Snakebite is a neglected tropical disease of global importance affecting at least 2.5 million people with more than 100,000 deaths annually.1, 2 Morbidity and mortality are high in countries such as Myanmar, where recent hospital data reported 15,000 to 20,000 cases per year with case-fatality ratio of 10.9%.3 Experience elsewhere suggests that hospital-based data may underestimate the actual burden of snakebite by more than two-thirds.4, 5
To assess outcomes of snakebite cases at Mandalay General Hospital, we established a clinical data collection system. This major hospital serves as a regional referral center for snakebite. In this region of Myanmar, Eastern Russell’s Viper (ERV; Daboia siamensis) snakebite is of the utmost importance given the high incidence of acute kidney injury (AKI) following envenoming.6, 7
The primary purpose of this clinical audit, which represents one arm of an Australian Department of Foreign Affairs and Trade–funded foreign aid project to improve the outcomes of snakebite patients in Myanmar,8 is to provide accurate information to local health authorities to improve health care policies and resource allocation. In addition, we wanted to examine the clinical variables that affect the development of AKI following ERV envenoming. We report 12 months of observational data pertaining to ERV snakebites.
Results
A total of 965 patients presented to Mandalay General Hospital after snakebites during the 12-month period. Data for 17 patients were incomplete, leaving 948 for analysis. Bites were attributed to ERV in 686 cases (72.4%), cobra (Naja kaouthia and Naja mandalayensis) in 17 (1.8%), “green snake” (Trimeresurus albolabris) in 61 (6.4%), krait (Bungarus spp.) in 4 (0.4%), other snakes including nonvenomous species in 35 (3.7%), and unknown snakes in 145 (15.3%). In most cases, the dead snake was brought to the hospital and identified by medical staff. In the others, the diagnostic clinical syndrome combined with recognition by the patient of the familiar "mwe bwe" (ERV, Daboia siamensis) was accepted as sufficient identification. This report concentrates on ERV cases given that envenoming from this species alone accounts for 70% of all patients requiring acute nephrological care in Myanmar.9
Patients were typically male (64.9%) and had been bitten on the lower limbs during farm work. Median age was 34 (interquartile range [IQR] 24). Appropriate first aid (pressure pad and immobilization) was rarely applied. Tight tourniquets were applied commonly (77.8%); other interventions included incision (5.8%) and tattooing (8.9%). The first point of health care contact for most was either a rural health center or township hospital (82.8%), and traditional healers were consulted first in 13.9%. The median time from bite to arrival at a health care facility was 1.5 hours (IQR 2.19); the median time from bite to administration of the first dose of antivenom was 2 hours (IQR 3.5).
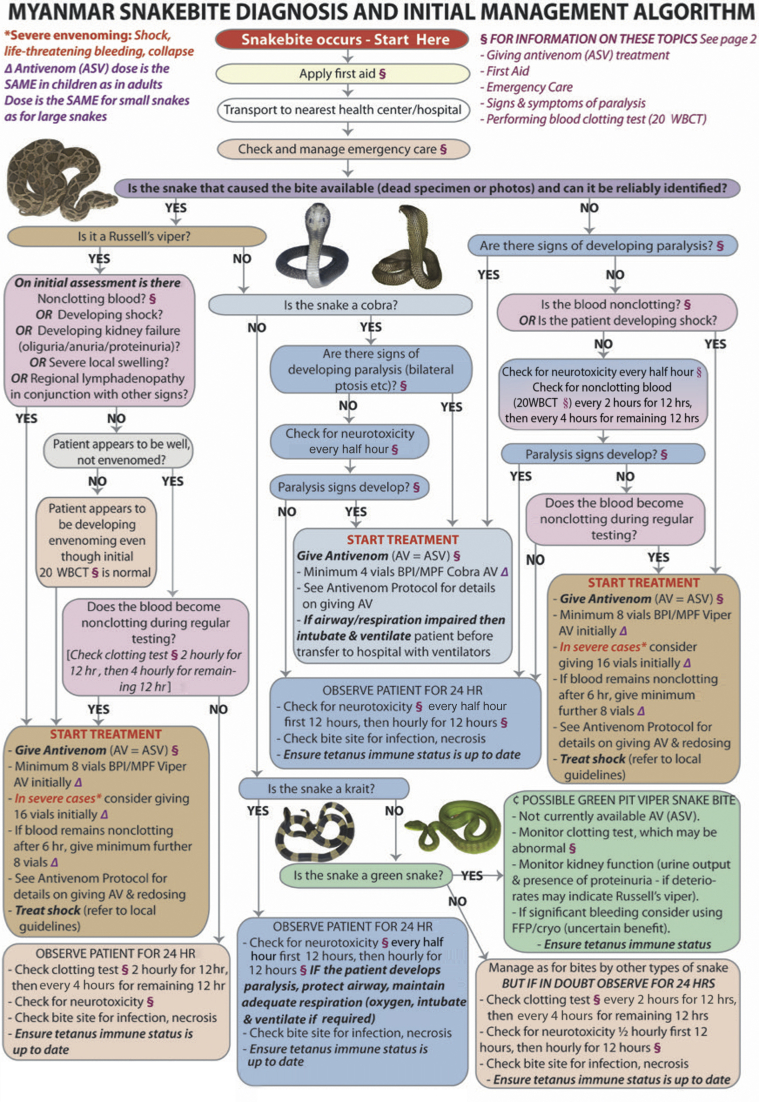
Almost all patients received antivenom (679, 98.9%); 295 cases (43%) received treatment considered compliant with national guidelines (initial dose of 8 vials of Burma Pharmaceutical Industry ERV monovalent antivenom, - F[ab']2 fragments of equine hyperimmune plasma, for patients with significant features of ERV envenoming, see Figure 1). A common but noncompliant pattern in the remaining cases involved 1 to 2 vials given at a small health care facility followed by transfer to a larger facility where more antivenom was given.
