Abstract
Several lines of evidence support the hypothesis that abnormally elevated brain levels of kynurenic acid (KYNA), a metabolite of the kynurenine pathway (KP) of tryptophan degradation, play a pathophysiologically significant role in schizophrenia and other major neurodevelopmental disorders. Studies in experimental animals suggest that KP impairments in these diseases may originate already in utero since prenatal administration of KYNA’s bioprecursor kynurenine leads to biochemical and structural abnormalities, as well as distinct cognitive impairments, in adulthood. As KP metabolism during pregnancy is still insufficiently understood, the present study was designed to examine the de novo synthesis of KYNA and 3-hydroxykynurenine (3-HK), an alternative biologically active product of kynurenine degradation, in tissue slices obtained from pregnant mice on gestational day (GD) 18. Fetal brain and liver, placenta, and maternal brain and liver were collected, and the tissues were incubated in vitro in the absence or presence of micromolar concentrations of kynurenine. KYNA and 3-HK were measured in the extracellular milieu. Basal and newly produced KYNA was detected in all cases. As KYNA formation exceeded 3-HK production by 2–3 orders of magnitude in placenta and maternal brain, and as very little 3-HK neosynthesis was detectable in fetal brain tissue, detailed follow-up experiments focused on KYNA only. Fetal brain produced 3–4 times more KYNA than maternal brain and placenta, though less than maternal and fetal liver. No significant differences were observed using tissues obtained on GD 14 compared to GD 18. Pharmacological inhibition of KYNA’s main biosynthetic enzymes, kynurenine aminotransferase (KAT) I and II, revealed qualitative and quantitative differences between the tissues, with a preferential role of KAT I in fetal and maternal brain and of KAT II in fetal and maternal liver. Findings using tissue slices from KAT II knockout mice confirmed these conclusions. Together, these results clarify the dynamics of KP metabolism during pregnancy and provide the basis for the conceptualization of interventions aimed at manipulating cerebral KP function in the prenatal period.
Keywords: Development, Kynurenine, Schizophrenia
Introduction
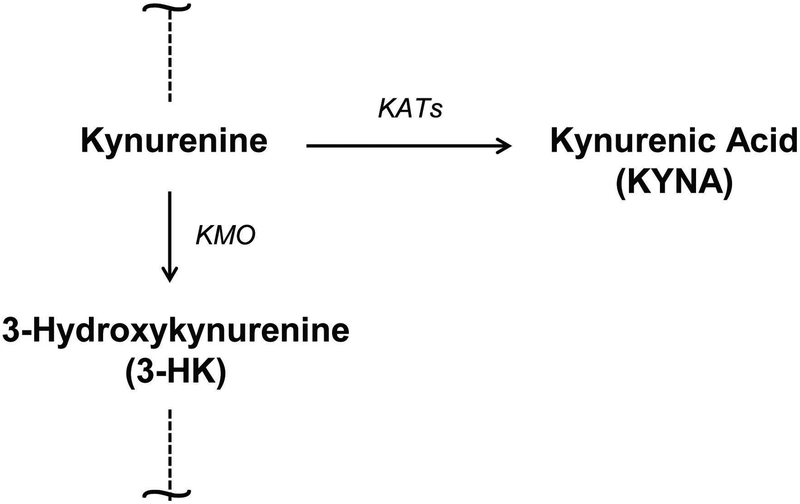
The kynurenine pathway (KP) is the main catabolic route of the essential amino acid tryptophan in mammals. Kynurenine, the first stable KP metabolite (Fig. 1), generates several neuroactive metabolites downstream [1,2]. One of these compounds, kynurenic acid (KYNA), which is produced primarily by irreversible enzymatic transamination of kynurenine [3,4], is an endogenous inhibitor of α7 nicotinic acetylcholine (α7nACh) and N-methyl-D-aspartate (NMDA) receptors and also affects other specific targets [5,6]. In humans, rats, and mice, four kynurenine aminotransferases (KAT I-IV), are able to catalyze the formation of KYNA, with KATI and especially KAT II considered to be most relevant for its rapid synthesis in brain and liver of adult rats and humans [4,7]. Notably, no mechanisms for KYNA degradation appear to exist in mammals [1], and newly synthesized KYNA is promptly liberated into the extracellular milieu [8–10]. In a separate branch of the KP, kynurenine is first degraded to the free radical generator 3-hydroxykynurenine (3-HK) by kynurenine 3-monooxygenase (KMO), and then further to a series of biologically active metabolites, eventually leading to the formation of the abundant cofactor NAD+ [1,2]. Kynurenine therefore occupies a pivotal position in the KP, making the ratio between newly produced KYNA and 3-HK an informative marker of the function of the entire metabolic cascade.
Figure 1:
Simplified illustration of the branching point of the kynurenine pathway of tryptophan degradation. KATs: kynurenine aminotransferases; KMO: kynurenine 3-monooxygenase.
Malfunction of the KP has been plausibly linked to the pathophysiology of several diseases, including a number of notable psychiatric and neurological disorders [1,2,11–15]. Studied most extensively in this regard, KYNA levels (but not 3-HK levels) are elevated in post-mortem brain and cerebrospinal fluid of persons with schizophrenia, a major brain disease that originates early in life [16–20]. In experimental animals, increased brain KYNA concentrations cause several cognitive impairments, which are remarkably similar to those seen in people with schizophrenia [1,21–25]. Notably, and in line with the neurodevelopmental etiology of the disease, prolonged administration of kynurenine to pregnant rats results in elevated brain KYNA (but not 3-HK) levels, as well as distinct cognitive abnormalities, in adult offspring [26].
Interestingly, the mammalian brain normally contains several-fold higher concentrations of kynurenine, KYNA and 3-HK during the fetal period than at any postnatal stage [27–31]. As the cerebral levels of all three of these KP metabolites decrease precipitously immediately after birth [28,31], it is tempting to surmise that they normally serve distinct roles in prenatal brain development. This assumption is indirectly supported by evidence that an impairment of KP metabolism during the prenatal period has adverse consequences [26,32–38], but has not been sufficiently evaluated so far.
In contrast to maternally derived kynurenine and 3-HK, maternal KYNA is not able to cross the placenta [39]. As the administration of tryptophan or kynurenine to pregnant dams readily raises KYNA levels in the fetal brain [26,32,39,40], and as the placenta plays only a minor role in this process [39], fetal KYNA must be synthesized locally. The present study was designed to provide further insights by elaborating the characteristics of the prenatal conversion of kynurenine to KYNA and 3-HK, respectively, ex vivo. To this end, we collected maternal brain and liver, placenta, and fetal brain and liver from pregnant mice on gestational day (GD) 18, and in some cases on GD 14. Slices from the various tissues were then incubated in vitro in the absence or presence of kynurenine, and basal levels and neosynthesis of KYNA and 3-HK were assessed in the extracellular milieu. By elucidating the features of two critical KP branches during pregnancy, our experimental results provided valuable new insights into the dynamics, and by implication the function, of prenatal KP metabolism.
