Abstract
Purpose:
We aimed to determine which early EEG features and feature combinations most accurately predicted short-term neurobehavioral outcomes and survival in children resuscitated after cardiac arrest.
Methods:
This was a prospective single-center observational study of infants and children resuscitated from cardiac arrest who underwent conventional EEG monitoring with standardized EEG scoring. Logistic regression evaluated the marginal effect of each EEG variable or EEG variable combinations on the outcome. The primary outcome was neurobehavioral outcome (Pediatric Cerebral Performance Category score) and the secondary outcome was mortality. We identified the models with the highest areas under the receiver operating characteristic curve (AUC), evaluated the optimal models using a 5-fold cross-validation approach, and calculated test characteristics maximizing specificity.
Results:
89 infants and children were evaluated. Unfavorable neurologic outcome (Pediatric Cerebral Performance Category score 4–6) occurred in 44 subjects (49%) including mortality in 30 subjects (34%). A model incorporating a four-level EEG Background Category (normal, slow-disorganized, discontinuous or burst-suppression, or attenuated-flat), Stage 2 Sleep Transients (present or absent), and Reactivity-Variability (present or absent) had the highest AUC. Five-fold cross-validation for the optimal model predicting neurologic outcome indicated a mean AUC of 0.75 (range 0.70–0.81) and for the optimal model predicting mortality indicated a mean AUC of 0.84 (range 0.76–0.97). The specificity for unfavorable neurologic outcome and mortality were 95% and 97%, respectively. The positive predictive value for unfavorable neurologic outcome and mortality were both 86%.
Conclusions:
The specificity of the optimal model using a combination of early EEG features was high for unfavorable neurologic outcome and mortality in critically ill children after cardiac arrest. However, the positive predictive value was only 86% for both outcomes. Therefore, EEG data must be considered together with the overall clinical context when used for neuroprognostication early after cardiac arrest.
Keywords: EEG, Cardiac Arrest, Pediatric, Outcome
Introduction
In-hospital cardiac arrest (CA) occurs in over 10,000 children per year in the United States, and neurobehavioral morbidity is high among survivors.1–8 Early assessment of brain injury severity is important for neuroprognostication and potentially for subject stratification upon entry into neuroprotection trials. Studies in children after CA have elucidated several clinical variables and biomarkers correlated with mortality and unfavorable neurobehavioral outcomes.9–13 However, clinical and resuscitation variables do not directly assess brain function and therefore may not optimally predict neurobehavioral outcomes. In contrast, electroencephalographic (EEG) data assess cortical function, and EEG monitoring is commonly employed after CA resuscitation for seizure identification.14,15 Several early post-CA EEG features have been associated with neurologic outcome at hospital discharge.15–27 However, many of these studies involved small retrospective cohorts, occurred prior to the era of contemporary critical care which may have changed outcomes, and used data collected from EEG reports rather than standardized assessment of the raw EEG tracing. Furthermore, EEG data comparison across studies is difficult since many of these studies evaluated variable EEG features using non-standardized EEG terminology.
In this study, we aimed to determine which early EEG features or feature combinations optimally predicted short-term neurobehavioral outcomes and survival in a large, contemporary, and consecutive cohort of children after resuscitation from CA. This work serves as a foundation for developing early, comprehensive, and evidence-based multi-modal prediction models of neurobehavioral outcome.
Methods
This was a prospective observational single-center study of consecutive infants and children treated in the Pediatric Intensive Care Unit of a single tertiary care hospital between September 2013 and February 2016. Informed consent was obtained from guardians of patients for data collection. Data were collected using the Research Electronic Data Capture (REDCap).28 and consisted of prospectively defined demographic, CA, resuscitation, post CA care, EEG, and outcome variables. This study was approved by the Institutional Review Board at the Children’s Hospital of Philadelphia.
Consistent with recent guidelines29 and consensus statements,14 clinical practice at our institution is to perform EEG monitoring in all patients with encephalopathy following CA to identify seizures. EEG monitoring was initiated using portable Grass-Telefactor video-EEG systems with 21 gold-over-silver scalp surface electrodes positioned according to the international 10–20 system and affixed with collodion adhesive using standard technical specifications.30 Full EEG tracings were saved for research purposes.
After clinical management, standardized EEG scoring was performed for this study by a pediatric electroencephalographer at the earliest available timepoint. The EEG variables (Table 2) were derived from the Standardized Critical Care EEG Terminology published by the American Clinical Neurophysiology Society.31 Electroencephalographic seizures were defined as paroxysmal events that were different from the background, lasted longer than 10 seconds, had a temporal-spatial evolution in morphology, frequency, and amplitude, and had a plausible electrographic field. Electroencephalographic status epilepticus was defined as ≥50% of a one-hour epoch containing electroencephalographic seizures. These seizure definitions are consistent with our prior critical care EEG studies.32–34 and published definitions.35 Most of these EEG features have good to substantial inter-rater agreement,36,37 including among children after CA.38 In addition to the standardized terminology, the EEG Background Category was categorized as: (1) normal (including normal discontinuity in neonates); (2) slow-disorganized (including mild excessive discontinuity in neonates); (3) discontinuous (including substantial excessive discontinuity in neonates); (4) burst-suppression; or (5) attenuated-featureless. This EEG categorization system was utilized in prior critical care EEG studies.17,32–34,39,40 Further, a study in which four pediatric electroencephalographers assessed EEGs from children post-CA demonstrated very-high (kappa 0.89) inter-rater agreement for the EEG Background Category.38
Table 2.
EEG characteristics and outcomes. Data are presented as N (%). White rows display EEG variables using standardized critical care EEG variables from the American Clinical Neurophysiology Society. Gray rows display EEG variables using combined response categories created in this study.
