Abstract
Lung cancer is one of the commonest cancers in the world. More than 70% of lung cancer patients are diagnosed with non-small cell lung cancer (NSCLC). Major histocompatibility complex class II (MHC class II), an important component in antigen presenting process, usually expresses on professional antigen presenting cells (APCs), and it can be induced by interferon-γ (IFN-γ). MHC class II can be expressed by NSCLC cells. In NSCLC patients, the expression of MHC class II can be correlated with the outcome of anti-programmed death-1 (anti-PD-1) therapy. This review summarizes MHC class II expression in NSCLC and the correlation between MHC class II and NSCLC diagnosis, prognosis and therapy.
Keywords: major histocompatibility complex class II, non-small cell lung cancer, immune therapy
Introduction
Lung cancer is one of the commonest cancers in the world.1 Mortality from lung cancer is the highest among all cancers in male and female.2 Lung cancer can be divided into non-small cell lung cancer (NSCLC) and small cell lung cancer (SCLC). NSCLC patients account for more than 70% of lung cancer patients.3 Nowadays, early stage NSCLC patients usually tend to be cured by surgery,4 however, most patients with advanced NSCLC cannot be operated on.5 Chemotherapy can be applied for patients in advanced stages.6 However, fewer than half NSCLC patients could benefit from chemotherapy due to tumor intrinsic and acquired resistance to chemotherapy drugs.7 Target therapy provides a method for treating advanced stage patients, such as epidermal growth factor receptor tyrosine kinase inhibitor (EGFR-TKI) therapy. But only patients who have the appropriate mutation can get benefit from it.5,8 Recently, immunotherapy for cancer has mademuch progress, and many strategies to modulate host antitumor immunity have been studied and applied for cancer treatment.9 For example, therapeutics targeting on immune checkpoints, such as cytotoxic T lymphocyte antigen-4 (CTLA-4) and programmed death-1 (PD-1)/programmed death-ligand 1 (PD-L1), have been explored in different clinical trials, even though some problems need to be further investigated.10–12
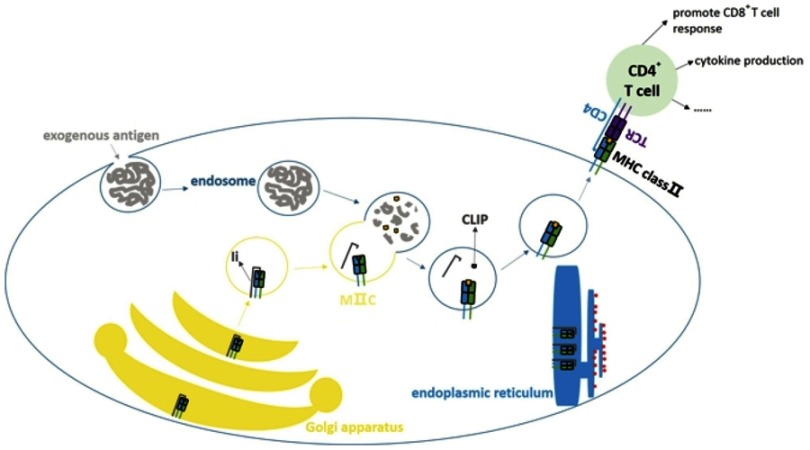
Major histocompatibility complex class II (MHC class II), also called human leukocyte antigen class II (HLA class II), involves an antigen presenting process, especially exogenous antigens (Figure 1). It is important in activating CD4+ T cells. MHC class II expresses on professional antigen presenting cells (APCs) and thymic epithelial cells constitutively, and it can also be induced by interferon-γ (IFN-γ) on other cells.13 Due to its important role in immune reaction, it has been studied in cancers such as, NSCLC,14 melanoma,15 colorectal cancer,16 thyroid cancer,17 breast cancer18 and so on. MHC class II is correlated with the cancer prognosis and it is meaningful for selecting patients for immunotherapy.19 In this review, we studied MHC class II and focused on it in NSCLC.
Figure 1.
Classical antigen presenting by MHC class II molecule. MHC class II is an important part in antigen presenting process especially in presentation of exogenous antigens. APCs can take up antigens through phagocytosis, pinocytosis and other ways. Protein antigens will go into the endosomes. The α chain and β chain of MHC class II are dimerized in endoplasmic reticulum and combine with Ia-associated invariant chain (Ii) to form (αβIi)3 as nonamer. The nonamer will be sent to Golgi apparatus and then form the MHC class II compartment (MIIC). In MIIC the Ii will be decomposed, but class II-associated invariant chain peptide (CLIP) will still be preserved. The endosome merges with MIIC, CLIP will be removed with assistance of HLA-DM (not shown in figure) and the peptide of antigen will be loaded on MHC class II subsequently. Then it can be presented to CD4+ T cells to activate them and trigger the latter reactions.
