Abstract
Aim
α-Lipoic acid exerts a powerful antioxidant effect by acting as a free radical scavenger and inducing endogenous antioxidants such as vitamin E and glutathione. In the present study, we examined the effects of α-lipoic acid on cardiac dysfunction in rat hearts with aortocaval fistulae.
Main methods
Aortocaval fistulae were created between the abdominal aorta and inferior vena cava in male rats. Hemodynamic parameters were measured 14 days after surgery using an intravascular pressure transducer, and then these hearts were harvested for tissue weight measurement, pathological evaluation, and mRNA isolation.
Results
In vehicle-treated rats, left ventricular end-diastolic pressure and left ventricular weight significantly increased at 14 days after fistula creation. Fistula-creation resulted in expression of 4-hydroxy-2-nonenal, NADPH oxidase subunit p67phox and BNP mRNA in a time-dependent manner in the left ventricle.
Long-term treatment (initiated 2 days before surgery, and continued for 14 days after fistula creation; days -2 to 14) with α-lipoic acid (30 mg/kg/day) markedly suppressed the increases in left and right ventricular weight, and left ventricular end-diastolic pressure. α-Lipoic acid treatment from days -2 to 14 prominently prevented the expression of 4-hydroxy-2-nonenal and NADPH oxidase subunit p67phox, and significantly raised BNP mRNA levels. Short-term treatment with α-lipoic acid from day - 2 to 7 was effective in preventing cardiac enlargement and dysfunction, similar to long-term treatment, but treatment from days 7–14 was not effective.
Conclusions
Treatment with α-lipoic acid can prevent cardiac hyperplasia and dysfunction, probably by inhibiting superoxide production and enhancing BNP mRNA expression in an early phase after fistula creation.
Keywords: Biochemistry, Aortocaval fistula, α-Lipoic acid, 4-Hydroxy-2-nonenal, BNP
1. Introduction
Oxidative processes play an important role in the pathogenesis of congestive heart failure. The metabolite 4-hydroxy-2-nonenal is recognized as a reliable marker of oxidative stress, and is known to increase in the myocardium of heart failure patients [1]. Some clinical trials, such as the Nurses' Health Study and the Established Populations for Epidemiologic Studies of the Elderly [2, 3], have reported that antioxidants may reduce risk of coronary heart disease. However, the initial Heart Outcomes Prevention Evaluation (HOPE) and HOPE–The Ongoing Outcomes (HOPE-TOO) study demonstrated that supplementation with the antioxidant vitamin E did not prevent cardiovascular events in patients with cardiac history [4]. These findings suggest that antioxidant vitamin supplementation is of limited effectiveness for cardiovascular disease.
α-Lipoic acid, a dithiol compound, has strong antioxidant properties. α-Lipoic acid has been shown to scavenge singlet oxygen, hydrogen peroxide, peroxynitrite, and nitric oxide [5]. In addition to its direct reactive oxygen scavenging properties, α-lipoic acid is known to chelate redox-active metal ions such as iron, copper, and zinc [6]. It has also been reported that α-lipoic acid improves mitochondrial function, and reduces oxidative stress by regulating genes associated with anti-oxidative and anti-inflammatory signaling [7, 8]. Thus, α-lipoic acid has direct and indirect antioxidant properties, and exerts potential therapeutic effects for cardiovascular diseases. In addition, several clinical trials have demonstrated therapeutic benefits of α-lipoic acid in various diseases [8, 9, 10, 11, 12, 13].
We previously reported that oxidative stress was markedly elevated in a rat model with aortocaval fistula [14]. In this study, we investigated the effect of α-lipoic acid on deterioration of cardiac function after fistula creation, and mechanisms of its action. We also evaluated whether therapeutic efficacy of α-lipoic acid is influenced by time of initiation of therapy, and term of therapy after fistula creation.
2. Materials and methods
2.1. Animals and experimental design
Ten-week old male Sprague-Dawley rats were obtained from Japan SLC, Inc. (Shizuoka, Japan). All animals were maintained at the departmental animal care facility of Osaka University of Pharmaceutical Sciences in accordance with recommendations for animal research based on the guidelines of the Declaration of Helsinki. Rats were housed in a light controlled room with a 12-hour light/dark cycle, and were allowed ad libitum access to food and water. Experimental protocols and animal care methods were approved by the Experimental Animal Research Committee at Osaka University of Pharmaceutical Sciences.
Several experiments were carried out according to the protocol indicated below. In the first experiments, we harvested the left ventricles at 0, 3, 7, 10, and 14 days after aortocaval fistula creation. We carried out immunohistochemistry to detect 4-hydroxy-2-nonenal, and quantitated brain natriuretic peptide (BNP) and NADPH oxidase subunit mRNA in left ventricles by quantitative real time polymerase chain reaction. In the 2nd experiment, fistula-created rats were divided into 4 groups. Each group received one of the following treatment regimens; (1) α-lipoic acid (30 mg/kg/day) treatment initiated 2 days before and continued until 14 days after aortocaval fistula creation (days -2 to 14), (2) α-lipoic acid treatment spanning days -2 to 7, (3) α-lipoic acid treatment spanning days 7–14, and (4) treatment with vehicle (a solution consisting 20% ethanol and 80% corn oil). Sham operated rats were dosed with vehicle from 2 days before to 14 days after surgery. All animals were subcutaneously administered α-lipoic acid or vehicle.
2.2. Aortocaval fistula procedure
An aortocaval fistula was created as described, to induce volume overload [14]. Briefly, the abdominal aorta and inferior vena cava of rats anesthetized with sodium pentobarbital (50 mg/kg i.p.) were carefully exposed. The abdominal aorta was clamped just caudal to the left renal artery and then punctured with an 18-gauge disposable needle between the renal artery and the aortic bifurcation. The needle was inserted into the inferior vena cava and withdrawn, and then the aortic puncture was sealed with cyanoacrylate glue. The creation of fistulae was verified by observation of swelling of the vena cava, and by mixing of arterial and venous blood. Identical sham operations were performed in control rats without constructing fistulae. All procedures were performed by the same individual.
2.3. Cardiac hemodynamics and morphometric analysis
Cardiac hemodynamics were measured 14 days after surgery using an intravascular pressure transducer (model SPR-294A Millar Micro-Tip catheter transducer, Millar Instruments, Houston, TX, USA). Rats were anesthetized with pentobarbital (50 mg/kg i.p.) as described [15]. The transducer was inserted into the right cervical vein and advanced to the right ventricle to determine right ventricular systolic pressure. A catheter was then introduced into the right carotid artery to measure aortic systolic and diastolic pressure. The catheter was then advanced into the left ventricle to determine left ventricular systolic, and end-diastolic pressure, and peak positive and negative dP/dt. Shunt patency was visually confirmed before sacrificing the rats. Blood was sampled, and then whole hearts were weighed. Right and left atria and ventricles were separated and weighed. Left ventricles were then immediately fixed with 10% formalin. Fixed left ventricles were embedded in paraffin and cut into 4-μm-thick sections. Tissue sections were stained with hematoxylin-eosin or Sirius Red. The shortest diameters of cardiomyocytes were measured by light microscopy (×400 magnification) only in nucleated transverse sections stained with hematoxylin-eosin in at least 30 areas of each section. Myocardial fibrosis of the left ventricle was determined by measuring red stained areas in 10 fields per section using Image J. The amount of fibrosis was then used to calculate % fibrosis.