Figure 1.
Myanmar national snakebite protocol. 20 WBCT, 20-minute whole blood clotting–test; ASV or AV, snakebite antivenom; BPI/MPF, Burma Pharmaceutical Industry/Myanmar Pharmaceutical Factory. Algorithm Copyright © 2018 Prof. Julian White. Snake photographs Copyright © 2018 Mark O’Shea. For page 2 of this management algorithm, please see White et al.8
In this study, AKI was defined pragmatically as a composite endpoint of either requirement for dialysis or, in the absence of requirement for dialysis, a peak serum creatinine level of >120 μmol/l in men or >100 μmol/l in women and a pattern of rising serial creatinine consistent with AKI.
The clinical consequences of envenoming are listed in Table 1. AKI was extremely common, manifesting in 488 patients (71% of entire cohort). Of these 488, dialysis (predominantly haemodialysis) was required in 213 (31% of entire cohort), whereas the other 275 patients (40% of entire cohort) suffered a pathological rise in serum creatinine but did not need dialysis (median peak serum creatinine 245.5 μmol/l [IQR 332] in male patients, 260.5 μmol/l [IQR 322] in female patients). Female patients were 1.8 times more likely than male patients to develop AKI (P < 0.01). AKI developed more frequently in older patients, with odds ratio (OR) of 5.5 (11.4 for survivors) in those >64 years compared with those <15 years (P < 0.01).
Table 1.
Clinical features of 686 cases of Russell’s Viper envenoming
| Clinical features | Number (% of 686) | AKI group (% of 488) | No-AKI group (% of 198) |
|---|---|---|---|
| Acute kidney injury | 488 (71) | ||
| Coagulopathy | 465 (67) | 373 (76) | 92 (47) |
| Thrombocytopenia | 461 (67) | 414 (85) | 47 (24) |
| Capillary leak | 240 (35) | 216 (44) | 24 (12) |
| Pulmonary edema | 16 | 14 | 2 |
| Periorbital edema | 118 | 106 | 12 |
| Conjunctival edema | 91 | 82 | 9 |
| Generalized edema | 15 | 14 | 1 |
| Shock | 103 (15) | 92 (19) | 11 (6) |
| Bite site infection | 74 (11) | 51 (11) | 23 (12) |
| Local necrosis | 44 (6.4) | 33 (7) | 11 (6) |
| Gastrointestinal bleeding | 38 (5.5) | 33 (7) | 5 (3) |
| Septicemia | 29 (4.2) | 26 (5) | 3 (2) |
| Panhypopituitism | 19 (2.7) | 19 (4) | 0 |
| Ophthalmoplegia | 2 (0.29) | 2 (0.4) | 0 |
| None | 59 (8.6) |
In this study, AKI was defined pragmatically as a composite endpoint of either requirement for dialysis or, in the absence of requirement for dialysis, a peak serum creatinine level of >120 μmol/l in men or >100 μmol/l in women and a pattern of rising serial creatinine consistent with AKI.
Multivariate analysis (Table 2) showed that the time interval from bite to antivenom administration (irrespective of the initial dosage of antivenom) was the strongest predictor of subsequent AKI (OR 1.7 when antivenom was given at 1–2 hours compared with 0–1 hour, P < 0.05; OR 3.2 at 2–3 hours compared with 0–1 hour, P < 0.01; OR 4.2 at 3–4 hours compared with 0–1 hour, P < 0.01; OR 12.4 at 4–5 hours compared with 0–1 hour, P < 0.01). This effect was observed across the 2 AKI subgroups as defined by dialysis requirement or serum creatinine rise without need for dialysis. Early administration of antivenom was also associated with shorter duration of coagulopathy (for patients receiving antivenom at 10 hours compared with those at 0–1 hour, P < 0.001).
