Abstract
Polycystic kidney disease (PKD) is characterized by slowly expanding renal cysts that damage the kidney, typically resulting in renal failure by the fifth decade. The most common cause of death in these patients, however, is cardiovascular disease. Expanding cysts in PKD induce chronic kidney injury that is accompanied by immune cell infiltration, including macrophages, which we and others have shown can promote disease progression in PKD mouse models. Here, we show that monocyte chemoattractant protein-1 [MCP-1/chemokine (C-C motif) ligand 2 (CCL2)] is responsible for the majority of monocyte chemoattractant activity produced by renal PKD cells from both mice and humans. To test whether the absence of MCP-1 lowers renal macrophage concentration and slows disease progression, we generated genetic knockout (KO) of MCP-1 in a mouse model of PKD [congenital polycystic kidney (cpk) mice]. Cpk mice are born with rapidly expanding renal cysts, accompanied by a decline in kidney function and death by postnatal day 21. Here, we report that KO of MCP-1 in these mice increased survival, with some mice living past 3 mo. Surprisingly, however, there was no significant difference in renal macrophage concentration, nor was there improvement in cystic disease or kidney function. Examination of mice revealed cardiac hypertrophy in cpk mice, and measurement of cardiac electrical activity via ECG revealed repolarization abnormalities. MCP-1 KO did not affect the number of cardiac macrophages, nor did it alleviate the cardiac aberrancies. However, MCP-1 KO did prevent the development of pulmonary edema, which occurred in cpk mice, and promoted decreased resting heart rate and increased heart rate variability in both cpk and noncystic mice. These data suggest that in this mouse model of PKD, MCP-1 altered cardiac/pulmonary function and promoted death outside of its role as a macrophage chemoattractant.
Keywords: cardiovascular disease, cpk mice, monocyte chemoattractant protein-1, polycystic kidney disease
INTRODUCTION
Polycystic kidney disease (PKD) is the fourth leading cause of renal failure in the United States and one of the most common life-threatening genetic diseases (8). The autosomal dominant form of PKD (ADPKD), which is caused by mutations in one of two genes, PKD1 or PKD2 (45), affects up to 1 in 500–1,000 births. ADPKD is ∼10 times more common than sickle cell disease, 15 times more common than cystic fibrosis, and 20 times more common than Huntington’s disease (20). The autosomal recessive form of PKD (ARPKD), which is caused by defects in the PKHD1 gene, is less common (~1 to 20,000 births) and primarily affects neonates and young children (46, 75). PKD is characterized by the presence of renal cysts, which expand throughout the lifetime of the patient. This expansion promotes the destructive compression of the surrounding tubules and microvasculature, resulting in a progressive loss of renal function and, eventually, end-stage renal disease (ESRD) (22). Approximately half of patients with PKD who survive to the fifth decade lose kidney function, requiring either dialysis or transplant. The most common cause of death in these patients, however, is cardiovascular disease, which includes ischemic heart disease, hypertensive heart disease, aneurysms, and congestive heart failure (17, 52). As such, PKD is an ideal prototype of cardiorenal syndrome, in which chronic renal dysfunction induces dysfunction in the heart (74).
The cpk mouse (Cys1cpk/cpk or cpk/cpk) is a widely used, rapidly progressing model of PKD (19, 51, 53, 54). These mice, which have a homozygous defect in the cilia-associated gene Cys1 (26, 77), are born with renal cysts that rapidly expand, with an accompanying loss of kidney function and death by approximately postnatal day 21 (PN21). Because PN21 kidneys consist primarily of cysts with very little parenchyma and kidney function, it has been assumed that these animals die from renal failure. The possibility that cpk mice have cardiovascular disease, which may be responsible for their short lifespan, has not been examined.
PKD kidneys, including those from humans and PKD models like cpk mice, have elevated levels of macrophages (30, 50, 65, 78). We and others have shown that these immune cells promote cyst cell proliferation, cyst expansion, and disease progression (30, 65), suggesting that macrophages may be good targets for therapies to slow disease progression. In the absence of injury, renal macrophages in normal adult mice are predominantly embryonically/neonatally derived (16, 25, 61). As is the case after acute kidney injury of normal mice (15, 80), the elevated levels of macrophages in PKD most likely arise from both monocyte recruitment and proliferation of resident macrophages, although the contribution from each source to the total number of these cells is unknown. This present study was based originally on the hypothesis that an effective blockade of macrophage recruitment might lower renal macrophage concentration and ameliorate pathological effects of their presence.
A number of studies have pointed to MCP-1 [also known as chemokine (C-C motif) ligand 2 (CCL2)] as a primary macrophage recruitment factor in PKD. MCP-1 is best known as a monocyte chemoattractant, although it can also influence multiple cell types in other ways (7, 21, 33, 38, 58, 69) and can function as an inflammatory cytokine (12, 72). MCP-1 is commonly found after tissue injury of almost any type and is present at elevated levels in the kidneys and urine of both patients with ADPKD and PKD model rodents (9, 11, 81, 83).
Here, studies that assessed MCP-1 expression in PKD have been extended to include human ARPKD as well as additional mouse models of PKD. We show that MCP-1 is responsible for a large majority of the total monocytye chemoattractant activity produced by both ADPKD cyst cells and cells from the cystic kidneys of cpk mice. To examine the effects of MCP-1 on macrophage concentration and disease progression, we bred cpk/+ and MCP-1 knockout (KO) mice (Ccl2−/− mice) to produce animals with a complete deficiency in this cytokine. Notably, cystic cpk animals deficient for MCP-1 had significantly extended survival times compared with wild-type (WT) mice deficient for MCP-1. However, unexpectedly, there was no improvement in cystic disease, kidney function, or differences in macrophage concentration in these animals. Instead, the results presented here point to a role for this cytokine as a risk factor for altered cardiac and pulmonary function and death in cpk mice.
MATERIALS AND METHODS
Mice.
C57BL/6J-cpk/+ mice, which were obtained from a colony established at the University of Kansas Medical Center from stock originally purchased from Jackson Laboratory, were bred with a Ccl2 KO strain in the C57BL/6 congenic background (no. 004434, Jackson Laboratory) to generate double-heterozygous mice (cpk/+:Ccl2+/−). Subsequently, these mice were used to derive cpk/+:Ccl2+/+ and cpk/+:Ccl2−/− substrains, which were maintained and used to generate all animals used in this study. KO of MCP-1 in cpk/cpk:Ccl2−/− mice (n = 3 from three different sets of parents) was validated and confirmed by PCR and sequencing of the Neo cassette inserted into exon 2 of the null allele using primers in intron 1 (5′-TGACAGTCCCCAGAGTCACA-3′) and a Neo cassette-specific primer (5′-TGCCTGCTTGCCGAATATCA-3′). Sequences from all mice were identical and showed only a 5-amino acid overlap with WT MCP-1 at the mature NH2 terminus. Details of this validation are available upon request. All animal experiments were approved by the Institutional Animal Care and Use Committee.
Tissue harvest.
Mice were euthanized by CO2 or isoflurane exposure and opening of the chest cavity, and body weight was recorded. Hearts, kidneys, and lungs were harvested and weighed. Hearts were either placed directly into formalin or cut in half longitudinally, and halves were flash frozen or placed into 1 ml RNAlater (ThermoFisher Scientific) and placed at 4°C for a day and then stored at −80°C. The right kidney was halved, and half was placed into RNAlater and the other half was flash frozen. The left kidney was placed into formalin, which was changed to 70% ethanol after 24–48 h. Lungs were dried in a 65°C oven for 3–5 days, at which time they were reweighed, and their dry weight was recorded. For histology and immunohistochemistry of lung tissue, mice were anesthetized with isoflurane, and the chest cavity was opened before whole body perfusion through a 26-gauge needle inserted into the right ventricle of the heart. A small opening was cut into the left ventricle to allow fluid outflow. Using a flow rate of 0.7 ml/min, we perfused ice-cold PBS for 10 min followed by cold formalin for 10 min to fix the tissues. Lungs were removed and placed into formalin, which was changed to 70% ethanol after 24 h.
Histology and immunohistochemistry.
Sections (4 μm) from formalin-fixed, paraffin-embedded kidneys (cross-section at the hilum), hearts (midventricle), or lungs were stained with hematoxylin and eosin (Richard-Allan Scientific, Kalamazoo, MI), and images were captured on an Olympus BX41 microscope with the ×2 objective using a Spot Idea camera and Spot Imaging software (v5.2.5, Spot Imaging). Cystic indexes were quantified from kidney cross-sections using ImageJ64 (NIH), as previously described (65). The left ventricular area of the heart and right ventricular thickness were measured using ImageJ (60). Heart sections were stained with picrosirius red (no. 24901, Polysciences, Warrington, PA) and examined under bright-field and polarized light using an Olympus BX41 microscope.
For immunohistochemistry, kidney, heart, or lung sections were deparaffinized and steamed in 0.01 M citrate buffer (pH 6.0) for 20 min (steamer no. HS900, Black & Decker, Madison, WI). Sections were then incubated in 3% H2O2 followed by serum from the host animal, in which the relevant secondary antibody was generated. Samples were incubated with anti-MCP-1 (catalog no. HPA019163, Sigma-Aldrich, St. Louis, MO), anti-CD68 (kidneys and lungs, ab125212, Abcam, Cambridge, MA), or anti-F4/80 (hearts, M4150, Spring Bioscience, Pleasanton, CA) overnight at 4°C or 1 h at room temperature. The appropriate secondary antibodies (ImmPRESS, Vector Laboratories, Burlingame, CA) were then applied for 30 min at room temperature followed by an incubation with DAB substrate (Vector Laboratories) and hematoxylin counterstain before visualization by light microscopy. Macrophages were counted in 10 nonoverlapping high-powered fields using a ×40 objective on an Olympus BX41 microscope.
Quantitative RT-PCR.
Kidney and heart tissue samples were lysed by the addition of RLT lysis buffer from the RNeasy Miniprep kit (Qiagen, Valencia, CA) and homogenized using a Pro200 homogenzier (Cole-Palmer, Vernon Hills, IL). Samples were further dispersed using QIAshredder columns (Qiagen, Valencia, CA). Total RNA from these lysates was purified according to the RNeasy Miniprep kit directions and then analyzed by the KUMC Genome Sequencing Facility for quality, determined with an Agilent 2100 bioanalyzer. RNAs with an RNA integrity number (RIN) of at least 7 were used for quantitative RT-PCR. cDNA was synthesized using a kit (no. 4369914) from ThermoFisher Scientific, and quantitative RT-PCR analysis was performed on a Bio-Rad CFX96 real-time PCR machine using SYBR green mix (no. A25742, ThermoFisher Scientific). Primer sequences are shown in Table 1. The efficiency of each primer pair was determined using the DART-PCR program (47). Using the gene-specific efficiencies, we calculated the mRNA relative abundance according to methods previously described by Pfaffl (48) and normalized to the mean of the reference gene used.
Table 1.
RT-PCR primers
| Forward | Reverse | |
|---|---|---|
| Mouse | ||
| Acta1 | 5′-CCCAAAGCTAACCGGGAGAAG-3′ | 5′-CCAGAATCCAACACGATGCC-3′ |
| Acta2 | 5′-GTCCCAGACATCAGGGAGTAA-3′ | 5′-TCGGATACTTCAGCGTCAGGA-3′ |
| Actb | 5′-GGCTGTATTCCCCTCCATCG-3′ | 5′-CCAGTTGGTAACAATGCCATGT-3′ |
| Atp2a2 | 5′-GAGAACGCTCACACAAAGACC-3′ | 5′-CAATTCGTTGGAGCCCCAT-3′ |
| Ccl2 | 5′-TAAAAACCTGGATCGGAACCAAA-3′ | 5′-GCATTAGCTTCAGATTTACGGGT-3′ |
| Ccl4 | 5′-TTCCTGCTGTTTCTCTTACACCT-3′ | 5′-CTGTCTGCCTCTTTTGGTCAG-3′ |
| Col1a1 | 5′-CTGGCGGTTCAGGTCCAAT-3′ | 5′-TTCCAGGCAATCCACGAGC-3′ |
| Col3a1 | 5′-CTGTAACATGGAAACTGGGGAAA-3′ | 5′-CCATAGCTGAACTGAAAACCACC-3′ |
| Cxcl3 | 5′-AGGCCCCAGGCTTCAGATAAT-3′ | 5′-AATGCAGGTCCTTCATCATGGT-3′ |
| Gapdh | 5′-CCACTCACGGCAAATTCAAC-3′ | 5′-GTAGACTCCACGACATACTCA-3′ |
| Il-1b | 5′-GCAACTGTTCCTGAACTCAACT-3′ | 5′-ATCTTTTGGGGTCCGTCAACT-3′ |
| Il-6 | 5′-TAGTCCTTCCTACCCCAATTTCC-3′ | 5′-TTGGTCCTTAGCCACTCCTTC-3′ |
| Myh6 | 5′-GCCCAGTACCTCCGAAAGTC-3′ | 5′-GCCTTAACATACTCCTCCTTGTC-3′ |
| Myh7 | 5′-ACTGTCAACACTAAGAGGGTCA-3′ | 5′-TTGGATGATTTGATCTTCCAGGG-3′ |
| Nos2 | 5′-GTTCTCAGCCCAACAATACAAGA-3′ | 5′-GTGGACGGGTCGATGTCAC-3′ |
| Nppa | 5′-GCTTCCAGGCCATATTGGAG-3′ | 5′-GGGGGCATGACCTCATCTT-3′ |
| Nppb | 5′-GAGGTCACTCCTATCCTCTGG-3′ | 5′-GCCATTTCCTCCGACTTTTCTC-3′ |
| Ppara | 5′-AACCTCGAGTGTCGAATATGTGG-3′ | 5′-AGACGAATAGTTCGCCGAAAG-3′ |
| Pparg | 5′-CCACCAACTTCGGAATCAGCT-3′ | 5′-TTTGTGGATCCGGCAGTTAAGA-3′ |
| Tgfb1 | 5′-TGGCGAGCCTTAGTTTGGA-3′ | 5′-TCGACATGGAGCTGGTGAAA-3′ |
| Tnfa | 5′-ACCCTCACACTCAGATCATCTTC-3′ | 5′-TGGTGGTTTGCTACGACGT-3′ |
| Trpc3 | 5′-TCGAGAGGCCACACGACTA-3′ | 5′-CTGGACAGCGACAAGTATGC-3′ |
| Trpc6 | 5′-AGCCAGGACTATTTGCTGATGG-3′ | 5′-AACCTTCTTCCCTTCTCACGA-3′ |
| Vim | 5′-CGTCCACACGCACCTACAG-3′ | 5′-GGGGGATGAGGAATAGAG-3′ |
| Zc3h12a | 5′-ACGAAGCCTGTCCAAGAATCC-3′ | 5′-TAGGGGCCTCTTTAGCCACA-3′ |
| Human | ||
| CCL2 | 5′-AGCAGCAAGTGTCCCAAAGA-3′ | 5′-TTGGGTTTGCTTGTCCAGGT-3′ |
| OAZ1 | 5′-CACCATGCCGCTCCTAAG-3′ | 5′-GAGGGAGACCCTGGAACTCT-3′ |
See text for gene descriptions.
