Abstract
Ischemic heart disease is a growing worldwide epidemic. Improvements in medical and surgical therapies have reduced early mortality after acute myocardial infarction and increased the number of patients living with chronic heart failure. The irreversible loss of functional cardiomyocytes puts these patients at significant risk of ongoing morbidity and mortality after their index event. Recent evidence suggests that inflammation is a key mediator of postinfarction adverse remodeling in the heart. In this review, we discuss the cardioprotective and deleterious effects of inflammation and its mediators during acute myocardial infarction. We also explore the role of mesenchymal stem cell therapy to limit secondary injury and promote myocardial healing after myocardial infarction.
Keywords: immunomodulation, inflammation, mesenchymal stem cell, myocardial infarction, myocardial repair
INTRODUCTION
Ischemic heart disease is the number one cause of death worldwide accounting for over nine million deaths annually (117). Atherosclerotic plaque formation in epicardial coronary arteries leads to progressively diminished myocardial blood flow and ultimately results in myocardial injury or infarction (MI) through plaque rupture or critical supply-demand mismatch (174). Inflammation has long been known to play a vital role in both the progression of atherosclerosis, as well as secondary injury after myocardial damage (113, 122). Despite its foundational role in wound healing, an overwhelming inflammatory response after MI can have significant deleterious effects on cardiomyocytes and lead to worse patient outcomes (15). Improved understanding in the interactions between cells, extracellular matrix (ECM), and signaling molecules within the injured myocardium have allowed development of novel experimental therapies. These therapies seek to target the intricate balance between proinflammatory and anti-inflammatory pathways in an attempt to limit ischemic injury and prevent subsequent development of heart failure (34, 107, 154). Mesenchymal stem cells (MSCs), in particular, have emerged as potent paracrine modulators of inflammation that promote myocardial healing after infarction (68, 108, 138). The goals of this review are to discuss the cardioprotective and deleterious effects of inflammation and its individual mediators during acute MI and explore the immunomodulatory role of MSC therapy to limit secondary damage after MI.
INFLAMMATION FACILITATES HEALING AFTER ACUTE MYOCARDIAL INFARCTION
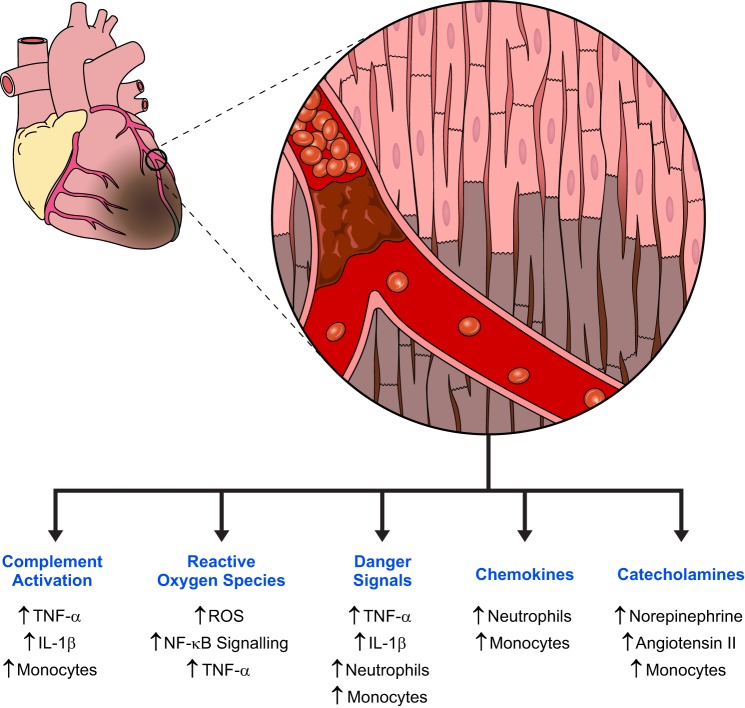
Myocardial injury and infarction is associated with an inflammatory cascade that is essential for debris removal and scar formation (58, 120). In an ST-elevation myocardial infarction (STEMI), sudden and complete coronary artery occlusion creates an acutely hypoxic environment known as an ischemic zone (80). Onset of cell death begins within 30 min to an hour after cessation of blood flow through a combination of necrosis and apoptosis (178). Necrosis in MI involves disruption of mitochondrial membranes driven by calcium transport dysregulation, secondary to anaerobic acidosis, while apoptosis can occur through both the intrinsic and extrinsic pathways (32, 129). The large amounts of cellular debris released by infarcted myocardium trigger a vigorous inflammatory response (Fig. 1).
Fig. 1.
Overview of inflammatory contributors after acute myocardial infarction. Acute occlusion of epicardial coronary arteries leads to massive cell death within hours of plaque rupture. This triggers multiple signaling pathway activations through innate immune system mechanisms, release of reactive oxygen species (ROS), and systemic neurohormonal signaling. These pathways ultimately result in the release of proinflammatory cytokines and recruitment of cellular mediators of inflammation, such as neutrophils and monocytes. These mediators are critical for sustaining an inflammatory response and eventual healing of the infarcted myocardium.
Complement Activation Initiates the Postinfarction Inflammatory Cascade
The release of cytosolic cellular constituents results in complement activation within 2 h of an acute MI (13, 141, 180, 195). The presence of mitochondrial membrane components, such as cardiolipin, outside the cell activate the classical complement pathway by directly binding complement component 1q (C1q) (149). This results in activation of the C1 complex (C1qC1rC1s), which cleaves both C4 and C2. C4b and C2a make up C3 convertase, which cleaves C3 to make C5 convertase (C4bC2aC3b), which subsequently cleaves C5 to facilitate chemotactic recruitment of neutrophils and monocytes (47, 75). Classical monocytes (Ly-6Chi in mouse, CD14++CD16– in humans), in particular, are activated by the complement pathway and propagate the initial inflammatory cascade through secretion of large quantities of proinflammatory cytokines, including interleukin 1β (IL-1β) and tumor necrosis factor-α (TNF-α) (51, 116, 128, 153). Infiltrating neutrophils augment this response by supporting further recruitment and activation of classical monocytes (60, 164).
Reactive Oxygen Species Augment Inflammatory Signaling after Myocardial Infarction
Timely early reperfusion therapy is the standard of care for patients presenting with STEMI and reestablishes nutritive blood flow to reversibly injured myocardium (77, 130). Numerous epidemiologic studies over the past two decades have shown reduced morbidity and mortality with early percutaneous coronary intervention (PCI) for acutely occluded coronary arteries (172). However, the ischemia-reperfusion process can also increase the generation of reactive oxygen species (ROS) within the affected myocardium upon reestablishment of blood flow (14, 168). Cardiomyocytes contain large numbers of mitochondria that can potentially generate ROS through the electron transport chain. In physiological conditions, 2% of oxygen consumed is converted to highly reactive superoxide, which is rapidly reduced by intrinsic antioxidant mechanisms (186). During acute ischemia, however, there is a backlog of electrons in the electron transport chain due to the lack of its final electron acceptor that results in leakage of electrons from the mitochondria (30). These electrons react with residual oxygen molecules to form supraphysiological quantities of superoxide. Simultaneously, ischemia depletes cellular ADP in favor of ATP and AMP through the action of adenylate kinase. Upon reperfusion, the combination of increased oxygen availability and lack of available ADP further increases electron leakage and ultimately results in generation of additional ROS (30, 97).
ROS have been implicated in causing a wide range of deleterious effects in the myocardium (42, 99, 191). During acute MI, exposure of cardiac fibroblasts and infiltrating neutrophils to excess ROS results in the activation of ASK1 mitogen-activated protein (MAP) kinase kinase kinase and p38 MAP kinase within these cells (29, 37, 76). These pathways lead to downstream activation of the master transcription factor nuclear factor-κB (NF-κB) and directs production of TNF-α (76, 196). Additionally, ROS triggers cardiac mast cell degranulation in the perivascular space to release large amounts of TNF-α and histamines (57, 93). TNF-α induces further ROS production through mitochondrial uncoupling and amplifies NF-κB activation (73). TNF-α also influences production of other proinflammatory cytokines, such as IL-1β and IL-6, to help sustain the inflammatory response after MI (31, 36, 62, 71, 88).
Danger Signals Facilitates Innate Immune Response to Myocardial Infarction
Necrotic cells within infarcting myocardium release specific molecules, known as damage-associated molecular patterns (DAMPs), into the extracellular matrix and bloodstream that serve as danger signals to activate the host innate immune system (119). These ligands consist of various nucleic acids, ECM components, and intracellular proteins that are recognized by a family of evolutionarily conserved pattern recognition receptors known as Toll-like receptors (TLRs) (89). Major DAMPs released after MI include heat shock proteins (HSPs) 60 and 70, high mobility group box 1 protein (HMGB1), ECM fragments (consisting of hyaluronic acid and fibronectin), mitochondrial DNA (mtDNA), and messenger RNA (mRNA). HSPs, HMGB1 and ECM fragments are recognized by circulating TLR2 and TLR4,while mtDNA and mRNA are recognized by TLR9 and TLR3, respectively (40, 131, 169). Activation of TLRs result in recruitment of the adaptor protein myeloid-differentiation primary response protein 88 (MyD88), which facilitates a proinflammatory response through activation of NF-κB (1, 9). Furthermore, danger signals, such as IL-1α released by necrotic cardiomyocytes have also been shown to directly activate MyD88 in cardiac fibroblasts, independent of TLR signaling (109). This results in the release of proinflammatory cytokines, including TNF-α and IL-1β, which facilitate recruitment and activation of myeloid cells within the infarct site (33, 183, 193).
Neutrophils and Monocytes are Recruited to Acutely Infarcted Myocardium
The first cellular mediators of inflammation to arrive in the infarcted myocardium are neutrophils. NF-κB activation and release of complement pathway fragment C5a after myocardial infarction result in the synthesis of various CCL and CXCL family chemokines (56). CXCL family chemokines, in particular CXCL8, have been shown facilitate recruitment of neutrophils to the infarcted myocardium (24). As the prototypic neutrophil chemotactic factor, CXCL8 binds the CXCR1 and CXCR2 surface receptors to recruit neutrophils along its chemotactic gradient (8). Simultaneously, endothelial activation results in upregulation of key leukocyte adhesion molecules, including ICAM-1, P-selectin, and E-selectin (135). Neutrophil L-selectin recognizes these adhesion molecules and facilitates neutrophil rolling and transmigration through junctional adhesion molecule family proteins at the infarct site (189, 190). This process guides the characteristic pattern of neutrophil infiltration seen 6 to 8 h after an acute MI.
Despite the large numbers of neutrophils recruited, they do not appear to be the primary inflammatory mediator of post-MI repair. Recent evidence suggests that monocytes serve as central participants in this process, and neutrophils play an important role in facilitating their recruitment (23). Proinflammatory classical monocytes are recruited early after MI by CCL family chemokines CCL2 and CCL7 in a CCR2-dependent fashion (41). Once recruited, these monocytes propagate the inflammatory response through ongoing production of IL-1β and TNF-α and give rise to proinflammatory M1 macrophages, which clear cellular debris and damaged ECM through phagocytosis and proteolysis (51, 152).
Neurohormonal Signaling Sustains Monocyte Response after Myocardial Infarction
Monocytes are produced in steady state from bone marrow hematopoietic progenitor cells in response to macrophage colony-stimulating factor. More than half of the body’s undifferentiated monocytes are stored in reserve within the subcapsular red pulp of the spleen and released in response to acute myocardial injury (181). While CCL2 and CCL7 signaling is sufficient for recruitment of circulating and bone marrow monocytes, splenic monocyte mobilization appears to depend on the combination of CCR2 and ANG II signaling (101, 170, 184). Circulating ANG II levels are increased after MI through activation of the renin-angiotensin-aldosterone system. This occurs as a result of both direct renin release from peri-infarct zones of the myocardium, as well as secondary signaling from a sudden decrease in myocardial contractility and cardiac output (38, 49). This, in part, facilitates the release of splenic classical proinflammatory monocytes, which are rapidly recruited to infarcted myocardium (102).
Splenic monocyte reserves are rapidly depleted after acute MI, and ongoing monocyte production is maintained, in part, through sympathoadrenergic signaling. Myocardial ischemia increases myocardial interstitial and serum catecholamine levels through both local reflex sympathetic release of norepinephrine and systemic release of epinephrine and norepinephrine, resulting in a net increase in vascular tone (96, 158). These catecholamines signal the bone marrow niche cells to release hematopoietic stem cells and progenitor cells into circulation and sustain monocyte production within the spleen (50). Classical proinflammatory monocytes are subsequently released into circulation to both maintain and augment the inflammatory response within the postinfarct myocardium (148).
