Abstract
Intervertebral disc degeneration (IDD) is associated with the nucleus pulposus (NP) cells inflammation and apoptosis. Previous studies have shown that glycyrrhizin (GL) is a valid inhibitor of the high-mobility group box-1 gene (HMGB1) which expressed much higher in an inflammatory condition. However, it is not known whether GL protects against IDD by the inhibition of HMGB1. To study the effect and mechanism of glycyrrhizin on intervertebral disc degeneration. We analyzed the expression of HMGB1 in different degree of degenerate disc tissues. Interleukin 1 beta (IL-1β) was used in stimulating the NP cells to degeneration. We used recombined human HMGB1 to resist the function of GL to explore whether GL acted via the target of HMGB1. Our study showed that the expression of HMGB1 markedly increased in severely degenerated disc tissues. IL-1β promoted the progress of IDD, and the stimulation of GL could reverse the effects of IL-1β. Moreover, p38 and p-JNK were significantly suppressed by GL stimuli. These results suggested that GL prevented NP degradation via restraining inflammation and cell apoptosis by inhibition of HMGB1 via p38/p-JNK signaling pathway. GL may become a novel cytokine for the therapy of IDD in the future.
Keywords: Nucleus pulposus cells, intervertebral disc degeneration, glycyrrhizin, HMGB1, inflammation
Introduction
Intervertebral disc degeneration (IDD) is a pathological process change in both biochemical composition and architecture, which can lead to disk herniation and low back pain [1-3]. IDD can cause catabolism of the extracellular matrix (ECM) leading to reduced water content and a loss of the disc height [4,5]. Nucleus pulposus (NP) cells are the main cells in the intervertebral disc, which are helpful in retaining water and the creation of the ECM [6]. The vitality of the NP cells has a meaningful effect on disc stability. In the progress of disc metabolism, proinflammatory cytokines are reported to take an important role in ECM breakdown. Levels of interleukin (IL)-1β, IL-6 and tumor necrosis factor (TNF)-α have been found to significantly arise with IDD [7,8]. Therefore, a better understanding of the molecular mechanisms of the cells vitality and inflammation of the IDD is essential for clinic treatment.
The high-mobility group box-1 gene (HMGB1) produces a protein which acts as a cytokine, can be released from nucleus to cytosol and even extracellular space upon inflammatory and other stimulation to mediate inflammation in multiple injury models [9,10]. HMGB1 stimulates the release of tumor necrosis factor (TNF) and other products of activated macrophages [11]. On the other hand, monocytes release HMGB1 with the activation of inflammatory stimuli [12]. HMGB1 also can be released in injured or necrotic situations, which is a major stimulus of necrosis induced inflammation [13]. Recently, HMGB1 has been investigated to be highly expressed in a lipopolysaccharide-induced rat IDD model [14]. However, it remains previously unknown the thorough mechanism of HMGB1 in the progress of IDD.
The upregulation of HMGB1 is directly associated with the pathogenesis of various human diseases. It is believed that the control of inflammation could downregulate the tissue damage inflicted by inflammatory mediators. Therefore, it is considered as a promising therapeutic target. For inhibiting HMGB1 activity, glycyrrhizin (GL) a natural triterpene acts as an effective and direct inhibitor of HMGB1, which has been routinely used in several in vitro and in vivo models [15-18]. Based on these preliminary findings, this project aimed to explore the use of GL in anti-inflammation of the progress in IDD and the potential mechanism of the way it affects HMGB1. This study may supply a novel target in the therapeutic strategy with the intervertebral disc disease.
Materials and methods
Patient tissue samples
Human disc tissue samples were collected from patients undergoing disc herniation surgery. This project was approved by the Ethics Committee of The Affiliated Hospital of Xuzhou Medical University. We obtained informed consent from all the patients or relatives before the surgeries. Degenerative disc tissues were donated by 15 patients (mean age, 47; age range, 37-63; 8 males, 7 females) in total. We just took the NP non-detached with the endplates. We divided the samples into two groups according to the Pfirrmann score of disc degeneration [19]. Grade II or III belong to the Mild group, and Grade IV or V belong to the Severe group. Tissues were conserved in liquid nitrogen for the following protein or RNA isolations.
