Abstract
Environmental and occupational exposure to industrial chemicals has been linked to toxic and carcinogenic effects in animal models and human studies. However, current toxicology testing does not thoroughly explore the endocrine disrupting effects of industrial chemicals, which may have low dose effects not predicted when determining the limit of toxicity. The objective of this study was to evaluate the endocrine disrupting potential of a broad range of chemicals used in the petrochemical sector. Therefore, 139 chemicals were classified for reproductive toxicity based on the United Nations Globally Harmonized System for hazard classification. These chemicals were evaluated in PubMed for reported endocrine disrupting activity, and their endocrine disrupting potential was estimated by identifying chemicals with active nuclear receptor endpoints publicly available databases. Evaluation of ToxCast data suggested that these chemicals preferentially alter the activity of the estrogen receptor (ER). Four chemicals were prioritized for in vitro testing using the ER-positive, immortalized human uterine Ishikawa cell line and a range of concentrations below the reported limit of toxicity in humans. We found that 2,6-di-tert-butyl-p-cresol (BHT) and diethanolamine (DEA) repressed the basal expression of estrogen-responsive genes PGR, NPPC, and GREB1 in Ishikawa cells, while tetrachloroethylene (PCE) and 2,2’-methyliminodiethanol (MDEA) induced the expression of these genes. Furthermore, low-dose combinations of PCE and MDEA produced additive effects. All four chemicals interfered with estradiol-mediated induction of PGR, NPPC, and GREB1. Molecular docking demonstrated that these chemicals could bind to the ligand binding site of ERα, suggesting the potential for direct stimulatory or inhibitory effects. We found that these chemicals altered rates of proliferation and regulated the expression of cell proliferation associated genes. These findings demonstrate previously unappreciated endocrine disrupting effects and underscore the importance of testing the endocrine disrupting potential of chemicals in the future to better understand their potential to impact public health.
Keywords: endocrine disrupting chemical (EDC), environmental chemical, estrogen receptor, risk assessment, uterus
1. Introduction
The chemicals used in petroleum manufacturing have greatly advanced the abundance of usable petroleum products, but these chemicals simultaneously present direct and indirect environmental dangers as water, air, and soil pollutants if emissions are not well controlled [1]. Petroleum manufacturing is necessary to support the global need for fuel, facilitating the transport of people, food, and goods. Moreover, products derived from the oil and gas sector are critical for the generation of electricity and the production of many commercial materials, including plastics, food preservatives, synthetic consumer products, and several household items. However, the process of refining crude oil into usable products requires the use of many hazardous chemicals, some of which have been shown to be toxic in human studies and animal models [2, 3]. Moreover, the potential for these chemicals to more subtly impact the reproductive system by altering the actions of endocrine hormones is not well understood.
An endocrine disruptor, or endocrine-disrupting chemical (EDC), is an exogenous substance or mixture that alters the function(s) of the endocrine system and consequently causes adverse health effects in an intact organism or its progeny [4]. EDCs are of unique concern for public health due to their demonstrated effects at very low concentrations in animal models [5, 6]. It is noteworthy that epidemiological studies have reported modest or inconsistent results, which likely reflect study limitations, the combined effect of chemical mixtures, and the absence of an unexposed control group [7]. The molecular mechanisms by which chemicals can exert their EDC effects are various and include: mimicking or inhibiting the activity of endogenous hormones, altering the levels of endogenous hormones by changing the rate of their production and metabolism, competing with hormone binding proteins, altering the levels of co-activators, or persistent effects through epigenetic reprogramming.
In the reproductive system, EDCs can disrupt physiology by altering the functions of the hypothalamic-pituitary-gonadal axis (HPG axis), a system of hormone-producing glands that controls reproductive function. Previous studies evaluating the effects of select petrochemicals and the chemicals used in petroleum manufacturing have demonstrated that exposure to these chemicals can negatively impact female reproductive tract functions in humans and animal models [8–12]. Long-term exposure to benzene in mice caused ovarian atrophy with granulosa cell tumors found in mice exposed to the highest dose studied [13]. Elevated levels of benzene in the ovarian follicular fluid from patients undergoing IVF was associated with fewer oocytes retrieved and altered levels of follicle stimulating hormone and estradiol [14]. Exposure to polycyclic aromatic hydrocarbons (PAH), emitted during petroleum manufacturing, resulted in gonadal dysfunction and reduced egg number in fish [15]. In mouse models, early-life exposure to PAHs depleted ovarian germ cell numbers [16]. However, studies exploring the endocrine disrupting potential of chemicals used in petroleum manufacturing on uterine biology are limited.
In addition to classifying the toxic and carcinogenic effects of chemicals, it is important to understand the endocrine disrupting potential of these chemicals and the biological pathways involved. The objectives of this study are to (1) review the current literature reporting the EDC effects of 139 chemicals used in the petrochemical industry, (2) predict the EDC potential of the 139 chemicals by evaluating nuclear receptor endpoints reported in the Endocrine Disruptor Screening Program (EDSP) and ToxCast databases, (3) evaluate the EDC activity of select prioritized chemicals (2,2’-methyliminodiethanol, diethanolamine, tetrachloroethylene, and 2,6-di-tert-butyl-p-cresol) by evaluating changes to endogenous gene expression in a uterine cell line, and (4) visualize the gene networks impacted by those selected chemicals. These results can be used to prioritize chemicals for in vivo confirmation studies. Moreover, the chemicals used in petroleum manufacturing are widely used in other commercial processes, suggesting exposure studies may be applicable to various industrial workers and surrounding communities.
2. Materials & Methods
2.1. Literature Search.
A list of 139 chemicals with reproductive health hazard properties used in petrochemical industry was developed based on several regulatory and advisory lists, including: Malaysia’s Department of Occupational Safety and Health’s Industry Code of Practice (ICOP), Germany’s Substance Database (GESTIS), Japan’s National Institute of Technology and Evaluation (NITE), and the European Chemicals Agency (ECHA) (Supplemental Table 1). The reproductive classification was based on the Globally Harmonized System (GHS) for classification and labeling. Chemicals were classified by category: 1A-known reproductive toxicant, 1B-presumed reproductive toxicant, and 2-suspected reproductive toxicant. ICOP and ECHA provide regulatory list. GESTIS and NITE provide advisory lists. The chemicals selected for this study are primarily used in various work units in the petrochemical sector, including the laboratories, process areas, and wastewater treatment areas. We conducted a literature review in PubMed February 2019 using the web browser Google Chrome and following query: “[chemical name] and ‘endocrine disruptor’” or “[CAS #] and ‘endocrine disruptor’”, notating the total number of published studies. The published studies for each chemical were evaluated for relevance (Supplemental Table 2). A relevant hit was defined as a published study that directly investigated the endocrine disrupting effects of the given chemical rather than include the chemical as part of the experimental design. For example, some studies included the chemical as a solvent, diluent, or did not directly study the effect of the chemical. We utilized the EDSP dashboard to identify chemicals with potential or known adverse endocrine-related effects (Supplemental Table 2) [17, 18]. Like ToxCast, the EDSP utilizes high-throughput assays to screen chemicals for their potential interaction with the estrogen, androgen, and thyroid hormone systems. Chemical interactions with hormone receptors are modeled in the EDSP using ToxCast Model Predictions and Consensus CERAPP QSAR ER Model Predictions [19]. Each chemical was also quired in the ToxCast dashboard, which covers 1,000 high-throughput endpoints for over 9,000 chemicals, for active nuclear receptor endpoints, focusing on nuclear receptors with known functions in the reproductive system: estrogen receptor (ER), androgen receptor (AR), glucocorticoid receptor (GR), and progesterone receptor (PR) (Supplemental Table 2) [20–22]. A subsequent literature search was performed for the chemicals with active nuclear receptor endpoints by the search terms: “[chemical name] and ‘estrogen receptor’” or “[CAS #] and ‘estrogen receptor’”. All relevant hits were noted. Chemicals with reported estrogen receptor activity determined by a variety of reporter assays (receptor binding, agonist/antagonist activity, response element binding) as documented in the ToxCast database and demonstrating a paucity of data related to their EDC effects were prioritized for in vitro experiments. Studies were performed with the following chemicals: 2,2’-methyliminodiethanol (MDEA), diethanolamine (DEA), Tetrachloroethylene (PCE), and 2,6-di-tert-butyl-p-cresol (BHT).
2.2. Reagents.
RPMI-1640 was purchased from Gibco (Thermo Fisher Scientific, Waltham, MA). Fetal bovine serum (FBS) was purchased from Sigma-Aldrich (St. Louis, MO) and stripped (charcoal-dextran treated) FBS was purchased from Gemini Bio-Products (Sacramento, CA). Human TaqMan real-time PCR (RT-PCR) primer-probes were purchased from Applied Biosystems (Thermo Fisher Scientific). Estrone (E1; CAS No. 53-16-7; ≥ 99.0% pure), Estriol (E3; CAS No. 50-27-1; ≥ 97% pure), tetrachloroethylene (PCE; CAS No. 127-18-4; ≥ 99.0% pure), 2,2’-methyliminodiethanol (MDEA; CAS No. 105-59-9; ≥ 99.0% pure), diethanolamine (DEA; CAS No.111-42-2; ≥ 98.0% pure), butylated hydroxytoluene (BHT; CAS No. 128-37-0; ≥ 99.0% pure), and Bisphenol A (BPA; CAS No. 80-05-7; 100% pure) were purchased from Sigma Aldrich. PCE, MDEA, and DEA, were dissolved in methanol. E1 and BHT were dissolved in dimethyl sulfoxide (DMSO). E3 and BPA were dissolved in ethanol. Tamoxifen (CAS No. 10540-29-1; 99.35% pure) was purchased from MP Biomedicals (Solon, OH) and was dissolved in ethanol. ICI 182,780 (CAS No. 129453-61-8; ≥ 99.0%) was purchased from Tocris (Minneapolis, MN) and dissolved in methanol. Estradiol (E2 ; 1,3,5(10)-Estratrien-3,17β-Diol; CAS No. 50-28-2; ≥ 99.0%) was purchased from Steraloids, Inc. (Newport, RI) and dissolved in ethanol. The estrogen receptor alpha (ERα) antibody was purchased from Cell Signaling Technologies (Catalog #8644S; Danvers, MA); the estrogen receptor beta (ER β) antibody was purchased from Thermo Fisher Scientific (Catalog #PA1–310B; Waltham, MA); the β-actin antibody was purchased from Millipore (Catalog #MAB1501; Temecula, MA); and secondary antibodies were purchased from LI-COR Biosciences (Lincoln, NE).
2.3. Cell Culture.
Immortalized human uterine endometrial adenocarcinoma (Ishikawa) cells obtained from ATCC (Manassas, VA) were cultured in RPMI-1640 medium supplemented with 5% heat-inactivated FBS [23]. Ishikawa cells were authenticated by short-tandem repeat analysis utilizing the DNA Analysis Facility at Yale University. The day prior to treatment, media was changed to phenol red-free RPMI-1640 medium supplemented with 5% charcoal-stripped, heat-inactivated FBS. Ishikawa cells were treated with chemicals over a range of doses below the reported limit of toxicity (Figure 2B). As there is no reported exposure limit for MDEA, cells were exposed to MDEA at the same range of doses used for DEA, PCE, and BHT. The dose of estradiol used to treat Ishikawa cells was chosen based on the reported IC50 of estradiol for human ERα [24–26]. Vehicle treatments utilized the same volume of chemical diluent (methanol, dimethyl sulfoxide, or ethanol).
