Significance
Accurate prediction of community responses to global change drivers (GCDs) is critical given the effects of biodiversity on ecosystem services. There is consensus that human activities are driving species extinctions at the global scale, but debate remains over whether GCDs are systematically altering local communities worldwide. Across 105 experiments that included over 400 experimental manipulations, we found evidence for a lagged response of herbaceous plant communities to GCDs caused by shifts in the identities and relative abundances of species, often without a corresponding difference in species richness. These results provide evidence that community responses are pervasive across a wide variety of GCDs on long-term temporal scales and that these responses increase in strength when multiple GCDs are simultaneously imposed.
Keywords: community composition, global change experiments, herbaceous plants, species richness
Abstract
Global change drivers (GCDs) are expected to alter community structure and consequently, the services that ecosystems provide. Yet, few experimental investigations have examined effects of GCDs on plant community structure across multiple ecosystem types, and those that do exist present conflicting patterns. In an unprecedented global synthesis of over 100 experiments that manipulated factors linked to GCDs, we show that herbaceous plant community responses depend on experimental manipulation length and number of factors manipulated. We found that plant communities are fairly resistant to experimentally manipulated GCDs in the short term (<10 y). In contrast, long-term (≥10 y) experiments show increasing community divergence of treatments from control conditions. Surprisingly, these community responses occurred with similar frequency across the GCD types manipulated in our database. However, community responses were more common when 3 or more GCDs were simultaneously manipulated, suggesting the emergence of additive or synergistic effects of multiple drivers, particularly over long time periods. In half of the cases, GCD manipulations caused a difference in community composition without a corresponding species richness difference, indicating that species reordering or replacement is an important mechanism of community responses to GCDs and should be given greater consideration when examining consequences of GCDs for the biodiversity–ecosystem function relationship. Human activities are currently driving unparalleled global changes worldwide. Our analyses provide the most comprehensive evidence to date that these human activities may have widespread impacts on plant community composition globally, which will increase in frequency over time and be greater in areas where communities face multiple GCDs simultaneously.
Human activities are driving unprecedented changes in many factors that may affect the composition and functioning of plant communities. Determining the factors that cause alterations in plant community structure is critical, as important ecosystem functions and services are influenced by plant community composition (1, 2). Changes in resource availability (e.g., atmospheric carbon dioxide [CO2], nitrogen [N], precipitation patterns) may have large consequences for plant community structure worldwide (3). Yet, our ability to interpret and predict plant community responses to global change is complicated by many factors, such as the type of global change driver (GCD) and the environmental context. Observational and experimental evidence has demonstrated disparate and seemingly conflicting patterns of species richness responses to environmental change across a variety of independent studies, metaanalyses, and large data syntheses (4–11). As such, there is continued debate over whether local-scale biodiversity loss is a worldwide trend (12–14). Moreover, recent studies (15, 16) advocate the use of multivariate metrics (e.g., Bray–Curtis dissimilarity) that account for not only changes in species number, but also species identities and relative abundances to provide a more comprehensive picture of composition responses to GCDs.
Both biotic (e.g., shifts in competitive dominance or susceptibility to herbivores) and abiotic (e.g., environmental filtering) processes (17–19) have been invoked to explain how GCDs affect plant community richness and composition at local scales, and it seems reasonable to expect that plant community responses will vary across a broad array of GCDs (2, 15). Resource additions (e.g., nutrient additions) are predicted to reduce plant species richness and alter plant community composition due to changes in competitive interactions among species for the remaining limiting resources (e.g., water or light) (7, 8, 20). In contrast, increased environmental stress may have varying effects on plant community composition by either shifting or increasing niche availability. For example, repeated removal of plant material through haying (a common land use change in many herbaceous systems) may increase species richness by increasing light availability and favoring species that can tolerate removal of aboveground material. In contrast, increased drought or temperature stress may decrease plant species richness, as many species may not be able to persist under these novel conditions (7, 21). In addition to the type of driver manipulated, the number of simultaneously imposed GCDs may also impact community responses. Previous studies have shown that plant community responses may be greater under multiple simultaneously imposed GCDs (22–24). In contrast, both empirical evidence and theoretical evidence suggest that ecosystem function responses have been shown to dampen with increasing numbers of simultaneously imposed GCDs (25, 26) due to a canceling out of positive and negative effects on functions, such as productivity and nutrient cycling. Based on these conflicting results, determining a generalizable pattern of the effects of multiple GCDs on community responses is needed.
