Abstract
Cathepsin K (CTSK) is an important protease responsible for degrading type I collagen, osteopontin, and other bone matrix proteins. The mutations in the CTSK gene can cause pycnodysostosis (OMIM 265800), a rare autosomal recessive bone dysplasia. Patients with pycnodysostosis have been reported to present specific dental abnormalities; however, whether these dental abnormalities are related to dysfunctional CTSK has never been reported. Here we investigated the histologic changes of cementum and alveolar bone in a pycnodysostosis patient, caused by novel compound heterozygous mutations in the CTSK gene (c.87 G>A p.W29X and c.848 A>G p.Y283C). The most impressive manifestations in tooth were extensive periradicular high-density clumps with unclear periodontal space by orthopantomography examination and micro–computed tomography scanning analysis. Hematoxylin/eosin and toluidine blue staining and atomic force microscopy analysis showed that the cementum became significantly thickened, softened, and full of cementocytes. The disorganized bone structure was the main character of alveolar bone. The p.W29X mutation may represent the loss-of-function allele with an earlier termination codon in the precursor CTSK polypeptide. Residue Y283 is highly conserved among papain-like cysteine proteases. Three-dimensional structure modeling analysis found that the loss of the hydroxybenzene residue in the Y283C mutation would interrupt the hydrogen network and possibly affect the self-cleavage of the CTSK enzyme. Furthermore, p.Y283C mutation did not affect the mRNA and protein levels of overexpressed CTSK in COS-7 system but did reduce CTSK enzyme activity. In conclusion, the histologic and ultrastructural changes of cementum and alveolar bone might be affected by CTSK mutation via reduction of its enzyme activity (clinical trial registration: ChiCTR-TNC-10000876).
Keywords: pycnodysostosis, cathepsin K, molecular biology, tooth, dental cementum, alveolar bone
Introduction
Cathepsin K (CTSK; OMIM 601105) is a member of the papain-like cysteine protease family. It is highly expressed in osteoclasts and plays a critical role during the osteoclast-mediated bone resorption. In osteoclasts, CTSK is responsible for degrading bone matrix proteins, such as type I collagen, osteopontin, and osteonectin (Hou et al. 1999; Schilling et al. 2007; Mujawar et al. 2009). The mutations of the CTSK gene may cause a rare autosomal recessive bone dysplasia, pycnodysostosis (OMIM 265800; Gelb et al. 1996). Patients with pycnodysostosis usually show the typical features of short stature, increased bone density, frequently pathologic fractures, and osteolysis of the distal phalanges (Xue et al. 2011). In addition, dental abnormalities—including severe dental crowding and malocclusion, delayed eruption of permanent teeth, and persistence of deciduous teeth—are also observed in patients with pycnodysostosis (Xue et al. 2011). However, the detailed pathohistologic characters of dental abnormalities related to dysfunctional CTSK have never been reported.
Here, on the basis of a pycnodysostosis patient with novel compound heterozygous mutations of the CTSK gene, we highlight the histologic changes in his teeth, especially cementum and surrounding tissues, and demonstrate molecular pathogenesis of the novel missense mutation p.Y283C.
Methods
Ethics
The proband and other 14 family members were included in this study. Written informed consents were obtained from the patient and family members. The study was authorized by the Ethics Committee, School of Stomatology, Fourth Military Medical University, Xi’an, China (No. 2009-011) and the Chinese Clinical Trial Registry (registration no. ChiCTR-TNC-10000876). The manuscript was prepared in compliance with the STROBE checklist.
Genomic DNA Preparation and Mutation Analysis
To rule out the possibility of chromosomal abnormalities, peripheral blood was obtained from the proband for karyotype analysis. Blood samples were also obtained from 14 other family members and 50 unrelated normal controls. Genomic DNA was extracted using QIAamp DNA blood mini kit (Qiagen Inc., Chatsworth, CA, USA). The coding exons and exon/intron splice junctions of the CTSK gene were amplified by polymerase chain reaction (PCR). Primers and PCR amplification conditions were previously described (Donnarumma et al. 2007). PCR products were purified with DNA Fragment Quick Purification/Recover Kit (DingGuo, Beijing, China) and sequenced with an ABI 377 Sequencer (Perkin-Elmer Corp., Norwalk, CT, USA). Sequence variants were identified using DNAStar, MegAlign 5.01 (Demonstration System DNAStar, Inc., Madison, WI, USA).
Mutant Structure Analysis
To illustrate the effects of the novel missense mutation p.Y283C, we analyzed the mutant structure based on the crystal structure of human CTSK. A molecular model of p.Y283C was constructed with the SWISS-MODEL server and Swiss-PdbViewer 4.04, which retrieved the template structure 7pckA (Sivaraman et al. 1999). Structural graphics and visualization were based on the SWISS-MODEL server and Swiss-Pdb Viewer. The CTSK orthologs were screened using the NCBI HomoloGene database (http://www.ncbi.nlm.nih.gov/homologene/68053), and the damaging effects of the mutation were predicted by the SIFT algorithm.