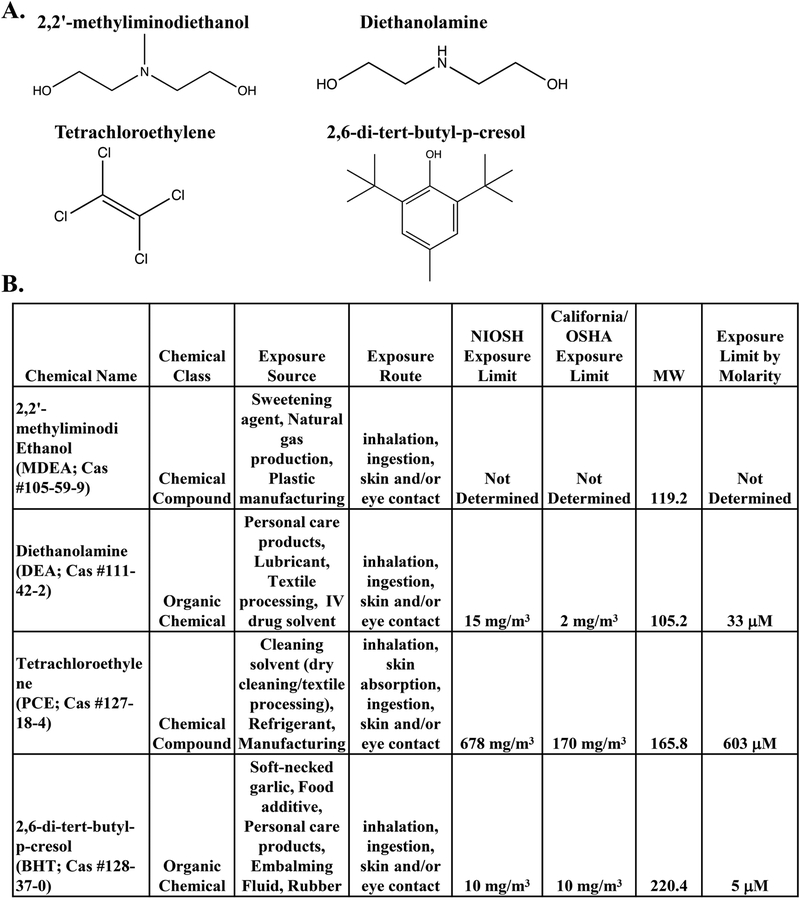
FIGURE 2. Description of the prioritized chemicals: BHT, DEA, PCE, and MDEA.
(A) The chemical structures of the compounds tested in this study: 2,2’-methyliminodiethanol (MDEA), diethanolamine (DEA), tetrachloroethylene (PCE), and 2,6-di-tert-butyl-p-cresol (BHT). (B) Description of the sources, routes, and limits of exposure for MDEA, DEA, PCE, and BHT. Exposure limits are determined as the 8-hr permissible exposure limit with time-weighted average.
2.4. RNA Isolation and Quantitative Real-Time PCR.
Total mRNA was isolated from Ishikawa cells treated for 6 hrs using the Qiagen RNeasy mini kit with RNase-Free DNase treatment on-column according to the manufacturer’s protocol (Qiagen, Valencia, CA). RNA quality and concentration were assessed by spectrophotometry with the NanoDrop One (Thermo Fisher Scientific). RNA was subjected to cut-off values of >1.8 for the A260/A280 ratio and >1.6 for the A260/A230 ratio. Quantitative RT-PCR (qRT-PCR) was performed in a 10 μl reaction with 100 ng RNA using a one-step reaction on the Bio Rad CFX384 thermal cycler or CFX Connect thermal cycler using predesigned primer-probe sets (Supplemental Table 3; Thermo Fisher Scientific). The thermocycling parameters for each reaction were: 48°C for 30 min, 95°C for 10 min, followed by 40 cycles of 95°C for 15 sec and 60°C for 60 sec. The amplification signal from the reference gene peptidylprolyl isomerase B (PPIB), which was unaffected by chemical treatment, was used to normalize the amplification signals from each gene primer-probe. All qRT-PCR analysis was determined from 5 biological replicates, which were each evaluated as technical duplicates.
2.5. Protein Isolation and Western Blotting.
Ishikawa cells were lysed in Tris-glycine sodium dodecyl sulfate (SDS) buffer supplemented with β-mercaptoethanol (BME). The protein concentration of each sample was measured using the Pierce 660 nM protein assay kit (Thermo Fisher Scientific). SDS-polyacrylamide gel electrophoresis was used to separate equal amounts of protein from cell extracts. Separated proteins were transferred to a nitrocellulose membrane, which was blocked with 7.5% non-fat milk in Tris-buffered saline (TBS) for one hr at room temperature then incubated overnight with primary antibodies against ERα (1:1000) or ERβ (1:1000) and β-actin (1:5000) in 5% skim milk in TBS Tween-20 (TBS-T) at 4°C (Supplemental Table 4). The following day, membranes were incubated with fluorescent conjugated secondary antibodies for one hr at room temperature, and the immunoreactivity was visualized on the LI-COR imaging system (LI-COR Biosciences). The observed protein level in each lane was normalized to the level of reference protein β-actin for that sample.
2.6. Cell Proliferation Assay.
Ishikawa cells were plated at an equal density of 2 × 105 per well in a 6-well plate. Once cells adhered (approximately 4 hrs), media was changes to phenol-red free RPMI with charcoal-stripped, heat-inactivated FBS and cells were treated with vehicle, 10 nM estradiol (E2), 100 nM BHT, 100 nM DEA, 100 nM MDEA, or 100 nM PCE. Medium and chemical treatment was replaced every 48 hr. The number of cells per well was counted 48 and 96 hrs later using the Countess II Automated Cell Counter (Thermo Fisher Scientific). Each biological replicate was counted twice, and the results were averaged.
2.7. Gene Networks and Data Visualization with Cytoscape.
Chemical-associated genes were extracted from the Comparative Toxicogenomics Database (CTD), a manually curated knowledge database on the interaction between chemical–gene, chemical–disease and gene–disease [27]. Each resulting gene list was functionally analyzed for overrepresented diseases or functions using Ingenuity Pathway Analysis software (Build 463341M, Version 42012434, Qiagen). The p-value of the overlap was calculated by the right-tail Fisher’s Exact Test. The relationship between chemical-associated genes and enriched diseases and functions was visualized using Cytoscape, an open-source software platform for integration, analysis and visualization of networked data [28].
2.8. Molecular Modeling.
Three ERα structures were considered as receptor configurations for molecular docking: (1) the receptor is in estradiol bound but inactive conformation (pdb ID: 1a52), (2) the receptor is in estradiol and the coactivator peptide src-1 bound agonist conformation, and (3) the receptor is in 4-hydroxytamoxifen bound antagonist conformation. The ligand binding site of proteins were prepared for docking using the Receptor_Setup module (Release 3.2.0.2) with the help of bound ligands (estradiol or 4-hydroxy tamoxifen) while docking studies were carried out using the FRED module (Release 3.2.0.2) of the OpenEye software package (OpenEye Scientific Software, Inc., Santa Fe, NM, U.S.A.; www.eyesopen.com). The scoring was done using the default ChemGauss4 scoring function (of the FRED module) which was based on the shape of the ligand, hydrogen bonding between ligand and receptor, hydrogen bonding interactions with implicit solvent, and metal-chelator interactions. The residues of ERα that interact with endogenous ligands and chemicals were visualized in the ball-and-stick form using the software program Ligplot [29].
2.9. Statistical Analysis.
Data represent the average of at 3–5 biological replicates and are presented as means ± SEM. Statistical significance was determined by one-way analysis of variance with Tukey’s post-hoc analysis using Graph Pad Prism software version 7.0. Significance was determined as *p<0.05 or **p<0.01.
3. Results
3.1. Chemical GHS Classification and Literature Review.
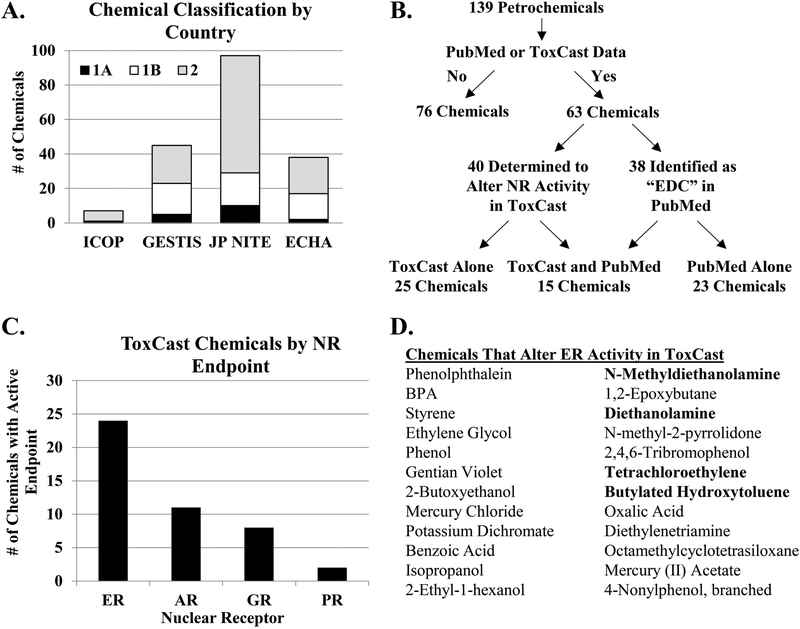
To evaluate the potential impact to human reproduction and development, 139 chemicals used in petroleum manufacturing were classified according to their reproductive toxicity. The GHS system of classification for reproductive toxicity was determined from human and animal data using an evidence weighted, numerical system of 1A, 1B, or 2 based on known, presumed, or suspected effects on reproduction or development, respectively [30]. Chemicals are classified as category 1A based on human data or category 1B based on animal data, while chemicals classified as category 2 have limited human or animal studies. The chemicals were classified by four governmental agencies: ICOP (Malaysia), GESTIS (Germany), JP NITE (Japan), and ECHA (Europe). The JP NITE had the most extensive classification of the 139 chemicals, with 10 classified as 1A, 19 classified as 1B, and 68 classified as 2 (Figure 1A). ICOP, GESTIS, and ECHA classified fewer chemicals overall, and most of the chemicals were ranked category 2 by these agencies. The lack of classification for many of the chemicals suggests an absence of experimental data regarding the potential reproductive toxicity of these environmental chemicals. In fact, our literature search determined that only 38 of the identified 139 chemicals used in petroleum manufacturing had been previously associated with the term “endocrine disruptor” (Figure 1B). We found a total of 1537 articles in PubMed for these 38 chemicals, although >70% of these articles were related to studies with BPA. Furthermore, not all of the associated articles directly tested the EDC activity of that chemical. For example, methanol and toluene had 9 and 3 unique PubMed articles, respectively, linking the chemical or CAS No. to the term “endocrine disruptor”, but no relevant studies that tested these chemicals. In the case of benzene, 13 articles were found by PubMed search but only 1 actually tested the EDC activity [31]. A secondary search of benzene and the term “estrogen receptor” identified 58 articles. Again, most studies utilized benzene during chemical extraction, thereby not directly testing the activity of benzene, or evaluated a chemical derivative that contained the benzene moiety. The paucity of data further establishes the need for EDC testing.
