Abstract
The impact of sexual dimorphism and mitophagy on hepatic mitochondrial adaptations during the treatment of steatosis with physical activity are largely unknown. Here, we tested if deficiencies in liver-specific peroxisome proliferative activated-receptor-γ coactivator-1α (PGC-1α), a transcriptional coactivator of biogenesis, and BCL-2/ADENOVIRUS EIB 19-kDa interacting protein (BNIP3), a mitophagy regulator, would impact hepatic mitochondrial adaptations (respiratory capacity, H2O2 production, mitophagy) to a high-fat diet (HFD) and HFD plus physical activity via voluntary wheel running (VWR) in both sexes. Male and female wild-type (WT), liver-specific PGC-1α heterozygote (LPGC-1α), and BNIP3 null mice were thermoneutral housed (29–31°C) and divided into three groups: sedentary-low-fat diet (LFD), 16 wk of (HFD), or 16 wk of HFD with VWR for the final 8 wk (HFD + VWR) (n = 5–7/sex/group). HFD did not impair mitochondrial respiratory capacity or coupling in any group; however, HFD + VWR significantly increased maximal respiratory capacity only in WT and PGC-1α females. Males required VWR to elicit mitochondrial adaptations that were inherently present in sedentary females including greater mitochondrial coupling control and reduced H2O2 production. Females had overall reduced markers of mitophagy, steatosis, and liver damage. Steatosis and markers of liver injury were present in sedentary male mice on the HFD and were effectively reduced with VWR despite no resolution of steatosis. Overall, reductions in PGC-1α and loss of BNIP3 only modestly impacted mitochondrial adaptations to HFD and HFD + VWR with the biggest effect seen in BNIP3 females. In conclusion, hepatic mitochondrial adaptations to HFD and treatment of HFD-induced steatosis with VWR are more dependent on sex than PGC-1α or BNIP3.
Keywords: liver, metabolism, mitochondrial respiratory capacity, mitophagy, reactive oxygen species
INTRODUCTION
Hepatic steatosis, the excessive storage of hepatic lipids, largely tracks with obesity and is closely associated with type 2 diabetes and metabolic syndrome (33). There is currently no accepted pharmacologic intervention available for hepatic steatosis. In contrast, lifestyle interventions including diet-induced weight loss and increased physical activity/exercise with or without weight loss effectively treat the condition (21, 46); however, the mechanisms by which this occurs are not fully elucidated. Investigating the key contributors to hepatic steatosis and mechanisms by which physical activity can treat the condition is warranted. The roles of hepatic mitochondrial biogenesis, turnover, and respiratory capacity remain controversial in the pathogenesis and treatment of hepatic steatosis. In animal models, molecular modulation of hepatic mitochondrial function and fat oxidation regulate susceptibility to steatosis while longitudinal assessments of high-fat diet (HFD)-induced steatosis in rodents show that steatosis leads to compensatory increases in markers of mitochondrial flux (52, 54). Similar results have been found in cross-sectional studies comparing healthy controls to patients with steatosis (27, 57). Despite this, we and others have clearly shown that positive mitochondrial adaptations [increases in content, fatty acid oxidation (FAO), or enzyme activity] following exercise and physical activity interventions are associated with both protection and treatment of hepatic steatosis (5, 35, 46).
Physical activity or exercise improves hepatic metabolic health in part through the induction of peroxisome proliferative activated-receptor-γ coactivator-1α (PGC-1α), a transcriptional coactivator that regulates gluconeogenesis, mitochondrial biogenesis, and TCA cycle flux and increases fat oxidation (14, 16, 32, 51). We have shown hepatic adenoviral overexpression of PGC-1α elicits reduced hepatic lipids with a concomitant increase in hepatocyte mitochondrial FAO, maximal respiratory capacity, and electron transport system (ETS) protein expression (38, 39). Conversely, loss of PGC-1α elicits reduced hepatic mitochondrial content, respiration and fat oxidation, gluconeogenesis, and enhanced fasting-induced steatosis (7, 13, 15, 34). Since PGC-1α is intricately linked to exercise/physical activity and hepatic mitochondrial health, it is important to examine whether full PGC-1α expression is required for beneficial physical activity-induced mitochondrial adaptations to treat hepatic steatosis.
In addition to modulating mitochondrial biogenesis, increased physical activity or regular exercise putatively improves hepatic mitochondrial function by recycling damaged or low-functioning mitochondria through activation of mitophagy, a mitochondrial specific form of macroautophagy (24, 26). Studies suggest a coordinated dynamic link between PGC-1α and mitophagy (29). Exercise-induced PGC-1α expression leads to corresponding increases in mitophagy flux that are reduced with PGC-1α deficiency (60), indicating coordinated regulation of mitochondrial biogenesis and turnover to maintain a healthy pool. Interestingly, our recent data suggest that a reduction in hepatic PGC-1α increases basal mitophagy flux (62), further underscoring the importance of understanding the role of PGC-1α in regulating mitochondrial dynamics.
Disruption of macroautophagy and mitophagy is thought to contribute to hepatic steatosis by increasing accumulation of dysfunctional organelles, resulting in subsequent increases in reactive oxygen species (ROS) formation, inflammation, and apoptosis (2). Indeed, a previous study examining the deficiency in a critical receptor-mediated mitophagy pathway BCL-2/ADENOVIRUS EIB 19-kDa interacting protein (BNIP3) showed increased hepatic ROS, inflammation, reduced FAO and respiration, and hepatic lipid accumulation (19). Furthermore, recent data from our laboratory showed reduced mitochondrial respiratory function in male BNIP3 mice when compared with wild-type (WT) controls (62). Additional evidence exists that HFD alters hepatic mitochondrial dynamics and mitophagy (42); however, it remains unknown how the loss of the BNIP3-mediated mitophagy proteins alters hepatic mitochondrial adaptations to HFD and physical activity for treatment of HFD-induced steatosis.
Here we examined the effects of mitochondrial adaptations driven by a 16-wk HFD in both male and female mice. We assessed WT animals and those with deficiencies in hepatic PGC-1α [liver-specific PGC-1α heterozygotes (LPGC-1α)] or a loss of BNIP3 (BNIP3 null mice). Half of the mice received voluntary running wheels for the final 8 wk of the HFD (from 8 to 16 wk) to examine if PGC-1α- and BNIP3-mediated pathways are critical for treatment of an expected HFD-induced steatosis with increased physical activity. Female mice are known to be protected from steatosis (23), and we have shown they have enhanced mitochondrial respiratory capacity with lower mitophagy compared with male counterparts (62). Thus both sexes were examined to determine possible sexual dimorphism in these physiological processes. We also elected to perform these studies under thermoneutral conditions, which profoundly increase the capacity for HFDs to induce steatosis and hepatic injury (18). We hypothesized that loss of PGC-1α or BNIP3 would enhance HFD-induced steatosis and mitochondrial derangements and that VWR would fail to treat these pathologies due to reductions in mitochondrial biogenesis and mitophagy. In contrast, we hypothesized that female WT would be protected from steatosis due to enhanced mitochondrial respiratory capacity but that this protection may be mitigated with loss of hepatic PGC-1α and BNIP3.
MATERIALS AND METHODS
Ethical approval.
The animal protocol was approved by the Institutional Animal Care and Use Committee at the University of Kansas Medical Center and Kansas City Veterans Affairs Medical Center (animal protocol number 2015–2271). All experiments were carried out in accordance with the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health (NIH Guide, 8th ed., 2011) and adhere to the American Physiological Society’s Guiding Principles in the Care and Use of Vertebrate Animals in Research and Training. Mice were anaesthetized with pentobarbital sodium (100 mg/kg) before a terminal procedure.
Animals.
The liver-specific PGC-1α heterozygous (LPGC-1α) and BNIP3 null mice (BNIP3) were developed as previously described (11, 13). Briefly, male WT C57Bl/6J mice were bred with female PGC-1α homozygote floxed mice on a C57Bl/6J background [B6N.129(FVB)-Ppargc1atm2.1Brsp/J] (Jackson Laboratory, Bar Harbor, ME) resulting in female PGC1α heterozygote floxed mice. The female PGC-1α heterozygotes were then bred with male albumin reporter-driven Cre [B6.CgTg(Albcre)21Mgn/J] to produce pups with liver-specific heterozygosity for PGC-1α. We elected to use a physiologically relevant heterozygote PGC-1α knockdown because it is a transcriptional coactivator and it has been previously shown that this approach results in reduced expression of key genes controlling mitochondrial biogenesis and mitochondrial function, while maintaining appropriate glucose homeostasis (13, 15). In contrast, we elected to use a complete BNIP3 knockout because its known action is limited to mitophagy. PGC-1α+/+ littermates of the LPGC-1α+/− breeding paradigm were used as WT controls for all genotypes. Eight-week-old male and female WT, LPGC-1α+/−, and BNIP3−/− mice (n = 5–7 per sex, per strain) were bred and maintained on a C57Bl/6J background. Mice were housed at thermoneutrality (29–31°C) on a reverse light cycle (dark 10:00–22:00) with ad libitum access to water and low-fat diet (LFD; D12110704: 10% kcal fat, 3.5% kcal sucrose, and 3.85 kcal/g; Research Diets, New Brunswick, NJ) or HFD (D12451; 45% kcal fat, 17% kcal sucrose, 1% cholesterol wt/wt, 4.68 kcal/g; Research Diets) for 16 wk. Animal weights and food intake were measured at baseline and weekly throughout the 16-wk study. Body composition was measured at the beginning and end of the study via MRI (model 900; EchoMRI, Houston, TX). HFD + VWR mice were allowed free access to running wheels from weeks 8–16 during the HFD to determine if physical activity would reduce steatosis. VWR distances were recorded with an automated system that communicated data to a central hub via Bluetooth (ENV-047V; Med Associates, Fairfax, VT). This body of work utilized an independent cohort of WT, LPGC-1α, and BNIP3 null mice not examined in previous studies.
Tissue collection and mitochondrial isolation.
Livers of anesthetized mice were quickly excised, and portions were either flash frozen in liquid N2 or placed into 8 ml ice-cold mitochondrial isolation buffer (in mM; 220 mannitol, 70 sucrose, 10 Tris, and 1 EDTA, pH adjusted to 7.4 with KOH) and homogenized. Hepatic mitochondria were isolated as described previously (39, 62). Briefly, livers were transferred to a 15-ml glass tube on ice and homogenized with a Teflon pestle. Homogenates were transferred to a 50-ml conical tube and centrifuged (4°C, 10 min, 1500 g). Supernatant was strained through fresh gauze and transferred into a 30-ml round-bottom tube and centrifuged (4°C, 10 min, 8,000 g). The pellet was resuspended in 6 ml isolation buffer using glass-on-glass Dounce homogenizer for three to four passes and centrifuged (4°C, 10 min, 6,000 g). The pellet was again resuspended in 4 ml isolation buffer containing 0.1% fatty acid-free BSA using Dounce homogenization and centrifuged (4°C, 10 min, 4,000 g). The final isolated mitochondrial pellet was resuspended in 500–750 μl modified MiR05 mitochondrial respiration buffer (0.5 mM EGTA, 3 mM MgCl2, 60 mM KMES, 20 mM glucose, 10 mM KH2PO4, 20 mM HEPES, 110 mM sucrose, and 0.1% BSA, adjusted pH to 7.1 with KOH). Protein concentrations were determined by bicinchoninic acid assay.
