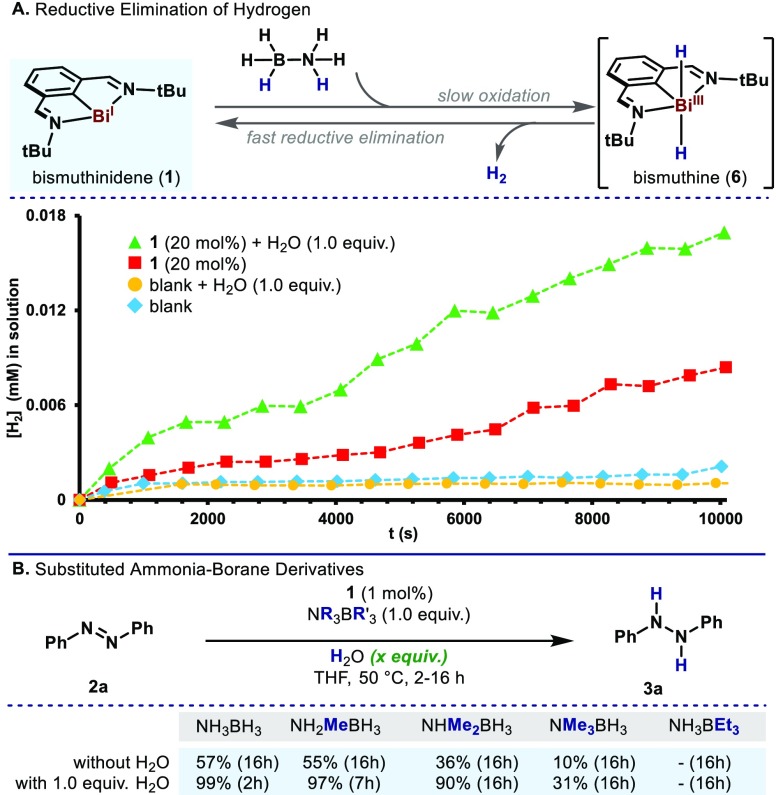
The unprecedented catalytic activity of such Bi(I) complexes led us to explore the operative mechanism governing this homogeneous transformation (Scheme 1).22 First, the ability of 1 for catalytic dehydrogenation of AB was tested. Thus, 1.0 equiv of AB was mixed with 0.2 equiv of Bi(I) complex in THF-d8 and the formation of H2 in solution was monitored (Scheme 1A, red). In absence of Bi(I) complex, no H2 was observed after 150 min,23 indicating that Bi(I) promotes a slow dehydrogenation of AB (Scheme 1A, blue and yellow). Furthermore, during our optimization studies we noticed a dramatic change in rate when water was added (Table 1, entry 8). With this result in mind, we tested the effect of H2O in the dehydrogenation of AB catalyzed by Bi(I). Indeed, the addition of 1.0 equiv of H2O caused an increase in rate for the formation of H2 (Scheme 1A, green). On the basis of Dostál observations,16 we speculated that the formation of H2 is derived from a highly unstable bismuthine (6).24 The positive effect of H2O is proposed to arise from H-bonding from AB and water, thus facilitating a plausible oxidation of 1 to 6, which upon rapid H2 extrusion regenerates species 1. Indeed, to further evaluate the effect of H2O in the reaction, a series of experiments with alkylated derivatives of AB were carried out (Scheme 1B). When the reaction was performed with NMe3BH3 as reducing agent, only a 10% of 3a was obtained after 16 h. Moreover, when NH2MeBH3 and NHMe2BH3 complexes were employed, a 55% and 36% of 3a was obtained, respectively. These results clearly indicate protons of AB play a key role in the transfer hydrogenation. Subsequently, the same reactions were performed in the presence of 1.0 equiv of H2O. Interestingly, with NMe3BH3 31% of 3a was observed, improving the yield of the anhydrous reaction. With NHMe2BH3 and 1.0 equiv H2O the yield was improved to 90% after 16 h, and with NH2MeBH3 complex the reaction time was dramatically reduced to fully convert 2a to 3a. These results support the experimental observations in Scheme 1A. As control, the reaction in the presence of NH3BEt3 did not lead to conversion of the starting material even after 16 h, which indicates the relevance of the hydride source of AB.
