Abstract
The adaptive immune system plays a crucial role in anti-tumor immune responses. Enhancement of T cell responses through checkpoint blockade has become a major therapeutic avenue of intervention for several tumors. Because it shapes immune responses and regulates their amplitude and duration, the microbiota has a substantial impact on anti-tumor immunity. Innate lymphoid cells (ILCs) comprise a heterogeneous population of lymphocytes devoid of antigen-specific receptors that mirror T helper cells in their ability to secrete cytokines that activate immune responses. Ongoing studies suggest that ILCs contribute to anti-tumor responses. Moreover, since ILCs are present at barrier surfaces, they are stimulated by the microbiota and, reciprocally, influence the composition of the microbiota by regulating the surface barrier microenvironment. Thus, ILC-microbiota cross-talk may in part underpin the effects of the microbiota on anti-tumor responses. In this article, we review current evidence linking ILCs to cancer and discuss the potential impact of ILC-microbiota cross-talk in anti-tumor immune responses.
1. Introduction
In the last twenty years, it has become accepted that the immune system plays a crucial role in tumor editing. The immune system can eliminate immunogenic tumors, confine them in a dormant state, or allow the escape of non-immunogenic variants that grow unrestrained [1]. Enhancement of anti-tumor immune responses through checkpoint blockade has become a major therapeutic avenue of intervention for several tumors [2].
Because of its role in shaping immune responses, the microbiota has become the focus of much attention in anti-tumor immunity and immunotherapy. The microbiota consists of a symbiotic ecological community of trillions of microorganisms that reside in the skin, gastrointestinal, respiratory and urinogenital tracts [3]. The microbiota regulates physiological functions, such as metabolism of nutrient components and generation of key bioactive molecules like vitamins [4–6]. By occupying surfaces that are access points for pathogens, the microbiota outcompetes pathogens for space and food, thereby protecting the host from infections. Importantly, the microbiota also plays a major role in shaping the immune system and regulating quality, magnitude and duration of immune responses, including anti-tumor responses [7–12].
Barrier surfaces are also the home of innate lymphoid cells (ILCs), a group of heterogenous lymphocytes that lack recombinant associated gene (RAG)-dependent antigen-specific receptors and specialize in the production of effector cytokines and chemokines in response to various stimuli in the microenvironment [13]. Since ILCs are important components of the immune system and extensively cross-talk with the microbiota, there is increasing interest in how ILCs modulate tumor initiation and progression, as well as their ability to impact immunotherapy. Here, we review the current studies on the role of ILCs in cancer, the impact of microbiota in cancer immunotherapy and discuss the plausible pro-tumorigenic and anti-tumorigenic implications of microbiota-ILC cross-talk.
2. ILCs and Natural Killer cells
ILCs develop in the fetal liver and bone marrow from the common lymphoid progenitor but, in contrast to T cells and B cells, are devoid of antigen-specific receptors. Based on the signature cytokines they secrete, their unique arrays of cell surface markers, and the transcription factors that relegate their fate, ILCs are broadly classified into 3 types: ILC1s, ILC2s, and ILC3s, which are considered the innate counterparts of Th1, Th2 and Th17 cells [13, 14]. Natural killer (NK) cells are cytolytic innate lymphocytes and therefore deemed the innate counterparts of CD8 T cells [13].
ILC1s overlap significantly with conventional NK cells, such that distinguishing the two is often problematic. Both cell types produce IFN-γ in response to IL-12, IL-18 and IL-15 and express shared cell surface markers including NKp46 and NK1.1in C57BL/6 mice and other strains carrying a similar Nkrp1c allele [15]. However, they do differ in some respects, for instance, ILC1s and NK cells reside in distinct locations. ILC1s are tissue resident cells and hence are largely confined to tissues, including the gut mucosa, salivary glands, liver, and adipose tissue, whereas NK cells are found in the blood and can recirculate between the blood and lymphoid or non-lymphoid tissues [16, 17]. Another criteria frequently used to distinguish ILC1s from NK cells, especially in mice, is based on the transcription factors that drive their development. Hobit [18] and T-bet [19] govern ILC1 development, whereas NK cells rely on EOMES and T-bet [20–22]. A third criteria for distinguishing between the two is based on their differing cytolytic potential; NK cells generate more cytolytic mediators, like perforin and granzymes, than do ILC1s [23]. Finally, although ILC1s and NK cells express a very similar panoply of surface markers, differential expression of CD49a, CD73 and CD61 can help segregate the two cell types [19, 24, 25]. Both ILC1s and NK cells play crucial roles in combating intracellular pathogens through secretion of IFN-γ. ILC1s provide an immediate tissue source of IFN-γ during infections, which is sustained by the subsequent migration of NK cells from the blood into the tissue [19, 25, 26]. NK cells also lyse virally infected cells and tumor cells, whereas the lytic function of ILC1s is thought to be limited, although this concept has not been experimentally demonstrated.
ILC2s populate adipose tissue, lungs, gut, liver, and skin in the steady state. They lack lineage markers and typically express KLRG1, Sca-1, CD25, CD127 and IL1RL1 (also known as IL-33R or ST2) on the cell surface [27]. The transcription factors GATA3 and RORα drive ILC2 differentiation [28, 29] and mature ILC2s secrete IL-5, IL-9, IL-13 and amphiregulin upon stimulation with IL-33, IL-25 and/or TSLP. Collectively, these characteristics render ILC2s the innate counterparts of Th2 [30]. ILC2s play a role in host response against helminths, but their sustained activation can be detrimental in allergies and asthma [31, 32].
ILC3s are primarily found in the intestine and secondary lymphoid organs in the steady state. They express IL-23R on the surface and depend on the transcription factor RORγT for their differentiation [13, 33]. ILC3s produce IL-22 and IL-17 in response to IL-23 and IL-1 and hence are considered the innate counterparts of Th17. IL-22 stimulates epithelial cell antimicrobial and repair mechanisms [34, 35], whereas IL-17 promotes granulopoiesis and recruitment of neutrophils [36, 37]. ILC3s help maintain intestinal homeostasis and provide host defense against extracellular bacteria and fungi [38–40]. In mouse, three subsets of ILC3s have been identified that have unique features but share overlapping developmental pathways and functions. CCR6+NKp46− ILC3s produce more IL-17 than IL-22 and express MHC class II, which induces the generation of a tolerogenic CD4+ T cell response against commensal microbes that buttresses intestinal homeostasis [41]. In the fetus, CCR6+NKp46− ILC3s correspond to lymphoid tissue inducers (LTi) cells, which drive the generation of lymphoid tissues during development [42, 43]. CCR6− NKp46+ ILC3s and their immediate CCR6−NKp46− precursors specialize in the production of IL-22.
3. The emerging role of ILCs in cancer immunoediting
The role of NK cells in cancer immunosurveillance has been extensively studied over the years and has recently drawn even more attention because NK cell-based therapies have such marked potential for treating cancer [44, 45]. A number of studies have provided evidence for this. Antibody-mediated depletion of NK cells results in increased tumor growth in various experimental models [46–48]. Enhanced tumor growth has been observed in mice with defects in NK cell receptors and functions [49–52]. In humans, early studies reported fewer NK cells and/or activity in patients with tumors than in healthy individuals and noted that NK cell infiltration into solid tumors is quite infrequent [53]. Lower frequency or impaired function of NK cells positively correlates with poor prognosis of head and neck [54, 55], pharyngeal [56] and colorectal cancers [57, 58]. NK cell activating receptors, such as NKp30 and NKp46, are expressed at lower levels in patients with acute myeloid leukemia than healthy individuals [59]. Recently, activation of NK cells through blockade of the inhibitory receptor NKG2A was shown to enhance anti-tumor responses elicited by checkpoint blockade or cancer vaccines in both experimental and clinical cancer [60]. Moreover, NK cells have been shown to effectively control experimental tumors that have been treated with drugs capable of inducing senescence, suggesting a potential synergism between NK cells and chemotherapy [61].
Moving beyond the established role of NK cells in cancer immunosurveillance and immunotherapy, the impact of ILCs in cancer is now being fully explored. ILC1s expressing granzyme B and TRAIL were detected in a MMTV-PyMT mammary tumor model, where they lysed tumor cells [62]. These unconventional ILC1s were IL-15-dependent but Nfil3-independent, in contrast to NK cells and conventional ILCs that depend on Nfil3 [62]. Since tumor growth was accelerated in Il15−/− mice but remained unaltered in Nfil3−/− mice, unconventional ILC1s rather than NK cells seemed to dominate the anti-tumor response in this model [62]. Recently, it was shown that human ILC1s and NK cells express an activating receptor, NKp44, which endows them with the ability to recognize tumor cells that secrete platelet derived growth factor (PDGF)-D. Detection of PDGFD triggers the secretion of IFN-γ and TNF-α that, together, slow the proliferation of tumor cells and promote increased expression of MHC class I and adhesion molecules, which facilitates their elimination by the immune system. Moreover, NKp44-PDGF-D interaction potentiates immune checkpoint blockade [63].
Given that IFN-γ hinders proliferation [64, 65], promotes MHC expression, and induces the release of proinflammatory chemokines that attract CD8 T cells [66, 67], NK cells and ILC1s are generally viewed as anti-tumorigenic (Fig.1). However, IFN-γ has been shown to foster tumor development in some situations. IFN-γ induces colonization, boosts proliferation and renders various tumor cells more aggressive [68–70]. In addition, IFN-γ promotes the expression of enzyme indoleamine 2,3–dioxygenase (IDO), which has immunomodulatory functions and facilitates the recruitment of myeloid derived suppressor cells (MDSC), which inhibit T cell response against tumors [71, 72]. Moreover, under certain conditions, such as SMAD4-deficiency, IFN-γ becomes somewhat irrelevant as NK cells and ILC1s alter their phenotype and express molecules implicated in immunoregulation, including the inhibitory receptor TIGIT and the ectonucleotidase CD73, which generates the inhibitory mediator adenosine [73]. A regulatory and poorly cytotoxic ILC population resembling NK cells and ILC1s was reported in human high grade serous tumors. These regulatory ILCs inhibited expansion and function of tumor infiltrating T cells in vitro [74]. Thus, anti-tumor and pro-tumor activities of ILC1s are probably context dependent.