MHC class II molecule
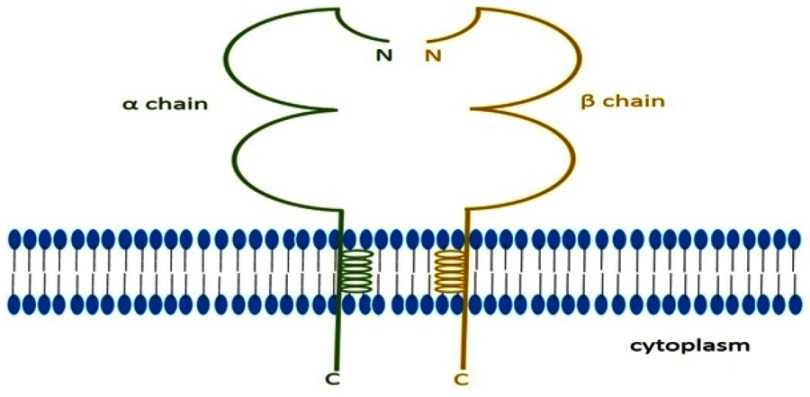
MHC class II is a group of protein which is encoded by HLA class II gene, and it has three classical molecules (HLA-DR, HLA-DQ, HLA-DP) and two nonclassical molecules (HLA-DM, HLA-DO). The classical molecules express on the cell surface and compose the antigen presenting molecules. Nonclassical molecules express in cytoplasm and involve modulating antigens binding with classical molecules.20 MHC class II, a heterodimer molecule, consists of α chain and β chain (Figure 2), and both chains are glycosylated and polymorphic.13 MHC class II usually expresses on professional APCs, including dendritic cells (DCs), B cells and macrophages, and it also expresses on thymic epithelial cells. Sometimes it can also been found on malignant tumor cells.14,21,22 IFN-γ can induce different cancer cell lines to express MHC class II, including large cell lung carcinoma cell lines, squamous cell lung carcinoma cell lines, etc.23
Figure 2.
Classical MHC class II molecule. MHC class II molecule is a heterodimer molecule which is encoded by HLA class II gene. It has two chains named α chain and β chain, and every chain has extracellular region, transcellular region and intracellular region.
MHC class II in antitumor immunity
As the downregulation of MHC class I would assist cancer cells escape from specific cytotoxic T cells mediated cytolysis,24 MHC class I was considered an important part in antitumor immunoreaction. Tumor antigen specific CD4+ T cell also plays an important role in inducing and maintaining antitumor immunity, including assisting CD8+ T cells, secreting cytokines which are essential for T cell differentiation and function.25 Because activation of antigen specific CD4+ T cells requires MHC class II to present the antigen, MHC class II is as significant as MHC class I in antitumor immunity. MHC class II constitutively expresses on APCs, however, APCs in the tumor microenvironment are frequently immunosuppressive and fail to activate T cells.26 This will limit the function of T cells. Nevertheless MHC class II can be induced to express on tumor cells by IFN-γ which means tumor cells may have the ability to present antigens. Alternatively, most immunogenic tumor antigens are inside the tumor cells, generally MHC class II cannot present intracellular antigen. However, according to current research it had been found that intracellular antigens could be presented by tumor cells. And tumor antigen specific CD4+ T cells could recognize tumor cells directly through the mechanism that had not been fully understood.25,27,28 A recent analysis even found that tumor mutations with high affinity to MHC class II would cause selective pressure in tumorigenesis, which also indicated that MHC class II-restricted antigen presentation was important in antitumor immunity.29 However, MHC class II-restricted antigen presentation may not always have positive effects on antitumor immunity. A study in mice found that nonmutated self-epitopes that might be overexpressed in tumor cells could be presented by MHC class II and be recognized by Tregs, which could suppress the antitumor response of the host.30 Another study showed that the antigen presented by tumor cells through MHC class II could alter tumor antigen specific T cells into Tregs and restrict the ability to eliminate tumor cells.31
Encoding gene of MHC class II and NSCLC
MHC class II is encoded by HLA class II gene which is the most polymorphic gene system in humans. Differences in frequency of HLA alleles between cancer patients and healthy people have been found. These differences correlate with the risk of carcinogenesis, cancer biological behavior, response to treatment and so on. These have been detected in many cancers. For instance, HLA-DRB1*1301/02 frequency was lower in patients compared to that in control, while HLA-DQB1*03 frequency was higher in HPV (+) cervical carcinoma.32 Lower frequency of HLA-DRB1*1302 was found in Han nationality lung cancer patients from North China and it was also detected in Japanese lung cancer patients.33,34 And patients of Han nationality from North China had higher frequency of HLA-DRB1*0401, HLA-DRB1*0402 and HLA-DRB1*1201 than healthy controls, which might indicate that these were susceptible factors of lung cancer.33 In Japanese, HLA-DRB1*14 group was found absent in lung cancer patients while the frequency of HLA-DRB1*0901 was significantly increased.34 HLA-DQA1*03 was associated with risk of adenocarcinoma, and it was also correlated to squamous cell carcinoma.35,36 HLA-DRB1*07, HLA-DQ02, HLA-DQ07 frequency was significantly decreased in NSCLC. And HLA-DRB1*07 was significantly higher in patients at an early stage than those at an advanced stage.37 Omer Araz et al found NSCLC patients who had better response to gemcitabine and cisplatin combination chemotherapy regimen had significantly higher frequency of HLA-DRB1*13, HLA-DQ5, HLA-DQ7.38
Besides, some single nucleotide polymorphisms (SNPs) in HLA class II gene were also detected in lung cancer. Rs2179920, a SNP located in intergenic region near HLA-DPB1, was significantly associated with EGFR mutation positive adenocarcinoma.39 Rs2395185 in HLA class II gene might be involved in gene-household air pollution interaction which might impact lung cancer risk in Asian never smoking female lung cancer patients.40 Both rs3129871 located in HLA-DRA and rs2187668 located in HLA-DQA1 had association with lung cancer risk, while former was a protective factor and latter a susceptible factor.41 SNPs were studied not only in classical MHC class II gene, but also in nonclassical MHC class II gene. SNP rs2071554, a missense variation in first exon of HLA-DOB was associated with poor survival in advanced patients. And in silico analysis suggested that it might damage the protein function.42
As many studies have shown HLA class II gene is different between cancer patients and healthy controls, HLA class II gene analysis may provide predictive information for lung cancer risk, prognosis and response to treatment.33,38,42 However, HLA genes have intrinsic differences between different regions and nationalities, which makes it necessary to do further research in large NSCLC samples in different regions and nationalities. Until now, the mechanisms of different genes causing these different consequences have not been well understood. MHC class II molecules encoded by different HLA class II genes may have different affinity to antigens and presenting ability, which can influence the host immune surveillance and tumor elimination. However, the mechanisms still need to be further analyzed and validated.
