Abstract
Exhaustive single-leg exercise has been suggested to reduce time to task failure (Tlim) during subsequent exercise in the contralateral leg by exacerbating central fatigue development. We investigated the influence of acetaminophen (ACT), an analgesic that may blunt central fatigue development, on Tlim during single-leg exercise completed with and without prior fatiguing exercise of the contralateral leg. Fourteen recreationally active men performed single-leg severe-intensity knee-extensor exercise to Tlim on the left (Leg1) and right (Leg2) legs without prior contralateral fatigue and on Leg2 immediately following Leg1 (Leg2-CONTRA). The tests were completed following ingestion of 1-g ACT or maltodextrin [placebo (PL)] capsules. Intramuscular phosphorus-containing metabolites and substrates and muscle activation were assessed using 31P-MRS and electromyography, respectively. Tlim was not different between Leg1ACT and Leg1PL conditions (402 ± 101 vs. 390 ± 106 s, P = 0.11). There was also no difference in Tlim between Leg2ACT-CONTRA and Leg2PL-CONTRA (324 ± 85 vs. 311 ± 92 s, P = 0.10), but Tlim was shorter in Leg2ACT-CONTRA and Leg2PL-CONTRA than in Leg2CON (385 ± 104 s, both P < 0.05). There were no differences in intramuscular phosphorus-containing metabolites and substrates or muscle activation between Leg1ACT and Leg1PL and between Leg2ACT-CONTRA and Leg2PL-CONTRA (all P > 0.05). These findings suggest that levels of metabolic perturbation and muscle activation at Tlim are not different during single-leg severe-intensity knee-extensor exercise completed with or without prior fatiguing exercise of the contralateral leg. Despite contralateral fatigue, ACT ingestion did not alter neuromuscular responses, muscle metabolites, or exercise performance.
Keywords: intramuscular metabolites, intramuscular substrates, nonlocal muscle fatigue, 31P-magnetic resonance spectroscopy, Paracetamol
INTRODUCTION
The mechanisms of exercise-induced fatigue can be attributed to processes within the central nervous system, termed central fatigue, and within the contractile elements of the working muscle, termed peripheral fatigue. It is now recognized that peripheral and central fatigue development are interlinked, in part, via group III/IV muscle afferent feedback (25). Empirical support for a role of group III/IV muscle afferent feedback in modulating the mechanisms of neuromuscular fatigue is provided by reports that inhibition of group III/IV muscle afferent feedback, via lumbar intrathecal administration of fentanyl, lowers central fatigue development and results in increased skeletal muscle metabolic perturbation [greater and/or more rapid increases in ADP and Pi accumulation and declines in phosphocreatine (PCr) and pH] and, thus, peripheral fatigue development (1, 2, 8, 10–12, 39–41). Conversely, prior fatiguing single-limb exercise has been reported to accentuate central fatigue development and lead to lower peripheral fatigue development during subsequent fatiguing exercise in a contralateral or nonlocal (previously rested) muscle group, when group III/IV muscle afferent feedback would be expected to be elevated (3, 22, 23, 26, 34, 41). However, the underlying mechanisms of nonlocal muscle fatigue, including the effect of prior fatiguing single-limb exercise on skeletal muscle metabolic perturbation during subsequent fatiguing exercise in a contralateral or nonlocal muscle group, have yet to be resolved (see Ref. 23 for review). Moreover, while lumbar intrathecal administration of fentanyl and prior fatigue of a contralateral or nonlocal muscle group can alter group III/IV muscle afferent feedback and the physiological bases of exercise-induced neuromuscular fatigue (1–3, 8, 10–12, 22, 26, 28, 29, 34), the effect of such interventions on exercise performance is equivocal. Specifically, while some studies indicate that exercise performance is altered in these situations, i.e., enhanced with fentanyl (3) or impaired following contralateral fatigue (14, 22, 29, 34, 42), others report no significant effect (1, 2, 8, 10, 12, 16, 21, 35, 43, 46).
An emerging body of evidence suggests that oral ingestion of acetaminophen (ACT) can blunt the development of exercise-induced neuromuscular fatigue and improve exercise capacity and/or performance (19, 30–32). It is generally accepted that the principal mechanism of action of ACT is the inhibition of cyclooxygenase, the enzyme that catalyzes the synthesis of prostaglandins from arachidonic acid (4). Since prostaglandins sensitize nociceptors (37, 38) and since blocking cyclooxygenase attenuates group III/IV muscle afferent discharge during dynamic exercise (24), these mechanisms might account for reports of increased work output for the same level of perceived pain and exertion (19, 30) and elevated muscle activation (31, 32) during exercise after ACT ingestion. Therefore, ACT administration might be ergogenic by reducing, but not abolishing, the net magnitude of group III/IV muscle afferent feedback, leading to a blunting of exercise-induced central fatigue. Since ACT appears to attenuate exercise-induced neuromuscular fatigue by abating aspects of central fatigue development (19, 30–32), ACT might be more effective at lowering exercise-induced neuromuscular fatigue following prior exhaustive exercise in a contralateral limb. However, the effects of ACT ingestion on exercise-induced fatigue development and its underlying mechanisms following prior exercise in a contralateral limb have yet to be investigated.
The purpose of this study was to investigate the effects of ACT ingestion on exercise-induced neuromuscular fatigue and some of its underlying mechanisms during single-leg severe-intensity knee-extensor exercise completed with and without prior exhaustive severe-intensity knee-extensor exercise in the contralateral leg. It was hypothesized that 1) prior exhaustive exercise would impair subsequent exercise tolerance in the contralateral leg by lowering muscle activation and the degree of muscle metabolic perturbation [changes in muscle pH and PCr ([PCr]), ADP ([ADP]), and Pi ([Pi]) concentrations] that could be attained, 2) ACT ingestion would enhance single-leg knee-extensor exercise tolerance by increasing muscle activation [higher surface electromyogram (EMG)] and permitting a greater degree of muscle metabolic perturbation, and 3) completion of prior exercise by the contralateral leg would lead to a greater enhancement of exercise tolerance following ACT ingestion.
MATERIALS AND METHODS
Subjects.
Fourteen active men [age 23.8 (SD 4.7) yr, height 1.80 (SD 0.10) m, body mass 81.6 (SD 14.9) kg] volunteered to participate in the study. All procedures were approved by the Ethics Committee of the Department of Sport and Health Sciences, University of Exeter. The study conformed to the principles of the World Medical Association Declaration of Helsinki. Subjects completed a health questionnaire that was checked by a medical doctor to ensure that the subjects could safely consume ACT before performing exhaustive exercise. The questionnaire incorporated questions pertaining to known allergies to medications, current intake of medication, and prior use of ACT, as well as any history of illnesses, cigarette and illegal drug use, alcohol consumption, and chronic illnesses (personal and family history). Before each visit, subjects were required to refrain from caffeine (for ≥12 h), strenuous exercise and alcohol (for ≥24 h), and analgesics and any form of anti-inflammatory drug (for the duration of the experiment) and to arrive in a fully rested, hydrated state. With the exception of these restrictions, subjects were instructed to maintain their usual diet and exercise regimen during the study. All tests were performed at a similar time of day (±2 h).
Preexperimental procedures.