Monocytes Facilitate Myocardial Healing and Fibrosis
Monocyte recruitment to the infarcted myocardium is biphasic, with classical proinflammatory monocytes being the predominant monocyte subset recruited within the first 48 h (125, 150). By 5 days postinfarction, however, nonclassical anti-inflammatory monocytes (Ly-6Clo in mouse, CD14+CD16++ in humans) and macrophages begin to dominate the peri-infarct regions (132). These cells arrive through both differentiation of classical monocytes into nonclassical macrophages and CX3CR1-dependent recruitment of circulating nonclassical monocytes (74). They are critical for the reparative stage following acute MI and secrete prohealing cytokines, including transforming growth factor-β (TGF-β), IL-10, and vascular endothelial growth factor (92, 132, 173). These factors facilitate the transdifferentiation of cardiac fibroblasts into α-smooth muscle actin-expressing myofibroblasts, degradation of damaged ECM, deposition of collagen for initiation of scar formation, and stabilization of the infarct through release of fibronectin (51). This remodeling helps to stiffen the myocardium and prevent potential rupture that may result from ongoing dilation (194).
Mediators of Inflammation May Be Cytoprotective after Myocardial Injury
Many studies have shown that both NF-κB and TNF-α signaling can have cardioprotective effects in the setting of acute MI. Activation of NF-κB occurs through the proteasomal degradation of its inhibitor, inhibitor of κBα (IκBα). IκB kinase (IKK) phosphorylates the signal response domain of IκBα in response to stimuli-driven signaling and leads to its ubiquitination (118). The β-subunit of IKK, IKKβ, has been shown to promote cardiomyocyte survival in hypoxic conditions through repression of the proapoptotic Bcl-2 family protein BNIP3 (11, 146, 159). Furthermore, in vivo studies have shown that TNF-α can have contradicting effects on the postinfarct myocardium through different signaling pathways: activation of TNF receptor 1 (TNFR1) appears to be cardiotoxic, while activation of TNF receptor 2 (TNFR2) appears to be cardioprotective (198). Finally, other innate immune mediators, such as TLR signaling, can also help maintain myocardial contractility and prevent cardiomyocyte apoptosis in the setting of acute MI (25, 35, 199).
PROLONGED INFLAMMATION IS DETRIMENTAL TO THE POSTINFARCT MYOCARDIUM
The acute loss of contractile myocardium associated with MI creates a change in ventricular loading conditions to promote ventricular dilatation, cardiomyocyte hypertrophy, and fibrosis (139, 167). However, research on the relationship between infarct size and left ventricular remodeling has identified a group of patients with adverse remodeling that seems out of proportion to their initial infarct size (157, 188). Instead, there is increasing evidence to suggest inflammation is not limited to the infarcted myocardium, and systemic imbalances in the postinfarct inflammatory cascade can exacerbate adverse remodeling beyond the infarct site (98, 126). Clinically, subsets of patients with higher degrees of inflammation, as measured by serum C-reactive protein levels, appeared to be at higher risk for mechanical complications, ventricular aneurysms, and mortality (6, 7).
A number of studies have examined the role of proinflammatory cytokines in adverse ventricular remodeling (133, 134, 137). TNF-α, in particular, has been shown to induce cardiomyocyte apoptosis and subsequent adverse ventricular remodeling in the infarcted heart (43, 44, 45, 86). Sustained TNF-α signaling depletes cytoprotective proteins for both the intrinsic and extrinsic apoptosis pathways within cardiomyocytes. Additionally, its interaction with cardiomyocyte TNFR1 can directly promote cell death through recruitment of TNFR1-associated DEATH domain protein (TRADD) and receptor-interacting serine/threonine-protein kinase 1 (RIP1) (72, 124). TRADD and RIP1 form complexes with TNF receptor-associated factor 2 and/or Fas-associated protein with death domain to, in turn, activate JNKs and inhibit cellular FLICE-inhibitory protein (c-FLIP) (3). JNK signaling promotes generalized proteolysis and caspase-independent cell death, while inhibition of c-FLIP activates caspase 8 to trigger caspase-dependent cell death (114). This ultimately results in further loss of cardiomyocytes with worsening ventricular loading conditions and, consequently, increased degrees of adverse remodeling (175).
TNF-α also has profound effects on ECM remodeling after MI. Collagen, as the chief constituent of myocardial ECM, provides structural support to cells in the cardiac microenvironment (52, 53). Postinfarct, TNF-α activates myocardial matrix metalloproteinases (MMPs) and dysregulates tissue inhibitors of MMPs to breakdown extracellular collagen (106, 162). This results in wall thinning with increased ventricular compliance and contributes to adverse ventricular remodelling after MI (18). Animal models of TNF-α inhibition have shown promise in reducing adverse ventricular remodeling through downregulation of MMP-9 and MMP-13 (17, 79). Further efforts are under way to modulate TNF-α and other proinflammatory signaling pathways in hopes of reducing morbidity and mortality after acute MI (136).
MESENCHYMAL STEM CELLS AND IMMUNOMODULATION FOR THE POSTINFARCT HEART
MSCs refer to a set of plastic-adherent multipotent stem cells located in adult tissues (e.g., bone marrow) that are self-renewing and can give rise to multiple mesoderm lineages. These cells are easy to isolate in large quantities, to culture in vitro, and have capacity to differentiate into adipocytes, osteoblasts, and chondrocytes (66). These unique properties have led to tremendous interest in their use for cardiac regeneration. Multiple animal and human studies have been conducted with the goal of using MSCs to replace cardiomyocytes lost during acute myocardial injury (161).
MSCs Differentiate into Cardiomyocytes In Vitro
The in vitro differentiation of MSCs into beating cardiomyocytes was first described by Makino et al. in 1999 (112). These early experiences used cytidine analogs such as 5-azacytidine (5-aza) to induce nonspecific DNA demethylation (20). Spontaneously beating cells were identified by direct observation and demonstrated cardiomyocyte-like structure, protein expression, and action potentials. These cells also express increased amounts of connexin 43 and have been shown in murine models to increase overall connexin 43 expression within infarcted myocardium (5, 103). Transplantation of these treated MSCs in a rat myocardial injury model also demonstrated reductions in scar area at 5 wk after transplantation (87, 176). However, safety concerns regarding the use of 5-aza have led to studies looking for alternative methods of differentiation MSCs into cardiomyocytes. Current efforts are under way to explore the use of microRNAs, growth factors, cytokines, and three-dimentional microenvironments to induce cardiomyocyte differentiation of MSCs (69).
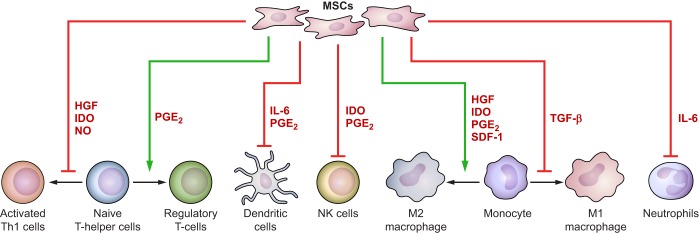
Despite this enthusiasm, it was subsequently observed that MSCs implanted into infarct sites do not stably engraft and are rapidly lost from the myocardium (70, 78, 104). Furthermore, very little, if any, in vivo differentiation to cardiomyocytes has been observed in transplanted MSCs (100, 144). Nevertheless, multiple studies have shown that MSC therapy improves ventricular function, reduces adverse remodeling, and improves functional status in animals and patients after acute MI (28). This benefit appears to be secondary to the nonprogenitor functions of MSCs, namely, paracrine signaling and cell-cell interactions (22, 64, 82, 85, 140). These functions allow implanted MSCs to alter the host immune response and promote endogenous tissue healing after MI to drive improved patient outcomes (Fig. 2).
Fig. 2.
Mesenchymal stem cells (MSCs) exert paracrine effects on cellular mediators of inflammation. MSCs secrete large numbers paracrine signaling molecules that affect the maturation, proliferation, and activity of various cellular mediators of inflammation. When delivered after an acute myocardial infarction, these functions help to accelerate the transition from inflammation to infarct healing.
Benefits of MSC Therapy Do Not Rely on Myocardial Delivery
Although early MSC studies relied on intracoronary or intramyocardial injection, MSCs have also been delivered in animal studies and human trials through peripheral intravenous infusion. In support of their paracrine functions, the efficacy of MSC therapy does not depend on engraftment of cells to the myocardium (94, 108). Several studies have shown that despite lower infarct zone engraftment and higher pulmonary retention of MSCs with intravenous injection relative to intramyocardial implantation, improvements in left ventricular ejection fraction (LVEF) were seen with both (59). This further supports that MSC injection may affect the systemic inflammatory process that follows an acute MI rather than function directly as progenitor cells. Nevertheless, a meta-analysis published by Kanelidis et al. (84) showed that intramyocardial injection of MSCs through catheter-based transendocardial stem cell injection offered additional benefits over intravenous infusion, suggesting that their presence within the myocardium may offer additional benefits over their systemic immunomodulatory functions.
MSCs Are Activated by the Inflammatory Niche
Because of their low level expression of major histocompatibility complex (MHC) class I and nonexpression of MHC class II molecules, MSCs are generally considered to be immune evasive (4). They require an inflammatory niche to activate their immunomodulatory effects on lymphocyte activation, proliferation, and differentiation (91). In vivo, MSCs home preferentially to infarcted myocardium after acute MI and are subsequently activated within the postinfarct inflammatory environment (127, 143). High concentrations of proinflammatory cytokines, such as INF-γ, TNF-α, IL-1α, and IL-1β work synergistically to activate MSCs and polarize them toward immunosuppressive phenotypes (105, 147). Recent evidence also suggests that cell-mediated cytotoxicity toward MSCs may further induce their immunomodulatory properties (63). Efforts are currently being made to preactivate MSCs in vitro to optimize their in vivo effects after therapeutic infusion (26, 142).
MSCs Participate in Paracrine Signaling to Improve Outcomes after Myocardial Infarction
Paracrine signaling by MSCs is achieved through secretion of soluble factors, including TGF-β, hepatocyte growth factor (HGF), stromal cell-derived factor-1, nitric oxide, heme oxygenase-1, IL-6, PGE2 and indoleamine 2,3-dioxygenase (IDO) (19, 65, 110, 177). These factors facilitate the immunomodulatory effects of MSCs by suppressing the recruitment, activation, and proliferation of proinflammatory lymphocytes and promoting production of immunomodulatory regulatory T cells. They also play a key role in polarizing macrophages toward the reparative M2 phenotype and inhibiting maturation of dendritic cells. Additionally, recent studies have shown that MSCs also secrete exosomes to influence immune cells and cardiomyocytes (90, 115). When introduced after a myocardial infarction, these MSC-secreted factors act synergistically to decrease proinflammatory activation, reduce cardiomyocyte apoptosis, and improve postinfarction angiogenesis within the infarcted tissue (185). A summary of the immunomodulatory effects of key MSC-secreted paracrine mediators is presented in Table 1.
Table 1.
Key immunomodulatory paracrine factors secreted by MSCs
| Factor | Function | References |
|---|---|---|
| HGF | Induction of immunomodulatory monocytes and macrophages Suppression of proinflammatory CD4+ T cells |
(27, 46, 61, 83, 151) |
| HO-1 | Decreases production of proinflammatory cytokines | (171, 197) |
| IDO | Inhibits T-cell and NK-cell activation and proliferation Promotes M2 macrophage polarization |
(55, 121, 166) |
| IL-6 | Pleiotropic effects on immune cells Induces secretion of PGE2 Inhibits dendritic cell maturation Inhibits neutrophil and lymphocyte apoptosis |
(16, 48, 145, 192) |
| NO | Inhibits T-cell proliferation | (54, 155) |
| PGE2 | Enhances inhibitory function of regulatory T cells Inhibits NK-cell activation and proliferation Inhibits dendritic cell maturation Promotes M2 macrophage polarization |
(12, 165, 166, 182) |
| SDF-1 | Recruitment of M2 macrophages Recruitment of progenitor cells |
(21, 179) |
| TGF-β | Decreases leukocyte adhesion and migration Reduces macrophage secretion of proinflammatory cytokines |
(67, 163, 187) |
HGF, hepatocyte growth factor; HO-1, heme oxygenase-1; IDO, indoleamine 2,3-dioxygenase; IL-6, interleukin-6; MSCs, mesenchymal stem cells; NO, nitric oxide; PGE2, prostaglandin E2; SDF-1, stromal cell-derived factor-1; TGF-β, transforming growth factor-β.
