Abstract
AMD pathobiology was irreversibly changed by the recent discovery of extracellular cholesterol-containing deposits in the subretinal space, between the photoreceptors and retinal pigment epithelium (RPE), called subretinal drusenoid deposits (SDDs). SDDs strikingly mirror the topography of rod photoreceptors in human macula, raising the question of whether an equivalent process results in a deposition related to foveal cones. Herein we propose that AMD's pathognomonic lesion—soft drusen and basal linear deposit (BLinD, same material, diffusely distributed)—is the leading candidate. Epidemiologic, clinical, and histologic data suggest that these deposits are most abundant in the central macula, under the fovea. Strong evidence presented in a companion article supports the idea that the dominant ultrastructural component is large apolipoprotein B,E–containing lipoproteins, constitutively secreted by RPE. Lipoprotein fatty acids are dominated by linoleate (implicating diet) rather than docosahexaenoate (implicating photoreceptors); we seek within the retina cellular relationships and dietary drivers to explain soft druse topography. The delivery of xanthophyll pigments to highly evolved and numerous Müller cells in the human fovea, through RPE, is one strong candidate, because Müller cells are the main reservoir of these pigments, which replenish from diet. We propose that the evolution of neuroglial relations and xanthophyll delivery that underlie exquisite human foveal vision came with a price, that is, soft drusen and sequela, long after our reproductive years.
Keywords: age-related macular degeneration, drusen, lipoproteins, cholesterol, retinal pigment epithelium, Bruch's membrane, macula, fovea, Müller cells, xanthophyll pigment
Age-related macular degeneration (AMD) is a major cause of legal blindness in the elderly, approachable through multidisciplinary research involving human tissues and patients. Our views of AMD have advanced substantially through clinical imaging in the last decade, culminating in the Classification of Atrophy Meetings Working Group, which is redefining atrophy to incorporate pathology newly revealed by optical coherence tomography.1 AMD pathobiology was irreversibly changed by the recent discovery and characterization of a layer of extracellular deposits in the subretinal space, between the photoreceptors and RPE, called subretinal drusenoid deposits (SDDs).2–5 One striking feature of SDDs is that they mirror the topography of rod photoreceptors in human macula,6,7 leading to the question of whether there is an equivalent process resulting in a deposition related to foveal cones.
A central thesis of this review is that the biogenesis of soft drusen, the pathognomonic and specific extracellular deposit of AMD8 and basal linear deposit (BLinD), a diffusely distributed form of the same material, is the leading candidate for such a process. Further, because the fatty acids in cholesterol-rich Bruch's membrane (BrM) lipoproteins, the dominant ultrastructural component of soft drusen, are rich in the fatty acid linoleate (from diet) rather than in docosahexaenoate (from photoreceptors),9 we seek within the neurosensory retina the cellular relationships and dietary factors that can explain the topography of soft drusen and BLinD in older adults. The delivery of xanthophyll pigments (XPs) to the highly evolved Müller cells supporting foveal cones is one strong candidate. These speculative but testable hypotheses potentially unify many disparate data sets.
Essentials From the Biology of Macula
A neurovascular unit, conceptualized originally for brain and then for inner retina,10,11 comprises microvessels, neurons, glia, pericytes, and extracellular matrix that link blood flow to the metabolic demands of neurons. The cells and tissues most prominently affected by AMD pathology are those of the outer retinal neurovascular unit,12 that is, photoreceptors, retinal pigment epithelium (RPE), Müller cells (in neurosensory retina), and the choriocapillaris (ChC) endothelium (in the choroidal vasculature) (Fig. 1, top). Together these cells are served by the choroid, which has the highest blood flow in the body and is regulated in part by the autonomic nervous system and intrinsic choroidal neurons,13 and contains clinically visible extracellular lipid depots.14 Between RPE and ChC is a laminated subendothelial extracellular matrix called Bruch's membrane, which functions as a vessel wall laid out flat, in parallel to vascular lumens,15 rather than circumferentially around them.
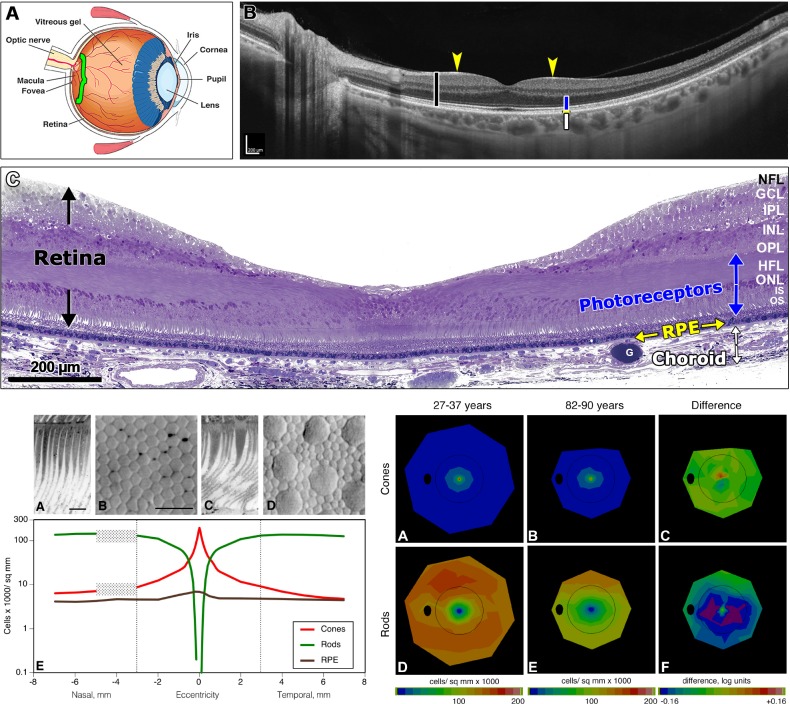
Figure 1.
Neurobiology and aging of macula. Top: (A) Cross-section of a human eye. Green bracket shows area in (B), including optic nerve head and macula. (B) Swept-source optical coherence tomography of a living human neurosensory retina (black bar) and choroid (white bar), showing the 6-mm-diameter macula and adjacent optic nerve. The retina contains multiple bands of alternating high and low reflectivity that coincide in part with the anatomic layers. Blue bar delimits layers occupied by photoreceptors and interleaved Müller glia (M). Arrows indicate the fovea. The choroidal vasculature contains lumens of large vessels and is bounded externally by the sclera. (C) High-resolution histology of the fovea, in the center of macula and responsible for high acuity vision. The retina, photoreceptor layers, and choroid are indicated by black, blue, and white arrows, respectively. A foveal pit is created by inner retinal neurons, Müller cells, and accompanying retinal vasculature being swept to the side of the visual axis. The RPE is a simple cuboidal epithelium that sits on Bruch's membrane, the inner wall of the choroidal vasculature. Osmium postfixation, epoxy embedding, 1-μm-thick section, toluidine blue stain. Lower left: Photoreceptor mosaic and topography of outer retinal cells. (A) Foveal cone inner and outer segments, longitudinal section. (B) Foveal cone inner segments in a flat-mounted retina of a 34-year-old donor, Nomarski differential interference contrast optics and video. (C) Nonfoveal cone and rod inner segments, longitudinal section. (D) Cone inner segments (large) and rod inner segments (small) in the same eye. (E) Number of cones (C), rods (R), and RPE per square millimeter of retinal surface in nasal and temporal retina, in young adults, as a function of eccentricity from the foveal center in mm. Peak densities of cones, rods, and RPE in young adults are 200,000/mm2, 150,000/mm2, and 10,000/mm2, respectively. With increasing eccentricity, cone density decreases, and rod density increases, becoming equal at ∼0.55 mm. The RPE exhibits central peak with a shallow gradient. Scale bars: 10 μm. Hatched rectangle, optic nerve head. Dashed lines, limits of macula. Lower right: Topography of cones and rods in aging human retina,64 shown as a fundus of a left eye. Black oval, optic nerve; ring, limits of macula. In (C) and (F), warm colors mean that older group has higher mean density than younger group and cool colors mean that older group has lower mean density than younger group. A yellow-green map means that differences between groups are small. (A) Cones, 27- to 36-year-old donors. (B) Cones, 82- to 90-year-old donors. (C) Log mean difference in cone density between younger adults and older adults is small and inconsistent. (D) Rods, 27- to 36-year-old donors. (E) Rods, 82- to 90-year-old donors. (F) Log mean difference in rod density between younger adults and older adults is greatest at 0.5 mm to 3 mm from fovea. Purple signifies that the log mean difference (aged-young) was < −0.16 log units, that is, that aged eyes had 31% fewer cells than young eyes. G, globule; GCL, ganglion cell layer; HFL, Henle fiber layer; INL, inner nuclear layer; IPL, inner plexiform layer; IS, inner segments; NFL, nerve fiber layer; ONL, outer nuclear layer; OPL, outer plexiform layer; OS, outer segments. Top: reprinted with permission from Tian L, Kazmierkiewicz KL, Bowman AS, Li M, Curcio CA, Stambolian DE. Transcriptome of the human retina, retinal pigmented epithelium and choroid. Genomics. 2015;105:253–264. © 2015 Elsevier Inc. Bottom: reprinted with permission from Jackson GR, Curcio CA, Sloan KR, Owsley C. Photoreceptor degeneration in aging and age-related maculopathy. In: Penfold PL, Provis JM, eds. Macular Degeneration. Berlin: Springer-Verlag; 2005:45–62.
The RPE is a monolayer of cuboidal polygonal cells embedded between photoreceptors and BrM. Strong apical to basolateral polarization makes the RPE a key player in maintaining homeostasis of photoreceptors apically and ChC basally and in the pathology of SDDs apically and drusen basally. The outer blood-retina barrier is maintained by junctional complexes on the RPE, the basolateral surface of which faces the systemic circulation. The inner part of the blood-retina barrier is maintained by endothelial cells and Müller glia around retinal capillaries.
The human macula, 6 mm in diameter, is defined in neurobiology as an area with a continuous layer of ganglion cells16 and in epidemiology as the area included in the Early Treatment of Diabetic Retinopathy Study grid for grading color fundus photographs.17 Because the macula constitutes ∼3% of the total retina area (of ∼1000 mm2), human eye studies that pool macula and periphery in assays based on whole eyecups overlook potentially important macula-specific signals. Older literature sometimes equates macula and fovea; the fovea is one subregion of the macula. It takes a whole macula to support a fovea.