Primary cells from cystic kidneys: growth and conditioned media.
All human kidney cells and tissue were supplied by the Institutional Review Board-approved PKD Biomaterials Research Core Laboratory at the University of Kansas Medical Center (KUMC). Primary cultures of ADPKD cyst cells were supplied by the PKD Biomaterials Research Core Laboratory at KUMC and cultured in “APDKD cyst cell media” [DMEM/F-12 (cellgro 15-090-CV, Mediatech, Manassas, VA)] supplemented with 5% FBS, 15 mM HEPES, 5 µg/ml insulin, 5 µ/ml transferrin, and 5 ng/ml sodium selenite (ITS; BD Biosciences, Bedford, MA) plus penicillin (100 U/ml) and streptomycin (130 µg/ml; Pen/Strep). Conditioned media (CM) were obtained from ADPKD cells (3.7 × 106 cells/15-cm plate) incubated in ADPKD cyst cell media for 24 h followed by washing once with PBS and replacing with DMEM (D6429, Sigma) containing 10% FBS, 2 mM additional glutamine, and Pen/Strep (complete media) for 1 day. CM was then collected from these cells, and cellular debris was removed by centrifugation at 600 g for 10 min.
Preparation of primary cells from cystic cpk mouse kidneys to produce CM.
Cystic kidneys from PN16 cpk mice were collected and placed in ice-cold PBS containing 2× Pen/Strep for transfer. Under sterile conditions, kidneys were placed in a culture dish and diced with a razor to generate fragments of ~2 mm3, which were then transferred into a 50-ml tube containing 15 ml cold DMEM with Pen/Strep. The diced tissue was digested by the addition of 5 ml collagenase (4 mg/ml, collagenase type IV, LS004189, Worthington), quickly warmed by incubation in a 37°C water bath for 5 min, and then further incubated at 37°C for 35 min with shaking (150 rpm). Collagenase was neutralized by the addition of 20 ml DMEM containing 10% FBS. The partly digested tissue was strained using a 40-µm strainer and then cultured with ADPKD cyst cell media for 3 days to allow attachment of kidney cells. The tissue was removed by aspiration, and kidney cells were allowed to grow until confluent (3–4 days). Media were replaced with complete media, cells were incubated for 3 days, and CM was collected and cleared by centrifugation at 600 g for 10 min.
Migration and neutralizing assays.
Cell migration assays were performed using 24-well transwells with 8-µm pore size uncoated polycarbonate membranes (catalog no. 3422, Costar). Lower wells contained 600 µl of either complete media only (to measure random movement), CM produced by ADPKD cyst cells, CM produced by cells from cystic kidneys of cpk mice, or purified human (50 ng/ml) or mouse (100 ng/ml) MCP-1 diluted in complete media as a positive control. CM was diluted fivefold in complete media for migration assays. Upper wells contained 100 µl of THP-1 monocytes (1 × 106 cells/ml) in complete media. These cells are nonadherent and, in transwell migration assays, will not stick to the filter after migration but will move into the media contained in the lower wells below the filter. After an overnight incubation (18 h), media and nonmigratory cells were removed by aspiration from upper well followed by the addition of EDTA (20 µl of 20 mM EDTA in PBS) and incubation for 20 min at 4°C. PBS (100 µl) was added to each upper well before removal of the inserts and collection of migrated cells into microcentrifuge tubes. Cells were pelleted by centrifugation (600 g, 5 min), and, after removal of media, cell pellets were frozen at −80°C before lysis in buffer containing CyQUANT GR dye (no. C7026, ThermoFisher Scientific) according to the manufacturer’s directions. Quantitative measurement of fluorescence was performed using a Synergy 2 microplate reader (BioTEK Instruments, Winooski, VT). Neutralization of MCP-1 in CM was carried out by preincubating the species-appropriate anti-MCP-1 blocking antibodies (MAB479 for the mouse MAB679 for the human, R&D Systems, Minneapolis, MN) or tisotype-matched Ig controls (mouse IgG2b for human CM and rat IgG2b for mouse CM, no. 14-4732-85 and no. 14-4031-85, respectively, eBioscience/ThermoFisher Scientific) with CM for 1 h at 37°C with agitation before the direct use of CM in migration assays.
Blood urea nitrogen measurement.
Blood was collected at the time of euthanization. Serum was isolated from each sample (centrifugation at 2,500 g for 8 min at 4°C followed by another spin of the supernatant for 8 min), and blood urea nitrogen (BUN) was measured using QuantiChrom urea assay kit (BioAssay Systems, Hayward, CA).
Electrocardiography.
Eighteen-day-old mice were anesthetized with isoflurane (2% induction, 1% maintenance) and placed in the supine position on a heating pad to maintain physiological temperature (monitored by rectal probe). 29-gauge ECG needle electrodes (ADInstruments, Colorado Springs, CO) were inserted into the limbs in a lead II configuration, and ECG was recorded for 15–30 min using an Animal BioAmp/PowerLab setup with LabChart data acquisition software (ADInstruments). ECG intervals were derived from the raw signal averaged over 200 consecutive beats aligned at the R wave using the LabChart ECG Analysis module. We used the Mitchell et al. (41) method for the determination of QT interval corrected for heart rate (HR; QTc). ST amplitude was measured 10 ms from R wave alignment (66). For HR variability (HRV) analysis, the standard deviation of the RR interval (SDRR) was calculated from 2 min of consecutive, stable ECG signal using the LabChart HRV analysis module.
Statistics.
Survival data between two groups were compared using the log-rank test calculated with Prism (v7.0, GraphPad, La Jolla, CA). Measured parameters from the ECG recordings were compared between four groups using one-way ANOVA calculated with Prism. Other data were compared between two groups using a two-tailed t-test calculated with Prism. P values of <0.05 were considered significant, as indicated in the figures. Data are presented as means ± SD.
RESULTS
Elevated expression of MCP-1, produced primarily by tubule epithelial cells, is a common feature of PKD.
Previous studies have shown elevated levels of MCP-1/CCL2 protein and/or transcript expression in ADPKD kidneys versus noncystic human kidneys (NHKs) as well as in cystic versus noncystic kidneys of multiple PKD rodent models (9, 11, 81, 83). To extend these findings, we used immunohistochemistry to assess MCP-1 expression in human ARPKD kidneys, human ADPKD kidneys, and NHKs (Fig. 1A). As previously reported for human ADPKD kidneys (9) and kidneys for rodent PKD models, MCP-1 in human ARPKD kidneys was present at elevated levels compared with NHKs (Fig. 1A) and localized primarily to tubule epithelial cells, particularly those lining cyst walls, as well as in scattered interstitial cells.
Fig. 1.
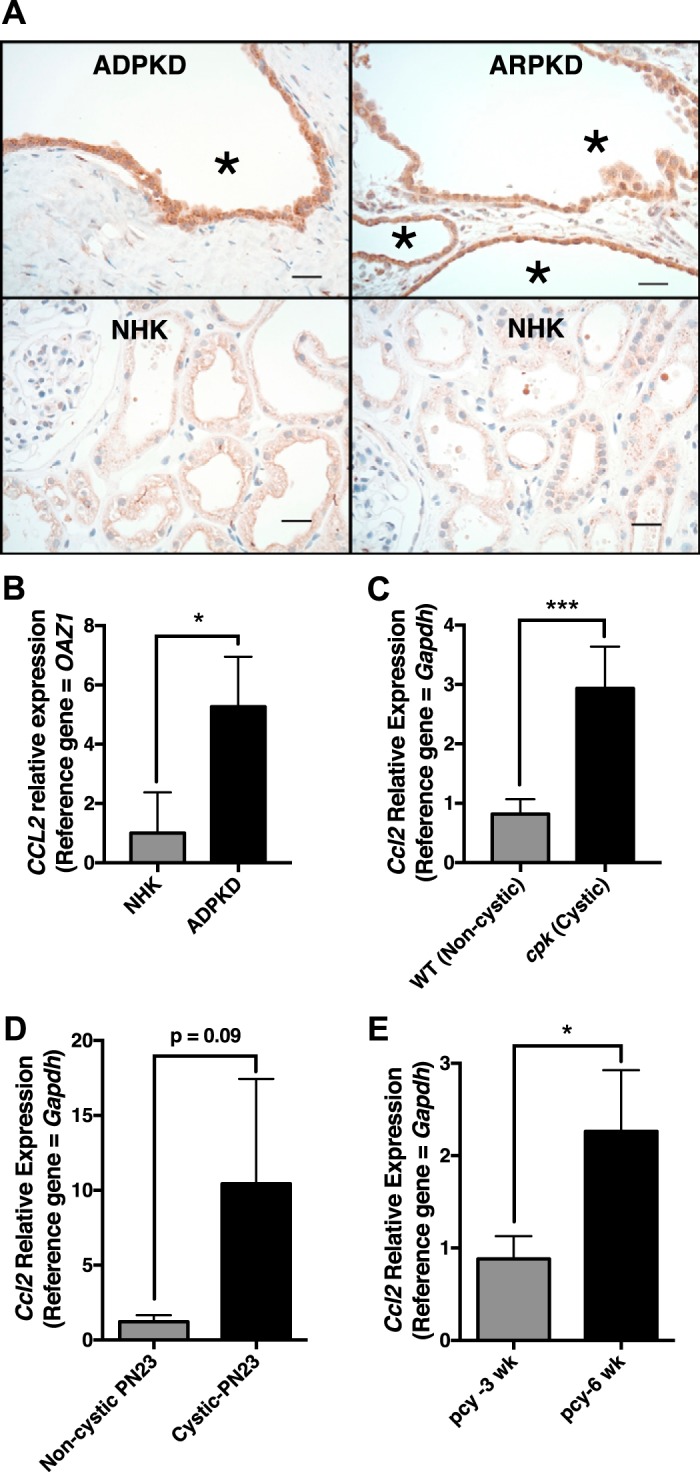
Elevated expression of monocyte chemoattractant protein (MCP)-1, produced primarily by tubule epithelial cells, is a common feature of polycystic kidney disease (PKD). A: formalin-fixed tissues from human autosomal dominant PKD (ADPKD), human autosomal recessive PKD (ARPKD), or noncystic human kidneys (NHK) were sectioned and stained by immunohistochemistry using an antibody to MCP-1. Scale bars = 25 µm. *Cystic space. B: chemokine (C-C motif) 2 (CCL2) expression levels were determined by quantitative RT-PCR using RNA isolated from NHKs and human ADPKD kidneys (n = 3 each). *P < 0.05. C: quantitative RT-PCR showing Ccl2 expression in the kidneys of cystic cpk mice (PN18) and their noncystic siblings (n = 5 each). ***P = 0.0002. D: quantitative RT-PCR showing Ccl2 expression in the kidneys of noncystic Pkd1flox/+:Pkhd1-Cre and cystic Pkd1flox/flox:Pkhd1-Cre mice at PN23 (n = 3 each). E: quantitative RT-PCR showing Ccl2 expression in the kidneys of pcy mice at 3 and 6 wk (n = 3 each). *P < 0.05. PN, postnatal day.
We also carried out quantitative RT-PCR using RNA isolated from human ADPKD and NHK tissues (Fig. 1B), kidneys from cystic cpk mice and their age-matched, WT littermates (Fig. 1C), Pkd2flox/flox;Pkhd1-Cre mice (Fig. 1D), and late cystic (PN42) and early cystic (PN21) kidneys of pcy mice (Fig. 1E) (68). CCL2/Ccl2 transcripts were elevated in cystic kidneys relative to noncystic kidneys and were increased in late versus early cystic disease in pcy mice. Collectively, these data support the notion that MCP-1/CCL2 protein and transcripts are elevated in the cystic kidneys of PKD of all origins. The finding of MCP-1 localization predominantly in the epithelium also supports the conclusions of similar previous studies (9, 11, 83), suggesting that this chemokine is produced primarily by tubular epithelial cells lining the cysts and, to a lesser degree, by cells within the interstitium.
MCP-1 is the primary monocyte recruitment factor made by primary renal cyst cells from ADPKD patients and cells from cystic cpk mouse kidneys.