Here, we examined results from 105 experiments conducted in grasslands around the world that together provide data on over 400 experimental manipulations of GCDs to determine whether we could identify general community response patterns across different types of manipulations, the magnitude of the manipulations imposed, or the attributes of the ecosystems where the experiments were conducted. In contrast to prior analyses, which have examined patterns of community change based on observational data (5, 16, 27), we focused on experiments, because they provide an important baseline (control plots) that is critical for the accurate assessment of community responses to GCDs by separating stochastic community shifts from global change effects. By identifying generalities where they exist across complex community patterns, we can make tangible progress toward prediction of future community responses to GCDs occurring worldwide, which is needed to develop strategies for maintaining the communities on which many ecosystem services rely.
Methods
We used hierarchical Bayesian modeling to examine how herbaceous plant communities responded to global change manipulations in 438 experimental treatments encompassed within 105 experiments at 52 sites around the world using the Community Responses to Resource Experiments (CoRRE) database (https://corredata.weebly.com/) (SI Appendix, section 2). The CoRRE database was assembled from plant species composition data collected by hundreds of researchers in field experiments across all continents except Antarctica and includes 285,019 species occurrence records of 2,843 species from 26,788 time points in experiments ranging in duration from 3 to 31 y (Table 1 and SI Appendix, section 3). Global change treatments included resource additions and removals (e.g., nutrient additions, increased atmospheric CO2, irrigation, drought) as well as nonresource manipulations (e.g., increased temperature, burning, mowing, herbivore removals), and were designed to simulate predicted future global change scenarios in different areas of the globe. We measured plant community responses in treatments relative to controls using 2 commonly used metrics of community difference: (i) ln response ratios (lnRR) of plant species richness (i.e., species number without regard to identity) and (ii) species composition responses in multivariate space using Bray–Curtis dissimilarities (encompassing shifts in plant species identities and their relative abundances). We also briefly present results from 2 additional richness metrics: percentage difference of plant species richness from control to treatment plots and lnRR of effective species number (eH). Because these 2 metrics show qualitatively identical results to lnRR of richness, we focus on lnRR of richness here for most analyses. For all metrics, we investigated the temporal nature of the observed differences over the length of each experiment as well as whether these effects varied based on the site-level (gamma) diversity or productivity of each experiment.
Table 1.
Summary statistics of experiments (n = 105) included in the data synthesis
| Variable | Minimum | Mean | Maximum |
| Experiment length (no. of y) | 3 | 8 | 31 |
| No. of manipulations | 1 | 2 | 5 |
| Gamma diversity (no. of species) | 3 | 31 | 79 |
| Aboveground biomass (g m−2 y−1) | 1.5 | 349 | 1,415 |
| Mean annual precipitation (mm) | 183 | 714 | 1,526 |
| Mean annual temperature (°C) | −12 | 8 | 22 |
Methods discusses variable descriptions.
Results and Discussion
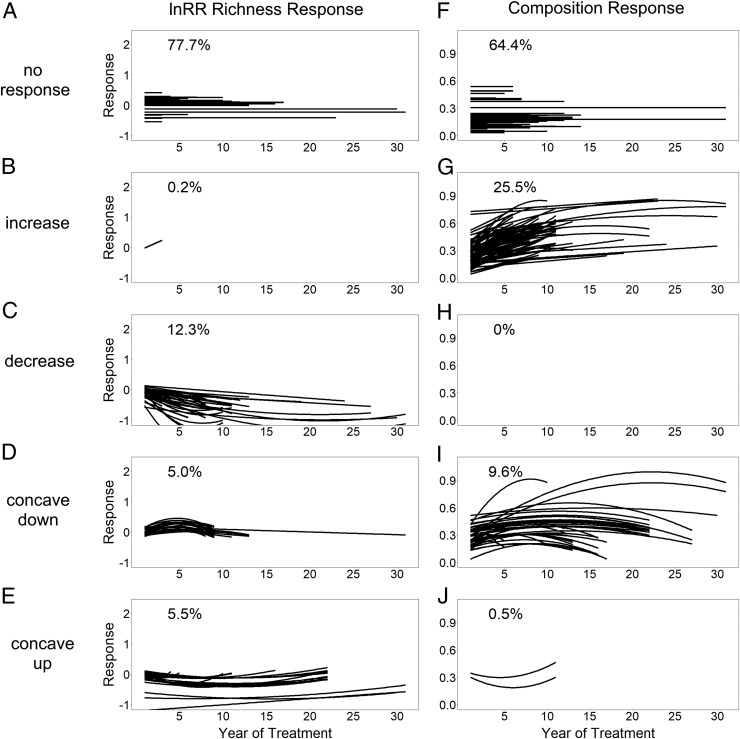
In experiments less than 10 y in duration, we found that plant communities are relatively resistant to global change manipulations, with 79.5 and 77.0% of treatments showing no richness or composition response, respectively (Fig. 1 A and F and Table 2). In contrast, in long-term (≥10-y) experiments, fewer manipulations (50%) showed no difference in species richness (Table 2). Importantly, 70.7% of long-term manipulations exhibited composition responses (Table 2), and some communities experienced almost complete turnover after 1 to 2 decades (composition responses close to 1.0) (Fig. 1). The increased prevalence of community responses in long-term experiments highlights the need for long-term data collection to better identify community responses to GCDs. In approximately half of the cases (54.5%) where experimental manipulations caused a composition shift through time, it occurred without a corresponding richness response. Consequently, the multivariate plant community composition responses observed here often reflect differences in species evenness, reordering of species ranks based on relative abundances, or species replacement (turnover) (15). Future consideration of these detailed community responses is warranted to (i) examine the temporal hierarchy of the response (i.e., is there an ordering to differences in evenness, reordering of species ranks, and turnover) (2) and (ii) move beyond using only richness differences as a metric of biodiversity (16). Studying these detailed community shifts will provide important insight into how alterations in ecosystem function with GCDs relate to compositional aspects of biodiversity.
