Abstract
The Kresge Hearing Research Institute-3 (KHRI-3) antibody binds to a guinea pig inner ear supporting cell antigen (IESCA) and causes hearing loss. To gain insight into the mechanism of antibody-induced hearing loss, we used antibody immunoaffinity purification to isolate the IESCA, which was then sequenced by mass spectroscopy, revealing 10 guinea pig peptides identical to sequences in human choline transporter-like protein 2 (CTL2). Full-length CTL2 cDNA sequenced from guinea pig inner ear has 85.9% identity with the human cDNA. Consistent with its expression on the surface of supporting cells in the inner ear, CTL2 contains 10 predicted membrane-spanning regions with multiple N-glycosylation sites. The 68 and 72 kDa molecular forms of inner ear CTL2 are distinguished by sialic acid modification of the carbohydrate. The KHRI-3 antibody binds to an N-linked carbohydrate on CTL2 and presumably damages the organ of Corti by blocking the transporter function of this molecule. CTL2 mRNA and protein are abundantly expressed in human inner ear. Sera from patients with autoimmune hearing loss bind to guinea pig inner ear with the same pattern as CTL2 antibodies. Thus, CTL2 is a possible target of autoimmune hearing loss in humans.
Keywords: antibody, antigen, auditory, autoimmunity, cochlea, deafness, choline, membrane, transport
Introduction
Autoimmune damage to the inner ear is suspected to be a cause of rapidly progressing hearing loss (McCabe, 1979). Antibodies to a 68-72 kDa inner ear antigen in the sera of affected individuals (Harris and Sharp, 1990; Moscicki et al., 1994; Gottschlich et al., 1995; Disher et al., 1997) correlate with response to immunosuppressive therapy (Moscicki et al., 1994; Gottschlich et al., 1995). If such antibodies do cause hearing loss, identification of the target antigen and elucidation of the mechanism by which the antibody causes hearing loss would be useful in the diagnosis and management of autoimmune hearing loss (AHL). Two groups identified heat shock protein 70 (HSP70) as a candidate target antigen (Billings et al., 1995; Bloch et al., 1995); however, HSP70 has not held up as a high-probability inner ear target antigen (Billings et al., 1998; Trune et al., 1998; Yeom et al., 2003). We developed monoclonal antibodies to cells isolated from the guinea pig (GP) organ of Corti (Zajic et al., 1991) and observed that hearing loss developed in mice carrying the Kresge Hearing Research Institute-3 (KHRI-3) hybridoma (Nair et al., 1995), provoking us to study this antibody and its antigen in more depth. In the guinea pig organ of Corti, the KHRI-3 antibody binds to supporting cells with a distinctive “wine-glass” pattern. Infusion of purified antibody into the cochlea yields in vivo binding to supporting cells in the organ of Corti that is accompanied by loss of outer hair cells, loss of hearing, and redistribution of the antigen in the regions of hair cell loss (Nair et al., 1995, 1997, 1999). Sera from suspected AHL patients often have antibody that binds to guinea pig inner ear supporting cells with the same distinctive wine-glass staining pattern as that observed with the monoclonal antibody (Disher et al., 1997). The presence of antibody with the same pattern of binding to supporting cells in sera from AHL patients implies that a similar mechanism may be at work in humans with rapidly progressive hearing loss.
To understand the mechanisms of KHRI-3 antibody-induced hearing loss, it is necessary to identify and characterize the inner ear supporting cell antigen (IESCA). We isolated the IESCA by immunoaffinity and then gel-purified and sequenced it by dual mass spectroscopy. In the affinity-purified material, 10 peptides were identified that are identical in sequence to human CTL2. Antisera to a conserved CTL2 peptide bind to the KHRI-3 IESCA. Thus, the KHRI-3 IESCA is the guinea pig homolog of human CTL2. In the human inner ear, CTL2 mRNA and protein are expressed, indicating a phylogenetically conserved inner ear function for this protein. The results indicate that CTL2 is a target of antibody-mediated hearing loss, that antibody binding disturbs the function of this molecule, and that proper function of CTL2 is required for hair cell survival.
Materials and Methods
Antibodies. Serum-free KHRI-3 monoclonal antibody was produced in the Cellmax Artificial Capillary System and purified on protein-G columns (Pierce, Rockford, IL) as described previously (Nair et al., 1997). Rabbit antisera were raised to a phylogenetically conserved antigenic segment in the N-terminal domain of CTL2 (Table 1). Two rabbits (R228 and R229) were each immunized four times with 500 μg of the synthetic CTL2 peptide (Princeton Biochemicals, Langome, PA). Antisera were tested for approximate antibody titer by ELISAs against the immunizing peptide, as described previously (Zajic et al., 1991; Nair et al., 1995). Protein-G-purified KHRI-3 IgG1 antibody and protein-A-Sepharose (Pierce)-purified IgG from the rabbit antisera to CTL2 were coupled to cyanogen bromide (CNBr)-activated Sepharose 4B beads (Sigma, St. Louis, MO) in a ratio of 5 mg of antibody to 1 gm of beads.
Table 1.
Peptide sequences obtained by MS-MS sequencing of the 68 and 72 kDa IESCA bands a
|
|
Sequencing run 1 |
Sequencing run 2 |
||
|---|---|---|---|---|
| Peptide |
68 and 72 kDa |
68 kDa |
72 kDa |
|
| 1 | (K)YDPTFK | (K)YDPTFKGPIYNR | ||
| 2 | (K)VIYPTDSR | (R)KVIYPTDSR | (R)KVIYPTDSR | |
| 3 | (K)CASPLVLLEFQCPTPQICVEK | (K)CASPLVLLEFQCPTPQICVEK | (K)CASPLVLLEFQCPTPQICVEK | |
| 4 | (K)GVAEVLR | |||
| 5 | (R)VYLHLR | (R)VYLHLR | ||
| 6 | (R)ILIAIALIK | |||
| 7 | (R)CQFAFYGGESGYHR | (R)CQFAFYGGESGYHR | (R)CQFAFYGGESGYHR | |
| 8 | (R)NAFFLLMR | (R)NAFFLLMR | (R)NAFFLLMR | |
| 9 | (K)VTDFLFLLGK | |||
| 10 |
|
(R)NDGSAERPYFMSSTLK |
(R)NDGSAERPYFMSSTLK |
|
The letter in parentheses signifies that the preceding amino acid was either lysine (K) or arginine (R).
