Abstract
Background:
Characterization of pharmacokinetics (PK) is lacking for vaginal progesterone in pregnancy. Dosing of vaginal progesterone for preterm birth prevention has been empirical. Due to pregnancy-related changes in vaginal and uterine blood flow, hepatic metabolism, renal clearance, and endogenously elevated serum progesterone, studies outside of pregnancy may not be applicable. The lack of the PK profile of vaginally administered progesterone in pregnancy limits the ability to define the exposure:response relationship needed to optimize dosing, which has implications for its use in research and clinical care regarding management of short cervix, prevention of recurrent preterm birth, and prevention of recurrent miscarriage.
Objective:
This was a study to establish the feasibility of using serum progesterone to establish basic pharmacokinetic parameters of vaginal progesterone in pregnancy for preterm birth prevention.
Study Design:
This is a prospective study of 6 low risk singletons 18 0/7- 23 6/7 weeks’ with BMI 20-40. Exclusion criteria were current vaginitis, abnormal pap smear, prescription medication use, cervical length ≤25mm, prior preterm birth, and contraindication to progesterone. Participants received a single dose of 200mg micronized vaginal progesterone and serum progesterone levels were evaluated every two hours from 0 to 12hours and then 24 hours post-dose. Primary outcome was concentration/time profile of serum progesterone.
Results:
Median maternal age was 27 [21.5-33.3] years, median BMI was 26.5 [23.3-29.0] kg/m2, and median gestational age was 22.9 [21.0-23.4] weeks.Median baseline serum progesterone was 47[40 to 52] ng/ml, median peak concentration was 54 [48 to 68]ng/ml, median time to peak was 12 [4 to 15]hours. There was a trend in rising serum progesterone over baseline with a median ΔCmax of 11ng/ml and interquartile range 2 to 22. Median percent change from baseline was an increase by 24% IQR [4% to 53%]. However, there was no clear elimination phase and the median area under the curve was 112ng*h/ml with an IQR of −43 to 239.
Conclusion:
Unlike in non-pregnant individuals, administration of vaginal progesterone in pregnant individuals only minimally impacts systemic exposure. There is a limited trend of rising serum progesterone over baseline levels with significant inter-individual variability. Serum progesterone is unlikely to be a good candidate for establishing pharmacokinetics or dosing of vaginal progesterone in pregnancy for preterm birth prevention.
INTRODUCTION
Vaginal progesterone therapy has been demonstrated to reduce the risk of preterm birth1–3. However, the benefit is not universal and among women with short cervix up to 30% may still have an early preterm birth4,5. Given the limited understanding of the pharmacology or mechanism of action of vaginal progesterone, we have little insight into this discrepant response, whether its related to patient characteristics and comorbidities outside of a clinical trial setting, or individual variability in dose:response relationship of vaginal progesterone. Pharmacokinetics is the study of the body does to a drug-absorption, distribution, metabolism, and clearance of a medication, and often presented as the concentration of a medication in a tissue compartment (ie serum, CSF etc), over time following a specified dosing regimen. Pharmacodynamics is the description of what the drug does to the body-eg the change in some physiologic endpoint such as heart rate relative to the concentration of drug in the blood. Dosing for vaginal progesterone for infertility therapy was established using serum progesterone for pharmacokinetic studies and changes in endometrial thickness or endometrial histology to evaluate pharmacodynamics6–8. The goal of progesterone therapy in in vitro fertilization (IVF) is for luteal phase support and development of an adequate secretory endometrial transformation for implantation; which is clearly distinct from the goals of progesterone therapy in preterm birth prevention. Dosing regimens for treatment of a short cervix are empiric based on IVF therapy, without consideration of the medication’s pharmacology. The most commonly used medications used in women with a short cervix are Prometrium (tablet) 200 mg daily or Crinone (4% or 8% gel)1.
