Abstract
Adrenocortical carcinomas (ACCs) are rare tumors with scant treatment options for which new treatments are required. The mTOR pathway mediates the intracellular signals of several growth factors, including the insulin-like growth factors (IGFs), and therefore represents a potential attractive pathway for the treatment of several malignancies including ACCs. Several mTOR inhibitors, including sirolimus, temsirolimus and everolimus, have been clinically developed. This review summarizes the results of the studies evaluating the expression of the mTOR pathway components in ACCs, the effects of the mTOR inhibitors alone or in combination with other drugs in preclinical models of ACCs and the early experience with the use of these compounds in the clinical setting. The mTOR pathway seems a potential target for treatment of patients with ACC, but further investigation is still required to define the potential role of mTOR inhibitors alone or in combination with other drugs in the treatment of ACC patients.
Keywords: adrenal, neuroendocrinology, growth factors, endocrine cancers
Introduction
Adrenocortical carcinomas (ACCs) are rare tumors with scant treatment options for which new treatments are required (1, 2, 3). The limited efficacy of conventional antineoplastic treatment in ACCs increases the need for novel effective treatment options. During the past 15 years, progress in understanding the pathogenesis of tumors has encouraged the development of so-called ‘targeted drugs’, which are compounds that specifically interfere with molecular mechanisms involved in tumor cell growth and/or tumor vascular supply, leading to major advances in oncology (4, 5).
Targeted drugs include compounds interfering with growth factor receptors and their related signaling pathways. Mammalian target of rapamycin (mTOR) is a protein kinase of the phosphoinositide 3 kinase (PI3Ks)/protein kinase B (PKB or AKT) signaling pathway, which forms multimolecular intracellular complexes and functions as a gatekeeper of metabolism, as well as cell growth. mTOR receives signals from sensors of cell stress, intracellular nutrient levels and several growth factors, including vascular endothelial growth factor (VEGF), insulin-like growth factors (IGFs), epidermal growth factor (EGF) and platelet-derived growth factor (PDGF) (6, 7, 8, 9). mTOR can be part of the two functional complexes mTORC1 and mTORC2. Upon the binding of several growth factors to their cognate tyrosine kinase receptors, AKT is phosphorylated and activated, which in turn leads to the activation of mTOR as part of the mTORC1 complex. Activated mTORC1 complex regulates cell proliferation via the activation of mRNA translation and is mediated mainly via two downstream components, i.e. p70 ribosomal protein S6 kinase 1 (S6K1) and eukaryotic translation initiation factor 4E binding proteins (4EBP1). A more extensive description of this pathway has been previously reported and a schematic representation of the pathway is shown in Fig. 1 (10, 11, 12, 13). The mTORC2 complex regulates cytosketeton function and seems to be involved in the activation of AKT function (10, 11). Several drugs inhibiting the mTORC1 complex have been developed as anticancer treatment including sirolimus, temsirolimus and everolimus (traditional mTOR inhibitors), which have been approved for the treatment of different malignancy such as renal cell carcinoma and pancreatic neuroendocrine tumors (14, 15). More recently some compounds which also target the mTORC2 complex have been proposed as anticancer treatment (i.e. OSI-027, AZD2014) (10, 11). Alterations of growth factors and their cognate receptors are considered to be involved in the pathogenesis of ACCs (16, 17, 18, 19, 20, 21). Therefore, compounds interfering with tumor angiogenesis and growth factor signaling pathways represent a potential novel treatment option for the management of patients with ACCs. The mTOR pathway, being involved in both these processes, could represent a potential target for treatment of these malignancies (2, 22). Moreover, the most common molecular alteration observed in ACCs is the increased expression of IGF2 mRNA, which is reported in up to 90% of cases (22). Therefore, IGF2 has been suggested to be involved in the pathogenesis of ACCs and represents a potential target for treatment in this malignancy. Since the mTOR pathway is one of the main mediators of the intracellular effects of IGFs, the study of the mTOR pathway in ACCs has been considered attractive as potential target for treatment and to better understand the pathogenesis of these tumors (10).
Figure 1.
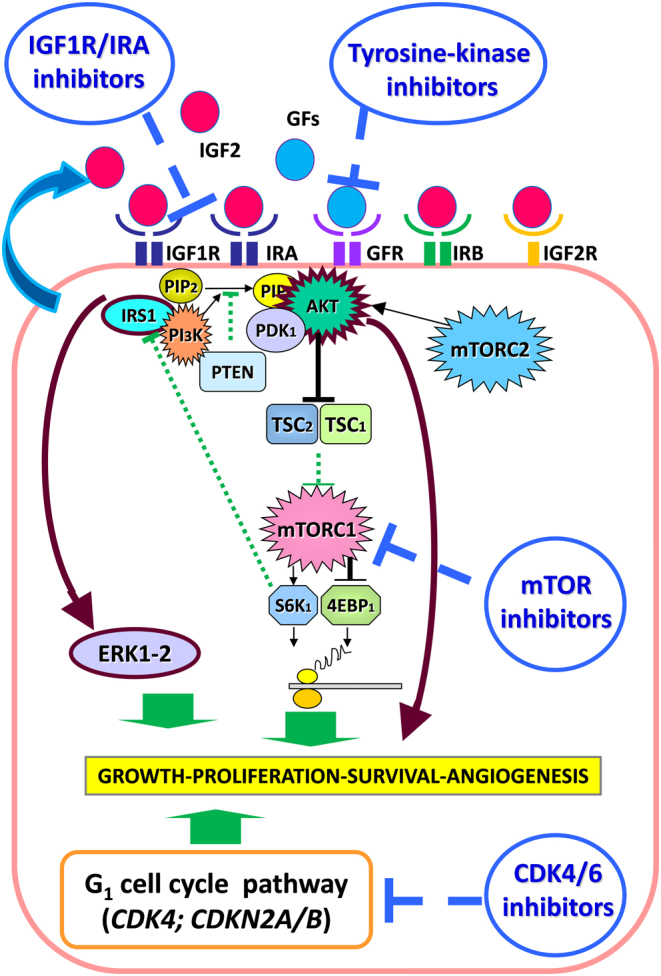
Schematic representation of the potential molecular pathways representing potential targets for treatment in patients with ACC, based on the results presented in the current review. GFs, growth factors; GFR, growth factor receptor. Brown lines show two potential escape pathways to the treatment with mTOR inhibitors: AKT and ERK activation.
This review aims at summarizing the results of the studies evaluating the expression of the mTOR pathway components in ACCs, the effects of the mTOR inhibitors alone or in combination with other drugs in preclinical models of ACCs and the early experience with the use of these compounds in the clinical setting. Our research group largely contributed to the current knowledge on the subject.