Scheme 1. (A) Dehydrogenation of AB with Bi(I) and (B) Transfer Hydrogenation Using Different Amine Borane Complexes.
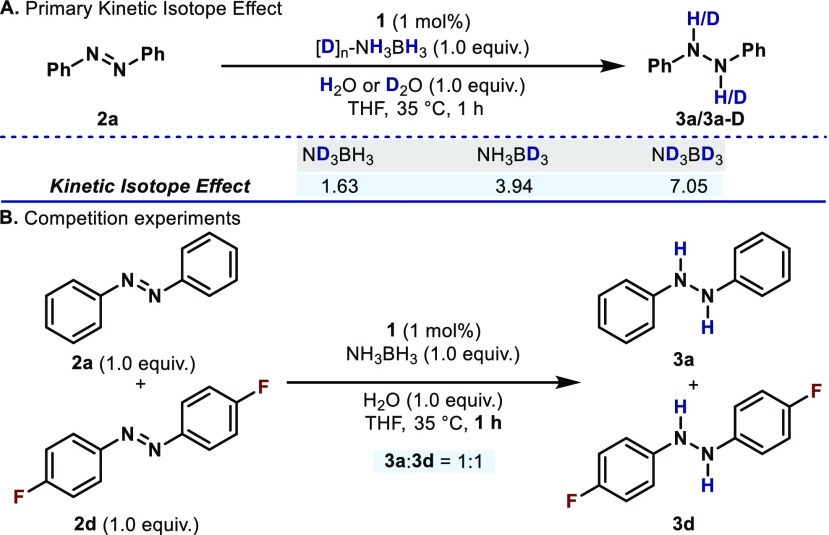
To further evaluate the formal oxidation en route to bismuthine 6, primary kinetic isotope effects were measured using deuterium labeled AB (Scheme 2A). When the reaction was performed with 1.0 equiv ND3BH3 and 1.0 equiv of D2O (rapid D-exchange between H2O and ND3BH3 would result in misleading KIE values) a primary KIE value of 1.63 was obtained. With 1.0 equiv of labeled NH3BD3, a higher KIE value of 3.94 is observed. Finally, when the reaction was performed with 1.0 equiv ND3BD3, a large KIE value of 7.05 was obtained. These results suggest a mechanistic scenario in which both N–H and B–H bonds are broken in the rate-determining step (RDS).25 Furthermore, a competition experiment between two-electronically distinct azoarenes was performed (Scheme 2B). When 1.0 equiv of 2a and 1.0 equiv of 2d were mixed with 1.0 equiv of AB in the presence of 1 mol % of 1, a 1:1 mixture of 3a and 3d was obtained after 1 h, suggesting that azoarenes are not participating in the RDS of this transformation.26 These experiments point out to an scenario in which Bi(I) and AB are both involved in the RDS. However, the rate acceleration observed when H2O is present in the system suggests that H2O might interact with AB through H-bonding, and also participate in the RDS.27
Scheme 2. (A) Kinetic Isotope Effect Experiments; (B) Competition Experiment between Two Electronically Different Azoarenes.