MHC class II expression and NSCLC
Research on MHC class II expression have been done among different cancers and its expression on tumor-infiltrating immune cells has been investigated. In pediatric adrenocortical tumors, HLA-DPA1 mainly expressed on tumor-infiltrating macrophages and DCs which was related to a better prognosis.43
MHC class II also expressed on tumor cells. Loss of MHC class II expression on lymphoma cells was associated with poor prognosis in diffuse large B-cell lymphoma.20 Expression of HLA-DR on colorectal cancer cells was found to be associated with the presence of tumor infiltrating lymphocytes (TILs) and peritumoral lymphocytes and it might link to a better prognosis in stage C colorectal cancer.16 Sconocchia et al reported that MHC class II expression on colorectal cancer cells was a favorable prognostic factor, but there were still some discrepancies.21 MHC class II expression on cervical adenocarcinoma cells was associated with a lower recurrence and a better disease-specific survival in cervical adenocarcinoma patients.44 Papillary thyroid carcinoma patients with HLA-DR, HLA-DQ expression on cancer cells had a lower rate of lymph nodule metastasis and required a lower dosage of 131I for treatment than HLA-DQ negative patients.17 Lazzaro et al found expression of HLA-DR on cancer cells was higher in breast medullary carcinoma compared to breast ductal carcinoma and medullary carcinoma had a relatively better prognosis.18 Expression level of HLA-DQ on melanoma cells was lower than HLA-DR in primary and metastatic melanoma.15
Expression of MHC class II also has been studied in NSCLC. In many NSCLC cell lines, expression of MHC class II had been detected. Using immunofluorescent HLA-DR, -DQ, -DP expression had been detected in A549, SKMES1, CALU6, A427 cell lines.45 MHC class II could be induced by IFN-γ in TKB-3 and TKB-4 cell lines and the induced MHC class II expressed on cell surface instead of cytoplasm.46 MHC class II expression in adenocarcinoma, squamous cell carcinoma and large cell carcinoma was detected in surgery samples by immunohistochemistry (IHC). It demonstrated that MHC class II expressed on both TILs and lung cancer cells.46 Another study showed high expression of MHC class II on tumor cells was associated with high MHC class II expression on TILs, and expression of MHC class II on adenocarcinoma cells was higher than non-adenocarcinoma cells.14 As for prognosis, MHC class II expression on TILs was associated with a better recurrence-free survival (RFS) and overall survival (OS).14 Ohri et al analyzed 40 NSCLC patients’ specimens by IHC and found non-macrophage expression of HLA-DR in tumor islets was significantly elevated in patients who had better prognoses.47 HLA-DPB1 expression detected in biopsy tissue also had positive correlation with good prognosis in lung cancer patients.48
According to the studies above, it can be concluded that MHC class II can express on both tumor infiltrating immune cells and tumor cells in NSCLC and other cancers. It had shown that MHC class II expressing on tumor infiltrating immune cells was related to better prognosis.14,43 The reason for this could be considered as better immune surveillance in tumor microenvironment, but this should be further investigated. In most cases, MHC class II expression on tumor cells was correlated to better prognosis. As researchers found more TILs in MHC class II positive cancer, the reason of better prognosis might be that MHC class II could present tumor antigens and recruit lymphocytes to tumor microenvironment to restrict cancer progression. Meanwhile it had also been detected that MHC class II expressed on tumor cells could promote the production of proinflammatory cytokines.21 It could cause a proinflammatory microenvironment for better antitumor reaction and would be another reason to explain the better prognosis. However, accurate mechanisms still need further study.