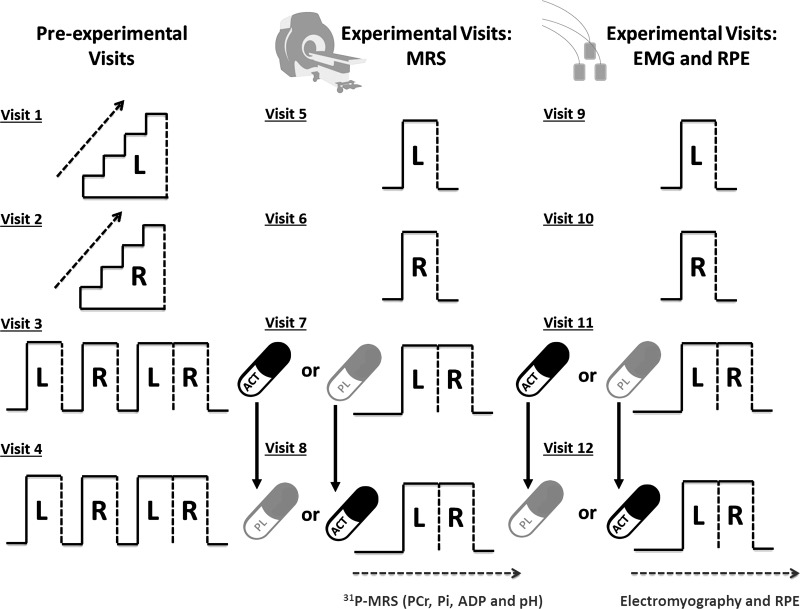
Subjects visited the laboratory on 12 occasions over an 8- to 12-wk period to complete the experimental testing, with ≥72 h separating consecutive tests (Fig. 1). The experimental testing incorporated four preexperimental trials (visits 1–4) and eight experimental trials (visits 5–12). Visits 1–4 were completed within a replica of an MRI scanner (with no magnetic field present). Initially, subjects completed a single-limb incremental test on the left leg (visit 1, Leg1) and right leg (visit 2, Leg2) to task failure to establish the limb-specific work rates that would be applied in subsequent experimental visits (see below). After these preliminary tests, subjects completed a familiarization session on visits 3 and 4 that comprised a single-leg severe-intensity constant work rate (CWR) test to task failure with the left leg (Leg1), a single-leg severe-intensity CWR test to task failure with the right leg (Leg2), and a crossover test, where the Leg1 protocol was repeated and immediately followed by the trial to assess contralateral fatigue in Leg2 (Leg2-CONTRA protocol). Severe-intensity exercise is defined as being above critical power (33). In these preliminary tests, the Leg1, Leg2, and Leg2 contralateral (Leg2-CONTRA) protocols were interspersed with 10 min of passive recovery.
Fig. 1.
Protocol schematic. Visits 1–4 were completed within a replica of the MRI scanner. Subjects completed a single-leg incremental test on the left (L) leg (visit 1, Leg1) and right (R) leg (visit 2, Leg2). On visits 3 and 4, subjects completed a familiarization session, which comprised a single-leg severe-intensity constant work rate (CWR) test to task failure with Leg1 and Leg2 and a crossover test where the Leg1 protocol was repeated and immediately followed by the Leg2 protocol (interspersed with 10 min of passive recovery). During visits 5 and 6, subjects completed the Leg1 and Leg2 protocols, respectively, without oral consumption of capsules. On visits 7 and 8, subjects commenced the crossover test 45 min following consumption of 1 g of maltodextrin (PL) and 45 min following consumption of 1 g of acetaminophen (ACT). Visits 5–8 were completed within the bore of an MRI scanner for assessment of intramuscular phosphorus-containing substrates and metabolites and then replicated within a replica of the MRI scanner (visits 9–12) to assess muscle electromyography (EMG) and ratings of perceived exertion (RPE). Dashed vertical lines represent the limit of tolerance [i.e., time to task failure (Tlim)] for each trial and/or leg. MRS, magnetic resonance spectroscopy; PCr, phosphocreatine.
Experimental procedures.
During visits 5 and 6, subjects completed the Leg1 and Leg2 protocols without oral consumption of capsules (Leg1CON and Leg2CON, respectively). On visits 7 and 8, subjects completed the crossover limb tests described above 45 min following consumption of 1 g of maltodextrin [placebo (PL)] to determine time to task failure (Tlim) values for Leg1 (Leg1PL) and Leg2-CONTRA (Leg2PL-CONTRA) and 45 min following consumption of 1 g of ACT to determine Tlim values for Leg1 (Leg1ACT) and Leg2-CONTRA (Leg2ACT-CONTRA). PL and ACT were administered in the form of two identically colored capsules. PL consisted of maltodextrin powder in gelatin capsules designed to have an appearance similar to ACT without analgesic or antipyretic effects. The oral consumption of PL and ACT ~45 min before commencement of exercise was selected to broadly coincide with attainment of the peak plasma ACT concentration ([ACT]), which occurs ~60 min after ACT ingestion (4, 17), at the onset of the Leg2-CONTRA tests. The PL and ACT conditions were administered double-blind in a counterbalanced crossover experimental design. Visits 5–8 were completed within the bore of an MRI scanner for assessment of exercise-induced changes in intramuscular phosphorus-containing substrates and metabolites. Visits 5–8 were replicated in visits 9–12 within a replica of the MRI scanner (with no magnetic field present) to assess muscle EMG and ratings of perceived exertion (RPE).
Experimental setup.
Exercise tests were performed with subjects in a prone position within the bore of a 1.5-T superconducting magnet (Gyroscan Clinical Intera, Philips, The Netherlands) using a custom-built ergometer for assessment of intramuscular [PCr], [Pi], [ADP], and pH (visits 5–8) or within a replica of the MRI scanner for preliminary testing (visits 1–4) and assessment of EMG and RPE responses (visits 9–12). The subject’s feet were fastened securely to padded foot braces using Velcro straps and connected to the ergometer load baskets via a rope-and-pulley system. The sprocket arrangement was such that when a bucket containing nonmagnetic weights was attached, it provided a concentric-only resistive load, allowing for the performance of rhythmic knee-extension exercise. Single-leg knee extensions over a distance of ∼0.22 m were performed continuously at a constant frequency, which was set in unison with the magnetic pulse sequence (40 pulses/min) to ensure that the quadriceps muscle was in the same phase of contraction during each magnetic resonance pulse acquisition. To prevent displacement of the quadriceps relative to the magnetic resonance spectroscopy (MRS) coil, Velcro straps were also fastened over the subject's thighs, hips, and lower back.
Experimental protocol.
To determine peak work rate for each leg, the subjects initially completed single-leg incremental knee-extensor exercise on visits 1 and 2 until they were unable to continue the prescribed work rate, as described previously (44). The load for the initial increment was 4 kg, which was increased by 0.5 kg/min thereafter until Tlim. Tlim was recorded when the subjects were unable to sustain the required contraction frequency for three consecutive repetitions. After these initial tests, the subjects were familiarized with the different exercise tests that comprised the experimental testing protocol. During these visits, a limb-specific, severe-intensity work rate, which was expected to elicit Tlim in ~5–8 min, was prescribed for each subject. The work rate initially selected was 80% of the peak work rate attained in the incremental test, and depending on responses in the familiarization tests, this was adjusted for each individual to give the desired exercise duration during subsequent tests.
The experimental exercise protocol consisted of CWR single-leg severe-intensity knee extension to Tlim. Initially, the subjects completed single-leg knee-extension exercise for each limb individually over two separate laboratory visits. Subsequently, to investigate the influence of ACT on contralateral leg fatigue, the subjects completed single-leg knee-extension exercise until task failure with Leg1 followed consecutively (<3 s) by the identical task with the contralateral leg (i.e., Leg2). These crossover tests to assess contralateral fatigue in Leg2 were completed 60 min following consumption of PL and ACT over two separate laboratory visits. For all trials, the subjects received strong verbal encouragement to continue for as long as possible, but they were given no feedback on the elapsed time.
