Abstract
Objective
Curcumin is the well-known compound which is extracted from turmeric powder, the dried rhizome of the Curcuma longa Linn. This have been used for the treatment of various disorders including inflammation. In this study we have analyzed the effect of curcumin on arthritis induced by adjuvant in rats, considering changes in methionine sulfoxide reductase A (MSRA) expression and antioxidant enzymes levels.
Methods
Five groups of adult male Wistar rats (n=10), were randomly selected as control, placebo, experimental 1, 2 and 3. The induction of arthritis was carried out by injection of 0.1 ml adjuvant in plantar region. The first experimental group received no curcumin treatment, whereas the experimental two and three received curcumin (1 and 2 g/kg daily) respectively, for fourteen days. MSRA gene expression was assessed by real-time PCR and protein levels of MSRA, SOD, CAT and GPx were analyzed via ELISA method.
Results
The results showed no significant weight changes among the groups during the experimental period and the paw swelling caused by adjuvant was recovered within fourteen days of treatment with curcumin. However, the levels of enzymes such as superoxide dismutase, catalase and glutathione peroxidase were increased by a dose dependent manner. These results also illustrated that the gene expression and protein level of MSRA in groups treated with curcumin increased significantly (p≤0.05).
Conclusion
We concluded that the curcumin can be used against inflammation. The increasing level of MSRA can be due to the antioxidant effect of curcumin. The enzymatic level changes (MSRA, SOD, CAT and GPx) may interfere with the aging process and delay it.
Keywords: curcumin, anti-inflammation, MSRA
Introduction
Curcumin (diferuloylmethane), a yellow pigment in the spice turmeric which is used for decades for medicinal purposes and a wide range of research has been developed for better understanding of various properties of this miraculous compound specially in Asian countries.1,2 However, curcumin is used in treatment of several disorders such as Rheumatoid Arthritis (RA), infection diseases, atherosclerosis and Cancers.3,4 The pharmacodynamics and pharmacokinetics of curcumin have been examined in animals and in humans. Curcumin illustrates its anti-inflammatory effects by downregulation of inflammatory transcription factors (like nuclear factor kB), enzymes (like cyclooxygenase 2 and 5 lipoxygenase) and cytokines (like tumor necrosis factor, interleukin 1 and 6).1,5,6 Because of the crucial role of inflammation in most chronic diseases, the potential effect of curcumin has been examined in neoplastic, neurological, antiproliferative, anti-invasive, and antiangiogenic agent cardiovascular, pulmonary and metabolic diseases. Various pharmacological aspects of curcumin in vitro and in vivo are discussed by other scientist.7,8
Rheumatoid arthritis (RA) is characterized by chronic synovial inflammation and hyperplasia, and progressive destruction of cartilage and bone, ultimately leading to irreversible joint damage. Curcumin also possesses antirheumatic and anti-arthritic properties which has been shown to inhibit collagen and growth of synovial fibroblasts obtained from patients with RA by the induction of apoptosis via the up-regulation of pro-apoptotic Bax as well as down-regulation of anti-apoptotic Bcl-2 and the XIAP.9–11
In general, the orally used drugs for the treatment of RA are absorbed via gastrointestinal tract. Their prototype or active metabolites are accumulated in the target tissue/cells to regulate immune balance or counteract inflammatory reaction to help to relief of arthritis. However, there are still some natural products such as ginsenoside Rb1, berberine, madecassoside and curcumin which would substitute to these drugs. These natural components though have low bioavailability; they are indeed capable to reduce RA.12–14 There is no reasonable explanation for these pharmacodynamics–pharmacokinetics paradoxes.15
Oxygen free radicals are molecules comprising one or more unpaired electrons in their outermost shells, forming in aerobic cellular metabolism. Reactive free radicals could bind to different molecules and lead to damage to nucleic acids, proteins, and lipid membranes. Under physiological conditions, free radicals can be affected and destroyed by the antioxidant defense system. Various natural antioxidants are able to scavenge free radicals and prevent oxidative damage.16,17 Natural antioxidants such as methionine sulfoxide reductase (MSR), catalase, superoxide dismutase (SOD), and glutathione peroxidase can exist in enzymatic antioxidants form. In addition, there are food-derived antioxidants such as vitamin A, C, E, carotenoids, and curcumin etc.17–19
Referred to previous paragraph, reactive oxygen species (ROS) such as superoxide anions (O2-), hydroxyl radicals (OH-) and hydrogen peroxide (H2O2) contribute to damage to biomolecules like DNA, protein and membrane lipids. Superoxide is converted to hydrogen peroxide via SOD. Either Catalase or Glutathione peroxidase converted hydrogen peroxide to water.20,21
As mentioned above, aerobic respiration causes the production of reactive oxygen species (ROS), which leads to the oxidation of methionine. This is followed by the generation of diastereomeric mixtures named Methionine-S-sulfoxide (Met-S-SO) and Methionine-R-sulfoxide (Met-R-SO). Two essential enzymes are needed with combined action for reduction of these sulfoxides, with Methionine-S-sulfoxide reductase (MSRA) [for Met-S-SO] and Methionine-R-sulfoxide reductase (MSRB) [for Met-R-SO]. Suggested functions which can enumerate for these enzymes include repair of protein oxidation damages, regulation of protein function and elimination of oxidants via reversible formation of methionine sulfoxides.22,23
Methionine bound residues in proteins are highly prone to oxidation by reactive oxygen species (ROS), resulting in formation of methionine sulfoxide [Met (O)] residues. However, this oxidation can be repaired by methionine sulfoxide reductase (MSRA), which replaced met(O) with met.24 The MSRA protein is highly expressed in liver, kidney, pigment epithelial cells of the retina, macrophages, cerebellum, and brain neurons. These tissues/cells are the most vulnerable ones to oxidative stress damages. Thus, the MSRA enzyme suppression could lead to loss of antioxidant defense, bringing about oxidative damage and a shorter lifespan. To assess the antioxidant function of MSRA in mammals and its possible influence on lifespan, they created a strain of mouse lacking the MSRA protein.23
Despite several studies related to effect of curcumin on inflammation resulted from arthritis, there are few studies about antioxidant properties of curcumin.
