Abstract
The androgen precursors, dehydroepiandrosterone (DHEA) and DHEA sulfate (DHEAS) are produced in high amounts by the adrenal cortex primarily in humans and a few other primates. The human adrenal also secretes 11-oxygenated androgens (11-oxyandrogens), including 11β-hydroxyandrostenedione (11OHA4), 11-ketoandrostenedione (11KA4), 11β-hydroxytestosterone (11OHT) and 11-ketotestosterone (11KT), of which 11OHT and 11KT are bioactive androgens. The 11-oxyandrogens, particularly 11KT, have been recognized as biologically important testicular androgens in teleost fishes for decades, but their physiological contribution in humans has only recently been established. Beyond fish and humans, however, the presence of 11-oxyandrogens in other species has not been investigated. This study provides a comprehensive analysis of a set of C19 steroids, including the traditional androgens and 11-oxyandrogens, across 18 animal species. As previously shown, serum DHEA and DHEAS were much higher in primates than all other species. Circulating 11-oxyandrogens, especially 11KT, were observed in notable amounts in male, but not in female trout, consistent with gonadal origin in fish. The circulating concentrations of 11-oxyandrogens ranged from 0.1 to 10 nM in pigs, guinea pigs and in all the primates studied (rhesus macaque, baboon, chimpanzee and human) but not in rats or mice, and 11OHA4 was consistently the most abundant. In contrast to fish, serum 11KT concentrations were similar in male and female primates for each species, despite significantly higher circulating testosterone in males, suggesting that 11KT production in these species is not testis-dependent and primarily originates adrenal-derived 11-oxyandrogen precursors.
Key terms: adrenal, steroids, 11-oxyandrogens, primates
1. Introduction
The adrenal secretion of abundant quantities of the androgen precursors, dehydroepiandrosterone (DHEA) and its sulfate (DHEAS), is a phenomenon limited to humans and some non-human primates [1–11]. The adrenal zona reticularis (ZR) of Old World primates [9] has an expression pattern of steroidogenic enzymes that makes it the site of DHEA and DHEAS biosynthesis in these species [3, 12, 13]. Notably, in adult humans, DHEAS circulates at prodigious concentrations, vastly exceeding the levels of other steroid hormones [14–16]. Past research has shown that the adrenal production of these 19-carbon (C19) steroids is negligible or absent in common laboratory and domestic animals such as mice, rats, hamsters, pigs, guinea pigs, dogs, sheep and cattle [4, 11, 17].
Recent liquid chromatography-tandem mass spectrometry (LC-MS/MS) studies have provided evidence for human adrenal production of 11-oxygenated androgens (11-oxyandrogens) and their potential role in androgen-related disorders such as premature adrenarche, congenital adrenal hyperplasia, castration-resistant prostate cancer and polycystic ovary syndrome [18–22]. These 11-oxyandrogens include derivatives of androstenedione (A4) and testosterone (T), including 11β-hydroxyandrostenedione (11OHA4), 11-ketoandrostenedione (11KA4), 11β-hydroxytestosterone (11OHT) and 11-ketotestosterone (11KT) [15, 23]. Of these, 11KT is a potent androgen, with bioactivity comparable to that of T [15, 19, 20, 24]. For over six decades 11KT has been recognized as a biologically important gonadal androgen in teleost fishes where it was shown to mediate induction of sexual male-type behavior, onset of spermatogenesis and female-to-male sex reversal [25–34]. In humans, however, the potential contribution of 11KT to physiology and disease, particularly in women and children, has only recently been documented [15, 20–22, 35]. Beyond fish and humans, the evolutionary phylogeny of 11-oxyandrogens across other species has not been studied. The goal of the current study was to provide a characterization of the 11-oxyandrogens in a variety of species, ranging from fish to humans, using LC-MS/MS.
2. Materials and Methods
2.1. Animal sera
Sera from the following animal classes were studied: Osteichthyes (trout), Amphibia (frog), Reptilia (alligator), Aves (chicken) and Mammalia (laboratory and domesticated animals, and primates). Sera from animals were obtained as detailed in Table 1. Additional sera from laboratory and domesticated animals, and non-human primates were also procured from the Unit for Laboratory Animal Medicine (ULAM), University of Michigan and commercially from BioIVT (Hicksville, NY). Animals used in this study, with the exception of alligators, were of reproductive age. The sera from ULAM were obtained after all procedures were approved by the Institutional Animal Care and Use Committees at the University of Michigan. The protocols used for serum procurement followed the Public Health Service guidelines for the humane care and use of experimental animals. After approval from the institutional review boards at the University of Michigan, serum was obtained from men and women between ages 20–40 years who had morning (8am–10am) blood draws in an outpatient setting, as part of routine medical care for minor health condition or as part of annual exams (Table 1).
Table 1A.
Sources of animal sera
| Source | Institution | Species | Number of animals provided | |
|---|---|---|---|---|
| Female | Male | |||
| Irene Salinas | University of New Mexico | Trout | 13 | 3 |
| Michael Criscitiello | Texas A&M University | Frog | 3 | 3 |
| Ruth Elsey | Rockefeller Wildlife Refuge | Alligator | 6 | 6 |
| Catherine VandeVoort; John Capitanio | California Primate Research Center | Rhesus macaque | 6 | 5 |
| Anne Dorrance | Michigan State University | Rat | 8 | 8 |
| ULAM | University of Michigan | Rat | 4 | 0 |
| Mouse | 5 | 5 | ||
| Alan Conley | University of California-Davis | Dog | 10 | 9 |
| Horse | 4 | 0 | ||
| Bret McNabb; | University of California-Davis | Cow | 7 | 6 |
| Joan Rowe | Sheep | 6 | 6 | |
| Barry Ball | University of Kentucky | Horse | 0 | 4 |
| Trish Berger; Russell Hovey | University of California-Davis | Pig | 6 | 8 |
| Adina Turcu | University of Michigan | Human | 25 | 23 |
| William Rainey | University of Michigan | Human | 15 | 14 |
Additional sera from adult laboratory and domesticated animals, and non-human primates were also purchased from BioIVT (Hicksville, NY).