The mTOR pathway in normal adrenals
In the normal adrenal gland, a layer-specific protein expression pattern of the major components of the mTOR pathway has been found, suggesting a uncharacterized role of the mTOR pathway in particular adrenal functions. For example, the stronger expression of several components (i.e total-mTOR, total-/phospho-4EBP1 and total-/phospho-S6K1) of the mTOR pathway in the zona reticularis could suggest a role of this pathway in androgen production, and the stronger expression of these components in the zona glomerulosa may be related to angiotensin II-induced activation of the mTOR pathway (23). An anti-secretory effect (e.g. inhibition of cortisol production) of mTOR inhibitors in ACC cell lines has been reported (24), although up to date, signs or symptoms of hypoadrenalism with the use of mTOR inhibitors in the clinical setting have not been clearly described (14). Further studies are required to clarify the specific role of the mTOR pathway in regulating steroid production.
Expression of the main components of the mTOR pathway in adrenocortical tumors
The expression of the main components of the mTOR pathway in adrenocortical tumors (ACTs) has been investigated in few studies (10, 25, 26, 27). Only one study evaluated the mRNA expression of mTOR, S6K1 and 4EBP1in a cohort of ACCs, demonstrating that the mRNA expression of S6K1 was significantly lower in ACCs than in benign ACTs (adrenocortical adenomas (ACAs)) (28). A highly variable protein expression of the main components of the mTOR pathway has been described in ACCs, and phospho-mTOR, phospho-S6K1 as well as phospho-4EBP1 were reported to be significantly expressed in 10–32, 30–59 and 40–60% of cases respectively (25, 26, 28). This is summarized in Table 1. In the study by Nakamura et al., the mean protein expression of several components of the mTOR pathway was lower in ACC samples than in ACA or normal adrenal samples, although the statistics were not reported (26). Similarly in a study from our group, the protein expression of total and phospho-mTOR, total and phospho-S6K1 and total and phospho-4EBP1 was lower in ACCs compared to ACAs, although this difference was statistically significant only for total S6K1, and probably due to the small sample size (28). Even though these studies adopted different antibodies and methodologies, they all reported that the expression of the main phospho-proteins of the mTOR pathway are not constantly found in these tumors, suggesting that this pathway is activated only in a subgroup ACCs. These data are partially in contrast with the study by Doghman et al. who reported that mTOR signaling is active in childhood ACTs (29). These contrasting data further support the increasing body of evidence which suggests that adult ACCs and childhood ACTs are different entities. Based on these data, the mTOR pathway should not be expected to be widely involved in the pathogenesis of ACCs but might be involved in a subset of them.
Table 1.
Studies reporting the protein expression (evaluated by immunohistochemistry) of the main components of the mTOR pathway in adrenocortical tumors. ACA, adrenocortical adenomas; ACC, adrenocortical carcinomas; IHC, immunohistochemistry; NA, normal adrenals.
| Author | Number of ACCs | Methodology | Type of antibodies | Phospho-Akt: positive ACC cases (%) | mTOR: positive ACC cases (%) | Phospho-mTOR: positive ACC cases (%) | p70 S6 Kinase: positive ACC cases (%) | Phoapho-p70 S6 Kinase: positive ACC cases (%) | Phospho-S6 Ribosomal Protein: positive ACC cases (%) | 4E-BP1: positive ACC cases (%) | Phospho-4E-BP1: positive ACC cases (%) | Phospho-Raptor: positive ACC cases (%) | Comparison with ACA | Comparison with NA | Consideration on prognosis | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nakamura et al. (26) | 41 | Standard IHC procedure. Specimens were categorized into six groups (0, 0%; 1, 1–5%; 2, 6–25%; 3, 26–50%; 4, 51–75%; 5, 76–100%). | Phospho-Akt (Ser473) monoclonal; phospho-mTOR (Ser2448) polyclonal; Phoapho-p70 S6 Kinase (Thr389) monoclonal; Phospho-S6 Ribosomal Protein (Ser240/244) polyclonal; Phospho-4E-BP1(Thr70) polyclonal | Not reported | Not evaluated | Not reported | Not evaluated | Not reported | Not reported | Not evaluated | Not reported | Not evaluated | With the exception of phospho-Akt, all the evaluated proteins were found to have a mean expression tendentially lower in ACC than in ACA, although the significance is not reported | All the evaluated proteins were found to have a mean expression tendentially lower in ACC than in NA, although the significance is not reported | Not reported | |
| De Martino et al. (28) | 20 | Standard IHC procedure. The score was calculated by the sum of the intensity score and the proportion of the stained cells; this provided a score between 0 and 6. The proportion score was as follows: 0 = no positivity (or <10%); +1 ≤ 1/3 tumor cell positivity; +2 = 1/3–2/3 tumor cell positivity; and +3 = more than 2/3 tumor cell positivity. The intensity score was as follows: +1 weak staining; +2 intermediate staining; and +3 strong staining. The score 0 was considered as negative, 2–3 as low, 4–5 as intermediate, and 6 as high. Finally, adrenocortical tumors were dichotomously grouped as having intermediate to high expression of the evaluated protein and phosphoproteins (IHC score ≥4) or not (IHC score <4). The reported percentages of positive ACC cases refer to the percentage of cases having intermediate to high expression of the evaluated proteins. | mTOR monoclonal; Phospho-mTOR (Ser2448) monoclonal; p70 S6 Kinase monoclonal; Phoapho-p70 S6 Kinase (Thr389) monoclonal; 4E-BPl monoclonal; Phospho-4E-BPl(Ser65) monoclonal | Not evaluated | 60 | 10 | 25 | 30 | Not evaluated | 75 | 60 | Not evaluated | All the evaluated proteins were found to have a mean expression tendentially lower in ACC than in ACA. This difference was significant only for p70 S6 Kinase (P = 0.009) | Not evaluated | In ACC group, a higher p70 S6 Kinase (P = 0.04) and phospho-4EBP1 (P = 0.04) protein expression were observed in tumors having a mitotic count <5 | |
| Germano et al. (25) | 58 | Tissue microarrays. Staining was assessed for all but one antibodies, using a binary scoring system based on the evaluation of cytoplasmic/membrane staining: score 0 = no staining, score 1 = positive staining. | Phospho-Akt (Ser473) polyclonal; phospho-mTOR (Ser2448) monoclonal; phospho-p70 S6K (Thr389) monoclonal; phospho-4EBP1 (Thr37/46) polyclonal; phospho-Raptor (Ser792) polyclonal | 55 | Not evaluated | 32 | Not evaluated | 59 | Not evaluated | Not evaluated | 40 | 29 | Not evaluated | Not evaluated | Phosho-mTOR expression was negative in tumors with high Weiss score (P = 0.025), and in all oncocytic ACC cases |
Two studies investigated the potential prognostic value of the expression of some components of the mTOR pathway in ACTs (Table 1). We showed that S6K1 mRNA and protein expression are lower in ACCs than in ACAs, and ACC samples with a lower phospho-S6K1 and/or phospho-4EBP1 protein expression had a significantly higher Weiss score than others. Additionally ACCs with a higher mitotic count (>5) presented a lower total S6K1 and phospho-4EBP1 protein expression (28). Recently Germano et al. observed a negative phospho-mTOR staining in tumors with high Weiss score (25). In childhood ACTs, generally known to have a less aggressive phenotype than adult ACCs, Doghman et al. reported a positive expression of some components of mTOR pathway (29). These data suggested that a subset of less differentiated ACCs could have an inactivation of the mTOR pathway. Therefore, the downregulation of the mTOR pathway in ACCs warrants further investigation as a potential prognostic factor.