Transient Expression of Wild-type and Mutant CTSK Gene in COS-7 Cells
The full-length cDNAs of the wild-type CTSK gene were cloned into the pcDNA3.1(+) vector, and the site mutation p.Y283C was generated by following the general methods (GenScript, Nanjing, China). COS-7 cells were maintained and propagated in DMEM containing 10% fetal bovine serum (Life Technologies, Inc., Carlsbad, CA, USA) in a humidified incubator at 37 °C with 5% CO2. One day before transfection, the cells were trypsinized and plated into 6-well cell culture dishes. When the cells reached a density of 70% ~ 80%, they were transfected with 2 μg of plasmid DNA in 6 μL of Lipofectamine 2000 Transfection Reagent according to the manufacture’s protocol (Invitrogen, San Diego, CA, USA). In all cases, COS-7 cells without transfection or mock transfected with Lipofectamine 2000 Transfection Reagent were used as control. The cells were harvested at 72 h after transfection.
Total RNA extraction and reverse transcription PCR
According to the manufacture’s protocol, total RNA was extracted using the E.Z.N.A.TM Total RNA Kit I (Omega, Norcross, GA, USA), and the RNA was reverse transcribed by PrimeScriptRT Reagent Kit (TAKARA, Japan). The cDNA was amplified using primer pairs specific for CTSK and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) by PCR and real-time quantitative PCR (qPCR), respectively. Sequences of primer pairs specific for CTSK included forward: 5′CCTCTCTTGGT GTCCATACA3′, reverse: 5′ATCTCTCTGTACCCTCTGC A3′ (490 base pairs [bp] for PCR) and qPCR forward primer: 5′GAGGTGGTTCAGAAGATGAC3′, qPCR reverse primer: 5′CCCAACAGGAACCACACT3′ (169 bp, for qPCR). The same primer pair was used for amplifying GAPDH (forward: 5′ ATGGGGAAGGTGAAGGTCG3′, reverse: 5′GGGGTCATT GATGGCAACAATA3′, 108 bp) by PCR and qPCR. The PCR conditions were 30 cycles of 95 °C for 30 s, 60 °C for 30 s, and 72 °C for 30 s. qPCR reactions were performed in a volume of 20 μL by SYBRPremix Ex Taq II (TAKARA, Japan). PCR conditions were performed as the suggestions in ABI 7500 real-time PCR system. The melt curve was included at the end of each PCR reaction.
Western blot
Western blot was performed according to our previously described method (Yang et al. 2013). Antibodies included rabbit anti-human CTSK polyclonal antibody (dilution 1:500, ProteinTech Group, Chicago, IL, USA), mouse anti-GAPDH monoclonal antibody (dilution 1:7,000, ProteinTech Group), and fluorophore-labeled goat anti-mouse or goat anti-rabbit secondary antibody (dilution 1:10,000, LI-COR, Lincoln, NE, USA). Bands were detected and quantified on an Odyssey image system (LI-COR).
Detection of the CTSK activities
Total protein cell extracts were prepared, and AMC concentrations were detected using CTSK activity fluorescence quantitative detection kit (Genmed Scientifics Inc., USA), according to the manufacture protocol. First, mixing the total protein cell extracts with the substrate z-Leu-Arg-7-amido-4-methylcoumarin (z-LR-AMC) at 37°C for 60 min (pH 5.5). Odanacatib (Selleckchem, Houston, TX, USA; final concentration 50 nM), a CTSK-specific inhibitor, was added to verify the involvement of CTSK and exclude other cathepsin activity. Then the Microplate reader (Synergy HT, Bioteck, VT, USA) detected the fluorescence intensity released by CTSK decomposition product AMC (excitation wavelength, 360 nm; emission wavelength, 460 nm). According to the standard curve, the concentrations of AMC from different samples were obtained. Finally, CTSK activities were calculated using the following formula:
Detection of the Dental Changes
Micro–computed tomography scanning
The involved left maxillary third molar was removed during the process of sequestrectomy. The tooth was collected and fixed in 4% paraformaldehyde for 7 d. Then a micro–computed tomography (micro-CT) scanner (Siemens Inveon Micro CT, Siemens AG, Germany) was used to determine the structure of the tooth. The scanning procedure was completed using 80 kV (500 μA), a 500-ms exposure time, and a scan angle of 360°. The acquired raw scanning data were then reconstructed into a 3-dimensional dataset using Inveon Research Workplace (Siemens AG).
Histologic analysis
After micro-CT examination, the patient’s tooth was dehydrated and embedded without decalcification. The specimens were cut mesial-distally at a thickness of 50 μm with Leica SP2600 (Leica Microsystems Nussloch GmbH, Nussloch, Germany). The sections were stained with hematoxylin and eosin and toluidine blue and observed under Leica DM6000B (Leica Microsystems Nussloch GmbH).
Atomic force microscopy
Three mutant specimens from the patient’s tooth and 3 control specimens from a healthy premolar extracted for the reason of orthodontics were examined by atomic force microscopy (AFM). For each specimen, at least 3 different areas (with 2-μm diameters) were measured. All the AFM images were acquired in the Peak force quantitative nanomechanical mapping (QNM) mode, using a commercial AFM MultiMode VIII (Bruker, Santa Barbara, CA, USA), at a scan frequency of 0.5 Hz with optimized feedback parameters. Ultrasharp antimony doped Si cantilevers (rectangular, MPP-13100-10, Bruker) with a typical resonance frequency of 525 KHz and a spring constant of 200 N/m were applied. The resolution of all the original AFM images was 512 × 512 pixels per image. The images were processed and analyzed with the commercial software Scanning Probe Image Processor (SPIPTM, Image Metrology ApS, version 5.1.3, Lyngby, Denmark).
