Summary
Compared with the noble-metal surface-enhanced Raman scattering (SERS) substrates activated by the surface plasmon resonance (SPR)-induced electromagnetic mechanism (EM), the relative low sensitivity and stability of the chemical mechanism (CM)-based substrates are the biggest obstacles to their applications. Herein, we report that quasi-metallic VO2 nanosheet arrays can be used as a sensitive and stable SERS substrate. The lowest detectable limit of analyte adsorbed on the VO2 nanosheets achieves 10−10 M and the maximum Raman enhancement factor (EF) reaches 6.7 × 107, which is comparable with that of the noble metals. The experimental and theoretical results demonstrate that the SERS performance of the VO2 nanosheets comes from the strong interfacial interactions based on charge transfer and the vigorous SPR effects. Our research results demonstrate that quasi-metals are very promising SERS detection platforms and reveal that CM, like EM, contributes significantly to the SERS activity of quasi-metals.
Subject Areas: Physical Chemistry, Electromagnetics, Materials Property, Nanomaterials
Graphical Abstract
Highlights
-
•
Surface-enhanced Raman scattering (SERS) on quasi-metallic VO2
-
•
High SERS enhancement factor and low limit of detection have been achieved
-
•
Synergistic effect of electromagnetic enhancement and chemical enhancement
Physical Chemistry; Electromagnetics; Materials Property; Nanomaterials
Introduction
Since Fleischmann and Van Duyne et al. groundbreakingly discovered that weak Raman signals of molecules can be drastically amplified by surface-enhanced Raman scattering (SERS) on rough silver surface (Fleischmann et al., 1974, Jeanmaire and Van Duyne, 1977, Moskovits, 1978), this technology already has become a very important label-free detection method in trace and even single-molecule levels (Nie and Emory, 1997, Kneipp et al., 1997). As a highly sensitive, non-contact, and non-destructive technology, SERS has been widely adopted in environmental detection (Li et al., 2010, Mulvihill et al., 2008), biological imaging (Palonpon et al., 2013, Kneipp et al., 2008, Qian et al., 2008), medical diagnostics (Schlucker, 2014, Wang et al., 2012), fingerprint molecular distinguishing (Lin et al., 2009, Alvarez-Puebla and Liz-Marzan, 2012), catalytic reaction monitoring, and other fields (Peksa et al., 2015, Li et al., 2015). The traditional SERS substrate materials are based on noble-metal nanostructures with roughened surfaces (Taylor et al., 2013, Zhang et al., 2013, Kanipe et al., 2016, Phan-Quang et al., 2015), which can greatly enhance the Raman signal intensity of analyte adsorbed on the substrate surfaces with a factor of 106 or higher. Local field enhancement-induced surface plasmon resonance (SPR), especially the emergence of a large number of “hot spots” (high-intensity electromagnetic field regions formed at nanoscale gaps) (Zhu et al., 2016), is considered as a well-known enhancement mechanism of Raman scattering, that is, electromagnetic mechanism (EM). However, EM-based noble-metal SERS substrates are generally subjected to the considerable complex and precise preparation processes, which makes them lack the structural controllability and signal repeatability of the substrates. In addition, they also suffer from the high cost, poor biocompatibility, neglectable photocorrosion, and so on. Another universally accepted enhancement mode, chemical mechanism (CM), mainly refers to the process of charge transfer between SERS substrates and molecules adsorbed on their surfaces (Zhang et al., 2015, Quagliano, 2004, Li et al., 2013). Charge transfer will cause molecule resonance, which will greatly increase the polarizability of adsorbed molecules, and the corresponding Raman scattering cross-section will increase, resulting in the enhancement of SERS signal (Qiu et al., 2015, Cong et al., 2015, Zheng et al., 2017). Although researchers have accepted the concept that SERS is a result of EM and CM combining, it is generally believed that CM plays only a minor role since charge transfer is a short-range action.
After the first generation of SERS technology based on the noble-metal materials dominated by EM, the non-noble-metal substrates based on CM have been developed vigorously in the last 10 years, such as semiconductor nanostructures including Cu2O (Lin et al., 2017, Lin et al., 2018), Si (Wang et al., 2011), W18O49 (Cong et al., 2015), TiO2 (Qi et al., 2014), MoO2 (Zhang et al., 2017), conductive polymers (Yilmaz et al., 2017), and metal-organic framework compounds (Sun et al., 2019), which constitute the second-generation of SERS substrates. Interestingly, amorphous semiconductors have recently been found to have better SERS performance than crystalline ones (Wang et al., 2019; Wang et al., 2017; Li et al., 2018a, Li et al., 2018b). Compared with noble-metal SERS substrates, nanostructural semiconductors possess tailorable band structure and more abundant resonance modes, and therefore can controlled detect target analyte with corresponding excitation-wavelength (532, 633, 785 nm, etc.). In addition to these features, semiconductor SERS substrates also have superior biocompatibility, easy-to-control morphology, and more abundant surface states and active sites. However, for the two most important parameters of SERS, Raman enhancement factors (EFs) and lowest detectable limit of analyte, CM-based semiconducting materials are generally much lower than that of noble metals driven by EM. Furthermore, compared with noble metals, the chemical stability and thermal stability of semiconducting materials are often poor, especially when they are exposed to the excitation light of Raman spectrometer; they are often oxidized or decomposed, thus losing their SERS activity. For example, although W18O49 (Cong et al., 2015) and Cu2O (Lin et al., 2017, Lin et al., 2018) have recently achieved excellent EFs of 105–106 levels, they are easily oxidized by O2 in the air, thus unavoidably losing their SERS capability. Therefore, it is an urgent problem to find low-cost non-noble-metal SERS substrate materials with high sensitivity, stability, and large-area signal uniformity for the practical application.
Based on the above-mentioned analysis, we hope to find highly sensitive non-noble-metal SERS active materials and explore whether CM and EM have comparable contributions to their SERS effects. Transition metal oxides often contain abundant family members with different valences (e.g., WO3, WO2.9, WO2.8, WO2.72, WO2) (Manthiram and Alivisatos, 2012, Xi et al., 2012a, Xi et al., 2012b). Among them, the high-valence species are often insulators or wide-band-gap semiconductors, whereas the low-valence members often have good conductivity and localized-SPR effects owing to their abundant d-orbital free electrons, that is to say, low-valence members often have both certain metallicity and semiconductivity, or quasi-metallicity. It is well known that strong localized SPR and appropriate energy level distribution are the essential conditions for EM and CM enhancements, respectively; therefore, if we can find a quasi-metallic metal oxide that has both strong charge transfer and localized SPR effects as SERS materials, it is possible to obtain high sensitivity due to the combination of EM and CM.
