Abstract
Cryoelectron microscopy and mutational analyses have shown that type 1 ryanodine receptor (RyR1) amino acid residues RyR1-E3893, -E3967, and -T5001 are critical for Ca2+-mediated activation of skeletal muscle Ca2+ release channel. De novo missense mutation RyR1-Q3970K in the secondary binding sphere of Ca2+ was reported in association with central core disease (CCD) in a 2-yr-old boy. Here, we characterized recombinant RyR1-Q3970K mutant by cellular Ca2+ release measurements, single-channel recordings, and computational methods. Caffeine-induced Ca2+ release studies indicated that RyR1-Q3970K formed caffeine-sensitive, Ca2+-conducting channel in HEK293 cells. However, in single-channel recordings, RyR1-Q3970K displayed low Ca2+-dependent channel activity and greatly reduced activation by caffeine or ATP. A RyR1-Q3970E mutant corresponds to missense mutation RyR2-Q3925E associated with arrhythmogenic syndrome in cardiac muscle. RyR1-Q3970E also formed caffeine-induced Ca2+ release in HEK293 cells and exhibited low activity in the presence of the activating ligand Ca2+ but, in contrast to RyR1-Q3970K, was activated by ATP and caffeine in single-channel recordings. Computational analyses suggested distinct structural rearrangements in the secondary binding sphere of Ca2+ of the two mutants, whereas the interaction of Ca2+ with directly interacting RyR1 amino acid residues Glu3893, Glu3967, and Thr5001 was only minimally affected. We conclude that RyR1-Q3970 has a critical role in Ca2+-dependent activation of RyR1 and that a missense RyR1-Q3970K mutant may give rise to myopathy in skeletal muscle.
Keywords: central core disease, homology modeling, ryanodine receptor, sarcoplasmic reticulum, single-channel recording
INTRODUCTION
The skeletal muscle (RyR1) and cardiac muscle (RyR2) ryanodine receptor Ca2+ release channels are responsible for intracellular Ca2+ release from sarcoplasmic reticulum (SR) during muscle action potential. The RyRs are homotetramers of ~5,000 amino acid subunits that have multiple regulatory sites for physiological and pharmacological effectors (6, 13, 19). RyRs are activated by micromolar Ca2+ and millimolar ATP and caffeine and inhibited by millimolar Ca2+ and Mg2+. Calcium release through RyRs is critical for skeletal and cardiac muscle contraction, and functional changes in the RyR protein complexes have been implicated in human pathologies. A large number of missense mutations are associated in RyR1 with malignant hyperthermia and central core disease (CCD) and are associated in RyR2 with catecholaminergic polymorphic ventricular tachycardia (10–12, 14, 30). Structural analyses of RyRs by crystallography and cryoelectron microscopy (cryo-EM) have provided detailed insights into RyR structural changes caused by disease-linked mutations and channel regulatory ligands (4, 5, 16, 24–26, 34–36).
The near-atomic resolution of the structure of purified RyR1 using cryo-EM and three-dimensional image analysis revealed binding sites for the three RyR1 channel agonists Ca2+, ATP, and caffeine (4). The Ca2+-binding site comprises amino acid residues Glu3893, Glu3967, and Thr5001 that directly interact with Ca2+ and residues His3895 and Gln3970 that are part of the secondary binding sphere of Ca2+. Mutagenesis and single-channel recordings showed that the three amino acids directly interacting with Ca2+ play a critical role in Ca2+-mediated activation of RyR1 (31). Single and double RyR1-E3893 and RyR1-E3967 mutants were not activated by Ca2+, and RyR1-T5001 mutant reduced the apparent affinity for Ca2+.
De novo CCD-linked mutation RyR1-Q3970K was identified in a 2-yr-old boy who was able to walk but not to run (28). Morphological analysis indicated few cores and focal loss of cross striations. Here, we tested the hypothesis that the CCD-associated RyR1-Q3970K mutant and RyR1-Q3970E mutant, corresponding to missense RyR2-Q3925E mutant, displayed an altered Ca2+-dependent regulation. RyR2-Q3925E was reported to be associated with arrhythmogenic syndrome in cardiac muscle (18). Both RyR1-Q3970K and RyR1-Q3970E mutants formed caffeine-sensitive, Ca2+-conducting channels in HEK293 cells and displayed low channel open probability in the presence of three RyR1-activating ligands, Ca2+, ATP, and caffeine, in single-channel measurements. The results suggest that altered regulation of RyR1-Q3970K by Ca2+ and ATP may give rise to skeletal muscle myopathy and imply that RyR2-Q3925E corresponding to RyR1-Q3970E is associated with cardiac muscle myopathy.
MATERIALS AND METHODS
Materials.
Protease inhibitors were obtained from Sigma-Aldrich (St. Louis, MO), jetPRIME transfection reagent from Polyplus (New York, NY), and phospholipids from Avanti Polar Lipids (Alabaster, AL).
Preparation of mutant channels.
Full-length rabbit RyR1 was cloned into the mammalian expression vector pCMV5 (8). Rabbit RyR1-Q3970K and -Q3970E mutations corresponding to human RyR1-Q3969 were introduced by the QuikChange site-directed mutagenesis method (Agilent, Santa Clara, CA) using Pfu-turbo DNA polymerase and mutagenic oligonucleotides (7). Amino acid numbering is described using the rabbit sequence (29). Wild-type (WT) and mutant RyR1s were transiently expressed in HEK293 cells using jetPRIME according to the manufacturer’s instructions. Crude membrane isolates were prepared from the transfected HEK293 cells in the presence of protease inhibitors and 1 mM oxidized glutathione (GSSG) as described (33).
SDS-PAGE and immunoblot analysis.
Proteins in crude membrane isolates of HEK293 cells (20 µg protein/lane) were separated using 3–12% gradient SDS-PAGE, transferred overnight to nitrocellulose membranes at 4°C, and probed using primary rabbit anti-RyR1 polyclonal antibody 6425, prepared by ψProSci (Poway, CA) (31). Immunoblots were developed using horseradish peroxidase-linked secondary anti-rabbit IgG antibody (Cell Signaling, Danvers, MA), and RyR1 proteins were quantified using Bio-Rad ChemiDoc MP Imaging System and ImageQuant TL analysis software.
Cellular Ca2+ release.