MRS measurements.
31P-MRS data, with a spectral width of 1,500 Hz and 1,000 data points, were acquired every 1.5 s. Phase cycling with four phase cycles led to a spectrum being acquired every 6 s. The subsequent spectra were quantified by peak fitting using the AMARES fitting algorithm in the jMRUI (v3) software package. Absolute values of [PCr] and [Pi] were subsequently calculated from the PCr-to-ATP and Pi-to-ATP ratios, with the assumption of 8.2 mM ATP. Intracellular pH was calculated using the chemical shift of the Pi spectra relative to the PCr peak. [ADP] was calculated as described by Kemp et al. (27). In all cases, relative amplitudes were corrected for partial saturation resulting from the short repetition time relative to T1 relaxation time via a spectrum consisting of 24 averages that was acquired with a TR of 20 s before the commencement of exercise testing.
Electromyography.
Throughout visits 9–12, muscle activity of the right and left vastus lateralis was recorded using active bipolar bar electrodes with a single differential configuration (model DE2.1, DelSys, Boston, MA). Initially, the leg was shaved and cleaned with alcohol to minimize skin impedance. The electrodes were placed over the respective muscle bellies parallel to the longitudinal axis of each muscle (Surface EMG for Non-Invasive Assessment of Muscles guidelines). Double-sided adhesive tape and a hypoallergenic medical tape were used to ensure stability of the EMG sensor. The position of the EMG electrodes was measured with respect to the location of the patella and the anterior superior iliac spine and marked with indelible ink to ensure placement in the same location on subsequent visits. The ground electrode was placed over the patella of the respective leg. The EMG signals were preamplified (×1,000), band-pass-filtered (20–450 Hz; Bagnoli-8, DelSys), and then transferred to a computer with a sampling frequency of 2 kHz. EMG data were recorded continuously and digitized synchronously with 16-bit resolution via an analog-to-digital converter (±5-V range, CED 1401 power, Cambridge Electronic Design, Cambridge, UK) using Spike2 software (Cambridge Electronic Design). During these trials, RPE was measured at 2-min intervals from the onset of exercise using Borg’s 6–20 scale (9).
Data analysis.
Baseline values for [PCr], [Pi], [ADP], and pH were defined as the mean values measured over the final 60 s of rest (i.e., before initiation of the severe-intensity exercise bout). Baseline values for Leg2 during the crossover protocol (for both PL and ACT) were calculated during the final 60 s of exhaustive Leg1 exercise. End-exercise values for these variables were defined as the mean values measured over the final 30 s of exercise. The changes (Δ) in [PCr], [Pi], [ADP], and pH across the protocol were then calculated as the difference between end-exercise and baseline values. [PCr], [Pi], and [ADP] are expressed as absolute concentrations and as percent change relative to resting baseline (i.e., 100%). The overall rate of change for [PCr], [Pi], [ADP], and pH was calculated as the difference between end-exercise and baseline values divided by Tlim. EMG was average-rectified and normalized to the first 30 s of each trial (aEMG). For analysis, Tlim values obtained from visits 5–8 were used. Visits 9–12 were used to overlay EMG and RPE responses on 31P-MRS data.
Statistics.
Differences in Tlim, baseline and end-exercise aEMG, and muscle [PCr], [Pi], [ADP], and pH between control limbs (i.e., Leg1 vs. Leg2) were assessed using paired-samples t-tests. A two-way (time × condition) repeated-measures ANOVA was employed to test for differences in the profiles of muscle [PCr], [Pi], [ADP], and pH, aEMG (using 30-s mean values), and RPE (using 120-s mean values). Where the ANOVA revealed a significant main or interaction effect, post hoc tests were completed using Bonferroni’s correction. For calculation of effect size, partial η2 was used for omnibus tests. Cohen's d was used to calculate the effect size for paired t-tests and post hoc comparisons. Where sphericity was violated, a Greenhouse-Geisser correction factor was applied. For all tests, results were considered statistically significant when P < 0.05. Data are presented as means (SD) unless otherwise indicated. All statistical analyses were conducted using IBM SPSS Statistics version 24.
RESULTS
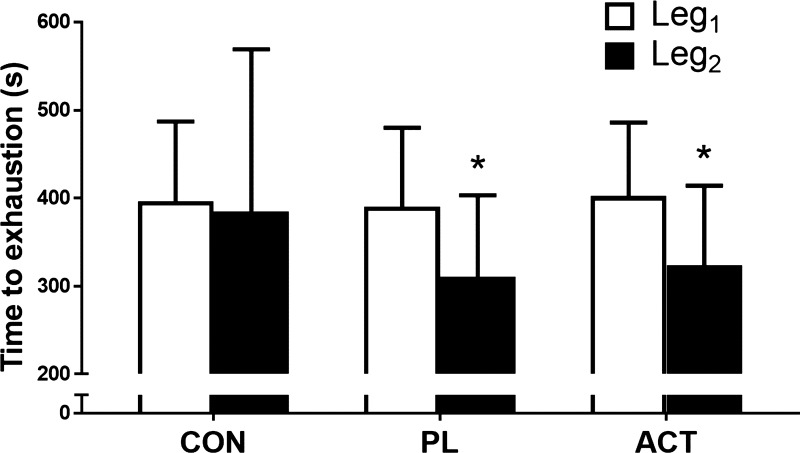
There was no difference in Tlim during the Leg1CON [396 (SD 105) s] and Leg2CON [385 (SD 104) s] protocols [P = 0.20, d = 0.10, coefficient of variation = 2.0 (SD 1.7) %; Fig. 2]. Moreover, there were no differences in [PCr], [Pi], [ADP], pH (Table 1, Fig. 3), aEMG amplitude (Table 2, Fig. 5), and RPE (Fig. 6) between Leg1CON and Leg2CON at any time (all P > 0.05). Compared with Leg2CON, Tlim was reduced by 19% when Leg2 was preceded by exhaustive exercise in Leg1 following consumption of PL [Leg2CON and Leg2PL-CONTRA 385 (SD 104) and 311 (SD 92) s, respectively, P < 0.01, d = 0.76; Fig. 2].
Fig. 2.
Exercise tolerance [time to task failure (exhaustion)] in Leg1CON, Leg2CON, Leg1PL, Leg2PL-CONTRA, Leg1ACT, and Leg2ACT-CONTRA conditions. CON, control; PL, placebo; CONTRA, contralateral; ACT, acetaminophen. Values are means (SD). *Significantly different from Leg1 (P < 0.05).
Table 1.
