Abstract
During peripheral nervous system development, Schwann cells are precisely matched to the axons that they support. This is mediated by axonal neuregulins that are essential for Schwann cell survival and differentiation. Here, we show that sensory and motor axons rapidly release heparin-binding forms of neuregulin in response to Schwann cell-derived neurotrophic factors in a dose-dependent manner. Neuregulin release occurs within minutes, is saturable, and occurs from axons that were isolated using a newly designed chamber slide apparatus. Although NGF and glial cell line-derived neurotrophic factor (GDNF) were the most potent neurotrophic factors to release neuregulin from sensory neurons, GDNF and BDNF were most potent for motor neurons and were the predominant neuregulin-releasing neurotrophic factors produced by cultured Schwann cells. Comparable levels of neuregulin could be released at a similar rate from neurons after protein kinase C activation with the phorbol ester, phorbol 12-myristate 13-acetate, which has also been shown to promote the cleavage and release of neuregulin from its transmembrane precursor. The rapid release of neuregulin from axons in response to Schwann cell-derived neurotrophic factors may be part of a spatially restricted system of communication at the axoglial interface important for proper peripheral nerve development, function, and repair.
Keywords: neuregulin, neurotrophin, GDNF, Schwann cell, axon, protein kinase
Introduction
Normal development and function of the peripheral nervous system (PNS) requires spatially and temporally orchestrated communication between axons and their surrounding Schwann cells. During early development, the growth and differentiation of Schwann cells closely follow the outgrowth of axons from their cell bodies to their targets (Bhattacharyya et al., 1994). Later in development, axons interact differentially with Schwann cells to produce both myelinated and unmyelinated axons (Nilsson and Berthold, 1988; Bolin and Shooter, 1993; Little and Heath, 1994).
Emerging evidence indicates that signals from the outgrowing axon, including the growth and differentiation factor neuregulin (NRG), are important mediators of PNS development (Fischbach and Rosen, 1997; Jessen and Mirsky, 1999; Mirsky et al., 2002; Falls, 2003). Disruption of NRG signaling between axons and Schwann cells, by directly knocking out either the entire NRG-1 gene or the genes encoding its receptors, erbB2 and erbB3, leads to an almost complete loss of Schwann cells, followed thereafter by death of the sensory and motor neurons that they support (Meyer and Birchmeier, 1995; Riethmacher et al., 1997; Morris et al., 1999; Woldeyesus et al., 1999; Adlkofer and Lai, 2000; Garratt et al., 2000). These effects are likely attributable to the effects of NRG on Schwann cell proliferation and early survival (Ciutat et al., 1996; Grinspan et al., 1996; Trachtenberg and Thompson, 1996; Winseck et al., 2002). Later, selective blockade of erbB signaling in nonmyelinating Schwann cells produces a progressive sensory neuropathy with subsequent loss of DRG neurons (Chen et al., 2003).
NRGs synthesized from the NRG-1 gene are membrane-spanning precursors (pro-NRG) that undergo regulated cleavage events to produce either soluble, heparin-binding (Ig) forms or membrane-bound cysteine-rich domain (CRD) forms (Wang et al., 2001; Falls, 2003). The soluble, heparin-binding forms of NRG are highly expressed along developing sensory and motor axons in the PNS through their interactions with endogenous heparan sulfate proteoglycans (Loeb et al., 1999). These forms are essential for muscle spindle formation (Andrechek et al., 2002; Hippenmeyer et al., 2002) and maintaining high concentrations of acetylcholine receptors at mature neuromuscular junctions (Sandrock et al., 1997). We showed recently that the amount of heparin-binding NRG forms released at neuromuscular junctions requires neuromuscular activity and is promoted by postsynaptic muscle neurotrophic factor production (Loeb et al., 2002). This raises the exciting possibility that NRG is released from individual motor nerve terminals in response to postsynaptic neurotrophic factors so that those synapses that are more active will become more stable.
Soluble forms of NRG may also be important for peripheral nerve development, because NRG blocks normal and NMDA and denervation-induced Schwann cell programmed cell death (Kopp et al., 1997; Winseck et al., 2002). Because Schwann cells express neurotrophic factors, including nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), neurotrophin-3 (NT-3), NT-4, and glial cell line-derived neurotrophic factor (GDNF) (Funakoshi et al., 1993; Henderson et al., 1994; Griesbeck et al., 1995; Friedman et al., 1996), they could similarly promote the release of NRG from axons. Here we provide evidence that Schwann cell-derived neurotrophic factors induce the rapid release of heparin binding-forms of NRG from axons of sensory and motor neurons. This bidirectional communication provides a way by which neurons and Schwann cells can support their mutual survival and differentiation during development and during the process of nerve repair after injury or disease.
Materials and Methods
Recombinant neurotrophins, erbB antibodies, and NRG and neurotrophic factor antagonists. Recombinant NGF was from Invitrogen (Gaithersburg, MD). BDNF and NT-3 were from Regeneron (Tarrytown, NY), and GDNF was from Amgen (Thousand Oaks, CA) and R & D Systems (Minneapolis, MN). Monoclonal antibodies against erbB2 (Ab-17) and erbB3 (Ab-6) were from Neomarkers (Fremont, CA). Trk receptor fusion proteins (R & D Systems) were used at 10 ng/ml. The monoclonal GDNF blocking antibody was from Amgen, and the polyclonal GDNF blocking antibody (AF-212-NA) was from R & D Systems; both were used at 20 ng/ml. The soluble NRG antagonist, IgB4, is a fusion protein between the extracellular domain of erbB4 and human IgG (Chen et al., 1996). Recombinant protein was prepared by culturing transfected human embryonic kidney (HEK) cells in OPTI-MEM for 48 hr, and the media containing secreted IgB4 was concentrated 20-fold using Centricon-10 devices (Millipore, Bedford, MA). Antagonist activity was calibrated by blocking NRG-mediated erbB phosphorylation in L6 myoblasts. Conditioned media from nontransfected HEK cells was used as a negative control and showed no NRG-blocking activity. All other chemicals and reagents were from Sigma (St. Louis, MO).
Measurement of NRG release from cultured neurons. Currently there are no highly sensitive and specific antibodies against NRG that can measure minute physiologic concentrations of NRG by either Western blot or ELISA. To circumvent this, we developed a highly sensitive and specific biological assay for NRG, using tyrosine phosphorylation of the erbB receptors in cultured L6 myotubes.
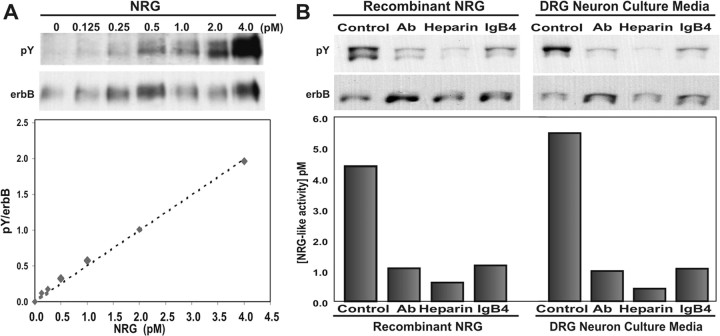
Test samples were prepared by concentrating neuron culture media 10-fold to 50 μl using Centricon-10 devices (Millipore) and added to 100 μl of L6 cell culture media (DMEM, 10% FCS, PCN/Strep, 2 nm glutamine) before adding to L6 myoblasts in 48-well plates (Costar, Corning, NY) and allowed to fuse and differentiate for 7-8 d. The test sample was added for 45 min at 37°C to allow NRG activation of erbB receptor tyrosine kinases, after which the cells were placed on ice and lysed with 100 μl of lysis buffer (50 mm Tris, 150 mm NaCl, 2 mm EDTA, 15 mm Na2HPO4, 1% Triton X-100, 0.1% SDS, 0.5% Na-deoxycholate, 10 mm sodium orthovanadate, 1 mm phenylmethylsulfonyl fluoride, and 20 μg/ml leupeptin). Total cell lysate was quantitatively immunoprecipitated overnight at 4°C using monoclonal antibodies against erbB2 (Neomarkers, Ab-17) and erbB3 (Neomarkers, Ab-6) with Protein-G Sepharose beads (Sigma). The beads were washed twice with 1 ml of lysis buffer and then once with 1 ml of PBS. Western blots were performed on 6% reducing gels using an antibody against phosphotyrosine (pY, 4G10; Upstate Biotechnology, Lake Placid, NY) (Li and Loeb, 2001) and then stripped and reprobed with a mixture of the erbB2 and erbB3 antibodies to measure the total amount of erbB protein present in each lysate. Optimization experiments comparing erbB protein recovery by immunoprecipitation with total cell lysate revealed that we pulled down nearly all of the erbB receptors. Immunoprecipitation of the NRG receptors with specific antibodies against erbB2 and erbB3 does not rule out the possibility that the other EGF receptor family members, such as erbB4 and the EGF receptor, coimmunoprecipitate with erbB2 and erbB3. Because this can lead to one or more extra bands on a phosphotyrosine Western blot, we reprobe the blot with the same erbB2 (top band) and erbB3 (bottom band) antibodies used for immunoprecipitation. Only the bands revealed by these antibodies are used for quantitation. Band intensity was quantified on a flatbed scanner with a transparency adapter using Meta-Morph image analysis software as described (Li and Loeb, 2001). The calculated ratio of tyrosine-phosphorylated erbB protein to total erbB protein provides a quantitative, linear, and highly reproducible measurement of NRG concentration (see Fig. 1 A).
