Abstract
The objective of this study was to determine whether miR-93, miR-29c, and miR-200c, which we previously reported to be downregulated in leiomyomas, target cell cycle regulatory proteins that influence cell proliferation. Based on TargetScan algorithm 3 cell cycle regulatory proteins namely, E2F transcription factor 1 (E2F1), Cyclin D1 (CCND1) and CDK2 which were predicted to be targets of these miRNAs were further analyzed. In 30 hysterectomy specimens, we found the expression of E2F1 and CCND1 messenger RNA (mRNA) was increased in leiomyoma as compared to matched myometrium, with no significant changes in CDK2 mRNA levels. There was a significant increase in the abundance of all 3 proteins in leiomyoma in comparison with matched myometrium. Using luciferase reporter assay, we demonstrated E2F1 and CCND1 are targets of miR-93 and CDK2 is a target of miR-29c and miR-200c. We confirmed these findings through transfection studies in which transfection of primary leiomyoma cells with miR-93 resulted in a significant decrease in the expression of E2F1 and CCND1 mRNA and protein levels, whereas knockdown of miR-93 had the opposite effect. Similarly, overexpression of miR-29c and miR-200c in leiomyoma cells inhibited the expression of CDK2 protein and mRNA, whereas knockdown of this microRNAs (miRNA) had the opposite effect. Transfection of miR-29c, miR-200c, and miR-93 in primary leiomyoma cells resulted in a time-dependent inhibition of cell proliferation and cell motility. These results collectively indicate that the 3 miRNAs known to be downregulated in fibroid tumors are critical in regulation of cell proliferation because of their effects on 3 key cell cycle regulatory proteins, which are overexpressed in uterine leiomyomas.
Keywords: microRNA, Leiomyoma, fibroids, cell proliferation
Introduction
The pathogenesis of leiomyomas, which are benign tumors affecting 40% to 70% of reproductive women, has been under intense investigation.1-3 Multiple studies using either microarray or next-generation sequencing have demonstrated dysregulation of a number of protein coding genes involved in cell proliferation and apoptosis that are crucial to fibroid growth and progression.3,4 MicroRNAs (miRNAs), which are small noncoding RNAs (20-22 nucleotides [nt]), are key posttranscriptional regulators of protein coding genes, and they exert their function through inhibition of translation or degradation of messenger RNA (mRNA).5 Aberrant expression of miRNAs is associated with a wide range of disorders, including tumorigenesis and tissue fibrosis.6,7 Previous studies, including our own high-throughput sequencing, identified the expression profile of a large number of miRNAs in leiomyoma and myometrium and provided support for altered expression and regulatory function of a number of them, including let 7, miR-21, miR-29, miR-200, and miR-25-93-106 cluster in leiomyoma and leiomyoma smooth muscle cells (LSMCs).8-15 More recently, we reported on altered expression of a number of long noncoding RNAs (lncRNAs) that are longer than 200 nt in leiomyomas.16 Some lncRNAs regulate the expression of miRNAs acting as a sponge.17
Our previous work demonstrated a decrease in the expression of miR-29c in leiomyoma which primarily regulates extracellular matrix (ECM)-related genes and epigenetic-related enzymes DNMT3A.12 Others have reported similar findings with miR-29b, which is also downregulated in fibroids.15 We also reported a decrease in the expression of miR-200c in leiomyoma14 and in SKLMS-1, a leiomyosarcoma cell line,18 compared with normal myometrium. MiR-200c is a key miRNA regulating epithelial to mesenchymal transformation (EMT),11,14,19 and it has been associated with a whole host of cancer types.14,20-24 Similar to the pattern of miR-29c and miR-200c expression, we reported that miR-93 levels are also downregulated in leiomyoma with an associated increase in its target namely Interleukin-8.13 The objective of this study was to determine whether the 3 miRNAs (miR-29c, miR-200c, and miR-93) whose expression are downregulated in leiomyoma target cell cycle–associated regulatory proteins, thereby influencing leiomyoma cell proliferation. We selected to investigate 3 cell cycle–associated proteins that are predicted targets of miR-29c, miR-200c, and miR-93 based on TargetScan algorithm. As such, we focused on cyclin-dependent kinase 2 (CDK2), which is a member of serine/threonine protein kinases, which, upon binding to cyclin E, is required for the transition of the cell from G1 phase to the S phase of the cell cycle.25 Based on TargetScan, CDK2 is predicted to be a target of miR-29c and miR-200c. Cyclin-dependent kinase 2 has been shown to be a target of miR-200c and miR-29 in other cell types.18,26-28 The next protein of interest was cyclin D1, which in human is encoded by the CCND1 gene.29 Cyclin D1 is synthesized during the G1 phase of the cycle and forms a complex with CDK4/6, thereby regulating progression of the cell from the G1 phase into the S phase of the cell cycle.29,30 Cyclin D1 has been shown to be a target of miR-93 in HL-1, a cardiac muscle cell line.31 E2F1 is a member of the E2F transcription factor family which plays a key role in G1 to S phase transition. This transcription factor binds to several cell cycle regulatory proteins, including the retinoblastoma family and cyclin A/CDK2 complexes.32 E2F1 is targeted by miR-93 in osteosarcoma cells33 and hepatocellular carcinoma.34 Based on TargetScan, both E2F1 and CCND1 are predicted targets of miR-93. In this present study, we determined the expression of these 3 key cell cycle regulatory proteins (CCND1, E2F1, and CDK2) in leiomyomas and compare it to matched myometrium. Our objective was to determine if these cell cycle regulatory proteins are targeted by these 3 miRNAS (miR-93, miR29c, and miR200c) all of which are downregulated in fibroids.
