Abstract
Mechanisms of synaptic plasticity in CNS circuits are commonly investigated using in vitro preparations such as brain slices or slice culture. During their preparation, slices are exposed to low temperatures, and electrophysiological measurements are sometimes made below physiological temperature. Because dendritic spines, which occur at the majority of excitatory synapses, are morphologically plastic, we investigated the influence of reduced temperature on their morphology and plasticity using live cell imaging of hippocampal slices from transgenic mice expressing a green fluorescent protein-based neuronal surface marker and electron microscopy of adult brain slices. Our data show that dendritic spines are highly sensitive to reduced temperature with rapid loss of actin-based motility followed at longer times by reversible loss of the entire spine structure. Thus, reduced temperature significantly affects synaptic morphology, which is in turn known to influence several key aspects of synaptic transmission. Evidence that hypothermia potentiates anesthesia and is associated with spine loss in hibernating animals further suggests that spine morphology may have a widespread influence on brain function.
Keywords: synaptic plasticity, cytoskeleton, hippocampus, green fluorescent protein, hibernation, anesthesia, time-lapse microscopy
Introduction
Several key aspects of synaptic transmission at excitatory synapses, including the distribution of postsynaptic glutamate receptors, postsynaptic ion fluxes, and the spread of presynaptically released neurotransmitter, are strongly influenced by the shapes of dendritic spines (Barbour and Häusser, 1997; Kullmann and Asztely, 1998; Nusser et al., 1998; Takumi et al., 1999; Majewska et al., 2000; Euler and Denk, 2001). Together with recent evidence indicating that spines are motile structures with a shape that can alter within seconds or minutes (Fischer et al., 1998; Dunaevsky et al., 2001; Trachtenberg et al., 2002; Roelandse et al., 2003), this suggests that changes in spine shape might alter synaptic transmission properties. Many studies of synaptic physiology and plasticity at excitatory synapses have been performed using in vitro brain slices prepared under conditions that involve cooling tissue to temperatures as low as 2°C with electrophysiological measurements made at reduced temperatures. However, studies on cellular dynamics have shown that actin-dependent motility, resulting in significant changes in cell morphology, is strongly attenuated at reduced temperatures, with a Q10 of ∼4 ranging from 29 to 39°C (Hartmann-Petersen et al., 2000). Similar values have been recorded for epithelial cells from various teleosts (Ream et al., 2003).
To investigate the possibility that reduced temperature may influence the motility and morphology of dendritic spines, we took advantage of transgenic mice expressing a green fluorescent protein (GFP)-linked neuronal surface marker that enables high resolution imaging of the dendrite surface in brain slice cultures (Roelandse et al., 2003). Our results show that decreased temperature has profound influence on dendritic spine morphology leading ultimately to their disappearance. These effects, which are reversible, suggest that studies of synaptic physiology performed at reduced temperatures may not accurately reflect events occurring in the brain.
Materials and Methods
Organotypic hippocampal slice cultures were prepared from postnatal day 8 (P8) transgenic animals expressing a plasma membrane targeted form of GFP (GFPtKras) that allows the surface structure of dendrites to be visualized in living cells (Roelandse et al., 2003). For confocal imaging, slices were mounted in purpose-built chambers (Life Imaging Services, Olten Switzerland) and observed under continuous perfusion with artificial CSF (ACSF; (in mm) 124 NaCl, 2.5 KCl, 2.0 MgSO4, 1.25 KH2PO4, 26 NaHCO3, 10 glucose, 4 sucrose, and 2.5 CaCl2 saturated with 95% O2/5% CO2) using a Yokogawa microlens Nipkow confocal system (PerkinElmer Life Sciences, Cambridge, UK). Images were acquired using a cooled CCD camera (PCO Computer Optics, Kelheim, Germany) and analyzed with MetaMorph software (Universal Imaging, West Chester, PA).