Cells culture and drug treatment
The tissues were minced and washed three times with a phosphate-buffered saline solution (PBS), and digested for 30 minutes in 0.25% trypsin solution at 37°C. Next, the samples were incubated in 0.15% type II collagenase at 37°C overnight. Transfer the cell solution onto a cell strainer with 100 µm pore sizes and resuspended in Dulbecco’s modified Eagle’s medium and Ham’s F-12 medium (DMEM/F12, Thermo Fisher, USA) containing 10% fetal bovine serum (FBS, Gibco, USA). The cells were seeded in six-well plates at 1 × 105 cells per well. The degeneration NP cells model is established by IL-1β (5 ng/ml, Biochrom AG) stimulus. After the cells attached to the culture plate, we explored the optimize concentration by using different concentrations of glycyrrhizin (0, 10 µM, 50 µM, 100 µM, 200 µM, Selleck), and optimize stimulus time by different time pots (0, 1 d, 2 d, 3 d). Recombined human HMGB1 (h-HMGB1, 0.23 µg/ml, Sigma) was used as an antagonist to counteract the function of glycyrrhizin.
Cell viability assay
NP cell viability was determined to utilize the CCK8 assay. Cells were seeded (1 × 104 cells/well) in 96-well plates and treated with different concentrations of GA (0-200 μM) for 24 h or different time ports (0-3 d, 100 μM). Subsequently, cell viability was determined using a CCK8 cell viability/cytotoxicity assay kit (C0009, Beyotime, China) according to the manufacturer’s instructions. Absorbance was then measured using a microtiter plate reader (Labsystems Multiskan, MS, Finland) at 570 nm.
Colony formation assay
NP cells (5 × 103 cells /well) in 24-well culture plates were incubated for 14 days when the colonies were visible. Crystal violet staining was performed, and the number of colonies was counted.
Reverse transcription-polymerase chain reaction (RT-PCR)
Total RNA was isolated from NP tissues or cells with TRIzol reagent (Invitrogen, USA) according to the manufacturer’s instructions. After that, RNA was reverse transcribed to cDNA with PrimeScript™ RT Master Mix (Applied Biosystems, USA). RT-PCR was performed to quantify HMGB1, type II collagen, TNF-α, IL-6/8, iNOS, caspase 3/8, and Glyceraldehyde phosphate dehydrogenase (GAPDH) mRNA expression levels. RT-PCR was conducted using SYBR Green Master Mix (Applied Biosystems, USA). GAPDH was used for normalization. The primers used for RT-PCR are shown in Table 1. Relative mRNA expression levels were calculated by the 2-ΔΔCt methods.
Table 1.
Primer sequences of the genes for RT-PCR
| Gene name | Forward (5’>3’) | Reverse (5’>3’) |
|---|---|---|
| HMGB1 | TATGGCAAAAGCGGACAAGG | CTTCGCAACATCACCAATGGA |
| Collagen II | TGGACGATCAGGCGAAACC | GCTGCGGATGCTCTCAATCT |
| TNF-α | CTACCATCACCGCACTGAGAT | GGTCACTTCACCATAGTGGACA |
| IL-6 | ACTCACCTCTTCAGAACGAATTG | CCATCTTTGGAAGGTTCAGGTTG |
| IL-8 | AGCCCAGAACACTGGTCTC | ACTCAGGATTTCAATGGTGCC |
| iNOS | TTCAGTATCACAACCTCAGCAAG | TGGACCTGCAAGTTAAAATCCC |
| Caspase 3 | GCCATCGTGGCTAAACAGGTA | GTTGGTGTTCATCCGCTTGC |
| Caspase 8 | CTGGAAGATGGTCGTACCCTG | GGTCTTGCCAGTGAGTGTCT |
| GAPDH | ACAACTTTGGTATCGTGGAAGG | GCCATCACGCCACAGTTTC |
RT-PCR, quantitative reverse-transcription polymerase chain reaction.
Western blot (WB) analysis
Tissues and NP cells were harvested in RIPA lysis buffer. Proteins were isolated using a Nuclear/Cytosol Fractionation Kit (Beyotime, China) according to the manufacturer’s instructions. Protein concentrations were measured with the Enhanced BCA Protein Assay Kit (Beyotime, China). After blocking with 5% skim milk for 1 hour at room temperature and probed overnight with anti-HMGB1 (Abcam, USA, 1:1000), anti-caspase 3/8 (Abcam, USA, 1:1000), anti-type II collagen (Millipore, USA, 1:1000), anti-p38 (Millipore, USA, 1:2000), anti-p-JNK (Abcam, USA, 1:1000), anti-TNF-α (Abcam, USA, 1:3000), anti-IL-6/8 (Abcam, USA, 1:1000), anti-iNOS (Abcam, USA, 1:1000), and anti-β-actin (Cell Signaling Technology, USA, 1:2000). After washing with PBST, the membrane was incubated with secondary antibody (Abcam, USA, 1:2000) for 2 h at room temperature. Protein bands were visualized and detected using the enhanced chemiluminescence system. β-Actin were used as the controls.