Fig. 1.
Experimental global change manipulations drive temporal differences in plant community composition. Richness responses (A–E) are measured as the lnRR of richness between treatment and control plots within a year; positive values indicate net species gains in treatment plots relative to control plots, while negative values indicate net species losses. lnRR richness response has a lower bound of −1 and no upper bound. Composition responses (F–J) are measured as the Euclidean distance between centroids of control and treatment plots within a year in a principle coordinates analysis based on a Bray–Curtis dissimilarity matrix; composition response is bounded by 0 and 1. Responses are grouped among 5 possible shapes indicated along the left sides of the panels. For all panels, lines correspond to models for 438 individual global change treatments responses across 105 experiments. For all lines, slopes and intercepts are plotted as 0 when 95% credible intervals of parameters include 0. Percentages are percentages of studies exhibiting a particular response shape across all experiments (i.e., not considering experiment length). Percentage responses for short-term vs. long-term experiments can be found in Table 2.
Table 2.
Summary of the response shape of the richness (lnRR and % difference richness), effective species number (lnRR eH), and composition differences across 438 treatments included in the data synthesis
| Response shape | lnRR richness % (no.) | % Difference richness (no.) | lnRR eH % (no.) | Composition difference % (no.) |
| <10 y | ||||
| No response | 87.0 (280) | 79.5 (256) | 80.7 (259) | 77.0 (248) |
| Linear increase | 0.3 (1) | 2.8 (9) | 2.5 (8) | 20.8 (67) |
| Delayed increase | 0.0 (0) | 0.0 (0) | 0.3 (1) | 0.0 (0) |
| Asymptotic increase | 0.0 (0) | 0.0 (0) | 0.6 (2) | 0.0 (0) |
| Linear decrease | 6.5 (21) | 9.0 (29) | 8.4 (27) | 0.0 (0) |
| Delayed decrease | 0.6 (2) | 0.3 (1) | 0.9 (3) | 0.0 (0) |
| Asymptotic decrease | 0.0 (0) | 0.6 (2) | 0.0 (0) | 0.0 (0) |
| Concave down | 5.0 (16) | 5.9 (19) | 6.2 (20) | 2.2 (7) |
| Concave up | 0.6 (2) | 1.9 (6) | 0.3 (1) | 0.0 (0) |
| ≥10 y | ||||
| No response | 50.0 (58) | 41.4 (48) | 44.0 (51) | 29.3 (34) |
| Linear increase | 0.0 (0) | 0.9 (1) | 1.7 (2) | 22.4 (26) |
| Delayed increase | 0.0 (0) | 0.0 (0) | 0.0 (0) | 4.3 (5) |
| Asymptotic increase | 0.0 (0) | 0.0 (0) | 0.0 (0) | 12.1 (14) |
| Linear decrease | 16.4 (19) | 19.0 (22) | 21.6 (25) | 0.0 (0) |
| Delayed decrease | 0.0 (0) | 0.0 (0) | 0.0 (0) | 0.0 (0) |
| Asymptotic decrease | 9.5 (11) | 13.8 (16) | 11.2 (13) | 0.0 (0) |
| Concave down | 5.2 (6) | 8.6 (10) | 7.8 (9) | 30.2 (35) |
| Concave up | 19.0 (22) | 16.4 (19) | 13.8 (16) | 1.7 (2) |
Shown are percentages (with numbers in parentheses) of responses falling into each of 9 shape categories split by experiment length into those less than 10 y (n = 322 responses) and those greater than or equal to 10 y (n = 116 responses) in length. Note that these percentages differ from those in Fig. 1, which presents percentages of each response shape across all experiments regardless of length. Methods discusses response variable descriptions.