2.2. Steroid Quantitation by LC-MS/MS
We quantified 8 C19 steroids using LC-MS/MS: DHEA, DHEAS, A4, T, 11OHA4, 11KA4, 11OHT and 11KT. Unlabeled and deuterium-labeled steroids were obtained from Sigma-Aldrich, Cerilliant, C/D/N isotopes, Steraloids and The National Heart, Lung and Blood Institute RTI International Metabolite Standards Synthesis Center (Supplemental Table 1). Steroid extraction by liquid-liquid extraction and quantitation was carried out as previously described [20].
Samples (10 μL) were injected via autosampler and resolved with a pair of Agilent 1260/1290 binary pump HPLCs via 2D liquid chromatography, first on a 10 mm × 3 mm, 3 μm particle size Hypersil Gold C4 loading column (Thermo Scientific, Waltham, Massachusetts) followed by a Kinetex 50 mm × 2.1 mm, 2.6 μm particle size biphenyl resolving column (Phenomenex, Torrance, CA). The mobile phases consisted of 0.2 mmol/L aqueous ammonium fluoride (A) and methanol with 0.2 mmol/L ammonium fluoride (B). Steroids were eluted using gradient specifications as described in Supplemental Tables 2 and 3. The column effluent was directed into the source of an Agilent 6495 triple quadrupole mass spectrometer using electrospray ionization in positive ion mode for Δ4 (A4, T and 11-oxyandrogens) and Δ5 (DHEA) or negative ionization mode for steroid sulfates (DHEAS) and analyzed using multiple reaction monitoring (MRM) mode (Supplemental Table 1). Quantitation was accomplished by comparing ion currents for the monitored ions with weighted (1/x) 12-point linear external calibration curves (r2 was >0.995) and corrected for specimen dilution and recovery of internal standards using ChemStation and MassHunter software (Agilent, Santa Clara, CA). Intra-assay and inter-assay coefficients of variation (CV) were assessed by measuring quality control pooled serum samples five times within a run and across five different runs, respectively, and were < 12% for all steroids. The lower limit of detection (LOD) and lower limit of quantitation (LOQ) for each steroid were defined by the minimum concentration achieving an extrapolated signal-to-noise ratio of 3 and 5, respectively. LOD ranged from 0.007 nM for A4 to 0.105 nM for DHEAS and LOQ ranged from 0.011 nM for A4 to 0.174 nM for DHEAS (Supplemental Table 1).
It should be noted that sex of the trout was not available on the receipt of the serum samples. The identification of the sex of the trout based on the 11KT concentrations after measurement by LC-MS/MS. Trout demonstrating substantial serum concentrations of 11KT were marked as ‘males’ on the basis of previous studies [25–34].
2.3. Statistical analysis
GraphPad Prism 7 (La Jolla, CA) was used for statistical analysis. Non-parametric Mann-Whitney U test or Wilcoxon matched-pairs signed rank test was used for two group comparisons. Kruskal-Wallis test was used for multiple group comparison. Significance was accepted at an alpha level of 0.05.
3. Results
3.1. Circulating concentrations of the classic adrenal androgens DHEA and DHEAS across different species
Negligible concentrations of DHEA and DHEAS were found in species ranging from fish to birds, as well as in rodents and other common laboratory and domesticated animals (Fig. 1, Table 2). Large amounts of circulating DHEA and DHEAS were observed only in primates (Fig. 1) (p < 0.001, each primate species vs. other non-primate species). While rhesus monkeys demonstrated the highest DHEA concentrations among primates (64.7 ± 10.8 nM), maximal circulating DHEAS was seen in humans (5215 ± 320 nM). A sub-analysis by sex showed that men have higher levels of both DHEA and DHEAS compared to women (p< 0.001; data not shown), in concordance with previous reports [36–39].
Fig. 1. Circulating concentrations of the classic adrenal androgens in adult animals across different species.
LC-MS/MS was utilized to quantify the serum concentrations of DHEA and DHEAS in 17 species. Males and females were pooled. Only primates were shown to produce high amounts of these androgen precursors. Data are denoted as mean ± SEM. Concentrations of DHEA and DHEAS are expressed in nmol/L (nM) and μmol/L (μM) respectively. DHEA, dehydroepiandrosterone; DHEAS, DHEA sulfate.
Table 2.
C19 Steroid concentrations in Trout.
| Steroid | Female (n=13) | Male (n=3) | p value |
|---|---|---|---|
| DHEA | 0.14 ± 0.01 | 0.24 ± 0.11 | 0.618 |
| DHEAS | 1.74 ± 0.22 | 3.11 ± 1.10 | 0.182 |
| A4 | 0.32 ± 0.04 | 0.45 ± 0.12 | 0.230 |
| T | 0.04 ± 0.01 | 0.06 ± 0.02 | 0.409 |
| 11OHA4 | 0.65 ± 0.14 | 0.09 ± 0.06 | 0.029 |
| 11KA4 | 0.53 ± 0.13 | 0.54 ± 0.28 | 0.880 |
| 11OHT | 0.02 ± 0.01 | 0.04 ± 0.04 | 0.116 |
| 11KT | 0.09 ± 0.03 | 15.5 ± 5.79 | 0.004 |
Data are denoted as mean ± SEM and concentrations are expressed in nmol/L (nM). Statistical significance was determined by nonparametric Mann–Whitney U test. P < 0.05 was considered statistically significant.
3.2. Circulating concentrations of the 11-oxyandrogens across different species
As observed with DHEA and DHEAS, all primates had >1 nM circulating concentrations of A4, 11OHA4 and 11KA4 (Supplemental Fig.1, Fig. 2), and lower amounts of the 11-oxygenated derivatives of A4 were detected in dogs, cattle, sheep and horses (Fig. 2). Among lower species, only serum from pigs and guinea pigs exhibited substantial amounts (0.1–1 nM) of 11-oxyandrogens. Circulating concentrations of 11OHA4 and 11KA4 in guinea pigs (5.5 ± 1.2 nM; 0.9 ± 0.2 nM, respectively) were similar to those seen in humans (5.6 ± 0.4 nM; 1.1 ± 0.1 nM respectively) (Fig. 2).
Fig. 2. Circulating concentrations of the 11-oxygenated derivatives of androstenedione in adult animals across different species.
LC-MS/MS was utilized to measure the serum concentrations of 11OHA4 and 11KA4 in 17 species. Males and females were pooled. In addition to all primates, guinea pigs exhibited significant serum amounts of both 11OHA4 and 11KA4. Data are denoted as mean ± SEM. Steroid concentrations are expressed in nmol/L (nM). 11OHA4, 11β-hydroxyandrostenedione; 11KA4, 11-ketoandrostenedione.