Immunocytofluorescence (IF) staining
Cultured NP cells (1 × 105/well) in each group were washed three times with PBS, fixed with 4% formaldehyde for 15 min at room temperature, and then blocked with 5% BSA for 30 min. Subsequently, the cells were incubated overnight at 4°C with a primary antibody against HMGB1 (Abcam, USA, 1:500) or type II collagen (Abcam, USA, 1:500), followed by Cy3-conjugated goat anti-rabbit IgG antibody (Abcam, USA, 1:200) for 2 h at room temperature. Nuclei were counterstained with DAPI (Beyotime, China, 1:500), and the cells were visualized using a fluorescence microscope (Zeiss, Germany).
Flow cytometry analysis
The apoptosis was determined using the Apoptosis Detection Kit (KeyGEN, Nanjing, China) based on the procedures provided by the manufacturer. NP cells were stained with Annexin V-FITC and propidium iodide (PI) in the dark for 30 minutes. Cells were analyzed using a fluorescence-activated cell sorting flow cytometer (BD Biosciences, San Jose, California, USA).
Statistical analysis
All data are normality displayed by the means ± standard deviations. Data compared between the two groups were analyzed using Student’s t-test. Comparison between multiple groups was done using One-way ANOVA test followed by Post Hoc Test (Least Significant Difference). P values < 0.05 were considered statistically significant.
Results
Expression of HMGB1 in human NP tissues with Pfirrmann grades
We isolated the protein and RNA from the patients’ disc tissues, carried out Western blot to measure the type II collagen and HMGB1 protein expression with different grades of disc degeneration. In addition, RT-PCR was taken to measure the mRNA expression of type II collagen and HMGB1 in the disc samples. We chose 4 samples from each patients group randomly, and the results showed that the type II collagen protein was significantly decreased in the Severe group (Figure 1A). Meanwhile, HMGB1 gene was markedly upregulated in the Severe group compared to the Mild group (Figure 1). Sequences of primers used for RT-PCR are shown in Table 1. These results showed that the HMGB1 expression increased with the disc got into much severe degeneration.
Figure 1.
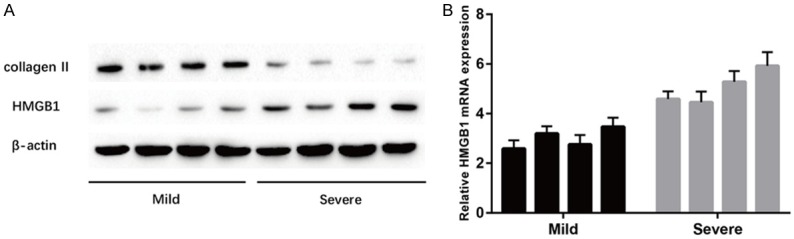
Expression of HMGB1 in human NP tissues with different Pfirrmann grades. A. Results of protein expression of collagen II and HMGB1 in human NP tissues with different Pfirrmann grades were determined by Western blotting. B. Results of mRNA expression of HMGB1 was determined by RT-PCR. All the data in Mild group are significantly different from the Severe group, and P value < 0.05.
Effect of GL on cell viability
We next considered whether GL might enhance the proliferated properties of NP cells. CCK8 assay was used to measure the cells viability under the different concentration and time ports GL stimulation. The result showed that 100 µM of GL obviously promoted the highest cells viability, meanwhile, cells cultured without GL seen as the control one (Figure 2A). But, we found no statistical differences among the different time ports after stimulation (Figure 2B). Therefore, we choose two days’ 100 µM GL stimulation in the following experiment.
Figure 2.