FIGURE 1. Classification of chemicals used in petroleum manufacturing.
(A) Reproductive toxicity classifications of 139 chemicals provided by Petronas according to Malaysia’s Industry Code of Practice (ICOP), Germany’s Substance Database GESTIS, Japan’s National Institute of Technology and Evaluation (JP-NITE), and the European Chemicals Agency (ECHA). (B) Venn diagram illustrates the classification of the chemicals as determined by the PubMed literature search and the nuclear receptor (NR) activity as reported by the ToxCast database. (C) The number of chemicals with active endpoints in assays pertaining to the estrogen receptor (ER), androgen receptor (AR) glucocorticoid receptor (GR), and progesterone receptor (PR) are graphed. (D) Chemicals that alter ER activity based on the ToxCast database are listed. The chemicals in bold print indicate chemicals prioritized for further evaluation.
To determine whether the 139 chemicals have the potential to act as endocrine disruptors, each chemical was evaluated in the EDSP and ToxCast databases for predicted hormone receptor binding and activity in nuclear receptor (NR) assays. We found 96 chemicals present in the EDSP database (Supplemental Table 2). Of these 96 chemicals, 6 had predicted agonist or antagonist activity for ER or AR, 17 chemicals displayed information related to the Consensus CERAPP QSAR ER Model Predictions, and 19 chemicals demonstrated bioactivity in ER and/or AR assays. Although there was some degree of overlap between the information found in the EDSP and ToxCast databases, some chemicals were only found in one database. For example, 35 chemicals were found in the EDSP database but not ToxCast database, and 6 chemicals were found in the ToxCast database but not the EDSP database. Moreover, certain chemicals were found to have bioactive ER endpoints in the EDSP database but not the ToxCast database (e.g. CAS Nos. 112-30-1 and 110-;85-0), suggesting the importance of evaluating bioactivity in multiple databases. The search of the ToxCast database revealed that 40 of the 139 chemicals altered the activity of the nuclear receptors in reporter assays (Figure 1B). Further analysis of these assays indicated that ER was the most commonly affected nuclear receptor (Figure 1C). We found that 24 of the 40 chemicals altered the activity of ER, as reported by ToxCast (Figure 1D). Due to the predominance of reported ER activity with the chemicals evaluated, we performed an additional literature search in PubMed for the 24 chemicals identified to alter ER activity and the term “estrogen receptor.” We identified 4 chemicals that had limited published data related to their EDC and estrogen receptor activity (0–4 relevant published studies) and prioritized these chemicals for in vitro experimentation. The four chemicals chosen for experimentation were 2,2’-methyliminodiethanol (MDEA), diethanolamine (DEA), tetrachloroethylene (PCE), and 2,6-di-tert-butyl-p-cresol (BHT), and the chemical structures of the compounds used in this study are shown in Figure 2A. In addition to their use in petroleum manufacturing, these chemicals are found in food additives, personal care products, natural gas production, plastic manufacturing, textile processing, dry cleaning reagents, embalming fluid, and other manufacturing processes. The exposure source, route, and limit were determined for each chemical (Figure 2B). The routes of exposure include inhalation, ingestion, and skin or eye contact. The 8-hr permissible exposure limit with time-weighted average as determined by the National Institute for Occupational Safety and Health (NIOSH) and the State of California’s Occupational Safety and Health Administration are listed. NIOSH exposure limits were converted to molarity and are listed.
3.2. In vitro exposure of selected chemicals and expression of endogenous estrogen-responsive genes.
The four prioritized chemicals demonstrated activity at high concentrations in ER bioassays found in the ToxCast database and/or work by others [32]. These studies provide information on their potential estrogenic activity on reporter constructs transfected into kidney and ovarian cell types. Because these reporter assays limit the biological complexity that can be incorporated into the assay readout, we utilized the ER-positive Ishikawa cells treated with vehicle (0 nM), reference ER agonists and antagonists, and the chemicals BHT, DEA, PCE, or MDEA at concentrations of 1, 10, 100, and 1000 nM for 6 hrs to measure endogenous gene regulation [32, 33]. Ishikawa cells are ideal for measuring the estrogenic response of xenoestrogens and have been proposed as a replacement for the classic uterotrophic in vivo assay [34]. Moreover, Ishikawa cells are derived from the human uterine endometrium, a cell-type not traditionally included in toxicity testing. Isolated mRNA from treated Ishikawa cells was evaluated by qRT-PCR for the expression of three classic estrogen-responsive genes: the progesterone receptor (PGR), growth regulation by estrogen in breast cancer 1 (GREB1), and natriuretic peptide precursor C (NPPC), which are reported to be regulated by estradiol in Ishikawa cells [35–37]. In agreement with previous reports, estradiol (E2) and the endogenous estrogens, estrone (E1), and estriol (E3), induced the expression of all three genes at the concentrations employed compared to vehicle control (Figure 3A). Maximal induction of target gene mRNA by the endogenous estrogens was achieved at the 100 nM concentration, whereas increasing the treatment concentration to1000 nM did not further augment transcript levels. However, the maximal induction of gene transcripts in response to BPA was achieved at 1000 nM, although transcript levels of PGR, GREB1, and NPPC were lower following BPA exposure compared to estradiol at comparable concentrations. BPA is a weaker estrogen compared to estradiol, so it was expected that maximal induction would be achieved at a higher concentration [38, 39]. The selective estrogen receptor modulator tamoxifen, which acts as a pure antagonist in the breast but not in the endometrium, significantly induced gene expression at the highest concentration [40]. These findings are in agreement with others that have shown that 1000 nM tamoxifen can induce estrogen-responsive genes in the human endometrial Ishikawa cell line [35]. The pure antiestrogen ICI 182, 780 did not induce the expression of any of the estrogen-responsive genes, instead selectively lowering the relative mRNA expression at concentrations of 1, 10, and 100 nM [41].
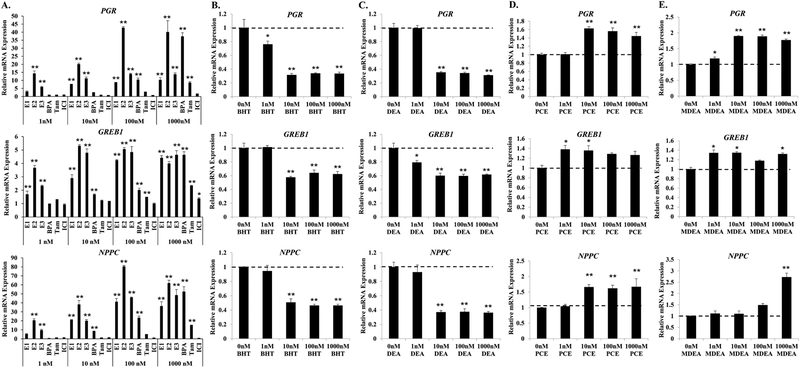
FIGURE 3. BHT, DEA, PCE, and MDEA exposure and transcript levels of endogenous estrogen-responsive genes in Ishikawa cells.
Transcript levels of the progesterone receptor (PGR), growth regulation by estrogen in breast cancer 1 (GREB1), and natriuretic peptide C (NPPC) mRNA were evaluated by qRT-PCR in Ishikawa cells treated for 6 hr with vehicle (0 nM) or 1, 10, 100, or 1000 nM (A) positive and negative reference chemicals, (B) BHT, (C) DEA, (D) PCE, or (E) MDEA. Values were normalized to the reference gene peptidylprolyl isomerase B (PPIB) and set relative to vehicle. Bar graphs represent means of at least five biological replicates ± SEM. *p<0.05, **p<0.01 as determined by ANOVA with Tukey’s post-hoc analysis when compared to vehicle or 0 nM treatment.
When compared to no treatment, exposure to BHT or DEA repressed the basal expression of all three estrogen-responsive genes (Figure 3B and 3C). The lowest dose resulting in significant changes to gene expression was 1 nM for both chemicals. Maximal inhibition of PGR, GREB1, and NPPC expression by BHT and DEA was achieved at 10 nM. Both PCE and MDEA induced the expression of PGR, GREB1, and NPPC mRNA (Figure 3D and 3E). The minimal dose for significant induction varied by chemical and gene. GREB1 was significantly induced by 1 nM of PCE or MDEA. PCE significantly up-regulated the expression of PGR and NPPC mRNA at 10 nM. PGR mRNA was induced by MDEA at 1 nM, while expression of NPPC was induced only at the highest dose of MDEA. These chemicals induced the transcript levels of estrogen receptor alpha (ERα) and beta (ERβ) following 6hr treatment (Supplemental Figure 1A). Western blot analysis revealed variable effects on relative protein levels, although both BHT and DEA repressed ERβ expression 48 hrs after treatment (Supplemental Figure 1B). These data indicate that BHT, DEA, PCE, and MDEA can alter the expression of endogenous estrogen target genes, potentially through altering the expression of ER. Importantly, these chemicals regulate endogenous gene expression at concentrations lower than the recommended exposure limit for humans.
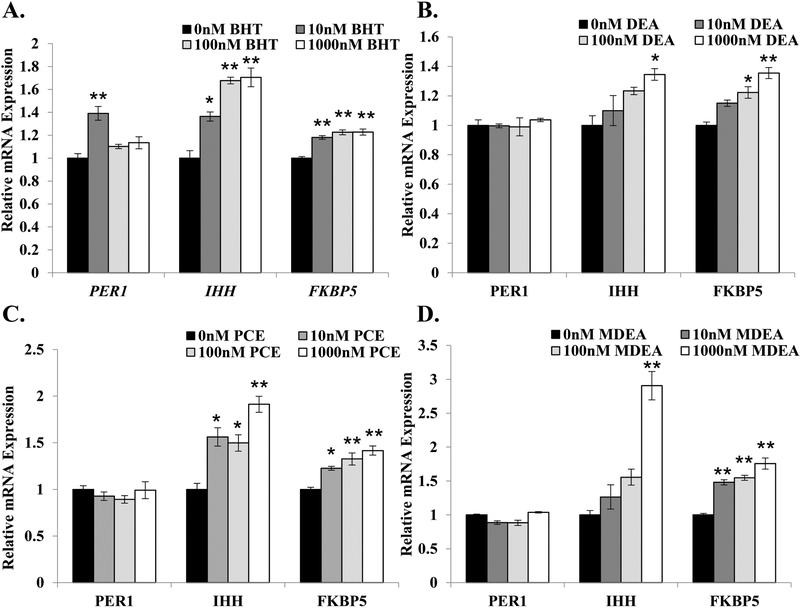
To evaluate whether the prioritized chemicals were selective for estrogenic activities or display promiscuous EDC activity, Ishikawa cells were treated with vehicle (0 nM), BHT, DEA, PCE, or MDEA at concentrations of 10, 100, and 1000 nM for 6 hrs, and isolated mRNA was analyzed for the expression of three glucocorticoid- and progesterone-responsive genes: period circadian protein homolog 1 (PER1), Indian hedgehog homolog (IHH), and FK506 binding protein 5 (FKBP5). As shown in Figure 4, the four chemicals altered the expression of PER1, IHH, and FKBP5 to varying degrees. Although the expression of PER1 was only regulated in response to 10 nM BHT (Figure 4A), all four chemicals significantly up-regulated the expression of IHH and FKBP5 in a dose-dependent manner (Figure 4A–D). These results indicate that BHT, DEA, PCE, and MDEA can alter the expression of genes regulated by other endocrine hormones in human uterine cells.
