Abstract
Osteoporosis and osteopenia impact more than 54 million Americans, resulting in significant morbidity and mortality. Alterations in bone remodeling are the hallmarks for osteoporosis, and thus the development of novel treatments that will prevent or treat bone diseases would be clinically significant, and improve the quality of life for these patients. Bone remodeling involves the removal of old bone by osteoclasts and the formation of new bone by osteoblasts. This process is tightly coupled, and is essential for the maintenance of bone strength and integrity. Since the osteoclast is the only cell capable of bone resorption, the development of drugs to treat bone disorders has primarily focused on reducing osteoclast differentiation, maturation, and bone resorption mechanisms, and there are few treatments that actually increase bone formation. Evidence from observational, experimental, and clinical studies demonstrate a positive link between naturally occurring compounds and improved indices of bone health. While many natural extracts and compounds are reported to have beneficial effects on bone, only resveratrol, sulforaphane, specific phenolic acids and anthocyanins, have been shown to both increase bone formation and reduce resorption through their effects on the bone epigenome. Each of these compounds alters specific aspects of the bone epigenome to improve osteoblast differentiation, reduce osteoblast apoptosis, improve bone mineralization, and reduce osteoclast differentiation and function. This review focuses on these specific natural compounds and their epigenetic regulation of bone remodeling.
Keywords: anthocyanins, DNA methylation, ferulic acid, non-coding RNA, osteoblast, osteoclast, resveratrol, sulforaphane, syringic acid
Graphical Abstract
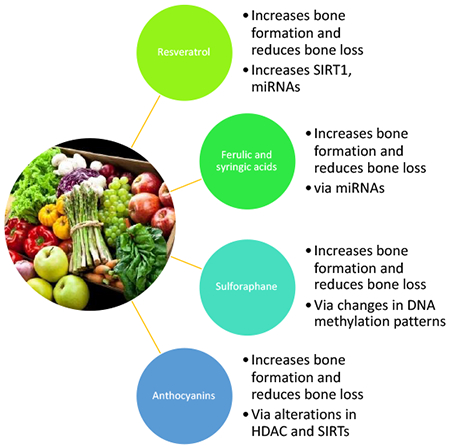
3.0. Introduction
Bone formation in mammals develops from three specific cell types through a variety of signaling mechanisms [1–4]. The facial bones in the skull are formed by the neural crest, while the axial skeleton of the trunk, base of the skull, parietal bones, ribs and vertebrae are formed from the paraxial mesoderm. The lateral plate mesoderm, a pair of neurula-stage mesodermal sheets that are located lateral to the intermediate mesoderm, forms the appendicular limb skeleton [1–4]. In the face and skull, osteogenesis occurs through the process of intramembranous ossification where mesenchymal stem cell derived osteoblasts condense and directly form bone [2]. The remainder of the boney skeleton is formed by secondary ossification of cartilage templates by the process of endochondral ossification [1–4]. During endochondral ossification bone develops through the intermediate step of replacing hyaline cartilage. The cartilage does not form bone, but instead serves as a template that is entirely replaced by new bone, and therefore this process takes a longer time than intramembranous ossification [1–4]. Once formed, the processes of bone modeling and remodeling involve bone formation or bone resorption on bone surface. Both processes impact the structure of bone, however remodeling also impacts microdamage, mineralization, and collagen cross-linking [4].
3.1. Bone remodeling
Bone remodeling in vertebrates is a tightly coupled and dynamic process in which old bone is removed by osteoclasts, and new bone is formed by osteoblasts [5–7]. This process is highly integrated and is essential for the maintenance of bone strength and integrity [8, 9]. Bone remodeling assures the plasticity of bone and coupling imbalances may lead to increased bone mass (osteopetrosis) or reduced bone mass (osteoporosis).
Osteoblasts are the primary bone producing cells and differentiate derived from mesenchymal stem cells (MSCs) [1,10]. Osteoblasts biosynthesize extracellular proteins including collagen, osteocalcin and other proteins that make up the major components of bone [10]. These cells play a central role in the ossification process by secreting the extracellular matrix proteins and increasing the mineralization of the bone matrix [1, 10]. The mineralized bone matrix provides strength to the skeleton, as well as supplies the necessary minerals and growth factors.
The differentiation of MSCs to osteoblast lineage cells is regulated by many transcription factors, including Runt-related transcription factor 2 (Runx2) which has many targets and upstream regulators, including Sp7/Osterix [2–3, 11]. Experiments using transgenic Runx2- and Sp7/Osx-null mutant rodents, showed a complete lack of bones, while in humans, these mutations are associated with serious bone disorders [3, 12]. Other upstream regulators of osteoblast differentiation also include Wnt/notch, Sox9, Msx2 and hedgehog signaling, and other co-activators include bone morphogenic proteins and parathyroid hormone. Osteoblast differentiation is further supported by vitamin D and histone deacetylases (HDACs) [3,12]. Mature osteoblasts also play a pivotal role in the regulation of osteoclast differentiation (osteoclastogenesis), blood Ca2+ homeostasis, and the control of Ca2+ release by osteoclasts. Thus, determining the mechanisms that regulate osteoblast differentiation and function are of critical importance for the clinical prevention and management of osteopenia and osteoporosis, as well as common skeletal disorders that are associated with diminished osteoblast production and function, such as in seen in chronic alcoholism [2,3,11].
Osteoclasts are large multinucleate bone cells that are derived from monocyte/macrophage lineage precursor cells [13]. Osteoclasts are the only cells that are capable of degrading mineralized bone, and are important for bone remodeling and calcium homeostasis [13–15]. The bone resorption activities of osteoclasts are regulated by a wide variety of factors and signaling pathways, including bone morphogenic proteins, calcitonin, the receptor activator of nuclear factor kappa-B (NF-κβ) ligand (RANKL), Wingless/Integrated signaling pathway (Wnt), interleukins (IL)-1, IL-6, IL-11, macrophage colony-stimulating factor (M-CSF), and parathyroid hormone (PTH) [13–15]. Excessive osteoclast activity plays an important role in the development of many bone pathologies including osteoporosis, osteopetrosis, rheumatoid arthritis, bone loss in alcoholism, and metastatic tumor disease [13–15]. Since the osteoclast is the only cell able to induce bone resorption, investigations of bone disease pathology have focused primarily on the mechanisms of osteoclast differentiation, maturation and function, which has led to the development of drugs that reduce osteoclastogenesis, such as the bisphosphonates.
3.2. Diseases of bone remodeling
In adults, bone loss may be induced by an increase of bone remodeling, where there is a high turnover of bone due to increased resorption [9]. Bone loss may also be caused by uncoupling of bone remodeling, where there is an increased in bone resorption due to increased osteoclast activity, and reduced formation (as in alcoholism) [9, 16]. The third type of bone loss involves reduced bone remodeling when bone resorption, although reduced, still outpaces formation [9, 16]. Uncoupled bone remodeling leads to very rapid (e.g., months) bone loss as compared with bone loss resulting from a remodeling imbalance (years) [9, 16]. Interestingly, heavy alcohol consumption also reduces bone mineral density, impairs bone quality and increases fracture risk by uncoupling bone formation [16]. Ethanol exposure decreases the indices of osteoblast activity and differentiation in cultured human osteoblasts, but also increases the activity of osteoclasts thereby uncoupling remodeling [16]. These data are consistent with findings from chronic alcoholics [17]. During uncoupling of bone remodeling, the proliferation and function of osteoblasts is reduced, while the activity of osteoclasts is increased, thus increased bone resorption is not replaced with newly bone formation.
Osteoporosis is the most common bone remodeling disease that results from an increase in bone resorption and a reduction in bone formation, leading to an increased risk of fragility fractures [18–20]. Osteoporosis and osteopenia affect over 54 million Americans, with estimated healthcare costs of > $20 billion USD annually [21, 22]. Osteoporosis has become a global epidemic, and current estimates suggest that > 200 million people are currently living with this disease [22]. Statistics from the International Osteoporosis Foundation suggest that one in three women over the age of 50 years, and one in five men will suffer from osteoporotic fractures during their lifetime, resulting in loss of independence, health and productivity [22]. However, there are many other serious remodeling bone disorders, including Paget’s disease, that affects ~1 million Americans; renal osteodystrophy associated with dialysis therapy; alcohol-associated bone loss; osteopetrosis and non-inherited (dietary) rickets (rare) [23, 24].
Osteopetrosis refers to a group of rare heritable metabolic bone disorders that affect > 14,000 Americans annually [24]. The genetic mutations associated with osteopetrosis are associated with genes involved in the differentiation and function of osteoclasts, leading to abnormal or too few osteoclasts. These diseases usually present in clinic as abnormally dense bone that is prone to fracture [24]. The symptoms and severity of osteopetrosis vary greatly depending on the age of onset and type, but most cases include abnormal cortical bone morphology, and abnormal rib and pelvis bone ossification, as well as abnormal cranial nerve morphology [24]. There are several major types of osteopetrosis that are normally diagnosed by their pattern of inheritance and include: autosomal dominant, autosomal recessive, or X-linked [24]. In this disease, there is an impairment of bone modeling and remodeling due to the failure of osteoclasts to resorb bone. This alteration in bone resorption leads to an abnormal increase in bone mass, but an increased risk of fracture [24]. Treatment for osteopetrosis is based on the individual patient and specific symptoms but includes nutritional support, vitamin D, gamma interferon, bone marrow transplant and other surgeries. Therefore, a better understanding of the molecular and epigenetic mechanisms that regulate bone remodeling will significantly impact these patients as well, and the development of safe and effective drug or dietary treatments would be clinically important.
3.3. Treatment of bone remodeling disorders
3.3.1. Bisphosphonates
Since the osteoclast is the only cell capable of bone resorption, analyses of osteoclast differentiation, maturation, regulation, and bone resorption mechanisms have remained the central focus for new therapeutic approaches for osteoporosis, and are the primary target of the bisphosphonates [25–28]. The development of the bisphosphonates (BPPs; also known as diphosphonates) from inorganic pyrophosphates occurred in the late 1960s. Studies have shown that BPPs are selectively absorbed onto the bone mineral surface where they inhibit the function of osteoclasts, and thereby reduce bone resorption [25–28]. The BPPs are made up of two groups: the non-amino (non-nitrogen) containing compounds (etidronate and clondronate) with interfere with ATP-dependent intracellular pathways and the amino (nitrogen) containing compounds, such as pamidronate, alendronate, ibandronate and zoledronate [27–28]. These compounds inhibit key enzymes in the mevalonate pathway such as farnesyl pyrophosphate synthase that is required for the post-translational prenylation of GTPase which is essential for intracellular signaling in osteoclasts [25–28]. The BPPs have played a fundamental role in the management of osteoporosis but have also some value in treating osteogenesis imperfecta, Paget’s disease and bone disorders associated with cancer [26–28].
Adverse events associated with BPP use include increased risk of osteonecrosis of the jaw, atypical femur fractures, hypocalcemia, gastro-intestinal side effects and atrial fibrillation [25–28]. In addition, anti-resorption agents, such as bisphosphonates, are reported to also inhibit bone formation by osteoblasts [25–28]. This is problematic since bone strength and integrity are dependent on a balance of both bone formation and resorption, and this balance is determined primarily by the function of both osteoblasts and osteoclasts [26]. Therefore, future development of new therapeutic options for bone loss should focus on both bone resorption and bone formation.
3.3.2. Bone anabolic agents
Currently, there are few treatments known to build bone, but these include the anabolic agents, estrogen replacement therapies, and intermittent parathyroid hormone (IPTH) [29–32]. While all of these treatments are available, they have serious adverse events associated with their use, and thus are not routinely recommended for the treatment of osteoporosis and other bone remodeling disorders [31]. IPTH is only approved for treatment of severe osteoporosis in the U.S., due to serious adverse events, and its potential to increase the development of osteosarcoma [32]. Several anabolic agents have been approved for use in severe cases of osteoporosis, but the use of these agents is restricted to only those patients in which other anti-osteoporosis drugs have failed, or in patients that are intolerant to these agents or have conditions that are contraindicated with other osteoporosis agents, as well as glucocorticoid-induced osteoporosis [33, 34]. The US Food and Drug Administration has approved a number of new anabolic agents for osteoporosis, including teriparatide and abaloparatide [34]. Both teriparatide and abaloparatide have been shown to significantly reduce vertebral and non-vertebral fractures in clinical trials, however since both are administered by subcutaneous injection, their daily use by patients is more difficult than oral drugs [34]. The adverse events associated with these two drugs are similar however, abaloparatide has somewhat higher incidence of serious adverse events drugs including heart palpitations and severe nausea, while teriparatide has a higher incidence of hypercalcemia and injection site hematomas. Both drugs have similar risks for other adverse events and are carcinogenic (bone tumors) in rodents [34]. One new anabolic drug, Romozosumab, is also under review by the FDA. Romozosumab is a monoclonal antibody that has been shown to bind to, and inhibit, the activity of sclerostin, thereby reducing bone resorption and increasing bone formation [35]. Sclerostin is a glycoprotein produced in osteocytes that, when activated, inhibits canonical Wnt signaling and bone formation. When Romozosumab binds to sclerostin it increases the interaction of the Wnt ligands with their co-receptors, resulting in increased in bone formation and improved bone mineral density (BMD). In clinical trials, Romozosumab, in combination with alendronate, has been shown to significantly reduce the risk of fracture in postmenopausal women after 12 months of treatment [35]. However, considering that the use of these agents is restricted, as well as the frequency and potential serious nature of the adverse events associated with these new drugs, and the fact that they require daily injections or a doctor’s visit, new safer preventative and/or therapeutic agents for bone loss are urgently needed.
4.0. Epigenetic regulation of bone formation
Along with dietary and lifestyle factors involved in bone health, genetic and epigenetic factors also play a role in the regulation of bone remodeling (Figure 1) [36–38]. The mechanisms by which organisms are able to alter gene expression and modify cellular processes in response to external and internal environmental stimuli including aging, diet, mechanical stress, obesity-related adipokines and inflammatory cytokines are known as epigenetic regulation [36–37]. Epigenetics is defined as changes in the genome that are heritable, and alterations of gene expression and/or function occur without alterations in underlying genomic DNA [37]. Over the past ten years, numerous investigations have suggested that epigenetic alterations play a primary role in normal bone formation and function, as well as in bone remodeling and pathogenesis [36–38]. Growing evidence suggests that epigenetic dysregulation and inappropriate gene expression or silencing may be the missing links between genetic and environmental factors and the increased risk of osteoporosis and bone fracture [38]. Epigenetic regulation of bone formation refers to the mechanisms that impact gene expression such as posttranslational histone modifications, miRNA-mediated post-transcriptional regulation and DNA methylation [reviewed in 36–38].
Figure 1.