However, we have designed this study to find out whether or not curcumin can exert its effect on arthritis via interfering with the gene expression and protein level of MSRA and other antioxidant enzymes such as SOD, Cat and GPx.
Materials and methods
In this study 50 male Wistar rats, weighing about 210–225 g were obtained from the animal research facilities of the Fasa University of Medical Sciences. The rats were kept in a fully ventilated room under the standard conditions of temperature, light and humidity, they were fed with standard plates and water ad libitum.
All animal experiments were carried out by following the animal ethical guidelines and regulation of Fasa University of Medical Sciences (Ethical code number IR.FUMS.REC.1395.196).
Rats were randomly divided into five groups of ten each as follows:
Control group received normal food and water
Placebo group in addition to normal diet and water received vehicle (saline)
The experimental rats (arthritis) were sub-divided into three groups:
Arthritis induced rats received normal diet and water with no curcumin treatment(Exp.1)
Arthritis induced rats treated with 1 g/kg of curcumin (Exp. 2)
Arthritis induced rats treated with 2 g/kg of curcumin (Exp. 3)
The rats were treated with curcumin by gavage feeding for fourteen days after inflammation was confirmed caused by adjuvant injection.
Induction of arthritis
Freundʼs Complete Adjuvant (FCA) was obtained from Sigma-Aldrich Company. The adjuvant induced arthritis (AIA) models were carried out by the method described in other studies. Briefly, the model was induced by a single injection of 0.1 ml of FCA into the plantar skin of the hind paw. Arthritis induced from day 1 in experimental animals by injection of 0.1 ml complete Freundʼs adjuvant (FCA consisted of 6 mg mycobacterium butyricum being suspended in heavy paraffin oil to give a concentration of 6 mg/ml) in sub plantar region of hind paw.25,26
paw volume measured on first, 7th and 14th days interval. The mean changes of edema in each group (n=10) were compared with control. Body weight measured weakly in all groups by digital balance.
RNA extraction
Fresh portion of rat liver dissected and placed in the RNase-free microtubes and then RNAs were extracted from tissues using TRIZOL reagent according to the instructions of manufacturer and were qualified by measuring absorbance on A260/280 nm by NanoDrop (Thermo scientific ND-2000c USA).
cDNA synthesis and quantitative real-time PCR(qRT-PCR)
Total RNAs from control, placebo and experimental groups were reverse transcribed using TAKARA Synthesis Kit (Takara Japan) and the manufacturer’s instructions. Real-time PCR was performed using SYBR* Premix ExTaqTM II (Takara co., Ltd), produced cDNA and proper primers (Table 1). The housekeeping gene (β-actin) was used for normalizing the amplification. Relative changes in gene expression were calculated using the ΔΔCt (threshold cycle) method. Fold change values were calculated using the formula 2−ΔΔCt.
Table 1.
primer sequences
| Gene | Sequence of the selected primer pair (nucleotide) |
|---|---|
| Β-actin | Forward: 5ʹ-AAGGCCAACCGTGAAAAGAT-3ʹ Reverse: 5ʹ-ACCAGAGGCATACAGGGAC-3ʹ |
| MSRA | Forward: 5ʹ-TTGGAATGGGCTGCTTCTGG-3ʹ Reverse: 3ʹ-GTAGGTGGGATTGCGTGTGT-5ʹ |
Anti-oxidant enzymes level analysis
The antioxidant level was determined spectrophotometrically using MSRA, SOD, CAT and GPx analysis ELISA kits (ZelBio Germany).