Full characterization of drug pharmacokinetics, a bedrock of modern therapeutics, is lacking in vaginal progesterone in pregnancy. While the strongest evidence for benefit of vaginal progesterone is in management of short cervix2,9, vaginal progesterone has been studied and found to be beneficial for use in pregnancy for a variety of indications including prevention of recurrent miscarriage10, prevention of recurrent preterm birth3. Although the pharmacokinetics and pharmacodynamics have been extensively for the purpose of infertility therapy, there is also no established pharmacodynamic endpoint akin to endometrial thickness used for infertility that is established for vaginal progesterone therapy for short cervix, recurrent preterm birth prevention, or prevention of recurrent miscarriage11. This handicaps attempts to identify dose regimens without exhaustive empiric testing of various dose regimens. Studies in non-pregnant women demonstrate that relative to other routes of administration, vaginal progesterone resulted in a relatively lower peak serum progesterone but higher endometrial progesterone level suggesting a “uterine first pass effect” to vaginally administered progesterone6,12,13. In non-pregnant individuals although the time to peak serum progesterone levels differs, overall bioavailability between oral and vaginal progesterone is similar14. However, the bioavailability of vaginal progesterone is posited to be dependent on absorption rather than clearance8,15, thus the relatively increased vaginal and uterine blood flow in pregnancy could have significant implications for absorption, either leading to increased systemic absorption due to overall increased vaginal absorption or an increased uterine first pass effect and potentially reduced systemic absorption.
Additionally, there is a significant endogenous elevation of serum progesterone in pregnancy16, higher than the mean systemic levels achieved with vaginal progesterone administration in non-pregnant individuals15,17–20. Given the endogenously elevated levels of serum progesterone in pregnancy, its use as a pharmacokinetic marker in pregnancy has not been established. The purpose of this pharmacokinetic study was to establish the feasibility of using serum progesterone to define basic pharmacokinetic parameters of vaginal progesterone in the second trimester of pregnancy.
METHODS
This is a prospective study to establish the baseline pharmacokinetics of vaginal progesterone in the second trimester of pregnancy in low risk singletons. This study was approved by the Thomas Jefferson University IRB and registered in clinicaltrials.gov ().
Participants
Inclusion criteria included pregnant singletons ≥18years old, gestational age 18 0/7- 23 6/7 weeks, BMI 20-40, no prior preterm birth. Exclusion criteria included: contraindication to progesterone therapy, adverse reaction to progesterone, medical comorbidity requiring daily medication including hypertension, diabetes, opioid use disorder, thyroid disease, major fetal anomaly or known chromosomal anomaly, symptoms of vaginal bleeding, preterm labor, or rupture of membranes, any prior progesterone use in the pregnancy, active vaginitis, illicit substance use, known or suspected malignancy of breast or genital organs, abnormal pap smear including HPV+, and cervical length ≤25mm.
Study Protocol
This study was conducted in the Thomas Jefferson University Clinical Research Unit. Participants were advised to avoid sexual intercourse or any vaginal products as well as any grapefruit products 24 hours prior to and during the study period. Participants underwent baseline vital signs, weight, and screening to confirm eligibility. At hour zero, serum progesterone was drawn and then 200mg micronized vaginal progesterone suppository (Virtus Pharmaceutical, Bristol, PA) was placed in the posterior fornix. Micronized vaginal progesterone is bioidentical to human progesterone hormone and the micronization increases its bioavailability11,21. Subsequently, serum progesterone was drawn every two hours from 2-12 hours and then at 24 hours. The time frame for serum progesterone sampling was based on studies in non-pregnant individuals demonstrating peak serum progesterone levels within 4-8 hours15,17,19,20. In addition to serum progesterone levels, baseline demographic information was collected along with survey for side effects.
Outcome Measures
Primary outcome was the plasma concentration/time profile of serum progesterone. Progesterone concentration assayed with validated electrochemiluminescence immunoassay (Cobas e 602, Roche, Indianapolis, IN) with a reported precision coefficient of variation (CV) of 2.4%22. Area under the curve (AUC) calculated with trapezoid technique from hour 0 to hour 24 post dose. Other outcomes include basic pharmacokinetic parameters such as peak serum progesterone concentration (Cmax), peak change from baseline serum progesterone level Δmax), time to peak serum progesterone level (Tmax). Compartmental analysis with single compartment model using NONMEM software (ICON development Solutions, Ellicot City, MD) was planned. In contrast to noncompartmental analysis that illustrates a simple concentration/time plot, compartmental analysis takes into consideration the distribution of a medication in body tissues (compartments) and can be used for computer modeling and simulation. Additionally, Pearson correlation coefficient was used to determine whether there was a relationship between AUC and demographic variables including age, race, BMI. SPSS v. 25.0 was used for statistical analysis. P<0.05 was considered significant
Sample Size
This was a study to establish the feasibility of using serum progesterone to establish basic pharmacokinetic parameters of vaginal progesterone in pregnancy. An empiric sample of 6 was targeted.
