Abstract
Although the physiological basis of erythermalgia, an autosomal dominant painful neuropathy characterized by redness of the skin and intermittent burning sensation of extremities, is not known, two mutations of Nav1.7, a sodium channel that produces a tetrodotoxin-sensitive, fast-inactivating current that is preferentially expressed in dorsal root ganglia (DRG) and sympathetic ganglia neurons, have recently been identified in patients with primary erythermalgia. Nav1.7 is preferentially expressed in small-diameter DRG neurons, most of which are nociceptors, and is characterized by slow recovery from inactivation and by slow closed-state inactivation that results in relatively large responses to small, subthreshold depolarizations. Here we show that these mutations in Nav1.7 produce a hyperpolarizing shift in activation and slow deactivation. We also show that these mutations cause an increase in amplitude of the current produced by Nav1.7 in response to slow, small depolarizations. These observations provide the first demonstration of altered sodium channel function associated with an inherited painful neuropathy and suggest that these physiological changes, which confer hyperexcitability on peripheral sensory and sympathetic neurons, contribute to symptom production in hereditary erythermalgia.
Keywords: dorsal root ganglion, hyperpolarization, voltage clamp, tetrodotoxin sensitive, neuropathic pain, skeletal sodium channel
Introduction
Sensory neurons in dorsal root ganglia (DRG) express multiple voltage-gated sodium channels including Nav1.7 (Klugbauer et al., 1995; Sangameswaran et al., 1997), Nav1.8 (Akopian et al., 1996; Sangameswaran et al., 1996), and Nav1.9 (Dib-Hajj et al., 1998; Tate et al., 1998), which play important roles in regulating their excitability (Matzner and Devor, 1994; Cummins et al., 1998, 1999; Renganathan et al., 2001). Nav1.7 is selectively expressed in DRG and sympathetic ganglia (Black et al., 1996; Toledo-Aral et al., 1997) and is abundant in small-diameter DRG neurons (Black et al., 1996, 2004), including nociceptors (Djouhri et al., 2003a). Recombinant Nav1.7 produces a fast-inactivating tetrodotoxin-sensitive (TTX-S) current (Klugbauer et al., 1995; Sangameswaran et al., 1997) and displays slow repriming and slow closed-state inactivation that poise it to respond to small, slow depolarizations (Cummins et al., 1998; Herzog et al., 2003).
It is now well established that dysregulated expression of sodium channel genes, for example Nav1.3, can produce changes in sodium currents within spinal sensory neurons that contribute to neuropathic pain (Cummins and Waxman, 1997; Black et al., 1999; Hains et al., 2004). Recently, Nav1.7 expression in DRG neurons has been shown to increase after carrageenan-induced inflammation of rat hindpaw (Black et al., 2004). The dynamic regulation of Nav1.7 suggests that this channel contributes to neuronal hyperexcitability leading to inflammatory pain.
In contrast to dysregulated sodium channel expression, to date there have been no demonstrations of changes in sodium currents attributable to mutations associated with pain. Familial primary erythermalgia is a rare, dominantly inherited painful neuropathy that is manifested as burning pain and redness of the extremities (van Genderen et al., 1993). Layzer (2001) hypothesized that sensitized C-fibers and the axon reflex underlie these symptoms. A segment of chromosome 2, which is known to contain sodium channel genes, has been linked to primary erythermalgia (Drenth et al., 2001). Subsequently, Yang et al. (2004) showed that two independent mutations in SCN9A, which encodes Nav1.7, are linked to this disorder. The two substitutions produce a change of isoleucine 848 to threonine (I848T) and leucine 858 to histidine (L858H).
We investigated the effect of the I848T and L858H mutations on the biophysical properties of hNav1.7. Both mutations cause a significant hyperpolarizing shift in the V1/2 of activation of the mutant channel, which was accompanied by a larger ramp current. Our data are consistent with a role of Nav1.7 in DRG neuron hyperexcitability in erythermalgia.
Materials and Methods
Plasmids. The plasmid carrying the human Nav1.7 cDNA insert was described previously (Klugbauer et al., 1995). The TTX-S determinant residue of Nav1.7, tyrosine 362, was changed by site-directed mutagenesis to a serine to render the channel resistant to TTX (Nav1.7R) (Herzog et al., 2003). The I848T and L858H were individually introduced into Nav1.7R using the Quick Change XL site-directed mutagenesis kit (Stratagene, La Jolla, CA) with two mutagenic primers that were designed according to the manufacturer recommendations.