In 1990, my colleagues and I presented a comprehensive two-dimensional map of cone and rod density in seven short postmortem retinas prepared as unstained whole mounts (Fig. 1, bottom left).18 This technique enables visualization, topography, and morphologic detail of inner segments, while largely eliminating histologic processing artifacts and counting errors. With accurately localized foveal centers and computerized microscopy, it is possible to determine photoreceptor density over the entire retina. In the human macula, rods outnumber cones: a rod-dominant perifovea surrounds a cone-only foveola that is 0.8 mm in diameter. The foveola has a high peak density (>150,000 mm2) and a sharp decline with eccentricity. Rods are also numerous (peak >150,000 mm2) in an elliptical crest at 2- to 5-mm eccentricity that encircle the optic nerve head nasally.18 Macular cone topography in this study has been replicated repeatedly in vivo by adaptive optics scanning laser ophthalmoscopy imaging.19,20 Similar laboratory approaches have been recently applied to cell density and autofluorescence of human macular RPE.21 A demonstrated peak cell density of ∼7200/mm2 RPE cells and a shallow eccentricity-dependent decline is consistent with previous literature.22,23
Neurons of the inner nuclear and ganglion cell layers are numerous and are displaced from the foveal center to create the foveal pit.24 Approximately 95% of macular ganglion cells are midget cells, that is, compact neurons responsible for transmitting signal for high acuity and color vision to the brain.25 There are at least two ganglion cells for each foveal cone within the central several degrees of vision.18,26–28 Each foveal cone contacts two midget bipolar cells, splitting the output signal along “private lines” signifying light ON and light OFF to corresponding midget ganglion cells.29 This unique circuitry means that 40% of human retinal ganglion cells are offset from photoreceptor inner segments defining their receptive fields in visual space.18 The Henle fiber layer (Fig. 1, top) thus comprises 14% of retinal thickness and contains inner fibers of rod and cone photoreceptors (interleaved with Müller glia and up to 600 μm in length) extending centrifugally to contact bipolar neurons.16,27,30–33
Müller glia span the retina between external and internal limiting membranes. In the macula Müller cells equal or exceed the number of foveal cones34,35 and are Z-shaped owing to the Henle fiber layer. Extramacular Müller cells are vertical and are outnumbered by photoreceptors. Among their many functions,36 Müller glia deliver vitamin A derivatives required by phototransduction selectively to cones37,38 (although this function has been explored in only mice to date). Recent evidence supports macular Müller cells as the principal reservoir of the yellow xanthophyll pigments (XP) lutein and zeaxanthin,39–43 which are replenished by diet (see Aging of Macula below). Owing to their hydrophobicity, XPs localize to the interior of cell membranes. XP is detectable in vivo with behavioral tests involving color matching (heterochromatic flicker photometry)44 and noninvasive imaging (e.g., two-wavelength autofluorescence, fluorescence lifetimes).45,46
Defining the Spaces Between the Layers
Between the photoreceptors and the apical RPE is the subretinal space, a closed physiologic compartment.47 It is bounded inwardly by the external limiting membrane, a plane of heterotypic junctional complexes between photoreceptors and Müller cells and outwardly by junctional complexes among the RPE cells.48 Within this compartment is the delicate interphotoreceptor matrix, which includes specialized domains ensheathing every cone and rod photoreceptor49,50 to mediate intercellular transfers and adhesion.51
As defined in a companion article,9 a three-layer definition BrM allows a tissue compartment and potential space between the RPE-basal lamina and the inner collagenous layer, called the sub–RPE-basal lamina space.9 Drusen are thus focal deposits located between the RPE-basal lamina and the inner collagenous layer of BrM, in the sub–RPE-basal lamina space. Basal linear deposit is a thin layer of soft druse material, in the same compartment as drusen. Together BLinD and soft drusen comprise the Oil Spill on aging BrM (by this definition)52 and constitute AMD's specific deposits. SDDs are also extracellular, located between the photoreceptors and RPE. While common in AMD, SDDs also occur in monogenic inherited disorders involving BrM and inherited and acquired disorders of retinoid processing.53
Aging of Macula
As shown by population-based epidemiology studies based on color fundus photography, aging is the greatest risk factor for AMD, followed by family history and smoking.54,55 Intraocular factors most relevant to AMD initiation and progression are the disposition of drusen and hyperpigmentation,56,57 suggesting that focusing on cell and tissue pathology and pathways underlying these signs will yield insights into molecular pathogenesis.
The relative rate of rod and cone loss characterizes each degeneration affecting photoreceptors,58–60 and within the neurovascular unit, photoreceptor health is a readout of the support system.61 In normal eyes from human donors aged 27 to 92 years in which inner segments have been counted in whole mounts for accuracy, rods decline 30% at 0.5- to 3-mm eccentricity, and cones remain stable (Fig. 1, bottom).62 The rod-vulnerable region is closer to the fovea than the ring of high rod density and autofluorescence attributable to RPE lipofuscin (i.e., lysosome-related, long-lasting inclusion bodies rich in vitamin A derivatives)21,63 and in fact hugs the rod-free area. Other studies enumerating cells in sectioned tissues agree on relative sparing of foveal cones in aging,22 which continues well into advanced AMD.64,65 Rod-mediated dark adaptation, which is limited by retinoid delivery,66 is slowest within the central 6° of macula (of 12° tested in a small sample).67
Major age-related macular changes relevant to AMD besides rod loss and cone resilience are the stability of RPE cell numbers despite lipofuscin accumulation and mitochondrial degeneration.21,22,68–70 Sprouting of rod terminals in aging are surprisingly more prominent in peripheral retina than in the macula.71 BrM undergoes cross-linking, thickening, calcification, and lipidization.72–78 Macular soft and hard drusen accumulate in the sub–RPE-BL space.79,80 Choriocapillaris apposition to BrM declines,77 along with increased immunoreactivity for membrane attack complex (terminal component of the complement cascade),81 loss of autonomic nerve fibers,82 and thinning of the choroid.77
Among aging-related factors that could impact rod survival in central macula (Fig. 1, bottom right), we focused on BrM and the sub–RPE-BL space, where AMD pathology is prominent. We investigated BrM lipidization, because a straightforward connection from there to druse lipids, arguably the first druse component described,83–87 seemed possible. Lipid accumulation in vessel walls can be informed by the pathophysiology of atherosclerotic cardiovascular disease88 as well as the clinical success in reducing its public health burden.89
Soft Drusen Composition Resembles Both Plasma Lipoproteins and Outer Segments
A companion review article9 details histochemical, ultrastructural, direct assay, gene expression, and cell culture evidence that membranous debris, the principal component of soft drusen as defined by Sarks et al.,90,91 derives from large lipoproteins containing apolipoproteins B and E, secreted basolaterally by the RPE into BrM. Lipoproteins are multimolecular complexes that resemble oil droplets solubilized for transport through aqueous media with a surface of proteins (apolipoproteins and others), phospholipid, and unesterified cholesterol. Very low-density lipoprotein (of hepatic origin, parent to “bad cholesterol” low-density lipoprotein [LDL]) and dietary chylomicrons (of intestinal origin) are two well studied examples; the brain has high-density lipoprotein (HDL) rich in apolipoprotein E (apoE). As elaborated separately,9 the predominant ultrastructural component in soft drusen was initially called “membranous debris,” which in turn is partly preserved “lipoprotein-derived debris.” By using lipid-preserving ultrastructural techniques originally used for elucidating the deposition of plasma LDL in arterial intima,92,93 it has been possible to see the core-and-surface morphology of lipoproteins that accumulate with age in human BrM, with highest concentration in the macula. When assayed with high-performance liquid chromatography, isolated lipoproteins and BrM extracts are rich in esterified and unesterified cholesterol. Importantly, fatty acids are dominated by linoleate (implicating dietary sources) and not docosahexaenoate (not implicating photoreceptor outer segments). Recently, a culture system of primary porcine RPE was shown to lay down a continuous layer of deposits containing lipid and hydroxyapatite, depending on the substrate the cells were grown on, without supplementation of outer segments and taking up only components of culture medium, which include plasma lipoproteins. These findings give credence to the notion of a diet-driven system.94 Further, clinical imaging has documented that drusen grow, and RPE migrates anteriorly into the retina, thus no longer maintaining the druse,95,96 indicating that presence of drusen implies a certain level of RPE functionality. The current model for BrM lipoproteins is that they have two principal sources, plasma lipoproteins delivering lipophilic essentials and phagocytized outer segments, and they provide a mechanism for the RPE to offload unneeded lipids to the systemic circulation and avoid lipotoxicity. Details are available in our companion article.9
Using Topography to Dissect Mechanisms
Adhering to the philosophy that improved care for AMD patients is best served by following the biology,97 we next consider biologic antecedents for these pathways in neurosensory retina. According to the terrain theory of vision,98 the sampling of visual space by neurons in each species' retina is influenced by the species' normal habitat. Mammalian retinas have an area of high neuronal density specialized for visual acuity, onto which images are registered by coordinated head and eye movements.99 Conversely, neuronal density and spatial resolution is low in peripheral retina, because space for neurons receiving visual input in the brain is limited and peripheral retina has evolved for functions like motion detection. Retinal neurobiology has taught us that cell populations have emergent properties not appreciated by study of individual cells.99 Topography is thus a powerful independent variable for dissecting pathogenic mechanisms, while also completing a trajectory from clinical manifestations back to the evolutionary biology that brought humans a macula in the first place.
Topographic considerations can prioritize mechanisms for follow-up. For example, light exposure is often mentioned in the AMD context, yet retinal illuminance is homogeneous to 50° eccentricity100,101 and not obviously related to the distribution of AMD's characteristic pathology. We have previously considered whether a strong macula-to-periphery gradient (7:1) of esterified cholesterol in aged BrM is related to gradients in photoreceptor density and concluded that these are too small (2.7 for rods, 1.9 for cones).75 Drawing from hemodynamic considerations in the regional vulnerability of large vessels for atherosclerosis, we also have considered ChC blood flow impacting BrM capacity to retain lipoproteins.102 However, existing laboratory studies of blood flow have technical limitations,103,104 and new in vivo methods are under active development.105,106 Here we ask whether dietary delivery of lipophilic essentials required by macular cells could influence drusen biogenesis.
Do Soft Drusen Have a Predilection for the Macula?
Drusen and hyperpigmentation are the two largest intraocular risk factors for progression to neovascularization and atrophy. Epidemiology, restricted to the 30° view of color fundus cameras,17,107 has shown that drusen conferring the greatest progression risk reside in the central 1-mm-diameter subfield of the Early Treatment of Diabetic Retinopathy Study grid.57,108–111 Risk is markedly heightened when normalized for this subfield's small area. Similarly, longitudinal clinical studies using optical coherence tomography have determined that high progression risk associated with drusen volume within the central 3 mm diameter is not increased by using the central 5 mm diameter.112,113 Via clinical ophthalmoscopy, color fundus photography, and ultrastructural clinicopathologic correlation, Sarks et al.114 have stated that soft drusen arise within the “inner macula” and are preferentially depleted, relative to hard drusen, by prophylactic laser therapy.
Pathology data supporting a predilection of soft drusen for macula exist but are sparse, because few studies have been designed to test this possibility. In a large series of autopsy eyes analyzed by ex vivo imaging and histology, low-profile “serogranular drusen” in macula86 have been contrasted to globular drusen in periphery.115 More peripheral drusen than macular drusen contain apolipoprotein immunoreactivity, whereas all drusen in all regions contain cholesterol forms, suggesting higher lipid concentration in macular drusen.116 In drusen microdissected from nine eyes of seven AMD donors, soft drusen are found only in the macula,117 and are less likely to be harvested intact, and more likely to have overlying basal laminar deposit, high coverage by RPE, and interiors dominated by a homogeneous membranous material consistent with the Sarks' descriptions.117 In a high-resolution histology survey of nonneovascular AMD eyes, BLinD is thickest under the fovea.4 These suggestive results should be fortified with new high-resolution histology and clinical imaging covering the entire retina, which is now possible. In particular, more data supporting scarcity or absence of soft drusen in the periphery could definitively establish specificity for foveal biology.
Considering Dietary Delivery of Xanthophyll Pigment
Our model of soft drusen biogenesis suggests that fatty acids from diet are combined with unesterified cholesterol both from diet and from outer segments to create large lipoproteins secreted by RPE in BrM.9 Fatty acids in RPE-secreted BrM lipoproteins are enriched in linoleate (implicating diet as a source) and not docosahexaenoate (from photoreceptor outer segments),118,119 raising the question of what major dietary pathway(s) supply fatty acids and for what purpose. One possibility is the delivery of vitamin A derivatives for phototransduction. The paucity of well-studied bisretinoid A2E in macula relative to periphery120–125 (unlike mouse126) hints at a unique division of labor between RPE and Müller cells in vitamin A homeostasis, which remains to be explored further. Here we suggest that delivery of XP to Müller cells is a plausible biologic purpose for a large influx of dietary lipids through RPE that is specific to central macula.
The XPs lutein, zeaxanthin, and meso-zeaxanthin127 are highly concentrated in the fovea, decline by an order of magnitude within 2° of fixation, reach very low levels at the edge of the macula, and remain low throughout the retina.128–130 The distribution of XPs can be envisioned as three concentric zones centered on the fovea: zeaxanthin is central-most (foveola, 350 μm diameter), overlapped by meso-zeaxanthin (foveal avascular zone, 500 μm diameter); these two are surrounded by lutein (foveal-parafoveal annulus of outer diameter, 2.0 mm).131 The role of XP in vision is actively investigated under several mechanistic hypotheses. The protection hypothesis suggests that XP protects the retina from cumulative damage of ambient blue light via antioxidant properties.132 The optical hypothesis suggests that XP improves visual performance and visual comfort by attenuating chromatic aberration and light scatter, via short-wavelength light filtering and dichroism.133,134 The neural efficiency hypothesis suggests that XP improves vision by a direct interaction with neurons,135 perhaps conferring an evolutionary advantage.133 XP biology has expanded beyond the macula to the brain, where these compounds are detected and studied in reference to cognition and aging.136,137 XP is of clinical significance because dietary supplements containing lutein and zeaxanthin are recommended for some patients with intermediate AMD.138
Recent evidence supports macular Müller cells as the principal cellular reservoir of XP. High XP concentration in the Henle fiber layer, foveal center,128,139–141 and inner plexiform layer is well explained by the morphology of individual Müller glia.32,142,143 The persistent finding of rings and shoulders in addition to a strong central peak is consistent with Müller cell side branches in the synaptic layers.144–146 In macular telangiectasia type 2, XP absence is associated with histologically confirmed degeneration of foveal Müller cells.39,40 Patients with Sjögren-Larsson syndrome exhibit loss of clinically detectable XP and presence of inner retinal cysts suggestive of Müller cell degeneration.41,147,148 Surgically excised lamellar hole epiretinal membranes are enriched both in Müller cell markers and in XP.42,43 Autofluorescence imaging suggests that XP persists in central geographic atrophy,149–151 even after photoreceptor death, because Müller cells remain.33,64 Attributing strong XP signal to only cone axons in the Henle fiber layer152 overlooks the numerous rod and Müller processes also in this layer. In 1984 Snodderly et al.128 rejected Müller cells as a reservoir, in part because little evidence at the time suggested so many foveal glia. Subsequent studies in monkeys34 and humans153 indicate equal numbers of Müller glia and macular photoreceptors, at the limited sites examined. This hypothesis does not exclude XPs or their binding proteins also localizing to other cells,154,155 including cone axons, or the possibility of transfers between Müller cells and photoreceptors.
