Abstract
While addiction to drugs of abuse represents a significant health problem worldwide, the behavioral and neural mechanisms that underlie addiction and relapse are largely unclear. The concept of the “dark side of addiction”, developed and explored by George Koob and colleagues, describes a systematic decrease in reward-related processing following drug self-administration, and subsequent recruitment of anti-reward (i.e. stress) systems. Indeed, the activation of central nervous system (CNS) stress-response systems by drugs of abuse is contributory not only to mood and anxiety-related disorders, but critical to both the maintenance of addiction and relapse following abstinence. In both human and animal studies, compounds that activate the bed nucleus of the stria terminalis (BNST) have roles in stress-related behaviors and addiction processes. The activation of pituitary adenylate cyclase activating peptide (PACAP) systems in the BNST mediates many consequences of chronic stressor exposure that may engage in part downstream corticotropin releasing hormone (CRH) signaling. Similar to footshock stress, the BNST administration of PACAP or the PAC1 receptor-specific agonist maxadilan can facilitate relapse following extinction of cocaine-seeking behavior. Further, in the same paradigm, the footshock-induced relapse could be attenuated following BNT pretreatment with PAC1 receptor antagonist PACAP6–38, implicating PACAP systems as critical components underlying stress-induced reinstatement. In congruence with previous work, the PAC1 receptor internalization and endosomal MEK/ERK signaling appear contributory mechanisms to the addiction processes. The studies offer new insights and approaches to addiction and relapse therapeutics.
Introduction
The addiction to drugs of abuse, such as tobacco, alcohol, opioids, and illicit drugs is a growing problem that affects the welfare of individuals worldwide. A diagnosis of substance use disorders is based upon a pathological set of behaviors related to the use of a given substance, including impaired control, social impairment, risky use, and pharmacological indicators, such as withdrawal and tolerance. Substance abuse is typically characterized by recurring episodes of relapse following extended drug-free periods (Koob and Volkow, 2010; Erb, Shaham and Stewart, 1998). The debilitating condition represents a state whereby drug seeking and subsequent self-administration, dominate a user’s thoughts and behaviors. For some individuals, the tendency to abuse drugs continues despite detrimental side effects, such as health problems and lost work productivity (Feit and Taylor, 2015). Multiple factors contribute to substance abuse, including the dysregulation of reward systems, environmental stimuli, and vulnerable genes (Jasinska et al., 2014; Levran et al., 2014). The tendency of users to relapse severely hinders the adequate treatment of substance abuse disorders. Understanding the behavioral and physiological mechanisms of relapse processes in substance abuse disorders is critical to determine viable targets for relapse prevention.
In chronic users, drug-taking behavior progresses in a three-stage cycle that includes the “dark side of addiction” (see below; Koob & Le Moal, 2005; Koob, 2010): 1) reinforcing effects (i.e. binge/intoxication), 2) negative affect (i.e. withdrawal), and 3) anticipation (i.e. craving/relapse). Notably, brain regions associated with emotional responding may be involved in these phases of addiction. For example, the central amygdala (CeA), demonstrates a key negative-reinforcement function in the acute reinforcing effects of drugs of abuse. Intra-CeA infusion of dopamine D1 receptor antagonists block cocaine self-administration (Caine et al., 1995), and lesions of the CeA block oral alcohol consumption (Möller et al., 1997). As the CeA and bed nucleus of the stria terminalis (BNST) share similar morphologies, and are in close proximity, we and others have suggested that the BNST may also contribute to negative reinforcement of drug taking.
Again, a primary challenge in the treatment of substance abuse is the tendency of users to relapse following acute or extended periods of abstinence. On average, over 60% of substance abusers will return to drug use within a year of receiving treatment (Scott et al., 2011; Higgins et al., 2012), and while many factors may contribute to drug relapse, one critical factor may be stressor exposure (Sinha et al., 2006). Relapse rates, for example, are often higher following stressful events (Breese et al., 2011). In laboratory rats, exposure to stressful stimuli can produce the “reinstatement” of drug-seeking behaviors following periods of abstinence. Hence, rats are initially allowed to self-administer drugs of abuse, (i.e. cocaine) via an operant response such as a lever press, and after this acquisition period, responding is extinguished when subsequent lever presses no longer deliver the drug reinforcer. After extinction, responding returns following exposure to a stressor, such as unpredictable footshock. Relapse-like returns in behavioral responding for the drug may also be observed after drug re-exposure (drug-priming), and drug cues, although these forms of relapse may involve differing underlying neurocircuitry (Miles et al., 2017; Mantsch et al., 2015; Erb & Stewart, 1999; Carroll & Comer, 1996).
Drugs of abuse can simultaneously activate both reward and stress circuits, and while stress itself may cause relapse, the underlying mechanisms are unclear. However, the activation of central and peripheral stress responses likely becomes a part of the context of drug taking. Stress might therefore create a “renewal effect” and cause relapse of responding when it occurs again after extinction has been conducted in the absence of stress (see Bouton, 2014; Schepers, 2017; Miles et al., 2017), which Bouton and colleagues have called the contextual hypothesis of stress-induced reinstatement. Upon stressor exposure, several stress-response systems are activated, including the hypothalamic pituitary adrenal (HPA) axis and the sympathetic nervous system (SNS), as well as behavioral responding, such as the neural circuits important for emotional behavior such as fear and anxiety. Brain areas involved in regulating these physiological and emotional processes may undergo neurochemical and morphological plasticity changes following stressor exposure, and these changes may underlie several mental health disorders (such as post-traumatic stress disorder (PTSD) and depression; Hammack et al., 2010). Indeed, the activation of these systems may also be critical to both the maintenance and relapse of addiction.
BNST in Stress and Addiction Processes
The BNST forms part of the anterior aspect of a continuum called the central extended amygdala (Alheid and Heimer, 1988; Heimer and Alheid, 1991; but see also Swanson and Petrovich, 1998) that has been widely implicated in mediating behavioral responses to anxiety and stressor exposure in both clinical and laboratory studies (Choi et al., 2008; Walker et al., 2003; Avery et al., 2016). Anatomically, the BNST is located ventral to the septum and extends both above and below the anterior commissure (Dong et al., 2001); it is often divided into anterior and posterior regions by fibers of the stria terminalis (Ju & Swanson, 1989). The anterior region of the BNST can be further divided into several sub-regions including the anterolateral, anteromedial, oval, dorsomedial, and magnocellular nuclei (Dong et al., 2001). The posterior, in contrast, is sub-divided into only three sub-regions, the principal, transverse, and interfascicular nuclei. Both the anterior and posterior BNST have distinct efferent and afferent intra-BNST projections, as well as projections to distal CNS targets. While the sub-regions of the BNST may differentially regulate stress responding, the anterolateral division has been largely associated with the behavioral consequences of stressor exposure (Choi et al., 2007).
The phenotypic heterogeneity of BNST neurons may be critical in mediating the stress and behavioral responses. Neurons within the different BNST sub-nuclei endogenously express numerous receptors and neuropeptides; the oval nucleus (BNSTov) in particular has been shown to express the stress peptides corticotropin releasing hormone (CRH) and pituitary adenylate cyclase activating peptide (PACAP), among others. In addition, the BNSTov receives peptidergic inputs from the CeA (an area responsible for autonomic and behavioral components of emotion); the basolateral amygdala (BLA, necessary for eliciting fear responses), parabrachial nucleus (PBn, a sensory integration center), hippocampus, and medial prefrontal cortex (mPFC). The BNST also projects to the paraventricular nucleus (PVN), limbic structures, and brainstem nuclei that mediate emotional behavior (see Crestani et al., 2013). Hence, the BNST is thought to represent a nexus of communication networks relaying information among limbic structures and emotional processing centers, and therefore may be a central mediator of stress-related behaviors (Roman et al., 2014; Hammack and May, 2015; Walker et al., 2010; Walker and Davis, 2008; Herman et al., 1996; Herman, 2012; Duvarci et al., 2009; Gungor and Paré, 2016). However, as repeated or chronic stress exposures may facilitate changes in BNST cytoarchitecture and neurotransmitter/neuropeptide expression, the resulting neuroplasticity may be the underlying driver of maladaptive stress- and addiction-related responses (i.e., stress induced relapse). In a 28- day study of unpredictable stress in rats, for example, there was an apparent increase in BNST volume and stress-associated anxiety-like behavior (Pego et al., 2008; McEwen and Chattarji, 2007). However, not all BNST sub-regions may demonstrate similar levels of neuroplasticity to stressor challenges; some BNST nuclei may be more sensitive than others with differential responses and consequences. Our previous work, for example, demonstrated increased PACAP (Figure 1; discussed below) and PAC1 receptor transcript levels in the anterior dorsolateral BNST (BNSTdl), but not the ventral BNST (vBNST) following a repeated variate stress paradigm in rats (see also, Hammack et al., 2009). Similarly, the same stress paradigm can increase uniquely BNSTdl CRH mRNA expression, which has been associated with enhanced anxiety-like behavior (Lee and Davis, 1997; Schulkin et al., 1998). Hence, BNSTdl PACAP and CRH signaling may be tethered and activated upon stress and addiction/relapse processes.