Mitochondrial respiration.
Mitochondrial oxygen consumption (pmol·s−1·ml−1) and H2O2 (pmol·s−1·ml−1) flux were simultaneously measured using the Oroboros O2K-Fluorometer (O2K; Oroboros Instruments) as described previously (28, 62) and analyzed using DatLab 7 (Oroboros Instruments). After calibration, isolated mitochondria and appropriate substrates were added to the Oroboros chamber. All mitochondrial respiration experiments were completed with a chamber temperature of 30°C in the modified MiR05 mitochondrial respiration buffer described above with the addition of malate (2 mM), free CoA (63.5 μM), and l-carnitine (2.5 mM) at a total volume of 2 ml. This modified MiR05 mitochondrial respiration buffer was utilized without the addition of lactobionic acid and taurine because they have antioxidant capacity and could possibly interfere with our H2O2 emission measurements. Coupled maximal respiration rates in isolated mitochondrial were measured utilizing l-palmitoylcarnitine (10 μM) as a substrate. Specific maximal respiration activities through complexes I (state 3) and I + II (state 3S) were determined after the addition of adenosine 5′-disphosphate (ADP; 2.5 mM) followed by succinate (10 mM). Maximal uncoupled respiration was determined with titrations of carbonyl cyanide-p-trifluoromethoxyphenylhydrazone (FCCP; 1 μM). All data were normalized to total mitochondrial protein content within each chamber. The coupling control ratio (basal/state 3) was calculated to infer uncoupling, as lower ratio values indicate increased coupling (44). H2O2:O2 ratios were calculated (state 3S respiration/state 3S H2O2 emission) to infer the quantity of H2O2 emission at a given respiratory rate.
Liver triacylglycerol analysis.
Hepatic TG concentration was determined using a commercially available kit (TR0100; Sigma, St. Louis, MO), as described previously (47).
Reduced and oxidized glutathione determination.
Reduced (GSH) and oxidized (GSSG) glutathione concentrations were assessed fluorometrically using the methods developed by Hissin and Hilf (25) as described previously (50).
Citrate synthase.
Citrate synthase activity was determined in hepatic whole homogenate as described previously (56).
Western blotting.
Mitochondrial isolation was completed as described above. Frozen liver tissue was homogenized using a TissueLyzer II (Qiagen, Germantown, MD) bead homogenizer in a buffer containing (2 × 2 min, 20 Hz) 50 mM HEPES, 12 mM sodium pyrophosphate, 100 mM sodium fluoride, 10 mM EDTA, phosphatase inhibitors (Phosphatase Inhibitor Cocktail 2, P5726 and 3, P0044; Sigma-Aldrich), and 1% Triton X-100. Western ready Laemmli samples were produced from liver tissue homogenate and isolated hepatic mitochondria. SDS-PAGE was used to separate samples, which were then transferred to polyvinylidene difluoride membrane and probed with primary antibodies at a concentration of 1:2,000. BNIP3 (3769S), Parkin (4211S), phospho-DRP1 Ser637 (pDRP1, 6319), DRP1 (8570S), and LC3A/B (12741S) antibodies were purchased from Cell Signaling Technology (Danvers, MA) Densitometry was used to quantify individual protein bands with Image Laboratory software (Bio-Rad Laboratories, Hercules, CA). All values were normalized to total protein or mitochondrial protein using 0.1% amidoblack (Sigma-Aldrich) staining as previously described (39).
mRNA expression.
RNA was extracted using an RNeasy mini-kit following the manufacturer’s instructions (74104; Qiagen, Hilden, DE) and cDNA was prepared as previously described (38). A QuantStudio 3 Real-Time PCR System (ThermoFisher Scientific, Waltham, MA) and SYBR green mouse primers (Sigma Aldrich) (Supplemental Table S1; all Supplemental material for this article is available at https://doi.org/10.6084/m9.figshare.7824083.v2) were used for real-time quantitative PCR analysis. All mRNA values were normalized to the housekeeping gene, cyclophilin B.
Serum assays.
Serum cholesterol, triglycerides (TGs), nonesterified fatty acids (NEFA), alkaline phosphatase (ALP), alanine aminotransferase (ALT), and aspartate aminotransferase (AST) were analyzed using the Cobas c311 analyzer (Roche Diagnostics, Indianapolis, IN) as reported previously (62).
Gene expression analysis.
Liver gene expression profiles were assessed via RNA-sequencing as previously described (63). Total RNA was isolated from liver using a combination of TRI reagent and RNeasy mini columns (Qiagen, Valencia, CA), including on-column DNAse digestion. Isolation of polyA RNA and construction of barcoded RNA-seq libraries was performed using TruSeq reagents according to the manufacturer’s protocols (Illumina). Quantification of the RNAseq libraries was done using Qubit dsDNA high-sensitivity reagents, which were diluted, denatured, and sequenced using Illumina methodology (HiSeq 2500, 50-bp single reads). Following sequencing and demultiplexing, reads were trimmed for adapters, filtered based on Phred quality score, and aligned to the rat genome using the STAR aligner. Resulting .bam files were imported in Seqmonk for gene level quantification. RNA-seq quality metrics including proportion of reads aligning to genic regions were calculated. Differential expression and other analysis including principal component PCA were performed using packages in base R and the limma-voom pipeline (49). Pairwise comparisons between LFD and HFD, and HFD and HFD + VWR groups within each sex were performed and differentially expressed genes were identified (P < 0.05, and minimum + 2-fold change). Multiple testing corrections were done using the false discover rate method. Additional analyses were performed using packages in the R statistical software, Enrichr and JVenn software tools (3, 8, 31).
Statistical analysis.
To assess the relationship between genotype (WT, LPGC-1α, and BNIP3), diet (LFD, HFD, and HFD + VWR), and sex on hepatic mitochondrial adaptations, we first identified putative outliers for each of the outcome measures within each treatment group using the Grubbs method (α = 0.05) (22). Identified outliers using the Grubbs method were each manually inspected to determine the nature of their outlying value. Outlying values suspected to be due to an erroneous measurement were excluded from downstream statistical analyses (58).
A series of three-way ANOVA models were fit independently to each outcome measurement and used to model the relationship between genotype, diet, and sex across different measures of hepatic mitochondrial adaptation. ANOVA models included main effect terms for genotype, treatment, and sex, along with two-way and three-way interaction terms across these factors. We were also interested in comparing measures of mitochondrial adaptation between specific subgroups of mice defined by their genotype, diet, and sex. To facilitate such comparisons, we used the parameter estimates obtained from the three-way ANOVA models along with linear contrasts representing each of the comparisons of interest. Among the 153 total pairwise comparisons (for example:
where 18 represents the number of treatment combinations), 81 were identified as comparisons of interest. These 81 comparisons represented assessments of measures of mitochondrial adaptation across treatments among mice of the same genotype, across sex among mice of the same genotype, and only comparing LPGC-1α or BNIP3 to respective WT controls. To address the issue of multiple testing, a Bonferroni correction was performed to control the family-wise error rate at 5% across the 81 comparisons of interest. Furthermore, simultaneous 95% confidence intervals were created for each pairwise comparison. Comparisons with a Bonferroni corrected P < 0.05, or equivalently those for which the simultaneous 95% confidence interval did not include 0, were considered statistically significant. Data analysis was performed using the R statistical programming language version 3.5.1 (https://r-project.org). Data are reported as means ± SE.
RESULTS
Animal characteristics.
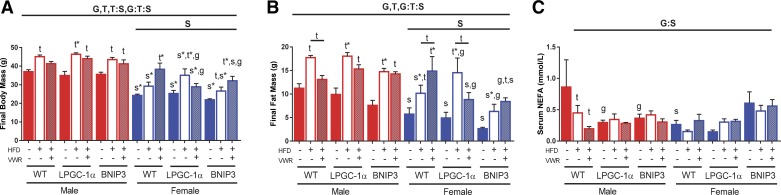
Figure 1 and Supplemental Table S2 contain detailed anthropometric information regarding genotype, sex, and treatment effects. Briefly, males showed significantly higher overall body and fat mass (P < 0.05, Fig. 1, A and B), average weekly energy intake, and serum cholesterol compared with females (P < 0.05, Supplemental Table S2). HFD significantly increased body mass after 16 wk compared with LFD in WT males but not females (P = 0.054, Fig. 1A). HFD + VWR caused a nonsignificant reduction of body mass compared with HFD in WT males (Fig. 1A). In contrast, HFD + VWR increased body mass in WT females (Fig. 1A). HFD significantly increased body mass in LPGC-1α and BNIP3 males and females (P < 0.05, Fig. 1A). Like WT females, HFD + VWR increased body mass compared with HFD in BNIP3 females (Fig. 1A). HFD + VWR did not significantly lower body mass in LPGC-1α or BNIP3 males or females (Fig. 1A).
Fig. 1.
Body mass and fat mass are impacted by sex, genotype, and treatment condition. Final body mass (A), fat mass (B), and serum nonesterified fatty acids (NEFA) (C) were measured in wild-type (WT), L peroxisome proliferative activated-receptor-γ coactivator 1α (LPGC-1α), and BCL-2/ADENOVIRUS EIB 19-kDa interacting protein (BNIP3) mice. Data are presented as means ± SE (n = 5–7). HFD, high-fat diet; VWR, voluntary wheel running; G, significant main effect for genotype; T, significant main effect of treatment; S, significant main effect for sex; g, genotype within sex within treatment; t, treatment within sex within genotype compared with low-fat diet control; s, sex within genotype within treatment condition; G:S, genotype by sex interaction; G:T, genotype by treatment interaction, T:S; treatment by sex interaction; G:T:S, genotype by treatment by sex interaction. *P < 0.05, significance post Bonferroni correction.
HFD significantly increased fat mass in WT males and females (P < 0.05, Fig. 1B). Like total body mass outcomes, HFD + VWR had opposing sex effects as it significantly decreased fat mass in WT males compared with HFD but significantly increased fat mass in females (P < 0.05, Fig. 1B). HFD increased fat mass in LPGC-1α and BNIP3 males and females compared with the LFD group (Fig. 1B). HFD + VWR lowered fat mass in LPGC-1α males and females compared with their HFD counterparts, yet this effect was absent in BNIP3 males and females (Fig. 1B).