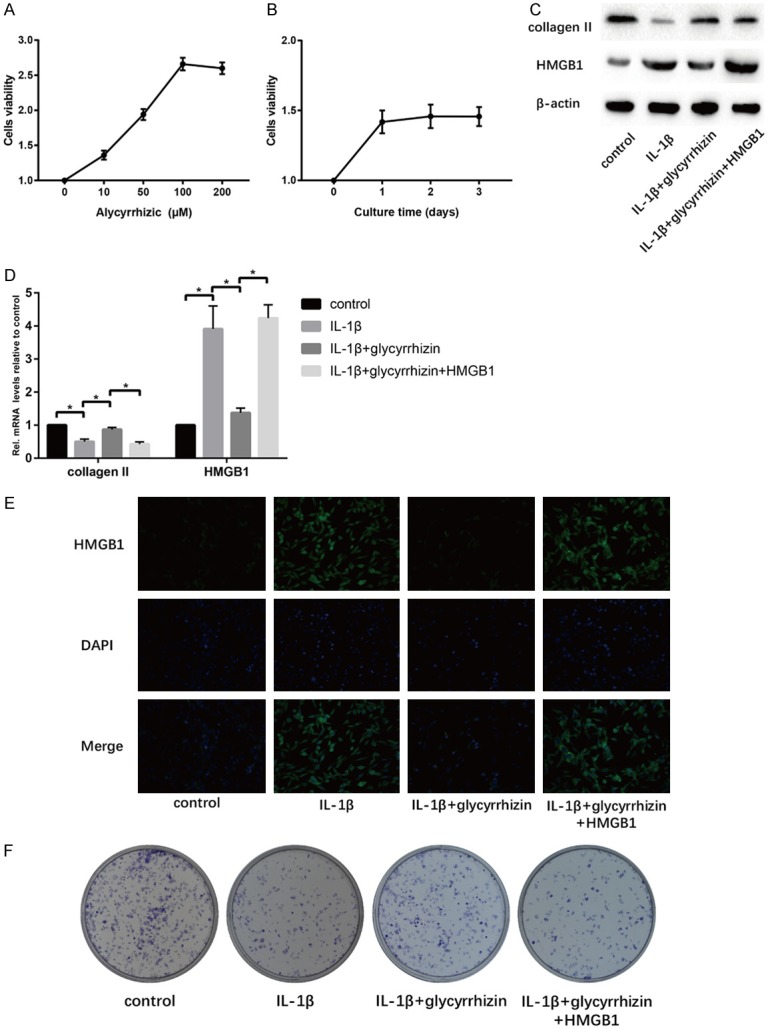
Effect of GL treatment on IL-1β-induced senescent NP cells in vitro. A. Cells were treated with GL (0, 10, 50, 100, 200 μM) for 24 h. Viability was determined by CCK8 assay. B. Cells were treated with GL (100 μM) for various time (0, 1, 2, 3 d). Viability was determined by CCK8 assay. C. Results of protein expression of collagen II, HMGB1 and β-actin in four groups were determined by Western blotting. D. Results of mRNA expression of collagen II and HMGB1 in four groups were determined by RT-PCR. E. Cell IF was performed to determine the expression of HMGB1 in cells of the four groups. F. Cell proliferation was analyzed by colony formation assay in four groups (“*” means there is a statistical difference with the two groups, P value < 0.05).
Effect of GL on HMGB1 in senescent NP cells in vitro
IL-1β (10 ng/ml) was applied into the establishment of NP cells degeneration model according to the previous method [20]. We explored the type II collagen expression to analyzed the senescent degree of NP cells, and HMGB1 expression both in protein and mRNA levels. The results showed that IL-1β significantly decreased the type II collagen expression and increased the HMGB1 expression at the same time compared to the control group (Figure 2C, 2D). On the contrary, GA-treated cells showed significantly higher type II collagen expression and lower HMGB1 expression compared to the IL-1β group (Figure 2C-E). However, h-HMGB1 could also reverse this protective effect of GL (Figure 2C-E). From the colony formation assay, GL showed the ability in enhancing the proliferation of NP cells compared with the IL-1β group (Figure 2F).
GL suppresses inflammation on senescent NP cells in vitro
We analyzed the TNF-α, IL-6, IL-8 and iNOS expression with western blot and RT-PCR. We found that these inflammatory cytokines expression increased in senescent NP cells compared with the control one. The results showed the GL could suppress the inflammation in the NP cell degenerated model (Figure 3). In order to test whether HMGB1 is the target of GL, r-HMGB1 was used to resist the function of GL. Hopefully, obtained data showed the inflammatory effect of GL vanished by the addition of r-HMGB1.
Figure 3.