Figure 1.
Cultured neurons release minute quantities of soluble heparin-binding NRG. A, Western blot of immunoprecipitated erbB2 and erbB3 proteins from L6 myotubes treated with increasing amounts of NRG produces a sensitive and quantitative bioassay for NRG. The blot was probed with anti-phosphotyrosine (pY) antibodies and then stripped and reprobed with antibodies to erbB2 and erbB3 (erbB). Quantitation of this blot reveals the ratio of tyrosine-phosphorylated erbB protein to total erbB protein (pY/erbB) to be linear. The dashed line represents the linear regression of this data described by y = 0.4999x, R2 = 0.9936. B, Specificity of this NRG assay was shown using reagents that block the erbB receptor phosphorylation induced by recombinant NRG (5 pm) as well as sensory neuron culture media. Conditioned culture media was pretreated with an NRG-blocking antibody (1:10), soluble heparin (10 mg/ml), or a soluble fusion protein of erbB4 and human IgG (1:10) and then assayed for NRG as in A. Each reagent inhibited NRG-induced erbB tyrosine phosphorylation from the neuron cultures in a pattern identical to recombinant NRG, indicating that sensory neurons secrete predominately the soluble Ig form of NRG.
Neuronal cultures. Sensory neurons were obtained from embryonic day (E) 12 chicken dorsal root ganglia (DRG) as described (Banker, 1998) and cultured in serum-free media (L15, PCN/Strep, 0.6% glucose, 2 mm glutamine, 0.06% NaHCO3) supplemented with N2, B27, and 10 ng/ml NGF (Invitrogen). Ventral spinal cord cultures containing motor neurons were obtained from E5 chicken ventral spinal cord as described (Loeb and Fischbach, 1997) and grown in serum-free media (L15, PCN/Strep, 0.3% glucose, 2 mm glutamine, basal medium Eagle (BME) vitamin mix, and 54 μg/ml imidazole) supplemented with 6 μg/ml E11 chick muscle extract and N2 (Invitrogen). After trituration of the tissue, the cells were plated at 1.25 × 105 cells per square centimeter in 24-well plates coated with 15 μg/ml laminin (Invitrogen) and 10 μg/ml poly-d-lysine (Sigma). The sensory neuron cultures were treated with 1.75 μm Ara-C (Sigma) on alternate days (24 hr on, 24 hr off, 24 hr on, 24 hr off) to remove excess fibroblast and glial contamination.
Schwann cell cultures and conditioned media. Schwann cells from E18 chicken sciatic nerves were pooled from several animals and incubated at 37°C for 30 min in HBSS containing calcium and magnesium, with 0.1% collagenase (Worthington, Freehold, NJ) and 0.25% trypsin without EDTA (Invitrogen). The dissociation solution was removed and replaced with 1 ml of DMEM containing 10% horse serum (Invitrogen), and the nerves were triturated. DMEM (10 ml) was added, and the cells were pelleted by centrifugation and resuspended in serum-free culture media (DMEM, PCN/Strep, 2 mm glutamine, 0.3% glucose, BME vitamin mix, 50 μg/ml ascorbic acid, 5 μg/ml insulin, 50 μg/ml transferrin, 1 μg/ml Na selenite) and plated at a density of 1.0 × 105 cells per square centimeter in T75 flasks coated with collagen (BD Biosciences, San Jose, CA). To obtain Schwann cell-conditioned media (SCCM), the regular culture media was removed after 2 d, and the flasks were washed gently two times in 10 ml of DMEM and replaced with DMEM containing PCN/Strep and 0.1% BSA to collect conditioned media 48 hr later. Cellular debris was removed by centrifugation, and the media was concentrated 20-fold using Centricon-10 devices (Millipore) and passed through a 0.45 μm filter.
Campenot-style compartmented culture system. To measure small amounts of NRG from axons, we modified the Campenot chamber slide culture system. These devices have been used to spatially isolate distal axons from neuron cell bodies (Campenot et al., 1994, 2003; MacInnis and Campenot, 2002). The traditional system uses a Teflon divider attached to a collagen- or laminin-coated plastic substrate with silicone grease so that axons plated into a central compartment can extend axons that penetrate the grease barrier and enter the adjacent, separated compartments. We constructed chambers cut from 2-mm-thick sheets of solid silicone rubber (Grace Biolabs, Bend, OR) modeled after the Camp9 chamber from Tyler Research (Edmonton, Canada) (see Fig. 7A). Camp9 chambers were laid over the rubber sheet, and a scalpel was used to cut out an exact replica. These were then sterilized, stored in 70% ethanol, and allowed to air dry completely before use. They were applied to tissue culture dishes of various sizes that were coated with 30 μg/ml collagen (BD Biosciences), 20 μg/ml poly-d-lysine (Sigma), and 20 μg/ml laminin (Invitrogen) and allowed to air dry completely (Loeb and Fischbach, 1997). Because they were constructed from clear 2-mm-thick silicone, axons growing underneath the barrier were easily observed (see Fig. 7C). Chicken DRG sensory neurons were plated into the central compartment (C), and axons grew underneath the barrier into the outer compartments (U and T) for 4 d. One side (T) was treated with either NGF (100 ng/ml) or BSA for 2 hr at 37°C, whereas the other side (U) was used as a control. The media from eight chambers was pooled and assayed for the release of NRG with the L6 bioassay.
Figure 7.
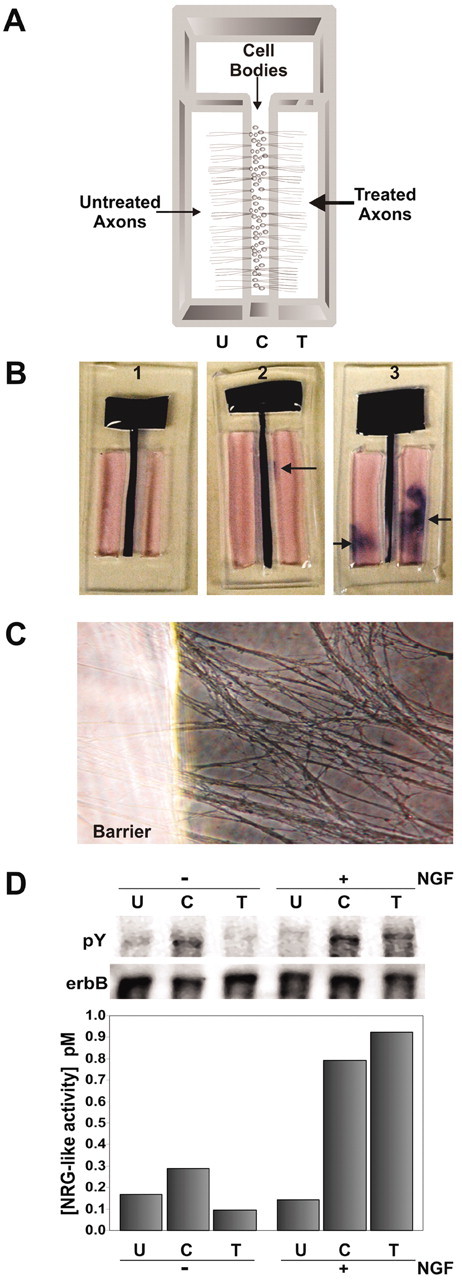
Focal release of NRG from axons. A, Schematic diagram of a compartmented Campenot-style chamber is shown where neurons were plated into the center compartment “C” and allowed to grow into both treated “T” and untreated “U” outer compartments. After 3d, the neurons extend axons equally into the outer compartments, whereas their cells bodies remain in the center. B, All culture chambers were tested individually for leakage between compartments at the end of each experiment by visual inspection of a tracker dye showing no leakage (1), minor leakage (2), or severe leakage (3). Only media from those chambers with no leakage were used for analysis. C, An example of axons growing under the clear silicone barrier (left) and into the outer compartment (right) is shown. D, These chamber slides were used to assess the effects of 100 ng/ml NGF for 2 hr on axons from the treated side compared with both the untreated and central compartments (right 3 lanes). Measurement of NRG in each of the three compartments revealed that distal axons in the NGF-treated compartment rapidly released NRG compared with the untreated side. NRG release was also promoted in the center compartment, containing proximal axons and cell bodies, suggesting a retrograde signaling event from the distal axons. Control culture chambers without NGF treatment (left 3 lanes) did not release as much NRG into either the T or C compartments. Media from a total of eight culture chambers was pooled for NGF treatment, and four culture chambers were pooled for the negative control. The same pattern of NRG release was seen in replicate experiments.