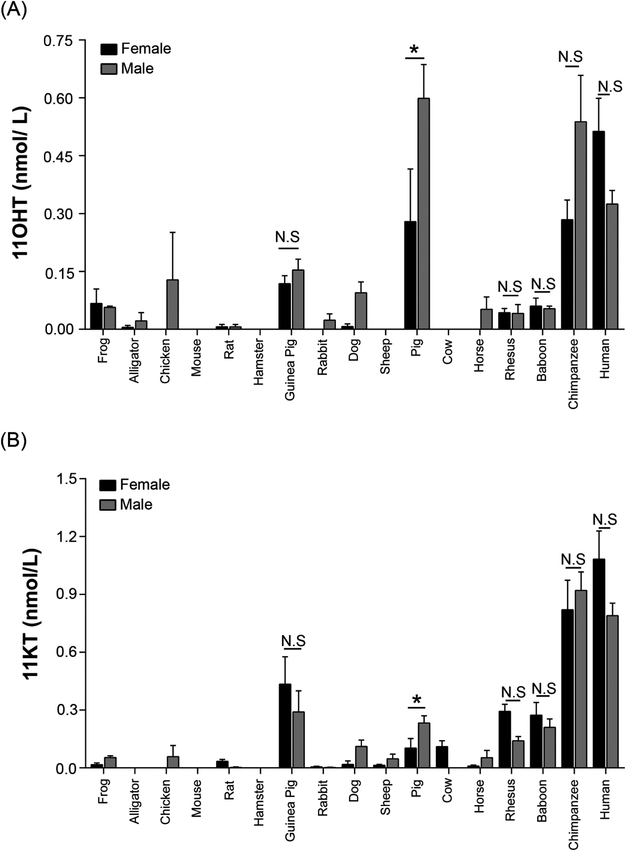
Amongst tetrapods, primates, particularly chimpanzees and humans, produced both 11OHT and 11KT (Fig. 3). Interestingly, with the exception of pigs and guinea pigs, these bioactive 11-oxyandrogens were negligible or absent in the other tetrapods studied. While pigs exhibited high 11OHT (0.4 ± 0.1 nM) with concentrations comparable to those observed in humans (0.4 ± 0.1 nM), guinea pigs demonstrated 11KT concentrations (0.4 ± 0.1 nM) similar to those seen in the Old World monkeys (rhesus and baboon) (~0.2 nM). These 11KT values were, however, significantly lower than in humans (0.9 ± 0.1 nM) (p < 0.001) (Fig. 3). Notably, unlike T, serum concentrations of 11OHT and 11KT were similar between sexes across tetrapods (Fig. 4 and Supplemental Fig.2).
Fig. 3. Circulating concentrations of the bioactive 11-oxyandrogens in adult animals across different species.
LC-MS/MS was utilized to measure the serum concentrations of 11OHT and 11KT in 17 species. Males and females were pooled. Primates, pigs and guinea pigs demonstrated substantial serum concentrations of 11OHT and 11KT. Data are denoted as mean ± SEM. Steroid concentrations are expressed in nmol/L (nM). 11OHT, 11β-hydroxytestosterone; 11KT, 11-ketotestosterone.
Fig. 4. Circulating concentrations of the bioactive 11-oxyandrogens in adult females and males of 17 species.
Data are denoted as mean ± SEM. Steroid concentrations are expressed in nmol/L (nM). 11OHT, 11β-hydroxytestosterone; 11KT, 11-ketotestosterone. Non-parametric Mann-Whitney U test was used to compare the steroid concentrations between females and males. *P<0.05; N.S, not significant.
The maximal concentrations of 11KT across species were observed in the male trout, with levels 59-fold higher than those seen in men (0.8 ± 0.1 nM) (p < 0.001); however, male trout had lower circulating T concentrations than men (p < 0.05) (Fig. 5B, Table 2). In contrast to humans and other primates, female trout produced negligible amounts of 11KT (Fig. 5A, Table 2).
Fig.5. Comparison of circulating levels of (A) 11KT and (B) Testosterone in females vs. males for trout and primates.
(A) While male trout synthesized substantially higher concentrations of 11KT as compared to female, no clear sex differences were observed for 11KT. (B) Testosterone, on the other hand, was synthesized in significantly elevated concentrations in male primates vs. females. Additionally, we observed that female primates produced similar quantities of 11KT and Testosterone. Data are denoted as mean ± SEM. Steroid concentrations are expressed in nmol/L (nM). 11KT, 11- ketotestosterone. Non-parametric Wilcoxon matched-pairs signed rank test was used to compare 11KT vs. Testosterone in the same sex. **P<0.01; ***P<0.001; N.S, not significant.
Female primates produced similar quantities of 11KT and T (Fig. 5A). While male trout synthesized substantially higher concentrations of 11KT as compared to T (258-fold, p< 0.05), T was elevated than 11KT in all male primates (Fig. 5B). The concentrations of 11KT in males and female primates, however, were not significantly different (p = 0.205).
4. Discussion
Adrenal steroidogenesis varies among species based on differences in the expression and activities of steroidogenic enzymes in the different adrenocortical zones. This is particularly the case for adrenal production of androgens and their precursors. In primates, adrenal biosynthesis of DHEA and DHEAS occurs within the ZR, a zone which exhibits abundant cytochrome b5 (CYB5) and sulfotransferase type 2A1 expression, but which lacks 3β-hydroxysteroid dehydrogenase type 2 (HSD3B2) [6, 7, 13, 40, 41]. CYB5 is an allosteric regulator known to enhance the 17,20-lyase activity of CYP17 (17α-hydroxylase/17,20-lyase) and to facilitate the conversion of 17α-hydroxypregnenolone to DHEA in the ZR in primates via the Δ5 pathway [7, 41, 42]. The low expression of HSD3B2 in the ZR limits the enzymatic competition with CYP17, thus promoting the flow of substrates towards DHEA and DHEAS.