Caffeine-induced Ca2+ release in HEK293 cells was measured using the fluorescence Ca2+ indicator Fluo-4. Briefly, HEK293 cells expressing wild-type (WT) or mutant RyR1s were grown on coverslips and incubated with membrane-permeable Fluo-4-AM (31). After washing away excess extracellular Fluo-4 AM, cellular Ca2+ release was triggered by the addition of ~8 mM caffeine and recorded in individual cells using Sola SEII Light Engine and NIS Elements Software (Nikon Instruments, Melville, NY). We probed nine coverslips for each genotype (WT, Q3970E, and Q3970K). On each coverslip, 30–50 cells were examined.
Single-channel recordings.
Single-channel activities of WT and mutant RyR1s were recorded using the planar lipid bilayer method (31). Crude membrane isolates were added to the cis (cytosolic) chamber of a bilayer apparatus. Channel activities were measured using 0.25 M KCl and 20 mM K-HEPES, pH 7.4 on both sides of the bilayer, 2 µM trans (SR luminal) with indicated cis (cytosolic) Ca2+ concentrations and additions. The trans side of the bilayer was defined as ground. Electrical signals were filtered at 2 kHz, digitized at 10 kHz, and analyzed at 50% threshold setting (31). Data acquisition and analysis of 2-min recordings were performed using pClamp software (Molecular Devices, San Jose, CA).
Computational methods.
The structures of the Ca2+-binding site in RyR1-Q3970E and RyR1-Q3970K mutants were modeled by performing in silico amino acid substitutions in closed (PDB: 5TAQ) and open (PDB: 5TAL) RyR1-WT cryo-EM structures in the presence of Ca2+, ATP, and caffeine. Prior to mutagenesis, the missing residues and atoms were relocated in both open and closed RyR1 structures through homology modeling. Subsequently, in silico mutations at chosen residue positions in RyR1 were performed using the Mutagenesis tool in PyMOL molecular visualization suite (https://pymol.org/2/) (3). During mutagenesis, the side-chain orientations of the substituted residues were selected based on 1) backbone dependency and 2) minimum clash score. The mutant RyR1 structures RyR1-Q3970E and RyR1-Q3970K were subjected to energy minimization by employing steepest descent and conjugate gradient algorithms in the GROMACS 4 package (9). Thus, generated optimal structures of RyR1-WT, RyR1-Q3970E, and RyR1-Q3970K in both closed and open conformations were analyzed and compared for loss/gain of interactions in Ca2+-binding pocket, up to the second coordination sphere of Ca2+, as described previously (31).
Biochemical assays and data analysis.
Free Ca2+ concentrations were determined using a Ca2+-selective electrode. Free Ca2+ concentrations following the addition of 2 mM ATP were calculated using MaxChelator (https://web.stanford.edu/~cpatton/downloads.htm) and constants from the Theo Schoenmakers’ Chelator (27). Data are presented as the mean ± SE. Differences between samples were analyzed using ANOVA followed by Tukey’s test, where P < 0.05 was considered significant.
RESULTS
In addition to three RyR1 amino acid residues (Glu3893, Glu3967, and Thr5001) that directly bind Ca2+ (31), Gln3970 is part of the secondary coordination sphere of the Ca2+-binding site conserved in RyR1 and RyR2 (Fig. 1) (4). To assess the significance of Gln3970 in Ca2+-dependent activation of RyR1 in skeletal muscle central core disease pathology (28), we expressed RyR1-Q3970K in HEK293 cells and characterized recombinant mutant proteins by using a cellular Ca2+ release assay, single-channel recordings, and computational methods. We also prepared and characterized RyR1-Q3970E mutant, which corresponds to the RyR2-Q3925E mutant identified in a patient with arrhythmogenic syndrome (18). Some RyR1-WT data were previously published using the methods described in the present report (31).
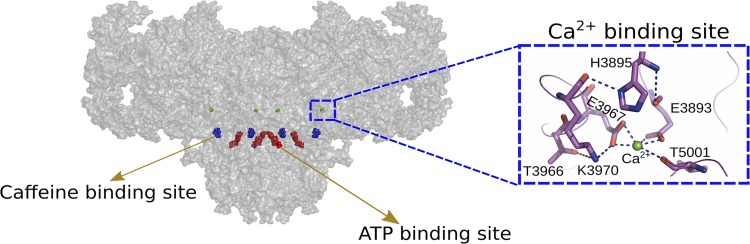
Fig. 1.
Location of Ca2+-, ATP-, and caffeine-binding sites of open type 1 ryanodine receptor-wild type (RyR1-WT; PDB code 5TAL). Protein structure is shown as a transparent surface. Ca2+-, ATP-, and caffeine-binding sites are shown in green, red, and blue, respectively. Inset: structure of Ca2+-binding site of open RyR1-Q3970K mutant in the presence of Ca2+, ATP, and caffeine. [Modified from Xu et al. (31) with permission.]
Characterization RyR1-Q3970K and -Q3970E in HEK293 cells.