2.4. Immunohistochemistry
Fixed left ventricles were embedded in paraffin and cut into 4 μm sections. Immunohistochemistry was performed as described previously [14]. Briefly, sections were incubated with the mouse monoclonal antibody against 4-hydroxy-2-nonenal (1:50; Japan Institute for the Control of Ageing, Shizuoka, Japan), which indicates production of reactive oxygen species. The levels of 4-hydroxy-2-nonenal in left ventricles were semi-quantitatively measured by measuring positively stained areas in 10 fields per section using Image J. The area of positive staining was used to calculate % positive staining/area. All histological analysis was carried out by a single examiner, who was blinded with respect to the experimental group to which each sample belonged.
2.5. Quantitative real-time PCR
Total RNA was extracted from rat left ventricle using the TRIzol method (Invitrogen, Carlsbad, CA), and further purified with an RNeasy Mini Kit (Qiagen, Valencia, CA), according to each manufacturer's protocol. RNA was quantitated by spectrophotometry using a NanoDrop ND-1000 spectrophotometer (NanoDrop Technologies), and the ratio of optical densities at 260 and 280 nm (OD260/OD280) was used to evaluate purity (UV absorption ratio, A260/A280 > 1.9) of the nucleic acid samples. Purified total RNA (1.2 μg) was subsequently reverse-transcribed with High Capacity cDNA Reverse Transcription Kits (Life Technologies Japan, Tokyo, JAPAN). Quantitative real-time PCR was performed with a LightCycler 480 System using LightCycler 480 Probes Master kit (Roche Applied Science, Indianapolis, IN) with gene-specific Taqman® Gene expression assays for BNP; Rn00580641_m1, gp91phox; Rn00576710_m1, p22phox; Rn00577357_m1, p47phox; Rn00586945_m1, p67phox; Rn01759078_m1, (Applied Biosystems, South San Francisco, CA). A comparative CT method was used for relative quantification of RNA expression [16]. GAPDH, Rn01775763_g1, was used as an internal control for normalization.
2.6. Drugs
α-Lipoic acid, purchased from Nacalai Tesque (Kyoto, Japan), was dissolved in a solution consisting of 20% ethanol and 80% corn oil. Other chemicals were obtained from Nacalai Tesque and Wako Pure Chemical Industries (Osaka, Japan).
2.7. Statistical analysis
All values were expressed as means ±S.E.M. Relevant data were processed by EXSUS software, version 8.0.1 (CAC Exicare, Tokyo, Japan). For statistical analysis, we used one-way analysis of variance followed by Dunnett's multiple comparison tests. Differences were considered significant at a value of P < 0.05.
3. Results
3.1. Left ventricular 4-hydroxy-2-nonenal production after fistula creation
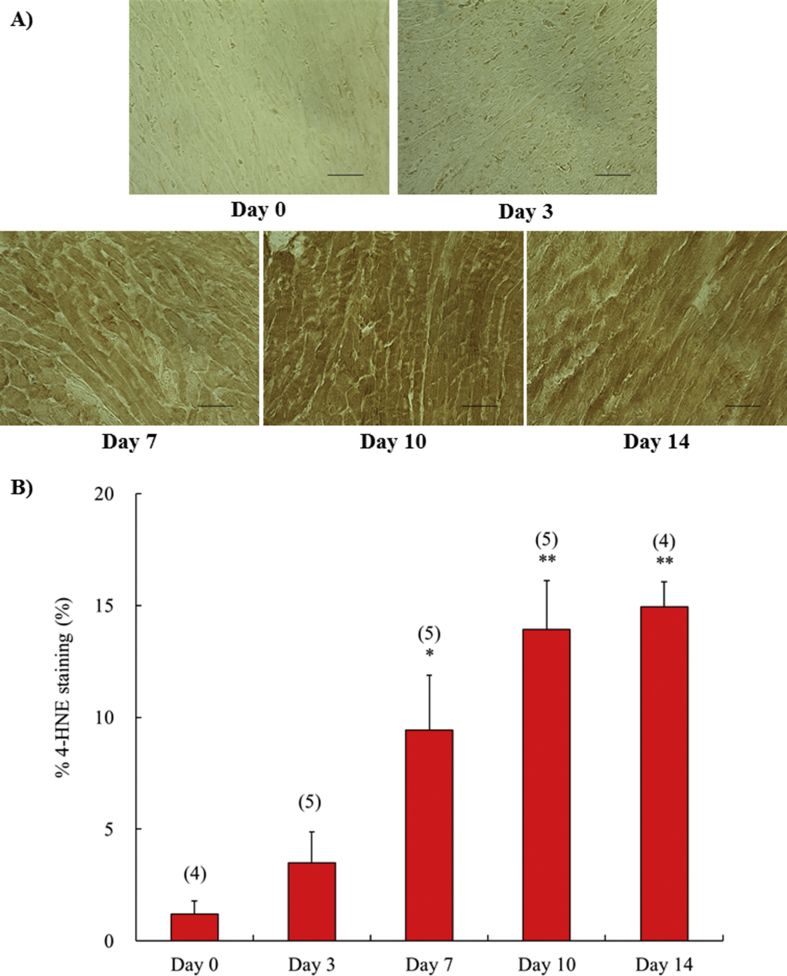
In a previous study [14], we found that left ventricular oxidative stress significantly increased at day 14 after fistula creation, compared with the sham operated group. As shown in Fig. 1, we detected a time-dependent change in left ventricular oxidative stress by immunohistochemical quantitation of cells positive for 4-hydroxy-2-nonenal. Positive staining for 4-hydroxy-2-nonenal began to increase at day 7 and continued through day 14.
Fig. 1.
The time course of the expression of 4-hydroxy-2-nonenal in left ventricle at 0, 3, 7, 10 and 14 days after surgery. Representative immunohistochemical staining showing 4-hydroxy-2-nonenal in left ventricle obtained from fistula created rat (A). All figures are depicted at 400×magnification. The scale represents 50 μm. Semi-quantification of 4-hydroxy-2-nonenal expression in left ventricle (B). Each value represents the mean ± S.E.M. of number of animals in parentheses. **P < 0.01, *P < 0.05, compared with day 0. 4-HNE, 4-hydroxy-2-nonenal.