MHC class II expression in peripheral blood was also analyzed in lung cancer patients.34,49–52 HLA-DR9 was found to have higher frequency in peripheral blood of lung cancer patients, while HLA-DR6 was found to have lower frequency in lung cancer patients than healthy controls by serological MHC typing.34 Another study showed lung cancer patients with an increased HLA-DR (+) cells in peripheral blood had a poor prognosis. Further analysis showed the poor prognosis was correlated with squamous cell carcinoma but not adenocarcinoma.49 Nakamura et al found blood HLA-DR (%) was higher in nodule metastatic patients.50 HLA-DR7 on peripheral blood lymphocyte might decrease the risk of lung cancer in Caucasians.51 Early diagnosis for lung cancer is a vital problem that has not been fully studied. Kanangat et al reported that the serum level of HLA-DQA1, HLA-DQ (26kDa band), HLA-DRB and HLA-DMB was different between early stage adenocarcinoma patients and benign nodule patients. Level of HLA-DQA1 28kDa band was significantly increased and HLA-DQA1 33-35kDa band was decreased in adenocarcinoma. These conclusions suggested that these molecules might be used for adenocarcinoma early diagnosis.52 However,the sample size of former research was not large and other pathological types of NSCLC should be included.
MHC class II expression can be changed by some molecules.53–55 CIITA is an important regulating factor for promoting MHC class II expression.56 It was a non-DNA-binding coactivator which participated in coordinating the recruitment of the factors which were related to chromatin remodeling, transcription initiation and transcription elongation.56 A study had shown that expression of MHC class II on B cells and DCs were apparently decreased in CIITA deficient mice.57 It has been demonstrated that MHC class II expression induced by IFN-γ was mediated by CIITA.58 TGF-β suppressed MHC class II induced by IFN-γ also through restricting CIITA mRNA accumulation.59 In another study, when researchers used IFN-γ to stimulate lung adenocarcinoma cell line and bronchial epithelial cell line, it showed different abundance of a CIITA splice variant in two cell lines and different level of MHC class II on cell membrane. Further analysis found this variant could change the expression of genes encoding chaperons which regulated MHC class II assembly and transportation to reduce the surface expression of MHC class II.53 Retinoblastoma protein (Rb) was also involved in MHC class II expression. IFN-γ-induced MHC class II expression was absent or highly repressed in Rb-defective NSCLC cells. Histone deacetylase (HDAC) inhibitor MS-275 can rescue the expression of MHC class II in Rb-defective NSCLC cells.54 In cell line, EGFR-TKI and ligand blocking antibodies could upregulate the level of CIITA and the cells with pretreatment of EGFR blocking antibodies showed an augmentation of IFN-γ-induced MHC class II expression,55 which suggested that EGFR inhibitor might not only suppress proliferation but also facilitate host immune reaction against tumor.
MHC class II may be considered as a biomarker to predict patients’ response to some treatments. Some researchers had shown PGI2 had antitumor effect.60,61 In two overexpressed prostacyclin synthase (PGIS) mouse lung cancer models, researchers found that only in the model with a high expression of MHC class II, PGI2 could cause CD4+ T cells infiltration elevating and show antitumor effect. Thus MHC class II should be considered as a potential biomarker for PGI2 therapy response.62 NSCLC patients with higher frequency of HLA-DRB1*13, HLA-DQ5, HLA-DQ7 would have a better response to gemcitabine and cisplatin combination chemotherapy regimen.38
MHC class II and NSCLC immunotherapy
MHC class II and anti-PD-1/PD-L1 therapy
At present, anti-PD-1/PD-L1 monoclonal antibodies have shown promising effects for NSCLC.10 For instance, nivolumab has shown clinical meaningful effect and an acceptable safety profile for NSCLC.11,12 Although it was reported tumor expression of PD-L1 could be a biomarker for anti-PD-1 response, more than half the patients with PD-L1 high expression had a poor response to nivolumab.63,64 Therefore, it is important to find more biomarkers on patient selection. A study in melanoma reported that HLA-DR expression on melanoma cells correlated with response to anti-PD-1 therapy, which suggested HLA-DR might be a marker for anti-PD-1 therapy.19 Since MHC class II also expressed in NSCLC cells,14,46 it was worth studying the expression of MHC class II in NSCLC to find whether it could be used as a biomarker independently or be accompanied with other markers to screen lung cancer patients before immunotherapy.
MHC class II vaccine
MHC class II vaccine consists of tumor cells which are genetically modified to express MHC class II and costimulatory molecules.3 It could activate tumor-reactive CD4+ T cells. Subsequently, effective and memory CD8+ T cells would be generated.65 Ia associated invariant chain (Ii) could prevent endogenous antigens from loading on MHC class II in antigen presenting process. Thompson et al found that Ii (–) MHC class II vaccine could activate type 1 CD4+ T cells more effectively than Ii (+) APCs.65 Myeloid-derived suppressor cells (MDSCs) can inhibit host adaptive immune reaction.66 But in vitro study of NSCLC, MHC class II vaccine could activate tumor-specific, IFN-γ secreting CD4+ T cells even with the presence of MDSCs.3 Subsequently vitro studies showed that this kind of vaccine had cross-reaction among different major histopathological types of NSCLC and activated T cells were MHC class II restricted and tumor specific.3,67
Summary and prospective
As an important molecule in antigen presentation, MHC class II has been studied in NSCLC.14,46–48 As we know, MHC class II gene is highly polymorphic. Different alleles and SNPs have been found between NSCLC patients and healthy controls. However, the reasons for this have not been fully understood. What is more, MHC class II expression was detected in both NSCLC tumor tissue and peripheral blood. As for NSCLC treatment, diverse monoclonal antibodies have been applied to NSCLC in clinical trials and MHC class II deserves to be validated whether it can be a biomarker for NSCLC immunotherapy. NSCLC MHC class II vaccine has shown its antitumor effect in vitro studies. It may provide a new therapeutic for advanced stage patients after verifying its antitumor effect and safety in cancer models and clinical trials. Although there have been more and more studies on MHC class II, the MHC class II in NSCLC has not been fully understood and needs further study.