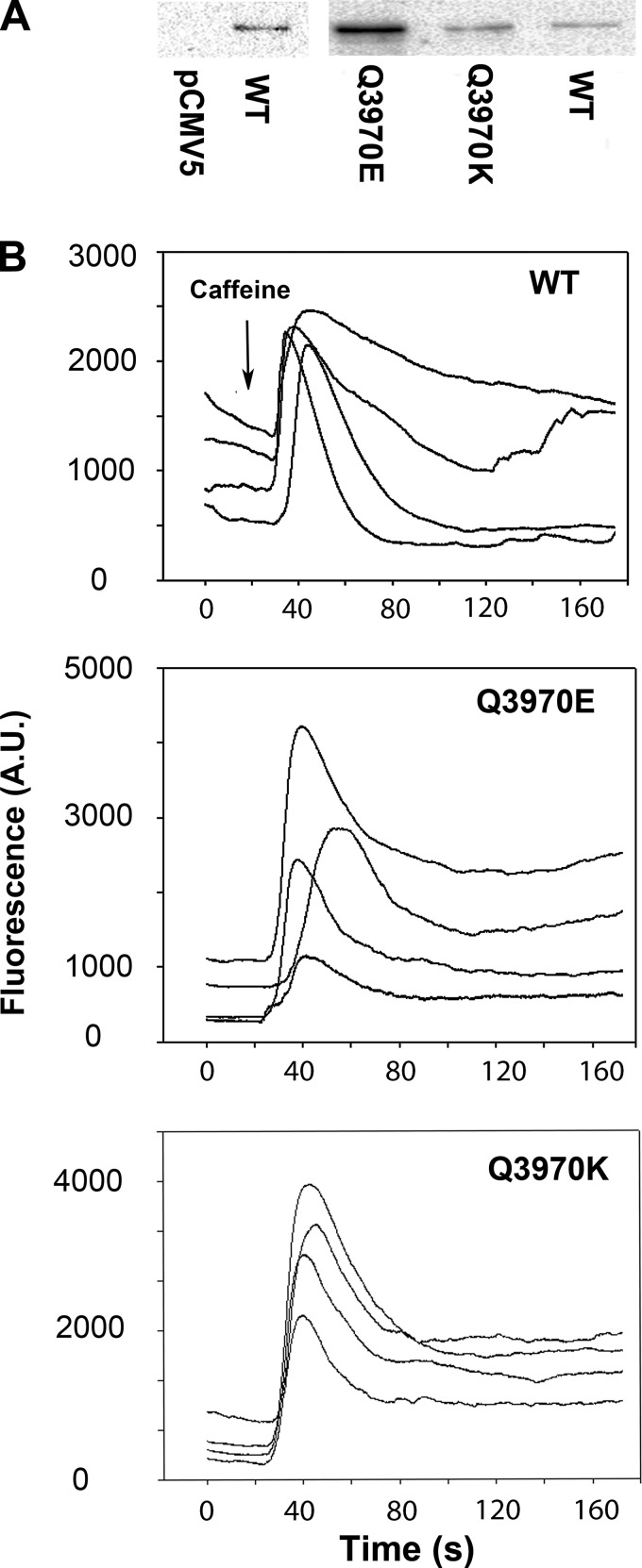
Quantitative analysis of immunoblots showed specificity of the RyR1 polyclonal antibody for RyR1 (Fig. 2A, left). Protein levels of RyR1-Q3970E exceeded WT by 10- to 20-fold, whereas RyR1-Q3970K was twofold greater than WT (Fig. 2A, right). HEK293 cells expressing RyR1-E3893 or RyR1-E3967 also expressed at elevated levels compared with WT (31). The functional expression of RyR1 mutants was determined using fluorescent Ca2+ indicator Fluo-4 and RyR1 agonist caffeine (Fig. 2B). Both RyR1-Q3970K and RyR1-Q3970E exhibited caffeine-dependent intracellular Ca2+ transients in intact HEK293 cells, indicating the expression of Ca2+-conducting channels. A variable caffeine-induced Ca2+ release was observed in 40 ± 6% of HEK293 cells (n = 9) transfected with RyR1-WT. As previously reported (31), the variable response may have resulted from uneven exposure to caffeine and removal of released Ca2+ from the cells. The number of RyR1-Q3970E and RyR1-Q3970K cells responding to caffeine was significantly less than WT (12 ± 5%, n = 9, P < 0.005) and 14 ± 4%, n = 9, P < 0.005), respectively, despite the expression of elevated protein levels of both mutants compared with WT.
Fig. 2.
Immunoblot and caffeine-induced Ca2+ release by HEK293 cells expressing wild-type (WT) and mutant type 1 ryanodine receptors (RyR1s). A: immunoblots of HEK293 cells transfected with pCMV5 vector and RyR1-WT expression vector (left), and 565 kDa RyR1-Q3970E, RyR1-Q3970K, and RyR1-WT (right). The blots identified the RyR1 protein band by its absence from the pCMV5 vector-transfected sample (left). B: Ca2+ transients in HEK293 cells expressing RyR1-WT (top), RyR1-Q3970E (middle), and RyR1-Q3970K (bottom) as changes of Fluo-4 fluorescence before and following the addition of 8 mM caffeine (arrow) to the bath solution. AU, arbitrary units.
Single-channel analysis of mutant RyR1s.
The lipid bilayer method was used to determine the regulation of RyR1-Q3970E and RyR1-Q3970K by endogenous activating ligands Ca2+ and ATP and exogenous ligand caffeine. Membrane isolates were fused with lipid bilayers, and single-channels were recorded using 0.25 M KCl on both sides of the bilayer, taking advantage of the impermeability of RyR1 to Cl− and high permeability to K+ compared with Ca2+.
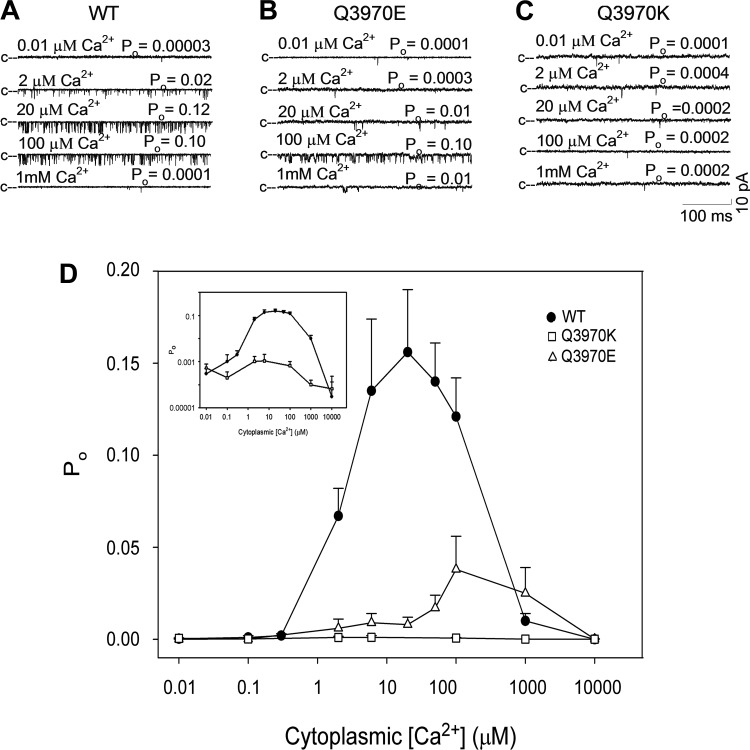
Single open-channel probability (Po) of WT and mutant RyR1s was determined at various cis cytosolic Ca2+ concentrations. Figure 3 shows representative single-channel traces of RyR1-WT (Fig. 3A), RyR1-Q3970E (Fig. 3B), and RyR1-Q3970K (Fig. 3C) at cytosolic Ca2+ ranging from 0.01 µM to 1 mM. RyR1-WT exhibited a typical bimodal Ca2+-dependent activation and inactivation profile with peak at ~20 µM Ca2+, which indicated the presence of high-affinity Ca2+ activation and low-affinity Ca2+ inactivation sites (Fig. 3D). RyR1-Q3970E exhibited a low Po compared with WT and shifted Ca2+ activation rightward. RyR1-Q3970K exhibited very low open probability (Po) at all tested Ca2+ concentrations. A bimodal Ca2+ activation/inactivation profile comparable to WT suggested that the Q3970K mutation did not markedly alter RyR1 Ca2+ affinity at activation and inactivation sites (Fig. 3D, inset). The single-channel K+ conductance for both mutants was essentially the same as for RyR1-WT (776 ± 17 pS, n = 8, for WT; 763 ± 7 pS, n = 7, for Q3970E; and 720 ± 23 pS, n = 4, for Q3970K; not shown).