The current study demonstrates that circulating DHEA and DHEAS are an order of magnitude lower in non-primate species as compared to Old World monkeys, apes and humans, which is in agreement with previous reports [4, 10, 11, 17, 43, 44]. The negligible levels of DHEA and DHEAS in the common laboratory animals, including rats and mice, have been attributed to the lack of adrenal expression of CYP17 [35, 45–47]. The low 17,20-lyase adrenal activity in rabbit and dog as compared to primates, and the preferential hydroxylase activity of the hamster CYP17 enzyme over the lyase reaction might explain the low secretion of DHEA in these species [48, 49]. Hornsby et al. demonstrated that HSD3B2 activity was 10-fold higher in bovine adrenocortical cells than in fetal human adrenocortical cells, diverting the Δ5 steroid flux into the Δ4 pathway at the pregnenolone → progesterone step, thus obstructing the synthesis of DHEA and DHEAS [50, 51]. The retention of HSD3B2 in the inner cortical zone in these species might also contribute to low DHEA concentrations [52, 53]. The current study confirms that efficient adrenal production of DHEA/DHEAS is limited to primates, all of which exhibit a steroidogenically unique ZR, with an enzyme profile that promotes the Δ5 androgenic pathway.
These findings also suggest that amongst non-primate tetrapods, only pigs and guinea pigs are capable of synthesizing 11-oxyandrogens. Early studies indicated that in pig, both the testis and the adrenal can produce 11OHA4 and the testis alone synthesizes 11OHT [54–56]. Porcine CYP17 is able to catalyze both the Δ4- and Δ5- lyase reactions without the need for CYB5, promoting the synthesis of A4 and 11OHA4 at the expense of DHEA [56–60]. Unlike human CYP17, guinea pig lyase activity of CYP17 preferentially metabolizes 17α-hydroxyprogesterone, a Δ4 steroid, to A4 [61–65], which is then rapidly converted into 11OHA4 by 11β-hydroxylase [64, 66]. 11OHA4 can be further metabolized to the other 11-oxyandrogens as previously described [67, 68]. Interestingly, we found that the concentrations of 11OHA4 and 11KA4 in guinea pig sera are comparable to those seen in primates. This phenomenon could perhaps be attributed in part to the decreased sensitivity of the guinea pig glucocorticoid receptor to glucocorticoids, leading to enhanced adrenocorticotropic hormone (ACTH) [69–72]. Elevated ACTH, in turn, stimulates the adrenal output of not only cortisol but also of C19 steroids, including 11-oxyandrogens [15, 22].
Recent reports have highlighted the production 11-oxyandrogens in humans [15, 18, 20, 22, 73]. Our data demonstrate that other primates, such as Old World monkeys and apes, also synthesize these steroids. In primates, the 11-oxyandrogens are likely adrenal-derived, because their synthesis depends on the 11β-hydroxylation of A4 and T via 11β-hydroxylase, an enzyme which is almost solely expressed in the adrenal gland in these species [23, 74]. Although it has been previously proposed that 11KT might be a direct product of the gonad in humans [75], the low gonadal expression of 11β-hydroxylase suggests that the contribution of gonads to the synthesis of 11-oxyandrogens is minimal. Moreover, 11OHA4 is a poor substrate for 17β-hydroxysteroid dehydrogenase type 3, the testicular isoenzyme responsible for conversion of A4 to T [21]. To gauge the origin of 11KT in primates, we compared the concentrations of T and 11KT between sexes. As previously found in humans [22], 11KT concentrations were similar in both sexes in all primate species, despite significantly higher circulating T in males. Importantly, 11KT and T circulated at comparable levels in females. Collectively, this data suggests that in primates, 11KT is likely synthesized from the peripheral metabolism of adrenal-derived 11OHA4 as suggested previously [15, 23, 68]. This contrasts with the teleost fishes, where 11-oxyandrogens represent the principal testicular androgen because of the gonadal expression of 11β-hydroxylase [76–81].
The clinical importance of 11-oxyandrogens warrants identifying potential animal models to further study their production and role in physiology. Herein, we have demonstrated that 11-oxyandrogens are produced in multiple primate species. Using primates as research models, however, limits most mechanistic studies owing to the costs and availability of these animals. Of note, amongst non-primate animals, pigs and guinea pigs synthesize 11-oxyandrogens, have circulating levels similar to those seen in primates, and therefore might serve as suitable models. Further investigations are, nonetheless, required to ascertain the utility of these animals as appropriate model systems to better understand the biosynthesis, regulation and the physiological role of the 11-oxyandrogens.
Supplementary Material
Table 1B.
Total number of animals included in the study
| Species | Species Name | Female | Male |
|---|---|---|---|
| Trout | Oncorhynchus mykiss | 13 | 3 |
| Frog | Xenopus laevis | 3 | 3 |
| Alligator | Alligator mississippiensis | 6 | 6 |
| Chicken | Gallus gallus | 3 | 5 |
| Mouse | Mus musculus | 5 | 5 |
| Rat | Rattus norvegicus domesticus | 11 | 8 |
| Hamster | Mesocricetus auratus | 5 | 3 |
| Guinea Pig | Cavia porcellus | 5 | 3 |
| Rabbit | Oryctolagus cuniculus | 6 | 6 |
| Dog | Canis lupus familiaris | 10 | 9 |
| Sheep | Ovis aries | 9 | 9 |
| Pig | Sus scrofa domestica | 9 | 7 |
| Cow | Bos taurus | 10 | 9 |
| Horse | Equus ferus caballus | 11 | 11 |
| Rhesus macaque | Macaca mulatta | 11 | 10 |
| Baboon | Papio hamadryas | 3 | 3 |
| Chimpanzee | Pan troglodytes | 5 | 5 |
| Human | Homo sapiens | 40 | 37 |
Highlights.
11-oxygenated androgens, as well as DHEA and DHEAS were quantified using LC-MS/MS in 18 animal species.
The serum concentration of the classic adrenal androgens DHEA and DHEAS in sexually mature non-primate mammals are an order of magnitude lower than that found in Old World monkeys, apes and humans.
Other than trout, only guinea pigs, pigs and primates had significant levels of circulating 11-oxygenated androgens.
Although circulating concentration of testosterone was significantly higher in male vs. female primates, circulating 11KT levels were comparable between the sexes in all primates.
Testosterone and 11KT circulated at similar concentrations in reproductive age women.