MSCs Affect Leukocyte Activation, Proliferation, and Maturation
In addition to their systemic secretory functions, transplanted MSCs have been shown to interact with both innate and adaptive immune system leukocytes to facilitate targeted immunosuppression (160). Their main actions in the innate immune system involve monocyte/macrophage trafficking and polarization. In one study, MSCs have been shown to reduce mobilization of classical proinflammatory monocytes from the spleen through reduction of ventricular CCL-2 expression during acute myocardial inflammation (123). Other studies have shown that MSC infusion after MI reduces the proportion of proinflammatory M1 macrophages within the infarcted myocardium and simultaneously drive polarization of monocytes toward the alternatively activated M2 state (39). This reduces the amount of proinflammatory cytokines (including TNF-α and IL-1β) within the infarcted myocardium. The presence of M2 macrophages further produces soluble factors, including TGF-β, IL-10, HGF, PGE2, and IDO to suppress the proinflammatory state (111). Taken together, these findings suggest that MSC administration after MI can accelerate the transition from inflammation to infarct healing.
Activated MSCs have also been shown to suppress the adaptive immune system. They secrete high levels of chemokines to attract T cells, B cells, macrophages, and dendritic cells, and can influence them through contact-mediated signaling and locally secreted factors (147). Through a series of studies, MSCs have been shown to express Fas ligand (FasL) to directly trigger T-cell apoptosis via the Fas/FasL pathway, as well as facilitate inhibition of T-cell proliferation through the programmed death 1 pathway (2, 10). Though the role of adaptive immunity in MI is unclear, autoreactivity may play a role in progression of adverse remodeling and progression of heart failure (156). More detailed studies are required to understand the postinfarct interplay between the innate and adaptive immune systems.
MSC Therapy Show Benefits in Human Clinical Trials
One of the early randomized controlled trials of MSC therapy in humans was reported by Chen et al. in 2004 (28). They randomized 69 patients presenting with acute MI to receive PCI with or without intracoronary injection of autologous bone marrow MSCs. The MSCs were delivered at an average of 18 days after the initial PCI intervention, and patients who received MSCs had an average 14% improvement in LVEF at 6 mo (P < 0.01) relative to control patients. Smaller infarct sizes on PET perfusion imaging and decreased LV adverse remodeling by LV volume measurements on echocardiography were also observed. These promising findings have led to multitudes of other studies to assess the safety and efficacy of MSC therapy in humans.
The safety of MSC therapy for acute MI was specifically explored in a meta-analysis published by Lalu et al. (95) in 2018. By looking across 11 human MSC studies (with a total of 509 patients) for acute MI and 12 studies (with 639 patients) for ischemic heart failure, they summarized that there were no associations between MSC therapy and acute adverse events relative to the control groups. They did find an unexplained increased risk of delayed neurologic adverse events with MSC administration (odds ratio 3.79, P < 0.05), although they simultaneously noted limitations in the interpretation of this signal due to the lack of detailed adverse event reporting in original trials. Overall, MSC therapy significantly improved LVEF of patients included in this meta-analysis and especially within the acute MI subcohort. This suggests that MSC therapy may offer favorable risk-benefit profiles for the secondary treatment of acute myocardial infarction.
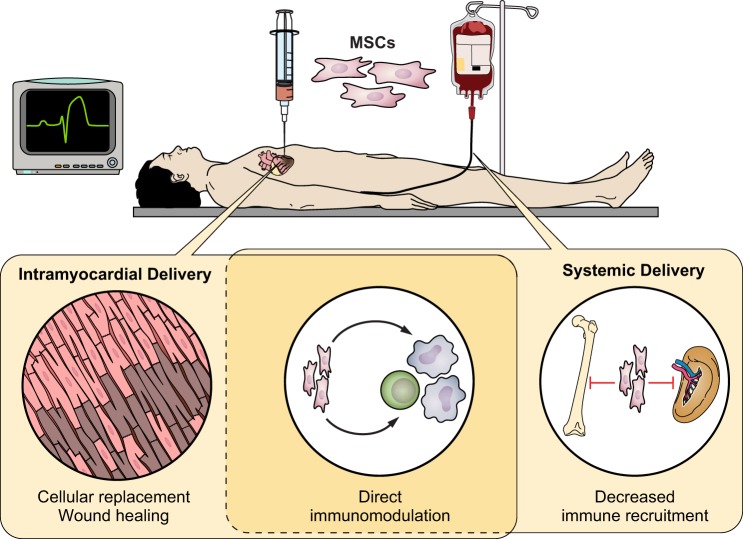
In a separate meta-analysis, Jeong et al. (81) explored the efficacy of MSC therapy in ischemic heart disease in 14 published randomized placebo-controlled trials encompassing 950 patients. They noted that MSC treatment improves LVEF by 3.84% (95% CI 2.32–5.35) relative to the control group with corresponding decreases in adverse remodeling reflected in reduced LV end-systolic volume and end-diastolic volumes. These benefits persisted out to 2 yr from time of MSC implantation. Furthermore, MSC therapy improved patient function with improved scores on the 6-min walk test at 6 mo. Nonsignificant trends were also seen in favor of MSC-treated patients for reduced mortality and heart failure rehospitalization. These findings echo other findings and suggest that MSC therapy is beneficial, although larger and more rigorous trials are needed to quantify its safety and efficacy (Fig. 3).
Fig. 3.
The role of mesenchymal stem cells for treatment of acute myocardial infarction. Intramyocardial or systemic intravenous delivery of mesenchymal stem cells after an acute myocardial infection can promote myocardial healing through multiple mechanisms. Early in vitro evidence has shown that MSCs may have some capacity to differentiate into cardiomyocytes and replenish cells lost with myocardial injury. Further evidence has shown that MSCs participate in paracrine signaling to prevent cardiomyocyte apoptosis, decrease recruitment of proinflammatory cells, and drive monocytes to take on an anti-inflammatory prohealing phenotype over the classical proinflammatory phenotype. These properties have resulted in MSCs offering promise of reduced left ventricular adverse remodeling and improved patient functional status in early human clinical trials.
Conclusion
The inflammatory reaction following an acute myocardial infarction is a “double-edged sword” that results in both beneficial and detrimental effects. The acute postinfarct inflammation is necessary to clear away debris and to initiate healing with scar formation. However, persistent immune activation can worsen myocardial damage and drive adverse cardiac remodeling. Immunomodulatory therapy with MSCs may hold promise as a treatment option. This novel therapy can help attenuate the severity of inflammation and polarize mediators of inflammation toward a reparative phenotype. Early clinical experiences have suggested both safety and efficacy with its use for ischemic heart disease. Further laboratory studies are necessary to understand the detailed cellular and molecular interplay between MSCs, cardiomyocytes, and the immune system after myocardial infarction, and larger clinical studies are needed to better characterize the full risk-benefit profile of its use as a viable therapeutic option.
DISCLOSURES
R. C. Arora has received an unrestricted educational grant from Pfizer Canada, Inc., and honoraria from Mallinckrodt Pharmaceuticals for work unrelated to this work. All authors declare that there are no conflicts of interest, financial or otherwise, related to this work.
AUTHOR CONTRIBUTIONS
W.Y., E.A.-ER., S.S., and S.D. conceived and designed research; W.Y. prepared figures; W.Y., E.A.-ER., S.S., and S.D. drafted manuscript; L.A.K., R.C.A., and S.D. edited and revised manuscript; and W.Y., E.A.-ER., S.S., L.A.K., R.C.A., and S.D. approved final version of manuscript.
ACKNOWLEDGMENTS
This work was supported by Canadian Institutes of Health Research Grant MOP142265 (to S. Dhingra).
REFERENCES
- 1.Akira S, Hoshino K. Myeloid differentiation factor 88-dependent and -independent pathways in Toll-like receptor signaling. J Infect Dis 187, Suppl 2: S356–S363, 2003. doi: 10.1086/374749. [DOI] [PubMed] [Google Scholar]
- 2.Akiyama K, Chen C, Wang D, Xu X, Qu C, Yamaza T, Cai T, Chen W, Sun L, Shi S. Mesenchymal-stem-cell-induced immunoregulation involves FAS-ligand-/FAS-mediated T-cell apoptosis. Cell Stem Cell 10: 544–555, 2012. doi: 10.1016/j.stem.2012.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amgalan D, Chen Y, Kitsis RN. Death receptor signaling in the heart: cell survival, apoptosis, and necroptosis. Circulation 136: 743–746, 2017. doi: 10.1161/CIRCULATIONAHA.117.029566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ankrum JA, Ong JF, Karp JM. Mesenchymal stem cells: immune evasive, not immune privileged. Nat Biotechnol 32: 252–260, 2014. doi: 10.1038/nbt.2816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Antonitsis P, Ioannidou-Papagiannaki E, Kaidoglou A, Papakonstantinou C. In vitro cardiomyogenic differentiation of adult human bone marrow mesenchymal stem cells. The role of 5-azacytidine. Interact Cardiovasc Thorac Surg 6: 593–597, 2007. doi: 10.1510/icvts.2007.157875. [DOI] [PubMed] [Google Scholar]
- 6.Anzai T. Post-infarction inflammation and left ventricular remodeling: a double-edged sword. Circ J 77: 580–587, 2013. doi: 10.1253/circj.CJ-13-0013. [DOI] [PubMed] [Google Scholar]
- 7.Anzai T, Yoshikawa T, Shiraki H, Asakura Y, Akaishi M, Mitamura H, Ogawa S. C-reactive protein as a predictor of infarct expansion and cardiac rupture after a first Q-wave acute myocardial infarction. Circulation 96: 778–784, 1997. doi: 10.1161/01.CIR.96.3.778. [DOI] [PubMed] [Google Scholar]
- 8.Apostolakis S, Vogiatzi K, Amanatidou V, Spandidos DA. Interleukin 8 and cardiovascular disease. Cardiovasc Res 84: 353–360, 2009. doi: 10.1093/cvr/cvp241. [DOI] [PubMed] [Google Scholar]
- 9.Arslan F, de Kleijn DP, Pasterkamp G. Innate immune signaling in cardiac ischemia. Nat Rev Cardiol 8: 292–300, 2011. doi: 10.1038/nrcardio.2011.38. [DOI] [PubMed] [Google Scholar]
- 10.Augello A, Tasso R, Negrini SM, Amateis A, Indiveri F, Cancedda R, Pennesi G. Bone marrow mesenchymal progenitor cells inhibit lymphocyte proliferation by activation of the programmed death 1 pathway. Eur J Immunol 35: 1482–1490, 2005. doi: 10.1002/eji.200425405. [DOI] [PubMed] [Google Scholar]
- 11.Baetz D, Regula KM, Ens K, Shaw J, Kothari S, Yurkova N, Kirshenbaum LA. Nuclear factor-κB-mediated cell survival involves transcriptional silencing of the mitochondrial death gene BNIP3 in ventricular myocytes. Circulation 112: 3777–3785, 2005. doi: 10.1161/CIRCULATIONAHA.105.573899. [DOI] [PubMed] [Google Scholar]
- 12.Baratelli F, Lin Y, Zhu L, Yang SC, Heuzé-Vourc’h N, Zeng G, Reckamp K, Dohadwala M, Sharma S, Dubinett SM. Prostaglandin E2 induces FOXP3 gene expression and T regulatory cell function in human CD4+ T cells. J Immunol 175: 1483–1490, 2005. doi: 10.4049/jimmunol.175.3.1483. [DOI] [PubMed] [Google Scholar]
- 13.Bavia L, Lidani KC, Andrade FA, Sobrinho MI, Nisihara RM, de Messias-Reason IJ. Complement activation in acute myocardial infarction: An early marker of inflammation and tissue injury? Immunol Lett 200: 18–25, 2018. doi: 10.1016/j.imlet.2018.06.006. [DOI] [PubMed] [Google Scholar]
- 14.Bolli R, Marbán E. Molecular and cellular mechanisms of myocardial stunning. Physiol Rev 79: 609–634, 1999. doi: 10.1152/physrev.1999.79.2.609. [DOI] [PubMed] [Google Scholar]