As a very interesting transition metal oxide with intermediate valence state, VO2 nanostructures have been deeply studied and widely applied in fabrication of intelligent temperature-controlled films and electronic devices with ultralow power consumption due to its magical phase transition of insulator-conductor properties (Qazilbash et al., 2007, Strelcov et al., 2009, Morrison et al., 2014). VO2 has also recently been reported as a smart glass and high performance battery material (Hao et al., 2018, Li et al., 2019). However, the SERS properties are seldom studied. Herein, we report that quasi-metallic VO2 nanosheet arrays grown on ordinary glass with 16 cm2 scale can be used as an effective SERS substrate material with outstanding Raman enhancement effect. As a result of the dual enhancement of EM and CM of the VO2 substrate, the lowest detectable limit to the typical Raman probe molecule Rhodamine 6G (R6G) on the VO2 nanosheet arrays can be as low as 10−10 M level and the maximum EF is up to 6.7 × 107, which are even compared with the commercialized Au SERS substrates. From the perspective of application and fundamental science, our results demonstrate that quasi-metals can be used as ultrasensitive and stable SERS platforms and reveal that CM and EM both contribute equally to the total SERS performance of quasi-metallic materials.
Results
Selection and Structural Design of SERS Substrate Candidate
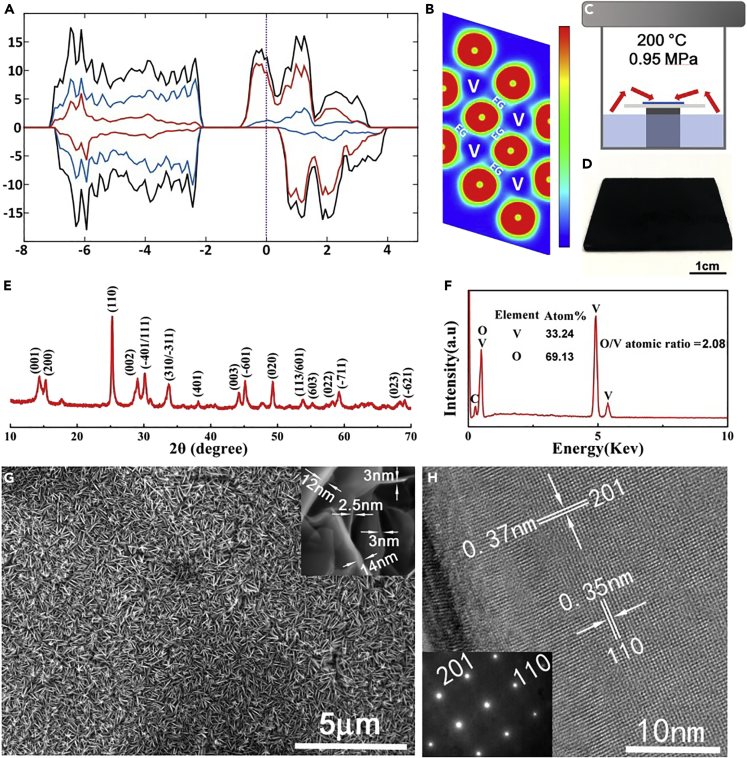
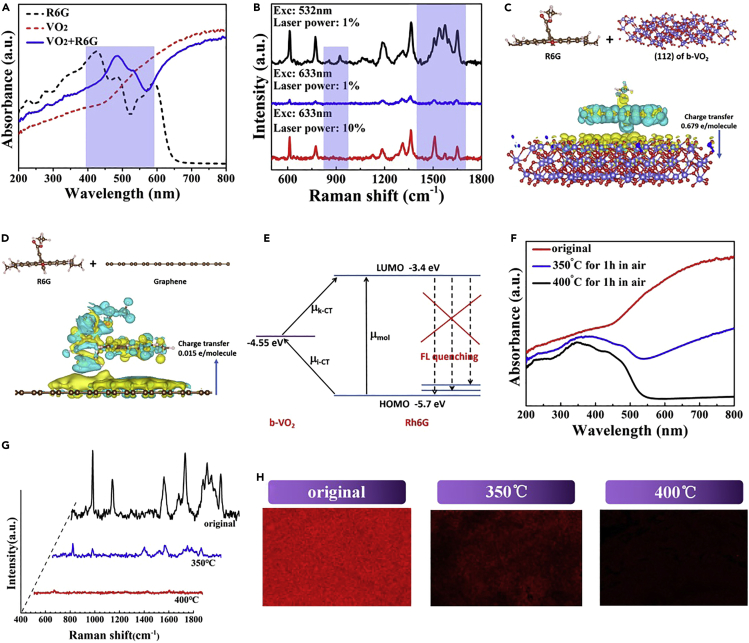
As a very interesting transition metal oxide, VO2 has many crystal structures, such as m-VO2 (monoclinic), t-VO2 (triclinic), r-VO2 (rutile), and a-VO2 (tetragonal). Among them, m-VO2 contains a metastable b-VO2 (bronze phase) (Whittaker et al., 2009). We chose b-VO2 as the candidate for a new SERS active material because theoretical calculations forecast that this structure has an obvious quasi-metallic feature, which is different from the most studied m-VO2, which exhibits typical semiconductor characteristics at room temperature. As shown in Figure 1A, the results of simulated density functional theory (DFT) calculations clearly show that b-VO2 present a definite quasi-metallic characteristic. It can be seen that the calculated band gap is about 1.15 eV. At the same time, the highest occupied states of the b-VO2 near the Fermi level are composed of V 3d orbitals and O 2p orbitals. Combined with these characteristics, therefore, b-VO2 can be considered as a typical quasi-metal. Furthermore, the free electron gas distribution, which was achieved by conducting the electron localization functions (ELF) calculation, also suggests that there are non-zero free electron tunnels between V atoms (Figure 1B). These tunnels of free electrons could form non-polar V-V metallic bonds, giving b-VO2 conducting properties. From the perspective of SERS, the quasi-metal characteristic of b-VO2 suggests that it may have the synergistic enhancement effect of charge-transfer-induced CM and localized SPR-driven EM.