In WT males, HFD significantly reduced serum NEFA compared with LFD while HFD + VWR lowered NEFA further (Fig. 1C). Interestingly, LFD LPGC-1α and BNIP3 males had reduced serum NEFA compared with WT controls (P < 0.05, Fig. 1C). Moreover, WT LFD females had significantly less serum NEFA than males (Fig. 1C). Aside from WT males, no other treatment effects were found with respect to serum NEFA (Fig. 1C). VWR + HFD increased serum cholesterol in WT females above the HFD group (Supplemental Table S2). Serum TG differences were primarily driven by genotype and not treatment (Supplemental Table S2). As expected, the HFD and VWR + HFD increased average weekly energy intake across genotypes (main effect; P < 0.05, Table S2). Average running distance ranged from ~6–8 km/night in all VWR groups except the PGC-1α female mice who averaged >10 km/night (Supplemental Table S2). Overall, there were numerous significant sex, treatment, and genotype effects on anthropometrics; however, the responses to treatment were primarily sex dependent, with WT males and females treated with HFD + VWR having the most divergent responses.
Physical activity-induced changes in hepatic mitochondrial respiration are primarily driven by sex.
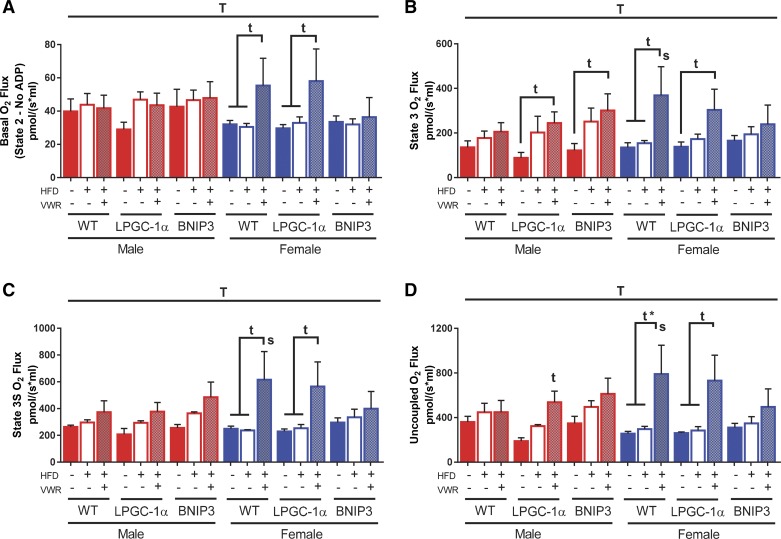
Utilizing palmitoylcarnitine as a substrate, our findings show maximal respiratory rate was generally unaffected by a 16-wk HFD in both WT male and female mice under basal, state 3, state 3S, or uncoupled (FCCP) conditions (Fig. 2, A–D). Interestingly, while HFD + VWR treatment again had no significant effect on respiration in WT males, it elicited a significant increase in mitochondrial respiration in WT females under basal (P < 0.05), state 3 (P < 0.05), state 3S (P < 0.05), and FCCP (P < 0.05, Padj < 0.05) conditions (Fig. 2, A–D). LFD LPGC-1α males tended to have a slightly reduced maximal respiratory rates in all states compared with WT that was modestly increased with HFD (Fig. 2, A–D). HFD + VWR significantly increased state 3 and uncoupled respiration compared with LFD in LPGC-1α males (P < 0.05, Fig. 2, B and D). LPGC-1α females on LFD and HFD had similar respiratory rates; however, like the WT females, respiration was significantly increased with HFD + VWR in all states (P < 0.05, Fig. 2, A–D). BNIP3 males displayed significantly increased state 3 respiratory capacity in the HFD + VWR group (P < 0.05, Fig. 2B).
Fig. 2.
Female mice have elevated maximal hepatic mitochondrial respiration with high-fat diet (HFD) plus physical activity via voluntary wheel running (VWR). Maximal hepatic mitochondrial respiratory capacity was examined in wild-type (WT), L peroxisome proliferative activated-receptor-γ coactivator 1α (LPGC-1α), and BCL-2/ADENOVIRUS EIB 19-kDa interacting protein (BNIP3) mice using the Oxygraph O2k-Fluorometer high-resolution respirometer in isolated liver mitochondrial samples using palmitoylcarnitine (PCarn) as a substrate. Basal (A), ADP-stimulated (B; State 3), ADP-stimulated + succinate (C; State 3S), and uncoupled (D) maximal respiratory capacity were examined. Maximal respiratory capacity values were normalized to mitochondrial protein content within the chamber as determined via the BCA assay. Data are presented as means ± SE (n = 5–7). T, significant main effect of treatment; t, treatment within sex within genotype compared with low-fat diet control; s, sex within genotype within treatment condition. *P < 0.05, significance post Bonferroni correction.
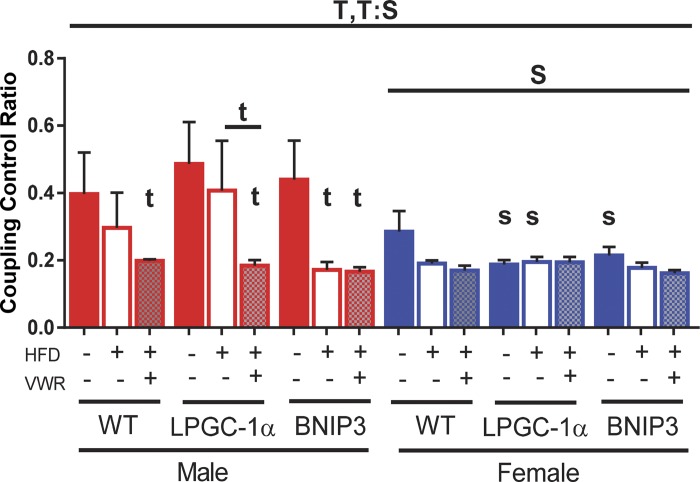
Coupling control ratios were lower in females across all genotypes (main effect; P < 0.05, Fig. 3) compared with males. Interestingly, HFD + VWR significantly reduced the coupling control ratio (more coupled) in WT males resulting in levels similar to WT females (P < 0.05, Fig. 3). HFD and HFD + VWR similarly improved coupling control ratio in WT females compared with LFD (Fig. 3). Coupling control was reduced with HFD and HFD + VWR in BNIP3 males when compared with LFD controls, matching inherent levels in BNIP3 female counterparts (P < 0.05, Fig. 3). Moreover, HFD + VWR also reduced the coupling control ratio in LPGC-1α males (P < 0.05 vs. LFD and HFD) resulting in levels that matched females. Female LPGC-1α and BNIP3 mice showed no additional improvement in coupling control with HFD or physical activity compared with LFD (Fig. 3). In summary, HFD + VWR significantly increased maximal mitochondrial respiratory capacity in female WT and LPGC-1α mice in all respiratory states. Loss of BNIP3 in female mice blunted this adaptation. Females had significantly improved coupling control ratios across all genotypes, while male WT and LPGC-1α mice required HFD + VWR to lower coupling control ratios (improved coupling efficiency) to levels comparable to females. Conversely, BNIP3 males displayed this improvement with both HFD and HFD + VWR.
Fig. 3.
Female mice have increased hepatic mitochondrial coupling compared with males. Coupling control ratios were calculated (basal/state 3). Data are presented as means ± SE (n = 5–7). HFD, high-fat diet; VWR, voluntary wheel running; WT, wild type; LPGC-1α, L peroxisome proliferative activated-receptor-γ coactivator 1α; BNIP3, BCL-2/ADENOVIRUS EIB 19-kDa interacting protein; T, significant main effect of treatment; S, significant main effect for sex; t, treatment within sex within genotype compared with low-fat diet control; s, sex within genotype within treatment condition.
Sex and physical activity impact hepatic mitochondrial H2O2 emission.
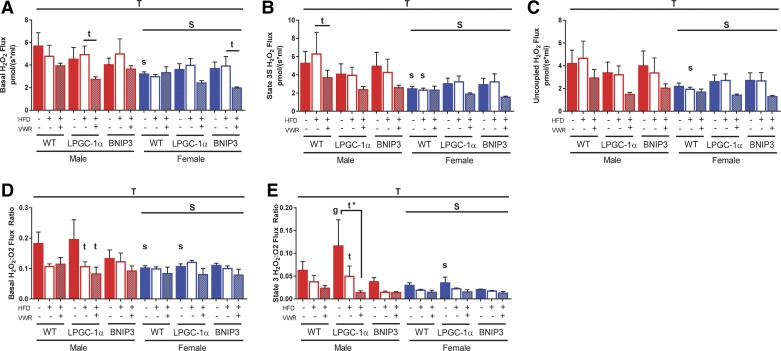
We simultaneously measured mitochondrial H2O2 emission with respiration to determine if HFD or HFD + VWR would alter ROS production in isolated liver mitochondria. Overall, females had a significant reduction in H2O2 emission (basal, state 3S, and uncoupled) when compared with males (main effect of sex; P < 0.05, Fig. 4, A–C), which is consistent with our previous results on LFD (62). HFD had little impact on WT males or females compared with LFD, however, physical activity (HFD + VWR) caused nonsignificant reductions H2O2 emission in WT males (basal, state 3S, and uncoupled; Fig. 4, A–C). This effect was not seen in WT females presumably because of already low emission levels (Fig. 4, A–C). As seen in our previous study on LFD (62), deficiencies in PGC-1α or BNIP3 were not sufficient to drive increased H2O2 emission following a chronic HFD in either male or female mice (Fig. 4, A–C). However, HFD + VWR elicited a both significant and nonsignificant reductions in H2O2 emission for male and female LPGC-1α and BNIP3 mice in all respiratory states (Fig. 4, A–C).
Fig. 4.
Physical activity reduces male hepatic mitochondrial H2O2 production. With the use of the O2k-Fluorometer, simultaneous H2O2 emission was also measured for basal (A), state 3S (B), and uncoupled states (C) in the isolated mitochondria with palmitoylcarnitine (PCarn) as a substrate. Values were normalized to mitochondrial protein content within the chamber as determined via the BCA assay. Electron leak was assessed utilizing the basal H2O2:O2 (D) and state 3 H2O2:O2 flux (E) ratios. Data are presented as means ± SE (n = 5–7). HFD, high-fat diet; VWR, voluntary wheel running; WT, wild type;LPGC-1α, L peroxisome proliferative activated-receptor-γ coactivator 1α; BNIP3, BCL-2/ADENOVIRUS EIB 19-kDa interacting protein; T, significant main effect of treatment; S, significant main effect for sex; g, genotype within sex within treatment; t, treatment within sex within genotype compared with low-fat diet control; s, sex within genotype within treatment condition. *P < 0.05, significance post Bonferroni correction.
