Abstract
Head and neck cancer is one of the most prevalent cancers around the world. Head and neck squamous cell carcinoma (HNSCC) accounts for nearly 90% of head and neck cancer. In recent years, significant advances have been made in immunotherapy for HNSCC. Although some clinical trials targeting immune checkpoints have shown success, the molecular mechanism for regulation of programmed death 1 (PD-1) and its ligand (PD-L1) is partially understood. In an effort to explore the effect of activation of signal transducers and activators of transcriptions (STAT3) on PD-1/PD-L1, the expression and correlation between phosphorylation of STAT3 and PD-1/PD-L1 were determined with immunostaining of human and mouse HNSCC tissue sections. PD-1/PD-L1 overexpression was found to be significantly associated with p-STAT3 in human and mouse HNSCC. Targeting STAT3 by a small molecule effectively inhibited the expression of PD-L1 in the CAL27 cell line. Furthermore, we found that blockade of STAT3 signaling downregulated PD-1/PD-L1 in a Tgfbr1/Pten 2cKO HNSCC mouse model. These findings suggest that STAT3 signaling plays an important role in PD-1/PD-L1 regulation and the antitumor immune response of HNSCC.
Keywords: immune checkpoint, mice model, S3I-201, cytokine, immune escape, immunotherapy
Introduction
Head and neck squamous cell carcinoma (HNSCC) is a major form of head and neck cancer, with >450,000 patients diagnosed every year (Jemal et al. 2011). In recent years, substantial advances have been made in developing therapeutic approaches for HNSCC, including radiotherapy, chemotherapy, and especially immunotherapy (Ferris 2015).
The signal transducers and activators of transcription (STAT) signaling pathway plays a critical role in biological processes (Bromberg and Darnell 2000). The abnormal activation of STAT signaling is implicated in many disease conditions, including cancer, asthma, and diabetes (Miklossy et al. 2013). The STAT family is constituted with 7 cytoplasmic transcription proteins: STAT1, STAT2, STAT3, STAT4, STAT5A, STAT5B, and STAT6 (Mullen and Gonzalez-Perez 2016). In cancer, STAT3 phosphorylation leads to activation of downstream target genes, resulting in increased cell proliferation, cell autophagy, tumor angiogenesis, and tumor immune evasion (Vogt and Hart 2011). Activation of STAT3 signaling in HNSCC can promote immune escape and cancer stem cell production (Bu et al. 2015; Bu et al. 016). S3I-201 is a chemical probe inhibitor of STAT3 activity, which disrupts STAT3-STAT3 complex formation (Siddiquee et al. 2007).
Programmed death 1 (PD-1; CD279) and its ligand PD-L1 (B7-H1 or CD274) play an important role in the maintenance of immune homeostasis (Okazaki and Honjo 2007). In the tumor microenvironment, cancer cells can utilize this pathway to evade T cell–mediated tumor-specific immunity (Freeman et al. 2000). PD-1 is an immune checkpoint molecule expressed by activated T cells, B cells, NK cells, and monocytes (Okazaki and Honjo 2007). In T cells, nuclear translocation of nuclear factor of activated T cells (NFAT) and binding of nuclear factor of activated T cells, cytoplasmic 1 (NFATc1), to the programmed cell death 1 (PDCD1) promoter is required for PD-1 transcription (Oestreich et al. 2008). PD-1 expression in T cells leads to inhibition of T-cell proliferation and cytokine secretion. Additionally, the expression of PD-L1 has been identified in multiple cancer types, such as HNSCC, melanoma, and lung cancer (Yu et al. 2015; Daud et al. 2016; Mansfield et al. 2016). Cell-intrinsic PI3K-AKT-mTOR signaling can upregulate the expression of PD-L1 in cancer cells (Chang et al. 2015). The interaction between PD-1 and its ligand PD-L1 inhibits T-cell proliferation, survival, and effector function, which limit antigen-specific T-cell responses and antitumor immunity (Bellmunt et al. 2017). Meanwhile, it reduces INF-γ production in the tumor microenvironment (Bellmunt et al. 2017). In clinical trials, therapies based on targeting PD-1 and PD-L1 have shown promising success in various cancers (Sullivan and Flaherty 2015; Hong et al. 2016). Even though an anti-PD-1 drug was recently approved by the U.S. Food and Drug Administration to treat advanced head and neck cancer, molecular mechanisms for regulation of PD-1 and PD-L1 are still far from clear.
In our previous study, we demonstrated that targeting STAT3 signaling reversed the immunosuppressive state by decreasing the number of immature myeloid cells in a Tgfbr1/Pten 2cKO mouse model (Bu et al. 2016). However, whether STAT3 signaling regulates immune checkpoint molecules PD-1 and PD-L1 in HNSCC is still unclear. In this study, we examined the levels of phosphorylated STAT3 (p-STAT3), PD-1, and PD-L1 in a human HNSCC tissue microarray and mouse specimen. We found that the activation of STAT3 signaling (substance) is significantly associated with the expression of PD-L1 in tumor cells and that the activation of STAT3 signaling (mesenchyme) is significantly associated with expression of PD-1 in stromal cells. Additionally, we confirmed that targeting STAT3 signaling effectively inhibits the PD-1/PD-L1 axis in an HNSCC (Tgfbr1/Pten 2cKO) mouse model.