Materials and Methods
Chemicals
KYNA, ascorbic acid, aminooxyacetic acid (AOAA) and glutamine were purchased from Sigma-Aldrich (St. Louis, MO, USA). L-Kynurenine sulfate (“kynurenine”; purity: 99.4%) was obtained from Sai Advantium (Hyderabad, India). The selective KAT II inhibitor BFF-122 [(S)-(−)-9-(4-aminopiperazine-1-yl)-8-fluoro-3-methyl-6-oxo-2,3,5,6-tetrahydro-4H-1-oxa-3a-azaphenalene-5-carboxylic acid] was kindly provided by Dr. Y. Kajii (Mitsubishi-Tanabe Pharma Corporation, Yokohama, Japan). The KMO inhibitor Ro 61–8048 was a generous gift from Dr. W. Fröstl (Novartis, Basel, Switzerland). All other chemicals were obtained from various commercial suppliers and were of the highest available purity.
Animals
Wild-type (WT) FVB/N mice (8–9 weeks) were purchased from Jackson Laboratory (Bar Harbor, ME, USA). KAT II KO FVB/N mice were generated as previously described [41]. Male and female mice were bred in house up to 48 h, and the presence of a copulation plug was confirmed on GDs 1 or 2. The male was then removed, and the female was left undisturbed until the day of the experiment.
Animals were maintained on a 12 h/12 h light/dark cycle in a temperature-controlled room in the animal facility of the Maryland Psychiatric Research Center. Access to food and water was provided ad libitum. All experiments were approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Maryland School of Medicine and followed the ‘Principles of Laboratory Animal Care’ (NIH publication No. 86–23, 1996).
Tissue preparation and incubation
On the day of the experiment, pregnant mice (n = 3–6 per group) were euthanized with CO2, and maternal and fetal brain and liver, as well as placenta, were rapidly removed and placed in oxygenated Krebs-Ringer buffer (117.6 mM NaCl, 4.6 mM KCl, 2.4 mM CaCl2, 2.4 mM MgSO4, 3.1 mM NaH2PO4, 26.2 mM NaHCO3, 11.1 mM glucose, pH 7.4). Ascorbic acid (500 μM) was added to the buffer to avoid non-enzymatic oxidation of kynurenine and its metabolites.
Tissue slices (0.4-mm-thick) were prepared from the maternal forebrain, the entire placenta, the whole fetal brain, one lobe of the maternal liver and the whole fetal liver, using a McIIwain chopper (Mickle Laboratory Engineering, Gomshall, UK). The tissues were immediately immersed in Krebs-Ringer buffer for 20 min, and one slice each from maternal brain, liver or placenta was placed into a well containing 200 μL of buffer, using a multi-well culture plate. Fetal brain and liver slices were directly taken from the oxygenated buffer with a pipette and transferred into the wells (final volume: 200 μL) [42]. Twenty μL of buffer or of a solution containing kynurenine (final concentration: 10 μM or 100 μM) were added, and the tissues were incubated for 1 h at 37°C in a shaking water bath. Separate wells were incubated with kynurenine in the absence of tissue. The culture plate was then placed on ice, and the reaction was terminated by adding 25 μL of 2 N HCl and 25 μL of 25% perchloric acid to each well. The medium was rapidly removed and centrifuged (6,000 × g, 5 min), and the supernatant was subjected to HPLC analysis for KYNA or 3-HK measurement (see below). Tissue slices were homogenized in 200 μL of ultrapure water and frozen at −80°C for protein determination. All assays were performed in duplicates or triplicates. De novo KYNA or 3-HK production was determined by subtracting both endogenous levels (incubation of tissue in the absence of kynurenine) and levels measured following incubation of kynurenine alone (no tissue).
The effect of KAT inhibition was determined using the non-specific aminotransferase inhibitor AOAA (1 mM), the KAT I inhibitor glutamine (1 mM) or the specific KAT II inhibitor BFF-122 (1 mM) (all final concentrations). These compounds were added (20 μL) to the incubation mixture together with kynurenine (10 μM). Controls were obtained by incubating the compounds without tissue.
KYNA analysis
For KYNA determination, 20 μL of the supernatant were applied to a 3 μm C18 reverse phase HPLC column (100 mm × 4 mm; Dr. Maisch GmbH, Ammerbuch, Germany), using a mobile phase containing 50 mM sodium acetate and 3% acetonitrile (pH adjusted to 6.2 with glacial acetic acid) at a flow rate of 0.5 ml/min. Zinc acetate (0.5 M, not pH adjusted) was delivered post-column by a peristaltic pump (Dionex AXP, Thermo Fisher, Waltham, MA, USA) at a flow rate of 0.1 ml/min. In the eluate, KYNA was detected fluorimetrically (excitation: 344 nm, emission: 398 nm; S200a fluorescence detector; Perkin Elmer, Waltham, MA, USA). The retention time of KYNA was approximately 14 min.
3-HK analysis
For 3-HK determination, 20 μL of the supernatant were applied to a 3 μm C18 reverse phase HPLC column (HR-80; 80 mm × 4.6 mm; Thermo Fisher Scientific, Waltham, MA, USA). The mobile phase consisted of 1.5% acetonitrile, 0.9% trimethylamine, 0.59% phosphoric acid, 0.27 mM EDTA and 8.9 mM sodium heptane sulfonic acid. 3-HK was eluted at a flow rate of 0.5 ml/min and detected electrochemically using an HTEC 500 detector (Eicom, San Diego, CA, USA; oxidation potential: + 0.5 V). The retention time of 3-HK was approximately 11 min.
KMO enzyme activity
To determine KMO activity, fetal (1:5, w/v) and maternal (1:25, w/v) brains were homogenized in 100 mM Tris–HCl buffer (pH 8.1) containing 10 mM KCl and 1 mM EDTA. Eighty μL of the preparation were then incubated for 40 min at 37°C in a solution containing 1 mM NADPH, 3 mM glucose-6-phosphate, 1 U/ml glucose-6 phosphate dehydrogenase, 100 μM L-kynurenine, 10 mM KCl and 1 mM EDTA in a total volume of 200 μL. The reaction was stopped by the addition of 50 μL of 6% perchloric acid. Blanks were obtained by adding the KMO inhibitor Ro 61–8048 (100 μM) to the incubation solution. After centrifugation (16,000 × g, 15 min), 20 μL of the resulting supernatant were subjected to HPLC analysis to measure 3-HK, as described above.
Protein measurement
Protein was measured according to the method of Lowry et al. [43] using bovine serum albumin as a standard.