For analysis of adult brain slices, hippocampi from mature wild-type mice (n = 3; all 5 weeks of age) were dissected in ice-cold oxygenated buffer (in mm: 234 NaHCO3, 1 NaH2PO4, 8 MgSO4, and 10 glucose, pH 7.4) and sliced at 400 μm using a McIlwain tissue chopper (Mickle Engineering, Gomshall, UK). Slices were incubated on 0.4 μm porous culture plate inserts (Millipore, Bedford, MA) for 60 min at room temperature in ACSF (in mm: 117 NaCl, 5.3 KCl, 26 NaHCO3, 1 NaH2PO4, 1.5 MgSO4, 2.5 CaCl2, and 10 glucose, pH 7.4, equilibrated with 95% O2/5% CO2) with Ca2+ removed to avoid excitotoxic damage (Feig and Lipton, 1990). Subsequently, incubation was continued at either 37 or 4°C for 9 hr in ACSF saturated with 95% O2/5% CO2 after which slices were prepared for DiO labeling or electron microscopy by microwave-assisted fixation (Jensen and Harris, 1989). Briefly, slices were transferred into prewarmed fixative [2% formaldehyde, 6% glutaraldehyde, and 2 mm CaCl2 in 0.1 m cacodylate buffer (CCB), pH 7.3] and irradiated at maximum power (1000 W) for 9 sec in a Bio-Rad (Hercules, CA) H2500 microwave processor. The postirradiation temperature, measured using a built-in temperature probe situated in the fixation solution, was 35-50°C.
For DiO labeling, slices were embedded in 3% agarose, cut transversely at 400 μm using a Leica VT1000 second microtome (Leica, Glattbrugg, Switzerland), and shot with DiO-coated tungsten particles (0.7 μm diameter) using a Gene Gun (Bio-Rad) as described previously (Gan et al., 2000). For electron microscopy analysis, fixed slices were washed in 0.1 m CCB, postfixed with 1% osmium tetroxide and 1.5% potassium ferro-cyanide in 0.1 m CCB, pH 7.3, and stained with 4% ethanolic uranyl acetate. Serial ultra-thin sections of area CA1 were stained for 30 min with 6% aqueous uranyl acetate and then for 15 min with 0.4% lead citrate in 0.04 m NaOH.
Quantification of synaptic contacts in electron microscopy samples was performed by counting markers of synaptic structure (presynaptic clusters of synaptic vesicles in axons; postsynaptic densities in dendrites) in randomly selected images of area CA1 in slices kept at 4 or 37°C in each case covering 120 μm2. For slices maintained at 37°C, presynaptic and postsynaptic marker numbers were equal so that numbers in Figure 3 are given as synapses. For slices kept at 4°C, the frequency of presynaptic and postsynaptic markers differed (see Results), so vesicle clusters and postsynaptic densities (PSDs) were counted separately.
Figure 3.
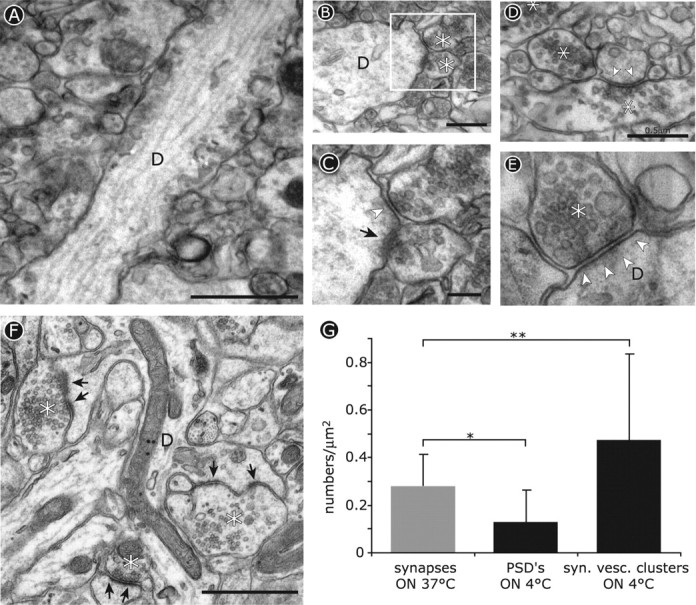
Ultrastructural changes induced by cooled tissue slices to 4°C. A-E, Electron micrographs of dendrites in acutely cut brain slices from adult mice after incubation overnight at 4°C. The asterisks indicate presynaptic boutons, and arrows indicate the postsynaptic density. A, Longitudinal section of a dendrite (D) in area CA1 lacking dendritic spines on its surface. Scale bar, 1 μm. B, Synapses made directly onto the shafts of dendrites after cooling. Scale bar, 0.5 μm. C, Detail of the boxed area in B showing decreased staining of the postsynaptic density (arrowhead) at one synapse compared with its neighbor (arrow). Scale bar, 0.2 μm. D, Presynaptic specializations are maintained after cooling, whereas synaptic vesicle clusters are present at sites lacking a clear postsynaptic density (arrowheads). Scale bar, 0.5 μm. E, Synapses with marked presynaptic terminals and synaptic junctions often lack a clearly stained postsynaptic density after cooling. Scale bar, 0.2 μm. F, Ultrastructure of a control slice from the same tissue block maintained at 37°C overnight. The asterisks indicate presynaptic boutons, and the arrows indicate PSDs. Scale bar, 1 μm. G, Quantification of synaptic contact numbers in 37°C slices (gray bar) compared with indicators of synaptic structure (postsynaptic densities and synaptic vesicle clusters) in slices incubated at 4°C. *p < 0.025; **p < 0.05.