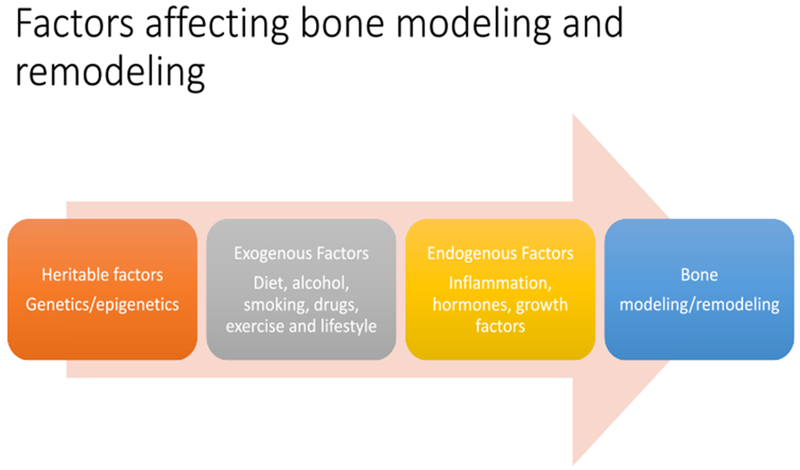
Heritable, endogenous and exogenous factors affecting bone modeling/remodeling in vertebrates.
4.1. DNA methylation
Briefly, DNA methylation involves the transfer of a methyl group from S-adenosyl-L-methionine, a methyl precursor, to the 5 position of cytosines in CpG dinucleotides [36–39]. DNA methylation silences gene expression by preventing key transcription factors from binding, and thus altering a wide range of cellular processes in bone. The silencing of transcription by DNA methylation also increases the recruitment of methyl CpG-binding transcriptional repressors inducing a condensed chromatin state [36–39]. DNA methylation is catalyzed by DNA methyltransferases (DNMTs), which are encoded by four DNMT genes: DNMT1, DNMT2, DNMT3A and DNMT3B [38, 40]. In bone, published studies have suggested that DNA methylation plays an important role in the control of genes related to both osteoblast and osteoclast differentiation, including Wnt, RANK/RANKL, and other key signaling pathways [reviewed in 36, 40]. Epigenetic modifications, such as reversible histone modification and DNA methylation, regulate gene transcription and are associated with bone diseases, including osteoporosis [41–43].
4.2. Histone modifications
Histone modifications are enzymatically catalyzed by histone methyltransferases (HMTs), histone demethylases (HDMs) histone acetyltransferases (HATs), and histone deacetylases (HDACs) [36–37, 44–49; Figure 2]. These enzymes post-translationally modify the N-terminal of histones and these include modifications in acetylation, methylation, phosphorylation, biotinylation and ubiquitination [36–37, 44–47]. Histone methyltransferases add methyl groups to lysine and/or arginine residues in histones, while HDMs remove the methyl groups. Histone acetyltransferases catalyze the addition of an acetyl group to lysine residues of histones and increases transcription. Whereas HDACs repress transcription by deacetylation, altering the structural confirmation of histones and increasing the affinity of the lysine side chain for negatively charged DNA, and increasing chromatin condensation [36–37, 45–46]. In mammals, there are 18 histone deacetylase enzymes that are subdivided into different classes according to their homology with yeast HDACs [36–37, 45–47]. Class I includes the HDACs 1, 2, 3, and 8, which are located in the nucleus, and ubiquitously expressed in human cell lines and tissues. Class II are subdivided into two subclasses: IIa (HDAC 4, 7, and 9) and IIb (HDAC 6 and 10) and can move between the nucleus and cytoplasm. Class III HDACs, also known as the sirtuins [45–46]. The sirtuins (silent information regulator 2-Sir2, SIRTs) are a group of highly conserved nicotinamide adenine dinucleotide (NAD)-dependent enzymes [45–46]. SIRTs deacetylate histones and non-histone proteins, including important transcription factors, proteins and enzymes that play a role in chromatin architecture, gene expression, as well as cellular metabolism [36–37, 45–47]. There are currently seven known SIRTs (SIRT1-7) in mammals, of which SIRT1 is located in the nucleus and SIRT3 is located in the mitochondria [45–46]. In terms of bone, HDACs deacetylate lysine residues of non-histone proteins, including osterix/sp7, Runx2 and other transcription factors affecting their stability and/or cellular localization to influence gene expression [36–39].
Figure 2.
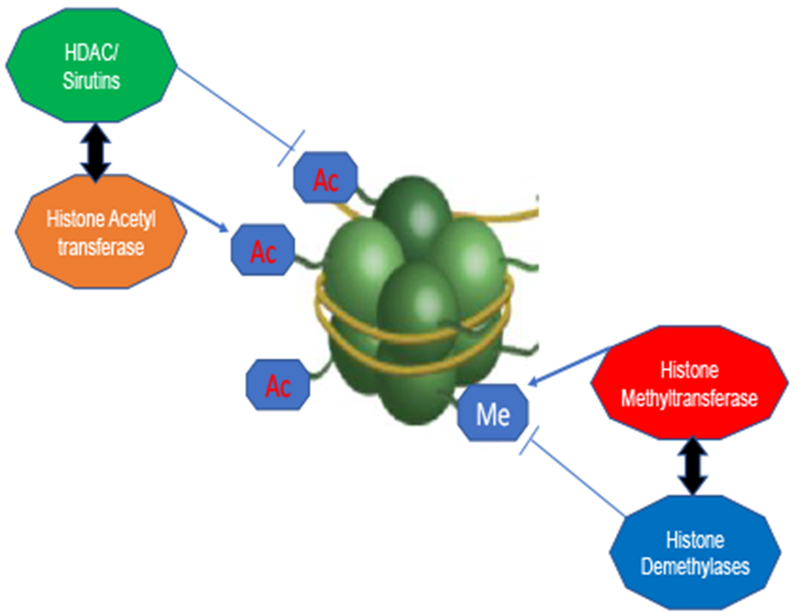
A simplified schematic diagram depicting an overview of histone-modifying enzymes, focusing primarily on histone deacetylases (HDACs), histone acetyltransferases (HATs), histone methyl transferases (HTMs), and histone demethylases (HDMs) and their enzymatic effects on chromatin structure. Sirtuins are a subclass of HDACs (class III) and are a NAD+-dependent deacetylases.
In recent years, the differentiation of mesenchymal stem cells to pre-mature and mature osteoblasts has been shown to also be regulated by epigenetic mechanisms, including the alteration nucleosome organization by methylation/acetylation of the N-terminal of histone 3 lysine 27 (H3K27) at the transcriptional start site of osteoblast specific genes [44, 48–49]. These enzymatic modifications alter promoter accessibility and the transcription of key genes needed for osteoblast differentiation. The differentiation of MSCs to pre-mature and mature osteoblasts is associated with the upregulation of Runx2, osteocalcin, and OPN [48–49]. These changes in gene expression in MSCs is mediated in part by the Polycomb group (PcG) protein complexes by epigenetic alterations of chromatin [44,48–49]. PcG protein complexes act as transcriptional repressors, and Polycomb repressive complex 1 interacts with chromatin remodelers to induce a repressive heterochromatin state [48–49]. In humans, Polycomb repressive complex 2 contains three subunits: enhancer of Zeste Homology 2 (EZH2), suppressor of Zeste 12, and embryonic ectoderm development [47]. The histone methyltransferase, EZH2, trimethylates histone 3 at lysine 27 (H3K27me3). This trimethylation leads to the recruitment of PRC1 and condenses chromatin, as well as repressing gene expression. During osteoblast differentiation, Runx2 and osteocalcin are repressed in the presence of EZH2 through a significant increase of H3K27me3 activity. Dudakovic et al., [48–49] have shown that inhibition of EZH2 activity increases MSC commitment and their differentiation in vitro, and in wild type mice, pharmacological inhibition of EZH2 increased bone formation. Thus, EZH2 activity is necessary for osteoblast differentiation and skeletal development. This group has further demonstrated that this epigenetic mechanism is also involved in bone loss associated with estrogen depletion, as inhibition of EZH2 prevented bone loss in ovariectomized mice [49]. Godfrey et al., [44] have further demonstrated that specific osteoblast promoters are altered from a suppressed state (H3K27me3) to an active state (H3K27ac) during differentiation. These modifications of histone 3 (H3) are orchestrated by the Polycomb Repressive Complex (PRC2) via EZH2, and the Brg1 associated complex (BAF) via BAF45A. The PRC2 complex is primarily responsible for the deposition of H3K27me3, while BAF is linked to the H3K27ac modification through chromatin remodeling [48–49].
4.3. Non-coding RNAs
The final category of epigenetic modifiers includes the small noncoding RNAs (ncRNAs) [38, 50–51]. These are small functional RNA molecules that are transcribed by DNA but are not translated into proteins. Of the ncRNAs, the microRNA (miRNA) function to regulate post-translational gene expression by reducing their target mRNAs and/or by inhibiting mRNA translation [38, 50–51]. These miRNAs are also involved in the regulation of specific genes involved in cell differentiation and apoptosis [38]. An accumulating body of evidence suggest that miRNAs play a critical role in the regulation of various biological processes, including bone homeostasis [50]. MicroRNAs appear to be involved in the regulation of osteoblast and osteoclast differentiation, as well as bone resorption [50–53; Figure 3]. Human studies have shown that the expression of several miRNAs is significantly increased in the serum of patients with osteoporotic fractures, and these miRNAs are known to enhance osteogenic differentiation [53]. Interestingly, miRNAs are some of the most common RNA species in serum, thus it is possible that they may be useful as biomarkers for diagnostic purposes of specific disease states, and therefore are interesting targets for drug development [54]. A recent study has shown that miR-23a Cl, a key non-coding miRNA, played a critical role the promotion of osteoblast differentiation, and thereby controlled skeletogenesis [44]. These researchers showed that knock-down of miR-23a-Cl in mice resulted in an increased expression of BAF45a and Runx2, as well as a reduced expression of EZH2. Overall, these data suggest that knockdown of miR-23a Cl in this mouse model significantly increased osteoblast differentiation and bone formation [44].
Figure 3.
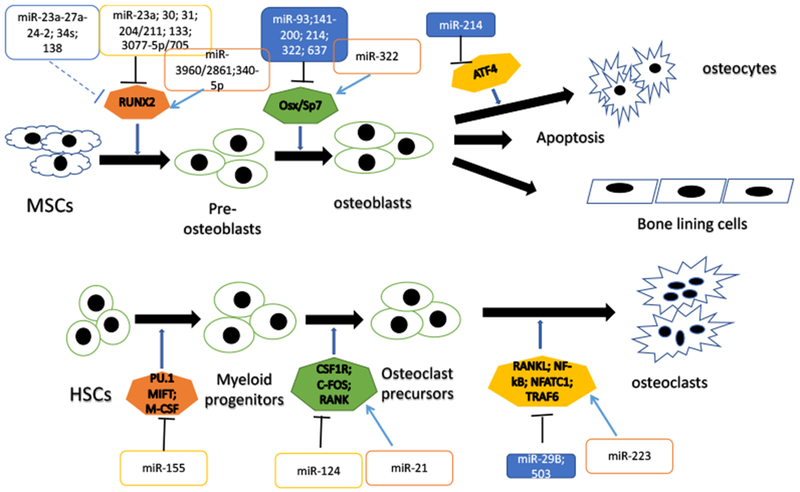
A schematic diagram of osteoblast and osteoclast differentiation from mesenchymal stem cells (MSCs) and hematopoietic stem cells (HSCs), outlining some of the miRNAs involved in these processes and their effects on important transcription factors and genes involved. The arrows represent activation or active processes, the dashed lines represent indirect inactivation and the solid black lines represent direct inactivation.
5.0. Methods
Over the past ten years, the impact of natural compounds on the epigenome has become the focus of investigations in cancer biology, neurology, nephrology and bone research. For this review, searches for studies detailing the effects of dietary and natural compounds on bone remodeling with an experimentally confirmed epigenetic mechanism of action were conducted in numerous electronic databases including MEDLINE, PubMed CENTRAL, NAPRALERT, Google Scholar and Scopus electronic databases from conception until December 31, 2018, without language restrictions, using relevant keywords in both free text and Medical Subject Headings (MeSH terms) format. Terms for the searches of the scientific and medical literature included anthocyanins, anthocyanidins, bone, natural compounds/products, herbal medicines, dietary compounds, ferulic acid, resveratrol, syringic acid, osteoblast, osteoclast, epigenetic, miRNA, MSCs, histone, HDAC, SIRT, and DNA methylation. The Boolean connectors used included AND, OR and NOT, and publications in all languages were reviewed. Searches of the alternative literature were conducted in UIC repositories, catalogues (UIC) for books, abstracts and websites (OpenGray, GetNet International) as well as conference proceedings of both national and international. Two researchers (GBM and NR) independently extracted data from the studies using and disagreement between them was resolved by consensus with a third researcher (TOL). Data was derived from all studies that described natural or dietary compounds with impact on bone remodeling AND epigenetic mechanisms of action. These natural compounds included: resveratrol, sulforaphane, ferulic acid, syringic acid, anthocyanins/anthocyanidins, delphinidin, delphinidin-3-glucoside, cyanidin and cyanidin-3-glucoside. The available data that correlated the effects of these compounds on any aspect of bone remodeling AND epigenetic mechanisms of action was analyzed. A critical analysis of these data was performed, and possible future directions for this research were suggested.
6.0. Results
6.1. Dietary interventions for bone remodeling
Evidence from observational, experimental and clinical studies strongly suggest a positive link between high fruit and vegetable consumption and the indices of bone health [55–58]. Just using plum as one example, there are more than 100 published studies (including clinical trials) in PubMed alone all indicating that these fruits have significant impact on bone health. Furthermore, numerous investigations have demonstrated that many naturally occurring compounds, including flavonoids, polyphenolic and other compounds reduce bone resorption and increase bone mineral density in in vitro, in vivo and in human studies [reviewed in 57–59]. Most of these reviews have focused on the effects of natural plant-based extracts and pure compounds in cultured osteoblasts and animal models of osteoporosis, and the involvement of anabolic signaling pathways including bone morphogenic proteins, and estrogen receptor signaling pathways [58–59]. However, very few natural compounds have actually been investigated for their effects on the epigenome in bone remodeling, and the epigenetic regulation of bone remodeling by naturally occurring compounds has not been the focus of previously published reviews.
6.2. Dietary compounds as epigenetic modulators of bone remodeling
6.2.1. Resveratrol (Figure 4)
Figure 4.
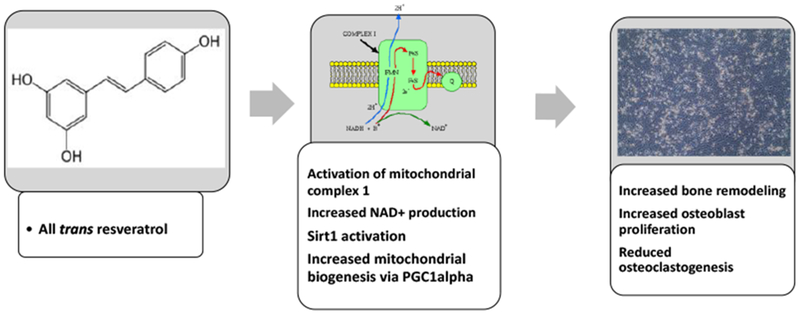
Resveratrol impacts bone remodeling by increasing SIRT1 and ATP production in osteoblasts. Treatment of osteoblast with resveratrol, activates mitochondrial complex I in the electron transport chain, increasing production of NAD+ and ATP formation, leading to an alteration in the NAD+/NADH ratio and thereby activating SIRT1 and increasing osteoblast differentiation and function.