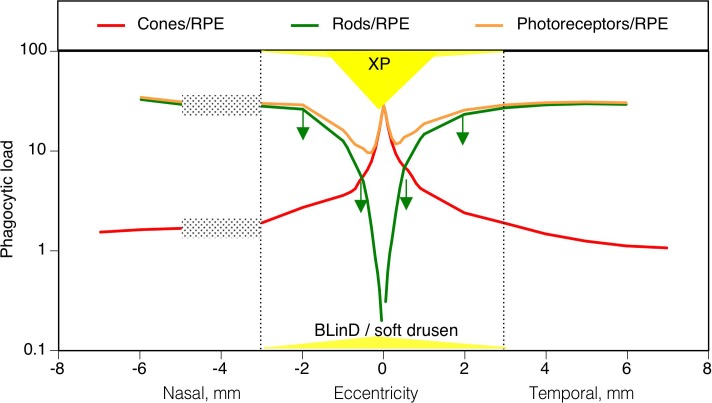
Figure 2 plots on the same eccentricity axes several mechanisms that may contribute to the apparent vulnerability of parafoveal rods and resilience of foveal cones in aging. The phagocytic load on RPE is represented by a ratio of photoreceptors/mm2 to RPE/mm2 and is computed for cones and rods, separately and summed. This ratio is high at the cone peak, lower in the parafovea, and higher again at the edge of the macula. Yet foveal cones survive into late AMD, and in aging the rods die in an annulus of vulnerability where phagocytic load is lower than elsewhere. These considerations suggest that phagocytic load is less of a factor in AMD initiation than is commonly believed, as previously concluded for monkeys.23 Figure 2 also shows that BLinD/soft drusen in nonneovascular AMD are found across the macula, being slightly thicker at the foveal center than elsewhere. The annulus of age-related rod loss localizes to the shoulder of this lipid-rich area in BrM. Foveal cones directly overlie lipid deposits but in contrast to rods are sustained by Müller cells harboring XPs, which are present throughout the foveal center and across the annulus of vulnerability.
Figure 2.
Topography of human macular photoreceptors, retinal pigment epithelium, XP, and BLinD/soft drusen. Dotted lines indicate the limits of the 6-mm-diameter macula. Stippled rectangles indicate the optic nerve head. Green downward arrows indicate the annulus of deepest rod loss in aging (Fig. 1, bottom right, deep blue). The lateral extents of XP and BLinD/soft drusen are drawn to scale on the eccentricity axis and not-to-scale on the y-axis. Data come from different eyes of younger adults: photoreceptors/mm2 (Ref. 18); RPE/mm2 (Ref. 18); XP129; thickness of BLinD/soft drusen in AMD eyes.4
Factors underlying XP absorption, distribution, metabolism, and excretion are actively investigated and include intestinal absorption and intracellular transport in enterocytes, receptors and transporters in retina and brain target tissues, and participation by the microbiome.156 Available evidence suggests that retinal XPs are turned over, that is, delivered and removed, on a time scale of months. A detailed time course of XP repletion in two volunteers has shown a steady increase for 140 days after initiation of supplementation, followed by a plateau or slight decrease to 1 year or more.157 Many studies report that dietary supplementation results in higher MPOD (measured behaviorally or via several imaging technologies) at the earliest time points tested, typically 2 to 4 months and depending on the dose and baseline XP levels in test subjects.131,158–169 XP removal is less well documented, with limited data suggesting stable, slightly decreasing, or baseline MPOD.157,169 Mature macaque monkeys consuming a semipurified diet lacking XP lose yellow pigment; these animals were examined after years on this regimen and therefore the minimum time for XP depletion is unknown.170 Interestingly, XP-deficient animals have hypopigmented spots in the fovea that could represent either drusen or lipoidal degeneration of individual RPE cells, both of which are found in monkeys.171–174
We thus hypothesize that XP is a marker for Müller cell protection and enhancement of foveal cone function for acute vision. We also hypothesize that an influx of HDL-mediated XP delivery through RPE provides a major source of fatty acids in BrM lipoproteins, which are a source of peroxidizable, cytotoxic lipids and a barrier to transport between the ChC and outer retinal cells.
HDL (Plasma and Genes) and Xanthophylls
Accumulation of XP in the macula begins with consumed foods, digestion, absorption, and transport in plasma, and ultimately capture, transcellular transfer, and stabilization within retinal cell membranes.127,132 On a sufficient diet, HDL is the major lipoprotein transporter of lutein (52%) and zeaxanthin (44%); LDL is the major transporter of α- and β-carotene and lycopene.175 The best studied cellular receptor for plasma HDL is scavenger receptor B-I (SRB-I).176 Evidence for a facilitated, SRB-I–mediated transport mechanism for lutein absorption in human intestinal enterocytes has been summarized.132 Animal models lacking apoA-I (principal protein of HDL) lack lutein in retina and retain it in brain, suggesting specific targeting and uptake mechanisms.177 Evidence accruing for ocular cells includes selective reduction of XP uptake (compared to LDL) by depletion of SRB-I activity in RPE-derived cell lines178,179 and capacity for XP binding by interphotoreceptor retinoid-binding protein (IRBP) within the subretinal space.180 In contrast to lutein and zeaxanthin, meso-zeaxanthin is rare in common dietary sources and appears to be converted within the RPE from dietary lutein by a newly recognized function of RPE65, the well-known retinol isomerase of the visual cycle.181
Our overall hypothesis adds a new perspective to recurring reports that elevated levels of plasma HDL modestly increase risk (1.15–2.3 fold) for incident early AMD, in population182–186 and case-control studies,187 and not in a large cohort of advanced AMD.188 This effect of AMD is opposite to the well-documented association of elevated plasma HDL with reduced risk for cardiovascular disease. It is possible that elevated HDL level leads to increased XP uptake by the RPE, in turn increasing both the amount available for delivery to photoreceptors and excess lipids to be offloaded to ChC, in RPE-secreted lipoproteins. Yet, positive association between plasma concentrations of lutein and HDL does not always imply an association between detectable retinal XP and plasma HDL concentration.189
XP delivery also adds a new perspective to the association of genes of plasma HDL metabolism with AMD, including APOE, CETP, ABCA1, LIPC, and SCARB1.190–196 DNA sequence variants in LIPC and ABCA1 associate with intermediate and large drusen.197 The International Age-related Macular Degeneration Genomics Consortium cohort of 16,144 cases, with 17,832 controls,195 has been used in Mendelian randomization analyses of genes associated with plasma lipid classes on AMD risk. In one study,198 three of five variants reached genome-level significance (LIPC × 2; CETP), placing AMD intermediate between cardiovascular disease (5/17 variants) and Alzheimer (1/4 variants) in lipid gene effects. Another study using this data set has shown that HDL-increasing alleles CETP and LIPC have opposite effects on AMD risk.199 A meta-analysis of 21 studies suggests that elevated HDL is a risk factor for any and early AMD.200 A sequence variant in SCARB1 (gene encoding SRB-I) is positively associated with plasma lutein but not with behaviorally measured XP.196
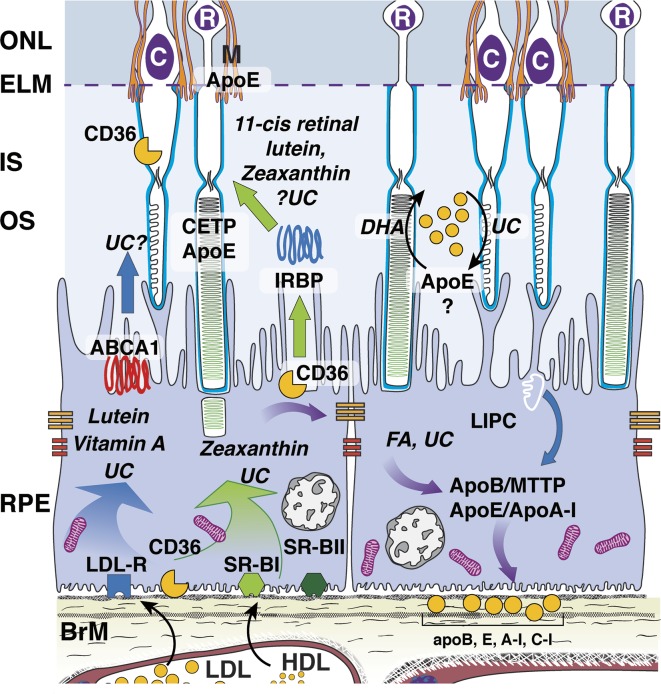
Not all variants in HDL genes result in higher plasma HDL. Our AMD pathobiology model suggests that genetic variations either modulate concentrations of plasma HDL, intraocularly modulating retinal uptake, intercellular transfer, and membrane stabilization, or both. Many of these gene products are expressed in outer retina (Fig. 3), consistent with an intraretinal HDL system based on apoE, like brain.4 Immunolocalization of HDL-related genes, using validated antibodies in polarized RPE (in vivo or high-fidelity culture), includes apoE (outer segments, RPE, Müller cells,94,201–204 drusen,116,190,201 SDD)117; ABCA1 (ATP-binding cassette transporter), RPE cell bodies205–208; CETP (cholesteryl ester transfer protein), outer segments, and outer plexiform layer205; LIPC (hepatic lipase), all retinal neurons plus RPE, and not in Müller cells192; SRB-I, RPE expression,209,210 activity,178,211 and localization.202,206 Lutein has been detected in subretinal fluid removed from rhegmatogenous retinal detachments,212 consistent with transfer between RPE and retina. Both systemic and ocular mechanisms of HDL-mediated XP delivery could be simultaneously operative. More data are certainly needed.
Figure 3.
Outer retinal lipid recycling pathways potentially involved in XP transport. Lutein and zeaxanthin, vitamin A, and some unesterified cholesterol (UC) enter RPE via receptor-mediated uptake of plasma lipoproteins, especially at the LDL and SRB-I receptors. These are transferred to photoreceptors by poorly understood mechanisms that may involve interphotoreceptor retinol-binding protein (IRBP, center), an apoE-based small HDL particle (right), or diffusion (not shown). Because lipids in Bruch membrane lipoproteins are rich in the fatty acid (FA) linoleate (and not docosahexaenoate [DHA]), soft drusen are proposed as a downstream consequence to constitutive dietary delivery of lipophilic essentials to macular cells, and the RPE-mediated recycling of unneeded lipids from these sources and from outer segments, to the circulation via large lipoprotein particles containing apoB and apoE. Lipoproteins accumulate in the sub–RPE-basal lamina largely because of impaired egress through the Bruch's membrane-choriocapillary complex. This is part of a larger system thought to include physiologic release of molecules contributing to subretinal drusenoid deposit (not shown).4,102 Reproduced with permission from Spaide RF, Ooto S, Curcio CA. Subretinal drusenoid deposits AKA pseudodrusen [published online ahead of print May 30, 2018]. Surv Ophthalmol. doi:10.1016/j.survophthal.2018.05.005. © 2018 Published by Elsevier Inc.