Inner ear tissues. All animal studies were reviewed and approved by the University of Michigan Committee on Use and Care of Animals. In addition, the Division of Research Grants of the National Institutes of Health-National Institute on Deafness and Other Communication Disorders reviewed and approved all studies described in this report. To prepare inner ear extracts, guinea pig temporal bones were collected immediately after euthanasia, and the bullas were opened and placed in PBS on ice. The bone of the otic capsule was quickly removed, and the organ of Corti and vestibular sensory organs were collected in lysis buffer [Dulbecco's PBS (Invitrogen, Carlsbad, CA) containing 0.5% NP-40 and a mixture of protease inhibitors (Roche Diagnostics, Mannheim, Germany)] and immediately homogenized as described previously (Zajic et al., 1991; Disher et al., 1997). For immunofluorescence (IF) experiments, guinea pigs were decapitated, bullas were removed, and the otic capsules were opened and fixed in 2% paraformaldehyde in phosphate buffer, pH 7.2, for 2 hr. The cochleas were washed; dissected free of the bony capsule, spiral ligament, stria vascularis, and tectorial membrane; permeabilized with 0.3% Triton X-100; and incubated in 3% normal goat serum for 30 min before incubation with antibodies. The guinea pig vestibular system was dissected and prepared for immunofluorescence as described previously (Ptok et al., 1991). Fresh human inner ear vestibular tissue was obtained from patients undergoing translabyrinthine procedures for removal of acoustic tumors or nerve section for intractable vertigo. Patients gave previous informed written consent for the use of their tissue. The study was approved by the University of Michigan Institutional Review Committee for the Protection of Human Research Subjects. The human tissues were fixed in 2% paraformaldehyde in phosphate buffer, pH 7.2, for 2 hr and washed three times with PBS. The cupula or otoconial membranes were carefully removed, and the crista ampularis or macula was incubated with 3% goat serum, washed, and incubated overnight at 4°C in anti-CTL2 serum diluted 1:100. The tissue was washed three times in PBS and incubated for 45 min at room temperature in affinity-purified tetramethylrhodamine isothiocyanate-conjugated goat anti-rabbit antibody from Jackson ImmunoResearch (West Grove, PA). After a final wash, the tissues were mounted in Prolong Antifade Mounting Media (Molecular Probes, Eugene, OR) and examined. For RNA extraction, inner ear tissues (guinea pig or human) were dissected in cold RNAlater (Ambion, Austin, TX), sheared through an 18 gauge needle five times, and homogenized by centrifugation through a QIAShredder (Qiagen, Valencia, CA). Total RNA was extracted from guinea pig cochlea, human vestibular tissues, and human cell lines with the RNeasy Mini and Midi kits (Qiagen), respectively.
Immunoprecipitation. Cochlear or vestibular tissue lysates were mixed with CNBr beads coupled to either KHRI-3 or CTL2 antibody (20 μl per ear), incubated overnight at 4°C, and centrifuged to recover the beads. In some experiments, extracts were incubated overnight with anti-CTL2 antibody and antigen-antibody complexes were precipitated by addition of 30 μl of protein A-agarose (Sigma) for 2 hr at 4°C. The precipitates were collected by centrifugation at 5000 × g for 5 min, washed three times, resuspended in sample buffer for either reducing or nonreducing conditions, boiled, and subjected to electrophoresis.
SDS-PAGE. Cochlear extracts, immunoprecipitated antigen, and molecular weight standards were prepared in sample buffer (Zajic et al., 1991; Disher et al., 1997), boiled for 5 min, and separated by electrophoresis using 3% stacking gels with 8 or 10% separating gels. Samples for dual mass spectrometric (MS-MS) sequencing were isolated using 1-mm-thick gels. All reagents used for sequencing studies were prepared in HPLC-grade water.
Immunoaffinity purification and sequencing of the IESCA. We used KHRI-3-CNBr beads either in an immunoaffinity column or in an immunoprecipitation paradigm to isolate the IESCA for MS-MS peptide sequence analysis. In the first experiment, protein extracts from 12 ears were divided into two aliquots, and each was mixed with 1 ml of beads coupled to 1.25 mg of purified KHRI-3 monoclonal antibody. The antibody-coupled beads were washed with binding buffer (lysis buffer, described above), mixed with guinea pig cochlear lysate, and gently rocked overnight at 4°C. The beads were transferred to a 10 ml Poly-Prep Chromatography Column (Bio-Rad, Richmond, CA), and the antigen was eluted with 100 mm glycine-HCl, pH 2.8, containing 0.5% NP-40, collected in 1 ml fractions, immediately neutralized with 1 m sodium phosphate, pH 8.0, and concentrated using Millipore (Bedford, MA) Ultrafree centrifugal filters. The concentrated sample was reduced and electrophoresed in a 1-mm-thick 8% SDS-PAGE gel. Part of the sample was Western blotted with KHRI-3 to identify the two colloidal Coomassie blue-stained protein bands in the gel that migrated at 68 and 72 kDa. These bands were cut from the gel and pooled. A region of the same size was cut from an empty lane as a background control. A second affinity-purification experiment was performed and pooled with the previous sample. Control slices and gel slices containing bands of interest were washed two times for 3 min each with 50% acetonitrile (CH3CN) in water, frozen, and sent to the Microchemistry Facility of Harvard University (Cambridge, MA) for MS-MS peptide sequencing.
In a second peptide-sequencing experiment, the IESCA was immunoprecipitated. Extracts from 12 ears were incubated overnight with 240 μl of KHRI-3-CNBr beads (∼300 μg of purified antibody) at 4°C and washed five times with 1 ml of binding buffer. After the last wash, the beads were resuspended in 120 μl of reducing buffer (2% SDS, 10% glycerol, 5% 2-mercaptoethanol in 62.5 mm Tris-Cl, pH 6.8), boiled, and briefly centrifuged. The supernatant was electrophoresed, and the gel was stained with colloidal Coomassie blue. Bands migrating at 68 and 72 kDa were cut out, placed in separate tubes, and processed for MS-MS sequencing, as described above.
Western blotting. Proteins from electrophoresis gels were transferred to nitrocellulose membranes at a constant voltage of 25 V in transfer buffer (20% methanol, 150 mm glycine, 20 mm Tris buffer). The membranes were blocked and probed with primary and secondary antibody as described previously (Zajic et al., 1991; Disher et al., 1997).
Enzymatic deglycosylation. For enzymatic deglycosylation, one ear equivalent of KHRI-3 affinity-purified antigen (30 μl) in sodium phosphate buffer, pH 7.6, was mixed with 3 μl of 10% denaturing buffer (5% SDS, 10% β-mercaptoethanol), boiled for 10 min, cooled, and treated with 1:10 vol 10% NP-40 to counter the effects of SDS and ensure proper enzyme activity (Blass et al., 1998). This mixture was then digested in separate tubes at 37°C overnight with 50 mU of neuraminidase, 0.5 U of O-glycosidase (Roche Diagnostics, Indianapolis, IN), 1000 U of N-glycosidase F (PNGase F; New England Biolabs, Beverly, MA), or both neuraminidase and O-glycosidase. Control digestions of fetuin confirmed the enzyme activity. The samples were then boiled with 5× nonreducing buffer and subjected to SDS-PAGE and Western blotting.
Reverse transcription-PCR. For the reverse transcription (RT) reaction, 2 μg of total RNA was used to synthesize cDNA. Both oligo-dT and random primers were used with Moloney murine leukemia virus reverse transcriptase (Invitrogen) according to the manufacturer's instructions. All primers were designed using Primer3 software (http://www-genome.wi.mit.edu/cgi-bin/primer/primer3_www.cgi). To amplify the CTL2 cDNA, six sets of sense and antisense primers were designed based on the human CTL2 gene sequence. To sequence the guinea pig CTL2 cDNA, we used a back-translation algorithm [Swiss Prot Expert Protein Analysis System (ExPASy); Swiss Institute of Bioinformatics; http://us.expasy.org/] (Falquet et al., 2002) to deduce the possible guinea pig coding region from the amino acid sequence. This information was then used to design the initial four primer sets (Table 2), after which a walking primer strategy was implemented. We then designed long-range guinea pig primers based on these known sequences. PCR was performed using 0.5 μl of cDNA, 200 μm deoxy-NTPs, a 300 nm concentration of each primer, 1× PCR buffer, 1.5 mm MgCl2, and 2.6 U of Expand High-Fidelity DNA Polymerase (Roche Diagnostics). After denaturation at 94°C for 1 min, the reaction was performed for 30-35 cycles at 94°C for 1 min, at 56-65°C (depending on the primer set) for 1 min, and at 72°C for 2 min. For amplification of long sequences (i.e., >1 kb), we used the Advantage cDNA Kit and Polymerase Mix (Clontech, Cambridge, UK).