3.2. Left ventricular NADPH oxidase subunit mRNA expression after fistula creation
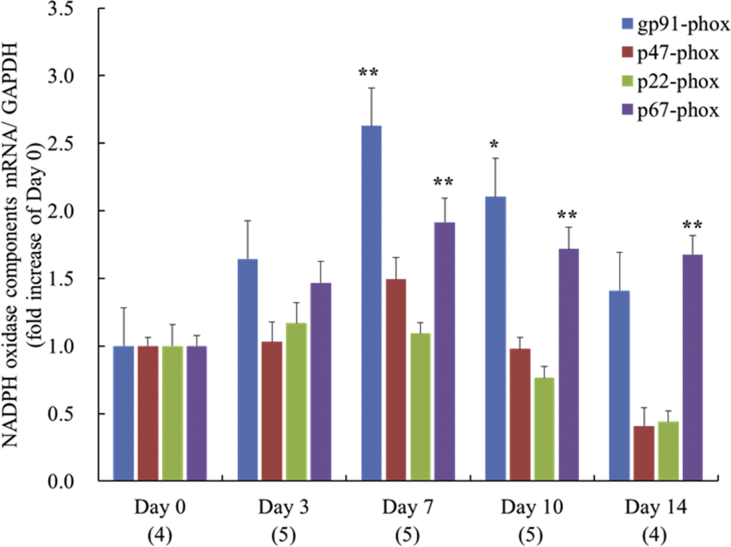
Expression of gp91phox mRNA significantly increased at day 7 and 10, but decreased to the level of day 0 by day 14. Expression of p67phox mRNA was significantly elevated from day 7 to day 14, while p22phox and p47phox mRNA expression did not significantly increase through day 14 (Fig. 2).
Fig. 2.
The time course of the expression of NADPH oxidase subunit mRNA, gp91phox, p22phox, p47phox and p67phox at 0, 3, 7, 10 and 14 days after surgery in left ventricle. Each value represents the mean ± S.E.M. **P < 0.01, *P < 0.05, compared with day 0.
3.3. Left ventricular brain natriuretic peptide mRNA expression after fistula creation
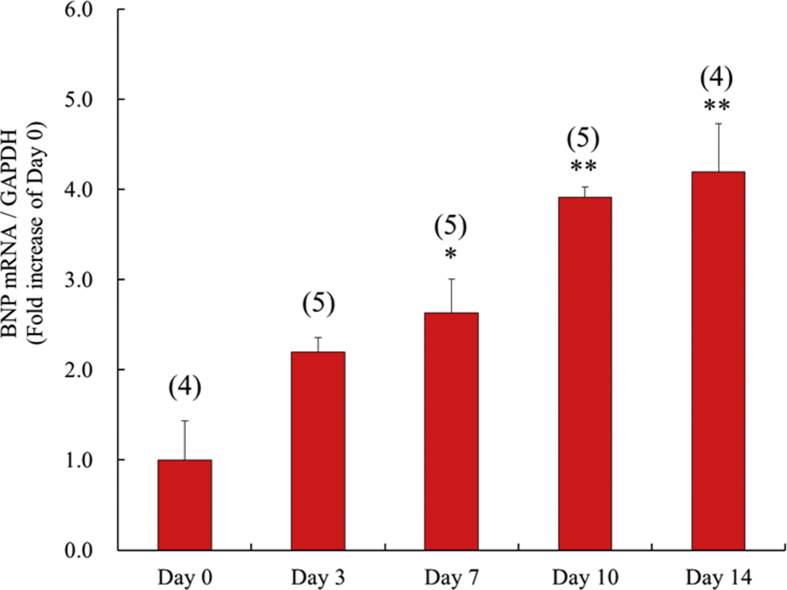
BNP mRNA expression began to rise significantly on day 7 after fistula creation. Elevated BNP expression continued through day 14 (Fig. 3).
Fig. 3.
The time course of the expression of brain natriuretic peptide mRNA at 0, 3, 7, 10 and 14 days after surgery in left ventricle. Each value represents the mean ± S.E.M. of number of animals in parentheses. **P < 0.01, *P < 0.05, compared with day 0. BNP, brain natriuretic peptide.
3.4. Body and heart weights after fistula creation, and effects of α-lipoic acid treatment
Creation of fistulae resulted in increased right and left atrium weight, and right and left ventricular weight compared with the sham operated group (Table 1). Increases in right and left ventricular weight were prevented by α-lipoic acid treatment from days -2 to 14 compared with the vehicle treated fistula group. Short term treatment with early initiation (days -2 to 7) also markedly suppressed gains in right and left ventricular weight. Delayed initiation treatment (days 7–14) failed to suppress the weight increases. Fistula formation tended to reduce body weight compared to sham operated rats. Treatment with α-lipoic acid from days -2 to 14 or from days -2 to 7 but not from days 7–14 tended to suppress this body weight loss.
Table 1.
Time-window response of body weight and heart weight at 14 days after fistula creation.
| Sham | Fistula | Days -2 to 7 | Days 7–14 | Days -2 to 14 | |
|---|---|---|---|---|---|
| n | 5 | 10 | 6 | 7 | 9 |
| BW (g) | 325.6 ± 10.5 | 299.8 ± 10.3 | 326.2 ± 4.5 | 309.1 ± 4.1 | 321.8 ± 5.6 |
| Right atrium/BW (mg/g) | 0.11 ± 0.02 ** | 0.35 ± 0.02 | 0.27 ± 0.03 | 0.28 ± 0.03 * | 0.27 ± 0.02 ** |
| Left atrium/BW (mg/g) | 0.13 ± 0.02 ** | 0.33 ± 0.03 | 0.26 ± 0.03 | 0.27 ± 0.01 | 0.25 ± 0.03 * |
| Right ventricle/BW (mg/g) | 0.45 ± 0.01 ** | 0.88 ± 0.03 | 0.73 ± 0.04 ** | 0.83 ± 0.03 | 0.73 ± 0.03 ** |
| Left ventricle/BW (mg/g) | 2.00 ± 0.04 ** | 2.85 ± 0.05 | 2.39 ± 0.10 ** | 2.81 ± 0.06 | 2.60 ± 0.07 * |
Each value represents the mean ± SEM. BW indicates body weight. **P < 0.01, *P < 0.05, compared with Fistula group.
3.5. Cardiac hemodynamics after fistula creation, and effects of α-lipoic acid treatment
The parameters of cardiac hemodynamics in the vehicle treated fistula group included markedly increased right ventricular systolic pressure (RVSP) and left ventricle end diastolic pressure (LVEDP), compared with the sham operated group (Table 2). α-Lipoic acid treatment from days -2 to 14 or from days -2 to 7, but not from days 7–14, significantly suppressed increases in RVSP and LVEDP.
Table 2.
Time-window response of cardiac hemodynamics at 14 days after fistula creation.
| Sham | Fistula | Days -2 to 7 | Days 7–14 | Days -2 to 14 | |
|---|---|---|---|---|---|
| n | 5 | 7 | 6 | 7 | 9 |
| Heart rate (beats/min) | 450.1 ± 20.2 | 389.2 ± 25.7 | 401.3 ± 10.1 | 446.0 ± 14.8 | 444.2 ± 8.4 |
| Mean atrial pressure (mmHg) | 92.7 ± 11.4 | 78.2 ± 9.3 | 87.8 ± 3.6 | 92.0 ± 4.5 | 95.5 ± 3.9 |
| Right ventricular systolic pressure (mmHg) | 38.8 ± 4.3 * | 62.6 ± 7.0 | 41.2 ± 4.2 * | 46.1 ± 3.8 | 41.9 ± 3.2 * |
| Left ventricular systolic pressure (mmHg) | 110.9 ± 11.1 | 105.5 ± 9.8 | 107.8 ± 3.8 | 112.5 ± 5.3 | 118.9 ± 3.4 |
| Left ventricular end-diastolic pressure (mmHg) | 3.8 ± 0.6 ** | 8.5 ± 1.0 | 4.7 ± 0.8 * | 9.2 ± 0.7 | 4.6 ± 0.7 ** |
| Peak positive dP/dt (mmHg/s) | 9264 ± 1018 | 8937 ± 1142 | 11173 ± 542 | 10080 ± 551 | 10763 ± 455 |
| Peak negative dP/dt (mmHg/s) | 7136 ± 826 | 6846 ± 1261 | 7440 ± 408 | 7587 ± 371 | 7450 ± 244 |
Each value represents the mean ± SEM. **P < 0.01, *P < 0.05, compared with Fistula group.