Muscle metabolic responses in Leg1CON, Leg1PL, Leg1ACT, Leg2CON, Leg2PL-CONTRA, and Leg2ACT-CONTRA conditions
| Leg1CON | Leg1PL | Leg1ACT | Leg2CON | Leg2PL-CONTRA | Leg2ACT-CONTRA | |
|---|---|---|---|---|---|---|
| [PCr] | ||||||
| Baseline, % | 100 (0) | 100 (0) | 100 (0) | 100 (0) | 92 (5)* | 93 (4)* |
| 120 s, % | 70 (8) | 70 (8) | 71 (7) | 71 (8) | 62 (9)* | 63 (7)* |
| End exercise, % | 42 (9) | 41 (9) | 41 (8) | 44 (8) | 45 (7) | 44 (8) |
| Rate of change, mmol/s | −0.06 (0.01) | −0.06 (0.03) | −0.06 (0.02) | −0.05 (0.03) | −0.06 (0.04) | −0.06 (0.03) |
| [Pi] | ||||||
| Baseline, % | 100 (0) | 100 (0) | 100 (0) | 100 (0) | 125 (24)* | 126 (23) |
| 120 s, % | 310 (66) | 313 (71) | 306 (62) | 312 (66) | 316 (70) | 318 (64) |
| End exercise, % | 590 (149) | 590 (137) | 594 (156) | 588 (177) | 459 (110)* | 460 (109)* |
| Rate of change, mmol/s | 0.05 (0.02) | 0.05 (0.02) | 0.05 (0.02) | 0.05 (0.02) | 0.05 (0.02) | 0.05 (0.02) |
| [ADP] | ||||||
| Baseline, % | 100 (0) | 100 (0) | 100 (0) | 100 (0) | 200 (78)* | 201 (77)* |
| 120 s, % | 404 (161) | 415 (183) | 400 (148) | 412 (170) | 538 (176) | 516 (154)* |
| End-exercise, % | 1,028 (386) | 1,036 (421) | 1,046 (409) | 1,024 (401) | 980 (316) | 978 (312) |
| Rate of change, µmol/s | 0.15 (0.08) | 0.15 (0.09) | 0.14 (0.07) | 0.15 (0.09) | 0.17 (0.10) | 0.15 (0.09) |
| pH | ||||||
| Baseline | 7.04 (0.01) | 7.03 (0.02) | 7.05 (0.04) | 7.04 (0.03) | 7.04 (0.03) | 7.05 (0.02) |
| 120 s | 6.96 (0.09) | 6.94 (0.07) | 6.92 (0.08) | 6.95 (0.08) | 6.93 (0.10) | 6.94 (0.08) |
| End-exercise | 6.77 (0.18) | 6.76 (0.15) | 6.76 (0.16) | 6.83 (0.15) | 6.83 (0.20) | 6.80 (0.15) |
Values are means (SD) of 14 male subjects who performed single-leg severe-intensity knee-extensor exercise to task failure on the left (Leg1) and right (Leg2) legs without prior contralateral fatigue and on Leg2 immediately following Leg1 (Leg2-CONTRA). PL, placebo; ACT, acetaminophen; [PCr], phosphocreatine concentration; [Pi], Pi concentration; [ADP], ADP concentration.
P < 0.05 vs. Leg2CON.
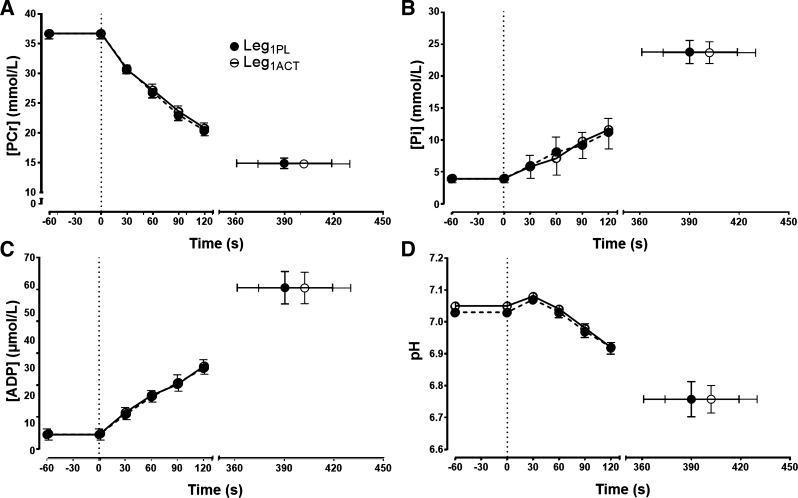
Fig. 3.
Intramuscular phosphocreatine (PCr) concentration ([PCr]; A), Pi concentration ([Pi]; B), ADP concentration ([ADP]; C), and pH (D) during severe-intensity single-leg knee-extensor exercise in the left leg following ingestion of placebo (Leg1PL) and acetaminophen (Leg1ACT). Values are group means ± SE.
Table 2.
EMG responses of the vastus lateralis in Leg1CON, Leg1PL, Leg1ACT, Leg2CON, Leg2PL-CONTRA, and Leg2ACT-CONTRA conditions
| Leg1CON | Leg1PL | Leg1ACT | Leg2CON | Leg2PL-CONTRA | Leg2ACT-CONTRA | |
|---|---|---|---|---|---|---|
| EMGRMS amplitude | ||||||
| Baseline, mV | 0.04 (0.01) | 0.04 (0.02) | 0.04 (0.02) | 0.04 (0.02) | 0.05 (0.02)* | 0.05 (0.02)* |
| End exercise, % | 229 (54) | 224 (43) | 238 (51) | 234 (52) | 226 (58) | 242 (52) |
| 120 s, % | 150 (27) | 160 (25) | 166 (26) | 158 (29) | 155 (32) | 158 (34) |
Values are means (SD) of 10 subjects who performed single-leg severe-intensity knee-extensor exercise to task failure on the left (Leg1) and right (Leg2) legs without prior contralateral fatigue and on Leg2 immediately following Leg1 (Leg2-CONTRA). PL, placebo; ACT, acetaminophen; RMS, root mean square.
P < 0.05 vs. Leg2CON.
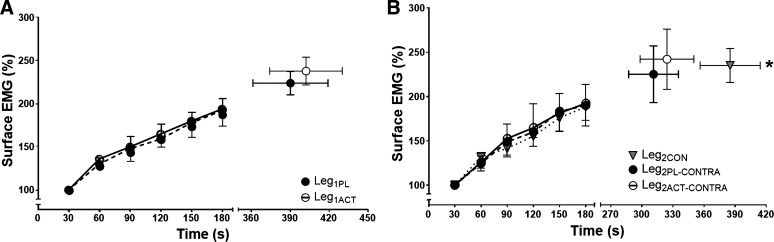
Fig. 5.
A: surface EMG of the vastus lateralis during severe-intensity single-leg knee-extensor exercise in the left leg following ingestion of placebo (Leg1PL) and acetaminophen (Leg1ACT). B: surface EMG of the vastus lateralis during severe-intensity single-leg knee-extensor exercise in the right control leg (Leg2CON) and in Leg2 following prior exhaustive exercise in Leg1 after ingestion of placebo (Leg2PL-CONTRA) and acetaminophen (Leg2ACT-CONTRA). Mean values for average rectified EMG during each muscle contraction were calculated and averaged over each 30-s period. Values are group means ± SE relative to the first 30 s of each trial. *Time to task failure (Tlim) significantly different from Leg2PL-CONTRA and Leg2ACT-CONTRA (P < 0.05).
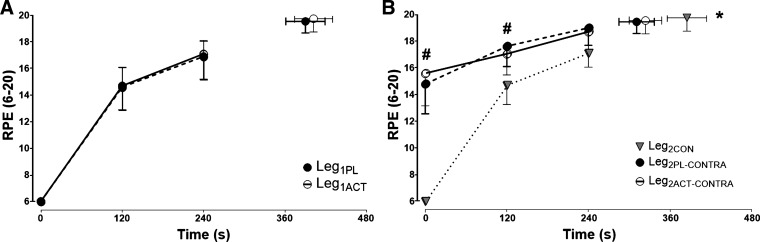
Fig. 6.
A: ratings of perceived exertion (RPE) during severe-intensity single-leg knee-extensor exercise of the left leg following ingestion of placebo (Leg1PL) and acetaminophen (Leg1ACT). B: RPE during severe-intensity single-leg knee-extensor exercise of the right control leg (Leg2CON) and the right leg following prior exhaustive exercise in the left leg after ingestion of placebo (Leg2PL-CONTRA) and acetaminophen (Leg2ACT-CONTRA). Values are group means ± SE. *Time to task failure (Tlim) significantly different from Leg2PL-CONTRA and Leg2ACT-CONTRA (P < 0.05); #RPE significantly different from Leg2CON (P < 0.05).