When considering all manipulations regardless of experiment length, we find that the community responses to global change manipulations varied in both direction and magnitude (Fig. 1). When richness responded to experimental manipulations (22.3% of all manipulations), it generally declined either linearly or asymptotically (Fig. 1 and Table 2). Similarly, when composition responded to experimental manipulations (35.6% of all manipulations), it generally increased in dissimilarity from control plots (Fig. 1 and Table 2). Interestingly, in a small subset of the cases studied here (10.5% of richness and 10.1% of composition responses), community responses to global change manipulations were parabolic, with the minimum or maximum of the curve occurring within the study period, suggesting that the initial community responses in these sites eventually dampen over time (Fig. 1 and Table 2). These parabolic trends were more often detected in the long-term experiments and treatments that manipulated 2 or more factors. For richness responses, these parabolic trends were nearly equally split among those that were concave up, indicative of initial richness losses that later recovered due to immigration of new species or recovery of previously lost species, and those that were concave down, indicative of initial richness gains that later declined. In contrast, the parabolic trends in composition response were nearly all concave down, demonstrating an initial divergence of treatment and control plots followed by convergence. The few cases of long-term convergence between treatment and control plots stemmed from a shift in control plots toward the altered state exhibited in the treatments (SI Appendix, section 5). Overall, these parabolic trends caused by a shift in communities in control plots suggest that human activities may currently be impacting the environment at a scale beyond the scope of some experimental treatments, as has previously been demonstrated in global observational data syntheses (5, 8, 25).
Across sites, we found that site-level productivity was positively related to richness increases in response to global change manipulations, while gamma diversity (site-level species number) had no effect on the direction or magnitude of the richness or composition responses (SI Appendix, section 4). Hence, high-productivity ecosystems seem more responsive to GCDs, possibly due to the greater availability of resources, and therefore niche space, in such systems (28) or the greater ability of species in these systems to respond to GCDs due to higher growth rates in productive herbaceous systems (29). The greater community responsiveness at high-productivity sites may contribute to the maintenance of ecosystem function, as species with traits adapted to the novel environmental conditions presented by global change scenarios increase in abundance in these communities (30). However, higher abundances of species that are not functionally similar to the existing community (2, 3, 5) would likely result in altered ecosystem function.
Declines in species richness are often attributed to decreased niche dimensionality with alleviation of resource limitations (17) or increased environmental filtering (19), while richness increases may be due to invasions or increased environmental heterogeneity (31). We did observe richness differences in a few cases that may be attributable to these mechanisms. For example, multiple resource additions may decrease niche dimensionality, leading to dominance of a few competitive species and therefore richness declines (20). In contrast, multiple resource additions can shift an ecosystem’s stoichiometry to alter the relative availability of the most limiting resource and thus, competitive interactions, thereby reducing species loss (32). Furthermore, resource additions may increase species invasions by relaxing environmental filters (33), again reducing species loss. Nevertheless, in the majority of cases, we found that global change treatments altered community composition with no corresponding richness responses. These results highlight the fact that, by not accounting for species identity, species richness does not entirely capture community responses to GCDs (16). Indeed, species richness can stay constant even with complete turnover in the identities of species within a community. Therefore, multivariate metrics of species abundances are needed to assess complex community responses to GCDs (15).
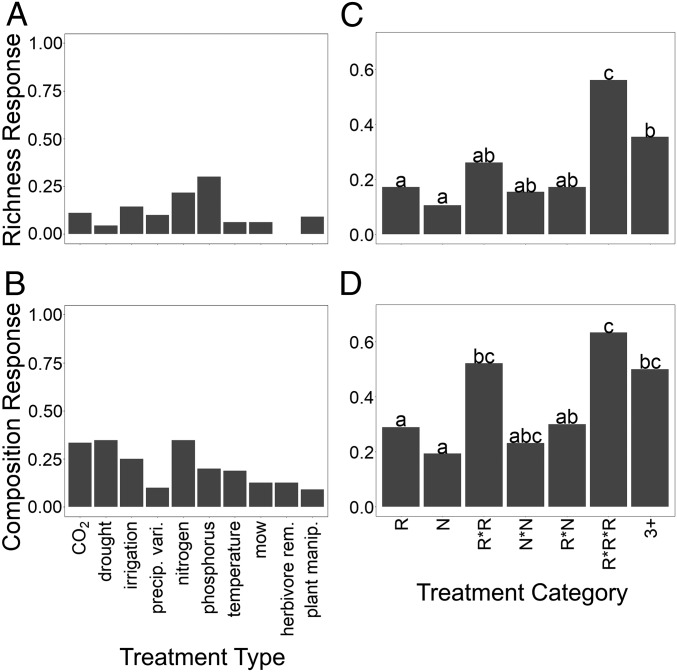
Interestingly, we did not find differences in richness or composition responses based on the type of GCD applied (Fig. 2 and Table 3). Our results differ from previous metaanalyses that show stronger richness losses with N additions than other GCDs (7). However, we did find that global change manipulations that simultaneously manipulated 3 or more GCDs were significantly more likely to show richness and composition responses than treatments that only manipulated 1 or 2 GCDs (Fig. 2 and Table 3). These results are consistent with previous studies examining community responses to GCDs (22–24), but contrast with trends observed for ecosystem function responses to multiple GCDs from 2 previous studies, which tend to show damped responses with increasing factors manipulated (25, 26). This difference highlights the need to examine how differences in community composition relate to altered ecosystem function (2, 15, 25).