We next quantified H2O2 emission relative to respiration in both basal (basal H2O2:O2) and state 3S (state 3S H2O2:O2) to infer quantity of H2O2 emission at a given respiratory rate. These conditions again revealed lower emission levels in females compared with males regardless of genotype (main effect; P < 0.05; Fig. 4, D and E, respectively). Similar to results seen for coupling control ratio, HFD and HFD + VWR caused nonsignificant reductions in the basal and state 3 H2O2:O2 ratios compared with LFD in WT males (Fig. 4E), suggesting that HFD leads to enhanced mitochondrial basal and ADP-dependent function and concomitant reduction in H2O2. This effect was not observed in females, likely due to their inherently low levels of H2O2 emission. LPGC-1α males also showed reduced basal and state 3 H2O2:O2 flux with HFD and HFD + VWR treatment compared with LFD (P < 0.05, Fig. 4, D and E). BNIP3 males had similar, albeit nonsignificant, reductions in basal and state 3 H2O2:O2 flux with HFD and HFD + VWR treatment compared with LFD counterparts (Fig. 4, D and E). Again, these effects were attenuated in BNIP3 and LPGC-1α females. Overall, deficiencies in PGC-1α and BNIP3 did not elicit alterations in mitochondrial H2O2 on a HFD. Instead, differences were primarily driven by sex-dependent reductions in females.
Sex, diet, and physical activity impact HFD-induced hepatic steatosis.
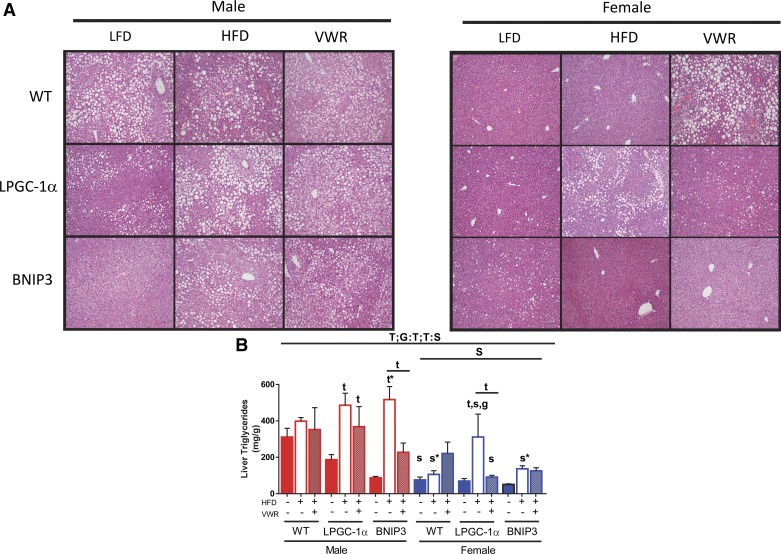
We also assessed liver TG and GSH and GSSG to examine the impact of diet, activity, and genotype on hepatic lipid storage and antioxidant capacity. Unexpectedly, WT males had elevated TG on a LFD that was only slightly increased with HFD and treatment with VWR did not lower liver TG (Fig. 5B). Importantly, it is likely that elevated liver TG on a LFD is a product of thermoneutral housing (18). Liver TGs were higher in males than females across all groups (main effect; P < 0.05, Fig. 5B). WT females had 75% (P < 0.05), 73% (P < 0.05), and 37% lower liver TG in all three groups (LFD, HFD, and HFD + VWR, respectively) compared with males (Fig. 5B). These sex-specific differences were clearly highlighted in liver hematoxylin and eosin images (Fig. 5A). While WT females on a HFD were protected against HFD-induced steatosis, physical activity (HFD + VWR) elicited an unexpected twofold increase in liver TG compared with LFD (Fig. 5B). However, it is important to note that these levels were still lower than males on a HFD (Fig. 5B). Overall, male LPGC-1α and BNIP3 had lower liver TG compared with WT on a LFD, but they were more susceptible to HFD-induced increases in liver TG compared with WT males (61 and 83% respective increases in liver TG compared with LFD controls) (P < 0.05, Fig. 5B). This HFD-induced elevation in liver TG for both LPGC-1α and BNIP3 males was attenuated by physical activity treatment (HFD + VWR) (24 and 56% respective increases in liver TG compared with LFD controls) (P < 0.05 for BNIP3, Fig. 5B). Similar to WT females, BNIP3 females were protected against HFD-induced increases in liver TG, while LPGC-1α mice displayed higher susceptibility (Fig. 5B). HFD + VWR reduced liver TG in female LPGC-1α but had no effect on BNIP3 (Fig. 5B). In summary, females were protected from HFD-induced steatosis when compared with males while physical activity attenuated steatosis in male LPGC-1α and BNIP3 mice compared with their HFD counterparts. Interestingly, HFD + VWR increased liver TG in only WT females while the LPGC-1α and BNIP3 females did not adapt through a similar mechanism.
Fig. 5.
Female mice are protected from hepatic steatosis. Liver lipid content was assessed for wild-type (WT), L peroxisome proliferative activated-receptor-γ coactivator 1α (PGC-1α) and BCL-2/ADENOVIRUS EIB 19-kDa interacting protein (BNIP3) mice using hematoxylin and eosin staining (A) using a Nikon 80i microscope at ×10 magnification and utilizing a biochemical liver TG assay (B). Data are presented as means ± SE (n = 5–7). HFD, high-fat diet; VWR, voluntary wheel running; LFD, low-fat diet; G, significant main effect for genotype; T, significant main effect of treatment; S, significant main effect for sex; g, genotype within sex within treatment; t, treatment within sex within genotype compared with low-fat diet control; s, sex within genotype within treatment condition; G:T, genotype by treatment interaction, T:S; treatment by sex interaction. *P < 0.05, significance post Bonferroni correction.
Females also had a significant overall reduction in the GSH:GSSG ratio (main effect: P < 0.05, Supplemental Fig. S1) suggesting reduced oxidative stress when compared with males. This effect was largely driven by a significant reduction in GSH (P < 0.05 for sex, Supplemental Fig. S1). There were no diet nor genotype effects on the GSH:GSSG ratio. These data support the notion that females are protected from HFD-induced steatosis and have reduced oxidative burden.
Sex and physical activity impact markers of liver injury.
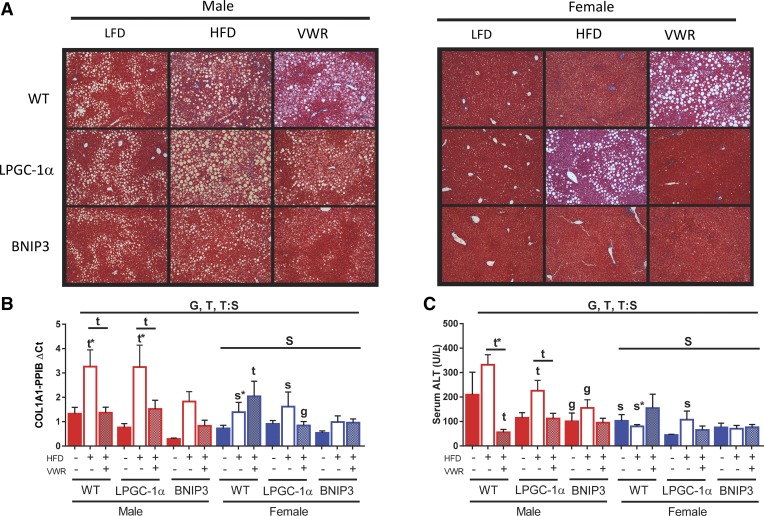
Chronic HFD induces hepatic steatosis and pathways that initiate fibrosis and disease progression while physical activity via VWR can treat these processes (36). Our data suggest overall protection of females from HFD-induced liver damage, which is supported by histology via trichrome staining (Fig. 6A). Thus we examined hepatic gene expression for collagen type I alpha 1 chain (COL1A1) a purported marker of fibrosis. Overall, females had significantly lower COL1A1 expression (main effect; P < 0.05, Fig. 6B). WT females had 45% lower COL1A1 expression on LFD than WT males (Fig. 6B). In WT males, HFD increased Col1A1 1.5-fold versus LFD controls (P < 0.05, Fig. 6B) and was completely abrogated with the addition of VWR. WT females also had a near doubling of COL1A1 expression on HFD compared with LFD (Fig. 6B). Interestingly, HFD + VWR elicited a 2.8- and 1.46-fold increase of COL1A1 compared with LFD and HFD, respectively, in WT females (P < 0.05 for LFD), which tracked with increased liver TG. Male LPGC-1α and BNIP3 mice COL1A1 expression patterns mimicked that of the WT males, except for BNIP3 having overall reduced expression. LPGC-1α and BNIP3 female livers also tended to have HFD-induced increases in COL1A1 expression while HFD + VWR lowered hepatic COL1A1 expression in LPGC-1α but not BNIP3 females.
Fig. 6.
Physical activity reduces indexes of liver damage in male mice. Liver fibrosis/collagen deposition was examined for wild-type (WT), L peroxisome proliferative activated-receptor-γ coactivator 1α (PGC-1α)and BCL-2/ADENOVIRUS EIB 19-kDa interacting protein (BNIP3) mice using trichrome staining (A). Collagen gene expression (COL1A1) was determined using RT-PCR (B). Relative gene expression was normalized to cyclophilin B (PPIB). Hepatic function was measured via serum alanine aminotransferase (ALT) (C). All data are presented as means ± SE (n = 5–7). HFD, high-fat diet; VWR, voluntary wheel running; G, significant main effect for genotype; T, significant main effect of treatment; S, significant main effect for sex; g, genotype within sex within treatment; t, treatment within sex within genotype compared with low-fat diet control; s, sex within genotype within treatment condition; G:S, genotype by sex interaction; T:S; treatment by sex interaction. *P < 0.05, significance post Bonferroni correction.
Serum ALT, a serum marker of liver damage, mimicked the COL1A1 pattern of increasing with HFD and a restoration with HFD + VWR in males, regardless of genotype (Fig. 6C). Interestingly, WT males were the most responsive to physical activity on the HFD, improving ALT with 73 and 83% reductions compared with the LFD control and HFD groups (P < 0.05, Fig. 6C). Once again, females had overall reduced ALT (P < 0.05, Fig. 6C) with minimal responsiveness to HFD and HFD + VWR, and no genotypic differences. We further measured genes encoding for α-smooth muscle actin (α-SMA), monocyte chemoattractant protein 1 (CCL2), glutathione peroxidase 1 (GPX1), and tumor necrosis factor-α (TNF-α) markers of hepatic fibrosis, injury, and inflammation (Supplemental Table S3). Interestingly, all inflammatory and fibrotic gene markers were indicative of HFD-induced hepatic stress in males that was abrogated with VWR. However, WT and BNIP3 females had increases in these gene markers with HFD and HFD + VWR. Conversely, LPGC-1α females had a similar response to males. In conclusion, females have reduced indexes of fibrosis and oxidative stress compared with males, regardless of treatment. HFD + VWR reduces these indexes in male mice compared with the HFD group. This effect is opposite in females, where HFD + VWR increases indexes of fibrosis and oxidative stress with concomitant increases in liver TG, in WT. Finally, our data suggest that the loss of mitophagy via BNIP3 may protect from liver injury and fibrosis following chronic HFD feeding.