Materials and Methods
Human HNSCC Tissue Microarray
Human HNSCC specimens were obtained from the Department of Oral and Maxillofacial–Head and Neck Oncology, School and Hospital of Stomatology, Wuhan University. Tissue microarrays (T-12-412 and T-15-411) are made by Shanghai OUTDO Biotech CO, Ltd. This study was approved by the School and Hospital of Stomatology of Wuhan University Medical Ethics Committee, and informed consent was routinely obtained from the patients. A total of 122 cases of HNSCC tissue specimens were utilized in this study.
Immunohistochemistry
The immunostaining of tissue sections was performed as described previously (Yu et al. 2015). The following antibodies were used: anti-p-STAT3, anti-PD-1, and anti-PD-L1 from CST and anti-INFγ from Abcam.
Cell Culture
The human tongue squamous cell carcinoma cell line CAL27 and FaDu were purchased from the American Type Culture Collection, and the cells were grown in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum in a humidified atmosphere of 95% air and 5% CO2 at 37 °C.
Mice and Treatment
All the mice were bred inducible tissue-specific Tgfbr1/Pten 2cKO mice (K14-CreERtam+/-; Tgfbr1flox/flox; Ptenflox/flox) in the FVBN/CD1/129/C57 mixed background and genotyped. All mice were maintained in specific pathogen-free facility, and experiment procedures were performed in accordance with the guidelines of the Institutional Animal Care and Use Committee of Wuhan University.
S3I-201 (NSC74859) was purchased from Selleck Chemicals and stored at 4 °C. The drug was dissolved in dimethyl sulfoxide for use according to the manufacturer’s recommendations. Phosphate-buffered saline (PBS) was used as a negative control for tumorigenesis experiments. After oral gavage of tamoxifen for 5 subsequent days, the Tgfbr1/Pten 2cKO mice were randomly divided into a control group (PBS, 100 μL, intraperitoneal; n = 6 mice) and a treatment group (5 mg/kg S3I-201, intraperitoneal; n = 6 mice). S3I-201 or PBS was injected at day 14, and mice were maintained for 4 wk. General inspection and monitoring were performed every day for all mice. The tumor volumes were measured with a micrometer caliper and photographed twice a week. The endpoint was determined according to a systematic evaluation by the facility veterinarian. The mice were euthanized at the end of this study, and the tumors were harvested and fixed in paraffin overnight or frozen at −80 °C for subsequent immunohistochemical staining or Western blot, respectively.
Flow Cytometry
The single-cell suspension was prepared from the spleen, lymph node, blood, and tumor of Tgfbr1/Pten 2cKO mice. The anti-mouse antibody PE-conjugated PD-1 (eBioscience) and isotype-matched IgG control (eBioscience) were used in this study. Dead cells were excluded with 7AAD (Invitrogen) staining. Flow cytometric data analysis was performed with FlowJo software (Tree Star).
Western Blot
Tumors from Tgfbr1/Pten 2cKO mice were carefully dissected (n = 6, respectively). A total of 30 µg of protein from each sample was denatured and then subjected to 12% SDS-polyacrylamide gel electrophoresis followed by transfer onto polyvinylidene fluoride membranes (Millipore Corporation). Next, the blots were stained with an enhanced chemiluminescence detection kit (West Pico; Thermo). The following antibodies were used for Western blot analysis: p-STAT3 (CST), STAT3 (CST), PD-1 (Santa Cruz Biotechnology), and PD-L1 (Gene Tex). β-Actin or GAPDH was used as a loading control.
Scoring System
Human HNSCC tissue microarray and mouse tissue slices were scanned with an Aperio ScanScope CS scanner (Vista) with a background substrate for each slice and quantified with Aperio Quantification software (version 9.1) for membrane, nuclear, or pixel quantification. An area of interest was selected in either the epithelial or the cancerous area for scanning and quantification. The histoscore of membrane and nuclear staining was calculated as a percentage of different positive cells with the following formula: (3+) × 3 + (2+) × 2 + (1+) × 1. The histoscore of pixel quantification was calculated as the total intensity/total cell number. The threshold for scanning of different positive cells was set according to the standard controls provided by Aperio.
Statistical Analysis
Data were analyzed with GraphPad Prism version 5.0 for Windows (GraphPad Software Inc.). The relationship among the expressions of several proteins was analyzed by the Spearman rank correlation test. Kaplan-Meier was used to analyze the survival curve, and the log-rank test was used to detect the differences of overall survival. P values <0.05 were considered as significant.
