Abstract
The olfactory bulb plays a critical role in odor discrimination and in processing olfactory cues controlling social behavior in mammals. Given that the pituitary adenylate cyclase-activating polypeptide (PACAP) type 1 receptor (PAC1) is highly expressed in the olfactory bulb, we examined its role in regulating olfaction and social investigation. We found that olfactory detection of nonsocial stimuli was similar in PAC1-deficient mice and wild-type (WT) littermates. In contrast, PAC1-deficient mice displayed markedly abnormal social behaviors. PAC1-deficient mice exhibited a faster decrease in social investigation after repeated exposure to social cues or ovariectomized female urine compared with WT mice. Moreover, PAC1-deficient females exhibited delayed affiliative behavior when housed with novel males, and PAC1-deficient males displayed excessive sexual mounting toward both females and males as well as reduced aggression and increased licking and grooming toward intruder males. In aggregate, these results uncover PAC1 signaling as an important factor in the development and/or functioning of neural pathways associated with pheromone processing and the regulation of social interactions in mice. In turn, these studies raise the potential clinical relevance of PACAP signaling dysfunctions in neuropsychiatric disorders characterized by social reciprocity impairments such as autism spectrum disorders.
Keywords: behavior, peptide, olfaction, sex, receptor, memory
Introduction
In rodents, the main olfactory epithelium and the vomeronasal organ (VNO), through their respective projections to the main olfactory bulb (MOB) and accessory olfactory bulb (AOB), are involved in recognizing volatile and nonvolatile odorants, which in turn regulate a variety of behaviors, including social interactions (Powers and Winans, 1975; Wysocki et al., 1980; Halpern, 1987; Del Punta et al., 2002; Stowers et al., 2002). Although these distinct olfactory systems are known to regulate multiple complex social and sexual behaviors, underlying neurochemical mechanisms remain poorly understood. These CNS regions are particularly difficult to manipulate because of their location or small size. However, using knock-out and transgenic approaches, recent studies suggest that specific central neuropeptidergic pathways play critical roles in olfactory discrimination and social behavior, including corticotropin releasing factor (Heinrichs et al., 1997), oxytocin (Ferguson et al., 2002), gastrin-releasing peptide (Yamada et al., 2000, 2001), and vasopressin (Wersinger et al., 2002; Bielsky et al., 2004) systems. The receptors for these neuropeptides are expressed in the olfactory bulb or the amygdala and hypothalamus (structures involved in emotional responses), suggesting that behavioral changes observed in mutant mice reflect alterations in the olfactory nuclei or regions of higher level information processing (Insel and Winslow, 1998; Popik and van Ree, 1998; Pfaff, 2001; Ferguson et al., 2002). Indeed, animal studies have led to a better understanding of the role of oxytocin and vasopressin in the regulation of complex social behavior (Young, 2002) and to the search for genetic alterations of peptidergic systems in human disorders such as autism (Marui et al., 2004; Wassink et al., 2004).
The pituitary adenylate cyclase-activating polypeptide (PACAP) belongs to a peptide family that includes vasoactive intestinal peptide (VIP), secretin, glucagon, and growth hormone-releasing factor (Arimura, 1998) and acts via three G-protein-coupled receptors: VPAC1 and VPAC2 display high affinity for both VIP and PACAP, whereas PACAP receptor 1 (PAC1) binds only PACAP with high affinity (Harmar et al., 1998). A role for the PACAP ligand-receptor system in olfactory function and social affiliation is supported by a variety of evidence. PACAP-immunoreactive fibers densely innervate the MOB, especially the glomerular layer, and the anterior olfactory bulb, whereas PACAP-expressing cell bodies are found among the MOB mitral cells and in the lateral part of the anterior olfactory nucleus (Hannibal, 2002). Moreover, PAC1 receptor mRNA is expressed in granule, mitral, and glomerular cell layers of the olfactory bulb (Jaworski and Proctor, 2000). Finally, AOB mitral cells project to hypothalamic areas through the medial amygdala (Lehman et al., 1980). All of these efferent structures are also rich in PACAP binding sites and receptor mRNA (Hashimoto et al., 1996). Whereas PACAP signaling components are expressed in many locations along the odorant pathway, a role for the peptide in regulating olfactory information or olfactory-dependant social and sexual behavior has never been demonstrated. In the present study, we assessed olfactory function in PAC1-deficient mice using conditioned and nonconditioned tests of olfactory habituation, food exploration, and social and sexual behavior.
Materials and Methods
Animals. PAC1-/- (Jamen et al., 2000b) and wild-type (WT) CB57BL/6J times 129/Sv mice were received at 3 months of age (F4) and were used to generate 12 heterozygote couples (F1′). These mice were used to generate 10 PAC1-/- males, 10 PAC1-/- females, 12 WT males, and 11 WT females (F2′; referred to hereafter as the first set). PAC1-/- mice exhibit ∼60% mortality at 4 weeks of age (Jamen et al., 2000a), and fertility of PAC1-/- females is severely reduced (Jamen et al., 2000b). Thus, to obtain a greater number of animals, six additional matings of heterozygote females (F1′) times PAC1-/- males (F2′) were performed, from which 10 PAC-/- males and 10 heterozygote males were retained for additional analyses (the second set). Mice were weaned at 4 weeks, and same-sex littermates were housed together in cages (two to four per cage) until ∼6 months of age. For bromodeoxyuridine (BrdU) analysis, another set of mice (-/- and +/+ littermates; 6 months of age) issued from (F2′) heterozygote times (F2′) heterozygote matings (F3′) was used.
Because PACAP knock-out mice have been shown to exhibit excessive jumping in a novel environment (Hashimoto et al., 2001), a behavior we also observed in PAC1-/- males, mice used for behavioral testing were left in their home cage whenever possible. The 12 hr light/dark cycle was used (lights on at 7:00 A.M.). All experiments were performed between 10:00 A.M. and 6:00 P.M. and were in accordance with the guidelines of the University of Medicine and Dentistry of New Jersey.
Genotyping. Genotyping was performed on tail snips of 4-week-old mice using primers as described previously (Jamen et al., 2000b). Subject mice were assigned a number and were tested randomly. The code was broken at the end of the day after behavioral testing.
Mate affiliative behavior of PAC-/- and WT females. Six-month-old PAC1-/- and WT females were housed together (five PAC1-/- and five WT mice per cage) 2 weeks before testing to synchronize all females in anestrus. Then, females (10 PAC-/- and 11 WT littermates) were housed in individual cages and paired with a WT male. The next morning and on the two subsequent days, mate affiliation (male and female laying side-by-side) was evaluated.
Analysis of male behavior. Males from the first and second sets of mice were housed individually for 1 week before the first behavioral test: social investigation toward ovariectomized (OVX) CD-1 females (Charles River, Wilmington, MA). At the end of this test, male subjects were returned to their home cage with an OVX CD-1 female until the mice were killed. During subsequent testing, the companion OVX CD-1 mouse and the feeding tray were removed temporarily from the home cages. Additional tests of mice of the first set were: second test of social investigation toward CD-1 females, social investigation of an anesthetized male mouse target, urine investigation, odor-food exploration test, and odor memory-discrimination test. Additional tests of mice of the second set were: second test of social investigation toward CD-1 females, odor-food exploration test, odor memory-discrimination test, and intruder test. The sequence of behavioral tests in male mice and age during testing is indicated in Table 1.
Table 1.
Sequence of behavioral tests
|
Age (months) |
First set (littermates from +/− and +/− matings) |
Second set (littermates from +/− and −/− matings) |
|---|---|---|
| 6-7 | Social investigation toward CD-1 female, four times for 1 min each | Social investigation toward CD-1 female, four times for 1 min each |
| Social investigation toward CD-1 anesthetized male, two times for 1 min each | ||
| 7-8 | Social investigation toward CD-1 female, two times for 4 min each (30 min interval) | Social investigation toward CD-1 female, two times for 4 min each (30 min interval) |
| 8-9 | Social investigation toward CD-1 female, two times for 4 min each (100 min, 240 min, or 20 hr intervals) | |
| 10-12 | Odor-food exploration tests | |
| 13-14 | Odor memory and discrimination | Female urine investigation |
| 14-15 |
|
Intruder test |
Social investigation toward an ovariectomized female. OVX CD-1 females were presented to the subject males in their home cages. The first test consisted of four successive 1 min presentations of the same female with a 10 min interval. A significant decrease in investigation time during subsequent exposures indicates that mice were able to recognize females presented previously. Investigation included licking, smelling, and following the OVX female. To assess specificity of social investigation, males were presented with a new OVX female 10 min after the fourth trial. One month later, a second test consisted of two successive 4 min presentations of the same OVX female with a 30 min interval. One week later, animals were tested with different intervals (100 min, 240 min, and 20 hr) to assess differences in duration of social memory. The 10 PAC1+/- and 10 PAC1-/- males tested previously were divided into two groups: six PAC1+/- and four PAC1-/- males were tested at 100 min, and the following day, the remaining four PAC1+/- and six PAC1-/- mice were tested at 240 min. The latter six PAC-/- mice were also tested after 20 hr to check for full recovery.
Social investigation of an anesthetized male mouse target. Target CD-1 male mice were anesthetized with an intraperitoneal injection of Nembutal (54 mg/kg) at least 10 min before presentation. The target mouse was placed in the center of a clean cage on a 2 cm sawdust layer. Subject mice were then introduced individually into the cage corner, and the duration of social investigation (sniffing and contact with the target) was measured during 4 min. After this first trial, the target mouse was placed in a new clean cage. Thirty minutes after the first presentation, the subject mouse was again introduced to the target in the new cage, and the duration of social investigation was recorded again.