One of the dietary compounds most studied for bone remodeling is resveratrol. Resveratrol (3,5,4’-trihydroxystilbene; RES) is a naturally occurring polyphenolic compound that is found in red wine, red grape skins, acai berry, peanuts, and many other plant species, and is reported to have positive effects on health and increase life span [59–65]. Numerous investigations have reported on the wide range of pharmacological effects associated with RES, including as a treatment for metabolic and bone disorders, diabetes, cancer, cardiovascular, inflammatory and neurodegenerative disorders [62–65].
In terms of clinical trials, RES was first shown to be of benefit in bone remodeling by Ornstrup et al., in 2014 [66]. This group performed a randomized placebo controlled involving 74 middle aged obese men with metabolic syndrome. The patients were treated with high dose RES (1 g/day); low dose RES (150 mg/day) or placebo, and the primary outcome measured was a change in bone ALP. The results of the study showed that treatment with RES (1 gm/day) significantly increased ALP and lumbar spine bone mineral density in obese men, but had no effects at the hip [66]. In a second study, Bo et al. [67] performed a randomized, placebo-controlled, double-blind study involving 192 patients with type 2 diabetes. The patients were randomized in three arms, a RES 500 mg/day; RES40 mg/day or placebo and treatment continued for six months. Outcomes measured included bone ALP, bone mineralization, bone mineral content, as well as calcium, phosphorus, and 25-hydroxy vitamin D concentrations in the serum. After 6 months of treatment, the serum levels of calcium were increased, and bone ALP levels were higher in the RES treated groups. Interestingly, RES 500 mg/day also increased serum levels of 25-hydroxy vitamin D. The study further demonstrated that supplementation with RES 500 mg reduced bone mineral density loss in patients with type 2 diabetes [67]. These clinical trials are supported by numerous in vivo studies demonstrating that resveratrol improves bone mineral density, bone microarchitecture and remodeling [68–73]. In animal studies, RES treatments increased osteoblastogenesis by upregulating the estrogen receptor-dependent bone morphogenetic protein 2 (BMP-2) which reduced bone loss in ovariectomized rats [70–71]. In osteoporosis models, RES suppresses bone resorption by reducing the expression of nuclear factor kappa-β ligand (RANKL), that is significantly up-regulated in osteoporosis and a major cause of bone resorption [12]. In bone, RANKL is secreted by osteoblasts and binds to the RANK receptor on osteoclast precursor cells and mature osteoclasts, thereby activating osteoclastogenesis [72]. In 2011, Shakibaei et al., [73] demonstrated that RES suppressed RANKL induced NF-κβ activation, and thereby prevented the differentiation and activation of multinucleated osteoclasts. Resveratrol treatment also activated the osteogenic factors Runx2 and SIRT1, as well as enhanced bone formation. These researchers further demonstrated that RANKL increased NF-κβ signaling and p300 functions in osteoclasts, while treatment with RES reversed RANKL-pSOO-NF-κβ activation, and thereby reduced bone resorption [73]. In terms of other biological targets, RES altered the expression of intracellular mediators including cyclooxygenases, estrogen receptors, MAPK, mTOR and PI3K/Akt, microRNAs, NF-κβ, phosphodiesterases, and several protein kinases [74–76]. All of these mediators are known to modulate multiple biochemical and signaling pathways that effect a variety of cellular processes such as cell cycle regulation, metabolism, post-translational modifications and inflammatory responses [77]. Furthermore, RES has also been shown to regulate modifications in DNA and histone proteins, thereby impacting gene expression and silencing of some important cellular processes including apoptosis, genomic imprinting, chromosome activation and stem cell pluripotency [78–79]. Several other epigenetic mechanisms are also reported to be affected by RES, HDAC activation, DNA methylation, and lysine-specific demethylase-1 (LSD1) [65].
Interestingly, RES has structural similarity to diethylstilbestrol and acts as an estrogen receptor agonist [59]. RES is reported impact osteoporosis by not only reducing osteoclast proliferation, but also by increasing osteoblast differentiation and function [62,80–81]. In 2004, Lian et al. [80] reported that a portion of MSCs differentiated to adipocytes in presence of the sirtuin 1 (SIRT1) inhibitor nicotinamide, but pre-treatment of these cells with RES prevented adipocyte differentiation. Furthermore, the transcription factor Runx2 was also inhibited by nicotinamide (SIRT1 inhibitor), and RES treatments were able to reverse these effects in osteoblasts [80]. Thus, RES is a SIRT1 activator and increases Runx2 expression, as well as osteoblast differentiation and function. RES treatment of cultured C3H10T1/2 mesenchymal cells also reduced adipocyte development and increased the expression of osteoblast markers [81]. However, treatment with nicotinamide, a SIRT1 inhibitor, increased adipocyte number and the expression of adipocyte markers [81]. In addition, treatment of rat primary bone marrow stromal cells, with RES increased the expression of expression of osteoblast markers and bone mineralization [82]. This was also observed in human and mouse MSCs, as treatment that is involved in the recruitment and differentiation of osteoblasts, with RES also stimulated osteoblast differentiation and proliferation [82–85]. In 2011, Lee et al., [85] reported that treatment of human periodontal ligament cells with RES and isonicotinamide stimulated osteoblastic differentiation in a concentration-dependent manner and increased the expression of mRNAs encoding for alkaline phosphatase, osteopontin, osteocalcin, osterix and Runx2, as well as induced calcium deposition. In the same cell line, treatment with the SIRT1 inhibitor, sirtinol (a SIRT1 inhibitor), nicotinamide or RNA interference-induced gene silencing suppressed bone mineralization and further reduced the expression of osteoblast marker mRNAs [85].
Mechanistically, RES treatments increased adenosine monophosphate kinase (AMPK) activity, suppressed the phosphorylation of Akt, Smad 1/5/8 and c-Jun N-terminal kinase, and the activation of NF-κβ [85]. Tseng et al., [83] further demonstrated that RES exerts its effects on human MSCs primarily through regulation of the SIRT1/F0X03A and estrogen signaling pathways. This group showed that RES activated SIRT1 in MSCs, which then increased F0X03A-dependent transcriptional activity, and Runx2 gene transcription, demonstrating that SIRT1 activation increases Runx2 [83]. In 2012, Shakibaei et al., [84] reported that nicotinamide induced Runx2 acetylation in MSCs was reversed after pretreatment with RES, suggesting that RES-activated SIRT1, which in turn deacetylated and activated Runx2. Knockdown of the SIRT1 protein in MSCs inhibited the effects of RES, demonstrating the involvement of SIRT1 [84]. This group also used immunoprecipitation and western blotting to demonstrate a functional and physical interaction between Runx2 and SIRT1, suggesting that SIRT1 directly deacetylates Runx2 [73]. Another in vitro study reported that hydrogen peroxide (H2O2)-induced apoptosis in mouse osteoblast MC3T3-E1 cells and this treatment inhibited SIRT1 and Bcl-2 expression; but increased p53 acetylation, and Bax and caspase 9 activities [86]. Treatment of the MC3T3-E1 cells with RES reversed the Fl202-induced apoptosis, p53 acetylation and caspase 9 activation [86]. Thus, RES reduced H2O2-induced apoptosis in osteoblasts through its effects as a SIRT1 activator [86]. More recently, in a study by Zhao et al., [87]. RES treatment of mice increased osteoblast activity and bone mineralization, as well as alkaline phosphatase (ALP) and eNOS levels. Moreover, RES activated SIRT1 which in turn stimulated BMP2 release via eNOS [87]. Knockdown studies in mice further demonstrated that SIRT1 deletion reduced ALP, BMP2 and eNOS [87]. In hFOB human osteoblasts, RES reversed serum starvation-induced apoptosis and increased both HDAC1 and SIRT1 mRNA expression [88–89]. In addition, in osterix/sp7:mCherry medaka, RES treatment increased osx/sp7/Runx2 expression and osteoblastogenesis [89].
One of the most interesting epigenetic effects of RES is its activation of SIRT1, which is an NAD+-dependent deacetylase [90; Figures 4 & 5]. Treatment of cells with RES, activated the mitochondrial complex I in the electron transport chain which causes excessive production of NAD+, increases ATP formation and increases the NAD+/NADH ratio leading to the modification of chromatin-associating proteins and the activation of SIRT1 [91]. The biological activities of SIRT1/3 in bone have been reviewed, and shown to be associated with a wide range of biological effectors that regulate numerous proteins and factors including the peroxisome proliferator-activated receptor-gamma coactivator-1alpha (PGC-1α), Forkhead box and bone morphogenic proteins [90]. Resveratrol’s effects on PGC-1α, a transcription coactivator protein known to interact with a wide variety of transcription factors involved in biological responses such as thermogenesis, mitochondrial biogenesis, and muscle and bone formation, suggest that RES also increases mitochondrial biogenesis [92]. Studies have further shown that RES upregulates mitochondrial biogenesis by the activation of SIRT1-induced PGC-1α [92]. Also interesting is the fact that RES may also reduce the level of SIRTs, especially if used for prolonged periods of time, or at high doses, suggesting that there is a negative feedback system could regulate the excessive sirtuin activity [91–92].
Figure 5.
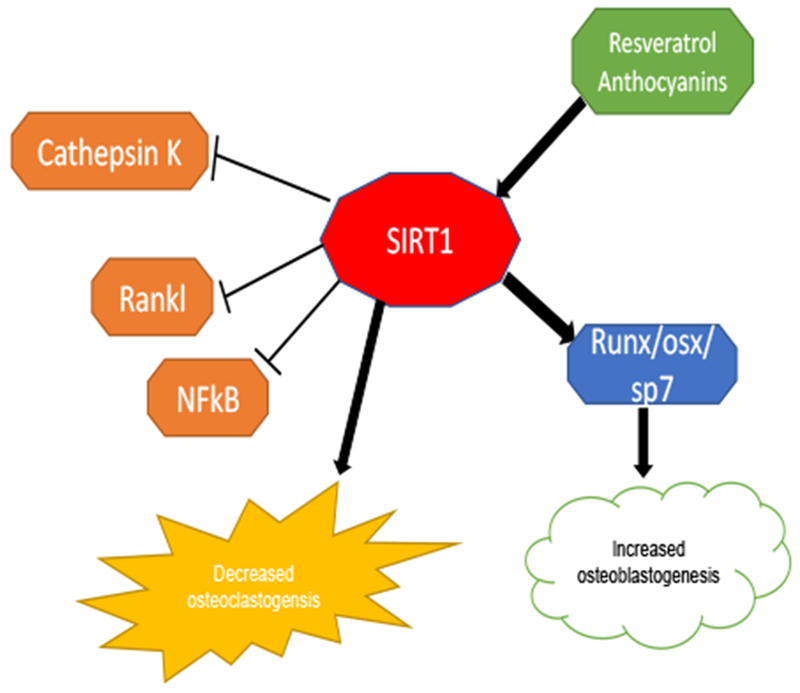
Schematic diagram of the effects of resveratrol and anthocyanins/anthocyanidins (delphinidin and delphinidin-3-glucoside) on SIRT1 activation and increased osteoblastogenesis. These compounds also reduced osteoclastogenesis by reducing the expression of RANKL, NF-κβ and cathepsin K.
In serum-starved cultured human hFOB osteoblasts, the expression of SIRT1/3 and PGC-1α mRNA was reduced and the cells entered apoptosis [93]. Flowever, treatment with RES rescued hFOB cells from apoptosis and significantly increased the expression of PGC-1α, and SIRT1/3 mRNAs suggesting that RES stimulated mitochondrial biogenesis in human hFOB osteoblasts and reversed apoptosis [93]. In 2019, Wang et al., demonstrated that RES treatments of MSCs increased SIRT1 binding with the Polycomb complex protein BMI-1 (BMI-1) and reduced BMI-1 acetylation in a dose-dependent manner [94]. RES also inhibited MSC apoptosis and promoted their differentiation into osteoblasts, all of which was associated with SIRT1 upregulation and nuclear translocation of BMI-1 [94].
6.2.2. Sulforaphane
Sulforaphane (SFN; Figure 6) is an organosulfur compound biosynthesized in food plants from its precursor glucoraphanin by the action of the enzyme myrosinase, that is activated after damage or cutting the plant [95–96]. Glucoraphanin is present in high concentrations in cruciferous vegetables such as broccoli and cabbages, and very high concentrations in the sprouts of cauliflower and broccoli [96]. SFN has been extensively investigated for its antimicrobial and anticarcinogenic activities, and is reported to be of potential therapeutic benefit for type 1 and 2 diabetes, osteoarthritis, and rheumatoid arthritis [98–103]. SFN was also reported to protect cartilage from damage, reduce synovial hyperplasia, reduce matrix-degrading proteases, as well as RANKL-dependent differentiation of osteoclasts by suppressing NF-κβ, and activating the nuclear factor erythroid 2-related factor 2 (NRF2) transcription factor [104–107]. In bone, SFN mimics dimethyl sulfoxide (DMSO), as DMSO is known to cause phenotypic changes and differentiation of osteoblasts by acting on the epigenome [108]. In 2012, Thaler et al., [108] reported that DNA-demethylation was important in osteoblasts for modulating gene expression and cellular differentiation. In 2016, this group further showed that SNF triggered osteoblast differentiation by increasing DNA-demethylation [109]. In addition, due to the molecular similarities of SFN with DMSO, SFN was shown to improve bone by acting through an epigenetic mechanism that stimulates ten-eleven translocation 1 (Tet1)/Tet2-dependent hydroxy-methylation of DNA to reactivate gene expression [109]. In this study, SFN increased DNA demethylation by way of Tet1 and Tet2 and increased pre-osteoclast differentiation by increasing mineralization of the extracellular matrix, as well as the expression of genes and transcription factors-Rynx2, Col1a1, Bglap2, Sp7, Atf4 and Alpl. In osteocytes and mouse calvarial explants, SFN decreased RANKL expression and, in preosteoclasts, SFN induced apoptosis by up-regulation of the Tet1/Fas/Caspase 8 and Caspase 3/7 pathway [109]. This study further demonstrated that treatment of sham-operated mice with SFN for five weeks significantly increased metaphyseal cancellous bone volume and trabecular number in proximal tibias, however trabecular thickness was not increased. Furthermore, treatment of these animals with SFN did not appear to impact cortical thickness [109]. Thus, the increased bone volume/trabecular volume levels observed in SFN treated mice were due changes in trabecular number and not due to a thickening of the trabeculae by enhanced bone apposition [109]. However, SFN treatment of OVX mice reduced bone loss due to estrogen-deficiency as observed by a reduction in the OVX-dependent decrease of trabecular number. These effects of SFN were correlated with an increase in bone volume (~20%) and a higher trabecular number in both normal and OVX mice. The results of this study suggested that SFN induced epigenetic alterations that stimulated osteoblastogenesis and reduced bone resorption, thereby shifting the balance in favor of bone formation [109].