Results
Activation of STAT3 Transcription Factor Associated with the PD-1/PD-L1 Signaling Axis in Patients with HNSCC
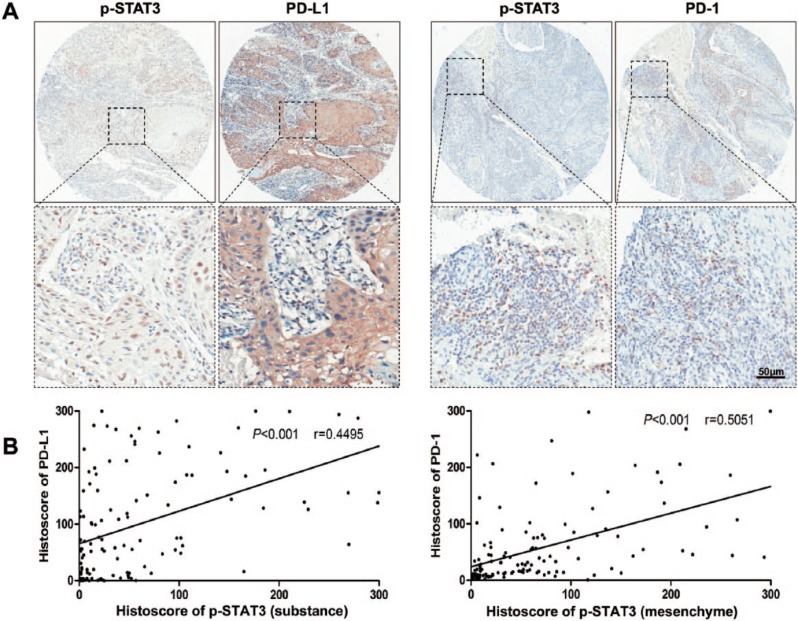
In our previous study, we confirmed the high phosphorylation level of STAT3 in patients with HNSCC as compared with oral mucosa (Bu et al. 2015). In the current study, we determined phosphorylated STAT3 levels in 122 HNSCC patients with immunostaining (Fig. 1A). The presence of p-STAT3 was observed in the cancer cells (substance) with strong staining in HNSCC tissue samples. Similarly, the strong staining was observed in tumor microenvironmental cells (mesenchyme). Considering the role of STAT3 transcription factor for tumor immune escape, we investigated the protein levels of PD-1 and PD-L1 in the HNSCC tissue microarray (Fig. 1A). To identify whether p-STAT3 affects levels of immune checkpoint molecules PD-1 and PD-L1, the Spearman rank correlation coefficient test and linear tendency test were conducted on the histoscore. Indeed, we found substantial levels of p-STAT3 associated with PD-L1 (P < 0.001, r = 0.4495; Fig. 1B). Moreover, the mesenchymal expression of p-STAT3 was statistically associated with PD-1 (P < 0.001, r = 0.5051; Fig. 1B). These results suggest that p-STAT3 might play a role in PD-1/PD-L1 regulation in HNSCC.
Figure 1.
p-STAT3 and PD-1/PD-L1 expression levels in human HNSCC. (A) Representative immunostaining pictures of p-STAT3 (substance), PD-L1, p-STAT3 (mesenchyme), and PD-1 in human HNSCC. Scale bar = 50 µm. (B) Linear regression analysis of p-STAT3 (substance) and PD-L1 histoscore of immunostaining in a human HNSCC tissue microarray; P < 0.001, r = 0.4495. (C) linear regression analysis of p-STAT3 (mesenchyme) and PD-1 histoscore of immunostaining in a human HNSCC tissue microarray; P < 0.001, r = 0.5051. HNSCC, head and neck squamous cell carcinoma; PD-1, programmed death 1; PD-L1, programmed death 1 ligand; STAT, signal transducers and activators of transcription; p-STAT3, phosphorylated STAT3.
Next, we investigated the impact of p-STAT3 on HNSCC prognosis. Kaplan-Meier analysis was performed. First, we calculated the cutoff point by the Cutoff Finder tool (Budczies et al. 2012). Cutoff optimization by correlation with survival in 122 HNSCC cases and plot of the differences in survival time are shown in Appendix Figure 1. The cutoff point of 58.88 was determined for p-STAT3 substance levels. The median histoscore (83.91) was used as the cutoff point for the p-STAT3 mesenchymal level. However, the results of Kaplan-Meier analysis suggested that both p-STAT3 (epithelia) and p-STAT3 (mesenchyme) levels could not be considered potential prognostic factors (Appendix Fig. 2).
Activation of STAT3 Transcription Factor Regulates PD-L1 Expression In Vitro
To determine the effects of STAT3 on PD-L1 expression, we inhibited phosphorylation of STAT3 in the CAL27 and FaDu cell line. We found that downregulation of p-STAT3 caused significantly decreased expression of PD-L1 in a dose-dependent manner (Fig. 2).
Figure 2.
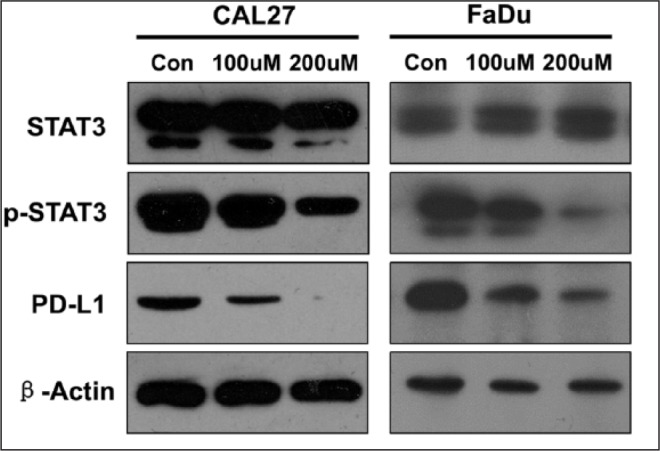
The expression level of PD-L1 was measured by Western blotting in a head and neck squamous cell carcinoma cell line (Cal 27 and FaDu), with and without S3I-201. β-Actin was used as the internal protein loading control. PD-L1, programmed death 1 ligand; STAT, signal transducers and activators of transcription; p-STAT3, phosphorylated STAT3.