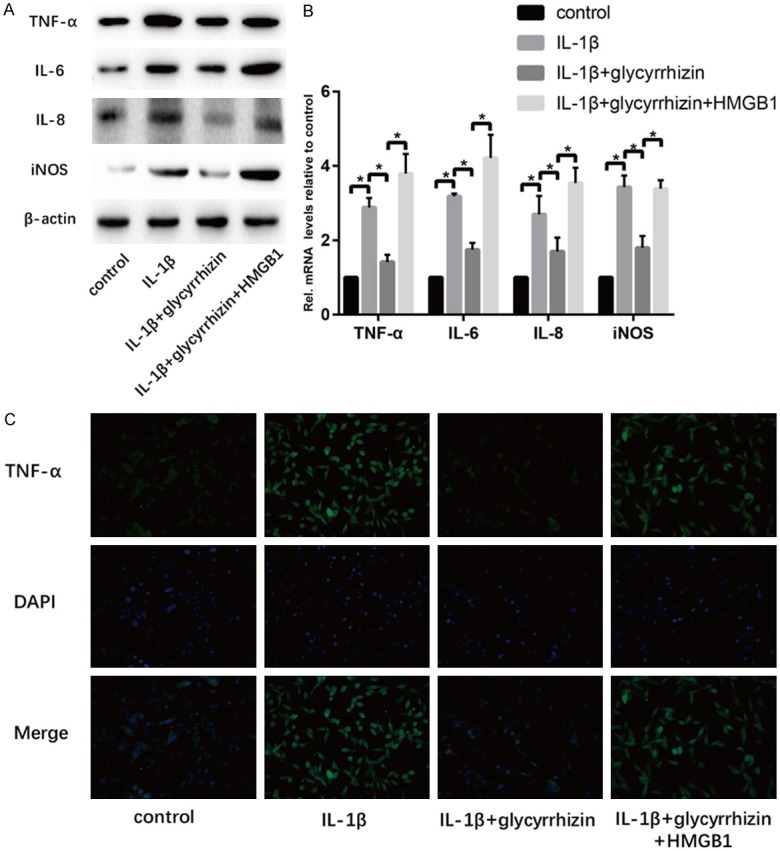
GL treatment suppresses inflammation on IL-1β-induced senescent NP cells in vitro. A. Results of protein expression of TNF-α, IL-6, IL-8, iNOS and β-actin in four groups were determined by Western blotting. B. Results of mRNA expression of TNF-α, IL-6, IL-8, and iNOS in four groups were determined by RT-PCR. C. Cell IF was performed to determine the expression of TNF-α in cells of the four groups (“*” means there is a statistical difference with the two groups, P value < 0.05).
GL suppresses apoptosis on senescent NP cells in vitro
The p38/p-JNK signal has been accepted as an important pathway to mediate apoptosis during cell fate. Figure 4 shows that the expression of p38, p-JNK, as well as the apoptosis-associated gene caspase 3/8, decreased significantly with the GL simulation compared to the IL-1β group. The flow cytometry result showed the GL group had a less number of total apoptosis cells compared with the IL-1β group. And the ex0genous r-HMGB1 played an adverse role in the anti-apoptosis progress of GL stimulation in the degenerated NP cells.
Figure 4.
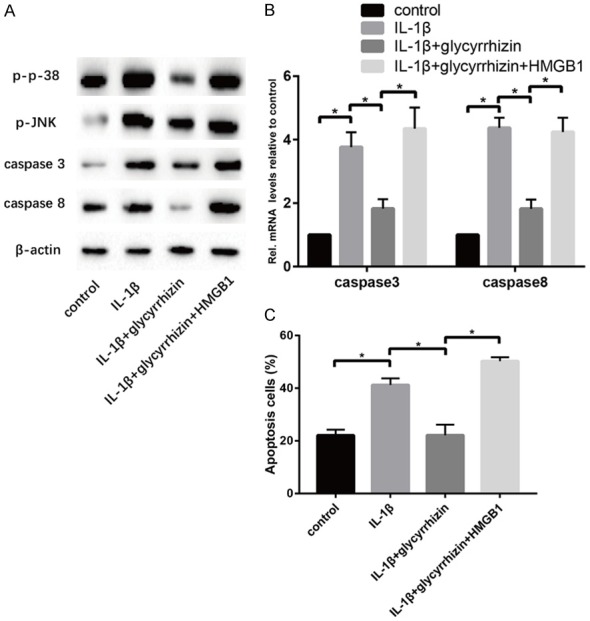
GL treatment suppresses apoptosis on IL-1β-induced senescent NP cells in vitro. A. Results of protein expression of p38, p-JNK, caspase 3, caspase 8 and β-actin in four groups were determined by Western blotting. B. Results of mRNA expression of caspase3 and caspase8 in four groups were determined by RT-PCR. C. Apoptosis levels in cells of four groups were detected by flow cytometry (“*” means there is a statistical difference with the two groups, P value < 0.05).
Discussion
IDD is one of the most universal degenerative diseases among aging populations, in which intervertebral discs usually undergo both morphological and biomechanical changes [21]. The NP seen as a most important structure in the intervertebral disc maintains the stability of the intervertebral disc and buffering the impact of external force on the spine, as well as to produce the extracellular matrix and secrete pro-inflammatory cytokines. IDD is associated with NP cells change, including increased cell death and degradation of the extracellular matrix [4]. Numbers of causes have been proved to play a role in the pathogenesis of IDD, such as calcification of endplate, excessive mechanical loading, spine infection, and aging. For the complicated mechanism of IDD, inflammation and cell apoptosis are widely accepted as the specific features during its process [22].
