Abstract
ToxR and TcpP, two winged helix-turn-helix (w-HTH) family transcription factors, co-activate expression of the toxT promoter in Vibrio cholerae. ToxT then directly regulates a number of genes required for virulence. In addition to co-activation of toxT, ToxR can directly activate the ompU promoter and repress the ompT promoter. Based on a previous study suggesting that certain wing residues of ToxR are preferentially involved in toxT co-activation compared to direct ompU activation, we employed alanine-scanning mutagenesis to determine which residues in the wing of ToxR are required for activation of each promoter. All of the ToxR wing residues tested that were critical for transcriptional activation of toxT and/or ompU were also critical for DNA binding. While some ToxR wing mutants had reduced interaction with TcpP, that reduced interaction did not correlate with a specific defect in toxT activation. Rather, such mutants also affected ompU activation and DNA binding. Based on these findings we conclude that the primary role of the wing of ToxR is to bind DNA, along with the DNA recognition helix of ToxR, and this function is required both for direct activation of ompU and co-activation of toxT.
Introduction
ToxR and TcpP are transmembrane transcription factors that coordinately activate transcription of toxT, the gene encoding the master virulence regulator in V. cholerae. TcpP is the direct activator of toxT, binding to the toxT promoter from -53 to -38, just upstream of the -35 element [1]. Although, TcpP is able to activate intermediate levels of toxT expression when overexpressed in the absence of ToxR [2, 3], ToxR is required for TcpP-mediated expression of toxT at endogenous expression levels. ToxR binds the toxT promoter from -96 to -83 [4], approximately three helical turns upstream of the TcpP binding site, enhancing TcpP-mediated activation of the toxT promoter. In addition to acting as a co-activator of toxT, ToxR is also able to directly activate the ompU promoter and repress the ompT promoter [4–6].
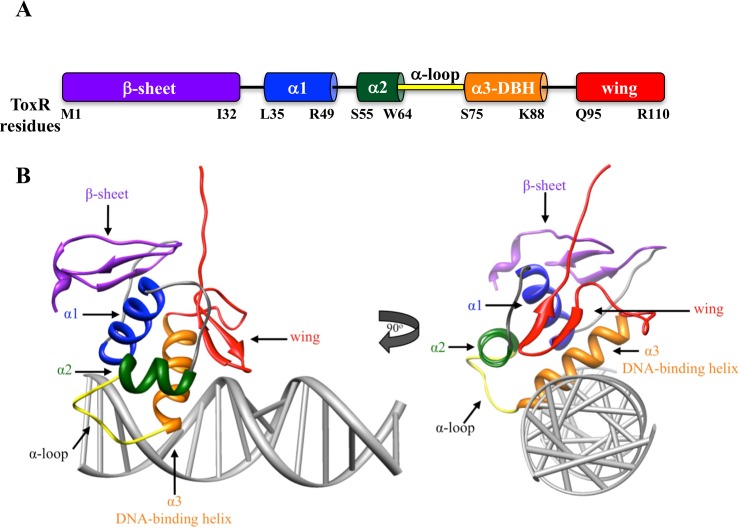
ToxR and TcpP both have cytoplasmic domains homologous to the w-HTH (winged helix-turn-helix) family of transcription factors [7]. Most w-HTH proteins have an N-terminal regulatory domain and a C-terminal w-HTH domain. However, both ToxR and TcpP have an N-terminal w-HTH domain, which is linked to a C-terminal periplasmic domain through a single-pass transmembrane domain. The periplasmic domain of ToxR is involved in, but not required for dimerization [8–13]. The periplasmic domain of TcpP regulates stability and is proteolytically degraded under non-inducing conditions [14–17]. Likewise, the periplasmic domain of ToxR is the target of proteolysis under conditions of alkaline pH and nutrient limitation [18]. The functions and stability of ToxR and TcpP periplasmic domains are enhanced by the periplasmic proteins ToxS and TcpH, respectively [8, 14–17, 19, 20]. The w-HTH domains of ToxR and TcpP bind to the toxT promoter and activate transcription. The w-HTH domain consists of an N-terminal β-sheet, 3 α-helixes including the DNA-binding helix (α3) that is inserted into the major groove of the DNA, and a C-terminal wing (Fig 1). The N-terminal β-sheet can be involved in protein-protein interaction as well as stabilizing the hydrophobic core [7, 21–23]. The first two α-helixes form part of the hydrophobic core as well as interact with the DNA backbone helping to stabilize protein-DNA interactions [21, 24, 25]. Between the second α-helix (α2) and the DNA-binding helix (α3) is the α-loop. The α-loop of w-HTH proteins is hypothesized to interact with RNA polymerase (RNAP, [7]). The α-loop of ToxR is critical for direct activation of the ompU promoter while less essential for co-activation of the toxT promoter, as would be predicted for a domain that interacts with RNAP [26]. The wing of w-HTH proteins consists of a β-strand hairpin, which inserts into the minor grove of the DNA, thereby enhancing binding to the promoter [21, 25, 27]. The wing of w-HTH proteins is often also involved in dimerization [21, 28, 29].
Fig 1. Domain arrangement of ToxR and modeled structure of ToxR on DNA highlighting those domains.
A) Based on homology to other w-HTH proteins [7] the residues defining each domain of ToxR are highlighted. B) A modeled structure of ToxR bound to DNA was generated with the I-TASSER modeling program (http://zhanglab.ccmb.med.umich.edu/I-TASSER/) and the crystal structure of other w-HTH family members [26]. Binding of ToxR to DNA was modeled using the NMR structure of PhoB bound to DNA [25]. Domains were highlighted with the same color scheme used in part A.
Dimerization is critical for activation of w-HTH transcription factors and can be mediated by interactions between w-HTH domains, the N-terminal regulatory domain (of w-HTH proteins containing this domain) and the promoter architecture [21, 23, 28, 30, 31]. w-HTH proteins have been found to dimerize in three different orientations. Both PhoB and OmpR have been shown to dimerize in a head-to-tail orientation on the promoter. In this orientation, the wing of the upstream w-HTH protein interacts with the β-sheet of the downstream w-HTH [21, 28]. Alternatively, Mycobacterium tuberculosis PhoP has been shown by crosslinking to dimerize in a head-to-head orientation with the two β-sheets interacting [30]. OmpR can also be found in head-to head orientations [23], although formation of OmpR dimers in head-to-head or head-to-tail orientation may be primarily dependant on the orientation of the OmpR binding site [31]. Finally, HSF in Kluyveromyces lactis interacts in a tail-to-tail orientation in which the two wings interact [29]. In this system the wings are only involved in protein-protein interaction and do not appear to interact with the DNA [29]. Both ToxR and TcpP form homodimers [8–13, 32] as well as ToxR-TcpP heterodimers [2, 26, 33]. However, the orientation and interface(s) of these dimers has not been elucidated.
When ToxR is bound to the toxT promoter, a hypersensitivity site is created at the TcpP binding site [34] indicating an alteration of the structure of the toxT promoter upon ToxR binding. This hypersensitivity site indicates that upon ToxR binding to the toxT promoter, the TcpP-binding site may become more accessible, allowing for enhanced TcpP binding to the promoter. Although, it should be noted when membranes containing both ToxR and TcpP were used for DNAse I footprinting studies, only the ToxR-protected region was observed [34]. This suggests ToxR may bind DNA and tether TcpP in place for RNAP recruitment/stimulation [2, 26].
Additionally, the toxT promoter is repressed by H-NS, and ToxR is critical for toxT activation in the presence of H-NS, as it can increase expression of toxT over 10-fold, whereas in the absence of H-NS, ToxR only increases expression of toxT two to three-fold [35]. Together, this indicates that one of the primary roles of ToxR may be to relieve H-NS repression, but that ToxR also plays an additional roll(s) in activation beyond relieving H-NS repression. ToxR also likely influences the localization of the toxT promoter by recruiting it to the membrane thereby enhancing access of TcpP to the toxT promoter [36]. Although a soluble form of the cytoplasmic domain of ToxR is able to activate transcription of ompU, membrane localization is required for ToxR co-activation of toxT [37].