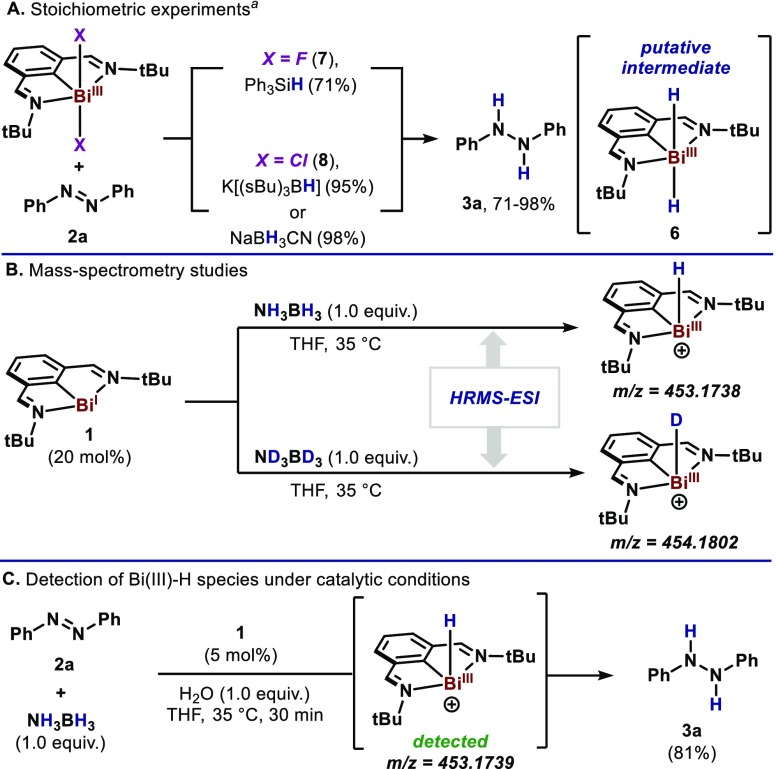
Bi(III)–H compounds are known to be highly unstable and reactive, which complicates their characterization as potential intermediates.16a However, a series of experiments were designed to elucidate the presence of such elusive species (Scheme 3A). Hence, when the reaction was performed with fluorobismuthine 7 and Ph3SiH, 3a was obtained in 71% yield. Equally, when 8 was mixed with 2.0 equiv K-Selectride or NaBH3CN, 3a was obtained in 95% and 98% yield respectively, together with Bi(I),19 indicating that both H in 3a derive from the hydridic sources. Importantly, all these reactions afforded Bi(I) (1) and H2 when no azobenzene was present in the mixture.16 While efforts to detect these intermediates by NMR spectroscopy were unsuccessful, we decided to investigate the dehydrogenation of AB by HRMS techniques. Indeed, when 1 is mixed with 5.0 equiv of AB, a peak corresponding to [6–H]+ (C16H24BiN2+, experimental: m/z = 453.1738; simulated: m/z = 453.1737) was observed (Scheme 3B), thus suggesting the formation of Bi(III)-hydride species.28 Similarly, when AB was replaced by its deuterated analog, a mass for the Bi(III) deuteride was detected (C16H23DBiN2+, experimental: m/z = 454.1802; simulated: m/z = 454.1801. Additionally, the same Bi(III) hydride species was detected when the transfer hydrogenation of 2a was performed under catalytic conditions and analyzed by HRMS (Scheme 3C). Taken together, these results indicate formation of Bi(III) hydrides in the dehydrogenation of AB,16 which further react in the presence of azoarene 2a to obtain 3a. Although different scenarios could be foreseen from such hydridic intermediate, further computational and spectroscopic evidence is needed to fully understand its role in the formation of 3a. Indeed, these studies are now being pursued in our laboratory.
Scheme 3. (A) Stoichiometric and (B and C) Mass Spectrometry Studies.
2.0 equiv of reducing agent.
In conclusion, this work demonstrates the capacity of bismuth compounds to be engaged in catalytic redox transformations. The described protocol, which is a unique example of Bi(I) catalysis, resulted useful for the transfer hydrogenation of azoarenes and nitroarenes with AB as hydrogen surrogate. Preliminary mechanistic investigations suggest the intermediacy of highly reactive and elusive Bi(III) hydrides. These results constitute a unique proof-of-concept of a pnictogen operating between oxidation states I and III to mimic transformations typically performed by TM-catalysts.
Acknowledgments
Financial support for this work was provided by Max-Planck-Gesellschaft, Max-Planck-Institut für Kohlenforschung and Fonds der Chemischen Industrie (FCI-VCI). We thank Prof. Dr. A. Fürstner for insightful discussions and generous support. O.P. thanks the Alexander von Humboldt foundation for a postdoctoral research fellowship.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/jacs.9b00594.