Figure 1.
Electric Structures, Synthesis, Crystal Structure, Particle Morphology, and Microstructure of b-VO2
(A) Electronic density of states for b-VO2.
(B) The calculated ELF of b-VO2. Green to red indicates the gradually increased charge localization.
(C) Schematic illustrating the synthesis of the metallic b-VO2 nanosheet array on glass.
(D) The prepared substrate covered with black b-VO2 nanosheets.
(E) XRD pattern of the b-VO2 sample.
(F) EDS component analysis of the b-VO2 sample.
(G) SEM images of the b-VO2 nanosheet arrays.
(H) HRTEM image of the b-VO2 nanosheets, showing the exposed crystal face is (112).
It is known that charge transfer between analyte and substrate is a short-range action (Park and Kim, 2010);, therefore, the enhancement effect based on CM is closely related to the scale and dimension of substrate materials. Recent studies on two-dimensional (2D) SERS active materials such as graphene and molybdenum disulfide nanosheets show that effective charge transfer between the adsorbed molecules and the substrates requires that the surface of the material be as flat as possible to effectively adsorb the molecules to be measured (Xie et al., 2009, Muehlethaler et al., 2016). At the same time, to obtain strong interactions of analytes and substrates, the thickness of these SERS active materials should be as small as possible to minimize the loss of charge carriers during the transfer process (Li et al., 2018a, Li et al., 2018b). On the other hand, the localized-SPR-based EM enhancement generally requires that the surface of the SERS substrate be as rough as possible, preferably containing a large number of nano-scale gaps, to generate a large number of high-intensity electromagnetic “hot spots” (Willets, 2014). From these two seemingly contradictory aspects, we expect to synthesize b-VO2 ultrathin nanosheet arrays, which not only meets the structural requirements of charge transfer, but also satisfies the demand of forming a large number of high-intensity electromagnetic “hot spots” among the nanosheet gaps.
Synthesis and Characterizations of VO2 Nanosheet Arrays
According to the previous analysis, a facile, scalable, and low-cost hydrothermal-assisted chemical vapor decomposition (HCVD) route has been designed for the in situ growth of the large-area b-VO2 ultrathin nanosheet arrays on glass (16 cm2 level). As shown in Figure 1C, an appropriate amount of vanadyl acetylacetonate (VAA) is dissolved in a certain volume of absolute ethanol to form a bright blue transparent solution. The resulting homogeneous solution is then transferred to a Teflon-lined high-pressure reactor, and a common glass sheet is placed above the liquid level as a growth platform for b-VO2 nanosheets. At 200°C, the closed reaction system is filled with ethanol vapor mixed with VAA molecules. Under this solvothermal condition, these tetravalent vanadium atoms contained in VAA will undergo alcoholysis to form crystal clusters of VO2, which are then deposited on glass surface and grown into arrayed nanosheets. The key to the success of this method is to maintain a reductive reaction atmosphere in the autoclave; otherwise it is very easy to generate V2O5 with a higher valence state. In the reactor, ethanol vapor exactly has a considerable strong reducibility under the heating conditions. Contrast experiments show that, if ethanol is replaced by deionized water, the obtained product is V2O5 nanobelts (Figure S1). As shown in Figure 1D, a glass sheet of 4 cm × 4 cm can be completely covered by a layer of black b-VO2 nanosheets after 12 h of HCVD reaction, which suggests that our method is very suitable for the growing of large-area VO2 nanosheet arrays.
The crystal phase of the obtained product was determined by X-ray diffraction (XRD) technology. The XRD pattern of the black sample can be accurately indexed as the monoclinic b-VO2 with the lattice parameters of a = 4.5968, b = 5.6844, c = 4.9133, and β = 89.398° (JCPDF No. 65-7960), and no other crystalline phase was found (Figure 1E). Energy dispersive spectrum (EDS) reveals that these samples contain only V and O elements, and the O/V ratio is about 2.08, which further proves that this sample is indeed VO2 (Figure 1F). The morphology and crystallographic orientation of the sample were investigated by field-emission scanning electron microscopy (SEM) and high-resolution transmission electron microscope (HRTEM). Overall, the black products are a lay of very uniform sheet-like nanostructures, which are vertically distributed on the glass surface (Figure 1G). High-magnification SEM images show that the thickness of these b-VO2 nanosheets are only 3–15 nm (inset in Figure 1G). Interestingly, there are many thin nanosheets interspersed between these thick nanosheets, a feature that is very helpful to adsorb more molecules on the surfaces and strongly promote charge transfer between nanosheets and analytes. Furthermore, the clear lattice fringes recorded in the HRTEM image demonstrate that these b-VO2 nanosheets possess a high crystallinity (Figure 1H). The lattice fringes with the interplanar spacing of 0.37 and 0.35 nm can be accurately referred to as the (201) and (110) crystal faces, respectively, which are also confirmed by the corresponding selected area electron diffraction pattern (inset in Figure 1H). Based on the information of the HRTEM image, it can be reasonably calculated that the exposed crystal face of the nanosheets is (112). Interestingly, this exposed crystal surface can interact strongly with the probe molecule R6G of SERS, which will be further demonstrated below. The specific BET (Brunauer-Emmett-Teller) surface area of the b-VO2 nanosheets was determined to be 44.8 m2 g−1 (Figure S2). In addition, Fourier transform infrared spectroscopy and Raman spectroscopy characterizations suggest that the surfaces of these nanosheets are very clean (Figures S3 and S4) and there was no signal of reaction residues except b-VO2, which is very important for trace detection because it can reduce the interference of the substrate itself.