Transfections. The hNav1.7 channels were cotransfected with the human β1 and β2 subunits (Lossin et al., 2002) into human embryonic kidney (HEK293) cells using the calcium phosphate precipitation method. HEK293 cells were grown under standard tissue culture conditions (5% CO2; 37°C) in DMEM supplemented with 10% fetal bovine serum. The calcium phosphate-DNA mixture was added to the cell culture medium and left for 3 hr, after which the cells were washed with fresh medium. Sodium currents were recorded 40-72 hr after transfection.
Whole-cell patch-clamp recordings. Whole-cell patch-clamp recordings were conducted at room temperature (∼21°C) using an EPC-10 amplifier and the Pulse program (v 8.5; HEKA Elektronik, Lambrecht/Pfalz, Germany). Fire-polished electrodes (0.8-1.5 MΩ) were fabricated from 1.7 mm VWR Scientific (West Chester, PA) capillary glass using a Sutter Instruments (Novato, CA) P-97 puller. Average access resistance was 1.4 ± 0.4 MΩ (mean ± SD; n = 85). Voltage errors were minimized using 80% series resistance compensation; the capacitance artifact was canceled using computer-controlled circuitry of the patch-clamp amplifier. Linear leak subtraction was used for all voltage-clamp recordings. Recordings were always started 3 min after establishing the whole-cell configuration. Membrane currents were filtered at 5 kHz and sampled at 20 kHz. The pipette solution contained the following (in mm): 140 CsF, 1 EGTA, 10 NaCl, and 10 HEPES, pH 7.3. The standard bathing solution was the following (in mm): 140 NaCl, 3 KCl, 1 MgCl2, 1 CaCl2, and 10 HEPES, pH 7.3. Data were analyzed using Pulsefit (HEKA Elektronik) and Origin (Microcal Software, Northampton, MA) software. Data sets used for statistical analysis were checked for normal distributions using a Shapiro-Wilks normality test.
Unless otherwise noted, statistical significance was determined (p < 0.05) using an unpaired t test. Results are presented as mean ± SEM and error bars in the figures represent SEs.
Results
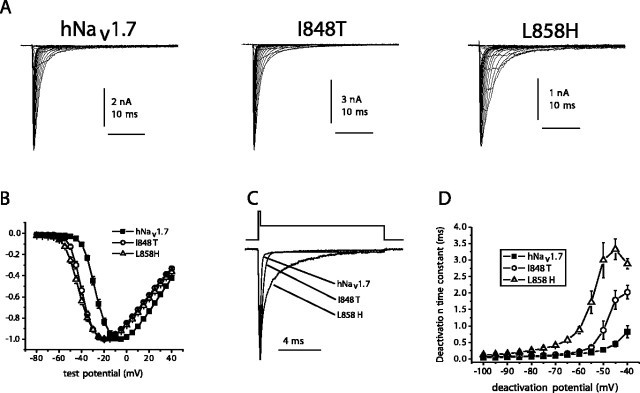
Wild-type (WT) hNav1.7R and the two mutant derivative channels I848T and L858H were transiently expressed along with hβ-1 and hβ-2 subunits in HEK293 cells. Figure 1A shows representative whole-cell currents. Although similar peak current densities were recorded from cells expressing WT (318 ± 43 pA/pF; n = 29) and I848T (350 ± 37 pA/pF; n = 27) channels, the current densities recorded from cells expressing L858H channels were significantly smaller (174 ± 30 pA/pF; n = 27). The voltage dependence of activation was examined using a series of depolarizing test pulses from -100 mV. Mutant channels activated at potentials 10-15 mV more negative than WT channels (Fig. 1B). The midpoint of activation (estimated by fitting the data with a Bolztman function) was significantly more negative for I848T currents (-38.4 ± 1.0 mV; n = 27) and L858H currents (-37.9 ± 0.9 mV; n = 27) than for WT currents (-24.6 ± 1.1 mV; n = 29). Although the midpoint of activation was almost identical for I848T and L858H channels, the threshold for activation appeared to be ∼5 mV more negative for L858H channels than for I848T channels.
Figure 1.
The I848T and L858H mutations of hNav1.7 alter activation and deactivation. A, Current traces recorded from representative HEK293 cells expressing either wild-type hNav1.7 or mutant channels, I848T or L858H. Cells were held at -100 mV, and currents were elicited with 50 msec test pulses to potentials ranging from -80 to 40 mV. B, Normalized peak current-voltage relationship for wild-type (filled squares; n = 29), I848T (open circles; n = 27), and L858H (open triangles; n = 27) channels. C, Representative tail currents of WT, I848T, and L858H channels. Cells were held at -100 mV and depolarized to -20 mV for 0.5 msec, followed by a repolarization to -50 mV to elicit tail currents. D, Time constants for tail current deactivation at repolarization potentials ranging from -40 to -100 mV for wild-type (filled squares; n = 7), I848T (open circles; n = 7), and L858H (open triangles; n = 7) hNav1.7 channels. Time constants were obtained with single exponential fits to the deactivation phase of the currents. Error bars represent SE.