RESULTS
Median maternal age was 27 [21.5-33.3] years, median BMI was 26.5 [23.3-29.0] kg/m2, and median gestational age was 22.9 [21.0-23.4] weeks. Baseline data (age, BMI, gestational age, baseline serum progesterone) were normally distributed based on Shapiro-Wilk test of normality. One participant (ID 1, Figure 1) had a peak plasma level (Cmax) which deviated significantly (>2SD) from other participants’, thus median values were used for summary statistics. Cmax was not normally distributed (Shapiro-Wilk test of normality p=.005).
Figure 1:

Change in serum progesterone concentration/time plot of 6 pregnant women 18–23 weeks’ gestation after 200mg micronized vaginal progesterone. Serum progesterone sampled every two hours from hour zero (pre dose) over 12 hours post dose and then at 24 hours post dose.
Overall, median baseline serum progesterone was 47[40 to 52] ng/ml, median peak concentration (Cmax) was 54 [48 to 68]ng/ml, median time to peak was 12.0 [4 to 15] hours. There was a trend in rising serum progesterone over baseline with a median ΔCmax of 11ng/ml and interquartile range 2 to 22ng/ml. Median percent change from baseline was an increase by 24% IQR [4% to 53%]. However, there was no clear elimination phase and median area under the curve was 112ng*h/ml with an IQR of −43 to 239ng*h/nl due to fluctuation above and below initial baseline serum progesterone value after micronized progesterone administration. Median change in serum progesterone from baseline concentration time plot in Figure 2. Compartmental analysis was not able to be conducted due to limited impact on serum progesterone levels and lack of elimination phase.
Figure 2:
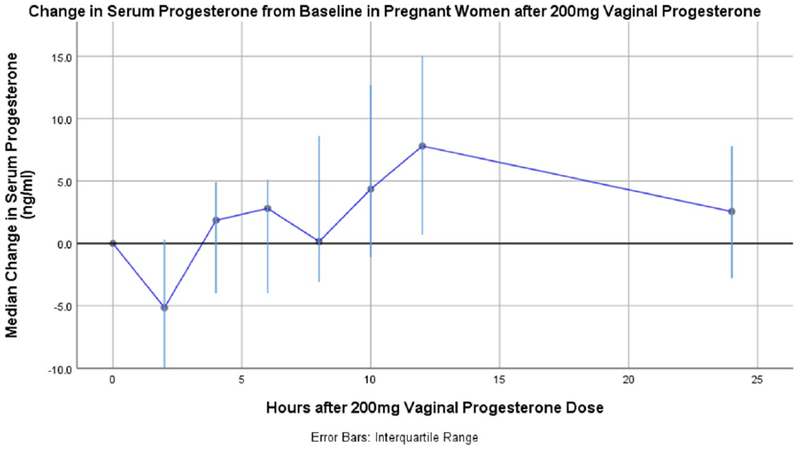
Median change in serum progesterone from baseline concentration/time plot of six pregnant women 18–23 week’s gestation after 200mg micronized vaginal progesterone.
There was no significant correlation between age (r=−0.20, p=0.97), BMI (r=−.126, p=0.81), gestational age (r=.12, p=.82), or race (r=−.15, p=0.77) and area under the curve.
One participant reported a mild headache, and another reported hives on legs thought to be related to bed sheets and not progesterone. There were no serious adverse effects.