In the era of personalized medicine, the description of the main components of the mTOR pathway in ACCs is an important step to explore, as their presence can be considered as potential markers for treatment with mTOR inhibitors. Considering that molecular biomarkers capable to predict the clinical response to mTOR inhibitors have not been clearly identified yet, the currently available studies suggest that a subset of patients have potential molecular evidence of mTOR pathway activation. However, further studies are required to explore whether these molecular events could predict an increased sensitivity to mTOR inhibitors.
Effects of mTOR inhibitors in ACCs
The testing of mTOR inhibitors in preclinical models of ACCs is a mandatory step to explore whether these compounds could represent a novel treatment opportunity for the management of ACCs. Few studies have evaluated the effects of different mTOR inhibitors, sirolimus, everolimus and/or temsirolimus on human ACC cancer cell lines (NCI-H295R, their clone HAC15 and SW13) and primary ACC cell cultures. Using different methodologies (Table 2), it was demonstrated that mTOR inhibitors inhibit the proliferation in ACC cell lines (including NCI-H295R) (22, 24, 25, 28, 29, 30, 31). These compounds had stronger anti-proliferative effects in the SW13 cell line than in NCI-H295R (25, 28, 29) and showed anti-proliferative effects in some but not all ACC primary cell cultures (28, 29, 30). However, it should be considered that while NCI-H295R cells are well accepted as a good model of ACCs, a debate is still open about the appropriateness of SW13 cells as a model for this type of cancer (32). Taking into account this and the other potential limitations of ACC cell lines as preclinical model of ACCs, the results of the current studies might suggest that among ACC patients it could be possible to find subgroups of patients with a higher sensitivity to mTOR inhibitors. The anti-proliferative effects of mTOR inhibitors in ACC cells seem to be associated with cell cycle inhibition and/or apoptosis induction, although these effects have been observed only at high of the concentrations tested (24, 30). Based on current data the anti-proliferative effects of mTOR inhibitors at concentrations that are potentially reachable in vivo seem to be predominantly cytostatic (24). An anti-secretory effect of sirolimus in ACC cells has also been reported (24). In mice, the inhibition of NCI-H295R xenograft growth has been reported using high everolimus dose (29). Additionally, sirolimus was found to significantly reduce cell survival and cortisol secretion only in selected ACC primary cultures (28). These data suggest that a subset of patients with ACCs might be more sensitive than others to this treatment. Therefore, further studies are warranted to find potential biomarkers predictive of response to treatment with mTOR inhibitors in ACCs. In this respect, the protein expression of the main components of the mTOR pathway was investigated in relation to the in vitro effects of mTOR inhibitors in ACC primary cultures (28). However, the expression of none of the evaluated proteins correlated with the in vitro response to these drugs (28). This absence of a correlation could be due to the low number of primary cultures used in this study. However, specifically designed clinical trials can appropriately evaluate for biomarkers predictive of response to treatments. Unfortunately, this type of clinical trials is extremely difficult to perform in such a rare cancer as ACCs. Therefore, progress in this direction can only be awaited from the results of clinical trials in other more common types of cancer. Once a clear predictive biomarker is identified in other cancers, its value in ACCs should be explored.
Table 2.
Studies evaluating the effects of different mTOR inhibitors in adrenocortical carcinoma cell lines.
| Author | Type of cells | mTOR inhibitor used | Methodology to evaluate inhibition of cell proliferation | Inhibition of cell proliferation | Type of drug combination | Methodology to evaluate effects of drug combination | Effects of drug combination | Confirmation in xenografts |
|---|---|---|---|---|---|---|---|---|
| Doghman et al. (29) | H295R; SW13; primary pediatric ACT cells | Everolimus | Cells were counted after 6 days of culture in the presence of the drug | Inhibition of cell proliferation | Not evaluated | Not evaluated | Not evaluated | H295R xenograft growth in NOD/SCID/yc null mice treated with placebo or with RAD001 (10/mg/kg/day). Tumor growth was significantly different in animals treated with the drug (P < 0.01) |
| De Martino et al. (24) | H295R; HAC15; SW13 | Sirolimus or evemsirolimus | After 24 h, 3, 6, and 9 days of treatment, the cells were harvested for DNA measurement | Sirolimus and temsirolimus significantly suppressed the cell growth in a doseand time-dependent manner | Anti-IGF2-neutralizing antibody | Cell viability assay (WST-1) | 72 h treatment with sirolimus combined with anti-IGF2 Abs almost totally blocked H295R cell proliferation (90% inhibition); sirolimus or anti-IGF2 antibody alone induced an inhibition in H295R cell proliferation of 64 and 42% respectively | Not evaluated |
| Mariniello et al. (30) | H295R; SW13; primary cells cultures | Everolimus | After 2 days of culture, cells were maintained overnight in low serum medium and drug incubation was started. Cell viability studies employing the 3-(4,5-dimethylthiazol-2-yl)-2,5 diphenyltetrazolium bromide (MTT) assay were performed after 24 and 72 hours of treatment | Dose-dependent cytotoxicity on SW13 and H295R. In primary adrenocortical cell cultures, a significant growth inhibitory response to the tested drugs was found in 3 of 5 cases | Sorafenib | MTT assay | The combination of everolimus with sorafenib produced a further reduction of SW13 cell viability, but not in H295R | Sorafenib monotherapy showed no significant activity on either SW13 and H295R xenografts, whereas administration of everolimus alone delayed SW13 tumor growth but was ineffective against H295R xenografts. Combination therapy produced remarkable tumor growth inhibitory effects on both SW13 and H295R xenografts; pharmacological treatments affected median survival of SW13 and H295R treated xenografts |
| De Martino et al. (28) | Human primary cultures | Sirolimus | The effects of sirolimus on cell survival in 7 human primary cultures of adrenocortical cancel were harvested for DNA measurement | Only one of 7 ACC primary culture showed a significant cell number reduction after sirolimus treatment | Not evaluated | Not evaluated | Not evaluated | Not evaluated |
| De Martino et al. (26) | H295R and SW13 | Sirolimus | After 6 days of treatment, the cells were harvested for DNA measurement | In both H295 and SW13, the selected concentrations of sirolimus significantly inhibited cell proliferation | Mitotane | DNA measurement | When mitotane was used at low concentrations (between 10−7 and 5 × 10−6 Μ), sirolimus had a statistically significant additive effect, when compared with mitotane alone | Not evaluated |
| Germano et al. (25) | SW13 and H295R | Everolimus | After 72 h treatment a cell viability assay (WST-1) was performed | Everolimus induced a dose-dependent decrease of cell viability in the two adrenal cancer cell | Pasireotide and mitotane | Cell viability assay (WST-1) | In the association of mitotane with everolimus, mitotane blocked everolimus activity in both SW-13 and H295R cell lines, independently of their responsiveness to mitotane, indicating an antagonistic effect of the two drugs, the combination of pasireotide and everolimus determined a synergistic cytotoxic effect in SW-13 cells and an antagonistic effect on cell growth in H295R cells | Not evaluated |
| De Martino et al. (31) | H295R and HAC15 | Sirolimus or everolimus | After 6 days of treatment in high or low serum concentration medium, the cells were harvested for DNA measurement | Sirolumus and everolimus inhibited cell proliferation in H295R and HAC15 cells in a dose dependent manner | Linsitinib | DNA measurement | Selected doses of sirolimus or everolimus combined with linsitinib 5 × 10−8 M had statistically significant additive effect on cell proliferation at some but not all the conditions tested | Not evaluated |
ACT, adrenocortical tumors.