Statistical analysis
All data are expressed as the mean ± SEM. Student’s t-test, one-way or two-way Anova followed by Bonferroni’s post-hoc test, were used to determine significance in all experiments. A p value of <0.05 was considered significant.
Results
Basal levels and de novo production of 3-HK and KYNA: maternal brain, placenta and fetal brain
We first compared the status of 3-HK and KYNA in maternal brain, placenta and fetal brain of WT mice at GD 18 under physiological conditions. Both metabolites were detected in the media of all three tissues following the 1-h incubation (Table 1). Under these basal conditions, 3-HK levels were several-fold higher in medium obtained from fetal brain than from either maternal brain or placenta (each p<0.001, two-way Anova followed by Bonferroni’s post-hoc test). Measured in the same samples as 3-HK, extracellular KYNA concentrations recovered from placental and fetal brain slices were much higher than those recovered from maternal brain tissue (p<0.001 and p<0.01, respectively; two-way Anova followed by Bonferroni’s post-hoc test). Notably, under these basal conditions, fetal brain slices released more 3-HK than KYNA (p<0.001, two-way Anova followed by Bonferroni’s post-hoc test), whereas the placenta released more KYNA than 3-HK (p<0.05, two-way Anova followed by Bonferroni’s post-hoc test). Medium obtained after the incubation of maternal brain slices showed no preference for either metabolite (Table 1).
Table 1:
Basal levels and de novo production of extracellular 3-HK and KYNA in maternal brain, placenta and fetal brain.
| Basal | 10 μM Kynurenineμ |
100 μM Kynurenine |
||
|---|---|---|---|---|
|
Maternal Brain |
3-HK (pmoles/h/mg protein) |
0.21 ± 0.03 | 0 | 0.18 ± 0.12 |
|
KYNA (pmoles/h/mg protein) |
0.13 ± 0.06 | 2.67 ± 0.56 | 22.50 ± 4.58 | |
| Placenta |
3-HK (pmoles/h/mg protein) |
0.33 ± 0.11 | 0 | 0.63 ± 0.20 |
|
KYNA (pmoles/h/mg protein) |
1.12 ± 0.23 | 1.23 ± 0.44 | 20.44 ± 4.06 | |
|
Fetal Brain |
3-HK (pmoles/h/mg protein) |
1.97 ± 0.27 | 0 | 0.04 ± 0.29 |
|
KYNA (pmoles/h/mg protein) |
0.93 ± 0.09 | 7.34 ± 1.23 | 88.78 ± 14.42 |
Basal extracellular levels and de novo production of 3-HK and KYNA in tissue slices obtained from GD 18 WT mice. Neosynthesis was assessed by incubation in the presence of 10 μM or 100 μM kynurenine and was calculated by subtracting basal levels. Data are the mean ± SEM (n=2–6). See text for experimental details and statistical analyses.
In all three tissues, de novo production of KYNA from kynurenine exceeded the neosynthesis of 3-HK (Table 1). Specifically, incubation with 10 μM and 100 μM of the common bioprecursor kynurenine increased KYNA levels linearly, whereas 3-HK formation was very limited. In fact, only small increases in extracellular 3-HK levels were seen even after the tissues were exposed to 100 μM kynurenine. Notably, incubation with kynurenine caused fetal brain slices to release several times more KYNA than the two other tissues (each p<0.001, two-way Anova followed by Bonferroni’s post-hoc test). No clear differences in the minor de novo production of 3-HK were observed between the tissues tested.
Separately, we determined the activity of KMO, which catalyzes 3-HK formation from kynurenine (Fig. 1), in tissue homogenates. This experiment revealed significantly lower enzyme activity in the fetal brain (0.20 ± 0.05 pmoles 3-HK/h/mg tissue; n=3) than in the maternal brain (2.59 ± 0.53 pmoles 3-HK/h/mg tissue; n=3; p<0.01, Student’s t-test), supporting the conclusion that the fetal brain has only a limited capacity to produce 3-HK from its direct bioprecursor.
In light of these findings, subsequent experiments focused exclusively on the neosynthesis of KYNA. To approximate physiological conditions in fetal tissues [28,44], 10 μM of kynurenine was routinely used to drive KYNA production in all following studies.
Basal levels and de novo production of KYNA: fetal and maternal liver
Next, we determined extracellular KYNA following the incubation of tissue slices from fetal and maternal liver (Table 2). Less KYNA was detected in the media obtained from fetal than from maternal tissue (p<0.01, Student’s t-test). Still, the basal extracellular KYNA levels recovered from fetal liver were substantially higher than those measured after the incubation of placental or fetal brain slices (each p<0.001, one-way Anova followed by Bonferroni’s post-hoc test) (cf. Table 1).
Table 2:
Basal levels and de novo production of extracellular KYNA in fetal and maternal liver.
| Fetal Liver | Maternal Liver | |
|---|---|---|
|
Basal (pmoles/h/mg protein) |
3.5 ± 0.4 | 9.7 ± 1.1 |
|
De novo production (pmoles/h/mg protein) |
40.0 ± 7.3 | 91.3 ± 19.1 |
Basal extracellular levels and de novo production of KYNA after incubation of tissue slices from fetal and maternal liver of GD 18 WT mice with 10 μM kynurenine. De novo production indicates values obtained after the subtraction of basal levels. Data are the mean ± SEM (n=4). See text for experimental details and statistical analyses.
Incubation of fetal and maternal liver slices with 10 μM kynurenine significantly raised extracellular KYNA levels in both cases (each p<0.01 vs. basal levels, Student’s t-test), and the effect was greater in maternal tissue (p<0.05, Student’s t-test). However, as observed for basal values, even KYNA neosynthesis in the fetal liver greatly exceeded de novo production in the placenta and in the fetal brain (each p<0.001, one-way Anova followed by Bonferroni’s post-hoc test).
Pharmacological inhibition of KYNA synthesis
To investigate the role of KAT enzymes in KYNA neosynthesis, we tested the effects of the non-specific aminotransferase inhibitor AOAA, glutamine (which inhibits KAT I), and the specific KAT II inhibitor BFF-122 [7,45,46].