Activation of STAT3 Transcription Factor Associated with the PD-1/PD-L1 Signaling Axis in a Tgfbr1/Pten 2cKO Mice Model
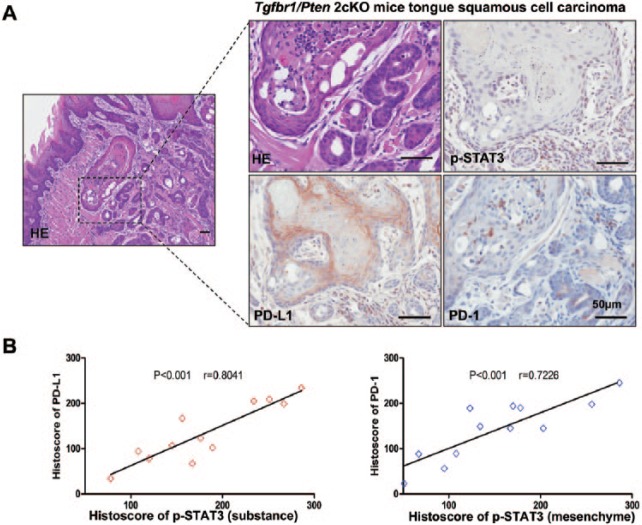
In our previous studies, we reported an immunosuppressive state in our tumor-bearing Tgfbr1/Pten 2cKO mice, and overexpression of PD-1 and PD-L1 was observed in the tumor microenvironment (Yu et al. 2015). To determine whether activation of STAT3 was associated with the PD-1/PD-L1 signaling axis in Tgfbr1/Pten 2cKO mice, we examined the expression levels of p-STAT3, PD-1, and PD-L1 in tumor-bearing mice. We observed that tumor cells and stromal cells have higher levels of p-STAT3 in the tumor microenvironment (Fig. 3A). Additionally, we observed increased expression of PD-1 in stromal cells and PD-L1 in tumor cells (Fig. 3A). We next performed correlation analysis according to the histoscore for immunostaining. We found that the substance p-STAT3 levels were significantly higher and statistically correlated with PD-L1 (P < 0.001, r = 0.8041; Fig. 3B, left). Similarly, the mesenchymal levels of p-STAT3 were statistically associated with PD-1 (P < 0.001, r = 0.7226; Fig. 3B, right). These data suggest that activation of STAT3 might lead to the PD-1/PD-L1 expression.
Figure 3.
p-STAT3 and PD-1/PD-L1 levels in a Tgfbr1/Pten 2cKO mice model. (A) Representative immunostaining pictures of p-STAT3, PD-L1, and PD-1 in Tgfbr1/Pten 2cKO HNSCC mice. Scale bars = 50 µm. (B) Linear regression analysis of p-STAT3 (substance) and PD-L1 histoscore of immunostaining in Tgfbr1/Pten 2cKO HNSCC mice; P < 0.001, r = 0.8041. (C) Linear regression analysis of p-STAT3 (mesenchyme) and PD-1 histoscore of immunostaining in Tgfbr1/Pten 2cKO HNSCC mice; P < 0.001, r = 0.7226. HE, hematoxylin and eosin; HNSCC, head and neck squamous cell carcinoma; PD-1, programmed death 1; PD-L1, programmed death 1 ligand; STAT, signal transducers and activators of transcription; p-STAT3, phosphorylated STAT3.
Inhibition of p-STAT3 Reduced Tumor Growth in a Tgfbr1/Pten 2cKO Mice Model
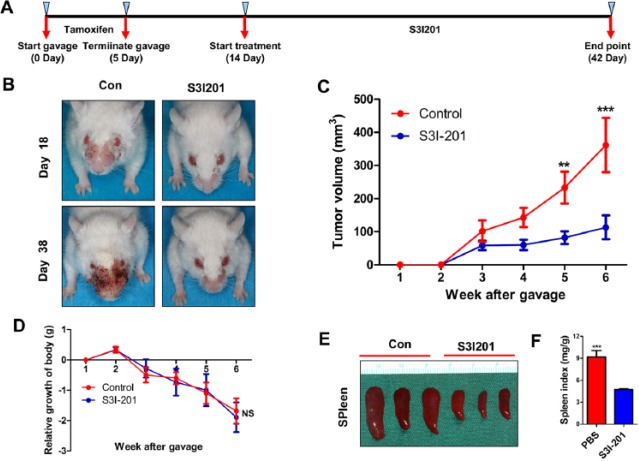
To determine whether inhibition of phosphorylation of STAT3 has any tumor-suppressive effect in vivo, Tgfbr1/Pten 2cKO mice were injected with S3I-201 (STAT3 inhibitor) or PBS intraperitoneally (n = 6 mice; Fig. 4A). We observed decreased tumor volume in the S31-201-treated group versus the control group (Fig. 4B, C). Moreover, S31-201 treatment did not cause additional toxicity (Fig. 4D). Next, we assessed the effect of S31-201 on the spleen index in Tgfbr1/Pten 2cKO mice and found that it significantly suppressed splenomegalia (Fig. 4E). Quantification of these results further demonstrated that the decrease in p-STAT3 levels with S31-201 treatment improved the spleen index (Fig. 4F).