Another possible role for ToxR in co-activation of TcpP is recruitment of TcpP to the toxT promoter through ToxR-TcpP interaction. ToxR-TcpP interaction can be observed by DSP crosslinking in V. cholerae membranes [2, 26] and a membrane-localized E. coli bacterial two-hybrid (BACTH) system [26]. ToxR-TcpP interaction likely occurs via the wing of TcpP, since mutations in the wing of TcpP can disrupt ToxR-TcpP interaction resulting in a defect in transcriptional activation [2]. The wing of TcpP plays a dual role, since residues in the wing of TcpP also are required for binding to the toxT promoter [2, 34]. The wing of ToxR may also be involved in ToxR-TcpP interaction, as ToxR-P101L was preferentially defective for toxT activation, relative to ompU activation, and was defective in interaction with TcpP by DSP crosslinking [26]. It is possible ToxR-TcpP interaction is maintained upon binding the toxT promoter, allowing ToxR to stabilize TcpP binding to the promoter, thereby enhancing transcriptional activation. Alternatively, ToxR-TcpP interaction could be disrupted upon ToxR binding to DNA, allowing each protein to then bind the promoter independently. Finally, it is possible that ToxR/TcpP interaction is maintained upon DNA binding by ToxR. In this scenario, TcpP would be tethered in place such that it could productively interact with RNA polymerase without binding DNA. This model is supported by the observation that a poor DNA-binding mutant of TcpP, TcpP-H93L, can be rescued for toxT activation when co-expressed with ToxR [2]. This model is also supported by the observation that TcpP-mediated footprinting is lost in the presence of ToxR co-expression [34].
The role of the ToxR-wing in co-activation of toxT could be due to DNA binding and/or ToxR-TcpP interaction. DNA binding by the wing of ToxR is critical for activation of both toxT and ompU since mutations in the wing of ToxR (T99K or G104S) that inhibit DNA binding inhibit transcriptional activation of both promoters [26]. However, several mutants in the wing (P101L, and R103G) as well as mutants in the loop leading from the DNA-binding helix to the wing (D89E, K92E, S93P) are preferentially defective for transcription of toxT, compared to ompU [26]. This could be due to differences in binding to the two promoters, ToxR-TcpP interaction, or other as yet unknown factors. The goal of this study was to determine the role of each residue in the wing of ToxR in transcriptional activation of both toxT and ompU. The requirement of ToxR wing residues for ToxR-TcpP interaction and DNA binding was also assessed to determine what role the wing of ToxR plays in co-activation of toxT.
Results
The wing of ToxR is required for transcriptional activation of both toxT and ompU
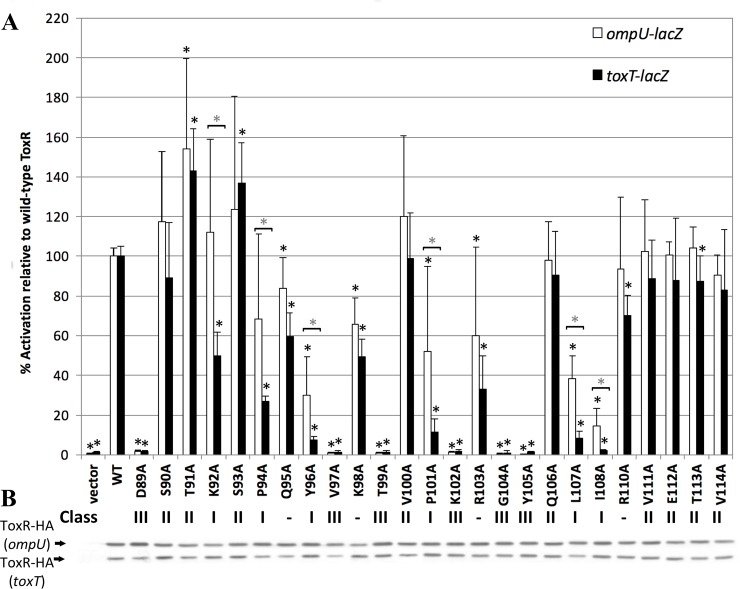
To determine the role of each wing residue in ToxR co-activation of toxT and direct activation of ompU, we mutated each wing residue to alanine, including residues in the loop between the DNA-binding helix (α3) and the wing (Fig 1). The range of potential wing residues from D89-V114 was based on homology of ToxR to molecules with known structures such as OmpR and PhoB {Krukonis, 2000 #5068}. Alanine scanning was used because residues in this region of ToxR can have different phenotypes depending on the amino acid to which they are mutated [26]. Each ToxR-wing mutant was expressed from a plasmid and tested for transcriptional activation of chromosomal ompU-lacZ (strain EK410) and toxT-lacZ (strain EK1072) (Fig 2) [26, 37]. Since each ToxR derivative was tagged at the C-terminus (periplasmic domain) with an HA tag, expression of each ToxR mutant was assessed in both the ompU-lacZ and toxT-lacZ reporter strains with an anti-HA antibody (Fig 2B).
Fig 2. Requirement of each residue in the ToxR wing on toxT and ompU activation.
A) ToxR-HA wing mutants were expressed from pBluescriptSK- (pSK) in the presence of pREP4 to regulate expression. The level of activation both the toxT-lacZ and ompU-lacZ chromosomal reporters for each mutant was measured by β-galactosidase assay and normalized to wild-type ToxR. * p<0.001923 using the Students’ T-test and a cut-off for significance at 0.05/26 according to the Bonferroni correction for multiple comparisons. * above a [indicates ToxR mutants that are at least 2-fold more defective for toxT activation than ompU activation. * p<0.05 using the Students’ T-test. B) Stability of ToxR-HA was monitored by Western blot using a monoclonal anti-HA. All strains were tested at least six times on at least two different days. Where appropriate, the mutant class is listed below each substitution. ToxR-Q95A, ToxR-K98A, ToxR-R103A, and ToxR-R110A did not fit into any of the mutant class designations.
Of the 25 residues tested, six were preferentially required for toxT co-activation as compared to ompU activation (K92, P94, Y96, P101, L107, and I108, Fig 2). Residues preferentially required for toxT co-activation were defined by mutation of that residue resulting in a 2-fold decrease in toxT co-activation relative to ompU activation and designated Class I mutants (Fig 3 green). Three of the six residues (Y96, L107, and I108) were required for both toxT co-activation and ompU activation as mutation of these residues resulted in less than 50% activation of ompU (Fig 2). These three residues are particularly critical for co-activation of toxT, since mutation of any of these residues decreased toxT transcriptional activation to 10% or less of wild-type. Based on in silico modeling, these residues are clustered at the hinge of the wing adjacent to the β-strands (Fig 3A). The two prolines present in the wing, P94 and P101, are partially required for ompU activation (70% and 50% activity respectively, when mutated), but critical for toxT co-activation (26% and 11% activity respectively, when mutated). K92 is not required for ompU activation (maintained ~100% activity when mutated), but is involved in toxT co-activation (50% activity), similar to a previously described ToxR-K92E mutant [26]. None of the wing residues were preferentially required for ompU activation relative to toxT activation (Fig 2).
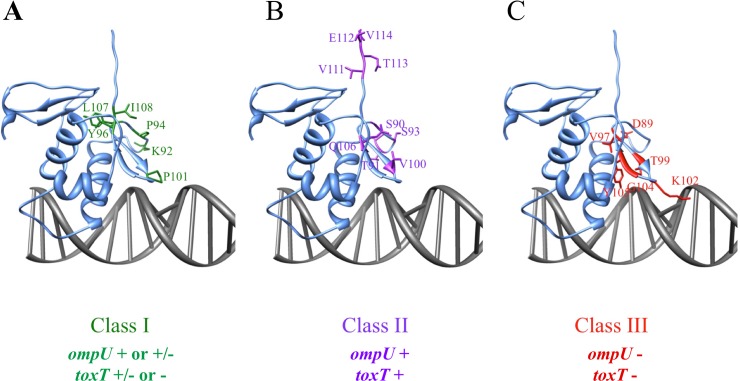
Fig 3. Assignment of three classes of ToxR wing mutants.
ToxR w-HTH domain was previously modeled using I-TASSER (http://zhanglab.ccmb.med.umich.edu/I-TASSER/)[26]. This previously-generated model with then modeled onto DNA using Chimera and the NMR structure of the ToxR homolog PhoB bound to DNA {Yamane, 2008 #5306}. Mutants were divided into classes based on phenotype. A) Class I mutants (green) are greater than 2-fold defective for toxT activation relative to ompU activation when expressed from a plasmid (Fig 2). B) Class II mutants (purple) are not significantly defective for activation of either promoter. C) Class III mutants (red) are required for activation of both toxT and ompU promoters and DNA binding.