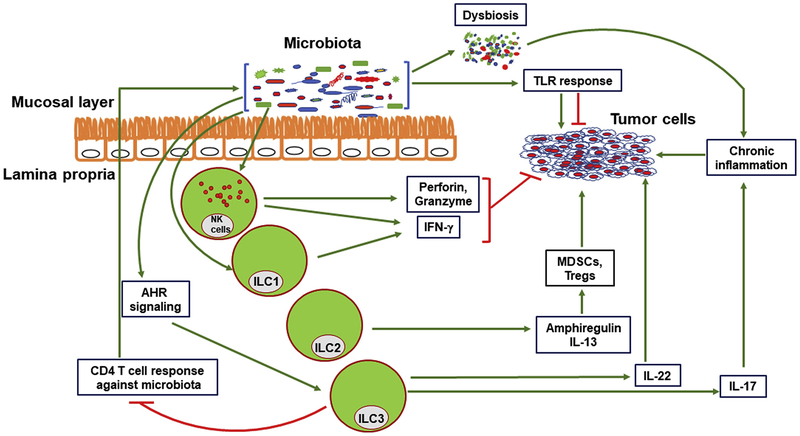
Figure 1. Direct and indirect effects of ILCs on cancer.
ILCs have both pro and anti-tumorigenic effect. ILC1s and NK cells block tumor cell growth through IFN-γ secretion. NK cells also lyse tumor cells through secretion of perforin and granzymes. IL-13 and amphiregulin secreted by ILC2s enhance MDSC and Treg functions respectively, facilitating immune evasion of tumors. ILC3 help maintaining a diverse microbiota and induce CD4 T cell tolerance for commensal bacteria, preventing dysbiosis and chronic inflammation that create a pro-tumorigenic microenvironment. Species in a healthy microbiota can also augment anti-cancer therapy. However, ILC3s can promote tumor growth by excessive secretion of IL-22. Through inappropriate release of IL-17, ILC3s also contribute to chronic inflammation that promotes tumorigenesis. Products from microbiota enhance TLR activation, which can promote or inhibit tumors depending on the context.
The roles of ILC2s and ILC3s in cancer have also been investigated. Since type-2 responses are generally associated with tumor progression, ILC2s may be pro-tumorigenic. Supporting this notion, the frequency of peripheral ILC2s was increased in patients with gastric cancer [75]. In mice, an increase in ILC2 number by IL-33 favored tumor progression in an experimental model of breast cancer [76]. Furthermore, IL-13 secretion by ILC2s activated MDSCs and triggered the differentiation of M2 macrophages, which are pro-tumorigenic (Fig.1) [76, 77]. ILC2s constitutively express Arginase-1 [78, 79], which may inhibit T cell responses [80, 81] by depriving L-arginine. Moreover, ILC2s secrete amphiregulin, which enhances the suppressive function of regulatory T cells (Tregs), thereby facilitating immunosuppression [82]. However, there is some evidence that IL-5 and IL-9 secreted by ILC2s may induce an anti-tumorigenic response. In the absence of either IL-5 or IL-9, increased tumor metastasis was observed in several different mouse models [83–85]. Thus, more studies are necessary to define the role of ILC2s in cancer.
Most reports suggest that ILC3s are pro-tumorigenic. Consistent with this proposal, the major activator of ILC3s, IL-23 was found to be highly expressed in human and mice colon tumors [86–88]. Mice deficient for IL-23R are resistant to B16F10 melanoma tumor growth [87]. Furthermore, colonic tumor growth was blunted in IL-23- and IL-23R-deficient mice undergoing spontaneous development of colon cancer [88]. ILC3 effector cytokines, IL-22 and IL-17, have also been shown to promote tumorigenesis (Fig.1) [89–91]. IL-22 promotes colorectal cancer in a Helicobacter hepaticus-induced model [91]. IL- 22 binding protein is a decoy receptor produced by dendritic cells that controls the bioavailability of IL-22 in the steady state. Lack of IL-22 binding protein and subsequent increase of IL-22 activity facilitated cancer development in a model of spontaneous colorectal cancer [92]. Overall, these reports suggest that sustained activation of ILC3s by IL-23 and inappropriate secretion of IL-17 and IL-22 may result in chronic intestinal inflammation that facilitates cancer. This condition may occur in patients with inflammatory bowel disease, in which sustained IL-23-mediated inflammation increases the risk of colon cancer [93]. However, an anti-tumorigenic role of ILC3s has also been reported in both experimental and clinical cancers. NKp46+ LTi cells inhibited tumor growth in a B16F10 subcutaneous melanoma model [94]. Upon activation by IL-12, these cells induced expression of adhesion molecules such as ICAM and VCAM on the tumor vasculature, which might facilitate infiltration of effector cells into the tumor tissue. ILC3s also enhanced the anti-tumorigenic effect of combined immune-chemotherapy by facilitating macrophage infiltration into the tumor and growth arrest in the B16F10 melanoma model [95]. Moreover, the presence of ILC3s was associated with a beneficial prognosis in human non-small cell lung cancer (NSCLC) [96]. Significant infiltration of ILC3s was evident in early stage tumors, whereas very few infiltrating ILC3s were detected in advanced stage-III tumors. ILC3s accumulated at the edge of tertiary lymphoid structures (TLS) and positively correlated with the density of TLS. Since these infiltrating ILC3s secrete lymphotoxins, they may contribute to the formation of denser TLS, which is predictive of a favorable prognosis [96].
In conclusion, ILC1s together with NK cells are viewed as anti-tumorigenic, whereas ILC2s are considered pro-tumorigenic. Depending on the context, ILC3s can assert both anti- and pro-tumorigenic effects. While the anti-tumor activity of NK cells can be harnessed for therapeutic purposes, ongoing studies in both mouse experimental models and human patients are needed to clarify whether ILCs are a viable target for tumor immunotherapy.
4. The impact of microbiota in immune responses against cancer
The microbiome can play both pro- and anti-carcinogenic roles. Bacteria like E. coli, Bacteroides fragilis and ε and γ proteobacteria secrete colibactin, B. fragilis toxin (BFT) and cytolethal distending toxin (CDT), respectively, which can damage host DNA either directly or indirectly by inducing reactive oxygen species [97–99]. These toxins have been associated with both clinical and experimental colon cancer [100, 101]. Other components of the microbiome, like H. pylori and B. fragilis secrete toxins like CagA and BFT respectively, which disrupt the β-catenin signaling pathway and drive cancer pathogenesis in the colon [102, 103]. By digesting or modifying the dietary components, gut bacteria also produce metabolites, such as hydrogen sulfide and secondary bile acids, that are carcinogenic (Fig.1) [104–106].
Given the role of healthy microbiota in controlling metabolism and inflammation, perturbations of the microbiota, known as dysbiosis, may increase the risk of cancer by causing dysregulated metabolism and chronic inflammation. Mechanistically, dysbiosis and inflammation can lead to increased expression of TLRs and other pattern recognition receptors in the gut mucosa, thereby enhancing gut responsiveness to bacterial products and generating a feed forward loop that exacerbates inflammation and favors tumor growth (Fig.1) [12, 107, 108].
A major impact of the microbiota on chemotherapy and immunotherapy for cancer has been recently demonstrated. By damaging the mucosal epithelium, chemotherapy facilitates the translocation of microbiota species into mesenteric lymph node and spleen [109]. In the case of cyclophosphamide treatment, leakage of microbiota through the intestinal barrier resulted in a beneficial enhancement of Th1 and Th17 response against cancer [109, 110]. Accordingly, mice depleted of microbiota by antibiotics treatment and germ-free mice both responded more poorly to this chemotherapy than did mice with normal microflora [109, 110].
Immunotherapy can also be potentiated by the presence of gut microflora. Mice with healthy gut flora responded more favorably to anti-CTLA-4 and anti-PD-1 treatment than did germ-free mice or mice with disturbed microbiota [111–113]. These observations were further corroborated by studies with cancer patients. A negative correlation between antibiotic use and a positive response to anti-PD-1 therapy was observed in patients with non-small cell lung cancer, metastatic melanoma and urothelial carcinoma. Furthermore, the fecal microbiota profiles of poor responders and non-responders to anti-PD-1 therapy differed from those of the responders [11, 12, 114]. Similarly, the efficacy of adjuvant therapies based on anti-IL-10 or CpG-oligodeoxyribonucleotides depends on the gut microbiota, as untreated mice responded better to these therapies than did antibiotic treated mice [115]. Altogether, these studies suggest that a healthy microbiota lowers the risk for cancer and can actually potentiate the effects of cancer chemotherapy and immunotherapy by enhancing anti-cancer immune responses (Fig.1). In contrast, dysbiosis or expansion of toxin-producing species can facilitate a chronic inflammation and epithelial damage that increase the risk of cancer.