Localized SPR Effect and Stability
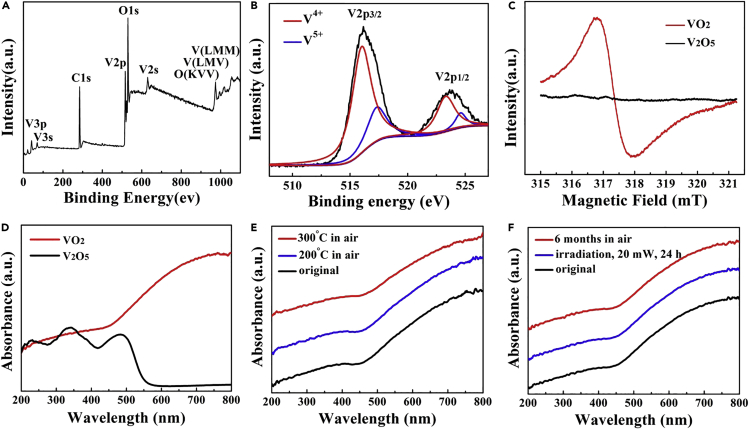
X-ray photoelectron spectroscopy (XPS) was used to identify the binding states and chemical compositions of these b-VO2 nanosheets. The XPS survey spectrum shows that these detected peaks at 515.2 and 631.3 eV can be identified as V 2p and V 2s of V4+, respectively (Figure 2A), which is characteristic of vanadium dioxide (Nethravathi et al., 2013). The peak at 529.6 eV can be assigned to O 1s of the O2−. The high-resolution XPS of V 2p, as shown in Figure 2B, could be well fitted into two spin-orbit doublets, corresponding to V4+ and V5+ oxidation states, respectively. The two characteristic strong peaks at 516.03 and 523.35 eV can be indexed to V4+, whereas the other two weak shoulder peaks at 517.38 and 524.61 eV can be attributed to V5+. According to the size of the peak areas, the concentration of V4+ on the sample surface is much higher than that of V5+, which confirms that the vanadium ion in the sample is basically tetravalent. At the same time, the strong electron paramagnetic resonance (EPR) spectrum signal directly demonstrated that the b-VO2 nanosheets have a large number of free electrons in its d-orbital (Figure 2C). In contrast, V2O5 nanosheets (morphology see Figure S5) obtained by oxidizing the VO2 nanosheets at 400°C in air were not detected by the effective ESR signals. These XPS and EPR results are highly consistent with the theoretical results mentioned earlier, which together prove that these b-VO2 nanosheets contain a high concentration of d-orbital free electrons.
Figure 2.
Valence States, EPR, and Ultraviolet-vis Absorption Characterizations of the b-VO2 Nanosheets
(A) XPS survey spectrum of the b-VO2 nanosheets.
(B) V 2p spectrum of the b-VO2 nanosheets, which demonstrates that most vanadium ion in the sample is tetravalent.
(C) EPR spectrum of the sample, displaying a strong free electron signal.
(D) Ultraviolet-vis absorption spectrum of the sample, showing a strong LSPR absorption from visible to NIR regions.
(E and F) The LSPR absorption of these samples are almost the same after being heated in air (E) and irradiated by laser and long-term preservation in air (F), suggesting the high thermal and chemical stability of the metallic b-VO2 nanosheets.
Importantly, these abundant d-orbital free electrons make the b-VO2 nanosheets exhibit strong localized-SPR effect from visible to near-infrared (NIR) regions (Figure 2D), which provides the possibility for EM enhancement of SERS to take place. In contrast, V2O5 nanosheets without free d-orbit electrons did not exhibit SPR behavior. Many intermediate valence transition metal oxide nanomaterials with strong localized-SPR are easy to be oxidized by oxygen in air, thus losing the SPR behavior and the corresponding SERS activities, which is another major obstacle to the practical application of semiconductor SERS substrates in addition to their low sensitivity. For example, plasmonic W18O49 nanostructure has been reported to have excellent SERS performance, and its Raman EF (105) is comparable with that of noble metals. However, for W18O49, even if heated at 100°C for 5 h in air, its SPR activity quickly disappears owing to oxidation (Figures S6 and S7). In contrast, the b-VO2 nanosheets show a high oxidation resistance (Figure 2E). Even if heated at 300°C in air for 1 h, the intensity and position of the plasma resonance absorption of these nanosheets did not change significantly, which is rare in transition metal oxides with intermediate valence states. This strong oxidation resistance has also been demonstrated by its high thermal stability (Figure S8). Considering that SERS substrates are exposed to the laser irradiation of Raman spectrometer, the high stability of the substrate materials is extremely important. In addition to the strong oxidation resistance, these b-VO2 nanosheets also exhibit long-term environmental and irradiated stability (Figure 2F). Even after exposure to air for 6 months, their SPR peaks did not change noticeably, which provides an opportunity for the commercialization of this material. The stability of these b-VO2 nanosheets was also confirmed by XRD and XPS characterization (Figures S9 and S10).