Acknowledgements
We thank Mr. Matthew Ullenbruch and Dr. Matthew Taylor (University of Michigan, Ann Arbor) for rat and mouse serum procurement, and Mr. Patrick O’Day for assistance with mass spectrometry assays. The extensive number of species studied could not have been accomplished without the generosity and enthusiasm of many colleagues including Dr. Irene Salinas for providing trout sera (Department of Biology, University of New Mexico); Drs. Catherine VandeVoort, John Capitanio and staff (California National Primate Research Center, UC Davis) for providing rhesus macaque sera; Drs. Bret McNabb and Joan Rowe (School of Veterinary Medicine, UC Davis) for providing sera from cow and sheep; Dr. Anne Dorrance (Department of Pharmacology and Toxicology, Michigan State University) for rat serum; Dr. Michael Criscitiello (College of Veterinary Medicine and Biomedical Sciences, Texas A&M University) for providing frog sera; Drs. Trish Berger and Russell Hovey (Department of Animal Science, UC Davis) for proving porcine sera and Dr. Barry Ball (Gluck Equine Research Center, University of Kentucky) for horse sera.
Funding: This work was supported by grants from the National Institutes of Health (NIH) R01DK069950 and R01DK43140 to W.E.R., R01GM086596 to R.J.A., 1K08DK109116 to A.F.T. and the National Center for Advancing Translational Sciences 2UL1TR000433 to J.R.
Financial Support: This work was supported by National Institutes of Health Grants R01DK069950 and R01DK43140 (to W.E.R.), R01GM086596 (to R.J.A.), and 1K08DK109116 (to A.F.T.) and by National Center for Advancing Translational Sciences Grant 2UL1TR000433 (to J.R.).
Steroid Abbreviations:
- 11-oxyandrogens
11-oxygenated androgens
- DHEA
Dehydroepiandrosterone
- DHEAS
DHEA sulfate
- A4
Androstenedione
- T
Testosterone
- 11OHA4
11β-Hydroxyandrostenedione
- 11KA4
11-Ketoandrostenedione
- 11OHT
11β-Hydroxytestosterone
- 11KT
11-Ketotestosterone
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure Statement: The authors have nothing to disclose.
REFERENCES
- [1].Short RV, The secretion of sex hormones by the adrenal gland, Biochem Soc Symp, 18 (1960) 59–84. [PubMed] [Google Scholar]
- [2].Cohn GL, Mulrow PJ, Androgen release and synthesis in vitro by human adult adrenal glands, J Clin Invest, 42 (1963) 64–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Rege J, Nanba AT, Auchus RJ, Ren J, Peng HM, Rainey WE, Turcu AF, Adrenocorticotropin Acutely Regulates Pregnenolone Sulfate Production by the Human Adrenal In Vivo and In Vitro, J Clin Endocrinol Metab, 103 (2018) 320–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Cutler GB Jr., Glenn M, Bush M, Hodgen GD, Graham CE, Loriaux DL, Adrenarche: a survey of rodents, domestic animals, and primates, Endocrinology, 103 (1978) 2112–2118. [DOI] [PubMed] [Google Scholar]
- [5].Ducharme JR, Forest MG, De Peretti E, Sempe M, Collu R, Bertrand J, Plasma adrenal and gonadal sex steroids in human pubertal development, J Clin Endocrinol Metab, 42 (1976) 468–476. [DOI] [PubMed] [Google Scholar]
- [6].Nguyen AD, Corbin CJ, Pattison JC, Bird IM, Conley AJ, The developmental increase in adrenocortical 17,20-lyase activity (biochemical adrenarche) is driven primarily by increasing cytochrome b5 in neonatal rhesus macaques, Endocrinology, 150 (2009) 1748–1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Mapes S, Corbin CJ, Tarantal A, Conley A, The primate adrenal zona reticularis is defined by expression of cytochrome b5, 17alpha-hydroxylase/17,20-lyase cytochrome P450 (P450c17) and NADPH-cytochrome P450 reductase (reductase) but not 3beta-hydroxysteroid dehydrogenase/delta5–4 isomerase (3beta-HSD), J Clin Endocrinol Metab, 84 (1999) 3382–3385. [DOI] [PubMed] [Google Scholar]
- [8].Smail PJ, Faiman C, Hobson WC, Fuller GB, Winter JS, Further studies on adrenarche in nonhuman primates, Endocrinology, 111 (1982) 844–848. [DOI] [PubMed] [Google Scholar]
- [9].Conley AJ, Pattison JC, Bird IM, Variations in adrenal androgen production among (nonhuman) primates, Semin Reprod Med, 22 (2004) 311–326. [DOI] [PubMed] [Google Scholar]
- [10].Snipes CA, Forest MG, Migeon CJ, Plasma androgen concentrations in several species of Old and New World monkeys, Endocrinology, 85 (1969) 941–945. [DOI] [PubMed] [Google Scholar]
- [11].Guillemette C, Hum DW, Belanger A, Levels of plasma C19 steroids and 5 alpha-reduced C19 steroid glucuronides in primates, rodents, and domestic animals, Am J Physiol, 271 (1996) E348–353. [DOI] [PubMed] [Google Scholar]
- [12].Narasaka T, Suzuki T, Moriya T, Sasano H, Temporal and spatial distribution of corticosteroidogenic enzymes immunoreactivity in developing human adrenal, Mol Cell Endocrinol, 174 (2001) 111–120. [DOI] [PubMed] [Google Scholar]
- [13].Endoh A, Kristiansen SB, Casson PR, Buster JE, Hornsby PJ, The zona reticularis is the site of biosynthesis of dehydroepiandrosterone and dehydroepiandrosterone sulfate in the adult human adrenal cortex resulting from its low expression of 3 beta-hydroxysteroid dehydrogenase, J Clin Endocrinol Metab, 81 (1996) 3558–3565. [DOI] [PubMed] [Google Scholar]
- [14].Parker LN, Odell WD, Control of adrenal androgen secretion, Endocr Rev, 1 (1980) 392–410. [DOI] [PubMed] [Google Scholar]