Several residue side chains in the wing region of ToxR are not required for transcriptional activation of either promoter (S90, T91, S93, V100, Q106, V111, E112, T113, and V114, Fig 2) and were designated Class II mutants (Fig 3B, purple). When these residues were mutated to alanine >80% of transcriptional activation of both promoters was maintained (Fig 2). The majority of these residues are either in the loop between the DNA-binding helix (α3) and the wing or after the β-hairpin of the wing (Fig 3). The only amino acid side-chain in the β-turn of the wing (residues V97-G104) not required for transcriptional activation of at least one promoter was V100, indicating that the β-hairpin is critical for transcriptional activation. This is expected since many w-HTH proteins insert the β-hairpin of the wing into the minor groove of promoter DNA [21, 25, 27].
As was expected, since the wing of ToxR is involved in DNA binding [26], several wing residues of ToxR are required for transcriptional activation of both ompU and toxT (D89, V97, T99, K102, G104, Y105, Fig 2). Mutation of these residues, designated Class III (Fig 3C), resulted in less than 5% activation of either ompU or toxT loci (Fig 2). This results in toxT activation as low as the empty vector control. The majority of these residues are predicted to be involved in DNA binding, either directly or indirectly, and mutations in T99 and G104 have been previously shown to disrupt DNA binding to both promoters [26]. All of the residues required for any detectable transcriptional activation of both promoters are found in the β-hairpin of the wing except D89, which is positioned near the end of the DNA-binding helix (Fig 3C).
Analysis of selected ToxR mutants expressed from the chromosome
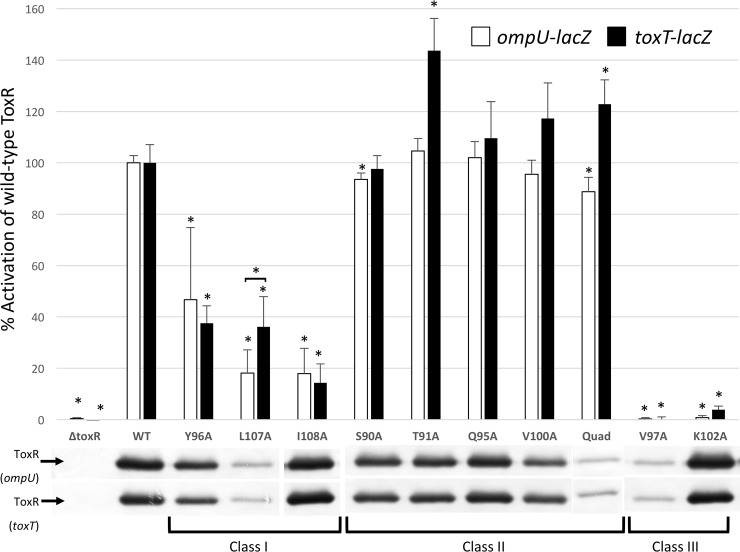
Since overexpression can result in altered phenotypes, we analyzed the requirement for certain ToxR wing residues for transcriptional activation using chromosomally expressed ToxR wing mutants. Thus, we measured activation of chromosomal toxT-lacZ and ompU-lacZ reporter from members of each class of ToxR mutant expressed from the native toxR locus (Fig 4). Three residues preferentially required for toxT co-activation under overexpression conditions, Y96, L107, and I108, were no longer preferentially required for toxT co-activation when expressed from the chromosome. L107 was even slightly more critical for ompU activation than toxT co-activation when endogenously expressed (p<0.005), although ToxR-L107A was unstable when expressed from the chromosome in the absence of the C-terminal HA tag. Q95 contributed to, but was not required for, toxT activation when expressed from a plasmid (ToxR-Q95A has 59% of wild-type activation), was able to activate both toxT and ompU promoters at wild-type levels when chromosomally expressed (Figs 2 and 4).
Fig 4. ToxR wing mutants expressed from the toxR chromosomal locus are not preferentially defective for toxT activation.
A) Selected ToxR wing mutants representing each class of mutant were placed on the chromosome and assayed for transcriptional activation of toxT-lacZ and ompU-lacZ by β-galactosidase assay. Class I mutants were at least 2-fold more defective for toxT activation than ompU activation when expressed from a plasmid, but equally defective for activation of both promoters when endogenously expressed. Class II mutants are not defective for activation of either promoter, and maintained ≥90% activity even when mutated. Class III mutants are defective for activation of both promoters. * p<0.0045 using the Students’ T-test and a cut-off for significance at 0.05/11 according to the Bonferroni correction for multiple comparisons. B) The stability of each ToxR wing mutant was monitored by Western blot using anti-ToxR antibodies.
All four class II mutants tested tolerated mutation to alanine and maintained transcriptional activation of both toxT and ompU promoters, whether expressed from the native toxR locus or a plasmid. To reveal any subtle effects on transcription by the four chromosomal class II mutants we constructed a quadruple mutant, ToxR-S90A/T91A/Q95A/V100A - designated ToxR-Quad, however this mutant was still fully able to activate both promoters (Fig 4). As was found with plasmid-based expression, both of the class III mutants tested (ToxR-V97A and ToxR-K102A) were still required for transcriptional activation of toxT and ompU when endogenously expressed.
These results illustrate that when one finds subtle phenotypes affecting activation of different promoters, assessing the affects with endogenous expression levels of the transcription activator can be informative. For these studies with un-tagged ToxR mutants expressed from the chromosome, the stability of three mutants appeared compromised, ToxR-V97A, ToxR-L107A and the ToxR-Quad mutant. However, since we used an anti-ToxR antibody to detect ToxR for this analysis, it remains possible that these mutations affected an epitope recognized by our antibody. V97 and L107 are predicted to be adjacent to one another at the hinge region of the ß-turn of the wing and may form a single epitope (Fig 3). This is why we used HA-tagged ToxR expressed from a plasmid for our initial studies.
Several ToxR wing residues are required for DNA binding
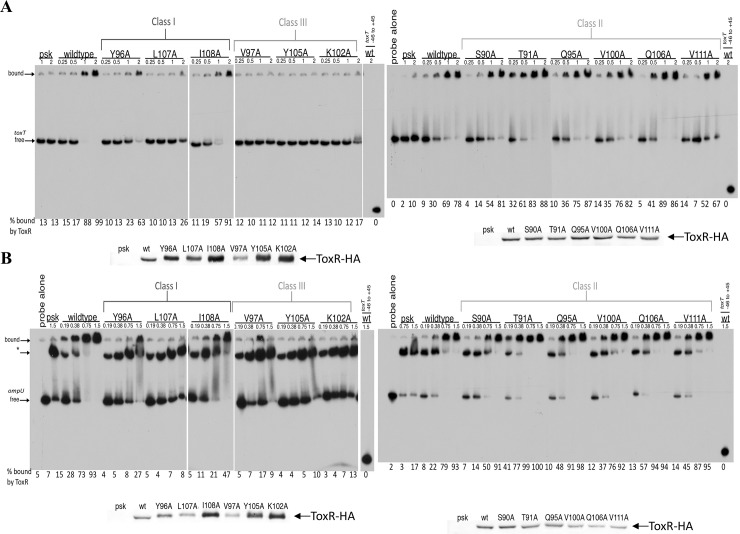
Since the wing of w-HTH proteins is often involved in binding to the minor groove of the DNA, we determined which ToxR wing residues are required for DNA binding. We selected ToxR wing mutants from each class of ToxR wing residues and measured their DNA binding by EMSA (Electrophoretic Mobility Shift Assay) using plasmid-based expression of ToxR-HA derivatives. Each ToxR derivative was expressed in V. cholerae membranes and purified membranes were used for EMSAs as described previously {Krukonis, 2000 #5068}. Shifting of the toxT and ompU promoters by wild-type ToxR was detectable at 0.5 mg/ml and 0.19 mg/ml respectively (Fig 5A and 5B). All of the class III mutants tested (ToxR wing residues required for both toxT and ompU transcriptional activation) showed a dramatic decrease in binding capacity. This included ToxR-V97A, ToxR-Y105A, and ToxR-K102A that had no toxT binding capacity (Fig 5A and S1 Fig) and no shifting to the fully bound ompU/ToxR complex (Fig 5B and S1 Fig). As noted previously [26], even extracts from cells carrying the empty vector pSK-Bluescript had residual ToxR-independent ompU shifting to an intermediate position in the gel denoted with *.