Fig. 3.
Effects of cytosolic Ca2+ on type 1 ryanodine receptor-wild type (RyR1-WT) and mutant channel open probability (Po). A–C: representative single-channel currents of RyR1-WT (A), RyR1-Q3970E (B), and RyR1-Q3970K (C) were recorded as downward deflections from the closed state (c–) in 250 mM symmetrical KCl at −20 mV with 2 µM sarcoplasmic reticulum (SR) luminal Ca2+ and the indicated cytosolic Ca2+ concentrations. D: Ca2+ dependence of RyR1-WT, RyR1-Q3970E, and RyR1-Q3970K channel open probabilities. Data are the mean ± SE of 3–21 recordings. Inset: open probabilities of WT and Q3970K in a logarithmic scale. [WT data in D were obtained from Xu et al. (31) under recording conditions used in the present report.]
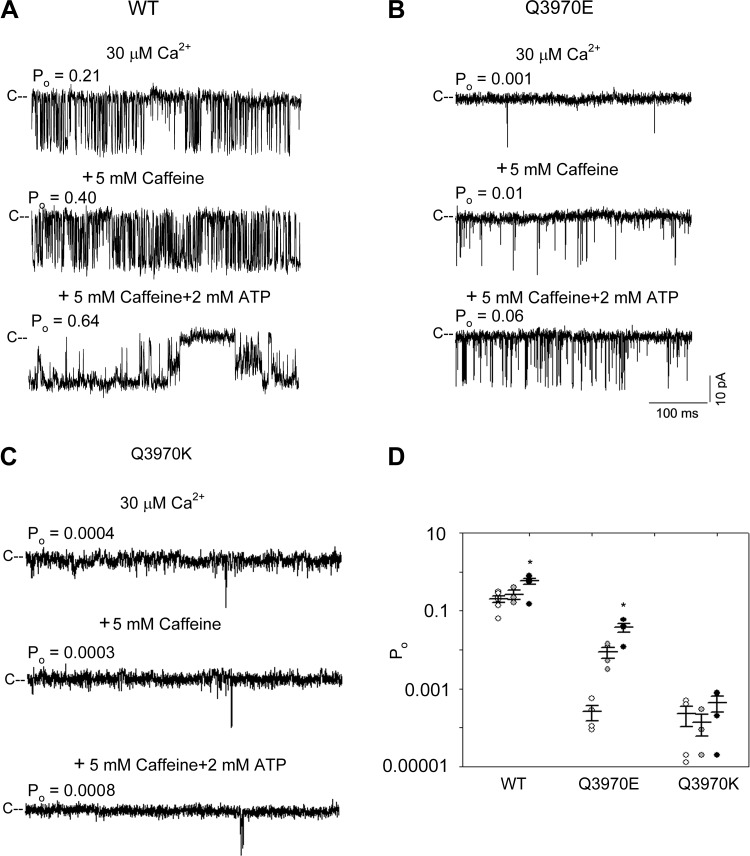
Next, we tested whether mutant RyR1s can be activated by the two RyR agonists caffeine and ATP (19). To compare single-channel activities with the RyR1 structural models determined by cryo-EM, Ca2+, caffeine, and ATP concentrations were used as reported by des Georges et al. (4). Single channels were recorded in the presence of 30 µM cytosolic Ca2+ at three different conditions: 1) without caffeine or ATP, 2) with 5 mM caffeine, and 3) with 5 mM caffeine and 2 mM ATP (Fig. 4, A–C). In the presence of 5 mM caffeine, single-channel open probabilities of WT was 1.3-fold higher than Ca2+ only (average Po were 0.20 and 0.26), and RyR1-Q3970E was 30-fold higher (Po were 0.0003 and 0.009) (Fig. 4D). In the presence of both caffeine and ATP single-channel open probabilities of WT was 2.85-fold higher than 30 µM Ca2+ (Po were 0.20 and 0.57), and RyR1-Q3970E was 123-fold (Po were 0.0003 and 0.037). No significant difference in Po was measured for RyR1-Q3970K in the presence of 5 mM caffeine or 5 mM caffeine and 2 mM ATP compared with 30 µM Ca2+ only.
Fig. 4.
Effects of caffeine and ATP on type 1 ryanodine receptor- wild type (RyR1-WT) and mutant channel open probabilities (Po). A–C: representative single-channel currents of RyR1-WT (A), RyR1-Q3970E (B), and RyR1-Q3970K (C) were recorded as downward deflections from the closed state (C–) in 250 mM symmetrical KCl at −20 mV. Luminal Ca2+ was 2 µM. Cytosolic solutions contained 30 µM Ca2+ (top traces), 30 µM Ca2+ and 5 mM caffeine (middle traces), and 30 µM Ca2+, 5 mM caffeine, and 2 mM ATP (bottom traces). Free Ca2+ was 5 µM in the presence of 5 mM caffeine and 2 mM ATP as described under materials and methods. D: channel open probability in the presence of 30 µM cytoplasmic Ca2+ (open symbols), 30 µM Ca2+ and 5 mM caffeine (shaded symbols), and 5 µM Ca2+ plus 5 mM caffeine and 2 mM ATP (closed symbols). Data are the mean ± SE of 3–6 recordings. *P < 0.05 compared with respective controls by one-way ANOVA followed by Tukey’s test. [WT data in D were obtained from Xu et al. (31) under recording conditions used in the present report.]