3.6. Mean diameter and ratio of fibrosis among myocytes in left ventricles with fistulae, and effects of α-lipoic acid treatment
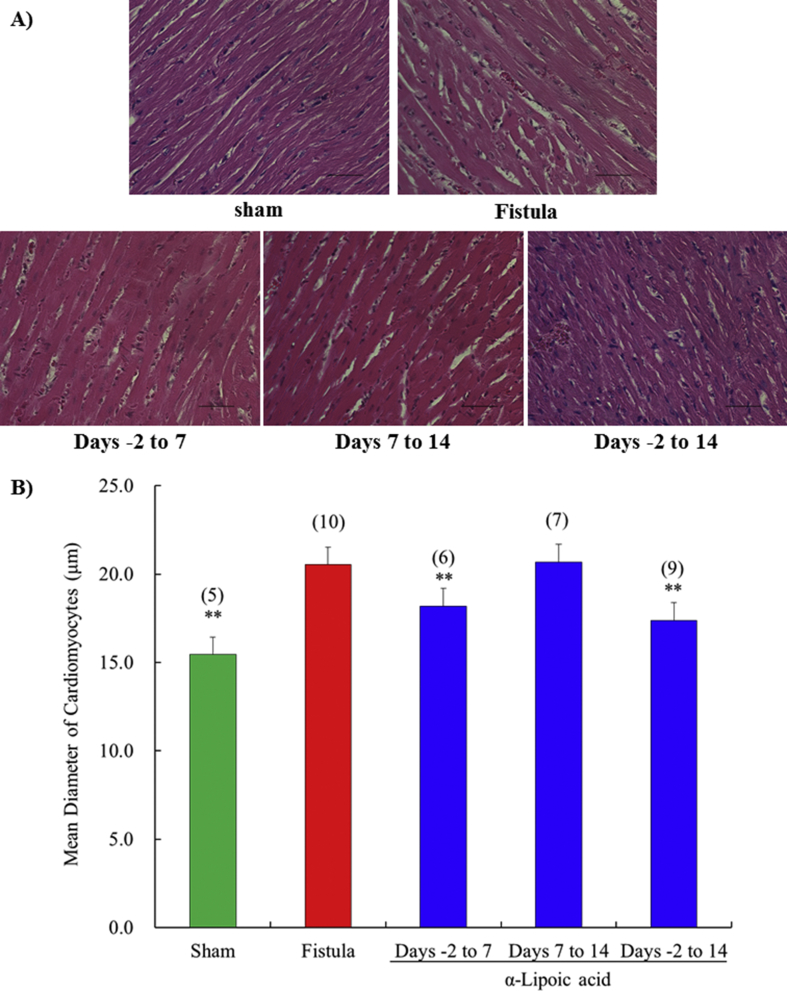
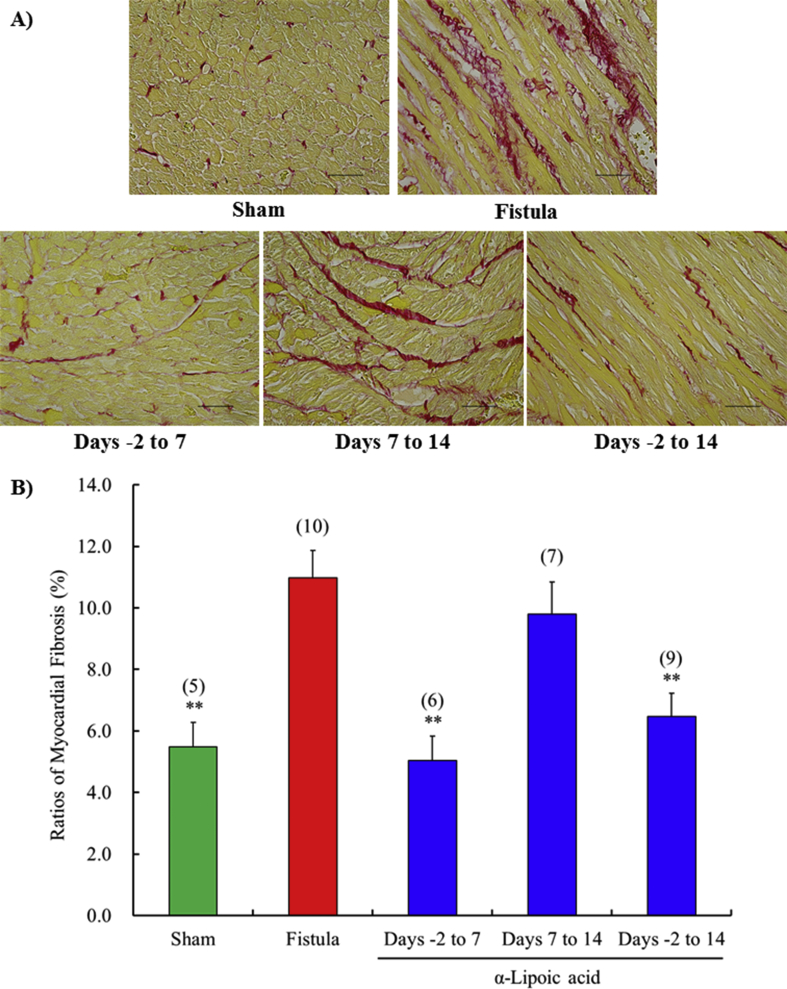
The mean diameters and % fibrosis of cardiomyocytes in the left ventricle were significantly increased at day 14 by fistula creation. The increased diameter and % fibrosis of cardiomyocytes was prevented by α-lipoic acid treatment from days -2 to 14 compared with the vehicle treated fistula group (Figs. 4 and 5). Short term treatment with early initiation (days -2 to 7) also markedly inhibited cardiomyocyte hypertrophy and % fibrosis. However, short term treatment with delayed initiation (days 7–14) failed to suppress them.
Fig. 4.
Time-window response of the diameter of cardiomyocytes in left ventricle at 14 days after surgery. Representative sections are stained with HE (A). All figures are depicted at 400×magnification. The scale represents 50 μm. The shortest diameters of cardiomyocytes were measured by light microscopy (B). Each value represents the mean ± S.E.M. of number of animals in parentheses. **P < 0.01, compared with fistula.
Fig. 5.
Time-window response of the myocardial fibrosis in left ventricle at 14 days after surgery. Representative sections are stained with Sirius Red (A). All figures are depicted at 400×magnification. The scale represents 50 μm. The ratio of myocardial fibrosis (B). Each value represents the mean ± S.E.M. of number of animals in parentheses. **P < 0.01, compared with fistula.