Statistical analysis
All the data was expressed as mean ± standard deviation (SD). The means of various parameters of control and experimental animals were compared using ANOVA & Tukey post-hoc test for statistical significance. P≤0.05 was considered to be statistically significant.
Results
Paw swelling
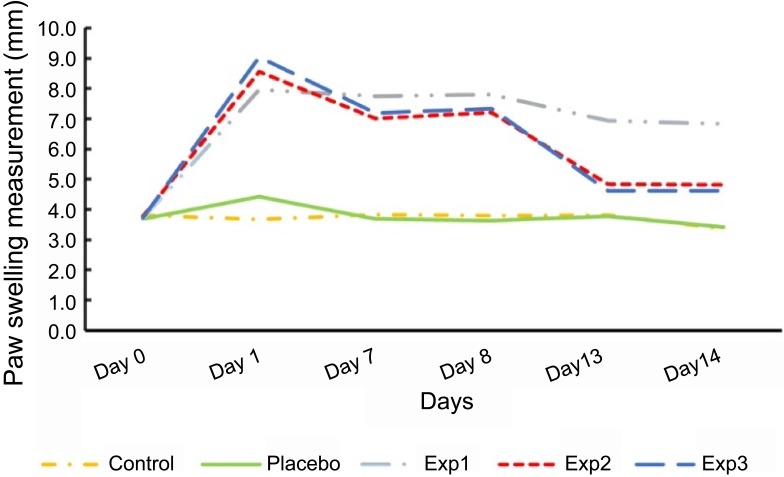
Our data demonstrated that control and placebo paw swelling did not change significantly over the experimental period (Figure 1). In contrast to this, paw measurements rose sharply in the first three days after injection of adjuvant in arthritis induced rats in three experimental groups (P≤0.05). This was followed by a continuous decrease after gavage with curcumin during the time frame in Exp. 2 and 3 (P≤0.05). As for Exp. 1, it was not the same. The swelling size was as big as 8 millimeters three days after induction.
Figure 1.
Effect of curcumin on paw measurements in arthritis induced rats. Exp. 1 group without any curcumin treatment. Exp. 2 group with 1 g/kg curcumin treatment. Exp. 3 group with 2 g/kg curcumin treatment. P≤0.05. Results are presented as mean±SD, n=10 (all Exp. groups are AIA models via FCA).
Weight changes
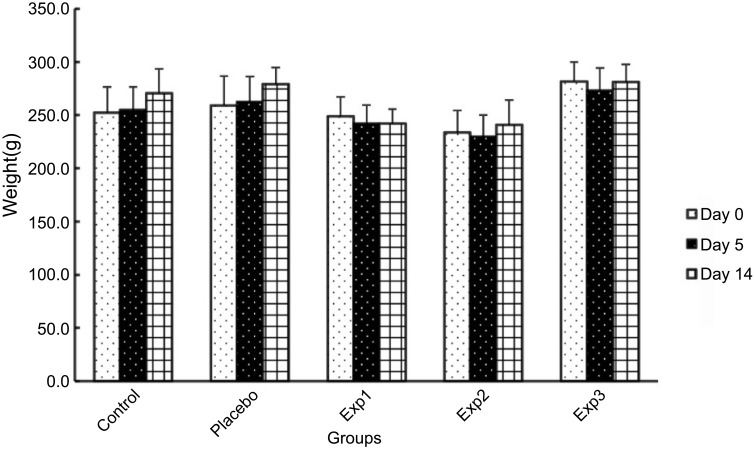
Experimental groups lost weight after arthritis induction in the first few days. However, the Exp. 2 and 3 recover the weight lost in last week of the experimental period, ultimately at the end the weight changes between the groups were not significant (Figure 2).
Figure 2.
Effect of curcumin on weight changes of arthritis induced rats. Exp. 1 group without any curcumin treatment. Exp. 2 group with 1 g/kg curcumin treatment. Exp. 3 group with 2 g/kg curcumin treatment. day0: weight of rats in day 0. day5: weight of rats in day 5. day14: weight of rats in day 14. p≤0.05. Results are presented as mean±SD, n=10 (all Exp. groups are AIA models via FCA).