DISCUSSION
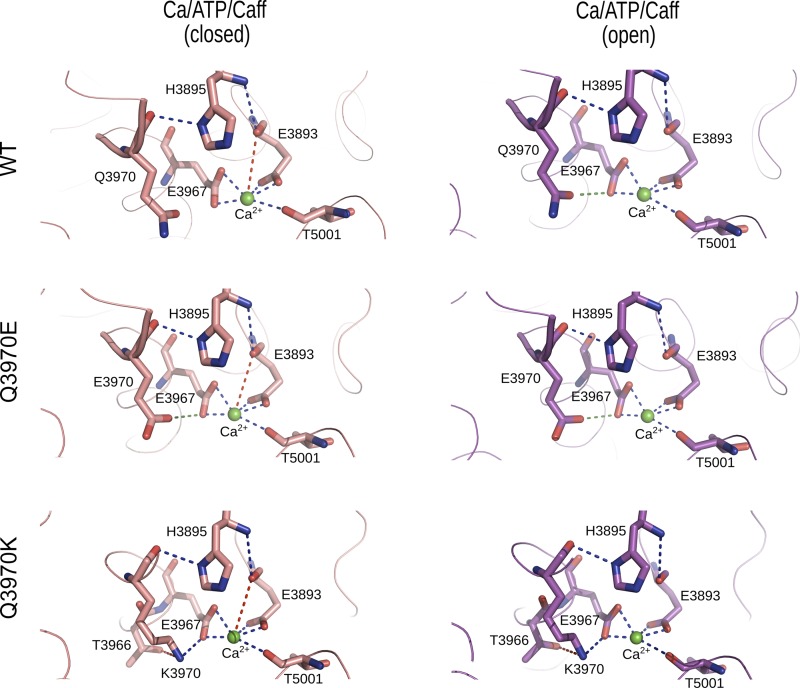
High-resolution cryo-EM and structural analysis of intact RyR1 have shown that RyR1-Q3970 is part of a secondary coordination sphere of Ca2+. De novo CCD-associated RyR1-Q3970K mutant exhibited a caffeine-induced Ca2+ response in HEK293 cells, indicating the expression of caffeine-sensitive, Ca2+-conducting channels. In single-channel measurements, activation by Ca2+, ATP, and caffeine was greatly reduced for RyR1-Q3970K compared with WT. This suggests that RyR1-Q3970K mutant channels underwent some structural changes outside the cellular environment or required cofactors lost during isolation. RyR1-Q3970E, which corresponds to RyR2-Q3925E associated with arrhythmogenic syndrome in cardiac muscle, also displayed a caffeine-induced Ca2+ release in HEK293 cells. RyR1-Q3970E was activated by Ca2+ with apparent reduced affinity and level of Ca2+ activation compared with WT. In contrast to RyR1-Q3970K, RyR1-Q3970E maintained ATP and caffeine activation. However, the maximal open probability of RyR1-Q3970E in the presence of all three activating ligands (Ca2+, caffeine, and ATP) was ~15-fold lower than WT. Together, these findings suggest that the two mutants formed loss-of-function channels in lipid bilayers. Computational analysis of the two mutants suggested structural rearrangements in the secondary binding sphere of Ca2+. On the other hand, both RyR1 mutations only minimally affected the interaction of Ca2+ with RyR1 amino acid residues Glu3893, Glu3967, and Thr5001 that directly interact with Ca2+ (Fig. 5).
Fig. 5.
Interactions of Ca2+ with wild type (WT) and mutant type 1 ryanodine receptors (RyR1s). Shown are predicted interactions of Ca2+ with WT and mutant RyR1s in the presence of Ca2+, ATP, and caffeine. Residues displaying electrostatic interactions with Ca2+ in the Ca2+/ATP/caffeine closed (5TAQ) and open (5TAL) states are depicted in stick representation. Backbone of RyR1 is shown as a ribbon. Ca2+ is shown as a green sphere. Strong electrostatic interactions (distance <3.2 Å) are shown as blue dashed lines and weak electrostatic interactions (distance <4 Å) are shown as red dashed lines between Ca2+ and RyR1 residues. Attractive and repulsive amino acid interactions (distance <3.5 Å) are depicted as blue and green dashed lines, respectively.
Central core disease is a congenital skeletal muscle myopathy causing gradual muscle weakness after birth. In human pathology, large cores in the center of skeletal muscle lacking mitochondria and oxidative enzymes are observed (10). Thus far, over 60 missense mutations in the RYR1 gene have been reported to be associated with CCD (21), and a number of human patients with these mutations are susceptible to malignant hyperthermia, another RYR1-linked skeletal myopathy. Using mutant RyR1 expressing dyspedic myotubes, two major types of RyR1 mutants were proposed to mediate CCD: 1) leaky RyR1 channel causing SR Ca2+ store depletion, and 2) loss-of-function RyR1 resulting in excitation-contraction “uncoupling”. In both cases, reduced Ca2+ release through the mutant RyR1s caused muscle weakness (1, 2, 15).
Single-channel recordings have shown that CCD-associated RyR1 mutations affect channel function by causing loss-of-function ion conductances or gain-of-function gating activities. Ion conductance mutants result in low or absent Ca2+ conductance and are localized in the pore-forming carboxyl-terminal domain (I4897T, G4898E, G4898R, and ΔV4926-I4927 in rabbit RyR1 sequence), but also include amino terminal domain mutants (R110W and R110W/L486V in rabbit RyR1 sequence) (15, 17, 32, 37). Gating mutants have essentially the same single-channel Ca2+ conductance but display an open probability in single-channel recordings that results in “leaky” RyR1 channels and SR Ca2+ store depletion. Examples of gain-of-function mutants are CCD-linked RyR1-ΔR4215-F4217 (17), minicore myopathy mutant RyR1-N2283H (37), and likely RyR1-R164C and -Y523S, where the latter two exhibited leakiness of stored Ca2+ in skeletal myotubes or HEK293 cells (1, 21). RyR1-Q3970K appears to be a rare loss-of-function gating mutant. Recombinant homotetramer of RyR1-Q3970K conducted Ca2+ in HEK293 cells and, in contrast to conductance mutants (32), had a K+ conductance comparable to that of WT in single-channel recordings. Ca2+ conductance and Ca2+/K+ permeability ratio could not be determined due to low open-channel probability of RyR1-Q3970K. The impact of the RyR1-Q3970K mutation on skeletal muscle excitation-contraction coupling may be further clarified by future knock-in mouse experiments. One possibility may be that the RyR1-Q3970K mutation will reduce the depolarization-induced Ca2+ release via an impaired secondary Ca2+-induced Ca2+ release mechanism.