Female urine investigation. Urine from three 9- to 12-month-old CD-1 OVX females was collected on the day of the experiment and diluted fourfold in water. Urine was presented to male subjects by placing 100 μl on a circle of filter paper (Whatman 1; Whatman, Ann Arbor, MI) inserted in a tube. The control tube contained water-impregnated paper. Each tube was placed randomly at one corner of the home cage without disturbing the animal. A test session consisted of three 2 min presentations of the same urine with a 10 min interval. A significant decrease in investigation time during the second or third exposures indicates that mice were able to recognize the urine that was presented previously. To assess specificity, the same animal was presented with urine collected from a different set of OVX mice. The time animals spent sniffing inside the tube was recorded.
Odor-food exploration test. The food exploration test consists of finding a piece of commercially available breakfast cereal (0.1 gm; Cheerios; General Mills, Minneapolis, MN; hereafter referred to as cookie), hidden under the sawdust. During this test, mice were allowed to feed ad libitum only between 2:00 and 8:00 P.M., so that test trials occurred after ∼16 hr of food deprivation. The experiment consisted of three habituation trials and three experimental sessions. In the habituation trial (days -2 to 0), mice were presented with a cup containing the cookie impregnated with 100 μl of strawberry scent (1:10) and covered with a 2 cm sawdust layer, which was removed by the mice to access and eat the cookie. Each experimental session (session I, days 1-4; session II, days 8-11; session III, days 15-18) consisted of four trials separated by 1 day. Mice were presented an empty cup covered with sawdust and a cup containing a strawberry-scented (1:10) cookie under the sawdust. To assess the subjects' ability for odor discrimination with decreasing scent concentration, on days 4, 11, and 18, 2 hr after the initial test, a cup containing a cookie with strawberry scent at 1:100, 1:1000, or 1:10,000 and a control cup were presented to the mice. Two hours after completing this test, a cup containing a cookie without strawberry scent and a control cup were presented. To prevent cookie recognition in this last test, mice were not allowed to eat the unscented cookie. Latency (time for cookie retrieval), total investigation (time sniffing and digging both cups), and percentage of odor discrimination (time sniffing and digging the cup containing the scented cookie/total investigation) were scored during experimental sessions.
Odor memory and discrimination. Odors (vanilla or strawberry at 1:10) were presented by placing 100 μl onto a circle of filter paper (Whatman 1) inserted in a tube. The control tube contained water-impregnated paper. Each tube was placed randomly at one corner of the home cage without disturbing the animal. A test session consisted of five 2 min odor presentations of the vanilla odor with a 10 min intertrial interval. The time animals spent sniffing inside the tube was recorded. A significant decrease in investigation time during subsequent exposures indicates odor recognition. To assess odor discrimination and specificity, the same animal was presented with strawberry odor at the end of the test. Then, trials separated by 1 week were performed by testing different concentrations of vanilla (1:100, 1:1000, or 1:10,000). Finally, mice were tested 1 week later with two consecutive odor presentations (1:10) separated by 240 min intervals.
Mounting and aggressive behavior. Mounting behavior of PAC1-/-, PAC-/+, and WT males toward OVX CD-1 females was analyzed during the social memory tests. Mounting behavior and territorial aggression toward males was also analyzed using the intruder paradigm (Buck, 1996; Stowers et al., 2002). Intruders were CD-1 males castrated [gonadectomy (GDX)] at 7 weeks. Fresh urine was obtained from two dominant CD-1 males (3-6 months of age). For the first trial, the castrated male intruder was introduced into the resident's cage for 4 min (presentations >5 min were avoided to prevent aggression from the intruder). One week later, a different castrated male intruder that had been swabbed with 50 μl of pure male urine on the back and urogenital region was used for testing in a 4 min trial. Nonaggressive social behavior included licking, smelling, following, and sexual mounting of the intruder. Smelling and following the intruder were considered social investigation. Aggressive social behavior toward the intruder included tail rattling and attacks (biting, tumbling, wrestling, and cornering).
BrdU labeling, immunohistochemistry, and quantitation. To evaluate mitotic activity of neuroprecursors in the ventricular zone lining the lateral ventricles, 6-month-old PAC1-/- and WT males were perfused 2 hr after four intraperitoneal injections (one injection every hour) of a solution of BrdU (200 mg/kg body weight; Sigma, St. Louis, MO). After overnight fixation, brains were protected in 20% sucrose and frozen on dry ice. Brains were cut in serial coronal sections with a cryostat (15 μm thick) and mounted on slides coated with 2-3 aminopropyltriethoxysilane. Six serial sections (75 μm apart) for each animal and including the anterior subventricular zones (SVZs) (SVZ portions starting ∼60 μm posterior to the level of the junction of the corpus callosum and ending ∼50 μm anterior to the level of the crossing of the anterior commissure) were processed for BrdU immunohistochemistry according to a standard technique. In brief, sections were treated with 0.1% trypsin/CaCl2 in 100 mm Tris for 20 min and then washed with PBS. After a 30 min treatment with 2N HCl to denature DNA, they were rinsed in sodium borate buffer (0.1 m, pH 8.5) and incubated overnight in monoclonal anti-BrdU (1: 100; Dako, Carpinteria, CA) in PBS/0.3% Triton X-100/1.5% horse serum, followed by 1 hr of incubation with a biotinylated anti-mouse secondary antibody. Staining was visualized using a Vectastain avidinbiotin complex kit and Vector SG peroxidase substrate (Vector Laboratories, Burlingame, CA). Sections were dehydrated, cleared, and coverslipped with DPX mountant (Fluka, Buchs, Switzerland). The total number of BrdU-labeled cells in the SVZs of the six sections in each animal was counted bilaterally with a microscope at 40×.
Statistical analysis. Unless stated otherwise, behavioral data were analyzed using ANOVA for repeated measures, with one between subject factor (genotype) and one within subject factor (time or concentration), followed by post hoc Sheffe test for multiple comparisons to establish significant differences between the means. The female affiliative behavior data were analyzed with the χ2 test. The intruder tests were analyzed with the t test. All data are expressed as mean ± SEM.
Results
Female affiliative behavior
Because social investigation by females toward new individuals is of shorter duration than by males (Ferguson et al., 2002), we assessed their social interaction by examining mate affiliation. Although most WT females exhibited side-by-side contact with males the morning after the introduction of the male in the cage, PAC1-/- females and their male companions were always resting separately at different corners of the cage after the first night. Side-by-side contact of all mice pairs was observed only on the third day (Table 2). This delay in social interaction suggests PAC1-/- females have a defect in the initiation of affiliative behavior, likely contributing to the reduced fertility we observed previously (Jamen et al., 2000a).
Table 2.
Affiliative behavior of PAC−/− and WT female mice
|
|
PAC−/− |
PAC+/+ |
χ2 |
p |
|---|---|---|---|---|
| Day 1 | 0/10 | 9/11 | 14.32 | 0.0002 |
| Day 2 | 5/10 | 9/11 | 2.39 | 0.12 |
| Day 3 |
10/10 |
11/11 |
|
|
Side-by-side contact behavior of PAC1−/− (n = 10) and PAC1+/+ (n = 11) female mice with a novel male. Data represent proportion of females exhibiting contact behavior with their male companion.
Social investigation in males
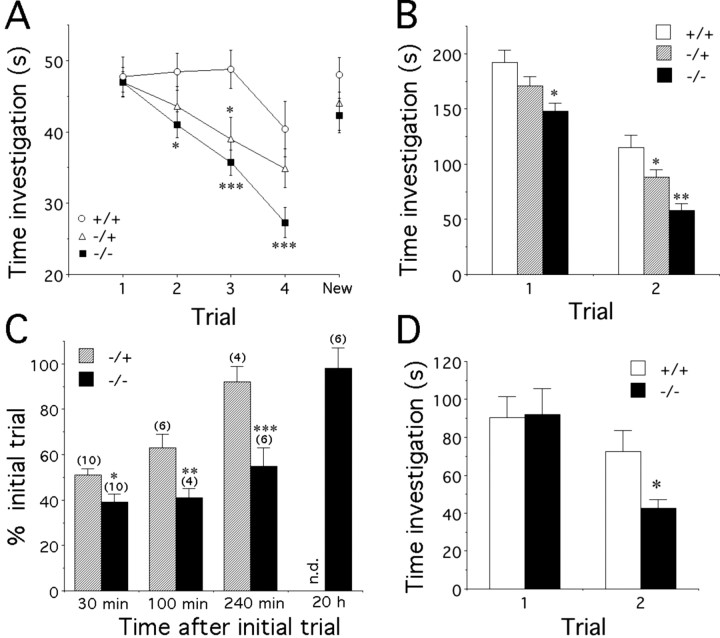
Social investigation in male mice was analyzed during four short (1 min) repeated pairings with the same OVX female (Fig. 1A). Although no difference was found at first presentation among the three genotypes, during the following trials, PAC-/- mice showed a significantly faster decline in the time spent investigating the OVX female compared with WT subjects, whereas PAC-/+ males exhibited an intermediate phenotype (genotype effect, F(2,38) = 5.8, p = 0.006; time effect, F(3,114) = 34.0, p < 0.0001; genotype times time interaction, F(6,114) = 3.2, p = 0.006). In addition, the time spent investigating a novel OVX female after the fourth trial was significantly increased compared with the fourth presentation in all groups (time effect, F(1,37) = 32.0, p < 0.0001; genotype times time interaction, F(2,37) = 1.4, p > 0.05). Thus, the decrease in time investigating the first animal was female specific, ruling out nonspecific satiation. This could reflect enhanced social memory toward the familiar female or altered habituation (see Discussion). One month later, social investigation in male mice was again analyzed during two longer (4 min) repeated pairings (Fig. 1B). All groups were able to recognize the female at the second presentation (time effect, F(1,38) = 31.0, p < 0.001; genotype times time interaction, F(2,38) = 0.99, p > 0.05). However, a clear genotype effect (F(2,38) = 12.8; p < 0.0001) indicated that PAC1-/- and PAC-/+ mice showed a significant decrease in total investigation time compared with WT mice (Fig. 1B).