COMMENT
Principal Findings
We have identified that even with endogenously elevated serum progesterone, vaginal progesterone administration results in a modest rise in serum progesterone over baseline Δmax of 11ng/ml and interquartile range 2 to 22), although the effect is very modest and inconsistent, with significant inter-individual variability. Overall, even with a trend of a modest peak increase from baseline, serum progesterone levels remained with the normal range for pregnancy. Although serum progesterone levels have been used to establish pharmacokinetic parameters and compare dosing of micronized vaginal progesterone outside of pregnancy, it is unlikely to be useful for such studies when used in pregnancy for preterm birth prevention.
Results in Context
Although other pharmacokinetic studies on micronized vaginal progesterone in pregnancy were not identified, we found Cmax and AUC from studies in non-pregnant women were similar to our values of change from baseline in pregnancy (Table 2). Our values regarding median baseline progesterone are consistent with reported norms; median serum progesterone in the second trimester of pregnancy is ~47.5ng/ml22. However with our study there is notably more inter-individual variation. Similarly sized studies in non-pregnant individuals also noted significant inter individual variability14,23. Coefficient of variation in our study was almost double that of studies outside of pregnancy (0.67 in Norman et al14 and 1.46 in our study). Of note, with vaginal progesterone therapy, Cmax in non-pregnant women was less than the normal levels of endogenous progesterone in the second trimester of pregnancy. In contrast to studies outside of pregnancy, most of which noted a Tmax <4hrs14,15,19, the Tmax in pregnancy was delayed until on average 12 hours.
Table 2:
Summary of literature on pharmacokinetic studies of micronized vaginal progesterone in non-pregnant women and this study on pregnant women in the second trimester. AUC: area under curve; Cmax: maximum concentration; Tmax: time to maximum concentration. Data presented as mean±SD or median[IQR]. AUC subscript indicates time over which AUC calculated after one dose.
| Study | Study Population | Dose | AUC (ng*h/ml) | Cmax (ng/ml) | Tmax (h) |
|---|---|---|---|---|---|
| Paulson 20148 | Healthy, premenopausal women (N=9) | 200mg micronized vaginal progesterone insert (PVI Ferring Pharmaceutials), rapid disintegrating | 138±35 (0-24) | 11.5±3.9 | 12.0±4.9 |
| Norman 199114 | Healthy, premenopausal women (N=10) | 400mg (Cyclogest suppository, Cox Pharmaceuticals) | 308±208(0-96) | 28±18.7 | 3.1 |
| Erny 198919 | Healthy, premenopausal women (N=6) | 100mg (Utrogestan) | 88.8 [49.12-151.7] (0-24) | 8.5 [5.8-12.6] | 2.8 [2-4] |
| Study Number 00532320 | Healthy post menopausal women (N=18 | 100mg (Utrogestan) | 209.6±113.7^(0-96) | 6.8±1.6^ | 9.0±7.1 |
| Archer 199515 | Healthy post menopausal women (N=10) | 100mg (Zetachron) | 165.6±76.9(0-24) | 14.5±4.6 | 3.2±12 |
| Boelig 2018 | Healthy pregnant singletons 18-23 weeks’ gestation (N=6) | 200mg (Virtus Pharmaceutical) | 112 [−43 to 239]* (0-24) | 11 [2 to 22]* | 12 [4 to 15] |
values are change in serum progesterone from baseline.^Converted from original value using 314.46g/mol
There are some notable findings in our results. First, after dosing there is change in serum progesterone both above and below baseline, likely related to natural hormonal variation. Indeed historical studies suggest although there is no consistent diurnal trend to serum progesterone in prior to the late third trimester24,25, there is short term variability in serum progesterone and serum progesterone may transiently decrease by ~15% within an hour after a meal25. Participants in this study were provided breakfast, lunch, and dinner; the progesterone declined one hour after breakfast. The vaginally administered progesterone would not have been systemically absorbed by that time based on the Tmax data provided and the rate of change of systemic levels. A previous study in non-pregnant individuals also noted there was sometimes decline from baseline after a dose20. Second, one participant had a significantly higher peak than the rest; this is consistent with studies outside of pregnancy14 which demonstrated a wide range of Cmax.
