Abstract
Background:
Ultra-processed food has low nutritional quality, is associated with development of chronic diseases, and may increase exposure to chemicals used in food packaging and production.
Objectives:
To assess associations of ultra-processed food consumption with exposure to phthalates and bisphenols, including newer replacements, in the general U.S. population.
Methods:
Among 2,212 National Health and Nutrition Examination Survey (NHANES) 2013–2014 participants (≥6 years), we classified items reported in a 24-hour dietary recall according to the NOVA food processing classification system and calculated energy intake from ultra-processed food. Urinary concentrations of mono-benzyl (MBzP), mono-(3-carboxypropyl) (MCPP), mono-(carboxyisononyl) (MCNP), mono-(carboxyisoctyl) (MCOP), and four metabolites of di(2-ethylhexyl) (∑DEHP) phthalates and bisphenols A, F, and S were measured in spot urine samples. We estimated percent changes in natural log creatinine-standardized concentrations per 10% higher energy from ultra-processed food in covariate-adjusted multivariable linear regression models. We examined effect measure modification by age group, race/ethnicity, and poverty:income ratio and assessed associations with minimally processed food intake.
Results:
In adjusted models, higher energy from ultra-processed food was associated with higher urinary concentrations of MCPP, MCNP, and MCOP but not MBzP, ∑DEHP, or bisphenols. Each 10% higher energy from ultra-processed food was associated with 8.0% (95% CI: 5.6%, 10.3%) higher urinary MCOP concentrations, with a stronger association among children than adolescents or adults. Ultra-processed sandwiches/hamburgers, French fries/other potato products, and ice cream/pops were associated with higher concentrations of multiple chemicals. Higher energy from minimally processed food was associated with lower concentrations of MCPP, MCNP, MCOP, and bisphenols A and F.
Discussion:
Ultra-processed food consumption may increase exposure to currently used phthalates. Additional research is needed to determine whether minimally processed food diets or changes in food production practices can reduce phthalate and bisphenol exposures and related health effects, particularly among children who are more vulnerable to toxicants and tend to consume more ultra-processed food than adults.
Keywords: Phthalates, bisphenol A, processed food, exposure assessment, endocrine disruptors
1. Introduction
Phthalates and bisphenols are multifunctional synthetic chemicals found in a wide array of consumer and industrial products. High molecular weight phthalates are used to make plastics flexible and durable and bisphenols are used in epoxy resins and polycarbonate plastics (Koch and Calafat 2009). The majority of Americans have detectable urinary concentrations of multiple phthalate metabolites and bisphenols (Centers for Disease Control and Prevention 2018). While these chemicals are rapidly eliminated via urinary excretion (Calafat et al. 2015), the omnipresence of exposure sources is of growing concern given that exposure to some phthalates and bisphenol A are associated with wide-ranging adverse health outcomes related to their ability to disrupt the endocrine system (Gore et al. 2015).
Ingestion is the predominant exposure route for high molecular weight phthalates and bisphenols (Christensen et al. 2012; Koch and Calafat 2009; Koch et al. 2013). Because they are not chemically-bound, phthalates and bisphenols used in food contact materials or food processing plastics can transfer to food (Koch and Calafat 2009). Higher urinary concentrations of several phthalate metabolites have been associated with consuming certain types of food, such as dairy, meat, spices, flour, wheat, or grains (Dong et al. 2017; Mervish et al. 2014; Sakhi et al. 2014; Sathyanarayana et al. 2013; Serrano et al. 2014a; Zota et al. 2016). Phthalate exposures have also been associated with food venues, including fast food restaurants (Watkins et al. 2014; Zota et al. 2016), school cafeteria lunches (Munoz et al. 2018), and dining out (Varshavsky et al. 2018). Canned food and beverage intake has been associated with higher urinary bisphenol A concentrations (Carwile et al. 2011; Hartle et al. 2016). Furthermore, several studies reported that adhering to a fresh food diet or consuming homegrown food was associated with lower urinary concentrations of certain phthalate metabolites and bisphenol A (Correia-Sa et al. 2018; Correia-Sa et al. 2017; Rudel et al. 2011; Serrano et al. 2014b). In contrast, a dietary intervention trial reported higher DEHP concentrations during the intervention due to DEHP contamination of some dairy products and spices (Sathyanarayana et al. 2013).
These prior studies suggest that processed and packaged foods are likely sources of high molecular weight phthalate and bisphenol A exposures. Recently, there is growing interest in “ultra-processed foods,” defined as ready-to-eat formulations manufactured with little or no whole foods. Ultra-processed foods tend to be of low overall nutritional value (Martinez Steele et al. 2016; Martinez Steele et al. 2017; Moubarac et al. 2017), which is of concern given that consumption is increasing globally (Monteiro et al. 2013). Prospective studies have reported that higher consumption of ultra-processed food was associated with obesity, hypertension, breast cancer, and shorter lifespan (Fiolet et al. 2018; Hall et al. 2019; Kim et al. 2019; Mendonca et al. 2017; Mendonca et al. 2016; Rico-Campa et al. 2019; Schnabel et al. 2019). Some of these studies have hypothesized that exposures to endocrine-disrupting chemicals may be a pathway by which ultra-processed foods are linked to adverse health outcomes (Fiolet et al. 2018; Kim et al. 2019). However, no studies have examined whether ultra-processed food consumption is related to increased biomarkers of phthalate or bisphenol exposure.
Furthermore, there is little information quantifying dietary sources of exposure to newer replacement phthalates and bisphenols. Biomonitoring studies suggest that exposures to di(2-ethylhexyl) phthalate (DEHP), butyl benzyl phthalate (BBzP), and bisphenol A have declined in the U.S. over recent years, while exposure to di-n-octyl phthalate (DOP), diisononyl phthalate (DiNP), diisodecyl phthalates (DiDP), bisphenol S, and bisphenol F have increased (Koch et al. 2017; Ye et al. 2015; Zota et al. 2014). Although human health studies are sparse, these may be cases of “regrettable substitution” (Howard 2014; Wolff et al. 2017) given that these compounds are structurally homologous to their predecessors and may have similar hormonal activity and endocrine disrupting effects (Attina and Trasande 2015; Rochester and Bolden 2015; Rosenmai et al. 2014; Trasande and Attina 2015). Thus, it is critical to understand dietary sources of exposure to both legacy and replacement phthalates and bisphenols to inform intervention strategies to reduce currently relevant exposures in a changing chemical landscape.
To address these gaps, our primary objective was to determine whether ultra-processed food consumption was associated with urinary concentrations of phthalate or bisphenol biomarkers, including newer replacement chemicals, in a population-based survey of the general U.S. population in 2013–2014. Because prior studies have found demographic differences in sources of phthalate and bisphenol exposure, we also examined effect measure modification (EMM) by age, race/ethnicity, and poverty to income ratio. Finally, we evaluated associations with minimally processed food consumption to determine if eating less processed food is associated with lower exposure to phthalates and bisphenols.
2. Materials and Methods
2.1. Study population
We used data collected during the 2013–2014 cycle of National Health and Nutrition Examination Survey (NHANES), a nationally-representative population-based survey of the non-institutionalized U.S. population. Our rationale for using data collected during the 2013–2014 cycle is that both dietary data and chemical exposure data were available at this time point, and these data reflect a relatively recent assessment of the U.S. food supply. During an evaluation at the NHANES mobile examination center, participants completed questionnaires and a 24-hour dietary recall and provided a spot urine sample. The study protocol was approved by the National Center for Health Statistics (NCHS) Research Ethics Review Board and participants (or parents/guardians for participants <18 years of age) gave signed informed consent (CDC 2013).
Our study was limited to 2,686 participants aged 6 years and older who NCHS selected as part of a random one-third subsample for chemical analysis. We restricted our analyses to those with complete dietary and chemical exposure data (n=2,406). Then, we excluded 194 participants with missing covariate information (8% of participants) of whom 193 did not report income and 1 did not complete the physical activity assessment. The characteristics of those included in the analytic sample versus those who were excluded were very similar with no statistically significant differences (data not shown). Our final analytic sample was 2,212 participants.
2.2. Food processing classification
Trained interviewers who were fluent in English or Spanish collected 24-hour dietary recalls in-person using the validated US Department of Agriculture (USDA) Automated Multiple-Pass Method (AMPM) (Blanton et al. 2006; Moshfegh et al. 2008). For children 6–11 years of age, an adult household member assisted with the interview. Participants reported a detailed list of foods and beverages consumed in the last 24 hours. To provide estimates for portion sizes, 3-dimensional measuring guides such as bowls and glasses were provided.