- 15.Bonvini RF, Hendiri T, Camenzind E. Inflammatory response post-myocardial infarction and reperfusion: A new therapeutic target? Eur Heart J 7, Suppl: I27–I36, 2005. doi: 10.1093/eurheartj/sui077. [DOI] [Google Scholar]
- 16.Bouffi C, Bony C, Courties G, Jorgensen C, Noël D. IL-6-dependent PGE2 secretion by mesenchymal stem cells inhibits local inflammation in experimental arthritis. PLoS One 5: e14247, 2010. doi: 10.1371/journal.pone.0014247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bradham WS, Moe G, Wendt KA, Scott AA, Konig A, Romanova M, Naik G, Spinale FG. TNF-α and myocardial matrix metalloproteinases in heart failure: relationship to LV remodeling. Am J Physiol Heart Circ Physiol 282: H1288–H1295, 2002. doi: 10.1152/ajpheart.00526.2001. [DOI] [PubMed] [Google Scholar]
- 18.Brower GL, Gardner JD, Forman MF, Murray DB, Voloshenyuk T, Levick SP, Janicki JS. The relationship between myocardial extracellular matrix remodeling and ventricular function. Eur J Cardiothorac Surg 30: 604–610, 2006. doi: 10.1016/j.ejcts.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 19.Bujak M, Frangogiannis NG. The role of TGF-β signaling in myocardial infarction and cardiac remodeling. Cardiovasc Res 74: 184–195, 2007. doi: 10.1016/j.cardiores.2006.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Burlacu A, Rosca AM, Maniu H, Titorencu I, Dragan E, Jinga V, Simionescu M. Promoting effect of 5-azacytidine on the myogenic differentiation of bone marrow stromal cells. Eur J Cell Biol 87: 173–184, 2008. doi: 10.1016/j.ejcb.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 21.Cao X, Han ZB, Zhao H, Liu Q. Transplantation of mesenchymal stem cells recruits trophic macrophages to induce pancreatic β-cell regeneration in diabetic mice. Int J Biochem Cell Biol 53: 372–379, 2014. doi: 10.1016/j.biocel.2014.06.003. [DOI] [PubMed] [Google Scholar]
- 22.Caplan AI. Mesenchymal stem cells: time to change the name! Stem Cells Transl Med 6: 1445–1451, 2017. doi: 10.1002/sctm.17-0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carbone F, Nencioni A, Mach F, Vuilleumier N, Montecucco F. Pathophysiological role of neutrophils in acute myocardial infarction. Thromb Haemost 110: 501–514, 2013. doi: 10.1160/TH13-03-0211. [DOI] [PubMed] [Google Scholar]
- 24.Cavalera M, Frangogiannis NG. Targeting the chemokines in cardiac repair. Curr Pharm Des 20: 1971–1979, 2014. doi: 10.2174/13816128113199990449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chao W. Toll-like receptor signaling: a critical modulator of cell survival and ischemic injury in the heart. Am J Physiol Heart Circ Physiol 296: H1–H12, 2009. doi: 10.1152/ajpheart.00995.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen H, Min XH, Wang QY, Leung FW, Shi L, Zhou Y, Yu T, Wang CM, An G, Sha WH, Chen QK. Pre-activation of mesenchymal stem cells with TNF-α, IL-1β, and nitric oxide enhances its paracrine effects on radiation-induced intestinal injury. Sci Rep 5: 8718, 2015. doi: 10.1038/srep08718. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 27.Chen PM, Liu KJ, Hsu PJ, Wei CF, Bai CH, Ho LJ, Sytwu HK, Yen BL. Induction of immunomodulatory monocytes by human mesenchymal stem cell-derived hepatocyte growth factor through ERK1/2. J Leukoc Biol 96: 295–303, 2014. doi: 10.1189/jlb.3A0513-242R. [DOI] [PubMed] [Google Scholar]
- 28.Chen SL, Fang WW, Ye F, Liu YH, Qian J, Shan SJ, Zhang JJ, Chunhua RZ, Liao LM, Lin S, Sun JP. Effect on left ventricular function of intracoronary transplantation of autologous bone marrow mesenchymal stem cell in patients with acute myocardial infarction. Am J Cardiol 94: 92–95, 2004. doi: 10.1016/j.amjcard.2004.03.034. [DOI] [PubMed] [Google Scholar]
- 29.Chen W, Frangogiannis NG. Fibroblasts in post-infarction inflammation and cardiac repair. Biochim Biophys Acta 1833: 945–953, 2013. doi: 10.1016/j.bbamcr.2012.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen YR, Zweier JL. Cardiac mitochondria and reactive oxygen species generation. Circ Res 114: 524–537, 2014. doi: 10.1161/CIRCRESAHA.114.300559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen Y, Pat B, Zheng J, Cain L, Powell P, Shi K, Sabri A, Husain A, Dell’italia LJ. Tumor necrosis factor-α produced in cardiomyocytes mediates a predominant myocardial inflammatory response to stretch in early volume overload. J Mol Cell Cardiol 49: 70–78, 2010. doi: 10.1016/j.yjmcc.2009.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chiong M, Wang ZV, Pedrozo Z, Cao DJ, Troncoso R, Ibacache M, Criollo A, Nemchenko A, Hill JA, Lavandero S. Cardiomyocyte death: mechanisms and translational implications. Cell Death Dis 2: e244, 2011. doi: 10.1038/cddis.2011.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chong AJ, Shimamoto A, Hampton CR, Takayama H, Spring DJ, Rothnie CL, Yada M, Pohlman TH, Verrier ED. Toll-like receptor 4 mediates ischemia/reperfusion injury of the heart. J Thorac Cardiovasc Surg 128: 170–179, 2004. doi: 10.1016/j.jtcvs.2003.11.036. [DOI] [PubMed] [Google Scholar]
- 34.Christia P, Frangogiannis NG. Targeting inflammatory pathways in myocardial infarction. Eur J Clin Invest 43: 986–995, 2013. doi: 10.1111/eci.12118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Coggins M, Rosenzweig A. The fire within: cardiac inflammatory signaling in health and disease. Circ Res 110: 116–125, 2012. doi: 10.1161/CIRCRESAHA.111.243196. [DOI] [PubMed] [Google Scholar]
- 36.Craig R, Larkin A, Mingo AM, Thuerauf DJ, Andrews C, McDonough PM, Glembotski CC. p38 MAPK and NF-κ B collaborate to induce interleukin-6 gene expression and release. Evidence for a cytoprotective autocrine signaling pathway in a cardiac myocyte model system. J Biol Chem 275: 23814–23824, 2000. doi: 10.1074/jbc.M909695199. [DOI] [PubMed] [Google Scholar]
- 37.Cuenda A, Rousseau S. p38 MAP-kinases pathway regulation, function and role in human diseases. Biochim Biophys Acta 1773: 1358–1375, 2007. doi: 10.1016/j.bbamcr.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 38.Dargie HJ, Byrne J. Pathophysiological aspects of the renin-angiotensin-aldosterone system in acute myocardial infarction. J Cardiovasc Risk 2: 389–395, 1995. doi: 10.1177/174182679500200502. [DOI] [PubMed] [Google Scholar]
- 39.Dayan V, Yannarelli G, Billia F, Filomeno P, Wang XH, Davies JE, Keating A. Mesenchymal stromal cells mediate a switch to alternatively activated monocytes/macrophages after acute myocardial infarction. Basic Res Cardiol 106: 1299–1310, 2011. doi: 10.1007/s00395-011-0221-9. [DOI] [PubMed] [Google Scholar]
- 40.de Haan JJ, Smeets MB, Pasterkamp G, Arslan F. Danger signals in the initiation of the inflammatory response after myocardial infarction. Mediators Inflamm 2013: 206039, 2013. doi: 10.1155/2013/206039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dewald O, Zymek P, Winkelmann K, Koerting A, Ren G, Abou-Khamis T, Michael LH, Rollins BJ, Entman ML, Frangogiannis NG. CCL2/monocyte chemoattractant protein-1 regulates inflammatory responses critical to healing myocardial infarcts. Circ Res 96: 881–889, 2005. doi: 10.1161/01.RES.0000163017.13772.3a. [DOI] [PubMed] [Google Scholar]
- 42.Dhalla NS, Elmoselhi AB, Hata T, Makino N. Status of myocardial antioxidants in ischemia-reperfusion injury. Cardiovasc Res 47: 446–456, 2000. doi: 10.1016/S0008-6363(00)00078-X. [DOI] [PubMed] [Google Scholar]
- 43.Dhingra S, Bagchi AK, Ludke AL, Sharma AK, Singal PK. Akt regulates IL-10-mediated suppression of TNFα-induced cardiomyocyte apoptosis by upregulating Stat3 phosphorylation. PLoS One 6: e25009, 2011. doi: 10.1371/journal.pone.0025009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dhingra S, Sharma AK, Arora RC, Slezak J, Singal PK. IL-10 attenuates TNF-α-induced NF-κB pathway activation and cardiomyocyte apoptosis. Cardiovasc Res 82: 59–66, 2009. doi: 10.1093/cvr/cvp040. [DOI] [PubMed] [Google Scholar]
- 45.Dhingra S, Sharma AK, Singla DK, Singal PK. p38 and ERK1/2 MAPKs mediate the interplay of TNF-α and IL-10 in regulating oxidative stress and cardiac myocyte apoptosis. Am J Physiol Heart Circ Physiol 293: H3524–H3531, 2007. doi: 10.1152/ajpheart.00919.2007. [DOI] [PubMed] [Google Scholar]
- 46.Di Nicola M, Carlo-Stella C, Magni M, Milanesi M, Longoni PD, Matteucci P, Grisanti S, Gianni AM. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood 99: 3838–3843, 2002. doi: 10.1182/blood.V99.10.3838. [DOI] [PubMed] [Google Scholar]
- 47.Distelmaier K, Adlbrecht C, Jakowitsch J, Winkler S, Dunkler D, Gerner C, Wagner O, Lang IM, Kubicek M. Local complement activation triggers neutrophil recruitment to the site of thrombus formation in acute myocardial infarction. Thromb Haemost 102: 564–572, 2009. doi: 10.1160/TH09-02-0103. [DOI] [PubMed] [Google Scholar]
- 48.Djouad F, Charbonnier LM, Bouffi C, Louis-Plence P, Bony C, Apparailly F, Cantos C, Jorgensen C, Noël D. Mesenchymal stem cells inhibit the differentiation of dendritic cells through an interleukin-6-dependent mechanism. Stem Cells 25: 2025–2032, 2007. doi: 10.1634/stemcells.2006-0548. [DOI] [PubMed] [Google Scholar]
- 49.Dostal DE, Baker KM. The cardiac renin-angiotensin system: conceptual, or a regulator of cardiac function? Circ Res 85: 643–650, 1999. doi: 10.1161/01.RES.85.7.643. [DOI] [PubMed] [Google Scholar]
- 50.Dutta P, Courties G, Wei Y, Leuschner F, Gorbatov R, Robbins CS, Iwamoto Y, Thompson B, Carlson AL, Heidt T, Majmudar MD, Lasitschka F, Etzrodt M, Waterman P, Waring MT, Chicoine AT, van der Laan AM, Niessen HW, Piek JJ, Rubin BB, Butany J, Stone JR, Katus HA, Murphy SA, Morrow DA, Sabatine MS, Vinegoni C, Moskowitz MA, Pittet MJ, Libby P, Lin CP, Swirski FK, Weissleder R, Nahrendorf M. Myocardial infarction accelerates atherosclerosis. Nature 487: 325–329, 2012. doi: 10.1038/nature11260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dutta P, Nahrendorf M. Monocytes in myocardial infarction. Arterioscler Thromb Vasc Biol 35: 1066–1070, 2015. doi: 10.1161/ATVBAHA.114.304652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Eghbali M, Blumenfeld OO, Seifter S, Buttrick PM, Leinwand LA, Robinson TF, Zern MA, Giambrone MA. Localization of types I, III and IV collagen mRNAs in rat heart cells by in situ hybridization. J Mol Cell Cardiol 21: 103–113, 1989. doi: 10.1016/0022-2828(89)91498-3. [DOI] [PubMed] [Google Scholar]
- 53.Fedak PW, Verma S, Weisel RD, Li RK. Cardiac remodeling and failure: from molecules to man (Part II). Cardiovasc Pathol 14: 49–60, 2005. doi: 10.1016/j.carpath.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 54.Fiorucci S, Mencarelli A, Distrutti E, Baldoni M, del Soldato P, Morelli A. Nitric oxide regulates immune cell bioenergetic: a mechanism to understand immunomodulatory functions of nitric oxide-releasing anti-inflammatory drugs. J Immunol 173: 874–882, 2004. doi: 10.4049/jimmunol.173.2.874. [DOI] [PubMed] [Google Scholar]