Oral progesterone has been studied in pregnant and non-pregnant women. Oral progesterone outside of pregnancy has similar systemic bioavailability as vaginal progesterone14 and daily oral progesterone therapy in pregnancy is associated with a two-fold increase in serum progesterone compared to placebo in the second and third trimester in one study and meta analysis26,27. Our results suggest that, in contrast to oral progesterone administration in pregnancy, the systemic bioavailability of vaginal progesterone administration is reduced in pregnancy, possibly due to altered uterine first pass effect. The pharmacokinetic profile of vaginal progesterone has been reported to be dependent on absorption rather than clearance8,15. With the increased vaginal and uterine blood flow in pregnancy uterine first pass effect may be even more marked, further limiting the systemic impact of vaginal progesterone.
Research Implications
Given that our results suggest the impact of vaginal progesterone on systemic serum progesterone is limited and vaginal progesterone has specifically been shown to be effective in the setting of short cervix, our data supports the theory that the mechanism of action of vaginal progesterone is local11 and thus local markers for vaginal progesterone absorption and efficacy should be utilized in future pharmacologic studies. Unfortunately methods to measure local progesterone uptake, such as endometrial or myometrial sampling, used in studies of non-pregnant individuals6,12,13 are not feasible for use in an ongoing pregnancy. If local progesterone uptake cannot be directly measured in pregnancy, indirect cervicovaginal markers of effect should be explored to better understand the pharmacology of vaginal progesterone in pregnancy and to develop rational dosing models. Studies on surrogate markers of vaginal micronized progesterone treatment effect are limited. One study evaluated changes in cervicovaginal inflammatory markers following 400mg daily micronized vaginal progesterone treatment in pregnant women with short cervix and found significant changes in IL-1B and IL-428. Another evaluated vaginal microbiome and found vaginal progesterone did not result in a change in local microbiome29. Neither study evaluated a dose:response relationship with these markers. Thus there remains a need for pharmacologic study of vaginal progesterone in the setting of preterm birth prevention with either direct or indirect endpoints to establish a dose:response relationship and generate a rational dosing schema.
Strengths and Limitations
This study has a number of strengths. This is the first published study examining the pharmacokinetics of micronized vaginal progesterone in the second trimester of singleton pregnancies. Strict inclusion criteria and similar baseline characteristics limited the role gestational age or BMI may have in influencing interpatient variability. We have demonstrated the limited systemic impact of vaginal progesterone in pregnancy, highlighting the likely local mechanism of action and need to identify local markers of vaginal progesterone efficacy.
There are some limitations to this study. The sample size is small, although similar to other published studies on vaginal progesterone pharmacokinetics12,23. Due to lack of available pharmacokinetic data in pregnancy, the timing of serum progesterone sampling was designed based on vaginal progesterone pharmacokinetics in non-pregnant individuals. Thus data on the change in serum progesterone from hours 12-24 post dose are limited. Additionally, we do not have data on steady state levels of progesterone with repeat administration and this additional information may be informative, although one study in twins did not find any significant change in serum progesterone after 4 weeks of vaginal progesterone therapy30. Finally, we were unable to conduct a paired control comparison to assess endogenous hourly changes in serum progesterone without any intervention.
Clinical Implications
Vaginal progesterone is not FDA approved for preterm birth prevention31 but is FDA approved for infertility therapies, and brands such as Crinone and Endometrin were approved for use in pregnancy with pharmacokinetic studies in non-pregnant individuals. National guidelines recommend vaginal progesterone therapy for preterm birth prevention in select populations and also acknowledge the limited data on optimal dosing or formulation1. Our results demonstrate vaginal progesterone administration results in a very limited impact on systemic serum progesterone levels, which may reflect a more marked uterine first pass effect in pregnancy. Caution should be used when applying pharmacokinetic studies outside pregnancy to the use of vaginal progesterone in pregnancy, especially in the second and third trimester. Future studies on dose dependent markers of vaginal progesterone efficacy need to be established to optimize our ability to dose vaginal progesterone and prevent preterm birth.
Conclusion
Serum progesterone is unlikely to be a reliable marker of vaginal progesterone pharmacokinetics or efficacy and future pharmacologic studies on local markers of absorption and effect need to be explored.