The kinetics of deactivation, which reflects the transition from the open to the closed state, of WT and mutant channels was also examined by eliciting tail currents at a range of potentials after briefly activating the channels (at -20 mV for 0.5 msec). Altered deactivation of skeletal muscle sodium channels is thought to contribute to the pathophysiology of paramyotonia congenita (Featherstone et al., 1998). I848T and L858H currents exhibited slower kinetics of deactivation (Fig. 1C). The time constant of deactivation (measured with single exponential fits) was slower at potentials ranging from -100 mV to -40 mV for the mutant channels. Interestingly, the effect on deactivation was much greater with the L858H mutation than with the I848T mutation at all deactivation voltages tested. For example, the deactivation time constants for I848T and L858H channels at -50 mV were approximately threefold and ∼10-fold, respectively, larger than that of WT channels.
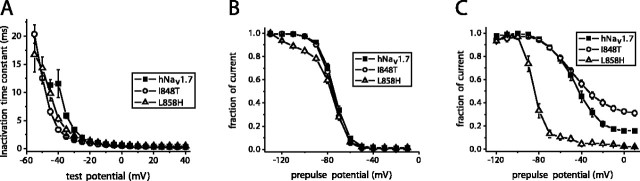
The fast-inactivation time constant, which provides a measure of the open-to-inactivated transition, was estimated using m3h Hodgkin and Huxley type fits to the current data. The time constants for fast inactivation between -40 and -20 mV were smaller for the mutant channels than for WT channels (Fig. 2A). However, the time constants of the three channels were similar at more depolarized potentials.
Figure 2.
The I848T and L858H mutations differentially alter inactivation of hNav1.7. A, Fast inactivation kinetics as a function of voltage for wild-type (filled squares; n = 8), I848T (open circles; n = 8), and L858H (open triangles; n = 8) hNav1.7 channels. Currents elicited as described in Figure 1 A were fit with Hodgkin-Huxley type m 3h model to estimate the inactivation time constants. B, Comparison of steady-state fast inactivation for wild-type (filled squares; n = 20), I848T (open circles; n = 19), and L858H (open triangles; n = 17) hNav1.7 channels. Currents were elicited with test pulses to 0 mV after 500 msec inactivating prepulses. C, Comparison of steady-state slow inactivation for wild-type hNav1.7 (filled squares; n = 9), I848T (open circles; n = 8), and L858H (open triangles; n = 9) hNav1.7 channels. Slow inactivation was induced with 30 sec prepulses, followed by 100 msec pulses to -120 mV to allow recovery from fast inactivation. A test pulse to 0 mV for 20 msec was used to determine the fraction of current available. Error bars represent SE.
In contrast to the dramatic differences in the voltage dependence of activation, the voltage dependence of steady-state fast inactivation was similar for WT, I848T, and L858H (Fig. 2B). The midpoint of fast inactivation (measured with 500 msec prepulses) was not significantly different for WT (-73.6 ± 1.1 mV; n = 20), I848T (-75.8 ± 1.1 mV; n = 19), and L858H (-76.1 ± 1.2 mV; n = 17) channels. The steady-state fast-inactivation curve for L858H channels deviated from that of the other channels at negative voltages (e.g., between -120 and -80 mV). This is likely attributable to differences in slow inactivation (see below).
Defective slow inactivation of skeletal muscle sodium channels has been proposed to play a role in hyperkalemic periodic paralysis, and therefore, we also examined the voltage dependence of steady-state slow inactivation of hNav1.7 currents. Thirty second prepulses, followed by 100 msec recovery pulses to -120 mV to allow recovery from fast inactivation, preceded the test pulse (to 0 mV for 20 msec) to determine the fraction of current available. Dramatic differences were observed for slow-inactivation properties of WT, I848T, and L858H currents (Fig. 2C). Surprisingly, the L858H mutation substantially enhanced slow inactivation of hNav1.7. This enhancement of slow inactivation is likely to account for the enhanced inactivation of L858H currents observed at negative potentials with the steady-state fast-inactivation protocol (Fig. 2B). In contrast, the I848T mutations impaired slow inactivation of hNav1.7 channels. At 0 mV, only 67 ± 6% of I848T channels (n = 8) are slow inactivated (determined from the fraction of channels available for activation as measured in Fig. 2C) compared with 84 ± 4% of WT channels (n = 8) and >97 ± 2% of L858H channels (n = 7). For both mutants, the percentage of channels that were slow inactivated at 0 mV was significantly different than for WT channels. Therefore, the two mutations identified in the Nav1.7 channels of patients with primary erythermalgia have differential effects on slow inactivation of hNav1.7 channels.