- [15].Rege J, Nakamura Y, Satoh F, Morimoto R, Kennedy MR, Layman LC, Honma S, Sasano H, Rainey WE, Liquid chromatography-tandem mass spectrometry analysis of human adrenal vein 19-carbon steroids before and after ACTH stimulation, J Clin Endocrinol Metab, 98 (2013) 1182–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Eisenhofer G, Peitzsch M, Kaden D, Langton K, Pamporaki C, Masjkur J, Tsatsaronis G, Mangelis A, Williams TA, Reincke M, Lenders JWM, Bornstein SR, Reference intervals for plasma concentrations of adrenal steroids measured by LC-MS/MS: Impact of gender, age, oral contraceptives, body mass index and blood pressure status, Clin Chim Acta, 470 (2017) 115–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Rivarola MA, Snipes CA, Migeon CJ, Concentration of androgens in systemic plasma of rats, guinea pigs, salamanders and pigeons, Endocrinology, 82 (1968) 115–121. [DOI] [PubMed] [Google Scholar]
- [18].O’Reilly MW, Kempegowda P, Jenkinson C, Taylor AE, Quanson JL, Storbeck KH, Arlt W, 11-Oxygenated C19 Steroids Are the Predominant Androgens in Polycystic Ovary Syndrome, J Clin Endocrinol Metab, 102 (2017) 840–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Pretorius E, Africander DJ, Vlok M, Perkins MS, Quanson J, Storbeck KH, 11-Ketotestosterone and 11-Ketodihydrotestosterone in Castration Resistant Prostate Cancer: Potent Androgens Which Can No Longer Be Ignored, PLoS One, 11 (2016) e0159867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Rege J, Turcu AF, Kasa-Vubu JZ, Lerario AM, Auchus GC, Auchus RJ, Smith JM, White PC, Rainey WE, 11-Ketotestosterone Is the Dominant Circulating Bioactive Androgen During Normal and Premature Adrenarche, J Clin Endocrinol Metab, 103 (2018) 4589–4598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Storbeck KH, Bloem LM, Africander D, Schloms L, Swart P, Swart AC, 11beta-Hydroxydihydrotestosterone and 11-ketodihydrotestosterone, novel C19 steroids with androgenic activity: a putative role in castration resistant prostate cancer?, Mol Cell Endocrinol, 377 (2013) 135–146. [DOI] [PubMed] [Google Scholar]
- [22].Turcu AF, Nanba AT, Chomic R, Upadhyay SK, Giordano TJ, Shields JJ, Merke DP, Rainey WE, Auchus RJ, Adrenal-derived 11-oxygenated 19-carbon steroids are the dominant androgens in classic 21-hydroxylase deficiency, Eur J Endocrinol, 174 (2016) 601–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Swart AC, Schloms L, Storbeck KH, Bloem LM, Toit T, Quanson JL, Rainey WE, Swart P, 11beta-hydroxyandrostenedione, the product of androstenedione metabolism in the adrenal, is metabolized in LNCaP cells by 5alpha-reductase yielding 11beta-hydroxy-5alpha-androstanedione, J Steroid Biochem Mol Biol, 138 (2013) 132–142. [DOI] [PubMed] [Google Scholar]
- [24].Campana C, Rege J, Turcu AF, Pezzi V, Gomez-Sanchez CE, Robins DM, Rainey WE, Development of a novel cell based androgen screening model, The Journal of steroid biochemistry and molecular biology, 156 (2016) 17–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Cardwell JR, Liley NR, Hormonal control of sex and color change in the stoplight parrotfish, Sparisoma viride, Gen Comp Endocrinol, 81 (1991) 7–20. [DOI] [PubMed] [Google Scholar]
- [26].Idler DR, Macnab HC, The biosynthesis of 11-ketotestosterone and 11-beta-hydroxytestosterone by Atlantic salmon tissues in vitro, Can J Biochem, 45 (1967) 581–589. [DOI] [PubMed] [Google Scholar]
- [27].Idler DR, Schmidt PJ, Ronald AP, Isolation and identification of 11-ketotestosterone in salmon plasma, Can J Biochem Physiol, 38 (1960) 1053–1057. [PubMed] [Google Scholar]
- [28].Kobayashi M, Aida K, Stacey NE, Induction of testis development by implantation of 11-ketotestosterone in female goldfish, Zool Sci 8(1991) 389–393. [Google Scholar]
- [29].Kobayashi M, Nakanishi T, 11-ketotestosterone induces male-type sexual behavior and gonadotropin secretion in gynogenetic crucian carp, Carassius auratus langsdorfii, Gen Comp Endocrinol, 115 (1999) 178–187. [DOI] [PubMed] [Google Scholar]
- [30].Miura T, Yamauchi K, Takahashi H, Nagahama Y, Hormonal induction of all stages of spermatogenesis in vitro in the male Japanese eel (Anguilla japonica), Proc Natl Acad Sci U S A, 88 (1991) 5774–5778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Nagahama Y, Miura T, Kobayashi T, The onset of spermatogenesis in fish, Ciba Found Symp, 182 (1994) 255–267; discussion 267–270. [DOI] [PubMed] [Google Scholar]
- [32].Pottinger TG, Pickering AD, The effects of 11-ketotestosterone and testosterone on the skin structure of brown trout, Salmo trutta L, Gen Comp Endocrinol, 59 (1985) 335–342. [DOI] [PubMed] [Google Scholar]
- [33].Simpson TH, Wright RS, A radioimmunoassay for 11-oxotestosterone: its application in the measurement of levels in blood serum of rainbow trout (S. Gairdneri), Steroids, 29 (1977) 383–398. [DOI] [PubMed] [Google Scholar]
- [34].Young G, Thorarensen H, Davie PS, 11-Ketotestosterone suppresses interrenal activity in rainbow trout (Oncorhynchus mykiss), Gen Comp Endocrinol, 103 (1996) 301–307. [DOI] [PubMed] [Google Scholar]
- [35].Nishihara M, Winters CA, Buzko E, Waterman MR, Dufau ML, Hormonal regulation of rat Leydig cell cytochrome P-45017 alpha mRNA levels and characterization of a partial length rat P-45017 alpha cDNA, Biochem Biophys Res Commun, 154 (1988) 151–158. [DOI] [PubMed] [Google Scholar]
- [36].Rege J, Karashima S, Lerario AM, Smith JM, Auchus RJ, Kasa-Vubu JZ, Sasano H, Nakamura Y, White PC, Rainey WE, Age-dependent Increases in Adrenal Cytochrome b5 and Serum 5-Androstenediol-3-sulfate, J Clin Endocrinol Metab, 101 (2016) 4585–4593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Guran T, Firat I, Yildiz F, Kaplan Bulut I, Dogru M, Bereket A, Reference values for serum dehydroepiandrosterone-sulphate in healthy children and adolescents with emphasis on the age of adrenarche and pubarche, Clin Endocrinol (Oxf), 82 (2015) 712–718. [DOI] [PubMed] [Google Scholar]