Results
We examined the influence of reduced temperature on dendritic spines using hippocampal slices from transgenic mice expressing a membrane-targeted GFP construct (GFPtKras), which enables the fine structure of dendrites to be recorded in living slices of brain tissue (Roelandse et al., 2003). Observations were made using both acutely cut slices from adult animals and cultured hippocampal slices maintained in vitro for >4 weeks when mature dendritic spines have been established (Frotscher et al., 1988; Roelandse et al., 2003).
Cultured slices maintained at 37°C show stable dendritic spines of mature morphology, which retain actin-based motility at the tips of spine heads (Roelandse et al., 2003). When the temperature was reduced to 24°C, spines initially retained the same motility as at 37°C (Fig. 1A, 0), but subsequently, motility gradually decreased (data not shown) and by 3 hr had stopped completely (Fig. 1A, 190). Cooling slices to still lower temperatures showed even more severe effects so that a brief exposure of cultures to 4°C for 30 min was sufficient to freeze spine motility entirely (Fig. 1B).
Figure 1.
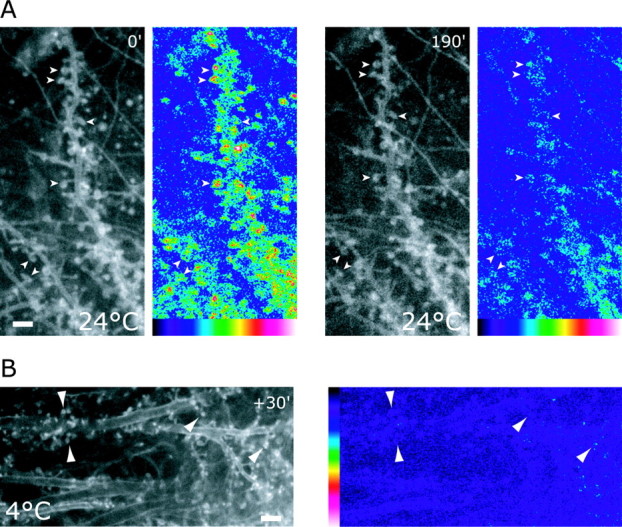
Culturing mature brain slices at room temperature (24°C) and at 4°C “freezes” spines. A, Shown are single images taken from two time series of two dendrites in a mature hippocampal slice culture taken at the beginning (0) of a cooling period to 24°C and 190 min later (190). To the right, the corresponding motility images of time series are shown with areas of high motility in red and white and background motility in blue and black. Images were acquired every 15 sec. Initially, when the cultures were kept at 24°C, spines were still motile (left; 0 min); however, over time, spine motility disappeared (right; 190). Scale bar, 2 μm. B, Brief exposure (30 min) of mature slice culture to 4°C stops spine motility. Dendrites were imaged at 24°C, and the corresponding motility image is shown at the right. Note the small spines. Scale bar, 3 μm.