Effect of ACT on single-leg exercise tolerance and contralateral leg fatigue.
There was no difference in Tlim between the Leg1CON [396 (SD 105) s], Leg1ACT [402 (SD 101) s], and Leg1PL [390 (SD 106) s] conditions (P = 0.55, η2 = 0.07; Fig. 2). Tlim values were significantly lower for Leg2PL-CONTRA and Leg2ACT-CONTRA than for Leg2CON (P < 0.01, η2 = 0.71; Fig. 2). However, there was no difference in Tlim between Leg2PL-CONTRA and Leg2ACT-CONTRA [311 (SD 92) and 324 (SD 85) s, respectively, P = 0.09, d = 0.15, coefficient of variation = 4.9 (SD 5.4) %; Fig. 2].
Muscle metabolic measurements.
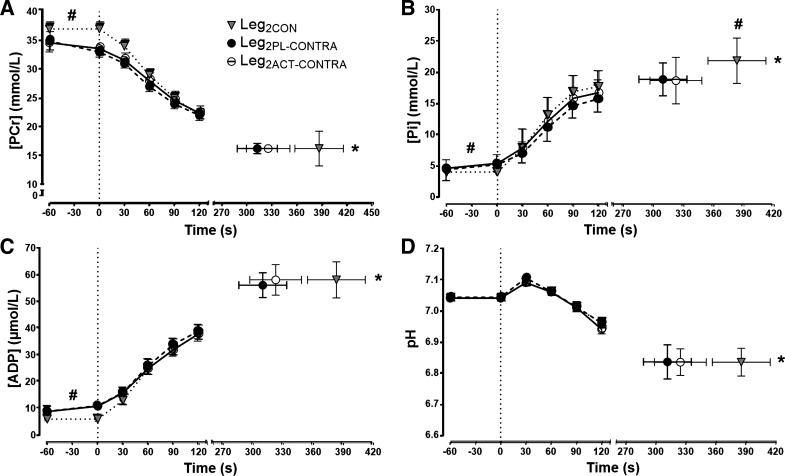
The [PCr], [Pi], [ADP], and pH profiles are illustrated in Fig. 3 for Leg1PL and Leg1ACT and in Fig. 4 for Leg2CON, Leg2PL-CONTRA, and Leg2ACT-CONTRA. There were no significant differences in [PCr], [Pi], [ADP], or pH at any time points between Leg1CON and Leg2CON (all P > 0.05; Table 1, Fig. 3). Similarly, there were no significant differences in end-exercise [PCr], [ADP], and pH between the Leg2CON, Leg2PL-CONTRA, and Leg2ACT-CONTRA conditions (Table 1, Fig. 4). However, end-exercise [Pi] was significantly lower in Leg2PL-CONTRA and Leg2ACT-CONTRA than in Leg2CON (P < 0.05, η2 = 0.89; Table 1, Fig. 4). Baseline [PCr] was significantly higher (P < 0.0001, η2 = 3.04), and [Pi] (P < 0.01, η2 = 2.13) and [ADP] (P < 0.01, η2 = 2.55; Table 1, Fig. 4) were significantly lower, in Leg2CON than Leg2PL-CONTRA and Leg2ACT-CONTRA, respectively. The rates of change for [Pi], [PCr], [ADP], and pH were not different between Leg2CON, Leg2PL-CONTRA, and Leg2ACT-CONTRA conditions.
Fig. 4.
Intramuscular phosphocreatine (PCr) concentration ([PCr]; A), Pi concentration ([Pi]; B), ADP concentration ([ADP]; C), and pH (D) during severe-intensity single-leg knee-extensor exercise in the right control leg (Leg2CON) and in the right leg following prior exhaustive exercise in the left leg after ingestion of placebo (Leg2PL-CONTRA) and ACT (Leg2ACT-CONTRA). Values are group means ± SE. *Time to task failure (Tlim) significantly different from Leg2PL-CONTRA and Leg2ACT-CONTRA (P < 0.05); #[Pi] significantly different from Leg2PL-CONTRA and Leg2ACT-CONTRA (P < 0.05).
Electromyography.
aEMG amplitude of vastus lateralis rose significantly from the first minute of exercise to end exercise in all conditions (P < 0.01, η2 = 3.8; Fig. 5). However, there were no differences in aEMG between Leg1CON, Leg1PL, and Leg1ACT at Tlim (Table 2, Fig. 5). End-exercise aEMG in Leg2CON was also not different from Leg2PL-CONTRA and Leg2ACT-CONTRA (Table 2, Fig. 5). However, absolute aEMG was elevated at the start of Leg2PL-CONTRA and Leg2ACT-CONTRA compared with Leg2CON (P < 0.01, η2 = 0.58; Table 2, Fig. 5).
Ratings of perceived exertion.
RPE increased in all trials following the onset of exercise (Fig. 6). However, there were no differences in RPE between Leg1CON, Leg1PL, and Leg1ACT at any time point (P = 0.72, η2 = 0.08; Fig. 6). The rate of rise and the end-exercise RPE were also not different between the Leg2CON trial and the Leg2PL-CONTRA and Leg2ACT-CONTRA trials (P = 0.66, η2 = 0.18). However, at the onset of exercise, RPE was significantly higher in Leg2PL-CONTRA and Leg2ACT-CONTRA than in Leg2CON (P < 0.01, η2 = 0.55; Fig. 6). Specifically, during the first 2 min of exercise, RPE was elevated 14% and 13% in Leg2PL-CONTRA and Leg2ACT-CONTRA, respectively, compared with Leg2CON (P < 0.01). There were no differences in RPE at any time points between Leg2PL-CONTRA and Leg2ACT-CONTRA (P = 0.60, η2 = 0.21; Fig. 6).
DISCUSSION
The principal original finding of this study was that while Tlim was lower during severe-intensity single-leg knee-extensor exercise after completion of prior fatiguing exercise in the contralateral leg, this effect was not mitigated by acute ACT ingestion. We found no differences in the rates of change or end-exercise values for skeletal muscle activation (via EMG), metabolic perturbation (via 31P-MRS), and perception of effort (via RPE) during exercise after prior contralateral leg fatigue following ACT and PL ingestion. Moreover, there were no differences in Tlim, skeletal muscle activation, metabolic perturbation, and RPE during single-leg exercise without completion of prior fatiguing exercise by the contralateral leg following ACT and PL ingestion. These findings do not support our experimental hypotheses and suggest that acute ingestion of 1 g of ACT does not improve Tlim, skeletal muscle activation, metabolic perturbation, or perceived exertion during single-leg severe-intensity knee-extensor exercise completed with or without prior fatiguing exercise by the contralateral leg.