Fig 5. Several wing residues of ToxR are required for DNA binding.
DNA binding by ToxR wing mutants was monitored using EMSA. V. cholerae membranes containing the ToxR wing mutants, but lacking TcpP, were bound to 32P end-labeled probes containing either the toxT promoter (A) or the ompU promoter (B). ToxR levels in each membrane preparation was monitored by anti-HA Western blot. Representative gels and quantification are shown from a minimum of two replicates. * indicates an intermediate ToxR-independent shifted ompU promoter that is also present with the pSK vector control extract [26].
The class I residues, which are involved in activation of both promoters, but preferentially affect toxT activation when expressed from a plasmid, were also required for DNA binding (Fig 5 and S1 Fig). High levels of membrane containing ToxR-Y96A or ToxR-I108A were required to shift the toxT and ompU promoters, indicating a dependence upon these residues for efficient DNA binding. Furthermore, these ToxR derivatives were more concentrated in their respective membrane preparations relative to wild-type ToxR, yet still required more membrane than wild-type ToxR to elicit similar gel shift activity (Fig 5, see anti-ToxR-HA Western blots). Mutation of ToxR-L107 resulted in an inability of ToxR-L107A to shift 50% of either probe even at the highest concentrations tested, indicating that this residue is a key residue for DNA binding (Fig 5). Thus, all of the ToxR wing residues tested for DNA binding that were involved in activation were also involved in promoter binding.
Of the class II mutants tested whose affected side-chains are not required for transcriptional activation of either promoter (ToxR-S90A, T91A, Q95A, V100A, Q106A, and V111A), none were defective for DNA binding either the toxT or ompU promoter (Fig 5 and S1 Fig). ToxR-T91A even had increased DNA binding efficiency to both the ompU and toxT promoters relative to wild-type ToxR. This corresponded to a slight, but significant (p<0.05) increase in transcriptional activation of both of these promoters (Fig 2). Based on all of the wing mutants tested for DNA binding, ToxR activity at both the toxT and ompU promoters corresponded to the ability of the ToxR wing to bind these promoters.
Some wing residues of ToxR affect TcpP interaction
Since previous studies have suggested ToxR and TcpP interaction plays a role in toxT activation [2, 26], we assessed the ability of several chromosomally-encoded ToxR mutants for their ability to interact with TcpP.
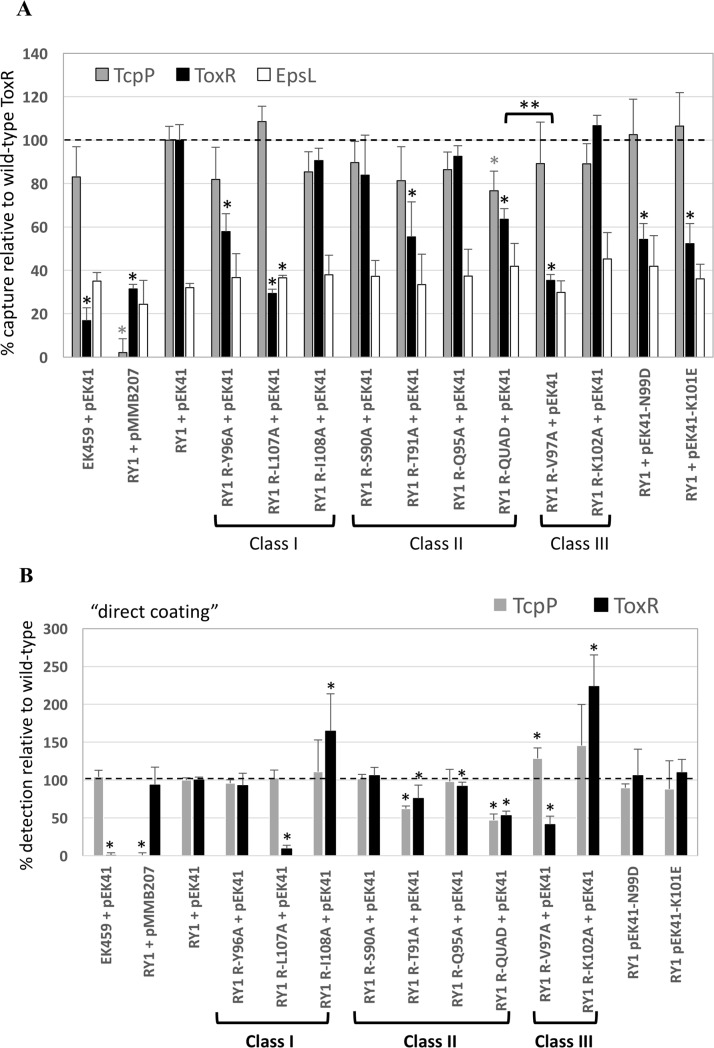
Two Class I mutants that expressed lower levels of toxT relative to ompU when expressed from a plasmid (Fig 2), ToxR-Y96A and ToxR-L107A, showed a 40% and 70% reduction in TcpP interaction (respectively) relative to wild-type ToxR using a ToxR co-capture assay where interacting membrane proteins are crosslinked with DSP, TcpP-HSV is immobilized onto plates using an anti-HSV mouse monoclonal antibody and the amount of ToxR co-captured is determined using a rabbit anti-ToxR polyclonal antibody (Fig 6, [2]). However, it should be noted, ToxR-L107A was barely detectable in whole cell extracts by direct coating of extracts onto a microtiter plate (Fig 6B). Thus, the fact that less ToxR-L107A was recruited is to be expected. However, it should be noted that the levels of both ToxR and TcpP in the ToxR-T91A membrane protein extract were also reduced relative to the wild-type ToxR extracts (Fig 6B and S2 Fig). This could explain the lower levels of ToxR co-capture by ToxR-T91A. On the other hand, ToxR-Y96A was expressed to similar levels as wild-type ToxR (Fig 6B and S2 Fig), but had a 40% defect in ToxR/TcpP interaction (Fig 6A), suggesting it has a bone fide defect in TcpP interaction.
Fig 6. Mutations in most ToxR wing residues do not affect ToxR-TcpP interaction.
Using membrane extracts expressing TcpP-HSV (from pEK41) and various chromosomally-expressed ToxR mutants, the ability of TcpP-HSV immobilized to a microtiter plate via anti-HSV antibodies to co-capture ToxR or the negative control inner membrane protein, EpsL was assessed. V. cholerae membranes were precipitated, resuspended at 2mg/ml protein and crosslinked with DSP prior to Triton X-100 solubilization of the membranes and ELISA capture of TcpP-HSV on mouse anti-HSV-coated 96-well plates. A) The levels of TcpP, ToxR and EspL were determined using specific rabbit anti-ToxR, rabbit anti-TcpP and rabbit anti-EpsL antibodies. Levels of each protein were normalized to the levels in a strain expressing wild-type TcpP-HSV and wild-type ToxR (set to 100%). The limit of detection for ToxR co-capture was about 17% based on a control capture assay with a TcpP-HSV+/ToxR- strain (EK459 + pEK41). For EpsL, the co-capture raw data ELISA signal was normalized to the ToxR ELISA signal. With this normalization, the EpsL signal in RY1 + pEK41 was 32% (relative to ToxR) and levels of EpsL co-capture did not vary +/- TcpP, indicating a lack of EpsL binding by TcpP-HSV.* p<0.0035 relative to wild-type TcpP or ToxR using the Students’ T-test and a cut-off for significance at 0.05/13 according to the Bonferroni correction for multiple comparisons. EK459 = V. cholerae ΔtoxRΔtcpP, RY1 = ΔtcpP. B) Membrane extracts directly coated onto 96-well plates without anti-HSV antibodies were used to assess total levels of TcpP and ToxR in each extract. * p<0.05 relative to wild-type TcpP or ToxR. ** p<0.001 between ToxR-QUAD and ToxR-V97A. A dotted line was added at the 100% point on the Y-axis.