Acknowledgment
This study was supported in part by grants from National Natural Science Foundation of China (81802255), Shanghai Pujiang Program (17PJD036) and a grant from Shanghai Municipal Commission of Health and Family Planning Program (20174Y0131), National Key Research & Development Project (2016YFC0902300), Major Disease Clinical Skills Enhancement Program of three year action plan for promoting clinical skills and clinical innovation in municipal hospitals, Shanghai Shen Kang Hospital Development Center Clinical Research Plan of SHDC (16CR1001A), “Dream Tutor“ Outstanding Young Talents Program (fkyq1901), Key Disciplines of Shanghai Pulmonary Hospital (2017ZZ02012), and a grant from Shanghai Science and Technology Commission (16JC1405900).
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108. doi: 10.3322/caac.21262 [DOI] [PubMed] [Google Scholar]
- 2.Chen W, Zheng R, Baade PD, et al Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–132. doi: 10.3322/caac.21338 [DOI] [PubMed] [Google Scholar]
- 3.Srivastava MK, Bosch JJ, Thompson JA, Ksander BR, Edelman MJ, Ostrand-Rosenberg S. Lung cancer patients’ CD4(+) T cells are activated in vitro by MHC II cell-based vaccines despite the presence of myeloid-derived suppressor cells. Cancer Immunol Immunother. 2008;57(10):1493–1504. doi: 10.1007/s00262-008-0490-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bendrat K, Stang A, Georgiev G, et al A compartment-specific transcriptome analysis reveals survival-relevant marker genes in the stroma fraction of squamous non-small cell lung cancer. J Biophotonics. 2012;5(4):367–377. doi: 10.1002/jbio.201100115 [DOI] [PubMed] [Google Scholar]
- 5.Cafarotti S, Lococo F, Froesh P, Zappa F, Andre D. Target Therapy in Lung Cancer. Adv Exp Med Biol. 2016;893:127–136. doi: 10.1007/978-3-319-24223-1_6 [DOI] [PubMed] [Google Scholar]
- 6.Du L, Morgensztern D. Chemotherapy for advanced-stage non-small cell lung cancer. Cancer J (Sudbury, Mass). 2015;21(5):366–370. doi: 10.1097/PPO.0000000000000141 [DOI] [PubMed] [Google Scholar]
- 7.Kim ES. Chemotherapy resistance in lung cancer. Adv Exp Med Biol. 2016;893:189–209. doi: 10.1007/978-3-319-24223-1_10 [DOI] [PubMed] [Google Scholar]
- 8.Solomon BJ, Mok T, Kim DW, et al First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med. 2014;371(23):2167–2177. doi: 10.1056/NEJMoa1408440 [DOI] [PubMed] [Google Scholar]
- 9.Lievense L, Aerts J, Hegmans J. Immune therapy. Adv Exp Med Biol. 2016;893:59–90. doi: 10.1007/978-3-319-24223-1_4 [DOI] [PubMed] [Google Scholar]
- 10.Xia B, Herbst RS. Immune checkpoint therapy for non-small-cell lung cancer: an update. Immunotherapy. 2016;8(3):279–298. doi: 10.2217/imt.15.123 [DOI] [PubMed] [Google Scholar]
- 11.Brahmer J, Reckamp KL, Baas P, et al Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373(2):123–135. doi: 10.1056/NEJMoa1504627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Borghaei H, Paz-Ares L, Horn L, et al Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373(17):1627–1639. doi: 10.1056/NEJMoa1507643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seliger B, Kloor M, Ferrone S. HLA class II antigen-processing pathway in tumors: molecular defects and clinical relevance. Oncoimmunology. 2017;6(2):e1171447. doi: 10.1080/2162402X.2016.1171447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.He Y, Rozeboom L, Rivard CJ, et al MHC class II expression in lung cancer. Lung Cancer. 2017;112:75–80. doi: 10.1016/j.lungcan.2017.07.030 [DOI] [PubMed] [Google Scholar]
- 15.Taramelli D, Fossati G, Mazzocchi A, Delia D, Ferrone S, Parmiani G. Classes I and II HLA and melanoma-associated antigen expression and modulation on melanoma cells isolated from primary and metastatic lesions. Cancer Res. 1986;46(1):433–439. [PubMed] [Google Scholar]
- 16.Walsh MD, Dent OF, Young JP, et al HLA-DR expression is associated with better prognosis in sporadic Australian clinicopathological stage C colorectal cancers. Int J Cancer. 2009;125(5):1231–1237. doi: 10.1002/ijc.24484 [DOI] [PubMed] [Google Scholar]
- 17.Jo YS, Lee JC, Li S, et al Significance of the expression of major histocompatibility complex class II antigen, HLA-DR and -DQ, with recurrence of papillary thyroid cancer. Int J Cancer. 2008;122(4):785–790. doi: 10.1002/ijc.23167 [DOI] [PubMed] [Google Scholar]