Gene expression and protein levels
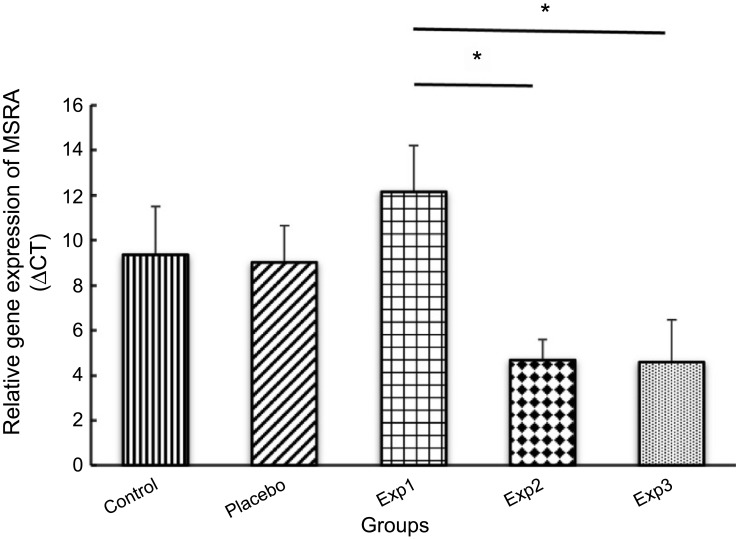
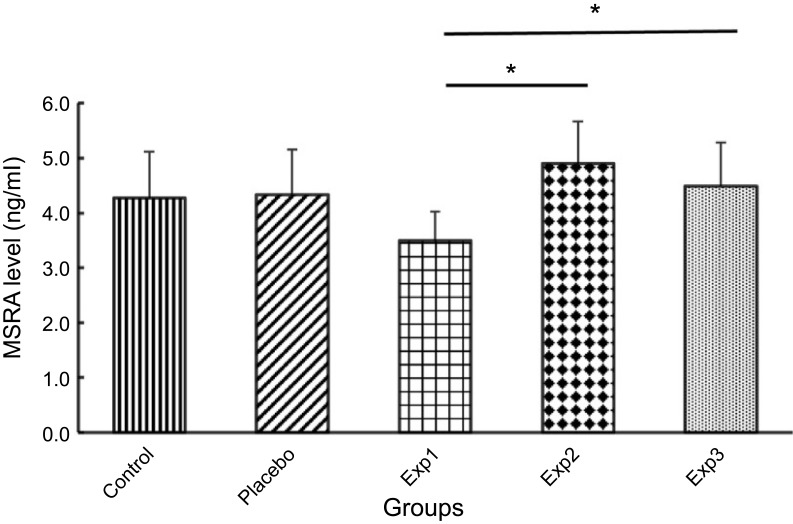
Gene expression and protein level in Exp. 1 decreased exponentially as compared with the control group. Our findings also showed that mRNA expression (Figure 3) and protein level (Figure 4) of MSRA in Exp. 2 and 3 was increased significantly (P≤0.05) when compared with the control and placebo.
Figure 3.
Effect of curcumin on gene expression of MSRA in arthritis induced rats (decreasing in the ΔCT shows the gene expression increment).
Figure 4.
Effect of curcumin on Methionine Sulfoxide Reductase A (MSRA) levels. Exp. 1 group without any curcumin treatment. Exp. 2 group with 1 g/kg curcumin treatment. Exp. 3 group with 2 g/kg curcumin treatment. *p≤0.05. Results are presented as mean±SD, n=10 (all Exp. groups are AIA models via FCA).
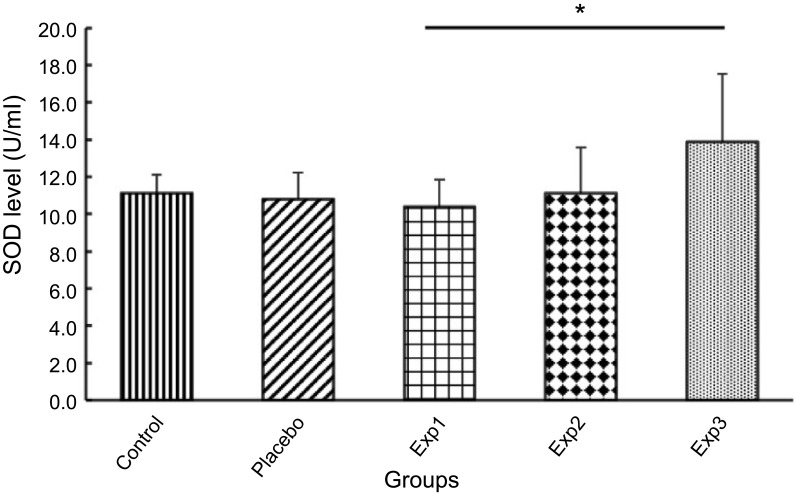
On the other hand, anti-oxidant enzymes such as SOD, CAT and GPx levels were assessed in rat’s plasma. Our results depicted that SOD level in Exp. 1 was reduced moderately. However, the Exp. 3 after treatment with curcumin showed a significant elevated levels of SOD compared to the other groups as exhibited in Figure 5.
Figure 5.
Effect of curcumin on Superoxide dismutase (SOD) level. Exp.1 group without any curcumin treatment. Exp. 2 group with 1 g/kg curcumin treatment. Exp. 3 group with 2 g/kg curcumin treatment. Results are presented as mean±SD, n=10, *p≤0.05 (all Exp. groups are AIA models via FCA).