5. The cross-talk between ILCs and microbiota
Because of their location at the barrier surfaces, ILC3s and the microbiota continuously cross-talk. ILC3s from germ-free mice produce less IL-22 than do mice with microflora [116]. Indeed, certain components of the microbiota, such as Lactobacillus reuteri, degrade nutritional tryptophan generating indol-derivatives that activate the aryl hydrocarbon receptor, a transcription factor expressed in ILC3s, which promotes the secretion of IL-22 [10, 117]. Similarly, segmented filamentous bacteria that colonize the gut induce IL-22 production in intestinal ILC3s by an IL-23-dependent mechanism [118]. Components of the microbiota induce macrophage production of IL-1β that, in turn, stimulates ILC3 secretion of GM-CSF, which promotes the maintenance of oral tolerance [119]. Similarly, the microbiota induces secretion of TNF-like ligand 1A by mononuclear phagocytes, which potentiates IL-22 production by ILC3s [120]. Commensal microbes also impact the metabolism of vitamin-A [121, 122], which promotes the generation of ILC3s. Vitamin-A deficiency resulted in diminished ILC3 numbers as well as defective gut homing and impaired ILC3 responses to C. rodentium infection [123, 124]. Signals from commensal microbes can stimulate mononuclear phagocytes and/or intestinal epithelial cells to secrete IL-1β, IL-23, IL-25 and TSLP [119, 125–128], which have been implicated in either blocking or promoting ILC3 functions [119, 125, 127, 129–131]. Furthermore, commensal microflora facilitates trafficking of ILC3s into the lungs of newborn mice, where they thwart pneumonia infection by secreting IL-22 [132]. Whether the microbiota affects ILC3 numbers is controversial. Adult germ-free mice have normal ILC3 numbers in the intestine [133, 134]. However, while the development of fetal NKp46−CCR6+ LTi cells may be microbiota-independent, the intestinal flora may at least promote the development of post-natal NKp46+CCR6−ILC3s [33]. Reciprocally, ILC3s can shape the microbiota through multiple mechanisms. By producing IL-22 that elicits the secretion of antimicrobial molecules by epithelial cells, ILC3s limit intestinal colonization by segmented filamentous bacteria [135–137] and perhaps other pathobionts [135, 136]. Corroborating the role of IL-22 in maintaining intestinal homeostasis, Zenewicz et al. reported that the microbiota of Il22−/− mice significantly differs from that of wild type mice in that it encompasses fewer members of some genera, including Lactobacillus, which is considered beneficial, and more of others like Helicobacter, which can be pathogenic. Moreover, wild type mice cohoused with Il22−/− mice long enough to adopt their altered microbiota suffered from exacerbated DSS-induced colitis, though the symptoms were more severe in the Il22−/− mice, reflecting both the protective role of IL-22 itself and the detrimentally altered microbiota [138]. It has been shown that ILC3s can also regulate the adaptive immune response against microbiota, thus promoting tolerance. Accordingly, mice that lack ILC3s had elevated levels of IgG and a heightened CD4+ T cell response against commensal microflora [139]. In addition, ILC3s can induce apoptosis of CD4+ T cells directed against commensal microbes in an MHC class II dependent manner [41]. Selective genetic ablation of MHC class II expression in ILC3s resulted in increased generation of commensal specific T cell and colonic inflammation, which was attenuated by administration of antibiotics. An inverse correlation between MHC class II expression in intestinal ILC3s and expansion of pathogenic Th17 cells in pediatric IBD patients further supports a role for ILC3s in regulating T cell responses against commensal microflora [41]. Collectively, these studies highlight the interdependency of ILC3s and the intestinal microbiota (Fig.1). While the microbiota dictates the function and the development of ILCs, ILCs contribute to the containment of various microbiota species and calibrate the tone of immune response against microbiota.
Emerging studies suggest a link between NK cells, ILC1s and the microbiota. The human fetal intestine has very few ILC1s, which only develop a few weeks after birth, suggesting that the microbiota supports ILC1 development [140, 141]. Moreover, NK cells from germ free mice produce very little IFN-γ and granzyme B in response to poly I:C stimulation. As a result, germ free mice fail to control murine cytomegalovirus infection as well as do specific pathogen free mice, although they harbor comparable numbers of NK cells [142]. Future studies are necessary to identify the microbiota species and/or their metabolites that impact ILC1s and NK cells. While intestinal and lung ILC2 numbers and functions are clearly induced by helminth infections [143, 144], a direct impact of the microbiota on ILC2 development has been controversial. While numbers and expression of surface receptors on lung ILC2s are similar between germ-free and specific pathogen free mice [145], expansion of ILC2s in the intestine of germ-free mice has been observed [146]. Further, infection of germ-free mice with norovirus attenuated the expansion of ILC2s and led to an increase in the ratio of ILC3s to ILC2s, suggesting trans-differentiation of ILC2s into ILC3s [146]. Moreover, elimination of microbiota by antibiotic treatment induced differentiation of ILC2s and ILC1s towards an ILC3 phenotype, revealing a crucial role for the microbiota in maintaining ILC1s and ILC2s [147]. Additionally, commensals maintain the fitness and functionality of ILC2s. Production of TSLP and IL-25 is regulated by commensals; in synergy with IL-33, TSLP and IL-25 play important roles in activating ILC2s. [126–128]. Altogether, these studies demonstrate the importance of the microflora in the development and maintenance of ILCs.
6. Concluding remarks
Relationships between ILCs and cancer, the microbiota and cancer, and ILCs and the microbiota have been established (Fig.2). These studies raise the possibility of complex interactions between ILCs, the microbiota and cancer that should be further explored. Several scenarios can be envisioned. In homeostatic settings, ILCs may be crucial to maintain a balance between pro- and anti-tumorigenic commensal bacteria; ILC- secreted cytokines may support a normal microflora, thereby preventing chronic inflammation and cancer. Concomitantly, ILCs may restrict the niche for commensal bacteria like E. coli and H. pylori that have been shown to be promote cancer development [101, 103]. ILC-secreted cytokines may also support particular bacteria, such as E. hirae, B. intestinihomini, B. fragilis, B. thetaiotamicron, B. breve and B. longum that have been shown to enhance cancer therapies [112, 113]. In pathologic settings, dysbiosis caused by antibiotic treatment or other factors may induce inappropriate release of cytokines by ILCs, particularly IL-17 and IL-22, which may contribute to chronic inflammation and predispose to cancer. Additionally, given the role of ILCs in recruiting immune cells and shaping adaptive immune responses, abnormal ILC responses to dysbiosis may also impact T cell responses, further contributing to chronic inflammation and cancer. In therapeutic settings, chemotherapy-induced damage of epithelial cells may induce ILC responses that, in turn, impact bacterial translocation and chemotherapy effectiveness. For example, it has been shown that methotrexate-induced epithelial damage elicits ILC3 production of IL-22, which limits damage of the epithelial barrier [148]. Whether ILC3s impact the effectiveness of methotrexate treatment remains unknown. Checkpoint blockade and other immunotherapies may also impact ILCs directly or indirectly, generating feedback loops that may impact microbiota and therapeutic efficacy. Thus, it is important to obtain a complete picture of ILCs profile in clinical cancer. Further research considering the interactions between ILCs, the microbiota and tumors may help delineate as yet undefined crucial steps in cancer progression and pave the way for therapeutic strategies that target ILCs.
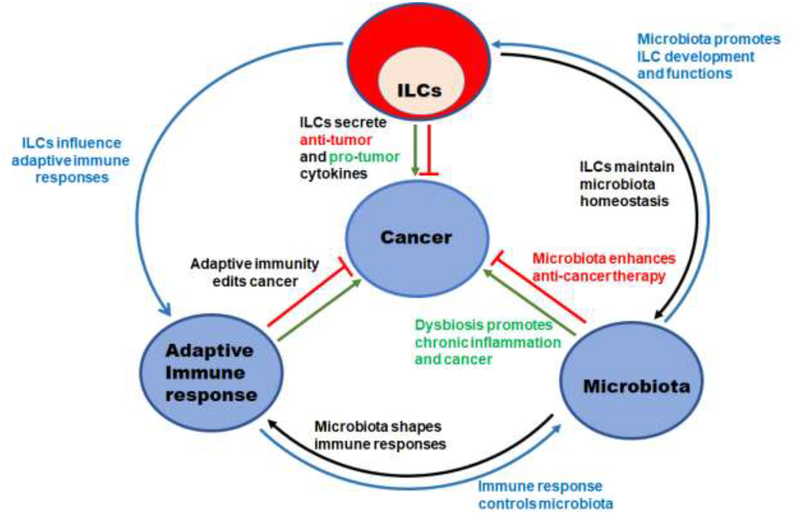
Figure 2. Schematic representation of the interactions between ILCs, adaptive immunity, microbiota and cancer.
ILCs can secrete pro- and anti-tumorigenic cytokines. ILCs can also impact anti-tumor adaptive immune response by secreting effector cytokines. ILCs contribute to the maintenance of a normal commensal microflora which reduces the risk of cancer and may potentiate anti-cancer chemotherapy and immune therapy. Reciprocally, microbiota drives ILCs development and function. Dysfunction of ILCs-microbiota interactions can lead to dysbiosis and chronic inflammation, resulting in a pro-tumorigenic microenvironment. Abnormal interactions between ILCs and adaptive immune responses may also induce a pro-tumorigenic environment through chronic inflammation and dysbiosis.
Acknowledgments
This work is supported by the US National Institutes of Health (UO1 AI095542, RO1 DE025884, and R01 AI134236.
We would like to thank Susan Gilfillan for critical comments
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement
The authors declare no commercial or financial conflict of interest.