Table 2.
Significant explanatory variables affecting AKI as determined by multivariate logistic regression
| Explanatory variables | Group | AKIa (surviving patients only in italics) |
|||
|---|---|---|---|---|---|
| Sig. cf. Ref.Group |
Odds ratio | Lower 95% CI | Upper 95% CI | ||
| Age groupb (cf. 0–15 yr) | 50–64 yr |
P < 0.05 P < 0.05 |
2.8 3.0 |
1.1 1.1 |
7.2 8.3 |
| Age group (cf. 0–15 yr) | >64 yr |
P < 0.01 P < 0.01 |
5.5 11.4 |
1.6 2.5 |
19.6 51.5 |
| Gender (cf. M) | F |
P < 0.01 P < 0.02 |
1.8 2.0 |
1.2 1.3 |
2.7 3.0 |
| Time bite to first AVc (cf. 0–1 h) | 1–2 h |
P < 0.05 P = 0.055 |
1.7 1.8 |
1.0 1.0 |
3.0 3.2 |
| Time bite to first AV (cf. 0–1 h) | 2–3 h |
P < 0.01 P < 0.01 |
3.2 2.8 |
1.5 1.3 |
6.8 6.2 |
| Time bite to first AV (cf. 0–1 h) | 3–4 h |
P < 0.01 P < 0.01 |
4.2 4.2 |
1.6 1.5 |
11.1 11.6 |
| Time bite to first AV (cf. 0–1 h) | 4–5 h |
P < 0.01 P < 0.01 |
12.4 12.5 |
2.5 2.3 |
62.9 67.3 |
| Time bite to first HCFd (cf. 0–1 h) | 4–5 h |
P = 0.055 P < 0.05 |
9.8 10.0 |
1.1 1.1 |
89.4 91.5 |
| Time bite to first HCF (cf. 0–1 h) | >10 h |
P < 0.02 P < 0.02 |
4.7 5.2 |
1.4 1.4 |
16.0 19.7 |
AKI, acute kidney injury; AV, antivenom; cf., compared with; CI, confidence interval; F, female; HCF, health care facility; M, male; Sig.cf.Ref.Group, significance compared with reference group.
-
aAKI, as defined as a composite endpoint of either requirement for dialysis or, in the absence of requirement for dialysis, a peak serum creatinine level of >120 μmol/l in men or >100 μmol/l in women and a pattern of rising serial creatinine consistent with AKI.
-
bAge group, years: 0–15 (ref.); 16–19; 20–29; 30–49; 50–64; >64.
-
cTime from bite to first antivenom administration, hours: 0–1 (ref.); 1–2; 2–3; 3–4; 4–5; 5–6; 6–10; >10.
-
dTime from bite to arrival at first HCF.
The development of AKI was an important clinical event given that AKI was associated significantly with mortality. The overall mortality was 12.2% (84 of 686) among the entire cohort of 686 ERV cases. More specifically, mortality was 20.2% (43 of 213) in those who required dialysis compared with 10.2% (28 of 275) in those with AKI but did not require dialysis (P = 0.002), and 6.6% (13 of 198) in those who did not develop AKI (P < 0.001).
Discussion
This study reveals the devastating scourge of snakebites in Myanmar. It highlights significant morbidity and mortality from ERV envenoming. The high rate of AKI (71%) was observed in a tertiary hospital caring for severely envenomed patients. The true rate of AKI consequent to all ERV bites may be lower, as not all patients require transfer to a tertiary hospital. Calculating the true risk of AKI requires accurate knowledge of snakebite incidence in the community. Our community-based survey of 2 rural townships in Mandalay indicated that the true incidence of snakebite in Myanmar may be twice as high as that derived from hospital data.S1 Evidently, a nationwide survey of all levels of the health care system is required.
Our finding that female patients were 1.8 times more likely than male patients to develop AKI after ERV envenoming warrants further investigation. Factors such as smaller body mass relative to venom load, nutritional status, pregnancy, and anemia may contribute to this gender disparity.