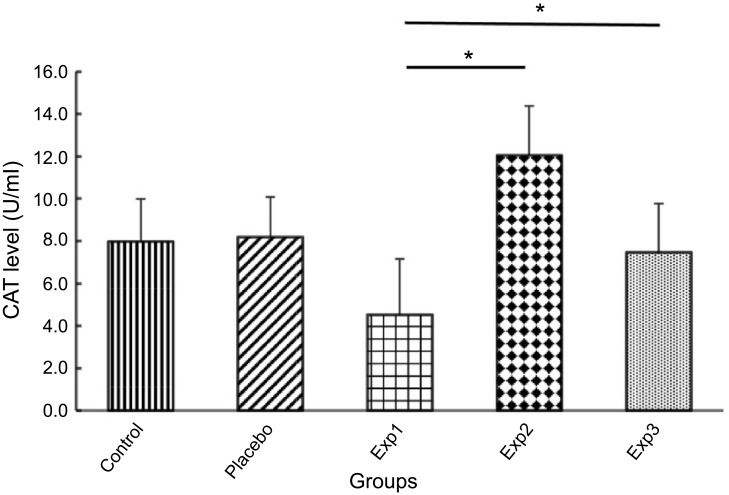
The CAT levels in Exp.1 has dramatically fall of 4.5 U/ml, Which was showed the least amount of CAT levels, but In contrast, the curcumin treated rats showed an increase CAT level exponentially in Exp. 2 and 3. Meanwhile, the increasing pattern was much higher in Exp. 2
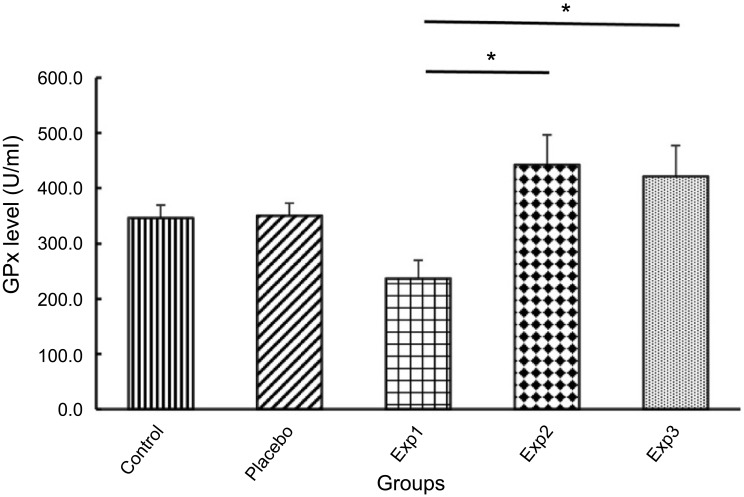
(p≤0.05) over Exp. 3 (Figure 6). We illustrated in Figure 7 that GPx level for control and placebo groups was approximately the same, whereas Exp. 1 decreased sharply, But the Exp. 2 and 3, after treatment with Curcumin, inversely raised in the GPx level significantly.
Figure 6.
Effect of curcumin on Catalase (CAT) level. Exp. 1 group without any curcumin treatment. Exp. 2 group with 1 gr/kg curcumin treatment. Exp. 3 group with 2 g/kg curcumin treatment. Results are presented as mean±SD, n=10, *p≤0.05 (all Exp. groups are AIA models via FCA).
Figure 7.
Effect of curcumin on Glutathione peroxidase (GPx) level. Exp. 1 group without any curcumin treatment. Exp. 2 group with 1 g/kg curcumin treatment. Exp. 3 group with 2 g/kg curcumin treatment. Results are presented as mean ±SD, n=10, *p≤0.05 (all Exp. groups are AIA models via FCA).
Discussion
The present study was assessed the anti-inflammatory role of curcumin related to arthritis which was induced in rats by the method mentioned. Curcumin is normally associated with the wide range of biological activities. Recently, multiple features such as anti-inflammatory and anti-oxidant effects have been identified for it. Curcumin prevented many age related diseases like arthritis, artherosclerosis, Alzheimer’s and Parkinson’s.21,27,28
Our findings demonstrated that curcumin’s effects were somehow dose-dependent. All experimental rats experienced a significant increase in paw measurements in first three days after adjuvant injection. It is interesting to note that both Exp. 2 and Exp. 3 groups which treated with curcumin (1 and 2 g/kg respectively) revealed a sharp continuous decrease in paw measurements to the end of experimental period. These results indicated that the change in paw swelling might contribute to the anti-inflammatory activity of curcumin. More recently, Zheng et al noticed that the paw swelling rates (PSRs) of rats treating by curcumin or methotrexate (MTX) [disease modifying antirheumatic therapy for RA] resulted a marked decline in AIA rats.26 Also, Castilhos et al estimated the possibility of arthritis induction as mechanical sensitivity and paw thickness of each rat. Therefore, rose in mechanical sensitivity and paw thickness were considered as sign of the inflammatory process.25
In our experiments the weight lost observed in experimental groups might stem from inflammation or loss of appetite. Apart from a decrease in rat’s weigh in the first half of the experiment, the Exp2 and Exp3 were then started to gain weight in the second half of the period compared with the Exp1(no treated group), though it was short of significance.