Fig. 2.
Across all datasets, the proportions of significant temporal plant community responses (lnRR richness and composition differences) to global change treatments do not vary by the type of single-factor global change manipulation imposed (A and B, respectively), but do vary by the number of treatments simultaneously imposed (C and D, respectively). Single-factor global change manipulations are categorized into treatment types (CO2 = increased atmospheric CO2; drought = reduced precipitation; irrigation = increased precipitation; precip. vari. = variation in precipitation timing but not amount; nitrogen = nitrogen additions; phosphorus = phosphorous additions; temperature = increased temperature; mow = mowing aboveground biomass; herbivore rem. = removal of above- and/or belowground herbivores; plant manip. = 1-time manipulation of plant through seed additions or diversity treatments at the start of the experiment). Treatment categories group treatments by the number and type of manipulations imposed (R = single resource; N = single nonresource; R × R = 2-way interactions with both treatments manipulating resources; N × N = 2-way interactions with both treatments manipulating nonresources; R × N = 2-way interactions with 1 resource and 1 nonresource manipulation; R × R × R = 3 or more way interactions with all treatments manipulating resources; 3+ = ≥3-way interactions with both resource and nonresource manipulations). Significant differences in the proportion of significant richness and composition responses among treatment categories are indicated by letters as determined by Fisher’s exact test for all pairwise combinations. a indicates significant differences in the proportion of richness or composition responses compared to results marked by b or c at P < 0.05 as determined by Fisher’s exact test. b indicates significant differences in the proportion of richness or composition responses compared to results marked by a or c at P < 0.05 as determined by Fisher’s exact test. c indicates significant differences in the proportion of richness or composition responses compared to results marked by a or b at P < 0.05 as determined by Fisher’s exact test.
Table 3.
Across all datasets, temporal plant community responses (lnRR richness and composition differences) to global change treatments do not vary by treatment type among single-resource or nonresource manipulations (richness: χ2 = 12.47, degrees of freedom [df] = 11, P = 0.330; composition: χ2 = 9.42, df = 11, P = 0.583), but do vary by treatment category among multifactorial manipulations (richness: χ2 = 21.85, df = 6, P = 0.001; composition: χ2 = 15.78, df = 6, P = 0.015)
| Treatment type/category | Total possible responses | No. of richness responses | Proportion significant richness responses | No. of composition responses | Proportion significant composition responses |
| Treatment type | |||||
| CO2 | 9 | 1 | 0.11 | 3 | 0.33 |
| Drought | 23 | 1 | 0.04 | 8 | 0.35 |
| Irrigation | 28 | 4 | 0.14 | 7 | 0.25 |
| Precipitation variability | 10 | 1 | 0.10 | 1 | 0.10 |
| N | 69 | 15 | 0.22 | 24 | 0.35 |
| Phosphorus | 20 | 6 | 0.30 | 4 | 0.20 |
| Other resource | 4 | 0 | 0.00 | 0 | 0.00 |
| Temperature | 16 | 1 | 0.06 | 3 | 0.19 |
| Mowing/clipping | 16 | 1 | 0.06 | 2 | 0.13 |
| Herbivore removal | 8 | 0 | 0.00 | 1 | 0.13 |
| Plant manipulation | 11 | 1 | 0.09 | 1 | 0.09 |
| Other nonresource | 6 | 3 | 0.50 | 4 | 0.67 |
| Treatment category | |||||
| Single resource | 163 | 28 | 0.17* | 47 | 0.29* |
| Single nonresource | 57 | 6 | 0.11* | 11 | 0.19* |
| Resource × resource | 46 | 12 | 0.26*† | 24 | 0.52†‡ |
| Nonresource × nonresource | 13 | 2 | 0.15*† | 3 | 0.23*†‡ |
| Resource × nonresource | 70 | 12 | 0.17*† | 21 | 0.30*† |
| 3+ Resources | 41 | 23 | 0.56‡ | 26 | 0.63‡ |
| No. + resource and nonresource | 48 | 17 | 0.35† | 24 | 0.50†‡ |
| Overall | 438 | 100 | 0.23 | 156 | 0.36 |
Numbers and proportions are of each treatment type/category that showed a significant temporal response to experimental global change manipulations. Across only long-term (≥10-y) datasets, temporal plant community responses to global change treatments do not vary by treatment type among single-resource or nonresource manipulations (richness: χ2 = 3.36, df = 10, P = 0.972; composition: χ2 = 4.21, df = 10, P = 0.938) or treatment category among multifactorial manipulations (richness: χ2 = 3.01, df = 6, P = 0.808; composition: χ2 = 1.39, df = 6, P = 0.967). Exclusion of treatment types or categories with fewer than 3 replicates did not qualitatively affect the results.