TABLE 1:
Pharmacokinetic parameters of 6 pregnant women 18–23 weeks’ after 200mg micronized vaginal progesterone. Serum progesterone sampled every two hours at hour zero (pre dose) over 12 hours post dose and then at 24 hours post dose. AUC: area under curve; Cmax: maximum concentration; Tmax: time to maximum concentration; IQR: interquartile range. Data presented as median [IQR].
| Median Age (years) | 27.0[21.5-33.3] |
| Median BMI(kg/m2) | 26.5 [23.3-29.0] |
| Median Gestational Age (wk) | 22.9 [21.0-23.4] |
| Median Baseline Serum Progesterone [IQR] (ng/ml) | 47[40 to 52] |
| Median Cmax [IQR] (ng/ml) | 54 [48 to 68] |
| Median Tmax [IQR] (hr) | 12 [4 to 15] |
| Median ΔCmax from Baseline [IQR] | 11 [2 to 22] |
| Median % change of ΔCmax from Baseline [IQR] | 24 [4 to 53] |
| Median AUC [IQR] ng*h/ml | 112 [−43 to 239] |
Condensation:
The pharmacokinetics of 200mg micronized vaginal progesterone were determined through serum progesterone. Given the limited change in serum concentrations and high variability, it is unlikely that serum concentrations can be used to optimize a dosing regimen or to serve as a surrogate marker for efficacy.
AJOG at a glance:
A. Why was this study conducted?
Dosing of micronized vaginal progesterone for preterm birth prevention is empiric and based on pharmacokinetic studies in non-pregnant individuals. However, given the elevated endogenous progesterone in pregnancy, altered vaginal/uterine blood flow, and altered hepatic metabolism/renal clearance, progesterone disposition and systemic exposure is expected to differ in pregnancy. This study was conducted to establish the feasibility of using serum progesterone to establish basic pharmacokinetic parameters of vaginal progesterone in pregnancy for preterm birth prevention.
B. Key Findings
Following the administration of 200mg micronized vaginal progesterone in pregnant singletons 18-23 weeks’ gestation, we attempted to define drug pharmacokinetics. A modest change was seen in serum progesterone concentrations but there was significant inter-individual variability.
C. What does this add to what is known?
Systemic progesterone concentrations do not appear to be useful in establishing a therapeutic dosing regimen for vaginal progesterone.
ACKNOWLEDGEMENTS
This study was funded by the Thomas Jefferson University Maternal Fetal Medicine Fellow Research Fund. Rupsa C. Boelig is supported by NIH grant T32GM008562.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures:
The authors report no conflicts of interest financial or otherwise. Rupsa C. Boelig is supported by NIH grant T32GM008562.
REFERENCES
- 1.Committee on Practice Bulletins—Obstetrics, The American College of Obstetricians and Gynecologists. Practice bulletin no. 130: prediction and prevention of preterm birth. Obstet Gynecol. 2012;120(4):964–973. doi: 10.1097/AOG.0b013e3182723b1b [DOI] [PubMed] [Google Scholar]
- 2.Romero R, Conde-Agudelo A, Da Fonseca E, et al. Vaginal Progesterone for Preventing Preterm Birth and Adverse Perinatal Outcomes in Singleton Gestations with a Short Cervix: A Meta-Analysis of Individual Patient Data. Am J Obstet Gynecol. 2018;218(2):161–180. doi: 10.1016/j.ajog.2017.11.576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Saccone G, Khalifeh A, Elimian A, et al. Vaginal progesterone compared to intramuscular 17-alpha-hydroxyprogesterone caproate for prevention of recurrent spontaneous preterm birth in singleton gestations: a systematic review and meta-analysis of randomized controlled trials. Ultrasound Obstet Gynecol. August 2016. doi: 10.1002/uog.17245 [DOI] [PubMed] [Google Scholar]
- 4.Dugoff L, Berghella V, Sehdev H, Mackeen AD, Goetzl L, Ludmir J. Prevention of Preterm Birth with Pessary in Singletons (PoPPS): a randomized controlled trial. Ultrasound Obstet Gynecol. September 2017. doi: 10.1002/uog.18908 [DOI] [PubMed] [Google Scholar]
- 5.Boelig RC, Hecht NBV. Cervical length <15mm is the most important risk factor for early preterm birth in women with short cervix treated with vaginal progesterone. In: Society for Maternal Fetal Medicine Annual Pregnancy Meeting ; 2018. [Google Scholar]