- 18.Lazzaro B, Anderson AE, Kajdacsy-Balla A, Hessner MJ. Antigenic characterization of medullary carcinoma of the breast: HLA-DR expression in lymph node positive cases. Appl Immunohistochem Mol Morphol. 2001;9(3):234–241. doi: 10.1097/00129039-200109000-00007 [DOI] [PubMed] [Google Scholar]
- 19.Johnson DB, Estrada MV, Salgado R, et al Melanoma-specific MHC-II expression represents a tumour-autonomous phenotype and predicts response to anti-PD-1/PD-L1 therapy. Nat Commun. 2016;7(10582). doi: 10.1038/ncomms10582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rimsza LM, Roberts RA, Miller TP, et al Loss of MHC class II gene and protein expression in diffuse large B-cell lymphoma is related to decreased tumor immunosurveillance and poor patient survival regardless of other prognostic factors: a follow-up study from the Leukemia and Lymphoma molecular profiling project. Blood. 2004;103(11):4251–4258. doi: 10.1182/blood-2003-07-2365 [DOI] [PubMed] [Google Scholar]
- 21.Sconocchia G, Eppenberger-Castori S, Zlobec I, et al HLA class II antigen expression in colorectal carcinoma tumors as a favorable prognostic marker. Neoplasia (New York, NY). 2014;16(1):31–42. doi: 10.1593/neo.131568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ruiter DJ, Bergman W, Welvaart K, et al Immunohistochemical analysis of malignant melanomas and nevocellular nevi with monoclonal antibodies to distinct monomorphic determinants of HLA antigens. Cancer Res. 1984;44(9):3930–3935. [PubMed] [Google Scholar]
- 23.Schwartz R, Momburg F, Moldenhauer G, Dorken B, Schirrmacher V. Induction of HLA class-II antigen expression on human carcinoma cell lines by IFN-Gamma. Int J Cancer. 1985;35(2):245–250. doi: 10.1002/ijc.2910350217 [DOI] [PubMed] [Google Scholar]
- 24.Bubenik J. MHC class I down-regulation: tumour escape from immune surveillance? (review). Int J Oncol. 2004;25(2):487–491. [PubMed] [Google Scholar]
- 25.Matsuzaki J, Tsuji T, Luescher I, et al Nonclassical antigen-processing pathways are required for MHC class II-restricted direct tumor recognition by NY-ESO-1-specific CD4(+) T cells. Cancer Immunol Res. 2014;2(4):341–350. doi: 10.1158/2326-6066.CIR-13-0138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wilke CM, Kryczek I, Zou W. Antigen-presenting cell (APC) subsets in ovarian cancer. Int Rev Immunol. 2011;30(2–3):120–126. doi: 10.3109/08830185.2011.567362 [DOI] [PubMed] [Google Scholar]
- 27.Schultz ES, Lethe B, Cambiaso CL, et al A MAGE-A3 peptide presented by HLA-DP4 is recognized on tumor cells by CD4+ cytolytic T lymphocytes. Cancer Res. 2000;60(22):6272–6275. [PubMed] [Google Scholar]
- 28.Friedman KM, Prieto PA, Devillier LE, et al Tumor-specific CD4+ melanoma tumor-infiltrating lymphocytes. J Immunother. 2012;35(5):400–408. doi: 10.1097/CJI.0b013e31825898c5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marty Pyke R, Thompson WK, Salem RM, Font-Burgada J, Zanetti M, Carter H. Evolutionary pressure against MHC class II binding cancer mutations. Cell. 2018;175(2):416–428.e413. doi: 10.1016/j.cell.2018.08.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ahmetlic F, Riedel T, Homberg N, et al Regulatory T cells in an endogenous mouse lymphoma recognize specific antigen peptides and contribute to immune escape. Cancer Immunol Res. 2019;7(4):600–608. doi: 10.1158/2326-6066.CIR-18-0419 [DOI] [PubMed] [Google Scholar]
- 31.Manlove LS, Berquam-Vrieze KE, Pauken KE, Williams RT, Jenkins MK, Farrar MA. Adaptive immunity to leukemia is inhibited by cross-reactive induced regulatory T Cells. J Immunol. 2015;195(8):4028–4037. doi: 10.4049/jimmunol.1501291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sastre-Garau X, Loste MN, Vincent-Salomon A, et al Decreased frequency of HLA-DRB1 13 alleles in Frenchwomen with HPV-positive carcinoma of the cervix. Int J Cancer. 1996;69(3):159–164. doi: [DOI] [PubMed] [Google Scholar]
- 33.Yang L, Wang LJ, Shi GL, et al Analysis of HLA-A, HLA-B and HLA-DRB1 alleles in Chinese patients with lung cancer. Genet Mol Res. 2010;9(2):750–755. doi: 10.4238/vol9-2gmr735 [DOI] [PubMed] [Google Scholar]
- 34.Tokumoto H. Analysis of HLA-DRB1-related alleles in Japanese patients with lung cancer–relationship to genetic susceptibility and resistance to lung cancer. J Cancer Res Clin Oncol. 1998;124(9):511–516. [DOI] [PubMed] [Google Scholar]