| Totals | Neurobehavioral Outcome | Mortality | |||||
|---|---|---|---|---|---|---|---|
| Unfavorable | Favorable | p-value | Die | Survive | p-value | ||
| 89 | 46 (52%) | 43 (48%) | 30 (34%) | 59 (66%) | |||
| EEG Background Category (5-level) | 0.001 | 0.000 | |||||
| Normal | 9 (10%) | 1 (2%) | 8 (19%) | 1 (3%) | 8 (14%) | ||
| Slow-Disorganized | 44 (49%) | 19 (41%) | 25 (58%) | 7 (23%) | 37 (63%) | ||
| Discontinuous | 18 (20%) | 10 (21%) | 8 (19%) | 6 (20%) | 12 (20%) | ||
| Burst-Suppression | 4 (4%) | 4 (9%) | 0 (0%) | 4 (13%) | 0 (0%) | ||
| Attenuated-Featureless | 14 (16%) | 12 (26%) | 2 (5%) | 12 (40%) | 2 (3%) | ||
| EEG Background Category (4-level) | 0.001 | 0.000 | |||||
| Normal | 9 (10%) | 1 (2%) | 8 (19%) | 1 (3%) | 8 (14%) | ||
| Slow-Disorganized | 44 (49%) | 19 (41%) | 25 (58%) | 7 (23%) | 37 (63%) | ||
| Discontinuous or Burst Suppression | 22 (25) | 14 (30%) | 8 (19%) | 10 (33%) | 12 (20%) | ||
| Attenuated-Featureless | 14 (16%) | 12 (26%) | 2 (5%) | 12 (40%) | 2 (3%) | ||
| Electrographic Seizures | 0.678 | 0.248 | |||||
| None | 82 (92%) | 41 (89%) | 41 (95%) | 26 (87%) | 56 (95%) | ||
| Seizure(s) | 1 (1%) | 1 (2%) | 0 (0%) | 1 (3%) | 0 (0%) | ||
| Status Epilepticus | 6 (7%) | 4 (9%) | 2 (5%) | 3 (10%) | 3 (5%) | ||
| Symmetric | 88 (99%) | 46 (100%) | 42 (98%) | 0.483 | 30 (100%) | 58 (98%) | 1.000 |
| Fastest Frequency (4-level) | 0.078 | 0.001 | |||||
| Attenuated | 11 (12%) | 9 (20%) | 2 (5%) | 9 (30%) | 2 (3%) | ||
| Delta | 52 (58%) | 26 (57%) | 26 (60%) | 17 (57%) | 35 (59%) | ||
| Delta + Theta | 24 27%) | 11 (24%) | 13 (30%) | 4 (13%) | 20 (34%) | ||
| Delta + Theta + Alpha | 2 (2%) | 0 (0%) | 2 (5%) | 0 (0%) | 2 (3%) | ||
| Fastest Frequency (3-level) | 0.083 | 0.001 | |||||
| Attenuated | 11 (12%) | 9 (20%) | 2 (5%) | 9 (30%) | 2 (3%) | ||
| Delta | 52 (58%) | 26 (57%) | 26 (60%) | 17 (57%) | 35 (59%) | ||
| Delta + Theta and/or Alpha | 26 (29%) | 11 (24%) | 15 (35%) | 4 (13%) | 22 (37%) | ||
| Continuity (8-level) | 0.002 | 0.000 | |||||
| Continuous | 49 (55%) | 20 (43%) | 29 (67%) | 8 (27%) | 41 (69%) | ||
| Nearly continuous with attenuation | 4 (4%) | 0 (0%) | 4 (9%) | 0 (0%) | 4 (7%) | ||
| Nearly continuous with suppression | 1 (1%) | 1 (2%) | 0 (0%) | 1 (3%) | 0 (0%) | ||
| Discontinuous with attenuation | 5 (6%) | 3 (7%) | 2 (5%) | 1 (3%) | 4 (7%) | ||
| Discontinuous with suppression | 12 (13%) | 6 (13%) | 6 (14%) | 4 (13%) | 8 (14%) | ||
| Burst-attenuation | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | ||
| Burst-suppression | 4 (4%) | 4 (9%) | 0 (0%) | 4 (13%) | 0 (0%) | ||
| Suppression | 14 (16%) | 12 (26%) | 2 (5%) | 12 (40%) | 2 (3%) | ||
| Continuity (4-level) | 0.002 | 0.000 | |||||
| Continuous or Nearly Continuous | 54 (60%) | 21 (46%) | 33 (76%) | 9 (30%) | 45 (76%) | ||
| Discontinuous with attenuation/suppression | 17 (19%) | 9 (20%) | 8 (19%) | 5 (17%) | 12 (20%) | ||
| Burst-attenuation/suppression | 4 (4%) | 4 (9%) | 0 (0%) | 4 (13%) | 0 (0%) | ||
| Suppression | 14 (16%) | 12 (26%) | 2 (5%) | 12 (40%) | 2 (3%) | ||
| Voltage | 0.001 | 0.000 | |||||
| Normal | 44 (49%) | 18 (39%) | 26 (60%) | 10 (33%) | 34 (58%) | ||
| Low | 27 (30%) | 12 (26%) | 15 (35%) | 5 (17%) | 22 (37%) | ||
| Suppressed | 18 (20%) | 16 (35%) | 2 (5%) | 15 (50%) | 3 (5%) | ||
| Stage II Transients (4-level) | 0.000 | 0.001 | |||||
| Present and normal | 14 (16%) | 1 (2%) | 13 (30%) | 0 (0%) | 14 (24%) | ||
| Present but abnormal | 9 (10%) | 2 (4%) | 7 (16%) | 1 (3%) | 8 (14%) | ||
| Absent | 65 (73%) | 43 (93%) | 22 (51%) | 29 (97%) | 36 (61%) | ||
| Awake only | 1 (1%) | 0 (0%) | 1 (2%) | 0 (0%) | 1 (2%) | ||
| Stage II Transients (2-level) | 0.000 | 0.000 | |||||
| Present (normal or abnormal) | 23 (26%) | 3 (7%) | 20 (47%) | 1 (3%) | 22 (37%) | ||
| Absent | 66 (74%) | 43 (93%) | 23 (53%) | 29 (97%) | 37 (63%) | ||
| Reactivity Present | 35 (39%) | 11 (24%) | 24 (56%) | 0.003 | 6 (20%) | 29 (49%) | 0.011 |
| Variability Present | 38 (43%) | 13 (28%) | 25 (58%) | 0.006 | 7 (23%) | 31 (53%) | 0.012 |
| Variability or Reactivity Present | 41 (46%) | 14 (30%) | 27 (63%) | 0.003 | 7 (23%) | 34 (58%) | 0.003 |
| Sporadic Epileptiform Discharges Present | 8 (9%) | 6 (13%) | 2 (5%) | 0.268 | 4 (13%) | 4 (7%) | 0.435 |
| Ictal-Interictal Continuum Patterns Present | 6 (7%) | 5 (11%) | 1 (2%) | 0.204 | 3 (10%) | 3 (5%) | 0.401 |
The primary outcome was neurobehavioral outcome, and the secondary outcome was mortality. Outcomes were assessed at discharge from the Pediatric Intensive Care Unit. Neurobehavioral outcome was assessed using the Pediatric Cerebral Performance Category (PCPC) score which is a validated six-point scale that categorizes functional impairment (1=normal, 2=mild disability, 3=moderate disability, 4=severe disability, 5=coma and vegetative state, and 6=death).41 Unfavorable neurobehavioral outcome was defined as discharge PCPC scores of 4–6. Like in prior studies,42 the mode of death was classified based on review of clinical notes into one of the following categories: brain death, withdrawal of technological support for poor neurologic prognosis, withdrawal of technologic support for refractory circulatory failure, or re-arrest without return of spontaneous circulation. Patients who were made “Do Not Attempt Resuscitate” were classified as withdrawal of technological support for poor neurologic prognosis or withdrawal of technologic support for refractory circulatory failure based on attending physician notes.