- 6.Tavaniotou A, Smitz J, Bourgain C, Devroey P. Comparison between different routes of progesterone administration as luteal phase support in infertility treatments. Hum Reprod Update. 2000;6(2):139–148. http://www.ncbi.nlm.nih.gov/pubmed/10782572. Accessed October 3, 2017. [DOI] [PubMed] [Google Scholar]
- 7.Pasquale SA, Bachmann GA, Foldesy RG, Blackwell RE, Levine JP. Peripheral progesterone (P) levels and endometrial response to various dosages of vaginally administered P in estrogen-primed women. Fertil Steril. 1997;68(5):810–815. doi: 10.1016/S0015-0282(97)00329-4 [DOI] [PubMed] [Google Scholar]
- 8.Paulson RJ, Collins MG, Yankov VI. Progesterone pharmacokinetics and pharmacodynamics with 3 dosages and 2 regimens of an effervescent micronized progesterone vaginal insert. J Clin Endocrinol Metab. 2014;99(11):4241–4249. doi: 10.1210/jc.2013-3937 [DOI] [PubMed] [Google Scholar]
- 9.Campbell S Prevention of spontaneous preterm birth: universal cervical length assessment and vaginal progesterone in women with a short cervix: time for action! Am J Obstet Gynecol. 2018;218(2):151–158. doi: 10.1016/j.ajog.2017.12.222 [DOI] [PubMed] [Google Scholar]
- 10.Haas DM, Hathaway TJ, Ramsey PS. Progestogen for preventing miscarriage in women with recurrent miscarriage of unclear etiology. Cochrane Database Syst Rev. 2018;(10). doi: 10.1002/14651858.CD003511.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O’Brien JM, Lewis DF. Prevention of preterm birth with vaginal progesterone or 17-alpha-hydroxyprogesterone caproate: A critical examination of efficacy and safety. Am J Obstet Gynecol. 2016;214(1):45–56. doi: 10.1016/j.ajog.2015.10.934 [DOI] [PubMed] [Google Scholar]
- 12.Cicinelli E, de Ziegler D, Bulletti C, Matteo MG, Schonauer LM, Galantino P. Direct transport of progesterone from vagina to uterus. Obstet Gynecol. 2000;95(3):403–406. http://www.ncbi.nlm.nih.gov/pubmed/10711552. Accessed October 3, 2017. [DOI] [PubMed] [Google Scholar]
- 13.Bulletti C, de Ziegler D, Flamigni C, et al. Targeted drug delivery in gynaecology: the first uterine pass effect. Hum Reprod. 1997;12(5):1073–1079. http://www.ncbi.nlm.nih.gov/pubmed/9194669. Accessed October 3, 2017. [DOI] [PubMed] [Google Scholar]
- 14.Norman T, Morse C, Dennerstein L. Comparative bioavailability of orally and vaginally-administered progesterone. Fertil Steril. 1991;56(6):1034–1039. doi: 10.1016/S0015-0282(16)54713-X [DOI] [PubMed] [Google Scholar]
- 15.Archer DF, Fahy GE, Viniegra-Sibal A, Anderson FD, Snipes W, Foldesy RG. Initial and steady-state pharmacokinetics of a vaginally administered formulation of progesterone. Am J Obstet Gynecol. 1995;173(2):471–478. doi: 10.1016/0002-9378(95)90268-6 [DOI] [PubMed] [Google Scholar]
- 16.Ogueh O, Clough A, Hancock M, Johnson MR. A longitudinal study of the control of renal and uterine hemodynamic changes of pregnancy. Hypertens Pregnancy. 2011;30(3):243–259. doi: 10.3109/10641955.2010.484079 [DOI] [PubMed] [Google Scholar]
- 17.Norman TR, Morse CA, Dennerstein L. Comparative bioavailability of orally and vaginally administered progesterone**Supported by Hoechst, Hounslow, United Kingdom. Fertil Steril. 1991;56(6):1034–1039. doi: 10.1016/S0015-0282(16)54713-X [DOI] [PubMed] [Google Scholar]
- 18.Miles RA, Paulson RJ, Lobo RA, Press MF, Dahmoush L, Sauer MV. Pharmacokinetics and endometrial tissue levels of progesterone after administration by intramuscular and vaginal routes: a comparative study. Fertil Steril. 1994;62(3):485–490. http://www.ncbi.nlm.nih.gov/pubmed/8062942. Accessed October 3, 2017. [DOI] [PubMed] [Google Scholar]
- 19.Erny R; Simoncini C; Chastlliere N; de Lingeres B Variations de la progesterone plamatique induites pa l’adminstration vaginale d’Utrogestan. J Gynecol Obs Reprod Biol. 1989;18(2):229–234. [PubMed] [Google Scholar]
- 20.(Reviewer) BK. Clinical Pharmacology and Biopharmaceutics Review: NDA 20–756 Crinone (progesterone gel). Presented at the: 1997.