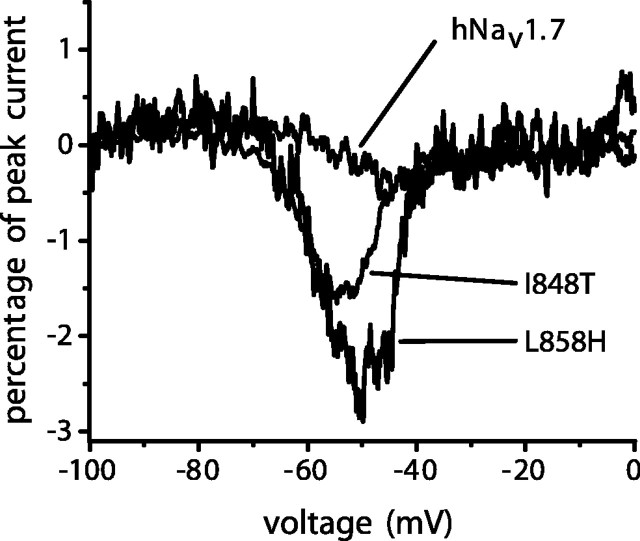
Finally, we examined the currents induced in hNav1.7 channels by slow ramp depolarizations. Significantly larger currents were elicited with slow ramp (0.2 mV/ms) depolarizations from -100 to +20 mV by either I848T or L858H channels compared with WT channels (Fig. 3). The ramp currents (expressed as a percentage of peak current) were 1.8 ± 0.2% for I848T channels (n = 19), 2.6 ± 0.3% for L858H channels (n = 16), and 0.6 ± 0.1% for WT channels (n = 16). The ramp currents in cells expressing I848T and L858H channels were pronounced between -70 and -40 mV (Fig. 3) and thus could contribute to subthreshold depolarizations and the initiation of action potentials.
Figure 3.
The I848T and L858H mutations enhance ramp currents of hNav1.7. Representative ramp currents elicited with 500 msec ramp depolarizations from -100 to 0 mV from HEK293 cells expressing wild-type, I848T, and L858H channels.
Discussion
Voltage-gated sodium channels mediate an increase in Na+ permeability during depolarization of membrane potential that underlies action potential electrogenesis. Although dysregulated sodium channel expression contributes to the pathophysiology of neuropathic pain (Matzner and Devor, 1994; Waxman et al., 2000), mutations of voltage-gated sodium channel α-subunits, which underlie a number of human and animal disorders (for review, see Goldin, 2001; Keating and Sanguinetti, 2001; Meisler et al., 2001; Cannon, 2002), were not associated with painful neuropathies until Yang et al. (2004) identified two mutations in SCN9A, the gene encoding Nav1.7, in patients with primary erythermalgia. In this study, we have characterized the functional consequences of these two hNav1.7 mutations, I848T and L858H.
The I848T and L858H mutations are located in the S4-S5 linker region of domain II (DIIS4-S5) of the channel. Biophysical analysis of the mutant channels revealed several differences compared with wild-type hNav1.7. Both mutations significantly shifted the voltage dependence of activation in the hyperpolarizing direction. Deactivation of hNav1.7 was also slowed by both mutations, although the effect was much larger with the L858H mutation. Both mutations also significantly increased the size of ramp currents produced by hNav1.7 channels in response to slow depolarizations between -70 and -40 mV, a range that probably encompasses resting potential of sensory neurons (Harper and Lawson, 1985; Caffrey et al., 1992). These changes in the functional properties of hNav1.7 are likely to contribute to increased excitability of spinal sensory neurons that express Nav1.7 and may underlie the abnormal pain sensations in patients with inherited erythermalgia.
The I848T and L858H mutations shifted the voltage dependence of activation of hNav1.7 by almost 15 mV in a hyperpolarizing direction. A shift of this magnitude is expected to decrease the threshold for action potential generation in sensory neurons and increase neuronal excitability. Both mutations also significantly slowed the rate of deactivation of hNav1.7. Impaired deactivation of skeletal muscle sodium channels (Nav1.4) has been hypothesized to contribute to abnormal muscle excitability (predominantly myotonia) in patients with paramyotonia congenita (Featherstone et al., 1998). Many of the Nav1.4 mutations that cause myotonia slow both the rate of fast inactivation and deactivation, and in computer simulations, this combination can induce a destabilization of repolarization after an action potential, leading to “myotonic runs” (Featherstone et al., 1998). Unlike these Nav1.4 mutations, the hNav1.7-I848T and -L858H did not slow the rate of fast inactivation, and therefore the hNav1.7 mutations might not destabilize repolarization.