All statistical analyses were performed using Stata 15.0 (College Station, TX). We report summary statistics as medians and interquartile ranges for continuous variables and counts and proportions for categorical variables. For some EEG variables, only a small number of subjects had certain EEG features. Therefore, we grouped some EEG features to create fewer categories for analysis such that each category had adequate sample size, as shown in Table 2. We performed the same analyses for unfavorable neurologic outcome and mortality. Logistic regression was used to evaluate the marginal effect of each EEG variable (or EEG variable combinations) on the outcome. Variables with p-value <0.1 were included in subsequent multivariable models. We compared areas under the receiver operating characteristic curves (AUC) between models. We assessed the collinearity between the variables in each model and excluded variables with a variation inflation factor greater than ten or less than 0.1.43 We identified the models with the highest AUC. The model with the fewest variables that had an AUC that did not differ significantly from the model with the highest AUC was chosen as the optimal model for further analysis. We evaluated the goodness of fit using the Hosmer-Lemeshow test. We evaluated the optimal model using a 5-fold cross-validation approach. The full cohort was randomly divided into five subgroups with a similar prevalence of unfavorable outcomes as in the original sample. Each time, one subgroup was used as the validation sample and the other four subgroups were used for model development. We report the mean and range of the AUC for the five validation cohorts.
We calculated test characteristics (specificity, sensitivity, positive predictive value, and negative predictive value) for the optimal model where cutoffs were selected to maximize specificity. Optimal specificity was favored because the aim of our model was to minimize false positives which might incorrectly label a patient with unfavorable outcome when they might in fact have a favorable outcome.
Results
Eighty-nine infants and children were evaluated. Table 1 provides subject characteristics. The median age was 2.1 (IQR: 0.27, 9.1) years. Fourteen subjects were neonates (<1 month of age) at a median of 7 (IQR: 3, 14 days). Fifty-six (63%) of subjects were male. CA occurred in-hospital in 58 subjects (65%) and out-of-hospital in 31 subjects (35%). The most common CA causes were shock in 38 subjects (43%) and respiratory failure in 34 subjects (38%). The median initial lactate was 5.0 (IQR: 2.8, 8.4), and the median lowest initial pH was 7.20 (IQR: 7.03, 7.29).
Table 1.
Clinical, cardiac arrest, and resuscitation characteristics.
| Variable | N (%) Median [IQR] |
|---|---|
| Age (years) | 2.1 [0.27, 9.1] |
| Male | 56 (63%) |
| Race | |
| White | 46 (52%) |
| Black | 19 (21%) |
| Other | 24 (27%) |
| Hispanic | 14 (16%) |
| Pre-Cardiac Arrest PCPC Score | |
| 1 = Normal | 57 (64%) |
| 2 = Mild Disability | 12 (13%) |
| 3 = Moderate Disability | 8 (9%) |
| 4 = Severe Disability | 9 (10%) |
| 5 = Coma or Vegetative State | 3 (3%) |
| Pre-Existing Condition | 68 (76%) |
| Pre-Cardiac Arrest Ventilation | 34 (38%) |
| Congenital Heart Disease | 33 (37%) |
| Respiratory Failure | 23 (26%) |
| Pre-Cardiac Arrest Vasoactive Infusions | 18 (20%) |
| Chronic Tracheostomy-Ventilator | 16 (18%) |
| In-Hospital Cardiac Arrest | 58 (65%) |
| In-Hospital Cardiac Arrest Location (N=58) | |
| Cardiac ICU | 24 (41 %) |
| Pediatric ICU | 15 (26%) |
| Emergency Department | 8 (14%) |
| Floor | 8 (14%) |
| Other | 3 (5%) |
| Witnessed Cardiac Arrest | 64 (72%) |
| Bystander CPR for Out-of-Hospital Cardiac Arrest (N=31) | 25 (81 %) |
| CPR Duration (minutes) (N=70) | 10 [4, 20] |
| Initial Rhythm | |
| Asystole | 16 (18%) |
| Pulseless Electrical Activity | 10 (11%) |
| Bradycardia | 33 (37%) |
| Ventricular Fibrillation or Tachycardia | 11 (12%) |
| Other/Unknown | 19 (21%) |
| Cardiac Arrest Cause (may have >1) | |
| Sudden Infant Death Syndrome | 5 (6%) |
| Drowning | 9 (10%) |
| Shock | 38 (43%) |
| Respiratory Failure | 34 (38%) |
| Trauma | 8 (9%) |
| Epinephrine Doses | |
| 0 | 14 (1 6%) |
| 1 | 15 (17%) |
| 2 | 10 (11%) |
| 3 | 18 (20%) |
| 4 | 7 (8%) |
| ≥5 | 20 (22%) |
| Unknown | 5 (6%) |
| Initial Lactate (N=82) | 5.0 [2.8, 8.4] |
| Lowest pH Initial 24 Hours After Cardiac Arrest (N=88) | 7.20 [7.03, 7.29] |
| Intubated | 67 (76%) |
| Induced Hypothermia | 10 (11%) |
| Pentobarbital Infusion | 3 (3%) |
| Benzodiazepine Infusion | 69 (78%) |
| Benzodiazepine Bolus | 56 (63%) |
| Glasgow Coma Scale - Eye Opening (N=77) | |
| Spontaneously | 12 (16%) |
| To Speech | 5 (6%) |
| To Pain | 4 (5%) |
| No Response | 56 (73%) |
| Glasgow Coma Scale - Best Verbal Response (N=77) | |
| Oriented | 0 (0%) |
| Confused | 2 (3%) |
| Inappropriate Words | 0 (0%) |
| Incomprehensible Sounds | 1 (1%) |
| No Response | 74 (96%) |
| Glasgow Coma Scale - Best Motor Score (N=76) | |
| Obeys Commands | 6 (8%) |
| Moves to Localized Pain | 3 (4%) |
| Flexion Withdrawal from Pain | 15 (20%) |
| Abnormal Flexion | 2 (3%) |
| Abnormal Extension | 0 (0%) |
| No Response | 50 (65%) |
| Mortality | 30 (34%) |
| Unfavorable Neurobehavioral Outcome | 44 (49%) |
CPR: cardiopulmonary resuscitation, ICU: intensive care unit, IQR: interquartile range, PCPC: pediatric cerebral performance category.