Figure 4.
Inhibition of phosphorylation of STAT3 reduced tumor growth in a Tgfbr1/Pten 2cKO mice model. (A) Schematic picture shows the tamoxifen gavage and drug delivery strategy of S3I-201 in Tgfbr1/Pten 2cKO mice. (B) Representative pictures of mice with head and neck tumors after treatment with S3I-201 or PBS on days 18 and 38. (C) Total volume of tumor was measured in the S3I-201 and control groups once a week. Data are presented as mean ± SEM (unpaired Student’s t test; **P < 0.01, ***P < 0.001). (D) Relative growth of body (g) was measured in the S3I-201 and control groups once a week. Data are presented as mean ± SEM (unpaired Student’s t test; NS, not significant). (E) Representative spleen pictures of Tgfbr1/Pten 2cKO mice after treatment with S3I-201 or PBS. (F) Spleen index shows that S3I-201 significantly inhibited compensatory growth of spleen in Tgfbr1/Pten 2cKO mice (1-way analysis of variance with post hoc Tukey test; ***P < 0.001). PBS, phosphate-buffered saline; STAT, signal transducers and activators of transcription.
PD-1/PD-L1 Expression Is Significantly Downregulated by Decreased p-STAT3 Levels in a Tgfbr1/Pten 2cKO Mice Model
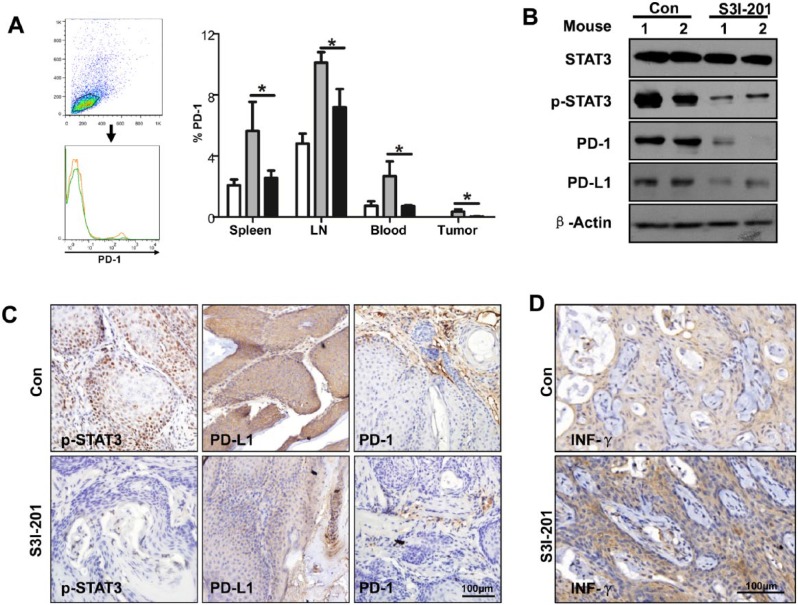
To identify effects on the PD-1/PD-L1 signaling axis in Tgfbr1/Pten 2cKO mice with S3I-201 treatment, we performed flow cytometry. We found that inhibition of phosphorylation of STAT3 remarkably decreased PD-1+ cells in the spleen, lymph node, blood, and tumor (Fig. 5A). Western blot assay revealed that the expression of PD-1 and PD-L1 was downregulated when the mice were treated with S3I-201 (Fig. 5B). Immunostaining of the tissue sections further demonstrated that inhibition of STAT3 phosphorylation reduces the expression of PD-1/PD-L1 in Tgfbr1/Pten 2cKO mice (Fig. 5C). INF-γ acts as an effector molecule, which could kill tumor cells (Lo Presti et al. 2017). So, we performed immunohistochemistry to assess the expression of INF-γ (Fig. 5D). We observed that inhibition of STAT3 phosphorylation promotes secretion of INF-γ in the tumor microenvironment. In summary, our results indicate that the immunosuppressive state can be reversed with S3I-201 treatment through downregulating the PD-1/PD-L1 axis in Tgfbr1/Pten 2cKO mice.
Figure 5.
S3I-201 treatment downregulates the PD-1/PD-L1 signaling axis in Tgfbr1/Pten 2cKO mice. (A) Representative flow cytometry showed decreased staining population of PD-1 in the S3I-201 treatment group and a quantitative PD-1+ cell population in spleen, LN, blood, and tumor in the S3I-201 treatment and control groups (1-way analysis of variance with post hoc Tukey test; *P < 0.05). (B) The intratumor levels of STAT3, p-STAT3, PD-1, and PD-L1 were measured by Western blotting in the S3I-201 treatment and control groups. β-Actin was used as the internal protein loading control. (C) Representative immunostaining pictures of p-STAT3, PD-L1, and PD-1 in tumor derive from Tgfbr1/Pten 2cKO mice in the S3I-201 treatment and control groups. Scale bar = 100 µm. (D) The expression level of INF-γ was accessed by immunohistochemistry in Tgfbr1/Pten 2cKO mice after treatment with S3I-201 or PBS. Scale bar = 100 µm. LN, lymph node; PBS, phosphate-buffered saline; PD-1, programmed death 1; PD-L1, programmed death 1 ligand; STAT, signal transducers and activators of transcription; p-STAT3, phosphorylated STAT3.