FIGURE 4. BHT, DEA, PCE, and MDEA exposure and transcript levels of endogenous glucocorticoid- and progesterone-responsive genes in Ishikawa cells.
Levels of periodic circadian regulator 1 (PER1), Indian hedgehog (IHH), and FK506 binding protein 5 (FKBP5) transcripts were evaluated by qRT-PCR in Ishikawa cells treated for 6 hr with vehicle (0 nM) or 1, 10, 100, or 1000 nM (A) BHT, (B) DEA, (C) PCE, or (D) MDEA. Values were normalized to the reference gene peptidylprolyl isomerase B (PPIB) and set relative to vehicle. Bar graphs represent means of at least five biological replicates ± SEM. *p<0.05, **p<0.01 as determined by ANOVA with Tukey’s post-hoc analysis.
3.3. Gene networks regulated by the selected chemicals.
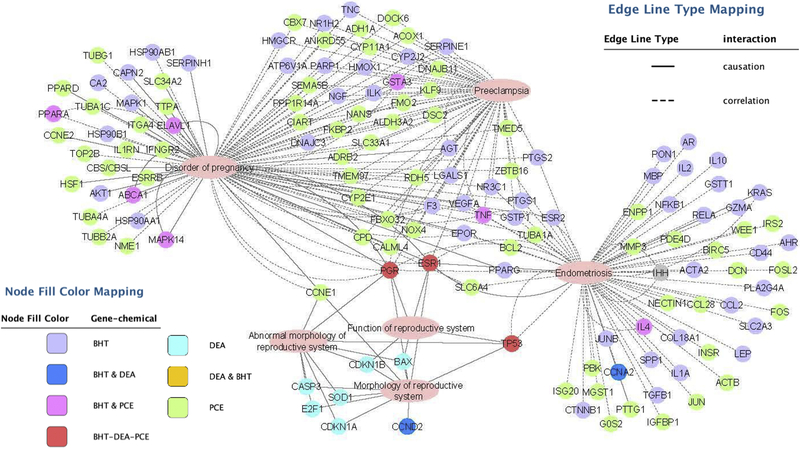
To evaluate the global effect of these chemicals on the genome, we utilized the Comparative Toxicogenomics Database (http://ctdbase.org). This database contains manually-curated information extracted from the published, peer-reviewed scientific literature, providing key information on the interactions of environmental chemicals with gene products and their effect on human disease. This database also includes real-world measurements and biological effects of environmental chemicals and human biomarkers [42]. Gene networks were created to visualize the relationship between the chemical-associated genes reported in the literature and diseases of the female reproductive tract (Figure 5). Genes reported to be regulated by BHT, DEA, PCE, and MDEA were identified by the Comparative Toxicogenomics Database. Gene lists were created for BHT, DEA, and PCE based on reported expression data. MDEA had no reported expression data and was excluded from gene network analysis. Additional genes determined to be regulated by BHT, DEA, and PCE in this study were included in the gene lists (PER1, IHH, FKBP5, ESR1, ESR2, CCNE1, and PRKCA). Gene lists were annotated by Ingenuity Pathway Analysis (IPA) for biological functions based on causative and correlative interactions, and functions related to the uterus and pregnancy were visualized (disorder of pregnancy, preeclampsia, endometriosis, abnormal/morphology of the reproductive system, and function of the reproductive system). Genes regulated by BHT and PCE were highly associated with disorder of pregnancy, preeclampsia, and endometriosis, while the genes associated with DEA were highly associated with the development and function of the reproductive system. These results indicate that the chemicals studied have the potential to alter uterine functions.
FIGURE 5. Gene networks associated with BHT, DEA, and PCE regulated genes.
Genes regulated by BHT, DEA, or PCE were identified using the Comparative Toxicogenomics Database and analyzed for enriched biological functions using Ingenuity Pathway Analysis (IPA) software. IPA distinguished the genes with functions related to the uterus and pregnancy and gene networks were created using Cytoscape. The node fill color indicates the chemical or chemicals that regulate the expression of each gene. Solid or broken lines indicate causative or correlative interactions, respectively.
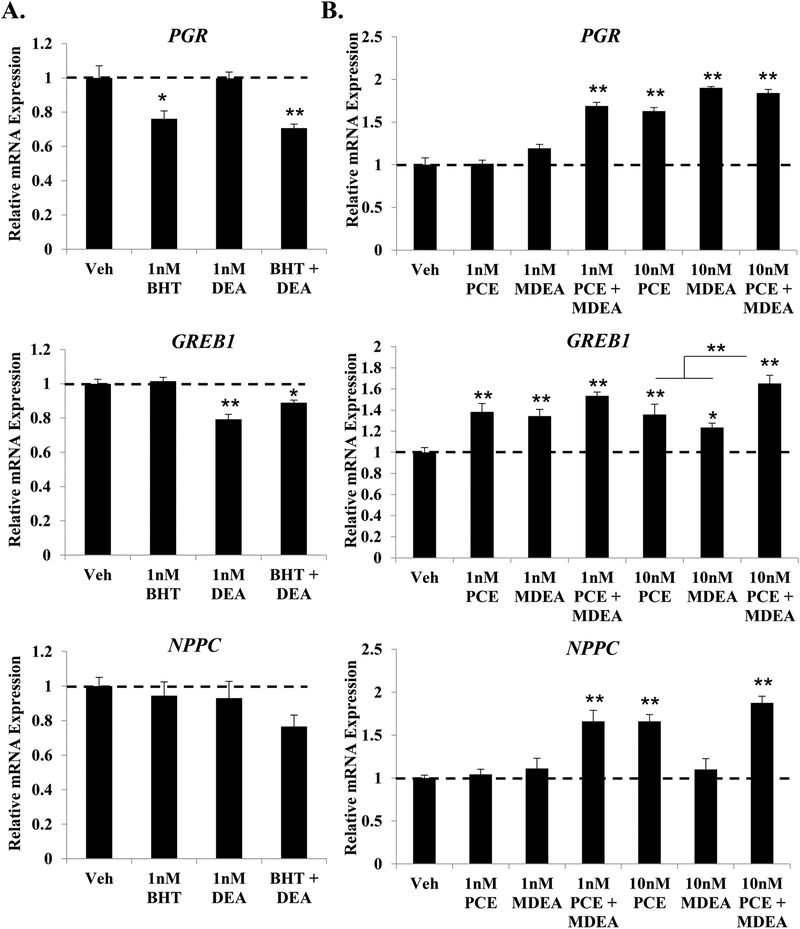
3.4. Impact of combination treatments.
Environmental exposures often occur as combinations of chemicals. To investigate the additive effects of these prioritized chemicals, Ishikawa cells were treated for 6 hrs with low-dose combinations of BHT and DEA or PCE and MDEA, and the expression of PGR, GREB1, and NPPC mRNA was evaluated by qRT-PCR from isolated RNA (Figure 6). Chemicals were combined based on their determined agonist or antagonist activity reported in Figure 3, and cells were treated with concentrations at or below the lowest dose that produced a transcriptional change. The combination of BHT and DEA did not significantly alter the pattern of gene expression reported for the single treatments (Figure 6A). The combination of 1 nM PCE and 1 nM MDEA significantly induced the expression of PGR and NPPC compared to the individual treatments at 1 nM (Figure 6B). The combination of 10 nM PCE and MDEA significantly enhanced the induction of GREB1 compared to each individual treatment. These data demonstrate that low-dose combinations of PCE and MDEA expand the exposure range at which these chemicals alter endogenous gene expression.
FIGURE 6. Low dose combinations of select chemicals.
Expression of the progesterone receptor (PGR), growth regulation by estrogen in breast cancer 1 (GREB1), and natriuretic peptide C (NPPC) mRNA were evaluated by qRT-PCR in Ishikawa cells treated for 6 hr with vehicle or low-dose combinations of (A) 1 nM BHT and DEA or (B) 1 nM or 10 nM PCE and MDEA. Values were normalized to the reference gene peptidylprolyl isomerase B (PPIB) and set relative to vehicle. Bar graphs represent means of at least five biological replicates ± SEM. *p<0.05, **p<0.01 as determined by ANOVA with Tukey’s post-hoc analysis.
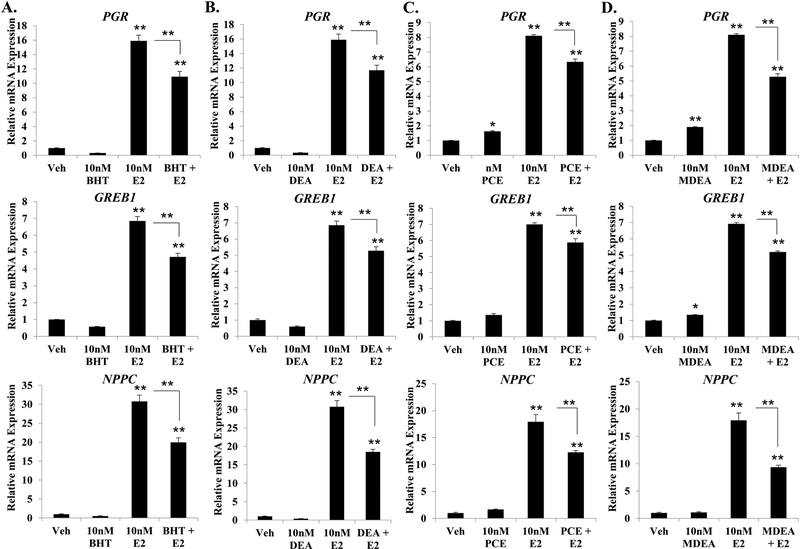
3.5. Regulation of estradiol-induced gene expression.
To determine whether the prioritized chemicals could alter estradiol-mediated gene regulation, Ishikawa cells were treated for 6 hrs with vehicle, 10 nM estradiol, 10 nM BHT, 10 nM DEA, 10 nM PCE, 10 nM MDEA, or a combination of estradiol and chemical. The expression of PGR, GREB1, and NPPC mRNA was then evaluated by qRT-PCR from isolated mRNA. As expected, estradiol treatment significantly induced the expression of all three genes (Figure 7). However, the magnitude of induction was significantly less when estradiol was combined with any of the selected chemicals. The greatest reduction in transcriptional activity was observed for NPPC when estradiol was co-treated with BHT, DEA, PCE, and MDEA, where the relative fold induction was reduced by 36, 40, 32, and 48%, respectively. These data indicate that the selected chemicals can alter estradiol-induced transcriptional activity in addition to altering basal expression of the estrogen-responsive genes.
FIGURE 7. Effect of BHT, DEA, PCE, and MDEA on estrogen-mediated gene regulation in Ishikawa cells.
Transcript levels of the progesterone receptor (PGR), growth regulation by estrogen in breast cancer 1 (GREB1), and natriuretic peptide C (NPPC) were evaluated by qRT-PCR in Ishikawa cells treated for 6 hr with vehicle, 10 nM E2, (A) 10 nM BHT, (B) 10 nM DEA, (C) 10 nM PCE, or (D) 10 nM MDEA in the presence and absence of 10 nM E2. Values were normalized to the reference gene peptidylprolyl isomerase B (PPIB) and set relative to vehicle. Bar graphs represent means of at least five biological replicates ± SEM. *p<0.05, **p<0.01 as determined by ANOVA with Tukey’s post-hoc analysis.