Conclusions
In this scenario, soft drusen and the Oil Spill on BrM in AMD develop over a lifetime of recycling unneeded lipids, many taken up with plasma HDL delivering XP to the choroid and impaired in egress by aged BrM-ChC. XP is a biomarker of Müller cell health and capacity for sustaining high-acuity, foveal cone-mediated vision, conferring a selective advantage for eyes with XP in mammalian evolution. XP may be just one representative of the nutritional support system of this superb human asset. We suggest that evolution of the neuroglial relations underlying exquisite foveal vision in humans came with a price, that is, soft drusen and their sequela, long after our reproductive years.
Limitations to this analysis are sparse experimental confirmation of hypotheses largely generated from human tissues and patients. We are fortunate that XP can now be measured noninvasively and objectively in vivo through imaging.45,146 Further, short-lived mouse models exhibiting relevant phenotypes are now available (XP accumulation,213 retentive matrix for RPE-secreted lipoproteins214–216). Our hypotheses, while speculative, bring together many lines of evidence and do not exclude other major extant hypotheses for AMD biology and may in fact occur in parallel. It is hoped that this conceptual framework can guide the exploration of clinical imaging data sets46,141,217 and high-throughput analytic assays of tissues.218,219
Acknowledgments
Supported by Grants R01 EY R01EY027948, EY021470, Heidelberg Engineering, Hoffman LaRoche, EyeSight Foundation of Alabama, and Research to Prevent Blindness.
Disclosure: C.A. Curcio, None
References
- 1.Sadda SR, Guymer R, Holz FG, et al. Consensus definition for atrophy associated with age-related macular degeneration on optical coherence tomography: CAM Report 3. Ophthalmology. 2017;125:537–548. doi: 10.1016/j.ophtha.2017.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zweifel SA, Spaide RF, Curcio CA, Malek G, Imamura Y. Reticular pseudodrusen are subretinal drusenoid deposits. Ophthalmology. 2010;117:303–312.e1. doi: 10.1016/j.ophtha.2009.07.014. [DOI] [PubMed] [Google Scholar]
- 3.Sarks J, Arnold J, Ho IV, Sarks S, Killingsworth M. Evolution of reticular pseudodrusen. Br J Ophthalmol. 2011;95:979–985. doi: 10.1136/bjo.2010.194977. [DOI] [PubMed] [Google Scholar]
- 4.Curcio CA, Messinger JD, Sloan KR, McGwin G, Jr, Medeiros NE, Spaide RF. Subretinal drusenoid deposits in non-neovascular age-related macular degeneration: morphology, prevalence, topography, and biogenesis model. Retina. 2013;33:265–276. doi: 10.1097/IAE.0b013e31827e25e0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Greferath U, Guymer RH, Vessey KA, Brassington K, Fletcher EL. Correlation of histologic features with in vivo imaging of reticular pseudodrusen. Ophthalmology. 2016;123:1320–1331. doi: 10.1016/j.ophtha.2016.02.009. [DOI] [PubMed] [Google Scholar]
- 6.Schmitz-Valckenberg S, Alten F, Steinberg JS, et al. Reticular drusen associated with geographic atrophy in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2011;52:5009–5015. doi: 10.1167/iovs.11-7235. [DOI] [PubMed] [Google Scholar]
- 7.Zarubina AV, Neely DC, Clark ME, et al. Prevalence of subretinal drusenoid deposits in older persons with and without age-related macular degeneration, by multimodal imaging. Ophthalmology. 2016;123:1090–1100. doi: 10.1016/j.ophtha.2015.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bressler SB, Bressler NM, Sarks SH, Sarks JP. Age-related macular degeneration: nonneovascular early AMD, intermediate AMD, and geographic atrophy. In: Ryan SJ, editor. Retina. St Louis, MO: Mosby; 2006. pp. 1041–1074. [Google Scholar]
- 9.Curcio CA. Soft drusen in age-related macular degeneration: biology and targeting, via the Oil Spill strategies. Invest Ophthalmol Vis Sci. 2018;59:AMD160–AMD181. doi: 10.1167/iovs.18-24882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hawkins BT, Davis TP. The blood-brain barrier/neurovascular unit in health and disease. Pharmacol Rev. 2005;57:173–185. doi: 10.1124/pr.57.2.4. [DOI] [PubMed] [Google Scholar]
- 11.Newman EA. Glial cell regulation of neuronal activity and blood flow in the retina by release of gliotransmitters. Philos Trans R Soc Lond B Biol Sci. 2015;370:20140195. doi: 10.1098/rstb.2014.0195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen W, Wang Z, Zhou X, Li B, Zhang H. Choroidal and photoreceptor layer thickness in myopic population. Eur J Ophthalmol. 2012;22:590–597. doi: 10.5301/ejo.5000092. [DOI] [PubMed] [Google Scholar]
- 13.Reiner A, Fitzgerald MEC, Del Mar N, Li C. Neural control of choroidal blood flow. Prog Retin Eye Res. 2017;64:96–130. doi: 10.1016/j.preteyeres.2017.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dolz-Marco R, Glover JP, Litts KM, et al. Choroidal and sub-retinal pigment epithelium caverns: multimodal imaging and correspondence with Friedman lipid globules. Ophthalmology. 2018;125:1287–1301. doi: 10.1016/j.ophtha.2018.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zouache MA, Eames I, Klettner CA, Luthert PJ. Form, shape and function: segmented blood flow in the choriocapillaris. Sci Rep. 2016;6:35754. doi: 10.1038/srep35754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Polyak SL. The Vertebrate Visual System. Chicago, IL: University of Chicago;; 1957. [Google Scholar]
- 17.Early Treatment Diabetic Retinopathy Study Research Group. Grading diabetic retinopathy from stereoscopic color fundus photographs—an extension of the modified Airlie House classification: ETDRS report number 10: Early Treatment Diabetic Retinopathy Study Research Group. Ophthalmology. 1991;98:786–806. [PubMed] [Google Scholar]
- 18.Curcio CA, Sloan KR, Kalina RE, Hendrickson AE. Human photoreceptor topography. J Comp Neurol. 1990;292:497–523. doi: 10.1002/cne.902920402. [DOI] [PubMed] [Google Scholar]
- 19.Zhang T, Godara P, Blanco ER, et al. Variability in human cone topography assessed by adaptive optics scanning laser ophthalmoscopy. Am J Ophthalmol. 2015;160:290–300.e1. doi: 10.1016/j.ajo.2015.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scoles D, Sulai Y, Langlo C, et al. In vivo imaging of human cone photoreceptor inner segments. Invest Ophthalmol Vis Sci. 2014;55:4244–4251. doi: 10.1167/iovs.14-14542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ach T, Huisingh C, McGwin G, Jr, et al. Quantitative autofluorescence and cell density maps of the human retinal pigment epithelium. Invest Ophthalmol Vis Sci. 2014;55:4832–4841. doi: 10.1167/iovs.14-14802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gao H, Hollyfield JG. Aging of the human retina: differential loss of neurons and retinal pigment epithelial cells. Invest Ophthalmol Vis Sci. 1992;33:1–17. [PubMed] [Google Scholar]
- 23.Snodderly DM, Sandstrom MM, Leung IY-F, Zucker CL, Neuringer M. Retinal pigment epithelial cell distribution in central retina of rhesus monkeys. Invest Ophthalmol Vis Sci. 2002;43:2815–2818. [PubMed] [Google Scholar]
- 24.Provis JM, Dubis AM, Maddess T, Carroll J. Adaptation of the central retina for high acuity vision: cones, the fovea and the avascular zone. Prog Retin Eye Res. 2013;35:63–81. doi: 10.1016/j.preteyeres.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dacey DM. Parallel pathways for spectral coding in primate retina. Annu Rev Neurosci. 2000;23:743–775. doi: 10.1146/annurev.neuro.23.1.743. [DOI] [PubMed] [Google Scholar]
- 26.Dacey DM. The mosaic of midget ganglion cells in the human retina. J Neurosci. 1993;13:5334–5344. doi: 10.1523/JNEUROSCI.13-12-05334.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Drasdo N, Millican CL, Katholi CR, Curcio CA. The length of Henle fibers in the human retina and a model of ganglion receptive field density in the visual field. Vis Res. 2007;47:2901–2911. doi: 10.1016/j.visres.2007.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Watson AB. A formula for human retinal ganglion cell receptive field density as a function of visual field location. J Vis. 2014;14(7):15. doi: 10.1167/14.7.15. [DOI] [PubMed] [Google Scholar]
- 29.Calkins DJ, Schein SJ, Tsukamoto Y, Sterling P. M and L cones in macaque fovea connect to midget ganglion cells by different numbers of excitatory synapses. Nature. 1994;371:70–72. doi: 10.1038/371070a0. [DOI] [PubMed] [Google Scholar]
- 30.Perry VH, Cowey A. The lengths of the fibres of Henle in the retina of macaque monkeys: implications for vision. Neuroscience. 1988;25:225–236. doi: 10.1016/0306-4522(88)90021-8. [DOI] [PubMed] [Google Scholar]
- 31.Curcio CA, Messinger JD, Mitra AM, Sloan KR, McGwin G, Jr, Spaide R. Human chorioretinal layer thicknesses measured using macula-wide high resolution histological sections. Invest Ophthalmol Vis Sci. 2011;52:3943–3954. doi: 10.1167/iovs.10-6377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Matet A, Savastano MC, Rispoli M, et al. En face optical coherence tomography of foveal microstructure in full-thickness macular hole: a model to study perifoveal Muller cells. Am J Ophthalmol. 2015;159:1142–1151.e3. doi: 10.1016/j.ajo.2015.02.013. [DOI] [PubMed] [Google Scholar]
- 33.Li M, Huisingh C, Messinger JD, et al. Histology of geographic atrophy secondary to age-related macular degeneration: a multilayer approach. Retina. 2018] doi: 10.1097/IAE.0000000000002182. [published online ahead of print May 11. [DOI] [PMC free article] [PubMed]
- 34.Burris C, Klug K, Ngo IT, Sterling P, Schein S. How Muller glial cells in macaque fovea coat and isolate the synaptic terminals of cone photoreceptors. J Comp Neurol. 2002;453:100–111. doi: 10.1002/cne.10397. [DOI] [PubMed] [Google Scholar]
- 35.Dacey DM, Packer O, Schalek R, et al. Connectomic reconstruction links human foveal cones to distinct circuitry in the center of the foveal pit. Invest Ophthalmol Vis Sci. 2017;58:1036–1036. [Google Scholar]
- 36.Reichenbach A, Bringmann A. New functions of Muller cells. Glia. 2013;61:651–678. doi: 10.1002/glia.22477. [DOI] [PubMed] [Google Scholar]
- 37.Mata NL, Radu RA, Clemmons RC, Travis GH. Isomerization and oxidation of vitamin a in cone-dominant retinas: a novel pathway for visual-pigment regeneration in daylight. Neuron. 2002;36:69–80. doi: 10.1016/s0896-6273(02)00912-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xue Y, Shen SQ, Jui J, et al. CRALBP supports the mammalian retinal visual cycle and cone vision. J Clin Invest. 2015;125:727–738. doi: 10.1172/JCI79651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Powner MB, Gillies MC, Tretiach M, et al. Perifoveal Müller cell depletion in a case of macular telangiectasia type 2. Ophthalmology. 2010;117:2407–2416. doi: 10.1016/j.ophtha.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Powner MB, Gillies MC, Zhu M, Vevis K, Hunyor AP, Fruttiger M. Loss of Muller's cells and photoreceptors in macular telangiectasia type 2. Ophthalmology. 2013;120:2344–2352. doi: 10.1016/j.ophtha.2013.04.013. [DOI] [PubMed] [Google Scholar]
- 41.Theelen T, Berendschot TT, Klevering BJ, Fuijkschot J, Hoyng CB, Willemsen MA. Multimodal imaging of the macula in hereditary and acquired lack of macular pigment. Acta Ophthalmol. 2014;92:138–142. doi: 10.1111/aos.12092. [DOI] [PubMed] [Google Scholar]