- 35.Kohno T, Kunitoh H, Shimada Y, et al Individuals susceptible to lung adenocarcinoma defined by combined HLA-DQA1 and TERT genotypes. Carcinogenesis. 2010;31(5):834–841. doi: 10.1093/carcin/bgq003 [DOI] [PubMed] [Google Scholar]
- 36.Kohno T, Kunitoh H, Mimaki S, et al Contribution of the TP53, OGG1, CHRNA3, and HLA-DQA1 genes to the risk for lung squamous cell carcinoma. J Thorac Oncol. 2011;6(4):813–817. doi: 10.1097/JTO.0b013e3181ee80ef [DOI] [PubMed] [Google Scholar]
- 37.Bulut I, Meral M, Kaynar H, Pirim I, Bilici M, Gorguner M. Analysis of HLA class I and II alleles regarding to lymph node and distant metastasis in patients with non-small cell lung cancer. Lung Cancer. 2009;66(2):231–236. doi: 10.1016/j.lungcan.2009.01.012 [DOI] [PubMed] [Google Scholar]
- 38.Araz O, Ucar EY, Meral M, et al Frequency of class I and II HLA alleles in patients with lung cancer according to chemotherapy response and 5-year survival. Clin Respir J. 2015;9(3):297–304. doi: 10.1111/crj.12143 [DOI] [PubMed] [Google Scholar]
- 39.Shiraishi K, Okada Y, Takahashi A, et al Association of variations in HLA class II and other loci with susceptibility to EGFR-mutated lung adenocarcinoma. Nat Commun. 2016;7:12451. doi: 10.1038/ncomms12451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hosgood HD 3rd, Song M, Hsiung CA, et al Interactions between household air pollution and GWAS-identified lung cancer susceptibility markers in the Female Lung Cancer Consortium in Asia (FLCCA). Hum Genet. 2015;134(3):333–341. doi: 10.1007/s00439-014-1528-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brenner DR, Brennan P, Boffetta P, et al Hierarchical modeling identifies novel lung cancer susceptibility variants in inflammation pathways among 10,140 cases and 11,012 controls. Hum Genet. 2013;132(5):579–589. doi: 10.1007/s00439-013-1270-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pu X, Hildebrandt MA, Lu C, et al Inflammation-related genetic variations and survival in patients with advanced non-small cell lung cancer receiving first-line chemotherapy. Clin Pharmacol Ther. 2014;96(3):360–369. doi: 10.1038/clpt.2014.89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pinto EM, Rodriguez-Galindo C, Choi JK, et al Prognostic significance of major histocompatibility complex class ii expression in pediatric adrenocortical tumors: a St. Jude and Children’s Oncology Group Study. Clin Cancer Res. 2016;22(24):6247–6255. doi: 10.1158/1078-0432.CCR-15-2738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Samuels S, Spaans VM, Osse M, et al Human leukocyte antigen-DR expression is significantly related to an increased disease-free and disease-specific survival in patients with cervical adenocarcinoma. Int J Gynecol Cancer. 2016;26(8):1503–1509. doi: 10.1097/IGC.0000000000000783 [DOI] [PubMed] [Google Scholar]
- 45.Redondo M, Ruiz-Cabello F, Concha A, et al Differential expression of MHC class II genes in lung tumour cell lines. Int J Immunogenet. 1998;25(6):385–391. [DOI] [PubMed] [Google Scholar]
- 46.Kamma H, Yazawa T, Ogata T, Horiguchi H, Iijima T. Expression of MHC class II antigens in human lung cancer cells. Virchows Arch B Cell Pathol Incl Mol Pathol. 1991;60(6):407–412. [DOI] [PubMed] [Google Scholar]
- 47.Ohri CM, Shikotra A, Green RH, Waller DA, Bradding P. The tissue microlocalisation and cellular expression of CD163, VEGF, HLA-DR, iNOS, and MRP 8/14 is correlated to clinical outcome in NSCLC. PLoS One. 2011;6(7):e21874. doi: 10.1371/journal.pone.0021874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Borczuk AC, Shah L, Pearson GD, et al. Molecular signatures in biopsy specimens of lung cancer. Am J Respir Crit Care Med. 2004;170(2):167–174. doi: 10.1164/rccm.200401-066OC [DOI] [PubMed] [Google Scholar]
- 49.Nakamura H, Saji H, Ogata A, et al Immunologic parameters as significant prognostic factors in lung cancer. Lung Cancer. 2002;37(2):161–169. [DOI] [PubMed] [Google Scholar]
- 50.Nakamura H, Kawasaki N, Hagiwara M, Saito M, Konaka C, Kato H. Cellular immunologic parameters related to age, gender, and stage in lung cancer patients. Lung Cancer. 2000;28(2):139–145. [DOI] [PubMed] [Google Scholar]
- 51.Romano PJ, Bartholomew M, Smith PJ, et al HLA antigens influence resistance to lung carcinoma. Hum Immunol. 1991;31(4):236–240. [DOI] [PubMed] [Google Scholar]