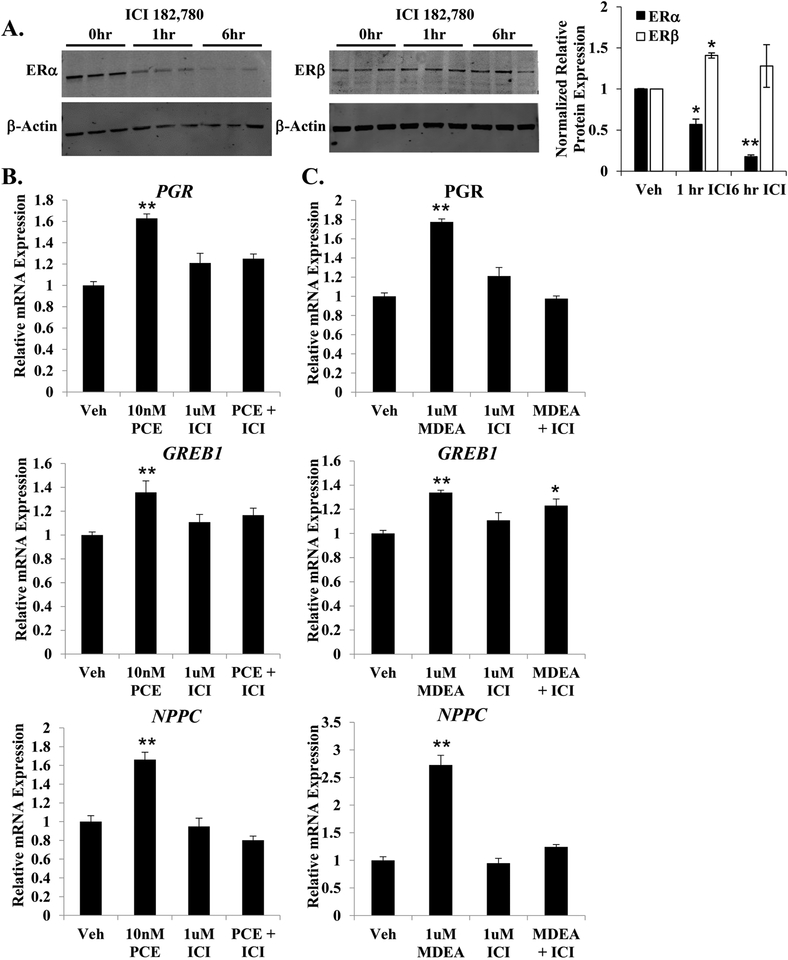
3.6. Effects mediated through estrogen receptor alpha.
To investigate the requirement of ERα for the basal agonist activity of PCE and MDEA, Ishikawa cells were pretreated for 1 hr with the ERα antagonist ICI 182,780 prior to the addition of PCE or MDEA. As expected, 1 μM ICI 182,780 specifically repressed the expression of ERα by 45% at 1 hr and >80% at 6 hr (Figure 8A). Ishikawa cells were treated for 6 hr with doses of PCE and MDEA determined to produce a maximal change in gene expression (Figure 3D and E), in the presence and absence of ICI 182, 780 pretreatment. The addition of 1 μM ICI 182,780 blocked the up-regulation of PGR, GREB1, and NPPC by PCE and MDEA (Figure 8B and C), suggesting that these chemicals require ERα to upregulate the expression of these estrogen-responsive genes.
FIGURE 8. PCE and MDEA signaling and ERα.
(A) Representative immunoblot of ERα and ERβ expression levels in Ishikawa cells treated for 0, 1, or 6 hr with 1 μM ICI 182,780. Data represent mean ± SEM from 3 independent experiments. Expression of β-actin was utilized as a loading control. (B) Expression of the progesterone receptor (PGR), growth regulation by estrogen in breast cancer 1 (GREB1), and natriuretic peptide C (NPPC) mRNA were evaluated by qRT-PCR in Ishikawa cells treated for 6 hr with 10 nM PCE or 1 μM MDEA with or without pretreatment with 1 μM ICI 182,780. Values were normalized to the reference gene peptidylprolyl isomerase B (PPIB) and set relative to vehicle. Bar graphs represent means of at least five biological replicates ± SEM. *p<0.05, **p<0.01 as determined by ANOVA with Tukey’s post-hoc analysis.
3.7. Simulating ERα bound by environmental chemicals.
To further support the molecular basis for the EDC activities of BHT, DEA, PCE, and MDEA, the selected chemicals were docked to the ligand-binding domain of ERα using the OpenEye software suite. Docking studies were performed with ERα in three unique conformations: inactive, agonist-bound (E2), and antagonist-bound (Supplemental Figure 2A). In the inactive form, helix-12 is completely displaced, exposing the ligand binding site [43]. When ligand-bound, Helix-12 partially covers the entrance to the ligand binding site [44]. The position adopted by Helix-12 in the agonist-bound form, in conjunction with Helix-3 and Helix-5, create the interacting surface for coregulator binding. However, when ERα is in the antagonist form, Helix-12 resides in the area used for coregulator binding [45].
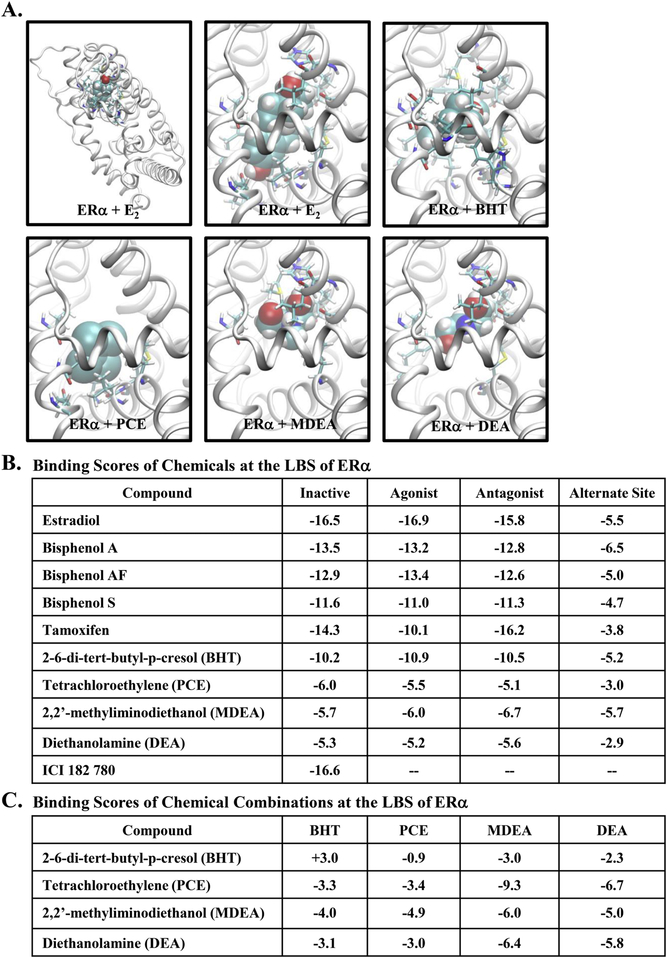
The endogenous ligand (estradiol) was successfully docked by the FRED module of the OpenEye software suite with all three confirmations, and the resulting structure was nearly identical to the X-ray crystal structure [44]. The space-fill representation of estradiol and the four chemicals docked to the ERα ligand-binding domain is shown (Figure 9A). The binding scores were determined for estradiol, known endocrine disruptors (Bisphenol A, Bisphenol AF, Bisphenol S), the selective estrogen receptor modulator tamoxifen, the estrogen receptor antagonist ICI 182, 780, and the four chemicals evaluated in this study (Figure 9B). The binding score for estradiol served as the reference, and binding scores for estrogenic and antiestrogenic compounds served as a comparison. The four experimental chemicals showed favorable total binding scores, and the binding score for BHT was most similar to the known endocrine disruptors and estradiol. None of the selected chemicals were able to be docked when the ligand-binding domain was first occupied by estradiol (data not shown). When estradiol occupies the ligand-binding domain, helix 12 caps the ligand-binding site in the agonist conformation, thereby precluding further binding at this site. Therefore, we determined binding scores for the SRC-1 binding site formed by helices 3, 5, and 12 when the ligand-binding site is occupied by estradiol (alternate site). The resulting binding scores for all compounds tested were relatively less favorable (see Figure 9B, the last column), indicating a rather low probability that any of the compounds would occupy the SRC-1 binding site when estradiol is bound. Finally, the binding scores were determined for the binding of the selected chemicals in combinations (Figure 9C). Interestingly, the binding score associated with the combined binding of PCE with MDEA was remarkedly favorable compared to the binding of PCE or MDEA alone.
FIGURE 9. Docking studies with the estrogen receptor ligand binding site.
(A) Ligand-docked conformations from the agonist conformation of ERα. E2 was accurately docked by the FRED module and the structure was nearly identical to the X-ray crystal structure and therefore, the docking score can be used as the reference. The complete structure of the ligand bound receptor was shown in the top left panel. Ligands were shown in the space-fill representation and the receptor was in the ribbon form, while interacting receptor residues were in the stick representation. (B) Docking scores for various compounds for three different receptor conformations (inactive, agonist, and antagonist forms). The final column was obtained for the compounds docked at the src-1 binding site to check whether they can be occupied the coregulator binding site thereby disrupting coregulator binding. Relatively small binding scores indicate that this site was not preferred by the compounds tested. (C) The chemicals listed in the “Compound” column were first docked in their best binding mode and listed binding scores were then evaluated for each of the chemical attempted to dock onto the remaining area of the ligand binding site.
To further our understanding of the interactions involved in chemical binding, we evaluated the various amino acid residues in the ligand binding pocket of ERα that may interact with the industrial chemicals when docked. When the endogenous hormone estradiol was combined with all three ERα confirmations (inactive, agonist, and antagonist), Arg-394 and Glu-353 residues were found to make potential hydrogen (H)-bonds with one hydroxyl group. In the inactive and agonist confirmations, the other hydroxyl group made H-bonds with His-524 or His-524 and Gly-512, respectively (Supplemental Figure 2B). The antagonist confirmation did not contain any H-bonds with this hydroxyl group and was found to have a lower binding score for this confirmation. Residues Met-343, Leu-346, Leu-349, Ala-350, Leu-367, Leu-384, and Met-388 all in Helix-3, along with Leu-391 (from Helix-5), Phe-404 (which does not belong to any secondary structural element), Met-421, Ile-424, and Leu-428 (from Helix-7), Gy-521 and Leu-525 (from Helix-11) were in contact with estradiol. The amino acid interactions varied for the BPA analogues, which are smaller in size than estradiol, BPA utilized both hydroxyl groups in making H-bonds with various residues of ERα. In addition to Leu-346, three residues, His-524, Gly-521, and Glu353 (involved in estradiol docking) were also involved in H-bonding with the hydroxyl groups of BPA (Supplemental Figure 2C). Our analysis found comparable binding scores for BPA in the agonist and antagonist confirmations of ERα, suggesting the ability of BPA to be associated with both agonist and antagonist activities. More than 75% of the residues associated with estradiol were also observed in ligand contacts with BPA. From the best docked poses, hydroxyl groups of BPAF were found to make only one (with Leu-387 or Gly-521) or no H-bonds with ERα (Supplemental Figure 2D) with only a slight preference for the agonist form. BPS displays one to three H-bonds with Arg-394, Leu-387, Glu-353, and Gly-521 residues with binding scores being comparable between the three confirmations of ERα (Supplemental Figure 2E). The docked confirmation of tamoxifen did not have any H-bonds, but a large number of ER residues were found to be in contact with the ligand (Supplemental Figure 2F).