3.7. 4-Hydroxy-2-nonenal production in left ventricles with fistulae, and effects of α-lipoic acid treatment
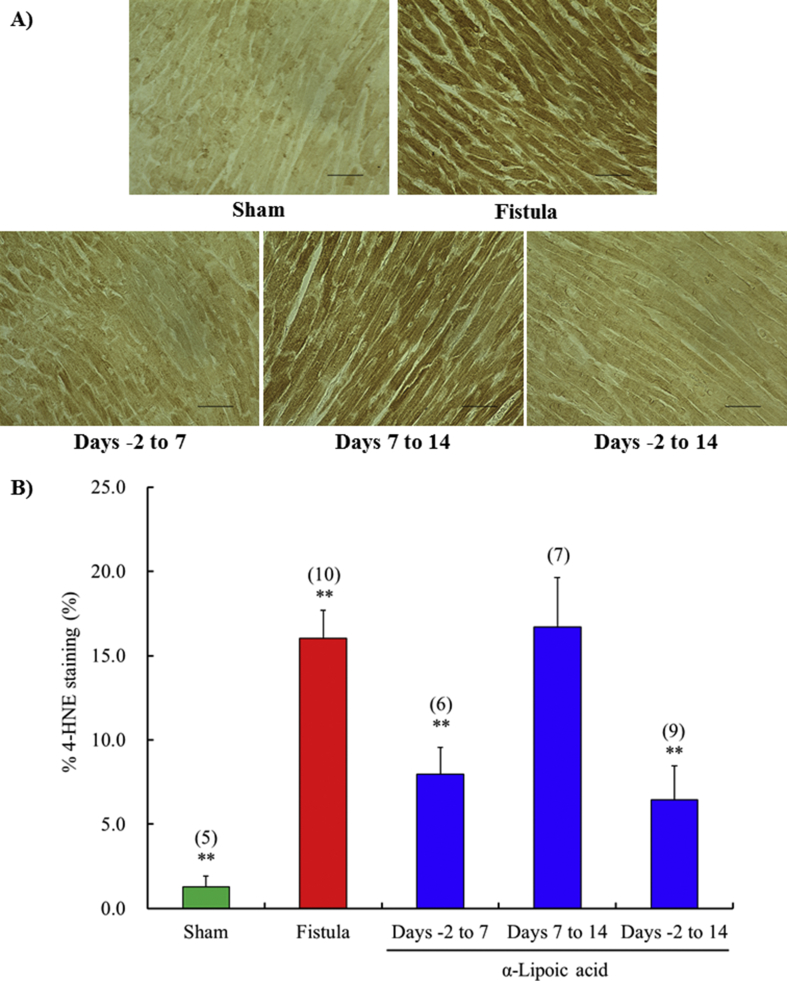
Production of 4-hydroxy-2-nonenal was prevented by α-lipoic acid treatment from days -2 to 14 compared with the vehicle treated fistula group (Fig. 6). Short-term treatment with early initiation (days -2 to 7) also markedly inhibited expression of 4-hydroxy-2-nonenal. Short term treatment with delayed initiation (days 7–14) failed to suppress increased production.
Fig. 6.
Time-window response of the expression of 4-hydroxy-2-nonenal in left ventricle at 14 days after surgery. Representative immunohistochemical staining showing 4-hydroxy-2-nonenal in left ventricle obtained from sham operated rat and fistula created rat with vehicle or α-lipoic acid at day 14 after surgery (A). All figures are depicted at 400×magnification. The scale represents 50 μm. Semi-quantification of 4-hydroxy-2-nonenal expression in left ventricle (B). Each value represents the mean ± S.E.M. of number of animals in parentheses. **P < 0.01, compared with fistula. 4-HNE, 4-hydroxy-2-nonenal.
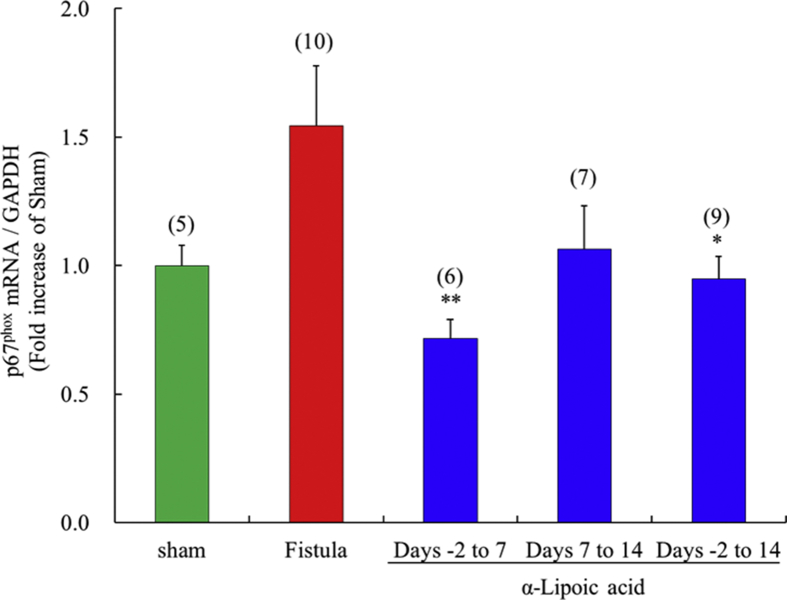
3.8. NADPH oxidase subunit p67phox mRNA expression in left ventricles with fistulae, and effects of α-lipoic acid treatment
α-Lipoic acid treatment from days -2 to 14 significantly inhibited p67phox mRNA expression compared with the vehicle treated fistula group (Fig. 7). Short term α-lipoic acid treatment with early initiation (days -2 to 7) significantly prevented raised p67phox mRNA expression, but short term treatment from days 7–14 had no effect.
Fig. 7.
Time-window response of the expression of p67phox mRNA in the fistula-created left ventricle. Each value represents the mean ± S.E.M. of number of animals in parentheses. **P < 0.01, *P < 0.05, compared with fistula.
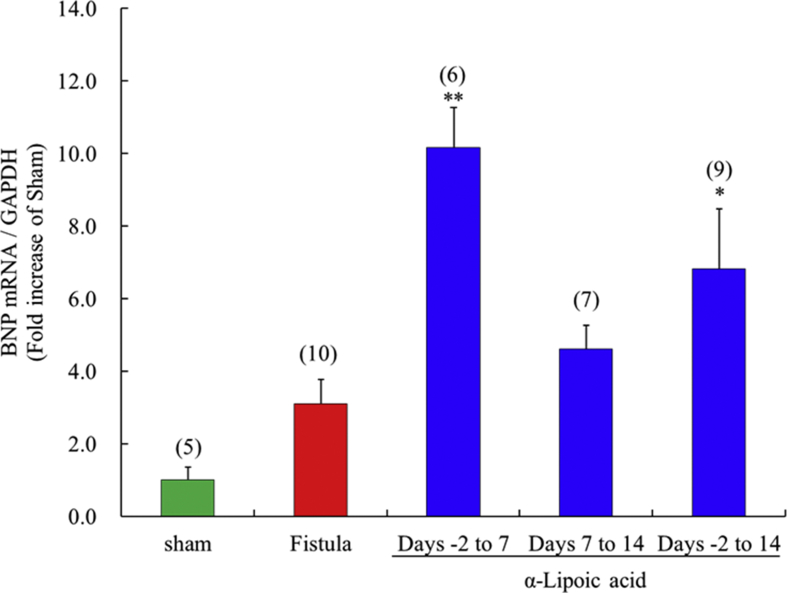
3.9. Brain natriuretic peptide mRNA expression in left ventricles with fistulae, and effects of α-lipoic acid treatment
α-Lipoic acid treatment from days -2 to 14 significantly increased brain natriuretic peptide mRNA expression compared with the vehicle treated fistula group (Fig. 8). Short term, early initiation treatment with α-lipoic acid (days -2 to 7) significantly increased brain natriuretic peptide mRNA expression, but treatment from days 7–14 had no effect.