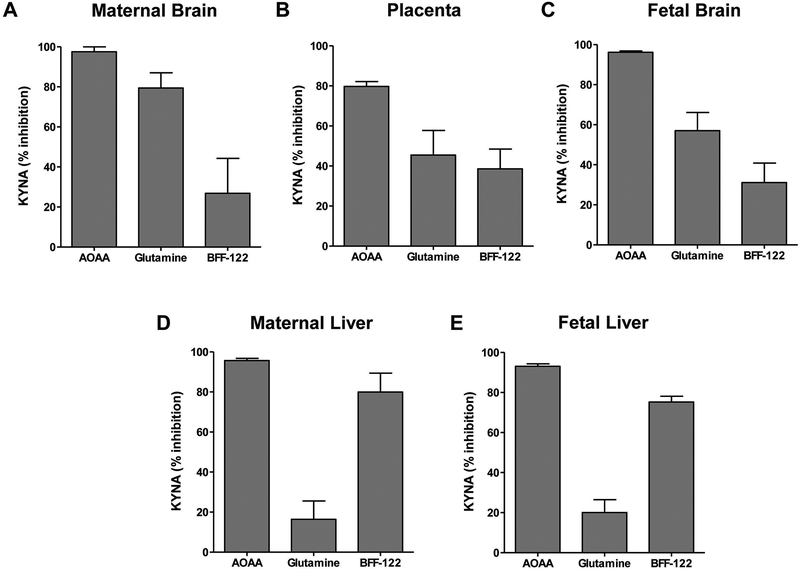
The pharmacological tools used revealed qualitative differences between the tissues analyzed (Fig. 2). Thus, whereas incubation in the presence of AOAA almost completely prevented the de novo production of KYNA in all tissues tested, KYNA synthesis in maternal and fetal brain was preferentially reduced by KAT I inhibition (75% and 57%, respectively; Fig. 2A,C). In contrast, KYNA formation in maternal and fetal liver was mainly KAT II-dependent, as indicated by 80% and 75% inhibition, respectively, in the presence of BFF-122 (Fig. 2D,E). The placenta showed a mixed profile, with glutamine causing 45% inhibition of KYNA synthesis and BFF-122 causing 39% inhibition (Fig. 2B).
Figure 2:
Effects of KAT inhibition on the neosynthesis of KYNA in slices from maternal brain (A), placenta (B), fetal brain (C), maternal liver (D) and fetal liver (E), obtained from WT mice at GD 18 (see Tables 1 and 2 for absolute values). Tissues were incubated in the presence of 10 μM kynurenine for 1 h at 37°C, and experiments were performed as described in the text. AOAA: general KAT inhibitor, glutamine: KAT I inhibitor; BFF-122: selective KAT II inhibitor. Final concentration of all 3 agents: 1 mM. Data are the mean ± SEM (n=4–5).
Studies using tissues from KAT II KO mice
Using standard experimental conditions, we next examined KYNA formation in the absence or presence of 10 μM kynurenine in tissue slices prepared from KAT II KO mice.
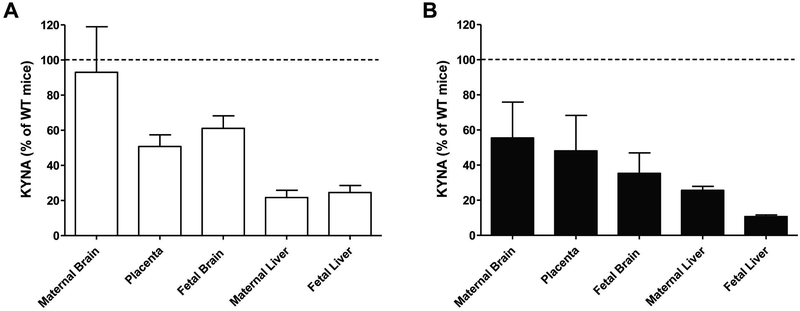
Following incubation of KO tissues under basal conditions, KYNA was always readily detectable in the extracellular milieu, albeit mostly at lower levels than in corresponding samples from WT mice (Fig. 3A). Specifically, slices prepared from KO mice produced about 2-fold (placenta and fetal brain) to 4-fold (fetal and maternal liver) less KYNA than the corresponding tissues from WT animals (cf. Tables 1 and 2). However, the amount of KYNA released from maternal brain slices was not different between WT and KAT II KO mice.
Figure 3:
Basal extracellular levels (A) and de novo KYNA production from kynurenine (10 μM; B), determined in the extracellular milieu of tissue slices from maternal brain, placenta, fetal brain, maternal liver and fetal liver of KAT II KO mice (GD 17/18) following incubation for 1 h at 37°C. Data are expressed as % of the levels in WT mice (see Tables 1 and 2 for absolute values). Experiments were performed as described in the text. De novo production was calculated by subtracting basal levels. Data are the mean ± SEM (n=3–5). See text for statistical analyses.
Incubation with kynurenine increased KYNA synthesis in fetal and maternal brain (each p<0.05, Student’s t-test), and in fetal and maternal liver (p<0.001. Student’s t-test) of KAT II KO mice, but had only a small, non-significant effect in the placenta. Overall, de novo KYNA production in all tissues was lower than in corresponding tissues from WT mice (Fig. 3B; cf. Tables 1 and 2), with the greatest reduction seen in fetal and maternal liver. The genotype difference reached statistical significance in fetal (p<0.01, Student’s t-test) and maternal (p<0.05, Student’s t-test) liver as well as in fetal brain (p<0.05, Student’s t-test).
Taken together with the pharmacological data, these results demonstrated that in mice KAT II is of relatively little relevance in the maternal brain and only partially contributes to KYNA formation in the placenta and the fetal brain, yet plays a major role in KYNA synthesis in fetal and maternal liver.
Basal levels and de novo production of KYNA at GD14
Finally, beginning to consider possible age-related differences during prenatal development, we conducted a pilot study examining KYNA production in WT mice at an earlier stage of gestation (GD 14). As in the preceding experiments, tissues were incubated for 1 h, alone or in the presence of 10 μM kynurenine, and KYNA was measured in the extracellular milieu. Basal KYNA levels, expressed in pmoles/mg protein, were 0.06 ± 0.03 (maternal brain), 0.58 ± 0.08 (placenta), 0.83 ± 0.15 (fetal brain), 2.91 ± 0.38 (fetal liver) and 8.54 ± 1.72 (maternal liver) (n=3 for each tissue). Statistical analyses did not reveal any differences between GD14 and GD18 in basal or newly formed KYNA in any of the tissues examined (p>0.05, Student’s t-tests).
Discussion
The present study demonstrated that maternal and fetal tissues, as well as placenta, all dissected from pregnant mice on GD 18, are capable of metabolizing kynurenine under physiological conditions in vitro. More specifically, our results revealed that the fetal brain, the major tissue of interest in our experiments, readily produces KYNA from kynurenine but is far less efficient in converting kynurenine to 3-HK. Most of our analyses therefore focused on KYNA formation. Using pharmacological and genetic approaches, we found significant differences between the processes that account for the de novo synthesis of KYNA in the various tissues. As kynurenine is the major catabolic product of the essential amino acid tryptophan, and as excessive prenatal exposure to kynurenine has adverse consequences later in life [26,32–35,37], we will discuss the implications of these results from both physiological and pathophysiological perspectives.