Although no hydrogen bonds were present in the best docked poses for BHT, a similar number of non-H-bonding ERα residues were found to be in contact with BHT as compared to estradiol, resulting in the highest binding scores of the four chemicals tested (Supplemental Figure 2G). For PCE and MDEA, only 8 non-H-bonding ERα residues were observed (Supplemental Figure 2H & I). Up to three H-bonds were found for MDEA (residues His-524, Thr-347, Leu-387, and Glu-353), which were dependent on receptor confirmation (Supplemental Figure 2I). Several non-H-bonding residues were found in the immediate vicinity of Arg-394, Glu-353, His-524 making a H-bond with one of the hydroxyl groups when DEA was docked (Supplemental Figure 2J). Finally, due to the large size of ICI 182, 780, only the inactive confirmation could be docked, leading to a binding score comparable to the agonist form of estradiol or the antagonist form of tamoxifen.
3.8. Impact on cell proliferation in immortalized human uterine cells.
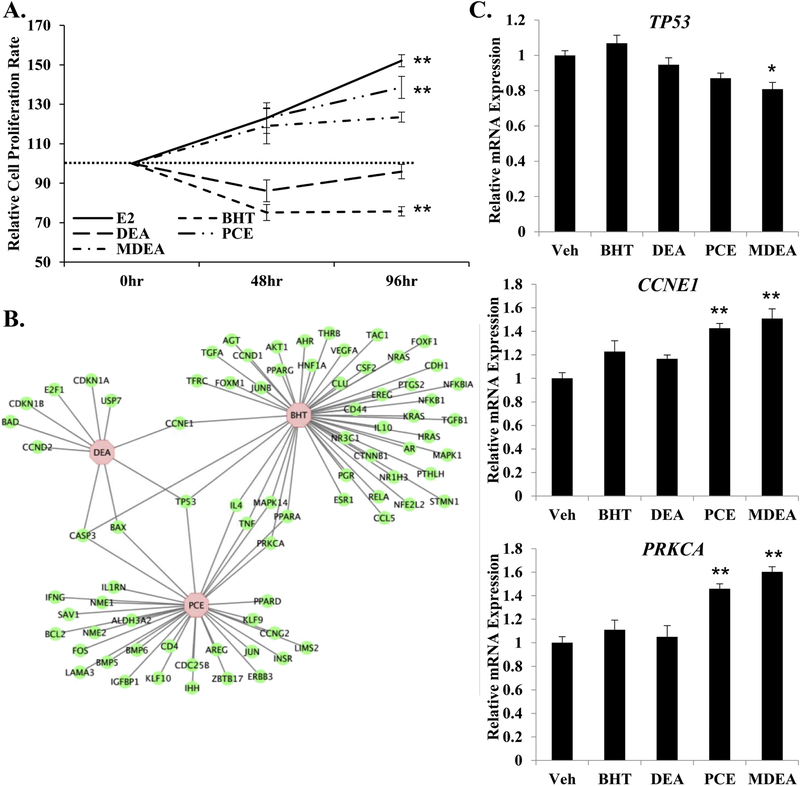
To examine the biological effects of the prioritized chemicals in Ishikawa cells, equal number of cells were plated and treated with vehicle, 10 nM E2, 100 nM BHT, 100 nM DEA, 100 nM PCE, or 100 nM MDEA. Cells were counted 48 and 96 hr after treatment. E2 and PCE significantly increased the rate of cell proliferation relative to vehicle at 96 hr, while BHT significantly decreased the relative rate of cell proliferation at 96 hr (Figure 10A). These data suggest that certain chemicals can functionally alter cell proliferation in human uterine cells. The gene lists created using the Comparative Toxicogenomics Database were analyzed using IPA software to identify regulated genes that are associated with cell proliferation (Figure 10B). These findings illustrate potential mechanisms by which the tested chemicals regulate cell proliferation. To further support the molecular basis for the observed changes in cell proliferation rates, the expression of tumor protein 53 (TP53), cyclin E1 (CCNE1), and protein kinase C alpha (PRKCA) was evaluated by qRT-PCR in cells treated with vehicle, 100 nM BHT, 100 nM DEA, 100 nM PCE, or 100 nM MDEA for 6 hrs. The only chemical that altered the expression of TP53 was MDEA. The proliferation inducing genes CCNE1 and PRKCA were both up-regulated by PCE and MDEA, agreeing with increased rates of cell proliferation following treatment with these chemicals.
FIGURE 10. Chemical exposure and cell proliferation in Ishikawa cells.
(A) Cell proliferation assays were performed on Ishikawa cells treated for 48 or 96 hrs with 10 nM E2, 100 nM BHT, 100 nM DEA, 100 nM PCE, or 100 nM MDEA. Numbers of cells were normalized to the vehicle-treated plates for that time-point. (B) Genes regulated by BHT, DEA, or PCE that are involved in proliferation of epithelial cells were determined by cross-referencing chemical-associated genes from the Comparative Toxicogenomics Database with gene functions from Ingenuity Pathway Analysis. Gene network was created using Cytoscape. (C) Expression of tumor protein 53 (TP53), cyclin E1 (CCNE1), and protein kinase C alpha (PRKCA) were evaluated by qRT-PCR in Ishikawa cells treated for 6 hr with vehicle, 100 nM BHT, 100 nM DEA, 100 nM PCE, or 100 nM MDEA. Values were normalized to the reference gene peptidylprolyl isomerase B (PPIB) and set relative to vehicle. Bar graphs represent means of at least five biological replicates ± SEM. *p<0.05, **p<0.01 as determined by ANOVA with Tukey’s post-hoc analysis.
4. Discussion
Although petroleum manufacturing utilizes a closed system, there is a risk for accidental exposure to individuals immediately involved in the petroleum refining process. Moreover, subsequent waste management and disposal can release chemical by-products into the air, water sources, and soil, potentially contaminating the surrounding environment [46]. Carcinogenicity and toxicity are well-defined endpoints when evaluating safety standards for chemical exposure [47]. Human studies have demonstrated that some chemicals used in petroleum manufacturing are reproductive toxicants, increasing the risk of infertility [48, 49]. However, exposure to these chemicals may also result in less pronounced physiological changes that are not always detectable by current toxicity testing paradigms or classification systems. For example, chemicals can alter metabolic or endocrine processes that negatively affect physiological functions, although the impact on these processes may not result in toxicity. Instead, these impacts can manifest as metabolic syndrome, unexplained infertility, or pregnancy loss. When considering the physiological impact of chemical exposure, overall health can be improved by identifying and prioritizing safer chemicals that minimize their toxic, carcinogenic, and endocrine disrupting potential.
Focusing on the nuclear receptor activity of the industrial chemicals, we found that many of the chemicals evaluated had reported activity on ER endpoints in the ToxCast database, specifically inducible reporter and receptor binding assays. However, there are limited published studies describing these chemicals as endocrine disruptors. The most well-characterized of the studied chemicals is DEA. It has been reported that DEA can antagonize the estrogen, androgen, and progesterone receptors in Ishikawa cells, the androgen receptor in human liver carcinoma HepG2 cells, and the estrogen receptor in human breast cancer MCF-7 cells [50, 51]. These studies demonstrate antagonism of transiently transfected reporter constructs containing a hormone response element at micromolar doses. To our knowledge, our study is the first report of DEA-mediated repression of endogenous estrogen-responsive genes.
The endocrine-disrupting effects of DEA have been linked to fertility and organismal development. Rats exposed to DEA through their drinking water exhibit degeneration of the seminiferous tubules with decreased sperm count and motility [52]. In vitro incubation of human sperm with DEA decreased motility and viability and caused morphological abnormalities in a dose-dependent manner [53]. In female rats, oral exposure to DEA at greater than 125 mg/kg/day resulted in post-implantation pregnancy loss, pup mortality, and reduced maternal and pup body weights [54]. However, another study showed that cutaneous exposure to DEA in rats did not result in fetal mortality but caused reduced maternal body weight, increased maternal kidney weight, and fetal skeletal abnormalities at 1500 mg/kg/day [55]. The proposed mechanism for the observed maternal and reproductive changes in mice was DEA-mediated repression of maternal choline levels. However, estrogen acts as an upstream regulator of choline biosynthesis, and it is possible that the deficits in maternal choline and the subsequent maternal and reproductive defects may be a result of DEA-mediated repression of estrogen activity [56, 57].
The apparent discrepancy between studies may reflect the use of different exposure routes, as dermal doses of DEA are not readily absorbed through the rat skin [58]. The international classification of DEA for reproductive toxicity is also inconsistent, where the Japan NITE has classified DEA as category 2 but ECHA, ICOP, and GESTIS failed to classify DEA as a reproductive toxicant. Classification by the NITE was based on data from a rat study demonstrating post-implantation loss, while ECHA stated that based on inconclusive data from a recent extended One-Generation Reproductive Toxicity Study, DEA does not warrant classification as a reproductive toxicant. We also found some discrepancy between the activity of DEA in the ToxCast assays and our modeled receptor binding. Where our study found the binding score of DEA to be weak compared to estradiol, ToxCast reported activity for DEA in the OT_ER_ERaERa_1440 assay, a receptor binding assay performed in HEK293T cells following 24 hr exposure. Again, differences in concentration and timing may underlie these apparent inconsistencies. Discordance between studies and regulatory groups suggests further studies are warranted on the endocrine disrupting potential of DEA, accounting for assay, concentration, and timepoint.
BHT is a synthetic antioxidant widely used as a food additive and in other fields to reduce free-radical induced damage, including personal care products, pharmaceuticals, plastic and rubber manufacturing, and petroleum products [59]. BHT exposure with reporter gene assays have demonstrated weak anti-androgenic activity in the human breast cancer cell line MDA-kb2 and estrogenic activity in the human embryonic kidney fibroblast cell line 293T [32, 60, 61]. However, our study is the first to demonstrate that BHT alters the expression of endogenous estrogen-responsive genes in a human uterine cell line. Interestingly, the ToxCast database reported an EC50 of 21.0 for BHT in the ATG_ERa_TRANS_up assay, a transfected trans-acting reporter gene assay evaluated following 24 hr of chemical exposure, and BHT was found to induce estrogen activity in 293T cells at concentrations greater than 50 μM [60]. However, our data revealed that BHT represses the expression of estrogen-responsive genes at lower concentrations. Many EDCs demonstrate nonmonotonic effects, including U or inverted U-shaped dose-response curves [62, 63]. It is likely that the biological consequences of exposure to these select chemicals are similarly dose-dependent. It is also possible that the exposure length and cellular context dictates the physiological response to hormone-similar chemicals. For example, estradiol produces a biphasic response in the uterus with early and late regulated genes, and the selective estrogen receptor modulator tamoxifen has contrasting actions in the uterus and breast [64–66]. It is important to consider the experimental context when comparing the response of chemicals.