Table 2.
PCR primer sets for sequencing human and guinea pig CTL2 cDNAs
|
1 |
CCACTTTCAAAGGACCCATTTAC |
CGAAGCACCTCAGCCACTC |
403 |
| 2 | CTCATCGCGATTGCACTCAT | CCGACTCACCACCGTAGAAG | 289 |
| 3 | GCCTTCTACGGTGGTGAGTC | CCCAACAGGAAGAGGAAGTC | 527 |
| 4 | ATGCCTTCTTCCTGCGCAT | TTTCTTGAGGGTGGAAGACAT | 347 |
| GP 5′ RACE | AGG ACG ATC ATC ACC CAG AC | ||
| GPSP1 | |||
| GP 5′ RACE | GCA TTC AGG AGG GTG AGG TA | ||
| GSPS2 | |||
| GP 5′ RACE | GGGCTGGCACACTTTACAAT | ||
| GPSP3 | |||
| GP 3′ RACE | GGGATTCTGGCTTTCTTCTT | ||
| GPSP5 | |||
| Long distance 2 | CTACCTCACGTACCTGAATGCTC | AATCATGATGTAGGCATTCCTATTAGG | |
| Guinea pig long distance | AGGTGATCTACCCCACTGATAGC | GGGACCCAGTAATAGTTGAGAGG | 1737 |
| RACE 5′ HSP1 | AGA TCT GGG GAG TGG GAC AT | 334 | |
| RACE 5′ HSP2 | TTT CGA GGG TCT CCA TGA GT | 200 | |
| RACE 5′ HSP3 | GTG GGG TAG ATC ACC TTT CG | 185 | |
| RACE 3′ HSP5 |
TCATCAGAGTGGCTGTCCTG |
|
359 |
The elongation step at 72°C was increased to 3 min for each cycle. PCR products were visualized on a 1.5% agarose gel using ethidium bromide fluorescence and were purified using the QIAquick PCR purification kit (Qiagen). Sequencing was by the University of Michigan DNA Sequencing Core.
Rapid amplification of cDNA ends. Both 5′ and 3′ rapid amplification of cDNA ends (RACE) were performed with the 5′/3′ RACE Kit (Roche Diagnostics). For 5′ RACE, an antisense primer, guinea pig-specific primer 1 (GPSP1) (Table 2), was used to make cDNA from GP cochlea total RNA using avian myeloblastosis virus (AMV)-RT. The purified cDNA was tailed with deoxy-ATP and terminal transferase. This tailed cDNA was then subjected to PCR using an oligo-dT-anchor primer and the antisense primer GPSP2 (Table 2), which was designed using the Primer3 program based on the previously determined GP sequence. A nested PCR was performed using this PCR product as the template with the GPSP3 antisense primer and the anchor primer. The same protocol was used for human inner ear and University of Michigan-Squamous Cell Carcinoma (UM-SCC)-11A RNA; these primers were designed from the known human CTL2 sequence human specific primer 1 (HuSP1)-HuSP3 (Table 2). For 3′ RACE, cDNA was made from total RNA of GP cochlea with an oligo-dT-anchor primer and AMV-RT and used as the template for PCR with the anchor primer and the primer GPSP5 (Table 2). Human inner ear and UM-SCC-11A RNA were subjected to the same procedure; all primers were designed from the known human CTL2 sequence HuSP5 (Table 2). To increase primer specificity, we used Perfect Match PCR Enhancer (Stratagene, La Jolla, CA) in all PCRs.
Results
Identification of CTL2
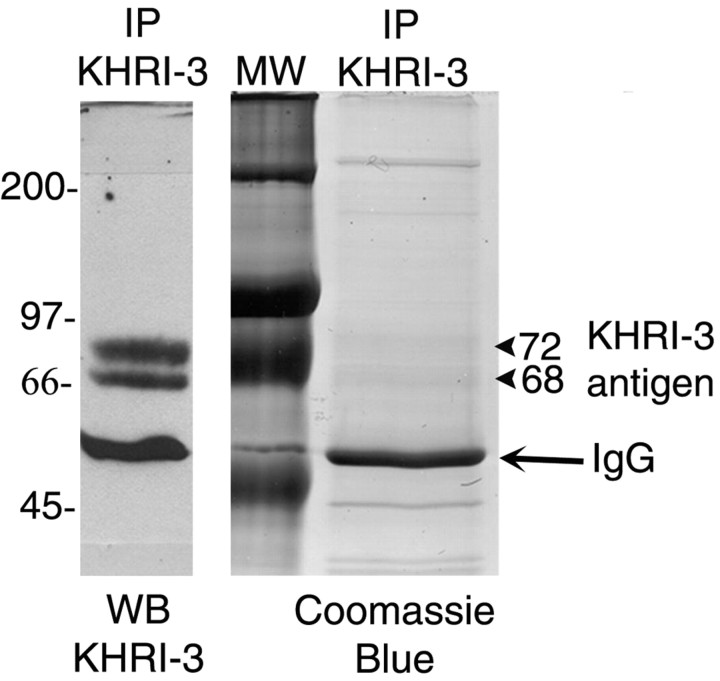
The IESCA defined by KHRI-3 was immunoaffinity-purified from guinea pig organ of Corti and analyzed by Western blot. The KHRI-3 antibody stains a protein doublet of 68 and 72 kDa on the Western blot (Fig. 1, left). The corresponding bands identified by colloidal Coomassie blue staining of the gel (Fig. 1, right) were combined for MS-MS sequencing. Six peptides identical to sequences predicted for human CTL2 (hCTL2), a member of the choline transporter-like protein family (O'Regan et al., 2000), were obtained (Table 1, column 2). To confirm the sequencing results and to determine whether both bands are derived from this protein, the 68 and 72 kDa bands were sequenced independently in a second experiment (Table 1, columns 3 and 4). In the three sequencing runs, we isolated 10 different guinea pig peptides that were identical to hCTL2 and numbered them from 1 to 10, based on their relative location in the predicted hCTL2 protein (Fig. 2, Table 1). As can be seen in the two columns of Sequencing Run 2 (Table 1), 6 of the 10 peptides were present in both the 68 and 72 kDa bands. Thus, both bands contain the guinea pig CTL2 protein.
Figure 1.
Gel purification of the immunoaffinity-purified (IP) inner ear supporting cell antigen. Left, Western blot (WB) of KHRI-3 immunoaffinity-purified inner ear antigen after electrophoresis under reducing conditions, stained with KHRI-3 antibody. Middle, Molecular weight (MW) markers. Right, Colloidal Coomassie blue staining of the PAGE gel showing the location of the 68 and 72 kDa bands corresponding to the KHRI-3 antigen.