All EEG recordings were initiated prior to or on the same day as the CA. The median time from return of spontaneous circulation to EEG initiation was 6.9 hours (IQR: 4.4, 11.5). A small number of patients who met other indications for EEG monitoring (i.e. encephalopathy of unknown etiology) were undergoing EEG monitoring prior to and during the CA. The median EEG duration was 48 hours (IQR: 20, 43). The EEG Background Category was normal in 9 subjects (10%), slow-disorganized in 44 subjects (49%), discontinuous in 18 subjects (20%), burst-suppression in 4 subjects (4%), and attenuated-featureless in 14 subjects (16%).
Unfavorable neurobehavioral outcome occurred in 44 subjects (49%) including mortality in 30 subjects (34%). The mode of death was brain death in 6 (20%) subjects, withdrawal of technological support for poor neurologic prognosis in 6 (20%) subjects, withdrawal of technologic support for refractory circulatory failure in 15 (50%) subjects, and re-arrest without return of spontaneous circulation in 3 (10%) subjects. Table 2 summarizes the associations between EEG features and unfavorable neurobehavioral outcome or mortality. EEG variables with p-value <0.1 in the univariate logistic regression models included worse EEG background category, low level of faster frequencies, absence of continuity, lower voltage, absence of stage 2 sleep transients, absence of reactivity, absence of variability, and absence of reactivity and/or variability. Electroencephalographic seizures category was not associated with unfavorable neurobehavioral outcome or mortality. Seven subjects (8%) had an electroencephalographic seizure, and 6 of the subjects with seizures (86%) had electroencephalographic status epilepticus. Seizures during any epoch were not associated with unfavorable neurobehavioral outcome [2/43 (4.7%) with favorable outcome vs. 5/46 (10.9%) with unfavorable outcome, p=0.44] or mortality [3/59 (5.1%) who did survive vs. 4/30 (13.3%) who did not survive, p=0.22].
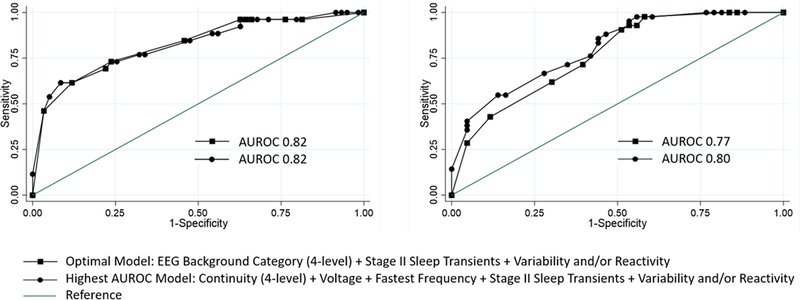
Table 3 provides the AUC for individual EEG features and combinatorial models and Supplemental Figure 1 shows the ROC curves for each of the models. We identified the same optimal model for neurologic outcome and mortality. This model included three variables [EEG Background Category (4-level) + Stage II Sleep Transients + Variability and/or Reactivity]. For unfavorable neurobehavioral outcome, the model provided an AUC of 0.77 (95% CI 0.67–0.86). The Hosmer-Lemeshow test yielded a p-value of 0.25 indicating good model fit. For mortality, the model provided an AUC of 0.82 (95% CI 0.72–0.92). The Hosmer-Lemeshow test yielded a p-value of 0.97 indicating good model fit. AUC for the three EEG variable model did not significantly differ from the model with the highest AUC that included more EEG variables for either neurologic outcome (p=0.33) or mortality (p=0.96). Figure 1 displays the ROC curves for the optimal model and the model with the highest AUC.
Table 3.
Area under the receiver operating characteristic curve (AUC) for models using EEG features in varying combinations for predicting unfavorable neurologic outcome and mortality.
| Model | Unfavorable Neurobehavioral Outcome AUC (95% CI) |
Mortality AUC (95% CI) |
|---|---|---|
| EEG Background Category (4-level) | 0.72 (0.62–0.81) | 0.79 (0.69–0.89) |
| Fastest Frequency (3-level) | 0.60 (0.50–0.70) | 0.70 (0.60–0.80) |
| Continuity (4-level) | 0.66 (0.56–0.76) | 0.75 (0.64–0.87) |
| Voltage | 0.66 (0.56–0.77) | 0.74 (0.63–0.85) |
| Sleep Transients (2-level) | 0.70 (0.62–0.78) | 0.67 (0.60–0.74) |
| Variability or Reactivity | 0.66 (0.56–0.76) | 0.67 (0.57–0.77) |
| Continuity (4-level) + Voltage | 0.69 (0.58–0.79) | 0.77 (0.65–0.89) |
| Continuity (4-level) + Voltage + Fastest Frequency | 0.69 (0.58–0.80) | 0.77 (0.64–0.89) |
| Continuity (4-level) + Voltage + Fastest Frequency + Stage II Sleep Transients | 0.80 (0.72–0.90) | 0.81 (0.72–0.91) |
| Continuity (4-level) + Voltage + Fastest Frequency + Variability and/or Reactivity | 0.72 (0.61–0.83) | 0.76 (0.64–0.89) |
| Continuity (4-level) + Voltage + Fastest Frequency + Stage II Sleep Transients + Variability and/or Reactivity | 0.80 (0.71–0.89) | 0.82 (0.72–0.93) |
| EEG Background Category (4-level) + Stage II Sleep Transients | 0.77 (0.68–0.87) | 0.83 (0/74–0.92) |
| EEG Background Category (4-level) + Variability and/or Reactivity | 0.74 (0.64–0.84) | 0.80 (0.69–0.90) |
| EEG Background Category (4-level) + Stage II Sleep Transients + Variability and/or Reactivity | 0.78 (0.68–0.87) | 0.83 (0.74–0.93) |
Figure 1.
Receiver operating characteristic curves for mortality (left) and unfavorable neurobehavioral outcome (right) showing the optimal (squares) and the model with the highest area under the receiver operating characteristic (AUROC) curve (circles).
The optimal model was evaluated further. Table 4 provides the estimated odds ratios from the optimal model. Table 5 provides the predicted probabilities of unfavorable neurobehavioral outcome and mortality for each of the possible model combinations, and Supplemental Figure 2 shows the sensitivity and specificity for each probability cutoff.
Table 4.
Logistic regression for the model incorporating EEG Background Category, sleep transients, and reactivity-variability for predicting unfavorable neurobehavioral outcome and mortality.
| EEG Variable | Unfavorable Neurobehavioral Outcome | Mortality | ||
|---|---|---|---|---|
| OR (95% CI) | p-value | OR (95% CI) | p-value | |
| EEG Category | ||||
| Normal | - | - | - | - |
| Slow-Disorganized | 2.68 (0.26–27.62) | 0.41 | 0.54 (0.04–7.02) | 0.64 |
| Discontinuous or Burst Suppression | 3.64 (0.30–43.43) | 0.31 | 1.62 (0.11–22.79) | 0.72 |
| Attenuated-Featureless | 9.62 (0.55–169.36) | 0.12 | 9.42 (0.44–200.02) | 0.15 |
| Stage 2 Sleep Transients | ||||
| Present | - | - | - | - |
| Absent | 6.46 (1.55–26.98) | 0.01 | 7.61 (0.76–75.9) | 0.08 |
| Reactivity-Variability | ||||
| Present | - | - | - | - |
| Absent | 1.58 (0.55–4.56) | 0.40 | 1.48 (0.44–4.96) | 0.53 |
Table 5.