Figure 1.
Social memory in PAC1-/-, PAC-/+, and WT mice. A, Time (seconds) spent by PAC1+/+ (n = 12), PAC1+/- (n = 10), and PAC1-/- (n = 19) male mice investigating the same ovariectomized female during each of four successive 1 min trials separated by 10 min. A fifth trial depicts the response to a novel female in a 1 min pairing 10 min after the fourth trial (post hoc analysis, *p < 0.05; ***p < 0.005 vs PAC1+/+). B, Time (seconds) spent by PAC1+/+ (n = 12), PAC1+/- (n = 10), and PAC1-/- (n = 19) male mice investigating the same ovariectomized female during two successive 4 min trials separated by 30 min (post hoc analysis, *p < 0.05; **p < 0.01 vs PAC1+/+). C, Effect of different intertrial intervals on ovariectomized female recognition by PAC1+/- and PAC1-/- male mice. Data depict mean percentage ± SEM of time spent investigating the ovariectomized female on the second exposure (4 min) compared with the time spent during the first presentation (4 min). Numbers in parentheses indicate the number of mice tested for each interval (unpaired t test, *p < 0.05; **p < 0.01; p < 0.005 vs PAC1+/-). D, Time (seconds) spent by PAC1+/+ (n = 6) and PAC1-/- (n = 6) male mice investigating the same anesthetized male during each of two successive 4 min trials separated by 30 min (unpaired t test, *p < 0.05 vs PAC1+/+).
We also compared the time spent by PAC1-/- and PAC1-/+ males investigating the stimulus female after different trial intervals (Fig. 1C). A previous study indicates that WT mice return to a full investigation time of female mice after a 90 min interval (Ferguson et al., 2000). Similarly, PAC1-/+ mice exhibited a reduced investigation time when retested after 30 or 100 min but not after 240 min, consistent with this previous result. In marked contrast, investigation by PAC1-/- mice remained reduced even after a 240 min interval (paired t test, p = 0.07 vs 30 min) but recovered fully after 20 hr (paired t test, p = 0.004 vs 30 min), suggesting that social investigation is markedly altered in PAC1-/- mutants.
Social investigation was also examined toward male targets using CD-1 males anesthetized to prevent movement, a potential component of social stimulation (Latane and Glass, 1968), and the development of aggressive male behavior. PAC1-/- males (Fig. 1D) investigated the male targets during the second presentation for less time than WT mice (genotype effect, F(1,10) = 1.1, p > 0.05; time effect, F(1,10) = 30.9, p = 0.0002; genotype times time interaction, F(1,10) = 6.7, p = 0.03), a result consistent with the decreased investigation toward females described above (Fig. 1C).
Urine investigation
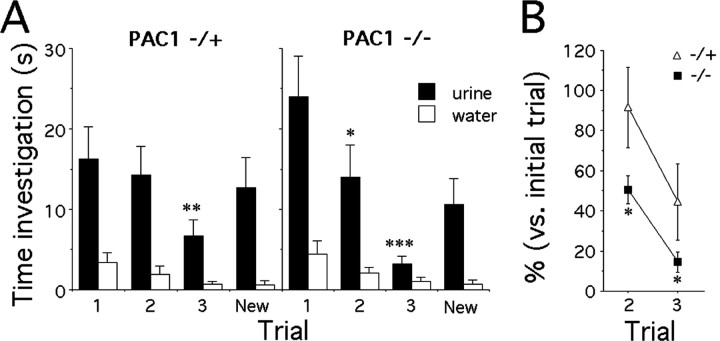
To determine whether changes in social investigation tests were caused by altered recognition of social scents contained in the urine of conspecific targets, PAC1-/+ and PAC1-/- males were subjected to repeated presentations of tubes containing water or urine collected from OVX CD-1 females (Fig. 2A). Both PAC1-/+ and PAC1-/- littermates showed declines in urine investigation after repeated exposure, but the decline was more rapid and pronounced in PAC1-/- males (genotype effect, F(1,14) = 0.9, p > 0.05; time effect, F(2,14) = 22.2, p < 0.0001; genotype times time interaction, F(2,14) = 4.4, p = 0.02). Because the levels of urine investigation at first presentation were different, although not significantly, data are also expressed as the percentage of initial trial to emphasize genotypic differences (Fig. 2B) (genotype effect, F(1,14) = 5.1, p = 0.04; time effect, F(1,14) = 24.9, p = 0.0002; genotype times time interaction, F(1,14) = 0.35, p > 0.05). Finally, both PAC1-/+ and PAC1-/- mice showed increased response time to a novel urine sample presented in the fourth trial, indicating they were able to identify this sample as different from the previous one (genotype effect, F(1,14) = 0.8, p > 0.05; time effect, F(1,14) = 15, p = 0.002; genotype times time interaction, F(1,14) = 0.8, p > 0.05). These observations suggest that the decreased investigation observed in the previous trials was specific to familiar urine, ruling out nonspecific satiation. Analysis of the responses to the control tube containing water and placed on the other side of the cage indicate that, despite a weak overall time effect (p = 0.035), neither a genotype nor a genotype times time effect were evident (Fig. 2A,B).
Figure 2.
Urine recognition in PAC1+/- and PAC1-/- male mice. A, Time spent by PAC1+/- (n = 8) and PAC1-/- (n = 8) male mice investigating the inside of a tube containing 25 μl of urine collected from three ovariectomized CD-1 female mice during three successive 1 min trials separated by 10 min. During the fourth trial, the response of males to urine collected from three different ovariectomized females was recorded 10 min after the third trial. The lack of significant response to the control tube containing water and placed on the other side of the cage indicates that the decline in time investigating the urine-containing tubes is specific (post hoc test, *p < 0.05; **p < 0.01; ***p < 0.001 vs first presentation). B, Time spent investigating urine-containing tubes on the second and third exposure compared with the time spent during the first presentation (unpaired t test, *p < 0.05 vs PAC1+/-).
Olfaction and scent habituation tests
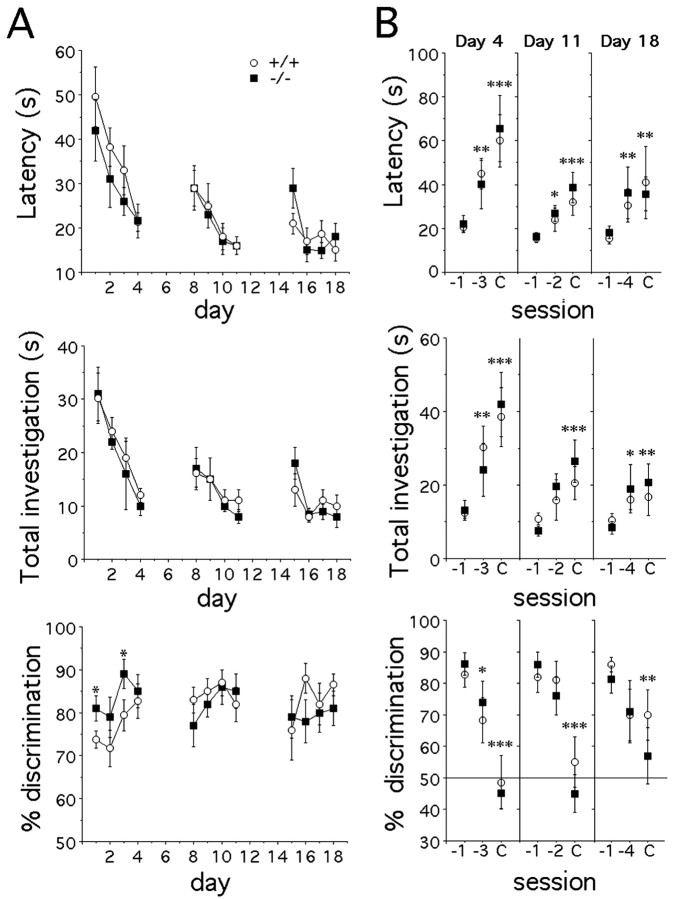
To determine whether deficits in social investigation might also be associated with changes in nonsocial olfaction, we examined mice in two tests of olfactory behavior. The first test consisted of finding a scented cookie hidden in a cup containing litter. Individually housed mice were food deprived for 16 hr before testing and were trained for 3 d to find the cookie with a high concentration of strawberry scent. Subsequently, mice were presented with a cup containing the scented cookie and an empty cup. The latency to locate the cookie and the total time of investigation did not differ in PAC1-/- and PAC1+/+ mice in the three experimental sessions (Fig. 3A). However, a difference in the percentage of time spent correctly discriminating scent from control was detected during the first experimental session (genotype effect, F(1,17) = 9.4, p = 0.007; time effect, F(3,51) = 3.0, p = 0.037; genotype times time interaction, F(3,51) = 0.3, p > 0.05), suggesting that PAC1-/- mice learned more rapidly to recognize the scented cookie during the training procedure. An alternative explanation may be that PAC1-/- mice are more effective at food-guided foraging. However, at the end of the first session, all mice exhibited similar abilities (Fig. 3A). To determine whether PAC1-/- mice have an altered olfactory detection threshold, we examined the effect of lowering the scent concentration. Although there was a reduction in odor discrimination with decreasing scent concentration, there were again no differences between the PAC1-/- and PAC1+/+ genotypes (Fig. 3B). Nevertheless, PAC1-/- mice may form better long-term memory than WT, as further suggested by testing mice 1 month later (data not shown).
Figure 3.