One of the major insights gained from the study was that the dynamics of KP metabolism on GD 18 differ both qualitatively and quantitatively, and in both the absence and the presence of kynurenine, between various tissues. Thus, slices from fetal brain liberated substantially more 3-HK than tissue from the placenta and the maternal brain into the extracellular milieu under basal conditions, defined here as a 1-h incubation in regular Krebs-Ringer buffer, but produced almost no 3-HK de novo when kynurenine was added to the incubation mixture. As the content of 3-HK in the mammalian brain is several-fold higher prenatally than postnatally [28,30], and as we detected only very little KMO activity in tissue homogenate prepared from fetal brain, these findings indicate that the rapid accumulation of 3-HK in the fetal brain seen following the administration of kynurenine to the dam in vivo [39,47] is very likely not due to local synthesis but a consequence of increased influx of the metabolite from the dam. This interpretation is in line with the fact that the large neutral amino acid transporter LAT-1, which readily recognizes both kynurenine and 3-HK [48], is highly enriched in the placenta [49]. The placenta is therefore able to accumulate – and then transmit to the fetus – maternally derived 3-HK. Notably, however, and especially under inflammatory conditions that can activate KMO [1], 3-HK may also enter the fetus after it is formed in the placenta itself [50–54].
Parallel analyses of KYNA produced very different results. Under basal conditions, similar amounts of extracellular KYNA were recovered after incubating slices from fetal brain and placenta, in both cases several times more than from maternal brain slices. However, placental slices were substantially less effective than fetal brain slices in producing KYNA from kynurenine de novo. Previous ex vivo work in mice [39], as well as studies using human placental extract [52], had demonstrated that perfusion with kynurenine and tryptophan, respectively, has only a limited effect on KYNA formation within the placenta. Moreover, in vivo work revealed that approximately 40% of KYNA that appeared to derive from the placenta in fact originated from residual maternal blood [39].
Taken together, these results indicate that KYNA synthesis within the placenta is not likely to play a defining role in regulating KYNA levels in the fetus. Notably, acute KYNA neosynthesis in the fetal brain also exceeded KYNA formation and release from maternal brain tissue slices 3–5-fold. Although the fetal liver, which was found here to have an even greater ability to form KYNA from kynurenine, may well contribute to the exceptionally high KYNA levels which are seen in the fetal brain in several mammalian species [27–31,44], these findings therefore support the idea that local biochemical events are largely responsible for determining the fate – and therefore the possible function – of KYNA in the fetal brain. Notably, as the blood-brain barrier, which is largely impermeable to KYNA in adulthood [48], is also functional during the prenatal period [55], even relatively large increases in maternal KYNA are unlikely to reach the fetal brain to a significant extent [39].
In view of the possible functional role of KYNA in the prenatal period, and the fact that a variety of stimuli and environmental insults affect KP metabolism in fetal tissues in vivo [44, 56–60], KYNA synthesis from kynurenine was studied in greater detail. In an initial approach, we examined the respective roles of the two best understood KATs, i.e. KAT I and KAT II, by pharmacological means, using AOAA, a non-specific inhibitor of pyridoxal-5-phosphate-dependent enzymes (including all KATs), glutamine (which inhibits KAT I) and the specific KATII inhibitor BFF-122 as tools [7,45,46]. In agreement with reports that KAT II activity is very low in the immature rat brain [28] and plays only a minor role in the adult mouse brain [7,10], these experiments revealed a major role of KAT I in both fetal and maternal brain tissue. Assessment of KYNA formation in the placenta, in contrast, did not show a clear difference between KAT I and KAT II, even though only the presence of KAT I had been documented in the placenta previously [52,61]. As anticipated [7,41], KYNA neosynthesis in both maternal and fetal liver was found to be almost exclusively catalyzed by KAT II.
Studies with tissues from KAT II KO mice were performed to complement these preliminary pharmacological studies using a genetic approach. In these experiments, we compared both basal release, i.e. the results of incubation in the absence of kynurenine, with the de novo formation of KYNA from kynurenine using tissues from WT and mutant mice. Generally, these studies confirmed that KYNA synthesis in both maternal and fetal liver is largely catalyzed by KAT II, whereas both basal and stimulated KYNA release in slices from maternal brain, fetal brain and placenta are less or – in the case of basal release from maternal brain – not KAT II-dependent.
Together, these findings indicate that the molecular and cellular mechanism(s) that account for KYNA formation during the prenatal period are remarkably complex. Notably, postnatally, the generation of KYNA is influenced by several factors, as KYNA production is stimulated by co-substrates of KAT, such as pyruvate or 2-oxoglutarate [62] and inhibited under hypoglycemic conditions and by amino acids that compete with kynurenine as substrates [63,64]. However, these regulatory mechanisms have not been examined during gestation. Ongoing studies in our laboratory are also designed to test possible additional contributions of the two KATs that were not examined here (KAT III and KAT IV; [4,7]), and to take into account that KYNA production from kynurenine can also occur non-enzymatically, i.e. by oxidative mechanisms [65]. Moreover, while the present study did not indicate significant differences in KYNA formation between tissues collected on GD 14 and GD 18, we are expanding the age range to conduct a comprehensive assessment of KYNA formation during gestation (starting at GD 10) and at several postnatal stages. In these studies, special emphasis will be placed on the developmental pattern of mechanisms that regulate KYNA synthesis and function [62–64], and on age-related differences in the metabolite’s putative receptor targets [5,6], under both physiological and pathological conditions.
KYNA-producing cells in the prenatal brain deserve particular attention. Although neurons are capable of synthesizing KYNA [66–69], there is consensus that non-neuronal cells, specifically astrocytes, are largely responsible for the de novo formation of KYNA in the mammalian brain in adulthood (see ref. 21 for review). In the developing rodent brain, astrocytes are first detected at GD 16 [70] and continue their maturation in the first postnatal weeks [71]. Perinatally, brain KYNA is therefore probably produced by, and released from, glial progenitor cells and, possibly, other cell types. Notably, as shown in vitro using human cortical slices obtained at mid-gestation, nanomolar concentrations of KYNA, in turn, affect progenitor cell proliferation, differentiation and survival [72], and may thus participate actively in cortical circuit formation during brain development [73].
As increased brain KYNA in the fetus and/or in the early postnatal period causes functional and behavioral abnormalities in adulthood [23,26,32–35,37], the present findings also have translational implications and relevance for schizophrenia and other brain disorders whose etiology can be traced to events early in life (see Introduction), despite potential differences between mouse and human pregnancy [74]. Thus, clarification of both enzymatic and non-enzymatic mechanisms that underlie perinatal KYNA formation in different tissues may lead to the conceptualization of fundamentally novel therapeutic interventions. Timely pharmacological treatments designed to mitigate deviant brain KYNA function perinatally may then be targeted to prevent or attenuate the detrimental effects caused by abnormal KP-related genes [47,75] and/or early pathogenic insults affecting KP metabolism [44,52,56–60].