The pathogenesis of AKI after ERV envenoming is incompletely known, but it is likely to be multifactorial, including microvascular fibrin deposition,S2 direct nephrotoxicity,S3 and hypotension.6 Until more effective therapies become available, antivenom will remain the mainstay of treatment. Our finding that a shorter delay before antivenom had a better outcome is in broad agreement with 2 other reports based on smaller cohorts of patients.3,S4
Over the past 4 years, Australian, UK, and Myanmar colleagues have helped Myanmar become self-sufficient in antivenom production8; however, increasing the production of antivenom may not be enough to improve clinical outcomes. In response to our finding of an association between time to antivenom and AKI, the Myanmar Ministry of Health is reviewing its policies about distributing more antivenom to rural health care centers and township hospitals that are within closer reach of snakebite patients.
A limitation of this study is the lack of independent identification of snakes; the ERV cohort was based on assumed snake identity. Venom detection testing was not available, and very few dead snakes brought in by patients were kept for identification, although those that were available were predominantly ERVs. This limitation reflects the realities of clinical practice, where experienced clinicians must make pragmatic decisions about the likely culprit snake. In Mandalay Division of Myanmar, snakebite patients presenting with incoagulable blood are most likely to have ERV envenoming. The only other snakes causing this effect are green pit vipers (genus Trimeresurus), whose envenoming is unresponsive to ERV antivenom, and only very rarely results in AKI.
Although we had observed a beneficial effect of shorter time to antivenom, administration of 8 vials of antivenom compared with fewer than 8 vials did not correlate with decreased likelihood of AKI on either univariate or multivariate analysis. In this regard, several points are worth considering. First, this was an observational study, not a controlled clinical trial. Confounding factors, such as antivenom availability and clinical bias, may have influenced the initial antivenom dose. Antivenom rationing was common in rural health facilities; it was likely that higher antivenom dose was reserved for patients judged to have severe envenoming. Second, antivenom-specific factors such as unreliable storage cold chain and variable neutralizing potency may have limited its clinical efficacy. Efforts are under way to address these concerns and to determine the optimal initial antivenom dose through controlled clinical trials.
Disclosure
All the authors declared no competing interests.
Acknowledgments
We thank the staff and patients at the Mandalay General Hospital who participated in this study. We thank the Myanmar Ministry of Health and Sports for supporting this project. Last, we thank the Australian Department of Foreign Affairs and Trade for funding this project. All patients provided consent for this study. In patients who were too unwell, consent was obtained from close relatives.
Footnotes
Supplementary Material
References
- 1.Harrison R.A., Hargreaves A., Wagstaff S.C. Snake envenoming: a disease of poverty. PLoS Negl Trop Dis. 2009;3:e569. doi: 10.1371/journal.pntd.0000569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization Prevalence of snakebite envenoming. 2017. https://www.who.int/snakebites/epidemiology/en/ Available at: Accessed June 27, 2019.
- 3.Myo-Khin, Theingi-Nyunt, Nyan-Tun-Oo Prognostic indicators in patients with snakebite: analysis of two-year data from a township hospital in central Myanmar. WHO South East Asia J Public Health. 2012;1:144–150. doi: 10.4103/2224-3151.206927. [DOI] [PubMed] [Google Scholar]
- 4.Fox S., Rathuwithana A.C., Kasturiratne A. Underestimation of snakebite mortality by hospital statistics in the Monaragala District of Sri Lanka. Trans R Soc Trop Med Hyg. 2006;100:693–695. doi: 10.1016/j.trstmh.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 5.Mohapatra B., Warrell D.A., Suraweera W. Snakebite mortality in India: a nationally representative mortality survey. PLoS Negl Trop Dis. 2011;5 doi: 10.1371/journal.pntd.0001018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Myint-Lwin, Warrell D.A., Phillips R.E. Bites by Russell's viper (Vipera russelli siamensis) in Burma: haemostatic, vascular, and renal disturbances and response to treatment. Lancet. 1985;2:1259–1264. doi: 10.1016/s0140-6736(85)91550-8. [DOI] [PubMed] [Google Scholar]
- 7.Warrell D.A. Snake venoms in science and clinical medicine. 1. Russell's viper: biology, venom and treatment of bites. Trans R Soc Trop Med Hyg. 1989;83:732–740. doi: 10.1016/0035-9203(89)90311-8. [DOI] [PubMed] [Google Scholar]
- 8.White J., Mahmood M.A., Alfred S. A comprehensive approach to managing a neglected, neglected tropical disease: The Myanmar Snakebite Project (MSP) Toxicon X. 2019;1:100001. doi: 10.1016/j.toxcx.2018.100001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mon Hla. Patterns of acute renal failure in Burma. In: Weatherall D.J., Ledingham J.G.G., Warrell D.A., editors. Vol. 18. Oxford University Press; Oxford, UK: 1987. p. 179. (Oxford Textbook of Medicine. Second Edition. Vol 2). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.