References
- [1].Schreiber RD, Old LJ, Smyth MJ, Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion, Science 331(6024) (2011) 1565–70. [DOI] [PubMed] [Google Scholar]
- [2].Wei SC, Duffy CR, Allison JP, Fundamental Mechanisms of Immune Checkpoint Blockade Therapy, Cancer Discov 8(9) (2018) 1069–1086. [DOI] [PubMed] [Google Scholar]
- [3].Wong SH, Kwong TNY, Wu CY, Yu J, Clinical applications of gut microbiota in cancer biology, Semin Cancer Biol (2018). [DOI] [PubMed] [Google Scholar]
- [4].Belzer C, Chia LW, Aalvink S, Chamlagain B, Piironen V, Knol J, de Vos WM, Microbial Metabolic Networks at the Mucus Layer Lead to Diet-Independent Butyrate and Vitamin B12 Production by Intestinal Symbionts, MBio 8(5) (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Guinane CM, Cotter PD, Role of the gut microbiota in health and chronic gastrointestinal disease: understanding a hidden metabolic organ, Therap Adv Gastroenterol 6(4) (2013) 295–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Magnusdottir S, Ravcheev D, de Crecy-Lagard V, Thiele I, Systematic genome assessment of B-vitamin biosynthesis suggests co-operation among gut microbes, Front Genet 6 (2015) 148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Hooper LV, Littman DR, Macpherson AJ, Interactions between the microbiota and the immune system, Science 336(6086) (2012) 1268–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Rooks MG, Garrett WS, Gut microbiota, metabolites and host immunity, Nat Rev Immunol 16(6) (2016) 341–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Atarashi K, Tanoue T, Oshima K, Suda W, Nagano Y, Nishikawa H, Fukuda S, Saito T, Narushima S, Hase K, Kim S, Fritz JV, Wilmes P, Ueha S, Matsushima K, Ohno H, Olle B, Sakaguchi S, Taniguchi T, Morita H, Hattori M, Honda K, Treg induction by a rationally selected mixture of Clostridia strains from the human microbiota, Nature 500(7461) (2013) 232–6. [DOI] [PubMed] [Google Scholar]
- [10].Cervantes-Barragan L, Chai JN, Tianero MD, Di Luccia B, Ahern PP, Merriman J, Cortez VS, Caparon MG, Donia MS, Gilfillan S, Cella M, Gordon JI, Hsieh CS, Colonna M, Lactobacillus reuteri induces gut intraepithelial CD4(+)CD8alphaalpha(+) T cells, Science 357(6353) (2017) 806–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Routy B, Le Chatelier E, Derosa L, Duong CPM, Alou MT, Daillere R, Fluckiger A, Messaoudene M, Rauber C, Roberti MP, Fidelle M, Flament C, Poirier-Colame V, Opolon P, Klein C, Iribarren K, Mondragon L, Jacquelot N, Qu B, Ferrere G, Clemenson C, Mezquita L, Masip JR, Naltet C, Brosseau S, Kaderbhai C, Richard C, Rizvi H, Levenez F, Galleron N, Quinquis B, Pons N, Ryffel B, Minard-Colin V, Gonin P, Soria JC, Deutsch E, Loriot Y, Ghiringhelli F, Zalcman G, Goldwasser F, Escudier B, Hellmann MD, Eggermont A, Raoult D, Albiges L, Kroemer G, Zitvogel L, Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors, Science 359(6371) (2018) 91–97. [DOI] [PubMed] [Google Scholar]
- [12].Gopalakrishnan V, Helmink BA, Spencer CN, Reuben A, Wargo JA, The Influence of the Gut Microbiome on Cancer, Immunity, and Cancer Immunotherapy, Cancer Cell 33(4) (2018) 570–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Colonna M, Innate Lymphoid Cells: Diversity, Plasticity, and Unique Functions in Immunity, Immunity 48(6) (2018) 1104–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Vivier E, Artis D, Colonna M, Diefenbach A, Di Santo JP, Eberl G, Koyasu S, Locksley RM, McKenzie ANJ, Mebius RE, Powrie F, Spits H, Innate Lymphoid Cells: 10 Years On, Cell 174(5) (2018) 1054–1066. [DOI] [PubMed] [Google Scholar]
- [15].Aust JG, Gays F, Mickiewicz KM, Buchanan E, Brooks CG, The expression and function of the NKRP1 receptor family in C57BL/6 mice, J Immunol 183(1) (2009) 106–16. [DOI] [PubMed] [Google Scholar]
- [16].Gao Y, Souza-Fonseca-Guimaraes F, Bald T, Ng SS, Young A, Ngiow SF, Rautela J, Straube J, Waddell N, Blake SJ, Yan J, Bartholin L, Lee JS, Vivier E, Takeda K, Messaoudene M, Zitvogel L, Teng MWL, Belz GT, Engwerda CR, Huntington ND, Nakamura K, Holzel M, Smyth MJ, Tumor immunoevasion by the conversion of effector NK cells into type 1 innate lymphoid cells, Nat Immunol 18(9) (2017) 1004–1015. [DOI] [PubMed] [Google Scholar]
- [17].Fuchs A, ILC1s in Tissue Inflammation and Infection, Front Immunol 7 (2016) 104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Mackay LK, Minnich M, Kragten NA, Liao Y, Nota B, Seillet C, Zaid A, Man K, Preston S, Freestone D, Braun A, Wynne-Jones E, Behr FM, Stark R, Pellicci DG, Godfrey DI, Belz GT, Pellegrini M, Gebhardt T, Busslinger M, Shi W, Carbone FR, van Lier RA, Kallies A, van Gisbergen KP, Hobit and Blimp1 instruct a universal transcriptional program of tissue residency in lymphocytes, Science 352(6284) (2016) 459–63. [DOI] [PubMed] [Google Scholar]
- [19].Klose CSN, Flach M, Mohle L, Rogell L, Hoyler T, Ebert K, Fabiunke C, Pfeifer D, Sexl V, Fonseca-Pereira D, Domingues RG, Veiga-Fernandes H, Arnold SJ, Busslinger M, Dunay IR, Tanriver Y, Diefenbach A, Differentiation of type 1 ILCs from a common progenitor to all helper-like innate lymphoid cell lineages, Cell 157(2) (2014) 340–356. [DOI] [PubMed] [Google Scholar]
- [20].Cortez VS, Robinette ML, Colonna M, Innate lymphoid cells: new insights into function and development, Curr Opin Immunol 32 (2015) 71–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Gordon SM, Chaix J, Rupp LJ, Wu J, Madera S, Sun JC, Lindsten T, Reiner SL, The transcription factors T-bet and Eomes control key checkpoints of natural killer cell maturation, Immunity 36(1) (2012) 55–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Daussy C, Faure F, Mayol K, Viel S, Gasteiger G, Charrier E, Bienvenu J, Henry T, Debien E, Hasan UA, Marvel J, Yoh K, Takahashi S, Prinz I, de Bernard S, Buffat L, Walzer T, T-bet and Eomes instruct the development of two distinct natural killer cell lineages in the liver and in the bone marrow, J Exp Med 211(3) (2014) 563–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Bernink JH, Peters CP, Munneke M, te Velde AA, Meijer SL, Weijer K, Hreggvidsdottir HS, Heinsbroek SE, Legrand N, Buskens CJ, Bemelman WA, Mjosberg JM, Spits H, Human type 1 innate lymphoid cells accumulate in inflamed mucosal tissues, Nat Immunol 14(3) (2013) 221–9. [DOI] [PubMed] [Google Scholar]
- [24].Cortez VS, Cervantes-Barragan L, Robinette ML, Bando JK, Wang Y, Geiger TL, Gilfillan S, Fuchs A, Vivier E, Sun JC, Cella M, Colonna M, Transforming Growth Factor-beta Signaling Guides the Differentiation of Innate Lymphoid Cells in Salivary Glands, Immunity 44(5) (2016) 1127–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Weizman OE, Adams NM, Schuster IS, Krishna C, Pritykin Y, Lau C, Degli-Esposti MA, Leslie CS, Sun JC, O’Sullivan TE, ILC1 Confer Early Host Protection at Initial Sites of Viral Infection, Cell 171(4) (2017) 795–808 e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Sun JC, Lanier LL, NK cell development, homeostasis and function: parallels with CD8(+) T cells, Nat Rev Immunol 11(10) (2011) 645–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Wagner M, Moro K, Koyasu S, Plastic Heterogeneity of Innate Lymphoid Cells in Cancer, Trends Cancer 3(5) (2017) 326–335. [DOI] [PubMed] [Google Scholar]
- [28].Halim TY, MacLaren A, Romanish MT, Gold MJ, McNagny KM, Takei F, Retinoic-acid-receptor-related orphan nuclear receptor alpha is required for natural helper cell development and allergic inflammation, Immunity 37(3) (2012) 463–74. [DOI] [PubMed] [Google Scholar]
- [29].Hoyler T, Klose CS, Souabni A, Turqueti-Neves A, Pfeifer D, Rawlins EL, Voehringer D, Busslinger M, Diefenbach A, The transcription factor GATA-3 controls cell fate and maintenance of type 2 innate lymphoid cells, Immunity 37(4) (2012) 634–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Kim BS, Artis D, Group 2 innate lymphoid cells in health and disease, Cold Spring Harb Perspect Biol 7(5) (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Price AE, Liang HE, Sullivan BM, Reinhardt RL, Eisley CJ, Erle DJ, Locksley RM, Systemically dispersed innate IL-13-expressing cells in type 2 immunity, Proc Natl Acad Sci U S A 107(25) (2010) 11489–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Neill DR, Wong SH, Bellosi A, Flynn RJ, Daly M, Langford TK, Bucks C, Kane CM, Fallon PG, Pannell R, Jolin HE, McKenzie AN, Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity, Nature 464(7293) (2010) 1367–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Sanos SL, Bui VL, Mortha A, Oberle K, Heners C, Johner C, Diefenbach A, RORgammat and commensal microflora are required for the differentiation of mucosal interleukin 22-producing NKp46+ cells, Nat Immunol 10(1) (2009) 83–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Pickard JM, Maurice CF, Kinnebrew MA, Abt MC, Schenten D, Golovkina TV, Bogatyrev SR, Ismagilov RF, Pamer EG, Turnbaugh PJ, Chervonsky AV, Rapid fucosylation of intestinal epithelium sustains host-commensal symbiosis in sickness, Nature 514(7524) (2014) 638–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Zheng Y, Valdez PA, Danilenko DM, Hu Y, Sa SM, Gong Q, Abbas AR, Modrusan Z, Ghilardi N, de Sauvage FJ, Ouyang W, Interleukin-22 mediates early host defense against attaching and effacing bacterial pathogens, Nat Med 14(3) (2008) 282–9. [DOI] [PubMed] [Google Scholar]