One of three Class II mutants tested that showed no defect in toxT activation, ToxR-T91A (Figs 2–4), also had a 45% reduction in TcpP interaction (Fig 6A). But, ToxR-T91A also had reduced ToxR and TcpP levels in whole extracts (Fig 6B). Finally, one of the two Class III mutants tested that lacked DNA-binding activity (Fig 5) and the ability to activate either the toxT or ompU promoter (Figs 2–4), ToxR-V97A, was >60% defective for TcpP interaction. However, ToxR-V97A was also reduced in ToxR expression levels in whole cell extracts (Fig 6B). One should note that ToxR-QUAD showed a similar reduced level of ToxR in whole cell extracts (Fig 6B), yet maintained more TcpP interaction than ToxR-V97A (Fig 6A, p<0.001).
Two previously characterized mutants of TcpP (TcpP-N99D and TcpP-K102E) with reduced interaction with ToxR [2] served as negative controls and had ~50% reductions in ToxR interaction in the current assay (Fig 6). Low levels of EpsL detection, an inner membrane component of the Type II secretion apparatus that does not interact with TcpP were not affected by ToxR mutations or TcpP-HSV in this assay (Fig 6A, white bars).
Discussion
Based on our findings, the wing of ToxR is required for DNA binding to both the toxT and ompU promoters. ToxR residues absolutely required for transcription activation of toxT and ompU (Fig 1, D89, V97, T99, K102, G104, and Y105) were also required for DNA binding (Fig 5 and [26]). Residues in the β-hairpin of the wing of ToxR are particularly critical for transcriptional activation and DNA binding (Figs 2–5). This is to be expected since this region of the wing is often inserted into the minor groove of the DNA [21, 25, 27]. Based on the modeled location of these residues (Fig 3), they may be involved in positioning of the wing relative to the rest of the w-HTH domain or directly interact with the DNA. K102 is predicted to protrude from the tip of the wing and likely interacts with nucleotides in the minor groove, similar to R219 in PhoB [21, 25]. ToxR-T99 and ToxR-Y105 correspond to PhoB-T217 and Y223 which interact with the backbone of DNA [21, 25]. ToxR-V97, ToxR-Y105, and ToxR-L107 may contribute to the hydrophobic core, since they correspond to residues that comprise the hydrophobic core in OmpR and PhoB [21, 24]. Furthermore, mutation of two of these residues (ToxR-V97A and ToxR-L107A) renders ToxR unstable (Fig 4), an anticipated phenotype of a mutant deficient in folding of the hydrophobic core. Mutation of a few residues near the hinge of the β-hairpin (ToxR-Y96, ToxR-L107 and ToxR-I108), where it joins the rest of the w-HTH domain, were preferentially required for toxT expression when expressed from a plasmid (Fig 2). However, these mutants lost the differential defect in toxT expression when expressed from the chromosomal toxR locus (Fig 4). It is likely that these residues are involved in positioning of the wing relative to the DNA-binding helix. I-TASSER and Chimera modeling of ToxR, based on other members of the w-HTH family [26], places many of these residues aimed towards the core of the protein indicating that they are likely involved in positioning of the wing.
D89, a residue in the loop leading from the DNA-binding helix (α3) to the β-hairpin of the wing was critical for DNA binding and activation of toxT and ompU (Figs 2, 4 and 5 and [26]). It is possible D89 contributes to the hydrophobic core of ToxR, but this is not likely since no residues in this loop have been shown to be part of the hydrophobic core in either OmpR or PhoB [21, 24]. We hypothesize D89 may be involved in positioning the wing relative to the DNA-binding helix.
Since mutation of residues C-terminal to amino acid 109 (ToxR-A109) had minimal effect on transcriptional activation of either promoter, it is likely that R110 marks the beginning of the variable linker region that tethers the w-HTH domain to the membrane [38].
Although some residues in the wing of ToxR were preferentially required for toxT activation, all of the residues tested lost this preferential requirement when expressed from the chromosome. Therefore, the preferential requirement of the wing for toxT activation is likely an effect of the overexpression system and not an actual increased dependence on the wing for toxT activation. Furthermore, the residues in the wing do not appear to preferentially be required for binding to one promoter or the other, since mutations leading to disruption of binding to the toxT promoter result in similar disruption of binding to the ompU promoter and the consensus sequences for ToxR binding to toxT and ompU are similar [4]. Additionally, a majority of the residues required for DNA binding are present in regions of the wing not likely to come into direct contact with the DNA, but instead are most likely involved in positioning of the wing such that it is able to insert into the minor groove. Together this shows that the role of the wing is to stabilize ToxR binding to promoter DNA.
Alanine substitutions in four ToxR residues were found to affect ToxR-TcpP interaction via a TcpP co-capture assay (Fig 6; ToxR-Y96A, ToxR-L107A, ToxR-T91A, and ToxR-V97A). We hypothesize general structural defects in some mutants may have resulted in disruption of the interaction with TcpP (V97A and L107A are both partially unstable and had some of the strongest defects in ToxR/TcpP interactions (Figs 4 and 6)). Alternatively, ToxR interacts with TcpP using some of the same wing residues required to bind DNA. In addition, Western blot analysis of protein extracts used in the capture assay and direct coating ELISAs to assess total protein content in these protein extracts showed three of four mutants defective for TcpP interaction had less ToxR available for TcpP interaction (Fig 6B and S2 Fig; ToxR-T91A, ToxR-V97A, and ToxR-L107A). The ToxR-T91A extract even had a reduced level of TcpP-HSV expression. This could account for the resulting decrease in ToxR/TcpP interaction for these mutants. ToxR-Y96A expressed similar levels of ToxR to wild-type ToxR and similar levels of TcpP-HSV, yet it still showed a 40% defect in TcpP interaction. ToxR-Y96A also had a preferential defect for toxT activation when expressed from a plasmid (Fig 2), but had no significant preferential defect in toxT activation when expressed from the chromosome (Fig 4). While these data argue against a direct role for the ToxR wing in TcpP interaction and TcpP recruitment to the toxT promoter, it is possible we have not yet identified a ToxR wing mutant allele that encodes a molecule fully capable of binding DNA, but defective for TcpP interaction.
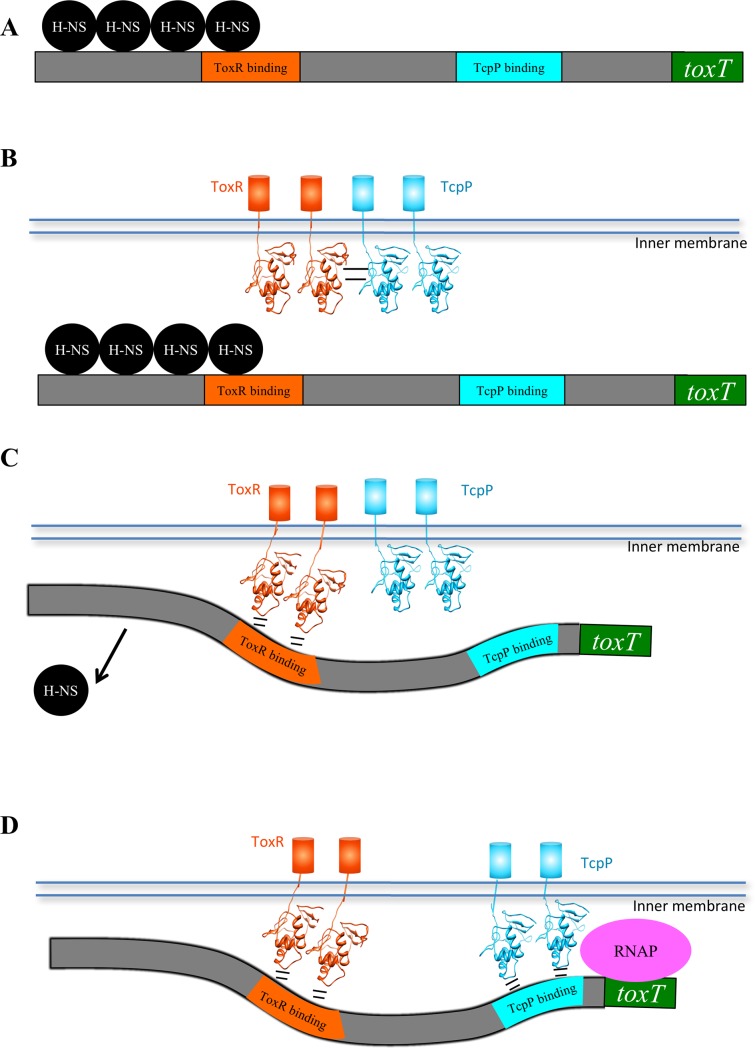
We propose that when ToxR binds the toxT promoter, TcpP is released to bind its neighboring TcpP-binding site on the toxT promoter. This “promoter delivery” model may allow ToxR to guide TcpP to its relatively weak TcpP-binding site (Fig 7C and 7D, [34]).