Experimental procedures and analytical data (1H and 13C NMR, HRMS) for all new compounds (PDF)
Author Contributions
† F.W. and O.P. contributed equally.
The authors declare no competing financial interest.
Supplementary Material
References
- a Hartwig J. F.Organotransition metal chemistry: From bonding to catalysis; University Science Books: Mill Valley, CA, 2010. [Google Scholar]; b Crabtree R. H.The Organometallic Chemistry of the Transition Metals; John Wiley & Sons: Hoboken, NJ, 2005. [Google Scholar]; c de Meijere A.; Diederich F.. Metal-Catalyzed Cross-Coupling Reactions; WILEY-VCH Verlag GmbH & Co. KGaA: Mörlenbach, Germany, 2004. [Google Scholar]
- a Zweig J. E.; Kim D. E.; Newhouse T. R. Methods Utilizing First-Row Transition Metals in Natural Product Total Synthesis. Chem. Rev. 2017, 117, 11680–11752. 10.1021/acs.chemrev.6b00833. [DOI] [PubMed] [Google Scholar]; b Su B.; Cao Z.-C.; Shi Z.-J. Exploration of Earth-Abundant Transition Metals (Fe, Co, and Ni) as Catalysts in Unreactive Chemical Bond Activations. Acc. Chem. Res. 2015, 48, 886–896. 10.1021/ar500345f. [DOI] [PubMed] [Google Scholar]
- a Bauer H.; Alonso M.; Färber C.; Elsen H.; Pahl J.; Causero A.; Ballmann G.; De Proft F.; Harder S. Imine hydrogenation with simple alkaline earth metal catalysts. Nat. Catal. 2018, 1, 40–47. 10.1038/s41929-017-0006-0. [DOI] [Google Scholar]; b Hill M. S.; Liptrot D. J.; Weetman C. Alkaline earths as main group reagents in molecular catalysis. Chem. Soc. Rev. 2016, 45, 972–988. 10.1039/C5CS00880H. [DOI] [PubMed] [Google Scholar]; c Kobayashi S.; Yamashita Y. Alkaline Earth Metal Catalysts for Asymmetric Reactions. Acc. Chem. Res. 2011, 44, 58–71. 10.1021/ar100101b. [DOI] [PubMed] [Google Scholar]
- a Weetman C.; Inoue S. The Road Travelled: After Main-Group Elements as Transition Metals. ChemCatChem 2018, 10, 4213–4228. 10.1002/cctc.201800963. [DOI] [Google Scholar]; b Chu T.; Nikonov G. I. Oxidative Addition and Reductive Elimination at Main-Group Element Centers. Chem. Rev. 2018, 118, 3608–3680. 10.1021/acs.chemrev.7b00572. [DOI] [PubMed] [Google Scholar]; c Yadav S.; Saha S.; Sen S. S. Compounds with Low-Valent p-Block Elements for Small Molecule Activation and Catalysis. ChemCatChem 2016, 8, 486–501. 10.1002/cctc.201501015. [DOI] [Google Scholar]; d Raţ C. I.; Soran A.; Varga R. A.; Silvestru C. C—H Bond Activation Mediated by Inorganic and Organometallic Compounds of Main Group Metals. Adv. Organomet. Chem. 2018, 70, 233–311. 10.1016/bs.adomc.2018.07.003. [DOI] [Google Scholar]; e Frey G. D.; Lavallo V.; Donnadieu B.; Schoeller W. W.; Bertrand Facile Splitting of Hydrogen and Ammonia by Nucelophilic Activation at a Single Carbon Center. Science 2007, 316, 439. 10.1126/science.1141474. [DOI] [PubMed] [Google Scholar]