- [36].Conti HR, Shen F, Nayyar N, Stocum E, Sun JN, Lindemann MJ, Ho AW, Hai JH, Yu JJ, Jung JW, Filler SG, Masso-Welch P, Edgerton M, Gaffen SL, Th17 cells and IL-17 receptor signaling are essential for mucosal host defense against oral candidiasis, J Exp Med 206(2) (2009) 299–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Stark MA, Huo Y, Burcin TL, Morris MA, Olson TS, Ley K, Phagocytosis of apoptotic neutrophils regulates granulopoiesis via IL-23 and IL-17, Immunity 22(3) (2005) 285–94. [DOI] [PubMed] [Google Scholar]
- [38].Fuchs A, Colonna M, Innate lymphoid cells in homeostasis, infection, chronic inflammation and tumors of the gastrointestinal tract, Curr Opin Gastroenterol 29(6) (2013) 581–7. [DOI] [PubMed] [Google Scholar]
- [39].Gladiator A, Wangler N, Trautwein-Weidner K, LeibundGut-Landmann S, Cutting edge: IL-17-secreting innate lymphoid cells are essential for host defense against fungal infection, J Immunol 190(2) (2013) 521–5. [DOI] [PubMed] [Google Scholar]
- [40].Xiong H, Keith JW, Samilo DW, Carter RA, Leiner IM, Pamer EG, Innate Lymphocyte/Ly6C(hi) Monocyte Crosstalk Promotes Klebsiella Pneumoniae Clearance, Cell 165(3) (2016) 679–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Hepworth MR, Fung TC, Masur SH, Kelsen JR, McConnell FM, Dubrot J, Withers DR, Hugues S, Farrar MA, Reith W, Eberl G, Baldassano RN, Laufer TM, Elson CO, Sonnenberg GF, Immune tolerance. Group 3 innate lymphoid cells mediate intestinal selection of commensal bacteria-specific CD4(+) T cells, Science 348(6238) (2015) 1031–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Shiu J, Piazuelo MB, Ding H, Czinn SJ, Drakes ML, Banerjee A, Basappa N, Kobayashi KS, Fricke WF, Blanchard TG, Gastric LTi cells promote lymphoid follicle formation but are limited by IRAK-M and do not alter microbial growth, Mucosal Immunol 8(5) (2015) 1047–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Eberl G, Marmon S, Sunshine MJ, Rennert PD, Choi Y, Littman DR, An essential function for the nuclear receptor RORgamma(t) in the generation of fetal lymphoid tissue inducer cells, Nat Immunol 5(1) (2004) 64–73. [DOI] [PubMed] [Google Scholar]
- [44].Fang F, Xiao W, Tian Z, NK cell-based immunotherapy for cancer, Semin Immunol 31 (2017) 37–54. [DOI] [PubMed] [Google Scholar]
- [45].Iannello A, Thompson TW, Ardolino M, Marcus A, Raulet DH, Immunosurveillance and immunotherapy of tumors by innate immune cells, Curr Opin Immunol 38 (2016) 52–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Smyth MJ, Thia KY, Street SE, Cretney E, Trapani JA, Taniguchi M, Kawano T, Pelikan SB, Crowe NY, Godfrey DI, Differential tumor surveillance by natural killer (NK) and NKT cells, J Exp Med 191(4) (2000) 661–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Hsu J, Hodgins JJ, Marathe M, Nicolai CJ, Bourgeois-Daigneault MC, Trevino TN, Azimi CS, Scheer AK, Randolph HE, Thompson TW, Zhang L, Iannello A, Mathur N, Jardine KE, Kirn GA, Bell JC, McBurney MW, Raulet DH, Ardolino M, Contribution of NK cells to immunotherapy mediated by PD-1/PD-L1 blockade, J Clin Invest 128(10) (2018) 4654–4668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Bottcher JP, Bonavita E, Chakravarty P, Blees H, Cabeza-Cabrerizo M, Sammicheli S, Rogers NC, Sahai E, Zelenay S, Reis e Sousa C, NK Cells Stimulate Recruitment of cDC1 into the Tumor Microenvironment Promoting Cancer Immune Control, Cell 172(5) (2018) 1022–1037 e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Glasner A, Ghadially H, Gur C, Stanietsky N, Tsukerman P, Enk J, Mandelboim O, Recognition and prevention of tumor metastasis by the NK receptor NKp46/NCR1, J Immunol 188(6) (2012) 2509–15. [DOI] [PubMed] [Google Scholar]
- [50].Halfteck GG, Elboim M, Gur C, Achdout H, Ghadially H, Mandelboim O, Enhanced in vivo growth of lymphoma tumors in the absence of the NK-activating receptor NKp46/NCR1, J Immunol 182(4) (2009) 2221–30. [DOI] [PubMed] [Google Scholar]
- [51].Deng W, Gowen BG, Zhang L, Wang L, Lau S, Iannello A, Xu J, Rovis TL, Xiong N, Raulet DH, Antitumor immunity. A shed NKG2D ligand that promotes natural killer cell activation and tumor rejection, Science 348(6230) (2015) 136–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Guerra N, Tan YX, Joncker NT, Choy A, Gallardo F, Xiong N, Knoblaugh S, Cado D, Greenberg NM, Raulet DH, NKG2D-deficient mice are defective in tumor surveillance in models of spontaneous malignancy, Immunity 28(4) (2008) 571–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Melero I, Rouzaut A, Motz GT, Coukos G, T-cell and NK-cell infiltration into solid tumors: a key limiting factor for efficacious cancer immunotherapy, Cancer Discov 4(5) (2014) 522–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Schantz SP, Ordonez NG, Quantitation of natural killer cell function and risk of metastatic poorly differentiated head and neck cancer, Nat Immun Cell Growth Regul 10(5) (1991) 278–88. [PubMed] [Google Scholar]
- [55].Wulff S, Pries R, Borngen K, Trenkle T, Wollenberg B, Decreased levels of circulating regulatory NK cells in patients with head and neck cancer throughout all tumor stages, Anticancer Res 29(8) (2009) 3053–7. [PubMed] [Google Scholar]
- [56].Schantz SP, Savage HE, Racz T, Taylor DL, Sacks PG, Natural killer cells and metastases from pharyngeal carcinoma, Am J Surg 158(4) (1989) 361–6. [DOI] [PubMed] [Google Scholar]
- [57].Tartter PI, Steinberg B, Barron DM, Martinelli G, The prognostic significance of natural killer cytotoxicity in patients with colorectal cancer, Arch Surg 122(11) (1987) 1264–8. [DOI] [PubMed] [Google Scholar]
- [58].Spacek J, Vocka M, Netikova I, Skalova H, Dundr P, Konopasek B, Zavadova E, Lubos P, Immunological examination of peripheral blood in patients with colorectal cancer compared to healthy controls, Immunol Invest 47(7) (2018) 643–653. [DOI] [PubMed] [Google Scholar]
- [59].Costello RT, Sivori S, Marcenaro E, Lafage-Pochitaloff M, Mozziconacci MJ, Reviron D, Gastaut JA, Pende D, Olive D, Moretta A, Defective expression and function of natural killer cell-triggering receptors in patients with acute myeloid leukemia, Blood 99(10) (2002) 3661–7. [DOI] [PubMed] [Google Scholar]
- [60].Andre P, Denis C, Soulas C, Bourbon-Caillet C, Lopez J, Arnoux T, Blery M, Bonnafous C, Gauthier L, Morel A, Rossi B, Remark R, Breso V, Bonnet E, Habif G, Guia S, Lalanne AI, Hoffmann C, Lantz O, Fayette J, Boyer-Chammard A, Zerbib R, Dodion P, Ghadially H, Jure-Kunkel M, Morel Y, Herbst R, Narni-Mancinelli E, Cohen RB, Vivier E, Anti-NKG2A mAb Is a Checkpoint Inhibitor that Promotes Anti-tumor Immunity by Unleashing Both T and NK Cells, Cell 175(7) (2018) 1731–1743 e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Hu J, Bernatchez C, Zhang L, Xia X, Kleinerman ES, Hung MC, Hwu P, Li S, Induction of NKG2D Ligands on Solid Tumors Requires Tumor-Specific CD8(+) T Cells and Histone Acetyltransferases, Cancer Immunol Res 5(4) (2017) 300–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Dadi S, Chhangawala S, Whitlock BM, Franklin RA, Luo CT, Oh SA, Toure A, Pritykin Y, Huse M, Leslie CS, Li MO, Cancer Immunosurveillance by Tissue-Resident Innate Lymphoid Cells and Innate-like T Cells, Cell 164(3) (2016) 365–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Barrow AD, Edeling MA, Trifonov V, Luo J, Goyal P, Bohl B, Bando JK, Kim AH, Walker J, Andahazy M, Bugatti M, Melocchi L, Vermi W, Fremont DH, Cox S, Cella M, Schmedt C, Colonna M, Natural Killer Cells Control Tumor Growth by Sensing a Growth Factor, Cell 172(3) (2018) 534–548 e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Gao J, Shi LZ, Zhao H, Chen J, Xiong L, He Q, Chen T, Roszik J, Bernatchez C, Woodman SE, Chen PL, Hwu P, Allison JP, Futreal A, Wargo JA, Sharma P, Loss of IFN-gamma Pathway Genes in Tumor Cells as a Mechanism of Resistance to Anti-CTLA-4 Therapy, Cell 167(2) (2016) 397–404 e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Muller-Hermelink N, Braumuller H, Pichler B, Wieder T, Mailhammer R, Schaak K, Ghoreschi K, Yazdi A, Haubner R, Sander CA, Mocikat R, Schwaiger M, Forster I, Huss R, Weber WA, Kneilling M, Rocken M, TNFR1 signaling and IFN-gamma signaling determine whether T cells induce tumor dormancy or promote multistage carcinogenesis, Cancer Cell 13(6) (2008) 507–18. [DOI] [PubMed] [Google Scholar]