Materials and Methods
Tissue Collection and Leiomyoma and Myometrial Smooth Muscle Cell Isolation
With prior approval from institutional review board (#036247), portions of uterine leiomyoma and matched myometrium were collected from patients (n = 30) who were scheduled to undergo hysterectomy at Harbor-UCLA Medical Center. The patients’ age ranged from 27 to 53 years (median = 43 ± 7.1), and they were not taking any hormonal medications for at least 3 months prior to surgery. All leiomyomas used in this study were 2 to 5 cm in diameter. Tissues were snap frozen and stored in liquid nitrogen for further analysis or used for isolation of LSMCs as previously described.12,13,35 Briefly, LSMCs were cultured in Dulbecco Modified Eagle Medium (DMEM) supplemented with 10% fetal bovine serum until reaching confluence with a change of media every 2 to 3 days. Cells at passages p1 to p3 were used for all experiments. Cell culture experiments were performed at least 3 times using LSMCs obtained from different patients. All supplies for isolation and cell culture were purchased from Sigma-Aldrich (St Louis, Missouri) and Fisher Scientific (Atlanta, Georgia).
Gain or Loss of Function of miR-93, miR-200c, and miR-29c
Leiomyoma smooth muscle cells were seeded at a cell density of 3.5 × 104/well in 6-well plates and at subconfluence transfected with 50 nM of pre-miR-93, pre-miR-200c, and pre-miR-29c, anti-miR-93, anti-miR-200c, anti-miR-29c, pre-miR negative control (NC), or anti-miR negative control (aNC) (Applied Biosystems, Foster city, California) for 24 to 96 hours using PureFection transfection reagent (System Biosciences, Inc, Mountain View, California), according to the manufacturer’s protocol.
RNA Isolation and Quantitative Real time-Polymerase Chain Reaction (RT-PCR) Analysis
Total RNA was extracted from LSMCs using TRIzol (Thermo Fisher Scientific, Waltham, Massachusetts), and their quantity and quality were determined (ND-1000 Spectrophotometer; NanoDrop Technologies, Wilmington, Delaware) as previously described.16,36,37 Subsequently, 1 μg was reverse transcribed using random primers according to the manufacturer’s guidelines (Applied Biosystems, Carlsbad, California). Primers for E2F1, CCND1, and CDK2 detection were designed by Primer Express Software (Applied Biosystems, Foster city, CA). Quantitative RT-PCR was performed using SYBR gene expression master mix (Applied Biosystems). Reactions were incubated for 10 minute at 95°C followed by 40 cycles for 15 seconds at 95°C and 1 minute at 60°C. The mRNA levels were determined using the Invitrogen (Carlsbad, CA) StepOne System with FBXW2 used for normalization.38 All reactions were run in triplicate, and relative expression was analyzed with the comparative cycle threshold method (2−ΔΔCT) according to the manufacturer (Applied Biosystems, Foster city, CA). Values were expressed as fold-change compared to the control group. The primer sequences used were as follows: E2F1 (sense, 5′-GGACTCTTCGGAGAACTTTCAGATC-3′; antisense, 5′-GGGCACAGGAAAACATCGA-3′); CCND1 (sense, 5′-GCCCTCTGTGCCACAGATGT-3′; antisense, 5′-CCCCGCTGCCACCAT-3′); CDK2 (sense, 5′-TTCCCCTCATCAAGAGCTATCTGT-3′; antisense, 5′-ACCCGATGAGAATGGCAGAA-3′); and FBXW2 (sense, 5′-CCTCGTCTCTAAACAGTGGAATAA-3′; antisense, 5′-GCGTCCTGAACAGAATCATCTA-3′).
Immunoblotting
Total protein isolated from leiomyoma and paired myometrium and LSMCs transfected with pre-miR-93, pre-miR-200c, pre-miR-29c, anti-miR-93, anti-miR-200c, anti-miR-29c, pre-miR negative (NC), and aNC were subjected to immunoblotting as previously described.37,39 Specific antibodies generated against E2F1 (sc-193; Santa Cruz Biotechnology, Dallas, Texas), CCND1 (60186-1-Ig; Proteintech Group, Inc, Chicago, Illinois), and CDK2 (sc-6248; Santa Cruz Biotechnology) with concentration of 1:1000, 1:000, and 1:250, respectively, were used to detect specific protein expression. The membranes were also stripped and probed with Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) antibody (Santa Cruz Biotechnology) to serve as loading control. The band densities were normalized by GAPDH using ImageJ program (http://imagej.nih.gov/ij/), and they are expressed as a ratio relative to the control group designated as 1.
Luciferase Reporter Assays
Leiomyoma smooth muscle cells were seeded in 6-well plates until reaching subconfluence and transiently cotransfected with 50 nM pre-miR-93, pre-miR-200c, pre-miR-29c oligonucleotides, or NC and a luciferase reporter plasmid (1 μg/well) containing 3′-untranslated region [UTR] sequences for E2F1, CCND1, or CDK2 (GeneCopoeia, Inc, Rockville, Maryland) using PureFection transfection reagent. Firefly and Renilla luciferase activities were measured after 48 hours of transfection using the dual-luciferase reporter assay system (Promega, Madison, Wisconsin). Firefly luciferase activity was normalized to Renilla luciferase activity, and the level of induction was reported as mean ± standard error of the mean (SEM) of 3 experiments performed in duplicate and compared with a ratio in cells transfected with NC independently set at 1.