- a Hounjet L. J.; Stephan D. W. Hydrogenation by Frustrated Lewis Pairs: Main Group Alternatives to Transition Metal Catalysts?. Org. Process Res. Dev. 2014, 18, 385–391. 10.1021/op400315m. [DOI] [Google Scholar]; b Stephan D. W. Frustrated Lewis Pairs: From Concept to Catalysis. Acc. Chem. Res. 2015, 48, 306–316. 10.1021/ar500375j. [DOI] [PubMed] [Google Scholar]; c Stephan D. W.; Erker G. Frustrated Lewis Pair Chemistry: Development and Perspectives. Angew. Chem., Int. Ed. 2015, 54, 6400–6441. 10.1002/anie.201409800. [DOI] [PubMed] [Google Scholar]
- a Power P. P. Main Group Elements as Transition Metals. Nature 2010, 463, 171–177. 10.1038/nature08634. [DOI] [PubMed] [Google Scholar]; b Melen R. L. Frontiers in Molecular p-block chemistry: From Structure to Reactivity. Science 2019, 363, 479–484. 10.1126/science.aau5105. [DOI] [PubMed] [Google Scholar]
- a Dunn N. L.; Ha M.; Radosevich A. T. Main Group Redox Catalysis: Reversible PIII/PV Redox Cycling at a Phosphorus Platform. J. Am. Chem. Soc. 2012, 134, 11330–11333. 10.1021/ja302963p. [DOI] [PubMed] [Google Scholar]; b McCarthy S. M.; Lin Y.-C.; Devarajan D.; Chang J. W.; Yennawar H. P.; Rioux R. M.; Ess D. H.; Radosevich A. T. Intermolecular N—H Oxidative Addition of Ammonia, Alkylamines, and Arylamines to a Planar σ3-Phosphorus Compound via an Entropy-Controlled Electrophilic Mechanism. J. Am. Chem. Soc. 2014, 136, 4640–4650. 10.1021/ja412469e. [DOI] [PubMed] [Google Scholar]; c Zhao W.; McCarthy S. M.; Lai T. Y.; Yennawar H. P.; Radosevich A. T. Reversible Intermolecular E—H Oxidative Addition to a Geometrically Deformed and Structurally Dynamic Phosphorous Triamide. J. Am. Chem. Soc. 2014, 136, 17634–17644. 10.1021/ja510558d. [DOI] [PubMed] [Google Scholar]; d Lin Y.-C.; Hatzakis E.; McCarthy S. M.; Reichl K. D.; Lai T.-Y.; Yennawar H. P.; Radosevich A. T. P—N Cooperative Borane Activation and Catalytic Hydroboration by a Distorted Phosphorous Triamide Platform. J. Am. Chem. Soc. 2017, 139, 6008–6016. 10.1021/jacs.7b02512. [DOI] [PMC free article] [PubMed] [Google Scholar]; e Nykaza T. V.; Cooper J. C.; Li G.; Mahieu N.; Ramirez A.; Luzung M. R.; Radosevich A. T. Intermolecular Reductive C—N Cross Coupling of Nitroarenes and Boronic Acids by PIII/PV=O Catalysis. J. Am. Chem. Soc. 2018, 140, 15200–15205. 10.1021/jacs.8b10769. [DOI] [PMC free article] [PubMed] [Google Scholar]; f Reichl K. D.; Dunn N. L.; Fastuca N. J.; Radosevich A. T. Biphilic Organophosphorus Catalysis: Regioselective Reductive Transposition of Allylic Bromides via PIII/PV Redox Cycling. J. Am. Chem. Soc. 2015, 137, 5292–5295. 10.1021/jacs.5b01899. [DOI] [PMC free article] [PubMed] [Google Scholar]; g Nykaza T. V.; Harrison T. S.; Ghosh A.; Putnik R. A.; Radosevich A. T. A Biphilic Phosphetane Catalyzes N—N Bond-Forming Cadogan Heterocyclization via PIII/PV=O Redox Cycling. J. Am. Chem. Soc. 2017, 139, 6839–6842. 10.1021/jacs.7b03260. [DOI] [PMC free article] [PubMed] [Google Scholar]; h Zhao W.; Yan P. K.; Radosevich A. T. A Phosphetane Catalyzes Deoxygenative Condensation of α-Keto Esters and Carboxylic Acids via PIII/PV=O Redox Cycling. J. Am. Chem. Soc. 2015, 137, 616–619. 