- 55.François M, Romieu-Mourez R, Li M, Galipeau J. Human MSC suppression correlates with cytokine induction of indoleamine 2,3-dioxygenase and bystander M2 macrophage differentiation. Mol Ther 20: 187–195, 2012. doi: 10.1038/mt.2011.189. [DOI] [PubMed] [Google Scholar]
- 56.Frangogiannis NG. Chemokines in ischemia and reperfusion. Thromb Haemost 97: 738–747, 2007. doi: 10.1160/TH07-01-0022. [DOI] [PubMed] [Google Scholar]
- 57.Frangogiannis NG, Lindsey ML, Michael LH, Youker KA, Bressler RB, Mendoza LH, Spengler RN, Smith CW, Entman ML. Resident cardiac mast cells degranulate and release preformed TNF-α, initiating the cytokine cascade in experimental canine myocardial ischemia/reperfusion. Circulation 98: 699–710, 1998. doi: 10.1161/01.CIR.98.7.699. [DOI] [PubMed] [Google Scholar]
- 58.Frangogiannis NG, Smith CW, Entman ML. The inflammatory response in myocardial infarction. Cardiovasc Res 53: 31–47, 2002. doi: 10.1016/S0008-6363(01)00434-5. [DOI] [PubMed] [Google Scholar]
- 59.Freyman T, Polin G, Osman H, Crary J, Lu M, Cheng L, Palasis M, Wilensky RL. A quantitative, randomized study evaluating three methods of mesenchymal stem cell delivery following myocardial infarction. Eur Heart J 27: 1114–1122, 2006. doi: 10.1093/eurheartj/ehi818. [DOI] [PubMed] [Google Scholar]
- 60.Frodermann V, Nahrendorf M. Neutrophil-macrophage cross-talk in acute myocardial infarction. Eur Heart J 38: 198–200, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Futamatsu H, Suzuki J, Mizuno S, Koga N, Adachi S, Kosuge H, Maejima Y, Hirao K, Nakamura T, Isobe M. Hepatocyte growth factor ameliorates the progression of experimental autoimmune myocarditis: a potential role for induction of T helper 2 cytokines. Circ Res 96: 823–830, 2005. doi: 10.1161/01.RES.0000163016.52653.2e. [DOI] [PubMed] [Google Scholar]
- 62.Gabriel AS, Martinsson A, Wretlind B, Ahnve S. IL-6 levels in acute and post myocardial infarction: their relation to CRP levels, infarction size, left ventricular systolic function, and heart failure. Eur J Intern Med 15: 523–528, 2004. doi: 10.1016/j.ejim.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 63.Galleu A, Riffo-Vasquez Y, Trento C, Lomas C, Dolcetti L, Cheung TS, Von Bonin M, Barbieri L, Halai K, Ward S, Weng L, Chakraverty R, Lombardi G, Watt FM, Orchard K, Marks DI, Apperley J, Bornhauser M, Walczak H, Bennett C, Dazzi F. Apoptosis in mesenchymal stromal cells induces in vivo recipient-mediated immunomodulation. Sci Transl Med 9: eaam7828, 2017. doi: 10.1126/scitranslmed.aam7828. [DOI] [PubMed] [Google Scholar]
- 64.Gallina C, Turinetto V, Giachino C. A new paradigm in cardiac regeneration: the mesenchymal stem cell secretome. Stem Cells Int 2015: 765846, 2015. doi: 10.1155/2015/765846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gallo S, Sala V, Gatti S, Crepaldi T. HGF/met axis in heart function and cardioprotection. Biomedicines 2: 247–262, 2014. doi: 10.3390/biomedicines2040247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gao F, Chiu SM, Motan DA, Zhang Z, Chen L, Ji HL, Tse HF, Fu QL, Lian Q. Mesenchymal stem cells and immunomodulation: current status and future prospects. Cell Death Dis 7: e2062, 2016. doi: 10.1038/cddis.2015.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Groh ME, Maitra B, Szekely E, Koç ON. Human mesenchymal stem cells require monocyte-mediated activation to suppress alloreactive T cells. Exp Hematol 33: 928–934, 2005. doi: 10.1016/j.exphem.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 68.Guo J, Lin GS, Bao CY, Hu ZM, Hu MY. Anti-inflammation role for mesenchymal stem cells transplantation in myocardial infarction. Inflammation 30: 97–104, 2007. doi: 10.1007/s10753-007-9025-3. [DOI] [PubMed] [Google Scholar]
- 69.Guo X, Bai Y, Zhang L, Zhang B, Zagidullin N, Carvalho K, Du Z, Cai B. Cardiomyocyte differentiation of mesenchymal stem cells from bone marrow: new regulators and its implications. Stem Cell Res Ther 9: 44, 2018. doi: 10.1186/s13287-018-0773-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gyöngyösi M, Blanco J, Marian T, Trón L, Petneházy O, Petrasi Z, Hemetsberger R, Rodriguez J, Font G, Pavo IJ, Kertész I, Balkay L, Pavo N, Posa A, Emri M, Galuska L, Kraitchman DL, Wojta J, Huber K, Glogar D. Serial noninvasive in vivo positron emission tomographic tracking of percutaneously intramyocardially injected autologous porcine mesenchymal stem cells modified for transgene reporter gene expression. Circ Cardiovasc Imaging 1: 94–103, 2008. doi: 10.1161/CIRCIMAGING.108.797449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hamid T, Guo SZ, Kingery JR, Xiang X, Dawn B, Prabhu SD. Cardiomyocyte NF-κB p65 promotes adverse remodelling, apoptosis, and endoplasmic reticulum stress in heart failure. Cardiovasc Res 89: 129–138, 2011. doi: 10.1093/cvr/cvq274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Haudek SB, Taffet GE, Schneider MD, Mann DL. TNF provokes cardiomyocyte apoptosis and cardiac remodeling through activation of multiple cell death pathways. J Clin Invest 117: 2692–2701, 2007. doi: 10.1172/JCI29134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Higuchi Y, Otsu K, Nishida K, Hirotani S, Nakayama H, Yamaguchi O, Matsumura Y, Ueno H, Tada M, Hori M. Involvement of reactive oxygen species-mediated NF-κB activation in TNF-α-induced cardiomyocyte hypertrophy. J Mol Cell Cardiol 34: 233–240, 2002. doi: 10.1006/jmcc.2001.1505. [DOI] [PubMed] [Google Scholar]
- 74.Hilgendorf I, Gerhardt LM, Tan TC, Winter C, Holderried TA, Chousterman BG, Iwamoto Y, Liao R, Zirlik A, Scherer-Crosbie M, Hedrick CC, Libby P, Nahrendorf M, Weissleder R, Swirski FK. Ly-6C high monocytes depend on Nr4a1 to balance both inflammatory and reparative phases in the infarcted myocardium. Circ Res 114: 1611–1622, 2014. doi: 10.1161/CIRCRESAHA.114.303204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hill JH, Ward PA. The phlogistic role of C3 leukotactic fragments in myocardial infarcts of rats. J Exp Med 133: 885–900, 1971. doi: 10.1084/jem.133.4.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hori M, Nishida K. Oxidative stress and left ventricular remodelling after myocardial infarction. Cardiovasc Res 81: 457–464, 2009. doi: 10.1093/cvr/cvn335. [DOI] [PubMed] [Google Scholar]
- 77.Ibanez B, James S, Agewall S, Antunes MJ, Bucciarelli-Ducci C, Bueno H, Caforio AL, Crea F, Goudevenos JA, Halvorsen S, Hindricks G, Kastrati A, Lenzen MJ, Prescott E, Roffi M, Valgimigli M, Varenhorst C, Vranckx P, Widimský P, ESC Scientific Document Group . 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation; The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J 39: 119–177, 2018. doi: 10.1093/eurheartj/ehx393. [DOI] [PubMed] [Google Scholar]
- 78.Iso Y, Spees JL, Serrano C, Bakondi B, Pochampally R, Song YH, Sobel BE, Delafontaine P, Prockop DJ. Multipotent human stromal cells improve cardiac function after myocardial infarction in mice without long-term engraftment. Biochem Biophys Res Commun 354: 700–706, 2007. doi: 10.1016/j.bbrc.2007.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Iyer RP, Jung M, Lindsey ML. MMP-9 signaling in the left ventricle following myocardial infarction. Am J Physiol Heart Circ Physiol 311: H190–H198, 2016. doi: 10.1152/ajpheart.00243.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Janse MJ, Cinca J, Moréna H, Fiolet JW, Kléber AG, de Vries GP, Becker AE, Durrer D. The “border zone” in myocardial ischemia. An electrophysiological, metabolic, and histochemical correlation in the pig heart. Circ Res 44: 576–588, 1979. doi: 10.1161/01.RES.44.4.576. [DOI] [PubMed] [Google Scholar]
- 81.Jeong H, Yim HW, Park HJ, Cho Y, Hong H, Kim NJ, Oh IH. Mesenchymal stem cell therapy for ischemic heart disease: Systematic review and meta-analysis. Int J Stem Cells 11: 1–12, 2018. doi: 10.15283/ijsc17061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jiang Y, Jahagirdar BN, Reinhardt RL, Schwartz RE, Keene CD, Ortiz-Gonzalez XR, Reyes M, Lenvik T, Lund T, Blackstad M, Du J, Aldrich S, Lisberg A, Low WC, Largaespada DA, Verfaillie CM. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature 418: 41–49, 2002. doi: 10.1038/nature00870. [DOI] [PubMed] [Google Scholar]
- 83.Kamimoto M, Mizuno S, Matsumoto K, Nakamura T. Hepatocyte growth factor prevents multiple organ injuries in endotoxemic mice through a heme oxygenase-1-dependent mechanism. Biochem Biophys Res Commun 380: 333–337, 2009. doi: 10.1016/j.bbrc.2009.01.080. [DOI] [PubMed] [Google Scholar]
- 84.Kanelidis AJ, Premer C, Lopez J, Balkan W, Hare JM. Route of delivery modulates the efficacy of mesenchymal stem cell therapy for myocardial infarction: a meta-analysis of preclinical studies and clinical trials. Circ Res 120: 1139–1150, 2017. doi: 10.1161/CIRCRESAHA.116.309819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Karantalis V, Hare JM. Use of mesenchymal stem cells for therapy of cardiac disease. Circ Res 116: 1413–1430, 2015. doi: 10.1161/CIRCRESAHA.116.303614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kaur K, Sharma AK, Singal PK. Significance of changes in TNF-α and IL-10 levels in the progression of heart failure subsequent to myocardial infarction. Am J Physiol Heart Circ Physiol 291: H106–H113, 2006. doi: 10.1152/ajpheart.01327.2005. [DOI] [PubMed] [Google Scholar]
- 87.Kawada H, Fujita J, Kinjo K, Matsuzaki Y, Tsuma M, Miyatake H, Muguruma Y, Tsuboi K, Itabashi Y, Ikeda Y, Ogawa S, Okano H, Hotta T, Ando K, Fukuda K. Nonhematopoietic mesenchymal stem cells can be mobilized and differentiate into cardiomyocytes after myocardial infarction. Blood 104: 3581–3587, 2004. doi: 10.1182/blood-2004-04-1488. [DOI] [PubMed] [Google Scholar]
- 88.Kawaguchi M, Takahashi M, Hata T, Kashima Y, Usui F, Morimoto H, Izawa A, Takahashi Y, Masumoto J, Koyama J, Hongo M, Noda T, Nakayama J, Sagara J, Taniguchi S, Ikeda U. Inflammasome activation of cardiac fibroblasts is essential for myocardial ischemia/reperfusion injury. Circulation 123: 594–604, 2011. doi: 10.1161/CIRCULATIONAHA.110.982777. [DOI] [PubMed] [Google Scholar]
- 89.Kawasaki T, Kawai T. Toll-like receptor signaling pathways. Front Immunol 5: 461, 2014. doi: 10.3389/fimmu.2014.00461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kordelas L, Rebmann V, Ludwig AK, Radtke S, Ruesing J, Doeppner TR, Epple M, Horn PA, Beelen DW, Giebel B. MSC-derived exosomes: a novel tool to treat therapy-refractory graft-versus-host disease. Leukemia 28: 970–973, 2014. doi: 10.1038/leu.2014.41. [DOI] [PubMed] [Google Scholar]
- 91.Krampera M. Mesenchymal stromal cell ‘licensing’: a multistep process. Leukemia 25: 1408–1414, 2011. doi: 10.1038/leu.2011.108. [DOI] [PubMed] [Google Scholar]