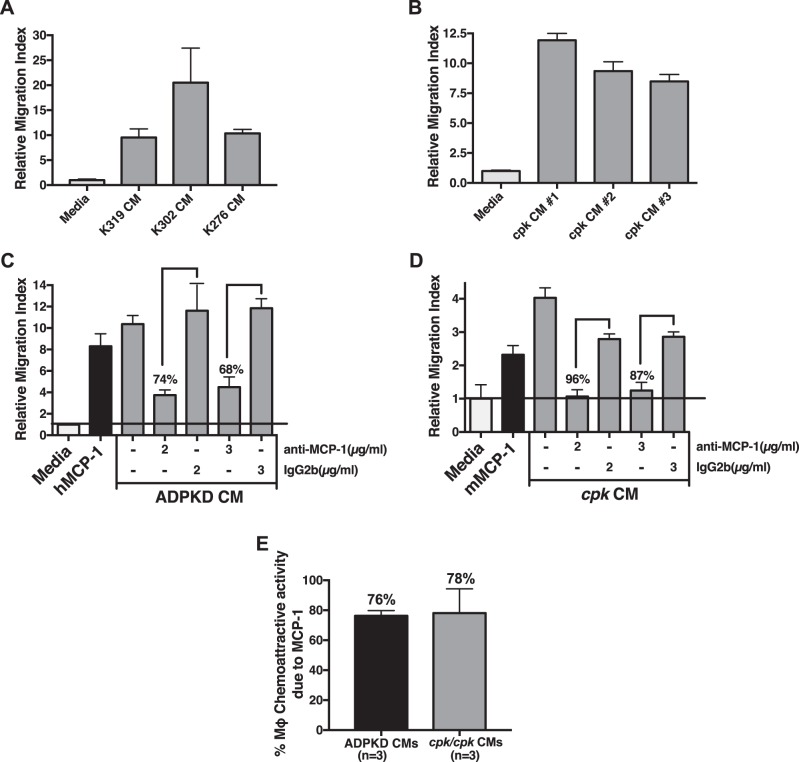
Tubular epithelial cells are known to produce a number of monocyte chemoattractants after injury, including MCP-1 (59, 81). We sought to determine the fractional contribution of MCP-1 to total monocyte chemoattractant activity produced by primary renal cyst cells. For this experiment, we used primary cells isolated from human ADPKD kidney cysts and those isolated from whole cystic cpk mouse kidneys. CM were collected from cultured primary cells and then assessed for total monocyte chemoattractant activity in transwell migration assays of human THP-1 monocytes. Compared with fresh media, CM had significantly elevated monocyte chemoattractant activity (Fig. 2, A and B) that was greater than purified human or mouse MCP-1 (50 ng/ml) tested in parallel (Fig. 2, C and D). To determine the fractional contribution of MCP-1 to total monocyte chemoattractant activity, each CM was preincubated with species-specific MCP-1-neutralizing antibodies or control Ig before being tested in transwell migration assays. At maximal effective doses of these neutralizing antibodies, the percent decline in migration due to MCP-1 was determined for multiple kidneys (Fig. 2, C and D). In this manner, the average contribution of MCP-1 was determined to be 76% from ADPKD CM and 78% from cystic cpk kidney CM (Fig. 2E). These data indicate that MCP-1 is responsible for a large majority of the total monocyte chemoattractant activity produced by cystic epithelial cells and cystic kidney cells.
Fig. 2.
Monocyte chemoattractant protein (MCP)-1 is the primary monocyte recruitment factor made by primary renal cyst cells from patients with autosomal dominant polycystic kidney disease (ADPKD) and cells from cystic cpk mouse kidneys. A: conditioned media (CM) collected from primary human ADPKD cyst cells isolated from different patients (patients K319, K302, and K276) cultured in vitro were tested for monocyte chemoattractant activity compared with that of fresh media using a transwell assay of THP-1 monocyte migration. B: cells were isolated from cystic kidneys of three different cpk mice (mouse 1, 2, and 3) and cultured in vitro. CM were collected from confluent cultures and tested for monocyte chemoattract activity, as described in A. C: to determine the fractional contribution of MCP-1 to total monocyte chemoattractant activity produced by human ADPKD cyst cells, CM collected from a primary human ADPKD cyst cell culture was preincubated (or not) with increasing concentrations of either human-specific MCP-1-neutralizing antibody (anti-MCP-1) or with control Ig of the same isotype (IgG2b) before being tested in a transwell assay of THP-1 monocyte migration. Media and media containing purified human (h)MCP-1 (50 ng/ml) were used in parallel, as negative and positive controls, respectively. At concentrations of 2 and 3 µg/ml of MCP-1-neutralizing antibody, migration was inhibited 74% and 68%, respectively, after subtraction of random migration obtained with media only, indicating that MCP-1 was responsible for ~71% of the total monocyte chemoattractant activity in this CM. D: contribution of MCP-1 to total monocyte chemoattractant activity produced by cystic cpk kidney cells was determined in a similar manner as described in C. CM were collected from primary cystic cpk kidney cells cultured in vitro and preincubated with mouse-specific MCP-1-neutralizing antibody or control antibody before transwell assays of THP-1 monocyte cell migration. Media and media containing purified mouse (m)MCP-1 (100 ng/ml) were used as negative and positive controls, respectively. At concentrations of 2 and 3 µg/ml MCP-1-neutralizing antibody, migration was inhibited 96% and 87%, respectively, after subtraction of random migration obtained with media only, indicating that MCP-1 was responsible for ~92% of the total monocyte chemoattractant activity in this CM. E: combined fractional contribution of MCP-1 to total monocyte chemoattractant activity determined from CM of ADPKD cyst cells isolated from three different kidneys and assayed as described in C and CM of cystic cpk kidney cells isolated from three different mice and assayed as described in D. Mϕ, macrophage.
Genetic deficiency of MCP-1 in cpk mice prolongs survival.
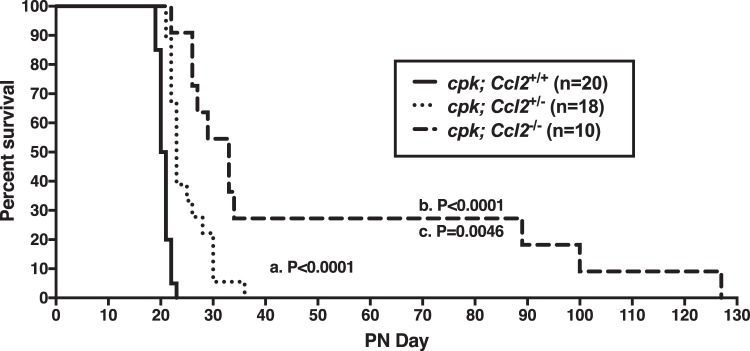
Since MCP-1 was shown to be the primary monocyte chemoattractant produced by cyst cells, we hypothesized that a deficiency of MCP-1 in cpk mice might reduce the number of macrophages recruited to the kidney and thereby restrain cyst expansion, similar to our previous study (65), where we systemically depleted macrophages in these mice. To test this hypothesis, heterozygous cpk/+ mice were bred to Ccl2-deficient mice of the same genetic background to generate homozygous (cystic) cpk mice that were either WT (cpk:Ccl2+/+), heterozygous (cpk:Ccl2+/−), or KO (cpk:Ccl2−/−) at the Ccl2 locus. Using these mice, we carried out a survival experiment. As expected, cpk:Ccl2+/+ mice died around PN21 (Fig. 3, solid line). The median survival time was significantly extended in cpk:Ccl2+/−mice to PN23 (P < 0.0001; Fig. 3, dotted line) and even further in cpk:Ccl2−/− mice to PN33 (P < 0.0001; Fig. 3, dashed line). In addition to surviving longer, cpk:Ccl2−/− mice appeared generally more comfortable, exhibiting no signs of distress. Remarkably, a few cpk:Ccl2−/− mice lived >89 days without apparent discomfort, despite hugely cystic kidneys.
Fig. 3.
Genetic deficiency of chemokine (C-C motif) ligand 2 (Ccl2) in cpk mice prolongs survival. Shown is a survival plot of cystic cpk mice that were either wild type at the Ccl2 locus (cpk:Ccl2+/+), heterozygous (cpk:Ccl2+/−), or null (cpk:Ccl2−/−). The median survival of cpk:Ccl2+/+ mice was 20.5 days, which was significantly extended in cpk:Ccl2+/− heterozygous mice to 23 days (P < 0.0001, as shown in a) and even further in cpk:Ccl2−/−-null mice to 33 days (P < 0.0001, as shown in b). The difference between median survival of cpk:Ccl2+/− heterozygous and cpk:Ccl2−/−-null mice was also significant (P = 0.01, as shown in c).
Absence of MCP-1 in cpk mice does not restrain renal cystic disease progression.
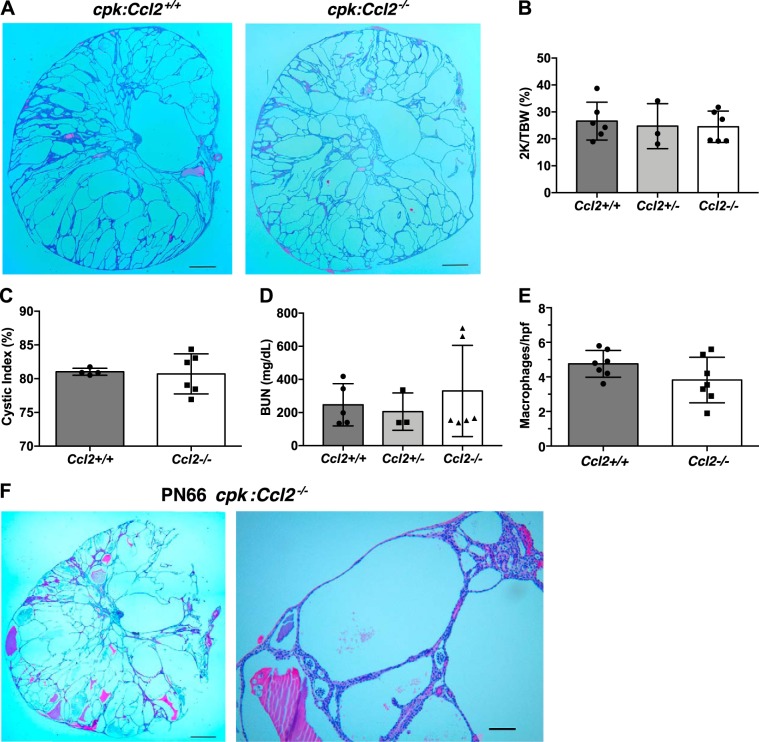
Renal cystic disease progression and relative macrophage levels were also assessed in cpk:Ccl2+/+ and cpk:Ccl2−/− mice. For these experiments, we euthanized animals at PN18, since cpk:Ccl2+/+ mice do not live past PN21 (Fig. 3). Despite the difference in survival between these two groups, surprisingly, kidneys from these two groups were histologically indistinguishable (Fig. 4A). Moreover, there was no difference in any of the disease progression parameters, including the two kidney-to-total body weight ratio (Fig. 4B), cystic index (Fig. 4C), and renal function as estimated by serum BUN (Fig. 4D). Serum BUN was approximately four to five times that of normal adult levels in all groups of mice. There appeared to be a small decline in the total number of macrophages in cpk:Ccl2−/− mouse kidneys relative to those of cpk:Ccl2+/+ mice, but this difference did not reach significance (Fig. 4E). To examine renal disease in the longer-lived cpk:Ccl2−/− mice, we euthanized one of the animals that was living with no apparent discomfort at PN66. Renal function was strikingly poor in this animal, with serum BUN exceeding 500 mg/dl. Grossly, the kidney parenchyma was essentially replaced by large, fluid-filled cysts, and small areas of apparent hemorrhage (not shown). Hematoxylin and eosin (H&E)-stained sections confirmed the gross impression, demonstrating large cysts filled with fluid, debris, hemorrhage, and very few remaining nephrons (Fig. 4F). These unexpected results indicate that MCP-1, despite being the major chemoattractant produced by cpk kidney cells (Fig. 2D), does not significantly influence total renal macrophage numbers or cystic disease progression in cpk mice. The fact that mice with astoundingly poor renal function exhibited prolonged survival suggests that renal failure per se is not the cause of death in cpk mice. Importantly, the data imply that the presence of MCP-1 in these animals promotes their decline and death.
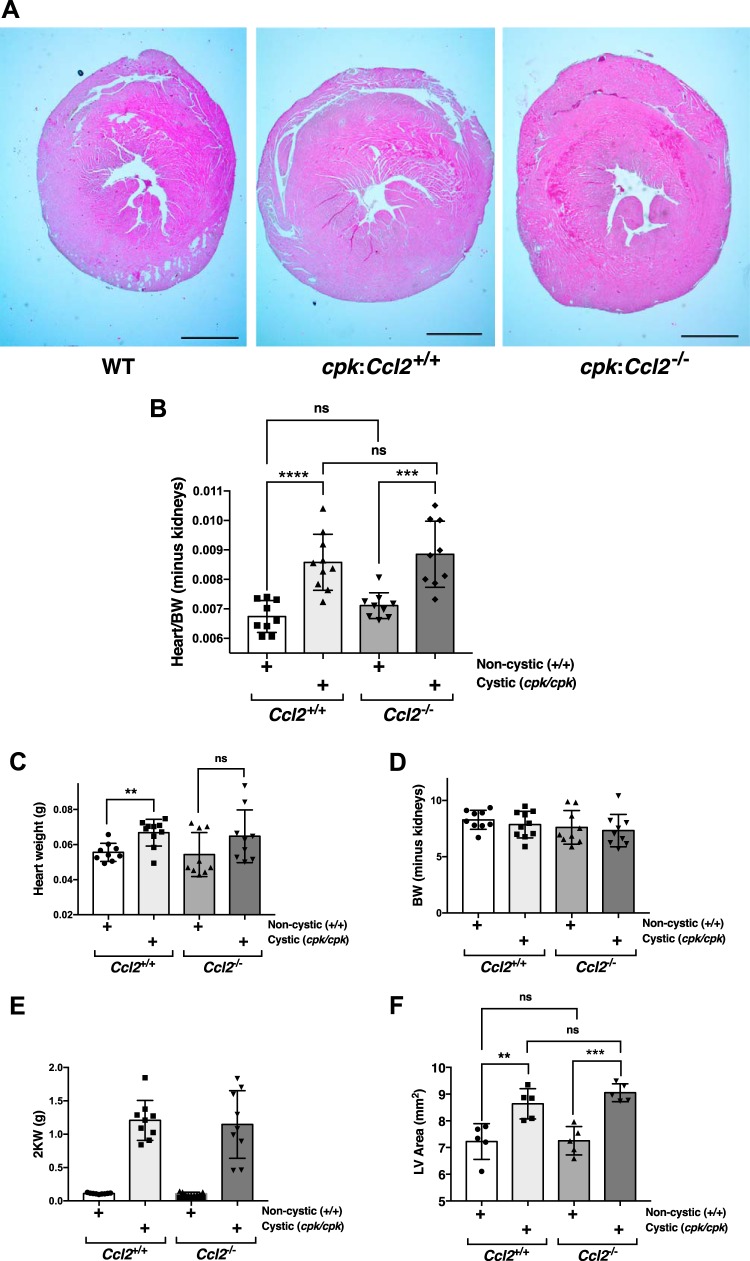
Fig. 4.