10.1021/ja511889y. [DOI] [PubMed] [Google Scholar]; i Nykaza T. V.; Ramirez A.; Harrison T. S.; Luzung M. R.; Radosevich A. T. Biphilic Organophosphorus-Catalyzed Intramolecular Csp2—H Amination: Evidence for a Nitrenoid in Catalytic Cadogan Cyclizations. J. Am. Chem. Soc. 2018, 140, 3103–3113. 10.1021/jacs.7b13803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arduengo A. J.; Stewart C. A. Low coordinate hypervalent phosphorus. Chem. Rev. 1994, 94, 1215–1237. 10.1021/cr00029a003. [DOI] [Google Scholar]
- de Marcillac P.; Coron N.; Dambier G.; Leblanc J.; Moalic J.-P. Experimental detection of α-particles from the radioactive decay of natural bismuth. Nature 2003, 422, 876–878. 10.1038/nature01541. [DOI] [PubMed] [Google Scholar]
- a Mehring M. From molecules to bismuth oxide-based materials: Potential homo- and heterometallic precursors and model compounds. Coord. Chem. Rev. 2007, 251, 974–1006. 10.1016/j.ccr.2006.06.005. [DOI] [Google Scholar]; b Briand G. G.; Burford N.. Coordination complexes of bismuth(III) involving organic ligands with pnictogen or chalcogen donors. In Adv. Inorg. Chem.; Academic Press: 2000; Vol. 50, pp 285–357. [Google Scholar]
- Mohan R. Green bismuth. Nat. Chem. 2010, 2, 336. 10.1038/nchem.609. [DOI] [PubMed] [Google Scholar]
- a Leonard N. M.; Wieland L. C.; Mohan R. S. Applications of bismuth(III) compounds in organic synthesis. Tetrahedron 2002, 58, 8373–8397. 10.1016/S0040-4020(02)01000-1. [DOI] [Google Scholar]; b Ollevier T.Bismuth-Mediated Organic Reactions; Springer: Berlin, Heidelberg, 2012. [Google Scholar]; c Hua R. Recent Advances in Bismuth-Catalyzed Organic Synthesis. Curr. Org. Synth. 2008, 5, 1–27. 10.2174/157017908783497518. [DOI] [Google Scholar]
- a Collins L. R.; van Gastel M.; Neese F.; Fürstner A. Enhanced Electrophilicity of Heterobimetallic Bi–Rh Paddlewheel Carbene Complexes: A Combined Experimental, Spectroscopic, and Computational Study. J. Am. Chem. Soc. 2018, 140, 13042–13055. 10.1021/jacs.8b08384. [DOI] [PubMed] [Google Scholar]; b Ren Z.; Sunderland T. L.; Tortoreto C.; Yang T.; Berry J. F.; Musaev D. G.; Davies H. M. L. Comparison of Reactivity and Enantioselectivity between Chiral Bimetallic Catalysts: Bismuth–Rhodium- and Dirhodium-Catalyzed Carbene Chemistry. ACS Catal. 2018, 8, 10676–10682. 10.1021/acscatal.8b03054. [DOI] [Google Scholar]
- a Ritschel B.; Poater J.; Dengel H.; Bickelhaupt F. M.; Lichtenberg C. Double C—H Activation of a Masked Cationic Bismuth Amide. Angew. Chem., Int. Ed. 2018, 57, 3825–3829. 10.1002/anie.201712725. [DOI] [PubMed] [Google Scholar]; b Casely I. J.; Ziller J. W.; Fang M.; Furche F.; Evans W. J. Facile Bismuth–Oxygen Bond Cleavage, C—H Activation, and Formation of a Monodentate Carbon-Bound Oxyaryl Dianion, (C6H2tBu2-3,5-O-4)2–. J. Am. Chem. Soc. 2011, 133, 5244–5247. 