- [38].de Peretti E, Forest MG, Pattern of plasma dehydroepiandrosterone sulfate levels in humans from birth to adulthood: evidence for testicular production, J Clin Endocrinol Metab, 47 (1978) 572–577. [DOI] [PubMed] [Google Scholar]
- [39].Sulcova J, Hill M, Hampl R, Starka L, Age and sex related differences in serum levels of unconjugated dehydroepiandrosterone and its sulphate in normal subjects, J Endocrinol, 154 (1997) 57–62. [DOI] [PubMed] [Google Scholar]
- [40].Rege J, Nakamura Y, Wang T, Merchen TD, Sasano H, Rainey WE, Transcriptome profiling reveals differentially expressed transcripts between the human adrenal zona fasciculata and zona reticularis, J Clin Endocrinol Metab, 99 (2014) E518–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Arlt W, Martens JW, Song M, Wang JT, Auchus RJ, Miller WL, Molecular evolution of adrenarche: structural and functional analysis of p450c17 from four primate species, Endocrinology, 143 (2002) 4665–4672. [DOI] [PubMed] [Google Scholar]
- [42].Auchus RJ, Lee TC, Miller WL, Cytochrome b5 augments the 17,20-lyase activity of human P450c17 without direct electron transfer, J Biol Chem, 273 (1998) 3158–3165. [DOI] [PubMed] [Google Scholar]
- [43].Townsley JD, Pepe GJ, Serum dehydroepiandrosterone and dehydroepiandrosterone sulphate in baboon (Papio Papio) pregnancy, Acta Endocrinol (Copenh), 85 (1977) 415–421. [DOI] [PubMed] [Google Scholar]
- [44].Conley AJ, Plant TM, Abbott DH, Moeller BC, Stanley SD, Adrenal androgen concentrations increase during infancy in male rhesus macaques (Macaca mulatta), Am J Physiol Endocrinol Metab, 301 (2011) E1229–1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Brock BJ, Waterman MR, Biochemical differences between rat and human cytochrome P450c17 support the different steroidogenic needs of these two species, Biochemistry, 38 (1999) 1598–1606. [DOI] [PubMed] [Google Scholar]
- [46].Hofmann FG, The concerted action upon a common substrate of steroid hydroxylases from the adrenal cortex and the testis, Biochim Biophys Acta, 58 (1962) 343–348. [DOI] [PubMed] [Google Scholar]
- [47].Perkins LM, Payne AH, Quantification of P450scc, P450(17) alpha, and iron sulfur protein reductase in Leydig cells and adrenals of inbred strains of mice, Endocrinology, 123 (1988) 2675–2682. [DOI] [PubMed] [Google Scholar]
- [48].Schiebinger RJ, Albertson BD, Barnes KM, Cutler GB Jr., Loriaux DL, Developmental changes in rabbit and dog adrenal function: a possible homologue of adrenarche in the dog, Am J Physiol, 240 (1981) E694–699. [DOI] [PubMed] [Google Scholar]
- [49].Cloutier M, Fleury A, Courtemanche J, Ducharme L, Mason JI, Lehoux JG, Characterization of the adrenal cytochrome P450C17 in the hamster, a small animal model for the study of adrenal dehydroepiandrosterone biosynthesis, DNA Cell Biol, 16 (1997) 357–368. [DOI] [PubMed] [Google Scholar]
- [50].Hornsby PJ, Aldern KA, Steroidogenic enzyme activities in cultured human definitive zone adrenocortical cells: comparison with bovine adrenocortical cells and resultant differences in adrenal androgen synthesis, J Clin Endocrinol Metab, 58 (1984) 121–127. [DOI] [PubMed] [Google Scholar]
- [51].Hornsby PJ, Biosynthesis of DHEAS by the human adrenal cortex and its age-related decline, Ann N Y Acad Sci, 774 (1995) 29–46. [DOI] [PubMed] [Google Scholar]
- [52].Dupont E, Zhao HF, Rheaume E, Simard J, Luu-The V, Labrie F, Pelletier G, Localization of 3 beta-hydroxysteroid dehydrogenase/delta 5-delta 4-isomerase in rat gonads and adrenal glands by immunocytochemistry and in situ hybridization, Endocrinology, 127 (1990) 1394–1403. [DOI] [PubMed] [Google Scholar]
- [53].Ishimura K, Yoshinaga-Hirabayashi T, Fujita H, Ishii-Ohba H, Inano H, Tamaoki B, Light and electron microscopic immunocytochemistry on the localization of 3 beta-hydroxysteroid dehydrogenase/isomerase in the bovine adrenal cortical cells, Histochemistry, 89 (1988) 35–39. [DOI] [PubMed] [Google Scholar]
- [54].Raeside JI, Renaud RL, Friendship RM, Isolation of 11 beta-hydroxylated androgens from testicular vein blood of the mature boar, Biochem Biophys Res Commun, 162 (1989) 1194–1199. [DOI] [PubMed] [Google Scholar]
- [55].Raeside JI, Renaud RL, Khalil MW, Formation of C19 11-hydroxysteroids by porcine Leydig cells, Biochem Cell Biol, 70 (1992) 174–176. [DOI] [PubMed] [Google Scholar]
- [56].Holzbauer M, Newport HM, Adrenal secretion rates and adrenal tissue concentrations of pregnenolone, progesterone, 11 beta OH-androstenedione and some other steroids in young pigs and dogs, J Physiol, 200 (1969) 821–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Robic A, Faraut T, Prunier A, Pathways and genes involved in steroid hormone metabolism in male pigs: a review and update, J Steroid Biochem Mol Biol, 140 (2014) 44–55. [DOI] [PubMed] [Google Scholar]
- [58].Nakajin S, Shinoda M, Haniu M, Shively JE, Hall PF, C21 steroid side chain cleavage enzyme from porcine adrenal microsomes. Purification and characterization of the 17 alpha-hydroxylase/C17,20-lyase cytochrome P-450, J Biol Chem, 259 (1984) 3971–3976. [PubMed] [Google Scholar]