To the best of our knowledge, the effects of compounds targeting the mTORC1 and 2 complex in ACC cell lines have not been explored yet. A recent study reported that n-3 polyunsaturated fatty acids prevent ACC growth by inhibiting mTORC1/2 in preclinical models of ACCs, which suggests that both mTORC complexes might play a role in ACC cell proliferation (33). Another compound that was reported to inhibit ACC growth in preclinical models of ACCs is the dual PI3-kinase/mTOR inhibitor NVP-BEZ235 (34). These new class of compounds require future investigations.
Relationship between the mTOR and the IGF pathways in ACCs
The relationship between the mTOR and the IGF pathways in ACCs has been scantly investigated (10). As the mTOR pathway mediates some of the IGF effects (10, 35, 36), it could be involved in mediating the pathogenic effects of IGFs in ACCs. Therefore it might be important to understand whether a differential expression of the main components of the IGF pathway could influence the in vitro sensitivity to mTOR inhibitors and whether there is a rational to combine drugs targeting the IGF and the mTOR pathways.
The relationship between the mTOR- and the IGF pathways in the NCI-H295R and SW13 ACC cell lines is addressed in a few studies (24, 28). These studies demonstrate that both ACC cell lines have a similar protein expression of IGF1R and the main components of the mTOR pathway, but both mRNA and protein expression of IGF2 were considerably higher in NCI-H295R compared with SW13. IGF1 significantly stimulated AKT and S6K1 phosphorylation in both NCI-H295R and SW13, demonstrating that the mTOR pathway acts as an intracellular mediator of IGFs in both human ACC cell lines (24). A schematic representation of the pathway is shown in Fig. 1. Therefore, the mTOR pathway could also be involved in mediating the proliferative effects of IGFs in ACC cell lines. However, the effects of the mTOR inhibitor sirolimus on the IGF-activated intracellular pathways were different between NCI-H295R and SW13 cells. At the experimental condition tested, IGF1 induced the activation of the AKT/mTOR pathway in both cell lines, but ERK activation was observed only in NCI-H295R. Sirolimus efficiently suppressed the mTORC1 activity in both cell lines. However, only in NCI-H295R cells, the inhibition of mTORC1 activity was associated with the activation of AKT, likely representing an escape pathway. This activation was further enhanced by IGF1 administration which also induced ERK stimulation in the sirolimus-treated NCI-H295R cells. Therefore, the NCI-H295R cell line seems to have two potential pathways of escape to treatment with traditional mTOR inhibitors: the AKT and ERK pathways (see Fig. 1 for the potential escape pathways) (35, 37). The activation of these escape pathways could be related, at least partially, to the IGF2 overexpression in NCI-H295R, which is not found in the SW13 cell model. Therefore, it could be speculated that high IGF2 expression could negatively influence the in vitro sensitivity of ACC cell lines to mTOR inhibitors, which supports the rationale to combine mTOR inhibitors and drugs specifically targeting the IGF pathway in ACCs (31). In another study everolimus has been reported to inhibit S6K1 phosphorylation in both NCI-H295R and SW13, to only slightly reduce AKT phosphorylation at the highest drug concentration used and to have no effect on ERK phosphorylation (30). High everolimus doses might reduce AKT phosphorylation sequestering of the mTOR as part of the mTORC1 complex and subsequently inhibiting the mTORC2 activity (10).
IGF2 overexpression is very common in ACC (about 80%) (18), whereas only a subset of ACC samples strongly expressed the components of the mTOR pathway, particularly the phospho-proteins (28). In the studies from our research group a subgroup of 16 ACC samples was characterized for protein expression of the main components of both mTOR and IGF pathway, including IGF2 (28, 31). Within this subgroup of ACC samples, we were not able to find correlations between these proteins (Table 3; personal unpublished data). Therefore, the expression of the main components of the mTOR and the IGF pathways seem not to be strongly related, which raises the questions whether in ACCs there is a dissociation between the expression of IGF2 and the activation of the classical IGF stimulated intracellular pathways, and whether the role of IGF2 in the pathogenesis of adult ACCs may have been overestimated, in agreement with some other recent speculations (38). However, it should also be considered that the complexity of the IGF system may have been underestimated since ACC expresses other components of the IGF pathway as well, such as the insulin receptor subtype A and the IGF2R (31, 36). These components have been scantly considered up today. As such, before finally declaring a ‘game over’ (38) for the role of IGF2 in adrenocortical tumorigenesis and as a potential target for novel treatment in ACC patients, it could be probably useful to return to the bench and try to better explore the IGF pathway in ACCs in its whole complexity.
Table 3.