Predicted probabilities of unfavorable neurobehavioral outcome and mortality for each of the possible model combinations organized by low to high probability of unfavorable neurobehavioral outcome.
| EEG Features | Probability (95% CI) | |||
|---|---|---|---|---|
| Background Category | Reactivity | Sleep | Unfavorable Neurobehavioral Outcome | Mortality |
| Normal | + | + | 0.06 (−0.07, 0.18) | 0.05 (−0.07, 0.18) |
| Normal | − | + | 0.09 (−0.11, 0.29) | 0.08 (−0.12, 0.27) |
| Slow-Disorganized | + | + | 0.14 (−0.02, 0.30) | 0.03 (−0.04, 0.10) |
| Discontinuous or Burst-Suppression | + | + | 0.18 (−0.07, 0.43) | 0.08 (−0.11, 0.28) |
| Slow-Disorganized | − | + | 0.21 (−0.03, 0.44) | 0.04 (−0.05, 0.14) |
| Discontinuous or Burst-Suppression | − | + | 0.26 (−0.07, 0.59) | 0.12 (−0.14, 0.38) |
| Normal | + | − | 0.28 (−0.18, 0.75) | 0.30 (−0.22, 0.82) |
| Attenuated-Featureless | + | + | 0.37 (−0.15, 0.89) | 0.35 (−0.32, 1.02) |
| Normal | − | − | 0.38 (−0.19, 0.96) | 0.39 (−0.24, 1.02) |
| Attenuated-Featureless | − | + | 0.48 (−0.04, 1.00) | 0.44 (−0.24, 1.12) |
| Slow-Disorganized | + | − | 0.51 (0.29, 0.74) | 0.19 (0.02, 0.36) |
| Discontinuous or Burst-Suppression | + | − | 0.59 (0.32, 0.86) | 0.41 (0.13, 0.69) |
| Slow-Disorganized | − | − | 0.63 (0.42, 0.83) | 0.25 (0.07, 0.44) |
| Discontinuous or Burst-Suppression | − | − | 0.69 (0.49, 0.90) | 0.51 (0.26, 0.77) |
| Attenuated-Featureless | + | − | 0.79 (0.49, 1.10) | 0.80 (0.50, 1.11) |
| Attenuated-Featureless | − | − | 0.86 (0.67, 1.04) | 0.86 (0.67, 1.04) |
Five-fold cross-validation for the optimal model predicting unfavorable neurobehavioral outcome indicated a mean AUC of 0.75 (range 0.70–0.81). Five-fold cross-validation for the optimal model predicting mortality indicated a mean AUCs of 0.84 (range 0.76–0.97). Table 6 provides the test characteristics for the optimal model for prediction of unfavorable neurobehavioral outcome and mortality.
Table 6.
Test Characteristics for the model incorporating EEG Background Category, sleep transients, and reactivity-variability for predicting unfavorable neurobehavioral outcome and mortality.
| Outcome | Outcome Prevalence (95% CI) |
Specificity (95% CI) |
Sensitivity (95% CI) |
Positive Predictive Value (95% CI) |
Negative Predictive Value (95% CI) |
|---|---|---|---|---|---|
| Unfavorable Neurobehavioral Outcome | 51.7 (40.8–62.4) | 95.3 (84.2–99.4) | 26.1 (14.3–41.1) | 85.7 (57.2–98.2) | 54.7 (42.7–66.2) |
| Mortality | 33.7 (24.0–44.5) | 96.6 (88.3–99.6) | 40.0 (22.7–59.4) | 85.7 (57.2–98.2) | 76.0 (64.7–85.1) |
Discussion
This prospective study of infants and children resuscitated from CA who underwent EEG monitoring evaluated early and standardized EEG features which could be used to assess the severity of brain injury and predict outcome. We specifically focused on early EEG features since early data would be most beneficial in stratifying patients by brain injury severity for future neuroprotection studies. Many EEG features, used individually and in combination, had a high specificity for unfavorable neurobehavioral outcome and mortality. Interestingly, the specificity of a simple model using a four-level EEG Background Category (normal, slow-disorganized, discontinuous or burst-suppression, or attenuated-flat), Stage 2 Sleep Transients (present or absent), and Reactivity-Variability (present or absent) predicted outcome as well as or better than more complicated models comprised of combinations of several individual EEG variables, likely because many of the EEG features were colinear.
During prognostication, incorrectly predicting an unfavorable outcome leading to withdrawal of technological support in a patient who might have had a good outcome might have grave consequences. Thus, a model predicting unfavorable outcomes (mortality or unfavorable neurobehavioral outcome) should aim to minimize false positives, thereby achieving high specificity. In our dataset, specificity refers to the percentage of subjects with a favorable outcome who are correctly identified by a negative test result. Thus, in our analysis, we selected cutoffs that would favor optimal specificity over sensitivity. Even with this cutoff selection, the positive predictive value for both unfavorable neurobehavioral outcome and mortality is only 86%. Thus, early EEG features alone should not be used to predict unfavorable outcome when guiding clinical decision making. However, these data suggest that multi-model prediction models would likely be strengthened by inclusion of EEG data. Furthermore, early EEG data could be useful for stratifying patients by severity of initial brain injury for future neuroprotection trials.