Using a [3H]ryanodine ligand-binding assay, Murayama et al. (22) showed that, in the presence of 1 M NaCl, RyR2-Q3925E, which corresponds to RyR1-Q3970E, shifted the Ca2+ activation curve to the right without reducing maximal activity at millimolar Ca2+. In our single-channel recordings, RyR1-Q3970E exhibited reduced affinity of Ca2+ activation and peak activity at 100 µM Ca2+ compared with WT at 20 µM Ca2+. The differences in peak activities may have resulted from isoform-specific differences between skeletal and cardiac muscle RyRs and the high salt concentration used in the [3H]ryanodine-binding assay. High salt concentrations are known to enhance RyR channel activity and levels of bound [3H]ryanodine (20, 23). In addition, we found for the first time that RyR1-Q3970E maintained ATP and caffeine activation, although open probability of RyR1-Q3970E in the presence of three channel activators Ca2+, ATP, and caffeine was low compared with WT. The impact of low open-channel probability on cardiac cellular Ca2+ signaling and the arrhythmogenic syndrome remain to be determined.
Our mutagenesis experiments have shown that glutamine substitution with lysine greatly reduced Ca2+-mediated channel activity, and substitution with glutamate, carrying an opposite charge to lysine, decreased the apparent affinity of activating Ca2+. Computational analysis using cryo-EM densities (4) determined the structure of the Ca2+-binding sites of Ca2+/ATP/caffeine-bound WT and mutant channels. In the closed RyR1-WT channel in the presence of activating ligands Ca2+, ATP, and caffeine, Gln3970 did not directly interact with Ca2+, whereas Ca2+ strongly interacted with the side-chains of Glu3893, Glu3967, and Thr5001. It is also apparent from the Ca2+-binding site in Ca2+/ATP/caffeine-bound closed WT and mutant channels that Ca2+ has weak interaction with the backbone carbonyl oxygen of Glu3893 (Fig. 5 and Table 1). Furthermore, our computational analysis indicated the presence of energetically favorable interactions between His3895 and Gln3970, and between His3895 and Glu3893. Rearrangements in the primary and secondary binding spheres of Ca2+ may have stabilized open WT channel states. These included loss of a weak interaction of Ca2+ with Glu3893 carbonyl oxygen and gain of repulsive interaction between Gln3970 and Glu3967 in open channel states.
Table 1.
Interactions between Ca2+ and amino acids of WT and mutant Ca2+ activation sites
| Amino Acid Interactions |
|||||||
|---|---|---|---|---|---|---|---|
| Ca2+ Interactions | Class 5TAQ (Closed) Distance, Å | Class 5TAL (Open) Distance, Å | Class 5TAQ (Closed) | Distance, Å | Class 5TAL (Open) | Distance, Å | Type |
| WT variant | |||||||
| E3893(OE1) | 2.4 | 2.3 | H3895(ND1) - Q3970(O) | 3.0 | H3895(ND1) - Q3970(O) | 3.0 | Attractive |
| E3893(OE2) | 2.1 | 2.2 | H3895(N) - E3893(O) | 3.5 | H3895(N) - E3893(O) | 3.5 | Attractive |
| E3893(O) | 3.8 | Q3970(OE1) - E3967(OE1) | 3.3 | Repulsive | |||
| E3967(OE1) | 2.5 | 2.4 | |||||
| E3967(OE2) | 2.2 | 2.3 | |||||
| T5001(O) | 2.6 | 2.5 | |||||
| Q3970E variant | |||||||
| E3893(OE1) | 2.4 | 2.3 | H3895(ND1) - E3970(O) | 3.0 | H3895(ND1) - E3970(O) | 3.0 | Attractive |
| E3893(OE2) | 2.1 | 2.2 | H3895(N) - E3893(O) | 3.5 | H3895 (N) - E3893 (O) | 3.5 | Attractive |
| E3893(O) | 3.8 | E3970(OE1) - E3967(OE1) | 3.3 | E3970(OE1) - E3967(OE1) | 3.0 | Repulsive | |
| E3967(OE1) | 2.5 | 2.4 | |||||
| E3967(OE2) | 2.2 | 2.3 | |||||
| T5001(O) | 2.6 | 2.5 | |||||
| Q3970K variant | |||||||
| E3893(OE1) | 2.4 | 2.3 | K3970(NZ) - T3966(OG1) | 3.9 | K3970(NZ) - T3966(OG1) | 3.8 | Attractive |
| E3893(OE2) | 2.1 | 2.2 | K3970(NZ) - E3967(OE2) | 2.9 | K3970(NZ) - E3967(OE2) | 2.6 | Attractive |
| E3893(O) | 3.8 | K3970(O) - H3895(ND1) | 3.0 | K3970(O) - H3895(ND1) | 3.0 | Attractive | |
| E3967(OE1) | 2.5 | 2.4 | H3895(N) - E3893(O) | 3.5 | H3895(N) - E3893(O) | 3.5 | Attractive |
| E3967(OE2) | 2.2 | 2.3 | |||||
| T5001(O) | 2.6 | 2.5 | |||||
Strong electrostatic interactions (distance <3.5Å) are shown in Fig. 5 as blue dashed lines and weak electrostatic interactions (distance >3.5Å) are shown as red dashed lines between Ca2+ and type 1 ryanodine receptor (RyR1) residues. Distances less than 3.5Å formed between inter-residues in RyR1-Q3970K are shown as blue dashed lines; repulsive interactions are shown as green dotted lines. WT, wild type.
In closed- and open-channel RyR1-Q3970E and RyR1-Q3970K structures, there were no marked changes in the interaction of Ca2+ with the side chains of Glu3893, Glu3967, and Thr5001 (Fig. 5 and Table 1). Neither the negatively charged glutamate in Q3970E nor the positively charged lysine in Q3970K directly interacted with Ca2+. However, in RyR1-Q3970E, there occurred a repulsive interaction between two negatively charged Glu3970 and Glu3967. Further, additional energetically favorable interactions formed by Lys3970 with its neighboring residues Thr3966 and Glu3967 were observed in RyR1-Q3970K (Fig. 5 and Table 1). These secondary structural changes and gain of interactions may be correlated to low channel activities as observed in RyR1-Q3970K single-channel recordings. Differences between the structural rearrangements in the Ca2+-binding site of Q3970K and Q3970E variants are consistent with the different single-channel activities of the two RyR1 mutants.