Results
Clinical Data
The proband was a 36-y-old man. His weight of 42 kg was 35.1% less than average. His height of 156 cm was 91.9% of normal range. A total of 14 different fractures occurred in the past 30 y. The patient showed skull deformities with frontal and parietal bossing, prominent eyes, hypoplastic facial bones, and symmetrical swellings on both sides of the submandibular region. Intraoral features included severe dental crowding and malocclusion, as well as a groove in the midline of the palate. The exposed wounds on the alveolar bone surface were >1 cm2 and combined with an oronasal fistula in the right side (Appendix Fig. 1). He also displayed short hands and feet with grooved nails, a narrow chest, and a deep groove in the lumbar area. Radiologic examinations showed generalized increase in bone density and multiple old fractures in bilateral lower limbs, hypoplasia of the clavicles, and osteolysis of the distal phalanges (Appendix Fig. 2).
Mutation Analysis of CTSK in the Pedigree
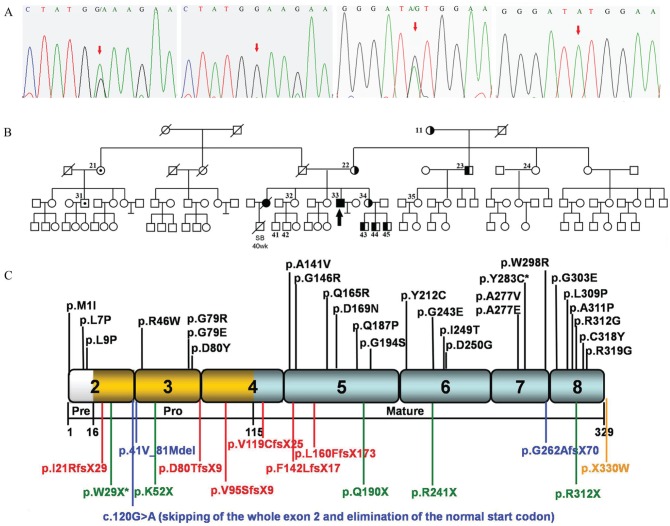
Sequence analysis revealed novel compound heterozygous mutations in CTSK. A single-base G-to-A transition at cDNA nucleotide 87 in exon 2 leading to a replacement of a tryptophan residue by a STOP codon (p.W29X) and a single-base A-to-G transition at cDNA nucleotide 848 in exon 7 resulting in a tyrosine to cysteine residue substitution (p.Y283C) were identified (the A of initiator ATG was taken as +1 position; GenBank accession no. NM_000396.2; Fig. 1A–D). Further analysis of the CTSK gene in the 14 family members revealed 7 maternal carriers of p.W29X and 2 paternal carriers of p.Y283C. The pedigree is shown in Figure 1E. To exclude the possibility that either of these changes was the result of single-nucleotide polymorphisms within the general Chinese population, 50 normal unrelated individuals were sequenced, and no such alteration of the gene was detected. Our mutation analysis in CTSK confirmed the diagnosis of pycnodysostosis.
Figure 1.
Sequence analysis of cathepsin K (CTSK) and pedigree.
(A) Sequencing results. The sequence chromatogram from
left to right shows nonsense mutation with G-to-A transition at cDNA
nucleotide 87 in exon 2 of the patient, normal exon 2 in control,
missense mutation with A-to-G transition at cDNA nucleotide 848 in exon
7 of the patient, and normal exon 7 in control (GenBank accession no.
NM_000396.2; the A of initiator ATG is taken as +1 position).
(B) CTSK gene analysis was performed
in the family members marked with subject numbers. The filled symbols
represent affected individuals; the arrow indicates the proband;
unfilled symbols represent unaffected individuals;  represents male
carrier of p.W29X;
represents male
carrier of p.W29X;  represents female
carrier of p.W29X;
represents female
carrier of p.W29X;  represents male
carrier of p.Y283C;
represents male
carrier of p.Y283C;  represents female
carrier of p.Y283C. SB means stillbirth. Slash indicates dead
individuals whose DNAs are unavailable for mutation analysis.
(C) The diagrammatic representation of the polypeptide.
It comprises a 15–amino acid preregion (white box), a 99-residue
proregion (gold boxes), and a 215–amino acid mature domain (gray boxes).
Missense mutations (black type) are represented at the top of the
diagram, while frame-shift mutations (red type), nonsense mutations
(green type), splicing mutations (blue type), and termination codon
mutations (yellow type) are at the bottom. The 2 mutations found in our
patient are marked with an asterisk (*).
represents female
carrier of p.Y283C. SB means stillbirth. Slash indicates dead
individuals whose DNAs are unavailable for mutation analysis.
(C) The diagrammatic representation of the polypeptide.
It comprises a 15–amino acid preregion (white box), a 99-residue
proregion (gold boxes), and a 215–amino acid mature domain (gray boxes).
Missense mutations (black type) are represented at the top of the
diagram, while frame-shift mutations (red type), nonsense mutations
(green type), splicing mutations (blue type), and termination codon
mutations (yellow type) are at the bottom. The 2 mutations found in our
patient are marked with an asterisk (*).