Cell Proliferation Assay
Leiomyoma smooth muscle cells were seeded at 1000 cells/well in 96-well plates and cultured for 48 hours. The cells were then transfected with 50 nM pre-miR-93, pre-miR-200c, and pre-miR-29c oligonucleotides or NC as described above. The rate of cell proliferation was determined using the MTT assay and cells were photographed. Briefly, 3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide (MTT), (Sigma, St Louis, MO) was added to the culture medium at a final concentration of 1 mg/mL and was incubated for 2 hours at 37°C. The medium was aspirated, and the formazan product was solubilized with dimethyl sulfoxide and the absorbance at 570 nm was determined and subtracted from the absorbance at 630 nm (background) for each well. The assay was performed in 6 replicates per condition, and it was repeated 3 times.
Cell Motility Assay
The cell motility activity was determined using Radius 24-well assay kit (Cell Biolabs, San Diego, California) consisting of a circular biocompatible gel in each well according to the manufacturer’s instructions. Briefly, LSMCs were seeded in the assay plates and were cultured for 48 hours and then were transfected with pre-miR-93, pre-miR-29c, pre-miR-200c, and NC as described above. After 72 hours of incubation, the biocompatible gels were removed, and the cells were incubated for additional 21 hours, and the images of migratory cells were captured using an Olympus IX70 microscope equipped with digital camera (Olympus Inc, Melville, New York).
Statistical Analysis
Wherever appropriate, the results were reported as mean ± SEM and analyzed by PRISM software (GraphPad, San Diego, California). Data set normality was determined by the Kolmogorov-Smirnov test. Comparisons involving 2 groups were analyzed using Student t tests. For comparisons involving multiple groups, analysis of variance followed by a Tukey honest significance difference (HSD) test for post hoc pairwise analysis was conducted. Statistical significance was established at P < .05.
Results
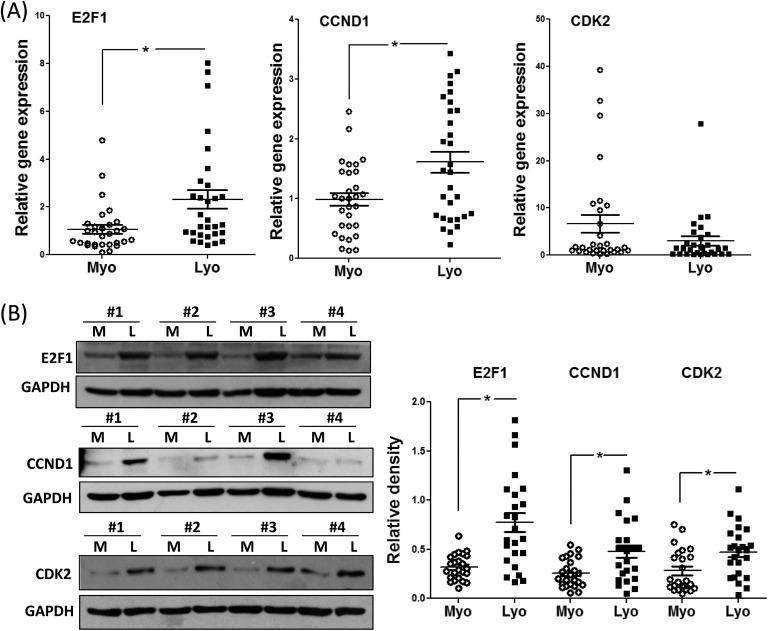
Initially, we determined the abundance of CCND1, E2F1, and CDK2 mRNA and protein in leiomyoma and matched myometrium in 30 hysterectomy specimens in subjects on no hormonal medications. As shown in Figure 1A, the expression of E2F1 and CCND1 mRNA was increased in leiomyoma as compared to matched myometrium; however, no significant changes were observed in CDK2 mRNA levels. Representative immunoblots and mean protein levels for CCND1, E2F1, and CDK2 are shown in Figure 1B. As demonstrated in Figure 1B, there was a significant increase in the abundance of all 3 proteins in leiomyoma as compared to matched myometrium. The differential expression of E2F1, CCND1, and CDK2 is independent of race and menstrual cycle phase (data not shown).
Figure 1.
A, Relative expression of E2F1, CCND1, and E2F1 messenger RNA (mRNA) in leiomyoma (Lyo) and matched myometrium (Myo; n = 30). B, Representative immunoblots for E2F1, CCND1, and cyclin-dependent kinase 2 (CDK2) in paired myometrium (M) and leiomyoma (L). Bar graph shows relative protein band densities (N = 24) in myometrium (Myo) and leiomyoma (Lyo). Results are presented as mean ± SEM and analyzed using paired Student t test. *P < .05.
We then determined whether the 3 cell cycle regulatory proteins are targets of miR-93, miR-29c, and miR-200c in leiomyomas using the luciferase assay (Figure 2). Figure 2A demonstrates the sequence alignment of seed regions of miRNAs with their target genes at the 3′-UTR region. In Figure 2B, we demonstrate that both E2F1 and CCND1 are targets of miR-93 in leiomyoma cells because overexpression of miR-93 in these cells reduced the luciferase activity of E2F1 and CCND1. Overexpression of miR-29c and miR-200c reduced the luciferase activity of CDK2, indicating miR-29c and miR-200c target CDK2 in leiomyoma cells (Figure 2C).
Figure 2.
A, The sequence alignment of seed regions of microRNAs (miRNAs) with their target genes at the 3′-UTR region. B, Relative luciferase activity in isolated leiomyoma smooth muscle cells (LSMCs) cotransfected with Renilla and firefly luciferase reporter carrying a 3′-UTR fragment of E2F1, CCND1, cyclin-dependent kinase 2 (CDK2), pre-miR-93, pre-miR-200c, pre-miR-29c, or control oligonucleotides (NC) for 48 hours. Relative luciferase activity is presented as the ratio of Firefly:Renilla as compared to NC, which was independently set as 1. Results are presented as mean ± SEM of at least 3 independent experiments with P values (*P < .05) indicated by corresponding lines.