In the present study, Tlim was shorter in the Leg2PL-CONTRA than the Leg2CON protocol, indicative of an earlier task failure after completion of exhaustive exercise in the contralateral leg compared with no prior fatiguing contralateral leg exercise. This observation is consistent with some (3, 14, 22, 29, 34, 42), but not all (16, 21, 35, 43, 46), previous studies reporting greater fatigue development after prior contralateral or nonlocal muscle fatigue. While the neuromuscular bases of contralateral fatigue development have yet to be fully resolved (23), there is evidence that greater central fatigue makes an important contribution to this phenomenon (3). In the current study, RPE was higher at baseline and over the initial stages of the Leg2PL-CONTRA test than the Leg2CON test, leading to an earlier attainment of peak RPE and Tlim, consistent with previous observations (3) and the notion that afferent feedback may contribute to increased pain and effort sensation (1, 20). Amann et al. (3) reported a lower EMG response at task failure and reduced peripheral fatigue development after prior contralateral leg fatigue. Although the EMG amplitude was not different at task failure in the current study between the Leg2CON and Leg2PL-CONTRA tests, baseline EMG was elevated in the Leg2PL-CONTRA condition, presumably due to isometric stabilization, leading to the earlier attainment of the same peak EMG amplitude. It should be noted here that EMG responses were normalized to the initial exercise values in the present study and in the study of Amann et al. (3). The greater muscle activation in the nonexercising contralateral leg during the baseline “resting” period in the Leg2PL-CONTRA condition was accompanied by lower muscle [PCr] and higher muscle [Pi] and [ADP] than in the Leg2CON condition. Since there were no differences in muscle [PCr] and [ADP] at Tlim and since the rates of change in [PCr] and [ADP] were not different between the Leg2CON and Leg2PL-CONTRA tests, the muscle [PCr] nadir and [ADP] peak were attained earlier in the Leg2PL-CONTRA test. These observations cohere with reports that the end-exercise values of muscle [PCr], [ADP], and pH are consistent when several bouts of exhaustive exercise of differing duration are completed within the severe-intensity domain (7, 45) and when Tlim is altered via prior passive heating of the legs (6) or by hyperoxic gas inhalation (45). Interestingly, however, and despite a higher baseline muscle [Pi] in the Leg2PL-CONTRA than the Leg2CON condition, muscle [Pi] was lower at task failure in the Leg2PL-CONTRA test. These novel observations suggest that the ergolytic effect of prior contralateral fatigue may be related, at least in part, to a limitation in the attainment of peak intramuscular [Pi].
It is unclear why prior contralateral leg fatigue limited the attainment of peak [Pi] in the Leg2PL-CONTRA condition compared with the Leg2CON condition, whereas the peak [ADP] and the nadir in pH and [PCr] were not different between these conditions. However, our observations of a limited peak perturbation of muscle [Pi], but not pH, [PCr], and [ADP], when group III/IV muscle afferent feedback would be expected to be elevated via prior contralateral fatigue (3) are in accord with studies from others who observed greater peak perturbation of muscle [Pi], but not pH, [PCr], and [ADP], when group III/IV muscle afferent feedback was abolished via lumbar intrathecal administration of fentanyl (8, 11, 12). Together, these complementary observations suggest that intramuscular phosphorus-containing metabolites and substrates may not respond in a uniform manner to manipulations in skeletal muscle group III/IV afferent feedback and that muscle [Pi] might be the more sensitive marker of muscle metabolic strain. However, it should be acknowledged that since within-test variability is greater for contracting skeletal muscle [Pi] than for pH, [PCr], and [ADP] (15), further research is required to verify these observations.
Although the completion of prior single-leg fatiguing exercise lowered Tlim during subsequent exercise in the contralateral leg in the current study, there were no differences between the Leg2ACT-CONTRA and Leg2PL-CONTRA conditions in Tlim, RPE, or muscle activation and phosphorus-containing metabolites and substrates. Similarly, and also in contrast to our hypothesis, acute ACT ingestion did not alter Tlim, RPE, or muscle activation, pH, [PCr], [ADP], or [Pi] during single-leg severe-intensity knee-extensor exercise completed without prior fatiguing exercise in the contralateral leg: these responses were similar between the Leg1CON, Leg1PL, and Leg1ACT conditions. These findings conflict with reports that acute ACT consumption can improve exercise performance by increasing work output for the same level of pain and effort sensation (19, 30) and by increasing muscle activation (31, 32).
Experimental considerations.
The lack of an ergogenic effect of ACT administration in the current study might be due to differences in the ACT administration procedure compared with previous studies reporting improved performance and delayed neuromuscular fatigue development (19, 30–32). In the present study, ACT was ingested 45 min before the start of the Leg1ACT test, which immediately transitioned to the Leg2ACT-CONTRA protocol, the primary focus of the current study. Since peak plasma [ACT] is attained ~60 min after oral ACT ingestion (4, 17), we elected to administer ACT such that peak plasma [ACT] was expected to coincide with the onset of the Leg2ACT-CONTRA, rather than the Leg1ACT, protocol. This might account for the lack of an ergogenic effect of ACT during the Leg1ACT protocol compared with other studies in which ACT was administered 60 min before the performance trial (19, 30–32). Therefore, we cannot exclude the possibility that earlier ACT ingestion (18), at the same or a greater dose (19, 30), might have resulted in improved single-leg severe-intensity exercise tolerance. However, interstudy differences in participant characteristics (i.e., training status, motivation, and responsiveness to analgesic medication) may have contributed to the differences in ergogenicity observed following ACT ingestion between the current study and some previous studies (19, 30–32).
In addition to differences in the ACT dosing procedure, the lack of an ergogenic effect of ACT administration in the current study might be linked to the nature of the fatiguing exercise test administered. Our subjects completed continuous single-leg severe-intensity knee-extensor exercise until task failure with no predetermined end point (i.e., an “open-loop” exercise test). This differs from situations in which ACT ingestion has been reported to be ergogenic, such as completion of a fixed-distance (16.1-km) time trial (30), a fixed number of maximal-effort repetitions (19, 31), or a fixed duration of maximal effort (32), all of which have a predetermined end point (i.e., a “closed-loop” exercise task). Moreover, since exercise-induced pain sensation is positively associated with exercise intensity (5, 13) and since ACT ingestion is suggested to be ergogenic by mitigating pain sensation (19, 30), this might account for the lack of improvement in performance in the longer-duration, continuous severe-intensity exercise test we employed compared with the improved exercise performance that has been reported during maximal-intensity exercise (19, 31, 32). With regard to contralateral fatigue development, we cannot exclude the possibility that ACT might have been effective at attenuating the effects of prior single-leg fatigue on Tlim during subsequent exercise if a greater degree of contralateral fatigue had been attained. For example, Tlim was lowered by 19% in Leg2PL-CONTRA compared with Leg2CON in the current study, whereas Amann et al. (3) reported a much larger (49%) reduction in Tlim following contralateral limb fatigue, which would have provided greater scope for an ergogenic effect with ACT ingestion. Moreover, since RPE is higher and Tlim is shorter at the same relative exercise intensity when a larger muscle mass is recruited (36), it is possible that ACT ingestion might have improved Tlim during exercise after prior fatigue, had a larger muscle mass been recruited in either the initial or the subsequent fatiguing exercise task. Further research is required to assess the exercise settings in which ACT administration is more or less likely to be ergogenic, including those with small compared with large muscle group exercise, in different exercise intensity domains, and with different pacing profiles (CWR compared with maximal and self-paced).
Perspectives and Significance
The completion of prior single-leg fatiguing exercise compromised exercise tolerance during subsequent exercise in the contralateral leg. This ergolytic effect of prior contralateral leg fatigue was accompanied by elevated baseline RPE, muscle activation, and [ADP] and lower baseline [PCr], leading to the earlier attainment of peak (RPE, muscle activation, and [ADP]) or nadir (muscle [PCr]) values in these variables and attainment of a submaximal end-exercise [Pi]. However, acute ACT ingestion was not effective at lowering perceived exertion, increasing muscle activation or intramuscular perturbation, or enhancing Tlim during single-leg severe-intensity exercise completed with or without prior fatigue in the contralateral leg. These findings do not support an ergogenic effect of analgesia during severe-intensity single-leg dynamic contractions.