As for MSRA expression, both of the Exp. 2 and 3 increased significantly. With stark contrast, the Exp. 1 gene expression, however, decreased exponentially which would induce by the inflammation. Studies have claimed that MSRA has antioxidant and antiaging properties. The oxidative damage caused by inflammation predicts the overexpression of MSRA could omit the oxidants.23,24 Our study confirms this prediction. Also the intracellular of ROS could be reduced through the scavenging process by MSRA.29 The second prediction is considered by the result of Moskivitz et al, who depicted that the reduction of MSRA activity in mice brings about shorter lifespan.23
This study used curcumin at two different doses of 1 and 2 mg/kg which both doses showed an anti-oxidant effect. Our study illustrated that MSRA activity increased significantly in Exp. 2 compared with Exp. 1, placebo and Control and this remained so for Catalase and GPx activity, while SOD activity rose sharply in Exp. 3. This meant that anti-oxidant activity of curcumin is highly dose dependent.
Curcumin may be used as an antiaging agent too. It would reduce the aging process and hair graying by increasing the MSRA expression and antioxidant enzymes’ level by reducing the H2O2 production through the SOD and CAT changes. It will be interesting to evaluate the molecular level and gene expression of aging biomarkers as well.
There have already been many researches which introduce the effects of curcumin on antioxidant enzymes. Earlier studies in 2012, have demonstrated that SOD3 (extracellular copper-zinc SOD) protects against destructive effects which roots from ROS.30,31 In 2014, Aziza reported that curcumin rose the activity of SOD, protecting the enzymatic antioxidants via denaturation.32 Zhu et al (2015) have shown that SOD and GSH levels were significantly higher in the ARPE-19 cells treated with curcumin than in the non-treated ones.21 Also, Tengku-Siti-Hajar Haryuna et al (2017) have illustrated that curcumin could increase the SOD expression significantly which was due to the curcumin activity as an anti-oxidant agent. The mechanism of this anti-oxidant activity related to radical scavenging property. Likewise, curcumin does this activity via upregulation of endogenous anti-oxidant enzymes’ expression like SOD, GSH and GSHPx.33,34 Other reports have illustrated that curcumin with lower doses can hinder the reduction the antioxidant enzyme GSH, whereas higher doses lead to a progressive decrease in GSH of red blood cells (with oxidative damage).35
A notable factor that has an impact on these results is the nature of curcumin properties, which may alter if different dosage of curcumin is used. It may act as an anti-oxidant in lower doses and inversely as a pro-oxidant in higher one. Meanwhile, this finding was consistent with the other studies mentioned above.36
However, there is a need to evaluate more different doses of curcumin to reach to a concrete conclusion.
Conclusion
In conclusion, the curcumin was able to reduce inflammation and increase enzymatic anti-oxidant activity via the increased expression of MSRA and the augmentation of some enzymes level such as MSRA, SOD, CAT and GPx. Also, it might be used against inflammation caused by arthritis at moderate doses.
Acknowledgments
We would like to acknowledge the Fasa University of Medical Sciences for providing us all the animal facilities and laboratory equipment for this study.
Disclosure
The authors report no conflicts of interest in this study.