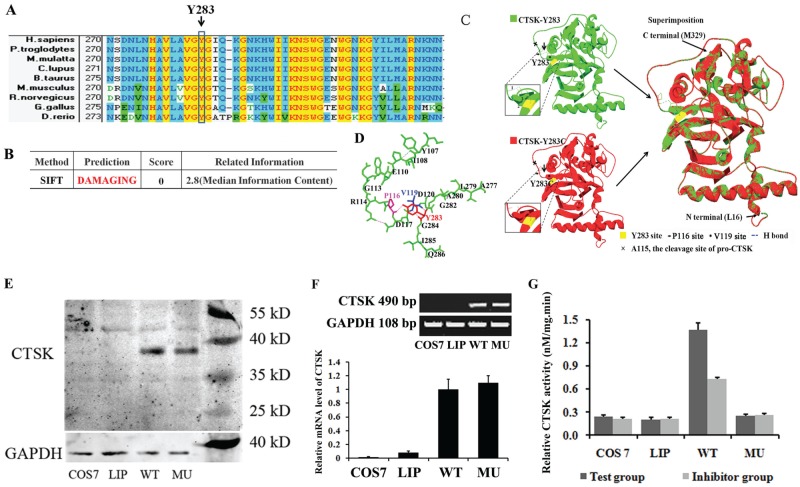
Furthermore, we screened the CTSK orthologs using the NCBI HomoloGene database (http://www.ncbi.nlm.nih.gov/homologene/68053). Residue Y283 is highly conserved among the 9 representative papain-like cysteine proteases in vertebrates (Fig. 2A).
Figure 2.
Cathepsin K (CTSK) mutant characteristics analysis. (A) Multiple-sequence alignment. The alignment of the CTSK with the corresponding segments in 9 species is shown. Y283C heterozygous missense mutation is at a highly conserved position in the CTSK; amino acids marked with column are highly conserved among all shown species. Background color illustration: transparent, nonsimilar; light blue, conservative; yellow, identical. (B) Damaging effects of the mutations predicted by the SIFT algorithm. (C) Modeling of the CTSK (16-329) p.Y283C mutation. The crystal structure of wild-type CTSK (16-329) is shown as CTSK-p.Y283 (PDB: 7pckA, green); site 283 is labeled in yellow; and 3 local H-bonds (1, 2, 3) are labeled by the blue dashed lines. The structure of the missense mutation p.Y283C is modeled by 7pckA and shown as CTSK-p.Y283C (red). The local region is 45° horizontally turned and magnified beside; superimposition of the wild-type CTSK structure with that of the Y283C model reveals the loss of the 3 hydrogen bonds between residue Y283 and residues P116 and V119 at the N-terminus of mature CTSK without affecting the backbone structure of the molecules. (D) The ribbon diagram shows the relation of hydrogen bonds in residues Y283 (red), P116 (purple), and V119 (blue). (E) Western blots. CTSK is shown in the upper panel, while glyceraldehyde-3-phosphate dehydrogenase (GAPDH) is shown in the lower panel. (F) Comparison of CTSK mRNA level. The reverse transcription polymerase chain reaction result is shown in the upper panel. The expected 490–base pair product was amplified from both wild-type and mutant mRNA. The SYBR-based quantitative polymerase chain reaction results are shown in the lower panel. (G) Fluorescence quantitative detection of CTSK activities. COS7, COS-7 cells without transfection; LIP, COS-7-cells mock transfected with Lipofectamine 2000; MU, COS-7-cells transfected with p.Y283C mutant CTSK gene; WT, COS-7 cells transfected with the wild-type CTSK gene.
SIFT was an analysis tool primarily applied to study the prediction of an amino acid substitution caused by nonsynonymous single-nucleotide polymorphisms (Kumar et al. 2009). The SIFT prediction score of substitution p.Y283C was reduced to 0.00 (Fig. 2B), which predicts a possible damaging of this alteration.
As shown in Figure 2C and D, the hydroxybenzene residue of p.Y283 could form 2 H-bonds with V119 and 1H-bond with P116. The 3 H-bonds may play an important role in restraining the local random coil structure near to the cleavage site (p.A115) of pro-CTSK. The loss of the hydroxybenzene residue in the Y283C mutation would interrupt the hydrogen network and possibly interfere with autoprocessing of the precursor molecule.
Experimental Expression Levels and Activity of Mutant CTSK
A protein band of 39 kDa, the approximate molecular mass for the pro-CTSK, was detected in homogenates of COS-7 cells transfected with the wild-type and p.Y283C mutant CTSK genes. The expression level of mutant CTSK was close to that of wild-type CTSK (0.933:1, P > 0.05; data not shown). No immunoreactivity was detected in homogenates of COS-7 cells without transfection or mock transfected with Lipofectamine 2000 Transfection Reagent (Fig. 2E). To determine the expression level of CTSK at the transcriptional level, we performed reverse transcription PCR analysis of mRNA (Fig. 2F). The expected 490-bp product was amplified from both wild-type and mutant mRNA, and no difference in the density of the amplified PCR products was observed. The result was coincident with that of qPCR analysis (Fig. 2F). The relative quantification from qPCR showed that the transient transfection resulted in a significantly increased mRNA level of CTSK. However, there was no difference between the wild-type and mutant mRNA level (P > 0.05).