Funding Sources
This work was supported by USPHS grant MH103222 (Silvio O. Conte Center for Translational Research).
Abbreviations:
- α7nACh
α7 nicotinic acetylcholine
- AOAA
aminooxyacetic acid
- BBB
Blood-brain barrier
- GD
Gestational day
- 3-HK
3-Hydroxykynurenine
- KAT
Kynurenine aminotransferase
- KMO
Kynurenine 3-monooxygenase
- KP
Kynurenine pathway
- KYNA
Kynurenic acid
- NMDA
N-methyl-D-aspartate
Footnotes
Statement of Ethics
Animal experiments conform to internationally accepted standards and have been approved by the appropriate institutional review body.
Disclosure Statement
The authors have no conflicts of interest to declare.
References
- 1.Schwarcz R, Bruno JP, Muchowski PJ, Wu HQ: Kynurenines in the mammalian brain: when physiology meets pathology. Nat Rev Neurosci 2012;13:465–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Badawy AA: Tryptophan availability for kynurenine pathway metabolism across the life span: Control mechanisms and focus on aging, exercise, diet and nutritional supplements. Neuropharmacology 2017;112:248–263. [DOI] [PubMed] [Google Scholar]
- 3.Guidetti P, Hoffman GE, Melendez-Ferro M, Albuquerque EX, Schwarcz R: Astrocytic localization of kynurenine aminotransferase II in the rat brain visualized by immunocytochemistry. Glia 2007;55:78–92. [DOI] [PubMed] [Google Scholar]
- 4.Han Q, Cai T, Tagle DA, Li J: Structure, expression, and function of kynurenine aminotransferases in human and rodent brains. Cell Mol Life Sci 2010;67:353–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stone TW, Stoy N, Darlington LG: An expanding range of targets for kynurenine metabolites of tryptophan. Trends Pharmacol Sci 2013;34:136–143. [DOI] [PubMed] [Google Scholar]
- 6.Moroni F, Cozzi A, Sili M, Mannaioni G: Kynurenic acid: a metabolite with multiple actions and multiple targets in brain and periphery. J Neural Transm (Vienna) 2012;119:133–139. [DOI] [PubMed] [Google Scholar]
- 7.Guidetti P, Amori L, Sapko MT, Okuno E, Schwarcz R: Mitochondrial aspartate aminotransferase: a third kynurenate-producing enzyme in the mammalian brain. J Neurochem 2007;102:103–111. [DOI] [PubMed] [Google Scholar]
- 8.Turski WA, Gramsbergen JB, Traitler H, Schwarcz R: Rat brain slices produce and liberate kynurenic acid upon exposure to L-kynurenine. J Neurochem 1989;52:1629–1636. [DOI] [PubMed] [Google Scholar]
- 9.Swartz KJ, During MJ, Freese A, Beal MF: Cerebral synthesis and release of kynurenic acid: an endogenous antagonist of excitatory amino acid receptors. J Neurosci 1990;10:2965–2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heredi J, Cseh EK, Berko AM, Veres G, Zadori D, Toldi J, Kis Z, Vecsei L, Ono E,Gellert L: Investigating KYNA production and kynurenergic manipulation on acute mouse brain slice preparations. Brain Res Bull 2019;146:185–191. [DOI] [PubMed] [Google Scholar]
- 11.Vecsei L, Szalardy L, Fulop F, Toldi J: Kynurenines in the CNS: recent advances and new questions. Nat Rev Drug Discov 2013;12:64–82. [DOI] [PubMed] [Google Scholar]
- 12.Heyes MP, Saito K, Crowley JS, Davis LE, Demitrack MA, Der M, Dilling LA, Elia J, Kruesi MJ, Lackner A, et al. : Quinolinic acid and kynurenine pathway metabolism in inflammatory and non-inflammatory neurological disease. Brain 1992;115 (Pt 5):1249–1273. [DOI] [PubMed] [Google Scholar]
- 13.Oxenkrug G: Serotonin-kynurenine hypothesis of depression: historical overview and recent developments. Curr Drug Targets 2013;14:514–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stone TW: Kynurenines in the CNS: from endogenous obscurity to therapeutic importance. Prog Neurobiol 2001;64:185–218. [DOI] [PubMed] [Google Scholar]
- 15.Chen Y, Guillemin GJ: Kynurenine pathway metabolites in humans: disease and healthy States. Int J Tryptophan Res 2009;2:1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schwarcz R, Rassoulpour A, Wu HQ, Medoff D, Tamminga CA, Roberts RC: Increased cortical kynurenate content in schizophrenia. Biol Psychiatry 2001;50:521–530. [DOI] [PubMed] [Google Scholar]
- 17.Erhardt S, Blennow K, Nordin C, Skogh E, Lindstrom LH, Engberg G: Kynurenic acid levels are elevated in the cerebrospinal fluid of patients with schizophrenia. Neurosci Lett 2001;313:96–98. [DOI] [PubMed] [Google Scholar]
- 18.Linderholm KR, Skogh E, Olsson SK, Dahl ML, Holtze M, Engberg G, Samuelsson M, Erhardt S: Increased levels of kynurenine and kynurenic acid in the CSF of patients with schizophrenia. Schizophr Bull 2012;38:426–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sathyasaikumar KV, Stachowski EK, Wonodi I, Roberts RC, Rassoulpour A, McMahon RP, Schwarcz R: Impaired kynurenine pathway metabolism in the prefrontal cortex of individuals with schizophrenia. Schizophr Bull 2011;37:1147–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Erhardt S, Schwieler L, Imbeault S, Engberg G: The kynurenine pathway in schizophrenia and bipolar disorder. Neuropharmacology 2017;112:297–306. [DOI] [PubMed] [Google Scholar]
- 21.Pocivavsek A, Notarangelo FM, Wu HQ, Bruno JP, Schwarcz R: Astrocytes as Pharmacological Targets in the Treatment of Schizophrenia: Focus on Kynurenic Acid Modeling the Psychopathological Dimensions of Schizophrenia. Elsevier, San Diego, CA, USA: 2016:423–443. [Google Scholar]