- [66].Martini M, Testi MG, Pasetto M, Picchio MC, Innamorati G, Mazzocco M, Ugel S, Cingarlini S, Bronte V, Zanovello P, Krampera M, Mosna F, Cestari T, Riviera AP, Brutti N, Barbieri O, Matera L, Tridente G, Colombatti M, Sartoris S, IFN-gamma-mediated upmodulation of MHC class I expression activates tumor-specific immune response in a mouse model of prostate cancer, Vaccine 28(20) (2010) 3548–57. [DOI] [PubMed] [Google Scholar]
- [67].Peng W, Liu C, Xu C, Lou Y, Chen J, Yang Y, Yagita H, Overwijk WW, Lizee G, Radvanyi L, Hwu P, PD-1 blockade enhances T-cell migration to tumors by elevating IFN-gamma inducible chemokines, Cancer Res 72(20) (2012) 5209–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Taniguchi K, Petersson M, Hoglund P, Kiessling R, Klein G, Karre K, Interferon gamma induces lung colonization by intravenously inoculated B16 melanoma cells in parallel with enhanced expression of class I major histocompatibility complex antigens, Proc Natl Acad Sci U S A 84(10) (1987) 3405–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Schurch C, Riether C, Amrein MA, Ochsenbein AF, Cytotoxic T cells induce proliferation of chronic myeloid leukemia stem cells by secreting interferon-gamma, J Exp Med 210(3) (2013) 605–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Zaidi MR, Davis S, Noonan FP, Graff-Cherry C, Hawley TS, Walker RL, Feigenbaum L, Fuchs E, Lyakh L, Young HA, Hornyak TJ, Arnheiter H, Trinchieri G, Meltzer PS, De Fabo EC, Merlino G, Interferon-gamma links ultraviolet radiation to melanomagenesis in mice, Nature 469(7331) (2011) 548–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Jurgens B, Hainz U, Fuchs D, Felzmann T, Heitger A, Interferon-gamma-triggered indoleamine 2,3-dioxygenase competence in human monocyte-derived dendritic cells induces regulatory activity in allogeneic T cells, Blood 114(15) (2009) 3235–43. [DOI] [PubMed] [Google Scholar]
- [72].Holmgaard RB, Zamarin D, Li Y, Gasmi B, Munn DH, Allison JP, Merghoub T, Wolchok JD, Tumor-Expressed IDO Recruits and Activates MDSCs in a Treg-Dependent Manner, Cell Rep 13(2) (2015) 412–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Cortez VS, Ulland TK, Cervantes-Barragan L, Bando JK, Robinette ML, Wang Q, White AJ, Gilfillan S, Cella M, Colonna M, SMAD4 impedes the conversion of NK cells into ILC1-like cells by curtailing non-canonical TGF-beta signaling, Nat Immunol 18(9) (2017) 995–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Crome SQ, Nguyen LT, Lopez-Verges S, Yang SY, Martin B, Yam JY, Johnson DJ, Nie J, Pniak M, Yen PH, Milea A, Sowamber R, Katz SR, Bernardini MQ, Clarke BA, Shaw PA, Lang PA, Berman HK, Pugh TJ, Lanier LL, Ohashi PS, A distinct innate lymphoid cell population regulates tumor-associated T cells, Nat Med 23(3) (2017) 368–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Bie Q, Zhang P, Su Z, Zheng D, Ying X, Wu Y, Yang H, Chen D, Wang S, Xu H, Polarization of ILC2s in peripheral blood might contribute to immunosuppressive microenvironment in patients with gastric cancer, J Immunol Res 2014 (2014) 923135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Jovanovic IP, Pejnovic NN, Radosavljevic GD, Pantic JM, Milovanovic MZ, Arsenijevic NN, Lukic ML, Interleukin-33/ST2 axis promotes breast cancer growth and metastases by facilitating intratumoral accumulation of immunosuppressive and innate lymphoid cells, Int J Cancer 134(7) (2013) 1669–82. [DOI] [PubMed] [Google Scholar]
- [77].Chanmee T, Ontong P, Konno K, Itano N, Tumor-associated macrophages as major players in the tumor microenvironment, Cancers (Basel) 6(3) (2014) 1670–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Monticelli LA, Buck MD, Flamar AL, Saenz SA, Tait Wojno ED, Yudanin NA, Osborne LC, Hepworth MR, Tran SV, Rodewald HR, Shah H, Cross JR, Diamond JM, Cantu E, Christie JD, Pearce EL, Artis D, Arginase 1 is an innate lymphoid-cell-intrinsic metabolic checkpoint controlling type 2 inflammation, Nat Immunol 17(6) (2016) 656–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Bando JK, Nussbaum JC, Liang HE, Locksley RM, Type 2 innate lymphoid cells constitutively express arginase-I in the naive and inflamed lung, J Leukoc Biol 94(5) (2013) 877–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Zea AH, Rodriguez PC, Culotta KS, Hernandez CP, DeSalvo J, Ochoa JB, Park HJ, Zabaleta J, Ochoa AC, L-Arginine modulates CD3zeta expression and T cell function in activated human T lymphocytes, Cell Immunol 232(1–2) (2004) 21–31. [DOI] [PubMed] [Google Scholar]
- [81].Rodriguez PC, Quiceno DG, Ochoa AC, L-arginine availability regulates T-lymphocyte cell-cycle progression, Blood 109(4) (2007) 1568–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Zaiss DM, van Loosdregt J, Gorlani A, Bekker CP, Grone A, Sibilia M, van Bergen en Henegouwen PM, Roovers RC, Coffer PJ, Sijts AJ, Amphiregulin enhances regulatory T cell-suppressive function via the epidermal growth factor receptor, Immunity 38(2) (2013) 275–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Ikutani M, Yanagibashi T, Ogasawara M, Tsuneyama K, Yamamoto S, Hattori Y, Kouro T, Itakura A, Nagai Y, Takaki S, Takatsu K, Identification of innate IL-5-producing cells and their role in lung eosinophil regulation and antitumor immunity, J Immunol 188(2) (2012) 703–13. [DOI] [PubMed] [Google Scholar]
- [84].Purwar R, Schlapbach C, Xiao S, Kang HS, Elyaman W, Jiang X, Jetten AM, Khoury SJ, Fuhlbrigge RC, Kuchroo VK, Clark RA, Kupper TS, Robust tumor immunity to melanoma mediated by interleukin-9-producing T cells, Nat Med 18(8) (2012) 1248–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Lu Y, Hong S, Li H, Park J, Hong B, Wang L, Zheng Y, Liu Z, Xu J, He J, Yang J, Qian J, Yi Q, Th9 cells promote antitumor immune responses in vivo, J Clin Invest 122(11) (2012) 4160–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Zhang L, Li J, Li L, Zhang J, Wang X, Yang C, Li Y, Lan F, Lin P, IL-23 selectively promotes the metastasis of colorectal carcinoma cells with impaired Socs3 expression via the STAT5 pathway, Carcinogenesis 35(6) (2014) 1330–40. [DOI] [PubMed] [Google Scholar]
- [87].Langowski JL, Zhang X, Wu L, Mattson JD, Chen T, Smith K, Basham B, McClanahan T, Kastelein RA, Oft M, IL-23 promotes tumour incidence and growth, Nature 442(7101) (2006) 461–5. [DOI] [PubMed] [Google Scholar]
- [88].Grivennikov SI, Wang K, Mucida D, Stewart CA, Schnabl B, Jauch D, Taniguchi K, Yu GY, Osterreicher CH, Hung KE, Datz C, Feng Y, Fearon ER, Oukka M, Tessarollo L, Coppola V, Yarovinsky F, Cheroutre H, Eckmann L, Trinchieri G, Karin M, Adenoma-linked barrier defects and microbial products drive IL-23/IL-17-mediated tumour growth, Nature 491(7423) (2012) 254–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Wang K, Karin M, The IL-23 to IL-17 cascade inflammation-related cancers, Clin Exp Rheumatol 33(4 Suppl 92) (2015) S87–90. [PubMed] [Google Scholar]
- [90].Wu S, Rhee KJ, Albesiano E, Rabizadeh S, Wu X, Yen HR, Huso DL, Brancati FL, Wick E, McAllister F, Housseau F, Pardoll DM, Sears CL, A human colonic commensal promotes colon tumorigenesis via activation of T helper type 17 T cell responses, Nat Med 15(9) (2009) 1016–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Kirchberger S, Royston DJ, Boulard O, Thornton E, Franchini F, Szabady RL, Harrison O, Powrie F, Innate lymphoid cells sustain colon cancer through production of interleukin-22 in a mouse model, J Exp Med 210(5) (2013) 917–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Huber S, Gagliani N, Zenewicz LA, Huber FJ, Bosurgi L, Hu B, Hedl M, Zhang W, O’Connor W Jr., Murphy AJ, Valenzuela DM, Yancopoulos GD, Booth CJ, Cho JH, Ouyang W, Abraham C, Flavell RA, IL-22BP is regulated by the inflammasome and modulates tumorigenesis in the intestine, Nature 491(7423) (2012) 259–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Kappelman MD, Farkas DK, Long MD, Erichsen R, Sandler RS, Sorensen HT, Baron JA, Risk of cancer in patients with inflammatory bowel diseases: a nationwide population-based cohort study with 30 years of follow-up evaluation, Clin Gastroenterol Hepatol 12(2) (2014) 265–73 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Eisenring M, vom Berg J, Kristiansen G, Saller E, Becher B, IL-12 initiates tumor rejection via lymphoid tissue-inducer cells bearing the natural cytotoxicity receptor NKp46, Nat Immunol 11(11) (2010) 1030–8. [DOI] [PubMed] [Google Scholar]