- 42.Pang CE, Maberley DA, Freund KB, et al. Lamellar hole-associated epiretinal proliferation: a clinicopathologic correlation. Retina. 2016;36:1408–1412. doi: 10.1097/IAE.0000000000001069. [DOI] [PubMed] [Google Scholar]
- 43.Obana A, Sasano H, Okazaki S, Otsuki Y, Seto T, Gohto Y. Evidence of carotenoid in surgically removed lamellar hole-associated epiretinal proliferation. Invest Ophthalmol Vis Sci. 2017;58:5157–5163. doi: 10.1167/iovs.17-22347. [DOI] [PubMed] [Google Scholar]
- 44.Wooten BR, Hammond BR, Jr, Land RI, Snodderly DM. A practical method for measuring macular pigment optical density. Invest Ophthalmol Vis Sci. 1999;40:2481–2489. [PubMed] [Google Scholar]
- 45.Dietzel M, Zeimer M, Heimes B, Claes B, Pauleikhoff D, Hense HW. Determinants of macular pigment optical density and its relation to age-related maculopathy: results from the Muenster Aging and Retina Study (MARS) Invest Ophthalmol Vis Sci. 2011;52:3452–3457. doi: 10.1167/iovs.10-6713. [DOI] [PubMed] [Google Scholar]
- 46.Sauer L, Schweitzer D, Ramm L, Augsten R, Hammer M, Peters S. Impact of macular pigment on fundus autofluorescence lifetimes. Invest Ophthalmol Vis Sci. 2015;56:4668–4679. doi: 10.1167/iovs.14-15335. [DOI] [PubMed] [Google Scholar]
- 47.Bunt-Milam AH, Saari JC, Klock IB, Garwin GS. Zonula adherentes pore size in the external limiting membrane of the rabbit retina. Invest Ophthalmol Vis Sci. 1985;26:1377–1380. [PubMed] [Google Scholar]
- 48.Williams DS, Arikawa K, Paallysaho T. Cytoskeletal components of the adherens junctions between the photoreceptors and the supportive Muller cells. J Comp Neurol. 1990;295:155–164. doi: 10.1002/cne.902950113. [DOI] [PubMed] [Google Scholar]
- 49.Johnson LV, Hageman GS, Blanks JC. Interphotoreceptor matrix domains ensheath vertebrate cone photoreceptor cells. Invest Ophthalmol Vis Sci. 1986;27:129–135. [PubMed] [Google Scholar]
- 50.Tien L, Rayborn ME, Hollyfield JG. Characterization of the interphotoreceptor matrix surrounding rod photoreceptors in the human retina. Exp Eye Res. 1992;55:297–306. doi: 10.1016/0014-4835(92)90194-w. [DOI] [PubMed] [Google Scholar]
- 51.Gonzalez-Fernandez F, Kittredge KL, Rayborn ME, et al. Interphotoreceptor retinoid-binding protein (IRBP), a major 124 kDa glycoprotein in the interphotoreceptor matrix of Xenopus laevis: characterization, molecular cloning and biosynthesis. J Cell Sci. 1993;105(pt 1):7–21. doi: 10.1242/jcs.105.1.7. [DOI] [PubMed] [Google Scholar]
- 52.Curcio CA, Johnson M, Rudolf M, Huang J-D. The oil spill in ageing Bruch's membrane. Br J Ophthalmol. 2011;95:1638–1645. doi: 10.1136/bjophthalmol-2011-300344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Spaide RF, Ooto S, Curcio CA. Subretinal drusenoid deposits a.k.a. pseudodrusen. 2018] doi: 10.1016/j.survophthal.2018.05.005. [published online ahead of print June 3. Surv Ophthalmol doi: [DOI] [PubMed]
- 54.Smith W, Assink J, Klein R, et al. Risk factors for age-related macular degeneration. Pooled findings from three continents. Ophthalmology. 2001;108:697–704. doi: 10.1016/s0161-6420(00)00580-7. [DOI] [PubMed] [Google Scholar]
- 55.Chakravarthy U, Wong TY, Fletcher A, et al. Clinical risk factors for age-related macular degeneration: a systematic review and meta-analysis. BMC Ophthalmol. 2010;10:31. doi: 10.1186/1471-2415-10-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Age-Related Eye Disease Study Research Group. The Age-Related Eye Disease Study severity scale for age-related macular degeneration: AREDS Report No. 17. Arch Ophthalmol. 2005;123:1484–1498. doi: 10.1001/archopht.123.11.1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Joachim N, Mitchell P, Burlutsky G, Kifley A, Wang JJ. The incidence and progression of age-related macular degeneration over 15 years: the Blue Mountains Eye Study. Ophthalmology. 2015;122:2482–2489. doi: 10.1016/j.ophtha.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 58.LaVail MM. Analysis of neurological mutants with inherited retinal degeneration. Invest Ophthalmol Vis Sci. 1981;21:638–657. [PubMed] [Google Scholar]
- 59.Jacobson SG, Voigt WJ, Parel J-M, et al. Automated light- and dark-adapted perimetry for evaluating retinitis pigmentosa. Ophthalmology. 1986;93:1604–1611. doi: 10.1016/s0161-6420(86)33522-x. [DOI] [PubMed] [Google Scholar]
- 60.Heckenlively JR. Retinitis Pigmentosa. Philadelphia, PA: J. B; 1988. Lippincott; [Google Scholar]
- 61.Curcio CA, Owsley C, Jackson GR. Spare the rods, save the cones in aging and age-related maculopathy. Invest Ophthalmol Vis Sci. 2000;41:2015–2018. [PubMed] [Google Scholar]
- 62.Curcio CA, Millican CL, Allen KA, Kalina RE. Aging of the human photoreceptor mosaic: evidence for selective vulnerability of rods in central retina. Invest Ophthalmol Vis Sci. 1993;34:3278–3296. [PubMed] [Google Scholar]
- 63.Wing GL, Blanchard GC, Weiter JL. The topography and age relationship of lipofuscin concentration in the retinal pigment epithelium. Invest Ophthalm Vis Sci. 1978;17:601–617. [PubMed] [Google Scholar]
- 64.Schaal KB, Freund KB, Litts KM, Zhang Y, Messinger JD, Curcio CA. Outer retinal tubulation in advanced age-related macular degeneration: optical coherence tomographic findings correspond to histology. Retina. 2015;35:1339–1350. doi: 10.1097/IAE.0000000000000471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Litts KM, Ach T, Hammack KM, et al. Quantitative analysis of outer retinal tubulation in age-related macular degeneration from spectral-domain optical coherence tomography and histology. Invest Ophthalmol Vis Sci. 2016;57:2647–2656. doi: 10.1167/iovs.16-19262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lamb TD, Pugh EN., Jr Dark adaptation and the retinoid cycle of vision. Prog Retin Eye Res. 2004;23:307–380. doi: 10.1016/j.preteyeres.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 67.Fraser RG, Tan R, Ayton LN, Caruso E, Guymer RH, Luu CD. Assessment of retinotopic rod photoreceptor function using a dark-adapted chromatic perimeter in intermediate age-related macular degeneration. Invest Ophthalmol Vis Sci. 2016;57:5436–5442. doi: 10.1167/iovs.16-19295. [DOI] [PubMed] [Google Scholar]
- 68.Del Priore LV, Kuo Y-H, Tezel TH. Age-related changes in human RPE cell density and apoptosis proportion in situ. Invest Ophthalmol Vis Sci. 2002;43:3312–3318. [PubMed] [Google Scholar]
- 69.Harman AM, Fleming PA, Hoskins RV, Moore SR. Development and aging of cell topography in the human retinal pigment epithelium. Invest Ophthalmol Vis Sci. 1997;38:2016–2026. [PubMed] [Google Scholar]
- 70.Feher J, Kovacs I, Artico M, Cavallotti C, Papale A, Balacco Gabrieli C. Mitochondrial alterations of retinal pigment epithelium in age-related macular degeneration. Neurobiol Aging. 2006;27:983–993. doi: 10.1016/j.neurobiolaging.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 71.Eliasieh K, Liets LC, Chalupa LM. Cellular reorganization in the human retina during normal aging. Invest Ophthalmol Vis Sci. 2007;48:2824–2830. doi: 10.1167/iovs.06-1228. [DOI] [PubMed] [Google Scholar]
- 72.Newsome DA, Huh W, Green WR. Bruch's membrane age-related changes vary by region. Curr Eye Res. 1987;6:1211–1221. doi: 10.3109/02713688709025231. [DOI] [PubMed] [Google Scholar]
- 73.Karwatowski WSS, Jeffried TE, Duance VC, Albon J, Bailey AJ, Easty DL. Preparation of Bruch's membrane and analysis of the age-related changes in the structural collagens. Br J Ophthalmol. 1995;79:944–952. doi: 10.1136/bjo.79.10.944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pauleikhoff D, Harper CA, Marshall J, Bird AC. Aging changes in Bruch's membrane: a histochemical and morphological study. Ophthalmology. 1990;97:171–178. [PubMed] [Google Scholar]
- 75.Curcio CA, Millican CL, Bailey T, Kruth HS. Accumulation of cholesterol with age in human Bruch's membrane. Invest Ophthalmol Vis Sci. 2001;42:265–274. [PubMed] [Google Scholar]
- 76.Haimovici R, Gantz DL, Rumelt S, Freddo TF, Small DM. The lipid composition of drusen, Bruch's membrane, and sclera by hot stage polarizing microscopy. Invest Ophthalmol Vis Sci. 2001;42:1592–1599. [PubMed] [Google Scholar]
- 77.Ramrattan RS, van der Schaft TL, Mooy CM, de Bruijn WC, Mulder PGH, de Jong PTVM. Morphometric analysis of Bruch's membrane, the choriocapillaris, and the choroid in aging. Invest Ophthalmol Vis Sci. 1994;35:2857–2864. [PubMed] [Google Scholar]
- 78.Sohn EH, Khanna A, Tucker BA, Abramoff MD, Stone EM, Mullins RF. Structural and biochemical analyses of choroidal thickness in human donor eyes. Invest Ophthalmol Vis Sci. 2014;55:1352–1360. doi: 10.1167/iovs.13-13754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sarks SH. Ageing and degeneration in the macular region: a clinico-pathological study. Br J Ophthalmol. 1976;60:324–341. doi: 10.1136/bjo.60.5.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.van der Schaft TL, Mooy CM, de Bruijn WC, Oron FG, Mulder PGH, de Jong PTVM. Histologic features of the early stages of age-related macular degeneration. Ophthalmology. 1992;99:278–286. doi: 10.1016/s0161-6420(92)31982-7. [DOI] [PubMed] [Google Scholar]
- 81.Mullins RF, Schoo DP, Sohn EH, et al. The membrane attack complex in aging human choriocapillaris: relationship to macular degeneration and choroidal thinning. Am J Pathol. 2014;184:3142–3153. doi: 10.1016/j.ajpath.2014.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jablonski MM, Iannaccone A, Reynolds DH, et al. Age-related decline in VIP-positive parasympathetic nerve fibers in the human submacular choroid. Invest Ophthalmol Vis Sci. 2007;48:479–485. doi: 10.1167/iovs.06-0972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Streeten BW. The sudanophilic granules of the human retinal pigment epithelium. Arch Ophthalmol. 1961;66:391–398. [Google Scholar]
- 84.Wolter JR, Falls HF. Bilateral confluent drusen. Arch Ophthalmol. 1962;68:219–226. doi: 10.1001/archopht.1962.00960030223013. [DOI] [PubMed] [Google Scholar]
- 85.Farkas TG, Sylvester V, Archer D, Altona M. The histochemistry of drusen. Am J Ophthalmol. 1971;71:1206–1215. doi: 10.1016/0002-9394(71)90964-0. [DOI] [PubMed] [Google Scholar]
- 86.Sarks SH. Council Lecture: drusen and their relationship to senile macular degeneration. Aust J Ophthalmol. 1980;8:117–130. doi: 10.1111/j.1442-9071.1980.tb01670.x. [DOI] [PubMed] [Google Scholar]
- 87.Pauleikhoff D, Zuels S, Sheraidah GS, Marshall J, Wessing A, Bird AC. Correlation between biochemical composition and fluorescein binding of deposits in Bruch's membrane. Ophthalmology. 1992;99:1548–1553. doi: 10.1016/s0161-6420(92)31768-3. [DOI] [PubMed] [Google Scholar]
- 88.Williams KJ, Tabas I. The response-to-retention hypothesis of early atherogenesis. Arterioscler Thromb Vasc Biol. 1995;15:551–561. doi: 10.1161/01.atv.15.5.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mensah GA, Wei GS, Sorlie PD, et al. Decline in cardiovascular mortality: possible causes and implications. Circ Res. 2017;120:366–380. doi: 10.1161/CIRCRESAHA.116.309115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sarks JP, Sarks SH, Killingsworth MC. Evolution of geographic atrophy of the retinal pigment epithelium. Eye. 1988;2:552–577. doi: 10.1038/eye.1988.106. [DOI] [PubMed] [Google Scholar]