The absence of monocyte chemoattractant protein-1 in cpk mice does not restrain cystic disease progression. cpk mice that were either wild type, heterozygous, or null at the chemokine (C-C motif) ligand 2 (Ccl2) locus were euthanized at postnatal day 18 before kidney and blood collection for analyses of cystic disease progression and kidney function. A: average-sized formalin-fixed kidneys from cpk:Ccl2+/+ and cpk:Ccl2−/− mice were sectioned and stained with hematoxylin and eosin. Images of these kidneys showed no apparent difference. Scale bar = 1 mm. B: the two-kidney/total body weight percentage (2K/TBW%) was determined for each animal analyzed and plotted as a function of genotype. C: the cystic index for cpk:Ccl2+/+ and cpk:Ccl2−/− mice was determined and plotted for each genotype. D: blood urea nitrogen (BUN) was determined for each mouse tested and plotted as a function of genotype. E: tissue sections of formalin-fixed kidneys from cpk:Ccl2+/+ and cpk:Ccl2−/− mice (n = 7 mice/group) were stained with the macrophage marker CD68, and macrophages were counted in 10 nonoverlapping high-powered fields (hpf) and plotted. F: a surviving cpk:Ccl2−/− mouse was euthanized at postnatal day 66 before kidney and blood collection for analyses of cystic disease progression and kidney function. The formalin-fixed kidney from this mouse was sectioned and stained with hematoxylin and eosin. Scale bars = 1 mm in the left image and 50 µm in the right image.
Gene expression of inflammation and fibrosis markers in cpk mouse kidneys.
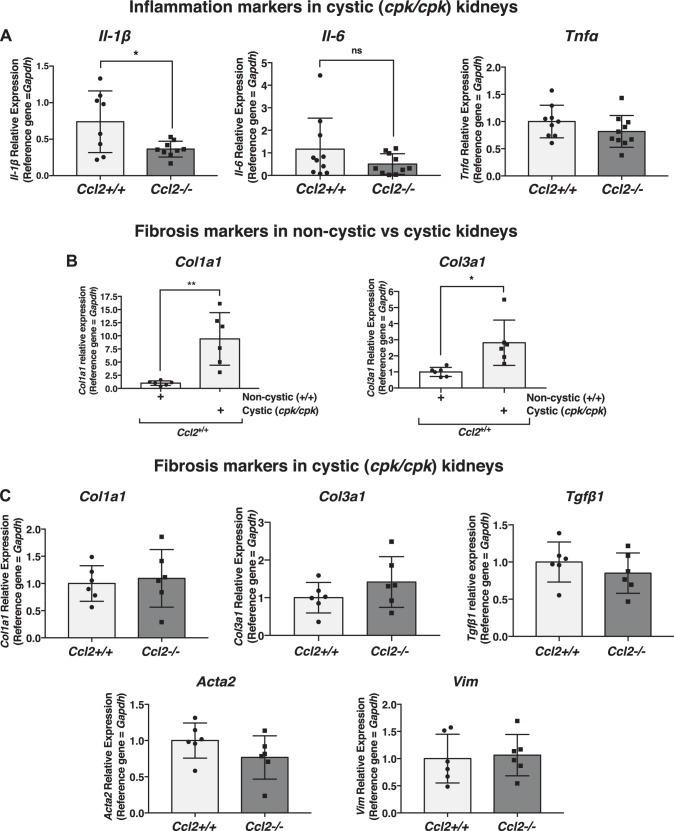
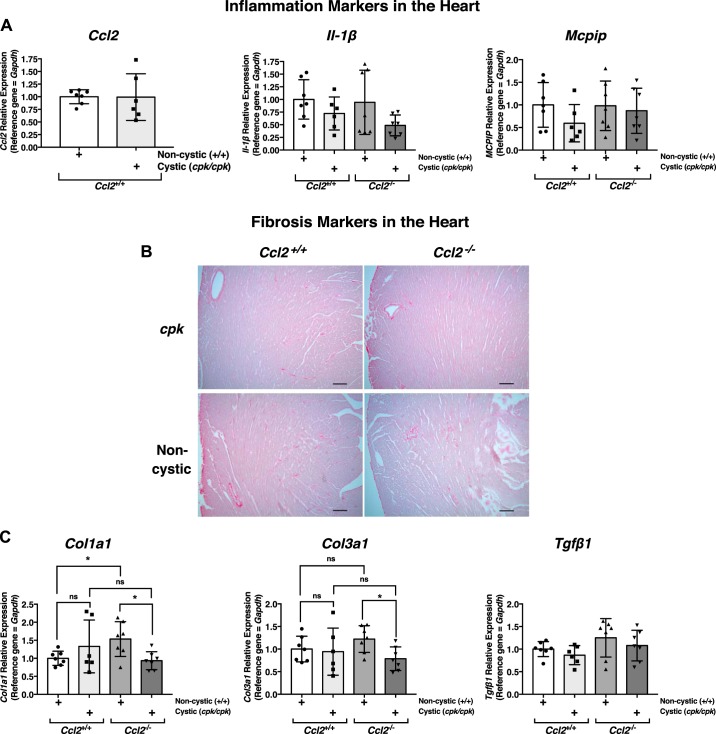
There are many examples in injury settings where the presence of MCP-1 has been shown to influence levels of inflammatory cytokines that could potentially influence survival (12, 14, 28, 36, 39, 70, 73). To obtain clues about inflammatory processes in cystic cpk mice, we assessed the relative transcript expression of a number of inflammation markers using quantitative RT-PCR of RNA isolated from whole cpk:Ccl2+/+ and cpk:Ccl2−/− kidneys from PN18 mice. These markers included Il-1β, Il-6, and Tnf (Fig. 5A). Of these, only Il-1β showed a significant difference in expression: approximately twofold higher in cpk:Ccl2+/+ versus cpk:Ccl2−/− kidneys. There was no significant difference in the relative expression of other inflammation markers, including inducible nitric oxide synthase (Nos2), Ccl4, and chemokine (C-X-C motif) ligand 3 (Cxcl3; data not shown). These data indicate that, outside of a single marker, Il-1β, the inflammatory transcript profiles of cpk:Ccl2+/+ and cpk:Ccl2−/− mouse kidneys appear to be similar.
Fig. 5.
Effects of chemokine (C-C motif) ligand 2 (Ccl2) deficiency on transcription markers of inflammation and fibrosis in cystic cpk mouse kidneys. A: quantitative RT-PCR of RNA isolated from cystic kidneys of cpk mice [postnatal day 18 (PN18)] that were either wild type (Ccl2+/+) or null at the Ccl2 locus (Ccl2−/−), showing the relative expression of the inflammation markers Il-1β, Il-6, and Tnfa. *P < 0.05. ns, not significant. B: quantitative RT-PCR of RNA isolated from kidneys of Ccl2+/+ mice (PN18) that were either noncystic (+/+) or cystic (cpk/cpk) at the cpk locus, showing the relative expression of the fibrosis markers collagen type I-α1 (Col1a1) and collagen type III-α1 (Col3a1). *P < 0.05; **P < 0.01. C: relative expression of the fibrosis markers Col1a1, Col3a1, transforming growth factor-β1 (Tgfb1), smooth muscle actin (Acta2), and vimentin (Vim) in cystic kidneys of cpk mice (PN18) that were either wild type (Ccl2+/+) or null at the Ccl2 locus (Ccl2−/−), as determined by quantitative RT-PCR of isolated RNA.
In cpk mice, evidence of renal fibrosis can be detected as early as PN10 (79). It was not surprising then that when the relative transcript levels of the fibrosis markers collagen type I-α1 (Col1a1) and collagen type III-α1 (Col3a1) were assessed by quantitative RT-PCR in cpk:Ccl2+/+ versus noncystic WT:Ccl2+/+ mouse kidneys, they were significantly elevated (Fig. 5B). To determine whether MCP-1 deficiency might influence the development of fibrosis in cpk kidneys, relative transcript expression of genes encoding not only collagen types I and III but also transforming growth factor-β1 (Tfgb1), smooth muscle actin (Acta2), and vimentin (Vim) were assessed in cpk:Ccl2+/+ versus cpk:Ccl2−/− kidneys. No differences in expression were found (Fig 5C), suggesting that MCP-1 deficiency does not affect fibrosis in cpk kidneys.
cpk mice have enlarged hearts, which are not altered by Ccl2 deficiency.
Since cpk mice did not appear to die from renal failure, other potential causes were investigated. We did not see evidence of hemorrhage in any body compartment, nor did we see any gross evidence of thrombosis. However, the hearts of cpk mice appeared enlarged. Cardiac hypertrophy occurs as an adaptive response to pressure overload and, under pathological conditions, can lead to deleterious events that ultimately result in heart failure and death. Indeed, cardiovascular disease is the most common cause of death in patients with ADPKD (17), and cardiovascular abnormalities have been documented in multiple mouse models of PKD (6, 31, 76). To our knowledge, though, possible cardiac manifestations in the cpk mouse have not been reported. To investigate this possibility, PN18 hearts from the following four groups were collected: noncystic (WT:Ccl2+/+), noncystic with Ccl2 KO (WT:Ccl2−/−), cpk:Ccl2+/+, and cpk:Ccl2−/−. Mice of all genotypes had four-chamber hearts, with no gross developmental abnormalities present. Analysis of heart weight relative to total body weight minus the weights of the two kidneys, however, demonstrated that the hearts of both cpk:Ccl2+/+ and cpk:Ccl2−/− mice were significantly enlarged relative to noncystic mice (Fig. 6, A and B). Confirmation that this difference was due to an increase in heart weight, rather than a decrease in body weight minus the weights of the two kidneys, was obtained by assessments of heart weight, body weight minus the weights of the two kidneys, and weights of the two kidneys (Fig. 6, C–E). There was no significant difference in heart enlargement between cpk:Ccl2+/+ and cpk:Ccl2−/− hearts. Among noncystic mice, there was also no difference in heart weights between WT:Ccl2+/+ and WT:Ccl2−/− mice. Measurement of H&E-stained sections from formalin-fixed, paraffin-embedded hearts of cpk:Ccl2+/+ and cpk:Ccl2−/− mice demonstrated left ventricular enlargement relative to noncystic mice (Fig. 5F). However, there was no difference in right ventricular thickness between these two cystic groups (not shown). There was also no difference in the histological appearance between WT:Ccl2+/+ and WT:Ccl2−/− hearts (not shown). We also assessed cardiac macrophage infiltration using immunohistochemistry for F4/80, a common macrophage marker. However, none of the hearts in any of the four groups showed any significant macrophage infiltrate, whereas antibody controls performed in parallel were positive (not shown). These results indicate that, while cystic cpk mice have enlarged hearts, Ccl2 deficiency does not significantly influence overall cardiac size, morphogenic differences, or macrophage infiltration.
Fig. 6.
cpk mice have enlarged hearts, which are not altered by chemokine (C-C motif) ligand 2 (Ccl2) deficiency. A: formalin-fixed hearts from wild-type (WT), cpk:Ccl2+/+, and cpk:Ccl2−/− mice (postnatal day 18) were sectioned and stained with hematoxylin and eosin. Scale bars = 1 mm. B: heart weight-to-total body weight (BW) ratio minus the weights of the kidneys was determined for each mouse (postnatal day 18) and analyzed for the following genotypes: WT;Ccl2+/+, WT;Ccl2−/−; cpk:Ccl2+/+, or cpk:Ccl2−/−; it was then plotted as a function of genotype. ***P < 0.001. ns, not significant. C: heart weights from the same mice used in B. **P < 0.01. D: body weight minus weights of the two kidneys from the same mice used in B. E: weights of the two kidneys (2K) from the same mice used in B. F: area of the left ventricle (LV) for mice of the indicated genotypes. **P < 0.01; ***P = 0.001.
Gene expression and fibrosis assessment in cpk mouse hearts.