Odor discrimination in a conditioned cue olfactory test. PAC1+/+ (n = 10) and PAC1-/- (n = 9) male mice were trained to recognize a cup containing a cookie with strawberry scent (1:10) during 3 d and were tested on consecutive days. A, Mice were then presented a cup covered with sawdust and a cup containing a cookie with strawberry scent (1:10) on four consecutive days (days 1-4, 8-11, and 15-18). Latency (time for cookie retrieval), total investigation (time sniffing and digging both cups), and percentage of odor discrimination (time sniffing and digging the cup containing the cookie/total investigation) were scored. Values are given as mean ± SEM. There were no differences between PAC1-/- and PAC1+/+ mice except for the percentage of discrimination on days 1-4 (unpaired t test, *p < 0.05 vs PAC1+/+). B, On days 4, 11, and 18, a cup containing a cookie with strawberry odor (-3:1:1000, -2:1:100, and -4:1:10,000) and a control cup were presented to mice 2 hr after presentation of a cup containing the cookie with strawberry odor (1:10; S-1) and a control cup. Two hours after completion of this test, a cup containing a cookie without strawberry scent (c) and a control cup were presented to the mice. There were no differences between PAC1-/- and PAC1+/+ mice. Odor discrimination in both genotypes was impaired when strawberry scent concentration was decreased (post hoc test, *p < 0.05; **p < 0.02; ***p < 0.001 vs -1).
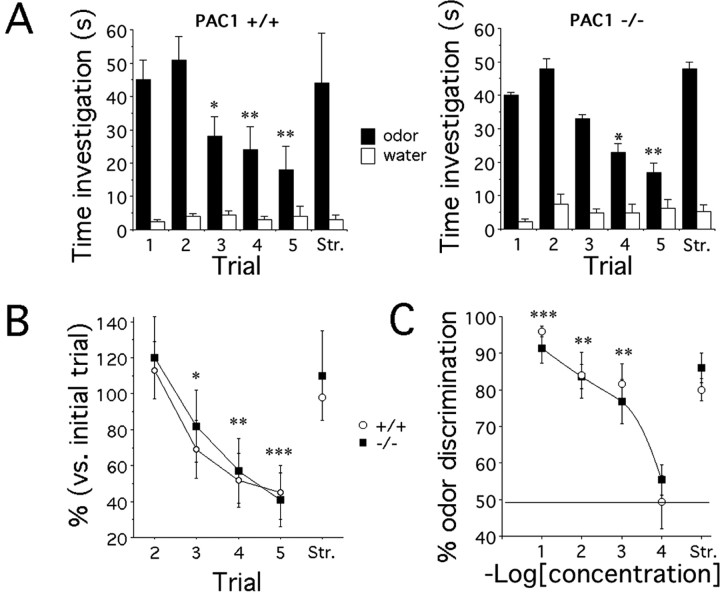
Although PAC1-/- and WT mice appear to have similar olfactory capacity, it is possible that genotype-specific differences in scent discrimination were masked by the conditioned olfactory testing paradigm. To address this concern, we performed a second test of nonsocial olfactory memory and specificity 2 months later. Although less sensitive than olfactory-guided foraging, differences in nonsocial olfaction can also be detected in untrained mice. PAC1-/- and WT mice exhibited parallel decreases in investigation time to repeated scent exposure (Fig. 4A), indicating similar abilities to recognize vanilla during trials 1-5 (genotype effect, F(1,13) = 0.1, p > 0.05; time effect, F(4,52) = 9.7, p = 0.0001; genotype times time interaction, F(4,52) = 0.24, p > 0.05). Similar responses to the novel strawberry scent at test termination indicated the specificity of odor recognition. Accordingly, there was no difference in nonsocial olfactory memory between PAC1-/- and PAC1+/+ mice when the results were expressed as percentage of the time investigating the odors compared with the first trial (Fig. 4B). Moreover, by using decreasing vanilla concentrations (Fig. 4C), we detected no differences in odor detection threshold between PAC1-/- and WT mice (genotype effect, F(1,53) = 0.4, p > 0.05; concentration effect, F(3,53) = 17, p = 0.0001; genotype times concentration interaction, F(3,53) = 0.8, p > 0.05). Finally, because differences in social memory among genotypes were detected previously after a 240 min interval, mice were tested 1 week later with two consecutive odor presentations separated by the same temporal delay. Although the investigation time during the second exposure was ∼50% less than the first, no genotype-dependent difference was found (data not shown). Together, these results suggest that PAC1 signaling does not influence odor discrimination or recently acquired memory linked to nonsocial olfactory functions.
Figure 4.
Performance in an odor habituation task in PAC1+/+ and PAC1-/- mice. A, Time spent by PAC1+/+ (n = 7) and PAC1-/- (n = 8) mice sniffing a tube containing vanilla (trials 1-5) or strawberry (new) odor diluted at 10-1. A control tube containing water was placed on the opposite corner of the cage (post hoc analysis, *p < 0.05; **p < 0.01 vs trial 1). B, Percentage of time spent investigating the odor during the different trials compared with the time spent investigating the vanilla odor during the first presentation (post hoc analysis, *p < 0.05; **p < 0.01; ***p < 0.001 vs first trial). No differences were observed between PAC1-/- and PAC1+/+ mice. C, Percentage of odor discrimination (time sniffing the tube containing the scent/time sniffing control and scent tubes) for decreasing concentrations of vanilla scent (10-1 to 10-4) or novel scent strawberry (Str; 10-1) tested at 1 week interval (post hoc analysis, **p < 0.01; ***p < 0.001 vs vanilla 10-4). The detection threshold for PAC1+/+ and PAC1-/- mice was identical.
Mounting and aggressive behavior
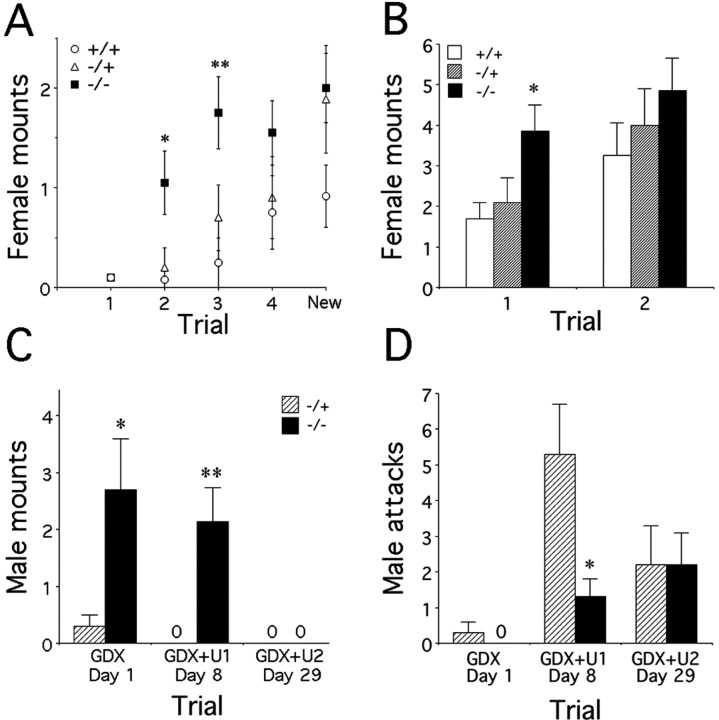
The mounting behavior of PAC1-/-, PAC-/+, and WT males toward OVX females was analyzed during the social investigation tests. When tested in 1 min trials separated by 10 min intervals, PAC1-/- males exhibited an increased number of mounts of the same OVX females during the second and third presentations (Fig. 5A) (genotype effect, F(2,38) = 5.9, p = 0.006; time effect, F(3,114) = 14.9, p = 0.0001; genotype times time interaction, F(6,114) = 2.2, p > 0.05). Similarly, when OVX females were presented 1 month later for 4 min trials, PAC1-/- mice exhibited earlier increased mounting behavior compared with WT (Fig. 5B) (genotype effect, F(2,38) = 3.38, p = 0.044; time effect, F(1,38) = 6.35, p = 0.016; genotype times time interaction, F(2,38) = 0.33, p > 0.05).
Figure 5.
Mounting and aggressive behavior in PAC1-/- males. A, Female mounting behavior by PAC1+/+ (n = 12), PAC1+/- (n = 10), and PAC1-/- (n = 19) male mice. Data depict mean ± SEM number of mounts of the same ovariectomized female during four successive 1 min trials separated by 10 min. The fifth trial (new) depicts the response to a novel female during a 1 min pairing 10 min after the fourth trial (post hoc analysis, *p < 0.02; **p < 0.01 vs PAC1+/+). B, Female mounting behavior of PAC1+/+ (n = 12), PAC1+/- (n = 10), and PAC1-/- (n = 19) male mice. Data depict mean ± SEM number of mounts of an ovariectomized female during two successive 4 min trials separated by 30 min. During the first trial, PAC1-/- mice exhibited increased mounting behavior compared with WT (post hoc analysis, *p < 0.02 vs PAC1+/+). C, D, Male mounting and aggressive behavior of PAC1+/- (n = 6) and PAC1-/- (n = 6) male mice. Data depict mean ± SEM number of mounts (C) or attacks (D) toward a gonadectomized CD-1 male mouse (GDX; tested on day 1) or a GDX male swabbed with 20 μl of fresh urine (U) collected from a dominant CD-1 male (GDX+U1, tested on day 8; GDX+U2, tested on day 29; t test, *p < 0.05; **p < 0.01 vs PAC1+/-).