To determine whether the p.Y283C mutation would damage the activity of CTSK, extracts from COS-7 cells transfected with the wild-type and p.Y283C mutant CTSK genes were subjected to fluorescence-based activity assay. The wild-type cells had an obvious activity of CTSK; CTSK-specific inhibitor odanacatib blocked at least 50% of its activity (P < 0.05). However, the activity in the p.Y283C mutant-transfected cells could not be detected, and its value was quite close to those of mock and blank transfects (P > 0.05; Fig. 2G).
Dental Abnormalities
The proband showed extensive periradicular high-density clumps with unclear periodontal space by orthopantomography examination (Fig. 3A). Teeth radiograph also showed unclear periodontal space, abnormal high-density around tooth roots, and stenosed pulp chambers (Fig. 3B–E).
Figure 3.
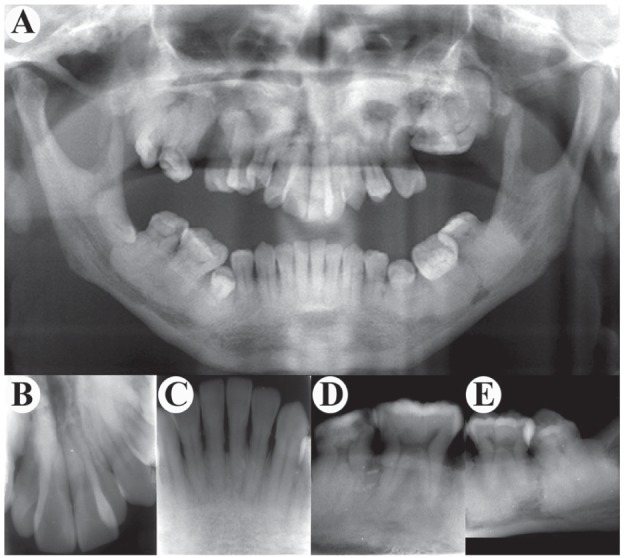
Radiologic findings of teeth. (A) Orthopantomography displays hypoplasia of maxilla and mandible with increased bone density and loss of normal trabeculae. It also shows a dense clump on the left maxillary molar area, obscure nasal cavity, maxillary sinuses, flattened mandibular angle, and the high radiodensity around the roots of lower molars. (B, C) Front teeth show relatively clear root boundaries. (D, E) Mandibular molars display narrowed pulp cavity, stenosis, or atresia in root canal. The most impressive feature is the extensive periradicular high-density clumps whose density appears to be the cortical bone.
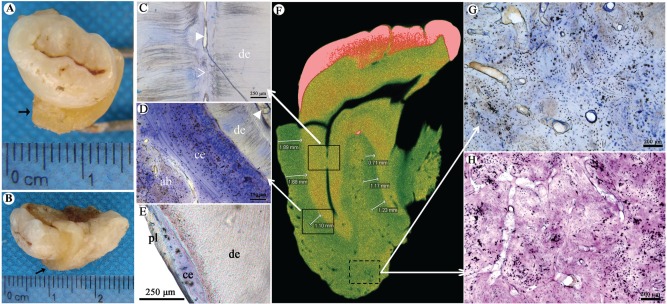
The affected left maxillary third molar was removed during the process of sequestrectomy. The tooth was found to be surrounded by a layer of hard tissue macroscopically (Fig. 4A, B). Phase contrast image, hematoxylin and eosin, and toluidine blue staining of the tooth showed that the root canal was narrow and partially sealed with calcified tissues (Fig. 4C). The cementum was significantly thickened around the roots without obvious boundary between cementum and alveolar bone. The thickened cementum was mainly made of cellular cementum, which was distributed from the cervical to apical part of root surface. More cementocytes with abnormal cellular process were found. Multilayer structure was found in the cellular cementum with darker staining gaps (Fig. 4D). The dentin did not show any obvious changes (Fig. 4C, D). The histologic appearance after toluidine blue staining of wild-type specimen is shown as Figure 2E.
Figure 4.
Abnormal bone and cementum structure. (A, B) The root is surrounded by a thick layer of hard tissue (marked with →). (C–E) Histologic appearance of tooth with toluidine blue staining. Panel C corresponds to the upper solid frame in panel F and shows that the root canal became narrowed and partially sealed with calcified tissues. de, dentin; solid arrowhead, root canal; clear arrowhead, atresia of root canal. Panel D corresponds to the lower solid frame in panel F and shows that the cementum is significantly thickened. There is no clear boundary between cementum and alveolar bone. The thickened cementum is mainly made of cellular cementum. More cementocytes with abnormal cellular process are found. ab, alveolar bone; ce, cementum; solid arrowhead, root canal. (E) Wild-type specimen shows thin cementum and periodontal ligament. pl, periodontal ligament. (F) Micro–computed tomography scanning of the patient’s tooth shows 2 roots of the left maxillary third molar with even and high-density image between the roots and alveolar bone (average thickness, 1.30 ± 0.42 mm). Arrows point to the different insets. (G, H) The changes of alveolar bone roughly corresponding to the dotted frame in panel F. (G) Toluidine blue staining shows disorganized bone structure with irregular medullary cavities scattered in the bone matrix. (H) Hematoxylin and eosin staining shows that structure of the cortical bone is irregularly interrupted by cellular components and matrix with different densities. Osteocytes show the irregularly cellular process.