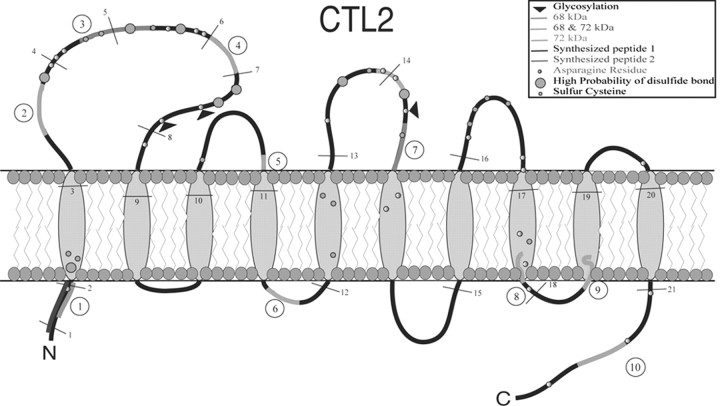
Figure 2.
Predicted structure of hCTL2. Shaded regions labeled with circled numerals from 1 to 10 indicate the location in the model of each of the 10 guinea pig peptides identified by MS-MS sequencing to be identical to human CTL2. The fine black lines that cross the backbone of the protein show the relative locations of the boundaries of the 22 exons. Circles indicate the approximate locations of Cys and Asn residues, respectively. Large circles indicate Cys residues likely to be involved in disulfide bonds. Predicted N-glycosylation sites in the extracellular loops were determined using Prosite (Falquet et al., 2002) and are shown with black arrowheads.
CTL2 is predicted to be a membrane-spanning protein
CTL2 is a member of a family of genes that are predicted to encode choline transporter-like proteins based on homology to CTL1 (O'Regan et al., 2000). The human CTL2 gene maps to chromosome 19p13.1, has a predicted open-reading frame of 22 exons (GenBank accession number AJ245621) (O'Regan et al., 2000), and encodes a putative protein of 706 aa. Using ExPASy (http://ca.expasy.org/sprot/) (Falquet et al., 2002), we determined that the amino acid sequence of hCTL2 predicts a structure with 10 helical transmembrane domains with the N- and C-terminal portions residing in the cytoplasmic domain. Other calculations allow the N terminus to be extracellular but with a greater entropy cost. Figure 2 presents a diagrammatic representation of the hCTL2 protein in the cell membrane, with the 10 peptides elucidated by sequencing shown as shaded regions numbered from 1 to 10. Circles indicate Asn and Cys residues. Four of the Asn residues are predicted to be glycosylated. One of these is in the cytoplasmic C-terminal domain. The three potential N-glycosylation sites in the extracellular domain are indicated by black triangles. The Cys residues predicted to be involved in disulfide bridges are indicated by large circles.
Identity of CTL2 and the IESCA defined by KHRI-3
To compare CTL2 and the IESCA defined by KHRI-3, we used rabbit antisera to a phylogenetically conserved and immunogenic CTL2-specific region in the N-terminal domain. The synthetic peptide contains the 13 aa in peptide 1 (Table 1) plus the next five upstream amino acids in the human CTL2 sequence (HGTPQKYDPTFKGPIYNR) (shown as a darkly shaded bar in the N terminus in Fig. 2). Rabbit antisera to the CTL2 peptide were tested by IF staining of guinea pig inner ear surface preparations, and the staining patterns were compared with that obtained with the KHRI-3 antibody (Fig. 3A). The IF pattern obtained with the anti-CTL2 sera is strikingly similar to the KHRI-3 staining of the phalangeal processes of the outer pillar cells, Deiters cells, and other supporting cells. Preimmune serum had no specific binding to guinea pig inner ear. A cell-surface location of the IESCA is consistent with KHRI-3 and anti-CTL2 IF staining at the membrane of supporting cells (Fig. 3) (Zajic et al., 1991; Nair et al., 1995, 1997, 1999).
Figure 3.
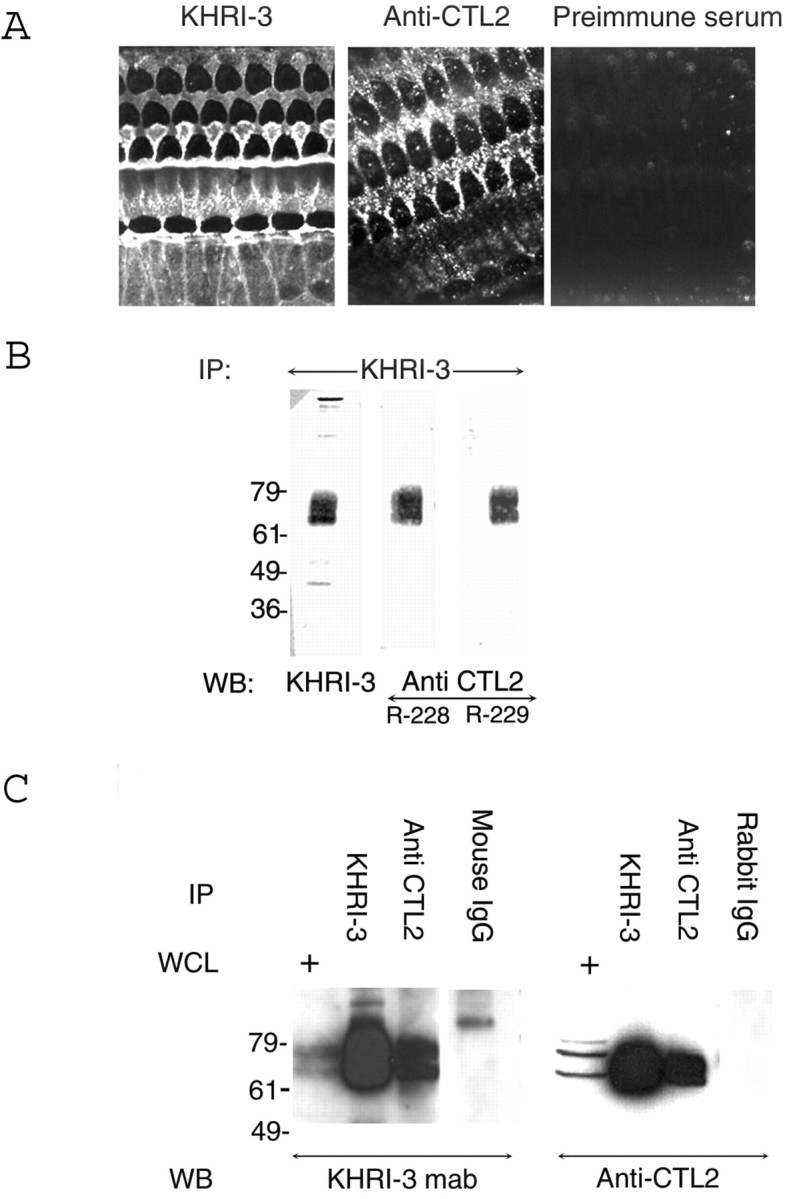
Staining patterns with KHRI-3 and rabbit anti-CTL2 antibodies. A, Immunofluorescence staining of guinea pig organ of Corti with KHRI-3 and CTL2 antibodies. Note the similarity of KHRI-3 antibody (left) and CTL2 antibody (right) staining of the phalangeal process of the pillar cells, Deiters cells, and other supporting cells. Both antibodies produce staining with a punctate pattern. B, Immunoprecipitation (IP) and Western blotting (WB) of KHRI-3 protein from guinea pig inner ear extracts. KHRI-3 immunoprecipitates were electrophoresed under reducing conditions and immunoblotted either with KHRI-3 (left) or with serum from CTL2-immunized rabbits (middle, rabbit 228; right, rabbit 229). C, Reciprocal immunoprecipitation and Western blotting with KHRI-3 and rabbit anti-CTL2 antibodies. Left, Western blotted with KHRI-3; right, Western blotted with anti-CTL2 (rabbit 229). The first lanes contain whole-cell lysate (WCL); the second lanes contain KHRI-3 immunoprecipitates; the third lanes contain anti-CTL2 immunoprecipitates; the fourth lanes contain mock immunoprecipitates after incubation with beads bound to either mouse or rabbit IgG. mab, Monoclonal antibody.