To rule out fluid leakage between compartments that could result in cross-contamination, we used a tracker dye for visual detection (see Fig. 7B), which was confirmed with the use of a radioactive tracer. Specifically, after conditioned media was removed from each compartment, new L15 media containing 0.2% trypan blue was routinely added to the central compartment, and the chambers were incubated for an additional 2 hr at 37°C. The chambers were then inspected visually for signs of leakage. Only the neuron-conditioned media from nonleaking compartments was pooled and analyzed. Typically, this system yields a 40-70% success rate. The sensitivity of the trypan blue leakage testing was confirmed using 32P-dCTP (0.25 μCi per chamber). The presence of radioactivity in outer compartments correlated perfectly with visual observation of blue dye penetration. Media from nonleaking compartments contained an average of 28.94 (±6.55) cpm, compared with the average blank with 26.75 (±4.05) cpm. Those that showed dye leakage contained between 604 and 3500 cpm.
Real-time, quantitative RT-PCR. Total RNA was collected from intact E18 chick sciatic nerve and from E18 chick Schwann cells cultured for 5 d using the RNeasy kit (Qiagen, Hilden, Germany). To reduce genomic DNA contamination, the column was treated with DNase (137.5 μg/ml) for 15 min. The concentration of mRNA eluted from the column was measured with Ribogreen (Molecular Probes), and 1 μg of RNA from each sample was reverse transcribed using Superscript II (Invitrogen). The neurotrophic factor primers used were as follows: NGF forward (5′-GACTGCCAGGACCAAGAGGA-3′) and reverse (5′-GGTGGCGGTGGTTTTGTC-3′), BDNF forward (5′-AGCCCAGTGAGGAAAACAAGG-3′) and reverse (5′-CATGTTTGCAGCATCCAGGT-3′), NT-3 forward (5′-CACCACTGTACCTCACAGAGGATT-3′) and reverse (5′-GATGATTTGTCCGTGACCCATA-3′), GDNF forward (5′-ATGCCAGAGGATTACCCAGATC-3′) and reverse (5′-TCTACGTTTGTGGCTGCACTTT-3′). We used glyceraldehyde-3-phosphate dehydrogenase (GAPDH) forward (5′-TGA TGG GTG TCA ACC ATG AGA-3′) and reverse (5′-TGG CAT GGA CAG TGG TCA TAA-3′). Each of these primer sets produced a single band of the correct size between 125 and 150 bp. We used Amplitaq Gold polymerase (Promega) with the following cycling parameters: 95°C for 10 min followed by 40 cycles of 95°C for 15 sec, 60°C for 10 sec, and 72°C for 50 sec. For real-time quantitative PCR, we used the SybrGreen system (Molecular Probes) on an MJ Research Opticon Machine and normalized to GAPDH.
Results
Cultured DRG sensory neurons release heparin-binding forms of NRG in response to secreted Schwann cell factors
To measure potentially minute amounts of NRG released by neurons, we optimized a bioassay to quantify erbB receptor (p185) phosphorylation in the L6 muscle cell line after stimulation with NRG (Loeb et al., 1998). This assay significantly amplifies the p185 signal by first selectively concentrating erbB2 and erbB3 receptors by immunoprecipitation followed by measurement of p185 activation with a phosphotyrosine Western blot. The signal is then normalized to the total amount of erbB2 and erbB3 protein in the sample to produce a quantitative and linear assay that can detect as little as 0.125 pm recombinant NRG (Fig. 1A). The specificity of this assay was confirmed in three different ways by blocking recombinant Ig-containing NRG with NRG antibodies, heparin, and a soluble erbB4 antagonist (IgB4) (Fig. 1B). As might be expected for Ig-containing forms of NRG, heparin was the most effective antagonist (Loeb and Fischbach, 1995). Using this assay, NRG activity was detected in conditioned media from primary cultures of embryonic DRG sensory neurons and was blocked by these same three agents (Fig. 1B). This suggests that these neurons release a biologically active, heparin-binding form of NRG.
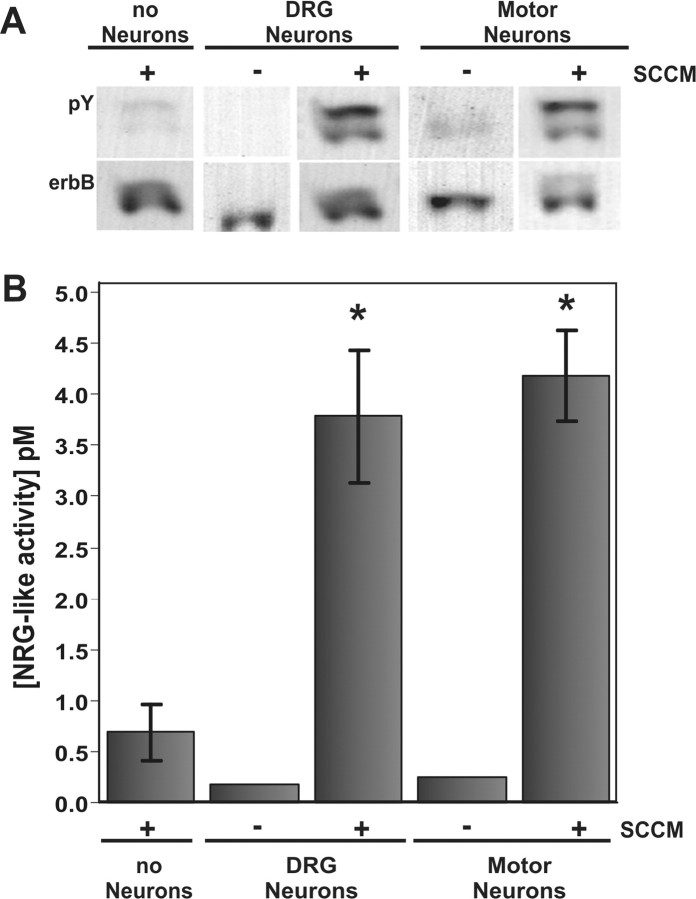
Given that NRG release is a regulated process (Loeb et al., 1998), we asked whether soluble factors produced by Schwann cells potentiate the release of NRG from sensory and motor neurons, both of which project their axons down the peripheral nerve. SCCM was applied to cultured sensory and motor neurons for 2 hr, and the doubly conditioned media from each was assayed for NRG using the above bioassay. An identical volume of SCCM not given to neurons was assayed in parallel. Exposure of the SCCM to both cultured sensory or motor neurons greatly increased the amount of NRG released over a 2 hr period (Fig. 2). Interestingly, the SCCM alone also contained detectable levels of NRG activity, but at significantly lower levels, consistent with a previous report of NRG activity from Schwann cells (Rosenbaum et al., 1997). Taken together, these results show that cultured Schwann cells produce soluble factors that stimulate neurons to release NRG.
Figure 2.
SCCM induces the rapid release of NRG from cultured neurons. A Western blot shown in A and then quantified in B showed that NRG release into both DRG sensory and motor neuron culture media was increased after exposure to SCCM for 2 hr. Although a small amount of NRG is present in the SCCM alone, primary sensory and motor neuron cultures released significantly more NRG in response to factors in the SCCM. This was performed in triplicate with the average and SD shown in the graph. Statistical significance for sensory (p = 0.0092) and motor (p = 0.0015) neurons was measured with a Student's t test.
Neurotrophic factors expressed in chick sciatic nerve and cultured Schwann cells promote the rapid release of NRG from sensory and motor neurons in a dose-dependent manner
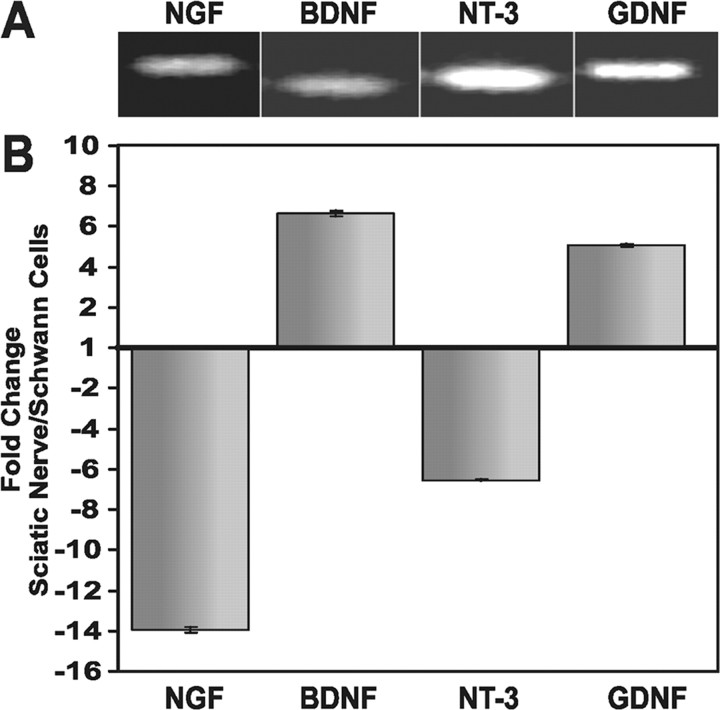
Exactly what Schwann cells make that promotes this rapid release of NRG is not known; however, the neurotrophic factors BDNF, NT-3, and GDNF may be good candidates because they have been shown to increase NRG mRNA and protein expression in cultured motor neurons (Loeb and Fischbach, 1997) and can activate protein kinase C (PKC) signaling, which has been shown to be sufficient to induce NRG release from transfected fibroblasts (Zirrgiebel et al., 1995; Loeb et al., 1998; Wooten et al., 2001). We performed real-time, quantitative RT-PCR on E18 chick sciatic nerve as well as on primary Schwann cells cultured from these nerves to determine whether these neurotrophic factors and NGF are expressed in Schwann cells (Fig. 3). To minimize contamination with other cell types present in intact sciatic nerve (Matsuoka et al., 1991; Zochodne and Cheng, 2000), we removed all visible fascia and blood vessels during the dissection. Real-time, quantitative RT-PCR revealed significant differences in Schwann cell neurotrophic factor expression, normalized to GAPDH, in vivo versus in vitro. Although transcripts for all four of these factors were indeed present in the intact sciatic nerve (Fig. 3A), marked changes in their expression levels were seen in the cultured Schwann cells (Fig. 3B). Specifically, NGF and NT-3 expression decreased 14- and 6-fold, respectively, and BDNF and GDNF expression increased 6- and 5-fold, respectively. This result not only demonstrates the presence of NGF, BDNF, NT-3, and GDNF in sciatic nerve and in cultured Schwann cells that could promote NRG release from neurons, but also shows a remarkable change in neurotrophic factor expression in Schwann cells removed from their normal environment in the intact sciatic nerve. Contributions from other cell types in the sciatic nerve cannot be excluded. The observed changes in neurotrophic factor expression after removal and culture of the nerve are remarkably similar to those seen in vivo after nerve transection or crush injury (Funakoshi et al., 1993; Hammarberg et al., 1996; Cai et al., 1998).