References
- 1.Hatcher H, Planalp R, Cho J, Torti FM, Torti SV. Curcumin: from ancient medicine to current clinical trials. Cell Mol Life Sci. 2008;65(11):1631–1652. doi: 10.1007/s00018-008-7452-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pulido-Moran M, Moreno-Fernandez J, Ramirez-Tortosa C, Ramirez-Tortosa M. Curcumin and health. Molecules. 2016;21(3):264. doi: 10.3390/molecules21030264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Noorafshan A, Ashkani-Esfahani S. A review of therapeutic effects of curcumin. Curr Pharm Des. 2013;19(11):2032–2046. [PubMed] [Google Scholar]
- 4.Tilak JC, Banerjee M, Mohan H, Devasagayam TP. Antioxidant availability of turmeric in relation to its medicinal and culinary uses. Phytother Res. 2004;18(10):798–804. doi: 10.1002/ptr.1553 [DOI] [PubMed] [Google Scholar]
- 5.Gao X, Kuo J, Jiang H, et al. Immunomodulatory activity of curcumin: suppression of lymphocyte proliferation, development of cell-mediated cytotoxicity, and cytokine production in vitro. Biochem Pharmacol. 2004;68(1):51–61. doi: 10.1016/j.bcp.2004.03.015 [DOI] [PubMed] [Google Scholar]
- 6.Surh YJ. Anti-tumor promoting potential of selected spice ingredients with antioxidative and anti-inflammatory activities: a short review. Food Chem Toxicol. 2002;40(8):1091–1097. [DOI] [PubMed] [Google Scholar]
- 7.Aggarwal BB, Sung B. Pharmacological basis for the role of curcumin in chronic diseases: an age-old spice with modern targets. Trends Pharmacol Sci. 2009;30(2):85–94. doi: 10.1016/j.tips.2008.11.002 [DOI] [PubMed] [Google Scholar]
- 8.Goel A, Kunnumakkara AB, Aggarwal BB. Curcumin as “Curecumin”: from kitchen to clinic. Biochem Pharmacol. 2008;75(4):787–809. doi: 10.1016/j.bcp.2007.08.016 [DOI] [PubMed] [Google Scholar]
- 9.Daily JW, Yang M, Park S. Efficacy of turmeric extracts and curcumin for alleviating the symptoms of joint arthritis: a systematic review and meta-analysis of randomized clinical trials. J Med Food. 2016;19(8):717–729. doi: 10.1089/jmf.2016.3705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ghosh S, Banerjee S, Sil PC. The beneficial role of curcumin on inflammation, diabetes and neurodegenerative disease: A recent update. Food Chem Toxicol. 2015;83:111–124. doi: 10.1016/j.fct.2015.05.022 [DOI] [PubMed] [Google Scholar]
- 11.Huang G, Xu Z, Huang Y, et al. Curcumin protects against collagen-induced arthritis via suppression of BAFF production. J Clin Immunol. 2013;33(3):550–557. doi: 10.1007/s10875-012-9839-0 [DOI] [PubMed] [Google Scholar]
- 12.Kim HA, Kim S, Chang SH, Hwang HJ, Choi YN. Anti-arthritic effect of ginsenoside Rb1 on collagen induced arthritis in mice. Int Immunopharmacol. 2007;7(10):1286–1291. doi: 10.1016/j.intimp.2007.05.006 [DOI] [PubMed] [Google Scholar]
- 13.Li H, Gong X, Zhang L, et al. Madecassoside attenuates inflammatory response on collagen-induced arthritis in DBA/1 mice. Phytomedicine. 2009;16(6–7):538–546. doi: 10.1016/j.phymed.2008.11.002 [DOI] [PubMed] [Google Scholar]
- 14.Wang Z, Chen Z, Yang S, et al. Berberine ameliorates collagen-induced arthritis in rats associated with anti-inflammatory and anti-angiogenic effects. Inflammation. 2014;37(5):1789–1798. doi: 10.1007/s10753-014-9909-y [DOI] [PubMed] [Google Scholar]
- 15.Yang Y, Wu X, Wei Z, et al. Oral curcumin has anti-arthritic efficacy through somatostatin generation via cAMP/PKA and Ca(2+)/CaMKII signaling pathways in the small intestine. Pharmacol Res. 2015;95–96:71–81. doi: 10.1016/j.phrs.2015.03.016 [DOI] [PubMed] [Google Scholar]
- 16.Aladag I, Eyibilen A, Guven M, Atis O, Erkorkmaz U. Role of oxidative stress in hearing impairment in patients with type two diabetes mellitus. J Laryngol Otol. 2009;123(9):957–963. doi: 10.1017/S0022215109004502 [DOI] [PubMed] [Google Scholar]
- 17.Haryuna TS, Munir D, Maria A, Bashiruddin J. The antioxidant effect of curcumin on cochlear fibroblasts in rat models of diabetes mellitus. Iran J Otorhinolaryngol. 2017;29(93):197–202. [PMC free article] [PubMed] [Google Scholar]
- 18.Phaniendra A, Jestadi DB, Periyasamy L. Free radicals: properties, sources, targets, and their implication in various diseases. Indian J Clin Biochem. 2015;30(1):11–26. doi: 10.1007/s12291-014-0446-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Quinonez-Flores CM, Gonzalez-Chavez SA, Del Rio Najera D, Pacheco-Tena C. Oxidative stress relevance in the pathogenesis of the rheumatoid arthritis: a systematic review. Biomed Res Int. 2016;2016:6097417. doi: 10.1155/2016/6097417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Golden TR, Hinerfeld DA, Melov S. Oxidative stress and aging: beyond correlation. Aging Cell. 2002;1(2):117–123. doi: 10.1046/j.1474-9728.2002.00015.x [DOI] [PubMed] [Google Scholar]