Discussion
In our previous study, we discovered that targeting STAT3 signaling reverses the immunosuppressive status in an HNSCC mouse model (Bu et al. 2016). To gain additional insights into the mechanism by which STAT3 signaling affects the tumor immune environment, we carried out this study and explored the relationship between STAT3 signaling and the PD-1/PD-L1 axis in HNSCC clinical samples and a Tgfbr1/Pten 2cKO mouse model. According to the histoscore of immunostaining of the human tissue microarray and tumor-bearing mice specimen, we found that increased levels of p-STAT3 are associated with PD-1 and PD-L1, suggesting that STAT3 signaling might regulate the expression of PD-1 and PD-L1. Next, we carried out an in vitro assay to confirm that STAT3 signaling influences the expression PD-L1 in the CAL27 cell line. Furthermore, our animal experiments with S31-201 treatment demonstrated that targeting STAT3 signaling significantly inhibits the PD-1/PD-L1 axis in the tumor microenvironment.
In the tumor microenvironment, PD-L1 expression in tumor cells inhibits the antitumor immune response (Mahoney et al. 2015). Also, the expression of PD-L1 in tumor cells has been classified into constitutive type and inducible type. In cancer, the blockade of negative immune checkpoints can augment the secretion of INF-γ by the T cells, which induces the expression of PD-L1 in cancer cells (Pardoll 2012). Additionally, some constitutive signaling pathway can regulate the endogenous level of PD-L1 in tumor cells, whereas activation of STAT3 signaling can enhance the expression of PD-L1 in melanoma cells (Jiang et al. 2013). Similarly, in our in vitro studies, we have shown that STAT3 signaling can regulate the expression of PD-L1 in an HNSCC cell line. Moreover, targeting STAT3 signaling reduced the expression of PD-L1 in tumor cells in an HNSCC mouse model.
PD-1, encoded by PDCD1, is primarily expressed in T cells, which controls T-cell exhaustion and tolerance in a variety of cancers (Nishimura et al. 1996). When the T cell is stimulated by antigens, the PD-1 expression will lead to altered expression of transcription factors (Blackburn et al. 2009). For PD-1 transcription in T cells, the nuclear translocation of NFAT and binding of NFATc1 to the PDCD1 promoter are required (Oestreich et al. 2008). Conversely, T-bet has been shown as a transcription suppressor for PD-1 expression (Ferris et al. 2016). Earlier reports have demonstrated that STAT3 signaling plays a critical role for T-cell differentiation (Yang et al. 2011). Up to now, multiple cytokines have been confirmed to regulate the expression of PD-1, including IL-6, IL-12, and INF-α (Terawaki et al. 2011). In unconventional T cells, such as natural killer T cells and γδ T cells, IL-12 activates STAT3, which is required for its maintenance (Wilson et al. 2015). INF-α-induced IL-10 expression in human CD4 T cells is regulated by the STAT3 signaling pathway (Govender et al. 2017). The IL-6/JAK/STAT3 signaling axis has been shown to promote HNSCC growth (Chakravarti et al. 2006). So, we hypothesized that STAT3 signaling regulates the expression of PD-1 in HNSCC. Indeed, in current study, we found that the overexpression of PD-1 is associated with p-STAT3 in a tissue microarray, suggesting that STAT3 signaling may regulate the expression of PD-1. Meanwhile, our animal experiment showed the blockade of STAT3 signaling decreased the expression of PD-1.
In our mouse model, targeting STAT3 signaling has been shown to enhance secretion of INF-γ in the tumor microenvironment. To evaluate whether PD-L1 was upregulated by INF-γ, we performed immunostaining and Western blot. We did not find overexpression PD-L1 in the S31-201-treated group as compared with control. It may be that targeting STAT3 directly inhibits constitutive PD-L1 expression in tumor cells (Pardoll 2012). Therefore, the downregulation of PD-L1 offsets INF-γ-induced PD-L1 expression. In general, targeting STAT3 signaling significantly downregulates PD-L1 in an HNSCC mice model.
Immuno-oncology is a rapidly growing field, and immune checkpoint inhibitors have the potential to be first-line drugs for many tumors (Buchbinder and Hodi 2016). Some inhibitors targeting PD-1 or PD-L1 are currently being investigated in clinical trials for HNSCC (www.ClinicalTrial.gov). Interestingly, on November 10, 2016, the U.S. Food and Drug Administration approved nivolumab (Opdivo; Bristol-Myers Squibb Company) for the treatment of recurrent or metastatic patients with HNSCC (Ferris et al. 2016). Besides, human papillomavirus (HPV) infection affects prognosis of patients with HNSCC. HPV-positive and HPV-negative HNSCC differed significantly based on the activity levels of the signaling pathway, including STAT3, NF-κB, AP1, and so on (Gaykalova et al. 2015; Isayeva et al. 2015; Verma et al. 2017). In the current study, we did not distinguish between signaling pathway expression in HPV-positive and HPV-negative HNSCC.
Taken together, the PD-1/PD-L1 signaling axis, whose expression is upregulated in human and mouse HNSCC, inhibits the antitumor immunity response (Yu et al. 2015). Moreover, overexpression of PD-1 and PD-L1 significantly correlates with phosphorylation of STAT3 in human and mice. Targeting STAT3 signaling reverses the immunosuppressive state in an HNSCC mouse model. Thus, our findings provide a potential mechanism of regulation for the PD-1/PD-L1 signaling axis in HNSCC.