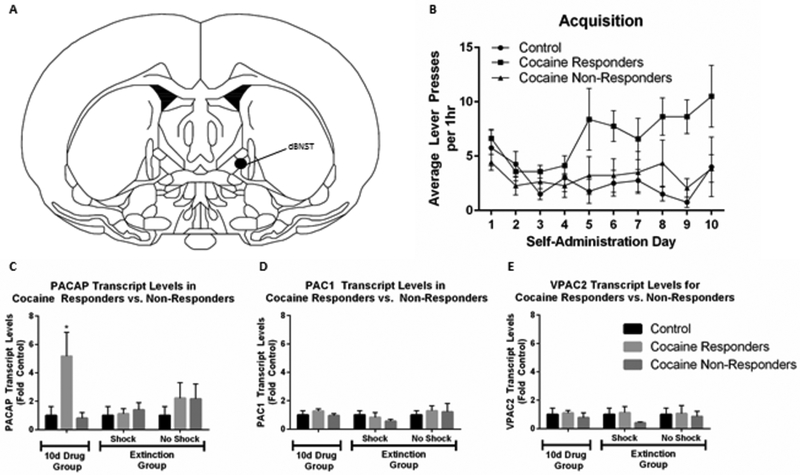
Figure 1.
Cocaine self-administration alters endogenous PACAP levels in the BNST. Adult male rats self-administered cocaine for 10d, and several underwent extinction training. dBNST was harvested for quantitative PCR analysis after 10d self-administration (Group 10d Drug), or after self-administration and then 20d Extinction Training (Group Extinction). All tissue samples were reverse transcribed with random hexamers to allow quantification and normalization across samples against 18S RNA. (A) Schematic depicting location of dorsal BNST punches. (B). Self-administration training on active lever; inactive lever responses remained uniformly low throughout acquisition (data not shown). (C) 10d cocaine self-administration increases PACAP in cocaine responders, but not non-responders in dBNST. (D, E) PAC1 and VPAC2 receptor transcript expression levels were not altered in these studies; transcript levels for CRH were also not changed (not shown). Reproduced with permission from Miles et al., 2017, Neuropsychopharmacology, 43, 978–986.
The Dark Side of Addiction
Although many brain regions differentially participate in the negative effects of withdrawal, including the nucleus accumbens (Walker and Koob, 2008), lateral habenula (Hikosaka, 2010), and central amygdala (Koob and Volkow, 2016), a critical anatomical substrate may be the extended amygdala (Heimer and Alheid, 1991), especially the BNST. As described earlier, the BNST is well situated to integrate stress regions with hedonic processing systems to produce in net, the negative emotional states associated with discontinued drug use. Koob and colleagues (Koob and Le Moal, 2001; Koob and Le Moal, 2008; Koob, 2015; Koob and Volkow, 2016) have argued that the BNST is an important part of an anti-reward system recruited by drugs of abuse, and sensitized over the course of addiction, which they refer to as the “dark side of addiction.” Developed to uniquely explain adaptations akin to opponent processes that occur in response to excessive activation of the brain reward system, the anti-reward system (which is thought to activate central stress circuits) becomes activated following excessive reward pathway activity (Koob & Le Moal, 2008). Furthermore, these researchers argue that two major adaptive changes occur following repeated drug use: 1) a within-system neural adaptation due to drug use that results in a reduction in the activity of reward circuits, and 2) the recruitment of anti-reward systems resulting from heightened activation of stress-related pathways, including those from the BNST, to limit or diminish reward. Indeed, Koob and colleagues (Koob, 2008; 2015; Koob and Le Moal, 1997; 2008) argue that the activation of CNS systems that contribute to the stress-related behavioral abnormalities may be engaged in part to combat the negative effects of abusive drugs and help restore body function, and that the recruitment of these systems underlies the loss of control observed as drug use transitions into addiction. The HPA axis (Goeders, 2002) and extended amygdala brain regions (Koob and Le Moal, 1997) are altered by drug self-administration, and as withdrawal symptoms develop and progress, the drug-induced neuroplasticity facilitates the brain stress systems to become more responsive than brain reward systems (however, see Koob, 2016; Ramsay et al., 2014). Stress neurocircuit activation of CRH (Koob and Le Moal, 2001), norepinephrine (Carlezon et al., 2000), and PACAP (Miles et al., 2017) during withdrawal are primary drivers of negative emotional states, and may drive an antireward system, such that neuroadaptations may occur after drug use that serve to limit reward (Koob and Le Moal, 1997; 2008).
Corticotropin Releasing Hormone (CRH)
CRH is a key regulator of stress responses (Bale and Vale, 2004; Dedic et al., 2018; Slominski et al., 2013) and causes the stimulation of glucocorticoid secretion (Spiess et al., 1981; Majzoub, 2006). CRH is released in the brain in response to stress (Koob, 1999; Dunn and Berridge, 1990), and initiates the HPA axis neuroendocrine stress response by binding to CRF1 receptors in the anterior pituitary upon stressor exposure (Vale et al., 1981; Bale and Vale, 2004). However, CRF1 receptors are abundant in stress-related brain regions, including the septum (Price et al., 2002; Sakanaka et al., 1988), hippocampus (Hauger et al., 2006), cerebellum (King and Bishop, 2002), neocortex (Radulovic et al., 1998), and thalamus (Tjong et al., 2010; Pett et al., 2000), and may mediate behavioral stress responses. Indeed, the BNST is an area of dense CRH expression (Herman et al., 1994) that may play a role in mediating stress responses. Administration of CRH produces the same physiological and behavioral effects as stressor exposure (see Dunn and Berridge, 1987; Bangasser and Kawasumi, 2015; Lezak et al., 2017). In a clinical setting, patients exhibiting symptoms of major depressive disorder (MDD), PTSD, and suicidal thoughts (Ressler et al., 2011; Arato et al., 1989; Baker et al., 1999; Nemeroff et al., 1991) have higher levels of CRH in their system than healthy counterparts. Depressed individuals also have heightened levels of CRH mRNA in extended amygdala areas (Merali et al., 2006). Furthermore, Dunn and colleagues demonstrated that central CRH administration to rodents produces the same physiological and behavioral effects as stressor exposure such as increased heart rate, low sex drive (Dunn and Berridge, 1987; 1990; see also McEwen, 1998; 2008; Lupien et al., 2009; Sapolsky, 2004), increased anhedonia and anorexic-like behaviors and reduced social interactions (Dunn and File, 1987).
CRH systems have also been proposed to play a role in the three stages of the addiction cycle (discussed above; Zorrilla et al., 2014), but may be particularly important for the withdrawal/negative affect stage (Koob, 2010; Richter and Weiss, 1999; Zorrilla et al., 2001). Notably, withdrawal from multiple drugs of abuse, including alcohol (Roberto et al., 2010), nicotine (George et al., 2007), opioids (Weiss et al., 2001), and cocaine (Richter and Weiss, 1999) increases extracellular CRH in rodents. Furthermore, infusions of CRH into the extended amygdala region appear to mimic consequences of stressor exposure after a period of drug exposure (Logril et al., 2011; Wise and Morales, 2010; George et al., 2007; Buffalari et al., 2012; Erb & Stewart, 1999; Shaham et al., 2000). Activation of the HPA axis during periods of withdrawal may also be CRH-activation dependent (Zorrilla et al., 2014), as small molecular CRH receptor antagonists reduce anxiety- and stress-like behaviors during withdrawal (Sommer et al., 2008). CRH R-1 knockout mice are also resistant to the effects of stress on increased drug-taking (Pastor et al., 2011; Molander et al., 2012), suggesting that CRH systems play a critical role in stress/withdrawal interactions. Many studies have also investigated the neural circuits underlying stress-induced reinstatement to drug-seeking (Kalivas & Volkow, 2016). Erb and Stewart (1999) for example, have implicated activation of CRH receptors in stress-induced relapse. Additionally, Shaham & colleagues (1997) demonstrated that an acute intracerebroventricular (ICV) injection of CRH mimicked stressor exposure and caused reinstatement, whereas the ICV injection of CRH receptor antagonists α-helical CRH, D-Phe CRH or CP-154.526 can block stress-induced reinstatement in heroin- or cocaine-trained rats, and reinstatement of drug-seeking behavior (Shaham et al. 1998). However, when tested in drug-primed animals, these antagonist manipulations had no effect, indicating an important role of CRH specifically in stress-induced reinstatement paradigms. Initially, the sites of these CRH actions were unclear; however, subsequent studies have implicated the BNST as a critical site for stress-induced reinstatement of drug- seeking behavior. The results of previous studies are consistent with hypotheses suggesting that BNST stress systems have a role in the vulnerability to relapse following stressor exposure (Miles et al., 2017; Koob & Le Moal, 2008); the BNST has been argued to play a key role in the anti-reward system that may be upregulated by repeated administration of drugs of abuse (Koob & Le Moal, 2008; 2001). Stressor exposure may promote relapse by activating some of the same anti-reward circuits associated with withdrawal (Koob, 2015). Both prior and current data suggest that recruitment of BNST PACAP and CRH circuits may be key features of the anti-reward system that sustains addiction and promotes relapse.