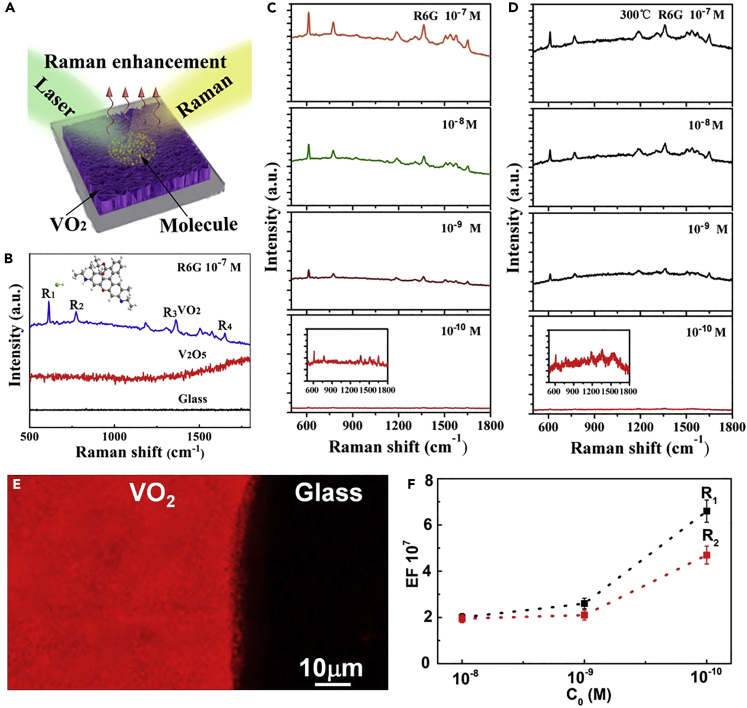
Enhanced Raman Scattering
Then, we tested the SERS properties of the b-VO2 nanosheet array substrate. As shown in Figure 3A, in all SERS measurements, the Raman exciting light is incident from above the substrate plane. In the experiments, the prepared probe molecule (R6G) solutions with specific concentrations (10−7–10−10 M) was added dropwise into the b-VO2 nanosheet arrays and dried at room temperature for 10 min before SERS measurement (see the Transparent Methods section for the specific process). Figure 3B shows that the b-VO2 nanosheet arrays exhibit excellent SERS activity for R6G with a concentration of 10−7 M (blue spectrum in Figure 3B). All Raman scattering peaks are clearly visible and highly consistent with the standard Raman spectrum of R6G reference material (Figure S11). The strongest four Raman scattering peaks at 612 (R1), 773 (R2), 1,363 (R3), and 1,652 cm−1 (R4) can be clearly observed, in which R1 and R2 can be indexed with the in-plane and out-of-plane bending motions of C and H atoms of the xanthenes skeleton, respectively; R3 and R4 can be referred to the C-C stretching vibrations of aromatic nucleus (Hildebrandt and Stockburger, 1984). To eliminate the contribution of glass to the properties of SERS since the nanosheets are grown on them, the R6G probe solutions were directly dripped onto the bare glass and tested for Raman signals. The results showed that no Raman signals were detected (black spectrum in Figure 3B), which definitely excludes the contribution of the glass in the SERS. When the quasi-metallic b-VO2 nanosheets were completely oxidized to the semiconducting V2O5 nanosheets without localized SPR effect (Figure S5), no effective SERS signals of probe molecules was detected except for the fluorescence background (red spectrum in Figure 3B), which further confirms that the enhanced Raman signals come from the quasi-metallic B-VO2 nanosheet arrays. On the other hand, contrastive experiments revealed that the bare b-VO2 nanosheet arrays without R6G solution only showed the typical Raman scattering peaks of themselves (Figure S12), indicating the enhanced Raman scattering peaks represented by R1, R2, R3, and R4 really originate from R6G molecules.
Figure 3.
SERS Measurements of R6G with the as-Prepared b-VO2 Nanosheets
(A) SERS measurement schematic diagram.
(B) Raman spectra of 10−7 M R6G aqueous solution obtained on b-VO2 nanosheets, bare glass, and V2O5 nanosheets.
(C) Gradually weakened Raman scattering signals recorded from Rh6G aqueous solution at four different concentration levels (10−7, 10−8, 10−9, 10−10 M), suggesting that the b-VO2 nanosheets have greatly enhanced Raman scattering, with a lowest detection limit of 10−10 M for analytes.
(D) These b-VO2 nanosheets still have high Raman enhancement effects even after 300°C of high-temperature heating in air.
(E) SERS mapping near an edge of a R6G/b-VO2 film.
(F) The average Raman EFs obtained by counting the peak intensities (R1 and R2) at three different concentration levels.
Figure 3C shows the SERS spectra of a series of R6G samples with different concentrations (10−7, 10−8, 10−9, 10−10 M). It can be seen that the b-VO2 substrate exhibits excellent SERS performance in a large concentration range. Even when the concentration of R6G is only 10−10 M, the distinguishable Raman signals still can be detected (signal-to-noise ratio greater than 5). Such a low detection limit allows this new SERS substrate to be used to trace even single-molecule detection of compounds with ultrahigh sensitivity. More importantly, this new SERS substrate based on the b-VO2 nanosheets has considerable high stability. As mentioned earlier, these b-VO2 nanosheets will not lose their localized-SPR effect even if heated at 300°C in air; accordingly, after the b-VO2 nanosheets were treated at 300°C for 5 h, it can be seen that their SERS intensities did not decrease noticeably and even 10−10 M analytes could still be detected (Figure 3D). For the practical applications of SERS, high stability of the SERS substrates is very important, because when transition metal oxides-based SERS substrates with intermediate valence state are exposed to the laser irradiation of Raman spectroscopy, they could often easily be oxidized or deformed, thus losing SERS activity or the reproducibility of the signals.
Raman scattering EF is generally considered to be the most important factor in evaluating the performances of SERS substrates. To visualize the EF of the VO2 nanosheet arrays, Figure 3E shows the Raman mapping image recorded from the edge of a R6G/VO2 layer. The obtained Raman mapping is clearly separated into two regions: the area covered with VO2 nanosheets showed strong Raman signals of R6G, whereas the R6G directly placed on the glass did not show any signals, suggesting the high uniform and strong SERS enhancement originated from the densely arranged VO2 nanosheets. To evaluate the Raman EF of the VO2 nanosheets more accurately, control experiments were carried out. In these experiments, on the VO2 nanosheet arrays, the SERS signal intensity of R1 and R2 scattering peaks of R6G with 10−8, 10−9, and 10−10 M was measured (integration time is 10 s). As references, on the bare glass, the normal Raman signal intensity of R1 and R2 scattering peaks of R6G with a much higher concentration (10−2 M) and integration time (4,000 s) was also measured under the same excitation light and operating mode. The calculated maximum EF is more than 6.7 × 107 (Figure 3F), which can be compared with the EFs of noble-metal substrates and is sufficient for single-molecule detection. As far as we know, the EF and detection limit of the quasi-metallic b-VO2 nanosheet arrays in various reported non-noble-metal SERS substrates (Table S1) are only lower than that of Mo(W)Te2 nanosheets with atomic layer thickness (Li et al., 2018a, Li et al., 2018b). But considering the difficulty of synthesis and quality control of the Mo(W)Te2 monatomic layers, these large-scale VO2 SERS substrates, which are easy to synthesize, have great advantages in practical applications.