Fig 7. Model of ToxR mediated co-activation of the toxT promoter.
A) Prior to activation, the toxT promoter is silenced by H-NS [35]. B) ToxR-TcpP interaction between the N-terminal β-sheet of ToxR and the wing of TcpP recruits TcpP to the toxT promoter. C) Upon ToxR binding to the toxT promoter, ToxR and TcpP no longer interact. ToxR binding to the promoter results in relieving H-NS repression, possibly bending the DNA to make the TcpP binding site more accessible [34], and recruiting the toxT promoter to the membrane [37]. D) This results in enhanced TcpP binding to the toxT promoter and transcriptional activation via TcpP interaction with RNA polymerase.
If ToxR were to maintain interaction with TcpP while bound to DNA, there are two likely ways in which ToxR and TcpP could interact based on the interaction of other w-HTH proteins: ToxR N-terminal β-sheet to TcpP N-terminal β-sheet or ToxR β-sheet to TcpP wing [21, 23, 28, 30, 31]. Previous studies have shown that the wing of TcpP is required for ToxR-TcpP interaction, since mutation of several residues of the wing of TcpP results in decreased ToxR interaction [2]. In this model, ToxR and TcpP would dimerize by interaction of the wing of TcpP with the β-sheet of ToxR (Fig 7B). If ToxR and TcpP interact in this orientation on the promoter it would place the wing of TcpP oriented upstream, away from the promoter, in the opposite orientation to other w-HTH proteins bound to their promoters [21, 25, 28]. Additionally, ToxR and TcpP bind to the toxT promoter three helical turns apart, making it difficult to envision how the w-HTH domains could interact when bound to the DNA [4], although ongoing studies indicate ToxR may in fact bind to these three intervening turns of the DNA helix (albeit with reduced affinity; ESK and Dr. Miquel Coll, unpublished observations). Finally, DNA binding by TcpP appears to be required for toxT activation [1] and the wing of TcpP is required for DNA binding [2, 34]. Thus, ToxR-TcpP interaction may enhance activation by recruiting TcpP to the toxT promoter along with ToxR, but this interaction may be disrupted upon ToxR DNA binding to allow TcpP to access its binding site downstream of ToxR on the toxT promoter (Fig 7). This would alleviate the need for ToxR and TcpP to interact while bound to DNA.
It has been previously shown that ToxR co-activation of toxT is due in part to alteration of the toxT promoter. ToxR relieves H-NS repression, recruits the promoter to the membrane, and exposes a DNAse I hypersensitivity site at the TcpP-binding site [34, 35, 37]. All of these functions are dependent on ToxR binding to the promoter, making the wing critical for ToxR co-activation of the toxT promoter. Based on this information we propose a model whereby ToxR-TcpP interaction recruits TcpP to the promoter. Upon ToxR binding to the toxT promoter (via the recognition helix and wing domain) the ToxR-TcpP interaction is disrupted. At this point H-NS repression is relieved due to displacement of H-NS by ToxR and TcpP is released in close proximity to its binding site on the toxT promoter. Given that at physiological concentrations TcpP is not as efficient at promoter binding as ToxR [34], the delivery of TcpP to the toxT promoter by ToxR may facilitate TcpP interactions with the toxT promoter (Fig 7). Single-molecule studies have also indicated that ToxR enhances the mobility of TcpP in the cell, facilitating its ability to reach the toxT promoter [36].
Materials and methods
Culture conditions
V. cholerae strains were routinely grown overnight in Vc LB (LB containing 5 g/L NaCl) at 37°C. Unless otherwise stated cultures were induced by dilution into Vc LB pH 6.5 and grown at 30°C. Cultures were grown in the presence of 100 μg/ml ampicillin, 25 μg/ml chloramphenicol, or 100 μg/ml streptomycin as needed.
Construction of strains and plasmids
ToxR wing mutants were generated by PCR using complimentary mutagenic primers as described previously [4]. Chromosomal ToxR wing mutants were created using chromosomal recombination of the suicide plasmid pKAS32 and selected for by loss of streptomycin resistance as described previously [39]. A list of strains and plasmids used can be found in S1 Table and a list of primers used in this study can be found in S2 Table.
Generation of an anti-ToxR antibody
A new anti-ToxR antibody was generated in two different rabbits by Covance using a His6-tagged ToxRcyt2 molecule containing the N-terminal 170 amino acids of ToxR including the winged-HTH domain and the linker domain prior to the transmembrane domain. Both animals gave a strong response to the antigen. This antibody was used to detect ToxR expressed from its chromosomal locus in ß-galactosidase assays (Fig 3) and capture assays (Fig 5).
β-galactosidase assay for transcriptional activation by ToxR
V. cholerae strains containing chromosomal ompU-lacZ or toxT-lacZ reporters [2, 3] were diluted 1:30 and induced as described above. ToxR expression from pSK-ToxR-HA constructs in the presence of the repressing plasmid pREP4 was induced by addition of 100μM IPTG. For all transcriptional activation assays, activation was assayed after 4 hours by β-galactosidase assay as described previously [40]. The OD600 was determined by spectrophotometry and used to normalize cultures for subsequent Western blot analysis using antibodies against HA (Covance) or ToxR (generated against the N-terminal 170 amino acids of ToxR).
Co-capture of ToxR with TcpP-HSV
Interactions between ToxR and TcpP were performed largely as previously described {Krukonis, 2003 #5067}{Morgan, 2011 #5620}, with some modifications. V. cholerae RY1 with each toxR allele of interest recombined at the normal toxR locus were transformed with plasmids expressing wild-type TcpP-HSV or the ToxR-interacting mutants TcpP-N99D or TcpP-K101E. Strains were diluted from an overnight culture 1:50 at 30°C and grown for 4–6 hrs at 30°C in 500ml LB containing 100μg/ml streptomycin, 25μg/ml chloramphenicol and 100μM IPTG. Membranes containing ToxR mutant proteins and/or TcpP-HSV proteins were prepared [41] and dialyzed into HEPES-buffered saline (HBS, 20mM HEPES pH = 7.0, 150 mM NaCl). Membranes were then treated for 30 minutes with 1 μl DNase I (New England Biolabs) in the presence of 2.5mM MgCl2 and 0.5 mM CaCl2. 2 mg/ml membrane proteins were then crosslinked using a 15-fold molar excess of DSP (Pierce) for 30 minutes at room temp, blocked with 50mM Tris pH = 7.4 and then solubilized in 1% Triton X-100 (Bio-Rad). The molarity of dialyzed membrane preparations was estimated by measuring the protein concentration and assuming a 50kD average protein size in the total membrane extract. Samples were then sonicated on ice (3 x 5 seconds) and 50μl membrane extracts were added to microtiter plates coated with mouse an anti-HSV antibody (Novagen, coated at 1:500 dilution in PBS). Membrane extract binding to the plate occurred overnight at 4°C. After washing five times with PBS, wells were incubated with 50μl a 1:250 dilution of rabbit anti-TcpP antibody, 1:1000 dilution rabbit anti-ToxR antibody (a kind gift from Dr. Victor DiRita) or 1:10,000 dilution rabbit anti-EpsL antibody (a kind gift from Dr. Maria Sandkvist). Primary antibody incubation proceeded overnight at 4°C. After five washes with PBS, wells were then incubated with a 1:3000 dilution of goat anti-rabbit-AP conjugated secondary antibody (Zymed) and binding was detected by addition of 100μl of the colorimetric substrate PNPP (Sigma) at 4 mg/ml following sequential washing with PBS (four times), ansd Tris-buffered saline (100mM Tris pH = 8.0, 150mM NaCl, one wash). Plates were read at ABS405. Relative levels of each ToxR mutant protein in the membrane extract were assessed by directly-coating samples on 96-well ELISA plates and using an anti-ToxR polyclonal antibody at a 1:1000 dilution. Relative levels of TcpP-HSV in each strain were assessed using the anti-TcpP monoclonal antibody at a 1:250 dilution. The amount of TcpP-HSV or ToxR captured is presented as % of wild-type. Samples diluted on the same day to the same relative concentrations used in the capture assay were boiled in SDS-sample buffer and run in a 12% SDS-PAGE gel for Western blot analysis of protein levels in the extracts. Statistical analysis was performed using the students’ t-test followed by a Bonferroni correction for multiple comparisons (0.05/11 comparisons = 0.0045), with six measurements from two different days relative to the strain expressing wild-type ToxR and wild-type TcpP-HSV. Levels of EpsL were expressed based on the raw ABS405 values and normalized to the raw ABS405 values of ToxR developed for the sample length of time (45 minute reaction) in the RY1 + pEK41 sample.