In animal models, many of the behavioral consequences of stressor exposure, including anxiogenic-like responses on elevated plus maze (Sarnyai et al., 2005; Navarro-Zaragoza et al., 2010), conditioned place aversion to drugs of abuse (Hand et al., 1988; Contarino and Papaleo, 2005) and reward thresholds produced by drug withdrawals, are reduced following CRH receptor antagonist administration (Bruijnzeel et al., 2010). Our lab and others (Tsukiyama et al., 2011; Hammack & May, 2014; Roman et al., 2014) have argued that in central circuits, PACAP may be upstream regulators of BNST CRH neurons and may therefore play a prominent role in modulating an extra-hypothalamic CRH function. Prior work has demonstrated that PACAP6–38 abates CRH production and transcripts in the BNST; hence, upstream BNST PACAP signaling may be necessary for CRH expression and function in a variety of stress-related circuits including the PVN in the hypothalamus. Finally, the functional associations between PACAP signaling and CRH were demonstrated in heterogeneous antagonist studies. Among many studies, the intracerebroventricular (ICV) administration of PACAP into rats, for example, reduced sensitivity to sucrose reward preference and intracranial self-stimulation (ICSS) tasks (Dore et al., 2013; Seiglie et al., 2015), and the effects could be blocked by CRH receptor antagonists. Hence, PACAP signaling may mediate the activation of the HPA axis in ways that may be associated or independent from CRH receptor signaling.
Pituitary adenylate cyclase activating peptide (PACAP)
First identified in ovine hypothalamic extracts for its ability to stimulate cyclic adenosine monophosphate (cAMP; Miyata et al., 1989) production in anterior pituitary cells, PACAP has since been identified as a pleiotropic peptide important in the homeostasis of many physiological systems, including the central regulation of stress-related circuits and function (Hammack & May, 2015; Stroth et al., 2011; Vaudry et al., 2000). As a neurotrophic factor, PACAP promotes cell survival of multiple neuron types, including progenitor cells, dorsal root ganglion cells, cerebellar granule cells, and peripheral sympathetic neurons in development or upon pro-apoptotic challenges (May et al., 2010; Stroth et al., 2011; 2013). As a neurotransmitter PACAP can increase neuronal excitability by gating a variety of channels via diverse signaling pathways. The peptide belongs to the vasoactive intestinal peptide (VIP) – secretin – growth-hormone releasing hormone (GHRH) – glucagon family (Miyata et al., 1989, 1990); the PACAP cDNA contains encodes a 176-amino-acid precursor that is posttranslationally processed into either the 38- or 27-amino-acid bioactive forms. For nearly all tissues, PACAP38 levels are typically 10- to 100-fold greater than those for PACAP27 (Vaudry et al., 2009). PACAPergic fibers innervate multiple stress-related regions, and high PACAP fiber densities have been identified in the hypothalamus, amygdala, and BNST. The high levels of PACAP expression in these limbic structures have implicated PACAP roles in stress responses (Hammack et al., 2009; Stroth et al., 2011). PACAP binds to three receptor subtypes; PACAP binds selectively at the PAC1 receptor, whereas both PACAP and VIP bind with near equal high affinity at VPAC1 and VPAC2 receptors (for reference, see May et al., 2010). Activation of PAC1 receptors can engage multiple signaling cascades including adenylyl cyclase/cAMP/PKA, phospholipase C/diacylglycerol/PKC/IP3, MEK/ERK and Akt. Further, PAC1 receptor isoform expression from the alternative splicing of the Hip and/or Hop cassettes into the segment encoding the third cytoplasmic loop have the potential of directing signaling specification and duration. VPAC receptors, by contrast, appear to be coupled primarily to cAMP pathways. Although the expression and wide distribution of PAC1 and/or VPAC receptors contribute to the broad and diverse actions of PACAP, notably the stress-and addition-related responses of PACAP appear to be mediated predominantly by the PACAP-selective PAC1 receptor.
PACAP & PAC1 receptors in emotional behaviors
As studies from several laboratories have shown that PACAP plays a crucial role in stress responding (for review, see Hammack et al., 2009; 2010; Lezak et al., 2014; Mustafa et al., 2015; Missig et al., 2014) we have hypothesized that PACAP may also participate in addiction processes. Indeed, repeated drug use exhibits many similarities to those of chronic stress, such as interpersonal conflicts, sleep deprivation, and loss of work productivity on a behavioral level, increased HPA axis activation, and increased PACAP mRNA transcripts on a physiological level. Hence, given that we have heavily implicated BNST PACAP systems as critical for many of the consequences of chronic stress (Hammack et al., 2009; Lezak et al., 2014; Missig et al., 2014; Roman et al., 2014; see below), PACAP may play a key role in mediating emotional responding following repeated drug use. PACAP and PAC1 receptors are highly expressed in areas that project to the HPA axis, and several studies have demonstrated the importance of PACAP in HPA signaling (Stroth et al., 2011). Furthermore, Stroth and colleagues (2011) demonstrated increased CRH transcription in response to PACAP in hypothalamic cells, and ICV PACAP administration increased CRH mRNA in the PVN (Hashimoto et al., 2011). Finally, PACAP null mice have impaired long-term HPA responses to chronic stressor, which is consistent with PACAP roles in HPA activation for stress responses (Vaudry et al., 2005; Hammack et al., 2010).
Our laboratory has demonstrated that an intra-BNST infusion of PACAP mimics the behavioral and physiological responses to stressor exposure (see Hammack et al., 2009; 2010; Miles et al., 2017). For example, when exposed to a seven-day chronic variate stress paradigm, rats exhibited anxiety- and anorexic-like behaviors that correlated with significant increases in PACAP transcript and protein levels, as well as PAC1 receptor transcripts in the BNST (Hammack et al., 2009). Bilateral intra-BNST infusion of PACAP on its own was sufficient to induce the same stress-related anorexic- and anxiety-like behaviors (Kocho-Schellenberg et al., 2014; Lezak et al., 2014; Roman et al., 2014). Indeed, BNST PACAP infusions in rats mimicked chronic stress-related responses in increased startle, anorexia and weight loss, and elevated circulating corticosterone levels (Hammack et al., 2009; Roman et al., 2014; Kocho-Schellenberg et al., 2014; Lezak et al., 2014). By contrast, bilateral chronic intra-BNST infusions of the PAC1/VPAC2 receptor antagonist PACAP6–38 reduced the stress-induced consequences of repeated variate stress, suggesting that endogenous BNST PACAP signaling is necessary for the stress responses (Roman et al., 2014). In aggregate, these data implicated PACAP as an important regulator of stress-related pathologies (Hammack et al., 2012; Hammack and May, 2015). Dysregulation of PACAP systems has also been implicated in stress-related disease in humans. Circulating PACAP levels and a single nucleotide polymorphism in the PAC1 receptor gene have both been associated with PTSD and other stress-related disorders in women (Ressler et al., 2011; Hashimoto et al., 2011; Hashimoto et al., 2016), to further implicate PACAP/PAC1 receptor function in stress- and anxiety-related states.
In addition to PACAP regulation of CRH production and transcripts, the close proximity of PACAP fiber terminals to the soma of CRH neurons provide physical evidence for direct PACAP – CRH links in the same stress circuits. PACAP-immunoreactive fiber terminals, for example, were identified to decorate the environs of CRH-expressing neurons in the PVN and BNST (Sherwood et al., 2000; Roman et al., 2014; Missig et al., 2014). Hence, the effects of PACAP may rest in part on downstream regulation of CRH expression and function, resulting in HPA activation for corticosterone release, even though increased plasma corticosterone levels itself may not necessarily require CRH receptor signaling (Meloni et al., 2016; Lezak et al., 2014; Norrholm et al., 2005). That CRH neurons represent one of the main targets of PACAP is further supported by studies with PACAP knockout mice, in which corticosterone release is diminished compared to wildtype mice, in response to chronic stressor exposure, open field or social defeat stress (Stroth & Eiden, 2010; Tsukiyama et al., 2011; Lehmann et al., 2013). Consistent with these mechanisms, Roman et al. (2014) showed that PACAP receptor antagonism via BNST PACAP6–38 administration during a chronic variate stress paradigm reduces corticosterone release. These effects are likely mediated by PAC1 receptors (rather than VPAC1 or VPAC2), as the PAC1 receptor specific agonist maxadilan, mimicked many PACAP-mediated stress effects in the extended amygdala (Missig et al., 2014; 2017; Clason et al., 2016).