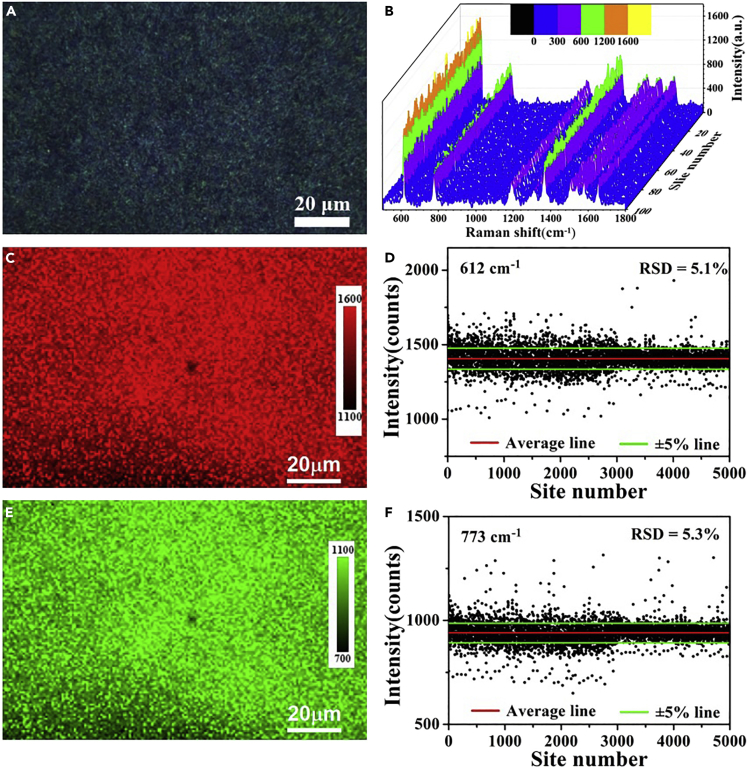
Uniformity and Repeatability of SERS Signals
In addition to the high sensitivity and EF, the large-range homogeneity and reproducibility of Raman signals is another important index to evaluate the performance and practicability of SERS substrate. The premise of obtaining uniform and repeatable signals is the uniformity of the substrate surface. As shown in Figure 4A, the optical photographs of one glass sheet covered with b-VO2 nanosheets show that the surface of the substrate is very uniform, which is highly consistent with the SEM characterizations described earlier. In the area shown in Figure 4A, 100 points are selected randomly for SERS detection, and the results show that the Raman spectra obtained by these points are highly consistent (Figure 4B), indicating that the present SERS substrate has excellent signal uniformity and reproducibility. The Raman mapping result of R1 scattering peak at 612 cm−1 recorded from 5,000 measuring points displays a uniform intensity distribution (Figure 4C), which further confirms the signal uniformity of the substrate in large scale. By counting the distribution of the intensity values of the 5,000 R1 peaks, the acquired relative standard deviation (RSD) is only 5.1% (Figure 4D). Similarly, the Raman mapping and statistical results of R2 scattering peak at 773 cm−1 also demonstrated the high signal uniformity of the b-VO2 substrate (Figure 4E), with RSD of about 5.3% (Figure 4F). Such high signal reproducibility makes it possible for the practical application of this new SERS substrate.
Figure 4.
Determination of the Signal Reproducibility and Uniformity of the Metallic b-VO2 Nanosheet Substrate
(A) Optical photograph of the substrate covered with b-VO2 nanosheets recorded from objective lenses of the Raman spectrometer.
(B) SERS signals collected from 100 randomly selected measuring points on the substrate.
(C) The SERS mapping at 612 cm−1 (R1) of 10−8 M R6G in the region shown in Figure 4A.
(D) The signal intensities at 612 cm−1 (R1) of 10−8 M R6G in the region shown in Figure 4A.
(E) The SERS mapping at 773 cm−1 (R2) of 10−8 M R6G in the region shown in Figure 4A.
(F) The SERS signal intensities at 773 cm−1 (R2) of 10−8 M R6G in the region shown in Figure 4A.
Universality as SERS Detection Platform
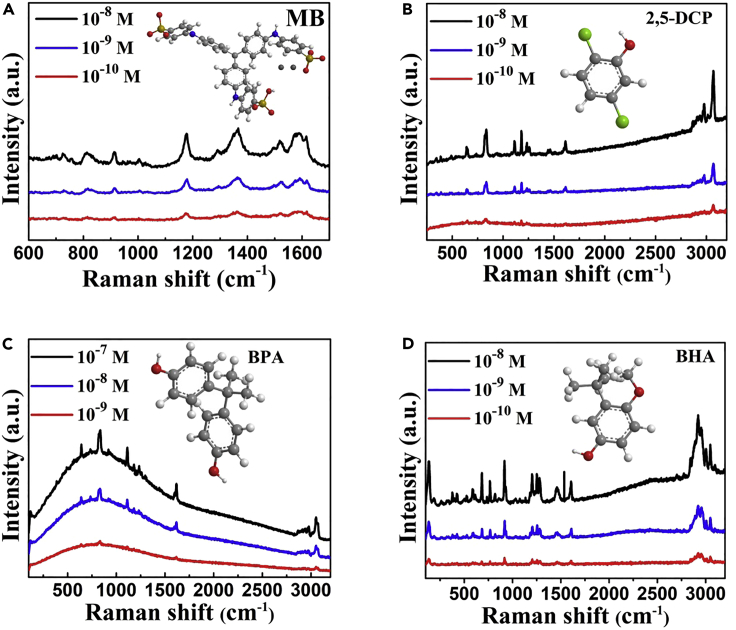
In addition to exhibiting the high sensitivity for the probe molecule of R6G, this quasi-metallic b-VO2 SERS substrate also exhibits very low detection limits for several other common dye molecules, such as methyl blue, methyl orange, and Rhodamine B. From 10−8 to 10−10 M, the resolvable Raman signals of the dye molecules can be measured from the nanosheet arrays (Figures 5A and S13). More importantly, the quasi-metallic VO2 substrate also exhibits excellent sensitivity to a range of highly concerned risk substances. Dichlorophenol, a common environmental hormone, has been proved to be highly carcinogenic and has attracted much attention from environmental monitoring departments in various countries. On this b-VO2 nanosheet arrays, the 2,5-dichlorophenol (2,5-DCP) molecules can be sensitively detected even if its concentration is only 10−10 M (Figure 5B). Another hazardous environmental hormone, bisphenol A (BPA), has been banned in the United States, the European Union, Canada, China, and so on. It is reported that BPA is an endocrine disruptor, which can mimic human hormones and may lead to adverse health effects. Like 2,5-DCP, BPA also can be sensitively detected at 10−10 M level on the b-VO2-based SERS substrate (Figure 5C). In addition, as a powerful antioxidant, butyl hydroxy anisd (BHA) has long been used to prevent food spoilage. However, recent studies have shown that it may have strong carcinogenicity and has been banned from food industry in Japan, China, and other countries. It is found that this VO2 SERS substrate also responds well to BHA (Figure 5D). These experimental results showed that the quasi-metallic b-VO2 nanosheet arrays are a versatile SERS active substrate to a series of chemicals.