Figure 3.
Neurotrophic factors are expressed in sciatic nerve and Schwann cell cultures. A, NGF, BDNF, NT-3, and GDNF mRNAs can be detected in intact chicken E18 sciatic nerve using RT-PCR. B, Real-time, quantitative PCR showed dramatic differences in relative expression between intact sciatic nerve and 5-d-old Schwann cell cultures as follows: NGF (14-fold decreased), BDNF (6-fold increased), NT-3 (6-fold decreased), and GDNF (5-fold increased).
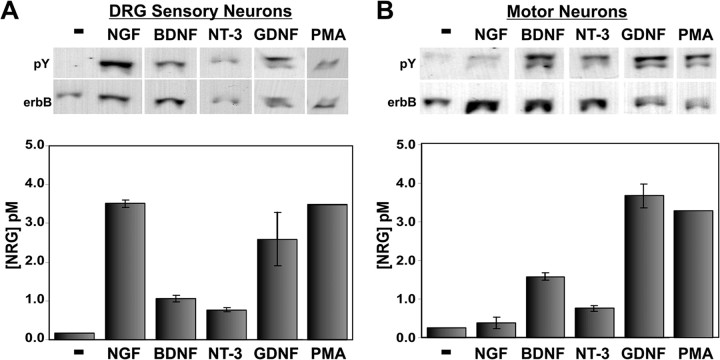
We next asked whether these neurotrophic factors were capable of inducing NRG release from sensory and motor neurons. DRG sensory neurons and motor neurons were exposed to NGF, BDNF, NT-3, or GDNF (100 ng/ml each) for 2 hr, and the culture media was assayed for NRG (Fig. 4). Nonconditioned media that was identical in every other way (including the presence of the neurotrophic factor) was used as a negative control; none of these factors alone had any effect on p185 induction in L6 cells (data not shown). For both types of neurons, these neurotrophic factors rapidly induced NRG release. The DRG sensory neurons released NRG most markedly in response to NGF or GDNF and less so with BDNF or NT-3. The motor neurons maximally released NRG when stimulated by GDNF or BDNF more than NT-3. The difference in the spectra of neurotrophic factor responsiveness appropriately reflects differences in the receptor systems expressed in each neuron type (Ernfors et al., 1993; McMahon et al., 1994; Copray and Kernell, 2000). Previous studies have shown that PKC activation may also be involved in NRG release (Burgess et al., 1995; Loeb et al., 1998). We therefore measured the amount of NRG released from both types of neurons with the phorbol ester, phorbol 12-myristate 13-acetate (PMA), over the same 2 hr period. PMA induced a maximal amount of NRG release comparable with NGF treatment of DRG sensory neurons and GDNF treatment of motor neurons, raising the possibility that these neurotrophic factors promote NRG release through activation of PKC.
Figure 4.
Neurotrophic factors induce NRG release from cultured sensory and motor neurons in distinct patterns. Western blots are shown for DRG sensory (A) and motor (B) neurons with quantitation of the amount of NRG released into neuronal culture media. Cultures were treated with either neurotrophic factors (100 ng/ml) or PMA (1 μm) for 2 hr. Although NGF and GDNF were most able to promote NRG release from sensory neurons, BDNF and GDNF were the two most potent factors for motor neurons. Activation of PKC with the phorbol ester PMA also induced maximal NRG release from both neuron types at a level comparable with neurotrophic factor treatment. Error bars correspond to 1 SD from three replicates.
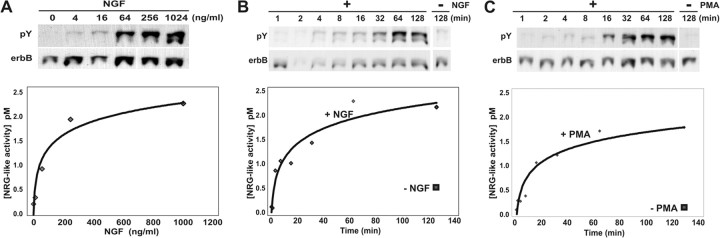
To determine whether this regulated release of NRG from neurons in culture is physiological, we performed time-course and dose-response studies of NGF on DRG sensory neurons. The release of NRG is dose dependent over a wide range of NGF concentrations. Half-maximal NRG release occurred at 64 ng/ml NGF and was saturable at 256 ng/ml NGF (Fig. 5A). The release of NRG was not only dose dependent, but also quite rapid. NGF induced detectable levels of NRG release from DRG sensory neurons in just 4 min (Fig. 5B). The amount of NRG released continued over time and was nearly saturated by 1 hr, with a calculated half-maximal effect at 15 min. Although additional NRG was released after 1 hr, it occurred at a considerably slower rate, which could be attributable to either a slower rate of NRG release or an increased rate of degradation. A similar rapid time course was also seen with PMA treatment (Fig. 5C), again raising the possibility that the neurotrophic factors promote NRG release through PKC activation. Both the dose-response and the time course clearly place the regulated release of NRG from neurons in the physiological range. Although the assay detected only pico-molar concentrations of NRG released into the culture media, this would correspond to significantly higher concentrations if it were restricted to the neuronal monolayer (≥10-fold).
Figure 5.
Neurotrophin-induced NRG release from neurons is rapid and dose dependent. A, Primary sensory neurons were exposed to increasing concentrations of NGF for 2 hr. Released NRG was measured by the Western blot bioassay (top) with quantitation (bottom). NGF (4 ng/ml) was sufficient to cause cultured neurons to release detectable amounts of NRG. The curve represents a nonlinear least squares fit best to the exponential equation y = 0.415ln(x) - 0.5637 and R2 = 0.9439. B, Sensory neuron cultures were treated with 100 ng/ml NGF for increasing lengths of time and NRG was measured as in A. Detectable levels of NRG (diamonds) appeared within 4 min and reached a plateau after 1 hr. Comparison is made with the amount of NRG released at 128 min without NGF exposure (square). The curve represents a nonlinear least squares best fit to an exponential equation y = 0.464ln(x) + 0.0098 and R2 = 0.9226. C, Sensory neuron cultures were treated with 1 μm PMA for increasing lengths of time, and NRG was measured as in A. As in B, detectable levels of NRG (diamonds) appeared within 4 min and reached a plateau after 1 hr. Comparison is made with the amount of NRG released at 128 min without PMA exposure (square). The curve represents a nonlinear least squares fit best to an exponential equation y = 0.3913ln(x) - 0.0953 and R2 = 0.9288.
Given that recombinant neurotrophic factors induced the rapid release of NRG from cultured neurons, we postulated that these neurotrophic factors might also be present in SCCM and tested this hypothesis using various specific neurotrophic factor antagonists. We used DRG sensory neurons to test this because these neurons can respond to each of the neurotrophic factors that we studied (Fig. 4). Because BDNF and GDNF were highly upregulated in the Schwann cell cultures compared with the sciatic nerve, we used two different antagonists for each of these. We used a polyclonal blocking antibody and a soluble TrkB receptor fused to the Fc portion of human IgG to block BDNF (TrkBFc) and two different blocking antibodies against GDNF. These were compared with soluble TrkC receptors fused to human IgG (TrkCFc) to block NT-3. As shown in Figure 6A, each of these antagonists was capable of effectively blocking its respective neurotrophic factor from inducing NRG release from DRG sensory neurons. When mixed with the SCCM, the TrkBFc, BDNF, and GDNF antibodies, but not the TrkCFc antagonist, significantly reduced SCCM-mediated NRG release from DRG sensory neurons (Fig. 6B). When antibodies against BDNF and GDNF were combined, they reduced SCCM-mediated NRG release to near baseline levels, more than any single factor alone. These results were statistically significant on triplicate measurements (p < 0.05) and confirmed in a second experiment. Although NGF was the most potent factor to release NRG from DRG neurons in Figure 4, recombinant BDNF and GDNF were also capable of releasing NRG and appear to be the predominate factors present in SCCM that stimulate neuronal NRG release.