High serum levels of HMGB1 have been found in many inflammatory events, such as rheumatoid arthritis [23], chronic kidney disease [24], and systemic lupus erythematosus [25]. A previous study has reported that HMGB1 can promote the inflammatory cytokines release via TLR signaling in human intervertebral disc cells [26]. So we hypothesis, inhibiting the activity of HMGB1 may minish pro-inflammatory signaling loops directly. Besides, we also want to probe into the effect of cell apoptosis by using the inhibitor of HMGB1. Interestingly but not surprisingly, well-known medicinal herbs have been proved as effective molecules inhibiting HMGB1 pathological activity. The first candidate in this group is glycyrrhizin (GL)-a natural triterpene found in Glycyrrhiza glabra and known for its anti-inflammatory and antiviral properties. GL was found to attenuate the transient spinal cord ischemic injury in rats by inhibiting the release of HMGB1 and decreasing inflammatory cytokines [18], to alleviate rat myocardial I/R-induced injury via direct inhibition of extracellular HMGB1 cytokine activity [27], and to reduce myocardial inflammation in mice [28].
From the result of the different expressions of HMGB1 gene in both Mild and Severe group, it is obviously that disc samples with much more degeneration have higher HMGB1 expression and the lower type II collagen expression. Regarding the NP cells culture, CCK8 assay is used to explore the optimized concentration and stimulation time of the GL. The result indicated the GL had an ability to promote the proliferation of NP cells with an optimized concentration. IL-1β was widely used to mimic the pathophysiology of IDD in vitro [29]. So we used this cells degenerated model to text several targets corresponding to the inflammation and cell apoptosis. It was shown that the inflammation markers level such as TNF-α, IL-6/8, and iNOS specifically increased with the IL-1β stimuli compared with the control one. However, it came to a reversion under the treatment of GL. As we expected, the anti-inflammatory function of GL to the NP cells crippled with the addition of r-HMGB1. Recent evidences have suggested that the exogenous HMGB1 is a potential proinflammatory cytokine in the progress of NP cells degeneration and the GL is an efficient inhibitor of it.
NP cell apoptosis is another factor in IDD. Thus, inhibiting the excessive apoptosis of NP cells may be a potential way to alleviate IDD. Ast the beginning of this project, it was proved that GL could promote NP cells proliferation. We wondered whether GL both have the capacity to suppress cell apoptosis. So we analyzed p38 and p-JNK, which are two key factors in the regulation of apoptosis. Previous studies have shown that JNK and p38 can participate in apoptosis by regulating oxidative stress and inflammatory response [30]. HMGB1 has been proved as a novel regulator of apoptosis via the JNK/p38 pathway in several fields [31,32]. Our findings also lead to strong support to the view that p38/p-JNK signaling pathway is activated by the increased expression of HMGB1. Hopefully, GL is a super inhibitor that suppresses the influence of HMGB1 to reduce the NP cells apoptosis in the IDD. In the next step, we have planned to explore a deeper mechanism that underlines the interaction between HMGB1 and p38/p-JUK signaling pathway in intervertebral disc degeneration.
In conclusion, it would be interesting to find a molecule that works by suppressing inflammation and cell apoptosis via inhibition of HMGB1 to attenuate the IDD. We systematically evaluated the role of GL treatment on the IDD model established by the NP cells IL-1β stimuli. Together, these results revealed a knowledge facilitating investigations that GL may be a useful method to treat the intervertebral disc diseases. We look forward to future studies which will explore this possible role of GL in vivo.
Conclusion
These results suggested that GL prevented NP degradation via restraining inflammation and cell apoptosis by inhibition of HMGB1 via p38/p-JNK signaling pathway. GL may become a novel cytokine for the therapy of IDD in the future.
Acknowledgements
Natural Science Foundation of China (31800810); Xuzhou city, Jiangsu province to promote the special fund for scientific and technological innovation post-subsidy record project (KH17045).
Disclosure of conflict of interest
None.