- 92.Kratofil RM, Kubes P, Deniset JF. Monocyte conversion during inflammation and injury. Arterioscler Thromb Vasc Biol 37: 35–42, 2017. doi: 10.1161/ATVBAHA.116.308198. [DOI] [PubMed] [Google Scholar]
- 93.Krystel-Whittemore M, Dileepan KN, Wood JG. Mast cell: a multi-functional master cell. Front Immunol 6: 620, 2016. doi: 10.3389/fimmu.2015.00620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kurtz A. Mesenchymal stem cell delivery routes and fate. Int J Stem Cells 1: 1–7, 2008. doi: 10.15283/ijsc.2008.1.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lalu MM, Mazzarello S, Zlepnig J, Dong YYR, Montroy J, McIntyre L, Devereaux PJ, Stewart DJ, David Mazer C, Barron CC, McIsaac DI, Fergusson DA. Safety and efficacy of adult stem cell therapy for acute myocardial infarction and ischemic heart failure (SafeCell Heart): a systematic review and meta-analysis. Stem Cells Transl Med 7: 857–866, 2018. doi: 10.1002/sctm.18-0120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lameris TW, de Zeeuw S, Alberts G, Boomsma F, Duncker DJ, Verdouw PD, Veld AJ, van Den Meiracker AH. Time course and mechanism of myocardial catecholamine release during transient ischemia in vivo. Circulation 101: 2645–2650, 2000. doi: 10.1161/01.CIR.101.22.2645. [DOI] [PubMed] [Google Scholar]
- 97.Lasley RD, Ely SW, Berne RM, Mentzer RM Jr. Allopurinol enhanced adenine nucleotide repletion after myocardial ischemia in the isolated rat heart. J Clin Invest 81: 16–20, 1988. doi: 10.1172/JCI113288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lee WW, Marinelli B, van der Laan AM, Sena BF, Gorbatov R, Leuschner F, Dutta P, Iwamoto Y, Ueno T, Begieneman MP, Niessen HW, Piek JJ, Vinegoni C, Pittet MJ, Swirski FK, Tawakol A, Di Carli M, Weissleder R, Nahrendorf M. PET/MRI of inflammation in myocardial infarction. J Am Coll Cardiol 59: 153–163, 2012. doi: 10.1016/j.jacc.2011.08.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lefer DJ, Granger DN. Oxidative stress and cardiac disease. Am J Med 109: 315–323, 2000. doi: 10.1016/S0002-9343(00)00467-8. [DOI] [PubMed] [Google Scholar]
- 100.Leiker M, Suzuki G, Iyer VS, Canty JM Jr, Lee T. Assessment of a nuclear affinity labeling method for tracking implanted mesenchymal stem cells. Cell Transplant 17: 911–922, 2008. doi: 10.3727/096368908786576444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Leuschner F, Dutta P, Gorbatov R, Novobrantseva TI, Donahoe JS, Courties G, Lee KM, Kim JI, Markmann JF, Marinelli B, Panizzi P, Lee WW, Iwamoto Y, Milstein S, Epstein-Barash H, Cantley W, Wong J, Cortez-Retamozo V, Newton A, Love K, Libby P, Pittet MJ, Swirski FK, Koteliansky V, Langer R, Weissleder R, Anderson DG, Nahrendorf M. Therapeutic siRNA silencing in inflammatory monocytes in mice. Nat Biotechnol 29: 1005–1010, 2011. doi: 10.1038/nbt.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Leuschner F, Panizzi P, Chico-Calero I, Lee WW, Ueno T, Cortez-Retamozo V, Waterman P, Gorbatov R, Marinelli B, Iwamoto Y, Chudnovskiy A, Figueiredo JL, Sosnovik DE, Pittet MJ, Swirski FK, Weissleder R, Nahrendorf M. Angiotensin-converting enzyme inhibition prevents the release of monocytes from their splenic reservoir in mice with myocardial infarction. Circ Res 107: 1364–1373, 2010. doi: 10.1161/CIRCRESAHA.110.227454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Li JY, Ke HH, He Y, Wen LN, Xu WY, Wu ZF, Zhao YM, Zhong GQ. Transplantation of mesenchymal stem cells modulated Cx43 and Cx45 expression in rats with myocardial infarction. Cytotechnology 70: 225–234, 2018. doi: 10.1007/s10616-017-0136-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Li L, Chen X, Wang WE, Zeng C. How to improve the survival of transplanted mesenchymal stem cell in ischemic heart? Stem Cells Int 2016: 9682757, 2016. doi: 10.1155/2016/9682757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Li W, Ren G, Huang Y, Su J, Han Y, Li J, Chen X, Cao K, Chen Q, Shou P, Zhang L, Yuan Z-R, Roberts AI, Shi S, Le AD, Shi Y. Mesenchymal stem cells: a double-edged sword in regulating immune responses. Cell Death Differ 19: 1505–1513, 2012. doi: 10.1038/cdd.2012.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Li YY, Feng YQ, Kadokami T, McTiernan CF, Draviam R, Watkins SC, Feldman AM. Myocardial extracellular matrix remodeling in transgenic mice overexpressing tumor necrosis factor-α can be modulated by anti-tumor necrosis factor α therapy. Proc Natl Acad Sci USA 97: 12746–12751, 2000. doi: 10.1073/pnas.97.23.12746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Liu J, Wang H, Li J. Inflammation and inflammatory cells in myocardial infarction and reperfusion injury: A double-edged sword. Clin Med Insights Cardiol 10: 79–84, 2016. doi: 10.4137/CMC.S33164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Luger D, Lipinski MJ, Westman PC, Glover DK, Dimastromatteo J, Frias JC, Albelda MT, Sikora S, Kharazi A, Vertelov G, Waksman R, Epstein SE. Intravenously delivered mesenchymal stem cells: systemic anti-inflammatory effects improve left ventricular dysfunction in acute myocardial infarction and ischemicc. Circ Res 120: 1598–1613, 2017. doi: 10.1161/CIRCRESAHA.117.310599. [DOI] [PubMed] [Google Scholar]
- 109.Lugrin J, Parapanov R, Rosenblatt-Velin N, Rignault-Clerc S, Feihl F, Waeber B, Müller O, Vergely C, Zeller M, Tardivel A, Schneider P, Pacher P, Liaudet L. Cutting edge: IL-1α is a crucial danger signal triggering acute myocardial inflammation during myocardial infarction. J Immunol 194: 499–503, 2015. doi: 10.4049/jimmunol.1401948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ma S, Xie N, Li W, Yuan B, Shi Y, Wang Y. Immunobiology of mesenchymal stem cells. Cell Death Differ 21: 216–225, 2014. doi: 10.1038/cdd.2013.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Majka M, Sułkowski M, Badyra B, Musiałek P. Concise review: mesenchymal stem cells in cardiovascular regeneration: emerging research directions and clinical applications. Stem Cells Transl Med 6: 1859–1867, 2017. doi: 10.1002/sctm.16-0484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Makino S, Fukuda K, Miyoshi S, Konishi F, Kodama H, Pan J, Sano M, Takahashi T, Hori S, Abe H, Hata J, Umezawa A, Ogawa S. Cardiomyocytes can be generated from marrow stromal cells in vitro. J Clin Invest 103: 697–705, 1999. doi: 10.1172/JCI5298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Marchant DJ, Boyd JH, Lin DC, Granville DJ, Garmaroudi FS, McManus BM. Inflammation in myocardial diseases. Circ Res 110: 126–144, 2012. doi: 10.1161/CIRCRESAHA.111.243170. [DOI] [PubMed] [Google Scholar]
- 114.Mariño G, Niso-Santano M, Baehrecke EH, Kroemer G. Self-consumption: the interplay of autophagy and apoptosis. Nat Rev Mol Cell Biol 15: 81–94, 2014. doi: 10.1038/nrm3735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Marote A, Teixeira FG, Mendes-Pinheiro B, Salgado AJ. MSCs-derived exosomes: Cell-secreted nanovesicles with regenerative potential. Front Pharmacol 7: 231, 2016. doi: 10.3389/fphar.2016.00231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Marx N, Neumann FJ, Ott I, Gawaz M, Koch W, Pinkau T, Schömig A. Induction of cytokine expression in leukocytes in acute myocardial infarction. J Am Coll Cardiol 30: 165–170, 1997. doi: 10.1016/S0735-1097(97)00116-2. [DOI] [PubMed] [Google Scholar]
- 117.Mathers C, Stevens GA, Mahanani WR, Fat DM, Hogan D. WHO Methods and Data Sources for Country-Level Causes of Death 2000–2016. Geneva, Switzerland: World Health Organization, 2018. [Google Scholar]
- 118.Mathes E, O’Dea EL, Hoffmann A, Ghosh G. NF-κB dictates the degradation pathway of IκBα. EMBO J 27: 1357–1367, 2008. doi: 10.1038/emboj.2008.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Matzinger P. The danger model: a renewed sense of self. Science 296: 301–305, 2002. doi: 10.1126/science.1071059. [DOI] [PubMed] [Google Scholar]
- 120.Mehta JL, Li DY. Inflammation in ischemic heart disease: response to tissue injury or a pathogenetic villain? Cardiovasc Res 43: 291–299, 1999. doi: 10.1016/S0008-6363(99)00132-7. [DOI] [PubMed] [Google Scholar]
- 121.Meisel R, Zibert A, Laryea M, Göbel U, Däubener W, Dilloo D. Human bone marrow stromal cells inhibit allogeneic T-cell responses by indoleamine 2,3-dioxygenase-mediated tryptophan degradation. Blood 103: 4619–4621, 2004. doi: 10.1182/blood-2003-11-3909. [DOI] [PubMed] [Google Scholar]
- 122.Melamed KH, Goldhaber SZ. Inflammation and myocardial infarction. Circulation 130: e334–e336, 2014. doi: 10.1161/CIRCULATIONAHA.114.010614. [DOI] [PubMed] [Google Scholar]
- 123.Miteva K, Pappritz K, El-Shafeey M, Dong F, Ringe J, Tschöpe C, Van Linthout S. Mesenchymal stromal cells modulate monocytes trafficking in coxsackievirus B3-induced myocarditis. Stem Cells Transl Med 6: 1249–1261, 2017. doi: 10.1002/sctm.16-0353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Monden Y, Kubota T, Inoue T, Tsutsumi T, Kawano S, Ide T, Tsutsui H, Sunagawa K. Tumor necrosis factor-α is toxic via receptor 1 and protective via receptor 2 in a murine model of myocardial infarction. Am J Physiol Heart Circ Physiol 293: H743–H753, 2007. doi: 10.1152/ajpheart.00166.2007. [DOI] [PubMed] [Google Scholar]
- 125.Mouton AJ, DeLeon-Pennell KY, Rivera Gonzalez OJ, Flynn ER, Freeman TC, Saucerman JJ, Garrett MR, Ma Y, Harmancey R, Lindsey ML. Mapping macrophage polarization over the myocardial infarction time continuum. Basic Res Cardiol 113: 26, 2018. doi: 10.1007/s00395-018-0686-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Mouton AJ, Rivera OJ, Lindsey ML. Myocardial infarction remodeling that progresses to heart failure: a signaling misunderstanding. Am J Physiol Heart Circ Physiol 315: H71–H79, 2018. doi: 10.1152/ajpheart.00131.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Nagaya N, Fujii T, Iwase T, Ohgushi H, Itoh T, Uematsu M, Yamagishi M, Mori H, Kangawa K, Kitamura S. Intravenous administration of mesenchymal stem cells improves cardiac function in rats with acute myocardial infarction through angiogenesis and myogenesis. Am J Physiol Heart Circ Physiol 287: H2670–H2676, 2004. doi: 10.1152/ajpheart.01071.2003. [DOI] [PubMed] [Google Scholar]
- 128.Nahrendorf M, Pittet MJ, Swirski FK. Monocytes: protagonists of infarct inflammation and repair after myocardial infarction. Circulation 121: 2437–2445, 2010. doi: 10.1161/CIRCULATIONAHA.109.916346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Nakagawa T, Shimizu S, Watanabe T, Yamaguchi O, Otsu K, Yamagata H, Inohara H, Kubo T, Tsujimoto Y. Cyclophilin D-dependent mitochondrial permeability transition regulates some necrotic but not apoptotic cell death. Nature 434: 652–658, 2005. doi: 10.1038/nature03317. [DOI] [PubMed] [Google Scholar]