The mutant hNav1.7 channels produced significantly larger currents in response to slow ramp depolarizations than wild-type channels. Increased overlap between the inactivation and activation curves, resulting from the large negative shift in the voltage dependence of activation (Fig. 1B) and unchanged voltage dependence of steady-state inactivation (Fig. 2B), may underlie the larger ramp currents. Impaired deactivation, which indicates that the open-to-closed transition is altered, is also likely to contribute to the increased ramp current amplitudes. At negative potentials (less than -45 mV), the closing rate is much larger than the inactivation rate, and therefore channel openings are more likely to be terminated by deactivation than by inactivation in this voltage range (Vandenberg and Bezanilla, 1991). Vandenberg and Bezanilla (1991) suggested that the deactivation rate of sodium channels limits the ability of sodium channels to open and reopen at negative potentials. Thus, slowing the deactivation rate would be expected to increase the number of openings and open times at negative potentials, and this could contribute to increased ramp current amplitudes. The fact that the L858H mutant exhibited the slowest deactivation kinetics and the largest ramp currents is consistent with this hypothesis. Because the ramp currents are evoked between -70 and -40 mV, close to the resting potential of DRG neurons (Harper and Lawson, 1985; Caffrey et al., 1992), the larger ramp currents in cells expressing mutant channels could amplify the response to small depolarizing inputs, increasing excitability. Nav1.7 channels are expressed at high levels in small, mostly nociceptive, sensory neurons (Djouhri et al., 2003a), suggesting that changes in activation, deactivation, and ramp current amplitude contribute to pain in erythermalgia patients with the I848T and L858H mutations.
The amino acid sequence of domain II S4-S5 linker in hNav1.7 and hNav1.4 are identical (Yang et al., 2004) and are highly conserved when the sequence of all known sodium channels are aligned (data not shown). The conservation of this sequence suggests that the DIIS4-S5 linker plays a similar role in different sodium channels. The I848T mutation in erythermalgia (Yang et al., 2004) corresponds to the I693T mutation in hNav1.4 of patients with episodic muscle weakness (Cannon, 2002). The T704M mutation in hNav1.4 causes hyperkalemic periodic paralysis, another muscle disorder characterized by episodic weakness (Cannon, 2002). The L858 in hNav1.7 corresponds to L703 in hNav1.4, which is immediately adjacent to the T704 residue. Thus, DIIS4-S5 mutations in both hNav1.7 and hNav1.4 are associated with hereditary neurological and muscle disorders, which suggests that this linker contributes to the determination of biophysical properties of sodium channels including voltage dependence of activation and deactivation.
The functional consequences of the I693T and T704M mutations on hNav1.4 have been characterized after expression in HEK293 cells (Plassart-Schiess et al., 1998; Hayward et al., 1999; Bendahhou et al., 2002) and are similar to the effects of the I848T mutation on hNav1.7. All of these mutations shift the voltage dependence of activation in a hyperpolarizing direction. The Nav1.4 mutations are thought to induce muscle weakness as a result of an enhanced persistent current (possibly attributable to window currents that result from the negative shift in the voltage dependence of activation) that depolarizes the muscle 5-10 mV, causing inactivation of the sodium currents and decreasing the ability of the muscle to fire action potentials (Lehmann-Horn et al., 1987; Cummins et al., 1993). The hNav1.4-I693T and hNav1.4-T704M mutations also significantly impair slow inactivation, and this impairment is thought to contribute to muscle weakness associated with these mutations (Ruff, 1994). However, whereas the I848T mutation impaired slow inactivation of hNav1.7, the L858H mutation of hNav1.7 enhanced slow inactivation. Because both mutations are associated with primary erythermalgia, this divergence might be interpreted as suggesting that slow inactivation is not important in its pathophysiology. Alternatively, there may be subtle differences in the symptoms of primary erythermalgia in patients with the I848T and L858H mutations attributable to the differential effect of the two mutations on slow inactivation.