EEG data can be obtained at bedside, either continuously or periodically, easily repeated, and provide functional information. Furthermore, EEG data is commonly obtained in critically ill patients including after CA to identify electroencephalographic seizures.14,29,44 Several contemporary studies have evaluated associations between EEG features and neurologic outcomes in children after CA. A single-center retrospective study of 128 consecutive children post-CA without therapeutic hypothermia who underwent EEG monitoring within one day of return of spontaneous circulation showed that the EEG background category was normal in 3%, slow-disorganized in 45%, discontinuous/burst-suppression in 19%, and attenuated-featureless in 33% of subjects. After controlling for covariates including clinical, CA, and resuscitation characteristics, for each incrementally worse EEG background category, the odds of death was 3.63 (95%CI, 2.18–6.0; p < 0.001) and the odds of unfavorable neurologic outcome at discharge was 4.38 (95%CI, 2.51–7.17; p = 0.001).17 This was similarly shown in another single-center retrospective study of 73 children post-CA with and without therapeutic hypothermia who underwent EEG monitoring within 72 hours of return of spontaneous circulation. After controlling for demographic and arrest characteristics, in the initial 12 hours, subjects with a normal or slow EEG backgrounds as opposed to burst-suppression or suppression backgrounds had more favorable outcomes. Subjects with normal EEG voltage, reactivity, or variability in the initial 12 hours also had more favorable outcomes. EEG data from later timepoints (12–24 hours and >24 hours) were not predictive of outcome.45 A single-center retrospective study of 41 children who had EEG data available after CA reported that EEG background suppression was associated with unfavorable outcome.27 A single-center retrospective study of 35 consecutive children managed with therapeutic hypothermia after CA who underwent EEG monitoring demonstrated that EEG backgrounds scored as unreactive, discontinuous, burst-suppression, or without discernable cerebral activity were associated with unfavorable outcomes, both during hypothermia and after return to normothermia.16 Another single-center retrospective study evaluated 34 subjects who were resuscitated from an in-hospital CA and underwent a routine 20-minute EEG during the first seven days following resuscitation. Although the patients in this study were influenced by clinician selection and were not managed with a standardized EEG monitoring pathway, the study identified associations between normal EEG background patterns and favorable outcome and between isoelectric EEG and unfavorable outcome.18 A single-center retrospective study of 34 children who underwent EEG monitoring after CA evaluating outcome at 6 months demonstrated that the presence of sleep spindles (including poorly formed sleep spindles) during the initial 24 hours after return of spontaneous circulation was associated with favorable outcomes. Spindles were present in 80% of children with favorable outcomes and only 8% of children with unfavorable outcomes.26
Seizures are reported in about 5–45% of subjects after CA,15,17,26,27,45 and EEG monitoring is often performed after CA for seizure identification.14,29 In this study, we found that seizures occurred in a slightly higher percentage of patients with unfavorable outcomes or mortality, but these associations were not significant. Other groups have similarly attempted to explore the association between electrographic seizures and outcome and reported that seizures were more common in subjects with unfavorable outcomes, but the differences were not statistically different. In the Topjian study, neither seizures nor status epilepticus were associated with mortality. However, status epilepticus was associated with unfavorable neurologic outcomes (87% unfavorable vs. 13% favorable, p=0.008).17 In the Ostendorf study,100% of 7 subjects with seizures had unfavorable outcomes, but the difference was not statistically significant.45 In the Brooks study, 78% of 9 subjects with seizures, 100% of 2 subjects with myoclonic status epilepticus, and 100% of 2 subjects with generalized periodic discharges had unfavorable outcomes, but the difference was not statistically significant.27 Similarly, in the Ducharme-Crevier study, 75% of 8 subjects with seizures had unfavorable outcomes, but the difference was not statistically significant.26 Thus, the impact of seizures on outcome remains uncertain. In cohorts of more heterogeneous acute encephalopathy etiologies, higher electroencephalographic seizure exposure has been associated with less favorable neurologic outcomes.33,46–48 Importantly, in all of these studies, clinicians aimed to intervene upon and manage identified electroencephalographic seizures, so the impact of a potentially higher seizure exposure in the absence of anti-seizure treatment cannot be assessed. It may be that in some children with more moderate injury, a higher electroencephalographic seizure burden can induce secondary brain injury that leads to less favorable neurologic outcomes.49
Other clinical and examination data may also be used for neuro-prognostication in children after CA. For example, studies have identified associations between unfavorable discharge outcomes and early abnormal neurologic examination signs (absent pupillary and motor responses),13 early post-CA hypotension,11 early elevated serum lactate levels,10 early depressed myocardial function,12 and abnormal brain computerized tomography scans.9 However, none of these measures predict outcomes independently.9–13 Importantly, when neurologists and intensivists predicted neurobehavioral outcomes from CA cases, the addition of early EEG data significantly improved prognostication accuracy.50 Our data support the idea that subsequent development of multi-modal models for neuro-prognostication in children should include early EEG data.
The study has several strengths when compared to the available literature. First, it prospectively evaluated a large, consecutive, and contemporary cohort of patients. Second, it used standardized scoring of the EEG tracing and did not rely solely on data obtained from clinical reports. Third, it assessed numerous EEG variables in addition to a categorical summary EEG variable. Fourth, although patients in this study underwent EEG monitoring, the early EEG variables evaluated could be obtained by a routine EEG. Fifth, the EEG scoring system used by the final model is relatively straight-forward and encompasses EEG features that have substantial inter-rater reliability.36,38
The study also has several limitations. First, we measured only short-term outcome using mortality and a simple outcome assessment tool (PCPC), and these outcomes may not reflect long-term patient-centered neurobehavioral outcomes. Studies utilizing longer-term and more detailed patient-centered neurobehavioral outcome assessments are needed. Second, EEG results were known to the clinical teams providing care which may have influenced goals of care decisions. To minimize the impact of this problem, we defined unfavorable neurobehavioral outcome in a broad manner (PCPC score 4–6) and not only death (PCPC score 6) to reduce the influence of family decisions regarding withdrawal of technological support on outcome categorization. A subject’s outcome would be categorized as unfavorable whether a family chose to withdraw or continue technological support of a child with severe disability, coma, or vegetative state (PCPC score 4–5). Third, we focused on the earliest available EEG after ROC given our aim of early prediction and early stratification of brain injury severity for future neuroprotective studies. However, assessing the EEG at later time points and evaluating for changes over time may be beneficial for prediction models of meaningful long-term neurobehavioral outcome. Fourth, although this was a large pediatric cardiac arrest cohort, the number of subjects could lead to model overfitting. Fifth, stage 2 sleep transients were included in the model with encephalopathic subjects, but it would be unclear how to implement this model if the patient were awake and thereby absent stage 2 sleep transient absence might be normal. Sixth, we included reactivity-variability in the final model, but further standardization of this assessment is needed51,52 as prior studies have shown limited inter-rater agreement for reactivity assessment.53–57 Future studies evaluating multi-modal prediction models will need to determine if inclusion of reactivity assessments add sufficient predictive value to be included despite these limitations.
In summary, many early EEG features, used individually and in combination, had a high specificity for unfavorable neurobehavioral outcome and mortality. Our model aimed for simplicity with the final model incorporating a four-level EEG Background Category (normal, slow-disorganized, discontinuous or burst-suppression, or attenuated-flat), Stage 2 Sleep Transients (present or absent), and Reactivity-Variability (present or absent). Our model also aimed for maximum specificity to limit the number of false positives when predicting unfavorable neurobehavioral outcome. The specificity of the optimal model was high for unfavorable neurobehavioral outcome (95%) and death (97%). However, the positive predictive value was only 86% for both outcomes. Therefore, early EEG data must be considered together with the overall clinical context. Further work is needed incorporating early and standardized EEG data along with clinical and examination data into comprehensive multi-modal models for neuroprognostication.