Relative expression of the inflammation markers Ccl2 and Il-1β, which were significantly elevated in cpk:Ccl2+/+ kidneys relative to those of WT:Ccl2+/+ and cpk:Ccl2−/− mice (Figs. 1C and 5A), were assessed in the hearts of these animals, as was the relative expression of MCP-induced protein (Mcpip; Zc3h12a), which is reportedly upregulated in the heart in response to cardiac-specific MCP-1 overexpression and has been reportedly associated with heart failure (82). Unlike the expression profile in the kidneys, neither Ccl2 nor Il-1β was upregulated in cpk:Ccl2+/+ hearts relative to hearts of WT:Ccl2+/+ or cpk:Ccl2−/− mice. In addition, there was no difference in cardiac expression of Mcpip among the four genotypes (Fig. 7A). The relative expression of other inflammation marker genes or those known to influence cardiac hypertrophy or function, including TNF-α (Tnfa), Il-6, transient receptor potential C3 (Trpc3), transient receptor potential C6 (Trpc6), Myc, FGF-23 (Fgf23), peroxisome proliferator-activated receptor (PPAR)-α (Ppara), PPAR-γ (Pparg), the inflammasome components NLR family pyrin domain containing 3 (Nlrp3) and caspase-1 (Casp1), and the macrophage marker F4/80 (Adgre1) were also assessed in these mouse hearts. In all cases, no differences in gene expression were detected (data not shown).
Fig. 7.
Effects of chemokine (C-C motif) ligand 2 (Ccl2) deficiency on gene expression and markers of fibrosis in cystic cpk mouse hearts. A: quantitative RT-PCR of RNA isolated from noncystic or cystic kidneys of cpk mice [postnatal day 18 (PN18)] that were either wild type (Ccl2+/+) or null at the Ccl2 locus (Ccl2−/−), showing the relative expression of the inflammation markers Ccl2, Il-1β, and monocyte chemoattractant protein-induced protein (Mcpip). B: heart sections from PN18 cpk and noncystic mice that were either Ccl2+/+ or Ccl2−/−, as indicated, were stained with picrosirus red and illuminated by bright field to visualize collagen type I-α1 (Col1a1) and collagen type III-α1 (Col3a1) fibrils. Scale bar = 100 µm. C: quantitative RT-PCR of RNA isolated from noncystic or cystic kidneys of cpk mice (PN18) that were either Ccl2+/+ or Ccl2−/−, showing the relative expression of the fibrosis markers Col1a1, Col3a1, and transforming growth factor-β1 (Tgfb1). *P < 0.05. ns, not significant.
Enlarged, hypertrophic hearts in a disease setting usually develop fibrosis, which can be detected by ~7 days after pathological pressure overload (63). To determine whether fibrosis was present in the enlarged hearts of cpk:Ccl2+/+ and cpk:Ccl2−/− mice, cardiac sections of each mouse genotype were stained with picrosirius red to allow detection of collagen types I and III fibrils (42). While the expected staining of the blood vessel walls was present in all samples, there was no evidence of fibrosis in these hearts (Fig. 7B). The relative expression of fibrosis marker genes (Col1a1, Col3a1, and Tgfb1) in the hearts from these mice were also assessed (Fig. 7C). There were no differences in expression of these genes, nor of the fibrosis marker gene Acta2 (data not shown). These results suggest that the hypertrophic hearts of both Ccl2+/+ and Ccl2−/− cystic cpk mice, at least at this stage of their lives (PN18), display no evidence of significant inflammation or fibrosis.
Fetal cardiac gene expression in cpk mice.
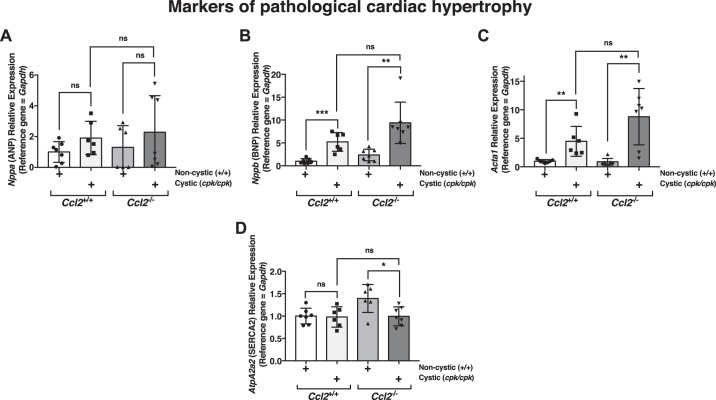
Characteristic changes in cardiac gene/protein expression occur in pathological hypertrophy in what is thought to be a compensatory response to protect the heart (4). These changes include the upregulation of fetal cardiac genes, such as those that encode atrial natriuretic peptide (ANP; Nppa), brain natriuretic peptide (BNP; Nppb), skeletal α-actin (Acta1), and β-myosin heavy chain (Myh7). There is also typically downregulation of genes that are normally expressed at higher levels in the adult, such as α-myosin heavy chain (Myh6) and SERCA2a (Atp2a2) (10, 27, 37). To determine whether this compensatory fetal gene program was present in cystic cpk mouse hearts and to determine the potential effects of Ccl2 deficiency, RNA was prepared from PN18 hearts of cpk:Ccl2+/+ and cpk:Ccl2−/− mice as well as WT:Ccl2+/+and WT:Ccl2−/− mice, and the relative expression of these fetal genes was assessed by quantitative RT-PCR (Fig. 8).
Fig. 8.
Fetal cardiac gene expression in cpk mice. Quantitative RT-PCR was carried out using RNA isolated from hearts of mice that were either noncystic (+/+) or cystic (cpk/cpk) at the cpk locus (postnatal day 18) and were either chemokine (C-C motif) ligand 2 (Ccl2)+/+ or Ccl2−/− to determine the pattern and relative expression of fetal genes associated with pathological hypertrophy [Nppa, which encodes atrial natriuretic peptide (ANP; A); Nppb, which encodes brain natriuretic peptide (BNP; B); Acta1, which encodes skeletal α-actin (C); and AtpA2a2, which encodes SERCA2 (D)]. *P < 0.05; **P < 0.01; ***P < 0.001. ns, not significant.
To examine the effects of cystic disease on the fetal cardiac gene program, relative expression in hearts from cystic versus noncystic mice was assessed for mice with both Ccl2+/+ and Ccl2−/− genotypes. Hearts from both groups of cystic mice showed a significant upregulation of the BNP gene relative to noncystic mice (~5.2-fold for Ccl2+/+ mice and ~4-fold for Ccl2−/− mice; Fig. 8B) and a trend for increased expression of the ANP gene (Fig. 8A). Upregulation of the skeletal α-actin gene was also seen in both groups as a result of cystic disease (~4.5-fold increase for Ccl2+/+ mice and 9.8-fold increase for Ccl2−/− mice; Fig. 8C). Assessment of the gene encoding SERCA2a revealed elevated expression (~1.4-fold) in noncystic Ccl2−/− mouse hearts compared with noncystic Ccl2+/+ mouse hearts, suggesting that MCP-1 acts directly or indirectly to influence the expression of this gene. Its expression was not altered when we compared cystic and noncystic Ccl2+/+ mice but was significantly downregulated in cystic Ccl2−/− mice compared with the noncystic counterpart group (~1.4-fold lower than WT:Ccl2−/− mice; Fig. 8D). There were no significant differences in the relative transcript levels of genes encoding α-myosin heavy chain or β-myosin heavy chain in the hearts of cystic versus noncystic animals (data not shown). Moreover, the ratio of α-myosin heavy chain gene expression to β-myosin heavy chain gene expression, an indicator of the predominant functional isoform, also did not show differences (data not shown). These results indicate that of the six gene expression changes in the fetal cardiac program that develop protectively during cardiac hypertrophy, two of them (elevated BNP and skeletal α-actin gene expression) occurred in the hearts of Ccl2+/+ cystic cpk animals at this stage of their lives (PN18) and three of them (elevated BNP and skeletal α-actin and reduced SERCA2a gene expression) occurred in Ccl2−/− cystic mouse hearts.
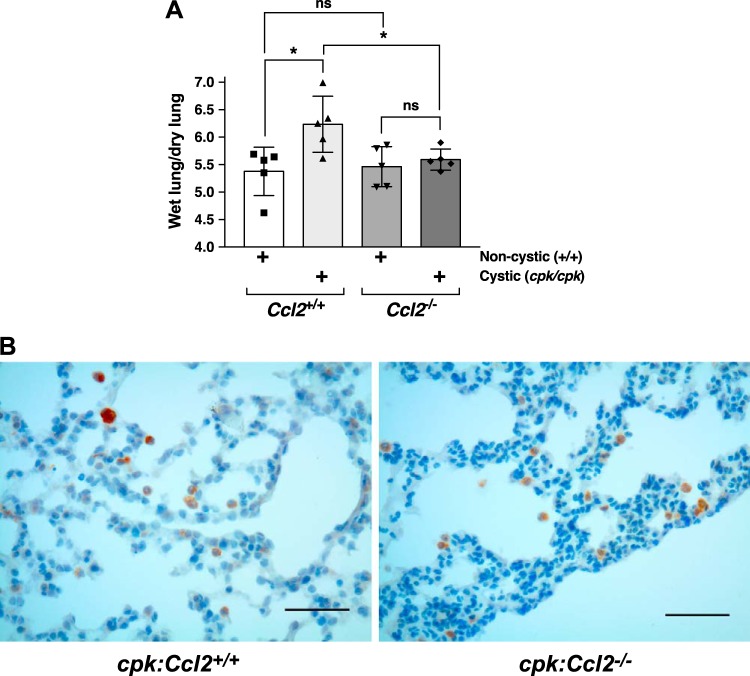
cpk animals develop pulmonary edema that is restrained by Ccl2 deficiency.
Cardiac dysfunction can be associated with pulmonary edema, and this condition can be measured experimentally by increased wet lung weight to dry lung weight (13). To determine whether there might be differences in this parameter, we collected lungs from PN18 mice of all genotypes and determined their wet weight-to-dry weight ratio. There was a significant increase in this value for the lungs of mice with cystic disease and intact MCP-1 (cpk:Ccl2+/+ vs. WT:Ccl2+/+ mice, ~16% increase), whereas this value did not change in Ccl2-deficient mice as a function of cystic disease (cpk:Ccl2−/−vs. WT:Ccl2−/− mice; Fig. 9A). Notably, there was also a significant increase for the lungs of cpk:Ccl2+/+ mice compared with those of cpk:Ccl2−/− mice (~12% increase). Examination of H&E-stained sections from formalin-fixed, paraffin-embedded lungs of cpk:Ccl2+/+ and cpk:Ccl2−/− mice showed no apparent difference in histological appearance (data not shown). Neither showed significant airway damage, vascular thickening, inflammation, congestion, or fluid within airspaces. Immunohistochemical stain for the macrophage marker CD68 showed occasional macrophages in the alveolar capillaries in both cpk:Ccl2+/+ and cpk:Ccl2−/− mouse lungs, but there did not appear to be a difference between them. A few collections of intra-alveolar macrophages could be found within the periphery of lungs from both mice (Fig. 9B). However, these did not appear more numerous in one group or other, and similar collections were seen in the lungs of noncystic mice (data not shown).
Fig. 9.
Cystic cpk animals develop pulmonary edema that is restrained by chemokine (C-C motif) ligand 2 (Ccl2) deficiency. A: lungs were collected from wild-type (WT):Ccl2+/+, cpk:Ccl2+/+, WT:Ccl2−/−, and cpk:Ccl2−/− mice (postnatal day 18) and were weighed immediately (wet lung weight). These were weighed again after drying (dry lung weight), and the wet lung weight-to-dry lung weight ratio was determined and plotted as a function of genotype. *P < 0.05. B: fixed lung sections from cpk:Ccl2+/+ and cpk:Ccl2−/− mice (postnatal day 18) that were stained with anti-CD68. Scale bars = 50 µm.
These results suggest that the presence of MCP-1, while not detectably influencing macrophage concentration in the lungs of cystic cpk mice relative to those that are MCP-1 deficient, promotes edema in this tissue, which may contribute to their early deaths.
Effects of cytogenesis and Ccl2 deficiency on cardiac electrical activity in cpk mice.
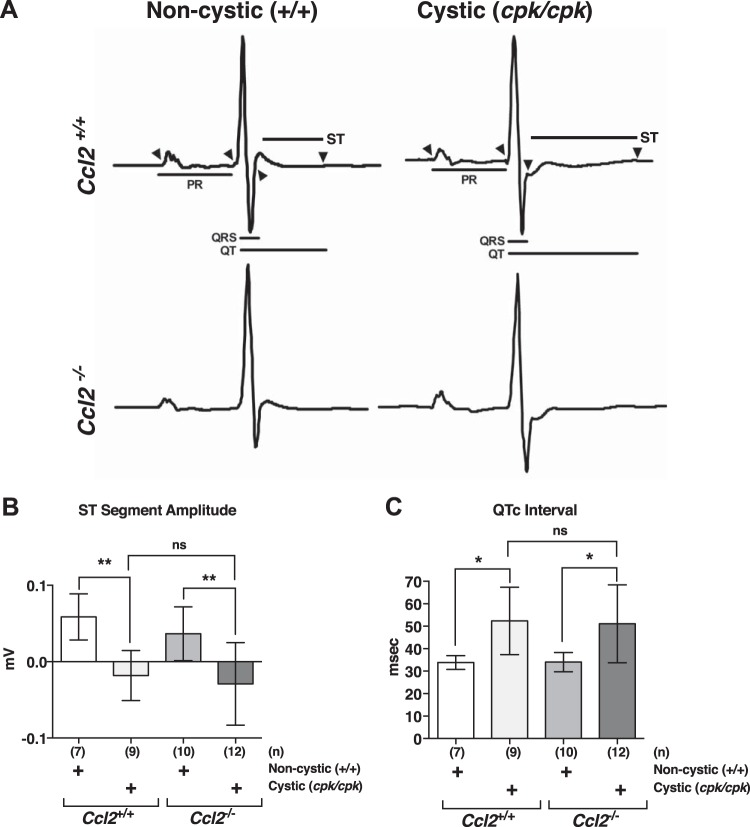
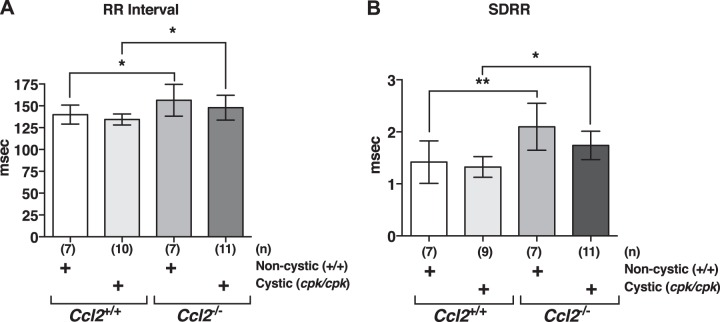
We measured cardiac electrical activity via ECG in cystic and noncystic mice that were either Ccl2+/+ or Ccl2−/− (Fig. 10A). Although there were no significant differences in PR interval (30.99 ± 0.75 ms for the WT:Ccl2+/+ group, 29.76 ± 0.68 ms for the cpk/Ccl2+/+ group, 32.09 ± 1.02 ms for the WT:Ccl2−/− group, and 31.51 ± 0.92 ms for the cpk/Ccl2−/− group, n = 7–12 mice/group, P > 0.05 in all cases) or QRS interval (9.08 ± 0.12 ms for the WT:Ccl2+/+ group, 9.33 ± 0.18 ms for the cpk/Ccl2+/+ group, 9.53 ± 0.25 for the WT:Ccl2−/− group, and 9.03 ± 0.24 ms for the cpk/Ccl2−/− group) among the groups, cpk/Ccl2+/+ and cpk/Ccl2−/− mice displayed marked repolarization abnormalities in the form of ST segment depression (Fig. 10B) and prolonged QTc interval (Fig. 10C) compared with WT:Ccl2+/+ and WT:Ccl2−/− mice, respectively. In addition, average resting HR of Ccl2−/− mice was reduced in cystic and noncystic groups, as indicated by increased RR interval (Fig. 11A).