KHRI-3 immunoprecipitates a protein detected by anti-CTL2 antisera
To confirm that CTL2 and the IESCA defined by KHRI-3 are the same molecule, proteins were immunoprecipitated from inner ear extracts with the KHRI-3 monoclonal antibody, separated by SDS-PAGE under nonreducing conditions, transferred to nitrocellulose, and identified by Western blot analysis with either KHRI-3 antibody or the anti-CTL2 sera from each of two immunized rabbits, R228 and R229. Identical staining patterns were obtained in Western blots with each of these antibodies (Fig. 3B). In a second immunoprecipitation experiment, guinea pig inner ear proteins were precipitated with either the KHRI-3 antibody coupled to CNBr beads or the rabbit anti-CTL2 serum (R229) coupled to CNBr beads. Precipitations with mouse IgG or rabbit IgG coupled to CNBr beads were used as controls. The immunoprecipitates were electrophoresed along with whole cochlear lysates, and the blots were probed either with KHRI-3 antibody (Fig. 3C, left) or with the R229 anti-CTL2 antiserum (Fig. 3C, right). KHRI-3 and the rabbit anti-CTL2 antibodies bind to 68 and 72 kDa bands (Fig. 3C) in the whole-cell lysate and also in the immunoprecipitates, indicating that both reagents identify the same proteins. There was no staining of the mock precipitates.
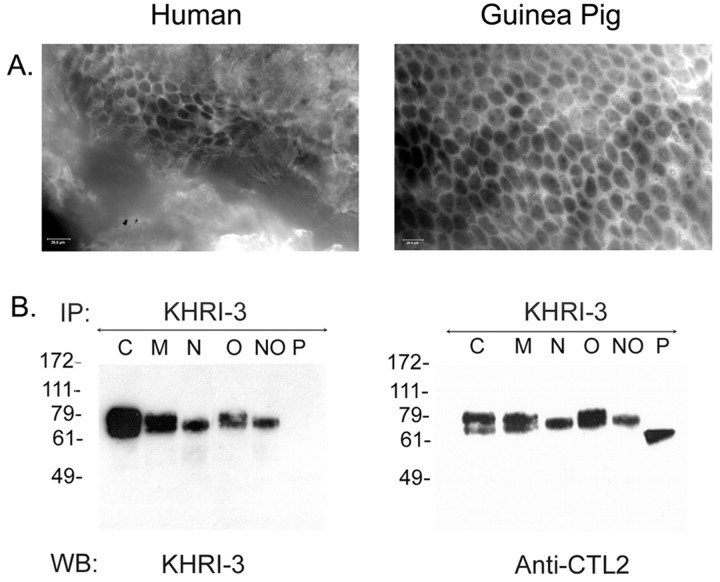
The IESCA detected by KHRI-3 antibody is expressed in a honeycomb pattern on supporting cells in the guinea pig vestibular system (Ptok et al., 1993). CTL2 protein expression in the human inner ear was determined by IF with the rabbit anti-CTL2 serum on vestibular tissues removed from patients undergoing therapeutic translabyrinthine surgical procedures. Similar preparations from the guinea pig vestibular system were used for comparison. CTL2 protein is prominently expressed on vestibular supporting cells (Fig. 4A) in a honeycomb pattern in both the human (left) and guinea pig (right). As in the organ of Corti, the bright staining of the supporting cells surrounds the dark areas where the sensory cells are located.
Figure 4.
CTL2 expression in human and guinea pig vestibular tissue and glycosylation of guinea pig CTL2. A, Immunofluorescence staining of human (left) and guinea pig (right) vestibular tissue with anti-CTL2 antibody. B, Western blots (WB) showing glycosylation of guinea pig CTL2. CTL2 was immunoprecipitated (IP) from guinea pig inner ear extract with KHRI-3 antibody, and aliquots were incubated with enzymes to remove specific carbohydrates, after which the digested samples were Western blotted with either KHRI-3 (left) or rabbit anti-CTL2 (right) antibodies. C lanes, Control protein without enzyme treatment; M lanes, mock-digested protein; N lanes, neuraminidase-digested protein; O lanes, O-glycosidase-digested protein; NO lanes, neuraminidase-digested and O-glycosidase-digested protein; P lanes, PNGase F-digested protein. Note that in the N lanes there is only one band with both antibodies after sialic acid removal, indicating that sialic acid modification may account for the doublet. It is also of interest that the KHRI-3 antibody fails to bind to the protein in the P lane (left) when the N-linked sugars are removed. The CTL2 antibody stains a 62 kDa protein in the P lanes, indicating that this is the deglycosylated core protein. These results verify the following: (1) the KHRI-3 antibody binds to an N-linked sugar, (2) the sialic acid residues account for the difference between lower and upper bands, and (3) the N-linked carbohydrate accounts for ∼10 kDa of the molecular mass.
Carbohydrate accounts for ∼10 kDa of the CTL2 mass
The KHRI-3 antibody binds to both bands of CTL2 from guinea pig inner ear (Figs. 1, 3) but not to IESCA from other species such as chicken or human (data not shown). In contrast, the rabbit anti-CTL2 peptide antibody binds to both bands of CTL2 in guinea pig inner ear extracts (Fig. 3) and also to the IESCA in human and guinea pig inner ear as shown by IF (Fig. 4A). To explain these observations, we postulated that KHRI-3 might recognize either a region of the CTL2 protein that is poorly conserved between species or that this antibody might bind to a carbohydrate modification that is unique to the guinea pig. CTL2 contains several predicted (Prosite) (Falquet et al., 2002) N-linked glycosylation sites (Fig. 2). In addition, because the CTL2 bands appear as a doublet and yet contain the same peptide sequences, we hypothesized that differences in carbohydrate modification might account for the two bands. To examine the nature and degree of the glycosylation, aliquots of KHRI-3 affinity-purified CTL2 protein from guinea pig inner ear were incubated with one or more specific enzymes as follows: neuraminidase (removes sialic acid residues), O-glycosidase (removes O-linked sugars), and PNGase F (removes all N-linked sugars), or with a mock enzyme solution. The digested samples were Western blotted with KHRI-3 monoclonal antibody (Fig. 4B, left) or with anti-CTL2 peptide antibody (Fig. 4B, right). The untreated (Fig. 4B, C lanes) and mock-digested (Fig. 4B, M lanes) CTL2 proteins appear as broad doublets of 68-72 kDa with both antibodies. In protein extracts treated with neuraminidase, the more slowly migrating band of the doublet disappears, leaving only the 68 kDa band (Fig. 4B, N lanes). O-glycosidase treatment did not decrease the molecular mass even when the sialic acid residues were removed by neuraminidase (necessary for O-glycosidase activity) (Fig. 4B, O and NO lanes). PNGase F treatment completely abolished KHRI-3 antibody binding (Fig. 4B, left P lane). However, the rabbit CTL2 antibody shows that the PNGase F-treated protein (Fig. 4B, right P lane) has a Mr of ∼62 kDa. Thus the guinea pig CTL2 protein is N-glycosylated but not O-glycosylated, and KHRI-3 antibody binds to an epitope on an N-linked carbohydrate. The sialic acid residues account for the difference between the lower and upper bands of the doublet, and the carbohydrate accounts for ∼10 kDa of the apparent Mr of the mature CTL2 protein.