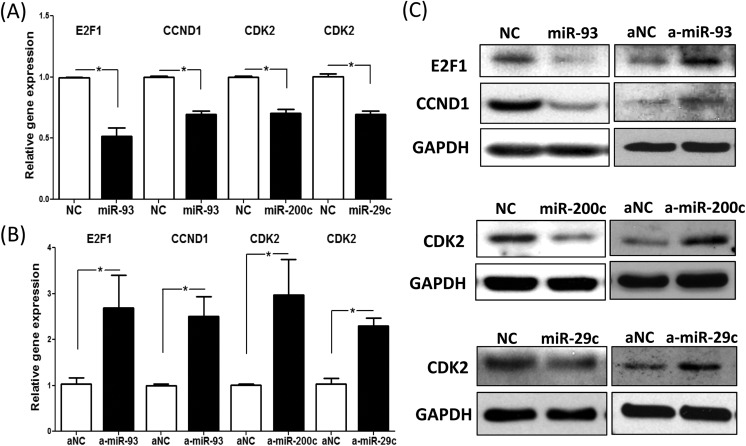
Upon establishing as to which cell cycle regulatory protein is regulated by which miRNA, we proceeded to over- and underexpress the miRNA of interest in leiomyoma cells through transfection of LSMCs with pre-miR or antisense oligo, respectively; then, we determined their effects on the expression of target mRNAs (Figure 3A and B) or protein (Figure 3C). As shown in Figure 3A and supporting the results from the luciferase assay (Figure 2), overexpression of miR-93 resulted in decreased expression of E2F1 and CCND1 mRNA levels (Figure 3A), whereas knockdown of miR-93 had the opposite effect (Figure 3B). Similarly, overexpression of miR-200c and miR-29c as expected resulted in decreased expression of CDK2 mRNA (Figure 3A), whereas knockdown of miR-200c and miR-29c had the opposite effect (Figure 3B). Protein data (Figure 3C) from the transfection studies were in line with mRNA data. As shown in Figure 3C, overexpression of miR-93 resulted in decreased E2F1 and CCND1 protein levels, whereas anti-miR-93 transfection induced E2F1 and CCND1 protein levels. These results were confirmed by transduction of lentivirus that contained doxycycline-inducible miR-93 insert (data not shown). Transfection of pre-miR-29c and pre-miR-200c led to reduced CDK2 protein levels, whereas anti-miR-29c and anti-miR-200c had the opposite effect (Figure 3C).
Figure 3.
Quantitative RT-PCR analysis of E2F1, CCND1, and cyclin-dependent kinase 2 (CDK2) messenger RNA (mRNA) expression in leiomyoma smooth muscle cells (LSMCs) following transfection with pre-miR-93 (miR-93, for E2F1 and CCND1), pre-miR-200c (miR-200c, for CDK2), pre-miR-29c (miR-29c, for CDK2), and control pre-miR oligonucleotides (NC) (A) or anti-miR-93 (a-miR-93, for E2F1 and CCND1), anti-miR-200c (a-miR-200c, for CDK2), anti-miR-29c (a-miR-29c, for CDK2), and control anti-miR oligonucleotides (aNC) (B) for 72 hours. Results are presented as mean ± SEM of at least 3 independent experiments with P values (*P < .05) indicated by corresponding lines. C, Western blot analysis of E2F1, CCND1, and CDK2 following transfection of LSMCs with control pre-miR oligonucleotides (NC), pre-miR-93 (miR-93, for E2F1 and CCND1), pre-miR-200c (miR-200c, for CDK2), pre-miR-29c (miR-29c, for CDK2) or control anti-miR oligonucleotides (aNC), anti-miR-93 (a-miR-93, for E2F1 and CCND1), anti-miR-200c (a-miR-200c, for CDK2), anti-miR-29c (a-miR-29c, for CDK2) for 96 hours. Results are representative of at least 3 independent cell preparations.
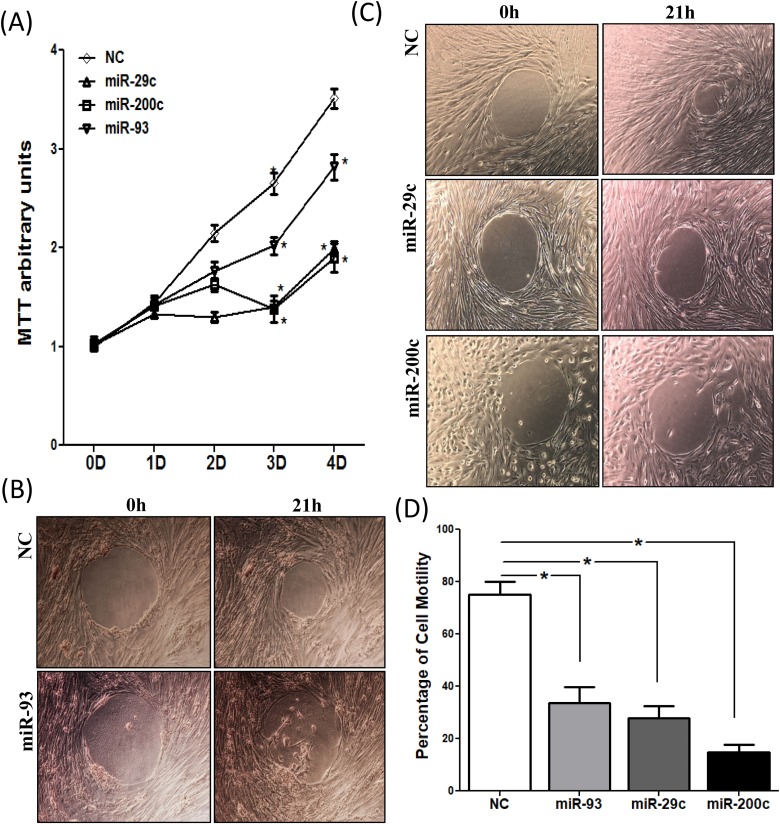
The significance of miR-29c, miR-200c, and miR-93 in regulating leiomyoma cell proliferation and motility is demonstrated in Figure 4. Transfection of pre-miR-29c, pre-miR-200c, and pre-miR-93 in primary leiomyoma cells resulted in a time-dependent inhibition of cell proliferation as measured by the MTT assay (Figure 4A), with a more profound inhibitory effect in response to miR-29c and miR-200c transfection as compared with miR-93. Upregulation of these 3 miRNAs inhibited cell motility as determined by cell motility assay as shown in Figure 4B to D.