- [59].Yanagibashi K, Hall PF, Role of electron transport in the regulation of the lyase activity of C21 side-chain cleavage P-450 from porcine adrenal and testicular microsomes, J Biol Chem, 261 (1986) 8429–8433. [PubMed] [Google Scholar]
- [60].Shet MS, Fisher CW, Tremblay Y, Belanger A, Conley AJ, Mason JI, Estabrook RW, Comparison of the 17 alpha-hydroxylase/C17,20-lyase activities of porcine, guinea pig and bovine P450c17 using purified recombinant fusion proteins containing P450c17 linked to NADPH-P450 reductase, Drug Metab Rev, 39 (2007) 289–307. [DOI] [PubMed] [Google Scholar]
- [61].Tremblay Y, Fleury A, Beaudoin C, Vallee M, Belanger A, Molecular cloning and expression of guinea pig cytochrome P450c17 cDNA (steroid 17 alpha-hydroxylase/17,20 lyase): tissue distribution, regulation, and substrate specificity of the expressed enzyme, DNA Cell Biol, 13 (1994) 1199–1212. [DOI] [PubMed] [Google Scholar]
- [62].Higuchi A, Kominami S, Takemori S, Kinetic control of steroidogenesis by steroid concentration in guinea pig adrenal microsomes, Biochim Biophys Acta, 1084 (1991) 240–246. [DOI] [PubMed] [Google Scholar]
- [63].Tagashira H, Kominami S, Takemori S, Kinetic studies of cytochrome P-45017 alpha, lyase dependent androstenedione formation from progesterone, Biochemistry, 34 (1995) 10939–10945. [DOI] [PubMed] [Google Scholar]
- [64].Belanger B, Couture J, Caron S, Bodou P, Fiet J, Belanger A, Production and secretion of C-19 steroids by rat and guinea pig adrenals, Steroids, 55 (1990) 360–365. [DOI] [PubMed] [Google Scholar]
- [65].Hyatt PJ, Bell JB, Bhatt K, Tait JF, Preparation and steroidogenic properties of purified zona fasciculata and zona reticularis cells from the guinea-pig adrenal gland, J Endocrinol, 96 (1983) 1–14. [DOI] [PubMed] [Google Scholar]
- [66].Belanger B, Fiet J, Belanger A, Effects of adrenocorticotropin on adrenal and plasma 11 beta-hydroxyandrostenedione in the guinea pig and determination of its relative androgen potency, Steroids, 58 (1993) 29–34. [DOI] [PubMed] [Google Scholar]
- [67].Bloem LM, Storbeck KH, Schloms L, Swart AC, 11beta-hydroxyandrostenedione returns to the steroid arena: biosynthesis, metabolism and function, Molecules, 18 (2013) 13228–13244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Swart AC, Storbeck KH, 11beta-Hydroxyandrostenedione: Downstream metabolism by 11betaHSD, 17betaHSD and SRD5A produces novel substrates in familiar pathways, Mol Cell Endocrinol, 408 (2015) 114–123. [DOI] [PubMed] [Google Scholar]
- [69].Liu L, Matthews SG, Adrenocortical response profiles to corticotrophin-releasing hormone and adrenocorticotrophin challenge in the chronically catheterized adult guinea-pig, Exp Physiol, 84 (1999) 971–977. [PubMed] [Google Scholar]
- [70].Keightley MC, Fuller PJ, Cortisol resistance and the guinea pig glucocorticoid receptor, Steroids, 60 (1995) 87–92. [DOI] [PubMed] [Google Scholar]
- [71].Keightley MC, Fuller PJ, Anomalies in the endocrine axes of the guinea pig: relevance to human physiology and disease, Endocr Rev, 17 (1996) 30–44. [DOI] [PubMed] [Google Scholar]
- [72].Keightley MC, Funder JW, Fuller PJ, Molecular cloning and sequencing of a guinea-pig pro-opiomelanocortin cDNA, Mol Cell Endocrinol, 82 (1991) 89–98. [DOI] [PubMed] [Google Scholar]
- [73].du Toit T, Bloem LM, Quanson JL, Ehlers R, Serafin AM, Swart AC, Profiling adrenal 11beta-hydroxyandrostenedione metabolites in prostate cancer cells, tissue and plasma: UPC(2)-MS/MS quantification of 11beta-hydroxytestosterone, 11keto-testosterone and 11keto-dihydrotestosterone, J Steroid Biochem Mol Biol, 166 (2017) 54–67. [DOI] [PubMed] [Google Scholar]
- [74].Turcu AF, Auchus RJ, Clinical significance of 11-oxygenated androgens, Curr Opin Endocrinol Diabetes Obes, 24 (2017) 252–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Imamichi Y, Yuhki KI, Orisaka M, Kitano T, Mukai K, Ushikubi F, Taniguchi T, Umezawa A, Miyamoto K, Yazawa T, 11-Ketotestosterone Is a Major Androgen Produced in Human Gonads, J Clin Endocrinol Metab, 101 (2016) 3582–3591. [DOI] [PubMed] [Google Scholar]
- [76].Feist G, Schreck CB, Fitzpatrick MS, Redding JM, Sex steroid profiles of coho salmon (Oncorhynchus kisutch) during early development and sexual differentiation, Gen Comp Endocrinol, 80 (1990) 299–313. [DOI] [PubMed] [Google Scholar]
- [77].Jiang JQ, Kobayashi T, Ge W, Kobayashi H, Tanaka M, Okamoto M, Nonaka Y, Nagahama Y, Fish testicular 11beta-hydroxylase: cDNA cloning and mRNA expression during spermatogenesis, FEBS Lett, 397 (1996) 250–252. [DOI] [PubMed] [Google Scholar]
- [78].Jiang JQ, Young G, Kobayashi T, Nagahama Y, Eel (Anguilla japonica) testis 11beta-hydroxylase gene is expressed in interrenal tissue and its product lacks aldosterone synthesizing activity, Mol Cell Endocrinol, 146 (1998) 207–211. [DOI] [PubMed] [Google Scholar]
- [79].Kindler PM, Philipp DP, Gross MR, Bahr JM, Serum 11-ketotestosterone and testosterone concentrations associated with reproduction in male bluegill (Lepomis macrochirus: Centrarchidae), Gen Comp Endocrinol, 75 (1989) 446–453. [DOI] [PubMed] [Google Scholar]
- [80].Leitz T, Reinboth R, The biosynthesis of 11-ketotestosterone by the testis of the Siamese fighting fish Betta splendens Regan (Anabantoidei, Belontiidae), Gen Comp Endocrinol, 66 (1987) 145–157. [DOI] [PubMed] [Google Scholar]
- [81].Mayer I, Borg B, Schulz R, Conversion of 11-ketoandrostenedione to 11-ketotestosterone by blood cells of six fish species, Gen Comp Endocrinol, 77 (1990) 70–74. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.