Cloning of the inner ear CTL2 cDNA
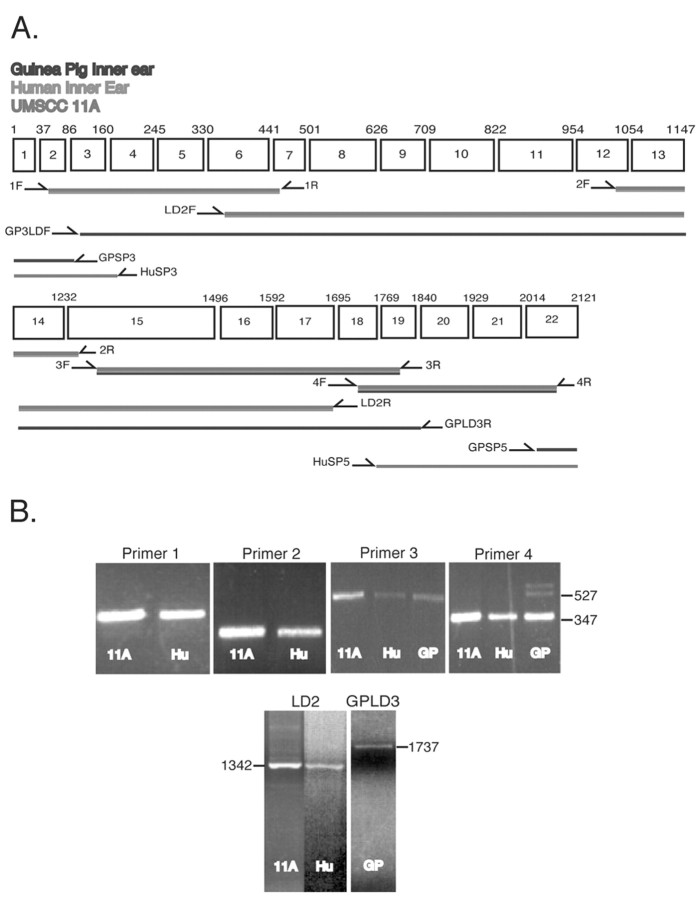
Because it is difficult to obtain large quantities of mRNA from inner ear tissue and also to examine the distribution of CTL2 in human cells, we screened cDNA samples from four human tumor cell lines plus normal human keratinocytes for CTL2 mRNA expression. We designed four sets of PCR primers (Table 2, Fig. 5A) based on the peptide sequences obtained from MS-MS sequencing and tested them first with cell-line cDNA. When we detected CTL2 expression, we then applied the primer sets to the inner ear cDNA. Theoretically these primers should amplify both human and guinea pig CTL2, because the primers are based on the original peptides. All four primer sets produced PCR products of the expected sequence with cDNA both from human epithelial UM-SCC-11A cells (11A) and from human inner ear (Hu) (Fig. 5B, panels 1-4). However, only primer sets 3 and 4 produced products with the guinea pig cDNA (Fig. 5B, panels 4 and 5), yielding sequences homologous to hCTL2. To extend the human sequence, the long-distance primers LD2F and LD2R were designed to span the region from primer sets 1 to 4 (Fig. 5A). These primers amplified cDNA from both human inner ear and UM-SCC-11A and produced the correct human CTL2 sequence for both samples (Fig. 5B, bottom, 11A and Hu lanes). We attributed the failure of primer sets 1 and 2 to amplify guinea pig inner ear cDNA to species differences in codon preference between GP and human mRNA. To overcome this problem a new GP-specific reverse primer based on a portion of the guinea pig cDNA sequence obtained with primer set 4 was designed. This primer, the GP long-distance reverse (GPLD3R) primer, begins at nucleotide 1851 (Fig. 5A). Then a new GP long-distance forward (GPLD3F) primer within peptide 3, beginning at nucleotide 115, was designed based on the GP peptide sequencing results and human sequence. This primer set successfully amplified a 1737 bp segment from GP inner ear cDNA (Fig. 5B, GP lane). Replicate long-distance PCR and sequencing runs confirmed the GP sequence. Using the known cDNA sequences for both species, a final set of GP-specific primers (GPSP3 and GPSP5) and a final set of human-specific primers (HuSP3 and HuSP5) were designed (Table 2) to obtain the remaining 3′ and 5′ sequences using RACE and thus complete the sequence of the GP CTL2 cDNA (GenBank accession number AY233002). This also confirmed that the expressed human product is identical to the expected hCTL2 sequence. The guinea pig CTL2 cDNA is 85.9% identical to hCTL2, and the predicted amino acid sequences of the proteins are 90.7% identical. The complete predicted amino acid sequences are shown in Figure 6 (a supplemental figure available at www.jneurosci.org). The guinea pig protein has 705 aa, one less than hCTL2. Asterisks under the sequences indicate amino acid identities for the two species. Differences in amino acid sequence are indicated by shading and also by absence of the asterisk below the sequences. The Cys residues predicted to be involved in disulfide bridges are shaded in pink, and the predicted N-glycosylation sites are shaded in green. Guinea pig CTL2 has five predicted N-glycosylation sites, whereas hCTL2 has four (three extracellular sites are shown by arrowheads in Figure 2; the fourth site is in the C-terminal cytoplasmic tail).
Figure 5.
Cloning of human and guinea pig CTL2 cDNA. A, Human CTL2 exon structure and the relative location of each primer pair for PCR amplification of cDNA from human and guinea pig inner ear and from human epithelial cells. Primers are numbered and designated either forward (F) or reverse (R) direction. LDF and LDR are long-distance forward and reverse primers, respectively. GPLDF and GPLDR are guinea pig-specific long-distance forward and reverse primers, respectively. GPSP3, GPSP5, HuSP3, and HuSP5 are the respective primers designed for guinea pig-specific and human-specific 3′ and 5′ RACE used to obtain the end sequences of each cDNA. B, Ethidium bromide-stained agarose gels showing the correctly sized PCR products from each of the primer sets for cDNA from UM-SCC-11A (11A), human inner ear (Hu), and guinea pig inner ear (GP). Sequencing confirmed that the PCR products contained appropriate CTL2 sequences.