We used the NOVA (a name, not an abbreviation) food processing classification system to classify each reported food and beverage in the 24-hour dietary recall (Monteiro et al. 2010). NOVA categories have 4 food processing levels: 1) unprocessed or minimally processed foods, 2) processed culinary ingredients, 3) processed foods, or 4) ultra-processed foods. The underlying rationale and details of the NOVA classification system have been published previously (Monteiro et al. 2010; Moubarac et al. 2017; Moubarac et al. 2014). Briefly, (1) “unprocessed or minimally processed foods” include plant and animal products, such as fruits, flours, and meat that are fresh or prepared without added substances such as sugar, salt, or oil. (2) “Processed culinary ingredients” encompass items derived from natural sources that are not typically consumed by themselves, but used as seasonings in the kitchen, e.g. honey, butter, salt. (3) “Processed foods” include unprocessed or minimally processed foods that have been prepared with processed culinary ingredients or preserved through various methods (e.g., canned vegetables and simple cheeses). (4) “Ultra-processed foods” include mass-produced, pre-packaged foods with industrial substances. Ingredients for ultra-processed foods rarely contain unprocessed or minimally processed foods, and often have additives that are uncommon in normal culinary preparations (e.g. flavorings, colorants, hydrolyzed protein, emulsifiers, and hydrogenated oils). Instant or ready-to-eat foods and the presence of artificial flavorings and preservatives are key characteristics of this food group. We provide examples of foods and beverages contained in each of the NOVA food processing categories in Supplemental Material, Table S1.
We downloaded the publicly available NHANES 2013–2014 individual food file (first day of dietary recall) in 2018, and merged this data with available information from the USDA Food and Nutrient Database for Dietary Studies (FNDDS) (U.S. Department of Agriculture 2016). Using steps outlined in previous studies (Martinez Steele et al. 2016; Martinez Steele and Monteiro 2017), we classified all foods and beverages reported by participants in the 24-hour dietary recall (N=38,303) according to the NOVA classification system by primarily using the NHANES dietary variables “main food description” and “additional food description.” Then, we made modifications to the classification using “source of food” and “combination food type” variables in NHANES individual food file. Most foods which were purchased from “restaurant fast food/pizza,” or “vending machine” or defined as “frozen meals” were classified as ultra-processed foods, in line with the definition of ultra-processed foods. If the main food description indicated that the food or beverage was made from a recipe, we assigned NOVA categories to the underlying ingredients (“standard reference description” variable), which we obtained from the USDA FNDDS. One author coded all of the items and a second author independently coded 20% of items to assure quality control.
We calculated percent of total energy intake from ultra- or minimally- processed foods using the approach outlined in previous studies (Martinez Steele et al. 2016; Martinez Steele et al. 2017). Briefly, we summed energy intake values of all foods and beverages consumed in the 24-hour period, and calculated the proportion of total energy intake from all four NOVA categories. For foods that were prepared from a recipe, we summed energy intake values for all underlying ingredients, and used the same method to calculate contributions of all NOVA categories to total energy. We obtained energy intake values for underlying ingredients from the USDA National Nutrient Database for Standard Reference 28 (U.S. Department of Agriculture 2017).
Additionally, we classified all ultra-processed foods and beverages into 18 food categories, by adapting the categories from Martinez Steele et al. 2016 (Supplemental Material, Table S2). To be consistent with previous studies, we classified most commercially-made breads as ultra-processed foods, because many breads did not have underlying ingredients in the NHANES database (Martinez Steele et al. 2016).
2.3. Biomarkers of phthalate and bisphenol exposures
We focused on chemicals potentially used in food packaging and processing including high molecular weight phthalates, bisphenol A, bisphenol F, and bisphenol S. Spot urine specimens were collected at the mobile examination center and stored at −20°C until analysis. Phthalate metabolites and total bisphenols (free plus conjugated) were quantified by the Centers for Disease Control and Prevention (CDC) National Center for Environmental Health laboratory using solid phase extraction coupled with high performance liquid chromatography–isotope dilution tandem spectrometry (Silva et al. 2007; Zhou et al. 2014). High molecular weight phthalate metabolites included in the current study were mono-benzyl (MBzP, a metabolite of BBzP), mono-(3-carboxypropyl) (MCPP, a nonspecific metabolite of several high and low molecular weight phthalates, including DOP), mono(carboxyisononyl) (MCNP, a metabolite of DiDP), mono(carboxyisoctyl) (MCOP, a metabolite of DiNP), and four metabolites of DEHP: mono(2-ethyl-5-carboxypentyl) (MECPP), mono(2-ethyl-5-hydroxyhexyl) (MEHHP), mono(2-ethyl-5-oxohexyl) (MEOHP), and mono(2-ethylhexyl) (MEHP) phthalates.
2.4. Covariates
We identified potential confounders from the literature and used directed acyclic graphs to identify variables on non-causal backdoor pathways between ultra-processed food consumption and chemical exposure. In NHANES, participants self-reported age, gender, race/ethnicity, income (used to calculate poverty to income ratio), total energy intake, and physical activity. Individuals ≥12 years of age reported frequency and duration of physical activity in a typical week. We calculated MET (metabolic equivalent of task)-hours per week for each individual by multiplying suggested MET scores for each activity and duration (CDC 2017). In multivariable analyses including children, we used number of days physically active as a continuous variable to adjust for physical activity because the physical activity questionnaire (which assessed frequency and duration of physical activity) was not administered to children <12 years of age. In age-stratified analyses, for adults and adolescents, we used a continuous MET score as a covariate.
Trained staff measured participants’ height and weight at the mobile examination center. We used this information to calculate body mass index (BMI, kg/m2), and classified participants as underweight (<18.5), normal weight (18.5–<25), overweight (25–<30), and obese (≥30). For children and adolescents, we used sex-specific BMI-for-age growth charts, and calculated underweight (<5th percentile), normal weight (5th–<85th percentile), overweight (85th–<95th percentile), and obese (≥95th percentile). Urinary creatinine was measured using the Roche/Hitachi Cobas 6000 Analyzer (CDC 2016).
2.5. Statistical analysis
We singly imputed biomarker values below the limit of detection from a truncated normal distribution (Buckley et al. 2016; Lubin et al. 2004). We calculated a ∑DEHP molar sum by adding the concentrations of four DEHP metabolites (MEHP, MECPP, MEHHP, and MEOHP), each inversely weighted by its molar weight. We multiplied this summed variable by the molar weight of MECPP to express ∑DEHP concentrations as μg/L of MECPP (Braun et al. 2017).
To account for urinary dilution, we calculated covariate-adjusted, creatinine-standardized concentrations as recommended by (O’Brien et al. 2016). Briefly, we modeled natural log creatinine as a function of its predictors reported previously, including age, gender, race/ethnicity, body mass index category, and height (Barr et al. 2005), and output predicted natural log creatinine values. We exponentiated the predicted value then divided the phthalate metabolite or bisphenol concentration by the ratio of the participant’s observed to predicted creatinine concentration. Given that distributions were skewed, we took the natural log of the covariate-adjusted, creatinine-standardized concentrations to approximate a normal distribution and reduce heteroscedasticity in our models. Based on O’Brien et al. (2016), we additionally adjusted the outcome models for natural log urinary creatinine to account for potential residual confounding.
We examined baseline characteristics of the participants and urinary phthalate metabolite and bisphenol concentrations by quartiles of percent of total energy intake from ultra-processed food using chi-squared tests for categorical variables and analysis of variance (ANOVA) for continuous variables.
We used multivariable linear regression models to examine whether proportion of total energy intake from ultra-processed food was associated with urinary concentrations of each natural log-transformed, covariate-adjusted, creatinine-standardized phthalate metabolite and bisphenol biomarker. Model 1 adjusted only for natural log urinary creatinine (O’Brien et al. 2016). Model 2 further adjusted for age (continuous), sex (male, female), race/ethnicity (non-Hispanic white, non-Hispanic black, Mexican American, Asian American, other), income (poverty to income ratio <130%, 130–<350%, ≥350%), total energy intake (continuous), physical activity (continuous), and BMI (underweight, normal weight, overweight, obese). From the regression models, we calculated percent difference in urinary phthalate metabolite and bisphenol concentrations using (e(β)-1) × 100% by ultra- or minimally processed food intake (Zota et al. 2016). We considered the estimates from this analysis as the main results.
We conducted several additional analyses to further explore relationships between phthalate metabolite and bisphenol concentrations with ultra-processed food consumption. First, we assessed dose-response relationships by fitting models with quartiles of total energy intake from ultra-processed food. Second, we tested for effect measure modification by age group (children aged 6–<12 years, adolescents aged 12–<20 years, and adults aged ≥20 years), race/ethnicity (non-Hispanic white, non-Hispanic black, Mexican-American, Asian-American, other race), and poverty to income ratio (<130%, 130–<350%, ≥350%). Third, we explored associations of ultra-processed food groups with chemical biomarkers by examining the percent difference in urinary phthalate metabolite and bisphenol concentrations associated with a one-percent higher total energy intake from each of 18 ultra-processed food and beverage groups. We used a one-percent difference because the median intake for individual categories of ultra-processed food was 4.5% (range: 0.5% to 11%). Fourth, we assessed associations of phthalate metabolite and bisphenol concentrations with minimally processed food consumption by repeating analyses above but using total energy intake from minimally processed food (rather than ultra-processed food) as independent variables.