Figure 4.
A, The rate of cell proliferation was determined after 4 days transfection of pre-miR-93, pre-miR-200c, pre-miR-29c, and control pre-miR oligonucleotides (NC) in leiomyoma smooth muscle cells (LSMCs) by MTT assay on the indicated days, with culture media changed every 2 days. Results are presented as mean ± SEM of 6 independent experiments. *P < .05. B and C, Photomicrographs of LSMCs transfected with pre-miR-93 (B), pre-miR-29c (C), pre-miR-200c (C) or the corresponding negative controls (NC) for 72 hours. The LSMC motility was determined after the biocompatible gels were removed for 21 hours. Motility assays were performed, in triplicates, using 3 independent cell preparations. D, Quantification of cell motility was analyzed by ImageJ and shown as mean ± SEM with P values (*P < .05) as compared to NC.
Discussion
Our results indicate that the miRNAs miR-93, miR-200c, and miR-29c, which were previously demonstrated to be downregulated in leiomyomas, target selectively key cell cycle regulatory proteins including CDK2, CCND1, and E2F1. Using the luciferase assay, we confirmed that miR-29c and miR-200c target CDK2 and miR-93 targets E2F1 and CCND1. As a result of downregulation of these miRNAs in leiomyoma, their target proteins, that is, CDK2, CCND1, and E2F1, were upregulated as demonstrated in this study. The significance of this group of miRNAs in leiomyoma cell proliferation was demonstrated through transfection of these miRNAs in isolated leiomyoma cells that resulted in a time-dependent decrease in cell proliferation and inhibition of cell motility as would be expected.
The regulation of cell cycle is highly complex and it is regulated through interaction of cyclins that form the regulatory subunit and CDKs, which are the catalytic subunits of an activated heterodimers.40 CDKs, through phosphorylation of target proteins, can activate or deactivate target proteins that control entry of cell into the next phase of cell cycle.40 In response to growth factors, cyclin D1 is produced, which binds to CDK4 or CDK6. Then, this complex phosphorylates the retinoblastoma susceptibility protein (Rb) that dissociates from the E2F/Rb complex, resulting in activation of E2F. The E2F family are transcription factors that are crucial in control of cell cycle.41 Activation of E2F induces transcription of genes, such as Cyclin E, Cyclin A, DNA polymerase, and thymidine kinase.42 Cyclin E binds to CDK2 enabling transition of cell from the G1 to S phase.40 G1 to S phase transition point is also known as a restriction point in the cell cycle and is a rate-limiting step in the cell cycle.40 Our results indicating increased expression of CDK2, E2F1, and cyclin D1 protein in fibroid tumors would suggest that there is an enhanced transition of fibroid cells from the G1 to S phase, which is consistent with the cell proliferation data presented. In contrast to EDF1 and CCND1, in case of CDK2, we did not find any changes in mRNA expression. This may be because the miRNAs targeting CDK2 may exert their effect through inhibition of protein translation rather than mRNA degradation.43
The miR-29 family, which consists of miR-29a, miR-29b, and miR-29c, share a common seed sequence with largely overlapping sets of predicted target genes; however, their differential expression and regulation suggest unique functional activities.44 This family of miRNAs is downregulated in various fibrotic disorders, and key ECM genes, including collagen subtypes and elastin, are targets of miR-29 regulatory function.37,45 Similar to our findings with miR-29c in fibroids,12 a recent report demonstrated reduced expression of miR-29b in leiomyoma as compared with matched myometrium, and restoring miR-29b expression in isolated LSMCs implanted in subrenal xenograft in a mouse model resulted in inhibition of ECM accumulation and rate of cell proliferation.15 MiR-29 family, as in fibroid tumors, is also downregulated in a number of malignancies including gastric,46,47 breast,48 osteosarcoma,49,50 and lymphomas.51,52 In gastric cancer cells, similar to our results in leiomyoma cells, enforced expression of miR-29a-3p inhibited the expression of CDK2, CDK4, and CDK6 and inhibited cell proliferation and cell migration.47 MiR-29b was shown to regulate cyclin D1 expression in adipocytes, and it is critical for adipogenic differentiation.53 Similarly, miR-29b was reported to inhibit intestinal mucosal growth by repressing CDK2 translation27 and targeted CDK6 in mantle cell lymphoma.52
The miR-200 family is normally expressed in cells of epithelial origin and not in fibroblasts of mesenchymal origin.54,55 This family of miRNAs suppresses tumors by inhibiting epithelial to mesenchymal transition.19 The role of miR-200 in EMT and tumor progression has been linked to bladder,24 breast,23 ovarian,22 pancreatic,21 melanoma,56 and stomach.57 Our own work showed downregulation of this miR-200c in leiomyomas, which was race dependent, with tumors from African Americans expressing lower levels of miR-200c in leiomyomas compared to Caucasians.14 In comparison with isolated leiomyoma cells, the expression of miR-200c was also lower in SKLMS-1, a leiomyosarcoma cell line.18 In this cell line, miR-200c was shown to target CDK2 and CCNE2, and its overexpression led to increased caspase 3/7 activity, thereby increasing apoptosis and reducing cell proliferation and migration, similar to our findings here with primary leiomyomas cells. Similar to leiomyoma cells, others have reported that miR-200c targets CDK2 and suppresses tumorigenesis in renal cell carcinoma26 and that miR-200b inhibits cell migration and invasion in non-small cell lung carcinoma cells by targeting FSCN1.58