- 130.O’Gara PT, Kushner FG, Ascheim DD, Casey DE Jr, Chung MK, de Lemos JA, Ettinger SM, Fang JC, Fesmire FM, Franklin BA, Granger CB, Krumholz HM, Linderbaum JA, Morrow DA, Newby LK, Ornato JP, Ou N, Radford MJ, Tamis-Holland JE, Tommaso CL, Tracy CM, Woo YJ, Zhao DX, Anderson JL, Jacobs AK, Halperin JL, Albert NM, Brindis RG, Creager MA, DeMets D, Guyton RA, Hochman JS, Kovacs RJ, Kushner FG, Ohman EM, Stevenson WG, Yancy CW; American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines . 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 127: e362–e425, 2013. doi: 10.1161/CIR.0b013e3182742c84. [DOI] [PubMed] [Google Scholar]
- 131.Oka T, Hikoso S, Yamaguchi O, Taneike M, Takeda T, Tamai T, Oyabu J, Murakawa T, Nakayama H, Nishida K, Akira S, Yamamoto A, Komuro I, Otsu K. Mitochondrial DNA that escapes from autophagy causes inflammation and heart failure. Nature 485: 251–255, 2012. [Erratum in Nature 490: 292, 2012]. doi: 10.1038/nature10992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ong SB, Hernández-Reséndiz S, Crespo-Avilan GE, Mukhametshina RT, Kwek XY, Cabrera-Fuentes HA, Hausenloy DJ. Inflammation following acute myocardial infarction: multiple players, dynamic roles, and novel therapeutic opportunities. Pharmacol Ther 186: 73–87, 2018. doi: 10.1016/j.pharmthera.2018.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ono K, Matsumori A, Shioi T, Furukawa Y, Sasayama S. Cytokine gene expression after myocardial infarction in rat hearts: possible implication in left ventricular remodeling. Circulation 98: 149–156, 1998. doi: 10.1161/01.CIR.98.2.149. [DOI] [PubMed] [Google Scholar]
- 134.Oral H, Sivasubramanian N, Dyke DB, Mehta RH, Grossman PM, Briesmiester K, Fay WP, Pagani FD, Bolling SF, Mann DL, Starling MR. Myocardial proinflammatory cytokine expression and left ventricular remodeling in patients with chronic mitral regurgitation. Circulation 107: 831–837, 2003. doi: 10.1161/01.CIR.0000049745.38594.6D. [DOI] [PubMed] [Google Scholar]
- 135.Palazzo AJ, Jones SP, Anderson DC, Granger DN, Lefer DJ. Coronary endothelial P-selectin in pathogenesis of myocardial ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol 275: H1865–H1872, 1998. doi: 10.1152/ajpheart.1998.275.5.H1865. [DOI] [PubMed] [Google Scholar]
- 136.Panahi M, Papanikolaou A, Torabi A, Zhang JG, Khan H, Vazir A, Hasham MG, Cleland JGF, Rosenthal NA, Harding SE, Sattler S. Immunomodulatory interventions in myocardial infarction and heart failure: a systematic review of clinical trials and meta-analysis of IL-1 inhibition. Cardiovasc Res 114: 1445–1461, 2018. doi: 10.1093/cvr/cvy145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Pasqui AL, Di Renzo M, Maffei S, Pastorelli M, Pompella G, Auteri A, Puccetti L. Pro/anti-inflammatory cytokine imbalance in postischemic left ventricular remodeling. Mediators Inflamm 2010: 974694, 2010. doi: 10.1155/2010/723589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Peng Y, Pan W, Ou Y, Xu W, Kaelber S, Borlongan CV, Sun M, Yu G. Extracardiac-lodged mesenchymal stromal cells propel an inflammatory response against myocardial infarction via paracrine effects. Cell Transplant 25: 929–935, 2016. doi: 10.3727/096368915X689758. [DOI] [PubMed] [Google Scholar]
- 139.Pfeffer MA. Left ventricular remodeling after acute myocardial infarction. Annu Rev Med 46: 455–466, 1995. doi: 10.1146/annurev.med.46.1.455. [DOI] [PubMed] [Google Scholar]
- 140.Phinney DG, Pittenger MF. Concise review: MSC-derived exosomes for cell-free therapy. Stem Cells 35: 851–858, 2017. doi: 10.1002/stem.2575. [DOI] [PubMed] [Google Scholar]
- 141.Pinckard RN, Olson MS, Giclas PC, Terry R, Boyer JT, O’Rourke RA. Consumption of classical complement components by heart subcellular membranes in vitro and in patients after acute myocardial infarction. J Clin Invest 56: 740–750, 1975. doi: 10.1172/JCI108145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Polchert D, Sobinsky J, Douglas G, Kidd M, Moadsiri A, Reina E, Genrich K, Mehrotra S, Setty S, Smith B, Bartholomew A. IFN-γ activation of mesenchymal stem cells for treatment and prevention of graft versus host disease. Eur J Immunol 38: 1745–1755, 2008. doi: 10.1002/eji.200738129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Ponte AL, Marais E, Gallay N, Langonné A, Delorme B, Hérault O, Charbord P, Domenech J. The in vitro migration capacity of human bone marrow mesenchymal stem cells: comparison of chemokine and growth factor chemotactic activities. Stem Cells 25: 1737–1745, 2007. doi: 10.1634/stemcells.2007-0054. [DOI] [PubMed] [Google Scholar]
- 144.Quinn C, Flake AW. In vivo differentiation potential of mesenchymal stem cells: Prenatal and postnatal model systems. Transfus Med Hemother 35: 239–247, 2008. doi: 10.1159/000129129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Raffaghello L, Bianchi G, Bertolotto M, Montecucco F, Busca A, Dallegri F, Ottonello L, Pistoia V. Human mesenchymal stem cells inhibit neutrophil apoptosis: a model for neutrophil preservation in the bone marrow niche. Stem Cells 26: 151–162, 2008. doi: 10.1634/stemcells.2007-0416. [DOI] [PubMed] [Google Scholar]
- 146.Regula KM, Baetz D, Kirshenbaum LA. Nuclear factor-κB represses hypoxia-induced mitochondrial defects and cell death of ventricular myocytes. Circulation 110: 3795–3802, 2004. doi: 10.1161/01.CIR.0000150537.59754.55. [DOI] [PubMed] [Google Scholar]
- 147.Ren G, Zhang L, Zhao X, Xu G, Zhang Y, Roberts AI, Zhao RC, Shi Y. Mesenchymal stem cell-mediated immunosuppression occurs via concerted action of chemokines and nitric oxide. Cell Stem Cell 2: 141–150, 2008. doi: 10.1016/j.stem.2007.11.014. [DOI] [PubMed] [Google Scholar]
- 148.Robbins CS, Chudnovskiy A, Rauch PJ, Figueiredo J-L, Iwamoto Y, Gorbatov R, Etzrodt M, Weber GF, Ueno T, van Rooijen N, Mulligan-Kehoe MJ, Libby P, Nahrendorf M, Pittet MJ, Weissleder R, Swirski FK. Extramedullary hematopoiesis generates Ly-6C(high) monocytes that infiltrate atherosclerotic lesions. Circulation 125: 364–374, 2012. doi: 10.1161/CIRCULATIONAHA.111.061986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Rossen RD, Michael LH, Hawkins HK, Youker K, Dreyer WJ, Baughn RE, Entman ML. Cardiolipin-protein complexes and initiation of complement activation after coronary artery occlusion. Circ Res 75: 546–555, 1994. doi: 10.1161/01.RES.75.3.546. [DOI] [PubMed] [Google Scholar]
- 150.Ruparelia N, Godec J, Lee R, Chai JT, Dall’Armellina E, McAndrew D, Digby JE, Forfar JC, Prendergast BD, Kharbanda RK, Banning AP, Neubauer S, Lygate CA, Channon KM, Haining NW, Choudhury RP. Acute myocardial infarction activates distinct inflammation and proliferation pathways in circulating monocytes, prior to recruitment, and identified through conserved transcriptional responses in mice and humans. Eur Heart J 36: 1923–1934, 2015. doi: 10.1093/eurheartj/ehv195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Rutella S, Bonanno G, Procoli A, Mariotti A, de Ritis DG, Curti A, Danese S, Pessina G, Pandolfi S, Natoni F, Di Febo A, Scambia G, Manfredini R, Salati S, Ferrari S, Pierelli L, Leone G, Lemoli RM. Hepatocyte growth factor favors monocyte differentiation into regulatory interleukin (IL)-102+IL-12low/neg accessory cells with dendritic-cell features. Blood 108: 218–227, 2006. doi: 10.1182/blood-2005-08-3141. [DOI] [PubMed] [Google Scholar]
- 152.Sager HB, Kessler T, Schunkert H. Monocytes and macrophages in cardiac injury and repair. J Thorac Dis 9, Suppl 1: S30–S35, 2017. doi: 10.21037/jtd.2016.11.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Samstad EO, Niyonzima N, Nymo S, Aune MH, Ryan L, Bakke SS, Lappegård KT, Brekke O-L, Lambris JD, Damås JK, Latz E, Mollnes TE, Espevik T. Cholesterol crystals induce complement-dependent inflammasome activation and cytokine release. J Immunol 192: 2837–2845, 2014. doi: 10.4049/jimmunol.1302484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Sanganalmath SK, Bolli R. Cell therapy for heart failure: a comprehensive overview of experimental and clinical studies, current challenges, and future directions. Circ Res 113: 810–834, 2013. doi: 10.1161/CIRCRESAHA.113.300219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Sato K, Ozaki K, Oh I, Meguro A, Hatanaka K, Nagai T, Muroi K, Ozawa K. Nitric oxide plays a critical role in suppression of T-cell proliferation by mesenchymal stem cells. Blood 109: 228–234, 2007. doi: 10.1182/blood-2006-02-002246. [DOI] [PubMed] [Google Scholar]
- 156.Sattler S, Fairchild P, Watt FM, Rosenthal N, Harding SE. The adaptive immune response to cardiac injury-the true roadblock to effective regenerative therapies? NPJ Regen Med 2: 19, 2017. doi: 10.1038/s41536-017-0022-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Schächinger V, Assmus B, Erbs S, Elsässer A, Haberbosch W, Hambrecht R, Yu J, Corti R, Mathey DG, Hamm CW, Tonn T, Dimmeler S, Zeiher AM; REPAIR-AMI investigators . Intracoronary infusion of bone marrow-derived mononuclear cells abrogates adverse left ventricular remodelling post-acute myocardial infarction: insights from the reinfusion of enriched progenitor cells and infarct remodelling in acute myocardial infarction (REPAIR-AMI) trial. Eur J Heart Fail 11: 973–979, 2009. doi: 10.1093/eurjhf/hfp113. [DOI] [PubMed] [Google Scholar]
- 158.Schömig A. Adrenergic mechanisms in myocardial infarction: cardiac and systemic catecholamine release. J Cardiovasc Pharmacol 12, Suppl 1: S1–S7, 1988. doi: 10.1097/00005344-198806121-00002. [DOI] [PubMed] [Google Scholar]
- 159.Shaw J, Zhang T, Rzeszutek M, Yurkova N, Baetz D, Davie JR, Kirshenbaum LA. Transcriptional silencing of the death gene BNIP3 by cooperative action of NF-κB and histone deacetylase 1 in ventricular myocytes. Circ Res 99: 1347–1354, 2006. doi: 10.1161/01.RES.0000251744.06138.50. [DOI] [PubMed] [Google Scholar]
- 160.Shi Y, Hu G, Su J, Li W, Chen Q, Shou P, Xu C, Chen X, Huang Y, Zhu Z, Huang X, Han X, Xie N, Ren G. Mesenchymal stem cells: a new strategy for immunosuppression and tissue repair. Cell Res 20: 510–518, 2010. doi: 10.1038/cr.2010.44. [DOI] [PubMed] [Google Scholar]
- 161.Singh A, Singh A, Sen D. Mesenchymal stem cells in cardiac regeneration: a detailed progress report of the last 6 years (2010-2015). Stem Cell Res Ther 7: 82, 2016. doi: 10.1186/s13287-016-0341-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Sivasubramanian N, Coker ML, Kurrelmeyer KM, MacLellan WR, DeMayo FJ, Spinale FG, Mann DL. Left ventricular remodeling in transgenic mice with cardiac restricted overexpression of tumor necrosis factor. Circulation 104: 826–831, 2001. doi: 10.1161/hc3401.093154. [DOI] [PubMed] [Google Scholar]
- 163.Smith WB, Noack L, Khew-Goodall Y, Isenmann S, Vadas MA, Gamble JR. Transforming growth factor-β1 inhibits the production of IL-8 and the transmigration of neutrophils through activated endothelium. J Immunol 157: 360–368, 1996. [PubMed] [Google Scholar]