GRANTS
This research was not sponsored by any funding body external to the University of Exeter. J. Fulford’s salary was supported via National Institute for Health Research Grant CRF/2016/10027 to the University of Exeter.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
P.T.M., S.J.B., A.V., and A.M.J. conceived and designed research; P.T.M., R.A.B., and J.F. performed experiments; P.T.M., R.A.B., and J.F. analyzed data; P.T.M., S.J.B., J.F., A.V., and A.M.J. interpreted results of experiments; P.T.M. and R.A.B. prepared figures; P.T.M., S.J.B., and A.M.J. drafted manuscript; P.T.M., S.J.B., J.F., and A.M.J. edited and revised manuscript; P.T.M., S.J.B., R.A.B., J.F., A.V., and A.M.J. approved final version of manuscript.
ACKNOWLEDGMENTS
Present address for S. J. Bailey: School of Sport, Exercise and Health Sciences, Loughborough University, Epinal Way, Loughborough, Leicestershire LE11 3TU, UK.
REFERENCES
- 1.Amann M, Blain GM, Proctor LT, Sebranek JJ, Pegelow DF, Dempsey JA. Implications of group III and IV muscle afferents for high-intensity endurance exercise performance in humans. J Physiol 589: 5299–5309, 2011. doi: 10.1113/jphysiol.2011.213769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amann M, Proctor LT, Sebranek JJ, Pegelow DF, Dempsey JA. Opioid-mediated muscle afferents inhibit central motor drive and limit peripheral muscle fatigue development in humans. J Physiol 587: 271–283, 2009. doi: 10.1113/jphysiol.2008.163303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amann M, Venturelli M, Ives SJ, McDaniel J, Layec G, Rossman MJ, Richardson RS. Peripheral fatigue limits endurance exercise via a sensory feedback-mediated reduction in spinal motoneuronal output. J Appl Physiol (1985) 115: 355–364, 2013. doi: 10.1152/japplphysiol.00049.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anderson BJ. Paracetamol (acetaminophen): mechanisms of action. Paediatr Anaesth 18: 915–921, 2008. doi: 10.1111/j.1460-9592.2008.02764.x. [DOI] [PubMed] [Google Scholar]
- 5.Astokorki AH, Mauger AR. Tolerance of exercise-induced pain at a fixed rating of perceived exertion predicts time trial cycling performance. Scand J Med Sci Sports 27: 309–317, 2017. doi: 10.1111/sms.12659. [DOI] [PubMed] [Google Scholar]
- 6.Bailey SJ, Wilkerson DP, Fulford J, Jones AM. Influence of passive lower-body heating on muscle metabolic perturbation and high-intensity exercise tolerance in humans. Eur J Appl Physiol 112: 3569–3576, 2012. doi: 10.1007/s00421-012-2336-6. [DOI] [PubMed] [Google Scholar]
- 7.Black MI, Jones AM, Blackwell JR, Bailey SJ, Wylie LJ, McDonagh ST, Thompson C, Kelly J, Sumners P, Mileva KN, Bowtell JL, Vanhatalo A. Muscle metabolic and neuromuscular determinants of fatigue during cycling in different exercise intensity domains. J Appl Physiol (1985) 122: 446–459, 2017. doi: 10.1152/japplphysiol.00942.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blain GM, Mangum TS, Sidhu SK, Weavil JC, Hureau TJ, Jessop JE, Bledsoe AD, Richardson RS, Amann M. Group III/IV muscle afferents limit the intramuscular metabolic perturbation during whole body exercise in humans. J Physiol 594: 5303–5315, 2016. doi: 10.1113/JP272283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Borg GAV. An Introduction to Borgs RPE Scale. New York: Movement Publications, 1985. [Google Scholar]
- 10.Broxterman RM, Layec G, Hureau TJ, Amann M, Richardson RS. Skeletal muscle bioenergetics during all-out exercise: mechanistic insight into the oxygen uptake slow component and neuromuscular fatigue. J Appl Physiol (1985) 122: 1208–1217, 2017. doi: 10.1152/japplphysiol.01093.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Broxterman RM, Layec G, Hureau TJ, Morgan DE, Bledsoe AD, Jessop JE, Amann M, Richardson RS. Bioenergetics and ATP synthesis during exercise: role of group III/IV muscle afferents. Med Sci Sports Exerc 49: 2404–2413, 2017. doi: 10.1249/MSS.0000000000001391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Broxterman RM, Hureau TJ, Layec G, Morgan DE, Bledsoe AD, Jessop JE, Amann M, Richardson RS. Influence of group III/IV muscle afferents on small muscle mass exercise performance: a bioenergetics perspective. J Physiol 596: 2301–2314, 2018. doi: 10.1113/JP275817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cook DB, O’Connor PJ, Eubanks SA, Smith JC, Lee M. Naturally occurring muscle pain during exercise: assessment and experimental evidence. Med Sci Sports Exerc 29: 999–1012, 1997. doi: 10.1097/00005768-199708000-00004. [DOI] [PubMed] [Google Scholar]
- 14.Doix AC, Lefèvre F, Colson SS. Time course of the cross-over effect of fatigue on the contralateral muscle after unilateral exercise. PLoS One 8: e64910, 2013. doi: 10.1371/journal.pone.0064910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Edwards LM, Tyler DJ, Kemp GJ, Dwyer RM, Johnson A, Holloway CJ, Nevill AM, Clarke K. The reproducibility of 31-phosphorus MRS measures of muscle energetics at 3 Tesla in trained men. PLoS One 7: e37237, 2012. doi: 10.1371/journal.pone.0037237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Elmer SJ, Amann M, McDaniel J, Martin DT, Martin JC. Fatigue is specific to working muscles: no cross-over with single-leg cycling in trained cyclists. Eur J Appl Physiol 113: 479–488, 2013. doi: 10.1007/s00421-012-2455-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Forrest JAH, Clements JA, Prescott LF. Clinical pharmacokinetics of Paracetamol. Clin Pharmacokinet 7: 93–107, 1982. doi: 10.2165/00003088-198207020-00001. [DOI] [PubMed] [Google Scholar]
- 18.Foster J, Mauger A, Thomasson K, White S, Taylor L. Effect of acetaminophen ingestion on thermoregulation of normothermic, non-febrile humans. Front Pharmacol 7: 54, 2016. doi: 10.3389/fphar.2016.00054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Foster J, Taylor L, Chrismas BCR, Watkins SL, Mauger AR. The influence of acetaminophen on repeated sprint cycling performance. Eur J Appl Physiol 114: 41–48, 2014. doi: 10.1007/s00421-013-2746-0. [DOI] [PubMed] [Google Scholar]
- 20.Gagnon P, Bussières JS, Ribeiro F, Gagnon SL, Saey D, Gagné N, Provencher S, Maltais F. Influences of spinal anesthesia on exercise tolerance in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 186: 606–615, 2012. doi: 10.1164/rccm.201203-0404OC. [DOI] [PubMed] [Google Scholar]
- 21.Grabiner MD, Owings TM. Effects of eccentrically and concentrically induced unilateral fatigue on the involved and uninvolved limbs. J Electromyogr Kinesiol 9: 185–189, 1999. doi: 10.1016/S1050-6411(98)00031-5. [DOI] [PubMed] [Google Scholar]
- 22.Halperin I, Copithorne D, Behm DG. Unilateral isometric muscle fatigue decreases force production and activation of contralateral knee extensors but not elbow flexors. Appl Physiol Nutr Metab 39: 1338–1344, 2014. doi: 10.1139/apnm-2014-0109. [DOI] [PubMed] [Google Scholar]