PACAP & PAC1 receptors in addiction-related behaviors
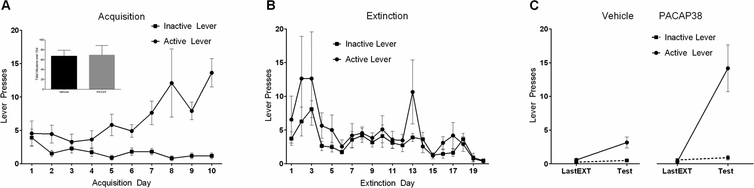
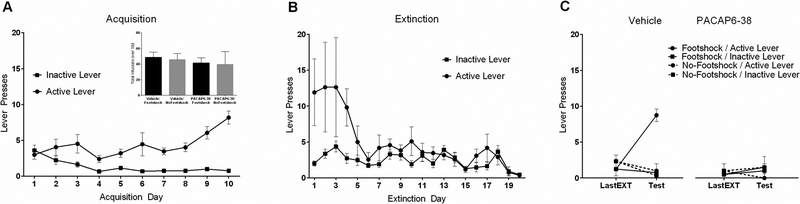
We recently demonstrated the effects of PACAP signaling on stress-induced reinstatement and drug-seeking (Miles et al., 2017). Rats with jugular vein catheter implants were trained to self-administer cocaine (3 mg/ml) on an active lever over 10 days (daily 1 h sessions) and the behavior was allowed to extinguish over the next 20 sessions. We found that acute footshock stress was able to reinstate the previously extinguished cocaine seeking behavior and that the response could be completely mimicked by PACAP infusion alone (no shock) into the BNST (Figure 2). Furthermore, the PACAP effects were completely recapitulated upon BNST infusions with the PAC1 receptor-specific agonist, maxadilan, demonstrating that the responses were generated solely by PAC1 receptor signaling and not by VPAC receptor activation (unpublished data). In addition, BNST infusions of the PAC1 receptor antagonist PACAP6–38, immediately before the footshock stressor exposure, attenuated reinstatement to drug seeking, implicating PACAP receptor signaling in the stress-induced reinstatement (Figure 3). Similar to the changes observed following chronic stressor exposure (Hammack et al., 2009), 10 days of cocaine self-administration training elevated BNST PACAP transcripts approximately 5-fold in the dorsal aspect of the anterolateral BNST (dBNST) in cocaine-responding animals compared to cocaine non-responding animals (Figure 1). Importantly, this increase in PACAP expression appeared to be a result of chronic cocaine self-administration as acute (1d) cocaine administration did not significantly alter PACAP mRNA levels compared to controls (Miles et al., 2017). Further, there were no differences in BNST PACAP transcript levels compared to controls following extinction training, indicating that the increases in PACAP transcript expression following chronic cocaine self-administration could be reversed by extinction, and that BNST PACAP transcript levels were not elevated by footshock for reinstatement (Miles et al., 2017). From chronic cocaine administration, the resulting neuroplasticity in the BNST may have augmented neuronal PACAP production and content such that footshock may have increased PACAP release and signaling without apparent changes in PACAP mRNA. Our laboratory, for example, has previously shown a change in both PACAP transcript and PACAP immunostaining in the dorsal aspect of the anterolateral BNST following chronic stress (Hammack et al., 2009; Missig et al., 2014; Roman et al., 2014); whether comparable changes are present following cocaine administration are currently under study. The chronic cocaine-induced increase in PACAP mRNA appeared unique; the transcript levels of CRH and VIP peptides, and VPAC1 VPAC2 and PAC1 receptors, appeared unchanged at all phases of the study paradigm. This appears consistent with previous work in which chronic stress produced a small increase in BNST PAC1 receptor transcripts, but not CRH, VIP or VPAC1/VPAC2 receptor transcripts (Lezak et al., 2014; Hammack et al., 2009).
Figure 2.
Intra-BNST PACAP38 causes reinstatement of extinguished cocaine seeking. (A) Self administration training. Animals in both the PACAP38 and Vehicle groups received the same amount of cocaine during acquisition (inset). (B) Extinction training. Extinction sessions occurred during the 20 days after self-administration training. Alarm signal on day 13 of extinction induced relapse response (active lever peak), demonstrating the effects of acute stress. (C) PACAP38 infusion caused significant reinstatement on the active lever (previously associated with cocaine delivery) during test for reinstatement compared with the last day of extinction (1 day before test). Reproduced with permission from Miles et al., 2017, Neuropsychopharmacology, 43, 978–986.
Figure 3.
Intra-BNST PACAP antagonist (PACAP6–38) attenuates stress-induced reinstatement in rats. (A) Self-administration training. Animals in all groups received the same amount of cocaine during acquisition (inset). (B) Extinction training. (C) Intra-BNST delivery of PACAP antagonist significantly attenuated shock-induced reinstatement. Only animals that received intra-BNST vehicle treatment before footshock demonstrated reinstatement of extinguished drug-seeking behavior. Reproduced with permission from Miles et al., 2017, Neuropsychopharmacology, 43, 978–986.
Although BNST CRH transcripts levels were not altered following chronic cocaine self-administration, the stress-induced increase in BNST PACAP transcript levels and immunoreactivity, and the apposition of BNST PACAP terminals onto BNST CRH neurons, implicate PACAP signaling onto all or discrete populations of CRH neurons (Marcinkiewcz et al., 2016) to affect stress and reinstatement activities. Interestingly, Missig et al. (2017) showed that chronic pain elevated PACAP transcript levels in the PBn neurons, but not those in CeA, BNST, or solitary nucleus (NTS) suggesting that changes in transcript expression may be stressor specific in different central nuclei. Again, comparable to previous work, acute stressor exposure or a single day of cocaine self-administration did not appear to increase PACAP transcript levels (Miles et al., 2017; Lezak et al., 2014) indicating that the regulation PACAP transcript appear unique in that only sustained emotional stress may drive its expression.
Stress-induced reinstatement requires PAC1 receptor endosomal MEK signaling
Previous studies have shown that the many effects of PACAP result not only from plasma membrane delimited Gαs/adenylyl cyclase or Gαq/phospholipase C signaling, but also from β-arrestin-mediated endosomal MEK/ERK activation. Internalized PAC1 receptor endosomal signaling has been shown to be critical in regulating membrane excitability in cardiac neurons; more pertinently, CeA PAC1 receptor internalization and endosomal MEK/ERK signaling appear to participate in thermal pain hypersensitivity and stress-related behaviors (Missig et al., 2017). Hence, we are currently examining whether similar PAC1 receptor mechanisms participate in reinstatement. In accord, cocaine experience may potentiate footshock-induced ERK activation (phosphorylated ERK, pERK) via a PAC1 receptor mechanism and blockade of endogenous BNST PACAP signaling with PACAP6–38 may attenuate stress-induced reinstatement in parallel with decreased pERK-immunoreactivity in the BNST. Furthermore, we hypothesize that PACAP-mediated BNST ERK activation will diminish upon pretreatments with clathrin-mediated endocytosis inhibitor Pitstop2 or MEK inhibitor PD98059; both inhibitor treatments may also block PACAP-induced reinstatement in drug-seeking behaviors. These ongoing experiments could identify common PAC1 receptor activation mechanisms in stress and relapse processes; results will further our understanding of CNS PACAP mechanisms and may suggest that adaptations to the BNST PACAP system may negatively affect the outcome of individuals striving to abstain from drug use.
Relation to human studies and clinical trials
Several studies have reported that during abstinence from drug use, stress exposure often occurs in the presence of contextual cues that had previously been associated with drug use (Epstein et al., 2009). These cues may cause an initial lapse in abstinence. Additionally, in a manner similar to the drug-cue induced reinstatement (better termed “renewal”; see Bouton, 2014), human studies have indicated that stressor exposure can lead to cue-induced drug craving (Moran-Santa Maria et al., 2015; Fox et al., 2014; Mantsch et al., 2015). Sinha and colleagues (1999; 2001) introduced a clinical trial based on the rodent stress-induced reinstatement paradigm and from these studies, stressor exposure, through guided imagery stress or Trier Social Stress Task, caused an increase in drug craving. Although systemic CRH injections into humans can induce physiological (i.e. increased cortisol release) and psychological (i.e. increased anxiety) stress responses, offering a potential approach to attenuate stress-induced relapse, none of the drugs to date appear effective (Sinha et al., 2007; Fox et al., 2012; McKee et al., 2014; Moran-Santa Maria et al., 2015); our recent studies on PACAP mechanisms in addiction may offer novel avenues for potential therapeutics. PACAP is ubiquitous and regulates several other physiological systems including cardiac function and vascular function, among others (Braas et al., 1998; Braas et al., 2004; Clason et al., 2016; Bertrand et al., 1996). However, the diversity of receptor isoforms as well as other targeting strategies could potentially help limit the actions of PACAP-related therapies to specific brain regions.