Fig. 10.
ECG abnormalities are present in hearts of cpk mice and are not altered by chemokine (C-C motif) ligand 2 (Ccl2) deficiency. A: representative averaged ECG waveforms for wild-type (WT)/Ccl2+/+ (top left), cpk/cpk:Ccl2+/+(top right), WT/Ccl2−/− (bottom left), and cpk/cpk:Ccl2−/− (bottom right) mice. Interval measurements are indicated by arrowheads showing interval boundaries. B: ST segment amplitude measured 10 ms after the R wave. **P < 0.01. C: QT interval corrected for heart rate (QTc). *P < 0.05. ns, not significant.
Fig. 11.
chemokine (C-C motif) ligand 2 (Ccl2)-deficient mice display enhanced heart rate variability (HRV) in both noncystic and cystic backgrounds. A: average resting RR interval. *P < 0.05. B: standard deviation of the RR interval (SDRR) derived from 2 min of continuous ECG recording. *P < 0.05; **P < 0.01.
As the predisposition to mortality from adverse cardiac events can be predicted by detecting a decrease in the intrinsic variability in HR over time, termed HRV, we measured HRV from the ECG recordings in cystic and noncystic mice. Both noncystic and cystic Ccl2−/− mice showed evidence of enhanced HRV with a significant elevation in SDRR compared with WT:Ccl2+/+ and WT:Ccl2−/− mice, respectively (Fig. 11B). These data indicate that disruption of Ccl2 leads to increased HRV, which is predicted to have a protective effect and is maintained in the cystic background despite the presence of left ventricular hypertrophy.
DISCUSSION
We have previously shown that renal macrophages promote disease progression in cpk mice and that specific reduction of their cell numbers by systemic clodronate treatment was sufficient to restrain cyst expansion and loss of kidney function (65). In the present study, we bred MCP-1-deficient cpk mice in an attempt to reduce renal macrophages by reducing their recruitment. Because we found that these animals had a prolonged survival, we were expecting also to find a reduced cystic burden and improved kidney function along with reduced macrophage numbers. However, there was no change in any of these parameters. These results suggest that cpk mice are not dying because of renal failure and that the presence of MCP-1 is promoting the decline of these animals.
Despite our findings here that MCP-1 is likely to be a major renal recruitment factor in cpk mouse kidney cells when grown in vitro, we found there was no significant reduction in renal macrophage concentration in cystic mice that were deficient for MCP-1 compared with those that were WT at the CCL2 locus. This finding may be due to the presence of other renal recruitment factors, the relative levels of which may be different in intact kidneys versus kidney cells grown in vitro, and/or the presence of resident renal macrophages, the numbers of which would be unaffected by recruitment. Another group has shown that partial inhibition of MCP-1 synthesis in a PKD rat model resulted in a partial reduction in renal macrophage numbers (83). However, there was no effect on renal cyst growth in these animals. The relative contribution of resident versus recruited macrophases to the total renal macrophage population in PKD has not been determined. However, only the level of infiltrating macrophages is likely to be affected by loss of recruitment factors, whereas both infiltrating and resident macrophage populations are likely to be lowered by systemic clodronate treatment, as carried out in our previous studies.
An important question arising from this study is: why do cpk:Ccl2−/− mice survive longer? To answer this question definitively, one must understand why cpk:Ccl2+/+ mice die by PN21. It has been assumed that cpk mice die from renal failure, since there is severe cystic disease and terrible renal function by this time. However, cpk:Ccl2−/− mice have similar cystic disease and renal function, at least as measured by BUN. Thus, it seems unlikely that cpk mice die from renal failure per se. Indeed, some of the cystic mice with MCP-1 KO show markedly elevated BUN and not only live for extended periods of time but also seem to be in no acute distress. It is possible that an unmeasured uremic toxin is responsible for death, and this toxin is somehow selectively eliminated in cpk:Ccl2−/− mice, but a potential mechanism for this is difficult to imagine. Regardless, the findings here indicate that the loss of MCP-1 protects from death and, by extension, suggests that MCP-1 promotes early death.
Since cardiovascular disease is the most common cause of death in patients with PKD (17, 52), we examined cpk hearts. Cardiovascular disease had not been assessed previously in cpk mice but has been demonstrated in multiple other mouse models of PKD (6, 31, 76). Thus, our finding here that cpk mice have enlarged hearts relative to their noncystic litter mates is not surprising, but the result establishes another example of PKD associated with cardiac disease.
Cardiac enlargement/hypertrophy typically develops as an adaptive response to functional overload. This can occur because of pathological pressures that occur in disease settings (pathological hypertrophy), such as hypertension, or because of chronic exercise training (physiological hypertrophy), as is often the case in athletes. The molecular mechanisms that promote pathological versus physiological hypertrophy and the resulting changes in gene expression are different (4). Pathological hypertrophy is initially compensatory but is ultimately maladaptive and a key risk factor for heart failure. It is commonly associated with reexpression of fetal cardiac genes (e.g., ANP, BNP, α-skeletal actin, and β-MHC) and downregulation of “adult” genes (e.g., α-MHC and SERCA2a). In the hearts of both cpk:Ccl2+/+ and cpk:Ccl2−/− mice, there was a partial reexpression of the fetal cardiac gene program (two of the six expression changes in this program for cpk:Ccl2+/+ mice and three of the six expression changes for cpk:Ccl2−/− mice) with a few differences. Whereas both cpk:Ccl2+/+ and cpk:Ccl2−/− mice showed cardiac upregulation of the genes for BNP and α-skeletal actin relative to their noncystic counterparts (WT:Ccl2+/+ and WT:Ccl2−/− mice, respectively), only cpk:Ccl2−/− mice showed decreased expression of the gene for SERCA2a. SERCA2a mediates Ca2+ uptake in the sarcoplasmic reticulum, thereby regulating both cardiac relaxation and contraction (32). Downregulation of SERCA2a in response to pressure overload is an energy-conserving mechanism (67), which is initially adaptive, similar to most of the gene expression changes that occur during reprogramming to the fetal cardiac program. However, as for most of the fetal program gene expression changes, the decreased levels of the SERCA2a gene, if maintained, ultimately is insufficient for effective cardiac function and, thereby, a promoter of heart failure (32). Thus, it is difficult to imagine that the transcriptional response of this gene in the hearts of cpk:Ccl2−/− mice relative to their noncystic counterparts, but not in the hearts of cpk:Ccl2+/+ mice, would contribute to their extended lifespan.
Other features associated with pathological hypertrophy include cardiac fibrosis, a feature that was not detected in cpk mouse hearts. After pressure overload in mice, cardiac enlargement occurs early (within 2 days), whereas fibrosis develops only later (detectable often by 7 days) (63). While some of the features of these mouse hearts (i.e., partial reexpression of fetal genes) suggest that they may be undergoing pathological hypertrophy, the full expression of features associated with this remodeling process may not yet be manifested because of their young age (PN18).
Assessment of the lungs in cystic and noncystic mice that were either Ccl2+/+ or Ccl2−/− revealed that only the short-lived cpk:Ccl2+/+ mice had elevated wet lung weight-to-dry lung ratios, indicative of pulmonary edema. While the presence of pulmonary edema can arise from cardiac dysfunction, other causes are also possible in these mice. Outside of its role as a chemoattractant, MCP-1 has been shown to interact directly with its receptor, chemokine (C-C motif) receptor 2 (CCR2), on endothelial cells to promote cytoskeletal rearrangements, resulting in the loosening of cell junctions and increased permeability (55, 56, 64). Through this mechanism, a previously study (64) has indicated that MCP-1 is an important regulator of the blood-brain barrier permeability that occurs during an inflammatory response after central nervous system conditions, including stroke, brain tumors, and traumatic brain injury. Similarly in the lung, MCP-1 acting through CCR2 was shown to induce endothelial retraction and leakiness both in vivo and in vitro (56). It may be that MCP-1 in cpk:Ccl2+/+ mice may act on endothelial cells in the lung to promote vascular leakiness, resulting in pulmonary edema. If this is true, then similar effects on the CCR2-expressing endothelium in other sensitive vascular compartments, such as the brain, might be expected, a possibility that will require further investigation to determine. Regardless of the cause, the pulmonary edema present in cpk:Ccl2+/+ mice is likely to promote morbidity in these animals and potentially contribute to their truncated lifespan.
There are known links between MCP-1 and cardiac dysfunction. Elevated levels of MCP-1 are found in cardiovascular disease, including other cardiorenal syndromes (23, 44), and, in experimental models of hypertrophy leading to cardiac dysfunction, MCP-1 has been reported to promote impaired diastolic function indirectly due to its chemoattractant activity (34). In that study, pressure overload of hearts promoted both hypertrophy and upregulation of cardiac MCP-1 transcript and protein levels, macrophage accumulation, and fibroblast proliferation, which led to myocardial fibrosis and diastolic dysfunction. Treatment with anti-MCP-1 neutralizing antibody was sufficient to causally link MCP-1 to macrophage accumulation and the accompanying fibroblast proliferation, myocardial fibrosis, and diastolic dysfunction, but not hypertrophy. It is unlikely that these processes are occurring in cpk:Ccl2+/+ hearts, however, since there is neither elevation of Ccl2 transcripts relative to those found in WT:Ccl2+/+ hearts nor evidence of macrophage accumulation and fibrosis.
Expression of the primary MCP-1 receptor, CCR2, has been demonstrated in hearts (82), and, while potential direct effects of MCP-1 on cardiac function have not been assessed, MCP-1 is known to influence the expression of inflammatory cytokines and factors in cardiomyocytes, which can affect cardiac function. MCP-1 treatment of cardiomyocytes in vitro has been shown to induce the expression of IL-6 and IL-1β (12), both of which can alter cardiomyocyte contractility (18, 71). In addition, cardiac-specific MCP-1 overexpression has been shown to induce the expression of MCPIP, which induces apoptotic cell death and has been proposed to be responsible for the heart failure that eventually develops in this transgenic model (82). There is no evidence that MCP-1 is eliciting these effects in cpk:Ccl2+/+ mice, however, since there was no difference in expression levels of Il-1b or MCPIP in the hearts of these mice versus those of cpk:Ccl2−/−, WT:Ccl2+/+, and WT:Ccl2−/− mice.
In accordance with the left ventricular hypertrophy observed in cpk mice, ECG analysis revealed marked changes in the repolarization phase, including depression of the ST segment and prolongation of QTc. Repolarization disturbances are commonly observed in models of left ventricular hypertrophy and heart failure (1, 40, 66). Interestingly, Ccl2−/− mice showed reduced resting HR and increased SDRR, an indicator of total autonomic variability on HR. Typically, higher levels of parasympathetic and/or lower sympathetic output to the heart are responsible for higher HRV. Lower HRV is a well-documented independent predictor of the risk of sudden cardiac death in healthy human populations (57) as well as in disease populations, including those with cardiovascular disease (3, 35), diabetes (62), and forms of kidney disease (24, 53). The immune system and sympathetic nervous system are tightly coupled entities that work in concert to regulate and respond to local inflammatory mediators (49). If circulating inflammatory cytokine levels are increased, this can signal the brain to trigger activation of the sympathetic nervous system as a stress response (5, 43, 49). In addition, the receptor for CCL2 itself, CCR2, has been shown to be present on peripheral neurons, and activation can stimulate and change sensitivity of neurons (2, 29). Therefore, disruption of MCP-1 signaling may have an indirect effect on sympathetic nervous system activity on the heart due to reduced inflammatory signaling, which may explain the reduced HR and increased HRV in Ccl2−/− mice. These effects may lead to the higher premature death rates in MCP-1-containing cystic mice compared with MCP-1-null cystic mice, a possibility that will need further investigation to verify.