- 52.Kanangat S, Seder CW, Pergande MR, et al Circulating histocompatibility antigen (HLA) gene products may help differentiate benign from malignant indeterminate pulmonary lesions. Hum Immunol. 2018;79(7):558–563. doi: 10.1016/j.humimm.2018.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chiu BL, Li CH, Chang CC. Selective modulation of MHC class II chaperons by a novel IFN-gamma-inducible class II transactivator variant in lung adenocarcinoma A549 cells. Biochem Biophys Res Commun. 2013;440(1):190–195. doi: 10.1016/j.bbrc.2013.09.066 [DOI] [PubMed] [Google Scholar]
- 54.Niesen MI, Blanck G. Rescue of major histocompatibility-DR surface expression in retinoblastoma-defective, non-small cell lung carcinoma cells by the MS-275 histone deacetylase inhibitor. Biol Pharm Bull. 2009;32(3):480–482. doi: 10.1248/bpb.32.480 [DOI] [PubMed] [Google Scholar]
- 55.Pollack BP, Sapkota B, Cartee TV. Epidermal growth factor receptor inhibition augments the expression of MHC class I and II genes. Clin Cancer Res. 2011;17(13):4400–4413. doi: 10.1158/1078-0432.CCR-10-3283 [DOI] [PubMed] [Google Scholar]
- 56.Reith W, LeibundGut-Landmann S, Waldburger JM. Regulation of MHC class II gene expression by the class II transactivator. Nat Rev Immunol. 2005;5(10):793–806. doi: 10.1038/nri1708 [DOI] [PubMed] [Google Scholar]
- 57.Chang CH, Guerder S, Hong SC, van Ewijk W, Flavell RA. Mice lacking the MHC class II transactivator (CIITA) show tissue-specific impairment of MHC class II expression. Immunity. 1996;4(2):167–178. [DOI] [PubMed] [Google Scholar]
- 58.Steimle V, Siegrist C, Mottet A, Lisowskagrospierre B, Mach B. Regulation of MHC class II expression by interferon-gamma mediated by the transactivator gene CIITA. Science (New York, NY). 1994;265(5168):106–109. doi: 10.1126/science.8016643 [DOI] [PubMed] [Google Scholar]
- 59.Lee YJ, Han Y, Lu HT, et al TGF-beta suppresses IFN-gamma induction of class II MHC gene expression by inhibiting class II transactivator messenger RNA expression. J Immunol. 1997;158(5):2065–2075. [PubMed] [Google Scholar]
- 60.Keith RL, Miller YE, Hudish TM, et al Pulmonary prostacyclin synthase overexpression chemoprevents tobacco smoke lung carcinogenesis in mice. Cancer Res. 2004;64(16):5897–5904. doi: 10.1158/0008-5472.CAN-04-1070 [DOI] [PubMed] [Google Scholar]
- 61.Keith RL, Miller YE, Hoshikawa Y, et al. Manipulation of pulmonary prostacyclin synthase expression prevents murine lung cancer. Cancer Res. 2002;62(3):734–740. [PubMed] [Google Scholar]
- 62.Li HY, McSharry M, Walker D, et al Targeted overexpression of prostacyclin synthase inhibits lung tumor progression by recruiting CD4+ T lymphocytes in tumors that express MHC class II. Oncoimmunology. 2018;7(5):e1423182. doi: 10.1080/2162402X.2018.1490854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rizvi NA, Mazieres J, Planchard D, et al Activity and safety of nivolumab, an anti-PD-1 immune checkpoint inhibitor, for patients with advanced, refractory squamous non-small-cell lung cancer (CheckMate 063): a phase 2, single-arm trial. Lancet Oncol. 2015;16(3):257–265. doi: 10.1016/S1470-2045(15)70054-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Taube JM, Klein A, Brahmer JR, et al Association of PD-1, PD-1 ligands, and other features of the tumor immune microenvironment with response to anti-PD-1 therapy. Clin Cancer Res. 2014;20(19):5064–5074. doi: 10.1158/1078-0432.CCR-13-3271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Thompson JA, Srivastava MK, Bosch JJ, Clements VK, Ksander BR, Ostrand-Rosenberg S. The absence of invariant chain in MHC II cancer vaccines enhances the activation of tumor-reactive type 1 CD4+ T lymphocytes. Cancer Immunol Immunother. 2008;57(3):389–398. doi: 10.1007/s00262-007-0381-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Almand B, Clark JI, Nikitina E, et al. Increased production of immature myeloid cells in cancer patients: a mechanism of immunosuppression in cancer. J Immunol. 2001;166(1):678–689. doi: 10.4049/jimmunol.166.1.678 [DOI] [PubMed] [Google Scholar]
- 67.Srivastava MK, Bosch JJ, Wilson AL, Edelman MJ, Ostrand-Rosenberg S. MHC II lung cancer vaccines prime and boost tumor-specific CD4+ T cells that cross-react with multiple histologic subtypes of nonsmall cell lung cancer cells. Int J Cancer. 2010;127(11):2612–2621. doi: 10.1002/ijc.25462 [DOI] [PMC free article] [PubMed] [Google Scholar]