The resident intruder paradigm is a useful test to assess male aggression and gender identification. WT mice are known to exhibit strong aggressive behavior toward castrated males swabbed with urine from dominant males but little or no aggression toward castrated males without urine scent (Buck, 1996; Stowers et al., 2002). Unexpectedly, PAC1-/- males exhibited significant mounting behavior toward castrated males, regardless of whether or not they bore dominant male urine scent (Fig. 5C). In contrast, PAC1-/+ mice exhibited responses typical of WT mice. In addition to inappropriate male mounting, PAC1-/- males exhibited markedly reduced attack behavior (Fig. 5D) accompanied by increased sniffing and licking behaviors (Table 3), suggesting an overall change in sex-related behaviors. In contrast, when presented again with castrated males bearing dominant male urine 3 weeks later, the changes in mounting, licking, and aggressive behavior observed in PAC1-/- mice were no longer observed.
Table 3.
Social investigation and aggressive behavior of PAC−/− and PAC−/+ male mice in the resident intruder paradigm
|
|
PAC−/− |
PAC−/+ |
p |
|---|---|---|---|
| Sniffing time (seconds) | |||
| GDX | 117 ± 13 | 132 ± 11 | NS |
| GDX plus U1 | 77 ± 10 | 30 ± 8 | 0.004 |
| GDX plus U2 | 100 ± 10 | 87 ± 9 | NS |
| Number of licking events | |||
| GDX | 0.57 ± 0.2 | 0 | 0.015 |
| GDX plus U1 | 0.1 ± 0.1 | 0.1 ± 0.1 | NS |
| GDX plus U2 | 0 | 0 | NS |
| Number of attacks | |||
| GDX | 0 | 0.3 ± 0.3 | NS |
| GDX plus U1 | 1.3 ± 0.5 (4 of 7) | 5.3 ± 1.4 (6 of 7) | 0.02 |
| GDX plus U2 | 2.2 ± 0.9 | 2.2 ± 1.1 | NS |
| Time attacks (seconds) | |||
| GDX | 0 | 0.4 ± 0.4 | NS |
| GDX plus U1 | 3.9 ± 13 | 34.1 ± 13.8 | 0.025 |
| GDX plus U2 | 14 ± 7 | 17 ± 9 | NS |
| Number of tail flicking events | |||
| GDX | 0 | 0.4 ± 0.2 | 0.055 |
| GDX plus U1 | 0.1 ± 0.1 | 0.6 ± 0.2 | 0.1 |
| GDX plus U2 |
0.75 ± 0.6 |
1.5 ± 0.7 |
NS |
The time spent in sniffing or attacking the intruder and the number of licking, attacking, or tail flicking events were evaluated in PAC1−/− (n = 7) and PAC1−/+ (n = 7) male mice. The intruder was a gonadectomized CD-1 mouse (tested on day 1) or a GDX male swabbed with 20 μl of fresh urine (U) collected from a dominant CD-1 male (GDX plus U1, tested on day 8; GDX plus U2, tested on day 29). Data (mean ± SEM) were analyzed with the Student's t test. NS, Not significant.
BrdU labeling in the SVZ
Because olfactory signals can increase neurogenesis and odor memory (Rochefort et al., 2002) and SVZ precursors, which express PAC1 receptors, generate granule cells that migrate not only to the MOB but also to the AOB (Bonfanti et al., 1997), we examined mitotic labeling of lateral ventricle precursors in PAC1-deficient mice. PACAP stimulates olfactory precursor neurogenesis and survival in vitro (Hansel et al., 2001), whereas we previously observed reduced mitotic labeling in vivo after acute PACAP intraventricular injections (Nicot and DiCicco-Bloom, 1999). Two hours after BrdU injection, there was no difference in the number of BrdU-labeled cells in the SVZ of PAC1-deficient and WT mice (PAC1-/-, 873 ± 58 (n = 5); PAC+/+, 875 ± 63 (n = 5). This short survival paradigm does not allow labeled precursors time to migrate to their targets, MOB or AOB (Bonfanti et al., 1997) and, therefore, these subpopulations were not assessed.
Discussion
Our observations indicate that PAC1-deficient mice exhibit aberrant social and sexual behaviors. PAC1-deficient females displayed reduced affiliative behavior, whereas PAC1-deficient males displayed a faster decrease in social investigation of OVX females or their urine after repeated exposure, as well as excessive mounting toward both females and males, and reduced aggression. Significantly, the alterations in odor-based social and sexual interactions in PAC1-deficient mice suggest marked deficits in recognizing and processing pheromonal cues. Finally, these striking changes in social behaviors were highly specific, because neither nonsocial odor discrimination nor odor memory were affected in PAC1-deficient mice, suggesting that PACAP systems play important regulatory roles in the processing of complex social interactions.
Role of PAC1 signaling in the regulation of social interactions
Previous studies using anosmia models in mice and rats (Alberts and Galef, 1971; Thor and Flannelly, 1977; Liebenauer and Slotnick, 1996) have shown that olfactory information is critical for establishing social behaviors and regulating the preference for conspecifics (Jones and Nowell, 1974). After disruption of olfactory systems, gender identification is dramatically altered, resulting in the failure to attack male intruders and in the initiation of male mounting behavior (Thor, 1980; Liebenauer and Slotnick, 1996). PAC1-deficient male mice exhibited a similar phenotype, showing markedly reduced aggression and increased sexual behavior toward males. This behavioral repertoire also reproduces some features observed in mice deficient in trp2 (a cation channel expressed in the vomeronasal system), which investigates males in a manner similar to female conspecifics (Stowers et al., 2002). However, PAC1 mutant mice exhibited residual aggression, suggesting that primary sensory signaling is not entirely defective. Nevertheless, gender identification was markedly abnormal in PAC1-deficient mice, suggesting that the recognition of specific male pheromone cues is severely hampered. Moreover, because PAC1-deficient male mice exhibited earlier mounting behavior toward ovariectomized females, the system(s) inhibiting initiation of mounting behavior also appears compromised when PACAP signaling is deficient.
Although sex recognition was altered in PAC1-deficient males, a decrease or delay in affiliative behavior with males was observed in PAC1-deficient females. Several reports suggest that PACAP plays a role in some aspects of female sexual behavior in mice. Pharmacological studies first suggested that PACAP advances female puberty onset, presumably through pituitary hormone regulation (Choi et al., 2000; Szabo et al., 2002). However, because male urinary proteins serve to accelerate female puberty onset (Mucignat-Caretta et al., 1995), the role of PACAP in pheromone processing identified here may be an additional mechanism. Furthermore, PACAP is also an important hypothalamic trigger of female sexual receptivity induced by steroids (Apostolakis et al., 2004). Moreover, whereas PACAP-deficient female mice have no defect in the estrous cycle, they exhibit a decrease in mating behavior and maternal care (Hashimoto et al., 2001; Shintani et al., 2002). These findings are consistent with our observations that PAC1-deficient females are barely fertile (Jamen et al., 2000a,b) and exhibit delayed affiliative behavior (our study), although additional studies are required to better delineate these functional defects. Together, these data suggest that PACAP, by regulating pheromone processing, sensory circuitry, and endocrine maturation, affects different components of sexual behavior and social affiliation.
In addition to abnormal gender identification, PAC1-deficent males exhibited strikingly altered social investigation of females on repeated presentation. Both PAC1-deficient males and WT littermates show decreased investigation during the repeated presentation of an OVX female (or its urine), indicating that they are able to recognize the familiar mouse (or odor). However, PAC1-deficient mice show a faster decline in investigation. Because this test is classically used to assess short-term social memory (Gheusi et al., 1994; Ferguson et al., 2002), our findings could be interpreted as a better ability of PAC1 knock-out mice to form short-term social memory. Although a social memory model has been used successfully to account for anosmic or knock-out mice that fail to exhibit normal declines in social investigation (Gheusi et al., 1994; Ferguson et al., 2002), this model may not be adequate for mice in which pheromonal and social cues are processed abnormally. Alternatively, PAC1-deficient males may be exhibiting a diminishing interest in investigating the same individual, an interpretation that is also consistent with a defect in central processing of social olfactory information. Indeed, even if social memory is enhanced in PAC1-/- mice, our results with other behavioral tests on PAC1-deficient mice also indicate altered social interactions, supporting the alternative model.
Potential mechanisms of PACAP in the processing of social signals in rodents
PACAP may act at several different levels of the CNS to mediate the effects we detect. First, it may directly regulate trp2 channel activity in the VNO-AOB pathway. Indeed, PACAP depolarizes sympathetic neurons partly via opening a nonselective cationic conductance channel potentially related to Trp2 (Beaudet et al., 2000). However, whether PACAP or PAC1 receptors are present in the VNO is currently unknown. The alterations in gender identification observed in PAC1-deficient mice may also occur downstream of the VNO-AOB projection, such as in the amygdala. The amygdala plays a key role in processing social cues both in rodents (Daenen et al., 2002) and humans (Baron-Cohen et al., 2000) and has been shown to express high levels of both PACAP and PAC1 receptors (Koves et al., 1991; Hashimoto et al., 1996). Other central brain structures regulating social behavior such as the lateral septum (Popik et al., 1992), basal nucleus of the stria terminalis, and medial preoptic area (Popik and van Ree, 1991) express PAC1 (Hashimoto et al., 1996) or PACAP (Hannibal, 2002) and may be involved as well. Future studies to identify the brain structures specifically involved could use local peptide injections and Fos immunoreactivity, as performed with oxytocin (Ferguson et al., 2001).