Given that the I693T mutation in Nav1.4 is associated with muscle weakness, how can the I848T mutation in Nav1.7 be associated with increased pain sensations? One explanation may derive from the fact that whereas mature muscle cells express only Nav1.4 channels, spinal sensory neurons express multiple sodium channel isoforms with distinct properties (Black et al., 1996). The Nav1.8 channel, which is expressed at high levels in many (but not all) spinal sensory neurons, has a very depolarized voltage dependence of inactivation compared with other channels such as Nav1.4 and Nav1.7 (Akopian et al., 1996) and plays a critical role in action potential firing in sensory neurons (Renganathan et al., 2001). If the Nav1.7-I848T mutation depolarizes the sensory neurons by 5-10 mV, as the Nav1.4 mutations associated with muscle weakness are thought to do in muscle (Lehmann-Horn et al., 1987; Cannon, 2000), the Nav1.8 channels are likely to still be available for activation. Depolarizations of similar magnitudes might produce reduced action potential activity in muscle but could lead to enhanced excitability in neurons that also express Nav1.8 because these cells would be closer to threshold for activation of Nav1.8 currents. Thus, mutations of identical residues in Nav1.7 and Nav1.4 that have similar effects on channel properties could have differential effects on excitability and cause distinct neurological disorders because of the presence of multiple sodium channels (including Nav1.8 which is not present in muscle) in spinal sensory neurons.
Non-nociceptive neurons are less likely to express Nav1.8 channels than nociceptive neurons (Djouhri et al., 2003b), and thus mutant Nav1.7 channels could possibly differentially alter excitability in nociceptive and non-nociceptive neurons. Moreover, although Nav1.7 currents exhibit similar properties in HEK293 cells and DRG neurons (Cummins et al., 1998; Herzog et al., 2003), it is also possible that the mutant and wild-type Nav1.7 channels are differentially modulated in DRG neurons, and this might contribute to the disease phenotype. For example, our studies in HEK293 cells were done with coexpression of the β1 and β2 subunits, but DRG neurons also express other β subunits such as β3 (Shah et al., 2000) that could impact the current properties. Nav1.4 and Nav1.7 currents exhibit distinct properties (Cummins et al., 1998) and could be differentially modulated by β subunits, and this could also contribute to the different disease phenotypes.
Our results highlight the fact that analogous mutations in sodium channels can result in dramatically different phenotypes and suggest that this difference may be attributable to the ensemble of other sodium channels (or channel subunits) that are expressed together with the mutant channel within DRG neurons. The changes in the electrophysiological properties of mutant hNav1.7 provide the first example of altered sodium channel function in a hereditary pain syndrome and suggest that targeting of sodium channels may provide a useful therapeutic strategy for this disorder.
Footnotes
This work was supported in part by the Medical Research Service and Rehabilitation Research Service, Department of Veterans Affairs and by a grant from the National Multiple Sclerosis Society. The Center for Neuroscience and Regeneration Research is a collaboration of the Paralyzed Veterans of America and the United Spinal Association with Yale University. T.R.C. was supported by a Biomedical Research grant from Indiana University School of Medicine.
Correspondence should be addressed to Dr. Stephen G. Waxman, Department of Neurology, LCI 707, Yale University School of Medicine, 333 Cedar Street, New Haven, CT 06510. E-mail: stephen.waxman@yale.edu.
Copyright © 2004 Society for Neuroscience 0270-6474/04/248232-05$15.00/0
References
- Akopian AN, Sivilotti L, Wood JN (1996) A tetrodotoxin-resistant voltage-gated sodium channel expressed by sensory neurons. Nature 379: 257-262. [DOI] [PubMed] [Google Scholar]
- Bendahhou S, Cummins TR, Kula RW, Fu YH, Ptacek LJ (2002) Impairment of slow inactivation as a common mechanism for periodic paralysis in DIIS4-S5. Neurology 58: 1266-1272. [DOI] [PubMed] [Google Scholar]
- Black JA, Dib-Hajj S, McNabola K, Jeste S, Rizzo MA, Kocsis JD, Waxman SG (1996) Spinal sensory neurons express multiple sodium channel alpha-subunit mRNAs. Mol Brain Res 43: 117-131. [DOI] [PubMed] [Google Scholar]