- 22.Akagbosu CO, Evans GC, Gulick D, Suckow RF, Bucci DJ: Exposure to kynurenic acid during adolescence produces memory deficits in adulthood. Schizophr Bull 2012;38:769–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Asp L, Holtze M, Powell SB, Karlsson H, Erhardt S: Neonatal infection with neurotropic influenza A virus induces the kynurenine pathway in early life and disrupts sensorimotor gating in adult Tap1−/− mice. Int J Neuropsychopharmacol 2010;13:475–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Erhardt S, Pocivavsek A, Repici M, Liu XC, Imbeault S, Maddison DC, Thomas MAR, Smalley JL, Larsson MK, Muchowski PJ, Giorgini F, Schwarcz R: Adaptive and Behavioral Changes in Kynurenine 3-Monooxygenase Knockout Mice: Relevance to Psychotic Disorders.Biol Psychiatry 2017;82:756–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.DeAngeli NE, Todd TP, Chang SE, Yeh HH, Yeh PW, Bucci DJ: Exposure to Kynurenic Acid during Adolescence Increases Sign-Tracking and Impairs Long-Term Potentiation in Adulthood. Front Behav Neurosci 2014;8:451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pocivavsek A, Thomas MA, Elmer GI, Bruno JP, Schwarcz R: Continuous kynurenine administration during the prenatal period, but not during adolescence, causes learning and memory deficits in adult rats. Psychopharmacology (Berl) 2014;231:2799–2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beal MF, Swartz KJ, Isacson O: Developmental changes in brain kynurenic acid concentrations. Brain Res Dev Brain Res 1992;68:136–139. [DOI] [PubMed] [Google Scholar]
- 28.Ceresoli-Borroni G, Schwarcz R: Perinatal kynurenine pathway metabolism in the normal and asphyctic rat brain. Amino Acids 2000;19:311–323. [DOI] [PubMed] [Google Scholar]
- 29.Cannazza G, Chiarugi A, Parenti C, Zanoli P, Baraldi M: Changes in kynurenic, anthranilic, and quinolinic acid concentrations in rat brain tissue during development. Neurochem Res 2001. ;26:511–514. [DOI] [PubMed] [Google Scholar]
- 30.Notarangelo FM, Pocivavsek A: Elevated kynurenine pathway metabolism during neurodevelopment: Implications for brain and behavior. Neuropharmacology 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Walker DW, Curtis B, Lacey B, Nitsos I: Kynurenic acid in brain and cerebrospinal fluid of fetal, newborn, and adult sheep and effects of placental embolization. Pediatr Res 1999;45:820–826. [DOI] [PubMed] [Google Scholar]
- 32.Pershing ML, Bortz DM, Pocivavsek A, Fredericks PJ, Jorgensen CV, Vunck SA, Leuner B, Schwarcz R, Bruno JP: Elevated levels of kynurenic acid during gestation produce neurochemical, morphological, and cognitive deficits in adulthood: implications for schizophrenia. Neuropharmacology 2015;90:33–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Forrest CM, Khalil OS, Pisar M, McNair K, Kornisiuk E, Snitcofsky M, Gonzalez N, Jerusalinsky D, Darlington LG, Stone TW: Changes in synaptic transmission and protein expression in the brains of adult offspring after prenatal inhibition of the kynurenine pathway. Neuroscience 2013;254:241–259. [DOI] [PubMed] [Google Scholar]
- 34.Khalil OS, Pisar M, Forrest CM, Vincenten MC, Darlington LG, Stone TW: Prenatal inhibition of the kynurenine pathway leads to structural changes in the hippocampus of adult rat offspring. Eur J Neurosci 2014;39:1558–1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pisar M, Forrest CM, Khalil OS, McNair K, Vincenten MC, Qasem S, Darlington LG, Stone TW: Modified neocortical and cerebellar protein expression and morphology in adult rats following prenatal inhibition of the kynurenine pathway. Brain Res 2014;1576:1–17. [DOI] [PubMed] [Google Scholar]
- 36.Pershing ML, Phenis D, Valentini V, Pocivavsek A, Lindquist DH, Schwarcz R, Bruno JP: Prenatal kynurenine exposure in rats: age-dependent changes in NMDA receptor expression and conditioned fear responding. Psychopharmacology (Berl) 2016;233:3725–3735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alexander KS, Pocivavsek A, Wu HQ, Pershing ML, Schwarcz R, Bruno JP: Early developmental elevations of brain kynurenic acid impair cognitive flexibility in adults: reversal with galantamine. Neuroscience 2013;238:19–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nilsen RM, Bjorke-Monsen AL, Midttun O, Nygard O, Pedersen ER, Ulvik A, Magnus P, Gjessing HK, Vollset SE, Ueland PM: Maternal tryptophan and kynurenine pathway metabolites and risk of preeclampsia. Obstet Gynecol 2012;119:1243–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Goeden N, Notarangelo FM, Pocivavsek A, Beggiato S, Bonnin A, Schwarcz R: Prenatal dynamics of kynurenine pathway metabolism in mice: Focus on kynurenic acid. Dev Neurosci 2017;39:519–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nicholls T, Nitsos I, Walker DW: Tryptophan metabolism in pregnant sheep: increased fetal kynurenine production in response to maternal tryptophan loading. Am J Obstet Gynecol 1999;181:1452–1460. [DOI] [PubMed] [Google Scholar]
- 41.Yu P, Di Prospero NA, Sapko MT, Cai T, Chen A, Melendez-Ferro M, Du F, Whetsell WO, Jr., Guidetti P, Schwarcz R, Tagle DA: Biochemical and phenotypic abnormalities in kynurenine aminotransferase II-deficient mice. Mol Cell Biol 2004;24:6919–6930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Boisset JC, Andrieu-Soler C, van Cappellen WA, Clapes T, Robin C: Ex vivo time-lapse confocal imaging of the mouse embryo aorta. Nat Protoc 2011;6:1792–1805. [DOI] [PubMed] [Google Scholar]
- 43.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ: Protein measurement with the Folin phenol reagent. J Biol Chem 1951;193:265–275. [PubMed] [Google Scholar]
- 44.Notarangelo FM, Schwarcz R: Restraint Stress during Pregnancy Rapidly Raises Kynurenic Acid Levels in Mouse Placenta and Fetal Brain. Dev Neurosci 2016;38:458–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Amori L, Guidetti P, Pellicciari R, Kajii Y, Schwarcz R: On the relationship between the two branches of the kynurenine pathway in the rat brain in vivo. J Neurochem 2009;109:316–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Speciale C, Wu HQ, Gramsbergen JB, Turski WA, Ungerstedt U, Schwarcz R: Determination of extracellular kynurenic acid in the striatum of unanesthetized rats: effect of aminooxyacetic acid. Neurosci Lett 1990;116:198–203. [DOI] [PubMed] [Google Scholar]
- 47.Beggiato S, Notarangelo FM, Sathyasaikumar KV, Giorgini F, Schwarcz R: Maternal genotype determines kynurenic acid levels in the fetal brain: Implications for the pathophysiology of schizophrenia. J Psychopharmacol 2018;32:1223–1232. [DOI] [PubMed] [Google Scholar]