Significant differences in the proportion of richness or composition responses compared to results marked by † or ‡ at P < 0.05 as determined by Fisher’s exact test.
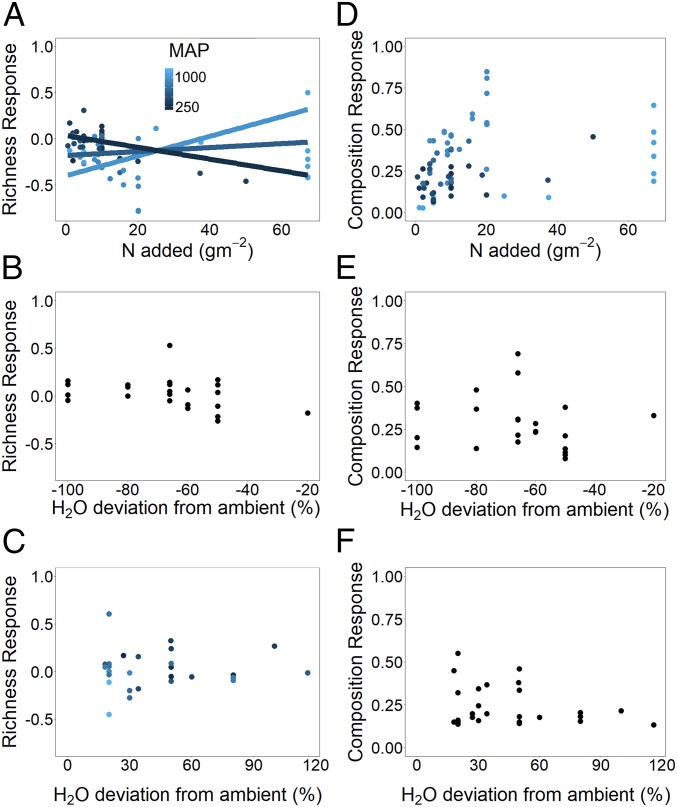
While on average, the effects of N addition on plant communities were not stronger than other global change treatments, we did find that the absolute level of N added interacted with mean annual precipitation (MAP) to influence richness responses (Fig. 3 and SI Appendix, section 6). Specifically, richness declined with increasing N added at sites with low MAP and increased with increasing N added at sites with high MAP (Fig. 3A and SI Appendix, section 6). In contrast, the magnitude of rainfall manipulations did not affect the richness or composition responses (Fig. 3 and SI Appendix, section 6). These results conflict with previous analyses of richness responses to N deposition, which show a decline in richness with increasing precipitation and N deposition (34). This discrepancy may be due to the high magnitude of N added in some of our experiments, more akin to nutrient runoff from agricultural fields than atmospheric deposition. Together, these results point toward colimitation of species richness across ecosystems (34, 35) and highlight the need to address potential threshold responses of community responses to resource manipulations.
Fig. 3.
Differences in (A–C) richness and (D–F) plant composition to the magnitude of (A and D) N addition treatments, (B and E) drought manipulation treatments, and (C and F) irrigation manipulation experiments. Points represent treatment responses for each experiment at each site in the final year of treatment, and lines indicate Bayesian regressions between treatment magnitude and richness or composition responses where significant. Points are colored by site-level MAP where the independent effect of MAP was significant, and lines are colored by MAP where the interactive effect between MAP and treatment magnitude was significant.