- [95].Moskalenko M, Pan M, Fu Y, de Moll EH, Hashimoto D, Mortha A, Leboeuf M, Jayaraman P, Bernardo S, Sikora AG, Wolchok J, Bhardwaj N, Merad M, Saenger Y, Requirement for innate immunity and CD90(+) NK1.1(−) lymphocytes to treat established melanoma with chemo-immunotherapy, Cancer Immunol Res 3(3) (2015) 296–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Carrega P, Loiacono F, Di Carlo E, Scaramuccia A, Mora M, Conte R, Benelli R, Spaggiari GM, Cantoni C, Campana S, Bonaccorsi I, Morandi B, Truini M, Mingari MC, Moretta L, Ferlazzo G, NCR(+)ILC3 concentrate in human lung cancer and associate with intratumoral lymphoid structures, Nat Commun 6 (2015) 8280. [DOI] [PubMed] [Google Scholar]
- [97].Garrett WS, Cancer and the microbiota, Science 348(6230) (2015) 80–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Putze J, Hennequin C, Nougayrede JP, Zhang W, Homburg S, Karch H, Bringer MA, Fayolle C, Carniel E, Rabsch W, Oelschlaeger TA, Oswald E, Forestier C, Hacker J, Dobrindt U, Genetic structure and distribution of the colibactin genomic island among members of the family Enterobacteriaceae, Infect Immun 77(11) (2009) 4696–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Nougayrede JP, Homburg S, Taieb F, Boury M, Brzuszkiewicz E, Gottschalk G, Buchrieser C, Hacker J, Dobrindt U, Oswald E, Escherichia coli induces DNA double-strand breaks in eukaryotic cells, Science 313(5788) (2006) 848–51. [DOI] [PubMed] [Google Scholar]
- [100].Arthur JC, Gharaibeh RZ, Muhlbauer M, Perez-Chanona E, Uronis JM, McCafferty J, Fodor AA, Jobin C, Microbial genomic analysis reveals the essential role of inflammation in bacteria-induced colorectal cancer, Nat Commun 5 (2014) 4724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Arthur JC, Perez-Chanona E, Muhlbauer M, Tomkovich S, Uronis JM, Fan TJ, Campbell BJ, Abujamel T, Dogan B, Rogers AB, Rhodes JM, Stintzi A, Simpson KW, Hansen JJ, Keku TO, Fodor AA, Jobin C, Intestinal inflammation targets cancer-inducing activity of the microbiota, Science 338(6103) (2012) 120–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Boleij A, Hechenbleikner EM, Goodwin AC, Badani R, Stein EM, Lazarev MG, Ellis B, Carroll KC, Albesiano E, Wick EC, Platz EA, Pardoll DM, Sears CL, The Bacteroides fragilis toxin gene is prevalent in the colon mucosa of colorectal cancer patients, Clin Infect Dis 60(2) (2015) 208–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Murata-Kamiya N, Kurashima Y, Teishikata Y, Yamahashi Y, Saito Y, Higashi H, Aburatani H, Akiyama T, Peek RM Jr., Azuma T, Hatakeyama M, Helicobacter pylori CagA interacts with E-cadherin and deregulates the beta-catenin signal that promotes intestinal transdifferentiation in gastric epithelial cells, Oncogene 26(32) (2007) 4617–26. [DOI] [PubMed] [Google Scholar]
- [104].Saracut C, Molnar C, Russu C, Todoran N, Vlase L, Turdean S, Voidazan S, Copotoiu C, Secondary bile acids effects in colon pathology. Experimental mice study, Acta Cir Bras 30(9) (2015) 624–31. [DOI] [PubMed] [Google Scholar]
- [105].Boleij A, Tjalsma H, Gut bacteria in health and disease: a survey on the interface between intestinal microbiology and colorectal cancer, Biol Rev Camb Philos Soc 87(3) (2012) 701–30. [DOI] [PubMed] [Google Scholar]
- [106].Yoshimoto S, Loo TM, Atarashi K, Kanda H, Sato S, Oyadomari S, Iwakura Y, Oshima K, Morita H, Hattori M, Honda K, Ishikawa Y, Hara E, Ohtani N, Obesity-induced gut microbial metabolite promotes liver cancer through senescence secretome, Nature 499(7456) (2013) 97–101. [DOI] [PubMed] [Google Scholar]
- [107].Paulos CM, Wrzesinski C, Kaiser A, Hinrichs CS, Chieppa M, Cassard L, Palmer DC, Boni A, Muranski P, Yu Z, Gattinoni L, Antony PA, Rosenberg SA, Restifo NP, Microbial translocation augments the function of adoptively transferred self/tumor-specific CD8+ T cells via TLR4 signaling, J Clin Invest 117(8) (2007) 2197–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Iida N, Dzutsev A, Stewart CA, Smith L, Bouladoux N, Weingarten RA, Molina DA, Salcedo R, Back T, Cramer S, Dai RM, Kiu H, Cardone M, Naik S, Patri AK, Wang E, Marincola FM, Frank KM, Belkaid Y, Trinchieri G, Goldszmid RS, Commensal bacteria control cancer response to therapy by modulating the tumor microenvironment, Science 342(6161) (2013) 967–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Daillere R, Vetizou M, Waldschmitt N, Yamazaki T, Isnard C, Poirier-Colame V, Duong CPM, Flament C, Lepage P, Roberti MP, Routy B, Jacquelot N, Apetoh L, Becharef S, Rusakiewicz S, Langella P, Sokol H, Kroemer G, Enot D, Roux A, Eggermont A, Tartour E, Johannes L, Woerther PL, Chachaty E, Soria JC, Golden E, Formenti S, Plebanski M, Madondo M, Rosenstiel P, Raoult D, Cattoir V, Boneca IG, Chamaillard M, Zitvogel L, Enterococcus hirae and Barnesiella intestinihominis Facilitate Cyclophosphamide-Induced Therapeutic Immunomodulatory Effects, Immunity 45(4) (2016) 931–943. [DOI] [PubMed] [Google Scholar]
- [110].Viaud S, Saccheri F, Mignot G, Yamazaki T, Daillere R, Hannani D, Enot DP, Pfirschke C, Engblom C, Pittet MJ, Schlitzer A, Ginhoux F, Apetoh L, Chachaty E, Woerther PL, Eberl G, Berard M, Ecobichon C, Clermont D, Bizet C, Gaboriau-Routhiau V, Cerf-Bensussan N, Opolon P, Yessaad N, Vivier E, Ryffel B, Elson CO, Dore J, Kroemer G, Lepage P, Boneca IG, Ghiringhelli F, Zitvogel L, The intestinal microbiota modulates the anticancer immune effects of cyclophosphamide, Science 342(6161) (2013) 971–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Sharma P, Allison JP, The future of immune checkpoint therapy, Science 348(6230) (2015) 56–61. [DOI] [PubMed] [Google Scholar]
- [112].Vetizou M, Pitt JM, Daillere R, Lepage P, Waldschmitt N, Flament C, Rusakiewicz S, Routy B, Roberti MP, Duong CP, Poirier-Colame V, Roux A, Becharef S, Formenti S, Golden E, Cording S, Eberl G, Schlitzer A, Ginhoux F, Mani S, Yamazaki T, Jacquelot N, Enot DP, Berard M, Nigou J, Opolon P, Eggermont A, Woerther PL, Chachaty E, Chaput N, Robert C, Mateus C, Kroemer G, Raoult D, Boneca IG, Carbonnel F, Chamaillard M, Zitvogel L, Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota, Science 350(6264) (2015) 1079–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Sivan A, Corrales L, Hubert N, Williams JB, Aquino-Michaels K, Earley ZM, Benyamin FW, Lei YM, Jabri B, Alegre ML, Chang EB, Gajewski TF, Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy, Science 350(6264) (2015) 1084–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Matson V, Fessler J, Bao R, Chongsuwat T, Zha Y, Alegre ML, Luke JJ, Gajewski TF, The commensal microbiome is associated with anti-PD-1 efficacy in metastatic melanoma patients, Science 359(6371) (2018) 104–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Sun Z, Fourcade J, Pagliano O, Chauvin JM, Sander C, Kirkwood JM, Zarour HM, IL10 and PD-1 Cooperate to Limit the Activity of Tumor-Specific CD8+ T Cells, Cancer Res 75(8) (2015) 1635–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Satoh-Takayama N, Vosshenrich CA, Lesjean-Pottier S, Sawa S, Lochner M, Rattis F, Mention JJ, Thiam K, Cerf-Bensussan N, Mandelboim O, Eberl G, Di Santo JP, Microbial flora drives interleukin 22 production in intestinal NKp46+ cells that provide innate mucosal immune defense, Immunity 29(6) (2008) 958–70. [DOI] [PubMed] [Google Scholar]
- [117].Zelante T, Iannitti RG, Cunha C, De Luca A, Giovannini G, Pieraccini G, Zecchi R, D’Angelo C, Massi-Benedetti C, Fallarino F, Carvalho A, Puccetti P, Romani L, Tryptophan catabolites from microbiota engage aryl hydrocarbon receptor and balance mucosal reactivity via interleukin-22, Immunity 39(2) (2013) 372–85. [DOI] [PubMed] [Google Scholar]