Finally, we conducted several sensitivity analyses: 1) adjusted for total energy intake from fat, 2) examined EMM by total energy intake from fast food to determine whether associations differed by food source, 3) excluded participants who were fasting for more than 12 hours (n=534), and 4) combined the minimally processed food and processed culinary ingredients categories given that both are derived from natural sources.
We considered associations to be statistically significant at p<0.05 for main effects and p<0.1 for effect measure modification. All analyses accounted for the complex survey design using appropriate survey weights. We conducted analyses using SAS version 9.4 (SAS Institute Inc.) and STATA 13.0 (StataCorp).
3. Results
In our analytic sample, the proportion of total energy intake from ultra-processed food ranged from 0 to 100%, with those in the highest quartile having 79.2 to 100% of total energy intake from ultra-processed food (Table 1). Participants in the highest quartile of ultra-processed food intake were more likely to be younger, non-Hispanic black and other race, have a poverty to income ratio <130%, and be obese than those in the lowest quartile (Table 1). The food groups with the greatest contribution to ultra-processed food consumption were soft drinks and fruit drinks (11.0% of total energy); ready-to-eat-pizza (9.4%); breads and tortillas (8.5%); ready-to-eat sandwiches and hamburgers (8.1%); reconstituted meat or fish products (8.1%); frozen and shelf-stable plate meals (6.5%); cakes, cookies, and pies (5.9%); and French fries and other potato products (5.9%). The proportion of total energy intake from minimally processed food ranged from 0 to 93.8%, with those in the highest quartile having 33.5%–93.8% of total energy intake from minimally processed food (Supplemental Material, Table S3). Urinary phthalate metabolites and bisphenols were detected in >95% of participants with the exception of MEHP (57.8%), bisphenol F (67.3%), and bisphenol S (88.9%) (Supplemental Material, Table S4).
Table 1.
Baseline participant characteristics according to quartiles of percent of total energy intake from ultra-processed food, National Health and Nutrition Examination Survey (NHANES) 2013–2014 (n=2,212)
| Characteristic | Quartile 1: 0-<46.7% (n=553) | Quartile 2: 46.7-<63.1% (n=553) | Quartile 3: 63.1-<79.2% (n=553) | Quartile 4: 79.2%−100% (n=553) | P-value |
|---|---|---|---|---|---|
| % of total energy intake from ultraprocessed food (median) | 35.3 | 55.6 | 70.4 | 87.2 | <0.001 |
| % of total energy intake from processed culinary ingredients (median) | 6.1 | 3.2 | 2.2 | 0 | <0.001 |
| % of total energy intake from processed food (median) | 17.4 | 12.1 | 7.5 | 0.9 | <0.001 |
| % of total energy intake from minimally processed food (median) | 39.5 | 25.9 | 17.2 | 6.7 | <0.001 |
| Age group | |||||
| Children (6-<12y) | 41 (11.9) | 75 (22.5) | 94 (30.2) | 107 (35.3) | |
| Adolescents (12<20y) | 60 (14.0) | 79 (19.3) | 115 (30.9) | 133 (35.8) | <0.001 |
| Adults (≥20y) | 452 (27.1) | 399 (27.3) | 344 (23.4) | 313 (22.0) | |
| Age, years | 45.9 ± 1.7 | 43.5 ± 1.2 | 38.3 ± 1.3 | 33.4 ± 1.0 | <0.001 |
| Female sex | 290 (24.5) | 271 (24.2) | 288 (24.7) | 325 (26.6) | 0.06 |
| Race/ethnicity | |||||
| Non-Hispanic white | 193 (23.8) | 223 (26.4) | 209 (24.6) | 236 (25.1) | |
| Non-Hispanic black | 90 (16.6) | 112 (23.4) | 147 (28.5) | 146 (31.4) | |
| Mexican American | 136 (23.7) | 160 (30.1) | 134 (24.4) | 122 (21.8) | <0.001 |
| Asian American | 118 (55.9) | 43 (18.8) | 35 (15.6) | 19 (9.6) | |
| Other | 16 (16.9) | 15 (20.5) | 28 (32.7) | 30 (29.8) | |
| Poverty level | |||||
| <130% | 188 (21.2) | 225 (25.4) | 214 (23.6) | 235 (29.7) | |
| 130-<350% | 184 (23.9) | 156 (22.5) | 187 (25.5) | 208 (28.0) | 0.007 |
| ≥350% | 181 (26.6) | 172 (29.9) | 152 (25.1) | 110 (18.3) | |
| Number of days physically activea | 3.4 ± 0.1 | 3.6 ± 0.1 | 3.9 ± 0.2 | 3.7 ± 0.1 | 0.10 |
| Body mass index, kg/m2 | 27.5 ± 0.4 | 28.5 ± 0.5 | 27.8 ± 0.3 | 27.7 ± 0.4 | 0.35 |
| Body mass index category | |||||
| Underweight | 13 (24.8) | 11 (25.8) | 14 (24.9) | 15 (24.4) | |
| Normal weight | 204 (26.2) | 181 (22.8) | 213 (25.5) | 213 (25.5) | 0.03 |
| Overweight | 185 (27.8) | 167 (27.2) | 146 (23.5) | 130 (21.5) | |
| Obese | 151 (19.3) | 194 (28.2) | 180 (25.4) | 195 (26.9) |
Note: Values are means ± standard errors for continuous variables and n (row percentages) for categorical variables. All analyses accounted for the complex survey design of NHANES by using appropriate sample weights.
For adolescents and adults (≥12 years of age), physical activity included number of days of vigorous and moderate work-related activity, walking or bicycling, and vigorous and moderate recreational activities in the past 7 days. For children (6–<12 years of age), physical activity was assessed by using number of days physically active in the past 7 days.
3.1. Associations of ultra-processed food consumption with urinary phthalate metabolite and bisphenol concentrations
In unadjusted comparisons, geometric mean covariate-adjusted, creatinine-standardized concentrations of bisphenol A and all phthalate metabolites except ∑DEHP were significantly higher with increasing quartiles of total energy intake from ultra-processed food (Table 2). In fully adjusted models, greater ultra-processed food intake was associated with higher urinary MCPP, MCNP, and MCOP concentrations (Table 3). The strongest relationship was between ultra-processed food consumption and MCOP; each 10% higher total energy intake from ultra-processed food was associated with 8.0% (95% CI: 5.6%, 10.3%) higher urinary MCOP concentrations.
Table 2.
Urinary phthalate metabolite and bisphenol concentrations (ug/g-creatinine) according to quartiles of percent of total energy intake from ultra-processed food, National Health and Nutrition Examination Survey 2013–2014 (n=2,212)
| Phthalate metabolite or bisphenol | Quartile 1: 0-<46.7% (n=553) | Quartile 2: 46.7-<63.1% (n=553) | Quartile 3: 63.1-<79.2% (n=553) | Quartile 4: 79.2%-100% (n=553) | P-value |
|---|---|---|---|---|---|
| Summed di(2-ethylhexyl) (∑DEHP)a | 22.7 (1.8) | 23.2 (1.1) | 24.3 (1.4) | 26.3 (1.4) | 0.08 |
| Mono-benzyl (MBzP) | 4.1 (0.3) | 3.9 (0.2) | 4.5 (0.3) | 5.6 (0.5) | 0.001 |
| Mono-(3-carboxypropyl) (MCPP) | 1.8 (0.1) | 2.1 (1.5) | 2.3 (0.2) | 2.8 (0.2) | 0.001 |
| Mono-(carboxyisononyl) (MCNP) | 2.3 (0.1) | 2.9 (0.2) | 3.0 (0.1) | 3.3 (0.2) | 0.001 |
| Mono-(carboxyisoctyl) (MCOP) | 16.7 (1.2) | 21.1 (1.5) | 21.8 (1.7) | 31.9 (1.7) | <0.001 |
| Bisphenol A | 1.2 (0.6) | 1.2 (0.7) | 1.4 (0.9) | 1.4 (0.6) | 0.004 |
| Bisphenol F | 0.5 (0.04) | 0.4 (0.03) | 0.4 (0.03) | 0.4 (0.03) | 0.43 |
| Bisphenol S | 0.5 (0.05) | 0.5 (0.03) | 0.5 (0.05) | 0.6 (0.05) | 0.06 |
Note: Values are geometric means (standard errors) of covariate-adjusted, creatinine-standardized concentrations.