Fig. 8.
Time-window response of the expression of brain natriuretic peptide mRNA in the fistula-created left ventricle. Each value represents the mean ± S.E.M. of number of animals in parentheses. **P < 0.01, *P < 0.05, compared with fistula. BNP, brain natriuretic peptide.
4. Discussion
The present study shows that treatment with α-lipoic acid can attenuate cardiac hypertrophy and fibrosis, and improve cardiac hemodynamic function in rats with surgically created fistulae. We found that the prophylactic efficacy of α-lipoic acid is associated with a decrease in some markers of oxidative stress, and an increase in BNP mRNA expression in the left ventricle in rats with induced fistulae. A more intriguing observation was that short-term α-lipoic acid treatment from day -2 to day 7 was also able to prevent increases in ventricular weight and decreases in ventricular performance to levels comparable with those seen in rats given α-lipoic acid from day -2 to day 14 after fistula creation. The oxidative stress in the left ventricle was significantly increased 7 days after fistula creation, but α-lipoic acid was able to inhibit this increase. Left ventricular BNP mRNA expression significantly increased 7 days after fistula creation, and α-lipoic acid further increased this expression. Thus, it seems likely that α-lipoic acid treatment ameliorates fistula induced cardiac hypertrophy, fibrosis, and cardiac hemodynamic dysfunction in the left ventricle by suppressing oxidative stress and increasing BNP expression in an early phase following fistula creation.
α-Lipoic acid has known therapeutic potential as a powerful antioxidant [17, 18]. Its therapeutic usefulness has been noted in animal models of diseases such as hypertension, ischemia/reperfusion-induced myocardial infarction, and diabetic nephropathy [19, 20, 21]. Furthermore, its clinical benefits in these conditions have been reported [22, 23, 24]. In animal models of volume-overloaded heart failure, such as rats with induced fistulae, there is evidence that prominent oxidative stress occurs in the left ventricle [14, 25]. Whether treatment with an antioxidant will have a beneficial effect for prevention of heart failure in the left ventricle of rats with surgically created fistulae remains unclear. In the present study, we demonstrated that α-lipoic acid prevented progression of left ventricular end-diastolic pressure, cardiac hypertrophy, and fibrosis by suppressing the elevation of oxidative stress markers, 4-hydroxy-2-nonenal, and mRNA expression of NADPH oxidase subunit p67phox. NADPH oxidase plays an essential role in superoxide production, development of myocardial hypertrophy, and interstitial cardiac fibrosis [26]. NADPH oxidase is a multi-subunit enzyme, comprising gp91phox, p22phox, p47phox, p67phox, p40phox, and Rac1 [27]. Since only mRNA expression of p67phox was upregulated at day 14 in the left ventricle of rats with induced fistulae, we investigated the effect of α-lipoic acid on p67phox expression. 4-hydroxy-2-nonenal is produced by lipid peroxidation, and is known to be an oxidative stress marker. Correlation between activation of NADPH oxidase, and production of 4-hydroxy-2-nonenal in left ventricular tissue after myocardial infarction in rabbits has also been reported [28]. These data suggest that superoxide derived from NADPH oxidase may be involved in the increased myocardial oxidative stress in volume-overloaded heart failure animal models. Szelényi et al [29] reported that the elevation of oxidative stress markers and BNP content in blood plays an important role in development of cardiac insufficiency in heart failure patients. BNP is a cardiac hormone produced predominantly by ventricular myocytes that plays an important role in regulating blood pressure and volume. Measurement of BNP is useful to support clinical judgments when diagnosing chronic heart failure. Increased expression of BNP in ventricular cardiomyocytes in response to oxidative stress has been reported [30]. In addition, ventricular BNP mRNA expression, and the level of plasma BNP have been prominently increased with creation of a fistula [14, 31]. Mouton et al [32] reported that BNP mRNA expression in the left ventricle correlates with cardiac hypertrophy in rats with volume overload. We noted that left ventricular BNP mRNA expression, as with superoxide production, was significantly increased at 7 days after fistula creation. α-Lipoic acid treatment further increased BNP mRNA expression in the left ventricle of rats with induced fistulae. Nevertheless, it prevented cardiac dysfunction in this study. This result is supported by the reports below. BNP has been shown to inhibit cardiac hypertrophy in cultured cardiomyocytes [33, 34]. Furthermore, increasing BNP levels by local adenoviral-driven expression was able to prevent cardiac remodeling and diastolic dysfunction in hypertensive rat hearts [35]. Taken together, these data suggest that inhibition of cardiac enlargement and fibrosis, and cardiac hemodynamic dysfunction by α-lipoic acid treatment is due to both suppression of NADPH oxidase dependent superoxide production, and elevation of left ventricular BNP mRNA expression in an early phase after fistula creation. It is well known that creation of aortocaval fistulae activates various neurohormones, including regulators of the adrenergic nervous system, the renin-angiotensin system, and endothelin-1, besides natriuretic hormone. These neurohormones act as compensatory mechanisms to maintain cardiac function, but they can also aggravate heart failure. α-lipoic acid has been reported to improve responses to these neurohormones in several disease conditions [36, 37, 38]. α-Lipoic acid may have been able to suppress the decline in cardiac function by acting on these neurohormones. Further study of the involvement of other cardioprotective mechanisms by lipoic acid, such as the renin-angiotensin system, sympathetic nervous system, and endothelin-1, will be necessary.
In the present study, we examined how the timing of initiation of α-lipoic acid treatment influenced its therapeutic efficacy. Our data clearly indicate that early initiation of α-lipoic acid treatment prior to fistula creation is important to suppress the cardiac morphometric and hemodynamic compromise. We also noted that delayed α-lipoic acid treatment after fistula creation was ineffective in suppressing development of cardiac dysfunction. We also demonstrated that short-term α-lipoic acid treatment from days -2 to 7 suppressed increased 4-hydoroxy-2-nonenal production and p67phox mRNA expression, and increased left ventricular BNP mRNA expression, while delayed α-lipoic acid treatment (days 7–14) could not prevent exacerbation of heart failure. We demonstrated that left ventricular 4-hydoroxy-2-nonenal production and p67phox mRNA expression increased in a time-dependent manner from day 7 to day 14 after fistula creation. These time-dependent changes in superoxide production after fistula creation may be closely related to the fact that early but not delayed α-lipoic acid treatment is effective in preventing development of cardiac dysfunction.