DNA mobility shift assay
DNA binding assays were performed as described previously [34]. Membranes were isolated from ΔtoxRΔtcpP V. cholerae strains (EK459) with pSK-toxR-HA alleles which had been induced for 6 hours in 1mM IPTG as described previously [41]. Membrane concentrations ranging from 0.19 to 2 mg/ml were incubated with either the ompU promoter probe (extending from -211 to +22 relative to the transcriptional start site), the toxT promoter probe (extending from -172 to +45 relative to the transcriptional start site), or the negative control probe (toxT promoter extending from-46 to +45 relative to the transcriptional start site). 3000 cpm of probe labeled with 32P-dATP was used in each reaction. Western blotting with anti-HA (Covance) antibody was used to monitor ToxR protein levels.
Modeling of ToxR wing residues
ToxR bound to DNA was modeled using the I-TASSER modeling program (http://zhanglab.ccmb.med.umich.edu/I-TASSER/) to determine ToxR structure based on the crystal structure of other w-HTH family members [26]. Binding of ToxR to DNA was modeled using the NMR structure of PhoB bound to DNA [25]. ToxR wing residues were modeled using the program Chimera based on the threaded structure of ToxR as determined previously by I-TASSER [26].
Supporting information
DNA binding by ToxR wing mutants was assayed by EMSA using V. cholerae membranes containing ToxR wing mutants, but lacking TcpP (See Fig 4). Band intensity of the shifted and unshifted fractions was measured by Image J. The percent shifted was defined as the unshifted fraction divided by the sum of the shifted and unshifted fractions. For ompU the intermediate ToxR-independent shifted fraction which shifts in the absence of ToxR was counted as unshifted. Percent shifted is plotted against the membrane protein concentration used as determined by Bradford Assay.
(PDF)
Levels reflect the results of the direct coating ELISA (Fig 5B) and show similar levels of EpsL in all extracts.
(PDF)
(PDF)
(PDF)
Acknowledgments
We would like to thank Dr. Maria Sandkvist for the anti-EpsL antibody and Dr. Victor DiRita for the anti-TcpP antibody. We also thank Drs. Miquel Coll and Albert Canals for insights into ToxR binding to the toxT promoter.
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
This work was supported by NIH NIAID R01 AI075087 to ESK, https://www.niaid.nih.gov/. This work was also supported by the Frederick G. Novy Fellowship from the University of Michigan Department of Microbiology and Immunology to SJM, https://medicine.umich.edu/dept/microbiology-immunology. This work was also supported by an internal Faculty Research Grant to ESK from the University of Detroit Mercy School of Dentistry. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Goss TJ, Seaborn CP, Gray MD, Krukonis ES. Identification of the TcpP-Binding Site in the toxT Promoter of Vibrio cholerae and the Role of ToxR in TcpP-Mediated Activation. Infect Immun. 2010;78(10):4122–33. IAI.00566-10 [pii] 10.1128/IAI.00566-10 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Krukonis ES, DiRita VJ. DNA binding and ToxR responsiveness by the wing domain of TcpP, an activator of virulence gene expression in Vibrio cholerae. Mol Cell. 2003;12(1):157–65. S1097276503002223 [pii]. . [DOI] [PubMed] [Google Scholar]
- 3.Häse CC, Mekalanos JJ. TcpP protein is a positive regulator of virulence gene expression in Vibrio cholerae. Proc Natl Acad Sci USA. 1998;95:730–4. 10.1073/pnas.95.2.730 PMCID: PMC18489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goss TJ, Morgan SJ, French EL, Krukonis ES. ToxR recognizes a direct repeat element in the toxT, ompU, ompT, and ctxA promoters of Vibrio cholerae to regulate transcription. Infect Immun. 2013;81(3):884–95. Epub 2013/01/09. 10.1128/IAI.00889-12 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crawford JA, Kaper JB, DiRita VJ. Analysis of ToxR-dependent transcription activation of ompU, the gene encoding a major envelope protein in Vibrio cholerae. Mol Microbiol. 1998;29:235–46. 10.1046/j.1365-2958.1998.00925.x [DOI] [PubMed] [Google Scholar]
- 6.Li CC, Crawford JA, DiRita VJ, Kaper JB. Molecular cloning and transcriptional regulation of ompT, a ToxR-repressed gene in Vibrio cholerae. Mol Microbiol. 2000;35(1):189–203. mmi1699 [pii]. 10.1046/j.1365-2958.2000.01699.x . [DOI] [PubMed] [Google Scholar]
- 7.Martinez-Hackert E, Stock AM. Structural relationships in the OmpR family of winged-helix transcription factors. J Mol Biol. 1997;269:301–12. 10.1006/jmbi.1997.1065 [DOI] [PubMed] [Google Scholar]
- 8.DiRita VJ, Mekalanos JJ. Periplasmic interaction between two membrane regulatory proteins, ToxR and ToxS, results in signal transduction and transcriptional activation. Cell. 1991;64:29–37. 10.1016/0092-8674(91)90206-e [DOI] [PubMed] [Google Scholar]
- 9.Ottemann KM, Mekalanos JJ. The ToxR protein of Vibrio cholerae forms homodimers and heterodimers. J Bacteriol. 1996;178:156–62. 10.1128/jb.178.1.156-162.1996 PMCID: PMC177633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fengler VH, Boritsch EC, Tutz S, Seper A, Ebner H, Roier S, et al. Disulfide Bond Formation and ToxR Activity in Vibrio cholerae. PLoS One. 2012;7(10):e47756 Epub 2012/11/13. 10.1371/journal.pone.0047756 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dziejman M, Kolmar H, Fritz HJ, Mekalanos JJ. ToxR co-operative interactions are not modulated by environmental conditions or periplasmic domain conformation. Mol Microbiol. 1999;31(1):305–17. 10.1046/j.1365-2958.1999.01173.x . [DOI] [PubMed] [Google Scholar]
- 12.Dziejman M, Mekalanos JJ. Analysis of membrane protein interaction: ToxR can dimerize the amino terminus of phage lambda repressor. Mol Microbiol. 1994;13(3):485–94. 10.1111/j.1365-2958.1994.tb00443.x . [DOI] [PubMed] [Google Scholar]
- 13.Ottemann KM, Mekalanos JJ. Analysis of Vibrio cholerae ToxR function by construction of novel fusion proteins. Mol Microbiol. 1995;15(4):719–31. 10.1111/j.1365-2958.1995.tb02380.x [DOI] [PubMed] [Google Scholar]
- 14.Matson JS, DiRita VJ. Degradation of the membrane-localized virulence activator TcpP by the YaeL protease in Vibrio cholerae. Proc Natl Acad Sci USA. 2005;102(45):16403–8. 10.1073/pnas.0505818102 PMCID: PMC1283431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beck NA, Krukonis ES, DiRita VJ. TcpH Influences Virulence Gene Expression in Vibrio cholerae by Inhibiting Degradation of the Transcription Activator TcpP. J Bacteriol. 2004;186(24):8309–16. 10.1128/JB.186.24.8309-8316.2004 PMCID: PMC532408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Teoh WP, Matson JS, DiRita VJ. Regulated intramembrane proteolysis of the virulence activator TcpP in Vibrio cholerae is initiated by the tail-specific protease (Tsp). Mol Microbiol. 2015;97(5):822–31. Epub 2015/05/23. 10.1111/mmi.13069 . [DOI] [PubMed] [Google Scholar]