Figure 6.
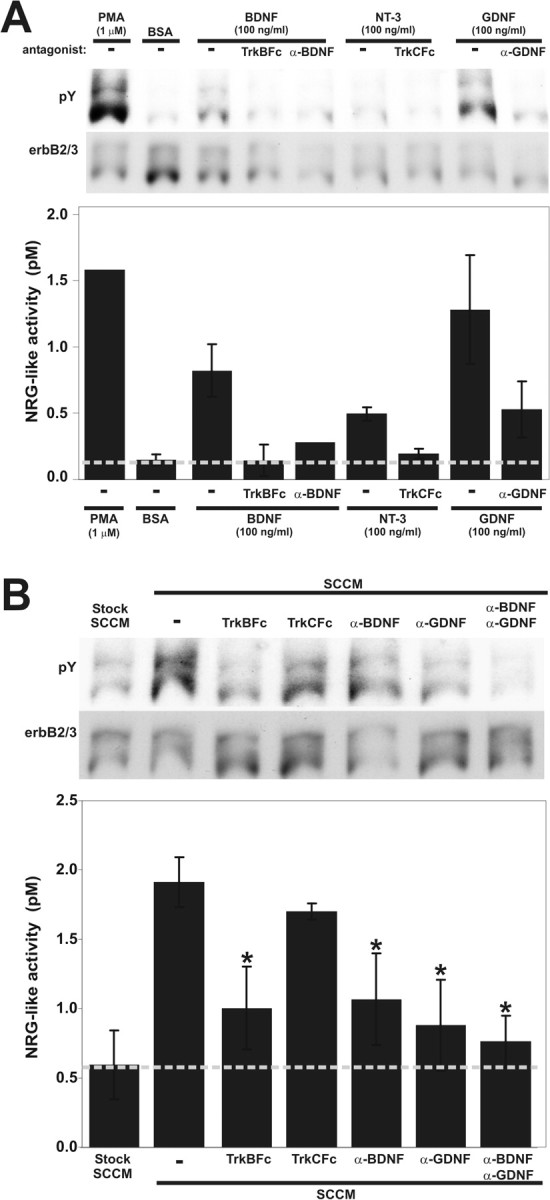
BDNF and GDNF are the predominant NRG-releasing factors produced by cultured Schwann cells. A, Specific antagonists block recombinant neurotrophic factor induction of NRG release from cultured neurons. Soluble fusion protein antagonists for BDNF (TrkBFc) and NT-3 (TrkCFc) as well as blocking antibodies against BDNF and GDNF were tested for their ability to block NRG release from DRG sensory neurons using 100 ng/ml BDNF, NT-3, or GDNF. A second α-GDNF antibody had similar blocking ability (data not shown). PMA (to stimulate protein kinase C) and BSA were used as positive and negative controls, respectively. NRG release was measured from duplicate samples with the bioassay as described previously and expressed as mean ± 1 SD. B, When the same antagonists were preincubated with SCCM, NRG release from DRG sensory neurons was effectively inhibited by TrkBFc and the BDNF and GDNF antibodies but not the TrkCFc antagonist. Combinations of the BDNF and GDNF antagonists maximally blocked the ability of SCCM to promote neuronal NRG release, suggesting an additive effect by BDNF and GDNF. Triplicate samples were measured with the bioassay, and statistical significance was calculated using a Student's t test (*p < 0.05).
Neurotrophin-induced NRG release occurs in axons
The above results demonstrate that NRG is rapidly released from neurons using physiological concentrations of neurotrophic factors present in Schwann cells. Nonetheless, for this to be a viable means of communication between axons and Schwann cells, it needs to be able to occur along the axon. To determine whether NRG is in fact released from axons, we used a modified Campenot-style compartmented culture system to isolate axons from their cell bodies. These devices are not without leakage (∼30-60% of the time), but only those compartments without any sign of leakage were included in the pooled analysis.
DRG sensory neurons plated in the central compartment (C) were allowed to extend their axons into two parallel outer compartments for 4 d (Fig. 7A). After this time, NGF (100 ng/ml) was added to the “treated” (T) axonal compartment for 2 hr at 37°C, after which NRG was measured from pooled, conditioned media from each of the three compartments combined from eight nonleaking chambers. Figure 7D shows that NGF induced the release of NRG from the treated axons (T) compared with both mock-treated axons on the control chamber slides and the untreated axons (U) on the opposite side of the same chamber slide. Interestingly, in addition to the marked increase of NRG in the treated compartment, an increase in NRG in the central compartment (C) of treated slides was also found. This was also seen in a second experiment. This suggests that not only is NRG released in response to direct neurotrophic factor signaling on axons, but it is also released more proximally through a retrograde mechanism of neurotrophic factor signaling to neuronal cell bodies and proximal axons.
Discussion
Rapid axoglial communication through Schwann cell neurotrophic factors and axonal NRGs
The formation of the peripheral nerve is a dynamic process that ultimately leads to a complete covering of motor and sensory axons by either myelinating or nonmyelinating Schwann cells, which are essential for efficient axonal signaling. During nerve development, Schwann cells migrate along axons and intensively proliferate, producing more Schwann cells than are actually needed. Subsequently, these “excess” Schwann cells are eliminated by programmed cell death in a manner similar to excess neurons being eliminated during development (Oppenheim, 1996; Caldero et al., 1998; Winseck et al., 2002). The search for axonal factors responsible for the proliferation of Schwann cells has led to the identification of a soluble, heparin-binding axonal activity initially named glial growth factor that was later found to belong to a group of alternatively spliced factors produced by the NRG1 gene (Marchionni et al., 1993; Lemke, 1996). Genetic studies in mice have shown that NRG signaling is critical for Schwann cell survival and peripheral nerve development. Here we have taken this one step further to show that soluble, heparin-binding forms of NRG are released from axons in a titratable manner, making this an ideal mode of communication that can help modulate this dynamic developmental process.
A plausible means by which localized axoglial communication can occur is summarized in supplemental Figure 1 (available at www.jneurosci.org/cgi/content/full/24/27/6218/DC1). We found that heparin-binding forms of NRG are rapidly released from motor and sensory axons in response to Schwann cell-secreted neurotrophic factors. This occurs in a dose-dependent manner, is saturable, and occurs in a cell-specific way so that motor and sensory neurons have differential spectra of responsiveness to different neurotrophic factors. This is not surprising, because each type of neuron expresses a distinct spectrum of neurotrophic factor receptors (Ernfors et al., 1993; Mu et al., 1993; McMahon et al., 1994; Wright and Snider, 1995; Baig and Khan, 1996; Bhattacharyya et al., 1997; Copray and Kernell, 2000; Rifkin et al., 2000). A similar amount of NRG was released with nearly identical kinetics after PKC activation with PMA, raising the possibility that neurotrophic factor-induced activation of PKC is responsible for NRG release from axons. A key feature of this signaling pathway is that it can occur along axons, which are in direct contact with Schwann cells. Once released, soluble forms of NRG can remain localized to sites of release by binding to heparan sulfate proteoglycans expressed along the developing nerve and at neuromuscular synapses (Loeb et al., 1999).
A rapid signaling system between axonal NRG and Schwann cell neurotrophic factors parallels the signaling system between motor nerve axons and muscle cells at neuromuscular synapses. We have shown previously that NRG protein expression in the synaptic basal lamina of the neuromuscular junction in vivo is prevented by blocking neuromuscular activity but can be “rescued” with neurotrophic factors (Loeb et al., 2002). The absence of NRG was not caused by a reduction in NRG mRNA but rather by a reduction in NRG released as a result of activity-dependent expression of muscle-derived neurotrophic factors (Funakoshi et al., 1993; Loeb et al., 2003). Just as this could be an important feedback loop to potentiate more active synapses through synapse-specific induction of acetylcholine receptor expression (Loeb, 2003), at the axoglial interface this mechanism may have equally important implications for Schwann cell proliferation, survival, and myelination (supplemental Fig. 1, available at www.jneurosci.org/cgi/content/full/24/27/6218/DC1).
Role of localized neurotrophic factor-NRG signaling in peripheral nerve development, maintenance, and disease Although much of the research emphasis in neurotrophic factors has centered on their roles as target-derived factors important for neuronal survival during development, neuronal survival is also highly dependent on glial-derived signals (Sepp and Auld, 2003). Disruption of NRG signaling in mice not only leads to an almost complete loss of Schwann cells, but shortly thereafter, all motor and sensory neurons die. This destruction is far more massive than that seen with any of the neurotrophic factor knock-out models that produced only limited death of specific and often small neuronal populations. This is not too surprising, because Schwann cells are a source of neurotrophic factors, including NGF, BDNF, NT-3, and GDNF (Funakoshi et al., 1993; Bolin and Shooter, 1994; Wetmore and Olson, 1995) and contact a far greater proportion of the axonal surface than do their targets. Because these factors induce the rapid release of NRG, it is possible that some of the neuronal cell death seen in the knock-outs occurred indirectly as a result of failed Schwann cell support.