PACAP and the Dark Side of Addiction
PACAP has been widely appreciated as a critical neuropeptide in regulating homeostasis in a variety of physiological systems, particularly those related to stress physiology. PACAP signaling activates both the physiological and behavioral responses to stress, and congruently, as several phases of addiction have been shown to activate stress response systems, our recent studies have been important in demonstrating that PACAP may have central contributory roles in drug-taking processes. As our lab and others have argued that PACAP may be upstream of CRH, we propose PACAP may be an important element initiating the plasticity that results in activation of the upregulated anti-reward system Koob and colleagues have called the Dark Side of Addiction. Notably, our work demonstrates for the first time the involvement of PACAP/PAC1 receptor activation in stress-induced reinstatement to drug seeking, and that PAC1 receptor endocytosis and endosomal MEK/ERK signaling may represent novel mechanisms in stress and relapse. These PACAP receptor mechanisms may represent new molecular targets for therapeutics and continued work in these models may reveal how PACAP signaling may contribute to the neuroplasticity that drives circuit dysregulation to facilitate psychiatric disorders such as addiction, PTSD, anxiety and other stress-related disorders. The advances have been rapid and in aggregate, the current prospects for deciphering PACAP/PAC1 receptor roles in stress-related disorders, including addiction and relapse are promising.
References
- Alheid GF, & Heimer L (1988). New perspectives in basal forebrain organization of special relevance for neuropsychiatric disorders: the striatopallidal, amygdaloid, and corticopetal components of substantia innominata. Neuroscience, 27(1), 1–39. [DOI] [PubMed] [Google Scholar]
- Alheid GF, Beltramino CA, De Olmos JS, Forbes MS, Swanson DJ, & Heimer L (1998). The neuronal organization of the supracapsular part of the stria terminalis in the rat: the dorsal component of the extended amygdala. Neuroscience, 84(4), 967–996. [DOI] [PubMed] [Google Scholar]
- Arató M, Bánki CM, Bissette G, & Nemeroff CB (1989). Elevated CSF CRF in suicide victims. Biological psychiatry, 25(3), 355–359. [DOI] [PubMed] [Google Scholar]
- Avery SN, Clauss JA, & Blackford JU (2016). The human BNST: functional role in anxiety and addiction. Neuropsychopharmacology, 41(1), 126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bale TL, & Vale WW (2004). CRF and CRF receptors: role in stress responsivity and other behaviors. Annu. Rev. Pharmacol. Toxicol, 44, 525–557. [DOI] [PubMed] [Google Scholar]
- Baker DG, West SA, Nicholson WE, Ekhator NN, Kasckow JW, Hill KK, … & Geracioti TD Jr (1999). Serial CSF corticotropin-releasing hormone levels and adrenocortical activity in combat veterans with posttraumatic stress disorder. American Journal of Psychiatry, 156(4), 585–588. [DOI] [PubMed] [Google Scholar]
- Bangasser DA, & Kawasumi Y (2015). Cognitive disruptions in stress-related psychiatric disorders: A role for corticotropin releasing factor (CRF). Hormones and behavior, 76, 125–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder EB, & Nemeroff CB (2010). The CRF system, stress, depression and anxiety—insights from human genetic studies. Molecular psychiatry, 15(6), 574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouton ME (2014). Why behavior change is difficult to sustain. Preventive medicine, 68, 29–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breese GR, Sinha R, & Heilig M (2011). Chronic alcohol neuroadaptation and stress contribute to susceptibility for alcohol craving and relapse. Pharmacology & therapeutics, 129(2), 149–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruijnzeel AW, Small E, Pasek TM, & Yamada H (2010). Corticotropin-releasing factor mediates the dysphoria-like state associated with alcohol withdrawal in rats. Behavioural brain research, 210(2), 288–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buffalari DM, Baldwin CK, Feltenstein MW, & See RE (2012). Corticotrophin releasing factor (CRF) induced reinstatement of cocaine seeking in male and female rats. Physiology & behavior, 105(2), 209–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caine SB, Heinrichs SC, Coffin VL, & Koob GF (1995). Effects of the dopamine D-1 antagonist SCH 23390 microinjected into the accumbens, amygdala or striatum on cocaine self-administration in the rat. Brain research, 692(1–2), 47–56. [DOI] [PubMed] [Google Scholar]
- Carlezon WA, Haile CN, Coopersmith R, Hayashi Y, Malinow R, Neve RL, & Nestler EJ (2000). Distinct sites of opiate reward and aversion within the midbrain identified using a herpes simplex virus vector expressing GluR1. Journal of Neuroscience, 20(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll ME, & Comer SD (1996). Animal models of relapse. Experimental and Clinical Psychopharmacology, 4(1), 11. [Google Scholar]
- Choi DC, Furay AR, Evanson NK, Ostrander MM, Ulrich-Lai YM, & Herman JP (2007). Bed nucleus of the stria terminalis subregions differentially regulate hypothalamic–pituitary–adrenal axis activity: implications for the integration of limbic inputs. Journal of Neuroscience, 27(8), 2025–2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clason TA, Girard BM, May V, & Parsons RL (2016). Activation of MEK/ERK signaling by PACAP in guinea pig cardiac neurons. Journal of Molecular Neuroscience, 59(2), 309–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contarino A, & Papaleo F (2005). The corticotropin-releasing factor receptor-1 pathway mediates the negative affective states of opiate withdrawal. Proceedings of the National Academy of Sciences of the United States of America, 102(51), 18649–18654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crestani CC, Alves FH, Gomes FV, Resstel L, Correa F, & Herman JP (2013). Mechanisms in the bed nucleus of the stria terminalis involved in control of autonomic and neuroendocrine functions: a review. Current neuropharmacology, 11(2), 141–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel SE, & Rainnie DG (2016). Stress modulation of opposing circuits in the bed nucleus of the stria terminalis. Neuropsychopharmacology, 41(1), 103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M, Walker DL, Miles L, & Grillon C (2010). Phasic vs sustained fear in rats and humans: role of the extended amygdala in fear vs anxiety. Neuropsychopharmacology, 35(1), 105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dedic N, Chen A, & Deussing JM (2018). The CRF family of neuropeptides and their receptors-mediators of the central stress response. Current molecular pharmacology, 11(1), 4–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dore R, Iemolo A, Smith KL, Wang X, Cottone P, & Sabino V (2013). CRF mediates the anxiogenic and anti-rewarding, but not the anorectic effects of PACAP. Neuropsychopharmacology, 38(11), 2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong HW, Petrovich GD, Watts AG, & Swanson LW (2001). Basic organization of projections from the oval and fusiform nuclei of the bed nuclei of the stria terminalis in adult rat brain. Journal of Comparative Neurology, 436(4), 430–455. [DOI] [PubMed] [Google Scholar]
- Dumont EC, Rycroft BK, Maiz J, & Williams JT (2008). Morphine produces circuit-specific neuroplasticity in the bed nucleus of the stria terminalis. Neuroscience, 153(1), 232–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn AJ, & Berridge CW (1987). Corticotropin-releasing factor administration elicits a stress-like activation of cerebral catecholaminergic systems. Pharmacology Biochemistry and Behavior, 27(4), 685–691. [DOI] [PubMed] [Google Scholar]
- Dunn AJ, & Berridge CW (1990). Physiological and behavioral responses to corticotropin-releasing factor administration: is CRF a mediator of anxiety or stress responses?. Brain research reviews, 15(2), 71–100. [DOI] [PubMed] [Google Scholar]
- Dunn AJ, & File SE (1987). Corticotropin-releasing factor has an anxiogenic action in the social interaction test. Hormones and behavior, 21(2), 193–202. [DOI] [PubMed] [Google Scholar]
- Duvarci S, Bauer EP, & Paré D (2009). The bed nucleus of the stria terminalis mediates inter-individual variations in anxiety and fear. Journal of Neuroscience, 29(33), 10357–10361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein DH, Willner-Reid J, Vahabzadeh M, Mezghanni M, Lin JL, & Preston KL (2009). Real-time electronic diary reports of cue exposure and mood in the hours before cocaine and heroin craving and use. Archives of general psychiatry, 66(1), 88–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erb S, & Stewart J (1999). A role for the bed nucleus of the stria terminalis, but not the amygdala, in the effects of corticotropin-releasing factor on stress-induced reinstatement of cocaine seeking. Journal of Neuroscience, 19(20), RC35–RC35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erb S, Shaham Y, & Stewart J (1998). The role of corticotropin-releasing factor and corticosterone in stress-and cocaine-induced relapse to cocaine seeking in rats. Journal of Neuroscience, 18(14), 5529–5536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feit MD, & Taylor OD (2015). Contemporary Substance Use Research. [Google Scholar]