- 91.Sarks JP, Sarks SH, Killingsworth MC. Evolution of soft drusen in age-related macular degeneration. Eye. 1994;8:269–283. doi: 10.1038/eye.1994.57. [DOI] [PubMed] [Google Scholar]
- 92.Guyton JR, Klemp KF. Ultrastructural discrimination of lipid droplets and vesicles in atherosclerosis: value of osmium-thiocarbohydrazide-osmium and tannic acid-paraphenylenediamine techniques. J Histochem Cytochem. 1988;36:1319–1328. doi: 10.1177/36.10.2458408. [DOI] [PubMed] [Google Scholar]
- 93.Frank JS, Fogelman AM. Ultrastructure of the intima in WHHL and cholesterol-fed rabbit aortas prepared by ultra-rapid freezing and freeze-etching. J Lipid Res. 1989;30:967–978. [PubMed] [Google Scholar]
- 94.Pilgrim MG, Lengyel I, Lanzirotti A, et al. Sub-retinal pigment epithelial deposition of drusen components including hydroxyapatite in a primary cell culture model. Invest Ophthalmol Vis Sci. 2017;58:708–719. doi: 10.1167/iovs.16-21060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Balaratnasingam C, Yannuzzi LA, Curcio CA, et al. Associations between retinal pigment epithelium and drusen volume changes during the lifecycle of large drusenoid pigment epithelial detachments. Invest Ophthalmol Vis Sci. 2016;57:5479–5489. doi: 10.1167/iovs.16-19816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sassmannshausen M, Steinberg JS, Fimmers R, et al. Structure-function analysis in patients with intermediate age-related macular degeneration. Invest Ophthalmol Vis Sci. 2018;59:1599–1608. doi: 10.1167/iovs.17-22712. [DOI] [PubMed] [Google Scholar]
- 97.Miller JW. Treatment of age-related macular degeneration: beyond VEG-F. Jpn J Ophthalmol. 2010;54:523–528. doi: 10.1007/s10384-010-0863-4. [DOI] [PubMed] [Google Scholar]
- 98.Hughes A. The topography of vision in mammals of contrasting life style: comparative optics and retinal organisation. In: Crescitelli F, editor. Handbook of Sensory Physiology. Berlin: Springer-Verlag;; 1977. pp. 613–756. [Google Scholar]
- 99.Wässle H, Boycott B. Functional architecture of the mammalian retina. Physiol Rev. 1991;71:447–480. doi: 10.1152/physrev.1991.71.2.447. [DOI] [PubMed] [Google Scholar]
- 100.Kooijman AC. Light distribution on the retina of a wide-angle theoretical eye. J Opt Soc Am. 1983;73:1544–1550. doi: 10.1364/josa.73.001544. [DOI] [PubMed] [Google Scholar]
- 101.Pflibsen KP, Pomerantzeff O, Ross RN. Retinal illuminance using a wide-angle model of the eye. J Opt Soc Am A. 1988;5:146–150. doi: 10.1364/josaa.5.000146. [DOI] [PubMed] [Google Scholar]
- 102.Pikuleva I, Curcio CA. Cholesterol in the retina: the best is yet to come. Prog Ret Eye Res. 2014;41:64–89. doi: 10.1016/j.preteyeres.2014.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Alm A, Bill A. Ocular and optic nerve blood flow at normal and increased intraocular pressures in monkeys (Macaca irus): a study with radioactively labelled microspheres including flow determinations in brain and some other tissues. Exp Eye Res. 1973;15:15–29. doi: 10.1016/0014-4835(73)90185-1. [DOI] [PubMed] [Google Scholar]
- 104.Nork TM, Kim CB, Shanmuganayagam D, Van Lysel MS, Ver Hoeve JN, Folts JD. Measurement of regional choroidal blood flow in rabbits and monkeys using fluorescent microspheres. Arch Ophthalmol. 2006;124:860–868. doi: 10.1001/archopht.124.6.860. [DOI] [PubMed] [Google Scholar]
- 105.Klufas MA, Yannuzzi NA, Pang CE, et al. Feasibility and clinical utility of ultra-widefield indocyanine green angiography. Retina. 2014;35:508–520. doi: 10.1097/IAE.0000000000000318. [DOI] [PubMed] [Google Scholar]
- 106.Ploner SB, Moult EM, Choi W, et al. Toward quantitative optical coherence tomography angiography: visualizing blood flow speeds in ocular pathology using variable interscan time analysis. Retina. 2016;36(suppl 1):S118–S126. doi: 10.1097/IAE.0000000000001328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hubbard LD, Danis RP, Neider MW, et al. Brightness, contrast, and color balance of digital versus film retinal images in the age-related eye disease study 2. Invest Ophthalmol Vis Sci. 2008;49:3269–3282. doi: 10.1167/iovs.07-1267. [DOI] [PubMed] [Google Scholar]
- 108.Wang Q, Chappell RJ, Klein R, et al. Pattern of age-related maculopathy in the macular area: The Beaver Dam eye study. Invest Ophthalmol Visual Sci. 1996;37:2234–2242. [PubMed] [Google Scholar]
- 109.Wang JJ, Rochtchina E, Lee AJ, et al. Ten-year incidence and progression of age-related maculopathy: the Blue Mountains Eye Study. Ophthalmology. 2007;114:92–98. doi: 10.1016/j.ophtha.2006.07.017. [DOI] [PubMed] [Google Scholar]
- 110.Chong EW, Amirul Islam FM, Robman LD, et al. Age-related macular degeneration phenotypes associated with mutually exclusive homozygous risk variants in CFH and HTRA1 genes. Retina. 2015;35:989–998. doi: 10.1097/IAE.0000000000000417. [DOI] [PubMed] [Google Scholar]
- 111.Delcourt C, Delyfer MN, Rougier MB, et al. Associations of complement factor H and smoking with early age-related macular degeneration: the ALIENOR study. Invest Ophthalmol Vis Sci. 2011;52:5955–5962. doi: 10.1167/iovs.10-6235. [DOI] [PubMed] [Google Scholar]
- 112.Yehoshua Z, Wang F, Rosenfeld PJ, Penha FM, Feuer WJ, Gregori G. Natural history of drusen morphology in age-related macular degeneration using spectral domain optical coherence tomography. Ophthalmology. 2011;118:2434–2441. doi: 10.1016/j.ophtha.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Abdelfattah NS, Zhang H, Boyer DS, et al. Drusen volume as a predictor of disease progression in patients with late age-related macular degeneration in the fellow eye. Invest Ophthalmol Vis Sci. 2016;57:1839–1846. doi: 10.1167/iovs.15-18572. [DOI] [PubMed] [Google Scholar]
- 114.Sarks SH, Arnold JJ, Sarks JP, Gilles MC, Walter CJ. Prophylactic perifoveal laser treatment of soft drusen. Aust N Z J Ophthalmol. 1996;24:15–26. doi: 10.1111/j.1442-9071.1996.tb01546.x. [DOI] [PubMed] [Google Scholar]
- 115.Lewis H, Straatsma BR, Foos RY. Chorioretinal juncture: multiple extramacular drusen. Ophthalmology. 1986;93:1098–1112. doi: 10.1016/s0161-6420(86)33615-7. [DOI] [PubMed] [Google Scholar]
- 116.Malek G, Li C-M, Guidry C, Medeiros NE, Curcio CA. Apolipoprotein B in cholesterol-containing drusen and basal deposits in eyes with age-related maculopathy. Am J Pathol. 2003;162:413–425. doi: 10.1016/S0002-9440(10)63836-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Rudolf M, Malek G, Messinger JD, Wang L, Clark ME, Curcio CA. Sub-retinal drusenoid deposits in human retina: organization and composition. Exp Eye Res. 2008;87:402–408. doi: 10.1016/j.exer.2008.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wang L, Li C-M, Rudolf M, et al. Lipoprotein particles of intra-ocular origin in human Bruch membrane: an unusual lipid profile. Invest Ophthalmol Vis Sci. 2009;50:870–877. doi: 10.1167/iovs.08-2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Bretillon L, Thuret G, Gregoire S, et al. Lipid and fatty acid profile of the retina, retinal pigment epithelium/choroid, and the lacrimal gland, and associations with adipose tissue fatty acids in human subjects. Exp Eye Res. 2008;87:521–528. doi: 10.1016/j.exer.2008.08.010. [DOI] [PubMed] [Google Scholar]
- 120.Bhosale P, Serban B, Bernstein PS. Retinal carotenoids can attenuate formation of A2E in the retinal pigment epithelium. Arch Biochem Biophys. 2009;483:175–181. doi: 10.1016/j.abb.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ablonczy Z, Higbee D, Anderson DM, et al. Lack of correlation between the spatial distribution of A2E and lipofuscin fluorescence in the human retinal pigment epithelium. Invest Ophthalmol Vis Sci. 2013;54:5535–5542. doi: 10.1167/iovs.13-12250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zemski Berry KA, Gordon WC, Murphy RC, Bazan NG. Spatial organization of lipids in the human retina and optic nerve by MALDI imaging mass spectrometry. J Lipid Res. 2014;55:504–515. doi: 10.1194/jlr.M044990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Adler L IV, Boyer NP, Anderson DM, et al. Determination of N-retinylidene-N-retinylethanolamine (A2E) levels in central and peripheral areas of human retinal pigment epithelium. Photochem Photobiol Sci. 2015;14:1983–1990. doi: 10.1039/c5pp00156k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Pallitto P, Ablonczy Z, Jones EE, et al. A2E and lipofuscin distributions in macaque retinal pigment epithelium are similar to human. Photochem Photobiol Sci. 2015;14:1888–1895. doi: 10.1039/c5pp00170f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Anderson DMG, Ablonczy Z, Koutalos Y, et al. Bis(monoacylglycero)phosphate lipids in the retinal pigment epithelium implicate lysosomal/endosomal dysfunction in a model of Stargardt disease and human retinas. Sci Rep. 2017;7:17352. doi: 10.1038/s41598-017-17402-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ablonczy Z, Higbee D, Grey AC, Koutalos Y, Schey KL, Crouch RK. Similar molecules spatially correlate with lipofuscin and N-retinylidene-N-retinylethanolamine in the mouse but not in the human retinal pigment epithelium. Arch Biochem Biophys. 2013;539:196–202. doi: 10.1016/j.abb.2013.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Bernstein PS, Li B, Vachali PP, et al. Lutein, zeaxanthin, and meso-zeaxanthin: the basic and clinical science underlying carotenoid-based nutritional interventions against ocular disease. Prog Retin Eye Res. 2016;50:34–66. doi: 10.1016/j.preteyeres.2015.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Snodderly DM, Auran JD, Delori FC. The macular pigment, II: spatial distribution in primate retinas. Invest Ophthalmol Vis Sci. 1984;25:674–685. [PubMed] [Google Scholar]
- 129.Hammond BR, Jr, Wooten BR, Snodderly DM. Individual variations in the spatial profile of human macular pigment. J Opt Soc Am A. 1997;14:1187–1196. doi: 10.1364/josaa.14.001187. [DOI] [PubMed] [Google Scholar]
- 130.Bone RA, Landrum JT, Mayne ST, Gomez CM, Tibor SE, Twaroska EE. Macular pigment in donor eyes with and without AMD: a case-control study. Invest Ophthalmol Vis Sci. 2001;42:235–240. [PubMed] [Google Scholar]
- 131.Nolan JM, Power R, Stringham J, et al. Enrichment of macular pigment enhances contrast sensitivity in subjects free of retinal disease: Central Retinal Enrichment Supplementation Trials, Report 1. Invest Ophthalmol Vis Sci. 2016;57:3429–3439. doi: 10.1167/iovs.16-19520. [DOI] [PubMed] [Google Scholar]
- 132.Loane E, Nolan JM, O'Donovan O, Bhosale P, Bernstein PS, Beatty S. Transport and retinal capture of lutein and zeaxanthin with reference to age-related macular degeneration. Surv Ophthalmol. 2008;53:68–81. doi: 10.1016/j.survophthal.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 133.Stringham JM, Garcia PV, Smith PA, et al. Macular pigment and visual performance in low-light conditions. Invest Ophthalmol Vis Sci. 2015;56:2459–2468. doi: 10.1167/iovs.14-15716. [DOI] [PubMed] [Google Scholar]