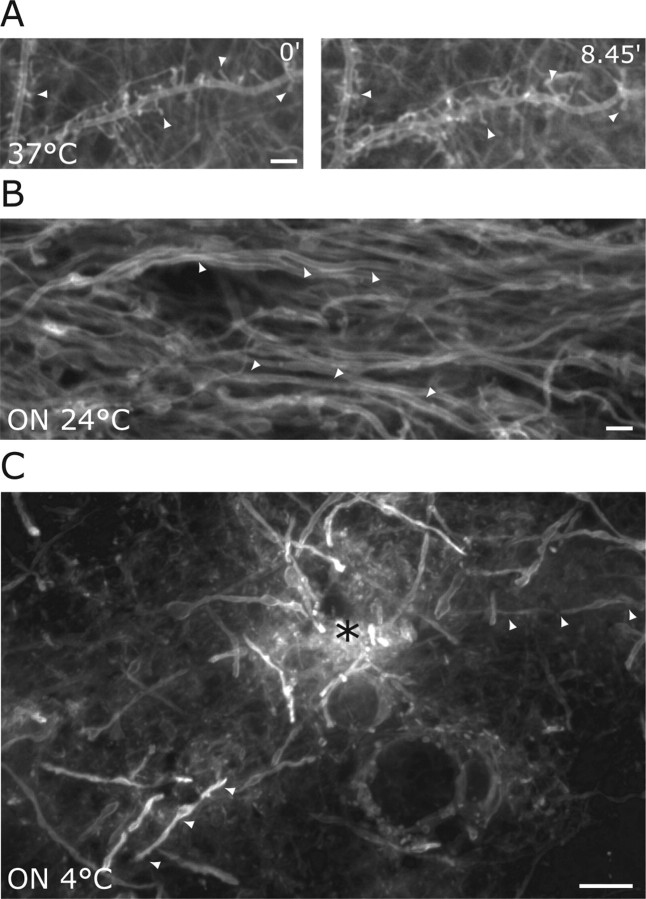
Dendrite structure in cultured slices maintained at 37°C remained stable throughout continuous recordings of >8 hr with no noticeable decline in motility or basic spine morphology (Fig. 2A, 0, 8.45). In contrast, when incubation at 24°C was continued overnight, dendrites showed striking structural regression so that the majority of spines had disappeared (Fig. 2B). To check that these effects were not limited to slices in culture or were an artifact of expressing the GFPtKras transgene, we examined acute cut slices from adult hippocampus in which dendrite structure was visualized using a gene gun to deliver the membrane marker DiO (DiOlistic labeling) (Gan et al., 2000). Incubating these adult slices overnight at 4°C resulted in the complete disappearance of dendritic spines (Fig. 2C).
Figure 2.
Prolonged cooling of mature brain slices leads to the disappearance of dendritic spines. A, Dendritic spines (arrowheads) in mature organotypic cultures at physiological temperatures remain present throughout continuous recordings up to 8 hr. Two images show a dendrite in a control culture maintained at 37°C at time 0 and 8 hr later (8.45). Scale bar, 3 μm. B, Several dendrites (arrowheads) in a mature slice culture that had been incubated overnight at 24°C lack the presence of the majority of spines on its surface. Scale bar, 5 μm. C, Acutely cut slices from the brains of adult mice were kept overnight at 4°C, fixed, and single neurons were labeled with a lipophilic dye (DiI). The asterisk indicates the approximate site of DiI entry in the slice. Dendrites lost their spines after the prolonged cooling to 4C° (arrowheads). Scale bar, 5 μm.
We also performed electron microscopy on adult brain slices that had been maintained overnight at either 4 or 37°C (Fig. 3). This confirmed that dendrites in tissue maintained at reduced temperature were indeed devoid of spines (Fig. 3A). Despite absence of spines, synapses were still present in slices maintained at 4°C with presynaptic terminals making synaptic contacts directly onto dendrite shafts (Fig. 3B-E). In addition to the absence of spines, synaptic contacts in cooled slices showed decreased staining of the postsynaptic density at sites where the presence of axon terminals containing vesicle clusters marked them clearly as synaptic contacts (Fig. 3B-E, arrowheads). We also noted an increase in the number of synaptic vesicle clusters along the axonal shaft (Fig. 3D). In contrast, control slices made from the same tissue samples but maintained overnight at 37°C showed the typical profusion of dendritic spines receiving synaptic contacts at regular intervals along the dendritic shaft (Fig. 3F). To quantify these observations, we counted PSDs and synaptic vesicle clusters as markers, respectively, of presynaptic and postsynaptic structures in warm and cold treated slices (Fig. 3G). For slices maintained at 37°C, both presynaptic and postsynaptic markers were present at all synaptic contacts so these were scored as synapses (Fig. 3G, gray bar). In contrast, counts of PSDs and vesicle clusters in slices maintained at 4°C confirmed that after cooling, postsynaptic density staining decreased, whereas synaptic vesicle cluster frequency increased (Fig. 3G, black bars).