As with miR-29c and miR-200c, the expression of miR-93 is reduced in leiomyomas; however, its host gene MCM7, a member of DNA helicases with a central role in initiation of DNA replication and cell cycle progression, is significantly increased in leiomyomas.13 Here, we demonstrate that miR-93 directly targets E2F1 and CCND1. The role of miR-93 in regulation of cell cycle–associated proteins and cell proliferation is cell and context dependent because, in contrast to leiomyoma cells, overexpression of miR-93 in gastric cancer cells activated CDK2 and aided in G1/S cell transition.59 In contrast to our findings in leiomyomas cells, Hazarika et al showed that overexpression of miR-93 in human umbilical vein cells (HUVEC) cell and CD2C12, a skeletal cell line, stimulated cell proliferation, and miR-93 and E2F1 levels were inversely related.60
In summary, leiomyomas are characterized by downregulation of miR-29c, miR-200c, and miR-93, as a result of which there is upregulation of their target cell cycle proteins, including CCND1, CDK2, and E2F1. Here, we show that miR-93 directly targets E2F1 and CCND1, and miR-29c and miR-200c target CDK2. These cell cycle proteins are key regulators of G1/S transition, which is a rate-limiting step of the cell cycle. The physiological significance of our findings is underscored by our data showing upregulation of miR-29c, miR-200c, and miR-93 in leiomyoma cells decreases cell proliferation and motility. Potential therapies that could correct the dysregulation of miR-29c, miR-200c, and miR-93 in leiomyomas restoring them to normal levels could potentially hold promise in inhibiting leiomyoma cell proliferation and fibroid progression.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by LA BioMed Research Committee Seed Grant Program (530592-01-00), LA BioMed Research Committee Bridge Grant Program (5311720100), and NIH R21 (HD088868).
References
- 1. Moravek MB, Bulun SE. Endocrinology of uterine fibroids: steroid hormones, stem cells, and genetic contribution. Curr Opin Obstet Gynecol. 2015;27(4):276–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Catherino WH, Eltoukhi HM, Al-Hendy A. Racial and ethnic differences in the pathogenesis and clinical manifestations of uterine leiomyoma. Semin Reprod Med. 2013;31(5):370–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Segars JH, Parrott EC, Nagel JD, et al. Proceedings from the Third National Institutes of Health International Congress on Advances in Uterine Leiomyoma Research: comprehensive review, conference summary, and future recommendations. Hum Reprod Update. 2014;20(3):309–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tsibris JC, Segars J, Coppola D, et al. Insights from gene arrays on the development and growth regulation of uterine leiomyomata. Fertil Steril. 2002;78(1):114–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Huntzinger E, Izaurralde E. Gene silencing by microRNAs: contributions of translational repression and mRNA decay. Nat Rev Genet. 2011;12(2):99–110. [DOI] [PubMed] [Google Scholar]
- 6. Schickel R, Boyerinas B, Park SM, Peter ME. MicroRNAs: key players in the immune system, differentiation, tumorigenesis, and cell death. Oncogene. 2008;27(45):5959–5974. [DOI] [PubMed] [Google Scholar]
- 7. Jiang X, Tsitsiou E, Herrick SE, Lindsay MA. MicroRNAs and the regulation of fibrosis. FEBS J. 2010;277(9):2015–2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fitzgerald JB, Chennathukuzhi V, Koohestani F, Nowak RA, Christenson LK. Role of microRNA-21 and programmed cell death 4 in the pathogenesis of human uterine leiomyomas. Fertil Steril. 2012;98(3):726–734.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Georgieva B, Milev I, Minkov I, Dimitrova I, Bradford AP, Baev V. Characterization of the uterine leiomyoma microRNAome by deep sequencing. Genomics. 2012;99(5):275–281. [DOI] [PubMed] [Google Scholar]
- 10. Luo X, Chegini N. The expression and potential regulatory function of microRNAs in the pathogenesis of leiomyoma. Semin Reprod Med. 2008;26(6):500–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chuang TD, Khorram O. miR-200c regulates IL8 expression by targeting IKBKB: a potential mediator of inflammation in leiomyoma pathogenesis. PloS One. 2014;9(4):e95370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chuang TD, Khorram O. Mechanisms underlying aberrant expression of miR-29c in uterine leiomyoma. Fertil Steril. 2016;105(1):236–245.e1. [DOI] [PubMed] [Google Scholar]