Although this analysis includes the effects of a wide variety of global change manipulations on plant communities, many combinations of GCDs potentially important to global change were underrepresented or missing from our analysis, reflective of their lack of study worldwide. These include combinations that are posited to have large impacts on the biosphere, such as the combined consequences of increased nutrient availability and altered precipitation patterns (36). Furthermore, the geographic scope of global change experiments is primarily constrained to the northern hemisphere (SI Appendix, section 3). Experiments that incorporate higher-order interactions at sites worldwide are critical for accurately predicting how communities will respond globally to predicted GCDs (25). Despite these limitations, our results clearly demonstrate that changes in plant community composition may be expected across a wide range of GCDs over the coming decades.
In conclusion, our comprehensive analysis finds that plant community structure is frequently altered by a broad array of GCDs and that these effects are largely only detectable over long (≥10-y) timescales. These community responses occurred at similar frequencies across the wide variety of GCDs examined in this study, but were more prevalent when 3 or more GCDs were manipulated simultaneously, representative of real-world situations where 1 GCD rarely operates in isolation. In about half of the cases where compositional responses were observed, they occurred without corresponding differences in species richness, indicating that coexistence mechanisms may be maintained in the face of changing environmental conditions or that competitive displacement is slower than the timescales of these experiments. Rather than species gains or losses, in many cases community responses seem to be due to the abundances of species tracking environmental conditions through reordering within the existing community or colonization from a regional species pool. Determining the functional consequences of these broad-scale community responses to GCDs demands investigation into the identities and traits of species that are most responsive to global environmental change (2, 37).
Supplementary Material
Acknowledgments
This work was conducted as a part of a Long-Term Ecological Research (LTER) Synthesis Group funded by NSF Grants EF-0553768 and DEB#1545288 through the LTER Network Communications Office and the National Center for Ecological Analysis and Synthesis, University of California, Santa Barbara. M.L.A. was supported by a fellowship from the Socio-Environmental Synthesis Center (SESYNC), which also provided computing support. SESYNC is funded by NSF Grant DBI-1052875. Funding for individual experiments included in this analysis can be found in SI Appendix, section 7.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
2Community Responses to Resource Experiments (CoRRE) Working Group Leader.
3CoRRE Working Group Member.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1819027116/-/DCSupplemental.
References
- 1.Cardinale B. J., et al. , The functional role of producer diversity in ecosystems. Am. J. Bot. 98, 572–592 (2011). [DOI] [PubMed] [Google Scholar]
- 2.Smith M. D., Knapp A. K., Collins S. L., A framework for assessing ecosystem dynamics in response to chronic resource alterations induced by global change. Ecology 90, 3279–3289 (2009). [DOI] [PubMed] [Google Scholar]
- 3.Franklin J., Serra-Diaz J. M., Syphard A. D., Regan H. M., Global change and terrestrial plant community dynamics. Proc. Natl. Acad. Sci. U.S.A. 113, 3725–3734 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chalcraft D. R., et al. , Scale-dependent responses of plant biodiversity to nitrogen enrichment. Ecology 89, 2165–2171 (2008). [DOI] [PubMed] [Google Scholar]
- 5.Dornelas M., et al. , Assemblage time series reveal biodiversity change but not systematic loss. Science 344, 296–299 (2014). [DOI] [PubMed] [Google Scholar]
- 6.Elahi R., et al. , Recent trends in local-scale marine biodiversity reflect community structure and human impacts. Curr. Biol. 25, 1938–1943 (2015). [DOI] [PubMed] [Google Scholar]
- 7.Murphy G. E. P., Romanuk T. N., A meta-analysis of declines in local species richness from human disturbances. Ecol. Evol. 4, 91–103 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Newbold T., et al. , Global effects of land use on local terrestrial biodiversity. Nature 520, 45–50 (2015). [DOI] [PubMed] [Google Scholar]
- 9.Sardans J., Rivas-Ubach A., Peñuelas J., The C:N:P stoichiometry of organisms and ecosystems in a changing world: A review and perspectives. Perspect. Plant Ecol. Evol. Syst. 14, 33–47 (2012). [Google Scholar]