Figure 6.
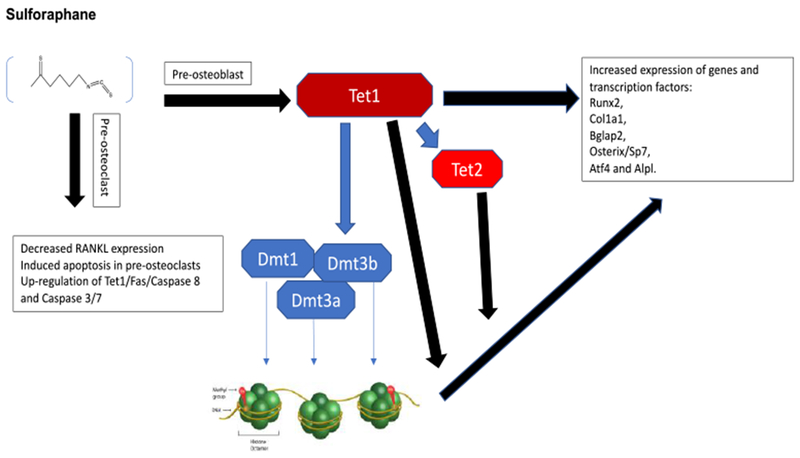
Sulforaphane increases bone formation and reduces bone resorption by promoting osteoblast differentiation via epigenetic alterations in DNA methylation and alterations of gene expression. Sulforaphane also induced pre-osteoclast apoptosis.
Other researchers have shown that SFN also stimulates osteoclast apoptosis by downregulating the expression of RANKL in osteocytes, thereby increasing the antiresorptive effects. In a 2014 study [110], activation of transcription factor NRF2 by SFN promoted the expression of two key antioxidant genes NAD(P)H dehydrogenase quinone 1 and peroxiredoxin-1, stimulating a sustained antioxidant response in osteoclast progenitors. Down-regulation of intracellular reactive oxygen species (ROS) levels reduced ROS-dependent RANKL expression in pre-osteoclasts thereby inhibiting osteoclast differentiation [110]. Thus, SFN is a dietary compound that alters DNA methylation patterns, thereby increasing osteoblastogenesis and reducing osteoclastogenesis contributing to its effects on bone formation [110]. However, unlike resveratrol, there are no clinical trials for sulforaphane, thus it is difficult to predict if this compound will be effective in humans. The effects of SFN on other epigenetic mechanisms such as ncRNA or HDAC/SIRT involvement have not been reported.
6.2.3. Ferulic acid
Ferulic acid (FA; also known as hydroxycinnamic acid; Figure 7) is a ubiquitous phenolic compound found in the cell walls of plants such as corn, rice, flax and oats, and has antioxidant activities [111–112]. FA is also an active ingredient of Chinese herbal medicines including the plant Angelica sinensis, and has been used as a traditional medicine for bone loss [111]. Published investigations demonstrate that FA inhibits osteoclast formation, as well as stimulates osteoblast differentiation and function, leading to enhanced bone formation both in vitro and in vivo [112–117]. In 2003, Sagar et al., published the only in vivo study of FA on bone health [114]. In this study, ovariectomized (OVX) female rats were treated with FA and/or 17α-ethinyl estradiol daily for 8 weeks. The results showed that showed that while OVX significantly reduced BMD, OVX rats treated with estrogen or ferulic acid showed a significant increase in BMD after 8 weeks of treatment [114]. Furthermore, treatment of RANKL-stimulated osteoclasts with FA reduced NF-κβ mRNA expression and other anti-inflammatory related genes, thereby inhibiting osteoclast differentiation and bone resorption [114].
Figure 7.
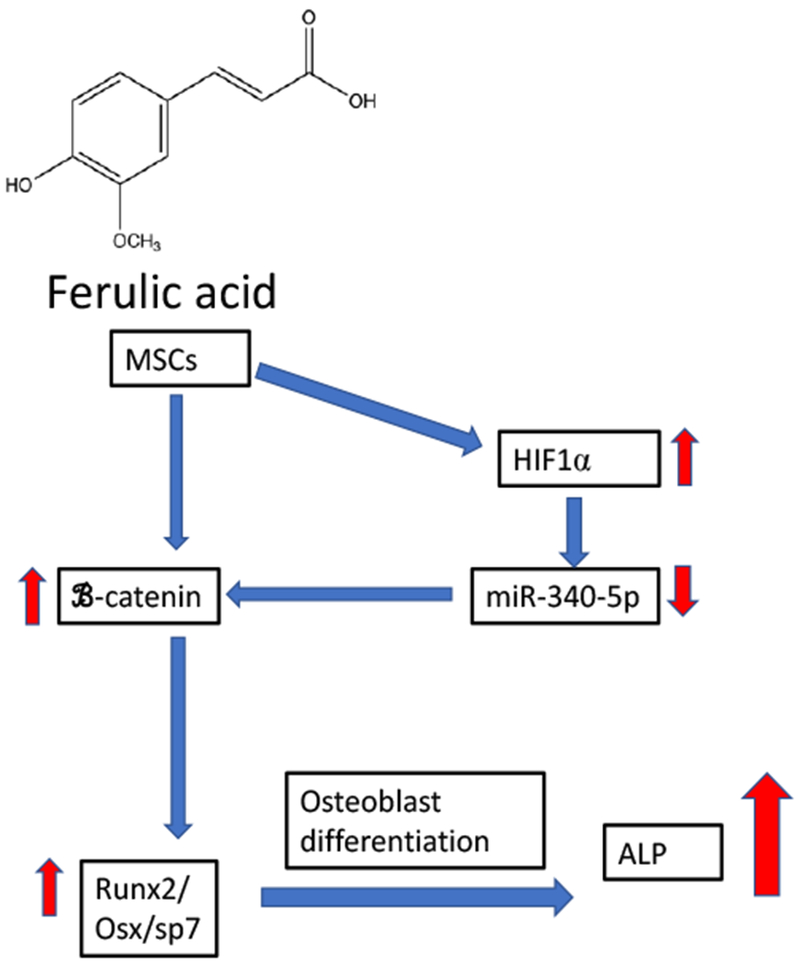
A flow chart of the effects of ferulic acid on mesenchymal stem cells (MSCs) and their differentiation into osteoblasts. Treatment of MSCs with ferulic acid activated HIF1α and down-regulated miR-340-5p expression. This increased β-catenin Wnt signaling resulting in an increased expression of Runx2 and osterix/sp7, two critical factors in osteoblast differentiation. These events lead to increased expression of alkaline phosphatase (ALP) in differentiated osteoblast.
In a 2016 ex vivo study, Sagar et al., [113] demonstrated that treatment of human CD14+ peripheral blood monocytes with FA (IC50 39 μM) significantly inhibited osteoclast formation, reduced differentiation of osteoclast progenitors, and bone resorption. In terms of mechanism of action, FA treatment inhibited RANKL-induced expression of dendritic cell-specific transmembrane protein (DC-STAMP), an important regulator of osteoclast fusion. In addition, the expression of matrix metalloproteinase-9 and cathepsin K, two osteoclast specific lysosomal proteases were significantly reduced in FA treated cells [113]. Furthermore, the number of mature osteoclasts was significantly reduced at 24 and 48 hrs after FA treatments, and an increase in apoptosis, caspase-3 activity and DNA-fragmentation were also observed suggesting that FA induced osteoclast apoptosis [113]. These authors concluded that FA inhibited osteoclast fusion by inhibiting DC-STAMP expression and induced apoptosis in mature osteoclasts via caspase-3 [113].
In 2015, Seo et al., demonstrated that FA reduced NF-κβ activation, as well as reduced the expression of iNOS, TNF-α and IL-6 in LPS-activated RAW 264.7 cells, thereby supporting the anti-inflammatory effects of FA [115]. In 2018, Doss et al., also reported on the effects of FA in cultured RAW 264.7 monocyte/macrophage cells [116]. In this study, RAW 264.7 cells were treated with increasing concentrations of FA with or without RANKL/M-CSF stimulation. The results demonstrated that FA treatment significantly attenuated RANKL-induced osteoclast differentiation as determined by SEM and TRAP staining analysis and reduced the bone resorption. In addition, FA treatments reduced RANKL-activated NF-κβ and the expression of NFATc1, c-Fos, TRAP, Cathepsin K and MMP-9, indicating that FA inhibits the RANKL/NF-κβ signaling pathway, thereby reducing osteoclast differentiation and the bone resorption activity of osteoclasts [116].
In 2018, Du et al., [117] investigated the epigenetic effects of FA in human bone marrow derived MSCs. In this study, FA treatment of MSCs up-regulated the expression of β-catenin, a transcription signaling pathway that is essential for MSC differentiation. These researchers demonstrated treatment of MSCs with FA upregulated HIF-1α, which in turn reduced the expression of miR-340-5p. This group further demonstrated that the Wnt/β-catenin signaling pathway was involved, as FA upregulated β-catenin, which was associated with the hypoxia-inducing effects of FA via miR-340-5p, which has HRE motifs in its promoter regions. FA repressed miR-340-5p expression through HIF-1α, which also shown to be an endogenous suppressor of β-catenin [117]. These investigators further demonstrated that the effects of FA were dependent on the inhibition of miR-340-5p for MSC differentiation. Induction of β-catenin signaling by FA was attenuated by miR-340-5p overexpression. In addition, this study also demonstrated that FA suppressed miR-340-5p via FI IF-1α, indicating that FA enhances the osteogenic potential of MSCs via epigenetic regulation of this miRNA [117; Fig. 7]. These data suggest that FA increases bone formation via an epigenetic mechanism that involves at least miR-340-5p. While FA shows potential for improving bone remodeling through alterations of miRNA, to date, these data have only been reported in vitro, and there is only one animal study suggesting that FA may reduce bone resorption. No clinical trials or studies in human osteoblasts/osteoclasts have been published to date. The effects of FA on other epigenetic mechanisms such as DNA methylation or HDAC/SIRT involvement have not been reported.
6.2.4. Syringic acid
Syringic acid (SA; Figure 8; IUPAC name: 4-hydroxy-3,5-dimethoxybenzoic acid) is another naturally occurring phenolic compound that is present in several edible plants including the fruit of the acai palm Euterpe oleracea, and the mycelium of the shiitake mushroom Lentinula edodes [118–119]. Previous studies have shown that SA has does not bind to the estrogen receptor but has antioxidant, radical scavenging and anti-inflammatory activities [120–123]. The effects of SA on bone metabolism and bone resorption have also been reported both in vitro and in vivo [124–126]. Treatment of cultured mouse osteoblasts with Drynariae rhizoma extracts (containing SA) increased their proliferation and differentiation [124].
Figure 8.
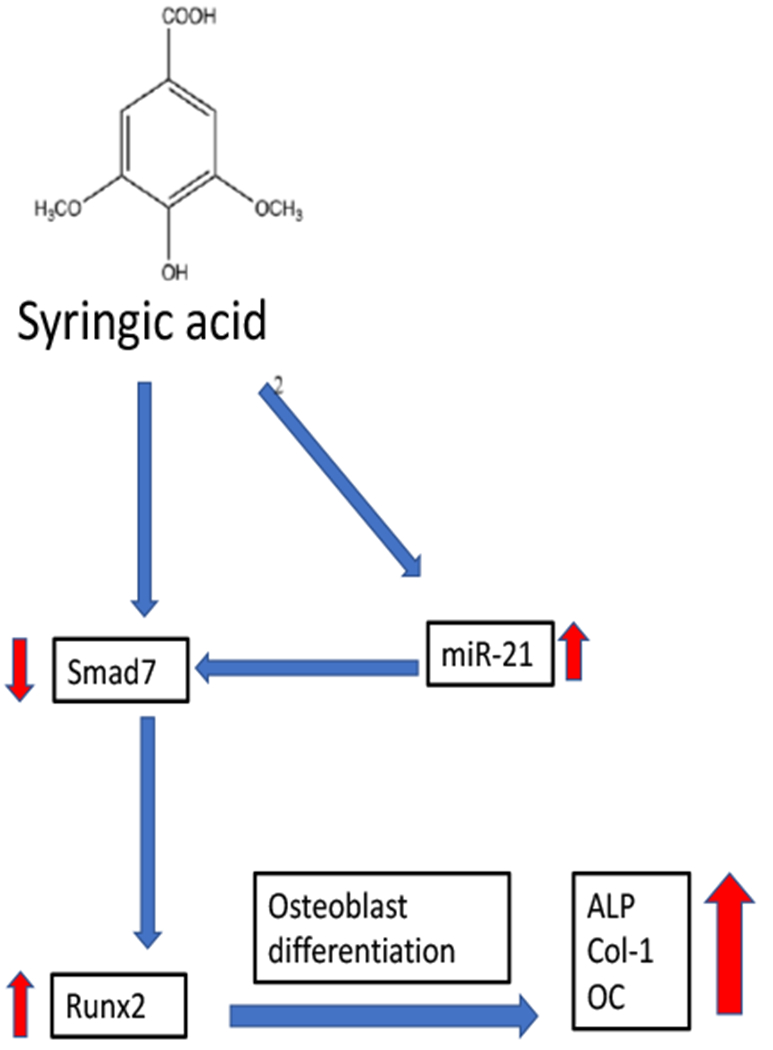
A flow chart of the effects of syringic acid on mesenchymal stem cells (MSCs) and their differentiation into osteoblasts. Treatment of MSCs with syringic acid upregulates miR-21 expression, and reduces Smad7 expression resulting in an increased expression of Runx2, a critical factor in osteoblast differentiation. These events lead to increased expression of the genes ALP, Col-1, and OC in differentiated osteoblast. ALP = alkaline phosphatase; Col-1 = type 1 collagen 1; OC = osteocalcin
In 2017, Tanaka et al. [125] investigated the effects of a diet containing SA on bone resorption and uterine wet weight in OVX mice fed with a diet containing 100 mg/kg SA for 10 weeks. The results of this study showed that treatment with SA had no effect on body weight, food intake, or uterine wet weight. However, bone mineral density, including cortical bone density, cancellous bone density, and total bone density were higher in the SA-treated mouse group as compared with the OVX-control mice. Histomorphometric analysis showed that SA treatments reduced the number of osteoclasts, but increased the number of osteoblasts, suggesting improved bone remodeling [125]. In bone marrow, SA treatments suppressed osteoclast differentiation, improved the markers of bone resorption, and the number of tartrate-resistant acid phosphatase (TRAP)-positive osteoclasts, as well as improved urinary DPD, and TRAP activity in OVX mice. An increase in the activity of bone alkaline phosphatase, a marker of bone formation, in the OVX mice was noted, suggesting that treatment with SA impacted both osteoblasts (bone formation) and osteoclasts (bone resorption) without having estrogenic effects on the uterus in OVX mice [125].