ACC samples characterized for protein expression of the main components of both the mTOR and IGF pathways.
| Pt N | IGFII protein expression | IGFIR protein expression | IGFIIR protein expression | mTOR protein expression | Phospho mTOR protein expression | 4EBP1 protein expression | Phospho-4EBP1 protein expression | S6K protein expression | Phospho S6K protein expression | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Score | Considerable expression* | Score | Considerable expression* | Score | Considerable expression* | Score | Considerable expression* | Score | Considerable expression* | Score | Considerable expression* | Score | Considerable expression* | Score | Considerable expression* | Score | Considerable expression* | |
| 1 | 4 | Yes | 5 | Yes | 4 | Yes | 4 | Yes | 2 | No | 4 | Yes | 4 | Yes | 0 | No | 0 | No |
| 2 | 6 | Yes | 4 | Yes | 5 | Yes | 3 | No | 0 | No | 4 | Yes | 3 | No | 0 | No | 4 | Yes |
| 3 | 6 | Yes | 3 | No | 4 | Yes | 0 | No | 0 | No | 4 | Yes | 4 | Yes | 2 | No | 4 | Yes |
| 4 | 3 | No | 6 | Yes | 5 | Yes | 5 | Yes | 0 | No | 4 | Yes | 4 | Yes | 4 | Yes | 0 | No |
| 5 | 5 | Yes | 5 | Yes | 5 | Yes | 6 | Yes | 0 | No | 2 | No | 4 | Yes | 2 | No | 0 | No |
| 6 | 6 | Yes | 6 | Yes | 5 | Yes | 5 | Yes | 0 | No | 4 | Yes | 3 | No | 0 | No | 2 | No |
| 7 | 4 | Yes | 5 | Yes | 6 | Yes | 6 | Yes | 4 | Yes | 6 | Yes | 5 | Yes | 3 | No | 0 | No |
| 8 | 6 | Yes | 3 | No | 5 | Yes | 2 | No | 0 | No | 3 | No | 4 | Yes | 3 | No | 2 | No |
| 9 | 4 | Yes | 3 | No | 5 | Yes | 5 | Yes | 0 | No | 4 | Yes | 3 | No | 4 | Yes | 0 | No |
| 10 | 3 | No | 4 | Yes | 5 | Yes | 4 | Yes | 0 | No | 4 | Yes | 2 | No | 0 | No | 4 | Yes |
| 11 | 6 | Yes | 4 | Yes | 5 | Yes | 3 | No | 0 | No | 4 | Yes | 2 | No | 3 | No | 4 | Yes |
| 12 | 5 | Yes | 6 | Yes | 6 | Yes | 6 | Yes | 2 | No | 4 | Yes | 5 | Yes | 4 | Yes | 2 | No |
| 13 | 4 | yes | 3 | No | 4 | Yes | 3 | No | 0 | No | 5 | Yes | 4 | Yes | 2 | No | 4 | Yes |
| 14 | 6 | Yes | 3 | No | 5 | Yes | 2 | No | 0 | No | 5 | Yes | 3 | No | 3 | No | NA | NA |
| 15 | 6 | Yes | 4 | Yes | 6 | Yes | 5 | Yes | 0 | No | 0 | No | 2 | No | 0 | No | 0 | No |
| 16 | 6 | Yes | 2 | No | 5 | Yes | 2 | No | 0 | No | 2 | No | 5 | Yes | 3 | No | 4 | Yes |
*Considerable expression defined as an intermediate to high expression as reported in De Martino et al. (28).
NA, not available.
Effects of mTOR inhibitors in combination with other drugs in ACTs
The data derived from the use of the mTOR inhibitors alone in preclinical studies (24, 25, 28-30), together with the expected heterogeneity of ACCs (25, 28, 39), suggest that caution is required before using this class of drugs in unselected ACC patients. Such caution was also suggested by preliminary clinical experience with the use of everolimus in some ACC patients with a late stage of disease (40). Unfortunately, due to the current lack of molecular biomarkers capable to predict the response to mTOR inhibitors in ACCs (25, 28), it is difficult to define selection criteria for ACC patients that are candidate for treatment with this class of drugs. Therefore, combination of mTOR inhibitors with other drugs, potentially active in ACCs, could be a more prudent clinical approach than the use of these inhibitors as monotherapy in unselected ACC patients.
Until recently, the IGF pathway was considered as the most attractive target for new treatment in ACCs (10, 41, 42) with a potential rationale to combine mTOR inhibitors with drugs targeting the IGF pathway (11, 24). Linsitinib (OSI-906) is an IGF1-R/insulin receptor blocker that has recently been tested in a phase III trial in ACC patients (43). It was shown that only a very small subgroup of patients seems to benefit from treatment with this drug, but the anticipated improvement in overall or progression-free survival was not observed. This observation again illustrates that ACCs is a very heterogenous disease. However, whether combining drugs that target the IGF system with other compounds, such as mTOR inhibitors, could be more effective requires further investigation. A recent study explored the in vitro effects of mTOR inhibitors in combination with linsitinib and showed that, particularly when cells were cultured in medium with low serum, combined treatment of mTOR inhibitors with linsitinib has additive growth inhibitory effects on ACC cells at pharmacological concentrations (31). This supports a potential role for treatment strategies combining mTOR inhibitors and drugs targeting the IGF pathway in ACCs. These results are in line with a recently published phase I study demonstrating that a subgroup (about 40%) of ACC patients treated with cixutumumab (IGF1R inhibitor) and temsirolimus (mTOR inhibitor) experienced long-term disease stabilization (longer than 6 months) (44).
Another attractive candidate for new combination treatment strategies in ACCs is mitotane, since this drug is currently considered as a key drug in the treatment of patients with advanced ACCs. Unfortunately the majority of studies suggest that about two-thirds of patients do not respond and/or do not tolerate this drug (1, 45, 46, 47). In ACC cell lines, two studies reported the effects of mTOR inhibitors in combination with mitotane (22, 25). One study demonstrated that sirolimus had some additive effects with mitotane, but only when mitotane was used at low concentration (22), whereas another study reported that mitotane blocked the anti-proliferative effects of everolimus (25). Although these studies are contrasting in their main final conclusions, both studies show that the effects of mitotane can, at least in some conditions, overcome the effects of the mTOR inhibitors thus limiting the usefulness of combining full doses of these two therapeutic agents. As mentioned earlier, the preclinical results show that the addition of sirolimus to low concentrations of mitotane has stronger anti-proliferative effects than mitotane alone (22). If these results could be translated to humans, they suggest that the addition of sirolimus might add to the antitumor action of mitotane, thereby reducing the mitotane dose required to obtain a desired clinical effect with potentially fewer side effects. In a clinical setting, mTOR inhibitors can be metabolized by the microsomal liver enzyme cytochrome P450 (CYP3A4/5). Drugs as mitotane are capable to induce these enzymes, and might increase the liver metabolization of mTOR inhibitors, potentially reducing the plasma concentration of these compounds to sub-therapeutic levels (44, 48). The combination of the mTOR inhibitor everolimus and the tyrosine kinase inhibitor sorafenib has been evaluated in preclinical models of ACCs in which it is shown that combined treatment was more effective than single drug treatment both in ACC cell lines and in xenograft models. These data support the rationale for combined treatment in this type of malignancy (30).