References
- 1.Rannou F, Lee TS, Zhou RH, Chin J, Lotz JC, Mayoux-Benhamou MA, Barbet JP, Chevrot A, Shyy JY. Intervertebral disc degeneration: the role of the mitochondrial pathway in annulus fibrosus cell apoptosis induced by overload. Am J Pathol. 2004;164:915–924. doi: 10.1016/S0002-9440(10)63179-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kadow T, Sowa G, Vo N, Kang JD. Molecular basis of intervertebral disc degeneration and herniations: what are the important translational questions? Clin Orthop Relat Res. 2015;473:1903–1912. doi: 10.1007/s11999-014-3774-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ma T, Guo CJ, Zhao X, Wu L, Sun SX, Jin QH. The effect of curcumin on NF-kappaB expression in rat with lumbar intervertebral disc degeneration. Eur Rev Med Pharmacol Sci. 2015;19:1305–1314. [PubMed] [Google Scholar]
- 4.Kepler CK, Ponnappan RK, Tannoury CA, Risbud MV, Anderson DG. The molecular basis of intervertebral disc degeneration. Spine J. 2013;13:318–330. doi: 10.1016/j.spinee.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 5.Vergroesen PP, Kingma I, Emanuel KS, Hoogendoorn RJ, Welting TJ, van Royen BJ, van Dieen JH, Smit TH. Mechanics and biology in intervertebral disc degeneration: a vicious circle. Osteoarthritis Cartilage. 2015;23:1057–1070. doi: 10.1016/j.joca.2015.03.028. [DOI] [PubMed] [Google Scholar]
- 6.Hayes AJ, Benjamin M, Ralphs JR. Extracellular matrix in development of the intervertebral disc. Matrix Biol. 2001;20:107–121. doi: 10.1016/s0945-053x(01)00125-1. [DOI] [PubMed] [Google Scholar]
- 7.Crockett MT, Kelly BS, van Baarsel S, Kavanagh EC. Modic type 1 vertebral endplate changes: injury, inflammation, or infection? AJR Am J Roentgenol. 2017;209:167–170. doi: 10.2214/AJR.16.17403. [DOI] [PubMed] [Google Scholar]
- 8.Johnson ZI, Schoepflin ZR, Choi H, Shapiro IM, Risbud MV. Disc in flames: roles of TNF-alpha and IL-1beta in intervertebral disc degeneration. Eur Cell Mater. 2015;30:104–116. doi: 10.22203/ecm.v030a08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang R, Harada T, Mollen KP, Prince JM, Levy RM, Englert JA, Gallowitsch-Puerta M, Yang L, Yang H, Tracey KJ, Harbrecht BG, Billiar TR, Fink MP. Anti-HMGB1 neutralizing antibody ameliorates gut barrier dysfunction and improves survival after hemorrhagic shock. Mol Med. 2006;12:105–114. doi: 10.2119/2006-00010.Yang. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tsung A, Sahai R, Tanaka H, Nakao A, Fink MP, Lotze MT, Yang H, Li J, Tracey KJ, Geller DA, Billiar TR. The nuclear factor HMGB1 mediates hepatic injury after murine liver ischemia-reperfusion. J Exp Med. 2005;201:1135–1143. doi: 10.1084/jem.20042614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Andersson U, Wang H, Palmblad K, Aveberger AC, Bloom O, Erlandsson-Harris H, Janson A, Kokkola R, Zhang M, Yang H, Tracey KJ. High mobility group 1 protein (HMG-1) stimulates proinflammatory cytokine synthesis in human monocytes. J Exp Med. 2000;192:565–570. doi: 10.1084/jem.192.4.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gardella S, Andrei C, Ferrera D, Lotti LV, Torrisi MR, Bianchi ME, Rubartelli A. The nuclear protein HMGB1 is secreted by monocytes via a non-classical, vesicle-mediated secretory pathway. EMBO Rep. 2002;3:995–1001. doi: 10.1093/embo-reports/kvf198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scaffidi P, Misteli T, Bianchi ME. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature. 2002;418:191–195. doi: 10.1038/nature00858. [DOI] [PubMed] [Google Scholar]
- 14.Rajan NE, Bloom O, Maidhof R, Stetson N, Sherry B, Levine M, Chahine NO. Toll-Like receptor 4 (TLR4) expression and stimulation in a model of intervertebral disc inflammation and degeneration. Spine (Phila Pa 1976) 2013;38:1343–1351. doi: 10.1097/BRS.0b013e31826b71f4. [DOI] [PubMed] [Google Scholar]
- 15.Sakamoto R, Okano M, Takena H, Ohtsuki K. Inhibitory effect of glycyrrhizin on the phosphorylation and DNA-binding abilities of high mobility group proteins 1 and 2 in vitro. Biol Pharm Bull. 2001;24:906–911. doi: 10.1248/bpb.24.906. [DOI] [PubMed] [Google Scholar]