Micro-CT scanning showed a band with high density around the lateral side of the root cervix (Fig. 4F). The average thickness of the band was about 1.30 ± 0.42 mm. Furthermore, histologic analysis on hard tissue section verified that this high-density band was derived from thickened cementum.
The disorganized bone structure was the main character of alveolar bone. There was no clear demarcation between the trabecular bone and the cortical bone. Few irregular medullary cavities were scattered in the bone matrix. The structure of the cortical bone was irregularly interrupted by cellular components and matrix with different densities. Osteocytes were shown with the irregularly cellular process (Fig. 4G, H).
Ultrastructure Changes in the Cementum
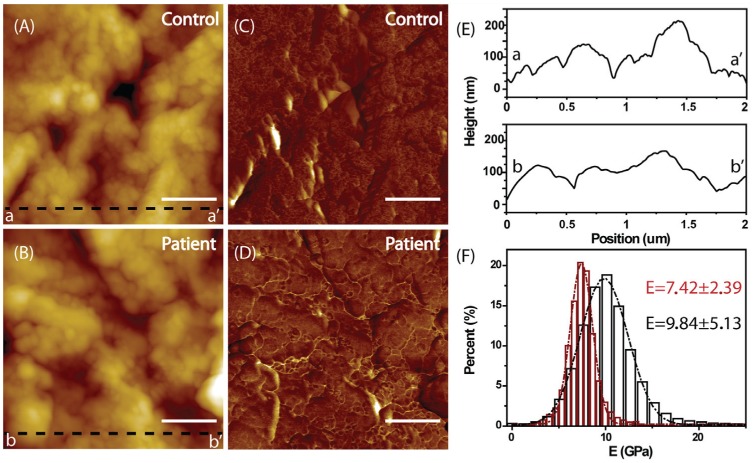
The AFM topographies of the healthy control’s and patient’s cementum samples are shown in Figure 5A and B, respectively. It is obvious that the protrusion size of healthy cementum (Fig. 5A) is smaller than those of the patient’s cementum (Fig. 5B). It could be further confirmed by the line profiles in Figure 5E. It was evident that the line profile bb′ (the patient’s) was much smoother than aa′ (the healthy control’s). By using the Peakforce QNM mode, it was possible to record not only the topography but also the mechanical properties of the measured samples (Rico et al. 2011; Shibata et al. 2011). Figure 5C and D shows the stiffness maps of the 2 cementum samples. In each image, the contrast did not indicate height any more but related to the Young’s modulus: the brighter color represents harder region. The corresponding distributions of the Young’s modulus of the 2 samples are shown in Figure 5F. The Young’s modulus value of the control’s cementum is 9.84 ± 5.13 GPa, which is close to the value of 11.0 ± 5.8 GPa previously reported in the elastic modulus of coronal cementum (Ho et al. 2009)—thus indirectly implying the reliability of this QNM technique. However, the Young’s modulus distribution for the patient’s cementum is much narrower with the value of 7.42 ± 2.39 GPa (P < 0.05), indicating the disease-associated softness of the cementum.
Figure 5.
Atomic force microscopy analysis. The topography and Young’s modulus of the healthy control’s (A, C) and the patient’s (B, D) cementum, respectively. The scale bar is 500 nm. (E) The line profiles corresponding to the dark dash lines aa′ (A) and bb′ (B). It is evident that the line profile bb′ (the patient’s) is much smoother than aa′ (the healthy control’s). (F) The corresponding Young’s modulus distributions of healthy (dark) and the patient’s cementum (red). The Young’s modulus distribution for the patient’s cementum is much narrower (7.42 ± 2.39 vs. 9.84 ± 5.13 GPa, P < 0.05), indicating the disease-associated softness of the cementum.
Discussion
CTSK was identified as the candidate gene of pycnodysostosis in 1996 (Gelb et al. 1996). According to our previous estimation, 35 different CTSK mutations have been reported in 60 pycnodysostosis families (Xue et al. 2011). Here we have confirmed the diagnosis of pycnodysostosis in our patient by sequence analysis, which is caused by novel compound mutations of the CTSK gene. The nonsense mutation p.W29X was predicted to terminate polypeptide synthesis in the proregion and thus probably eliminate the entire enzymatic activity. Compared with other reported truncated mutations (Gelb et al. 1996; Hou et al. 1999; Fujita et al. 2000; Fratzl-Zelman et al. 2004; Donnarumma et al. 2007; Arman et al. 2014), the p.W29X mutation may represent the loss-of-function allele with the earliest termination codon in the precursor CTSK polypeptide. The effects of the missense mutation p.Y283C on the function of CTSK were predicted by the SIFT algorithm and mutant structure modeling and were experimentally confirmed by transient expression of the mutants in COS-7 cells. Donnarumma et al. (2007) also examined the mutant CTSK protein (L7P, Q165R, G194S, I249T, D250G, G319C) expression levels by transient expression in COS-7 cells. They found that the missense mutation p.L7P in the N-terminal segment of the preregion of CTSK polypeptide significantly reduced CTSK expression, while mutations in the mature CTSK peptide (Q165R, G194S, I249T, D250G, and G319C) had little effects on the expression level of the protein. Similar to Donnarumma’s observation, both mRNA and protein expression levels of mutant p.Y283C were close to those of wild-type CTSK in our case. However, crystal structure and fluorescence quantitative assay indicated that the p.Y283C mutation may affect the self-cleavage of the CTSK enzyme, which in turn disrupts its enzymatic activation.