It was important to ensure that the disappearance of dendritic spines after prolonged cooling did not involve permanent damage to hippocampal neurons. To test this possibility, cultured slices that had been maintained overnight at 24°C were examined during and 24 hr after recovery at physiological temperature. Rewarming cooled cultures to 37°C resulted in immediate and rapid outgrowth of highly motile protrusions from the surface of dendrites (Fig. 4A-C) that resembled filopodia-like spine precursors on developing dendrites (Dailey and Smith, 1996; Roelandse et al., 2003). Quantifying the number of spines per 100 pixels of dendrite length in single planes from confocal images showed that the number of protrusions classified as spines dropped to approximately one-third of the control value after incubation overnight at 24°C (Fig. 4D) (p < 0.05; n = 73 dendrites at 37°C, 100 dendrites at 24°C). Spine recovery was remarkably effective so that spine density had returned to control levels 24 hr after rewarming to 37°C (Fig. 4D) (p < 0.05; n = 23 dendrites).
Figure 4.
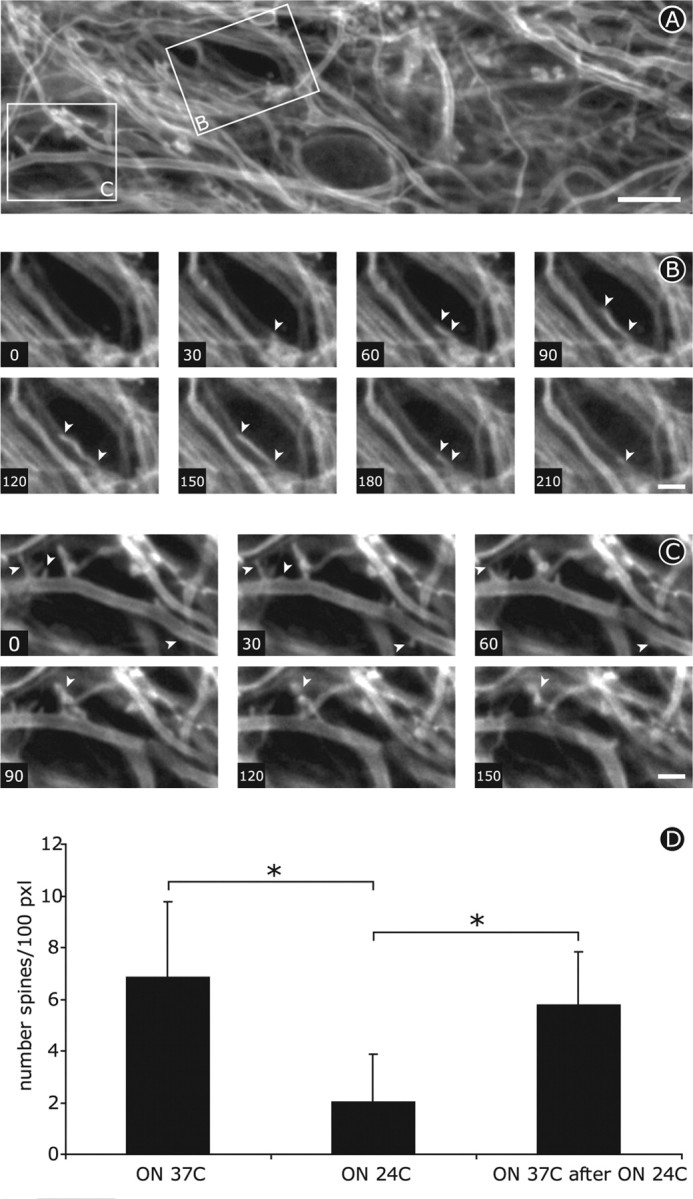
Subsequent rewarming of cooled slices to 37°C induces motile protrusions (arrowheads) to re-emerge from dendrite surface. A, First frame from time lapse of several dendrites in a culture that were maintained overnight at 24°C and subsequently at the beginning of rewarming to 37°C. The boxed areas are shown in B and C. Scale bar, 10 μm. B, C, Individual frames 30 sec apart taken from the same time lapse as shown in A. Subsequent rewarming of cooled slices to 37°C induces motile protrusions to rapidly extend and retract from dendrite surface. Scale bar: B, C, 5 μm. D, Quantification of the number of spines per 100 pixels dendrite length. Overnight cooling results in a significant but reversible reduction in the number of spines.