Molar sum of mono(2-ethyl-5-carboxypentyl) (MECPP), mono(2-ethyl-5-hydroxyhexyl) (MEHHP), mono(2-ethyl-5-oxohexyl) (MEOHP), and mono(2-ethylhexyl) (MEHP) phthalates.
Table 3.
Percent difference (95% confidence interval) in covariate-adjusted, creatinine-standardized urinary phthalate metabolite and bisphenol concentrations associated with ten percent higher total energy intake from ultra- or minimally- processed food, National Health and Nutrition Examination Survey 2013–2014 (n=2,212)
| Phthalate metabolite or bisphenol | Ultra-processed | Minimally processed | ||
|---|---|---|---|---|
| Model 1a | Model 2b | Model 1a | Model 2b | |
| Summed di(2-ethylhexyl) (∑DEHP)c | 2.1 (0.3, 3.9)* | 0.1 (−1.8, 2.1) | −2.8 (−4.8, −0.8) * | −1.0 (−3.5, 1.5) |
| Mono-benzyl (MBzP) | 6.0 (3.9, 8.2)* | 0.9 (−1.3, 3.2) | −7.9 (−11.6, −4.1)* | −3.2 (−7.3, 0.8) |
| Mono-(3-carboxypropyl) (MCPP) | 7.8 (5.9, 9.2)* | 5.3 (3.5, 7.2)* | −10.5 (−13.5, −7.4) * | −7.2 (−10.5, −3.7)* |
| Mono-(carboxyisononyl) (MCNP) | 6.4 (4.0, 8.7)* | 4.6 (2.5, 6.8)* | −9.0 (−12.8, −5.0)* | −6.1 (−9.9, −2.2)* |
| Mono-(carboxyisoctyl) (MCOP) | 9.9 (7.8, 12.1)* | 8.0 (5.6, 10.3)* | −12.8 (−16.4, −9.2)* | −10.1 (−13.9, −6.2)* |
| Bisphenol A | 3.5 (1.1, 5.9)* | 1.0 (−1.5, 3.2) | −6.4 (−9.2, −3.5)* | −3.6 (−6.1, −1.2)* |
| Bisphenol F | 5.2 (1.3, 9.1)* | 3.6 (−0.6, 7.9) | −10.0 (−15.0, −4.9)* | −6.9 (−11.9, −1.8)* |
| Bisphenol S | −0.2 (−3.7, 3.3) | −1.4 (−4.7, 1.9) | 1.6 (−2.3, 5.5) | 3.1 (−0.2, 6.4) |
Adjusted for natural log urinary creatinine.
Model 1 with additional adjustment for age, gender, race/ethnicity, poverty:income ratio, total energy intake, physical activity, and body mass index.
Molar sum of mono(2-ethyl-5-carboxypentyl) (MECPP), mono(2-ethyl-5-hydroxyhexyl) (MEHHP), mono(2-ethyl-5-oxohexyl) (MEOHP), and mono(2-ethylhexyl) (MEHP) phthalates.
p<0.05
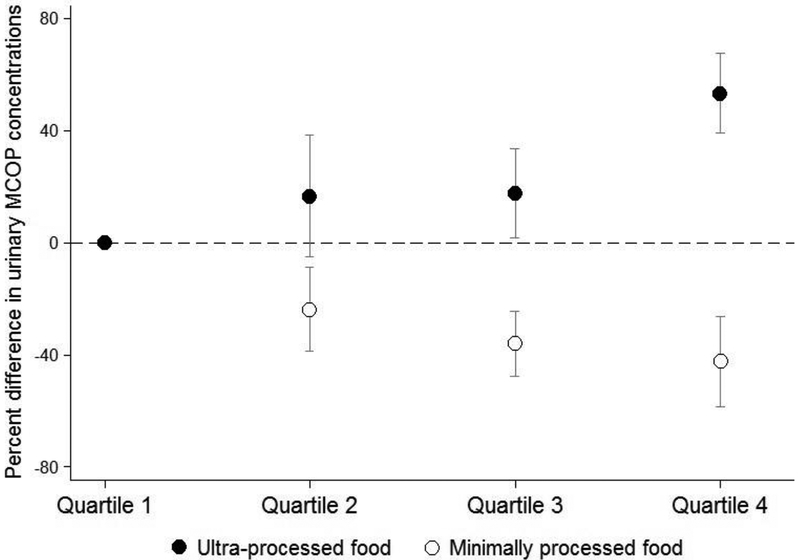
There was a monotonic dose-response relationship between higher quartile of ultra-processed food intake with higher concentrations of MCPP, MCNP, and MCOP (Supplemental Material, Table S5). Notably, urinary MCOP concentrations were 53.4% (95% CI: 39.3%, 67.5%) higher among participants in the fourth quartile of ultra-processed food intake compared to the lowest quartile (Figure 1). Associations of bisphenols with quartiles of ultra-processed food intake did not exhibit monotonic dose-response relationships (Supplemental Material, Table S5).
Figure 1.
Percent difference (95% confidence interval) in covariate-adjusted, creatinine-standardized urinary mono-(carboxyisoctyl) phthalate (MCOP) concentrations according to quartiles of total energy intake from ultra- or minimally processed food, National Health and Nutrition Examination Survey 2013–2014 (n=2,212)
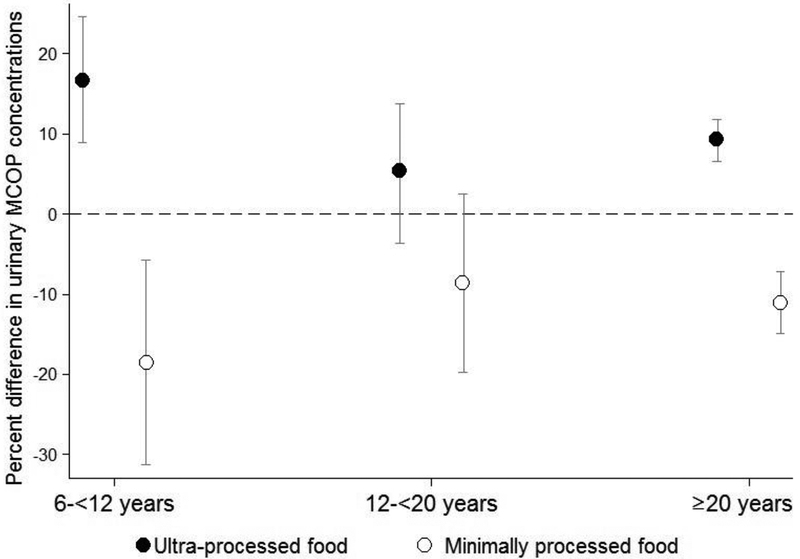
Associations of ultra-processed food intake with ∑DEHP and MCOP were significantly modified by age group (EMM p-value = 0.04), with larger percent differences in metabolite concentrations among children as compared with adolescents and adults (Supplemental Material, Table S6). Among children, each 10% higher total energy intake from ultra-processed food was associated with a 16.8% (95% CI: 8.9%, 24.6%) higher urinary MCOP concentrations (Figure 2). We did not observe statistically significant effect measure modification by race/ethnicity or poverty to income ratio (all EMM p-values >0.1, data not shown).
Figure 2.
Percent difference (95% confidence interval) in covariate-adjusted, creatinine-standardized urinary mono-(carboxyisoctyl) phthalate (MCOP) concentrations associated with ten percent higher total energy intake from ultra- or minimally processed food, stratified by age group, National Health and Nutrition Examination Survey 2013–2014 (n=2,212)
Phthalate metabolite and bisphenol concentrations were associated with total energy intake from several ultra-processed food groups (Table 4). Sandwiches and hamburgers; French fries and other potato products; ice cream and ice pops; and sauces, dressings, and gravies were generally associated with higher urinary MCPP, MCNP, and MCOP concentrations whereas salty snacks; sweet snacks; and instant and canned soups were associated with lower concentrations. Certain ultra-processed food groups were associated with higher urinary bisphenol A (soft and fruit drinks), bisphenol F (French fries and other potato products; milk-based desserts) and bisphenol S (pizza) concentrations or lower bisphenol F concentrations (pizza). No ultra-processed food group was associated with differences in MBzP concentrations, and there were no statistically significant associations of any phthalate or bisphenol biomarker with proportion of total energy intake from breads and tortillas; reconstituted meat or fish products; frozen and shelf-stable plate meals; cakes, cookies, and pies; milk-based drinks; breakfast cereals; yogurt; or other ultra-processed foods (data not shown).
Table 4.