Previous studies have shown that oxytocin, vasopressin, and mediating receptors play major roles in social recognition and social interactions, but not in nonsocial olfaction, by altering the pheromone-dependent vomeronasal-AOB-amygdala pathway or main olfactory bulb pathways (Dantzer, 1998; Ferguson et al., 2001). Oxytocinergic pathways are apparently required for the secondary olfactory processing that occurs in the olfactory bulb and the medial amygdala and downstream at the basal nucleus of the stria terminalis and medial preoptic area. Vasopressinergic pathways to the lateral septum are also important for social recognition in rodents (Landgraf et al., 1995; Dantzer, 1998). Whereas genetic deletions of oxytocin or V1aR and V1bR vasopressin receptors elicit reduced social memory (Ferguson et al., 2000; Wersinger et al., 2002; Bielsky et al., 2004), PAC1 receptor loss results in an enhanced decline in social investigation, suggesting that there may be functional antagonism between the two peptide systems. Because PACAP (Koves et al., 1991; Piggins et al., 1996; Hannibal, 2002) and PAC1 (Hashimoto et al., 1996) are also expressed in the medial amygdala and its efferent targets, there is an anatomical basis for oxytocin- or vasopressin-PACAP signaling interactions, with decreased PACAP signaling leading to enhanced oxytocin-vasopressin activity and altered social recognition. In further support of opposing peptide interactions are the contrary effects on anxiety induced by genetic deletions, with an absence of oxytocin resulting in increased anxiety (Mantella et al., 2003) and disruption of PACAP (Hashimoto et al., 2001) or PAC1 receptor (Otto et al., 2001) producing reduced anxiety. At the pharmacological level, peptide antagonism may be expected, because oxytocin receptors and V1Rs activate phospholipase C, Ca2+, and protein kinase C signaling through Gq/11 proteins (Ku et al., 1995; Barberis et al., 1998; Schoneberg et al., 1998; Birnbaumer, 2000). This sequence of signals can be antagonized in cells by activators of the protein kinase A pathway that act via G-proteins, including PAC1 receptor isoforms (Nicot and DiCicco-Bloom, 2001; Nicot et al., 2002). Thus, it is likely that normal social recognition and investigation requires a balance of neuropeptide signaling in olfactory-amygdala pathways.
Changes we detect in affiliative behaviors may also reflect interactions in PACAP-neurotransmitter systems in other brain regions. Specifically, the activities of V1aR in the ventral pallidum (Pitkow et al., 2001; Lim and Young, 2004; Lim et al., 2004) and oxytocin and dopamine (acting via D2 receptors) in the nucleus accumbens (Aragona et al., 2003; Liu and Wang, 2003) have been shown to enhance partner affiliation and social attachment in prairie voles. In rodents, there are dense PACAP-immunoreactive fibers (Hannibal, 2002) in ventral pallidum and heavy terminal fields exhibiting VIP, a PACAP-related peptide acting on PAC1, in nucleus accumbens (Sims et al., 1980). Moderate to high densities of PACAP-VIP binding sites (Martin et al., 1987; Masuo et al., 1992) are also present in all of these brain structures. Thus, disturbances of PACAP signaling in the balance in these neurochemical systems may underlie the decreased affiliation observed in PAC1- and PACAP-deficient mice. In the case of dopamine, interactions are supported by studies showing that PACAP activates tyrosine hydroxylase activity in nucleus accumbens homogenates (Moser et al., 1999).
Besides a role in established neurocircuitry, one could also propose that PACAP participates in forming or maintaining new granule cells involved in pheromone processing, as some newly generated SVZ precursors reach the AOB (Bonfanti et al., 1997). In the hippocampus (Shors et al., 2001) and MOB (Rochefort et al., 2002), recently generated neuronal precursors are thought to play a role in new memory formation, but their role in the AOB is unknown. Although PACAP regulates diverse functions during developmental neurogenesis (Nicot and DiCicco-Bloom, 1999, 2001; Hansel et al., 2001; Suh et al., 2001; Nicot et al., 2002; Waschek, 2002) and can alter adult SVZ mitosis after acute injection, there was no difference in acute BrdU labeling in adult SVZ of PAC1-deficient mice compared with WT littermates. Interestingly, when PAC1 male mice were retested with a male intruder 3 weeks later, they exhibited increased aggression and reduced mounting behavior compared with the first presentation, suggesting that mice can acquire new cues for gender identification. Significantly, the loss of odor recognition after olfactory bulb removal persists for only 3 weeks (Gheusi et al., 1994), whereas impaired social recognition after vomeronasal organ removal lasts only 1-2 weeks (for review, see Ferguson et al., 2002). These data suggest that after this period, neural plasticity and use of other cues or pathways allow mice to again recognize odors and conspecifics. These same mechanisms may occur in PAC1-deficient mice to recover gender identification. Alternatively, previous odor exposure may specifically stimulate new AOB cell generation, allowing identification of male conspecifics, a potential mechanism that is currently difficult to assess.
Lack of defects in nonsocial olfactory detection
PACAP ligand and receptor are expressed in developing neurons and basal cells of the olfactory epithelium (Hansel et al., 2001). Furthermore, the periglomerular and granule cells of the olfactory bulb involved in downstream coding of olfactory information express high levels of PAC1 receptor (Hashimoto et al., 1996) and receive innervation from PACAP-immunoreactive fibers (Hannibal, 2002). Despite these anatomical data supporting a role for PACAP in olfaction, PAC1-/- mice did not show a defect in odor discrimination (strawberry or vanilla), suggesting that PAC1 is dispensable for nonsocial olfaction in vivo. Moreover, the size and the cytoarchitecture of the olfactory bulb are apparently not affected in PAC1-deficient mice (our unpublished data), although the peptide stimulates olfactory neurogenesis in vitro (Hansel et al., 2001). This is in contrast with the dramatic reduction in olfactory bulb size and granule cell number in neural cell adhesion molecule knock-out mice, which exhibit impaired odor discrimination (Gheusi et al., 2000).
Although possible relationships between neurogenesis and memory formation remain a conceptual model, our observations on nonsocial olfactory function and BrdU labeling in PAC1 mutants provide some correlative support. Sensory input is critical for the generation and survival of granule and perigranular cells, because neurogenesis is decreased in odor-deprived or anosmic animals (Corotto et al., 1994; Petreanu and Alvarez-Buylla, 2002). In crickets, elegant studies have recently demonstrated a link between suppression of adult neurogenesis and impairment of olfactory memory (Scotto-Lomassese et al., 2003). In accordance with these findings, mice reared in standard conditions can retain odor traces for a period shorter than 120 min (Bluthe and Dantzer, 1993), whereas mice reared in odor-enriched environments retain odor traces for 240 min, an increase that correlates with increased survival of newly generated olfactory bulb neurons (Rochefort et al., 2002). In our experiments, PAC1-deficient and WT mice exhibited no differences in odor trace retention at 240 min and no difference in BrdU mitotic labeling, consistent with this model. Consequently, we conclude that PAC1 does not play a major role in nonsocial olfaction in adulthood.
A role of PAC1 signaling in the regulation of human social interactions?
In aggregate, our results identify PAC1 signaling as an important factor in processes underlying complex social behaviors in adult mice. The phenotype of PAC1-deficient animals suggests that alterations in social behavior depend on changes in pheromone detection and processing, whereas nonsocial information (recognition-discrimination of odorants) mediated by the main olfactory bulb is unaffected. These findings raise the possibility that PACAP systems are also involved in some aspects of human social behavior and its disorders, as previously suggested for oxytocin and vasopressin in autism (Insel and Winslow, 1998; Insel et al., 1999; Maestrini et al., 2000; Hollander et al., 2003; Wassink et al., 2004). Indeed, by comparing the social behavioral consequences of blocking the two peptide systems, one may propose that PACAP and oxytocin-vasopressin signaling elicit opposing effects at many brain loci, and that normal social memory and behavior require a delicate balance between these two pathways. With specific regard to the role of PACAP in pheromone detection, the vomeronasal organs in humans and related primates, such as Old World monkeys, are only vestigial, and genes encoding two major components of its signal transduction pathway have undergone inactivation (Zhang and Webb, 2003). However, functional brain imaging studies indicate that the human brain also processes sex pheromone signals (Savic et al., 2001), although potential roles for PACAP remain unexplored. More generally, the role of PACAP signaling in processes underlying complex social behavior now identify its ligand and receptor components as possible candidates for genetic susceptibility to the autism spectrum disorders.
Footnotes
This work was supported by National Institutes of Health Grant NS32401, the New Jersey Governor's Council on Autism, and the National Alliance for Autism Research. The PAC1-/- mice were initially generated with funds from the Centre National de la Recherche Scientifique, Unité Propre de Recherche (Montpellier, France). We thank Dr. Catalina Betancur for critical reading of this manuscript and helpful discussions and Malathi Akula, Lauren Tarentino, and James D'Ambola for their technical assistance.
Correspondence should be addressed to Emanuel M. DiCicco-Bloom at the above address, E-mail: diciccem@umdnj.edu.
A. Nicot's present address: Institut National de la Santé et de la Recherche Médicale E0350, 184 rue du faubourg Saint-Antoine, 75012 Paris, France.