Author Contributions
L.L. Bu, contributed to conception, design, data acquisition, and analysis, drafted the manuscript; G.T. Yu, contributed to conception, design, data analysis, and interpretation, drafted the manuscript; L. Wu, L. Mao, contributed to data acquisition, drafted the manuscript; W.W. Deng, contributed to data analysis, drafted the manuscript; J.F. Liu, contributed to data interpretation, drafted the manuscript; A.B. Kulkarni, W.F. Zhang, contributed to data analysis, critically revised the manuscript; L. Zhang, Z.J. Sun contributed to conception, design, and data analysis, drafted and critically revised the manuscript. All authors gave final approval and agree to be accountable for all aspects of the work.
Supplementary Material
Footnotes
A supplemental appendix to this article is available online.
This study was supported by the National Natural Science Foundation of China (81672667, 881672668, 81472528, 81472529, 81272963, 81272964) and the Program for New Century Excellent Talents in University (NCET-13-0439), Ministry of Education of China.
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
References
- Bellmunt J, Powles T, Vogelzang NJ. 2017. A review on the evolution of PD-1/PD-L1 immunotherapy for bladder cancer: the future is now. Cancer Treat Rev. 54:58–67. [DOI] [PubMed] [Google Scholar]
- Blackburn SD, Shin H, Haining WN, Zou T, Workman CJ, Polley A, Betts MR, Freeman GJ, Vignali DA, Wherry EJ. 2009. Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nat Immunol. 10(1):29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromberg J, Darnell JE., Jr. 2000. The role of stats in transcriptional control and their impact on cellular function. Oncogene. 19(21):2468–2473. [DOI] [PubMed] [Google Scholar]
- Bu LL, Yu GT, Deng WW, Mao L, Liu JF, Ma SR, Fan TF, Hall B, Kulkarni AB, Zhang WF, et al. 2016. Targeting STAT3 signaling reduces immunosuppressive myeloid cells in head and neck squamous cell carcinoma. Oncoimmunology. 5(5):e1130206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bu LL, Zhao ZL, Liu JF, Ma SR, Huang CF, Liu B, Zhang WF, Sun ZJ. 2015. STAT3 blockade enhances the efficacy of conventional chemotherapeutic agents by eradicating head neck stemloid cancer cell. Oncotarget. 6(39):41944–41958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchbinder EI, Hodi FS. 2016. Melanoma in 2015: immune-checkpoint blockade—durable cancer control. Nat Rev Clin Oncol. 13(2):77–78. [DOI] [PubMed] [Google Scholar]
- Budczies J, Klauschen F, Sinn BV, Gyorffy B, Schmitt WD, Darb-Esfahani S, Denkert C. 2012. Cutoff Finder: a comprehensive and straightforward web application enabling rapid biomarker cutoff optimization. PLoS One. 7(12):e51862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakravarti N, Myers JN, Aggarwal BB. 2006. Targeting constitutive and interleukin-6-inducible signal transducers and activators of transcription 3 pathway in head and neck squamous cell carcinoma cells by curcumin (diferuloylmethane). Int J Cancer. 119(6):1268–1275. [DOI] [PubMed] [Google Scholar]
- Chang CH, Qiu J, O’Sullivan D, Buck MD, Noguchi T, Curtis JD, Chen Q, Gindin M, Gubin MM, van der Windt GJ, et al. 2015. Metabolic competition in the tumor microenvironment is a driver of cancer progression. Cell. 162(6):1229–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daud AI, Wolchok JD, Robert C, Hwu WJ, Weber JS, Ribas A, Hodi FS, Joshua AM, Kefford R, Hersey P, et al. 2016. Programmed death-ligand 1 expression and response to the anti-programmed death 1 antibody pembrolizumab in melanoma. J Clin Oncol. 34(34):4102–4109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris RL. 2015. Immunology and immunotherapy of head and neck cancer. J Clin Oncol. 33(29):3293–3304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris RL, Blumenschein G, Jr., Fayette J, Guigay J, Colevas AD, Licitra L, Harrington K, Kasper S, Vokes EE, Even C, et al. 2016. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med. 375(19):1856–1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H, Fitz LJ, Malenkovich N, Okazaki T, Byrne MC, et al. 2000. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 192(7):1027–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaykalova DA, Manola JB, Ozawa H, Zizkova V, Morton K, Bishop JA, Sharma R, Zhang C, Michailidi C, Considine M, et al. 2015. NF-kappab and stat3 transcription factor signatures differentiate HPV-positive and HPV-negative head and neck squamous cell carcinoma. Int J Cancer. 137(8):1879–1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govender U, Corre B, Bourdache Y, Pellegrini S, Michel F. 2017. Type I interferon-enhanced IL-10 expression in human CD4 T cells is regulated by STAT3, STAT2, and BATF transcription factors. J Leukoc Biol. 101(5):1181–1190. [DOI] [PubMed] [Google Scholar]