- Fox HC, Seo D, Tuit K, Hansen J, Kimmerling A, Morgan PT, & Sinha R (2012). Guanfacine effects on stress, drug craving and prefrontal activation in cocaine dependent individuals: preliminary findings. Journal of psychopharmacology, 26(7), 958–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox HC, Morgan PT, & Sinha R (2014). Sex differences in guanfacine effects on drug craving and stress arousal in cocaine-dependent individuals. Neuropsychopharmacology, 39(6), 1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George O, Ghozland S, Azar MR, Cottone P, Zorrilla EP, Parsons LH, … & Koob GF (2007). CRF–CRF1 system activation mediates withdrawal-induced increases in nicotine self-administration in nicotine-dependent rats. Proceedings of the National Academy of Sciences, 104(43), 17198–17203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goeders NE (2002). The HPA axis and cocaine reinforcement. Psychoneuroendocrinology, 27(1): 13–33. [DOI] [PubMed] [Google Scholar]
- Gungor NZ, & Paré D (2016). Functional heterogeneity in the bed nucleus of the stria terminalisfunctional heterogeneity in the bed nucleus of the stria terminalis. Journal of Neuroscience, 36(31), 8038–8049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammack SE, & May V (2015). Pituitary adenylate cyclase activating polypeptide in stress-related disorders: data convergence from animal and human studies. Biological psychiatry, 78(3), 167–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammack SE, Cooper MA, & Lezak KR (2012). Overlapping neurobiology of learned helplessness and conditioned defeat: implications for PTSD and mood disorders. Neuropharmacology, 62(2), 565–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammack SE, Roman CW, Lezak KR, Kocho-Shellenberg M, Grimmig B, Falls WA, … & May V (2010). Roles for pituitary adenylate cyclase-activating peptide (PACAP) expression and signaling in the bed nucleus of the stria terminalis (BNST) in mediating the behavioral consequences of chronic stress. Journal of molecular neuroscience, 42(3), 327–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammack SE, Cheung J, Rhodes KM, Schutz KC, Falls WA, Braas KM, & May V (2009). Chronic stress increases pituitary adenylate cyclase-activating peptide (PACAP) and brain-derived neurotrophic factor (BDNF) mRNA expression in the bed nucleus of the stria terminalis (BNST): roles for PACAP in anxiety-like behavior. Psychoneuroendocrinology, 34(6), 833–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hand TH, Koob GF, Stinus L, & Le Moal M (1988). Aversive properties of opiate receptor blockade: evidence for exclusively central mediation in naive and morphine-dependent rats. Brain research, 474(2), 364–368. [DOI] [PubMed] [Google Scholar]
- Harmar AJ, Fahrenkrug J, Gozes I, Laburthe M, May V, Pisegna JR, … & Said SI (2012). Pharmacology and functions of receptors for vasoactive intestinal peptide and pituitary adenylate cyclase‐activating polypeptide: IUPHAR Review 1. British journal of pharmacology, 166(1), 4–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto H, Shintani N, Tanida M, Hayata A, Hashimoto R, & Baba A (2011). PACAP is implicated in the stress axes. Current pharmaceutical design, 17(10), 985–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto H, Shintani N, Ago Y, Hayata-Takano A, Nakazawa T, Hashimoto R, … & Baba A (2016). Implications of PACAP signaling in psychiatric disorders In Pituitary Adenylate Cyclase Activating Polypeptide—PACAP (pp. 757–766). Springer, Cham. [Google Scholar]
- Hauger RL, Risbrough V, Brauns O, & Dautzenberg FM (2006). Corticotropin releasing factor (CRF) receptor signaling in the central nervous system: new molecular targets. CNS & Neurological Disorders-Drug Targets (Formerly Current Drug Targets-CNS & Neurological Disorders), 5(4), 453–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilig M, Koob GF, Ekman R, & Britton KT (1994). Corticotropin-releasing factor and neuropeptide Y: role in emotional integration. Trends in neurosciences, 17(2), 80–85. [DOI] [PubMed] [Google Scholar]
- Heimer L, & Alheid GF (1991). Piecing together the puzzle of basal forebrain anatomy In The basal forebrain (pp. 1–42). Springer, Boston, MA. [DOI] [PubMed] [Google Scholar]
- Herman JP, Cullinan WE, & Watson SJ (1994). Involvement of the bed nucleus of the stria terminalis in tonic regulation of paraventricular hypothalamic CRH and AVP mRNA expression. Journal of neuroendocrinology, 6(4), 433–442. [DOI] [PubMed] [Google Scholar]
- Herman JP, Prewitt CMF, & Cullinan WE (1996). Neuronal circuit regulation of the hypothalamo-pituitary-adrenocortical stress axis. Critical Reviews™ in Neurobiology, 10(3–4). [DOI] [PubMed] [Google Scholar]
- Herman JP (2012). Neural pathways of stress integration: relevance to alcohol abuse. Alcohol research: current reviews, 34(4), 441. [PMC free article] [PubMed] [Google Scholar]
- Higgins ST, Silverman K, Sigmon SC, & Naito NA (2012). Incentives and health: an introduction. Preventive medicine, 55, S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hikosaka O (2010). The habenula: from stress evasion to value-based decision-making. Nature reviews neuroscience, 11(7), 503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasinska AJ, Stein EA, Kaiser J, Naumer MJ, & Yalachkov Y (2014). Factors modulating neural reactivity to drug cues in addiction: a survey of human neuroimaging studies. Neuroscience & Biobehavioral Reviews, 38, 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju G, & Swanson LW (1989). Studies on the cellular architecture of the bed nuclei of the stria terminalis in the rat: I. Cytoarchitecture. Journal of Comparative Neurology, 280(4), 587–602. [DOI] [PubMed] [Google Scholar]
- King JS, & Bishop GA (2002). The distribution and cellular localization of CRF-R1 in the vermis of the postnatal mouse cerebellum. Experimental neurology, 178(2), 175–185. [DOI] [PubMed] [Google Scholar]
- Kocho-Schellenberg M, Lezak KR, Harris OM, Roelke E, Gick N, Choi I, … & May V (2014). PACAP in the BNST produces anorexia and weight loss in male and female rats. Neuropsychopharmacology, 39(7), 1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF (1999). Corticotropin-releasing factor, norepinephrine, and stress. Biological psychiatry, 46(9), 1167–1180. [DOI] [PubMed] [Google Scholar]
- Koob GF (2008). A role for brain stress systems in addiction. Neuron, 59(1), 11–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF (2010). The role of CRF and CRF-related peptides in the dark side of addiction. Brain research, 1314, 3–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF (2016). The neurobiology of reward and stress and its relevance for understanding drug seeking and dependence symptomatology. The Oxford handbook of substance use and substance use disorders, 1, 166–191. [Google Scholar]
- Koob GF (2015). Medications for treatment of alcoholism that derive from the dark side of addiction. Canadian Journal of Addiction, 6(1), 27. [Google Scholar]
- Koob GF, & Le Moal M (1997). Drug abuse: hedonic homeostatic dysregulation. Science, 278(5335), 52–58. [DOI] [PubMed] [Google Scholar]
- Koob GF, & Le Moal M (2001). Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology, 24(2), 97. [DOI] [PubMed] [Google Scholar]
- Koob GF, & Le Moal M (2005). Plasticity of reward neurocircuitry and the ‘dark side’ of drug addiction. Nature Neuroscience, 8(11), 1442. [DOI] [PubMed] [Google Scholar]
- Koob GF, & Le Moal M (2008). Addiction and the brain antireward system. Annu. Rev. Psychol, 59, 29–53. [DOI] [PubMed] [Google Scholar]
- Koob GF, & Volkow ND (2010). Neurocircuitry of addiction. Neuropsychopharmacology, 35(1), 217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, & Volkow ND (2016). Neurobiology of addiction: a neurocircuitry analysis. The Lancet Psychiatry, 3(8), 760–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozicz T, Vigh S, & Arimura A (1998). The source of origin of PACAP-and VIP-immunoreactive fibers in the laterodorsal division of the bed nucleus of the stria terminalis in the rat. Brain research, 810(1–2), 211–219. [DOI] [PubMed] [Google Scholar]