- 164.Soehnlein O, Zernecke A, Eriksson EE, Rothfuchs AG, Pham CT, Herwald H, Bidzhekov K, Rottenberg ME, Weber C, Lindbom L. Neutrophil secretion products pave the way for inflammatory monocytes. Blood 112: 1461–1471, 2008. doi: 10.1182/blood-2008-02-139634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Spaggiari GM, Abdelrazik H, Becchetti F, Moretta L. MSCs inhibit monocyte-derived DC maturation and function by selectively interfering with the generation of immature DCs: central role of MSC-derived prostaglandin E2. Blood 113: 6576–6583, 2009. doi: 10.1182/blood-2009-02-203943. [DOI] [PubMed] [Google Scholar]
- 166.Spaggiari GM, Capobianco A, Abdelrazik H, Becchetti F, Mingari MC, Moretta L. Mesenchymal stem cells inhibit natural killer-cell proliferation, cytotoxicity, and cytokine production: role of indoleamine 2,3-dioxygenase and prostaglandin E2. Blood 111: 1327–1333, 2008. doi: 10.1182/blood-2007-02-074997. [DOI] [PubMed] [Google Scholar]
- 167.Sutton MG, Sharpe N. Left ventricular remodeling after myocardial infarction: pathophysiology and therapy. Circulation 101: 2981–2988, 2000. doi: 10.1161/01.CIR.101.25.2981. [DOI] [PubMed] [Google Scholar]
- 168.Sun Y. Myocardial repair/remodelling following infarction: roles of local factors. Cardiovasc Res 81: 482–490, 2009. doi: 10.1093/cvr/cvn333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Sun Y, Liu WZ, Liu T, Feng X, Yang N, Zhou HF. Role of Toll-like receptors in myocardial infarction. J Recept Signal Transduct Res 35: 420–422, 2015. doi: 10.3109/10799893.2014.993649. [DOI] [PubMed] [Google Scholar]
- 170.Swirski FK, Nahrendorf M, Etzrodt M, Wildgruber M, Cortez-Retamozo V, Panizzi P, Figueiredo JL, Kohler RH, Chudnovskiy A, Waterman P, Aikawa E, Mempel TR, Libby P, Weissleder R, Pittet MJ. Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science 325: 612–616, 2009. doi: 10.1126/science.1175202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Tang YL, Tang Y, Zhang YC, Qian K, Shen L, Phillips MI. Improved graft mesenchymal stem cell survival in ischemic heart with a hypoxia-regulated heme oxygenase-1 vector. J Am Coll Cardiol 46: 1339–1350, 2005. doi: 10.1016/j.jacc.2005.05.079. [DOI] [PubMed] [Google Scholar]
- 172.Terkelsen CJ, Christiansen EH, Sørensen JT, Kristensen SD, Lassen JF, Thuesen L, Andersen HR, Vach W, Nielsen TT. Primary PCI as the preferred reperfusion therapy in STEMI: it is a matter of time. Heart 95: 362–369, 2009. doi: 10.1136/hrt.2007.139493. [DOI] [PubMed] [Google Scholar]
- 173.Thomas G, Tacke R, Hedrick CC, Hanna RN. Nonclassical patrolling monocyte function in the vasculature. Arterioscler Thromb Vasc Biol 35: 1306–1316, 2015. doi: 10.1161/ATVBAHA.114.304650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Thygesen K, Alpert JS, White HD, Jaffe AS, Apple FS, Galvani M, Katus HA, Newby LK, Ravkilde J, Chaitman B, Clemmensen PM, Dellborg M, Hod H, Porela P, Underwood R, Bax JJ, Beller GA, Bonow R, Van Der Wall EE, Bassand JP, Wijns W, Ferguson TB, Steg PG, Uretsky BF, Williams DO, Armstrong PW, Antman EM, Fox KA, Hamm CW, Ohman EM, Simoons ML, Poole-Wilson PA, Gurfinkel EP, Lopez-Sendon J-L, Pais P, Mendis S, Zhu J-R, Wallentin LC, Fernandez-Aviles F, Fox KM, Parkhomenko AN, Priori SG, Tendera M, Voipio-Pulkki LM, Vahanian A, Camm AJ, De Caterina R, Dean V, Dickstein K, Filippatos G, Funck-Brentano C, Hellemans I, Kristensen SD, McGregor K, Sechtem U, Silber S, Tendera M, Widimsky P, Zamorano JL, Morais J, Brener S, Harrington R, Morrow D, Sechtem U, Lim M, Martinez-Rios MA, Steinhubl S, Levine GN, Gibler WB, Goff D, Tubaro M, Dudek D, Al-Attar N. Universal definition of myocardial infarction. Eur Heart J 28: 2525–2538, 2007. doi: 10.1093/eurheartj/ehm355. [DOI] [PubMed] [Google Scholar]
- 175.Tian M, Yuan YC, Li JY, Gionfriddo MR, Huang RC. Tumor necrosis factor-α and its role as a mediator in myocardial infarction: A brief review. Chronic Dis Transl Med 1: 18–26, 2015. doi: 10.1016/j.cdtm.2015.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Tomita S, Li RK, Weisel RD, Mickle DA, Kim EJ, Sakai T, Jia ZQ. Autologous transplantation of bone marrow cells improves damaged heart function. Circulation 100, Suppl: II247–II256, 1999. doi: 10.1161/01.CIR.100.suppl_2.II-247. [DOI] [PubMed] [Google Scholar]
- 177.Tompkins BA, Balkan W, Winkler J, Gyöngyösi M, Goliasch G, Fernández-Avilés F, Hare JM. Preclinical studies of stem cell therapy for heart disease. Circ Res 122: 1006–1020, 2018. doi: 10.1161/CIRCRESAHA.117.312486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Turillazzi E, Di Paolo M, Neri M, Riezzo I, Fineschi V. A theoretical timeline for myocardial infarction: immunohistochemical evaluation and Western blot quantification for interleukin-15 and monocyte chemotactic protein-1 as very early markers. J Transl Med 12: 188, 2014. doi: 10.1186/1479-5876-12-188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Unzek S, Zhang M, Mal N, Mills WR, Laurita KR, Penn MS. SDF-1 recruits cardiac stem cell-like cells that depolarize in vivo. Cell Transplant 16: 879–886, 2007. doi: 10.3727/096368907783338271. [DOI] [PubMed] [Google Scholar]
- 180.Väkevä A, Morgan BP, Tikkanen I, Helin K, Laurila P, Meri S. Time course of complement activation and inhibitor expression after ischemic injury of rat myocardium. Am J Pathol 144: 1357–1368, 1994. [PMC free article] [PubMed] [Google Scholar]
- 181.van der Laan AM, Ter Horst EN, Delewi R, Begieneman MP, Krijnen PA, Hirsch A, Lavaei M, Nahrendorf M, Horrevoets AJ, Niessen HW, Piek JJ. Monocyte subset accumulation in the human heart following acute myocardial infarction and the role of the spleen as monocyte reservoir. Eur Heart J 35: 376–385, 2014. doi: 10.1093/eurheartj/eht331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Vasandan AB, Jahnavi S, Shashank C, Prasad P, Kumar A, Prasanna SJ. Human Mesenchymal stem cells program macrophage plasticity by altering their metabolic status via a PGE2-dependent mechanism. Sci Rep 6: 38308, 2016. doi: 10.1038/srep38308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Vilahur G, Badimon L. Ischemia/reperfusion activates myocardial innate immune response: the key role of the toll-like receptor. Front Physiol 5: 496, 2014. doi: 10.3389/fphys.2014.00496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Wang NP, Erskine J, Zhang WW, Zheng RH, Zhang LH, Duron G, Gendreau J, Zhao ZQ. Recruitment of macrophages from the spleen contributes to myocardial fibrosis and hypertension induced by angiotensin II. J Renin Angiotensin Aldosterone Syst 18: 1470320317706653, 2017. doi: 10.1177/1470320317706653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Ward MR, Abadeh A, Connelly KA. Concise review: rational use of mesenchymal stem cells in the treatment of ischemic heart disease. Stem Cells Transl Med 7: 543–550, 2018. doi: 10.1002/sctm.17-0210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Webster KA. Mitochondrial membrane permeabilization and cell death during myocardial infarction: roles of calcium and reactive oxygen species. Future Cardiol 8: 863–884, 2012. doi: 10.2217/fca.12.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Werner F, Jain MK, Feinberg MW, Sibinga NE, Pellacani A, Wiesel P, Chin MT, Topper JN, Perrella MA, Lee ME. Transforming growth factor-β1 inhibition of macrophage activation is mediated via Smad3. J Biol Chem 275: 36653–36658, 2000. doi: 10.1074/jbc.M004536200. [DOI] [PubMed] [Google Scholar]
- 188.Westman PC, Lipinski MJ, Luger D, Waksman R, Bonow RO, Wu E, Epstein SE. Inflammation as a driver of adverse left ventricular remodeling after acute myocardial infarction. J Am Coll Cardiol 67: 2050–2060, 2016. doi: 10.1016/j.jacc.2016.01.073. [DOI] [PubMed] [Google Scholar]
- 189.Williams MR, Azcutia V, Newton G, Alcaide P, Luscinskas FW. Emerging mechanisms of neutrophil recruitment across endothelium. Trends Immunol 32: 461–469, 2011. doi: 10.1016/j.it.2011.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Woodfin A, Voisin MB, Beyrau M, Colom B, Caille D, Diapouli FM, Nash GB, Chavakis T, Albelda SM, Rainger GE, Meda P, Imhof BA, Nourshargh S. The junctional adhesion molecule JAM-C regulates polarized transendothelial migration of neutrophils in vivo. Nat Immunol 12: 761–769, 2011. doi: 10.1038/ni.2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Wu J, Xia S, Kalionis B, Wan W, Sun T. The role of oxidative stress and inflammation in cardiovascular aging. BioMed Res Int 2014: 615312, 2014. doi: 10.1155/2014/615312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Xu G, Zhang Y, Zhang L, Ren G, Shi Y. The role of IL-6 in inhibition of lymphocyte apoptosis by mesenchymal stem cells. Biochem Biophys Res Commun 361: 745–750, 2007. doi: 10.1016/j.bbrc.2007.07.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Yang Y, Lv J, Jiang S, Ma Z, Wang D, Hu W, Deng C, Fan C, Di S, Sun Y, Yi W. The emerging role of Toll-like receptor 4 in myocardial inflammation. Cell Death Dis 7: e2234, 2016. doi: 10.1038/cddis.2016.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Yano T, Miura T, Ikeda Y, Matsuda E, Saito K, Miki T, Kobayashi H, Nishino Y, Ohtani S, Shimamoto K. Intracardiac fibroblasts, but not bone marrow derived cells, are the origin of myofibroblasts in myocardial infarct repair. Cardiovasc Pathol 14: 241–246, 2005. doi: 10.1016/j.carpath.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 195.Yasojima K, Kilgore KS, Washington RA, Lucchesi BR, McGeer PL. Complement gene expression by rabbit heart: upregulation by ischemia and reperfusion. Circ Res 82: 1224–1230, 1998. doi: 10.1161/01.RES.82.11.1224. [DOI] [PubMed] [Google Scholar]
- 196.Yin H, Li P, Hu F, Wang Y, Chai X, Zhang Y. IL-33 attenuates cardiac remodeling following myocardial infarction via inhibition of the p38 MAPK and NF-κB pathways. Mol Med Rep 9: 1834–1838, 2014. doi: 10.3892/mmr.2014.2051. [DOI] [PubMed] [Google Scholar]
- 197.Zeng B, Chen H, Zhu C, Ren X, Lin G, Cao F. Effects of combined mesenchymal stem cells and heme oxygenase-1 therapy on cardiac performance. Eur J Cardiothorac Surg 34: 850–856, 2008. doi: 10.1016/j.ejcts.2008.05.049. [DOI] [PubMed] [Google Scholar]
- 198.Zhang Y, Zhao J, Lau WB, Jiao LY, Liu B, Yuan Y, Wang X, Gao E, Koch WJ, Ma XL, Wang Y. Tumor necrosis factor-α and lymphotoxin-α mediate myocardial ischemic injury via TNF receptor 1, but are cardioprotective when activating TNF receptor 2. PLoS One 8: e60227, 2013. doi: 10.1371/journal.pone.0060227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Zhu X, Zhao H, Graveline AR, Buys ES, Schmidt U, Bloch KD, Rosenzweig A, Chao W. MyD88 and NOS2 are essential for Toll-like receptor 4-mediated survival effect in cardiomyocytes. Am J Physiol Heart Circ Physiol 291: H1900–H1909, 2006. doi: 10.1152/ajpheart.00112.2006. [DOI] [PubMed] [Google Scholar]