Although we found that NGF, BDNF, NT-3, and GDNF were expressed in the intact sciatic nerve, Schwann cells cultured from these nerves had dramatic changes in neurotrophic factor expression, with a pronounced downregulation of NGF and NT-3 and upregulation of BDNF and GDNF. Similar changes in neurotrophic factor expression have been shown in the sciatic nerve after axotomy or nerve crush injury (Funakoshi et al., 1993; Hammarberg et al., 1996; Cai et al., 1998), suggesting that the regulated release of soluble NRG in injured or diseased nerves may also occur via the production of these neurotrophic factors. Although the cultured DRG neurons optimally responded to NGF to release NRG, they also responded to BDNF, GDNF, and NT-3; however, given the upregulation that we observed in BDNF and GDNF expression and the downregulation of NGF and NT-3 in cultured Schwann cells from intact sciatic nerve, it is not surprising that BDNF and GDNF were the principal NRG-releasing factors found from cultured Schwann cells. Exactly what regulates changes in neurotrophic factor expression in Schwann cells is not known. There is evidence, however, that neuronal NRG can regulate glial neurotrophic factor expression (Verdi et al., 1996; Hansen et al., 2001), raising the possibility of a reciprocal feedback loop between axonal NRG and Schwann cell neurotrophic factors.
Although the role of NRG in Schwann cell proliferation and survival is well documented (Dong et al., 1995; Levi et al., 1995; Meyer and Birchmeier, 1995; Grinspan et al., 1996; Mirsky et al., 1996; Syroid et al., 1996; Topilko et al., 1996; Baek and Kim, 1998; Jessen and Mirsky, 2002), the effect of NRG signaling within the peripheral nerve later in development is less clear. NRG expression increases in both motor and sensory neurons as the peripheral nerve matures (Loeb et al., 1999; our unpublished observations). In these later stages, individual Schwann cells need to decide whether or not to myelinate a given axon. There is evidence that neurotrophic factors are involved in this decision (Chan et al., 2001; Cosgaya et al., 2002). These studies show that BDNF promotes myelination both in vivo and in vitro. It remains to be seen whether this is caused by a direct effect of BDNF on the Schwann cells or an indirect effect by promoting NRG release from the neurons. Interestingly, GDNF, which leads to effective NRG release from both motor and sensory axons, can cause nonmyelinating Schwann cells to begin myelinating in adults (Hoke et al., 2003).
Our findings also have implications for understanding and treating diseases of the peripheral nerve. Overexpression of soluble NRG in mice results in malignant peripheral nerve sheath tumors (Huijbregts et al., 2003). In addition, various studies have shown dramatic changes in both neurotrophic factor and NRG expression after peripheral nerve injury that result in gene expression changes that pattern those seen during early development (Ernfors et al., 1993; Funakoshi et al., 1993; Carroll et al., 1997; Sobue et al., 1998). Therefore, signaling between neuron-derived NRG and Schwann cell-produced neurotrophic factors may have an important role in repairing peripheral nerves after injury. Consistently, both NRGs and neurotrophic factors have been tried therapeutically in humans and in animal models of PNS and CNS diseases that include amyotrophic lateral sclerosis, multiple sclerosis, epileptic seizures, Alzheimer's disease, Parkinson's disease, and traumatic spinal cord injuries (Seeburger and Springer, 1993; Unsicker, 1994; McMahon and Priestley, 1995; Marchionni et al., 1996; Cannella et al., 1998; Apfel, 1999; ter Laak et al., 2000; Coumans et al., 2001; Viehover et al., 2001; Massa et al., 2002; Sayer et al., 2002). Understanding the dynamics of NRG-neurotrophic factor signaling at the axoglial and neuromuscular interfaces will be important for the rational development of new treatments for these conditions, and this study is the first demonstration of a direct soluble growth factor-mediated axoglial communication system involving axonal NRG and Schwann cell neurotrophic factors.
NRG release from axons
A key observation here is that NRG-neurotrophic factor signaling can occur at the axoglial interface. In pursuing experiments to detect NRG release from isolated axons in culture, a large amount of pooled culture media from many such cultures was needed. In the process, we developed a simplified method to isolate neuronal cell bodies from their axons that was modeled on the Campenot chamber device (Campenot, 1977; Banker, 1998). Using this method, we have shown that neurotrophic factors promote the rapid release of NRG from axons and provide evidence for an additional retrograde signaling mechanism promoting NRG release from neuronal cell bodies and proximal axons in the middle chamber (Fig. 7D). Although the significance of retrograde NRG release remains to be determined, these findings are consistent with recent studies suggesting a role for retrograde neurotrophin signaling in axons (Ginty and Segal, 2002; MacInnis and Campenot, 2002).
Footnotes
This work was supported by National Science Foundation Grant IBN-0092623 (J.A.L.), National Multiple Sclerosis Society Grant RG-3410-A-2 (J.A.L.), and The Children's Research Center of Michigan (J.A.L.). We thank Regeneron and Amgen for kindly providing the recombinant neurotrophins and antibodies for this study. We also thank Yosef Yarden for the IgB4 NRG antagonist.
Correspondence should be addressed to Dr. Jeffrey A. Loeb, Department of Neurology and Center for Molecular Medicine and Genetics, Wayne State University, Elliman 3217, 421 East Canfield Avenue, Detroit, MI 48201. E-mail: jloeb@med.wayne.edu.
Copyright © 2004 Society for Neuroscience 0270-6474/04/246218-10$15.00/0
References
- Adlkofer K, Lai C (2000) Role of neuregulins in glial cell development. Glia 29: 104-111. [DOI] [PubMed] [Google Scholar]
- Andrechek ER, Hardy WR, Girgis-Gabardo AA, Perry RL, Butler R, Graham FL, Kahn RC, Rudnicki MA, Muller WJ (2002) ErbB2 is required for muscle spindle and myoblast cell survival. Mol Cell Biol 22: 4714-4722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apfel SC (1999) Neurotrophic factors and diabetic peripheral neuropathy. Eur Neurol 41 [Suppl 1]: 27-34. [DOI] [PubMed] [Google Scholar]
- Baek SY, Kim SU (1998) Proliferation of human Schwann cells induced by neu differentiation factor isoforms. Dev Neurosci 20: 512-517. [DOI] [PubMed] [Google Scholar]
- Baig MA, Khan MA (1996) The induction of neurotrophin and TRK receptor mRNA expression during early avian embryogenesis. Int J Dev Neurosci 14: 55-60. [DOI] [PubMed] [Google Scholar]
- Banker GG, Goslin K (1998) Culturing nerve cells, Ed 2. Cambridge, MA: MIT.
- Bhattacharyya A, Brackenbury R, Ratner N (1994) Axons arrest the migration of Schwann cell precursors. Development 120: 1411-1420. [DOI] [PubMed] [Google Scholar]
- Bhattacharyya A, Watson FL, Bradlee TA, Pomeroy SL, Stiles CD, Segal RA (1997) Trk receptors function as rapid retrograde signal carriers in the adult nervous system. J Neurosci 17: 7007-7016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolin LM, Shooter EM (1993) Neurons regulate Schwann cell genes by diffusible molecules. J Cell Biol 123: 237-243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolin LM, Shooter EM (1994) Characterization of a Schwann cell neurite-promoting activity that directs motoneuron axon outgrowth. J Neurosci Res 37: 23-35. [DOI] [PubMed] [Google Scholar]
- Burgess TL, Ross SL, Qian YX, Brankow D, Hu S (1995) Biosynthetic processing of neu differentiation factor. Glycosylation trafficking, and regulated cleavage from the cell surface. J Biol Chem 270: 19188-19196. [DOI] [PubMed] [Google Scholar]
- Cai F, Tomlinson DR, Fernyhough P (1998) Effect of sciatic nerve crush on local and target tissue production of neurotrophin-3 transcripts in rats. Neurosci Lett 252: 45-48. [DOI] [PubMed] [Google Scholar]
- Caldero J, Prevette D, Mei X, Oakley RA, Li L, Milligan C, Houenou L, Burek M, Oppenheim RW (1998) Peripheral target regulation of the development and survival of spinal sensory and motor neurons in the chick embryo. J Neurosci 18: 356-370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campenot RB (1977) Local control of neurite development by nerve growth factor. Proc Natl Acad Sci USA 74: 4516-4519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campenot RB, Draker DD, Senger DL (1994) Evidence that protein kinase C activities involved in regulating neurite growth are localized to distal neurites. J Neurochem 63: 868-878. [DOI] [PubMed] [Google Scholar]