- 17.Morgan SJ, French EL, Thomson JJ, Seaborn CP, Shively CA, Krukonis ES. Formation of an Intramolecular Periplasmic Disulfide Bond in TcpP Protects TcpP and TcpH from Degradation in Vibrio cholerae. J Bacteriol. 2015;198(3):498–509. Epub 2015/11/18. 10.1128/JB.00338-15 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Almagro-Moreno S, Kim TK, Skorupski K, Taylor RK. Proteolysis of Virulence Regulator ToxR Is Associated with Entry of Vibrio cholerae into a Dormant State. PLoS Genet. 2015;11(4):e1005145 Epub 2015/04/08. 10.1371/journal.pgen.1005145 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Midgett CR, Almagro-Moreno S, Pellegrini M, Taylor RK, Skorupski K, Kull FJ. Bile salts and alkaline pH reciprocally modulate the interaction between the periplasmic domains of Vibrio cholerae ToxR and ToxS. Mol Microbiol. 2017;105(2):258–72. 10.1111/mmi.13699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Almagro-Moreno S, Root MZ, Taylor RK. Role of ToxS in the proteolytic cascade of virulence regulator ToxR in Vibrio cholerae. Mol Microbiol. 2015;98(5):963–76. 10.1111/mmi.13170 . [DOI] [PubMed] [Google Scholar]
- 21.Blanco AG, Sola M, Gomis-Ruth FX, Coll M. Tandem DNA Recognition by PhoB, a Two-Component Signal Transduction Transcriptional Activator. Structure. 2002;10(5):701–13. [DOI] [PubMed] [Google Scholar]
- 22.Kenney LJ. Structure/function relationships in OmpR and other winged-helix transcription factors. Curr Opin Microbiol. 2002;5(2):135–41. S1369527402003107 [pii]. . [DOI] [PubMed] [Google Scholar]
- 23.Maris AE, Walthers D, Mattison K, Byers N, Kenney LJ. The response regulator OmpR oligomerizes via beta-sheets to form head-to-head dimers. J Mol Biol. 2005;350(5):843–56. S0022-2836(05)00612-1 [pii] 10.1016/j.jmb.2005.05.057 . [DOI] [PubMed] [Google Scholar]
- 24.Martinez-Hackert E, Stock AM. The DNA-binding domain of OmpR: crystal structures of a winged helix transcription factor. Structure. 1997;5(1):109–24. . [DOI] [PubMed] [Google Scholar]
- 25.Yamane T, Okamura H, Ikeguchi M, Nishimura Y, Kidera A. Water-mediated interactions between DNA and PhoB DNA-binding/transactivation domain: NMR-restrained molecular dynamics in explicit water environment. Proteins. 2008;71(4):1970–83. 10.1002/prot.21874 . [DOI] [PubMed] [Google Scholar]
- 26.Morgan SJ, Felek S, Gadwal S, Koropatkin NM, Perry JW, Bryson AB, et al. The two faces of ToxR: activator of ompU, co-regulator of toxT in Vibrio cholerae. Mol Microbiol. 2011;81(1):113–28. 10.1111/j.1365-2958.2011.07681.x PMCID: PMC3124598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dorman CJ, Dorman MJ. Control of virulence gene transcription by indirect readout in Vibrio cholerae and Salmonella enterica serovar Typhimurium. Environ Microbiol. 2017. 10.1111/1462-2920.13838 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harrison-McMonagle P, Denissova N, Martinez-Hackert E, Ebright RH, Stock AM. Orientation of OmpR monomers within an OmpR:DNA complex determined by DNA affinity cleaving. J Mol Biol. 1999;285(2):555–66. 10.1006/jmbi.1998.2375 [DOI] [PubMed] [Google Scholar]
- 29.Littlefield O, Nelson HCM. A new use for the 'wing' of the 'winged' helix-turn-helix motif in the HSF–DNA cocrystal. Nature Struct Biol. 1999;6(5):464–70. 10.1038/8269 [DOI] [PubMed] [Google Scholar]
- 30.Gupta S, Pathak A, Sinha A, Sarkar D. Mycobacterium tuberculosis PhoP recognizes two adjacent direct-repeat sequences to form head-to-head dimers. J Bacteriol. 2009;191(24):7466–76. Epub 2009/10/13. 10.1128/JB.00669-09 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rhee JE, Sheng W, Morgan LK, Nolet R, Liao X, Kenney LJ. Amino acids important for DNA recognition by the response regulator OmpR. J Biol Chem. 2008;283(13):8664–77. M705550200 [pii] 10.1074/jbc.M705550200 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang M, Liu Z, Hughes C, Stern AM, Wang H, Zhong Z, et al. Bile salt-induced intermolecular disulfide bond formation activates Vibrio cholerae virulence. Proc Natl Acad Sci USA. 2013;110(6):2348–53. Epub 2013/01/24. 10.1073/pnas.1218039110 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fan F, Liu Z, Jabeen N, Birdwell LD, Zhu J, Kan B. Enhanced interaction of Vibrio cholerae virulence regulators TcpP and ToxR under oxygen-limiting conditions. Infect Immun. 2014;82(4):1676–82. Epub 2014/02/05. IAI.01377-13 [pii] 10.1128/IAI.01377-13 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Krukonis ES, Yu RR, Dirita VJ. The Vibrio cholerae ToxR/TcpP/ToxT virulence cascade: distinct roles for two membrane-localized transcriptional activators on a single promoter. Mol Microbiol. 2000;38(1):67–84. mmi2111 [pii]. 10.1046/j.1365-2958.2000.02111.x . [DOI] [PubMed] [Google Scholar]
- 35.Nye MB, Pfau JD, Skorupski K, Taylor RK. Vibrio cholerae H-NS silences virulence gene expression at multiple steps in the ToxR regulatory cascade. J Bacteriol. 2000;182:4295–303. 10.1128/jb.182.15.4295-4303.2000 PMCID: PMC101945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Haas BL, Matson JS, DiRita VJ, Biteen JS. Single-molecule tracking in live Vibrio cholerae reveals that ToxR recruits the membrane-bound virulence regulator TcpP to the toxT promoter. Mol Microbiol. 2015;96(1):4–13. 10.1111/mmi.12834 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Crawford JA, Krukonis ES, DiRita VJ. Membrane localization of the ToxR winged-helix domain is required for TcpP-mediated virulence gene activation in Vibrio cholerae. Mol Microbiol. 2003;47(5):1459–73. 10.1046/j.1365-2958.2003.03398.x [DOI] [PubMed] [Google Scholar]
- 38.Osorio CR, Klose KE. A region of the transmembrane regulatory protein ToxR that tethers the transcriptional activation domain to the cytoplasmic membrane displays wide divergence among Vibrio species. J Bacteriol. 2000;182(2):526–8. 10.1128/jb.182.2.526-528.2000 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Skorupski K, Taylor RK. Broad-host-range positive selection vectors for allelic exchange. Gene. 1996;169:47–52. 10.1016/0378-1119(95)00793-8 [DOI] [PubMed] [Google Scholar]
- 40.Miller JH. Experiments in molecular genetics Cold Spring Harbor, N.Y.: Cold Spring Harbor Laboratory Press; 1972. [Google Scholar]
- 41.Miller VL, Taylor RK, Mekalanos JJ. Cholera toxin transcriptional activator ToxR is a transmembrane DNA binding protein. Cell. 1987;48:271–9. 10.1016/0092-8674(87)90430-2 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
DNA binding by ToxR wing mutants was assayed by EMSA using V. cholerae membranes containing ToxR wing mutants, but lacking TcpP (See Fig 4). Band intensity of the shifted and unshifted fractions was measured by Image J. The percent shifted was defined as the unshifted fraction divided by the sum of the shifted and unshifted fractions. For ompU the intermediate ToxR-independent shifted fraction which shifts in the absence of ToxR was counted as unshifted. Percent shifted is plotted against the membrane protein concentration used as determined by Bradford Assay.
(PDF)
Levels reflect the results of the direct coating ELISA (Fig 5B) and show similar levels of EpsL in all extracts.
(PDF)
(PDF)
(PDF)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.