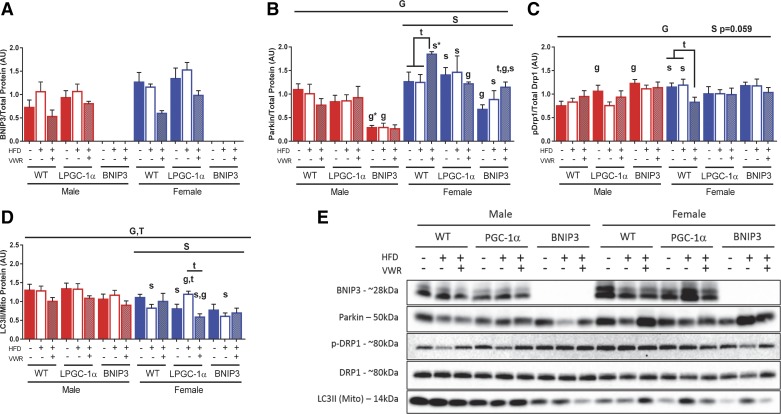
Female livers display greater mitophagy proteins.
We examined proteins associated with mitophagic pathways in whole homogenate (WH) and isolated mitochondrial (MITO) fractions from livers. Representative blots are shown in Fig. 7E. In WT male mice, HFD + VWR tended to reduce mitophagy related protein content in liver including BNIP3 (WH), Parkin (WH), and LC3II (MITO) compared with LFD and HFD conditions (Fig. 7, A, B, D, and E). Overall, WT females had elevated parkin (WH) content and reduced LC3II (MITO) content compared with male counterparts (Fig. 7, B and D). WT females also had reduced expression of BNIP3 and inhibitory pDrp1/Drp1 (Fig. 7, A and C) (WH) with HFD + VWR, while Parkin was increased compared with LFD (Fig. 7B). The loss of PGC-1α in females ablated any treatment-induced changes to Parkin compared with their respective WT control (Fig. 7B). Furthermore, LPGC-1α females have increased mitochondrial LC3II on a HFD compared with LFD, which was lowered with HFD + VWR (Fig. 7D). In contrast, LPGC-1α males tracked with respective WT controls in LC3II (MITO) (Fig. 7D). Female BNIP3 mice tended to have higher Parkin expression than males and remained responsive to HFD + VWR (Fig. 7B). However, the loss of BNIP3 reduced overall Parkin levels in both sexes when compared with WT or LPGC-1α counterparts (Fig. 7B). Generally, HFD + VWR was required for male mitochondrial LC3II content to match similar levels to those inherently seen in females (Fig. 7D). As LC3II is a primary marker of mitophagy activity, these data agree with our previous findings that VWR normalizes mitophagy activity in males to the level seen in females (62).
Fig. 7.
Hepatic mitophagy proteins are differentially expressed between sexes. To measure changes in mitophagy protein expression, BCL-2/ADENOVIRUS EIB 19-kDa interacting protein (BNIP3), Parkin, phoshpo-DRP1 (ser637), and total DRP1 (A, B, and C, respectively) were determined in liver whole homogenate while LC3II (D) was examined in isolated mitochondria. Western immunoblots (E) were normalized to total protein or mitochondrial protein depending on sample analyzed. All data are presented as means ± SE (n = 5–7). HFD, high-fat diet; VWR, voluntary wheel running; WT, wild type; G, significant main effect for genotype; T, significant main effect of treatment; S, significant main effect for sex; g, genotype within sex within treatment; t, treatment within sex within genotype compared with low-fat diet control; s, sex within genotype within treatment condition. *P < 0.05, significance post Bonferroni correction.
Sexual dimorphism of gene expression in response to HFD and HFD + VWR.
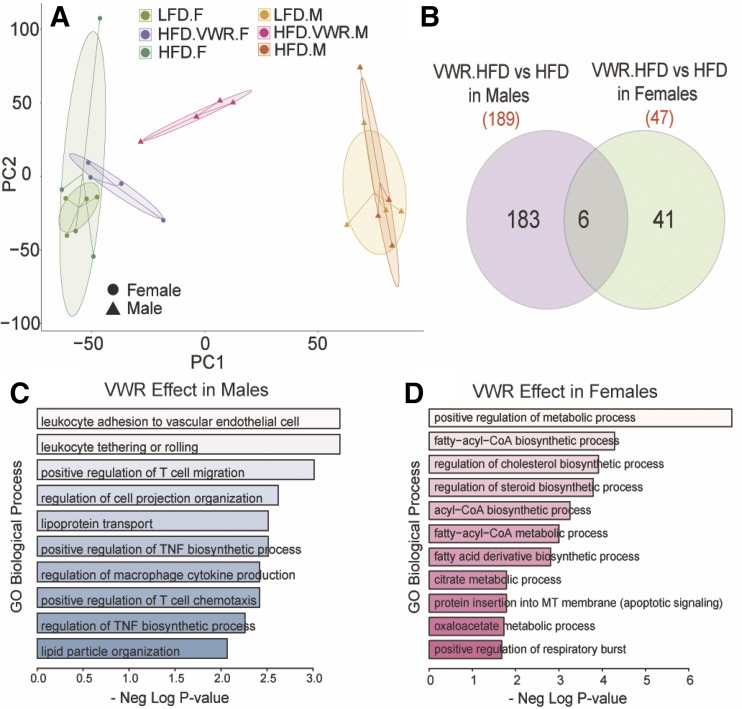
RNA-seq analysis of hepatic gene expression in WT mice was employed to evaluate global transcriptional profiles and to examine the relative effects of LFD, HFD, and HFD + VWR in male and female mice. Principal component analysis of all transcripts (counts/min >2) showed clear divergence of samples based on sex (along PC1) (Fig. 8A). Significant effect of diet was also observed on gene expression. HFD feeding altered the expression of 39 genes in males and 42 genes in females (P < 0.05, ±2-fold). More importantly, HFD + VWR had robust effects on gene expression in the liver of both males and females that were distinct, suggesting a clear sexual dimorphism. The addition of VWR to the HFD influenced expression of 189 genes in males and 47 genes in females (Fig. 8B). Enrichment of gene ontology biological processes using Enrichr also confirmed distinct effects of VWR. In males, HFD + VWR predominantly influenced genes involved in immune regulation, lipoprotein localization and lipid transport (CCL5, ITAG4, CCR2, and CIDEA; Fig. 8C). In contrast, female livers showed distinct regulation of fatty acyl CoA, steroid, cholesterol, and acyl-CoA biosynthesis genes (ACLY, FASN, ACACB, and ELOVL6; Fig. 8D). Despite the differences in the effect size of HFD + VWR between sexes, only HFD + VWR in males robustly shifted the expression of numerous genes (PCA; Fig. 8A). Complete lists of differentially expressed genes and gene onotology biological process terms are included in Supplemental Table S4.
Fig. 8.
Physical activity drives male hepatic gene expression toward females. To measure the sex-dependent gene expression responses to high-fat diet (HFD) and HFD plus physical activity via voluntary wheel running (VWR), we employed RNA-seq in only WT male and female livers. Principle components analysis was performed (A) unmasking sexually dimorphic transcriptional gene adaptations to HFD + VWR (Venn diagram) (B). Pathways of enhanced gene expression following HFD + VWR are listed for males (C) and females (D). GO, gene ontology.
DISCUSSION
Females are observed to have lower prevalence and susceptibility to the development of steatosis (23). Increased physical activity or exercise effectively prevents and treats hepatic steatosis (6, 43, 61). Chronic HFD, a commonly used method for inducing hepatic steatosis, may alter hepatic mitochondrial function through both mitochondrial biogenesis and mitophagy (29). Here we examined if female mice demonstrated different hepatic mitochondrial adaptations to HFD or treatment of HFD-induced steatosis with increased physical activity as compared with males. We utilized WT mouse models and those with reductions in mitochondrial biogenesis (liver specific PGC-1α heterozygotes) or mitophagy (BNIP3 null). Our primary findings are that WT, LPGC-1α, and BNIP3 females possess increased hepatic mitochondrial respiratory coupling control, lower H2O2 emission, and protection against steatosis and markers of fibrosis compared with males in all conditions. We also found that only female WT and LPGC-1α mice have increases in mitochondrial respiratory capacity in response to HFD + VWR. Furthermore, loss of BNIP3-mediated mitophagy blunted these responses in females while males had only nonsignificant increase in mitochondrial respiration with HFD + VWR. However, consistent with our previous results on a LFD (62), males required VWR to display improved mitochondrial coupling control ratio. This improvement in males following VWR tracked with reduced H2O2 emission and reduced evidence of liver fibrosis and serum ALT despite no resolution of steatosis, in contrast to our hypothesis. Also contradicting our initial hypothesis, deficiencies in PGC-1α or BNIP3 had only minor effects compared with sex-based differences.
Studies from our laboratory and others have shown that both acute and chronic HFD elicit reductions in mitochondrial function in mice, including maximal hepatic mitochondrial respiratory capacity (37, 38). As mentioned previously, our current results show that 16 wk of HFD did not elicit reductions in maximal respiratory capacity in either sex. We also saw that HFD did not increase hepatic steatosis in WT males. One possible explanation for these findings is the implementation of near thermoneutral housing conditions (30°C). Typical housing conditions are subthermoneutral for mice (~20–25°C), which dramatically increases resting energy expenditure by 30–50% (1) and likely also impacts hepatic mitochondrial energy demands and mitochondrial biogenesis and turnover. To date, few studies have examined liver metabolism in mice under thermoneutral housing conditions although it is known to significantly enhance the capability of HFDs to induce more pronounced liver injury (inflammation and fibrosis) (18). Thermoneutral housing may have specifically impacted mitochondrial adaptations in WT mice because it did not allow for HFD to cause a further increase in hepatic steatosis over LFD. Previous studies that have shown HFD-induced mitochondrial adaptations also report increased hepatic lipid storage, which may alter mitochondrial substrate utilization and the source of lipids that fuel liver metabolism, independently of increased dietary lipids (40, 53). In fitting with this concept, male LPGC-1α and BNIP3 mice did display significant HFD-induced steatosis that tracked with trends for increased basal, state 3S, and uncoupled respiratory capacity. To our knowledge, this is the first study examining murine hepatic mitochondrial respiratory adaptations to HFD and HFD + VWR at thermoneutral conditions and highlights potential interactions between temperature, genotype, diet, and physical activity.