- Campenot RB, Soin J, Blacker M, Lund K, Eng H, MacInnis BL (2003) Block of slow axonal transport and axonal growth by brefeldin A in compartmented cultures of rat sympathetic neurons. Neuropharmacology 44: 1107-1117. [DOI] [PubMed] [Google Scholar]
- Cannella B, Hoban CJ, Gao YL, Garcia-Arenas R, Lawson D, Marchionni M, Gwynne D, Raine CS (1998) The neuregulin, glial growth factor 2, diminishes autoimmune demyelination and enhances remyelination in a chronic relapsing model for multiple sclerosis. Proc Natl Acad Sci USA 95: 10100-10105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll SL, Miller ML, Frohnert PW, Kim SS, Corbett JA (1997) Expression of neuregulins and their putative receptors, ErbB2 and ErbB3, is induced during Wallerian degeneration. J Neurosci 17: 1642-1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan JR, Cosgaya JM, Wu YJ, Shooter EM (2001) Neurotrophins are key mediators of the myelination program in the peripheral nervous system. Proc Natl Acad Sci USA 98: 14661-14668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Rio C, Ji RR, Dikkes P, Coggeshall RE, Woolf CJ, Corfas G (2003) Disruption of ErbB receptor signaling in adult non-myelinating Schwann cells causes progressive sensory loss. Nat Neurosci 6: 1186-1193. [DOI] [PubMed] [Google Scholar]
- Chen X, Levkowitz G, Tzahar E, Karunagaran D, Lavi S, Ben-Baruch N, Leitner O, Ratzkin BJ, Bacus SS, Yarden Y (1996) An immunological approach reveals biological differences between the two NDF/heregulin receptors, ErbB-3 and ErbB-4. J Biol Chem 271: 7620-7629. [PubMed] [Google Scholar]
- Ciutat D, Caldero J, Oppenheim RW, Esquerda JE (1996) Schwann cell apoptosis during normal development and after axonal degeneration induced by neurotoxins in the chick embryo. J Neurosci 16: 3979-3990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copray S, Kernell D (2000) Neurotrophins and trk-receptors in adult rat spinal motoneurons: differences related to cell size but not to “slow/fast” specialization. Neurosci Lett 289: 217-220. [DOI] [PubMed] [Google Scholar]
- Cosgaya JM, Chan JR, Shooter EM (2002) The neurotrophin receptor p75NTR as a positive modulator of myelination. Science 298: 1245-1248. [DOI] [PubMed] [Google Scholar]
- Coumans JV, Lin TT, Dai HN, MacArthur L, McAtee M, Nash C, Bregman BS (2001) Axonal regeneration and functional recovery after complete spinal cord transection in rats by delayed treatment with transplants and neurotrophins. J Neurosci 21: 9334-9344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Z, Brennan A, Liu N, Yarden Y, Lefkowitz G, Mirsky R, Jessen KR (1995) Neu differentiation factor is a neuron-glia signal and regulates survival, proliferation, and maturation of rat Schwann cell precursors. Neuron 15: 585-596. [DOI] [PubMed] [Google Scholar]
- Ernfors P, Rosario CM, Merlio JP, Grant G, Aldskogius H, Persson H (1993) Expression of mRNAs for neurotrophin receptors in the dorsal root ganglion and spinal cord during development and following peripheral or central axotomy. Brain Res Mol Brain Res 17: 217-226. [DOI] [PubMed] [Google Scholar]
- Falls DL (2003) Neuregulins: functions, forms, and signaling strategies. Exp Cell Res 284: 14-30. [DOI] [PubMed] [Google Scholar]
- Fischbach GD, Rosen KM (1997) ARIA: a neuromuscular junction neuregulin. Annu Rev Neurosci 20: 429-458. [DOI] [PubMed] [Google Scholar]
- Friedman HC, Jelsma TN, Bray GM, Aguayo AJ (1996) A distinct pattern of trophic factor expression in myelin-deficient nerves of Trembler mice: implications for trophic support by Schwann cells. J Neurosci 16: 5344-5350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funakoshi H, Frisen J, Barbany G, Timmusk T, Zachrisson O, Verge VM, Persson H (1993) Differential expression of mRNAs for neurotrophins and their receptors after axotomy of the sciatic nerve. J Cell Biol 123: 455-465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garratt AN, Britsch S, Birchmeier C (2000) Neuregulin, a factor with many functions in the life of a Schwann cell. BioEssays 22: 987-996. [DOI] [PubMed] [Google Scholar]
- Ginty DD, Segal RA (2002) Retrograde neurotrophin signaling: Trk-ing along the axon. Curr Opin Neurobiol 12: 268-274. [DOI] [PubMed] [Google Scholar]
- Griesbeck O, Parsadanian AS, Sendtner M, Thoenen H (1995) Expression of neurotrophins in skeletal muscle: quantitative comparison and significance for motoneuron survival and maintenance of function. J Neurosci Res 42: 21-33. [DOI] [PubMed] [Google Scholar]
- Grinspan JB, Marchionni MA, Reeves M, Coulaloglou M, Scherer SS (1996) Axonal interactions regulate Schwann cell apoptosis in developing peripheral nerve: neuregulin receptors and the role of neuregulins. J Neurosci 16: 6107-6118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammarberg H, Piehl F, Cullheim S, Fjell J, Hokfelt T, Fried K (1996) GDNF mRNA in Schwann cells and DRG satellite cells after chronic sciatic nerve injury. NeuroReport 7: 857-860. [DOI] [PubMed] [Google Scholar]
- Hansen MR, Vijapurkar U, Koland JG, Green SH (2001) Reciprocal signaling between spiral ganglion neurons and Schwann cells involves neuregulin and neurotrophins. Hear Res 161: 87-98. [DOI] [PubMed] [Google Scholar]
- Henderson CE, Phillips HS, Pollock RA, Davies AM, Lemeulle C, Armanini M, Simmons L, Moffet B, Vandlen RA, Simpson LC (1994) GDNF: a potent survival factor for motoneurons present in peripheral nerve and muscle Science 266: 1062-1064.[ [DOI] [PubMed] [Google Scholar]; Erratum (1995) 267: 777] [Google Scholar]
- Hippenmeyer S, Shneider NA, Birchmeier C, Burden SJ, Jessell TM, Arber S (2002) A role for neuregulin 1 signaling in muscle spindle differentiation. Neuron 36: 1035-1049. [DOI] [PubMed] [Google Scholar]
- Hoke A, Ho T, Crawford TO, LeBel C, Hilt D, Griffin JW (2003) Glial cell line-derived neurotrophic factor alters axon Schwann cell units and promotes myelination in unmyelinated nerve fibers. J Neurosci 23: 561-567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huijbregts RP, Roth KA, Schmidt RE, Carroll SL (2003) Hypertrophic neuropathies and malignant peripheral nerve sheath tumors in transgenic mice overexpressing glial growth factor β3 in myelinating Schwann cells. J Neurosci 23: 7269-7280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessen KR, Mirsky R (1999) Schwann cells and their precursors emerge as major regulators of nerve development. Trends Neurosci 22: 402-410. [DOI] [PubMed] [Google Scholar]
- Jessen KR, Mirsky R (2002) Signals that determine Schwann cell identity. J Anat 200: 367-376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopp DM, Trachtenberg JT, Thompson WJ (1997) Glial growth factor rescues Schwann cells of mechanoreceptors from denervation-induced apoptosis. J Neurosci 17: 6697-6706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemke G (1996) Neuregulins in development. Mol Cell Neurosci 7: 247-262. [DOI] [PubMed] [Google Scholar]
- Levi A, Bunge R, Lofgren J, Meima L, Hefti F, Nikolics K, Sliwkowski M (1995) The influence of heregulins on human Schwann cell proliferation. J Neurosci 15: 1329-1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Loeb JA (2001) Neuregulin-heparin-sulfate proteoglycan interactions produce sustained erbB receptor activation required for the induction of acetylcholine receptors in muscle. J Biol Chem 276: 38068-38075. [DOI] [PubMed] [Google Scholar]
- Little GJ, Heath JW (1994) Morphometric analysis of axons myelinated during adult life in the mouse superior cervical ganglion. J Anat 184: 387-398. [PMC free article] [PubMed] [Google Scholar]
- Loeb JA (2003) Neuregulin: an activity-dependent synaptic modulator at the neuromuscular junction. J Neurocytol 32: 649-664. [DOI] [PubMed] [Google Scholar]
- Loeb JA, Fischbach GD (1995) ARIA can be released from extracellular matrix through cleavage of a heparin-binding domain. J Cell Biol 130: 127-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeb JA, Fischbach GD (1997) Neurotrophic factors increase neuregulin expression in embryonic ventral spinal cord neurons. J Neurosci 17: 1416-1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeb JA, Susanto ET, Fischbach GD (1998) The neuregulin precursor proARIA is processed to ARIA after expression on the cell surface by a protein kinase C-enhanced mechanism. Mol Cell Neurosci 11: 77-91. [DOI] [PubMed] [Google Scholar]
- Loeb JA, Khurana TS, Robbins JT, Yee AG, Fischbach GD (1999) Expression patterns of transmembrane and released forms of neuregulin during spinal cord and neuromuscular synapse development. Development 126: 781-791. [DOI] [PubMed] [Google Scholar]