- Lee Y, & Davis M (1997). Role of the hippocampus, the bed nucleus of the stria terminalis, and the amygdala in the excitatory effect of corticotropin-releasing hormone on the acoustic startle reflex. Journal of Neuroscience, 17(16), 6434–6446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann ML, Mustafa T, Eiden AM, Herkenham M, & Eiden LE (2013). PACAP-deficient mice show attenuated corticosterone secretion and fail to develop depressive behavior during chronic social defeat stress. Psychoneuroendocrinology, 38(5), 702–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levran O, Peles E, Randesi M, Li Y, Rotrosen J, Ott J, … & Kreek MJ (2014). Stress-related genes and heroin addiction: a role for a functional FKBP5 haplotype. Psychoneuroendocrinology, 45, 67–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lezak KR, Roelke E, Harris OM, Choi I, Edwards S, Gick N, … & Toufexis DJ (2014). Pituitary adenylate cyclase-activating polypeptide (PACAP) in the bed nucleus of the stria terminalis (BNST) increases corticosterone in male and female rats. Psychoneuroendocrinology, 45, 11–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lezak KR, Missig G, & Carlezon WA Jr (2017). Behavioral methods to study anxiety in rodents. Dialogues in clinical neuroscience, 19(2), 181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logrip ML, Koob GF, & Zorrilla EP (2011). Role of corticotropin-releasing factor in drug addiction. CNS drugs, 25(4), 271–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupien SJ, McEwen BS, Gunnar MR, & Heim C (2009). Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nature reviews neuroscience, 10(6), 434. [DOI] [PubMed] [Google Scholar]
- Majzoub JA (2006). Corticotropin-releasing hormone physiology. European Journal of Endocrinology, 155(suppl 1), S71–S76. [Google Scholar]
- Mantsch JR, Baker DA, Funk D, Lê AD, & Shaham Y (2016). Stress-induced reinstatement of drug seeking: 20 years of progress. Neuropsychopharmacology, 41(1), 335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcinkiewcz CA, Mazzone CM, D’Agostino G, Halladay LR, Hardaway JA, DiBerto JF, … & Tipton GJ (2016). Serotonin engages an anxiety and fear-promoting circuit in the extended amygdala. Nature, 537(7618), 97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS (1998). Protective and damaging effects of stress mediators. New England journal of medicine, 338(3), 171–179. [DOI] [PubMed] [Google Scholar]
- McEwen BS, & Chattarji S (2007). Neuroendocrinology of stress In Handbook of neurochemistry and molecular neurobiology (pp. 571–593). Springer; US. [Google Scholar]
- McEwen BS (2008). Central effects of stress hormones in health and disease: Understanding the protective and damaging effects of stress and stress mediators. European journal of pharmacology, 583(2–3), 174–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee SA, Potenza MN, Kober H, Sofuoglu M, Arnsten AF, Picciotto MR, … & Sinha R (2015). A translational investigation targeting stress-reactivity and prefrontal cognitive control with guanfacine for smoking cessation. Journal of psychopharmacology, 29(3), 300–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meloni EG, Venkataraman A, Donahue RJ, & Carlezon WA (2016). Bi-directional effects of pituitary adenylate cyclase-activating polypeptide (PACAP) on fear-related behavior and c-Fos expression after fear conditioning in rats. Psychoneuroendocrinology, 64, 12–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merali Z, Kent P, Du L, Hrdina P, Palkovits M, Faludi G, … & Anisman H (2006). Corticotropin-releasing hormone, arginine vasopressin, gastrin-releasing peptide, and neuromedin B alterations in stress-relevant brain regions of suicides and control subjects. Biological psychiatry, 59(7), 594–602. [DOI] [PubMed] [Google Scholar]
- Miles OW, Thrailkill EA, Linden AK, May V, Bouton ME, & Hammack SE (2017). Pituitary Adenylate Cyclase-Activating Peptide in the Bed Nucleus of the Stria Terminalis Mediates Stress-Induced Reinstatement of Cocaine Seeking in Rats. Neuropsychopharmacology, 43, 978–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Missig G, Roman CW, Vizzard MA, Braas KM, Hammack SE, & May V (2014). Parabrachial nucleus (PBn) pituitary adenylate cyclase activating polypeptide (PACAP) signaling in the amygdala: implication for the sensory and behavioral effects of pain. Neuropharmacology, 86, 38–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Missig G, Mei L, Vizzard MA, Braas KM, Waschek JA, Ressler KJ, … & May V (2017). Parabrachial pituitary adenylate cyclase-activating polypeptide activation of amygdala endosomal extracellular signal–regulated kinase signaling regulates the emotional component of pain. Biological psychiatry, 81(8), 671–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyata A, Arimura A, Dahl RR, Minamino N, Uehara A, Jiang L, … & Coy DH (1989). Isolation of a novel 38 residue-hypothalamic polypeptide which stimulates adenylate cyclase in pituitary cells. Biochemical and biophysical research communications, 164(1), 567–574. [DOI] [PubMed] [Google Scholar]
- Molander A, Vengeliene V, Heilig M, Wurst W, Deussing JM, & Spanagel R (2012). Brain-specific inactivation of the Crhr1 gene inhibits post-dependent and stress-induced alcohol intake, but does not affect relapse-like drinking. Neuropsychopharmacology, 37(4), 1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Möller C, Wiklund L, Sommer W, Thorsell A, & Heilig M (1997). Decreased experimental anxiety and voluntary ethanol consumption in rats following central but not basolateral amygdala lesions. Brain research, 760(1–2), 94–101. [DOI] [PubMed] [Google Scholar]
- Moran-Santa Maria MM, Baker NL, Ramakrishnan V, Brady KT, & McRae-Clark A (2015). Impact of acute guanfacine administration on stress and cue reactivity in cocaine-dependent individuals. The American journal of drug and alcohol abuse, 41(2), 146–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustafa T, Jiang SZ, Eiden AM, Weihe E, Thistlethwaite I, & Eiden LE (2015). Impact of PACAP and PAC1 receptor deficiency on the neurochemical and behavioral effects of acute and chronic restraint stress in male C57BL/6 mice. Stress, 18(4), 408–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro-Zaragoza J, Nunez C, Laorden ML, & Milanés MV (2010). Effects of corticotropin-releasing factor receptor-1 antagonists on the brain stress system responses to morphine withdrawal. Molecular pharmacology, 77(5), 864–873. [DOI] [PubMed] [Google Scholar]
- Nemeroff CB, Bissette G, Akil H, & Fink M (1991). Neuropeptide concentrations in the cerebrospinal fluid of depressed patients treated with electroconvulsive therapy: corticotrophin-releasing factor, β-endorphin and somatostatin. The British Journal of Psychiatry, 158(1), 59–63. [DOI] [PubMed] [Google Scholar]
- Norrholm SD, Das M, & Légrádi G (2005). Behavioral effects of local microinfusion of pituitary adenylate cyclase activating polypeptide (PACAP) into the paraventricular nucleus of the hypothalamus (PVN). Regulatory peptides, 128(1), 33–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastor R, Reed C, Burkhart-Kasch S, Li N, Sharpe AL, Coste SC, … & Phillips TJ (2011). Ethanol concentration-dependent effects and the role of stress on ethanol drinking in corticotropin-releasing factor type 1 and double type 1 and 2 receptor knockout mice. Psychopharmacology, 218(1), 169–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pego JM, Morgado P, Pinto LG, Cerqueira JJ, Almeida OFX, & Sousa N (2008). Dissociation of the morphological correlates of stress‐induced anxiety and fear. European Journal of Neuroscience, 27(6), 1503–1516. [DOI] [PubMed] [Google Scholar]
- Price ML, Kirby LG, Valentino RJ, & Lucki I (2002). Evidence for corticotropin-releasing factor regulation of serotonin in the lateral septum during acute swim stress: adaptation produced by repeated swimming. Psychopharmacology, 162(4), 406–414. [DOI] [PubMed] [Google Scholar]
- Radulovic J, Sydow S, & Spiess J (1998). Characterization of native corticotropin‐releasing factor receptor type 1 (cRFR1) in the rat and mouse central nervous system. Journal of neuroscience research, 54(4), 507–521. [DOI] [PubMed] [Google Scholar]
- Ramsay DS, Woods SC, & Kaiyala KJ (2014). Drug-induced regulatory overcompensation has motivational consequences: Implications for homeostatic and allostatic models of drug addiction. Temperature, 1(3), 248–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ressler KJ, Mercer KB, Bradley B, Jovanovic T, Mahan A, Kerley K, … & Ramirez M (2011). Post-traumatic stress disorder is associated with PACAP and the PAC1 receptor. Nature, 470(7335), 492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter RM, & Weiss F (1999). In vivo crf release in rat amygdala is increased during cocaine withdrawal in self‐administering rats. Synapse, 32(4), 254–261. [DOI] [PubMed] [Google Scholar]
- Roberto M, Cruz MT, Gilpin NW, Sabino V, Schweitzer P, Bajo M, … & Koob GF (2010). Corticotropin releasing factor–induced amygdala gamma-aminobutyric acid release plays a key role in alcohol dependence. Biological psychiatry, 67(9), 831–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roman CW, Lezak KR, Hartsock MJ, Falls WA, Braas KM, Howard AB, … & May V (2014). PAC1 receptor antagonism in the bed nucleus of the stria terminalis (BNST) attenuates the endocrine and behavioral consequences of chronic stress. Psychoneuroendocrinology, 47, 151–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakanaka M, Magari S, Shibasaki T, & Lederis K (1988). Corticotropin releasing factor‐containing afferents to the lateral septum of the rat brain. Journal of Comparative Neurology, 270(3), 404–415. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM (2004). Why zebras don’t get ulcers: The acclaimed guide to stress, stress-related diseases, and coping-now revised and updated. Holt paperbacks. [Google Scholar]