New potential targets for ACCs in addition to the IGF and mTOR pathways
Until today, most of the early clinical experience with targeted drugs, including drugs targeting the IGF pathway, failed to demonstrate the desired effects in patients with ACCs (2, 38, 43). This raises the question whether molecular events, potentially targetable with currently developed drugs, are present in at least a subset of ACC patients. Using hotspot gene sequencing and comparative genomic hybridization, the presence of a large number of mutations and copy number abnormalities of potential interest for therapeutic aims, were evaluated in a large group of adult ENSAT stage III-IV ACC samples. No relevant alteration in the evaluated components of the mTOR and IGF pathways were found with these techniques and no simple targetable molecular event emerged (21, 39). Therefore, based on genomic alterations, the cell cycle appeared to be the most relevant new potential therapeutic target for patients with advanced ACC (Fig. 1). Recent data from exome sequencing confirm that the cell cycle or WNT pathways might be future target for treatment in ACCs (20, 49). Further studies to explore the effects of these compounds in preclinical models of ACCs are warranted.
Overall current data underline that, despite the fact that during the last 10 years much progress has been made in describing the molecular alteration in ACCs, the translation of these progress from bench to the bedside with the aim to improve the treatment of patients with ACCs has not been easy, so far.
Conclusion and future directions
In conclusion, the mTOR pathway seems a potential target for treatment of a subset of patients with ACCs, but treatment strategies combining mTOR inhibitors with other drugs are expected be more effective than the use of mTOR inhibitors alone. Additionally, considering the potential heterogeneity of this malignancy, treatment strategies based on the selection of patients with a potentially higher chance to respond to mTOR inhibitors according to their tumor characteristics, might be more effective than the use of mTOR inhibitors in unselected patients. Unfortunately, molecular biomarkers capable to predict a clinical response to mTOR inhibitors have not been clearly identified yet. Therefore, further preclinical and clinical investigations are required to find new molecular biomarkers useful to predict tumor response to both conventional and novel treatments for patients with ACCs and to address the role of mTOR inhibitors, alone or in combination with other drugs, in selected subgroups of patients with these tumors. All these data could help to move into the direction of a more personalized approach to the treatment of ACCs, and hopefully this approach could lead to progress in the clinical management of this rare but aggressive disease.
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of this review.
Funding
This work did not receive any specific grant from any funding agency in the public, commercial or not-for-profit sector.
References
- 1.Fassnacht M, Dekkers OM, Else T, Baudin E, Berruti A, de Krijger R, Haak HR, Mihai R, Assie G, Terzolo M. European Society of Endocrinology Clinical Practice Guidelines on the management of adrenocortical carcinoma in adults, in collaboration with the European Network for the Study of Adrenal Tumors. European Journal of Endocrinology 2018. G1–G46. ( 10.1530/EJE-18-0608) [DOI] [PubMed] [Google Scholar]
- 2.Else T, Kim AC, Sabolch A, Raymond VM, Kandathil A, Caoili EM, Jolly S, Miller BS, Giordano TJ, Hammer GD. Adrenocortical carcinoma. Endocrine Reviews 2014. 282–326. ( 10.1210/er.2013-1029) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Creemers SG, Hofland LJ, Korpershoek E, Franssen GJ, van Kemenade FJ, de Herder WW, Feelders RA. Future directions in the diagnosis and medical treatment of adrenocortical carcinoma. Endocrine-Related Cancer 2016. R43–R69. ( 10.1530/ERC-15-0452) [DOI] [PubMed] [Google Scholar]
- 4.Piccart-Gebhart MJ, Procter M, Leyland-Jones B, Goldhirsch A, Untch M, Smith I, Gianni L, Baselga J, Bell R, Jackisch C, et al Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. New England Journal of Medicine 2005. 1659–1672. ( 10.1056/NEJMoa052306) [DOI] [PubMed] [Google Scholar]
- 5.Paez JG, Janne PA, Lee JC, Tracy S, Greulich H, Gabriel S, Herman P, Kaye FJ, Lindeman N, Boggon TJ, et al EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science 2004. 1497–1500. ( 10.1126/science.1099314) [DOI] [PubMed] [Google Scholar]
- 6.Hanna SC, Heathcote SA, Kim WY. mTOR pathway in renal cell carcinoma. Expert Review of Anticancer Therapy 2008. 283–292. ( 10.1586/14737140.8.2.283) [DOI] [PubMed] [Google Scholar]
- 7.Wan X, Helman LJ. The biology behind mTOR inhibition in sarcoma. Oncologist 2007. 1007–1018. ( 10.1634/theoncologist.12-8-1007) [DOI] [PubMed] [Google Scholar]
- 8.Konings IR, Verweij J, Wiemer EA, Sleijfer S. The applicability of mTOR inhibition in solid tumors. Current Cancer Drug Targets 2009. 439–450. ( 10.2174/156800909788166556) [DOI] [PubMed] [Google Scholar]
- 9.Le Tourneau C, Faivre S, Serova M, Raymond E. mTORC1 inhibitors: is temsirolimus in renal cancer telling us how they really work? British Journal of Cancer 2008. 1197–1203. ( 10.1038/sj.bjc.6604636) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Martino MC, Van Koetsveld PM, Pivonello R, Hofland LJ. Role of the mTOR pathway in normal and tumoral adrenal cells. Neuroendocrinology 2010. 28–34. ( 10.1159/000314280) [DOI] [PubMed] [Google Scholar]