As determined by our literature search, this is the first study describing PCE and MDEA as endocrine disruptors, although the ToxCast database reports active endpoints for both PCE and MDEA in the ERa_LUC-BG1 assay, an inducible reporter in a human ovary cell line. Interestingly, ICI 182,780 was found to inhibit induction of estrogen-responsive genes in PCE- and MDEA-treated cells, suggesting that PCE and MDEA exert their estrogenic effects through ERα. PCE exposure has been linked to increased risk of cervical cancer, spontaneous abortion, stillbirth, and placental abruption in humans, with several reports of fetal loss similarly observed in animal models [67–69]. Although there is no literature investigating the reproductive toxicity of MDEA, ECHA has reported reproductive toxicity when rats are orally gavaged with MDEA, including fewer implantation sites, early pregnancy loss, and reduced litter size. Our studies demonstrated that MDEA can alter the expression of estrogen-, progesterone- and glucocorticoid-responsive genes in immortalized human uterine cells. Therefore, MDEA represents a previously unappreciated endocrine disruptor.
In addition to exposure from petroleum manufacturing, the studied chemicals are widely utilized in other industries. For example, BHT is used by the food industry as a preservative, DEA is an ingredient in cosmetics and personal care products, PCE is commonly used to dry-clean fabrics, and MDEA is used to process natural gas. Due to their common use, it is possible that individuals are exposed to these compounds in combination rather than in isolation. We found that the estrogenic effect of PCE and MDEA in combination was more potent than the individual treatments alone. Interestingly, the binding score when the ligand-binding site of ERα was occupied by both PCE and MDEA was more similar to estradiol than the individual compounds in isolation, supporting our findings that low-dose combinations of PCE and MDEA are more effective at activating the estrogen receptor than either compound alone. Notably, all four chemicals antagonized estradiol-induced gene expression. Molecular docking indicated that estradiol was unable to bind when the ligand-binding site of ERα was first occupied by any of the select chemicals. It is possible that the chemicals behave as competitive antagonists in the presence of estradiol.
We found that these chemicals can alter the rate of proliferation in Ishikawa cells. Notably, MDEA increased rates of cell proliferation and repressed the expression of TP53, the anti-proliferative tumor suppressor protein p53 [70, 71]. Both PCE and MDEA induced expression of CCNE1, a regulator of the G1/S transition of the cell cycle, which is consistent with their ability to increase proliferation in Ishikawa cells [72–74]. PRKCA is part of a family of serine/threonine kinases that have been implicated in inhibition of both the G1/S and G2/M transitions of the cell cycle, as well as in regulation of p53 [75]. Both PCE and MDEA induced expression of PRKCA. This is the first report indicating that MDEA can alter the expression of proliferation-associated genes or rates of cellular proliferation. In the present study, we were unable to demonstrate BHT or DEA regulation of TP53 and CCNE1 as predicted by IPA software. These discrepancies may reflect differences in dosage, length of treatment, or tissue type from previous reported studies. Furthermore, the metabolic activation of these chemicals in vitro was not evaluated as part of this study, which may contribute to effects reported using in vivo models.
5. Conclusions
In summary, we have shown that the four environmental chemicals studied altered the expression of endogenous estrogen-responsive genes in human uterine cells. Importantly, these chemicals are not unique to the petroleum manufacturing process, and our results have broad application. Molecular docking suggests that these chemicals can bind to the ER ligand-binding domain, which may provide insight into their molecular mechanisms of action. These chemicals also have the potential to disrupt glucocorticoid and progesterone signaling. These results may reflect direct activation of the glucocorticoid and progesterone receptors or cross-talk between ER and other nuclear receptors [76–79]. Finally, we have demonstrated that the selected chemicals alter the rate of proliferation in Ishikawa cells, potentially by regulating the expression of proliferation-associated genes TP53, CCNE12 and PRKCA. Our in vitro findings provide an important basis for further characterizing the endocrine disrupting potential of these environmental chemicals. This study was limited to evaluating the regulation of three estrogen-responsive genes in one cell type, but future studies could incorporate a genome-wide approach, utilizing that concentrations that were found to produce endocrine disrupting effects. Evaluating endocrine disrupting activity of chemicals will provide a more comprehensive basis for hazard identification, which is an integral part of health risk assessment and may improve the health of individuals and communities.
Supplementary Material
Highlights:
The endocrine disrupting potential is not always captured by current toxicology testing paradigms.
Chemicals may alter the endocrine system at concentrations much lower than the published exposure limits.
139 chemicals were evaluated in United Nations Globally Harmonized System for chemical classification, a literature review, and search of publicly available toxicology databases
In vitro studies found novel estrogenic and anti-estrogenic activities of select chemicals at concentrations lower than the NIOSH exposure limit.
Acknowledgements
The authors wish to thank Andreanna Burman for technical help. This work was supported by the U.S. National Institutes of Health (NIH) National Institute of Environmental Health Sciences (NIEHS) grant R00 ES022983 to S.W. and by the Intramural Research Program of the NIH/NIEHS to L.P. through Z01 ES043010. The authors thank the Bureau of Educational and Cultural Affairs of the U.S. Department of State and the Malaysian-American Commission on Educational Exchange for the Fulbright Visiting Scholar Program at the Yale School of Public Health.
This research was supported by the National Institutes of Health/National Institute of Environmental Health Sciences [R00 ES022983] awarded to S.W. and the intramural program of the NIEHS (L.P.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare they have no actual or potential competing financial interests.
References
- 1.Kassotis CD, et al. , Endocrine-Disrupting Chemicals and Oil and Natural Gas Operations: Potential Environmental Contamination and Recommendations to Assess Complex Environmental Mixtures. Environ Health Perspect, 2016. 124(3): p. 256–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vignet C, et al. , Fish Reproduction Is Disrupted upon Lifelong Exposure to Environmental PAHs Fractions Revealing Different Modes of Action. Toxics, 2016. 4(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mielżyńska D, et al. , The influence of environmental exposure to complex mixtures including PAHs and lead on genotoxic effects in children living in Upper Silesia, Poland. Mutagenesis, 2006. 21(5): p. 295–304. [DOI] [PubMed] [Google Scholar]
- 4.Organization, W.H., IPCS global assessment of the state-of-the-science of endocrine disruptors, in Geneva, Switzerland: 2002. [Google Scholar]
- 5.Julien B, et al. , Evidence for estrogeno-mimetic effects of a mixture of low-dose pollutants in a model of ovariectomized mice. Environ Toxicol Pharmacol, 2018. 57: p. 34–40. [DOI] [PubMed] [Google Scholar]
- 6.Kinch CD, et al. , Low-dose exposure to bisphenol A and replacement bisphenol S induces precocious hypothalamic neurogenesis in embryonic zebrafish. Proc Natl Acad Sci U S A, 2015. 112(5): p. 1475–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee DH, Evidence of the Possible Harm of Endocrine-Disrupting Chemicals in Humans: Ongoing Debates and Key Issues. Endocrinol Metab (Seoul), 2018. 33(1): p. 44–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harville EW, et al. , The Gulf oil spill, miscarriage, and infertility: the GROWH study. Int Arch Occup Environ Health, 2018. 91(1): p. 47–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sirotkin AV and Harrath AH, Influence of oil-related environmental pollutants on female reproduction. Reprod Toxicol, 2017. 71: p. 142–145. [DOI] [PubMed] [Google Scholar]
- 10.Raji Y and Hart VO, Influence of prolonged exposure to Nigerian Bonny light crude oil on fertility indices in rats. Niger J Physiol Sci, 2012. 27(1): p. 55–63. [PubMed] [Google Scholar]
- 11.Roberts LG, Bevans AC, and Schreiner CA, Developmental and reproductive toxicity evaluation of toluene vapor in the rat. I. Reproductive toxicity. Reprod Toxicol, 2003. 17(6): p. 649–58. [DOI] [PubMed] [Google Scholar]
- 12.Fry DM, Reproductive effects in birds exposed to pesticides and industrial chemicals. Environ Health Perspect, 1995. 103 Suppl 7: p. 165–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maronpot RR, Ovarian toxicity and carcinogenicity in eight recent National Toxicology Program studies. Environ Health Perspect, 1987. 73: p. 125–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alviggi C, et al. , Association between intrafollicular concentration of benzene and outcome of controlled ovarian stimulation in IVF/ICSI cycles: a pilot study. J Ovarian Res, 2014. 7: p. 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vignet C, et al. , Fish Reproduction Is Disrupted upon Lifelong Exposure to Environmental PAHs Fractions Revealing Different Modes of Action. Toxics, 2016. 4(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lim J, et al. , The Mouse Fetal Ovary Has Greater Sensitivity Than the Fetal Testis to Benzo[a]pyrene-Induced Germ Cell Death. Toxicol Sci, 2016. 152(2): p. 372–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reif DM, et al. , Endocrine profiling and prioritization of environmental chemicals using ToxCast data. Environ Health Perspect, 2010. 118(12): p. 1714–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Willett CE, Bishop PL, and Sullivan KM, Application of an integrated testing strategy to the U.S. EPA endocrine disruptor screening program. Toxicol Sci, 2011. 123(1): p. 15–25. [DOI] [PubMed] [Google Scholar]
- 19.Mansouri K, et al. , CERAPP: Collaborative Estrogen Receptor Activity Prediction Project. Environ Health Perspect, 2016. 124(7): p. 1023–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dix DJ, et al. , The ToxCast program for prioritizing toxicity testing of environmental chemicals. Toxicol Sci, 2007. 95(1): p. 5–12. [DOI] [PubMed] [Google Scholar]
- 21.Whirledge SD, et al. , Uterine glucocorticoid receptors are critical for fertility in mice through control of embryo implantation and decidualization. Proc Natl Acad Sci U S A, 2015. 112(49): p. 15166–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Adams NR and DeMayo FJ, The Role of Steroid Hormone Receptors in the Establishment of Pregnancy in Rodents. Adv Anat Embryol Cell Biol, 2015. 216: p. 27–49. [DOI] [PubMed] [Google Scholar]
- 23.Whirledge S and Cidlowski JA, Estradiol antagonism of glucocorticoid-induced GILZ expression in human uterine epithelial cells and murine uterus. Endocrinology, 2013. 154(1): p. 499–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bolger R, et al. , Rapid screening of environmental chemicals for estrogen receptor binding capacity. Environ Health Perspect, 1998. 106(9): p. 551–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mueller SO, et al. , Phytoestrogens and their human metabolites show distinct agonistic and antagonistic properties on estrogen receptor alpha (ERalpha) and ERbeta in human cells. Toxicol Sci, 2004. 80(1): p. 14–25. [DOI] [PubMed] [Google Scholar]