Discussion
The inner ear glycoprotein immunoprecipitated from the guinea pig inner ear by the KHRI-3 antibody contains multiple peptides identical to those in human CTL2. Rabbit antisera to a CTL2-specific peptide bind to the IESCA isolated from the guinea pig inner ear by KHRI-3, and KHRI-3 binds to the inner ear protein precipitated by the antisera to CTL2, confirming that the IESCA and CTL2 are the same protein. The overall CTL2 sequence in guinea pigs and humans diverges, as expected for species that are evolutionarily distant from one another, but for the most part the sequences are identical at the DNA (86%) and protein (90%) levels, suggesting that the function is the same in both species. As in the guinea pig, CTL2 is abundantly expressed in the human inner ear. There is sufficient CTL2 mRNA in both guinea pig cochlea and human vestibular tissue to amplify the full sequence. Additionally, antibody to the N-terminal domain of CTL2 strongly stains the supporting cells in cochlear and vestibular tissues of the guinea pig and in human vestibular tissues. Furthermore, the pattern of CTL2 expression in the human ampulla is identical to that observed in the guinea pig vestibular system using either the KHRI-3 antibody (Ptok et al., 1993) or the rabbit anti-CTL2 peptide antibody. CTL2 protein is a predicted membrane protein, and immunofluorescence studies are consistent with this prediction.
An avian IESCA has also been described previously. This avian IESCA is a member of the receptor protein tyrosine phosphatase family that contains eight fibronectin repeats, a transmembrane domain, and an intracellular catalytic site (Kruger et al., 1999). Avian IESCA has an Mr of ∼220 kDa and is expressed on the apical surface of avian supporting cells with distribution on surface microvilli similar to what we described for the KHRI-3 antigen (Ptok et al., 1993). Both antigens (Kruger et al., 1999; Nair et al., 1999) exhibit altered expression and redistribution to areas of hair cell loss after inner ear trauma. Thus, both may be involved in scarring or repair processes. However, the mammalian KHRI-3 antigen is otherwise unrelated to the avian IESCA.
The function of CTL2 is unknown. CTL1 was cloned from cDNA in the electric organ of the torpedo fish to complement a yeast strain deficient in choline transport (O'Regan et al., 2000). CTL2, like CTL1, may serve as a choline transporter. Choline is required for biosynthesis of acetylcholine, which is a primary neurotransmitter in cholinergic nerve terminals. This may explain the abundant expression of CTL1 in the acetylcholine-rich electric organ of the Torpedo fish (O'Regan et al., 2000). Acetylcholine is also an important neurotransmitter in the inner ear, and CTL2 may be involved in uptake (recycling) of choline by supporting cells. Choline is also required for biosynthesis of phosphatidylcholine, a major component of membranes necessary for all cells. Choline does not permeate membranes; therefore, choline transporters are found in numerous tissues (Okuda and Haga, 2000). In previous studies of the IESCA in guinea pig inner ears infused with purified KHRI-3 antibody, we observed a redistribution of the antigen in regions of scar formation and hair cell loss (Nair et al., 1999). Presumably, as the supporting cells form scars to replace damaged hair cells, new membrane must be synthesized. The redistribution of CTL2 during scar formation is consistent with CTL2 having a role in choline transport for the purpose of membrane synthesis. An alternative possibility is that choline transporter-like proteins function in a capacity related to choline transport. CTL2, as well as CTL1 and CTL4, all contain lipid transporter homology domains called KOG1362 domains. Thus, these proteins may function to provide lipids essential for phosphatidylcholine or sphingophosphatidylcholine biosynthesis. The function of CTL2 is under investigation.
The KHRI-3 antibody binds to an N-linked carbohydrate moiety. In the CTL2 model shown in this paper, we predict that these carbohydrates are linked to asparagine residues N187 and N200 on extracellular loop 1 and/or N417 on extracellular loop 3. There are four predicted N-glycosylation sites in the human CTL2 molecule (five in the guinea pig), but the fourth asparagine, N697, is in the C-terminal domain, which our current model predicts to be in the cytoplasm. We know from our in vivo studies that binding of KHRI-3 to the carbohydrate leads to hair cell loss and ultimately hearing loss (Disher et al., 1997; Nair et al., 1999). We hypothesize that antibody blocks the function of the protein, perhaps by steric hindrance of the transporter pore or by blocking uptake of the transported molecule. This is consistent with the hypothesis that human antibodies, either to CTL2 or to other molecules that complex with it, might also result in damage to the inner ear or cause AHL. Why sensory cells die after this insult remains a mystery and suggests that the CTL2 complex provides something to supporting cells that is essential for hair cell survival. Because CTL2 is essential for hair cell survival, it is possible that genetic defects in CTL2 could also lead to hearing loss. CTL2 maps within an autosomal recessive, nonsyndromic hearing loss locus, DFNB15 (Chen et al., 1997), but it has not been assessed for mutation in DFNB15 families.
The predicted size of the 705 aa CTL2 protein is 80 kDa, which differs from the 68-72 kDa glycoproteins identified by CTL2 and KHRI-3 antibodies. Furthermore, the carbohydrate modifications are estimated to account for ∼10 kDa. There may be other post-translational modifications of CTL2 before the mature protein is inserted into the membrane. We noted an 80 kDa protein band that is detected by the CTL2 antiserum (Fig. 3C) but not by KHRI-3. This could be the nascent protein, which is then modified to form the final glycosylated and membrane-embedded form. The deglycosylation studies indicate that the core protein has an apparent Mr of 62 kDa, which is consistent with possible post-translational cleavage. However, we have not found smaller cleavage products, and the amino acid sequencing identified peptides over the entire length of the protein, arguing against such a modification. A possible alternative explanation for the reduced mass is protein folding. CTL2 has multiple transmembrane domains, which are helical and hydrophobic; thus in solution it is probably a globular protein. This could account for a reduced Mr relative to that predicted for a linear polypeptide (Westerhuis et al., 2000). One other possibility for the observed Mr of CTL2 is that there are alternative transcripts of the gene that encode different segments of the gene. Thus far, we have not found alternative transcripts in our PCR experiments with the cDNA. Extra bands were observed with primer set 4 (Fig. 5B), but the nature of these PCR products remains unknown because the sequences were not decipherable. These may represent mispriming with unknown guinea pig transcripts rather than alternative CTL2 transcripts. Northern blotting is usually used to identify other transcripts. However, this has been difficult to accomplish because of the relatively small amounts of mRNA available from either the human or guinea pig inner ear. In cDNA from lung, only a single transcript was found using three different probes corresponding to the 3′, middle, and 5′ ends of the transcript (our unpublished observations).
We conclude that CTL2 is the inner ear supporting cell antigen defined by KHRI-3. It is highly conserved and expressed in the inner ear of guinea pigs and humans, suggesting that it has the same function in both species. Sensory cells die after binding of an antibody to CTL2 molecules expressed on supporting cells; the explanation for this is unknown. Although the KHRI-3 monoclonal antibody to the carbohydrate causes hearing loss, we do not yet know whether an antipeptide antibody will have the same effect. Monoclonal antibodies with specificity for CTL2 peptides may answer this question. We postulate that antibody acts by blocking the transporter function of the molecule either by blocking the substrate receptor of the carbohydrate or by causing steric hindrance in the transporter pore. In either case, similar antibodies in humans could have the same effect and lead to antibody-mediated hearing loss.