Previous studies, including those from our laboratory, show VWR increases hepatic maximal mitochondrial respiratory capacity in male rodents (15, 20), adaptations that are likely needed to fuel increased fat oxidation and gluconeogenesis known to occur with exercise (59). Here we show that males have a slight increase in maximal respiratory capacity with HFD + VWR only in ADP-stimulated (state 3S). However, HFD + VWR elicited a significant and robust increase (~2–3 fold) in maximal respiration in WT and LPGC-1α females in all respiration states assessed. While HFD + VWR did not cause increased respiratory responses in BNIP3 females, their control ratios suggest they are universally more efficient. Intriguingly, this robust response to increased physical activity in the females contrasts our previous work where we saw no change in maximal respiratory capacity with 4 wk of VWR on a LFD (62). Thus it appears that physical activity and increased dietary fat interact to drive hepatic mitochondrial adaptations to a much greater magnitude in females than males. Our data suggest that the maximal adaptive response to HFD + VWR is in part driven by BNIP3-mediated mitophagy because the ability of HFD + VWR to drive up mitochondrial respiratory capacity was abated in BNIP3 mice. However, the adaptive mitochondrial coupling control efficiency of BNIP3 mice suggests that alternative pathways (Nix, Parkin, etc.) exist to maintain mitochondrial function when BNIP3-mediated mitophagy pathways are disrupted. These pathways may be sex dependent as our previous results showed that hepatic mitophagy flux is lower in females versus male mice (62), which is consistent with a previous study showing the same sex effects in neurons (10).
Previous studies suggest females possess inherent differences in hepatic mitochondrial metabolism that are paired with protection from diet-induced steatosis (23, 48). Indeed, we have previously shown females had improved respiratory coupling control with increased protein expression of ETS on a LFD, results that were largely not modified by 4 wk of VWR at ~10 km/night (62). In our current study, sedentary WT females were protected from steatosis during a 16-wk HFD; however, this protection was not associated with measurable changes in mitochondrial respiratory capacity or ETS protein expression. However, the superior coupling control and reduced H2O2 in females suggest that their mitochondria can more efficiently utilize increased dietary lipids for energy production compared with males. In addition, our data suggest that a reduction of PGC-1α partially diminishes the female protection against HFD-induced hepatic steatosis, but this was not associated with increased hepatic injury. Interestingly, these findings match previous work showing that normal expression of PGC-1α is critical for estrogen signaling to antioxidant genes that protect against liver injury (4). As already discussed, only females showed pronounced changes in mitochondrial respiratory capacity (stated 3S and uncoupled) with HFD + VWR. It is possible that the female hormone estrogen is the primary driver of these female versus male adaptations. Previous reports show that removal of ovarian function removes protection against steatosis and cardiometabolic risk factors in females while estradiol supplementation restores protection (41, 66). Burgeoning evidence suggests these effects are partially driven by hepatic mitochondrial adaptations (23, 45, 64, 66), while a far greater body of work shows pronounced impact on other metabolic tissues known to be important for hepatic lipid storage (regulation of fat storage and lipolysis in subcutaneous adipose and skeletal muscle insulin sensitivity) (9). Additional work is needed to examine the hepatic specific mechanisms by which females are protected from steatosis and respond differently to VWR + HFD than males. An interesting finding was that highly physically active female WT mice on a HFD displayed increased liver TGs concomitant with increased body and fat mass. The increased hepatic TG storage may be a physical activity-induced adaptation, similar to the “athlete’s paradox” in which endurance athletes display elevated intramuscular TGs paired with high mitochondrial oxidative capacity. High physical activity places an energetic demand on the liver. Lipids derived from lipolysis of adipose tissue and from intrahepatic stores are oxidized to fuel the energy costly process of gluconeogenesis need to fuel contracting skeletal muscles. It is possible that females have an enhanced need to refuel intrahepatic lipid stores between bouts of physical activity due to the dramatically lower lipid stores compared with males. The evolutionary metabolic adaptability needed for gestation and lactation in female mice and how this relates to hepatic mitochondrial adaptations and fuel storage under different conditions (diet or physical activity) deserves further study.
Hepatic steatosis is closely linked with increased susceptibility for fibrosis (55), arising through increased inflammation, liver damage (increased ALT), and activation of hepatic stellate cells (12), which then lay down excessive extracellular matrix in the liver (65). In the current study, we observed steatosis in WT males on LFD that was not associated with fibrosis. However, while sedentary WT males on a HFD did not show marked increases in steatosis, they did show markers of fibrosis and increased circulating ALT, results that did not occur if mice were provided VWR for the final 8 wk of the HFD. These results support previous studies showing the ability of physical activity or aerobic exercise to reduce liver injury and fibrosis.
Both liver-specific PGC-1α heterozygote mice and BNIP3 null mice have been shown to have increased sensitivity to liver fibrosis secondary to oxidative stress (13, 19). Compared with WT males, LPGC-1α and BNIP3 males did not have worsened markers of fibrosis. Despite the inconsistency for increased physical activity to prevent steatosis in male WT, LPGC-1α, and BNIP3 mice, it dramatically lowered serum and liver markers of hepatic inflammation and fibrosis in all groups showing that its protective effects on liver health are not necessarily directly linked to reduced liver TG storage. While females had higher expression of TNF-α, no liver damage was present. Once again, females were conferred an overall protection from fibrosis and increased ALT compared with males, a result that is likely to be expected given the lack of steatosis. Surprisingly, loss of BNIP3 was not sufficient to exacerbate hepatic inflammation, fibrosis, and markers of damage on HFD. This could be due to activation of alternative pathways during the chronic HFD, which leads to enhanced coupling control and reduced state 3 H2O2:O2 flux ratio decreasing oxidative stress.
We also employed RNA sequencing in WT mice to further examine sexually dimorphic gene expression changes in response to HFD and HFD + VWR. Gene expression changes showed that HFD had a significant impact on changing a significant number of genes in both sexes. However, HFD + VWR elicited much larger transcriptional changes suggesting that in the context of increased dietary lipids physical activity evokes a significant metabolic adaptation in the liver. Interestingly, male livers altered expression of twice the number of genes than females in response to HFD + VWR at the same criteria. Moreover, PCA analysis revealed that HFD + VWR robustly shifted gene expression in males while females had only modest transcriptional adaptations. Gene ontology analysis revealed that female livers displayed an upregulation of mitochondrial and lipid metabolism pathways matching the robust mitochondrial respiratory adaptations in response to HFD + VWR found only in females. Signaling through the estrogen receptor-α has been shown to robustly mediate changes in pathways (lipid, mitochondrial, and steroid) (17), similar to what was evoked by HFD + VWR in females. Further work is needed to determine how exercise differentially impacts transcriptional regulation of hepatic metabolism between males and females as this likely underlies differences in mitochondrial phenotypes and susceptibility for steatosis.
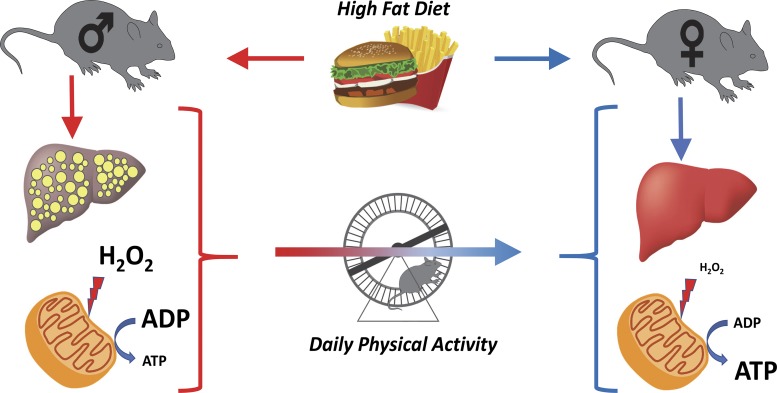
In summary, females possess improved hepatic mitochondrial respiratory coupling control, lower mitochondrial H2O2 emission, and protection against steatosis and fibrosis compared with males (Fig. 9). We also found that HFD feeding and increased physical activity interact to produce pronounced increases in basal and maximal respiratory capacity in female mice. However, these effects were reduced by the loss of BNIP3. Consistent with our previous finings (62), males required VWR to display improved mitochondrial coupling control ratio and mitophagy activity (Fig. 9). This improvement tracked with reduced H2O2 emission and reduced fibrosis/damage despite no resolution of steatosis. These VWR-induced change in males tracked with a shift in transcriptional adaptations toward a female phenotype (Fig. 9). These data strongly suggest that sex is the primary determinant for mitochondrial adaptations to a chronic HFD or HFD + VWR challenge in the study of diet-induced liver disease. Future studies are needed to explore the role of estrogen in the inherent mitochondrial differences and protection from fibrosis/steatosis observed in females.
Fig. 9.
Model of hepatic response to high-fat diet (HFD) and HFD plus physical activity via voluntary wheel running (VWR) in male and female mice. Male mice are more susceptible to HFD-induced hepatic steatosis than females. This is associated with increased hepatic mitochondrial H2O2 production and reduced mitochondrial coupling and efficiency. Females are protected from HFD-induced steatosis concomitant with low hepatic mitochondrial H2O2 production and increased coupling control. The addition of VWR in male mice drives their hepatic mitochondrial phenotype toward those of females by eliciting reduced steatosis and mitochondrial H2O2 production and increasing coupling.
GRANTS
This work was supported by Veterans Affairs Merit Review Grant 1I01BX002567-01 (to J. P. Thyfault) and also partially supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants R01-DK-088940 (to J. P. Thyfault) and K01-DK-112967-01 (to E. M. Morris), Institutional Development Award (IDeA) National Institute of General Medical Sciences Grant P20-GM-103418 (to J. P. Thyfault, E. M. Morris, C. J. Houchen, and C. S. McCoin), and Clinical and Translational Science Award TL1 Postdoctoral Training Grant TL1TR002368 (to C. S. McCoin). K. Shankar is supported in part by the U.S. Department of Agriculture-Agriculture Research Service Project 6251-51000-010-05S. Additional support was provided by the University of Kansas Medical Center Biomedical Research Training Program (to C. S. McCoin). The Genomics Core is supported by the University of Kansas–School of Medicine, the Kansas Intellectual and Developmental Disability Research Center (National Institute of Child Health and Human Development Grant U54-HD-090216), and the Molecular Regulation of Cell Development and Differentiation–COBRE (5P20GM104936-10).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
E.M.M. and J.P.T. conceived and designed research; C.S.M., A.V.S., J.A., K.N.F., and A.M. performed experiments; C.S.M., A.V.S., K.N.F., Q.X., D.C.K., A.M., and K.S. analyzed data; C.S.M., A.V.S., K.N.F., E.M.M., and J.P.T. interpreted results of experiments; C.S.M., A.V.S., K.N.F., C.J.H., and K.S. prepared figures; C.S.M. and A.V.S. drafted manuscript; C.S.M., A.V.S., J.A., K.N.F., Q.X., D.C.K., C.J.H., G.W.D.I., K.S., E.M.M., and J.P.T. edited and revised manuscript; C.S.M., A.V.S., J.A., K.N.F., Q.X., D.C.K., C.J.H., A.M., G.W.D. 2nd, K.S., E.M.M., and J.P.T. approved final version of manuscript.