- 13. Chuang TD, Luo X, Panda H, Chegini N. miR-93/106b and their host gene, MCM7, are differentially expressed in leiomyomas and functionally target F3 and IL-8. Mol Endocrinol. 2012;26(6):1028–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chuang TD, Panda H, Luo X, Chegini N. miR-200c is aberrantly expressed in leiomyomas in an ethnic-dependent manner and targets ZEBs, VEGFA, TIMP2, and FBLN5. Endocr Relat Cancer. 2012;19(4):541–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Qiang W, Liu Z, Serna VA, et al. Down-regulation of miR-29b is essential for pathogenesis of uterine leiomyoma. Endocrinology. 2014;155(3):663–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chuang TD, Khorram O. Expression Profiling of lncRNAs, miRNAs, and mRNAs and their differential expression in leiomyoma using next-generation RNA sequencing. Reprod Sci. 2018;25(2):246–255. [DOI] [PubMed] [Google Scholar]
- 17. Ransohoff JD, Wei Y, Khavari PA. The functions and unique features of long intergenic non-coding RNA. Nat Rev Mol Cell Biol. 2018;19(3):143–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chuang TD, Ho M, Khorram O. The regulatory function of miR-200c on inflammatory and cell-cycle associated genes in SK-LMS-1, a leiomyosarcoma cell line. Reprod Sci. 2015;22(5):563–571. [DOI] [PubMed] [Google Scholar]
- 19. Abba ML, Patil N, Leupold JH, Allgayer H. MicroRNA regulation of epithelial to mesenchymal transition. J Clin Med. 2016;5(1):E8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Panda H, Pelakh L, Chuang TD, Luo X, Bukulmez O, Chegini N. Endometrial miR-200c is altered during transformation into cancerous states and targets the expression of ZEBs, VEGFA, FLT1, IKKbeta, KLF9, and FBLN5. Reprod Sci. 2012;19(8):786–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kong D, Li Y, Wang Z, et al. miR-200 regulates PDGF-D-mediated epithelial-mesenchymal transition, adhesion, and invasion of prostate cancer cells. Stem Cells. 2009;27(8):1712–1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hu X, Macdonald DM, Huettner PC, et al. A miR-200 microRNA cluster as prognostic marker in advanced ovarian cancer. Gynecol Oncol. 2009;114(3):457–464. [DOI] [PubMed] [Google Scholar]
- 23. Tryndyak VP, Beland FA, Pogribny IP. E-cadherin transcriptional down-regulation by epigenetic and microRNA-200 family alterations is related to mesenchymal and drug-resistant phenotypes in human breast cancer cells. Int J Cancer. 2010;126(11):2575–2583. [DOI] [PubMed] [Google Scholar]
- 24. Adam L, Zhong M, Choi W, et al. miR-200 expression regulates epithelial-to-mesenchymal transition in bladder cancer cells and reverses resistance to epidermal growth factor receptor therapy. Clin Cancer Res. 2009;15(16):5060–5072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hochegger H, Takeda S, Hunt T. Cyclin-dependent kinases and cell-cycle transitions: does one fit all? Nat Rev Mol Cell Biol. 2008;9(11):910–916. [DOI] [PubMed] [Google Scholar]
- 26. Wang X, Chen X, Han W, et al. miR-200c targets CDK2 and suppresses tumorigenesis in renal cell carcinoma. Mol Cancer Res. 2015;13(12):1567–1577. [DOI] [PubMed] [Google Scholar]
- 27. Xiao L, Rao JN, Zou T, et al. miR-29b represses intestinal mucosal growth by inhibiting translation of cyclin-dependent kinase 2. Mol Biol Cell. 2013;24(19):3038–3046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Qi H, Liu Y, Li S, et al. Activation of AMPK attenuated cardiac fibrosis by inhibiting CDK2 via p21/p27 and miR-29 family pathways in rats. Mol Ther Nucleic Acids. 2017;8:277–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Baldin V, Lukas J, Marcote MJ, Pagano M, Draetta G. Cyclin D1 is a nuclear protein required for cell cycle progression in G1. Genes Dev. 1993;7(5):812–821. [DOI] [PubMed] [Google Scholar]
- 30. Kim JK, Diehl JA. Nuclear cyclin D1: an oncogenic driver in human cancer. J Cell Physiol. 2009;220(2):292–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zhang J, Qin L, Han L, et al. Role of MicroRNA-93 I in pathogenesis of left ventricular remodeling via targeting cyclin-D1. Med Sci Monit. 2017;23:3981–3988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yamasaki L. Role of the RB tumor suppressor in cancer. Cancer Treat Res. 2003;115:209–239. [DOI] [PubMed] [Google Scholar]
- 33. Montanini L, Lasagna L, Barili V, et al. MicroRNA cloning and sequencing in osteosarcoma cell lines: differential role of miR-93. Cell Oncol (Dordr). 2012;35(1):29–41. [DOI] [PubMed] [Google Scholar]
- 34. Li Y, Tan W, Neo TW, et al. Role of the miR-106b-25 microRNA cluster in hepatocellular carcinoma. Cancer Sci. 2009;100(7):1234–1242. [DOI] [PubMed] [Google Scholar]
- 35. Chuang TD, Khorram O. Tranilast inhibits genes functionally involved in cell proliferation, fibrosis, and epigenetic regulation and epigenetically induces miR-29c expression in leiomyoma cells. Reprod Sci. 2017;24(9):1253–1263. [DOI] [PubMed] [Google Scholar]
- 36. Chuang TD, Khorram O. Glucocorticoids regulate MiR-29c levels in vascular smooth muscle cells through transcriptional and epigenetic mechanisms. Life Sci. 2017;186:87–91. [DOI] [PubMed] [Google Scholar]