- 134.Nolan JM, Loughman J, Akkali MC, et al. The impact of macular pigment augmentation on visual performance in normal subjects: COMPASS. Vision Res. 2011;51:459–469. doi: 10.1016/j.visres.2010.12.016. [DOI] [PubMed] [Google Scholar]
- 135.Renzi LM, Hammond BR., Jr The relation between the macular carotenoids, lutein and zeaxanthin, and temporal vision. Ophthalmic Physiol Opt. 2010;30:351–357. doi: 10.1111/j.1475-1313.2010.00720.x. [DOI] [PubMed] [Google Scholar]
- 136.Erdman JW, Jr, Smith JW, Kuchan MJ, et al. Lutein and brain function. Foods. 2015;4:547–564. doi: 10.3390/foods4040547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Mohn ES, Erdman JW, Jr, Kuchan MJ, Neuringer M, Johnson EJ. Lutein accumulates in subcellular membranes of brain regions in adult rhesus macaques: relationship to DHA oxidation products. PLoS One. 2017;12:e0186767. doi: 10.1371/journal.pone.0186767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Age-Related Eye Disease Study 2 Research Group. Lutein + zeaxanthin and omega-3 fatty acids for age-related macular degeneration: the Age-Related Eye Disease Study 2 (AREDS2) randomized clinical trial. JAMA. 2013;309:2005–2015. doi: 10.1001/jama.2013.4997. [DOI] [PubMed] [Google Scholar]
- 139.Snodderly DM, Brown PK, Delori FC, Auran JD. The macular pigment. I. Absorbence spectra, localization, and discrimination from other yellow pigments in primate retinas. Invest Ophthalmol Vis Sci. 1984;25:660–673. [PubMed] [Google Scholar]
- 140.Trieschmann M, van Kuijk FJ, Alexander R, et al. Macular pigment in the human retina: histological evaluation of localization and distribution. Eye (Lond) 2008;22:132–137. doi: 10.1038/sj.eye.6702780. [DOI] [PubMed] [Google Scholar]
- 141.Meyer Zu Westrup V, Dietzel M, Pauleikhoff D, Hense HW. The association of retinal structure and macular pigment distribution. Invest Ophthalmol Vis Sci. 2014;55:1169–1175. doi: 10.1167/iovs.13-12903. [DOI] [PubMed] [Google Scholar]
- 142.Distler C, Dreher Z. Glia cells of the monkey retina, II: Müller cells. Vision Res. 1996;36:2381–2394. doi: 10.1016/0042-6989(96)00005-3. [DOI] [PubMed] [Google Scholar]
- 143.Govetto A, Bhavsar KV, Virgili G, et al. Tractional abnormalities of the central foveal bouquet in epiretinal membranes: clinical spectrum and pathophysiological perspectives. Am J Ophthalmol. 2017;184:167–180. doi: 10.1016/j.ajo.2017.10.011. [DOI] [PubMed] [Google Scholar]
- 144.Delori FC, Goger DG, Keilhauer C, Salvetti P, Staurenghi G. Bimodal spatial distribution of macular pigment: evidence of a gender relationship. J Opt Soc Am A Opt Image Sci Vis. 2006;23:521–538. doi: 10.1364/josaa.23.000521. [DOI] [PubMed] [Google Scholar]
- 145.Dietzel M, Zeimer M, Heimes B, Pauleikhoff D, Hense HW. The ringlike structure of macular pigment in age-related maculopathy: results from the Muenster Aging and Retina Study (MARS) Invest Ophthalmol Vis Sci. 2011;52:8016–8024. doi: 10.1167/iovs.11-7610. [DOI] [PubMed] [Google Scholar]
- 146.Balaratnasingam C, Chae B, Remmer MH, et al. The spatial profile of macular pigments is related to the topological characteristics of the foveal avascular zone. Invest Ophthalmol Vis Sci. 2015;56:7859–7865. doi: 10.1167/iovs.15-17532. [DOI] [PubMed] [Google Scholar]
- 147.van der Veen RL, Fuijkschot J, Willemsen MA, Cruysberg JR, Berendschot TT, Theelen T. Patients with Sjogren-Larsson syndrome lack macular pigment. Ophthalmology. 2010;117:966–971. doi: 10.1016/j.ophtha.2009.10.019. [DOI] [PubMed] [Google Scholar]
- 148.Fuijkschot J, Cruysberg JR, Willemsen MA, Keunen JE, Theelen T. Subclinical changes in the juvenile crystalline macular dystrophy in Sjogren-Larsson syndrome detected by optical coherence tomography. Ophthalmology. 2008;115:870–875. doi: 10.1016/j.ophtha.2007.05.063. [DOI] [PubMed] [Google Scholar]
- 149.Sunness JS, Bressler NM, Tian Y, Alexander J, Applegate CA. Measuring geographic atrophy in advanced age-related macular degeneration. Invest Ophthalmol Vis Sci. 1999;40:1761–1769. [PubMed] [Google Scholar]
- 150.Dysli C, Wolf S, Zinkernagel MS. Autofluorescence lifetimes in geographic atrophy in patients with age-related macular degeneration. Invest Ophthalmol Vis Sci. 2016;57:2479–2487. doi: 10.1167/iovs.15-18381. [DOI] [PubMed] [Google Scholar]
- 151.Sauer L, Klemm M, Peters S, et al. Monitoring foveal sparing in geographic atrophy with fluorescence lifetime imaging ophthalmoscopy: a novel approach. Acta Ophthalmol. 2017;96:257–266. doi: 10.1111/aos.13587. [DOI] [PubMed] [Google Scholar]
- 152.Redmond TM. RPE65 takes on another role in the vertebrate retina. Proc Natl Acad Sci U S A. 2017;114:10818–10820. doi: 10.1073/pnas.1715064114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Syrbe S, Kuhrt H, Gartner U, et al. Muller glial cells of the primate foveola: an electron microscopical study. Exp Eye Res. 2017;167:110–117. doi: 10.1016/j.exer.2017.12.004. [DOI] [PubMed] [Google Scholar]
- 154.Li B, Vachali P, Frederick JM, Bernstein PS. Identification of StARD3 as a lutein-binding protein in the macula of the primate retina. Biochemistry. 2011;50:2541–2549. doi: 10.1021/bi101906y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Bhosale P, Larson AJ, Frederick JM, Southwick K, Thulin CD, Bernstein PS. Identification and characterization of a pi isoform of glutathione S-transferase (GSTP1) as a zeaxanthin-binding protein in the macula of the human eye. J Biol Chem. 2004;279:49447–49454. doi: 10.1074/jbc.M405334200. [DOI] [PubMed] [Google Scholar]
- 156.Bohn T, Desmarchelier C, Dragsted LO, et al. Host-related factors explaining interindividual variability of carotenoid bioavailability and tissue concentrations in humans. Mol Nutr Food Res. 2017;61:1600685. doi: 10.1002/mnfr.201600685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Landrum JT, Bone RA, Joa H, Kilburn MD, Moore LL, Sprague KE. A one year study of the macular pigment: the effect of 140 days of a lutein supplement. Exp Eye Res. 1997;65:57–62. doi: 10.1006/exer.1997.0309. [DOI] [PubMed] [Google Scholar]
- 158.Berendschot TT, Goldbohm RA, Klopping WA, van de Kraats J, van Norel J, van Norren D. Influence of lutein supplementation on macular pigment, assessed with two objective techniques. Invest Ophthalmol Vis Sci. 2000;41:3322–3326. [PubMed] [Google Scholar]
- 159.Bernstein PS, Zhao DY, Sharifzadeh M, Ermakov IV, Gellermann W. Resonance Raman measurement of macular carotenoids in the living human eye. Arch Biochem Biophys. 2004;430:163–169. doi: 10.1016/j.abb.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 160.Neuringer M, Sandstrom MM, Johnson EJ, Snodderly DM. Nutritional manipulation of primate retinas, I: effects of lutein or zeaxanthin supplements on serum and macular pigment in xanthophyll-free rhesus monkeys. Invest Ophthalmol Vis Sci. 2004;45:3234–3243. doi: 10.1167/iovs.02-1243. [DOI] [PubMed] [Google Scholar]
- 161.Johnson EJ, Chung HY, Caldarella SM, Snodderly DM. The influence of supplemental lutein and docosahexaenoic acid on serum, lipoproteins, and macular pigmentation. Am J Clin Nutr. 2008;87:1521–1529. doi: 10.1093/ajcn/87.5.1521. [DOI] [PubMed] [Google Scholar]
- 162.Stringham JM, Hammond BR. Macular pigment and visual performance under glare conditions. Optom Vis Sci. 2008;85:82–88. doi: 10.1097/OPX.0b013e318162266e. [DOI] [PubMed] [Google Scholar]
- 163.Murray IJ, Makridaki M, van der Veen RL, Carden D, Parry NR, Berendschot TT. Lutein supplementation over a one-year period in early AMD might have a mild beneficial effect on visual acuity: the CLEAR study. Invest Ophthalmol Vis Sci. 2013;54:1781–1788. doi: 10.1167/iovs.12-10715. [DOI] [PubMed] [Google Scholar]
- 164.Dawczynski J, Jentsch S, Schweitzer D, Hammer M, Lang GE, Strobel J. Long term effects of lutein, zeaxanthin and omega-3-LCPUFAs supplementation on optical density of macular pigment in AMD patients: the LUTEGA study. Graefes Arch Clin Exp Ophthalmol. 2013;251:2711–2723. doi: 10.1007/s00417-013-2376-6. [DOI] [PubMed] [Google Scholar]
- 165.Bovier ER, Renzi LM, Hammond BR. A double-blind, placebo-controlled study on the effects of lutein and zeaxanthin on neural processing speed and efficiency. PLoS One. 2014;9:e108178. doi: 10.1371/journal.pone.0108178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Hammond BR, Fletcher LM, Roos F, Wittwer J, Schalch W. A double-blind, placebo-controlled study on the effects of lutein and zeaxanthin on photostress recovery, glare disability, and chromatic contrast. Invest Ophthalmol Vis Sci. 2014;55:8583–8589. doi: 10.1167/iovs.14-15573. [DOI] [PubMed] [Google Scholar]
- 167.Bovier ER, Hammond BR. A randomized placebo-controlled study on the effects of lutein and zeaxanthin on visual processing speed in young healthy subjects. Arch Biochem Biophys. 2015;572:54–57. doi: 10.1016/j.abb.2014.11.012. [DOI] [PubMed] [Google Scholar]
- 168.Iannaccone A, Carboni G, Forma G, Mutolo MG, Jennings BJ. Macular pigment optical density and measures of macular function: test-retest variability, cross-sectional correlations, and findings from the zeaxanthin pilot study of response to supplementation (ZEASTRESS-Pilot) Foods. 2016;5:E32. doi: 10.3390/foods5020032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Korobelnik JF, Rougier MB, Delyfer MN, et al. Effect of dietary supplementation with lutein, zeaxanthin, and omega-3 on macular pigment: a randomized clinical trial. JAMA Ophthalmol. 2017;135:1259–1266. doi: 10.1001/jamaophthalmol.2017.3398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Malinow MR, Feeney-Burns L, Peterson LH, Klein ML, Neuringer M. Diet-related macular anomalies in monkeys. Invest Ophthalmol Vis Sci. 1980;19:857–863. [PubMed] [Google Scholar]
- 171.Fine BS, Kwapien RP. Pigment epithelial windows and drusen: an animal model. Invest Ophthalmol Vis Sci. 1978;17:1059–1068. [PubMed] [Google Scholar]
- 172.Fine BS. Lipoidal degeneration of the retinal pigment epithelium. Am J Ophthalmol. 1981;91:469–473. doi: 10.1016/0002-9394(81)90234-8. [DOI] [PubMed] [Google Scholar]
- 173.Anderson MD, Dawson WW, Martinez-Gonzalez J, Curcio CA. Drusenoid lesions and lipid-filled retinal pigment epithelium cells in a rhesus macula. Vet Ophthalmol. 2006;9:201–207. doi: 10.1111/j.1463-5224.2006.00463.x. [DOI] [PubMed] [Google Scholar]
- 174.Yiu G, Wang Z, Munevar C, et al. Comparison of chorioretinal layers in rhesus macaques using spectral-domain optical coherence tomography and high-resolution histological sections. Exp Eye Res. 2018;168:69–76. doi: 10.1016/j.exer.2018.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Wang W, Connor SL, Johnson EJ, Klein ML, Hughes S, Connor WE. Effect of dietary lutein and zeaxanthin on plasma carotenoids and their transport in lipoproteins in age-related macular degeneration. Am J Clin Nutr. 2007;85:762–769. doi: 10.1093/ajcn/85.3.762. [DOI] [PubMed] [Google Scholar]
- 176.Zannis VI, Chroni A, Krieger M. Role of apoA-I, ABCA1, LCAT, and SR-BI in the biogenesis of HDL. J Mol Med. 2006;84:276–294. doi: 10.1007/s00109-005-0030-4. [DOI] [PubMed] [Google Scholar]
- 177.Connor WE, Duell PB, Kean R, Wang Y. The prime role of HDL to transport lutein into the retina: evidence from HDL-deficient WHAM chicks having a mutant ABCA1 transporter. Invest Ophthalmol Vis Sci. 2007;48:4226–4231. doi: 10.1167/iovs.06-1275. [DOI] [PubMed] [Google Scholar]