- 48.Fukui S, Schwarcz R, Rapoport SI, Takada Y, Smith QR: Blood-brain barrier transport of kynurenines: implications for brain synthesis and metabolism. J Neurochem 1991;56:2007–2017. [DOI] [PubMed] [Google Scholar]
- 49.Chrostowski MK, McGonnigal BG, Stabila JP, Padbury JF: LAT-1 expression in pre- and post-implantation embryos and placenta. Placenta 2009;30:270–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Badawy AA: Tryptophan metabolism, disposition and utilization in pregnancy. Biosci Rep 2015;35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ligam P, Manuelpillai U, Wallace EM, Walker D: Localisation of indoleamine 2,3-dioxygenase and kynurenine hydroxylase in the human placenta and decidua: implications for role of the kynurenine pathway in pregnancy. Placenta 2005;26:498–504. [DOI] [PubMed] [Google Scholar]
- 52.Manuelpillai U, Ligam P, Smythe G, Wallace EM, Hirst J, Walker DW: Identification of kynurenine pathway enzyme mRNAs and metabolites in human placenta: up-regulation by inflammatory stimuli and with clinical infection. Am J Obstet Gynecol 2005;192:280–288. [DOI] [PubMed] [Google Scholar]
- 53.Alberati-Giani D, Ricciardi-Castagnoli P, Kohler C, Cesura AM: Regulation of the kynurenine metabolic pathway by interferon-gamma in murine cloned macrophages and microglial cells. J Neurochem 1996;66:996–1004. [DOI] [PubMed] [Google Scholar]
- 54.Murthi P, Wallace EM, Walker DW: Altered placental tryptophan metabolic pathway in human fetal growth restriction. Placenta 2017;52:62–70. [DOI] [PubMed] [Google Scholar]
- 55.Goasdoue K, Miller SM, Colditz PB, Bjorkman ST: Review: The blood-brain barrier; protecting the developing fetal brain. Placenta 2017;54:111–116. [DOI] [PubMed] [Google Scholar]
- 56.Notarangelo FM, Wons KS, Schwarcz R: Prenatal LPS exposure preferentially increases kynurenine pathway metabolism in the fetal brain. Society for Neuroscience Abstract 2015;40:74.02. [Google Scholar]
- 57.Nicholls T, Nitsos I, Smythe G, Walker DW: Kynurenine production and catabolism in fetal sheep with embolized or nonembolized placentas. Am J Obstet Gynecol 2001;185:988–995. [DOI] [PubMed] [Google Scholar]
- 58.Sano M, Ferchaud-Roucher V, Kaeffer B, Poupeau G, Castellano B, Darmaun D: Maternal and fetal tryptophan metabolism in gestating rats: effects of intrauterine growth restriction. Amino Acids 2016;48:281–290. [DOI] [PubMed] [Google Scholar]
- 59.Baran H, Kepplinger B, Herrera-Marschitz M, Stolze K, Lubec G, Nohl H: Increased kynurenic acid in the brain after neonatal asphyxia. Life Sci 2001;69:1249–1256. [DOI] [PubMed] [Google Scholar]
- 60.Ceresoli-Borroni G, Schwarcz R: Neonatal asphyxia in rats: acute effects on cerebral kynurenine metabolism. Pediatr Res 2001;50:231–235. [DOI] [PubMed] [Google Scholar]
- 61.Milart P, Urbanska EM, Turski WA, Paszkowski T, Sikorski R: Kynurenine aminotransferase I activity in human placenta. Placenta 2001;22:259–261. [DOI] [PubMed] [Google Scholar]
- 62.Hodgkins PS, Wu HQ, Zielke HR, Schwarcz R: 2-Oxoacids regulate kynurenic acid production in the rat brain: studies in vitro and in vivo. J Neurochem 1999;72:643–651. [DOI] [PubMed] [Google Scholar]
- 63.Gramsbergen JB, Hodgkins PS, Rassoulpour A, Turski WA, Guidetti P, Schwarcz R: Brain-specific modulation of kynurenic acid synthesis in the rat. J Neurochem 1997;69:290–298. [DOI] [PubMed] [Google Scholar]
- 64.Schwarcz R, Poeggeler B, Rassoulpour A, Ceresoli-Borroni G, Hodgkins PS: Regulation of kynurenic acid levels in the developing rat brain. Amino Acids 1998;14:243–249. [DOI] [PubMed] [Google Scholar]
- 65.Blanco Ayala T, Lugo Huitron R, Carmona Aparicio L, Ramirez Ortega D, Gonzalez Esquivel D, Pedraza Chaverri J, Perez de la Cruz G, Rios C, Schwarcz R, Perez de la Cruz V : Alternative kynurenic acid synthesis routes studied in the rat cerebellum. Front Cell Neurosci 2015;9:178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Du F, Schmidt W, Okuno E, Kido R, Kohler C, Schwarcz R: Localization of kynurenine aminotransferase immunoreactivity in the rat hippocampus. J Comp Neurol 1992;321:477–487. [DOI] [PubMed] [Google Scholar]
- 67.Guillemin GJ, Cullen KM, Lim CK, Smythe GA, Garner B, Kapoor V, Takikawa O, Brew BJ: Characterization of the kynurenine pathway in human neurons. J Neurosci 2007;27:12884–12892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Heredi J, Berko AM, Jankovics F, Iwamori T, Iwamori N, Ono E, Horvath S, Kis Z, Toldi J, Vecsei L, Gellert L: Astrocytic and neuronal localization of kynurenine aminotransferase-2 in the adult mouse brain. Brain Struct Funct 2017;222:1663–1672. [DOI] [PubMed] [Google Scholar]
- 69.Roberts RC, Du F, McCarthy KE, Okuno E, Schwarcz R: Immunocytochemical localization of kynurenine aminotransferase in the rat striatum: a light and electron microscopic study. J Comp Neurol 1992;326:82–90. [DOI] [PubMed] [Google Scholar]
- 70.Bayraktar OA, Fuentealba LC, Alvarez-Buylla A, Rowitch DH: Astrocyte development and heterogeneity. Cold Spring Harb Perspect Biol 2014;7:a020362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Semple BD, Blomgren K, Gimlin K, Ferriero DM, Noble-Haeusslein LJ: Brain development in rodents and humans: Identifying benchmarks of maturation and vulnerability to injury across species. Prog Neurobiol 2013;106–107:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bagasrawala I, Zecevic N, Radonjic NV: N-Methyl D-Aspartate Receptor Antagonist Kynurenic Acid Affects Human Cortical Development. Front Neurosci 2016;10:435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Allen NJ, Lyons DA: Glia as architects of central nervous system formation and function. Science 2018;362:181–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Clark DA: The use and misuse of animal analog models of human pregnancy disorders. J Reprod Immunol 2014;103:1–8. [DOI] [PubMed] [Google Scholar]
- 75.Boros FA, Bohar Z, Vecsei L: Genetic alterations affecting the genes encoding the enzymes of the kynurenine pathway and their association with human diseases. Mutat Res 2018;776:32–45. [DOI] [PubMed] [Google Scholar]