In 2018, Arumugam et al., [126] investigated the epigenetic and molecular mechanisms by which SA exerts its effects on osteoblast differentiation in mouse mesenchymal stem cells (M-MSCs). At the cellular level, SA treatment of M-MSCs stimulated osteoblast differentiation, and increased alkaline phosphatase activity, as well as the accumulation of calcium deposits. At the molecular level, SA treatments promoted osteoblast differentiation by enhancing Runx2 expression and other marker genes including alkaline phosphatase, type I collagen, and osteocalcin. In terms of the epigenome, SA increased the expression of miR-21 and decreased one of its target genes, Smad7 [126]. Smad7 is known to be an antagonist of TGF-β/Smad signaling and negatively regulates Runx2. This study concluded that SA-stimulated the TGF-β/BMP signaling pathway in mouse MSCs, increased expression of miR-21 and decreased Smad7 expression leading to an increased expression of Runx2, which enhanced osteoblast differentiation [126]. Overall, these data suggest that SA may increase bone formation by increasing osteoblastogenesis through an epigenetic mechanism that involves at least miRNA-21 [126].
Similar to FA, while SA shows potential for improving bone remodeling by altering miR-21/Smad7, these data are in vitro, and there is only one animal study suggesting that SA reduces bone resorption in OVX mice [125]. No clinical trials or studies in human osteoblasts/osteoclasts have been published to date, so it is difficult to assess whether these data may be extrapolated to humans. Also similar to FA, effects of SA on other epigenetic mechanisms such as DNA methylation or HDAC/SIRTs have not been reported.
6.2.5. Anthocyanins
Anthocyanins/anthocyanidins are a group of water-soluble pigments present in fruits and vegetables, and are responsible for the vivid red, violet, blue, and purple colors of blueberries, vegetables, grapes and red wine [127–132]. Chemically, anthocyanins (ACNs) are derivatives of 2-phenylbenzopyrylium (flavylium cation, Figure 9), that consists of an aglycone (anthocyanidin), sugar(s), and in some minor cases acyl group(s) [132–133]. The anthocyanins and anthocyanidins (aglycones) consist of more than 500 compounds, of which the most common anthocyanins in the diet include those compounds having the aglycone moieties cyanidin (32%) followed by malvidin (22%), pelargonidin (19%), delphinidin (12%), petunidin (9%), and peonidin (6%) [132–133,135]. Anthocyanins commonly occur as 3-monosides, 3-biosides and 3-triosides, as well as 3,5-diglycosides and more rarely 3,7-diglycosides and are associated with the sugars: glucose < rhamnose < galactose <xylose < arabinose [132, 135]. Interestingly, anthocyanins are the most heavily consumed class of flavonoids in the diet, and in the United States, the estimated human daily intake ranges from 12 to 21 mg/day, however this amount has significantly declined since 1976, when it was ~180 mg/day [133–135].
Figure 9.
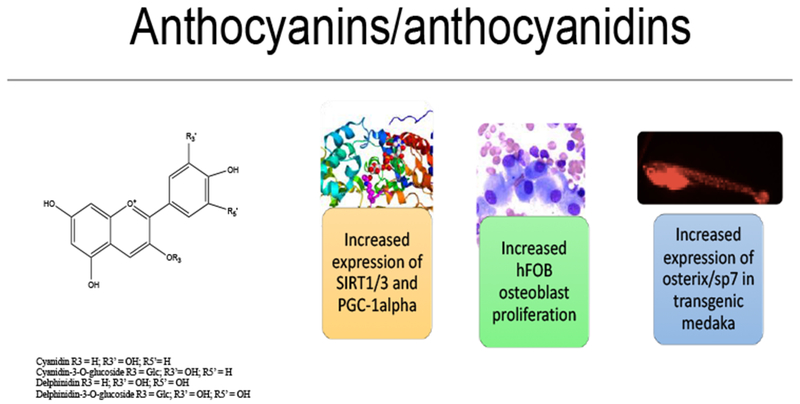
Anthocyanins from fruit extracts increase osteoblastogenesis and reduce osteoblast apoptosis in hFOB human osteoblasts and transgenic medaka by epigenetic regulation of SIRT1/3 and PGC-1α. In osterix/sp7:mCherry transgenic medaka treatment increased osteoblast differentiation and proliferation as well as the expression of osterix/sp7 and Runx2.
In 2012, an observational clinical study demonstrated an association between the habitual intake of flavonoids and bone mineral density (BMD) in a large (n = 3160) cohort of female twins in the UK [136]. In this study, the frequency of the ingestion of flavonoids, (including subclasses flavanones, anthocyanins, flavan-3-ols, polymers, flavonols, and flavones) were assessed and determined by semi-quantitative food frequency questionnaires and bone mineral density was also assessed [136]. The median intake of total flavonoids was 1.1 g/d, of which, the median anthocyanin intake was estimated at 13.7 mg/d, and the median flavanone intake was ~21.2 mg/day. The results of this study demonstrated that higher total intakes of flavonoids were positively associated with higher BMD in the spine. Higher anthocyanin intake resulted in the best effect, with a 0.034 and 0.029 g/cm2 increase in BMD at the spine and hip, respectively, showing an increase of 23.8% for the spine and 22.7% for hip-bone mineral density in these patients. Post-menopausal women with high anthocyanin intake also had significant improvements in BMD in the spine. The study concluded that higher intake of anthocyanins increased BMD at the spine and hip, and this effect was better in postmenopausal women [136].
Animal studies also indicate that high anthocyanin-containing fruits, such as plums, have a significant impact on bone health [137–143]. In 2001 Arjmandi et al., demonstrated that administration of dried plums containing high concentrations of anthocyanins to ovariectomized (OVX) rats reduced bone resorption, increased bone formation, and prevented bone loss [137]. In other studies, administration of dried plums to an osteopenic rat model of osteoporosis reduced bone loss, and in a male rat model of osteoporosis, administration of dried plums also prevented bone loss [138–139]. Devareddy et al., showed that administration of a blueberry-rich diet (high in anthocyanins/anthocyanidins) prevented BMD loss in a rat model of osteoporosis [140]. In 2010 Chen et al., [141] reported that a blueberry-rich diet increased bone formation in young rats, and further demonstrated that the serum from the blueberry-fed young rats increased osteoblast differentiation and reduce osteoclastogenesis in vitro [141]. In a 2011 study by Zhang et al., young rats fed a diet of blueberries showed a significantly reduction in OVX-induced bone loss [142]. Furthermore, when pre-osteoblasts were treated with serum isolated from blueberry-fed young rats there was an increase in osteoblast development and differentiation [142]. In a study by Kaume et al., treatment of OVX rats with a diet containing 5% freeze-dried blackberries also reduced bone resorption [143]. While all of the above studies used fruit extracts, all of these fruits, dried plums, blueberries and blackberries contain very high concentrations of anthocyanins/anthocyanidins [144].
In 2014, Moriwaki et al. reported that oral administration of the anthocyanin, delphinidin, reduced bone resorption in an OVX mouse model by the suppression of osteoclast formation [145]. They further reported that delphinidin treatment significantly inhibited the differentiation of cultured RAW264.7 cells into osteoclasts by suppressing the activity of NF-κβ, c-fos, and Nfatc1, important transcription factors for osteoclastogenesis [145].
In 2018, Raut et al., [146–147] investigated the effects of common anthocyanins and a blackcurrant (Ribes nigrum) extract (BCE) on osteoblastogenesis in human hFOB 1.19 osteoblasts and in transgenic osterix/sp7:mCherry medaka. The BCE contained four major anthocyanins: delphinidin-3-O-glucoside (D3G; Figure 9), delphinidin-3-O-rutinoside, cyanidin-3-O-glucoside, cyanidin-3-O-rutinoside. Both BCE and delphinidin-3-glucose increased hFOB osteoblast proliferation; rescued osteoblasts from serum-starvation-induced apoptosis by increasing Bcl-2 expression and reducing Bax mRNA expression. These compounds also altered HDAC1, HDAC3 expression, as well as increased SIRT1/3 and PGC-1α mRNA expression in apoptotic osteoblasts [146–147]. In osterix/Sp7:mCherry medaka, BCE and D3G treatments increased osteoblast proliferation by increasing osterix/Sp7IRunx2 expression [146–147, Fig. 5]. These data suggest that both BCE and D3G increased osteoblast proliferation and differentiation through epigenetic regulation of HDACs and by increasing mitochondrial biogenesis. In triple transgenic RANKL medaka, D3G reduced RANKL expression and improved bone mineral density [148–149]. In a similar study of Acai (Euterpe oleracea) fruit extract (AFE) and anthocyanidins, AFE and cyanidin-3-glucoside (C3G; Figure 9) increased osteoblast proliferation and reduced apoptosis in hFOB 1.19 osteoblasts induced by both serum and glucose starvation [150, 151]. Gene expression data showed that C3G altered HDAC1 expression, increased BCL-2, as well as reduced BAX mRNA expression and caspase 3/7 activity. AFE increased the proliferation of osteoblasts in double transgenic osx/Sp7:mCherry medaka by enhancing the expression of osx/Sp7 [150–151].
Both C3G and D3G also significantly upregulated the expression of peroxisome proliferator-activated receptor gamma coactivator-1 alpha (PGC-1α) mRNA in hFOB 1.19 osteoblasts [150–151]. PGC-1α has been shown regulate mitochondrial biogenesis, thus, these data suggest that anthocyanins may also enhance mitochondrial biogenesis in osteoblasts. The mitochondria generate energy for cells in the form of adenosine triphosphates (ATP), and are very sensitive to oxidative stress [152–153]. Numerous studies suggest that epigenetics also play a critical role in mitochondrial metabolism and functional integrity [153]. Interestingly, both osteoblasts and osteoclasts have high energy demands, are rich in mitochondria and, are prone to mitochondrial dysfunction due to their high susceptibility for reactive oxygen species [153]. Therefore, it is not surprising that bone disorders may be induced by mitochondrial dysfunction [154–157]. Thus, the research and development of dietary or other compounds would reverse this dysfunction, by altering the epigenome would be clinically significant.
7.0. Discussion
Bone remodeling is a tightly orchestrated and dynamic process involving the removal of old bone by osteoclasts and the formation of new bone by osteoblasts, and is critical for the maintenance of bone strength and integrity. Bone modeling and remodeling requires controlled regulation of the expression of specific genes in osteoblasts, osteoclasts and osteocytes. Research over the past ten years has shown that the process of bone remodeling is also regulated by epigenetic mechanisms, including DNA methylation, histone modification and non-coding RNAs. Furthermore, data suggest that dysregulation of the epigenetic functions in bone cells impacts both the function and differentiation of osteoblasts and osteoclasts, thereby contributing to pathogenic bone disorders, including osteoporosis and osteopetrosis.
7.1. Limitations of the research
Evidence from observational and experimental studies, as well as clinical trials have demonstrated a positive link between naturally occurring compounds and improved indices of bone health. Data from in vitro and in vivo studies have shown that the natural occurring compounds resveratrol, sulforaphane, ferulic and syringic acids and anthocyanins increase bone mineralization by increasing osteoblast function and differentiation, as well as reduce osteoclastogenesis, thereby recoupling bone remodeling through alterations of the bone epigenome. While these data are compelling, there are some limitations to this research that should be considered. First, with the exception of resveratrol and dietary anthocyanins, evidence from human studies and clinical trials are lacking for many compounds, making it difficult to extrapolate the existing data from in vitro and in vivo studies to bone remodeling in humans. In addition, for some phenolic compounds such as ferulic acid, much of the published data is in vitro only, and there are few animal studies published to confirm these in vitro results. Furthermore, the effects of these natural compounds on human osteoblasts or osteoclasts has not generally been investigated, and many studies have used only mouse osteoblasts/osteoclasts. While mouse osteoblasts are an acceptable model, it would be beneficial to at least demonstrate that the activity of natural compounds and their epigenetic mechanisms are similar in human cell lines. Obviously, the generation of data from clinical trials is an important future goal for the testing of these compounds. However, it is well recognized that clinical trials are extremely expensive and require a sufficient body of preclinical data to justify these costs and obtain the necessary funding to perform such studies.
Secondly, research of the epigenetic mechanisms involved in the actions of natural compounds on bone remodeling is still in its infancy. Novel technologies such as genome-wide sequencing and single cell ChIP analyses and RNA sequencing should be employed, and could propel this research forward to obtain epigenetic and transcriptional profiles of bone cells treated with these compounds. These methodologies would allow for the identification of specific single-cell variability and bone cell epigenetic mechanisms induced by natural and dietary compounds. Furthermore, the impact of natural compounds on emerging epigenetic mechanisms, including the alteration nucleosome organization by methylation/acetylation of the N-terminal of histone 3 lysine 27 (H3K27) have not yet been investigated. Considering the importance of these specific mechanisms for osteoblast differentiation and skeletal development, studies of H3K27 should be a focus for the future.
The third limitation of the research involves the immense complexity of bone remodeling and diseases of bone remodeling that are characterized by differences in severity and phases, as well as understanding the impact of other dietary, environmental and genetic influences. Most of the animal and human studies to date have not adequately taken these other factors into consideration. Future studies should be diligent in assessing some of these potentially confounding factors and their impact on study outcomes.
7.2. Limitations of this study
While this review is the first to our knowledge to be published on this theme, it however, presents certain limitations. First, although we searched for appropriate manuscripts in various databases, some relevant databases were not included (e.g. EMBASE) due to logistical constraints. However, we performed an intensive manual search of other relevant abstracts and articles and we do not believe that many papers were inadvertently overlooked. Where abstracts were obtained from presentations, we obtained copies of the presentations to evaluated methods and data analyses. It is possible that international abstracts and manuscripts, not abstracted in databases may have also been overlooked, however we do not believe that this altered the quality of the review.
7.3. Future outlook and potential clinical impact
Some of the most compelling observations from the published literature describe the impact of natural compounds on the re-coupling of bone remodeling. This is clinically significant in that current prescription drugs for osteoporosis and other bone diseases are usually only effective as bone anabolic or anti-resorption agents, and do not re-couple bone remodeling. Current evidence suggests that at least resveratrol and anthocyanins increase osteoblast differentiation, as well as reduce osteoclast differentiation and function to re-couple bone remodeling through alterations in the bone epigenome. Since these compounds have already been tested in clinic, further human studies should be undertaken to determine appropriate doses, length of treatment and long-term impact of these compounds alone or in combination on bone health. Furthermore, the published clinical data suggest that the anthocyanins maybe of value for reducing postmenopausal bone loss, and in vitro and in vivo data suggest that these compounds may re-couple bone remodeling. There are over 500 known anthocyanins/anthocyanidins compounds that could potentially be tested for the development of preventative treatments for osteoporosis and other diseases in which bone remodeling is uncoupled.
8.0. Conclusions
The mechanisms by which naturally occurring compounds impact bone remodeling are complex, however compelling data suggest that these compounds alter the bone epigenome through changes in DNA methylation, miRNAs and alterations in HDAC/SIRTs expression and function. Further work is needed as the current studies offer little insight on how epigenetic remodeling of bone-specific chromatin maintains bone mass in vivo. Understanding how natural compounds impact the bone epigenome is critical for the future development of novel therapeutic agents to prevent and treat bone loss. Finally, preliminary data also suggest that specific natural compounds may increase mitochondrial biogenesis and thereby reduce osteoblast apoptosis associated with aging and specific bone disorders. Considering the critical role of the mitochondria in aging and chronic diseases, investigating the effects of natural compounds on mitochondrial function and associated epigenetic alterations is another important focus for future research.