- 178.During A, Doraiswamy S, Harrison EH. Xanthophylls are preferentially taken up compared to beta-carotene by retinal cells via a scavenger receptor BI-dependent mechanism. J Lipid Res. 2008;49:1715–1724. doi: 10.1194/jlr.M700580-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Thomas SE, Harrison EH. Mechanisms of selective delivery of xanthophylls to retinal pigment epithelial cells by human lipoproteins. J Lipid Res. 2016;57:1865–1878. doi: 10.1194/jlr.M070193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Vachali PP, Besch BM, Gonzalez-Fernandez F, Bernstein PS. Carotenoids as possible interphotoreceptor retinoid-binding protein (IRBP) ligands: a surface plasmon resonance (SPR) based study. Arch Biochem Biophys. 2013;539:181–186. doi: 10.1016/j.abb.2013.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Shyam R, Gorusupudi A, Nelson K, Horvath MP, Bernstein PS. RPE65 has an additional function as the lutein to meso-zeaxanthin isomerase in the vertebrate eye. Proc Natl Acad Sci U S A. 2017;114:10882–10887. doi: 10.1073/pnas.1706332114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Klein R, Klein BE, Franke T. The relationship of cardiovascular disease and its risk factors to age-related maculopathy: the Beaver Dam Eye Study. Ophthalmology. 1993;100:406–414. doi: 10.1016/s0161-6420(93)31634-9. [DOI] [PubMed] [Google Scholar]
- 183.Klein R, Klein BE, Tomany SC, Cruickshanks KJ. The association of cardiovascular disease with the long-term incidence of age-related maculopathy: the Beaver Dam Eye Study. Ophthalmology. 2003;110:636–643. doi: 10.1016/S0161-6420(02)01448-3. [DOI] [PubMed] [Google Scholar]
- 184.van Leeuwen R, Klaver CC, Vingerling JR, et al. Cholesterol and age-related macular degeneration: is there a link? Am J Ophthalmol. 2004;137:750–752. doi: 10.1016/j.ajo.2003.09.015. [DOI] [PubMed] [Google Scholar]
- 185.Jonasson F, Fisher DE, Eiriksdottir G, et al. Five-year incidence, progression, and risk factors for age-related macular degeneration: the age, gene/environment susceptibility study. Ophthalmology. 2014;121:1766–1772. doi: 10.1016/j.ophtha.2014.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Saunier V, Merle BMJ, Delyfer MN, et al. Incidence of and risk factors associated with age-related macular degeneration: four-year follow-up from the ALIENOR Study. JAMA Ophthalmol. 2018;136:473–481. doi: 10.1001/jamaophthalmol.2018.0504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Hyman L, Schachat AP, He Q, Leske MC. Hypertension, cardiovascular disease, and age-related macular degeneration. Arch Ophthalmol. 2000;117:351–358. doi: 10.1001/archopht.118.3.351. [DOI] [PubMed] [Google Scholar]
- 188.Liu DJ, Peloso GM, Yu H, et al. Exome-wide association study of plasma lipids in >300,000 individuals. Nat Genet. 2017;49:1758–1766. doi: 10.1038/ng.3977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Loane E, Nolan JM, Beatty S. The respective relationships between lipoprotein profile, macular pigment optical density, and serum concentrations of lutein and zeaxanthin. Invest Ophthalmol Vis Sci. 2010;51:5897–5905. doi: 10.1167/iovs.09-4878. [DOI] [PubMed] [Google Scholar]
- 190.Klaver CC, Kliffen M, van Duijn CM, et al. Genetic association of apolipoprotein E with age-related macular degeneration. Am J Hum Genet. 1998;63:200–206. doi: 10.1086/301901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Souied EH, Benlian P, Amouyel P, et al. The epsilon4 allele of the apolipoprotein E gene as a potential protective factor for exudative age-related macular degeneration. Am J Ophthalmol. 1998;125:353–359. doi: 10.1016/s0002-9394(99)80146-9. [DOI] [PubMed] [Google Scholar]
- 192.Neale BM, Fagerness J, Reynolds R, et al. Genome-wide association study of advanced age-related macular degeneration identifies a role of the hepatic lipase gene (LIPC) Proc Natl Acad Sci U S A. 2010;107:7395–7400. doi: 10.1073/pnas.0912019107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Chen W, Stambolian D, Edwards AO, et al. Genetic variants near TIMP3 and high-density lipoprotein-associated loci influence susceptibility to age-related macular degeneration. Proc Natl Acad Sci U S A. 2010;107:7401–7406. doi: 10.1073/pnas.0912702107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Fauser S, Smailhodzic D, Caramoy A, et al. Evaluation of serum lipid concentrations and genetic variants at high-density lipoprotein metabolism loci and TIMP3 in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2011;52:5525–5528. doi: 10.1167/iovs.10-6827. [DOI] [PubMed] [Google Scholar]
- 195.Fritsche LG, Chen W, Schu M, et al. Seven new loci associated with age-related macular degeneration. Nat Genet. 2013;45:433–439. doi: 10.1038/ng.2578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.McKay GJ, Loane E, Nolan JM, et al. Investigation of genetic variation in scavenger receptor class B, member 1 (SCARB1) and association with serum carotenoids. Ophthalmology. 2013;120:1632–1640. doi: 10.1016/j.ophtha.2013.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Yu Y, Reynolds R, Fagerness J, Rosner B, Daly MJ, Seddon JM. Association of variants in the LIPC and ABCA1 genes with intermediate and large drusen and advanced age-related macular degeneration. Invest Ophthalmol Vis Sci. 2011;52:4663–4670. doi: 10.1167/iovs.10-7070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Burgess S, Davey Smith G. Mendelian randomization implicates high-density lipoprotein cholesterol-associated mechanisms in etiology of age-related macular degeneration. Ophthalmology. 2017;124:1165–1174. doi: 10.1016/j.ophtha.2017.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Fan Q, Maranville JC, Fritsche L, et al. HDL-cholesterol levels and risk of age-related macular degeneration: a multiethnic genetic study using Mendelian randomization. Int J Epidemiol. 2017;46:1891–1902. doi: 10.1093/ije/dyx189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Wang Y, Wang M, Zhang X, et al. The association between the lipids levels in blood and risk of age-related macular degeneration. Nutrients. 2016;8:663. doi: 10.3390/nu8100663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Anderson DH, Ozaki S, Nealon M, et al. Local cellular sources of apolipoprotein E in the human retina and retinal pigmented epithelium: implications for the process of drusen formation. Am J Ophthalmol. 2001;131:767–781. doi: 10.1016/s0002-9394(00)00961-2. [DOI] [PubMed] [Google Scholar]
- 202.Kurumada S, Onishi A, Imai H, Ishii K, Kobayashi T, Sato SB. Stage-specific association of apolipoprotein A-I and E in developing mouse retina. Invest Ophthalmol Vis Sci. 2007;48:1815–1823. doi: 10.1167/iovs.06-0902. [DOI] [PubMed] [Google Scholar]
- 203.Lee SJ, Kim JH, Chung MJ, et al. Human apolipoprotein E2 transgenic mice show lipid accumulation in retinal pigment epithelium and altered expression of VEGF and bFGF in the eyes. J Microbiol Biotechnol. 2007;17:1024–1030. [PubMed] [Google Scholar]
- 204.Levy O, Calippe B, Lavalette S, et al. Apolipoprotein E promotes subretinal mononuclear phagocyte survival and chronic inflammation in age-related macular degeneration. EMBO Mol Med. 2015;7:211–226. doi: 10.15252/emmm.201404524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Tserentsoodol N, Gordiyenko NV, Pascual I, Lee JW, Fliesler SJ, Rodriguez IR. Intraretinal lipid transport is dependent on high density lipoprotein-like particles and class B scavenger receptors. Mol Vis. 2006;12:1319–1333. [PubMed] [Google Scholar]
- 206.Duncan KG, Hosseini K, Bailey KR, et al. Expression of reverse cholesterol transport proteins ATP-binding cassette A1 (ABCA1) and scavenger receptor BI (SR-BI) in the retina and retinal pigment epithelium. Br J Ophthalmol. 2009;93:1116–1120. doi: 10.1136/bjo.2008.144006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Claudepierre T, Paques M, Simonutti M, et al. Lack of Niemann-Pick type C1 induces age-related degeneration in the mouse retina. Mol Cell Neurosci. 2010;43:164–176. doi: 10.1016/j.mcn.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 208.Ananth S, Gnana-Prakasam JP, Bhutia YD, et al. Regulation of the cholesterol efflux transporters ABCA1 and ABCG1 in retina in hemochromatosis and by the endogenous siderophore 2,5-dihydroxybenzoic acid. Biochim Biophys Acta. 2014;1842:603–612. doi: 10.1016/j.bbadis.2014.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Booij JC, van Soest S, Swagemakers SM, et al. Functional annotation of the human retinal pigment epithelium transcriptome. BMC Genomics. 2009;10:164. doi: 10.1186/1471-2164-10-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Li M, Jia C, Kazmierkiewicz KL, et al. Comprehensive analysis of gene expression in human retina and supporting tissues. Hum Mol Genet. 2014;23:4001–4014. doi: 10.1093/hmg/ddu114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Hayes KC, Lindsey S, Stephan ZF, Brecker D. Retinal pigment epithelium possesses both LDL and scavenger receptor activity. Invest Ophthalmol Vis Sci. 1989;30:225–232. [PubMed] [Google Scholar]
- 212.Chan C, Leung I, Lam KW, Tso MO. The occurrence of retinol and carotenoids in human subretinal fluid. Curr Eye Res. 1998;17:890–895. doi: 10.1076/ceyr.17.9.890.5141. [DOI] [PubMed] [Google Scholar]
- 213.Li B, Vachali PP, Gorusupudi A, et al. Inactivity of human beta,beta-carotene-9′,10′-dioxygenase (BCO2) underlies retinal accumulation of the human macular carotenoid pigment. Proc Natl Acad Sci U S A. 2014;111:10173–10178. doi: 10.1073/pnas.1402526111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Dinculescu A, Min SH, Dyka FM, et al. Pathological effects of mutant C1QTNF5 (S163R) expression in murine retinal pigment epithelium. Invest Ophthalmol Vis Sci. 2015;56:6971–6980. doi: 10.1167/iovs.15-17166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Stanton JB, Marmorstein AD, Zhang Y, Marmorstein LY. Deletion of EFEMP1 is protective against the development of sub-RPE deposits in mouse eyes. Invest Ophthalmol Vis Sci. 2017;58:1455–1461. doi: 10.1167/iovs.16-20955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Jiang M, Esteve-Rudd J, Lopes VS, et al. Microtubule motors transport phagosomes in the RPE, and lack of KLC1 leads to AMD-like pathogenesis. J Cell Biol. 2015;210:595–611. doi: 10.1083/jcb.201410112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Bernstein PS, Ahmed F, Liu A, et al. Macular pigment imaging in AREDS2 participants: an ancillary study of AREDS2 subjects enrolled at the Moran Eye Center. Invest Ophthalmol Vis Sci. 2012;53:6178–6186. doi: 10.1167/iovs.12-10275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Bhosale P, Li B, Sharifzadeh M, et al. Purification and partial characterization of a lutein-binding protein from human retina. Biochemistry. 2009;48:4798–4807. doi: 10.1021/bi9004478. [DOI] [PubMed] [Google Scholar]
- 219.Anderson DMG, Ablonczy Z, Koutalos Y, et al. High resolution MALDI imaging mass spectrometry of retinal tissue lipids. J Am Soc Mass Spectrom. 2014;25:1394–1403. doi: 10.1007/s13361-014-0883-2. [DOI] [PMC free article] [PubMed] [Google Scholar]