In summary, these data indicate that the presence of MCP-1 is death promoting in cpk mice. While the mechanisms by which this occurs are not certain, the data collected, thus far, suggest that multiple effects of this cytokine/chemokine, including the development of pulmonary edema in cystic mice and the detrimental influence on HR and HR, are possible contributors. Moreover, these effects of MCP-1 appear to be independent of its role as a chemoattractant and fibrosis-promoting agent. These results may have far-reaching implications regarding the contribution of MCP-1 to the morbidity and cardiac dysfunction in patients with PKD and possible therapeutic treatments that could influence mortality. In addition, because MCP-1 is present in many forms of disease accompanied by tissue injury, these findings may also have implications for the treatment of all cardiorenal diseases as well as diseases in other organ systems that are mechanistically linked to cardiovascular disease (e.g., inflammatory bowel disease and chronic obstructive pulmonary disease).
GRANTS
This work was supported by grants from the Polycystic Kidney Foundation, the Kansas City Area Life Sciences Institute (to K. I. Swenson-Fields. and T. A. Fields), and, in part, by National Institutes of Health Clinical and Translational Science Award Grants (UL1-TR-000001, formerly UL1-RR-033179) and UL1-TR-002366 [awarded to the University of Kansas Medical Center (KUMC)], and the internal Lied Basic Science Grant Program of the KUMC Research Institute KUMC Research Institute (to K. I. Swenson-Fields).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.M.S., T.A.F., M.J.W, and K. I. S-F. conception and design of research; S.M.S., J.D.M., R.R, J.D.P., D.P.W, D.F, Xiaoyan L., X.Z., T.A.F., J.A.V.. M.J.W. and K. I. S-F. performed experiments; S.M.S., Xiaogang L., T.A.F., J.A.V., M.J.W. and K. I. S-F. analyzed data; S.M.S., T.A.F., J.A.V., M.J.W. and K. I. S-F. interpreted results of experiments; S.M.S., T.A.F., J.A.V., M.J.W. and K. I. S-F. prepared figures; S.M.S., J.A.V. and K. I. S-F. drafted manuscript; T.A.F and K. I. S-F. edited and revised manuscript; S.M.S., J.D.M., R.R, J.D.P., D.P.W, D.F, Xiaogang L., Xiaoyan L., X.Z., T.A.F., J.A.V., M.J.W. and K. I. S-F. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Marsha Danley for technical assistance, Marcela Medrano for assistance with mouse breeding and genotyping, and Gail Reif for preparation of primary kidney cells and tissues. We also thank Jason Stubbs for helpful discussions.
REFERENCES
- 1.Armoundas AA, Wu R, Juang G, Marbán E, Tomaselli GF. Electrical and structural remodeling of the failing ventricle. Pharmacol Ther 92: 213–230, 2001. doi: 10.1016/S0163-7258(01)00171-1. [DOI] [PubMed] [Google Scholar]
- 2.Banisadr G, Gosselin RD, Mechighel P, Rostène W, Kitabgi P, Mélik Parsadaniantz S. Constitutive neuronal expression of CCR2 chemokine receptor and its colocalization with neurotransmitters in normal rat brain: functional effect of MCP-1/CCL2 on calcium mobilization in primary cultured neurons. J Comp Neurol 492: 178–192, 2005. doi: 10.1002/cne.20729. [DOI] [PubMed] [Google Scholar]
- 3.Barron HV, Lesh MD. Autonomic nervous system and sudden cardiac death. J Am Coll Cardiol 27: 1053–1060, 1996. doi: 10.1016/0735-1097(95)00615-X. [DOI] [PubMed] [Google Scholar]
- 4.Bernardo BC, Weeks KL, Pretorius L, McMullen JR. Molecular distinction between physiological and pathological cardiac hypertrophy: experimental findings and therapeutic strategies. Pharmacol Ther 128: 191–227, 2010. doi: 10.1016/j.pharmthera.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 5.Besedovsky H, del Rey A, Sorkin E, Dinarello CA. Immunoregulatory feedback between interleukin-1 and glucocorticoid hormones. Science 233: 652–654, 1986. doi: 10.1126/science.3014662. [DOI] [PubMed] [Google Scholar]
- 6.Boulter C, Mulroy S, Webb S, Fleming S, Brindle K, Sandford R. Cardiovascular, skeletal, and renal defects in mice with a targeted disruption of the Pkd1 gene. Proc Natl Acad Sci USA 98: 12174–12179, 2001. doi: 10.1073/pnas.211191098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cai K, Qi D, Hou X, Wang O, Chen J, Deng B, Qian L, Liu X, Le Y. MCP-1 upregulates amylin expression in murine pancreatic β cells through ERK/JNK-AP1 and NF-κB related signaling pathways independent of CCR2. PLoS One 6: e19559, 2011. doi: 10.1371/journal.pone.0019559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chebib FT, Torres VE. Autosomal dominant polycystic kidney disease: core curriculum 2016. Am J Kidney Dis 67: 792–810, 2016. doi: 10.1053/j.ajkd.2015.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen L, Zhou X, Fan LX, Yao Y, Swenson-Fields KI, Gadjeva M, Wallace DP, Peters DJ, Yu A, Grantham JJ, Li X. Macrophage migration inhibitory factor promotes cyst growth in polycystic kidney disease. J Clin Invest 125: 2399–2412, 2015. doi: 10.1172/JCI80467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chien KR, Knowlton KU, Zhu H, Chien S. Regulation of cardiac gene expression during myocardial growth and hypertrophy: molecular studies of an adaptive physiologic response. FASEB J 5: 3037–3046, 1991. doi: 10.1096/fasebj.5.15.1835945. [DOI] [PubMed] [Google Scholar]
- 11.Cowley BD Jr, Ricardo SD, Nagao S, Diamond JR. Increased renal expression of monocyte chemoattractant protein-1 and osteopontin in ADPKD in rats. Kidney Int 60: 2087–2096, 2001. doi: 10.1046/j.1523-1755.2001.00065.x. [DOI] [PubMed] [Google Scholar]
- 12.Damås JK, Aukrust P, Ueland T, Odegaard A, Eiken HG, Gullestad L, Sejersted OM, Christensen G. Monocyte chemoattractant protein-1 enhances and interleukin-10 suppresses the production of inflammatory cytokines in adult rat cardiomyocytes. Basic Res Cardiol 96: 345–352, 2001. doi: 10.1007/s003950170042. [DOI] [PubMed] [Google Scholar]
- 13.Dellacà RL, Zannin E, Sancini G, Rivolta I, Leone BE, Pedotti A, Miserocchi G. Changes in the mechanical properties of the respiratory system during the development of interstitial lung edema. Respir Res 9: 51, 2008. doi: 10.1186/1465-9921-9-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dewald O, Zymek P, Winkelmann K, Koerting A, Ren G, Abou-Khamis T, Michael LH, Rollins BJ, Entman ML, Frangogiannis NG. CCL2/monocyte chemoattractant protein-1 regulates inflammatory responses critical to healing myocardial infarcts. Circ Res 96: 881–889, 2005. doi: 10.1161/01.RES.0000163017.13772.3a. [DOI] [PubMed] [Google Scholar]
- 15.Duffield JS. Macrophages in kidney repair and regeneration. J Am Soc Nephrol 22: 199–201, 2011. doi: 10.1681/ASN.2010121301. [DOI] [PubMed] [Google Scholar]
- 16.Epelman S, Lavine KJ, Beaudin AE, Sojka DK, Carrero JA, Calderon B, Brija T, Gautier EL, Ivanov S, Satpathy AT, Schilling JD, Schwendener R, Sergin I, Razani B, Forsberg EC, Yokoyama WM, Unanue ER, Colonna M, Randolph GJ, Mann DL. Embryonic and adult-derived resident cardiac macrophages are maintained through distinct mechanisms at steady state and during inflammation. Immunity 40: 91–104, 2014. doi: 10.1016/j.immuni.2013.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fick GM, Johnson AM, Hammond WS, Gabow PA. Causes of death in autosomal dominant polycystic kidney disease. J Am Soc Nephrol 5: 2048–2056, 1995. [DOI] [PubMed] [Google Scholar]
- 18.Fontes JA, Rose NR, Čiháková D. The varying faces of IL-6: From cardiac protection to cardiac failure. Cytokine 74: 62–68, 2015. doi: 10.1016/j.cyto.2014.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fry JL Jr, Koch WE, Jennette JC, McFarland E, Fried FA, Mandell J. A genetically determined murine model of infantile polycystic kidney disease. J Urol 134: 828–833, 1985. doi: 10.1016/S0022-5347(17)47448-9. [DOI] [PubMed] [Google Scholar]
- 20.Gabow PA. Autosomal dominant polycystic kidney disease. N Engl J Med 329: 332–342, 1993. doi: 10.1056/NEJM199307293290508. [DOI] [PubMed] [Google Scholar]
- 21.Gharaee-Kermani M, Denholm EM, Phan SH. Costimulation of fibroblast collagen and transforming growth factor beta1 gene expression by monocyte chemoattractant protein-1 via specific receptors. J Biol Chem 271: 17779–17784, 1996. doi: 10.1074/jbc.271.30.17779. [DOI] [PubMed] [Google Scholar]
- 22.Grantham JJ, Mulamalla S, Swenson-Fields KI. Why kidneys fail in autosomal dominant polycystic kidney disease. Nat Rev Nephrol 7: 556–566, 2011. doi: 10.1038/nrneph.2011.109. [DOI] [PubMed] [Google Scholar]
- 23.Hatamizadeh P, Fonarow GC, Budoff MJ, Darabian S, Kovesdy CP, Kalantar-Zadeh K. Cardiorenal syndrome: pathophysiology and potential targets for clinical management. Nat Rev Nephrol 9: 99–111, 2013. doi: 10.1038/nrneph.2012.279. [DOI] [PubMed] [Google Scholar]
- 24.Herzog CA. Sudden cardiac death and acute myocardial infarction in dialysis patients: perspectives of a cardiologist. Semin Nephrol 25: 363–366, 2005. doi: 10.1016/j.semnephrol.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 25.Hoeffel G, Ginhoux F. Ontogeny of tissue-resident macrophages. Front Immunol 6: 486, 2015. doi: 10.3389/fimmu.2015.00486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hou X, Mrug M, Yoder BK, Lefkowitz EJ, Kremmidiotis G, D’Eustachio P, Beier DR, Guay-Woodford LM. Cystin, a novel cilia-associated protein, is disrupted in the cpk mouse model of polycystic kidney disease. J Clin Invest 109: 533–540, 2002. doi: 10.1172/JCI0214099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Izumo S, Nadal-Ginard B, Mahdavi V. Protooncogene induction and reprogramming of cardiac gene expression produced by pressure overload. Proc Natl Acad Sci USA 85: 339–343, 1988. doi: 10.1073/pnas.85.2.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jiang Y, Beller DI, Frendl G, Graves DT. Monocyte chemoattractant protein-1 regulates adhesion molecule expression and cytokine production in human monocytes. J Immunol 148: 2423–2428, 1992. [PubMed] [Google Scholar]
- 29.Jung H, Toth PT, White FA, Miller RJ. Monocyte chemoattractant protein-1 functions as a neuromodulator in dorsal root ganglia neurons. J Neurochem 104: 254–263, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Karihaloo A, Koraishy F, Huen SC, Lee Y, Merrick D, Caplan MJ, Somlo S, Cantley LG. Macrophages promote cyst growth in polycystic kidney disease. J Am Soc Nephrol 22: 1809–1814, 2011. doi: 10.1681/ASN.2011010084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim K, Drummond I, Ibraghimov-Beskrovnaya O, Klinger K, Arnaout MA. Polycystin 1 is required for the structural integrity of blood vessels. Proc Natl Acad Sci USA 97: 1731–1736, 2000. doi: 10.1073/pnas.040550097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kranias EG, Hajjar RJ. Modulation of cardiac contractility by the phospholamban/SERCA2a regulatome. Circ Res 110: 1646–1660, 2012. doi: 10.1161/CIRCRESAHA.111.259754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kruglov EA, Nathanson RA, Nguyen T, Dranoff JA. Secretion of MCP-1/CCL2 by bile duct epithelia induces myofibroblastic transdifferentiation of portal fibroblasts. Am J Physiol Gastrointest Liver Physiol 290: G765–G771, 2006. doi: 10.1152/ajpgi.00308.2005. [DOI] [PubMed] [Google Scholar]
- 34.Kuwahara F, Kai H, Tokuda K, Takeya M, Takeshita A, Egashira K, Imaizumi T. Hypertensive myocardial fibrosis and diastolic dysfunction: another model of inflammation? Hypertension 43: 739–745, 2004. doi: 10.1161/01.HYP.0000118584.33350.7d. [DOI] [PubMed] [Google Scholar]
- 35.La Rovere MT, Pinna GD, Maestri R, Mortara A, Capomolla S, Febo O, Ferrari R, Franchini M, Gnemmi M, Opasich C, Riccardi PG, Traversi E, Cobelli F. Short-term heart rate variability strongly predicts sudden cardiac death in chronic heart failure patients. Circulation 107: 565–570, 2003. doi: 10.1161/01.CIR.0000047275.25795.17. [DOI] [PubMed] [Google Scholar]
- 36.Lu B, Rutledge BJ, Gu L, Fiorillo J, Lukacs NW, Kunkel SL, North R, Gerard C, Rollins BJ. Abnormalities in monocyte recruitment and cytokine expression in monocyte chemoattractant protein 1-deficient mice. J Exp Med 187: 601–608, 1998. doi: 10.1084/jem.187.4.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.MacLellan WR, Schneider MD. Genetic dissection of cardiac growth control pathways. Annu Rev Physiol 62: 289–319, 2000. doi: 10.1146/annurev.physiol.62.1.289. [DOI] [PubMed] [Google Scholar]