- 11.Robbins HL, Hague A. The PI3K/Akt pathway in tumors of endocrine tissues. Frontiers in Endocrinology 2015. 188 ( 10.3389/fendo.2015.00188) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bahrami A, Khazaei M, Hasanzadeh M, ShahidSales S, Joudi Mashhad M, Farazestanian M, Sadeghnia HR, Rezayi M, Maftouh M, Hassanian SM, et al Therapeutic potential of targeting PI3K/AKT pathway in treatment of colorectal cancer: rational and progress. Journal of Cellular Biochemistry 2018. 2460–2469. ( 10.1002/jcb.25950) [DOI] [PubMed] [Google Scholar]
- 13.Eyre TA, Collins GP, Goldstone AH, Cwynarski K. Time now to TORC the TORC? New developments in mTOR pathway inhibition in lymphoid malignancies. British Journal of Haematology 2014. 336–351. ( 10.1111/bjh.12945) [DOI] [PubMed] [Google Scholar]
- 14.Hudes G, Carducci M, Tomczak P, Dutcher J, Figlin R, Kapoor A, Staroslawska E, Sosman J, McDermott D, Bodrogi I, et al Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. New England Journal of Medicine 2007. 2271–2281. ( 10.1056/NEJMoa066838) [DOI] [PubMed] [Google Scholar]
- 15.Yao JC, Shah MH, Ito T, Bohas CL, Wolin EM, Van Cutsem E, Hobday TJ, Okusaka T, Capdevila J, de Vries EG, et al Everolimus for advanced pancreatic neuroendocrine tumors. New England Journal of Medicine 2011. 514–523. ( 10.1056/NEJMoa1009290) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Volante M, Buttigliero C, Greco E, Berruti A, Papotti M. Pathological and molecular features of adrenocortical carcinoma: an update. Journal of Clinical Pathology 2008. 787–793. ( 10.1136/jcp.2007.050625) [DOI] [PubMed] [Google Scholar]
- 17.Fassnacht M, Kreissl MC, Weismann D, Allolio B. New targets and therapeutic approaches for endocrine malignancies. Pharmacology and Therapeutics 2009. 117–141. ( 10.1016/j.pharmthera.2009.03.013) [DOI] [PubMed] [Google Scholar]
- 18.Lerario AM, Moraitis A, Hammer GD. Genetics and epigenetics of adrenocortical tumors. Molecular and Cellular Endocrinology 2014. 67–84. ( 10.1016/j.mce.2013.10.028) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zheng S, Cherniack AD, Dewal N, Moffitt RA, Danilova L, Murray BA, Lerario AM, Else T, Knijnenburg TA, Ciriello G, et al Comprehensive pan-genomic characterization of adrenocortical carcinoma. Cancer Cell 2016. 723–736. ( 10.1016/j.ccell.2016.04.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Assie G, Letouze E, Fassnacht M, Jouinot A, Luscap W, Barreau O, Omeiri H, Rodriguez S, Perlemoine K, Rene-Corail F, et al Integrated genomic characterization of adrenocortical carcinoma. Nature Genetics 2014. 607–612. ( 10.1038/ng.2953) [DOI] [PubMed] [Google Scholar]
- 21.Lippert J, Appenzeller S, Liang R, Sbiera S, Kircher S, Altieri B, Nanda I, Weigand I, Gehrig A, Steinhauer S, et al Targeted molecular analysis in adrenocortical carcinomas: a strategy toward improved personalized prognostication. Journal of Clinical Endocrinology and Metabolism 2018. 4511–4523. ( 10.1210/jc.2018-01348) [DOI] [PubMed] [Google Scholar]
- 22.De Martino MC, van Koetsveld PM, Feelders RA, Lamberts SWJ, de Herder WW, Colao A, Pivonello R, Hofland LJ. Effects of combination treatment with sirolimus and mitotane on growth of human adrenocortical carcinoma cells. Endocrine 2016. 664–667. ( 10.1007/s12020-015-0818-0) [DOI] [PubMed] [Google Scholar]
- 23.Nazarewicz RR, Salazar G, Patrushev N, San Martin A, Hilenski L, Xiong S, Alexander RW. Early endosomal antigen 1 (EEA1) is an obligate scaffold for angiotensin II-induced, PKC-alpha-dependent Akt activation in endosomes. Journal of Biological Chemistry 2011. 2886–2895. ( 10.1074/jbc.M110.141499) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.De Martino MC, van Koetsveld PM, Feelders RA, Sprij-Mooij D, Waaijers M, Lamberts SW, de Herder WW, Colao A, Pivonello R, Hofland LJ. The role of mTOR inhibitors in the inhibition of growth and cortisol secretion in human adrenocortical carcinoma cells. Endocrine-Related Cancer 2012. 351–364. ( 10.1530/ERC-11-0270) [DOI] [PubMed] [Google Scholar]
- 25.Germano A, Rapa I, Duregon E, Votta A, Giorcelli J, Buttigliero C, Scagliotti GV, Volante M, Terzolo M, Papotti M. Tissue expression and pharmacological in vitro analyses of mTOR and sstr pathways in adrenocortical carcinoma. Endocrine Pathology 2017. 95–102. ( 10.1007/s12022-017-9473-8) [DOI] [PubMed] [Google Scholar]
- 26.Nakamura M, Miki Y, Akahira J, Morimoto R, Satoh F, Ishidoya S, Arai Y, Suzuki T, Hayashi Y, Sasano H. An analysis of potential surrogate markers of target-specific therapy in archival materials of adrenocortical carcinoma. Endocrine Pathology 2009. 17–23. ( 10.1007/s12022-009-9058-2) [DOI] [PubMed] [Google Scholar]
- 27.Fassnacht M, Weismann D, Ebert S, Adam P, Zink M, Beuschlein F, Hahner S, Allolio B. AKT is highly phosphorylated in pheochromocytomas but not in benign adrenocortical tumors. Journal of Clinical Endocrinology and Metabolism 2005. 4366–4370. ( 10.1210/jc.2004-2198) [DOI] [PubMed] [Google Scholar]
- 28.De Martino MC, Feelders RA, de Herder WW, van Koetsveld PM, Dogan F, Janssen JA, Waaijers AM, Pivonello C, Lamberts SW, Colao A, et al Characterization of the mTOR pathway in human normal adrenal and adrenocortical tumors. Endocrine-Related Cancer 2014. 601–613. ( 10.1530/ERC-13-0112) [DOI] [PubMed] [Google Scholar]
- 29.Doghman M, El Wakil A, Cardinaud B, Thomas E, Wang J, Zhao W, Peralta-Del Valle MH, Figueiredo BC, Zambetti GP, Lalli E. Regulation of insulin-like growth factor-mammalian target of rapamycin signaling by microRNA in childhood adrenocortical tumors. Cancer Research 2010. 4666–4675. ( 10.1158/0008-5472.CAN-09-3970) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mariniello B, Rosato A, Zuccolotto G, Rubin B, Cicala MV, Finco I, Iacobone M, Frigo AC, Fassina A, Pezzani R, et al Combination of sorafenib and everolimus impacts therapeutically on adrenocortical tumor models. Endocrine-Related Cancer 2012. 527–539. ( 10.1530/ERC-11-0337) [DOI] [PubMed] [Google Scholar]