- Loeb JA, Hmadcha A, Fischbach GD, Land SJ, Zakarian VL (2002) Neuregulin expression at neuromuscular synapses is modulated by synaptic activity and neurotrophic factors. J Neurosci 22: 2206-2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacInnis BL, Campenot RB (2002) Retrograde support of neuronal survival without retrograde transport of nerve growth factor. Science 295: 1536-1539. [DOI] [PubMed] [Google Scholar]
- Marchionni MA, Goodearl AD, Chen MS, Bermingham-McDonogh O, Kirk C, Hendricks M, Danehy F, Misumi D, Sudhalter J, Kobayashi K, Wroblewski D, Lynch C, Baldassare M, Hiles I, Davis JB, Hsuan J, Totty NF, Otsu M, McBurney RN, Waterfield MD (1993) Glial growth factors are alternatively spliced erbB2 ligands expressed in the nervous system. Nature 362: 312-318. [DOI] [PubMed] [Google Scholar]
- Marchionni MA, Kirk CJ, Isaacs IJ, Hoban CJ, Mahanthappa NK, Anton ES, Chen C, Wason F, Lawson D, Hamers FP, Canoll PD, Reynolds R, Cannella B, Meun D, Holt WF, Matthew WD, Chen LE, Gispen WH, Raine CS, Salzer JL, et al. (1996) Neuregulins as potential drugs for neurological disorders. Cold Spring Harb Symp Quant Biol 61: 459-472. [PubMed] [Google Scholar]
- Massa SM, Xie Y, Longo FM (2002) Alzheimer's therapeutics: neurotrophin small molecule mimetics. J Mol Neurosci 19: 107-111. [DOI] [PubMed] [Google Scholar]
- Matsuoka I, Meyer M, Thoenen H (1991) Cell-type-specific regulation of nerve growth factor (NGF) synthesis in non-neuronal cells: comparison of Schwann cells with other cell types. J Neurosci 11: 3165-3177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon SB, Priestley JV (1995) Peripheral neuropathies and neurotrophic factors: animal models and clinical perspectives. Curr Opin Neurobiol 5: 616-624. [DOI] [PubMed] [Google Scholar]
- McMahon SB, Armanini MP, Ling LH, Phillips HS (1994) Expression and coexpression of Trk receptors in subpopulations of adult primary sensory neurons projecting to identified peripheral targets. Neuron 12: 1161-1171. [DOI] [PubMed] [Google Scholar]
- Meyer D, Birchmeier C (1995) Multiple essential functions of neuregulin in development. Nature 378: 386-390. [DOI] [PubMed] [Google Scholar]
- Mirsky R, Stewart HJ, Tabernero A, Bradke F, Brennan A, Dong Z, Jessen KR (1996) Development and differentiation of Schwann cells. Rev Neurol (Paris) 152: 308-313. [PubMed] [Google Scholar]
- Mirsky R, Jessen KR, Brennan A, Parkinson D, Dong Z, Meier C, Parmantier E, Lawson D (2002) Schwann cells as regulators of nerve development. J Physiol (Paris) 96: 17-24. [DOI] [PubMed] [Google Scholar]
- Morris JK, Lin W, Hauser C, Marchuk Y, Getman D, Lee KF (1999) Rescue of the cardiac defect in ErbB2 mutant mice reveals essential roles of ErbB2 in peripheral nervous system development. Neuron 23: 273-283. [DOI] [PubMed] [Google Scholar]
- Mu X, Silos-Santiago I, Carroll SL, Snider WD (1993) Neurotrophin receptor genes are expressed in distinct patterns in developing dorsal root ganglia. J Neurosci 13: 4029-4041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson I, Berthold CH (1988) Axon classes and internodal growth in the ventral spinal root L7 of adult and developing cats. J Anat 156: 71-96. [PMC free article] [PubMed] [Google Scholar]
- Oppenheim RW (1996) Neurotrophic survival molecules for motoneurons: an embarrassment of riches. Neuron 17: 195-197. [DOI] [PubMed] [Google Scholar]
- Riethmacher D, Sonnenberg-Riethmacher E, Brinkmann V, Yamaai T, Lewin GR, Birchmeier C (1997) Severe neuropathies in mice with targeted mutations in the ErbB3 receptor. Nature 389: 725-730. [DOI] [PubMed] [Google Scholar]
- Rifkin JT, Todd VJ, Anderson LW, Lefcort F (2000) Dynamic expression of neurotrophin receptors during sensory neuron genesis and differentiation. Dev Biol 227: 465-480. [DOI] [PubMed] [Google Scholar]
- Rosenbaum C, Karyala S, Marchionni MA, Kim HA, Krasnoselsky AL, Happel B, Isaacs I, Brackenbury R, Ratner N (1997) Schwann cells express NDF and SMDF/n-ARIA mRNAs, secrete neuregulin, and show constitutive activation of erbB3 receptors: evidence for a neuregulin autocrine loop. Exp Neurol 148: 604-615. [DOI] [PubMed] [Google Scholar]
- Sandrock Jr AW, Dryer SE, Rosen KM, Gozani SN, Kramer R, Theill LE, Fischbach GD (1997) Maintenance of acetylcholine receptor number by neuregulins at the neuromuscular junction in vivo Science 276: 599-603. [DOI] [PubMed] [Google Scholar]
- Sayer FT, Oudega M, Hagg T (2002) Neurotrophins reduce degeneration of injured ascending sensory and corticospinal motor axons in adult rat spinal cord. Exp Neurol 175: 282-296. [DOI] [PubMed] [Google Scholar]
- Seeburger JL, Springer JE (1993) Experimental rationale for the therapeutic use of neurotrophins in amyotrophic lateral sclerosis. Exp Neurol 124: 64-72. [DOI] [PubMed] [Google Scholar]
- Sepp KJ, Auld VJ (2003) Reciprocal interactions between neurons and glia are required for Drosophila peripheral nervous system development. J Neurosci 23: 8221-8230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobue G, Yamamoto M, Doyu M, Li M, Yasuda T, Mitsuma T (1998) Expression of mRNAs for neurotrophins (NGF, BDNF, and NT-3) and their receptors (p75NGFR, trk, trkB, and trkC) in human peripheral neuropathies. Neurochem Res 23: 821-829. [DOI] [PubMed] [Google Scholar]
- Syroid DE, Maycox PR, Burrola PG, Liu N, Wen D, Lee KF, Lemke G, Kil-patrick TJ (1996) Cell death in the Schwann cell lineage and its regulation by neuregulin. Proc Natl Acad Sci USA 93: 9229-9234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ter Laak MP, Hamers FP, Kirk CJ, Gispen WH (2000) rhGGF2 protects against cisplatin-induced neuropathy in the rat. J Neurosci Res 60: 237-244. [DOI] [PubMed] [Google Scholar]
- Topilko P, Murphy P, Charnay P (1996) Embryonic development of Schwann cells: multiple roles for neuregulins along the pathway. Mol Cell Neurosci 8: 71-75. [DOI] [PubMed] [Google Scholar]
- Trachtenberg JT, Thompson WJ (1996) Schwann cell apoptosis at developing neuromuscular junctions is regulated by glial growth factor. Nature 379: 174-177. [DOI] [PubMed] [Google Scholar]
- Unsicker K (1994) Growth factors in Parkinson's disease. Prog Growth Factor Res 5: 73-87. [DOI] [PubMed] [Google Scholar]
- Verdi JM, Groves AK, Farinas I, Jones K, Marchionni MA, Reichardt LF, Anderson DJ (1996) A reciprocal cell-cell interaction mediated by NT-3 and neuregulins controls the early survival and development of sympathetic neuroblasts. Neuron 16: 515-527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viehover A, Miller RH, Park SK, Fischbach G, Vartanian T (2001) Neuregulin: an oligodendrocyte growth factor absent in active multiple sclerosis lesions. Dev Neurosci 23: 377-386. [DOI] [PubMed] [Google Scholar]
- Wang JY, Miller SJ, Falls DL (2000) The N-terminal region of neuregulin isoforms determines the accumulation of cell surface and released neuregulin ectodomain. J Biol Chem 276: 2841-2851. [DOI] [PubMed] [Google Scholar]
- Wetmore C, Olson L (1995) Neuronal and nonneuronal expression of neurotrophins and their receptors in sensory and sympathetic ganglia suggest new intercellular trophic interactions. J Comp Neurol 353: 143-159. [DOI] [PubMed] [Google Scholar]
- Winseck AK, Caldero J, Ciutat D, Prevette D, Scott SA, Wang G, Esquerda JE, Oppenheim RW (2002) In vivo analysis of Schwann cell programmed cell death in the embryonic chick: regulation by axons and glial growth factor. J Neurosci 22: 4509-4521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woldeyesus MT, Britsch S, Riethmacher D, Xu L, Sonnenberg-Riethmacher E, Abou-Rebyeh F, Harvey R, Caroni P, Birchmeier C (1999) Peripheral nervous system defects in erbB2 mutants following genetic rescue of heart development. Genes Dev 13: 2538-2548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wooten MW, Vandenplas ML, Seibenhener ML, Geetha T, Diaz-Meco MT (2001) Nerve growth factor stimulates multisite tyrosine phosphorylation and activation of the atypical protein kinase C's via an Src kinase pathway. Mol Cell Biol 21: 8414-8427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright DE, Snider WD (1995) Neurotrophin receptor mRNA expression defines distinct populations of neurons in rat dorsal root ganglia. J Comp Neurol 351: 329-338. [DOI] [PubMed] [Google Scholar]
- Zirrgiebel U, Ohga Y, Carter B, Berninger B, Inagaki N, Thoenen H, Lindholm D (1995) Characterization of TrkB receptor-mediated signaling pathways in rat cerebellar granule neurons: involvement of protein kinase C in neuronal survival. J Neurochem 65: 2241-2250. [DOI] [PubMed] [Google Scholar]
- Zochodne DW, Cheng C (2000) Neurotrophins and other growth factors in the regenerative milieu of proximal nerve stump tips. J Anat 196: 279-283. [DOI] [PMC free article] [PubMed] [Google Scholar]