Figure 5.
SERS Spectra of a Series of Common Environmental Pollutants
(A) Methyl blue (MB).
(B) 2,5-Dichlorophenol (2,5-DCP).
(C) Bisphenol A (BPA).
(D) Butyl hydroxy anisd (BHA).
Raman Enhancement Mechanism of the Quasi-Metallic VO2
Traditionally, the Raman enhancement of noble-metal SERS materials can be explained by EM based on the well-known localized-SPR effect, whereas the enhancement of semiconductor SERS materials can be attributed to the CM resulting from the charge transfer between the adsorbed molecules and the substrates (Li et al., 2010, Qian et al., 2008). Although it is now accepted that both enhancement mechanisms may take place together in one substrate, it is generally believed that the enhancement effect of CM is far less than that of EM owing to the short-range effectiveness of CM. However, our present research results break this traditional view, revealing that CM, like EM, also plays an important role in the enhancement of the total SERS performances when the substrate is quasi-metal. First, UV-vis absorption spectra are to used to examine whether there is charge transfer between the analyte molecule and the VO2 nanosheets. We measured the absorption spectra of pure R6G solution, clean VO2 nanosheets, and VO2 nanosheets adsorbed with R6G molecules, respectively. As shown in Figure 6A, compared with those of the pure R6G, the main absorption peaks of R6G adsorbed on VO2 nanosheets took place at a significant redshift (marked with color bar), suggesting the strong interaction and charge transfer probability between R6G and VO2. SERS based on CM is very sensitive to the wavelength changes of exciting light, and lasers of different wavelengths often lead to different molecular vibration modes, which are typical characteristics of CM enhancement. As shown in Figure 6B, the experimental results showed that the intensity of Raman scattering signals of R6G decreases obviously when the wavelength of exciting light changes from 532 to 633 nm, which can be reasonably attributed to the fact that the energy of 633-nm exciting light is obviously less than that of 532 nm. When the exciting power is increased by 20 times (633 nm), the Raman signal intensity of R6G is obviously increased, but it should be pointed out that the resonance mode of R6G molecule has significantly changed in several bands (marked with color bars), which further proves that there is a strong interaction between the analyte and the substrate. It was noted that, when graphene was used as the SERS substrate (its SERS activity has been reported [Xie et al., 2009]), only its own Raman signals have been detected and no effective Raman signals of R6G were detected (Figure S14), which indicated that the charge transfer between VO2 nanosheets and R6G is much larger than that between graphene and R6G. This phenomenon may be attributed to the chemical inertness of the graphene surface, whereas the surface of quasi-metal nanosheets synthesized by the solution method is much more active.
Figure 6.
Investigation of the Enhanced Mechanism of the b-VO2 Nanosheet Substrate
(A) Absorption spectra for R6G on VO2 compared with neat VO2 and R6G dye. The colored area denotes the R6G absorption band on VO2.
(B) Changing excitation wavelength has a great influence on SERS intensity and resonance mode.
(C and D) Side views of the electron density difference isosurfaces for R6G chemisorbed onto the (112) crystal face of b-VO2 (C) and graphene (D). Blue and yellow colors correspond to electron depletion and accumulation regions, respectively. The arrows indicate the direction of electron transfer.
(E) Energy level diagram and charge transfer transitions in the R6G-VO2 complex. μmol denotes the molecular transition. μi-CT and μk-CT denote the charge transfer transitions from the molecular ground states to VO2 and from VO2 to the molecular excited states, respectively.
(F) UV-vis absorption spectra of the vanadium oxide samples obtained by oxidation for different time periods.
(G) SERS spectra recorded from the vanadium oxide samples with different SPR effects, respectively.
(H) SERS mapping over the vanadium oxide samples with different SPR effects, respectively.
The interactions between the adsorbed R6G molecules and the VO2 nanosheets and subsequent charge transfer were investigated by DFT simulation (Figures 6C and 6D). The DFT calculations revealed that the binding energy of R6G and VO2 with exposed (112) crystal plane is 1.15 eV, whereas the binding energy of R6G and graphene is only 0.67 eV. At the same time, the results also show that the electron transfer from R6G to VO2 is 0.679 e/molecule, whereas that for graphene and R6G is only 0.015 e/molecule (from graphene to R6G). The obvious difference in the values of the binding energy and electron transfer demonstrated that the coupling effect of VO2 and R6G is much stronger than that of graphene and R6G. At the same time, from the charge density isosurfaces, the DFT simulations also showed that the electrons and holes generated by charge transfer form a strong dipole at the interface of R6G and VO2, which would strengthen the Raman scattering of R6G molecules on VO2 nanosheets. As a contrast, the forming dipole between VO2 and R6G is much stronger than that of graphene and R6G owing to the greater charge transfer, which further proves the strong coupling effect between VO2 and R6G. Furthermore, owing to the relatively high surface activity of VO2 nanosheets, the generated charge carriers are concentrated at the interface of VO2 and R6G, forming so-called quasi-covalent bonds (Li et al., 2018a, Li et al., 2018b), which would further enhance the coupling of VO2 and R6G in addition to the electrostatic force of dipole. Thus, the strong dipole effect and the consequent quasi-covalent bonds lead to the impressive SERS capability of the quasi-metallic VO2 nanosheets together.
In addition to the CM induced by the electrostatic coupling between R6G and VO2 discussed earlier, the outstanding SERS performance of the VO2 substrate also can be attributed to the photo-induced charge transfer (PICT) caused CM, as shown in Figure 6E. The energy levels of the highest occupied molecule orbital (HOMO) and the lowest occupied molecule orbital (LOMO) of R6G are −5.7 and −3.4 eV, respectively (Hildebrandt and Stockburger, 1984), whereas the Fermi level of b-VO2 nanosheets is 4.55 eV according to the characterization results of Kelvin probe force microscope as illustrated in Figure S15. The distribution of the levels is allowed to both the PICT transfer from the HOMO of R6G to the Fermi level of b-VO2, and the Fermi level of VO2 to the LOMO of R6G, which are beneficial to the SERS due to the wide energy range of charge transfer resonance. Therefore, the resonances greatly enhance the polarization tensor of the R6G molecule according to the well-known Herzberg-Teller vibronic coupling (Lombardi et al., 1986). It should be noted that the Fermi level of VO2 is almost a symmetrical match to the HOMO and LUMO of R6G (Figure 6E), which further promotes the PICT process, and the interference of fluorescence background of R6G is greatly reduced.