10.1021/ja201128d. [DOI] [PubMed] [Google Scholar]; c Kindra D. R.; Casely I. J.; Fieser M. E.; Ziller J. W.; Furche F.; Evans W. J. Insertion of CO2 and COS into Bi—C Bonds: Reactivity of a Bismuth NCN Pincer Complex of an Oxyaryl Dianionic Ligand, [2,6-(Me2NCH2)2C6H3]Bi(C6H2tBu2O). J. Am. Chem. Soc. 2013, 135, 7777–7787. 10.1021/ja403133f. [DOI] [PubMed] [Google Scholar]; d Nekoueishahraki B.; Sarish S. P.; Roesky H. W.; Stern D.; Schulzke C.; Stalke D. Addition of Dimethylaminobismuth to Aldehydes, Ketones, Alkenes, and Alkynes. Angew. Chem., Int. Ed. 2009, 48, 4517–4520. 10.1002/anie.200901215. [DOI] [PubMed] [Google Scholar]
- a Lichtenberg C. Well-Defined, Mononuclear BiI and BiII Compounds: Towards Transition-Metal-Like Behavior. Angew. Chem., Int. Ed. 2016, 55, 484–486. 10.1002/anie.201509234. [DOI] [PubMed] [Google Scholar]; b Ellis B. D.; Macdonald C. L. B. Stable compounds containing heavier group 15 elements in the +1 oxidation state. Coord. Chem. Rev. 2007, 251, 936–973. 10.1016/j.ccr.2006.07.007. [DOI] [Google Scholar]
- a Šimon P.; de Proft F.; Jambor R.; Růžička A.; Dostál L. Monomeric Organoantimony(I) and Organobismuth(I) Compounds Stabilized by an NCN Chelating Ligand: Syntheses and Structures. Angew. Chem., Int. Ed. 2010, 49, 5468–5471. 10.1002/anie.201002209. [DOI] [PubMed] [Google Scholar]; b Vránová I.; Alonso M.; Lo R.; Sedlák R.; Jambor R.; Růžička A.; Proft F. D.; Hobza P.; Dostál L. From Dibismuthenes to Three- and Two-Coordinated Bismuthinidenes by Fine Ligand Tuning: Evidence for Aromatic BiC3N Rings through a Combined Experimental and Theoretical Study. Chem. - Eur. J. 2015, 21, 16917–16928. 10.1002/chem.201502724. [DOI] [PubMed] [Google Scholar]; c Vránová I.; Dušková T.; Erben M.; Jambor R.; Růžička A.; Dostál L. Trapping of the N,C,N-chelated organobismuth(I) compound, [2,6-(Me2NCH2)2C6H3]Bi, by its coordination toward selected transition metal fragments. J. Organomet. Chem. 2018, 863, 15–20. 10.1016/j.jorganchem.2018.03.024. [DOI] [Google Scholar]; d Šimon P.; Jambor R.; Růžička A.; Dostál L. Oxidative Addition of Diphenyldichalcogenides PhEEPh (E = S, Se, Te) to Low-Valent CN- and NCN-Chelated Organoantimony and Organobismuth Compounds. Organometallics 2013, 32, 239–248. 10.1021/om3010383. [DOI] [Google Scholar]
- Balázs G.; Breunig H. J.; Lork E. Synthesis and Characterization of R2SbH, R2BiH, and R2Bi–BiR2 [R = (Me3Si)2CH]. Organometallics 2002, 21, 2584–2586. 10.1021/om020202z. [DOI] [Google Scholar]
- a Hardman N. J.; Twamley B.; Power P. P. (2,6-Mes2H3C6)2BiH, a Stable, Molecular Hydride of a Main Group Element of the Sixth Period, and Its Conversion to the Dibismuthene (2,6-Mes2H3C6)BiBi(2,6-Mes2C6H3). Angew. Chem., Int. Ed. 2000, 39, 2771–2773. . [DOI] [PubMed] [Google Scholar]; b Solyntjes S.; Bader J.; Neumann B.; Stammler H.-G.; Ignat’ev N.; Hoge B. Pentafluoroethyl Bismuth Compounds. Chem. - Eur. J. 2017, 23, 1557–1567. 10.1002/chem.201604910. [DOI] [PubMed] [Google Scholar]
- See Supporting Information for details.