9.0. Acknowledgement
This research was funded in part by a grant from the National Center for Complementary and Integrative Health at the National Institutes of Health (NIH; 1R21AT009452-01A1; GBM); a Schlumberger Foundation Fellowship award to TOL; and a Raman Post-Doctoral Fellowship by University Grants Commission, Govt. of India to NAR. The contents are solely the responsibility of the authors and do not necessarily represent the official views of the NCCIH or NIH, or the other funding agencies.
Abbreviations
- Akt
Protein kinase B
- ALP
alkaline phosphatase
- Atf4
Activating Transcription Factor 4
- BAF
Brg1 associated complex
- Bcl-2
B-cell lymphoma 2
- Bglap2
Bone Gla protein 2 (also known as Osteocalcin-2)
- BMD
Bone mineral density
- BMP-2
bone morphogenetic protein 2
- Cbfa-1
core-binding factor subunit alpha-1 (also known as Runx2)
- c-FOS
proto-oncogene
- Col1a1
gene for alpha-1 type I collagen
- CpG
regions of DNA where a cytosine nucleotide is followed by a guanine nucleotide
- DC-STAMP
Dendritic cell-specific transmembrane protein
- DNMT
DNA methyltransferases
- eNOS
endothelial nitric oxide synthase
- EZH2
Enhancer of Zeste Homology 2
- FA
Ferulic acid
- FOXO3A
Foxhead box O3
- H3K27
N-terminal of histone 3 lysine 27
- HATs
Histone acetyltransferases
- hFOB
human osteoblasts
- HDACs
Histone deacetylases
- HDMs
Histone demethylases
- HIF-1
Hypoxia-inducible factor 1-alpha
- HMTs
Histone methyltransferases
- IL
Interleukins (IL)-1, IL-6, IL-11
- LPS
lipopolysaccharide
- LSD1
lysine-specific demethylase-1
- MAPK
Mitogen-activated protein kinases
- M-CSF
Macrophage colony-stimulating factor
- miRNA
microRNA
- MMP-9
Matrix metallopeptidase 9
- MSC
mesenchymal stem cell
- mTOR
mammalian target of rapamycin
- ncRNA
non-coding RNAs
- NFATc1
Nuclear Factor of activated T cells 1
- NRF-2
Nuclear factor erythroid 2–related factor 2
- OVX
Ovarectomized
- P13K
phosphatidylinositol 3-kinase
- P53
Tumor protein p53
- PcG
Polycomb group of protein complexes
- PCR1, PCR2
Polycomb Repressive Complex 1 and 2
- PGC-1α
Peroxisome proliferator-activated receptor gamma coactivator 1-alpha
- PTH
Parathyroid hormone
- RANK
Receptor activator of nuclear factor kappa-B (NF-κβ)
- RANKL
Receptor activator of nuclear factor kappa-B (NF-κβ) ligand
- RES
Resveratrol
- Runx2
Runt-related transcription factor 2
- SA
syringic acid
- SFN
Sulforaphane
- SIRT
Sirtuins (silent information regulator 2- Sir2)
- Smad
Structurally similar Mothers Against Decapentaplegic
- Sp7/Osterix
Transcription factor Sp7, also called Osterix
- TET
Ten eleven translocation
- TNF-alpha
Tumor necrosis factor
- TRAP
Tartrate-resistant acid phosphatase
- Wnt
Wingless/Integrated signaling pathway
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: The authors declare that there are no conflicts of interest associated with this manuscript.
References
- 1.Olsen BR, Reginato AM, Wang W. Bone development. Annu Rev Cell Dev Biol. 2000;16:191–220. [DOI] [PubMed] [Google Scholar]
- 2.Nakashima K, Zhou X, Kunkel G, Zhang Z, Deng JM, Behringer RR, de Crombrugghe B. The novel zinc finger-containing transcription factor osterix is required for osteoblast differentiation and bone formation. Cell. 2002;108:17–29. [DOI] [PubMed] [Google Scholar]
- 3.Hojo H, Ohba S, He X, Lai LP, McMahon AP. Sp7/Osterix is restricted to bone-forming vertebrates where it acts as a Dlx co-factor in osteoblast specification. Developmental Cell Online Edition:2016/05/10 (Japan time), doi: 10.1016/j.devcel.2016.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rodda SJ, McMahon AP. Distinct roles for Hedgehog and canonical Wnt signaling in specification, differentiation and maintenance of osteoblast progenitors. Development. 2006;133:3231–3244. [DOI] [PubMed] [Google Scholar]
- 5.Ralston SH. Genetics of osteoporosis. Ann N Y Acad Sci. 2010; 1192(1): 181–189. [DOI] [PubMed] [Google Scholar]
- 6.Jiang SD, Dai LY, Jiang LS. Osteoporosis after spinal cord injury. Osteoporosis Int. 2006; 17(2): 180–192. [DOI] [PubMed] [Google Scholar]
- 7.Cortet B Effects of bone anabolic agents on bone ultrastructure. Osteoporosis Int. 2009;20:1097–1100. [DOI] [PubMed] [Google Scholar]
- 8.Karsenty G Transcriptional control of skeletogenesis. Annu Rev Genomics Hum Genet. 2008;9:183–196. [DOI] [PubMed] [Google Scholar]
- 9.Park-Min KH. Mechanisms involved in normal and pathological osteoclastogenesis. Cell Mol Life Sci. 2018;75(14):2519–2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Caetano-Lopes J, Canhão H, Fonseca JE. Osteoblasts and bone formation. Acta Reumatol Port. 2007;32(2): 103–110. [PubMed] [Google Scholar]
- 11.Raggatt LJ, Partridge NC. Cellular and molecular mechanisms of bone remodeling. J Biol. Chem. 2010;285:25103–25108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lapunzina P, Aglan M, Temtamy S, Caparros-Martin JA, Valencia M, Leton R, Martinez-Glez V, Elhossini R, Amr K, Vilaboa N, et al. Identification of a frameshift mutation in Osterix in a patient with recessive osteogenesis imperfecta. Am J Hum Genet. 2010;87:110–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ono T, Nakashima T. Recent advances in osteoclast biology. Histochem Cell Biol. 2018; 149(4):325–341. [DOI] [PubMed] [Google Scholar]
- 14.Asagiri M, Takayanagi H. The molecular understanding of osteoclast differentiation. Bone 2007;40:251–264. [DOI] [PubMed] [Google Scholar]
- 15.Nakamura I, Takashi N, Jimi E, Udagawa N, Suda T. Regulation of osteoclast function. Mod Rheumatol. 2012;22:167–177. [DOI] [PubMed] [Google Scholar]
- 16.Gaddini GW, Turner RT, Grant KA, Iwaniec UT. Alcohol: A simple nutrient with complex actions on bone in the adult skeleton. Alcohol Clin Exp Res. 2016;40(4):657–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Diez A, Puig J, Serrano S, Marinoso ML, Bosch J, Marrugat J, Mellibovsky L, Nogues X, Knobel H, Aubia J. Alcohol-induced bone disease in the absence of severe chronic liver damage. J Bone Miner Res 1994;9:825–831. [DOI] [PubMed] [Google Scholar]
- 18.Rizzoli R Postmenopausal osteoporosis: Assessment and management. Best Pract Res Clin Endocrinol Metab. 2018;32:739–757 [DOI] [PubMed] [Google Scholar]
- 19.Qiu Y, Yang W, Wang Q, Yan S, Li B, Zhai X. Osteoporosis in postmenopausal women in this decade: a bibliometric assessment of current research and future hotspots. Arch Osteoporos. 2018;13:121–125. [DOI] [PubMed] [Google Scholar]
- 20.Pouresmaeili F, Kamalidehghan B, Kamarehei M, Goh YM. A comprehensive overview on osteoporosis and its risk factors. Ther Clin Risk Manag. 2018;14:2029–2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Osteoporosis Fast Facts - National Osteoporosis Foundation. [Accessed 10/30/2108.]. https://cdn.nof.org/wp-content/uploads/2015/12/Osteoporosis-Fast-Facts.pdf.
- 22.Sozen T, Ozisik L, Basaran N. An overview and management of osteoporosis. Eur J Rheumatol. 2017;4:46–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu F, McDonald J. Disorders of bone remodeling. Annu Rev Pathol. 2011; 6: 121–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sobacchi C, Schulz A, Coxon FP, Villa A, Helfrich MH. Osteopetrosis: genetics, treatment and new insights into osteoclast function. Nat Rev Endocrinol. 2013;9(9):522–36. [DOI] [PubMed] [Google Scholar]
- 25.Cawthray J, Wasan E, Wasan K. Bone-seeking agents for the treatment of bone disorders. Drug DelivTransI Res. 2017;7:466–481. [DOI] [PubMed] [Google Scholar]
- 26.Skjødt MK, Frost M, Abrahamsen B. Side effects of drugs for osteoporosis and metastatic bone disease. Br J Clin Pharmacol. 2018;doi: 10.1111/bcp.13759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Davis S, Martyn-St James M, Sanderson J, Stevens J, Goka E, Rawdin A, Sadler S, Wong R, Campbell F, Stevenson M, Strong M, Selby P, Gittoes N. A systematic review and economic evaluation of bisphosphonates for the prevention of fragility fractures. Health Technol Assess. 2016. ;20(78): 1–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cremers S, Drake MT, Ebetino FH, Bilezikian JP, Russell RGG. Pharmacology of the bisphosphonates. Br J Clin Pharmacol. 2019; doi: 10.1111/bcp.13867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Seeman E, Martin TJ. Antiresorptive and anabolic agents in the prevention and reversal of bone fragility. Nat Rev Rheumatol. 2019; doi: 10.1038/s41584-019-0172-3 [DOI] [PubMed] [Google Scholar]
- 30.Sibonga JD, Iwaniec UT, Shogren KL, Rosen CJ, Turner RT. Effects of parathyroid hormone (1-34) on tibia in an adult rat model for chronic alcohol abuse. Bone. 2007;40:1013–1020. [DOI] [PubMed] [Google Scholar]
- 31.Gensure RC, Gardella TJ, Jüppner H. Parathyroid hormone and parathyroid hormone-related peptide, and their receptors. Biochem Biophys Res Commun. 2005; 18;328:666–678. [DOI] [PubMed] [Google Scholar]
- 32.Nikitovic D, Kavasi RM, Berdiacki A, Papachristou DJ, Tsiaoussis J, Spandidos DA, Tsatsakis A, Tzanakakis G. Parathyroid hormone/parathyroid hormone-related peptide regulate osteosarcoma cell functions: Focus on the extracellular matrix. Oncol Rep. 2016; 36: 1787–1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sibai T, Morgan EF, Einhorn T. Anabolic agents and bone quality. Clin Orthop Relat Res. 2011; 469: 2215–2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Boyce EG, Mai Y, Pham C. Abaloparatide: Review of a next-generation parathyroid hormone agonist. Ann Pharmacother. 2018;52(5):462–472. [DOI] [PubMed] [Google Scholar]
- 35.Saag KG, Petersen J, Brandi ML, Karaplis AC, Lorentzon M, Thomas T, Maddox J, Fan M, Meisner PD, Grauer A. Romosozumab or alendronate for fracture prevention in women with osteoporosis. N Engl J Med. 2017:12;377(15): 1417–1427. [DOI] [PubMed] [Google Scholar]
- 36.Hussain A, Jeffries M. Epigenetics and bone remodeling. Curr Osteoporos Rep. 2017; 15(5): 450–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ghaylor C, Weber FE. Epigenetic regulation of bone remodeling and its impact on osteoporosis. Int J Mol Sci. 2016;17(9). pii: E1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Feng Q, Zheng S, Zheng J. The emerging role of microRNAs in bone remodeling and its therapeutic implications for osteoporosis. Biosci Rep. 2018;38(3). pii: BSR20180453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reppe S, Datta H, Kaare M, Gautvik M. The Influence of DNA Methylation on bone cells. Curr Genomics. 2015; 16(6): 384–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Martin SL, Hardy TM, Tollefsbol TO. Medicinal chemistry of the epigenetic diet and caloric restriction. Curr Med Chem, 2013;20:4050–4059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Godfrey TC, Wildman BJ, Javad A, Legner C, Hassan M. Epigenetic remodeling and modification to preserve skeletogenesis in vivo. Connect Tissue Res. 2018; 59(Supp):52–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bergman Y, Cedar H. DNA methylation dynamics in health and disease. Nat Struct Mol Biol. 2014;21:274–281. [DOI] [PubMed] [Google Scholar]
- 43.Tate PH, Bird AP. Effects of DNA methylation on DNA-binding proteins and gene expression. Curr Opin Genet Dev. 1993;3(2):226–231. [DOI] [PubMed] [Google Scholar]
- 44.Godfrey T, Wildman B, Javad A, Lengner CJ, Hassan M. Epigenetic remodeling and modification to preserve skeletogenesis in vivo. Connect Tissue Res. 2018; 59(SUP1): 52–54. doi: 10.1080/03008207.2017.1408599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Altaf M, Saksouk N, Côté J. Histone modifications in response to DNA damage. Mutat Res. 2007;618(1-2):81–90. [DOI] [PubMed] [Google Scholar]
- 46.Meeran S, Ahmed A, Tollefsbol T. Epigenetic targets of bioactive dietary components for cancer prevention and therapy. Clin Epigenetics. 2010; 1 (3): 101–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Conte M, De Palma R, Altucci L. HDAC inhibitors as epigenetic regulators for cancer immunotherapy. Int J Biochem & Cell Biol. 2018;98:65–74. [DOI] [PubMed] [Google Scholar]
- 48.Dudakovic A, Camilleri ET, Paradise C, Samsonraj RM, Gluscevic M, Paggi C, Begun D, Khani F, Pichurin O, Ahmed FS, Elsayed R, Elsalanty M, Lawrence M, Karperin M, Reister S, Thaler R, Westendorf J, van Wijnen A. Enhancer of zeste homolog 2 (Ezh2) controls bone formation and cell cycle progression during osteogenesis in mice. J Biol Chem. 2018; 293(33): 12894–12907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dudakovic A, Camilleri ET, Riester SM, Paradise CR, Gluscevic M, O’Toole T, Thaler R, Evans JM, Yan H, Subramaniam M, Hawse JR, Stein GS, Montecino MA, McGee-Lawrence ME, Westendorf JJ, van Wijnen AJ. Enhancer of Zeste homolog 2 inhibition stimulates bone formation and mitigates bone loss caused by ovariectomy in skeletally mature mice. J. Biol. Chem. 2016;291: 24594–24606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tye CE, Boyd JR, Page NA, Falcone MM, Stein JL, Stein GS, Lian JB. Regulation of osteogenesis by long noncoding RNAs: An epigenetic mechanism contributing to bone formation. Connect Tissue Res. 2018;59(sup1):35–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Seeliger C, Karpinski K, Haug AT, Vester H, Schmitt A, Bauer JS.; van Griensven M. Five freely circulating miRNAs and bone tissue miRNAs are associated with osteoporotic fractures. J. Bone Miner. Res. 2014;29:1718–1728. [DOI] [PubMed] [Google Scholar]