- Black JA, Cummins TR, Plumpton C, Chen YH, Hormuzdiar W, Clare JJ, Waxman SG (1999) Upregulation of a silent sodium channel after peripheral, but not central, nerve injury in DRG neurons. J Neurophysiol 82: 2776-2785. [DOI] [PubMed] [Google Scholar]
- Black JA, Liu S, Tanaka M, Cummins TR, Waxman SG (2004) Changes in the expression of tetrodotoxin-sensitive sodium channels within dorsal root ganglia neurons in inflammatory pain. Pain 108: 237-247. [DOI] [PubMed] [Google Scholar]
- Caffrey JM, Eng DL, Black JA, Waxman SG, Kocsis JD (1992) Three types of sodium channels in adult rat dorsal root ganglion neurons. Brain Res 592: 283-297. [DOI] [PubMed] [Google Scholar]
- Cannon SC (2002) An expanding view for the molecular basis of familial periodic paralysis. Neuromuscul Disord 12: 533-543. [DOI] [PubMed] [Google Scholar]
- Cummins TR, Waxman SG (1997) Downregulation of tetrodotoxin-resistant sodium currents and upregulation of a rapidly repriming tetrodotoxin-sensitive sodium current in small spinal sensory neurons after nerve injury. J Neurosci 17: 3503-3514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummins TR, Zhou J, Sigworth FJ, Ukomadu C, Stephan M, Ptacek LJ, Agnew WS (1993) Functional consequences of a Na+ channel mutation causing hyperkalemic periodic paralysis. Neuron 10: 667-678. [DOI] [PubMed] [Google Scholar]
- Cummins TR, Howe JR, Waxman SG (1998) Slow closed-state inactivation: a novel mechanism underlying ramp currents in cells expressing the hNE/PN1 sodium channel. J Neurosci 18: 9607-9619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummins TR, Dib-Hajj SD, Black JA, Akopian AN, Wood JN, Waxman SG (1999) A novel persistent tetrodotoxin-resistant sodium current in SNS-null and wild-type small primary sensory neurons. J Neurosci 19: RC43(1-6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dib-Hajj SD, Tyrrell L, Black JA, Waxman SG (1998) NaN, a novel voltage-gated Na channel, is expressed preferentially in peripheral sensory neurons and down-regulated after axotomy. Proc Natl Acad Sci USA 95: 8963-8968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djouhri L, Newton R, Levinson SR, Berry CM, Carruthers B, Lawson SN (2003a) Sensory and electrophysiological properties of guinea-pig sensory neurones expressing Nav1.7 (PN1) Na+ channel alpha subunit protein. J Physiol (Lond) 546: 565-576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djouhri L, Fang X, Okuse K, Wood JN, Berry CM, Lawson SN (2003b) The TTX-resistant sodium channel Nav1.8 (SNS/PN3): expression and correlation with membrane properties in rat nociceptive primary afferent neurons. J Physiol (Lond) 550: 739-752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drenth JP, Finley WH, Breedveld GJ, Testers L, Michiels JJ, Guillet G, Taieb A, Kirby RL, Heutink P (2001) The primary erythermalgia-susceptibility gene is located on chromosome 2q31-32. Am J Hum Genet 68: 1277-1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Featherstone DE, Fujimoto E, Ruben PC (1998) A defect in skeletal muscle sodium channel deactivation exacerbates hyperexcitability in human paramyotonia congenita. J Physiol (Lond) 506: 627-638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldin A (2001) Resurgence of sodium channel research. Annu Rev Physiol 63: 871-894. [DOI] [PubMed] [Google Scholar]
- Hains BC, Saab CY, Klein JP, Craner MJ, Waxman SG (2004) Altered sodium channel expression in second-order spinal sensory neurons contributes to pain after peripheral nerve injury. J Neurosci 24: 4832-4839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper AA, Lawson SN (1985) Electrical properties of rat dorsal root ganglion neurones with different peripheral nerve conduction velocities. J Physiol (Lond) 359: 47-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward LJ, Sandoval GM, Cannon SC (1999) Defective slow inactivation of sodium channels contributes to familial periodic paralysis. Neurology 52: 1447-1453. [DOI] [PubMed] [Google Scholar]
- Herzog RI, Cummins TR, Ghassemi F, Dib-Hajj SD, Waxman SG (2003) Distinct repriming and closed-state inactivation kinetics of Nav1.6 and Nav1.7 sodium channels in mouse spinal sensory neurons. J Physiol (Lond) 551: 741-750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keating MT, Sanguinetti MC (2001) Molecular and cellular mechanisms of cardiac arrhythmias. Cell 104: 569-580. [DOI] [PubMed] [Google Scholar]