Footnotes
This work was supported by the Autoimmune Sensorineural Hearing Loss Research Fund, The Ruth and Lynn Townsend Fund, a gift from the Holden Foundation, research grants from the United States Public Health Service National Institutes of Health (NIH)-National Institute on Deafness and Other Communication Disorders (T32 DC00011, R01 DC02272, R01 DC03686, and R01 DC01634), and NIH Rheumatic Diseases Core Center Grant 1P30 AR048310. We thank Dr. Lawrence Tabak (Director, National Institute of Dental and Craniofacial Research) and Dr. Irwin Goldstein (Department of Biological Chemistry, University of Michigan) for invaluable advice and counsel in the methodology to analyze the contribution of the carbohydrate modifications and amino acid sequence of the inner ear supporting cell antigen.
Correspondence should be addressed to Dr. Thomas E. Carey, University of Michigan, 6020 Kresge Hearing Research Institute, 1301 East Ann Street, Ann Arbor, MI. E-mail: careyte@umich.edu.
Copyright © 2004 Society for Neuroscience 0270-6474/04/241772-08$15.00/0
References
- Billings PB, Keithley EM, Harris JP (1995) Evidence linking the 68 kilodalton antigen identified in progressive sensorineural hearing loss patient sera with heat shock protein 70. Ann Otol Rhinol Laryngol 104: 181-188. [DOI] [PubMed] [Google Scholar]
- Billings PB, Shin SO, Harris JP (1998) Assessing the role of anti-hsp70 in cochlear impairment. Hear Res 126: 210-213. [DOI] [PubMed] [Google Scholar]
- Blass S, Meier C, Vohr HW, Schwochau M, Specker C, Burmester GR (1998) The p68 autoantigen characteristic of rheumatoid arthritis is reactive with carbohydrate epitope specific autoantibodies. Ann Rheum Dis 57: 220-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloch DB, San Martin JE, Rauch SD, Moscicki RA, Bloch KJ (1995) Serum antibodies to heat shock protein 70 in sensorineural hearing loss. Arch Otolaryngol Head Neck Surg 121: 1167-1171. [DOI] [PubMed] [Google Scholar]
- Chen A, Wayne S, Bell A, Ramesh A, Srisailapathy CR, Scott DA, Sheffield VC, Van Hauwe P, Zbar RI, Ashley J, Lovett M, Van Camp G, Smith RJ (1997) New gene for autosomal recessive non-syndromic hearing loss maps to either chromosome 3q or 19p. Am J Med Genet 71: 467-471. [PubMed] [Google Scholar]
- Disher MJ, Ramakrishnan A, Nair TS, Miller JM, Telian SA, Arts HA, Sataloff RT, Altschuler RA, Raphael Y, Carey TE (1997) Human autoantibodies and monoclonal antibody KHRI-3 bind to a phylogenetically conserved inner-ear-supporting cell antigen. Ann NY Acad Sci 830: 253-265. [DOI] [PubMed] [Google Scholar]
- Falquet L, Pagni M, Bucher P, Hulo N, Sigrist CJ, Hofmann K, Bairoch A (2002) The PROSITE database, its status in 2002. Nucleic Acids Res 30: 235-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottschlich S, Billings PB, Keithley EM, Weisman MH, Harris JP (1995) Assessment of serum antibodies in patients with rapidly progressive sensorineural hearing loss and Meniere's disease. Laryngoscope 105: 1347-1352. [DOI] [PubMed] [Google Scholar]
- Harris JP, Sharp PA (1990) Inner ear autoantibodies in patients with rapidly progressive sensorineural hearing loss. Laryngoscope 100: 516-524. [DOI] [PubMed] [Google Scholar]
- Kruger RP, Goodyear RJ, Legan PK, Warchol ME, Raphael Y, Cotanche DA, Richardson GP (1999) The supporting-cell antigen: a receptor-like protein tyrosine phosphatase expressed in the sensory epithelia of the avian inner ear. J Neurosci 19: 4815-4827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe BF (1979) Autoimmune sensorineural hearing loss. Ann Otol Rhinol Laryngol 88: 585-589. [DOI] [PubMed] [Google Scholar]
- Moscicki RA, San Martin JE, Quintero CH, Rauch SD, Nadol Jr JB, Bloch KJ (1994) Serum antibody to inner ear proteins in patients with progressive hearing loss. Correlation with disease activity and response to corticosteroid treatment. JAMA 272: 611-616. [PubMed] [Google Scholar]
- Nair TS, Raphael Y, Dolan DF, Parrett TJ, Perlman LS, Brahmbhatt VR, Wang Y, Hou X, Ganjei G, Nuttall AL, Altschuler RA, Carey TE (1995) Monoclonal antibody induced hearing loss. Hear Res 83: 101-113. [DOI] [PubMed] [Google Scholar]
- Nair TS, Prieskorn DM, Miller JM, Mori A, Gray J, Carey TE (1997) In vivo binding and hearing loss after intracochlear infusion of KHRI-3 antibody. Hear Res 107: 93-101. [DOI] [PubMed] [Google Scholar]
- Nair TS, Prieskorn DM, Miller JM, Dolan DF, Raphael Y, Carey TE (1999) KHRI-3 monoclonal antibody-induced damage to the inner ear: antibody staining of nascent scars. Hear Res 129: 50-60. [DOI] [PubMed] [Google Scholar]
- Okuda T, Haga T (2000) Functional characterization of the human high-affinity choline transporter. FEBS Lett 484: 92-97. [DOI] [PubMed] [Google Scholar]
- O'Regan S, Traiffort E, Ruat M, Cha N, Compaore D, Meunier FM (2000) An electric lobe suppressor for a yeast choline transport mutation belongs to a new family of transporter-like proteins. Proc Natl Acad Sci USA 97: 1835-1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ptok M, Nair TS, Altschuler RA, Schacht J, Carey TE (1991) Monoclonal antibodies to inner ear antigens. II. Antigens expressed in sensory cell stereocilia. Hear Res 57: 79-90. [DOI] [PubMed] [Google Scholar]
- Ptok M, Nair T, Carey TE, Altschuler RA (1993) Distribution of KHRI 3 epitopes in the inner ear. Hear Res 66: 245-252. [DOI] [PubMed] [Google Scholar]
- Trune DR, Kempton JB, Mitchell CR, Hefeneider SH (1998) Failure of elevated heat shock protein 70 antibodies to alter cochlear function in mice. Hear Res 116: 65-70. [DOI] [PubMed] [Google Scholar]
- Westerhuis WH, Sturgis JN, Niederman RA (2000) Reevaluation of the electrophoretic migration behavior of soluble globular proteins in the native and detergent-denatured states in polyacrylamide gels. Anal Biochem 284: 143-152. [DOI] [PubMed] [Google Scholar]
- Yeom K, Gray J, Nair TS, Arts HA, Telian SA, Disher MJ, El-Kashlan H, Sataloff RT, Fisher SG, Carey TE (2003) Antibodies to HSP-70 in normal donors and autoimmune hearing loss patients. Laryngoscope 113: 1770-1776. [DOI] [PubMed] [Google Scholar]
- Zajic G, Nair TS, Ptok M, Van Waes C, Altschuler RA, Schacht J, Carey TE (1991) Monoclonal antibodies to inner ear antigens. I. Antigens expressed by supporting cells of the guinea pig cochlea. Hear Res 52: 59-71. [DOI] [PubMed] [Google Scholar]