- 52.Hackl M, Heilmeier U, Weilner S, Grillari J. Circulating microRNAs as novel biomarkers for bone diseases-Complex signatures for multifactorial diseases? Mol. Cell. Endocrinol. 2016;432:83–95. [DOI] [PubMed] [Google Scholar]
- 53.Meng P, Zhang D, Pan N, Sun N, Wang Q, Fan J, Zhou P, Zhu W, Jiang L. Identification of miR-194-5p as a potential biomarker for postmenopausal osteoporosis. Peer J. 2015; 3, e971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Panach L, Mifsut D, Tarin JJ, Cano A, Garcia-Perez MA. Serum circulating microRNAs as biomarkers of osteoporotic fracture. Calcif Tissue Int. 2015;97: 495–505. [DOI] [PubMed] [Google Scholar]
- 55.Wallace TC. Dried plums, prunes, and bone health: A comprehensive review. Nutrients 2017; 9:pii:E401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Arjmandi BH, Johnson SA, Pourafshar S, Navaei N, George KS, Hooshmand S, Chai SC, Akhavan NS. Bone-Protective effects of dried plum in postmenopausal women: Efficacy and possible mechanisms. Nutrients 2017;9(5).pii:E496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.An J, Yang H, Zhang Q, Liu C, Zhao J, Zhang L, Chen B. Natural products for treatment of osteoporosis: The effects and mechanisms on promoting osteoblast-mediated bone formation. Life Sci. 2016;147:46–58. [DOI] [PubMed] [Google Scholar]
- 58.Che CT, Wong M, Lam C. Natural products from Chinese medicines with potential benefits to bone health. Molecules 2016;21:239–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Baur JA, Sinclair DA. Therapeutic potential of resveratrol: the in vivo evidence. Nat Rev Drug Discov. 2006;54:93–506. [DOI] [PubMed] [Google Scholar]
- 60.Takaoka M The phenolic substances of white Hellebore (Veratrum grandiflorum Loes fil.) II. Nippon Kagaku Kaishi. 1939;60:1261–1264. [Google Scholar]
- 61.Mizutani K, Ikeda K, Kawai Y, Yamori Y. Resveratrol attenuates ovariectomy-induced hypertension and bone loss in stroke-prone spontaneously hypertensive rats. J Nutr Sci Vitaminol (Tokyo). 2000; 46:78–83. [DOI] [PubMed] [Google Scholar]
- 62.Tseng PC, Hou SM, Chen RJ, Peng H, Hsieh C, Kuo ML, Yen ML. Resveratrol Promotes osteogenesis of human mesenchymal stem cells by upregulating RUNX2 gene expression via the SIRT1/F0X03A Axis. J Bone Mineral Res 2011; 26:2552–2563. [DOI] [PubMed] [Google Scholar]
- 63.Ko JH, Sethi G, Um JY, Shanmugam MK, Arfuso F, Kumar AP, Bishayee A, Ahn KS. The Role of Resveratrol in Cancer Therapy. Int J Mol Sci. 2017; 18(12). pii: E2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wahab A, Gao K, Jia C, Zhang F, Tian G, Murtaza G, Chen J. Significance of Resveratrol in clinical management of chronic diseases. Molecules. 2017;22(8). pii: E1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fernandes GFS, Silva GDB, Pavan AR, Chiba DE, Chin CM, Santos JLD. Epigenetic regulatory mechanisms induced by resveratrol. Nutrients. 2017;9:1201–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ornstrup MJ, Harsløf T, Kjær TN, Langdahl BL, Pedersen SB. Resveratrol Increases Bone Mineral Density and Bone Alkaline Phosphatase in Obese Men: A Randomized Placebo-Controlled Trial. Journal of Clinical Endocrinol Metabol 2014; 99: 4720–4729. [DOI] [PubMed] [Google Scholar]
- 67.Bo S, Gambino R, Ponzo V, Cioffi I, Goitre I, Evangelista A, Ciccone G, Cassader M, Procopio M. Effects of resveratrol on bone health in type 2 diabetic patients. A double-blind randomized-controlled trial. Nutrition & Diabetes 2018; 8: 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lee AM, Shandala T, Nguyen L, Muhlhausler BS, Chen KM, Howe PR, Xian CJ. Effects of resveratrol supplementation on bone growth in young rats and microarchitecture and remodeling in ageing rats. Nutrients. 2014;6(12):5871–5887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Durbin SM, Jackson JR, Ryan MJ, Gigliotti JC, Alway SE, Tou JC. Resveratrol supplementation influences bone properties in the tibia of hindlimb-suspended mature Fisher 344 × Brown Norway male rats. Appl Physiol Nutr Metab. 2012;37(6):1179–1188. [DOI] [PubMed] [Google Scholar]
- 70.Su P, Tian Y, Yang C, Ma X, Wang X, Pei J, Qian A. Mesenchymal stem cell migration during bone formation and bone diseases therapy. Int J Mol Sci. 2018;19(8). pii: E2343.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mizutani K, Ikeda K, Kawai Y, Yamori Y. Resveratrol stimulates the proliferation and differentiation of osteoblastic MC3T3-E1 cells. Biochem Biophys Res Commun. 1998;253: 859–863. [DOI] [PubMed] [Google Scholar]
- 72.Danks L, Komatsu N, Guerrini MM, Sawa S, Armaka M, Kollias G, Nakashima T, Takayanagi H. RANKL expressed on synovial fibroblasts is primarily responsible for bone erosions during joint inflammation. Ann Rheum Dis. 2016;75(6):1187–95. [DOI] [PubMed] [Google Scholar]
- 73.Shakibaei M, Buhrmann C, Mobasheri A. Resveratrol-mediated SIRT-1 interactions with p300 modulate receptor activator of NF-κ-B Ligand (RANKL) activation of NF-κ-B signaling and Inhibit osteoclastogenesis in bone-derived cells J Biol Chem. 2011; 286:11492–11505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kulkarni SS, Cantó C. The molecular targets of resveratrol. Biochim. Biophys. Acta. 2015;1852:1114–1123. [DOI] [PubMed] [Google Scholar]
- 75.Lançon A, Kaminski J,Tili E, Michaille JJ, Latruffe N. Control of MicroRNA expression as a new way for resveratrol to deliver its beneficial effects. J Agric Food Chem. 2012;60: 8783–8789. [DOI] [PubMed] [Google Scholar]
- 76.Latruffe N, Langon A, Frazzi R, Aires V, Delmas D, Michaille JJ, Djouadi F, Bastin J, Cherkaoui-Malki M. Exploring new ways of regulation by resveratrol involving miRNAs, with emphasis on inflammation. Ann NY Acad Sci. 2015; 1348, 97–106. [DOI] [PubMed] [Google Scholar]
- 77.Britton RG, Kovoor C, Brown K. Direct molecular targets of resveratrol: Identifying key interactions to unlock complex mechanisms. Ann NY Acad Sci. 2015;1348:124–133. [DOI] [PubMed] [Google Scholar]
- 78.Farghali H, Kutinová CN, Leki’c N. Resveratrol and related compounds as antioxidants with an allosteric mechanism of action in epigenetic drug targets. Physiol Res. 2013;62:1–13. [DOI] [PubMed] [Google Scholar]
- 79.Lobo RA. Benefits and risks of estrogen replacement therapy. Am J Obstet. Gynecol. 1995; 173, 982–989. [DOI] [PubMed] [Google Scholar]
- 80.Lian JB, Javed A, Zaidi SK, Lengner C, Montecino M. Regulatory controls for osteoblast growth and differentiation: role of Runx/Cbfa/AML factors. Crit Rev Eukaryot Gene Expr. 2004; 14: 1–41. [PubMed] [Google Scholar]
- 81.Bäckesjö CM, Li Y, Lindgren U, Haldosen LA. Activation of SIRT1 decreases adipocyte formation during osteoblast differentiation of mesenchymal stem cells. J Bone Min Res. 2006;21:993–1002. [DOI] [PubMed] [Google Scholar]
- 82.Song LH, Pan W, Yu YH, Quarles LD, Zhou HH. Resveratrol prevents CsA inhibition of proliferation and osteoblastic differentiation of mouse bone marrow-derived mesenchymal stem cells through an ER/NO/cGMP pathway. Toxicol In Vitro. 2006;20: 915–922. [DOI] [PubMed] [Google Scholar]
- 83.Tseng PC, Hou SM, Chen RJ, Peng HW, Hsieh CF. Resveratrol promotes osteogenesis of human mesenchymal stem cells by upregulating RUNX2 gene expression via the SIRT1/F0X03A axis. J Bone Miner Res. 2011. ;26: 2552–2563. [DOI] [PubMed] [Google Scholar]
- 84.Shakibaei M, Shayan P, Busch F, Aldinger C, Buhrmann C. Resveratrol mediated modulation of Sirt-1/Runx2 promotes osteogenic differentiation of mesenchymal stem cells: Potential role of Runx2 deacetylation. PLoS ONE. 2012; 7(4): e35712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lee YM, Shin SI, Shin KS, Lee YR, Park BH, Kim EC. The role of sirtuin 1 in osteoblastic differentiation in human periodontal ligament cells. J Periodontal Res. 2011. ;46(6):712–721. [DOI] [PubMed] [Google Scholar]
- 86.He N, Zhu XW, He W, Zhao S, Zhao WY, Zhu C. Resveratrol inhibits the hydrogen dioxide-induced apoptosis via Sirt 1. Biosci, Biotechnol, Biochem. 2015;79:1779–1786. [DOI] [PubMed] [Google Scholar]
- 87.Zhao M, Ko SY, Garrett IR, Mundy GR, Gutierrez GE, Edwards JR. In addition, RES promoted skeletal growth in mice by increasing the SIRT1-bone morphogenic protein 2 longevity axis. Br J Pharmacol. 2018; 175(21 ):4183–4192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wicks SM, Huang S, Raut N, Yu TS, Winkler C, Ren ZT, Lee SM, Mahady GB. Resveratrol and dietary anthocyanins reduce osteoblast apoptosis, increase osteoblast proliferation, and reduce osteoclastogenesis in transgenic medaka. 24th International Conference of the Functional Foods Center - 12th International Symposium of ASFFBC, Functional Foods and Chronic Diseases: Science and Practice, September 20-21, 2018, Harvard Medical School, Boston, MA, USA Abstract. [Google Scholar]
- 89.Wicks SM, Raut N, Yu TS, Winkler C, Ren ZT, Lee SM, Mahady GB. Resveratrol and dietary anthocyanins reduce osteoblast apoptosis, increase osteoblast function and reduce osteoclastogenesis in transgenic medaka. American Society of Pharmacognosy annual meeting, Lexington KY, July 2018, Abstract. [Google Scholar]
- 90.Zainabadi K, Liu CJ, Caldwell ALM, Guarente L. SIRT1 is a positive regulator of in vivo bone mass and a therapeutic target for osteoporosis. PLoS One. 2017; 12(9):e0185236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Desquiret-Dumas V, Gueguen N, Leman G. Resveratrol induces a mitochondrial complex I-dependent increase in NADH oxidation responsible for sirtuin activation in liver cells. J Biol Chem. 2013;288(51):36662–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Liang H, Ward W. PGC-1α: a key regulator of energy metabolism. Adv. Physiol Edu. 2006;30:141–156. [DOI] [PubMed] [Google Scholar]
- 93.Raut N, Wicks SM, Yu TS, Winkler C, Ren ZT, Lee SM, Mahady GB. Dietary Anthocyanins and Resveratrol reduce osteoblast apoptosis, increase osteoblast function and differentiation in transgenic medaka via SIRT1/3 and PGC-1α. J Bone Biol. Osteoporosis. Invited manuscript, 2019, in press. [Google Scholar]
- 94.Wang H, Hu Z, Wu J, Mei Y, Zhang Q, Zhang H, Miao D, Sun W. Sirtl promotes osteogenic differentiation and increases alveolar bone mass via Bmi1 activation in mice. J Bone Miner Res. 2019;28:e3677. [DOI] [PubMed] [Google Scholar]
- 95.Fahey JW, Zhang Y, Talalay P. Broccoli sprouts: an exceptionally rich source of inducers of enzymes that protect against chemical carcinogens. Proc. Natl. Acad. Sci. U S A. 1997;94:10367–10372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Liang H, Yuan Q, Liu M. Simultaneous determination of glucoraphanin and sulforaphane in Brassica oleracea seeds by high-performance liquid chromatography with evaporative light-scattering detector. Nat. Prod. Res. 2013;27: 194–197. [DOI] [PubMed] [Google Scholar]
- 97.Zhang Y, Talalay P, Cho, CG, Posner A major inducer of anticarcinogenic protective enzymes from broccoli: isolation and elucidation of structure. Proc. Natl. Acad. Sci. U.S.A. 1992;89:2399–2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhang Z, Wang S, Zhou S, Yan X, Wang Y, Chen J, Mellen N, Kong M, Gu J, Tan Y, Zheng Y, Cai L. Sulforaphane prevents the development of cardiomyopathy in type 2 diabetic mice probably by reversing oxidative stress-induced inhibition of LKB1/AMPK pathway. J. Mol. Cell. Cardiol. 2014;77:42–52. [DOI] [PubMed] [Google Scholar]
- 99.Myzak MC, Karplus PA, Chung FL, Dashwood RH. A novel mechanism of chemoprotection by sulforaphane: inhibition of histone deacetylase. Cancer Res.2004;64:5767–5774. [DOI] [PubMed] [Google Scholar]
- 100.Rajendran P, Kidane AI, Yu TW, Dashwood WM, Bisson WH, Löhr CV, Ho E, Williams DE, Dashwood RH. HDAC turnover, CtIP acetylation and dysregulated DNA damage signaling in colon cancer cells treated with sulforaphane and related dietary isothiocyanates. Epigenetics. 2013; 8:612–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Takahashi N, Maeda K, Ishihara A, Uehara S, Kobayashi Y. Regulatory mechanism of osteoclastogenesis by RANKL and Wnt signals. Front Biosci (Landmark Ed). 2011; 16:21–30 [DOI] [PubMed] [Google Scholar]
- 102.Xue M, Qian Q, Adaikalakoteswari A, Rabbani N, Babaei-Jadidi R, Thornalley PJ. Activation of NF-E2-related factor-2 reverses biochemical dysfunction of endothelial cells induced by hyperglycemia linked to vascular disease. Diabetes. 2008; 57:2809–2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Facchini A, Stanic I, Cetrullo S, Borzl RM, Filardo G, Flamigni F. Sulforaphane protects human chondrocytes against cell death induced by various stimuli. J. Cell. Physiol. 2011;226:1771–1779. [DOI] [PubMed] [Google Scholar]