REFERENCES
- 1.Abreu-Vieira G, Xiao C, Gavrilova O, Reitman ML. Integration of body temperature into the analysis of energy expenditure in the mouse. Mol Metab 4: 461–470, 2015. doi: 10.1016/j.molmet.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amir M, Czaja MJ. Autophagy in nonalcoholic steatohepatitis. Expert Rev Gastroenterol Hepatol 5: 159–166, 2011. doi: 10.1586/egh.11.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bardou P, Mariette J, Escudié F, Djemiel C, Klopp C. jvenn: an interactive Venn diagram viewer. BMC Bioinformatics 15: 293, 2014. doi: 10.1186/1471-2105-15-293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Besse-Patin A, Léveillé M, Oropeza D, Nguyen BN, Prat A, Estall JL. Estrogen signals through peroxisome proliferator-activated receptor-γ coactivator 1α to reduce oxidative damage associated with diet-induced fatty liver disease. Gastroenterology 152: 243–256, 2017. doi: 10.1053/j.gastro.2016.09.017. [DOI] [PubMed] [Google Scholar]
- 5.Borengasser SJ, Rector RS, Uptergrove GM, Morris EM, Perfield JW 2nd, Booth FW, Fritsche KL, Ibdah JA, Thyfault JP. Exercise and omega-3 polyunsaturated fatty acid supplementation for the treatment of hepatic steatosis in hyperphagic OLETF rats. J Nutr Metab 2012: 268680, 2012. doi: 10.1155/2012/268680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brouwers B, Hesselink MK, Schrauwen P, Schrauwen-Hinderling VB. Effects of exercise training on intrahepatic lipid content in humans. Diabetologia 59: 2068–2079, 2016. doi: 10.1007/s00125-016-4037-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burgess SC, Leone TC, Wende AR, Croce MA, Chen Z, Sherry AD, Malloy CR, Finck BN. Diminished hepatic gluconeogenesis via defects in tricarboxylic acid cycle flux in peroxisome proliferator-activated receptor γ coactivator-1α (PGC-1α)-deficient mice. J Biol Chem 281: 19000–19008, 2006. doi: 10.1074/jbc.M600050200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen EY, Tan CM, Kou Y, Duan Q, Wang Z, Meirelles GV, Clark NR, Ma’ayan A. Enrichr: interactive and collaborative HTML5 gene list enrichment analysis tool. BMC Bioinformatics 14: 128, 2013. doi: 10.1186/1471-2105-14-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clegg D, Hevener AL, Moreau KL, Morselli E, Criollo A, Van Pelt RE, Vieira-Potter VJ. Sex hormones and cardiometabolic health: role of estrogen and estrogen receptors. Endocrinology 158: 1095–1105, 2017. doi: 10.1210/en.2016-1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Demarest TG, Waite EL, Kristian T, Puche AC, Waddell J, McKenna MC, Fiskum G. Sex-dependent mitophagy and neuronal death following rat neonatal hypoxia-ischemia. Neuroscience 335: 103–113, 2016. doi: 10.1016/j.neuroscience.2016.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Diwan A, Krenz M, Syed FM, Wansapura J, Ren X, Koesters AG, Li H, Kirshenbaum LA, Hahn HS, Robbins J, Jones WK, Dorn GW 2nd. Inhibition of ischemic cardiomyocyte apoptosis through targeted ablation of Bnip3 restrains postinfarction remodeling in mice. J Clin Invest 117: 2825–2833, 2007. doi: 10.1172/JCI32490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eng FJ, Friedman SL. Fibrogenesis I. New insights into hepatic stellate cell activation: the simple becomes complex. Am J Physiol Gastrointest Liver Physiol 279: G7–G11, 2000. doi: 10.1152/ajpgi.2000.279.1.G7. [DOI] [PubMed] [Google Scholar]
- 13.Estall JL, Kahn M, Cooper MP, Fisher FM, Wu MK, Laznik D, Qu L, Cohen DE, Shulman GI, Spiegelman BM. Sensitivity of lipid metabolism and insulin signaling to genetic alterations in hepatic peroxisome proliferator-activated receptor-γ coactivator-1α expression. Diabetes 58: 1499–1508, 2009. doi: 10.2337/db08-1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fernandez-Marcos PJ, Auwerx J. Regulation of PGC-1α, a nodal regulator of mitochondrial biogenesis. Am J Clin Nutr 93: 884S–890S, 2011. doi: 10.3945/ajcn.110.001917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fletcher JA, Linden MA, Sheldon RD, Meers GM, Morris EM, Butterfield A, Perfield JW 2nd, Rector RS, Thyfault JP. Fibroblast growth factor 21 increases hepatic oxidative capacity but not physical activity or energy expenditure in hepatic peroxisome proliferator-activated receptor γ coactivator-1α-deficient mice. Exp Physiol 103: 408–418, 2018. doi: 10.1113/EP086629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fletcher JA, Meers GM, Linden MA, Kearney ML, Morris EM, Thyfault JP, Rector RS. Impact of various exercise modalities on hepatic mitochondrial function. Med Sci Sports Exerc 46: 1089–1097, 2014. doi: 10.1249/MSS.0000000000000223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gao H, Fält S, Sandelin A, Gustafsson JA, Dahlman-Wright K. Genome-wide identification of estrogen receptor α-binding sites in mouse liver. Mol Endocrinol 22: 10–22, 2008. doi: 10.1210/me.2007-0121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Giles DA, Moreno-Fernandez ME, Stankiewicz TE, Graspeuntner S, Cappelletti M, Wu D, Mukherjee R, Chan CC, Lawson MJ, Klarquist J, Sünderhauf A, Softic S, Kahn CR, Stemmer K, Iwakura Y, Aronow BJ, Karns R, Steinbrecher KA, Karp CL, Sheridan R, Shanmukhappa SK, Reynaud D, Haslam DB, Sina C, Rupp J, Hogan SP, Divanovic S. Thermoneutral housing exacerbates nonalcoholic fatty liver disease in mice and allows for sex-independent disease modeling. Nat Med 23: 829–838, 2017. [Erratum in Nat Med 23: 1241, 2017.] doi: 10.1038/nm.4346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Glick D, Zhang W, Beaton M, Marsboom G, Gruber M, Simon MC, Hart J, Dorn GW II, Brady MJ, Macleod KF. BNip3 regulates mitochondrial function and lipid metabolism in the liver. Mol Cell Biol 32: 2570–2584, 2012. doi: 10.1128/MCB.00167-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Glick JL. Effects of exercise on oxidative activities in rat liver mitochondria. Am J Physiol 210: 1215–1221, 1966. doi: 10.1152/ajplegacy.1966.210.6.1215. [DOI] [PubMed] [Google Scholar]
- 21.Golabi P, Bush H, Younossi ZM. Treatment strategies for nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Clin Liver Dis 21: 739–753, 2017. doi: 10.1016/j.cld.2017.06.010. [DOI] [PubMed] [Google Scholar]
- 22.Grubbs FE. Procedures for detecting outlying observations in samples. Technometrics 11: 1–21, 1969. doi: 10.1080/00401706.1969.10490657. [DOI] [Google Scholar]
- 23.Hart-Unger S, Arao Y, Hamilton KJ, Lierz SL, Malarkey DE, Hewitt SC, Freemark M, Korach KS. Hormone signaling and fatty liver in females: analysis of estrogen receptor α mutant mice. Int J Obes 41: 945–954, 2017. doi: 10.1038/ijo.2017.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.He C, Bassik MC, Moresi V, Sun K, Wei Y, Zou Z, An Z, Loh J, Fisher J, Sun Q, Korsmeyer S, Packer M, May HI, Hill JA, Virgin HW, Gilpin C, Xiao G, Bassel-Duby R, Scherer PE, Levine B. Exercise-induced BCL2-regulated autophagy is required for muscle glucose homeostasis. Nature 481: 511–515, 2012. [Erratum in Nature 503: 146, 2013.] doi: 10.1038/nature10758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hissin PJ, Hilf R. A fluorometric method for determination of oxidized and reduced glutathione in tissues. Anal Biochem 74: 214–226, 1976. doi: 10.1016/0003-2697(76)90326-2. [DOI] [PubMed] [Google Scholar]
- 26.Kasperek GJ, Dohm GL, Barakat HA, Strausbauch PH, Barnes DW, Snider RD. The role of lysosomes in exercise-induced hepatic protein loss. Biochem J 202: 281–288, 1982. doi: 10.1042/bj2020281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koliaki C, Szendroedi J, Kaul K, Jelenik T, Nowotny P, Jankowiak F, Herder C, Carstensen M, Krausch M, Knoefel WT, Schlensak M, Roden M. Adaptation of hepatic mitochondrial function in humans with non-alcoholic fatty liver is lost in steatohepatitis. Cell Metab 21: 739–746, 2015. doi: 10.1016/j.cmet.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 28.Krumschnabel G, Fontana-Ayoub M, Sumbalova Z, Heidler J, Gauper K, Fasching M, Gnaiger E. Simultaneous high-resolution measurement of mitochondrial respiration and hydrogen peroxide production. Methods Mol Biol 1264: 245–261, 2015. doi: 10.1007/978-1-4939-2257-4_22. [DOI] [PubMed] [Google Scholar]
- 29.Kubli DA, Gustafsson ÅB. Mitochondria and mitophagy: the yin and yang of cell death control. Circ Res 111: 1208–1221, 2012. doi: 10.1161/CIRCRESAHA.112.265819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kuleshov MV, Jones MR, Rouillard AD, Fernandez NF, Duan Q, Wang Z, Koplev S, Jenkins SL, Jagodnik KM, Lachmann A, McDermott MG, Monteiro CD, Gundersen GW, Ma’ayan A. Enrichr: a comprehensive gene set enrichment analysis web server 2016 update. Nucleic Acids Res 44: W90–W97, 2016. doi: 10.1093/nar/gkw377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Laye MJ, Rector RS, Borengasser SJ, Naples SP, Uptergrove GM, Ibdah JA, Booth FW, Thyfault JP. Cessation of daily wheel running differentially alters fat oxidation capacity in liver, muscle, and adipose tissue. J Appl Physiol (1985) 106: 161–168, 2009. doi: 10.1152/japplphysiol.91186.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leite NC, Salles GF, Araujo AL, Villela-Nogueira CA, Cardoso CR. Prevalence and associated factors of non-alcoholic fatty liver disease in patients with type-2 diabetes mellitus. Liver Int 29: 113–119, 2009. doi: 10.1111/j.1478-3231.2008.01718.x. [DOI] [PubMed] [Google Scholar]