It is also known that dysfunctional CTSK affects osteogenesis and bone function. However, fewer bone histologic observations were reported in pycnodysostosis patients (Fratzl-Zelman et al. 2004; Chavassieux et al. 2008). The abnormal bone phenotypes include defective multinucleated osteoclasts, cartilage residual inclusion in the bone matrix, and depressed active bone formation. The disordered lamellar organization of the trabeculae was also reported. There have been no histologic observations of alveolar bones in pycnodysostosis patients. For the first time, we tried to find the features of alveolar bones in pycnodysostosis patient. The highly disordered bone matrixes were interlaced with uncalcified fibrous tissues without the clear boundary. The different histologic changes found here might contribute to the differences in bone formation and structure between iliac bone (endochondral ossification) and jaws (intramembranous ossification). However, the reason why no osteoclasts were found in the abnormal alveolar bone is unclear at this point and might be caused by individual variance among patients or the specificity of the jaw in formation and structure.
Cementum is a mineralized connective tissue that covers the surface of tooth root. In the present case, the impressive feature is the thickened cementum and abnormal cementum changes. Although the similar orthopantomographic characteristics of roots have been reported in other patients, the detailed description of these typical features was not mentioned (Bathi and Masur 2000; Dimitrakopoulos et al. 2007; Alves and Cantín 2014). According to a report from Stamfelj et al. (2008), a normal midpoint cementum thickness ranged between 5 and 800 µm, and maximal cementum thickness ranged between 25 and 1140 µm in maxillary molars. In our report, the maximal cementum thickness of the left maxillary third molar is far beyond 1140 µm. The increased cementocytes, cementoblasts, and dark-stained gap between cellular cementum layers suggested a demineralized or hypocalcified characteristic of cementum, and AFM topographies confirmed the softness of the cementum. This may be caused by inadequate collagen degradation. Cementum is composed of 90% collagen I and III and ground substance. The expression of CTSK has been localized in osteoclasts or odontoclasts around root (Linsuwanont et al. 2002; Tsuchiya et al. 2008). Here we found that mutant CTSK with lower enzymatic activity may reduce the efficiency of odontoclasts or cementoclasts in degradation of the newly formed cementum matrix.
Conclusion
The missense mutation p.Y283C in CTSK did not affect the mRNA and protein levels of CTSK; however, it reduced CTSK enzyme activity and caused the histologic and ultrastructural changes of cementum and alveolar bone. Our findings support the notion that CTSK may play an important role in the development of cementum and alveolar bone. The dysfunction of CTSK might affect the sequential degradation of cementum matrix protein and result in the hypomineralized cementum and disorganized alveolar bone in the affected subject.
Author Contributions
Y. Xue, X. Duan, contributed to conception, design, data acquisition, analysis, and interpretation, drafted and critically revised the manuscript; L. Wang, contributed to design, data analysis, and interpretation, drafted the manuscript; D. Xia, Q. Li, contributed to data analysis, drafted the manuscript; S. Gao, contributed to design, drafted and critically revised the manuscript; M. Dong, contributed to design and data analysis, drafted and critically revised the manuscript; T. Cai, contributed to conception, data analysis, and interpretation, critically revised the manuscript; S. Shi, contributed to conception, critically revised the manuscript; L. He, K. Hu, contributed to data acquisition and analysis, drafted the manuscript; T. Mao, contributed to conception and data acquisition, critically revised the manuscript. All authors gave final approval and agree to be accountable for all aspects of the work.
Supplementary Material
Acknowledgments
We thank the patient, his family members, and the controls for joining this research. We are also grateful for the technique support from Equipment Center of PLA Institute of Stomatological Research.
Footnotes
This work was supported in part by grants of the National Natural Science Foundation of China (NSFC 81271116 for X.D., NSFC 81300861 for Y.X.).
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
A supplemental appendix to this article is published electronically only at http://jdr.sagepub.com/supplemental.