Discussion
Our observations show that reduced temperature leads to a slowing and subsequent cessation of dendritic spine motility normally observed at physiological temperature. Prolonged incubation of slices at 24°C resulted in the disappearance of spines after prolonged incubation at 24°C, an effect that could occur within hours at 4°C. Because these effects were reversible when the temperature was returned to 37°C, cooling even for periods of hours does not appear to induce permanent damage to hippocampal neurons.
An obvious question arising from these results is whether these dramatic changes in synaptic morphology influence synaptic function. At least in the case of neurotransmitter cross-talk between synapses, existing evidence suggests that they do. A study of temperature effects on spillover of extrasynaptic glutamate showed that this effect is reduced at physiological temperature (33-36°C) compared with room temperature (21-23°C) (Asztely et al., 1997). These results were interpreted as showing that the extent of spillover depends on temperature-dependent changes in the path length of glutamate diffusion out of the synaptic cleft, a parameter generally considered to be determinative for neurotransmitter cross-talk (Clements, 1996; Isaacson, 2000). Indeed, it has been suggested that the deep invagination of the mammalian rod synapse is an adaptation to prevent spillover to neighboring rods (Rao-Mirotznik et al., 1998). Such an arrangement would limit spillover, thus enhancing neurotransmitter reuptake via glutamate transporters. This interpretation is consistent with the observation that spillover is reduced when slices are warmed from room temperature to 37°C (Asztely et al., 1997), although temperature-dependent effects on the reuptake mechanism itself may also play a role. Live cell imaging of the surface structure of dendrites and correlated electron microscopy have shown that, rather than the conventional image of synaptic contacts where the presynaptic and postsynaptic components are juxtaposed to one another at up to 50% of spine synapses, the spine encloses the presynaptic terminal to some degree (Roelandse et al., 2003). The regression of spine structure at reduced temperatures observed in our present experiments would reduce the extent of this enclosure and is thus consistent with the evidence that spillover increases at reduced temperature (Asztely et al., 1997).
An additional possible link between reduced temperature and synaptic morphology comes from the study of hibernating animals where brain temperature falls as low as 5°C (Hut et al., 2002) and is correlated with decreased synaptic transmission (Derij and Shtark, 1985; Krilowicz et al., 1989; Igelmund and Heinemann, 1995). Previous studies have indicated that this extreme hypothermia is associated with reversible loss of dendritic spines (Boycott, 1982; Popov et al., 1992), a result that is consistent with our finding that reduced temperature can lead to reversible loss of dendritic spines when tissue from mouse brain is cooled to room temperature. Together, these observations underline the high degree of plasticity retained by neuronal connections in the mature CNS.
Another link between synaptic motility and synaptic function is suggested by previous work in our laboratory showing that dendritic spine motility is blocked by volatile anesthetics at clinically relevant concentrations (Kaech et al., 1999). Cooling of the brain to 20°C in animals and humans, as commonly performed during surgical procedures, is known to produce effective anesthesia independently of chemical anesthetics (Antognini, 1993; Liu et al., 2001). Together with these previous observations, our present data support suggestions that actin-based mechanisms in dendritic spines may have widespread effects on brain function (Crick, 1982; Matus, 2000).
In both hibernating animals (Boycott, 1982; Popov et al., 1992) and the in vitro brain slices studied here, the most dramatic effects of reduced temperature were on dendritic spines. In our experiments, we also observed reduced staining of postsynaptic densities, whereas an increase in the numbers of synaptic vesicle clusters per unit area may indicate a partial loss of synaptic contacts. In contrast, the structure of the underlying dendrite shaft was essentially unaffected. A possible explanation for this striking difference in cold sensitivity lies in the contrast in cytoskeletal structure between the dendritic spine, which is based on filamentous actin, and the dendrite shaft where microtubules predominate (Matus et al., 1982; Halpain, 2000; Kaech et al., 2001). The high content of dynamic actin filaments in dendritic spines not only drives their high motility (Fischer et al., 1998) but also renders them sensitive to the low temperature, which inhibits actin filament dynamics resulting in reduced cell motility (Pollard, 1976; Hartmann-Petersen et al., 2000; Ream et al., 2003).