Copyright © 2004 Society for Neuroscience 0270-6474/04/248786-10$15.00/0
References
- Alberts JR, Galef Jr BG (1971) Acute anosmia in the rat: a behavioral test of a peripherally-induced olfactory deficit. Physiol Behav 6: 619-621. [DOI] [PubMed] [Google Scholar]
- Apostolakis EM, Lanz R, O'Malley BW (2004) Pituitary adenylate cyclase-activating peptide: a pivotal modulator of steroid-induced reproductive behavior in female rodents. Mol Endocrinol 18: 173-183. [DOI] [PubMed] [Google Scholar]
- Aragona BJ, Liu Y, Curtis JT, Stephan FK, Wang Z (2003) A critical role for nucleus accumbens dopamine in partner-preference formation in male prairie voles. J Neurosci 23: 3483-3490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arimura A (1998) Perspectives on pituitary adenylate cyclase activating polypeptide (PACAP) in the neuroendocrine, endocrine, and nervous systems. Jpn J Physiol 48: 301-331. [DOI] [PubMed] [Google Scholar]
- Barberis C, Mouillac B, Durroux T (1998) Structural bases of vasopressin/oxytocin receptor function. J Endocrinol 156: 223-229. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S, Ring HA, Bullmore ET, Wheelwright S, Ashwin C, Williams SC (2000) The amygdala theory of autism. Neurosci Biobehav Rev 24: 355-364. [DOI] [PubMed] [Google Scholar]
- Beaudet MM, Parsons RL, Braas KM, May V (2000) Mechanisms mediating pituitary adenylate cyclase-activating polypeptide depolarization of rat sympathetic neurons. J Neurosci 20: 7353-7361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielsky IF, Hu SB, Szegda KL, Westphal H, Young LJ (2004) Profound impairment in social recognition and reduction in anxiety-like behavior in vasopressin V1a receptor knockout mice. Neuropsychopharmacology 29: 483-493. [DOI] [PubMed] [Google Scholar]
- Birnbaumer M (2000) Vasopressin receptors. Trends Endocrinol Metab 11: 406-410. [DOI] [PubMed] [Google Scholar]
- Bluthe RM, Dantzer R (1993) Role of the vomeronasal system in vasopressinergic modulation of social recognition in rats. Brain Res 604: 205-210. [DOI] [PubMed] [Google Scholar]
- Bonfanti L, Peretto P, Merighi A, Fasolo A (1997) Newly-generated cells from the rostral migratory stream in the accessory olfactory bulb of the adult rat. Neuroscience 81: 489-502. [DOI] [PubMed] [Google Scholar]
- Buck LB (1996) Information coding in the vertebrate olfactory system. Annu Rev Neurosci 19: 517-544. [DOI] [PubMed] [Google Scholar]
- Choi EJ, Ha CM, Kim MS, Kang JH, Park SK, Choi WS, Kang SG, Lee BJ (2000) Central administration of an antisense oligodeoxynucleotide against type I pituitary adenylate cyclase-activating polypeptide receptor suppresses synthetic activities of LHRH-LH axis during the pubertal process. Brain Res Mol Brain Res 80: 35-45. [DOI] [PubMed] [Google Scholar]
- Corotto FS, Henegar JR, Maruniak JA (1994) Odor deprivation leads to reduced neurogenesis and reduced neuronal survival in the olfactory bulb of the adult mouse. Neuroscience 61: 739-744. [DOI] [PubMed] [Google Scholar]
- Daenen EW, Wolterink G, Gerrits MA, Van Ree JM (2002) The effects of neonatal lesions in the amygdala or ventral hippocampus on social behaviour later in life. Behav Brain Res 136: 571-582. [DOI] [PubMed] [Google Scholar]
- Dantzer R (1998) Vasopressin, gonadal steroids and social recognition. Prog Brain Res 119: 409-414. [DOI] [PubMed] [Google Scholar]
- Del Punta K, Leinders-Zufall T, Rodriguez I, Jukam D, Wysocki CJ, Ogawa S, Zufall F, Mombaerts P (2002) Deficient pheromone responses in mice lacking a cluster of vomeronasal receptor genes. Nature 419: 70-74. [DOI] [PubMed] [Google Scholar]
- Ferguson JN, Young LJ, Hearn EF, Matzuk MM, Insel TR, Winslow JT (2000) Social amnesia in mice lacking the oxytocin gene. Nat Genet 25: 284-288. [DOI] [PubMed] [Google Scholar]
- Ferguson JN, Aldag JM, Insel TR, Young LJ (2001) Oxytocin in the medial amygdala is essential for social recognition in the mouse. J Neurosci 21: 8278-8285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson JN, Young LJ, Insel TR (2002) The neuroendocrine basis of social recognition. Front Neuroendocrinol 23: 200-224. [DOI] [PubMed] [Google Scholar]
- Gheusi G, Bluthe RM, Goodall G, Dantzer R (1994) Social and individual recognition in rodents: methodological aspects and neurobiological bases. Behav Proc 33: 59-87. [DOI] [PubMed] [Google Scholar]
- Gheusi G, Cremer H, McLean H, Chazal G, Vincent JD, Lledo PM (2000) Importance of newly generated neurons in the adult olfactory bulb for odor discrimination. Proc Natl Acad Sci USA 97: 1823-1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halpern M (1987) The organization and function of the vomeronasal system. Annu Rev Neurosci 10: 325-362. [DOI] [PubMed] [Google Scholar]
- Hannibal J (2002) Pituitary adenylate cyclase-activating peptide in the rat central nervous system: an immunohistochemical and in situ hybridization study. J Comp Neurol 453: 389-417. [DOI] [PubMed] [Google Scholar]
- Hansel DE, May V, Eipper BA, Ronnett GV (2001) Pituitary adenylyl cyclase-activating peptides and α-amidation in olfactory neurogenesis and neuronal survival in vitro J Neurosci 21: 4625-4636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmar AJ, Arimura A, Gozes I, Journot L, Laburthe M, Pisegna JR, Rawlings SR, Robberecht P, Said SI, Sreedharan SP, Wank SA, Waschek JA (1998) International union of pharmacology. XVIII. Nomenclature of receptors for vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide. Pharmacol Rev 50: 265-270. [PMC free article] [PubMed] [Google Scholar]
- Hashimoto H, Nogi H, Mori K, Ohishi H, Shigemoto R, Yamamoto K, Matsuda T, Mizuno N, Nagata S, Baba A (1996) Distribution of the mRNA for a pituitary adenylate cyclase-activating polypeptide receptor in the rat brain: an in situ hybridization study. J Comp Neurol 371: 567-577. [DOI] [PubMed] [Google Scholar]
- Hashimoto H, Shintani N, Tanaka K, Mori W, Hirose M, Matsuda T, Sakaue M, Miyazaki J, Niwa H, Tashiro F, Yamamoto K, Koga K, Tomimoto S, Kunugi A, Suetake S, Baba A (2001) Altered psychomotor behaviors in mice lacking pituitary adenylate cyclase-activating polypeptide (PACAP). Proc Natl Acad Sci USA 98: 13355-13360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrichs SC, Min H, Tamraz S, Carmouche M, Boehme SA, Vale WW (1997) Anti-sexual and anxiogenic behavioral consequences of corticotropin-releasing factor overexpression are centrally mediated. Psychoneuroendocrinology 22: 215-224. [DOI] [PubMed] [Google Scholar]
- Hollander E, Novotny S, Hanratty M, Yaffe R, DeCaria CM, Aronowitz BR, Mosovich S (2003) Oxytocin infusion reduces repetitive behaviors in adults with autistic and Asperger's disorders. Neuropsychopharmacology 28: 193-198. [DOI] [PubMed] [Google Scholar]
- Insel TR, Winslow JT (1998) Serotonin and neuropeptides in affiliative behaviors. Biol Psychiatry 44: 207-219. [DOI] [PubMed] [Google Scholar]
- Insel TR, O'Brien DJ, Leckman JF (1999) Oxytocin, vasopressin, and autism: is there a connection? Biol Psychiatry 45: 145-157. [DOI] [PubMed] [Google Scholar]
- Jamen F, Rodriguez-Henche N, Pralong F, Jegou B, Gaillard R, Bockaert J, Brabet P (2000a) PAC1 null females display decreased fertility. Ann NY Acad Sci 921: 400-404. [DOI] [PubMed] [Google Scholar]
- Jamen F, Persson K, Bertrand G, Rodriguez-Henche N, Puech R, Bockaert J, Ahren B, Brabet P (2000b) PAC1 receptor-deficient mice display impaired insulinotropic response to glucose and reduced glucose tolerance. J Clin Invest 105: 1307-1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaworski DM, Proctor MD (2000) Developmental regulation of pituitary adenylate cyclase-activating polypeptide and PAC(1) receptor mRNA expression in the rat central nervous system. Brain Res Dev Brain Res 120: 27-39. [DOI] [PubMed] [Google Scholar]
- Jones RB, Nowell NW (1974) A comparison of the aversive and female attractant properties of urine from dominant and subordinate male mice. Med Weter 2: 141-144. [DOI] [PubMed] [Google Scholar]
- Koves K, Arimura A, Gorcs TG, Somogyvari-Vigh A (1991) Comparative distribution of immunoreactive pituitary adenylate cyclase activating polypeptide and vasoactive intestinal polypeptide in rat forebrain. Neuroendocrinology 54: 159-169. [DOI] [PubMed] [Google Scholar]
- Ku CY, Qian A, Wen Y, Anwer K, Sanborn BM (1995) Oxytocin stimulates myometrial GTPase and phospholipase C activities via coupling to Gq/11. Endocrinology 136: 1509-1515. [DOI] [PubMed] [Google Scholar]
- Landgraf R, Gerstberger R, Montkowski A, Probst JC, Wotjak CT, Holsboer F, Engelmann M (1995) V1 vasopressin receptor antisense oligodeoxynucleotide into septum reduces vasopressin binding, social discrimination abilities, and anxiety-related behavior in rats. J Neurosci 15: 4250-4258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latane B, Glass DC (1968) Social and nonsocial attraction in rats. J Pers Soc Psychol 9: 142-146. [DOI] [PubMed] [Google Scholar]