References
- Alves N, Cantín M. 2014. Clinical and radiographic maxillofacial features of pycnodysostosis. Int J Clin Exp Med. 7(3):492–496. [PMC free article] [PubMed] [Google Scholar]
- Arman A, Bereket A, Coker A, Kiper PÖ, Güran T, Ozkan B, Atay Z, Akçay T, Haliloglu B, Boduroglu K, et al. 2014. Cathepsin K analysis in a pycnodysostosis cohort: demographic, genotypic andphenotypic features. Orphanet J Rare Dis. 9:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bathi RJ, Masur VN. 2000. Pyknodysostosis: a report of two cases with a brief review of the literature. Int J Oral Maxillofac Surg. 29(6):439–442. [PubMed] [Google Scholar]
- Chavassieux P, Asser Karsdal M, Segovia-Silvestre T, Neutzsky-Wulff AV, Chapurlat R, Boivin G, Delmas PD. 2008. Mechanisms of the anabolic effects of teriparatide on bone: insight from the treatment of a patient with pycnodysostosis. J Bone Miner Res. 23(7):1076–1083. [DOI] [PubMed] [Google Scholar]
- Dimitrakopoulos I, Magopoulos C, Katopodi T. 2007. Mandibular osteomyelitis in a patient with pyknodysostosis: a case report of a 50-year misdiagnosis. J Oral Maxillofac Surg. 65(3):580–585. [DOI] [PubMed] [Google Scholar]
- Donnarumma M, Regis S, Tappino B, Rosano C, Assereto S, Corsolini F, Di Rocco M, Filocamo M. 2007. Molecular analysis and characterization of nine novel CTSK mutations in twelve patients affected by pycnodysostosis. Mutation in brief #961. Online. Hum Mutat. 28(5):524. [DOI] [PubMed] [Google Scholar]
- Fratzl-Zelman N, Valenta A, Roschger P, Nader A, Gelb BD, Fratzl P, Klaushofer K. 2004. Decreased bone turnover and deterioration of bone structure in two cases of pycnodysostosis. J Clin Endocrinol Metab. 89(4):1538–1547. [DOI] [PubMed] [Google Scholar]
- Fujita Y, Nakata K, Yasui N, Matsui Y, Kataoka E, Hiroshima K, Shiba RI, Ochi T. 2000. Novel mutations of the cathepsin K gene in patients with pycnodysostosis and their characterization. J Clin Endocrinol Metab. 85(1):425–431. [DOI] [PubMed] [Google Scholar]
- Gelb BD, Shi GP, Chapman HA, Desnick RJ. 1996. Pycnodysostosis, a lysosomal disease caused by cathepsin K deficiency. Science. 273(5279):1236–1238. [DOI] [PubMed] [Google Scholar]
- Ho SP, Senkyrikova P, Marshall GW, Yun W, Wang Y, Karan K, Li C, Marshall SJ. 2009. Structure, chemical composition and mechanical properties of coronal cementum in human deciduous molars. Dent Mater. 25(10):1195–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou WS, Brömme D, Zhao Y, Mehler E, Dushey C, Weinstein H, Miranda CS, Fraga C, Greig F, Carey J, et al. 1999. Characterization of novel cathepsin K mutations in the pro and mature polypeptide regions causing pycnodysostosis. J Clin Invest. 103(5):731–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar P, Henikoff S, Ng PC. 2009. Predicting the effects of coding non-synonymous variants on protein function using the SIFT algorithm. Nat Protoc. 4(7):1073–1081. [DOI] [PubMed] [Google Scholar]
- Linsuwanont B, Takagi Y, Ohya K, Shimokawa H. 2002. Localization of cathepsin K in bovine odontoclasts during deciduous tooth resorption. Calcif Tissue Int. 70(2):127–133. [DOI] [PubMed] [Google Scholar]
- Mujawar Q, Naganoor R, Patil H, Thobbi AN, Ukkali S, Malagi N. 2009. Pycnodysostosis with unusual findings: a case report. Cases J. 2:6544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rico F, Su C, Scheuring S. 2011. Mechanical mapping of single membrane proteins at submolecular resolution. Nano Lett. 11:3983–3986. [DOI] [PubMed] [Google Scholar]
- Schilling AF, Mülhausen C, Lehmann W, Santer R, Schinke T, Rueger JM, Amling M. 2007. High bone mineral density in pycnodysostotic patients with a novel mutation in the propeptide of cathepsin K. Osteoporos Int. 18(5):659–669. [DOI] [PubMed] [Google Scholar]
- Shibata M, Uchihashi T, Yamashita H, Kandori H, Ando T. 2011. Structural changes in bacteriorhodopsin in response to alternate illumination observed by high-speed atomic force microscopy. Angew Chem Int Ed Engl. 50(19):4410–4413. [DOI] [PubMed] [Google Scholar]
- Sivaraman J, Lalumiere M, Menard R, Cygler M. 1999. Crystal structure of wild-type human procathepsin K. Protein Sci.8:283–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamfelj I, Vidmar G, Cvetko E, Gaspersic D. 2008. Cementum thickness in multirooted human molars: A histometric study by light microscopy. AnnAnat. 190(2):129–139. [DOI] [PubMed] [Google Scholar]
- Tsuchiya M, Akiba Y, Takahashi I, Sasano Y, Kashiwazaki J, Tsuchiya S, Watanabe M. 2008. Comparison of expression patterns of cathepsin K and MMP-9 in odontoclasts and osteoclasts in physiological root resorption in the rat molar. Arch Histol Cytol. 71(2):89–100. [DOI] [PubMed] [Google Scholar]
- Xue Y, Cai T, Shi S, Wang W, Zhang Y, Mao T, Duan X. 2011. Clinical and animal research findings in pycnodysostosis and gene mutations of cathepsin K from 1996 to 2011. Orphanet J Rare Dis. 6:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang T, Zhang Y, Li Y, Hao Y, Zhou M, Dong N, Duan X. 2013. High amounts of fluoride induce apoptosis/cell death in matured ameloblast-like LS8 cells by downregulating Bcl-2. Arch Oral Biol. 58(9):1165–1173. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.