- 104.Clarke JD, Flsu A, Yu Z, Dashwood RH, Flo E. Differential effects of sulforaphane on histone deacetylases, cell cycle arrest and apoptosis in normal prostate cells versus hyperplastic and cancerous prostate cells. Mol. Nutr. Food Res. 2011. ;55; 999–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Davidson RK, Jupp O, de Ferrars R, Kay CD, Culley KL, Norton R, Driscoll C, Vincent TL, Donell ST, Bao Y, Clark IM. Sulforaphane represses matrixdegrading proteases and protects cartilage from destruction in vitro and in vivo. Arthritis Rheum. 2013;65, 3130–3140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kim SJ, Kang SY, Shin HH, Choi HS. Sulforaphane inhibits osteoclastogenesis by inhibiting nuclear factor-B. Mol Cell. 2005;20:364–370. [PubMed] [Google Scholar]
- 107.Ko JY, Choi YJ, Jeong GJ, Im Gl. Sulforaphane-PLGA microspheres for the intra-articular treatment of osteoarthritis. Biomaterials. 2013;34:5359–5368. [DOI] [PubMed] [Google Scholar]
- 108.Thaler R, Spitzer S, Karlic H, Klaushofer K, Varga F. DMSO is a strong inducer of DNA hydroxymethylation in pre-osteoblastic MC3T3-E1 cells. Epigenetics. 2012;7:635–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Thaler R, Maurizi A, Roschger P, Sturmlechner I, Khani F, Spitzer S, Rumpler M, Zwerina J, Karlic H, Dudakovic A, Klaushofer K, Teti A, Rucci N, Varga F, van Wijnen AJ. Anabolic and antiresorptive modulation of bone homeostasis by the epigenetic modulator Sulforaphane, a naturally occurring Isothiocyanate. J Biol Chem. 2016;291 (13):6754–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Gambari L, Lisignoli G, Cattini L, Manferdini C, Facchini A, Grassi F. Sodium hydrosulfide inhibits the differentiation of osteoclast progenitor cells via NRF2-dependent mechanism. Pharmacol. Res. 2014;87:99–112. [DOI] [PubMed] [Google Scholar]
- 111.Gong AG, Fluang VY, Wang HY, Lin HQ, Dong TT, Tsim KW. Ferulic acid orchestrates anti-oxidative properties of Danggui Buxue Tang, an ancient herbal decoction: elucidation by chemical knock-out approach. PLoS One 2016; 11, e0165486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lai YL, Yamaguchi M. Phytocomponent p-hydroxycinnamic acid stimulates bone formation and inhibits bone resorption in rat femoral tissues in vitro. Mol. Cell. Biochem. 2006;29: 45–52. [DOI] [PubMed] [Google Scholar]
- 113.Sagar T, Rantlha M, Kruger MC, Coetzee M, Deepak V. 2016 Ferulic acid impairs osteoclast fusion and exacerbates survival of mature osteoclasts. Cytotechnology 2016;68: 1963–1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sassa S, Kikuchi T, Shinoda H, Suzuki S, Kudo H, Sakamoto S. Preventive effect of ferulic acid on bone loss in ovariectomized rats. In Vivo 2003; 17, 277–280. [PubMed] [Google Scholar]
- 115.Seo MJ, Kim JK, Lee SH, Wang FF, Kang YR, Kang MH, Kwon YI, Jang HD. Ferulic acid ethyl ester attenuates inflammatory response by suppressing ROS-related NF-κβ signaling pathway in LPS-induced RAW264. 7 cells, FASEB J. 2015;29:913–915. [Google Scholar]
- 116.Doss HM, Samarpita S, Ganesan R, Rasool M. Ferulic acid, a dietary polyphenol suppresses osteoclast differentiation and bone erosion via the inhibition of RANKL dependent NF-κβ signaling pathway. Life Sci. 2018; 207:284–295. [DOI] [PubMed] [Google Scholar]
- 117.Du K, Li Z, Fang X, Cao T, Xu Y. Ferulic acid promotes osteogenesis of bone marrow-derived mesenchymal stem cells by inhibiting microRNA-340 to induce β-catenin expression through hypoxia. Eur J Cell Biol. 2017;96(6):496–503. [DOI] [PubMed] [Google Scholar]
- 118.Pacheco-Palencia LA, Mertens-Talcott S, Talcott ST. Chemical composition, antioxidant properties, and thermal stability of a phytochemical enriched oil from acai (Euterpe oleracea Mart.). J Agric Food Chem 2008;56:4631–4636. [DOI] [PubMed] [Google Scholar]
- 119.Itoh A, Isoda K, Kondoh M, Kawase M, Kobayashi M, Tamesada M, Yagi K. Hepatoprotective effect of syringic acid and vanillic acid on concanavalin a-induced liver injury. Biol Pharm Bull 2009;32:1215–1219. [DOI] [PubMed] [Google Scholar]
- 120.Itoh A, Isoda K, Kondoh M, Kawase M, Watari A, Kobayashi M, Tamesada M, Yagi K. Hepatoprotective effect of syringic acid and vanillic acid on CCI4-induced liver injury. Biol Pharm Bull 2010;33:983–987. [DOI] [PubMed] [Google Scholar]
- 121.Simoncini T, Lenzi E, Zöchling A, Gopal S, Goglia L, Russo E, Polak K, Casarosa E, Jungbauer A, Genazzani AD, Genazzani AR. Estrogen-like effects of wine extracts on nitric oxide synthesis in human endothelial cells. Maturitas 2011; 70:169–175. [DOI] [PubMed] [Google Scholar]
- 122.Nakajima Y, Sato Y, Konishi T. Antioxidant small phenolic ingredients in Inonotus obliquus (persoon) Pilat (Chaga). Chem Pharm Bull. 2007;55:1222–1226. [DOI] [PubMed] [Google Scholar]
- 123.Yan SL, Wang ZH, Yen HF, Lee YJ, Yin MC. Reversal of ethanol-induced hepatotoxicity by cinnamic and syringic acids in mice. Food Chem Toxicol 2016;98:119–126. [DOI] [PubMed] [Google Scholar]
- 124.Kang SN, Lee JS, Park JH, Cho JH, Park JH, Cho KK, Lee OH, Kim IS. In vitro anti-osteoporosis properties of diverse Korean Drynariae rhizoma phenolic extracts. Nutrients 2014;6:1737–1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Tanaka T, Kawaguchi N, Zaima N, Moriyama T, Fukuta Y, Shirasaka N. Anti-osteoporotic activity of a syringic acid diet in ovariectomized mice. J Nat Med. 2017;71:632–641. [DOI] [PubMed] [Google Scholar]
- 126.Arumugam B, Balagangadharan K, Selvamurugan N. Syringic acid, a phenolic acid, promotes osteoblast differentiation by stimulation of Runx2 expression and targeting of Smad7 by miR-21 in mouse mesenchymal stem cells. J Cell Commun Signal. 2018;12:561–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Cerletti C, De Curtis A, Bracone F, Digesu C, Morganti AG, lacoviello L, de Gaetano G, Donati MB. Dietary anthocyanins and health: data from FLORA and ATHENA EU projects. Br J Clin Pharmacol. 2017;83:103–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Mulabagal V, Calderón AI. Liquid chromatography/mass spectrometry based fingerprinting analysis and mass profiling of Euterpe oleracea (açaí) dietary supplement raw materials. Food Chemistry 2012; 134:1156–1164. [DOI] [PubMed] [Google Scholar]
- 129.Mulabagal V, Keller WJ, Calderon AI. Quantitative analysis of anthocyanins in Euterpe oleracea (açaí) dietary supplement raw materials and capsules by Q-TOF LC/MS. Pharmaceut Biol. 2012; 50:1289–1296. [DOI] [PubMed] [Google Scholar]
- 130.Smeriglio A, Barreca D, Bellocco E, Trombetta D. Chemistry, Pharmacology and Health Benefits of Anthocyanins. Phytother Res. 2016;30(8):1265–1286. [DOI] [PubMed] [Google Scholar]
- 131.Sehitoglu MH, Farooqi AA, Qureshi MZ, Butt G, Aras A. Anthocyanins: targeting of signaling networks in cancer cells. Asian Pac J Cancer Prev. 2014;15:2379–2381. [DOI] [PubMed] [Google Scholar]
- 132.Wallace TC, Giusti MM. Anthocyanins. Adv Nutr. 2015;6:620–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wu XL, Prior R. Identification and characterization of anthocyanins by high-performance liquid chromatography-electrospray ionization tandem mass spectrometry in common food in the United States: vegetables, nuts and grains. J. Agri Food Chem. 2006;53:3101–3113. [DOI] [PubMed] [Google Scholar]
- 134.Welch A, MacGregor A, Jennings A, Fairweather-Tait S, Spector T, Cassidy A. Habitual flavonoid intakes are positively associated with bone mineral density in women. J Bone Miner Res. 2012;27:1872–1878. [DOI] [PubMed] [Google Scholar]
- 135.Domitrovic R The molecular basis for the pharmacological activity of anthocyanins. Curr Med Chem. 2011;18:4454–4465. [DOI] [PubMed] [Google Scholar]
- 136.Welch A, MacGregor A, Jennings A, Fairweather-Tait S, Spector T, Cassidy A. Habitual flavonoid intakes are positively associated with bone mineral density in women. J Bone Min Res. 2012; 27:1872–1878. [DOI] [PubMed] [Google Scholar]
- 137.Arjmandi B, Lucas E, Juma S, Soliman A, Stoecker B, Khalil D, et al. Dried plums prevent ovariectomy-induced bone loss in rats. JANA. 2001;4:50–56. [Google Scholar]
- 138.Deyhim F, Stoecker BJ, Brusewitz GH, Devareddy L, Arjmandi BH. Dried plum reverses bone loss in an osteopenic rat model of osteoporosis. Menopause. 2005;12:755–62. [DOI] [PubMed] [Google Scholar]
- 139.Franklin M, Bu SY, Lerner MR, Lancaster EA, Bellmer D, Marlow D, et al. Dried plum prevents bone loss in a male osteoporosis model via IGF-I and the RANK pathway. Bone. 2006;39:1331–1342. [DOI] [PubMed] [Google Scholar]
- 140.Devareddy L, Hooshmand S, Collins JK, Lucas EA, Chai SC, Arjmandi BH. Blueberry prevents bone loss in ovariectomized rat model of postmenopausal osteoporosis. J Nutr Biochem. 2008;19:694–699. [DOI] [PubMed] [Google Scholar]
- 141.Chen JR, Lazarenko OP, Wu X, Kang J, Blackburn ML, Shankar K. Dietary-induced serum phenolic acids promote bone growth via p38 MAPK/beta-catenin canonical Wnt signaling. J Bone Miner Res. 2010;25:2399–2411. [DOI] [PubMed] [Google Scholar]
- 142.Zhang J, Lazarenko OP, Blackburn ML, Shankar K, Badger TM, Ronis MJ. Feeding blueberry diets in early life prevent senescence of osteoblasts and bone loss in ovariectomized adult female rats. PLoS One. 2011;6(9):e24486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Kaume L, Gadang V, Gilbert W, Wang L, Smith BJ, Devareddy L. Dose-dependent effects of blackberries in the prevention of bone loss in ovariectomized rats. J Bone Miner Res. 2010;25 Suppl 1:SU0431. [Google Scholar]
- 144.Wu XL, Prior R. Identification and characterization of anthocyanins by high-performance liquid chromatography-electrospray ionization tandem mass spectrometry in common food in the United States: vegetables, nuts and grains. J Agri Food Chem. 2006;53:3101–3113. [DOI] [PubMed] [Google Scholar]
- 145.Moriwaki S, Suzuki K, Muramatsu M, Nomura A, Inoue F, Into T, Yoshiko Y, Niida S. Delphinidin, one of the major anthocyanidins, prevents bone loss through the inhibition of excessive osteoclastogenesis in osteoporosis model mice. PLoS One. 2014;9(5):e97177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Raut N, Lawal TO, Wicks SM, Patel S, Mahady GB. Epigenetic regulation of osteoblastogenesis by blackcurrant fruit extracts in vitro and in vivo. Abstract. FASEB J. 2019;in press. [Google Scholar]
- 147.Raut N, Wicks SM, Yu TS, Winkler C, Ren Z, Lee SM, Mahady GB. Osteogenic effects of blackcurrant and acai extracts in human hFOB osteoblasts and transgenic medaka. 24th International Conference of the Functional Foods Center - 12th International Symposium of ASFFBC, Functional Foods and Chronic Diseases: Science and Practice, September 20-21,2018, Harvard Medical School, Boston, MA, USA Abstract. [Google Scholar]
- 148.Yu TS, Mahady GB, Winkler C. A medaka osteoporosis model for testing the activity of bone anabolic and anti-resorptive compounds. 8th Aquatic Animal Models of Human Disease Conference, January 7-12, 2017 University of Alabama, Birmingham, AL USA Abstract. [Google Scholar]
- 149.Imangali N, Mahady GB, Winkler C. Osteogenic effects of Delphinidin in a medaka model for osteoporosis. 22nd Biological Sciences Graduate Congress (BSGC) at National University of Singapore, 19-21 Dec 2017 Abstract. [Google Scholar]
- 150.Raut N, Lawal TO, Wicks SM, Patel S, Mahady GB. Acai fruit extracts and anthocyanins alter osteoblast proliferation and the expression of HDAC1 and osterix/Sp7 in vitro and in vivo. Abstract. FASEB 2019;in press. [Google Scholar]
- 151.Eggers-Woodard NA, Wicks SM, Raut N, Yu TS, Winkler C, Mahady GB, Carcache de Blanco EJ. Acai exhibits anti-diabetic and osteogenic activities in fish models. American Society of Pharmacognosy annual meeting, July 21—25, 2018 Lexington, KY Abstract. [Google Scholar]
- 152.Pietila M, Lehtonen S, Narhi M, Hassinen IE, Leskela HV, Aranko K, Nordstrom K, Vepsalainen A, Lehenkari P. Mitochondrial function determines the viability and osteogenic potency of human mesenchymal stem cells. Tissue Eng Part C. Methods. 2010; 16:435–445. [DOI] [PubMed] [Google Scholar]
- 153.Kalani A, Kamat PK, Voor MJ, Tyagi SC, Tyagi N. Mitochondrial Epigenetics in Bone Remodeling during hyperhomocysteinemia. Mol Cell Biochem. 2014;395: 89–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Tonna EA, Pillsbury N. Changes in the osteoblastic and mitochondrial population of aging periosteum. Nature. 1959; 183:337–338. [DOI] [PubMed] [Google Scholar]
- 155.Meyer MH, Meyer RA Jr. Altered expression of mitochondrial genes in response to fracture in old rats. Acta Orthop. 2006; 77:944–951. 39. [DOI] [PubMed] [Google Scholar]
- 156.Komarova SV, Ataullakhanov FI, Globus RK. Bioenergetics and mitochondrial transmembrane potential during differentiation of cultured osteoblasts. Am J Physiol Cell Physiol. 2000; 279:C1220–C1229. [DOI] [PubMed] [Google Scholar]
- 157.An JH, Yang JY, Ahn BY, Cho SW, Jung JY, Cho HY, Cho YM, Kim SW, Park KS, Kim SY, Lee HK, Shin CS. Enhanced mitochondrial biogenesis contributes to Wnt induced osteoblastic differentiation of C3H10T1/2 cells. Bone. 2010; 47:140–150. [DOI] [PubMed] [Google Scholar]