- 21.Solvay Pharmaceuticals I. Prometrium (progesterone, USP). 2009.
- 22.Progesterone III-Roche Cobas.; 2015.
- 23.Blake EJ, Norris PM, Dorfman SF, Longstreth J, Yankov VI. Single and multidose pharmacokinetic study of a vaginal micronized progesterone insert (Endometrin) compared with vaginal gel in healthy reproductive-aged female subjects. Fertil Steril. 2010;94(4):1296–1301. doi: 10.1016/j.fertnstert.2009.06.014 [DOI] [PubMed] [Google Scholar]
- 24.Runnebaum B; Rieben W; Bierwirth-von Munsterm Z Circadian variations in plasma progesterone in the luteal phase of the menstrual cycle and during pregnancy. Acta Endocrinol. 1972;69(4):731–738. [DOI] [PubMed] [Google Scholar]
- 25.Nakajima ST; McAuliffe T; Gibson M The 24-hour pattern of the levels of serum progesterone and immunoreactive human chorionic gonadotropin in normal early pregnancy. J Clin Endocrinol Metab. 1990;71(2):345–353. [DOI] [PubMed] [Google Scholar]
- 26.Ashoush S, El-Kady O, Al-Hawwary G, Othman A. The value of oral micronized progesterone in the prevention of recurrent spontaneous preterm birth: a randomized controlled trial. Acta Obstet Gynecol Scand. 2017;96(12):1460–1466. doi: 10.1111/aogs.13236 [DOI] [PubMed] [Google Scholar]
- 27.Boelig RC, Della Corte L, Ashoush S, et al. Oral progesterone for the prevention of recurrent preterm birth: systematic review and metaanalysis. Am J Obstet Gynecol MFM. 2019;1(1):1–13. doi: 10.1016/j.ajogmf.2019.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chandiramani M, Seed PT, Orsi NM, et al. Limited Relationship between Cervico-Vaginal Fluid Cytokine Profiles and Cervical Shortening in Women at High Risk of Spontaneous Preterm Birth. PLoS One. 2012;7(12):1–11. doi: 10.1371/journal.pone.0052412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kindinger LM, Bennett PR, Lee YS, et al. The interaction between vaginal microbiota, cervical length, and vaginal progesterone treatment for preterm birth risk. Microbiome. 2017;5(1):6. doi: 10.1186/s40168-016-0223-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Johnsson VL, Pedersen NG, Worda K, et al. Plasma progesterone, estradiol, and unconjugated estriol concentrations in twin pregnancies: Relation with cervical length and preterm delivery. Acta Obstet Gynecol Scand. 2018:0–2. doi: 10.1111/aogs.13464 [DOI] [PubMed] [Google Scholar]
- 31.Romero R, Stanczyk FZ. Progesterone is not the same as 17α-hydroxyprogesterone caproate: Implications for obstetrical practice. Am J Obstet Gynecol. 2013;208(6):421–426. doi: 10.1016/j.ajog.2013.04.027 [DOI] [PMC free article] [PubMed] [Google Scholar]