As well as supporting the idea that dendritic spine motility may influence synaptic function in the adult brain, our observations suggest that the results of physiological studies performed on in vitro brain slices at room temperature, especially after exposure to ice temperatures during preparation, are likely to significantly influence aspects of synaptic function, such as the number and distribution of postsynaptic receptor expression and neurotransmitter spillover that are known to be sensitive to changes in spine morphology.
Footnotes
We thank Fr. Andreas Luethi for critical reading of this manuscript.
Correspondence should be addressed to Dr. A. Matus, Friedrich Miescher Institute, P.O. Box 2543, 4002 Basel, Switzerland. E-mail: aim@fmi.ch.
Copyright © 2004 Society for Neuroscience 0270-6474/04/247843-05$15.00/0
References
- Antognini JF (1993) Hypothermia eliminates isoflurane requirements at 20° C. Anesthesiology 78: 1152-1156. [DOI] [PubMed] [Google Scholar]
- Asztely F, Erdemli G, Kullmann DM (1997) Extrasynaptic glutamate spillover in the hippocampus: dependence on temperature and the role of active glutamate uptake. Neuron 18: 281-293. [DOI] [PubMed] [Google Scholar]
- Barbour B, Häusser M (1997) Intersynaptic diffusion of neurotransmitter. Trends Neurosci 20: 377-384. [DOI] [PubMed] [Google Scholar]
- Boycott B (1982) Some further comments concerning dendritic spines. Trends Neurosci 5: 328-329. [Google Scholar]
- Clements JD (1996) Transmitter timecourse in the synaptic cleft: its role in central synaptic function. Trends Neurosci 19: 163-171. [DOI] [PubMed] [Google Scholar]
- Crick F (1982) Do dendritic spines twitch? Trends Neurosci 5: 44-46. [Google Scholar]
- Dailey ME, Smith SJ (1996) The dynamics of dendritic structure in developing hippocampal slices. J Neurosci 16: 2983-2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derij LV, Shtark MB (1985) Hibernators' brain: protein synthesis in the neocortex and the hippocampus. Comp Biochem Physiol B 80: 927-934. [DOI] [PubMed] [Google Scholar]
- Dunaevsky A, Blazeski R, Yuste R, Mason C (2001) Spine motility with synaptic contact. Nat Neurosci 4: 685-686. [DOI] [PubMed] [Google Scholar]
- Euler T, Denk W (2001) Dendritic processing. Curr Opin Neurobiol 11: 415-422. [DOI] [PubMed] [Google Scholar]
- Feig S, Lipton P (1990) N-methyl-d-aspartate receptor activation and Ca2+ account for poor pyramidal cell structure in hippocampal slices. J Neurochem 55: 473-483. [DOI] [PubMed] [Google Scholar]
- Fischer M, Kaech S, Knutti D, Matus A (1998) Rapid actin-based plasticity in dendritic spines. Neuron 20: 847-854. [DOI] [PubMed] [Google Scholar]
- Frotscher M, Kraft J, Zorn U (1988) Fine structure of identified neurons in the primate hippocampus: a combined Golgi/EM study in the baboon. J Comp Neurol 275: 254-270. [DOI] [PubMed] [Google Scholar]
- Gan WB, Grutzendler J, Wong WT, Wong RO, Lichtman JW (2000) Multicolor “DiOlistic” labeling of the nervous system using lipophilic dye combinations. Neuron 27: 219-225. [DOI] [PubMed] [Google Scholar]
- Halpain S (2000) Actin and the agile spine: how and why do dendritic spines dance? Trends Neurosci 23: 141-146. [DOI] [PubMed] [Google Scholar]
- Hartmann-Petersen R, Walmod PS, Berezin A, Berezin V, Bock E (2000) Individual cell motility studied by time-lapse video recording: influence of experimental conditions. Cytometry 40: 260-270. [DOI] [PubMed] [Google Scholar]
- Hut RA, Barnes BM, Daan S (2002) Body temperature patterns before, during, and after semi-natural hibernation in the Eur ground squirrel. J Comp Physiol [B] 172: 47-58. [DOI] [PubMed] [Google Scholar]