- Lehman MN, Winans SS, Powers JB (1980) Medial nucleus of the amygdala mediates chemosensory control of male hamster sexual behavior. Science 210: 557-560. [DOI] [PubMed] [Google Scholar]
- Liebenauer LL, Slotnick BM (1996) Social organization and aggression in a group of olfactory bulbectomized male mice. Physiol Behav 60: 403-409. [DOI] [PubMed] [Google Scholar]
- Lim MM, Young LJ (2004) Vasopressin-dependent neural circuits underlying pair bond formation in the monogamous prairie vole. Neuroscience 125: 35-45. [DOI] [PubMed] [Google Scholar]
- Lim MM, Wang Z, Olazabal DE, Ren X, Terwilliger EF, Young LJ (2004) Enhanced partner preference in a promiscuous species by manipulating the expression of a single gene. Nature 429: 754-757. [DOI] [PubMed] [Google Scholar]
- Liu Y, Wang ZX (2003) Nucleus accumbens oxytocin and dopamine interact to regulate pair bond formation in female prairie voles. Neuroscience 121: 537-544. [DOI] [PubMed] [Google Scholar]
- Maestrini E, Paul A, Monaco AP, Bailey A (2000) Identifying autism susceptibility genes. Neuron 28: 19-24. [DOI] [PubMed] [Google Scholar]
- Mantella RC, Vollmer RR, Li X, Amico JA (2003) Female oxytocin-deficient mice display enhanced anxiety-related behavior. Endocrinology 144: 2291-2296. [DOI] [PubMed] [Google Scholar]
- Martin JL, Dietl MM, Hof PR, Palacios JM, Magistretti PJ (1987) Autoradiographic mapping of [mono[125I]iodo-Tyr10, MetO17]vasoactive intestinal peptide binding sites in the rat brain. Neuroscience 23: 539-565. [DOI] [PubMed] [Google Scholar]
- Marui T, Hashimoto O, Nanba E, Kato C, Tochigi M, Umekage T, Kato N, Sasaki T (2004) Gastrin-releasing peptide receptor (GRPR) locus in Japanese subjects with autism. Brain Dev 26: 5-7. [DOI] [PubMed] [Google Scholar]
- Masuo Y, Ohtaki T, Masuda Y, Tsuda M, Fujino M (1992) Binding sites for pituitary adenylate cyclase activating polypeptide (PACAP): comparison with vasoactive intestinal polypeptide (VIP) binding site localization in rat brain sections. Brain Res 575: 113-123. [DOI] [PubMed] [Google Scholar]
- Moser A, Scholz J, Gansle A (1999) Pituitary adenylate cyclase-activating polypeptide (PACAP-27) enhances tyrosine hydroxylase activity in the nucleus accumbens of the rat. Neuropeptides 33: 492-497. [DOI] [PubMed] [Google Scholar]
- Mucignat-Caretta C, Caretta A, Cavaggioni A (1995) Acceleration of puberty onset in female mice by male urinary proteins. J Physiol (Lond) 486: 517-522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicot A, DiCicco-Bloom E (1999) PACAP inhibits mitosis in the subventricular zone of adult mice. Brain Res 848: 1-10.10612694 [Google Scholar]
- Nicot A, DiCicco-Bloom E (2001) Regulation of neuroblast mitosis is determined by PACAP receptor isoform expression. Proc Natl Acad Sci USA 98: 4758-4763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicot A, Lelievre V, Tam J, Waschek JA, DiCicco-Bloom E (2002) Pituitary adenylate cyclase-activating polypeptide and sonic hedgehog interact to control cerebellar granule precursor cell proliferation. J Neurosci 22: 9244-9254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto C, Martin M, Wolfer DP, Lipp HP, Maldonado R, Schutz G (2001) Altered emotional behavior in PACAP-type-I-receptor-deficient mice. Mol Brain Res 92: 78-84. [DOI] [PubMed] [Google Scholar]
- Petreanu L, Alvarez-Buylla A (2002) Maturation and death of adult-born olfactory bulb granule neurons: role of olfaction. J Neurosci 22: 6106-6113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaff D (2001) Precision in mouse behavior genetics. Proc Natl Acad Sci USA 98: 5957-5960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piggins HD, Stamp JA, Burns J, Rusak B, Semba K (1996) Distribution of pituitary adenylate cyclase activating polypeptide (PACAP) immunoreactivity in the hypothalamus and extended amygdala of the rat. J Comp Neurol 376: 278-294. [DOI] [PubMed] [Google Scholar]
- Pitkow LJ, Sharer CA, Ren X, Insel TR, Terwilliger EF, Young LJ (2001) Facilitation of affiliation and pair-bond formation by vasopressin receptor gene transfer into the ventral forebrain of a monogamous vole. J Neurosci 21: 7392-7396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popik P, van Ree JM (1991) Oxytocin but not vasopressin facilitates social recognition following injection into the medial preoptic area of the rat brain. Eur Neuropsychopharmacol 1: 555-560. [DOI] [PubMed] [Google Scholar]
- Popik P, van Ree JM (1998) Neurohypophyseal peptides and social recognition in rats. Prog Brain Res 119: 415-436. [DOI] [PubMed] [Google Scholar]
- Popik P, Vos PE, Van Ree JM (1992) Neurohypophyseal hormone receptors in the septum are implicated in social recognition in the rat. Behav Pharmacol 3: 351-358. [PubMed] [Google Scholar]
- Powers JB, Winans SS (1975) Vomeronasal organ: critical role in mediating sexual behavior of the male hamster. Science 187: 961-963. [DOI] [PubMed] [Google Scholar]
- Rochefort C, Gheusi G, Vincent JD, Lledo PM (2002) Enriched odor exposure increases the number of newborn neurons in the adult olfactory bulb and improves odor memory. J Neurosci 22: 2679-2689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savic I, Berglund H, Gulyas B, Roland P (2001) Smelling of odorous sex hormone-like compounds causes sex-differentiated hypothalamic activations in humans. Neuron 31: 661-668. [DOI] [PubMed] [Google Scholar]
- Schoneberg T, Kostenis E, Liu J, Gudermann T, Wess J (1998) Molecular aspects of vasopressin receptor function. Adv Exp Med Biol 449: 347-358. [DOI] [PubMed] [Google Scholar]
- Scotto-Lomassese S, Strambi C, Strambi A, Aouane A, Augier R, Rougon G, Cayre M (2003) Suppression of adult neurogenesis impairs olfactory learning and memory in an adult insect. J Neurosci 23: 9289-9296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shintani N, Mori W, Hashimoto H, Imai M, Tanaka K, Tomimoto S, Hirose M, Kawaguchi C, Baba A (2002) Defects in reproductive functions in PACAP-deficient female mice. Regul Pept 109: 45-48. [DOI] [PubMed] [Google Scholar]
- Shors TJ, Miesegaes G, Beylin A, Zhao M, Rydel T, Gould E (2001) Neurogenesis in the adult is involved in the formation of trace memories. Nature 410: 372-376. [DOI] [PubMed] [Google Scholar]
- Sims KB, Hoffman DL, Said SI, Zimmerman EA (1980) Vasoactive intestinal polypeptide (VIP) in mouse and rat brain: an immunocytochemical study. Brain Res 186:165-183. Stowers L, Holy TE, Meister M, Dulac C, Koentges G (2002) Loss of sex discrimination and male-male aggression in mice deficient for TRP2. Science 295: 1493-1500. [DOI] [PubMed] [Google Scholar]
- Suh J, Lu N, Nicot A, Tatsuno I, DiCicco-Bloom E (2001) PACAP is an anti-mitogenic signal in developing cerebral cortex. Nat Neurosci 4: 123-124. [DOI] [PubMed] [Google Scholar]
- Szabo F, Horvath J, Heinzlmann A, Arimura A, Koves K (2002) Neonatal PACAP administration in rats delays puberty through the influence of the LHRH neuronal system. Regul Pept 109: 49-55. [DOI] [PubMed] [Google Scholar]
- Thor DH (1980) Reciprocal homosexual mounting behavior in paired anosmic male rats. Psychol Rep 47: 349-350. [DOI] [PubMed] [Google Scholar]
- Thor DH, Flannelly KJ (1977) Peripheral anosmia and social investigatory behavior of the male rat. Behav Biol 20: 128-134. [DOI] [PubMed] [Google Scholar]
- Waschek JA (2002) Multiple actions of pituitary adenylyl cyclase activating peptide in nervous system development and regeneration. Dev Neurosci 24: 14-23. [DOI] [PubMed] [Google Scholar]
- Wassink TH, Piven J, Vieland VJ, Pietila J, Goedken RJ, Folstein SE, Sheffield VC (2004) Examination of AVPR1a as an autism susceptibility gene. Mol Psychiatry, in press. [DOI] [PubMed]
- Wersinger SR, Ginns EI, O'Carroll AM, Lolait SJ, Young III WS (2002) Vasopressin V1b receptor knockout reduces aggressive behavior in male mice. Mol Psychiatry 7: 975-984. [DOI] [PubMed] [Google Scholar]
- Wysocki CJ, Wellington JL, Beauchamp GK (1980) Access of urinary nonvolatiles to the mammalian vomeronasal organ. Science 207: 781-783. [DOI] [PubMed] [Google Scholar]
- Yamada K, Wada E, Wada K (2000) Male mice lacking the gastrin-releasing peptide receptor (GRP-R) display elevated preference for conspecific odors and increased social investigatory behaviors. Brain Res 870: 20-26. [DOI] [PubMed] [Google Scholar]
- Yamada K, Wada E, Wada K (2001) Female gastrin-releasing peptide receptor (GRP-R)-deficient mice exhibit altered social preference for male conspecifics: implications for GRP/GRP-R modulation of GABAergic function. Brain Res 894: 281-287. [DOI] [PubMed] [Google Scholar]
- Young LJ (2002) The neurobiology of social recognition, approach, and avoidance. Biol Psychiatry 51: 18-26. [DOI] [PubMed] [Google Scholar]
- Zhang J, Webb DM (2003) Evolutionary deterioration of the vomeronasal pheromone transduction pathway in catarrhine primates. Proc Natl Acad Sci USA 100: 8337-8341. [DOI] [PMC free article] [PubMed] [Google Scholar]