- Hong AM, Vilain RE, Romanes S, Yang J, Smith E, Jones D, Scolyer RA, Soon Lee C, Zhang M, Rose B. 2016. PD-L1 expression in tonsillar cancer is associated with human papillomavirus positivity and improved survival: implications for anti-PD1 clinical trials. Oncotarget. 7(47):77010–77020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isayeva T, Xu J, Ragin C, Dai Q, Cooper T, Carroll W, Dayan D, Vered M, Wenig B, Rosenthal E, et al. 2015. The protective effect of p16(INK4a) in oral cavity carcinomas: P16(Ink4A) dampens tumor invasion-integrated analysis of expression and kinomics pathways. Mod Pathol. 28(5):631–653. [DOI] [PubMed] [Google Scholar]
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. 2011. Global cancer statistics. CA Cancer J Clin. 61(2):69–90. [DOI] [PubMed] [Google Scholar]
- Jiang X, Zhou J, Giobbie-Hurder A, Wargo J, Hodi FS. 2013. The activation of MAPK in melanoma cells resistant to BRAF inhibition promotes PD-L1 expression that is reversible by MEK and PI3K inhibition. Clin Cancer Res. 19(3):598–609. [DOI] [PubMed] [Google Scholar]
- Lo Presti E, Toia F, Oieni S, Buccheri S, Turdo A, Mangiapane LR, Campisi G, Caputo V, Todaro M, Stassi G, et al. 2017. Squamous cell tumors recruit gammadelta T cells producing either IL17 or ifngamma depending on the tumor stage. Cancer Immunol Res. 5(5):397–407. [DOI] [PubMed] [Google Scholar]
- Mahoney KM, Rennert PD, Freeman GJ. 2015. Combination cancer immunotherapy and new immunomodulatory targets. Nat Rev Drug Discov. 14(8):561–584. [DOI] [PubMed] [Google Scholar]
- Mansfield AS, Aubry MC, Moser JC, Harrington SM, Dronca RS, Park SS, Dong H. 2016. Temporal and spatial discordance of programmed cell death-ligand 1 expression and lymphocyte tumor infiltration between paired primary lesions and brain metastases in lung cancer. Ann Oncol. 27(10):1953–1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miklossy G, Hilliard TS, Turkson J. 2013. Therapeutic modulators of stat signalling for human diseases. Nat Rev Drug Discov. 12(8):611–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullen M, Gonzalez-Perez RR. 2016. Leptin-induced jak/stat signaling and cancer growth. Vaccines (Basel). 4(3):pii:E26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura H, Agata Y, Kawasaki A, Sato M, Imamura S, Minato N, Yagita H, Nakano T, Honjo T. 1996. Developmentally regulated expression of the PD-1 protein on the surface of double-negative (CD4-CD8-) thymocytes. Int Immunol. 8(5):773–780. [DOI] [PubMed] [Google Scholar]
- Oestreich KJ, Yoon H, Ahmed R, Boss JM. 2008. NFATc1 regulates PD-1 expression upon T cell activation. J Immunol. 181(7):4832–4839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okazaki T, Honjo T. 2007. PD-1 and PD-1 ligands: from discovery to clinical application. Int Immunol. 19(7):813–824. [DOI] [PubMed] [Google Scholar]
- Pardoll DM. 2012. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 12(4):252–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiquee K, Zhang S, Guida WC, Blaskovich MA, Greedy B, Lawrence HR, Yip ML, Jove R, McLaughlin MM, Lawrence NJ, et al. 2007. Selective chemical probe inhibitor of Stat3, identified through structure-based virtual screening, induces antitumor activity. Proc Natl Acad Sci U S A. 104(18):7391–7396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan RJ, Flaherty KT. 2015. Immunotherapy: anti-PD-1 therapies-a new first-line option in advanced melanoma. Nat Rev Clin Oncol. 12(11):625–626. [DOI] [PubMed] [Google Scholar]
- Terawaki S, Chikuma S, Shibayama S, Hayashi T, Yoshida T, Okazaki T, Honjo T. 2011. IFN-alpha directly promotes programmed cell death-1 transcription and limits the duration of T cell-mediated immunity. J Immunol. 186(5):2772–2779. [DOI] [PubMed] [Google Scholar]
- Verma G, Vishnoi K, Tyagi A, Jadli M, Singh T, Goel A, Sharma A, Agarwal K, Prasad SC, Pandey D, et al. 2017. Characterization of key transcription factors as molecular signatures of HPV-positive and HPV-negative oral cancers. Cancer Med. 6(3):591–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt PK, Hart JR. 2011. Pi3k and STAT3: a new alliance. Cancer Discov. 1(6):481–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RP, Ives ML, Rao G, Lau A, Payne K, Kobayashi M, Arkwright PD, Peake J, Wong M, Adelstein S, et al. 2015. Stat3 is a critical cell-intrinsic regulator of human unconventional T cell numbers and function. J Exp Med. 212(6):855–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang XP, Ghoreschi K, Steward-Tharp SM, Rodriguez-Canales J, Zhu J, Grainger JR, Hirahara K, Sun HW, Wei L, Vahedi G, et al. 2011. Opposing regulation of the locus encoding IL-17 through direct, reciprocal actions of STAT3 and STAT5. Nat Immunol. 12(3):247–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu GT, Bu LL, Huang CF, Zhang WF, Chen WJ, Gutkind JS, Kulkarni AB, Sun ZJ. 2015. PD-1 blockade attenuates immunosuppressive myeloid cells due to inhibition of CD47/SIRPalpha axis in HPV negative head and neck squamous cell carcinoma. Oncotarget. 6(39):42067–42080. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.