5. Conclusion
α-Lipoic acid treatment prevented exacerbation of heart failure induced by aortocaval fistula, by inhibiting increased superoxide production and promoting left ventricular BNP mRNA expression. Short-term α-lipoic acid treatment started prior to fistula creation was effective in preventing the development of cardiac dysfunction. This short-term α-lipoic acid treatment could also inhibit NADPH oxidase-dependent superoxide production, and increase left ventricular BNP mRNA expression in an early phase after fistula creation. Our results suggest that antioxidant agents such as α-lipoic acid may exert a primary preventive effect in heart failure.
Declarations
Author contribution statement
Daisuke Kurumazuka: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Kento Kitada, Ryosuke Tanaka: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
Tatsuhiko Mori, Mamoru Ohkita, Masanori Takaoka, Yasuo Matsumura: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- 1.Nakamura K., Murakami M., Miura D., Yunoki K., Enko K., Tanaka M., Saito Y., Nishii N., Miyoshi T., Yoshida M., Oe H., Toh N., Nagase S., Kohno K., Morita H., Matsubara H., Kusano K.F., Ohe T., Ito H. Beta-blockers and oxidative stress in patients with heart failure. Pharmaceuticals. 2011;4:1088–1100. doi: 10.3390/ph4081088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stampfer M.J., Hennekens C.H., Manson J.E., Colditz G.A., Rosner B., Willett W.C. Vitamin E consumption and the risk of coronary disease in women. N. Engl. J. Med. 1993;328:1444–1449. doi: 10.1056/NEJM199305203282003. [DOI] [PubMed] [Google Scholar]
- 3.Losonczy K.G., Harris T.B., Havlik R.J. Vitamin E and vitamin C supplement use and risk of all-cause and coronary heart disease mortality in older persons: the Established Populations for Epidemiologic Studies of the Elderly. Am. J. Clin. Nutr. 1996;4:190–196. doi: 10.1093/ajcn/64.2.190. [DOI] [PubMed] [Google Scholar]
- 4.Lonn E., Bosch J., Yusuf S., Sheridan P., Pogue J., Arnold J.M., Ross C., Arnold A., Sleight P., Probstfield J., Dagenais G.R., HOPE and HOPE-TOO Trial Investigators Effects of long-term vitamin E supplementation on cardiovascular events and cancer: a randomized controlled trial. J. Am. Med. Assoc. 2005;293:1338–1347. doi: 10.1001/jama.293.11.1338. [DOI] [PubMed] [Google Scholar]
- 5.Moini H., Tirosh O., Park Y.C., Cho K.J., Packer L. R-α-Lipoic acid action on cell redox status, the insulin receptor, and glucose uptake in 3T3-L1 adipocytes. Arch. Biochem. Biophys. 2002;397:384–391. doi: 10.1006/abbi.2001.2680. [DOI] [PubMed] [Google Scholar]
- 6.Ou P., Tritschler H.J., Wolff S.P. Thioctic (lipoic) acid: a therapeutic metal-chelating antioxidant? Biochem. Pharmacol. 1995;50:123–126. doi: 10.1016/0006-2952(95)00116-h. [DOI] [PubMed] [Google Scholar]
- 7.Rochette L., Ghibu S., Richard C., Zeller M., Cottin Y., Vergely C. Direct and indirect antioxidant properties of α-lipoic acid and therapeutic potential. Mol. Nutr. Food Res. 2013;57:114–125. doi: 10.1002/mnfr.201200608. [DOI] [PubMed] [Google Scholar]
- 8.Park S., Karunakaran U., Jeoung N.H., Jeon J.H., Lee I.K. Physiological effect and therapeutic application of α-lipoic acid. Curr. Med. Chem. 2014;21:3636–3645. doi: 10.2174/0929867321666140706141806. [DOI] [PubMed] [Google Scholar]
- 9.Sola S., Mir M.Q., Cheema F.A., Khan-Merchant N., Menon R.G., Parthasarathy S., Khan B.V. Irbesartan and lipoic acid improve endothelial function and reduce markers of inflammation in the metabolic syndrome: results of the Irbesartan and Lipoic Acid in Endothelial Dysfunction (ISLAND) study. Circulation. 2005;111:343–348. doi: 10.1161/01.CIR.0000153272.48711.B9. [DOI] [PubMed] [Google Scholar]
- 10.Ziegler D., Schatz H., Conrad F., Gries F.A., Ulrich H., Reichel G. Effects of treatment with the antioxidant alpha-lipoic acid on cardiac autonomic neuropathy in NIDDM patients. Diabetes Care. 1997;20:369–373. doi: 10.2337/diacare.20.3.369. [DOI] [PubMed] [Google Scholar]
- 11.Alleva R., Tomasetti M., Sartini D., Emanuelli M., Nasole E., Di Donato F., Borghi B., Santarelli L., Neuzil J. alpha-Lipoic acid modulates extracellular matrix and angiogenesis gene expression in non-healing wounds treated with hyperbaric oxygen therapy. Mol. Med. 2008;14:175–183. doi: 10.2119/2007-00095.Alleva. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hegazy S.K., Tolba O.A., Mostafa T.M., Eid M.A., El-Afify D.R. Alpha-lipoic acid improves subclinical left ventricular dysfunction in asymptomatic patients with type 1 diabetes. Rev. Diabet. Stud. 2013;10:58–67. doi: 10.1900/RDS.2013.10.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koh E.H., Lee W.J., Lee S.A., Kim E.H., Cho E.H., Jeong E., Kim D.W., Kim M.S., Park J.Y., Park K.G., Lee H.J., Lee I.K., Lim S., Jang H.C., Lee K.H., Lee K.U. Effects of alpha-lipoic Acid on body weight in obese subjects. Am. J. Med. 2011;124 doi: 10.1016/j.amjmed.2010.08.005. 85.e1-8. [DOI] [PubMed] [Google Scholar]
- 14.Mori T., Kurumazuka D., Matsumoto C., Shirakawa H., Kimura S., Kitada K., Kobayashi K., Matsuda H., Hayashi T., Kitaura Y., Matsumura Y. Dietary salt restriction activates mineralocorticoid receptor signaling in volume-overloaded heart failure. Eur. J. Pharmacol. 2009;623:84–88. doi: 10.1016/j.ejphar.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 15.Mori T., Hayashi T., Sohmiya K., Okuda N., Shimomura H., Ohkita M., Matsumura Y., Yoshiyama M., Yoshikawa J., Kitaura Y. Mechanisms of combined treatment with celiprolol and candesartan for ventricular remodeling in experimental heart failure. Circ. J. 2005;69:596–602. doi: 10.1253/circj.69.596. [DOI] [PubMed] [Google Scholar]
- 16.Pfaffl M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gomes M.B., Negrato C.A. α-Lipoic acid as a pleiotropic compound with potential therapeutic use in diabetes and other chronic diseases. Diabetol. Metab. Syndr. 2014;6:80. doi: 10.1186/1758-5996-6-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Skibska B., Goraca A. The protective effect of lipoic acid on selected cardiovascular diseases caused by age-related oxidative stress. Oxid. Med. Cell Longev. 2015;2015:313021. doi: 10.1155/2015/313021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Melhem M.F., Craven P.A., Liachenko J., DeRubertis F.R. α-Lipoic acid attenuates hyperglycemia and prevents glomerular mesangial matrix expansion in diabetes. J. Am. Soc. Nephrol. 2002;13:108–116. doi: 10.1681/ASN.V131108. [DOI] [PubMed] [Google Scholar]