Percent difference (95% confidence interval) in covariate-adjusted, creatinine-standardized urinary phthalate metabolite and bisphenol concentrations associated with one percent higher total energy intake from ultra-processed food groups, National Health and Nutrition Examination Survey 2013–2014 (n=2,212)
| Ultra-processed food group | ∑DEHPa | MCPP | MCNP | MCOP | Bisphenol A | Bisphenol F | Bisphenol S |
|---|---|---|---|---|---|---|---|
| Soft and fruit drinks | −0.2 (−0.6, 0.1) | 0.3 (−0.3, 0.9) | 0.3 (−0.3, 0.9) | 0.1 (−0.7, 0.8) | 0.6 (0.1, 1.3)* | 0.8 (−0.3, 1.9) | 0.3 (−0.2, 0.8) |
| Pizza (ready-to-eat/heat) | −0.1 (−0.3, 0.1) | 0.3 (−0.1, 0.1) | 0.1 (−0.1, 0.4) | 0.4 (−0.1, 0.1) | −0.1 (−0.3, 0.2) | −0.7 (−0.1, - 0.01)* | 0.5 (0.1, 0.9)* |
| Sandwiches and hamburgers on a bun | 0.7 (0.1, 1.3)* | 1.6 (0.9, 2.2)* | 0.9 (0.4, 1.3)* | 1.8 (1.0,2.5)* | 0.3 (−0.2, 0.7) | 1.02 (−0.07, 2.1) | 0.41 (−2.1, 1.0) |
| French fries and other potato products | 0.1 (−0.5, 0.8) | 0.9 (−0.09, 1.8) | 0.06 (−0.06,1.4) | 1.5 (0.6, 2.3)* | 0.8 (−0.2, 1.7) | 1.8 (0.4, 3.1)* | −0.4 (−1.6, 0.8) |
| Salty snacks | −0.4 (−0.7, 0.06) | −0.9 (−1.5, −0.2)* | 0.05 (−0.8, 0.3) | −0.6 (−1.4, 0.2) | −0.001 (−0.5, 0.5) | −0.9 (−2.0, 0.2) | −0.7 (−1.6, 0.1) |
| Sweet snacks | −0.04 (−0.7, 0.6) | −1.1 (−2.2, 0.5) | −1.0 (−1.8, −0.3)* | −1.3 (−2.5, 0.5) | −0.7 (−1.3, 0.03) | −0.2 (−1.7, 1.3) | −0.3 (−1.4, 0.8) |
| Milk-based desserts | 0.2 (−0.5, 0.8) | 0.8 (−0.4, 2.0) | 0.05 (−0.3, 1.2) | 0.9 (−0.4, 2.3) | −0.3 (−0.1, 0.4) | 2.3 (0.2, 4.3)* | −0.8 (−1.6, 0.3) |
| Ice cream and ice pops | 0.5 (−0.1, 1.2) | 1.2 (−0.1, 2.6) | 1.5 (0.6, 2.5)* | 1.6 (0.2, 3.0)* | 0.1 (−0.8, 0.9) | −0.7 (−2.1, 0.6) | −0.9 (−2.2, 0.4) |
| Sauces, dressings, and gravies | −0.1 (−1.2, 0.9) | 1.2 (−0.5, 2.8) | 1.4 (0.08, 2.6)* | 1.6 (−0.1, 3.3) | −0.002 (−0.6, 0.7) | 1.7 (−1.2, 4.6) | 0.8 (−0.9, 2.5) |
| Instant and canned soups | −0.6 (−1.7, 0.5) | −1.6 (−3.4, 0.2) | −2.0 (−3.6, −0.4)* | −2.9 (−5.2, −0.4)* | 0.7 (−0.3, 1.7) | −1.1 (−3.9, 1.8) | −0.6 (−2.8, 1.8) |
Note: ∑DEHP, di(2-ethylhexyl) phthalate, MCPP, mono-(3-carboxypropyl) phthalate; MCNP, mono(carboxyisononyl) phthalate; MCOP, mono(carboxyisoctyl) phthalate. Models are adjusted for natural log urinary creatinine, age, gender, race/ethnicity, poverty:income ratio, total energy intake, physical activity, and body mass index.
Molar sum of mono(2-ethyl-5-carboxypentyl) (MECPP), mono(2-ethyl-5-hydroxyhexyl) (MEHHP), mono(2-ethyl-5-oxohexyl) (MEOHP), and mono(2-ethylhexyl) (MEHP) phthalates.
p<0.05
3.2. Associations of minimally processed food consumption with urinary phthalate metabolite and bisphenol concentrations
In unadjusted comparisons, concentrations of bisphenol A, bisphenol S, and all phthalate metabolites except ∑DEHP were significantly lower across increasing quartiles of total energy intake from minimally processed food (Supplemental Material, Table S7). In adjusted models, greater consumption of minimally processed food was associated with lower urinary concentrations of MCPP, MCNP, MCOP, and bisphenols A and F (Table 3). Similar to the analyses of ultra-processed foods, the strongest association was with MCOP: each 10% higher total energy intake from minimally processed food was associated with 10.1% (95% CI: −13.9%, −6.2%) lower urinary MCOP concentrations.
In analyses examining associations by quartile of minimally processed food intake, we observed monotonic relationships of lower levels of MCPP, MCNP, and MCOP with increasing quartile of minimally processed food (Supplemental Material, Table S8). For example, the highest quartile of minimally processed food intake was associated with −42.3% (−58.6%, −26.3%) lower urinary concentrations of MCOP compared to the lowest quartile (Figure 1). Bisphenols did not exhibit dose-response relationships, though the third quartile of minimally processed food intake was associated with lower concentrations of bisphenol A (percent difference: −15.2%, 95% CI: −25.5%, −4.8%) and bisphenol F (percent difference: −27.1%, 95% CI: −44.4%, −9.1%) (Supplemental Material, Table S8)
We observed statistically significant modification by age group for ∑DEHP and bisphenol A, with stronger associations among children for ∑DEHP (EMM p-value = 0.05) but stronger associations among adults for bisphenol A (EMM p-value = 0.07) (Supplemental Material, Table S9). Similar to our findings in analyses of ultra-processed foods, there was no statistically significant effect measure modification by race/ethnicity or poverty to income ratio (all EMM p-values >0.1, data not shown).
3.3. Sensitivity analyses
There were no substantial differences in magnitudes of association, or changes in statistical significance, compared to the original findings when we additionally adjusted for percent of total energy intake from fat (data not shown). Associations of ultra-processed food intake with phthalate metabolite and bisphenol concentrations were not modified by total energy intake from fast food (all EMM p-values >0.1). In analyses restricted to non-fasting NHANES participants, results were very similar to the primary analyses although positive associations of ultra-processed food with bisphenol F and of minimally processed food with bisphenol S became statistically significant (Supplemental Material, Table S10). Finally, findings were similar to the primary minimally processed food analyses when we combined the minimally processed food and processed culinary ingredients categories (Supplemental Material, Table S11).
4. Discussion
In this cross-sectional study of a representative sample of the U.S. population in 2013–2014, we found that higher ultra-processed food consumption was associated with higher urinary concentrations of MCPP, MCNP, and MCOP but not MBzP, ∑DEHP metabolites, or bisphenols. Individuals in the highest quartile of ultra-processed food consumption as a proportion of total energy intake had 25–50% higher urinary concentrations of MCPP, MCNP, and MCOP than those in the lowest quartile. Notably, we observed that individuals with higher consumption of minimally processed food had lower concentrations of the same phthalate metabolites and bisphenols A and F. To our knowledge, this study is the first to quantify relationships between ultra-processed food consumption and phthalate and bisphenol exposures.
While previous studies assessed intake of specific food items, our study adds to prior research by focusing on a broader category of highly processed foods defined a priori. Taking dairy as an example, whole or reduced milk and plain yogurt are classified as minimally processed whereas sweetened milk, sweetened yogurt, and ice cream are classified as ultra-processed. In our study, the ultra-processed food groups most strongly associated with phthalate exposures were sandwiches/hamburgers on a bun; French fries and other potato products; sweet snacks; ice cream/ice pops; and sauces, dressings, and gravies. Many of these foods are typically purchased at restaurant or fast food venues, which is consistent with prior studies reporting associations of phthalate exposures with consuming food away from home (Munoz et al. 2018; Varshavsky et al. 2018; Zota et al. 2016).
Concentrations of the phthalate metabolites associated with ultra-processed food consumption in the current study, MCPP, MCNP, and MCOP, are increasing over time in the U.S. while concentrations of DEHP metabolites and MBzP, which were not associated with ultra-processed foods, are decreasing (Koch et al. 2017; Zota et al. 2014). Our findings likely reflect these temporal changes in use of legacy and replacement phthalates, which may also contribute to differences in our results compared to older studies. Most other recent studies have also reported stronger associations of food or food venues with MCOP than with metabolites of DEHP (Varshavsky et al. 2018; Watkins et al. 2014; Zota et al. 2016) and weak or null associations of food sources with MBzP concentrations (Munoz et al. 2018; Rudel et al. 2011; Varshavsky et al. 2018). In our study, associations were strongest for MCOP, a metabolite of the DEHP substitute DiNP, which was the most commonly detected phthalate in a Norwegian study examining phthalates in food samples (Sakhi et al. 2014).