Supplementary Material
Supplemental Figure 1. Receiver operating characteristic curves for mortality (top) and unfavorable neurobehavioral outcome (bottom) showing the optimal (black squares), the model with the highest area under the receiver operating characteristic (AUROC) curve (black circles), and the other models (gray circles).
Supplemental Figure 2. Sensitivity (square) and specificity (circle) for mortality (top) and unfavorable neurobehavioral outcome (bottom).
Funding
Dr. Abend is funded by NIH K02NS096058.
Footnotes
Conflict of Interest
Dr. Abend receives funding from the NIH (NINDS), PCORI, and EFA which are all paid to his institution.
None of the other authors report conflicts of interest.
References
- 1.van Zellem L, Buysse C, Madderom M, et al. Long-term neuropsychological outcomes in children and adolescents after cardiac arrest. Intensive Care Med 2015;41:1057–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van Zellem L, Utens EM, Legerstee JS, et al. Cardiac Arrest in Children: Long-Term Health Status and Health-Related Quality of Life. Pediatr Crit Care Med 2015;16:693–702. [DOI] [PubMed] [Google Scholar]
- 3.Lopez-Herce J, Garcia C, Rodriguez-Nunez A, et al. Long-term outcome of paediatric cardiorespiratory arrest in Spain. Resuscitation 2005;64:79–85. [DOI] [PubMed] [Google Scholar]
- 4.Moler FW, Silverstein FS, Holubkov R, et al. Therapeutic Hypothermia after In-Hospital Cardiac Arrest in Children. N Engl J Med 2017;376:318–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berg RA, Nadkarni VM, Clark AE, et al. Incidence and Outcomes of Cardiopulmonary Resuscitation in PICUs. Crit Care Med 2016;44:798–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Knudson JD, Neish SR, Cabrera AG, et al. Prevalence and outcomes of pediatric in-hospital cardiopulmonary resuscitation in the United States: an analysis of the Kids’ Inpatient Database*. Crit Care Med 2012;40:2940–4. [DOI] [PubMed] [Google Scholar]
- 7.Slonim AD, Patel KM, Ruttimann UE, Pollack MM. Cardiopulmonary resuscitation in pediatric intensive care units. Crit Care Med 1997;25:1951–5. [DOI] [PubMed] [Google Scholar]
- 8.Girotra S, Spertus JA, Li Y, et al. Survival trends in pediatric in-hospital cardiac arrests: an analysis from Get With the Guidelines-Resuscitation. Circ Cardiovasc Qual Outcomes 2013;6:42–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Starling RM, Shekdar K, Licht D, Nadkarni VM, Berg RA, Topjian AA. Early Head CT Findings Are Associated With Outcomes After Pediatric Out-of-Hospital Cardiac Arrest. Pediatr Crit Care Med 2015;16:542–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Topjian AA, Clark AE, Casper TC, et al. Early lactate elevations following resuscitation from pediatric cardiac arrest are associated with increased mortality*. Pediatr Crit Care Med 2013;14:e380–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Topjian AA, French B, Sutton RM, et al. Early postresuscitation hypotension is associated with increased mortality following pediatric cardiac arrest. Crit Care Med 2014;42:1518–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Conlon TW, Falkensammer CB, Hammond RS, Nadkarni VM, Berg RA, Topjian AA. Association of left ventricular systolic function and vasopressor support with survival following pediatric out-of-hospital cardiac arrest. Pediatr Crit Care Med 2015;16:146–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abend NS, Topjian AA, Kessler SK, et al. Outcome prediction by motor and pupillary responses in children treated with therapeutic hypothermia after cardiac arrest. Pediatr Crit Care Med 2012;13:32–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Herman ST, Abend NS, Bleck TP, et al. Consensus statement on continuous EEG in critically ill adults and children, part I: indications. J Clin Neurophysiol 2015;32:87–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abend NS, Topjian A, Ichord R, et al. Electroencephalographic monitoring during hypothermia after pediatric cardiac arrest. Neurology 2009;72:1931–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kessler SK, Topjian AA, Gutierrez-Colina AM, et al. Short-term outcome prediction by electroencephalographic features in children treated with therapeutic hypothermia after cardiac arrest. Neurocrit Care 2011;14:37–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Topjian AA, Sanchez SM, Shults J, Berg RA, Dlugos DJ, Abend NS. Early Electroencephalographic Background Features Predict Outcomes in Children Resuscitated From Cardiac Arrest. Pediatr Crit Care Med 2016;17:547–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nishisaki A, Sullivan J 3rd, Steger B, et al. Retrospective analysis of the prognostic value of electroencephalography patterns obtained in pediatric in-hospital cardiac arrest survivors during three years. Pediatr Crit Care Med 2007;8:10–7. [DOI] [PubMed] [Google Scholar]
- 19.Pampiglione G, Harden A. Resuscitation after cardiocirculatory arrest. Prognostic evaluation of early electroencephalographic findings. Lancet 1968;1:1261–5. [DOI] [PubMed] [Google Scholar]
- 20.Tasker RC, Boyd S, Harden A, Matthew DJ. Monitoring in non-traumatic coma. Part II: Electroencephalography. Arch Dis Child 1988;63:895–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cheliout-Heraut F, Sale-Franque F, Hubert P, Bataille J. [Cerebral anoxia in near-drowning of children. The prognostic value of EEG]. Neurophysiol Clin 1991;21:121–32. [DOI] [PubMed] [Google Scholar]
- 22.Ramachandrannair R, Sharma R, Weiss SK, Cortez MA. Reactive EEG patterns in pediatric coma. Pediatr Neurol 2005;33:345–9. [DOI] [PubMed] [Google Scholar]
- 23.Mandel R, Martinot A, Delepoulle F, et al. Prediction of outcome after hypoxic-ischemic encephalopathy: a prospective clinical and electrophysiologic study. J Pediatr 2002;141:45–50. [DOI] [PubMed] [Google Scholar]
- 24.Pampiglione G, Chaloner J, Harden A, O’Brien J. Transitory ischemia/anoxia in young children and the prediction of quality of survival. Ann N Y Acad Sci 1978;315:281–92. [DOI] [PubMed] [Google Scholar]
- 25.Evans BM, Bartlett JR. Prediction of outcome in severe head injury based on recognition of sleep related activity in the polygraphic electroencephalogram. J Neurol Neurosurg Psychiatry 1995;59:17–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ducharme-Crevier L, Press CA, Kurz JE, Mills MG, Goldstein JL, Wainwright MS. Early Presence of Sleep Spindles on Electroencephalography Is Associated With Good Outcome After Pediatric Cardiac Arrest. Pediatr Crit Care Med 2017;18:452–60. [DOI] [PubMed] [Google Scholar]
- 27.Brooks GA, Park JT. Clinical and Electroencephalographic Correlates in Pediatric Cardiac Arrest: Experience at a Tertiary Care Center. Neuropediatrics 2018. [DOI] [PubMed] [Google Scholar]