- Igelmund P, Heinemann U (1995) Synaptic transmission and paired-pulse behaviour of CA1 pyramidal cells in hippocampal slices from a hibernator at low temperature: importance of ionic environment. Brain Res 689: 9-20. [DOI] [PubMed] [Google Scholar]
- Isaacson JS (2000) Spillover in the spotlight. Curr Biol 10: R475-R477. [DOI] [PubMed] [Google Scholar]
- Jensen FE, Harris KM (1989) Preservation of neuronal ultrastructure in hippocampal slices using rapid microwave-enhanced fixation. J Neurosci Methods 29: 217-230. [DOI] [PubMed] [Google Scholar]
- Kaech S, Brinkhaus H, Matus A (1999) Volatile anesthetics block actin-based motility in dendritic spines. Proc Natl Acad Sci USA 96: 10433-10437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaech S, Parmar H, Roelandse M, Bornmann C, Matus A (2001) Cytoskeletal microdifferentiation: a mechanism for organizing morphological plasticity in dendrites. Proc Natl Acad Sci USA 98: 7086-7092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krilowicz BL, Edgar DM, Heller HC (1989) Action potential duration increases as body temperature decreases during hibernation. Brain Res 498: 73-80. [DOI] [PubMed] [Google Scholar]
- Kullmann DM, Asztely F (1998) Extrasynaptic glutamate spillover in the hippocampus: evidence and implications. Trends Neurosci 21: 8-14. [DOI] [PubMed] [Google Scholar]
- Liu M, Hu X, Liu J (2001) The effect of hypothermia on isoflurane MAC in children. Anesthesiology 94: 429-432. [DOI] [PubMed] [Google Scholar]
- Majewska A, Tashiro A, Yuste R (2000) Regulation of spine calcium dynamics by rapid spine motility. J Neurosci 20: 8262-8268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matus A (2000) Actin-based plasticity in dendritic spines. Science 290: 754-758. [DOI] [PubMed] [Google Scholar]
- Matus A, Ackermann M, Pehling G, Byers HR, Fujiwara K (1982) High actin concentrations in brain dendritic spines and postsynaptic densities. Proc Natl Acad Sci USA 79: 7590-7594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusser Z, Lujan R, Laube G, Roberts JD, Molnar E, Somogyi P (1998) Cell type and pathway dependence of synaptic AMPA receptor number and variability in the hippocampus. Neuron 21: 545-559. [DOI] [PubMed] [Google Scholar]
- Pollard TD (1976) The role of actin in the temperature-dependent gelation and contraction of extracts of Acanthamoeba. J Cell Biol 68: 579-601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popov VI, Bocharova LS, Bragin AG (1992) Repeated changes of dendritic morphology in the hippocampus of ground squirrels in the course of hibernation. Neuroscience 48: 45-51. [DOI] [PubMed] [Google Scholar]
- Rao-Mirotznik R, Buchsbaum G, Sterling P (1998) Transmitter concentration at a three-dimensional synapse. J Neurophysiol 80: 3163-3172. [DOI] [PubMed] [Google Scholar]
- Ream RA, Theriot JA, Somero GN (2003) Influences of thermal acclimation and acute temperature change on the motility of epithelial wound-healing cells (keratocytes) of tropical, temperate and Antarctic fish. J Exp Biol 206: 4539-4551. [DOI] [PubMed] [Google Scholar]
- Roelandse M, Welman A, Wagner U, Hagmann J, Matus A (2003) Focal motility determines the geometry of dendritic spines. Neuroscience 121: 39-49. [DOI] [PubMed] [Google Scholar]
- Takumi Y, Ramirez-Leon V, Laake P, Rinvik E, Ottersen OP (1999) Different modes of expression of AMPA and NMDA receptors in hippocampal synapses. Nat Neurosci 2: 618-624. [DOI] [PubMed] [Google Scholar]
- Trachtenberg JT, Chen BE, Knott GW, Feng G, Sanes JR, Welker E, Svoboda K (2002) Long-term in vivo imaging of experience-dependent synaptic plasticity in adult cortex. Nature 420: 788-794. [DOI] [PubMed] [Google Scholar]