We did not observe associations of ultra-processed food consumption with concentrations of bisphenols, which are used in epoxy resins and polycarbonate plastics such as bottles and can linings (Cabaton et al. 2009; Carwile et al. 2009). The NOVA classification system categorizes canned vegetables and seafood as processed, not ultra-processed if there is no industrial substances, whereas instant and canned soups are considered ultra-processed. When we examined individual ultra-processed food groups, bisphenol A was associated with soft or fruit drink consumption but not instant or canned soups. Our findings are in contrast to a study of NHANES 2003–2008 participants which found that canned soup was strongly associated with bisphenol A concentrations while canned beverage consumption was not (Hartle et al. 2016). Differences in findings could be due to our focus on ultra-processed food, the inclusion of items stored in bottles or other containers besides cans, or to differences in the use of bisphenol A in food and beverage containers over time. Fast food consumption was not associated with bisphenol A concentrations in a previous report (Zota et al. 2016), also suggesting that bisphenols exposure may be more strongly related to container type than overall dietary patterns such as food venue or degree of processing.
While there is limited research on dietary sources of bisphenols S and F, they have been detected in foods and beverages including canned items and soda (Gallart-Ayala et al. 2011; Liao and Kannan 2013; Vinas et al. 2010). In the present study, higher consumption of French fries and other potato products and milk-based desserts was associated with higher bisphenol F concentrations, and individuals who consumed more minimally processed foods had lower bisphenol F concentrations. In contrast, bisphenol S was only associated with a single ultra-processed food group (pizza) and not with overall consumption of ultra- or minimally processed food. These findings align with a study measuring bisphenols in U.S. food samples that found bisphenols A and F at higher concentrations than bisphenol S (Liao and Kannan 2013), suggesting that bisphenol S is used less frequently in food contact or production materials. As noted above, additional research is needed to determine food sources of these and other bisphenol A analogues given that our focus on ultra-processed food consumption may not capture the primary dietary sources of bisphenols.
We found some unexpected associations; in particular, consuming instant/canned soup and sweet or salty snacks were inversely associated with MCPP, MCNP, or MCOP concentrations and consuming pizza was inversely associated with bisphenol F concentrations. We suspect these associations may be due to confounding whereby individuals who ate ultra-processed soups, snacks, or pizza were less likely to consume other categories of ultra-processed food or to be exposed to other phthalate sources. Alternatively, we conducted a large number of statistical tests and cannot rule out chance findings.
Our finding that higher consumption of minimally processed food was associated with lower urinary concentrations of several phthalates and bisphenols aligns with previous studies reporting lower concentrations of these chemicals among those consuming a healthy or fresh food diet (Correia-Sa et al. 2018; Correia-Sa et al. 2017; Rudel et al. 2011; Serrano et al. 2014b). For example, a dietary intervention study in which individuals consumed only foods not canned or packaged in plastic for three days observed substantial decreases in geometric mean concentrations of bisphenol A (66%) and DEHP metabolites (53–56%) from pre- to post-intervention (Rudel et al. 2011). In our study, individuals in the highest versus lowest quartile of minimally processed food consumption had 23–42% lower concentrations of MCPP, MCNP, MCOP, and bisphenol F, which were not measured by Rudel et al. In contrast, another dietary intervention trial found a substantial unexpected increase in urinary concentrations of DEHP metabolites among individuals during a complete dietary replacement aimed at reducing phthalate and bisphenol A exposures (Sathyanarayana et al. 2013). The increase was likely due to contaminated dairy and spices, suggesting that individual-level dietary modification may not be a feasible approach for reducing general population exposures given the ubiquity of these chemicals in the food supply and current gaps in knowledge regarding sources of contamination. Still, the strong relationship between minimally processed food intake and lower exposure to newer phthalates and bisphenols observed in our study indicates that a fresh food diet may reduce exposure to these replacement chemicals. Future dietary intervention research focusing on reducing consumption of ultra-processed food in favor of minimally processed food is needed to confirm this finding.
We observed a stronger association of urinary MCOP concentrations, a DiNP metabolite, with ultra-processed food consumption among children as compared with adolescents or adults. Each 10% higher percent total energy intake from ultra-processed foods was associated with 17% higher MCOP concentrations in children, compared to 5% higher concentrations among adolescents and 9% higher among adults. This finding may indicate that the types of ultra-processed food consumed by children have greater contamination with DiNP than foods consumed by older individuals, or may reflect age differences in phthalate metabolism. Two prior studies did not observe stronger associations of fast food consumption or dining out with phthalate exposure among children than adults (Varshavsky et al. 2018; Zota et al. 2016), possibly because offerings at fast food venues, restaurants, and cafeterias are more homogenous by age group compared with our analysis of overall dietary intake from all sources. The association of urinary ∑DEHP concentrations with ultra-processed food consumption was also modified by age group, though we were underpowered to detect statistically significant main effects. Larger studies are needed to fully investigate subgroup differences in associations.
The strong association of the DiNP metabolite MCOP with ultra-processed food in children is concerning given that DiNP has antiandrogenic effects, albeit less potent than DEHP (Chronic Hazard Advisory Panel on Phthalates and Phthalate Alternatives 2014), and is listed by California’s Proposition 65 as a known carcinogen (Office of Environmental Health Hazard Assessment 2019). The Chronic Hazard Advisory Panel on Phthalates and Phthalate Alternatives recommended a permanent ban on DiNP in children’s toys and childcare articles (Chronic Hazard Advisory Panel on Phthalates and Phthalate Alternatives 2014). It is notable that children and adolescents in our sample consumed a greater proportion of total energy intake from ultra-processed foods than adults. Thus, our findings support the American Academy of Pediatrics’ policy statement that recommends prioritizing fresh or frozen fruit and vegetable consumption and avoiding processed meats as an additional way to reduce chemical exposures in children (Trasande et al. 2018).
Our application of the NOVA classification system to detailed dietary information reported in the NHANES 24-hour recall allowed us to classify all foods consumed in the past day rather than relying on less detailed assessments focusing on usual intake (e.g., food frequency questionnaires). Although self-reported dietary intake is subject to misclassification, NHANES used standardized approaches to collect dietary data and the AMPM method has been validated (Blanton et al. 2006; Moshfegh et al. 2008). Still, there may have been some misclassification due to lack of detailed ingredient lists or product brands for some food items. We classified ultra-processed food to 18 food groups by adapting food group lists reported in previous studies (Martinez Steele et al. 2016; Martinez Steele et al. 2016) but classification was sometimes difficult to standardize due to limited detail in these lists. Future work categorizing ultra-processed foods should provide more information on what each food group is comprised of to facilitate such standardization.
An important strength of our study is the coordinated collection of urine samples and dietary information to represent exposures during approximately the same 24-hour period. These data provide the relevant temporal ordering for investigating sources of rapidly eliminated chemicals despite our cross-sectional study design. We analyzed data from the most recent NHANES cycle with available data in order to examine currently used phthalates and bisphenols, including both legacy and replacement phthalates and bisphenols. We also conducted a number of sensitivity analyses and found our results were robust to adjustment for total energy intake from fat and the exclusion of fasting participants.
It is difficult to disentangle ultra-processed food consumption from previously identified dietary predictors given that most of these foods and food sources are defined as ultra-processed according to the NOVA classification system. We found that observed ultra-processed food associations were not modified by fast food intake, suggesting that ultra-processed foods contribute to exposures regardless of whether or not they were purchased at a fast food restaurant. Nevertheless, we could not determine the source of food contamination in this observational study. Besides food processing, food packaging and handling may contribute to phthalate content of ultra-processed food. Future studies are needed to determine points along the production line that cause chemical contamination and to identify appropriate alternatives.
5. Conclusions
In summary, we found that ultra-processed food consumption was associated with increased urinary concentrations of several phthalate metabolites including MCPP, MCNP, and MCOP in a representative sample of the general U.S. population. Consuming minimally processed foods was associated with lower concentrations of these same phthalate metabolites as well as bisphenols A and F. Additional research is needed to determine whether minimally processed food diets or changes in food production practices can reduce phthalate and bisphenol exposures and related health effects, particularly among children who are more vulnerable to toxicants and tend to consume more ultra-processed food than adults.
Supplementary Material
Highlights.
Ultra-processed food is related to higher levels of phthalates but not bisphenols
Minimally processed food is related to lower levels of phthalates and bisphenols A and F
Magnitudes of some associations are stronger among children
Chemical exposures may contribute to adverse health effects of ultra-processed food
Acknowledgements
This study was supported by grant funding from the National Institutes of Health (JPB: P30 DK072488, R01 ES030078; CMR: K01 DK107782, R21 HL143089). The findings and conclusions in this manuscript are those of the authors and do not necessarily represent the official position of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing financial interests: The authors declare they have no actual or potential competing financial interests.