- a Kadam H. K.; Tilve S. G. Advancement in methodologies for reduction of nitroarenes. RSC Adv. 2015, 5, 83391–83407. 10.1039/C5RA10076C. [DOI] [Google Scholar]; b Orlandi M.; Brenna D.; Harms R.; Jost S.; Benaglia M. Recent Developments in the Reduction of Aromatic and Aliphatic Nitro Compounds to Amines. Org. Process Res. Dev. 2018, 22, 430–445. 10.1021/acs.oprd.6b00205. [DOI] [Google Scholar]; c Tafesh A. M.; Weiguny J. A Review of the Selective Catalytic Reduction of Aromatic Nitro Compounds into Aromatic Amines, Isocyanates, Carbamates, and Ureas Using CO. Chem. Rev. 1996, 96, 2035–2052. 10.1021/cr950083f. [DOI] [PubMed] [Google Scholar]
- 1,4-Dioxane proved superior for the reduction of nitroarenes. The addition of H2O proved highly detrimental, and large amounts of decomposition were obtained.
- For details on homogeneity tests and the use of radical traps, see Supporting Information.
- Staubitz A.; Robertson A. P. M.; Manners I. Ammonia-Borane and Related Compounds as Dihydrogen Sources. Chem. Rev. 2010, 110, 4079–4124. 10.1021/cr100088b. [DOI] [PubMed] [Google Scholar]
- The structure of species 6 is not clear, but on the basis of previous reports as well as on the structure of chlorobismuthine 8 (see ref (16)), we propose the hydride ligands to be in apical coordination sites as depicted in Scheme 1.
- a Bhattacharya P.; Krause J. A.; Guan H. Mechanistic Studies of Ammonia Borane Dehydrogenation Catalyzed by Iron Pincer Complexes. J. Am. Chem. Soc. 2014, 136, 11153–11161. 10.1021/ja5058423. [DOI] [PubMed] [Google Scholar]; b Chong C. C.; Hirao H.; Kinjo R. A Concerted Transfer Hydrogenolysis: 1,3,2-Diazaphospholene-Catalyzed Hydrogenation of N=N Bond with Ammonia-Borane. Angew. Chem., Int. Ed. 2014, 53, 3342–3346. 10.1002/anie.201400099. [DOI] [PubMed] [Google Scholar]
- No reaction between 1 and 2a or H2O was observed neither in catalytic nor stoichiometric conditions. See Supporting Information for details.
- Although 1 can dehydrogenate AB in the absence of H2O, the presence of 1.0 equiv of H2O accelerates the dehydrogenation step (Scheme 1A). In these cases, interaction of H2O and AB through H-bonding networks is postulated, which results in an effect of H2O in the rate-determining step. For references, see:; a Ingram D. J.; Headen T. F.; Skipper N. T.; Callear S. K.; Billing M.; Sella A. Dihydrogen vs Hydrogen Bonding in the Solvation of Ammonia Borane by Tetrahydrofuran and Liquid Ammonia. Phys. Chem. Chem. Phys. 2018, 20, 12200. 10.1039/C7CP08220G. [DOI] [PubMed] [Google Scholar]; b Belkova N. V.; Epstein L. M.; Filippov O. A.; Shubina E. S. Hydrogen and Dihydrogen Bonds in the Reactions of Metal Hydrides. Chem. Rev. 2016, 116, 8545. 10.1021/acs.chemrev.6b00091. [DOI] [PubMed] [Google Scholar]; c Stephens F. H.; Pons V.; Baker R. T. Ammonia-Borane: the Hydrogen source par excellence?. Dalton Trans. 2007, 2613. 10.1039/B703053C. [DOI] [PubMed] [Google Scholar]
- The same m/z is observed when the reaction of 8 with NaBH3CN is analyzed by HRMS after 4 h reaction time. This result further supports the notion that Bi(III)—H are indeed involved in the transfer hydrogenation.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.