- 28.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. Journal of biomedical informatics 2009;42:377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brophy GM, Bell R, Claassen J, et al. Guidelines for the evaluation and management of status epilepticus. Neurocrit Care 2012;17:3–23. [DOI] [PubMed] [Google Scholar]
- 30.Herman ST, Abend NS, Bleck TP, et al. Consensus statement on continuous EEG in critically ill adults and children, part II: personnel, technical specifications, and clinical practice. J Clin Neurophysiol 2015;32:96–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hirsch LJ, LaRoche SM, Gaspard N, et al. American Clinical Neurophysiology Society’s Standardized Critical Care EEG Terminology: 2012 version. J Clin Neurophysiol 2013;30:1–27. [DOI] [PubMed] [Google Scholar]
- 32.Abend NS, Gutierrez-Colina AM, Topjian AA, et al. Nonconvulsive seizures are common in critically ill children. Neurology 2011;76:1071–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wagenman KL, Blake TP, Sanchez SM, et al. Electrographic status epilepticus and long-term outcome in critically ill children. Neurology 2014;82:396–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Topjian AA, Gutierrez-Colina AM, Sanchez SM, et al. Electrographic status epilepticus is associated with mortality and worse short-term outcome in critically ill children. Crit Care Med 2013;41:215–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Beniczky S, Hirsch LJ, Kaplan PW, et al. Unified EEG terminology and criteria for nonconvulsive status epilepticus. Epilepsia 2013;54 Suppl 6:28–9. [DOI] [PubMed] [Google Scholar]
- 36.Abend NS, Gutierrez-Colina A, Zhao H, et al. Interobserver reproducibility of electroencephalogram interpretation in critically ill children. J Clin Neurophysiol 2011;28:15–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mani R, Arif H, Hirsch LJ, Gerard EE, LaRoche SM. Interrater reliability of ICU EEG research terminology. J Clin Neurophysiol 2012;29:203–12. [DOI] [PubMed] [Google Scholar]
- 38.Abend NS, Massey SL, Fitzgerald M, et al. Interrater Agreement of EEG Interpretation after Pediatric Cardiac Arrest Utilizing Standardized Critical Care EEG Terminology. Journal of Clinical Neurophysiology 2017;34:534–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Abend NS, Arndt DH, Carpenter JL, et al. Electrographic seizures in pediatric ICU patients: cohort study of risk factors and mortality. Neurology 2013;81:383–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang A, Arndt DH, Berg RA, et al. Development and validation of a seizure prediction model in critically ill children. Seizure 2015;25:104–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fiser DH, Long N, Roberson PK, Hefley G, Zolten K, Brodie-Fowler M. Relationship of pediatric overall performance category and pediatric cerebral performance category scores at pediatric intensive care unit discharge with outcome measures collected at hospital discharge and 1- and 6-month follow-up assessments. Crit Care Med 2000;28:2616–20. [DOI] [PubMed] [Google Scholar]
- 42.Du Pont-Thibodeau G, Fry M, Kirschen M, et al. Timing and Modes of Death after Pediatric Out-of-Hospital Cardiac Arrest Resuscitation. Resuscitation 2018. [DOI] [PubMed] [Google Scholar]
- 43.Kutner MH, Nachtsheim CJ, Neter J. Applied Linear Regression Models (4th ed.): McGraw-Hill; 2004. [Google Scholar]
- 44.Sanchez SM, Carpenter J, Chapman KE, et al. Pediatric ICU EEG monitoring: current resources and practice in the United States and Canada. J Clin Neurophysiol 2013;30:156–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ostendorf AP, Hartman ME, Friess SH. Early Electroencephalographic Findings Correlate With Neurologic Outcome in Children Following Cardiac Arrest. Pediatr Crit Care Med 2016;17:667–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Payne ET, Zhao XY, Frndova H, et al. Seizure burden is independently associated with short term outcome in critically ill children. Brain 2014;137:1429–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Topjian AA, Gutierrez-Colina AM, Sanchez SM, et al. Electrographic Status Epilepticus is Associated with Mortality and Worse Short-Term Outcome in Critically Ill Children. Crit Care Med 2013;41:215–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Abend NS, Wagenman KL, Blake TP, et al. Electrographic status epilepticus and neurobehavioral outcomes in critically ill children. Epilepsy Behav 2015;49:238–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pinchefsky EF, Hahn CD. Outcomes following electrographic seizures and electrographic status epilepticus in the pediatric and neonatal ICUs. Curr Opin Neurol 2017;30:156–64. [DOI] [PubMed] [Google Scholar]
- 50.Kirschen MP, Topjian AA, Hammond R, Illes J, Abend NS. Neuroprognostication after pediatric cardiac arrest. Pediatr Neurol 2014;51:663–8 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Amorim E, Gilmore EJ, Abend NS, et al. EEG Reactivity Evaluation Practices for Adult and Pediatric Hypoxic-Ischemic Coma Prognostication in North America. J Clin Neurophysiol 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fantaneanu TA, Tolchin B, Alvarez V, et al. Effect of stimulus type and temperature on EEG reactivity in cardiac arrest. Clin Neurophysiol 2016;127:3412–7. [DOI] [PubMed] [Google Scholar]
- 53.Wusthoff CJ, Sullivan J, Glass HC, et al. Interrater agreement in the interpretation of neonatal electroencephalography in hypoxic-ischemic encephalopathy. Epilepsia 2017;58:429–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hermans MC, Westover MB, van Putten MJ, Hirsch LJ, Gaspard N. Quantification of EEG reactivity in comatose patients. Clin Neurophysiol 2016;127:571–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Westhall E, Rosen I, Rossetti AO, et al. Interrater variability of EEG interpretation in comatose cardiac arrest patients. Clin Neurophysiol 2015;126:2397–404. [DOI] [PubMed] [Google Scholar]
- 56.Synek VM. Prognostically important EEG coma patterns in diffuse anoxic and traumatic encephalopathies in adults. J Clin Neurophysiol 1988;5:161–74. [DOI] [PubMed] [Google Scholar]
- 57.Young GB, McLachlan RS, Kreeft JH, Demelo JD. An electroencephalographic classification for coma. Can J Neurol Sci 1997;24:320–5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Receiver operating characteristic curves for mortality (top) and unfavorable neurobehavioral outcome (bottom) showing the optimal (black squares), the model with the highest area under the receiver operating characteristic (AUROC) curve (black circles), and the other models (gray circles).
Supplemental Figure 2. Sensitivity (square) and specificity (circle) for mortality (top) and unfavorable neurobehavioral outcome (bottom).