Overall, our experimental and computational findings suggest that RyR1-Q3970K and RyR1-Q3970E did not alter channel activity by modifying the strong interactions of Ca2+ with Glu3893, Glu3967, and Thr5001 but rather by causing secondary structural changes in the presence of the three activating ligands Ca2+, ATP, and caffeine. RyR1-Q3970 in the secondary coordination sphere of Ca2+ appears to be involved in Ca2+-dependent activation of RyR1. Our data also suggest that RyR1-Q3970K is likely a CCD-associated loss-of-function channel that conducts Ca2+, and functional characterization of this mutation in skeletal muscle will provide further insights into CCD pathology.
GRANTS
This study was supported by National Institutes of Health Grants GM-114015 and GM-123247 (to N. V. Dokholyan), AR-018687 (to G. Meissner), and P20 GM-103499 and UL1 TR-001450 (to N. Yamaguchi).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
G.M. and N.Y. conceived and designed research; V.R.C., L.X., H.G.A., D.A.P., and N.Y. performed experiments; V.R.C., L.X., H.G.A., D.A.P., N.V.D., G.M., and N.Y. analyzed data; V.R.C., L.X., H.G.A., D.A.P., N.V.D., G.M., and N.Y. interpreted results of experiments; V.R.C., L.X., and G.M. prepared figures; G.M. and N.Y. drafted manuscript; V.R.C., L.X., H.G.A., D.A.P., N.V.D., G.M., and N.Y. edited and revised manuscript; V.R.C., L.X., H.G.A., D.A.P., N.V.D., G.M., and N.Y. approved final version of manuscript.
REFERENCES
- 1.Avila G, Dirksen RT. Functional effects of central core disease mutations in the cytoplasmic region of the skeletal muscle ryanodine receptor. J Gen Physiol 118: 277–290, 2001. doi: 10.1085/jgp.118.3.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Avila G, O’Brien JJ, Dirksen RT. Excitation-contraction uncoupling by a human central core disease mutation in the ryanodine receptor. Proc Natl Acad Sci USA 98: 4215–4220, 2001. doi: 10.1073/pnas.071048198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.DeLano WL. The PyMOL Molecular Graphics System, version 1.5.0.4 New York: Schrödinger, 2011. https://pymol.org/2/. [Google Scholar]
- 4.des Georges A, Clarke OB, Zalk R, Yuan Q, Condon KJ, Grassucci RA, Hendrickson WA, Marks AR, Frank J. Structural basis for gating and activation of RyR1. Cell 167: 145–157.e17, 2016. doi: 10.1016/j.cell.2016.08.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Efremov RG, Leitner A, Aebersold R, Raunser S. Architecture and conformational switch mechanism of the ryanodine receptor. Nature 517: 39–43, 2015. doi: 10.1038/nature13916. [DOI] [PubMed] [Google Scholar]
- 6.Franzini-Armstrong C, Protasi F. Ryanodine receptors of striated muscles: a complex channel capable of multiple interactions. Physiol Rev 77: 699–729, 1997. doi: 10.1152/physrev.1997.77.3.699. [DOI] [PubMed] [Google Scholar]
- 7.Gao L, Balshaw D, Xu L, Tripathy A, Xin C, Meissner G. Evidence for a role of the lumenal M3-M4 loop in skeletal muscle Ca2+ release channel (ryanodine receptor) activity and conductance. Biophys J 79: 828–840, 2000. doi: 10.1016/S0006-3495(00)76339-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gao L, Tripathy A, Lu X, Meissner G. Evidence for a role of C-terminal amino acid residues in skeletal muscle Ca2+ release channel (ryanodine receptor) function. FEBS Lett 412: 223–226, 1997. doi: 10.1016/S0014-5793(97)00781-3. [DOI] [PubMed] [Google Scholar]
- 9.Hess B, Kutzner C, van der Spoel D, Lindahl E. GROMACS 4: algorithms for highly efficient, load-balanced, and scalable molecular simulation. J Chem Theory Comput 4: 435–447, 2008. doi: 10.1021/ct700301q. [DOI] [PubMed] [Google Scholar]
- 10.Jungbluth H, Treves S, Zorzato F, Sarkozy A, Ochala J, Sewry C, Phadke R, Gautel M, Muntoni F. Congenital myopathies: disorders of excitation-contraction coupling and muscle contraction. Nat Rev Neurol 14: 151–167, 2018. doi: 10.1038/nrneurol.2017.191. [DOI] [PubMed] [Google Scholar]
- 11.Landstrom AP, Dobrev D, Wehrens XH. Calcium signaling and cardiac arrhythmias. Circ Res 120: 1969–1993, 2017. doi: 10.1161/CIRCRESAHA.117.310083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lanner JT. Ryanodine receptor physiology and its role in disease. Adv Exp Med Biol 740: 217–234, 2012. doi: 10.1007/978-94-007-2888-2_9. [DOI] [PubMed] [Google Scholar]
- 13.Lanner JT, Georgiou DK, Joshi AD, Hamilton SL. Ryanodine receptors: structure, expression, molecular details, and function in calcium release. Cold Spring Harb Perspect Biol 2: a003996, 2010. doi: 10.1101/cshperspect.a003996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leenhardt A, Denjoy I, Guicheney P. Catecholaminergic polymorphic ventricular tachycardia. Circ Arrhythm Electrophysiol 5: 1044–1052, 2012. doi: 10.1161/CIRCEP.111.962027. [DOI] [PubMed] [Google Scholar]
- 15.Loy RE, Orynbayev M, Xu L, Andronache Z, Apostol S, Zvaritch E, MacLennan DH, Meissner G, Melzer W, Dirksen RT. Muscle weakness in Ryr1I4895T/WT knock-in mice as a result of reduced ryanodine receptor Ca2+ ion permeation and release from the sarcoplasmic reticulum. J Gen Physiol 137: 43–57, 2011. doi: 10.1085/jgp.201010523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ludtke SJ, Serysheva II, Hamilton SL, Chiu W. The pore structure of the closed RyR1 channel. Structure 13: 1203–1211, 2005. doi: 10.1016/j.str.2005.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lyfenko AD, Ducreux S, Wang Y, Xu L, Zorzato F, Ferreiro A, Meissner G, Treves S, Dirksen RT. Two central core disease (CCD) deletions in the C-terminal region of RYR1 alter muscle excitation-contraction (EC) coupling by distinct mechanisms. Hum Mutat 28: 61–68, 2007. doi: 10.1002/humu.20409. [DOI] [PubMed] [Google Scholar]