- 16.Mollica L, De Marchis F, Spitaleri A, Dallacosta C, Pennacchini D, Zamai M, Agresti A, Trisciuoglio L, Musco G, Bianchi ME. Glycyrrhizin binds to high-mobility group box 1 protein and inhibits its cytokine activities. Chem Biol. 2007;14:431–441. doi: 10.1016/j.chembiol.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 17.Ogiku M, Kono H, Hara M, Tsuchiya M, Fujii H. Glycyrrhizin prevents liver injury by inhibition of high-mobility group box 1 production by Kupffer cells after ischemia-reperfusion in rats. J Pharmacol Exp Ther. 2011;339:93–98. doi: 10.1124/jpet.111.182592. [DOI] [PubMed] [Google Scholar]
- 18.Gong G, Yuan LB, Hu L, Wu W, Yin L, Hou JL, Liu YH, Zhou LS. Glycyrrhizin attenuates rat ischemic spinal cord injury by suppressing inflammatory cytokines and HMGB1. Acta Pharmacol Sin. 2012;33:11–18. doi: 10.1038/aps.2011.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pfirrmann CW, Metzdorf A, Zanetti M, Hodler J, Boos N. Magnetic resonance classification of lumbar intervertebral disc degeneration. Spine (Phila Pa 1976) 2001;26:1873–1878. doi: 10.1097/00007632-200109010-00011. [DOI] [PubMed] [Google Scholar]
- 20.Kang L, Hu J, Weng Y, Jia J, Zhang Y. Sirtuin 6 prevents matrix degradation through inhibition of the NF-kappaB pathway in intervertebral disc degeneration. Exp Cell Res. 2017;352:322–332. doi: 10.1016/j.yexcr.2017.02.023. [DOI] [PubMed] [Google Scholar]
- 21.Andersson GB, An HS, Oegema TJ, Setton LA. Intervertebral disc degeneration. Summary of an AAOS/NIH/ORS workshop, September 2005. J Bone Joint Surg Am. 2006;88:895–899. doi: 10.2106/JBJS.F.00028. [DOI] [PubMed] [Google Scholar]
- 22.Molinos M, Almeida CR, Caldeira J, Cunha C, Goncalves RM, Barbosa MA. Inflammation in intervertebral disc degeneration and regeneration. J R Soc Interface. 2015;12:20150429. doi: 10.1098/rsif.2014.1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Andersson U, Harris HE. The role of HMGB1 in the pathogenesis of rheumatic disease. Biochim Biophys Acta. 2010;1799:141–148. doi: 10.1016/j.bbagrm.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 24.Chen Q, Guan X, Zuo X, Wang J, Yin W. The role of high mobility group box 1 (HMGB1) in the pathogenesis of kidney diseases. Acta Pharm Sin B. 2016;6:183–188. doi: 10.1016/j.apsb.2016.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Magna M, Pisetsky DS. The role of cell death in the pathogenesis of SLE: is pyroptosis the missing link? Scand J Immunol. 2015;82:218–224. doi: 10.1111/sji.12335. [DOI] [PubMed] [Google Scholar]
- 26.Fang F, Jiang D. IL-1beta/HMGB1 signalling promotes the inflammatory cytokines release via TLR signalling in human intervertebral disc cells. Biosci Rep. 2016;36 doi: 10.1042/BSR20160118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhai CL, Zhang MQ, Zhang Y, Xu HX, Wang JM, An GP, Wang YY, Li L. Glycyrrhizin protects rat heart against ischemia-reperfusion injury through blockade of HMGB1-dependent phospho-JNK/Bax pathway. Acta Pharmacol Sin. 2012;33:1477–1487. doi: 10.1038/aps.2012.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bangert A, Andrassy M, Muller AM, Bockstahler M, Fischer A, Volz CH, Leib C, Goser S, Korkmaz-Icoz S, Zittrich S, Jungmann A, Lasitschka F, Pfitzer G, Muller OJ, Katus HA, Kaya Z. Critical role of RAGE and HMGB1 in inflammatory heart disease. Proc Natl Acad Sci U S A. 2016;113:E155–E164. doi: 10.1073/pnas.1522288113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gorth DJ, Shapiro IM, Risbud MV. A new understanding of the role of IL-1 in age-related intervertebral disc degeneration in a murine model. J Bone Miner Res. 2019;34:1531–1542. doi: 10.1002/jbmr.3714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cai Y, Jiang W, Zhou AL, Zhou M, Xu L. Effect of oxymatrine on apoptosis of hippocampal neurons by p38/JNK signaling pathway. Zhongguo Zhong Yao Za Zhi. 2017;42:731–738. doi: 10.19540/j.cnki.cjcmm.2017.0020. [DOI] [PubMed] [Google Scholar]
- 31.Ye F, Chai W, Xie M, Yang M, Yu Y, Cao L, Yang L. HMGB1 regulates erastin-induced ferroptosis via RAS-JNK/p38 signaling in HL-60/NRAS(Q61L) cells. Am J Cancer Res. 2019;9:730–739. [PMC free article] [PubMed] [Google Scholar]
- 32.Zhou YH, Han QF, Wang LH, Liu T, Meng XY, Wu L, Li T, Jiao YR, Yao HC, Zhang DY. High mobility group box 1 protein attenuates myocardial ischemia reperfusion injury via inhibition of the p38 mitogen-activated protein kinase signaling pathway. Exp Ther Med. 2017;14:1582–1588. doi: 10.3892/etm.2017.4653. [DOI] [PMC free article] [PubMed] [Google Scholar]