- 20.Mervaala E., Finckenberg P., Lapatto R., Müller D.N., Park J.K., Dechend R., Ganten D., Vapaatalo H., Luft F.C. Lipoic acid supplementation prevents angiotensin II-induced renal injury. Kidney Int. 2003;64:501–508. doi: 10.1046/j.1523-1755.2003.00108.x. [DOI] [PubMed] [Google Scholar]
- 21.Deng C., Sun Z., Tong G., Yi W., Ma L., Zhao B., Cheng L., Zhang J., Cao F., Yi D. α-Lipoic acid reduces infarct size and preserves cardiac function in rat myocardial ischemia/reperfusion injury through activation of PI3K/Akt/Nrf2 pathway. PLoS One. 2013;8 doi: 10.1371/journal.pone.0058371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morcos M., Borcea V., Isermann B., Gehrke S., Ehret T., Henkels M., Schiekofer S., Hofmann M., Amiral J., Tritschler H., Ziegler R., Wahl P., Nawroth P.P. Effect of α-lipoic acid on the progression of endothelial cell damage and albuminuria in patients with diabetes mellitus: an exploratory study. Diabetes Res. Clin. Pract. 2001;52:175–183. doi: 10.1016/s0168-8227(01)00223-6. [DOI] [PubMed] [Google Scholar]
- 23.McMackin C.J., Widlansky M.E., Hamburg N.M., Huang A.L., Weller S., Holbrook M., Gokce N., Hagen T.M., Keaney J.F., Jr., Vita J.A. Effect of combined treatment with α-lipoic acid and acetyl-L-carnitine on vascular function and blood pressure in patients with coronary artery disease. J. Clin. Hypertens. 2007;9:249–255. doi: 10.1111/j.1524-6175.2007.06052.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Uyar I.S., Onal S., Akpinar M.B., Gonen I., Sahin V., Uguz A.C., Burma O. α-Lipoic acid attenuates inflammatory response during extracorporeal circulation. Cardiovasc J Afr. 2013;24:322–326. doi: 10.5830/CVJA-2013-067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gardner J.D., Murray D.B., Voloshenyuk T.G., Brower G.L., Bradley J.M., Janicki J.S. Estrogen attenuates chronic volume overload induced structural and functional remodeling in male rat hearts. Am. J. Physiol. Heart Circ. Physiol. 2010;29:H497–H504. doi: 10.1152/ajpheart.00336.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Murdoch C.E., Zhang M., Cave A.C., Shah A.M. NADPH oxidase-dependent redox signalling in cardiac hypertrophy, remodelling and failure. Cardiovasc. Res. 2006;71:208–215. doi: 10.1016/j.cardiores.2006.03.016. [DOI] [PubMed] [Google Scholar]
- 27.Babior B.M. NADPH oxidase: an update. Blood. 1999;93:1464–1476. [PubMed] [Google Scholar]
- 28.Qin F., Simeone M., Patel R. Inhibition of NADPH oxidase reduces myocardial oxidative stress and apoptosis and improves cardiac function in heart failure after myocardial infarction. Free Radic. Biol. Med. 2007;43:271–281. doi: 10.1016/j.freeradbiomed.2007.04.021. [DOI] [PubMed] [Google Scholar]
- 29.Szelényi Z., Á Fazakas, Szénási G., Kiss M., Tegze N., Fekete B.C., Nagy E., Bodó I., Nagy B., Molvarec A., Patócs A., Pepó L., Prohászka Z., Vereckei A. Inflammation and oxidative stress caused by nitric oxide synthase uncoupling might lead to left ventricular diastolic and systolic dysfunction in patients with hypertension. J Geriatr Cardiol. 2015;12:1–10. doi: 10.11909/j.issn.1671-5411.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim D.K., Choi E., Song B.W., Cha M.J., Ham O., Lee S.Y., Lee C.Y., Park J.H., Song H., Hwang K.C. Differentially regulated functional gene clusters identified in early hypoxic cardiomyocytes. Mol. Biol. Rep. 2012;39:9549–9556. doi: 10.1007/s11033-012-1819-1. [DOI] [PubMed] [Google Scholar]
- 31.Scheuermann-Freestone M., Freestone N.S., Langenickel T., Höhnel K., Dietz R., Willenbrock R. A new model of congestive heart failure in the mouse due to chronic volume overload. Eur. J. Heart Fail. 2001;3:535–543. doi: 10.1016/s1388-9842(01)00160-x. [DOI] [PubMed] [Google Scholar]
- 32.Mouton A.J., Ninh V.K., El Hajj E.C., El Hajj M.C., Gilpin N.W., Gardner J.D. Exposure to chronic alcohol accelerates development of wall stress and eccentric remodeling in rats with volume overload. J. Mol. Cell. Cardiol. 2016;97:15–23. doi: 10.1016/j.yjmcc.2016.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.He J.G., Chen Y.L., Chen B.L., Huang Y.Y., Yao F.J., Chen S.L., Dong Y.G. B-type natriuretic peptide attenuates cardiac hypertrophy via the transforming growth factor-β1/smad7 pathway in vivo and in vitro. Clin. Exp. Pharmacol. Physiol. 2010;37:283–289. doi: 10.1111/j.1440-1681.2009.05281.x. [DOI] [PubMed] [Google Scholar]
- 34.Chen Y., Yao F., Chen S., Huang H., Wu L., He J., Dong Y. Endogenous BNP attenuates cardiomyocyte hypertrophy induced by Ang II via p38 MAPK/Smad signaling. Die Pharmazie. 2014;69:833–837. [PubMed] [Google Scholar]
- 35.Cataliotti A., Tonne J.M., Bellavia D., Martin F.L., Oehler E.A., Harders G.E., Campbell J.M., Peng K.W., Russell S.J., Malatino L.S., Burnett J.C., Jr., Ikeda Y. Long-term cardiac pro-B-type natriuretic peptide gene delivery prevents the development of hypertensive heart disease in spontaneously hypertensive rats. Circulation. 2011;123:1297–1305. doi: 10.1161/CIRCULATIONAHA.110.981720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Takaoka M., Kobayashi Y., Yuba M., Ohkita M., Matsumura Y. Effects of α-lipoic acid on deoxycorticosterone acetate-salt-induced hypertension in rats. Eur. J. Pharmacol. 2001;424:121–129. doi: 10.1016/s0014-2999(01)01120-7. [DOI] [PubMed] [Google Scholar]
- 37.Heinisch B.B., Francesconi M., Mittermayer F., Schaller G., Gouya G., Wolzt M., Pleiner J. α-Lipoic acid improves vascular endothelial function in patients with type 2 diabetes: a placebo-controlled randomized trial. Eur. J. Clin. Investig. 2010;40:148–154. doi: 10.1111/j.1365-2362.2009.02236.x. [DOI] [PubMed] [Google Scholar]
- 38.de Queiroz T.M., Xia H., Filipeanu C.M., Braga V.A., Lazartigues E. α-Lipoic acid reduces neurogenic hypertension by blunting oxidative stress-mediated increase in ADAM17. Am. J. Physiol. Heart Circ. Physiol. 2015;309:H926–H934. doi: 10.1152/ajpheart.00259.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]