- 37. Chuang TD, Pearce WJ, Khorram O. miR-29c induction contributes to downregulation of vascular extracellular matrix proteins by glucocorticoids. Am J Physiol Cell Physiol. 2015;309(2):C117–C125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Almeida TA, Quispe-Ricalde A, Montes de Oca F, Foronda P, Hernandez MM. A high-throughput open-array qPCR gene panel to identify housekeeping genes suitable for myometrium and leiomyoma expression analysis. Gynecol Oncol. 2014;134(1):138–143. [DOI] [PubMed] [Google Scholar]
- 39. Khorram O, Chuang TD, Pearce WJ. Long-term effects of maternal undernutrition on offspring carotid artery remodeling: role of miR-29c. J Dev Orig Health Dis. 2015;6(4):342–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Musgrove EA. Cyclins: roles in mitogenic signaling and oncogenic transformation. Growth Factors. 2006;24(1):13–19. [DOI] [PubMed] [Google Scholar]
- 41. Wong JV, Dong P, Nevins JR, Mathey-Prevot B, You L. Network calisthenics: control of E2F dynamics in cell cycle entry. Cell Cycle. 2011;10(18):3086–3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Stanelle J, Stiewe T, Theseling CC, Peter M, Putzer BM. Gene expression changes in response to E2F1 activation. Nucleic Acids Res. 2002;30(8):1859–1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Valencia-Sanchez MA, Liu J, Hannon GJ, Parker R. Control of translation and mRNA degradation by miRNAs and siRNAs. Genes Dev. 2006;20(5):515–524. [DOI] [PubMed] [Google Scholar]
- 44. Kriegel AJ, Liu Y, Fang Y, Ding X, Liang M. The miR-29 family: genomics, cell biology, and relevance to renal and cardiovascular injury. Physiol Genomics. 2012;44(4):237–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wang H, Zhu Y, Zhao M, et al. miRNA-29c suppresses lung cancer cell adhesion to extracellular matrix and metastasis by targeting integrin beta1 and matrix metalloproteinase2 (MMP2). PloS One. 2013;8(8):e70192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Espinosa-Parrilla Y, Munoz X, Bonet C, et al. Genetic association of gastric cancer with miRNA clusters including the cancer-related genes MIR29, MIR25, MIR93, and MIR106: results from the EPIC-EURGAST study. Int J Cancer. 2014;135(9):2065–2076. [DOI] [PubMed] [Google Scholar]
- 47. Zhao Z, Wang L, Song W, et al. Reduced miR-29a-3p expression is linked to the cell proliferation and cell migration in gastric cancer. World J Surg Oncol. 2015;13:101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Rostas JW III, Pruitt HC, Metge BJ, et al. microRNA-29 negatively regulates EMT regulator N-myc interactor in breast cancer. Mol Cancer. 2014;13:200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Zhang W, Qian JX, Yi HL, et al. The microRNA-29 plays a central role in osteosarcoma pathogenesis and progression. Mol Biol (Mosk). 2012;46(4):622–627. [PubMed] [Google Scholar]
- 50. Di Fiore R, Drago-Ferrante R, Pentimalli F, et al. MicroRNA-29b-1 impairs in vitro cell proliferation, self-renewal and chemoresistance of human osteosarcoma 3AB-OS cancer stem cells. Int J Oncol. 2014;45(5):2013–2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Robaina MC, Mazzoccoli L, Arruda VO, et al. Deregulation of DNMT1, DNMT3B and miR-29 s in Burkitt lymphoma suggests novel contribution for disease pathogenesis. Exp Mol Pathol. 2015;98(2):200–207. [DOI] [PubMed] [Google Scholar]
- 52. Zhao JJ, Lin J, Lwin T, et al. microRNA expression profile and identification of miR-29 as a prognostic marker and pathogenetic factor by targeting CDK6 in mantle cell lymphoma. Blood. 2010;115(13):2630–2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Beezhold K, Klei LR, Barchowsky A. Regulation of cyclin D1 by arsenic and microRNA inhibits adipogenesis. Toxicol Lett. 2017;265:147–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Lu J, Getz G, Miska EA, et al. MicroRNA expression profiles classify human cancers. Nature. 2005;435(7043):834–838. [DOI] [PubMed] [Google Scholar]
- 55. Vrba L, Garbe JC, Stampfer MR, Futscher BW. Epigenetic regulation of normal human mammary cell type-specific miRNAs. Genome Res. 2011;21(12):2026–2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. van Kempen LC, van den Hurk K, Lazar V, et al. Loss of microRNA-200a and c, and microRNA-203 expression at the invasive front of primary cutaneous melanoma is associated with increased thickness and disease progression. Virchows Arch. 2012;461(4):441–448. [DOI] [PubMed] [Google Scholar]
- 57. Shinozaki A, Sakatani T, Ushiku T, et al. Downregulation of microRNA-200 in EBV-associated gastric carcinoma. Cancer Res. 2010;70(11):4719–4727. [DOI] [PubMed] [Google Scholar]
- 58. Xiao P, Liu W, Zhou H. miR-200b inhibits migration and invasion in non-small cell lung cancer cells via targeting FSCN1. Mol Med Rep. 2016;14(2):1835–1840. [DOI] [PubMed] [Google Scholar]
- 59. Kim YK, Yu J, Han TS, et al. Functional links between clustered microRNAs: suppression of cell-cycle inhibitors by microRNA clusters in gastric cancer. Nucleic Acids Res. 2009;37(5):1672–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Hazarika S, Farber CR, Dokun AO, et al. MicroRNA-93 controls perfusion recovery after hindlimb ischemia by modulating expression of multiple genes in the cell cycle pathway. Circulation. 2013;127(17):1818–1828. [DOI] [PMC free article] [PubMed] [Google Scholar]