- 10.Vellend M., et al. , Global meta-analysis reveals no net change in local-scale plant biodiversity over time. Proc. Natl. Acad. Sci. U.S.A. 110, 19456–19459 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Suding K. N., et al. , Functional- and abundance-based mechanisms explain diversity loss due to N fertilization. Proc. Natl. Acad. Sci. U.S.A. 102, 4387–4392 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cardinale B. J., Gonzalez A., Allington G. R. H., Loreau M., Is local biodiversity declining or not? A summary of the debate over analysis of species richness time trends. Biol. Conserv. 219, 175–183 (2018). [Google Scholar]
- 13.Gonzalez A., et al. , Estimating local biodiversity change: A critique of papers claiming no net loss of local diversity. Ecology 97, 1949–1960 (2016). [DOI] [PubMed] [Google Scholar]
- 14.Vellend M., et al. , Estimates of local biodiversity change over time stand up to scrutiny. Ecology 98, 583–590 (2017). [DOI] [PubMed] [Google Scholar]
- 15.Avolio M. L., et al. , A framework for quantifying the magnitude and variability of community responses to global change drivers. Ecosphere 6, 1–14 (2015). [Google Scholar]
- 16.Hillebrand H., et al. , Biodiversity change is uncoupled from species richness trends: Consequences for conservation and monitoring. J. Appl. Ecol. 55, 169–184 (2018). [Google Scholar]
- 17.Chesson P., Mechanisms of maintenance of species diversity. Annu. Rev. Ecol. Syst. 31, 343–366 (2000). [Google Scholar]
- 18.Grime J. P., Competitive exclusion in herbaceous vegetation. Nature 242, 344–347 (1973). [Google Scholar]
- 19.HilleRisLambers J., Adler P. B., Harpole W. S., Levine J. M., Mayfield M. M., Rethinking community assembly through the lens of coexistence theory. Annu. Rev. Ecol. Evol. Syst. 43, 227–248 (2012). [Google Scholar]
- 20.Harpole W. S., et al. , Addition of multiple limiting resources reduces grassland diversity. Nature 537, 93–96 (2016). [DOI] [PubMed] [Google Scholar]
- 21.Tilman D., El Haddi A., Drought and biodiversity in Grasslands. Oecologia 89, 257–264 (1992). [DOI] [PubMed] [Google Scholar]
- 22.Eskelinen A., Harrison S. P., Resource colimitation governs plant community responses to altered precipitation. Proc. Natl. Acad. Sci. U.S.A. 112, 13009–13014 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harpole W. S., Tilman D., Grassland species loss resulting from reduced niche dimension. Nature 446, 791–793 (2007). [DOI] [PubMed] [Google Scholar]
- 24.Reich P. B., Hungate B. A., Luo Y., Carbon-nitrogen interactions in terrestrial ecosystems in response to rising atmospheric carbon dioxide. Annu. Rev. Ecol. Evol. Syst. 37, 611–636 (2006). [Google Scholar]
- 25.Langley J. A., Hungate B. A., Plant community feedbacks and long-term ecosystem responses to multi-factored global change. AoB Plants 6, 1–12 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leuzinger S., et al. , Do global change experiments overestimate impacts on terrestrial ecosystems? Trends Ecol. Evol. 26, 236–241 (2011). [DOI] [PubMed] [Google Scholar]
- 27.Jones S. K., Ripplinger J., Collins S. L., Species reordering, not changes in richness, drives long-term dynamics in grassland communities. Ecol. Lett. 20, 1556–1565 (2017). [DOI] [PubMed] [Google Scholar]
- 28.Chase J. M., Leibold M. A., Ecological Niches: Linking Classical and Contemporary Approaches (University of Chicago Press, 2003). [Google Scholar]
- 29.Grime J. P., Plant Strategies and Vegetation Processes (John Wiley & Sons, 1979). [Google Scholar]
- 30.Liu H., et al. , Shifting plant species composition in response to climate change stabilizes grassland primary production. Proc. Natl. Acad. Sci. U.S.A. 115, 4051–4056 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shea K., Chesson P., Community ecology theory as a framework for biological invasions. Trends Ecol. Evol. 17, 170–176 (2002). [Google Scholar]
- 32.Reich P. B., Elevated CO2 reduces losses of plant diversity caused by nitrogen deposition. Science 326, 1399–1402 (2009). [DOI] [PubMed] [Google Scholar]
- 33.Suttle K. B., Thomsen M. A., Power M. E., Species interactions reverse grassland responses to changing climate. Science 315, 640–642 (2007). [DOI] [PubMed] [Google Scholar]
- 34.Simkin S. M., et al. , Conditional vulnerability of plant diversity to atmospheric nitrogen deposition across the United States. Proc. Natl. Acad. Sci. U.S.A. 113, 4086–4091 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hooper D. U., Johnson L., Nitrogen limitation in dryland ecosystems: Responses to geographical and temporal variation in precipitation. Biogeochemistry 46, 247–293 (1999). [Google Scholar]
- 36.Gough L., Osenberg C. W., Gross K. L., Collins S. L., Fertilization effects on species density and primary productivity in herbaceous plant communities. Oikos 89, 428–439 (2000). [Google Scholar]
- 37.Cadotte M. W., Arnillas C. A., Livingstone S. W., Yasui S. L. E., Predicting communities from functional traits. Trends Ecol. Evol. 30, 510–511 (2015). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.