- 21.Zhu W, Wu Y, Meng YF, et al. Effect of curcumin on aging retinal pigment epithelial cells. Drug Des Devel Ther. 2015;9:5337–5344. doi: 10.2147/DDDT.S84979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cabreiro F, Picot CR, Friguet B, Petropoulos I. Methionine sulfoxide reductases: relevance to aging and protection against oxidative stress. Ann N Y Acad Sci. 2006;1067:37–44. doi: 10.1196/annals.1354.006 [DOI] [PubMed] [Google Scholar]
- 23.Moskovitz J, Bar-Noy S, Williams WM, Requena J, Berlett BS, Stadtman ER. Methionine sulfoxide reductase (MsrA) is a regulator of antioxidant defense and lifespan in mammals. Proc Natl Acad Sci U S A. 2001;98(23):12920–12925. doi: 10.1073/pnas.231472998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ruan H, Tang XD, Chen ML, et al. High-quality life extension by the enzyme peptide methionine sulfoxide reductase. Proc Natl Acad Sci U S A. 2002;99(5):2748–2753. doi: 10.1073/pnas.032671199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Castilhos LG, Rezer JF, Ruchel JB, et al. Effect of Uncaria tomentosa extract on purinergic enzyme activities in lymphocytes of rats submitted to experimental adjuvant arthritis model. BMC Complement Altern Med. 2015;15:189. doi: 10.1186/s12906-015-0694-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zheng Z, Sun Y, Liu Z, Zhang M, Li C, Cai H. The effect of curcumin and its nanoformulation on adjuvant-induced arthritis in rats. Drug Des Devel Ther. 2015;9:4931–4942. doi: 10.2147/DDDT.S90147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jat D, Parihar P, Kothari SC, Parihar MS. Curcumin reduces oxidative damage by increasing reduced glutathione and preventing membrane permeability transition in isolated brain mitochondria. Cell Mol Biol (noisy-le-grand). 2013;59 Suppl:Ol1899–905. [PubMed] [Google Scholar]
- 28.Shailaja M, Damodara Gowda KM, Vishakh K, Suchetha Kumari N. Anti-aging role of curcumin by modulating the inflammatory markers in albino wistar rats. J Natl Med Assoc. 2017;109(1):9–13. doi: 10.1016/j.jnma.2017.01.005 [DOI] [PubMed] [Google Scholar]
- 29.Levine RL, Mosoni L, Berlett BS, Stadtman ER. Methionine residues as endogenous antioxidants in proteins. Proc Natl Acad Sci U S A. 1996;93(26):15036–15040. doi: 10.1073/pnas.93.26.15036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kwon MJ, Han J, Kim BH, Lee YS, Kim TY. Superoxide dismutase 3 suppresses hyaluronic acid fragments mediated skin inflammation by inhibition of toll-like receptor 4 signaling pathway: superoxide dismutase 3 inhibits reactive oxygen species-induced trafficking of toll-like receptor 4 to lipid rafts. Antioxid Redox Signal. 2012;16(4):297–313. [DOI] [PubMed] [Google Scholar]
- 31.Kwon M-J, Jeon Y-J, Lee K-Y, Kim T-Y. Superoxide dismutase 3 controls adaptive immune responses and contributes to the inhibition of ovalbumin-induced allergic airway inflammation in mice. Antioxid Redox Signal. 2012;17(10):1376–1392. doi: 10.1089/ars.2012.4572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aziza SAH, Abdel-Aal S, Mady H. Chemopreventive effect of curcumin on oxidative stress, antioxidant status, DNA fragmentation and caspase-9 gene expression in 1, 2-dimethylhydrazine-induced colon cancer in rats. Am J Biochem Mol Biol. 2014;4:22–34. doi: 10.3923/ajbmb.2014.22.34 [DOI] [Google Scholar]
- 33.Jagetia GC, Rajanikant GK. Curcumin stimulates the antioxidant mechanisms in mouse skin exposed to fractionated gamma-irradiation. Antioxidants (Basel). 2015;4(1):25–41. doi: 10.3390/antiox4010025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Malik P, Mukherjee TK. Structure-function elucidation of antioxidative and prooxidative activities of the polyphenolic compound curcumin. Chin J Biol. 2014;2014:1–8. doi: 10.1155/2014/396708 [DOI] [Google Scholar]
- 35.Banerjee A, Kunwar A, Mishra B, Priyadarsini KI. Concentration dependent antioxidant/pro-oxidant activity of curcumin studies from AAPH induced hemolysis of RBCs. Chem Biol Interact. 2008;174(2):134–139. doi: 10.1016/j.cbi.2008.05.009 [DOI] [PubMed] [Google Scholar]
- 36.Chen J, Wanming D, Zhang D, Liu Q, Kang J. Water-soluble antioxidants improve the antioxidant and anticancer activity of low concentrations of curcumin in human leukemia cells. Pharmazie. 2005;60(1):57–61. [PubMed] [Google Scholar]