- 34.Leone TC, Lehman JJ, Finck BN, Schaeffer PJ, Wende AR, Boudina S, Courtois M, Wozniak DF, Sambandam N, Bernal-Mizrachi C, Chen Z, Holloszy JO, Medeiros DM, Schmidt RE, Saffitz JE, Abel ED, Semenkovich CF, Kelly DP. PGC-1α deficiency causes multi-system energy metabolic derangements: muscle dysfunction, abnormal weight control and hepatic steatosis. PLoS Biol 3: e101, 2005. doi: 10.1371/journal.pbio.0030101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Linden MA, Fletcher JA, Morris EM, Meers GM, Laughlin MH, Booth FW, Sowers JR, Ibdah JA, Thyfault JP, Rector RS. Treating NAFLD in OLETF rats with vigorous-intensity interval exercise training. Med Sci Sports Exerc 47: 556–567, 2015. doi: 10.1249/MSS.0000000000000430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Linden MA, Sheldon RD, Meers GM, Ortinau LC, Morris EM, Booth FW, Kanaley JA, Vieira-Potter VJ, Sowers JR, Ibdah JA, Thyfault JP, Laughlin MH, Rector RS. Aerobic exercise training in the treatment of non-alcoholic fatty liver disease related fibrosis. J Physiol 594: 5271–5284, 2016. doi: 10.1113/JP272235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mantena SK, Vaughn DP Jr, Andringa KK, Eccleston HB, King AL, Abrams GA, Doeller JE, Kraus DW, Darley-Usmar VM, Bailey SM. High fat diet induces dysregulation of hepatic oxygen gradients and mitochondrial function in vivo. Biochem J 417: 183–193, 2009. doi: 10.1042/BJ20080868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Morris EM, Jackman MR, Meers GM, Johnson GC, Lopez JL, MacLean PS, Thyfault JP. Reduced hepatic mitochondrial respiration following acute high-fat diet is prevented by PGC-1α overexpression. Am J Physiol Gastrointest Liver Physiol 305: G868–G880, 2013. doi: 10.1152/ajpgi.00179.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Morris EM, Meers GM, Booth FW, Fritsche KL, Hardin CD, Thyfault JP, Ibdah JA. PGC-1α overexpression results in increased hepatic fatty acid oxidation with reduced triacylglycerol accumulation and secretion. Am J Physiol Gastrointest Liver Physiol 303: G979–G992, 2012. doi: 10.1152/ajpgi.00169.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Morris EM, Meers GM, Koch LG, Britton SL, Fletcher JA, Fu X, Shankar K, Burgess SC, Ibdah JA, Rector RS, Thyfault JP. Aerobic capacity and hepatic mitochondrial lipid oxidation alters susceptibility for chronic high-fat diet-induced hepatic steatosis. Am J Physiol Endocrinol Metab 311: E749–E760, 2016. doi: 10.1152/ajpendo.00178.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Palmisano BT, Zhu L, Eckel RH, Stafford JM. Sex differences in lipid and lipoprotein metabolism. Mol Metab 15: 45–55, 2018. doi: 10.1016/j.molmet.2018.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Papáčková Z, Daňková H, Páleníčková E, Kazdová L, Cahová M. Effect of short- and long-term high-fat feeding on autophagy flux and lysosomal activity in rat liver. Physiol Res 61, Suppl 2: S67–S76, 2012. [DOI] [PubMed] [Google Scholar]
- 43.Perseghin G, Lattuada G, De Cobelli F, Ragogna F, Ntali G, Esposito A, Belloni E, Canu T, Terruzzi I, Scifo P, Del Maschio A, Luzi L. Habitual physical activity is associated with intrahepatic fat content in humans. Diabetes Care 30: 683–688, 2007. doi: 10.2337/dc06-2032. [DOI] [PubMed] [Google Scholar]
- 44.Pesta D, Gnaiger E. High-resolution respirometry: OXPHOS protocols for human cells and permeabilized fibers from small biopsies of human muscle. Methods Mol Biol 810: 25–58, 2012. doi: 10.1007/978-1-61779-382-0_3. [DOI] [PubMed] [Google Scholar]
- 45.Qiu S, Vazquez JT, Boulger E, Liu H, Xue P, Hussain MA, Wolfe A. Hepatic estrogen receptor α is critical for regulation of gluconeogenesis and lipid metabolism in males. Sci Rep 7: 1661, 2017. doi: 10.1038/s41598-017-01937-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rector RS, Thyfault JP. Does physical inactivity cause nonalcoholic fatty liver disease? J Appl Physiol (1985) 111: 1828–1835, 2011. doi: 10.1152/japplphysiol.00384.2011. [DOI] [PubMed] [Google Scholar]
- 47.Rector RS, Thyfault JP, Morris RT, Laye MJ, Borengasser SJ, Booth FW, Ibdah JA. Daily exercise increases hepatic fatty acid oxidation and prevents steatosis in Otsuka Long-Evans Tokushima Fatty rats. Am J Physiol Gastrointest Liver Physiol 294: G619–G626, 2008. doi: 10.1152/ajpgi.00428.2007. [DOI] [PubMed] [Google Scholar]
- 48.Reue K. Sex differences in obesity: X chromosome dosage as a risk factor for increased food intake, adiposity and co-morbidities. Physiol Behav 176: 174–182, 2017. doi: 10.1016/j.physbeh.2017.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ritchie ME, Phipson B, Wu D, Hu Y, Law CW, Shi W, Smyth GK. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res 43: e47, 2015. doi: 10.1093/nar/gkv007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rivera H, Shibayama M, Tsutsumi V, Perez-Alvarez V, Muriel P. Resveratrol and trimethylated resveratrol protect from acute liver damage induced by CCl4 in the rat. J Appl Toxicol 28: 147–155, 2008. doi: 10.1002/jat.1260. [DOI] [PubMed] [Google Scholar]
- 51.Santos-Alves E, Marques-Aleixo I, Rizo-Roca D, Torrella JR, Oliveira PJ, Magalhães J, Ascensão A. Exercise modulates liver cellular and mitochondrial proteins related to quality control signaling. Life Sci 135: 124–130, 2015. doi: 10.1016/j.lfs.2015.06.007. [DOI] [PubMed] [Google Scholar]
- 52.Satapati S, Kucejova B, Duarte JA, Fletcher JA, Reynolds L, Sunny NE, He T, Nair LA, Livingston KA, Fu X, Merritt ME, Sherry AD, Malloy CR, Shelton JM, Lambert J, Parks EJ, Corbin I, Magnuson MA, Browning JD, Burgess SC. Mitochondrial metabolism mediates oxidative stress and inflammation in fatty liver. J Clin Invest 126: 1605, 2016. doi: 10.1172/JCI86695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Satapati S, Kucejova B, Duarte JA, Fletcher JA, Reynolds L, Sunny NE, He T, Nair LA, Livingston KA, Fu X, Merritt ME, Sherry AD, Malloy CR, Shelton JM, Lambert J, Parks EJ, Corbin I, Magnuson MA, Browning JD, Burgess SC. Mitochondrial metabolism mediates oxidative stress and inflammation in fatty liver. J Clin Invest 125: 4447–4462, 2015. [Erratum in J Clin Invest 126: 1605, 2016.] doi: 10.1172/JCI82204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Satapati S, Sunny NE, Kucejova B, Fu X, He TT, Méndez-Lucas A, Shelton JM, Perales JC, Browning JD, Burgess SC. Elevated TCA cycle function in the pathology of diet-induced hepatic insulin resistance and fatty liver. J Lipid Res 53: 1080–1092, 2012. doi: 10.1194/jlr.M023382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Singh S, Allen AM, Wang Z, Prokop LJ, Murad MH, Loomba R. Fibrosis progression in nonalcoholic fatty liver vs nonalcoholic steatohepatitis: a systematic review and meta-analysis of paired-biopsy studies. Clin Gastroenterol Hepatol 13: 643–654.e9, 2015. doi: 10.1016/j.cgh.2014.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Srere PA. Citrate synthase [EC 4.1.3.7. citrate oxaloacetate-lyase (CoA-acetylating)]. In: Methods in Enzymology: Citric Acid Cycle, edited by Lowenstein JM. New York: Academic Press, 1969, vol. 13, p. 3–11. [Google Scholar]
- 57.Sunny NE, Parks EJ, Browning JD, Burgess SC. Excessive hepatic mitochondrial TCA cycle and gluconeogenesis in humans with nonalcoholic fatty liver disease. Cell Metab 14: 804–810, 2011. doi: 10.1016/j.cmet.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tang AC, Nakazawa M, Romeo RD, Reeb BC, Sisti H, McEwen BS. Effects of long-term estrogen replacement on social investigation and social memory in ovariectomized C57BL/6 mice. Horm Behav 47: 350–357, 2005. doi: 10.1016/j.yhbeh.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 59.Thyfault JP, Morris EM. Intrinsic (genetic) aerobic fitness impacts susceptibility for metabolic disease. Exerc Sport Sci Rev 45: 7–15, 2017. doi: 10.1249/JES.0000000000000087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vainshtein A, Tryon LD, Pauly M, Hood DA. Role of PGC-1α during acute exercise-induced autophagy and mitophagy in skeletal muscle. Am J Physiol Cell Physiol 308: C710–C719, 2015. doi: 10.1152/ajpcell.00380.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.van der Heijden GJ, Wang ZJ, Chu ZD, Sauer PJ, Haymond MW, Rodriguez LM, Sunehag AL. A 12-week aerobic exercise program reduces hepatic fat accumulation and insulin resistance in obese, Hispanic adolescents. Obesity (Silver Spring) 18: 384–390, 2010. doi: 10.1038/oby.2009.274. [DOI] [PubMed] [Google Scholar]
- 62.Von Schulze A, McCoin CS, Onyekere C, Allen J, Geiger P, Dorn GW II, Morris EM, Thyfault JP. Hepatic mitochondrial adaptations to physical activity: impact of sexual dimorphism, PGC1α and BNIP3-mediated mitophagy. J Physiol 596: 6157–6171, 2018. doi: 10.1113/JP276539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wankhade UD, Zhong Y, Kang P, Alfaro M, Chintapalli SV, Thakali KM, Shankar K. Enhanced offspring predisposition to steatohepatitis with maternal high-fat diet is associated with epigenetic and microbiome alterations. PLoS One 12: e0175675, 2017. doi: 10.1371/journal.pone.0175675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Winn NC, Jurrissen TJ, Grunewald ZI, Cunningham RP, Woodford ML, Kanaley JA, Lubahn DB, Manrique-Acevedo C, Rector RS, Vieira-Potter VJ, Padilla J. Estrogen receptor-α signaling maintains immunometabolic function in males and is obligatory for 316: E156–E167, 2019. doi: 10.1152/ajpendo.00259.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wynn TA. Cellular and molecular mechanisms of fibrosis. J Pathol 214: 199–210, 2008. doi: 10.1002/path.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhu L, Brown WC, Cai Q, Krust A, Chambon P, McGuinness OP, Stafford JM. Estrogen treatment after ovariectomy protects against fatty liver and may improve pathway-selective insulin resistance. Diabetes 62: 424–434, 2013. doi: 10.2337/db11-1718. [DOI] [PMC free article] [PubMed] [Google Scholar]