References
- Attina TM; Trasande L Association of Exposure to Di-2-Ethylhexylphthalate Replacements With Increased Insulin Resistance in Adolescents From NHANES 2009–2012. J Clin Endocrinol Metab 2015;100:2640–2650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr DB; Wilder LC; Caudill SP; Gonzalez AJ; Needham LL; Pirkle JL Urinary creatinine concentrations in the U.S. population: implications for urinary biologic monitoring measurements. Environ Health Perspect 2005;113:192–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanton CA; Moshfegh AJ; Baer DJ; Kretsch MJ The USDA Automated Multiple-Pass Method accurately estimates group total energy and nutrient intake. J Nutr 2006;136:2594–2599 [DOI] [PubMed] [Google Scholar]
- Braun JM; Bellinger DC; Hauser R; Wright RO; Chen A; Calafat AM; Yolton K; Lanphear BP Prenatal phthalate, triclosan, and bisphenol A exposures and child visual-spatial abilities. Neurotoxicology 2017;58:75–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley JP; Engel SM; Braun JM; Whyatt RM; Daniels JL; Mendez MA; Richardson DB; Xu Y; Calafat AM; Wolff MS; Lanphear BP; Herring AH; Rundle AG Prenatal Phthalate Exposures and Body Mass Index Among 4- to 7-Year-old Children: A Pooled Analysis. Epidemiology 2016;27:449–458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabaton N; Dumont C; Severin I; Perdu E; Zalko D; Cherkaoui-Malki M; Chagnon MC Genotoxic and endocrine activities of bis(hydroxyphenyl)methane (bisphenol F) and its derivatives in the HepG2 cell line. Toxicology 2009;255:15–24 [DOI] [PubMed] [Google Scholar]
- Calafat AM; Longnecker MP; Koch HM; Swan SH; Hauser R; Goldman LR; Lanphear BP; Rudel RA; Engel SM; Teitelbaum SL; Whyatt RM; Wolff MS Optimal Exposure Biomarkers for Nonpersistent Chemicals in Environmental Epidemiology. Environ Health Perspect 2015;123:A166–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carwile JL; Luu HT; Bassett LS; Driscoll DA; Yuan C; Chang JY; Ye X; Calafat AM; Michels KB Polycarbonate bottle use and urinary bisphenol A concentrations. Environ Health Perspect 2009;117:1368–1372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carwile JL; Ye X; Zhou X; Calafat AM; Michels KB Canned soup consumption and urinary bisphenol A: a randomized crossover trial. JAMA 2011;306:2218–2220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC. National Health and Nutrition Examination Survey. 2013–2014 Interviewer Procedures Manual. 2013
- CDC. National Health and Nutrition Examination Survey. 2013–2014 Data Documentation, Codebook, and Frequencies. Albumin & Creatinine - Urine (ALB_CR_H). 2016
- CDC. National Health and Nutrition Examination Survey. 2013–2014 Data Documentation, Codebook, and Frequencies. Physical Activity (PAQ_H). 2017
- Centers for Disease Control and Prevention. Fourth National Report on Human Exposure to Environmental Chemicals, Updated Tables (March, 2018). 2018
- Christensen KL; Lorber M; Koslitz S; Bruning T; Koch HM The contribution of diet to total bisphenol A body burden in humans: results of a 48 hour fasting study. Environ Int 2012;50:7–14 [DOI] [PubMed] [Google Scholar]
- Chronic Hazard Advisory Panel on Phthalates and Phthalate Alternatives. Final Report. Bethesda MD: U.S. Consumer Product Safety Commission; 2014 [Google Scholar]
- Correia-Sa L; Kasper-Sonnenberg M; Palmke C; Schutze A; Norberto S; Calhau C; Domingues VF; Koch HM Obesity or diet? Levels and determinants of phthalate body burden - A case study on Portuguese children. Int J Hyg Environ Health 2018;221:519–530 [DOI] [PubMed] [Google Scholar]
- Correia-Sa L; Kasper-Sonnenberg M; Schutze A; Palmke C; Norberto S; Calhau C; Domingues VF; Koch HM Exposure assessment to bisphenol A (BPA) in Portuguese children by human biomonitoring. Environ Sci Pollut Res Int 2017;24:27502–27514 [DOI] [PubMed] [Google Scholar]
- Dong R; Zhou T; Zhao S; Zhang H; Zhang M; Chen J; Wang M; Wu M; Li S; Chen B Food consumption survey of Shanghai adults in 2012 and its associations with phthalate metabolites in urine. Environ Int 2017;101:80–88 [DOI] [PubMed] [Google Scholar]
- Fiolet T; Srour B; Sellem L; Kesse-Guyot E; Alles B; Mejean C; Deschasaux M; Fassier P; Latino-Martel P; Beslay M; Hercberg S; Lavalette C; Monteiro CA; Julia C; Touvier M Consumption of ultra-processed foods and cancer risk: results from NutriNet-Sante prospective cohort. BMJ (Clinical research ed) 2018;360:k322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallart-Ayala H; Moyano E; Galceran MT Fast liquid chromatography-tandem mass spectrometry for the analysis of bisphenol A-diglycidyl ether, bisphenol F-diglycidyl ether and their derivatives in canned food and beverages. J Chromatogr A 2011;1218:1603–1610 [DOI] [PubMed] [Google Scholar]
- Gore AC; Chappell VA; Fenton SE; Flaws JA; Nadal A; Prins GS; Toppari J; Zoeller RT EDC-2: The Endocrine Society’s Second Scientific Statement on Endocrine-Disrupting Chemicals. Endocrine reviews 2015;36:E1–e150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall KD; Ayuketah A; Brychta R; Cai H; Cassimatis T; Chen KY; Chung ST; Costa E; Courville A; Darcey V; Fletcher LA; Forde CG; Gharib AM; Guo J; Howard R; Joseph PV; McGehee S; Ouwerkerk R; Raisinger K; Rozga I; Stagliano M; Walter M; Walter PJ; Yang S; Zhou M Ultra-Processed Diets Cause Excess Calorie Intake and Weight Gain: An Inpatient Randomized Controlled Trial of Ad Libitum Food Intake. Cell Metab 2019; [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartle JC; Navas-Acien A; Lawrence RS The consumption of canned food and beverages and urinary Bisphenol A concentrations in NHANES 2003–2008. Environ Res 2016;150:375–382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard GJ Chemical alternatives assessment: the case of flame retardants. Chemosphere 2014;116:112–117 [DOI] [PubMed] [Google Scholar]
- Kim H; Hu EA; Rebholz CM Ultra-processed food intake and mortality in the USA: results from the Third National Health and Nutrition Examination Survey (NHANES III, 1988–1994). Public Health Nutr 2019:1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch HM; Calafat AM Human body burdens of chemicals used in plastic manufacture. Philos Trans R Soc Lond B Biol Sci 2009;364:2063–2078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch HM; Lorber M; Christensen KL; Palmke C; Koslitz S; Bruning T Identifying sources of phthalate exposure with human biomonitoring: results of a 48h fasting study with urine collection and personal activity patterns. Int J Hyg Environ Health 2013;216:672–681 [DOI] [PubMed] [Google Scholar]
- Koch HM; Ruther M; Schutze A; Conrad A; Palmke C; Apel P; Bruning T; Kolossa-Gehring M Phthalate metabolites in 24-h urine samples of the German Environmental Specimen Bank (ESB) from 1988 to 2015 and a comparison with US NHANES data from 1999 to 2012. Int J Hyg Environ Health 2017;220:130–141 [DOI] [PubMed] [Google Scholar]
- Liao C; Kannan K Concentrations and profiles of bisphenol A and other bisphenol analogues in foodstuffs from the United States and their implications for human exposure. Journal of agricultural and food chemistry 2013;61:4655–4662 [DOI] [PubMed] [Google Scholar]
- Lubin JH; Colt JS; Camann D; Davis S; Cerhan JR; Severson RK; Bernstein L; Hartge P Epidemiologic evaluation of measurement data in the presence of detection limits. Environ Health Perspect 2004;112:1691–1696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez Steele E; Baraldi LG; Louzada ML; Moubarac JC; Mozaffarian D; Monteiro CA Ultra-processed foods and added sugars in the US diet: evidence from a nationally representative cross-sectional study. BMJ Open 2016;6:e009892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez Steele E; Monteiro CA Association between Dietary Share of Ultra-Processed Foods and Urinary Concentrations of Phytoestrogens in the US. Nutrients 2017;9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez Steele E; Popkin BM; Swinburn B; Monteiro CA The share of ultra-processed foods and the overall nutritional quality of diets in the US: evidence from a nationally representative cross-sectional study. Popul Health Metr 2017;15:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendonca RD; Lopes AC; Pimenta AM; Gea A; Martinez-Gonzalez MA; Bes-Rastrollo M Ultra-Processed Food Consumption and the Incidence of Hypertension in a Mediterranean Cohort: The Seguimiento Universidad de Navarra Project. American journal of hypertension 2017;30:358–366 [DOI] [PubMed] [Google Scholar]