- Sarnyai Z, Bíró É, Gardi J, Vecsernyés M, Julesz J, & Telegdy G (1995). Brain corticotropin-releasing factor mediates ‘anxiety-like’behavior induced by cocaine withdrawal in rats. Brain research, 675(1–2), 89–97. [DOI] [PubMed] [Google Scholar]
- Schepers S (2017). Renewal in the context of stress: A potential mechanisms of stress-induced reinstatement. UVM Graduate College Dissertation and Theses. [Google Scholar]
- Schulkin J, Gold PW, & McEwen BS (1998). Induction of corticotropin-releasing hormone gene expression by glucocorticoids: implication for understanding the states of fear and anxiety and allostatic load. Psychoneuroendocrinology, 23(3), 219–243. [DOI] [PubMed] [Google Scholar]
- Scott CK, Dennis ML, Laudet A, Funk RR, & Simeone RS (2011). Surviving drug addiction: the effect of treatment and abstinence on mortality. American Journal of Public Health, 101(4), 737–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- See RE, Fuchs RA, Ledford CC, & McLAUGHLIN JOSELYN (2003). Drug addiction, relapse, and the amygdala. Annals of the New York Academy of Sciences, 985(1), 294–307. [DOI] [PubMed] [Google Scholar]
- Seiglie MP, Smith KL, Blasio A, Cottone P, & Sabino V (2015). Pituitary adenylate cyclase-activating polypeptide induces a depressive-like phenotype in rats. Psychopharmacology, 232(20), 3821–3831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaham Y, Funk D, Erb S, Brown TJ, Walker CD, & Stewart J (1997). Corticotropin-releasing factor, but not corticosterone, is involved in stress-induced relapse to heroin-seeking in rats. Journal of Neuroscience, 17(7), 2605–2614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaham Y, Erb S, Leung S, Buczek Y, & Stewart J (1998). CP-154,526, a selective, non-peptide antagonist of the corticotropin-releasing factor1 receptor attenuates stress-induced relapse to drug seeking in cocaine-and heroin-trained rats. Psychopharmacology, 137(2), 184–190. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Erb S, & Stewart J (2000). Stress-induced relapse to heroin and cocaine seeking in rats: a review. Brain Research Reviews, 33(1), 13–33. [DOI] [PubMed] [Google Scholar]
- Sherwood NM, Krueckl SL, & McRory JE (2000). The origin and function of the pituitary adenylate cyclase-activating polypeptide (PACAP)/glucagon superfamily. Endocrine Reviews, 21(6), 619–670. [DOI] [PubMed] [Google Scholar]
- Sinha R, Catapano D, & O’Malley S (1999). Stress-induced craving and stress response in cocaine dependent individuals. Psychopharmacology, 142(4), 343–351. [DOI] [PubMed] [Google Scholar]
- Sinha R (2001). How does stress increase risk of drug abuse and relapse? Psychopharmacology, 158(4), 343–359. [DOI] [PubMed] [Google Scholar]
- Sinha R, Garcia M, Paliwal P, Kreek MJ, & Rounsaville BJ (2006). Stress-induced cocaine craving and hypothalamic-pituitary-adrenal responses are predictive of cocaine relapse outcomes. Archives of general psychiatry, 63(3), 324–331. [DOI] [PubMed] [Google Scholar]
- Sinha R (2007). The role of stress in addiction relapse. Current psychiatry reports, 9(5), 388–395. [DOI] [PubMed] [Google Scholar]
- Slominski AT, Zmijewski MA, Zbytek B, Tobin DJ, Theoharides TC, & Rivier J (2013). Key role of CRF in the skin stress response system. Endocrine reviews, 34(6), 827–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer WH, Rimondini R, Hansson AC, Hipskind PA, Gehlert DR, Barr CS, & Heilig MA (2008). Upregulation of voluntary alcohol intake, behavioral sensitivity to stress, and amygdala crhr1 expression following a history of dependence. Biological psychiatry, 63(2), 139–145. [DOI] [PubMed] [Google Scholar]
- Spiess J, Rivier J, Rivier C, & Vale W (1981). Primary structure of corticotropin-releasing factor from ovine hypothalamus. Proceedings of the National Academy of Sciences, 78(10), 6517–6521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroth N, & Eiden LE (2010). Stress hormone synthesis in mouse hypothalamus and adrenal gland triggered by restraint is dependent on pituitary adenylate cyclase-activating polypeptide signaling. Neuroscience, 165(4), 1025–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroth N, Holighaus Y, Ait‐Ali D, & Eiden LE (2011). PACAP: a master regulator of neuroendocrine stress circuits and the cellular stress response. Annals of the New York Academy of Sciences, 1220(1), 49–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroth N, Kuri BA, Mustafa T, Chan SA, Smith CB, & Eiden LE (2013). PACAP controls adrenomedullary catecholamine secretion and expression of catecholamine biosynthetic enzymes at high splanchnic nerve firing rates characteristic of stress transduction in male mice. Endocrinology, 154(1), 330–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson LW, & Petrovich GD (1998). What is the amygdala?. Trends in neurosciences, 21(8), 323–331. [DOI] [PubMed] [Google Scholar]
- Timpl P, Spanagel R, Sillaber I, Kresse A, Reul JM, Stalla GK, … & Wurst W (1998). Impaired stress response and reduced anxiety in mice lacking a functional corticotropin-releasing hormone receptor 1. Nature genetics, 19(2), 162. [DOI] [PubMed] [Google Scholar]
- Tjong YW, Ip SP, Lao L, Wu J, Fong HH, Sung JJ, … & Che CT (2010). Neonatal maternal separation elevates thalamic corticotrophin releasing factor type 1 receptor expression response to colonic distension in rat. Neuro endocrinology letters, 31(2), 215–220. [PubMed] [Google Scholar]
- Tsukiyama N, Saida Y, Kakuda M, Shintani N, Hayata A, Morita Y, … & Hashimoto H (2011). PACAP centrally mediates emotional stress-induced corticosterone responses in mice. Stress, 14(4), 368–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vale W, Spiess J, Rivier C, & Rivier J (1981). Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and β-endorphin. Science, 1394–1397. [DOI] [PubMed] [Google Scholar]
- Van Pett K, Viau V, Bittencourt JC, Chan RK, Li HY, Arias C, … & Sawchenko PE (2000). Distribution of mRNAs encoding CRF receptors in brain and pituitary of rat and mouse. Journal of Comparative Neurology, 428(2), 191–212. [DOI] [PubMed] [Google Scholar]
- Vaudry D, Gonzalez BJ, Basille M, Yon L, Fournier A, & Vaudry H (2000). Pituitary adenylate cyclase-activating polypeptide and its receptors: from structure to functions. Pharmacological reviews, 52(2), 269–324. [PubMed] [Google Scholar]
- Vaudry D, Hamelink C, Damadzic R, Eskay RL, Gonzalez B, & Eiden LE (2005). Endogenous PACAP acts as a stress response peptide to protect cerebellar neurons from ethanol or oxidative insult. Peptides, 26(12), 2518–2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaudry D, Falluel-Morel A, Bourgault S, Basille M, Burel D, Wurtz O, … & Vaudry H (2009). Pituitary adenylate cyclase-activating polypeptide and its receptors: 20 years after the discovery. Pharmacological reviews, 61(3), 283–357. [DOI] [PubMed] [Google Scholar]
- Walker DL, & Davis M (2008). Role of the extended amygdala in short-duration versus sustained fear: a tribute to Dr. Lennart Heimer. Brain Structure and Function, 213(1–2), 29–42. [DOI] [PubMed] [Google Scholar]
- Walker BM, & Koob GF (2008). Pharmacological evidence for a motivational role of κ-opioid systems in ethanol dependence. Neuropsychopharmacology, 33(3), 643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker DL, Toufexis DJ, & Davis M (2003). Role of the bed nucleus of the stria terminalis versus the amygdala in fear, stress, and anxiety. European journal of pharmacology, 463(1–3), 199–216. [DOI] [PubMed] [Google Scholar]
- Weiss F, Ciccocioppo R, Parsons LH, Katner S, Liu XIU, Zorrilla EP, … & Richter RR (2001). Compulsive Drug‐Seeking behavior and relapse. Annals of the New York Academy of Sciences, 937(1), 1–26. [DOI] [PubMed] [Google Scholar]
- Wise RA, & Morales M (2010). A ventral tegmental CRF–glutamate–dopamine interaction in addiction. Brain research, 1314, 38–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorrilla EP, Valdez GR, & Weiss F (2001). Changes in levels of regional CRF-like-immunoreactivity and plasma corticosterone during protracted drug withdrawal in dependent rats. Psychopharmacology, 158(4), 374–381. [DOI] [PubMed] [Google Scholar]
- Zorrilla EP, & Koob GF (2010). Progress in corticotropin-releasing factor-1 antagonist development. Drug discovery today, 15(9–10), 371–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorrilla EP, Logrip ML, & Koob GF (2014). Corticotropin releasing factor: a key role in the neurobiology of addiction. Frontiers in neuroendocrinology, 35(2), 234–244. [DOI] [PMC free article] [PubMed] [Google Scholar]