- 23.Halperin I, Chapman DW, Behm DG. Non-local muscle fatigue: effects and possible mechanisms. Eur J Appl Physiol 115: 2031–2048, 2015. doi: 10.1007/s00421-015-3249-y. [DOI] [PubMed] [Google Scholar]
- 24.Hayes SG, Kindig AE, Kaufman MP. Cyclooxygenase blockade attenuates responses of group III and IV muscle afferents to dynamic exercise in cats. Am J Physiol Heart Circ Physiol 290: H2239–H2246, 2006. doi: 10.1152/ajpheart.01274.2005. [DOI] [PubMed] [Google Scholar]
- 25.Hureau TJ, Romer LM, Amann M. The “sensory tolerance limit”: a hypothetical construct determining exercise performance? Eur J Sport Sci 18: 13–24, 2018. doi: 10.1080/17461391.2016.1252428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johnson MA, Sharpe GR, Williams NC, Hannah R. Locomotor muscle fatigue is not critically regulated after prior upper body exercise. J Appl Physiol (1985) 119: 840–850, 2015. doi: 10.1152/japplphysiol.00072.2015. [DOI] [PubMed] [Google Scholar]
- 27.Kemp GJ, Roussel M, Bendahan D, Le Fur Y, Cozzone PJ. Interrelations of ATP synthesis and proton handling in ischaemically exercising human forearm muscle studied by 31P magnetic resonance spectroscopy. J Physiol 535: 901–928, 2001. doi: 10.1111/j.1469-7793.2001.00901.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kennedy DS, Fitzpatrick SC, Gandevia SC, Taylor JL. Fatigue-related firing of muscle nociceptors reduces voluntary activation of ipsilateral but not contralateral lower limb muscles. J Appl Physiol (1985) 118: 408–418, 2015. doi: 10.1152/japplphysiol.00375.2014. [DOI] [PubMed] [Google Scholar]
- 29.Kennedy A, Hug F, Sveistrup H, Guével A. Fatiguing handgrip exercise alters maximal force-generating capacity of plantar-flexors. Eur J Appl Physiol 113: 559–566, 2013. doi: 10.1007/s00421-012-2462-1. [DOI] [PubMed] [Google Scholar]
- 30.Mauger AR, Jones AM, Williams CA. Influence of acetaminophen on performance during time trial cycling. J Appl Physiol (1985) 108: 98–104, 2010. doi: 10.1152/japplphysiol.00761.2009. [DOI] [PubMed] [Google Scholar]
- 31.Morgan PT, Bowtell JL, Vanhatalo A, Jones AM, Bailey SJ. Acute acetaminophen ingestion improves performance and muscle activation during maximal intermittent knee extensor exercise. Eur J Appl Physiol 118: 595–605, 2018. doi: 10.1007/s00421-017-3794-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morgan PT, Vanhatalo A, Bowtell JL, Jones AM, Bailey SJ. Acetaminophen ingestion improves muscle activation and performance during a 3-min all-out cycling test. Appl Physiol Nutr Metab 44: 434–442, 2019. doi: 10.1139/apnm-2018-0506. [DOI] [PubMed] [Google Scholar]
- 33.Poole DC, Burnley M, Vanhatalo A, Rossiter HB, Jones AM. Critical power: an important fatigue threshold in exercise physiology. Med Sci Sports Exerc 48: 2320–2334, 2016. doi: 10.1249/MSS.0000000000000939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rattey J, Martin PG, Kay D, Cannon J, Marino FE. Contralateral muscle fatigue in human quadriceps muscle: evidence for a centrally mediated fatigue response and cross-over effect. Pflugers Arch 452: 199–207, 2006. doi: 10.1007/s00424-005-0027-4. [DOI] [PubMed] [Google Scholar]
- 35.Regueme SC, Barthèlemy J, Nicol C. Exhaustive stretch-shortening cycle exercise: no contralateral effects on muscle activity in maximal motor performances. Scand J Med Sci Sports 17: 547–555, 2007. doi: 10.1111/j.1600-0838.2006.00614.x. [DOI] [PubMed] [Google Scholar]
- 36.Rossman MJ, Venturelli M, McDaniel J, Amann M, Richardson RS. Muscle mass and peripheral fatigue: a potential role for afferent feedback? Acta Physiol (Oxf) 206: 242–250, 2012. doi: 10.1111/j.1748-1716.2012.02471.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rueff A, Dray A. Sensitization of peripheral afferent fibres in the in vitro neonatal rat spinal cord-tail by bradykinin and prostaglandins. Neuroscience 54: 527–535, 1993. doi: 10.1016/0306-4522(93)90272-H. [DOI] [PubMed] [Google Scholar]
- 38.Schaible HG, Ebersberger A, Natura G. Update on peripheral mechanisms of pain: beyond prostaglandins and cytokines. Arthritis Res Ther 13: 210, 2011. doi: 10.1186/ar3305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sidhu SK, Weavil JC, Mangum TS, Jessop JE, Richardson RS, Morgan DE, Amann M. Group III/IV locomotor muscle afferents alter motor cortical and corticospinal excitability and promote central fatigue during cycling exercise. Clin Neurophysiol 128: 44–55, 2017. doi: 10.1016/j.clinph.2016.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sidhu SK, Weavil JC, Thurston TS, Rosenberger D, Jessop JE, Wang E, Richardson RS, McNeil CJ, Amann M. Fatigue-related group III/IV muscle afferent feedback facilitates intracortical inhibition during locomotor exercise. J Physiol 596: 4789–4801, 2018. doi: 10.1113/JP276460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sidhu SK, Weavil JC, Venturelli M, Garten RS, Rossman MJ, Richardson RS, Gmelch BS, Morgan DE, Amann M. Spinal μ-opioid receptor-sensitive lower limb muscle afferents determine corticospinal responsiveness and promote central fatigue in upper limb muscle. J Physiol 592: 5011–5024, 2014. doi: 10.1113/jphysiol.2014.275438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Takahashi K, Maruyama A, Hirakoba K, Maeda M, Etoh S, Kawahira K, Rothwell JC. Fatiguing intermittent lower limb exercise influences corticospinal and corticocortical excitability in the nonexercised upper limb. Brain Stimul 4: 90–96, 2011. doi: 10.1016/j.brs.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 43.Todd G, Petersen NT, Taylor JL, Gandevia SC. The effect of a contralateral contraction on maximal voluntary activation and central fatigue in elbow flexor muscles. Exp Brain Res 150: 308–313, 2003. doi: 10.1007/s00221-003-1379-7. [DOI] [PubMed] [Google Scholar]
- 44.Vanhatalo A, Fulford J, Bailey SJ, Blackwell JR, Winyard PG, Jones AM. Dietary nitrate reduces muscle metabolic perturbation and improves exercise tolerance in hypoxia. J Physiol 589: 5517–5528, 2011. doi: 10.1113/jphysiol.2011.216341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vanhatalo A, Fulford J, DiMenna FJ, Jones AM. Influence of hyperoxia on muscle metabolic responses and the power-duration relationship during severe-intensity exercise in humans: a 31P magnetic resonance spectroscopy study. Exp Physiol 95: 528–540, 2010. doi: 10.1113/expphysiol.2009.050500. [DOI] [PubMed] [Google Scholar]
- 46.Zijdewind I, Zwarts MJ, Kernell D. Influence of a voluntary fatigue test on the contralateral homologous muscle in humans? Neurosci Lett 253: 41–44, 1998. doi: 10.1016/S0304-3940(98)00609-0. [DOI] [PubMed] [Google Scholar]