- Klugbauer N, Lacinova L, Flockerzi V, Hofmann F (1995) Structure and functional expression of a new member of the tetrodotoxin-sensitive voltage-activated sodium channel family from human neuroendocrine cells. EMBO J 14: 1084-1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Layzer RB (2001) Hot feet: erythromelalgia and related disorders. J Child Neurol 16: 199-202. [DOI] [PubMed] [Google Scholar]
- Lehmann-Horn F, Kuther G, Ricker K, Grafe P, Ballanyi K, Rudel R (1987) Adynamia episodica hereditaria with myotonia: a non-inactivating sodium current and the effect of extracellular pH. Muscle Nerve 10: 363-374. [DOI] [PubMed] [Google Scholar]
- Lossin C, Wang DW, Rhodes TH, Vanoye CG, George Jr AL (2002) Molecular basis of an inherited epilepsy. Neuron 34: 877-884. [DOI] [PubMed] [Google Scholar]
- Matzner O, Devor M (1994) Hyperexcitability at sites of nerve injury depends on voltage-sensitive Na+ channels. J Neurophysiol 72: 349-359. [DOI] [PubMed] [Google Scholar]
- Meisler MH, Kearney J, Ottman R, Escayg A (2001) Identification of epilepsy genes in human and mouse. Annu Rev Genet 35: 567-588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plassart-Schiess E, Lhuillier L, George Jr AL, Fontaine B, Tabti N (1998) Functional expression of Ile693Thr Na+ channel mutation associated with paramyotonia congenita in a human cell line. J Physiol (Lond) 507: 721-727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renganathan M, Cummins TR, Waxman SG (2001) Contribution of Nav1.8 sodium channels to action potential electrogenesis in DRG neurons. J Neurophysiol 86: 629-640. [DOI] [PubMed] [Google Scholar]
- Ruff RL (1994) Slow Na+ channel inactivation must be disrupted to evoke prolonged depolarization-induced paralysis. Biophys J 66: 542-545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangameswaran L, Delgado SG, Fish LM, Koch BD, Jakeman LB, Stewart GR, Sze P, Hunter JC, Eglen RM, Herman RC (1996) Structure and function of a novel voltage-gated, tetrodoxtoxin-resistant sodium channel specific to sensory neurons. J Biol Chem 271: 5953-5956. [DOI] [PubMed] [Google Scholar]
- Sangameswaran L, Fish LM, Koch BD, Rabert DK, Delgado SG, Ilnicka M, Jakeman LB, Novakovic S, Wong K, Sze P, Tzoumaka E, Stewart GR, Herman RC, Chan H, Eglen RM, Hunter JC (1997) A novel tetrodotoxin-sensitive, voltage-gated sodium channel expressed in rat and human dorsal root ganglia. J Biol Chem 272: 14805-14809. [DOI] [PubMed] [Google Scholar]
- Shah BS, Stevens EB, Gonzalez MI, Bramwell S, Pinnock RD, Lee K, Dixon AK (2000) Beta3, a novel auxiliary subunit for the voltage-gated sodium channel, is expressed preferentially in sensory neurons and is upregulated in the chronic constriction injury model of neuropathic pain. Eur J Neurosci 12: 3985-3990. [DOI] [PubMed] [Google Scholar]
- Tate S, Benn S, Hick C, Trezise D, John V, Mannion RJ, Costigan M, Plumpton C, Grose D, Gladwell Z, Kendall G, Dale K, Bountra C, Woolf CJ (1998) Two sodium channels contribute to the TTX-R sodium current in primary sensory neurons. Nat Neurosci 1: 653-655. [DOI] [PubMed] [Google Scholar]
- Toledo-Aral JJ, Moss BL, He ZJ, Koszowski AG, Whisenand T, Levinson SR, Wolf JJ, Silossantiago I, Halegoua S, Mandel G (1997) Identification of PN1, a predominant voltage-dependent sodium channel expressed principally in peripheral neurons. Proc Natl Acad Sci USA 94: 1527-1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberg CA, Bezanilla F (1991) A sodium channel gating model based on single channel, macroscopic ionic, and gating currents in the squid giant axon. Biophys J 60: 1511-1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Genderen PJ, Michiels JJ, Drenth JP (1993) Hereditary erythermalgia and acquired erythromelalgia. Am J Med Genet 45: 530-532. [DOI] [PubMed] [Google Scholar]
- Waxman SG, Dib-Hajj S, Cummins TR, Black JA (2000) Sodium channels and their genes: dynamic expression in the normal nervous system, dysregulation in disease states. Brain Res 886: 5-14. [DOI] [PubMed] [Google Scholar]
- Yang Y, Wang Y, Li S, Xu Z, Li H, Ma L, Fan J, Bu D, Liu B, Fan Z, Wu G, Jin J, Ding B, Zhu X, Shen Y (2004) Mutations in SCN9A, encoding a sodium channel alpha subunit, in patients with primary erythermalgia. J Med Genet 41: 171-174. [DOI] [PMC free article] [PubMed] [Google Scholar]