- Mendonca RD; Pimenta AM; Gea A; de la Fuente-Arrillaga C; Martinez-Gonzalez MA; Lopes AC; Bes-Rastrollo M Ultraprocessed food consumption and risk of overweight and obesity: the University of Navarra Follow-Up (SUN) cohort study. Am J Clin Nutr 2016;104:1433–1440 [DOI] [PubMed] [Google Scholar]
- Mervish N; McGovern KJ; Teitelbaum SL; Pinney SM; Windham GC; Biro FM; Kushi LH; Silva MJ; Ye X; Calafat AM; Wolff MS; Bcerp. Dietary predictors of urinary environmental biomarkers in young girls, BCERP, 2004–7. Environ Res 2014;133:12–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteiro CA; Levy RB; Claro RM; Castro IR; Cannon G A new classification of foods based on the extent and purpose of their processing. Cad Saude Publica 2010;26:2039–2049 [DOI] [PubMed] [Google Scholar]
- Monteiro CA; Moubarac JC; Cannon G; Ng SW; Popkin B Ultra-processed products are becoming dominant in the global food system. Obes Rev 2013;14 Suppl 2:21–28 [DOI] [PubMed] [Google Scholar]
- Moshfegh AJ; Rhodes DG; Baer DJ; Murayi T; Clemens JC; Rumpler WV; Paul DR; Sebastian RS; Kuczynski KJ; Ingwersen LA; Staples RC; Cleveland LE The US Department of Agriculture Automated Multiple-Pass Method reduces bias in the collection of energy intakes. The American journal of clinical nutrition 2008;88:324–332 [DOI] [PubMed] [Google Scholar]
- Moubarac JC; Batal M; Louzada ML; Martinez Steele E; Monteiro CA Consumption of ultra-processed foods predicts diet quality in Canada. Appetite 2017;108:512–520 [DOI] [PubMed] [Google Scholar]
- Moubarac JC; Parra DC; Cannon G; Monteiro CA Food Classification Systems Based on Food Processing: Significance and Implications for Policies and Actions: A Systematic Literature Review and Assessment. Curr Obes Rep 2014;3:256–272 [DOI] [PubMed] [Google Scholar]
- Munoz I; Colacino JA; Lewis RC; Arthur AE; Meeker JD; Ferguson KK Associations between school lunch consumption and urinary phthalate metabolite concentrations in US children and adolescents: Results from NHANES 2003–2014. Environ Int 2018;121:287–295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien KM; Upson K; Cook NR; Weinberg CR Environmental Chemicals in Urine and Blood: Improving Methods for Creatinine and Lipid Adjustment. Environ Health Perspect 2016;124:220–227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Office of Environmental Health Hazard Assessment. Chemicals Known to the State to Cause Cancer or Reproductive Toxicity. Safe Drinking Water and Toxic Enforcement Act of 1986. Sacramento, CA: California Environmental Protection Agency; 2019 [Google Scholar]
- Rico-Campa A; Martinez-Gonzalez MA; Alvarez-Alvarez I; Mendonca RD; de la Fuente-Arrillaga C; Gomez-Donoso C; Bes-Rastrollo M Association between consumption of ultra-processed foods and all cause mortality: SUN prospective cohort study. BMJ (Clinical research ed) 2019;365:l1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochester JR; Bolden AL Bisphenol S and F: A Systematic Review and Comparison of the Hormonal Activity of Bisphenol A Substitutes. Environ Health Perspect 2015;123:643–650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenmai AK; Dybdahl M; Pedersen M; Alice van Vugt-Lussenburg BM; Wedebye EB; Taxvig C; Vinggaard AM Are structural analogues to bisphenol a safe alternatives? Toxicol Sci 2014;139:35–47 [DOI] [PubMed] [Google Scholar]
- Rudel RA; Gray JM; Engel CL; Rawsthorne TW; Dodson RE; Ackerman JM; Rizzo J; Nudelman JL; Brody JG Food packaging and bisphenol A and bis(2-ethyhexyl) phthalate exposure: findings from a dietary intervention. Environ Health Perspect 2011;119:914–920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakhi AK; Lillegaard IT; Voorspoels S; Carlsen MH; Loken EB; Brantsaeter AL; Haugen M; Meltzer HM; Thomsen C Concentrations of phthalates and bisphenol A in Norwegian foods and beverages and estimated dietary exposure in adults. Environ Int 2014;73:259–269 [DOI] [PubMed] [Google Scholar]
- Sathyanarayana S; Alcedo G; Saelens BE; Zhou C; Dills RL; Yu J; Lanphear B Unexpected results in a randomized dietary trial to reduce phthalate and bisphenol A exposures. Journal of exposure science & environmental epidemiology 2013;23:378–384 [DOI] [PubMed] [Google Scholar]
- Schnabel L; Kesse-Guyot E; Alles B; Touvier M; Srour B; Hercberg S; Buscail C; Julia C Association Between Ultraprocessed Food Consumption and Risk of Mortality Among Middle-aged Adults in France. JAMA Intern Med 2019; [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano SE; Braun J; Trasande L; Dills R; Sathyanarayana S Phthalates and diet: a review of the food monitoring and epidemiology data. Environ Health 2014a;13:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano SE; Karr CJ; Seixas NS; Nguyen RH; Barrett ES; Janssen S; Redmon B; Swan SH; Sathyanarayana S Dietary phthalate exposure in pregnant women and the impact of consumer practices. Int J Environ Res Public Health 2014b;11:6193–6215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva MJ; Samandar E; Preau JL Jr.; Reidy JA; Needham LL; Calafat AM Quantification of 22 phthalate metabolites in human urine. Journal of chromatography B, Analytical technologies in the biomedical and life sciences 2007;860:106–112 [DOI] [PubMed] [Google Scholar]
- Trasande L; Attina TM Association of exposure to di-2-ethylhexylphthalate replacements with increased blood pressure in children and adolescents. Hypertension 2015;66:301–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trasande L; Shaffer RM; Sathyanarayana S; Council On Environmental H Food Additives and Child Health. Pediatrics 2018;142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Department of Agriculture. USDA Food and Nutrient Database for Dietary Studies 2013–2014. in: Group F.S.R., ed. Beltsville, MD: Agricultural Research Service; 2016 [Google Scholar]
- U.S. Department of Agriculture. USDA National Nutrient Database for Standard Reference, Release 28. in: Laboratory N.D., ed. Beltsville, MD: Agricultural Research Service; 2017 [Google Scholar]
- Varshavsky JR; Morello-Frosch R; Woodruff TJ; Zota AR Dietary sources of cumulative phthalates exposure among the U.S. general population in NHANES 2005–2014. Environ Int 2018;115:417–429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinas P; Campillo N; Martinez-Castillo N; Hernandez-Cordoba M Comparison of two derivatization-based methods for solid-phase microextraction-gas chromatography-mass spectrometric determination of bisphenol A, bisphenol S and biphenol migrated from food cans. Anal Bioanal Chem 2010;397:115–125 [DOI] [PubMed] [Google Scholar]
- Watkins DJ; Eliot M; Sathyanarayana S; Calafat AM; Yolton K; Lanphear BP; Braun JM Variability and predictors of urinary concentrations of phthalate metabolites during early childhood. Environ Sci Technol 2014;48:8881–8890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff MS; Buckley JP; Engel SM; McConnell RS; Barr DB Emerging exposures of developmental toxicants. Curr Opin Pediatr 2017;29:218–224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye X; Wong LY; Kramer J; Zhou X; Jia T; Calafat AM Urinary Concentrations of Bisphenol A and Three Other Bisphenols in Convenience Samples of U.S. Adults during 2000–2014. Environ Sci Technol 2015;49:11834–11839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X; Kramer JP; Calafat AM; Ye X Automated on-line column-switching high performance liquid chromatography isotope dilution tandem mass spectrometry method for the quantification of bisphenol A, bisphenol F, bisphenol S, and 11 other phenols in urine. Journal of chromatography B, Analytical technologies in the biomedical and life sciences 2014;944:152–156 [DOI] [PubMed] [Google Scholar]
- Zota AR; Calafat AM; Woodruff TJ Temporal trends in phthalate exposures: findings from the National Health and Nutrition Examination Survey, 2001–2010. Environ Health Perspect 2014;122:235–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zota AR; Phillips CA; Mitro SD Recent Fast Food Consumption and Bisphenol A and Phthalates Exposures among the U.S. Population in NHANES, 2003–2010. Environ Health Perspect 2016;124:1521–1528 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.