- 18.Medeiros-Domingo A, Bhuiyan ZA, Tester DJ, Hofman N, Bikker H, van Tintelen JP, Mannens MM, Wilde AA, Ackerman MJ. The RYR2-encoded ryanodine receptor/calcium release channel in patients diagnosed previously with either catecholaminergic polymorphic ventricular tachycardia or genotype negative, exercise-induced long QT syndrome: a comprehensive open reading frame mutational analysis. J Am Coll Cardiol 54: 2065–2074, 2009. doi: 10.1016/j.jacc.2009.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meissner G. The structural basis of ryanodine receptor ion channel function. J Gen Physiol 149: 1065–1089, 2017. doi: 10.1085/jgp.201711878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meissner G, Rios E, Tripathy A, Pasek DA. Regulation of skeletal muscle Ca2+ release channel (ryanodine receptor) by Ca2+ and monovalent cations and anions. J Biol Chem 272: 1628–1638, 1997. doi: 10.1074/jbc.272.3.1628. [DOI] [PubMed] [Google Scholar]
- 21.Murayama T, Kurebayashi N, Yamazawa T, Oyamada H, Suzuki J, Kanemaru K, Oguchi K, Iino M, Sakurai T. Divergent activity profiles of type 1 ryanodine receptor channels carrying malignant hyperthermia and central core disease mutations in the amino-terminal region. PLoS One 10: e0130606, 2015. doi: 10.1371/journal.pone.0130606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murayama T, Ogawa H, Kurebayashi N, Ohno S, Horie M, Sakurai T. A tryptophan residue in the caffeine-binding site of the ryanodine receptor regulates Ca2+ sensitivity. Commun Biol 1: 98, 2018. doi: 10.1038/s42003-018-0103-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ogawa Y, Harafuji H. Osmolarity-dependent characteristics of [3H]ryanodine binding to sarcoplasmic reticulum. J Biochem 107: 894–898, 1990. doi: 10.1093/oxfordjournals.jbchem.a123144. [DOI] [PubMed] [Google Scholar]
- 24.Peng W, Shen H, Wu J, Guo W, Pan X, Wang R, Chen SR, Yan N. Structural basis for the gating mechanism of the type 2 ryanodine receptor RyR2. Science 354: aah5324, 2016. doi: 10.1126/science.aah5324. [DOI] [PubMed] [Google Scholar]
- 25.Samsó M, Feng W, Pessah IN, Allen PD. Coordinated movement of cytoplasmic and transmembrane domains of RyR1 upon gating. PLoS Biol 7: e85, 2009. doi: 10.1371/journal.pbio.1000085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Samsó M, Wagenknecht T, Allen PD. Internal structure and visualization of transmembrane domains of the RyR1 calcium release channel by cryo-EM. Nat Struct Mol Biol 12: 539–544, 2005. doi: 10.1038/nsmb938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schoenmakers TJ, Visser GJ, Flik G, Theuvenet AP. CHELATOR: an improved method for computing metal ion concentrations in physiological solutions. Biotechniques 12: 870–874, 1992. [PubMed] [Google Scholar]
- 28.Snoeck M, van Engelen BG, Küsters B, Lammens M, Meijer R, Molenaar JP, Raaphorst J, Verschuuren-Bemelmans CC, Straathof CS, Sie LT, de Coo IF, van der Pol WL, de Visser M, Scheffer H, Treves S, Jungbluth H, Voermans NC, Kamsteeg EJ. RYR1-related myopathies: a wide spectrum of phenotypes throughout life. Eur J Neurol 22: 1094–1112, 2015. doi: 10.1111/ene.12713. [DOI] [PubMed] [Google Scholar]
- 29.Takeshima H, Nishimura S, Matsumoto T, Ishida H, Kangawa K, Minamino N, Matsuo H, Ueda M, Hanaoka M, Hirose T, Numa S. Primary structure and expression from complementary DNA of skeletal muscle ryanodine receptor. Nature 339: 439–445, 1989. doi: 10.1038/339439a0. [DOI] [PubMed] [Google Scholar]
- 30.Treves S, Jungbluth H, Muntoni F, Zorzato F. Congenital muscle disorders with cores: the ryanodine receptor calcium channel paradigm. Curr Opin Pharmacol 8: 319–326, 2008. doi: 10.1016/j.coph.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 31.Xu L, Chirasani VR, Carter JS, Pasek DA, Dokholyan NV, Yamaguchi N, Meissner G. Ca2+-mediated activation of the skeletal-muscle ryanodine receptor ion channel. J Biol Chem 293: 19501–19509, 2018. doi: 10.1074/jbc.RA118.004453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xu L, Wang Y, Yamaguchi N, Pasek DA, Meissner G. Single-channel properties of heterotetrameric mutant RyR1 ion channels linked to core myopathies. J Biol Chem 283: 6321–6329, 2008. doi: 10.1074/jbc.M707353200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yamaguchi N, Xu L, Pasek DA, Evans KE, Meissner G. Molecular basis of calmodulin binding to cardiac muscle Ca2+ release channel (ryanodine receptor). J Biol Chem 278: 23480–23486, 2003. doi: 10.1074/jbc.M301125200. [DOI] [PubMed] [Google Scholar]
- 34.Yan Z, Bai X, Yan C, Wu J, Li Z, Xie T, Peng W, Yin C, Li X, Scheres SHW, Shi Y, Yan N. Structure of the rabbit ryanodine receptor RyR1 at near-atomic resolution. Nature 517: 50–55, 2015. doi: 10.1038/nature14063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yuchi Z, Van Petegem F. Ryanodine receptors under the magnifying lens: Insights and limitations of cryo-electron microscopy and X-ray crystallography studies. Cell Calcium 59: 209–227, 2016. doi: 10.1016/j.ceca.2016.04.003. [DOI] [PubMed] [Google Scholar]
- 36.Zalk R, Clarke OB, des Georges A, Grassucci RA, Reiken S, Mancia F, Hendrickson WA, Frank J, Marks AR. Structure of a mammalian ryanodine receptor. Nature 517: 44–49, 2015. doi: 10.1038/nature13950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhou H, Yamaguchi N, Xu L, Wang Y, Sewry C, Jungbluth H, Zorzato F, Bertini E, Muntoni F, Meissner G, Treves S. Characterization of recessive RYR1 mutations in core myopathies. Hum Mol Genet 15: 2791–2803, 2006. doi: 10.1093/hmg/ddl221. [DOI] [PubMed] [Google Scholar]