- 31.De Martino MC, van Koetsveld PM, Feelders RA, de Herder WW, Dogan F, Janssen JAMJL, Hofste Op Bruinink D, Pivonello C, Waaijers AM, Colao A, et al IGF and mTOR pathway expression and in vitro effects of linsitinib and mTOR inhibitors in adrenocortical cancer. Endocrine 2019. 673–684. ( 10.1007/s12020-019-01869-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang T, Rainey WE. Human adrenocortical carcinoma cell lines. Molecular and Cellular Endocrinology 2012. 58–65. ( 10.1016/j.mce.2011.08.041) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu J, Xu M, Zhao Y, Ao C, Wu Y, Chen Z, Wang B, Bai X, Li M, Hu W. n-3 polyunsaturated fatty acids abrogate mTORC1/2 signaling and inhibit adrenocortical carcinoma growth in vitro and in vivo. Oncology Reports 2016. 3514–3522. ( 10.3892/or.2016.4720) [DOI] [PubMed] [Google Scholar]
- 34.Doghman M, Lalli E. Efficacy of the novel dual PI3-kinase/mTOR inhibitor NVP-BEZ235 in a preclinical model of adrenocortical carcinoma. Molecular and Cellular Endocrinology 2012. 101–104. ( 10.1016/j.mce.2012.08.014) [DOI] [PubMed] [Google Scholar]
- 35.Liu P, Cheng H, Roberts TM, Zhao JJ. Targeting the phosphoinositide 3-kinase pathway in cancer. Nature Reviews: Drug Discovery 2009. 627–644. ( 10.1038/nrd2926) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Altieri B, Colao A, Faggiano A. The role of insulin-like growth factor system in the adrenocortical tumors. Minerva Endocrinologica 2019. 43–57. ( 10.23736/S0391-1977.18.02882-1) [DOI] [PubMed] [Google Scholar]
- 37.Kurmasheva RT, Huang S, Houghton PJ. Predicted mechanisms of resistance to mTOR inhibitors. British Journal of Cancer 2006. 955–960. ( 10.1038/sj.bjc.6603353) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Drelon C, Berthon A, Val P. Adrenocortical cancer and IGF2: is the game over or our experimental models limited? Journal of Clinical Endocrinology and Metabolism 2013. 505–507. ( 10.1210/jc.2012-3310) [DOI] [PubMed] [Google Scholar]
- 39.De Martino MC, Al Ghuzlan A, Aubert S, Assie G, Scoazec JY, Leboulleux S, Do Cao C, Libe R, Nozieres C, Lombès M, et al Molecular screening for a personalized treatment approach in advanced adrenocortical cancer. Journal of Clinical Endocrinology and Metabolism 2013. 4080–4088. ( 10.1210/jc.2013-2165) [DOI] [PubMed] [Google Scholar]
- 40.Fraenkel M, Gueorguiev M, Barak D, Salmon A, Grossman AB, Gross DJ. Everolimus therapy for progressive adrenocortical cancer. Endocrine 2013. 187–192. ( 10.1007/s12020-013-9878-1) [DOI] [PubMed] [Google Scholar]
- 41.Barlaskar FM, Spalding AC, Heaton JH, Kuick R, Kim AC, Thomas DG, Giordano TJ, Ben-Josef E, Hammer GD. Preclinical targeting of the type I insulin-like growth factor receptor in adrenocortical carcinoma. Journal of Clinical Endocrinology and Metabolism 2009. 204–212. ( 10.1210/jc.2008-1456) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Almeida MQ, Fragoso MC, Lotfi CF, Santos MG, Nishi MY, Costa MH, Lerario AM, Maciel CC, Mattos GE, Jorge AA, et al Expression of insulin-like growth factor-II and its receptor in pediatric and adult adrenocortical tumors. Journal of Clinical Endocrinology and Metabolism 2008. 3524–3531. ( 10.1210/jc.2008-0065) [DOI] [PubMed] [Google Scholar]
- 43.Fassnacht M, Berruti A, Baudin E, Demeure MJ, Gilbert J, Haak H, Kroiss M, Quinn DI, Hesseltine E, Ronchi CL, et al Linsitinib (OSI-906) versus placebo for patients with locally advanced or metastatic adrenocortical carcinoma: a double-blind, randomised, phase 3 study. Lancet: Oncology 2015. 426–435. ( 10.1016/S1470-2045(15)70081-1) [DOI] [PubMed] [Google Scholar]
- 44.Naing A, Lorusso P, Fu S, Hong D, Chen HX, Doyle LA, Phan AT, Habra MA, Kurzrock R. Insulin growth factor receptor (IGF-1R) antibody cixutumumab combined with the mTOR inhibitor temsirolimus in patients with metastatic adrenocortical carcinoma. British Journal of Cancer 2013. 826–830. ( 10.1038/bjc.2013.46) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Puglisi S, Perotti P, Cosentini D, Roca E, Basile V, Berruti A, Terzolo M. Decision-making for adrenocortical carcinoma: surgical, systemic, and endocrine management options. Expert Review of Anticancer Therapy 2018. 1125–1133. ( 10.1080/14737140.2018.1510325) [DOI] [PubMed] [Google Scholar]
- 46.Terzolo M, Angeli A, Fassnacht M, Daffara F, Tauchmanova L, Conton PA, Rossetto R, Buci L, Sperone P, Grossrubatscher E, et al Adjuvant mitotane treatment for adrenocortical carcinoma. New England Journal of Medicine 2007. 2372–2380. ( 10.1056/NEJMoa063360) [DOI] [PubMed] [Google Scholar]
- 47.Megerle F, Herrmann W, Schloetelburg W, Ronchi CL, Pulzer A, Quinkler M, Beuschlein F, Hahner S, Kroiss M, Fassnacht M, et al Mitotane monotherapy in patients with advanced adrenocortical carcinoma. Journal of Clinical Endocrinology and Metabolism 2018. 1686–1695. ( 10.1210/jc.2017-02591) [DOI] [PubMed] [Google Scholar]
- 48.van Erp NP, Guchelaar HJ, Ploeger BA, Romijn JA, Hartigh Jd, Gelderblom H. Mitotane has a strong and a durable inducing effect on CYP3A4 activity. European Journal of Endocrinology 2011. 621–626. ( 10.1530/EJE-10-0956) [DOI] [PubMed] [Google Scholar]
- 49.Juhlin CC, Goh G, Healy JM, Fonseca AL, Scholl UI, Stenman A, Kunstman JW, Brown TC, Overton JD, Mane SM, et al Whole-exome sequencing characterizes the landscape of somatic mutations and copy number alterations in adrenocortical carcinoma. Journal of Clinical Endocrinology and Metabolism 2015. 100 E493–E502. ( 10.1210/jc.2014-3282) [DOI] [PMC free article] [PubMed] [Google Scholar]



 This work is licensed under a
This work is licensed under a