- 38.Madrigal JL, Leza JC, Polak P, Kalinin S, Feinstein DL. Astrocyte-derived MCP-1 mediates neuroprotective effects of noradrenaline. J Neurosci 29: 263–267, 2009. doi: 10.1523/JNEUROSCI.4926-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mandrekar P, Ambade A, Lim A, Szabo G, Catalano D. An essential role for monocyte chemoattractant protein-1 in alcoholic liver injury: regulation of proinflammatory cytokines and hepatic steatosis in mice. Hepatology 54: 2185–2197, 2011. doi: 10.1002/hep.24599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Marionneau C, Brunet S, Flagg TP, Pilgram TK, Demolombe S, Nerbonne JM. Distinct cellular and molecular mechanisms underlie functional remodeling of repolarizing K+ currents with left ventricular hypertrophy. Circ Res 102: 1406–1415, 2008. doi: 10.1161/CIRCRESAHA.107.170050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mitchell GF, Jeron A, Koren G. Measurement of heart rate and Q-T interval in the conscious mouse. Am J Physiol Heart Circ Physiol 274: H747–H751, 1998. doi: 10.1152/ajpheart.1998.274.3.H747. [DOI] [PubMed] [Google Scholar]
- 42.Montes GS, Junqueira LC. The use of the Picrosirius-polarization method for the study of the biopathology of collagen. Mem Inst Oswaldo Cruz 86, Suppl 3: 1–11, 1991. doi: 10.1590/S0074-02761991000700002. [DOI] [PubMed] [Google Scholar]
- 43.Nance DM, Sanders VM. Autonomic innervation and regulation of the immune system (1987–2007). Brain Behav Immun 21: 736–745, 2007. doi: 10.1016/j.bbi.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Niu J, Kolattukudy PE. Role of MCP-1 in cardiovascular disease: molecular mechanisms and clinical implications. Clin Sci (Lond) 117: 95–109, 2009. doi: 10.1042/CS20080581. [DOI] [PubMed] [Google Scholar]
- 45.Ong AC, Harris PC. A polycystin-centric view of cyst formation and disease: the polycystins revisited. Kidney Int 88: 699–710, 2015. doi: 10.1038/ki.2015.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Onuchic LF, Furu L, Nagasawa Y, Hou X, Eggermann T, Ren Z, Bergmann C, Senderek J, Esquivel E, Zeltner R, Rudnik-Schöneborn S, Mrug M, Sweeney W, Avner ED, Zerres K, Guay-Woodford LM, Somlo S, Germino GG. PKHD1, the polycystic kidney and hepatic disease 1 gene, encodes a novel large protein containing multiple immunoglobulin-like plexin-transcription-factor domains and parallel beta-helix 1 repeats. Am J Hum Genet 70: 1305–1317, 2002. doi: 10.1086/340448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Peirson SN, Butler JN, Foster RG. Experimental validation of novel and conventional approaches to quantitative real-time PCR data analysis. Nucleic Acids Res 31: e73, 2003. doi: 10.1093/nar/gng073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29: e45, 2001. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pongratz G, Straub RH. The sympathetic nervous response in inflammation. Arthritis Res Ther 16: 504, 2014. doi: 10.1186/s13075-014-0504-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Prasad S, McDaid JP, Tam FW, Haylor JL, Ong AC. Pkd2 dosage influences cellular repair responses following ischemia-reperfusion injury. Am J Pathol 175: 1493–1503, 2009. doi: 10.2353/ajpath.2009.090227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Preminger GM, Koch WE, Fried FA, McFarland E, Murphy ED, Mandell J. Murine congenital polycystic kidney disease: a model for studying development of cystic disease. J Urol 127: 556–560, 1982. doi: 10.1016/S0022-5347(17)53911-7. [DOI] [PubMed] [Google Scholar]
- 52.Rahman E, Niaz FA, Al-Suwaida A, Nahrir S, Bashir M, Rahman H, Hammad D. Analysis of causes of mortality in patients with autosomal dominant polycystic kidney disease: a single center study. Saudi J Kidney Dis Transpl 20: 806–810, 2009. [PubMed] [Google Scholar]
- 53.Ranpuria R, Hall M, Chan CT, Unruh M. Heart rate variability (HRV) in kidney failure: measurement and consequences of reduced HRV. Nephrol Dial Transplant 23: 444–449, 2008. doi: 10.1093/ndt/gfm634. [DOI] [PubMed] [Google Scholar]
- 54.Ricker JL, Gattone VH II, Calvet JP, Rankin CA. Development of autosomal recessive polycystic kidney disease in BALB/c-cpk/cpk mice. J Am Soc Nephrol 11: 1837–1847, 2000. [DOI] [PubMed] [Google Scholar]
- 55.Roberts TK, Eugenin EA, Lopez L, Romero IA, Weksler BB, Couraud PO, Berman JW. CCL2 disrupts the adherens junction: implications for neuroinflammation. Lab Invest 92: 1213–1233, 2012. doi: 10.1038/labinvest.2012.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Roblek M, Protsyuk D, Becker PF, Stefanescu C, Gorzelanny C, Glaus Garzon JF, Knopfova L, Heikenwalder M, Luckow B, Schneider SW, Borsig L. CCL2 is a vascular permeability factor inducing CCR2-dependent endothelial retraction during lung metastasis. Mol Cancer Res 17: 783–793, 2019. 10.1158/1541-7786.MCR-18-0530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Routledge HC, Chowdhary S, Townend JN. Heart rate variability—a therapeutic target? J Clin Pharm Ther 27: 85–92, 2002. doi: 10.1046/j.1365-2710.2002.00404.x. [DOI] [PubMed] [Google Scholar]
- 58.Schecter AD, Berman AB, Yi L, Ma H, Daly CM, Soejima K, Rollins BJ, Charo IF, Taubman MB. MCP-1-dependent signaling in CCR2−/− aortic smooth muscle cells. J Leukoc Biol 75: 1079–1085, 2004. doi: 10.1189/jlb.0903421. [DOI] [PubMed] [Google Scholar]
- 59.Schlöndorff D, Nelson PJ, Luckow B, Banas B. Chemokines and renal disease. Kidney Int 51: 610–621, 1997. doi: 10.1038/ki.1997.90. [DOI] [PubMed] [Google Scholar]
- 60.Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9: 671–675, 2012. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sheng J, Ruedl C, Karjalainen K. Most tissue-resident macrophages except microglia are derived from fetal hematopoietic stem cells. Immunity 43: 382–393, 2015. doi: 10.1016/j.immuni.2015.07.016. [DOI] [PubMed] [Google Scholar]
- 62.Siscovick DS, Sotoodehnia N, Rea TD, Raghunathan TE, Jouven X, Lemaitre RN. Type 2 diabetes mellitus and the risk of sudden cardiac arrest in the community. Rev Endocr Metab Disord 11: 53–59, 2010. doi: 10.1007/s11154-010-9133-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Souders CA, Borg TK, Banerjee I, Baudino TA. Pressure overload induces early morphological changes in the heart. Am J Pathol 181: 1226–1235, 2012. doi: 10.1016/j.ajpath.2012.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Stamatovic SM, Keep RF, Kunkel SL, Andjelkovic AV. Potential role of MCP-1 in endothelial cell tight junction ‘opening’: signaling via Rho and Rho kinase. J Cell Sci 116: 4615–4628, 2003. doi: 10.1242/jcs.00755. [DOI] [PubMed] [Google Scholar]
- 65.Swenson-Fields KI, Vivian CJ, Salah SM, Peda JD, Davis BM, van Rooijen N, Wallace DP, Fields TA. Macrophages promote polycystic kidney disease progression. Kidney Int 83: 855–864, 2013. doi: 10.1038/ki.2012.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sysa-Shah P, Sørensen LL, Abraham MR, Gabrielson KL. Electrocardiographic characterization of cardiac hypertrophy in mice that overexpress the ErbB2 receptor tyrosine kinase. Comp Med 65: 295–307, 2015. [PMC free article] [PubMed] [Google Scholar]
- 67.Taegtmeyer H, Sen S, Vela D. Return to the fetal gene program: a suggested metabolic link to gene expression in the heart. Ann N Y Acad Sci 1188: 191–198, 2010. doi: 10.1111/j.1749-6632.2009.05100.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Takahashi H, Calvet JP, Dittemore-Hoover D, Yoshida K, Grantham JJ, Gattone VH II. A hereditary model of slowly progressive polycystic kidney disease in the mouse. J Am Soc Nephrol 1: 980–989, 1991. [DOI] [PubMed] [Google Scholar]
- 69.Tarzami ST, Calderon TM, Deguzman A, Lopez L, Kitsis RN, Berman JW. MCP-1/CCL2 protects cardiac myocytes from hypoxia-induced apoptosis by a Gαi-independent pathway. Biochem Biophys Res Commun 335: 1008–1016, 2005. doi: 10.1016/j.bbrc.2005.07.168. [DOI] [PubMed] [Google Scholar]
- 70.Thompson WL, Karpus WJ, Van Eldik LJ. MCP-1-deficient mice show reduced neuroinflammatory responses and increased peripheral inflammatory responses to peripheral endotoxin insult. J Neuroinflammation 5: 35, 2008. doi: 10.1186/1742-2094-5-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Van Tassell BW, Toldo S, Mezzaroma E, Abbate A. Targeting interleukin-1 in heart disease. Circulation 128: 1910–1923, 2013. doi: 10.1161/CIRCULATIONAHA.113.003199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Viedt C, Dechend R, Fei J, Hänsch GM, Kreuzer J, Orth SR. MCP-1 induces inflammatory activation of human tubular epithelial cells: involvement of the transcription factors, nuclear factor-κB and activating protein-1. J Am Soc Nephrol 13: 1534–1547, 2002. doi: 10.1097/01.ASN.0000015609.31253.7F. [DOI] [PubMed] [Google Scholar]
- 73.Viedt C, Vogel J, Athanasiou T, Shen W, Orth SR, Kübler W, Kreuzer J. Monocyte chemoattractant protein-1 induces proliferation and interleukin-6 production in human smooth muscle cells by differential activation of nuclear factor-κB and activator protein-1. Arterioscler Thromb Vasc Biol 22: 914–920, 2002. doi: 10.1161/01.ATV.0000019009.73586.7F. [DOI] [PubMed] [Google Scholar]
- 74.Virzi GM, Corradi V, Panagiotou A, Gastaldon F, Cruz DN, de Cal M, Clementi M, Ronco C. ADPKD: prototype of cardiorenal syndrome type 4. Int J Nephrol 2011: 490795, 2011. doi: 10.4061/2011/490795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ward CJ, Hogan MC, Rossetti S, Walker D, Sneddon T, Wang X, Kubly V, Cunningham JM, Bacallao R, Ishibashi M, Milliner DS, Torres VE, Harris PC. The gene mutated in autosomal recessive polycystic kidney disease encodes a large, receptor-like protein. Nat Genet 30: 259–269, 2002. doi: 10.1038/ng833. [DOI] [PubMed] [Google Scholar]
- 76.Wu G, Markowitz GS, Li L, D’Agati VD, Factor SM, Geng L, Tibara S, Tuchman J, Cai Y, Park JH, van Adelsberg J, Hou H Jr, Kucherlapati R, Edelmann W, Somlo S. Cardiac defects and renal failure in mice with targeted mutations in Pkd2. Nat Genet 24: 75–78, 2000. doi: 10.1038/71724. [DOI] [PubMed] [Google Scholar]
- 77.Yoder BK, Hou X, Guay-Woodford LM. The polycystic kidney disease proteins, polycystin-1, polycystin-2, polaris, and cystin, are co-localized in renal cilia. J Am Soc Nephrol 13: 2508–2516, 2002. doi: 10.1097/01.ASN.0000029587.47950.25. [DOI] [PubMed] [Google Scholar]
- 78.Zeier M, Fehrenbach P, Geberth S, Möhring K, Waldherr R, Ritz E. Renal histology in polycystic kidney disease with incipient and advanced renal failure. Kidney Int 42: 1259–1265, 1992. doi: 10.1038/ki.1992.413. [DOI] [PubMed] [Google Scholar]
- 79.Zeng F, Miyazawa T, Kloepfer LA, Harris RC. Deletion of ErbB4 accelerates polycystic kidney disease progression in cpk mice. Kidney Int 86: 538–547, 2014. doi: 10.1038/ki.2014.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhang MZ, Yao B, Yang S, Jiang L, Wang S, Fan X, Yin H, Wong K, Miyazawa T, Chen J, Chang I, Singh A, Harris RC. CSF-1 signaling mediates recovery from acute kidney injury. J Clin Invest 122: 4519–4532, 2012. doi: 10.1172/JCI60363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zheng D, Wolfe M, Cowley BD Jr, Wallace DP, Yamaguchi T, Grantham JJ. Urinary excretion of monocyte chemoattractant protein-1 in autosomal dominant polycystic kidney disease. J Am Soc Nephrol 14: 2588–2595, 2003. doi: 10.1097/01.ASN.0000088720.61783.19. [DOI] [PubMed] [Google Scholar]
- 82.Zhou L, Azfer A, Niu J, Graham S, Choudhury M, Adamski FM, Younce C, Binkley PF, Kolattukudy PE. Monocyte chemoattractant protein-1 induces a novel transcription factor that causes cardiac myocyte apoptosis and ventricular dysfunction. Circ Res 98: 1177–1185, 2006. doi: 10.1161/01.RES.0000220106.64661.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zoja C, Corna D, Locatelli M, Rottoli D, Pezzotta A, Morigi M, Zanchi C, Buelli S, Guglielmotti A, Perico N, Remuzzi A, Remuzzi G. Effects of MCP-1 inhibition by bindarit therapy in a rat model of polycystic kidney disease. Nephron 129: 52–61, 2015. doi: 10.1159/000369149. [DOI] [PubMed] [Google Scholar]