- 26.Weichman BM and Notides AC, Estradiol-binding kinetics of the activated and nonactivated estrogen receptor. J Biol Chem, 1977. 252(24): p. 8856–62. [PubMed] [Google Scholar]
- 27.Davis AP, et al. , The comparative toxicogenomics database: update 2013. Nucleic acids research, 2012. 41(D1): p. D1104–D1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shannon P, et al. , Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome research, 2003. 13(11): p. 2498–2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wallace AC, Laskowski RA, and Thornton JM, LIGPLOT: a program to generate schematic diagrams of protein-ligand interactions. Protein Eng, 1995. 8(2): p. 127–34. [DOI] [PubMed] [Google Scholar]
- 30.Moore NP, et al. , Guidance on classification for reproductive toxicity under the globally harmonized system of classification and labelling of chemicals (GHS). Crit Rev Toxicol, 2013. 43(10): p. 850–91. [DOI] [PubMed] [Google Scholar]
- 31.Tapella L, et al. , Benzene and 2-ethyl-phthalate induce proliferation in normal rat pituitary cells. Pituitary, 2017. 20(3): p. 311–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Judson RS, et al. , Integrated Model of Chemical Perturbations of a Biological Pathway Using 18 In Vitro High-Throughput Screening Assays for the Estrogen Receptor. Toxicol Sci, 2015. 148(1): p. 137–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dreier DA, Connors KA, and Brooks BW, Comparative endpoint sensitivity of in vitro estrogen agonist assays. Regul Toxicol Pharmacol, 2015. 72(2): p. 185–93. [DOI] [PubMed] [Google Scholar]
- 34.Miller MM, et al. , Editor’s Highlight: Development of an In vitro Assay Measuring Uterine-Specific Estrogenic Responses for Use in Chemical Safety Assessment. Toxicol Sci, 2016. 154(1): p. 162–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tamm-Rosenstein K, et al. , Changes in the transcriptome of the human endometrial Ishikawa cancer cell line induced by estrogen, progesterone, tamoxifen, and mifepristone (RU486) as detected by RNA-sequencing. PLoS One, 2013. 8(7): p. e68907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Deschenes J, et al. , Regulation of GREB1 transcription by estrogen receptor alpha through a multipartite enhancer spread over 20 kb of upstream flanking sequences. J Biol Chem, 2007. 282(24): p. 17335–9. [DOI] [PubMed] [Google Scholar]
- 37.Whirledge S, Senbanjo LT, and Cidlowski JA, Genistein disrupts glucocorticoid receptor signaling in human uterine endometrial Ishikawa cells. Environ Health Perspect, 2015. 123(1): p. 80–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim HS, et al. , Potential estrogenic effects of bisphenol-A estimated by in vitro and in vivo combination assays. J Toxicol Sci, 2001. 26(3): p. 111–8. [DOI] [PubMed] [Google Scholar]
- 39.Blair RM, et al. , The estrogen receptor relative binding affinities of 188 natural and xenochemicals: structural diversity of ligands. Toxicol Sci, 2000. 54(1): p. 138–53. [DOI] [PubMed] [Google Scholar]
- 40.Vogel VG, et al. , Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes: the NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trial. JAMA, 2006. 295(23): p. 2727–41. [DOI] [PubMed] [Google Scholar]
- 41.Wakeling AE, Dukes M, and Bowler J, A potent specific pure antiestrogen with clinical potential. Cancer Res, 1991. 51(15): p. 3867–73. [PubMed] [Google Scholar]
- 42.Davis AP, et al. , The Comparative Toxicogenomics Database: update 2017. Nucleic Acids Res, 2017. 45(D1): p. D972–D978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tanenbaum DM, et al. , Crystallographic comparison of the estrogen and progesterone receptor’s ligand binding domains. Proc Natl Acad Sci U S A, 1998. 95(11): p. 5998–6003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Delfosse V, et al. , Structural and mechanistic insights into bisphenols action provide guidelines for risk assessment and discovery of bisphenol A substitutes. Proc Natl Acad Sci U S A, 2012. 109(37): p. 14930–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shiau AK, et al. , The structural basis of estrogen receptor/coactivator recognition and the antagonism of this interaction by tamoxifen. Cell, 1998. 95(7): p. 927–37. [DOI] [PubMed] [Google Scholar]
- 46.Frosch RA and Gallopoulos NE, Strategies for Manufacturing. Scientific American, 1989. 261(3): p. 144–153. [Google Scholar]
- 47.Chhabra RS, et al. , Toxicity characterization of environmental chemicals by the US National Toxicology Program: an overview. Int J Hyg Environ Health, 2003. 206(4–5): p. 437–45. [DOI] [PubMed] [Google Scholar]
- 48.Mehlman MA and Legator MS, Dangerous and cancer-causing properties of products and chemicals in the oil refining and petrochemical industry--Part II: Carcinogenicity, mutagenicity, and developmental toxicity of 1,3-butadiene. Toxicol Ind Health, 1991. 7(3): p. 207–20. [DOI] [PubMed] [Google Scholar]
- 49.Balise VD, et al. , Systematic review of the association between oil and natural gas extraction processes and human reproduction. Fertil Steril, 2016. 106(4): p. 795–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kassotis CD, et al. , Endocrine-Disrupting Activity of Hydraulic Fracturing Chemicals and Adverse Health Outcomes After Prenatal Exposure in Male Mice. Endocrinology, 2015. 156(12): p. 4458–4473. [DOI] [PubMed] [Google Scholar]
- 51.Kassotis CD, et al. , Estrogen and Androgen Receptor Activities of Hydraulic Fracturing Chemicals and Surface and Ground Water in a Drilling-Dense Region. Endocrinology, 2014. 155(3): p. 897–907. [DOI] [PubMed] [Google Scholar]
- 52.Melnick R, NTP technical report on the toxicity studies of Diethanolamine (CAS No. 111-42-2) Administered Topically and in Drinking Water to F344/N Rats and B6C3F1 Mice. Toxicity report series, 1992. 20: p. 1–D10. [PubMed] [Google Scholar]
- 53.Panchal SR and Verma RJ, Spermatotoxic effect of diethanolamine: An in vitro study. Asian Pacific Journal of Reproduction, 2013. 2(3): p. 196–200. [Google Scholar]
- 54.Price CJ, et al. , Postnatal development of rat pups after maternal exposure to diethanolamine. Birth Defects Research Part B: Developmental and Reproductive Toxicology, 2005. 74(3): p. 243–254. [DOI] [PubMed] [Google Scholar]
- 55.Marty MS, et al. , Developmental Toxicity of Diethanolamine Applied Cutaneously to CD Rats and New Zealand White Rabbits. Regulatory Toxicology and Pharmacology, 1999. 30(3): p. 169–181. [DOI] [PubMed] [Google Scholar]
- 56.Young DL, Estradiol- and testosterone-induced alterations in phosphatidylcholine and triglyceride synthesis in hepatic endoplasmic reticulum. J Lipid Res, 1971. 12(5): p. 590–5. [PubMed] [Google Scholar]
- 57.Resseguie M, et al. , Phosphatidylethanolamine N-methyltransferase (PEMT) gene expression is induced by estrogen in human and mouse primary hepatocytes. The FASEB Journal, 2007. 21(10): p. 2622–2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mathews* JM, et al. , Diethanolamine absorption, metabolism and disposition in rat and mouse following oral, intravenous and dermal administration. Xenobiotica, 1997. 27(7): p. 733–746. [DOI] [PubMed] [Google Scholar]
- 59.Yehye WA, et al. , Understanding the chemistry behind the antioxidant activities of butylated hydroxytoluene (BHT): a review. Eur J Med Chem, 2015. 101: p. 295–312. [DOI] [PubMed] [Google Scholar]
- 60.Wada H, et al. , In vitro Estrogenicity of Resin Composites. Journal of Dental Research, 2004. 83(3): p. 222–226. [DOI] [PubMed] [Google Scholar]
- 61.Pop A, et al. , Individual and combined in vitro (anti)androgenic effects of certain food additives and cosmetic preservatives. Toxicology in Vitro, 2016. 32: p. 269–277. [DOI] [PubMed] [Google Scholar]
- 62.Vandenberg LN, et al. , Hormones and endocrine-disrupting chemicals: low-dose effects and nonmonotonic dose responses. Endocrine reviews, 2012. 33(3): p. 378–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Conolly RB and Lutz WK, Nonmonotonic dose-response relationships: mechanistic basis, kinetic modeling, and implications for risk assessment. Toxicological Sciences, 2004. 77(1): p. 151–157. [DOI] [PubMed] [Google Scholar]
- 64.Kedar R, et al. , Effects of tamoxifen on uterus and ovaries of postmenopausal women in a randomised breast cancer prevention trial. The Lancet, 1994. 343(8909): p. 1318–1321. [DOI] [PubMed] [Google Scholar]
- 65.Katzenellenbogen BS, et al. , Bioactivities, Estrogen Receptor Interactions, and Plasminogen Activator-inducing Activities of Tamoxifen and Hydroxytamoxifen Isomers in MCF-7 Human Breast Cancer Cells. Cancer Research, 1984. 44(1): p. 112–119. [PubMed] [Google Scholar]
- 66.Hewitt SC, et al. , Estrogen receptor-dependent genomic responses in the uterus mirror the biphasic physiological response to estrogen. Mol Endocrinol, 2003. 17(10): p. 2070–83. [DOI] [PubMed] [Google Scholar]
- 67.Carwile JL, et al. , Prenatal drinking-water exposure to tetrachloroethylene and ischemic placental disease: a retrospective cohort study. Environmental Health, 2014. 13(1): p. 72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rebecca BD, et al. , Early lifestage exposure and potential developmental susceptibility to tetrachloroethylene. Birth Defects Research Part B: Developmental and Reproductive Toxicology, 2010. 89(1): p. 50–65. [DOI] [PubMed] [Google Scholar]
- 69.Weiderpass E and Labrèche F, Malignant Tumors of the Female Reproductive System. Safety and Health at Work, 2012. 3(3): p. 166–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Haupt S, Raghu D, and Haupt Y, Mutant p53 Drives Cancer by Subverting Multiple Tumor Suppression Pathways. Frontiers in Oncology, 2016. 6(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ullrich S, et al. , The p53 tumor suppressor protein, a modulator of cell proliferation. J Biol Chem, 1992. 267(22): p. 15259–15262. [PubMed] [Google Scholar]
- 72.Resnitzky D, et al. , Acceleration of the G1/S phase transition by expression of cyclins D1 and E with an inducible system. Molecular and Cellular Biology, 1994. 14(3): p. 1669–1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Resnitzky D and Reed SI, Different roles for cyclins D1 and E in regulation of the G1-to-S transition. Molecular and Cellular Biology, 1995. 15(7): p. 3463–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ohtsubo M, et al. , Human cyclin E, a nuclear protein essential for the G1-to-S phase transition. Molecular and Cellular Biology, 1995. 15(5): p. 2612–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Etta L and F.D. D, Linking Protein Kinase C to Cell‐Cycle Control. European Journal of Biochemistry, 1997. 248(1): p. 1–9. [DOI] [PubMed] [Google Scholar]
- 76.Whirledge S, Xu X, and Cidlowski JA, Global Gene Expression Analysis in Human Uterine Epithelial Cells Defines New Targets of Glucocorticoid and Estradiol Antagonism1. Biology of Reproduction, 2013. 89(3): p. 66, 1-17-66, 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Whirledge S, et al. , Pioneer Factors FOXA1 and FOXA2 Assist Selective Glucocorticoid Receptor Signaling in Human Endometrial Cells. Endocrinology, 2017. 158(11): p. 4076–4092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Katzenellenbogen BS, Mechanisms of Action and Cross-Talk Between Estrogen Receptor and Progesterone Receptor Pathways. Journal of the Society for Gynecologic Investigation, 2000. 7(1_suppl): p. S33–S37. [DOI] [PubMed] [Google Scholar]
- 79.Kraus WL, Weis KE, and Katzenellenbogen BS, Inhibitory cross-talk between steroid hormone receptors: differential targeting of estrogen receptor in the repression of its transcriptional activity by agonist- and antagonist-occupied progestin receptors. Mol Cell Biol, 1995. 15(4): p. 1847–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.