- [118].Sano T, Huang W, Hall JA, Yang Y, Chen A, Gavzy SJ, Lee JY, Ziel JW, Miraldi ER, Domingos AI, Bonneau R, Littman DR, An IL-23R/IL-22 Circuit Regulates Epithelial Serum Amyloid A to Promote Local Effector Th17 Responses, Cell 163(2) (2015) 381–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Mortha A, Chudnovskiy A, Hashimoto D, Bogunovic M, Spencer SP, Belkaid Y, Merad M, Microbiota-dependent crosstalk between macrophages and ILC3 promotes intestinal homeostasis, Science 343(6178) (2014) 1249288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Castellanos JG, Woo V, Viladomiu M, Putzel G, Lima S, Diehl GE, Marderstein AR, Gandara J, Perez AR, Withers DR, Targan SR, Shih DQ, Scherl EJ, Longman RS, Microbiota-Induced TNF-like Ligand 1A Drives Group 3 Innate Lymphoid Cell-Mediated Barrier Protection and Intestinal T Cell Activation during Colitis, Immunity 49(6) (2018) 1077–1089 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Konieczna P, Ferstl R, Ziegler M, Frei R, Nehrbass D, Lauener RP, Akdis CA, O’Mahony L, Immunomodulation by Bifidobacterium infantis 35624 in the murine lamina propria requires retinoic acid-dependent and independent mechanisms, PLoS One 8(5) (2013) e62617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Grizotte-Lake M, Zhong G, Duncan K, Kirkwood J, Iyer N, Smolenski I, Isoherranen N, Vaishnava S, Commensals Suppress Intestinal Epithelial Cell Retinoic Acid Synthesis to Regulate Interleukin-22 Activity and Prevent Microbial Dysbiosis, Immunity 49(6) (2018) 1103–1115 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Spencer SP, Wilhelm C, Yang Q, Hall JA, Bouladoux N, Boyd A, Nutman TB, Urban JF Jr., Wang J, Ramalingam TR, Bhandoola A, Wynn TA, Belkaid Y, Adaptation of innate lymphoid cells to a micronutrient deficiency promotes type 2 barrier immunity, Science 343(6169) (2014) 432–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Kim MH, Taparowsky EJ, Kim CH, Retinoic Acid Differentially Regulates the Migration of Innate Lymphoid Cell Subsets to the Gut, Immunity 43(1) (2015) 107–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Longman RS, Diehl GE, Victorio DA, Huh JR, Galan C, Miraldi ER, Swaminath A, Bonneau R, Scherl EJ, Littman DR, CX(3)CR1(+) mononuclear phagocytes support colitis-associated innate lymphoid cell production of IL-22, J Exp Med 211(8) (2015) 1571–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Mosconi I, Geuking MB, Zaiss MM, Massacand JC, Aschwanden C, Kwong Chung CK, McCoy KD, Harris NL, Intestinal bacteria induce TSLP to promote mutualistic T-cell responses, Mucosal Immunol 6(6) (2013) 1157–67. [DOI] [PubMed] [Google Scholar]
- [127].Sawa S, Lochner M, Satoh-Takayama N, Dulauroy S, Berard M, Kleinschek M, Cua D, Di Santo JP, Eberl G, RORgammat+ innate lymphoid cells regulate intestinal homeostasis by integrating negative signals from the symbiotic microbiota, Nat Immunol 12(4) (2011) 320–6. [DOI] [PubMed] [Google Scholar]
- [128].Zaph C, Du Y, Saenz SA, Nair MG, Perrigoue JG, Taylor BC, Troy AE, Kobuley DE, Kastelein RA, Cua DJ, Yu Y, Artis D, Commensal-dependent expression of IL-25 regulates the IL-23-IL-17 axis in the intestine, J Exp Med 205(10) (2008) 2191–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Giacomin PR, Moy RH, Noti M, Osborne LC, Siracusa MC, Alenghat T, Liu B, McCorkell KA, Troy AE, Rak GD, Hu Y, May MJ, Ma HL, Fouser LA, Sonnenberg GF, Artis D, Epithelial-intrinsic IKKalpha expression regulates group 3 innate lymphoid cell responses and antibacterial immunity, J Exp Med 212(10) (2015) 1513–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Cella M, Fuchs A, Vermi W, Facchetti F, Otero K, Lennerz JK, Doherty JM, Mills JC, Colonna M, A human natural killer cell subset provides an innate source of IL-22 for mucosal immunity, Nature 457(7230) (2009) 722–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Cella M, Otero K, Colonna M, Expansion of human NK-22 cells with IL-7, IL-2, and IL-1beta reveals intrinsic functional plasticity, Proc Natl Acad Sci U S A 107(24) (2010) 10961–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Gray J, Oehrle K, Worthen G, Alenghat T, Whitsett J, Deshmukh H, Intestinal commensal bacteria mediate lung mucosal immunity and promote resistance of newborn mice to infection, Sci Transl Med 9(376) (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Lee JS, Cella M, McDonald KG, Garlanda C, Kennedy GD, Nukaya M, Mantovani A, Kopan R, Bradfield CA, Newberry RD, Colonna M, AHR drives the development of gut ILC22 cells and postnatal lymphoid tissues via pathways dependent on and independent of Notch, Nat Immunol 13(2) (2011) 144–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Sawa S, Cherrier M, Lochner M, Satoh-Takayama N, Fehling HJ, Langa F, Di Santo JP, Eberl G, Lineage relationship analysis of RORgammat+ innate lymphoid cells, Science 330(6004) (2010) 665–9. [DOI] [PubMed] [Google Scholar]
- [135].Bird L, Mucosal immunology: IL-22 keeps commensals in their place, Nat Rev Immunol 12(8) (2012) 550–1. [DOI] [PubMed] [Google Scholar]
- [136].Sonnenberg GF, Monticelli LA, Alenghat T, Fung TC, Hutnick NA, Kunisawa J, Shibata N, Grunberg S, Sinha R, Zahm AM, Tardif MR, Sathaliyawala T, Kubota M, Farber DL, Collman RG, Shaked A, Fouser LA, Weiner DB, Tessier PA, Friedman JR, Kiyono H, Bushman FD, Chang KM, Artis D, Innate lymphoid cells promote anatomical containment of lymphoid-resident commensal bacteria, Science 336(6086) (2012) 1321–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Qiu J, Guo X, Chen ZM, He L, Sonnenberg GF, Artis D, Fu YX, Zhou L, Group 3 innate lymphoid cells inhibit T-cell-mediated intestinal inflammation through aryl hydrocarbon receptor signaling and regulation of microflora, Immunity 39(2) (2013) 386–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Zenewicz LA, Yin X, Wang G, Elinav E, Hao L, Zhao L, Flavell RA, IL-22 deficiency alters colonic microbiota to be transmissible and colitogenic, J Immunol 190(10) (2013) 5306–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Hepworth MR, Monticelli LA, Fung TC, Ziegler CG, Grunberg S, Sinha R, Mantegazza AR, Ma HL, Crawford A, Angelosanto JM, Wherry EJ, Koni PA, Bushman FD, Elson CO, Eberl G, Artis D, Sonnenberg GF, Innate lymphoid cells regulate CD4+ T-cell responses to intestinal commensal bacteria, Nature 498(7452) (2013) 113–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Kramer B, Goeser F, Lutz P, Glassner A, Boesecke C, Schwarze-Zander C, Kaczmarek D, Nischalke HD, Branchi V, Manekeller S, Huneburg R, van Bremen T, Weismuller T, Strassburg CP, Rockstroh JK, Spengler U, Nattermann J, Compartment-specific distribution of human intestinal innate lymphoid cells is altered in HIV patients under effective therapy, PLoS Pathog 13(5) (2017) e1006373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Bernink JH, Krabbendam L, Germar K, de Jong E, Gronke K, Kofoed-Nielsen M, Munneke JM, Hazenberg MD, Villaudy J, Buskens CJ, Bemelman WA, Diefenbach A, Blom B, Spits H, Interleukin-12 and −23 Control Plasticity of CD127(+) Group 1 and Group 3 Innate Lymphoid Cells in the Intestinal Lamina Propria, Immunity 43(1) (2015) 146–60. [DOI] [PubMed] [Google Scholar]
- [142].Ganal SC, Sanos SL, Kallfass C, Oberle K, Johner C, Kirschning C, Lienenklaus S, Weiss S, Staeheli P, Aichele P, Diefenbach A, Priming of natural killer cells by nonmucosal mononuclear phagocytes requires instructive signals from commensal microbiota, Immunity 37(1) (2012) 171–86. [DOI] [PubMed] [Google Scholar]
- [143].Wojno ED, Monticelli LA, Tran SV, Alenghat T, Osborne LC, Thome JJ, Willis C, Budelsky A, Farber DL, Artis D, The prostaglandin D(2) receptor CRTH2 regulates accumulation of group 2 innate lymphoid cells in the inflamed lung, Mucosal Immunol 8(6) (2015) 1313–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Huang Y, Guo L, Qiu J, Chen X, Hu-Li J, Siebenlist U, Williamson PR, Urban JF Jr., Paul WE, IL-25-responsive, lineage-negative KLRG1(hi) cells are multipotential ‘inflammatory’ type 2 innate lymphoid cells, Nat Immunol 16(2) (2015) 161–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Monticelli LA, Sonnenberg GF, Abt MC, Alenghat T, Ziegler CG, Doering TA, Angelosanto JM, Laidlaw BJ, Yang CY, Sathaliyawala T, Kubota M, Turner D, Diamond JM, Goldrath AW, Farber DL, Collman RG, Wherry EJ, Artis D, Innate lymphoid cells promote lung-tissue homeostasis after infection with influenza virus, Nat Immunol 12(11) (2011) 1045–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Kernbauer E, Ding Y, Cadwell K, An enteric virus can replace the beneficial function of commensal bacteria, Nature 516(7529) (2014) 94–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Gury-BenAri M, Thaiss CA, Serafini N, Winter DR, Giladi A, Lara-Astiaso D, Levy M, Salame TM, Weiner A, David E, Shapiro H, Dori-Bachash M, Pevsner-Fischer M, Lorenzo-Vivas E, Keren-Shaul H, Paul F, Harmelin A, Eberl G, Itzkovitz S, Tanay A, Di Santo JP, Elinav E, Amit I, The Spectrum and Regulatory Landscape of Intestinal Innate Lymphoid Cells Are Shaped by the Microbiome, Cell 166(5) (2016) 1231–1246 e13. [DOI] [PubMed] [Google Scholar]
- [148].Aparicio-Domingo P, Romera-Hernandez M, Karrich JJ, Cornelissen F, Papazian N, Lindenbergh-Kortleve DJ, Butler JA, Boon L, Coles MC, Samsom JN, Cupedo T, Type 3 innate lymphoid cells maintain intestinal epithelial stem cells after tissue damage, J Exp Med 212(11) (2015) 1783–91. [DOI] [PMC free article] [PubMed] [Google Scholar]