In addition to the CM-based Raman enhancement mentioned earlier, EM enhancement also contributes significantly to the overall SERS performances of the quasi-metallic VO2 substrate. As illustrated in Figure S16, when these VO2 nanosheets were heated in air for a period of time, their colors changed dramatically. Accordingly, their plasma resonance absorption significantly reduced and ultimately became imperceptible (Figure 6F). Obviously, this is because the VO2 sample is gradually oxidized to V2O5 (Figures S17 and S18), thus losing a large number of d-orbital free electrons, and correspondingly its localized SPR is also weakened and eventually disappeared. As a direct result, the SERS performance of these oxidized samples is greatly reduced (Figure 6G). The Raman mapping results also show that the SERS effect of the sample weakens with the decrease of the SPR intensity (Figure 6H). On the contrary, when we increase the concentration of oxygen vacancies in the VO2 nanosheets by the chemical reduction method (by immersing the VO2 sample in strongly reducing NaBH4 aqueous solution), the corresponding SPR absorption increases gradually (Figure S19) and the corresponding SERS signal also increases gradually (Figure S20). Based on the results, we believe that the regular relationship between the localized-SPR strength and the corresponding SERS performance demonstrated that EM enhancement also exists in the quasi-metallic b-VO2-based SERS. To further prove the existence of EM, these nanosheets are coated with amorphous SiO2 (named as SiO2/VO2) to block charge-transfer-based CM enhancement by an ingenious synthesis experiment (Figure S21). Compared with bare VO2 nanosheets, the SERS signal intensity of analyte on the VO2/SiO2 still can be detected, as shown in (Figure S22), which undoubtedly proves the existence of EM. Conversely, this result also further verified the existence of CM. For the total Raman enhancement, by calculating the peak area of R1 recorded on VO2 and VO2/SiO2, respectively, it can be concluded that the contributions of CM and EM are about 57% and 43%, respectively, which revels that both CM and EM play an important role in enhancing the Raman scattering.
Conclusions
In summary, we demonstrate that quasi-metallic b-VO2 nanosheet arrays can be used as a highly sensitive and stable SERS substrate material. The lowest detectable limit of R6G probe on the VO2 substrate can achieve picomole levels and the optimal Raman EF is up to 6.7 × 107, which obviously outstrips the previously reported CM based solely on SERS active materials represented by semiconducting nanostructures, graphene, metal organic frameworks, and so on. The ultrasensitive SERS performances achieved on the quasi-metallic VO2 nanosheet arrays can be attributed to the strong analyte-substrate interactions and the vigorous localized-SPR effects of the VO2. The present research results indicate that the quasi-metallic VO2 nanosheet array is a very promising SERS detection platform and demonstrates that CM and EM together play a key role in the overall SERS performance of quasi-metals.
Limitations of the Study
At present, it is difficult to accurately evaluate the contributions of EM and CM in quasi-metal. Although we use a thin layer of amorphous SiO2 to isolate charge transfer, it may also affect EM and introduce new interface effects. A more perfect evaluation method needs to be established in the future to accurately identify the contribution of EM and CM to the properties of quasi-metal SERS.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
This work received financial support from the Science Foundation of Chinese Academy of Inspection and Quarantine (2019JK004) and the National Key Research and Development Program of China (2017YFF0210003).
Author Contributions
Conceptualization, G.X., Z.T.; Investigation, Z.T., H.B., Y.Y., Q.K., Y.L., and W.F.; Calculation, C.C., and W.Y.; Writing – Original Draft, Z.T. and G.X.; Writing – Review & Editing, Z.T., H.B., and G.X.
Declaration of Interests
The authors declare no competing interests.
Published: September 27, 2019
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2019.08.040.
Supplemental Information
References
- Alvarez-Puebla R.A., Liz-Marzan L.M. SERS detection of small inorganic molecules and ions. Angew. Chem. Int. Ed. 2012;51:11214–11223. doi: 10.1002/anie.201204438. [DOI] [PubMed] [Google Scholar]
- Cong S., Yuan1 Y.Y., Chen Z.G., Hou J.Y., Yang M., Su Y.L., Zhang Y.Y., Li L., Li Q.W., Geng F.X., Zhao Z.G. Noble metal-comparable SERS enhancement from semiconducting metal oxides by making oxygen vacancies. Nat. Commun. 2015;6:7800. doi: 10.1038/ncomms8800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleischmann M., Hendra P.J., McQuillan A.J. Raman spectra of pyridine adsorbed at a silver electrode. Chem. Phys. Lett. 1974;26:163–166. [Google Scholar]
- Hao Q., Li W., Xu H.Y., Wang J.W., Yin Y., Wang H.Y., Ma L.B., Ma F., Jiang X.C., Schmidt O.G. VO2/TiN plasmonic thermochromic smart coatings for room-temperature applications. Adv. Mater. 2018;30:1705421. doi: 10.1002/adma.201705421. [DOI] [PubMed] [Google Scholar]
- Hildebrandt P., Stockburger M. Surface-enhanced resonance Raman spectroscopy of Rhodamine 6G adsorbed on colloidal silver. J. Phys. Chem. 1984;88:5935–5944. [Google Scholar]
- Jeanmaire D.L., Van Duyne R.P. Surface Raman spectroelectrochemistry. part I. heterocyclic, aromatic, and aliphatic amines adsorbed on the anodized silver electrode. J. Electroanal. Chem. 1977;84:1–20. [Google Scholar]
- Kanipe K.N., Chidester P.P.F., Stucky G.D., Moskovits M. Large format surface-enhanced Raman spectroscopy substrate optimized for enhancement and uniformity. ACS Nano. 2016;10:7566–7571. doi: 10.1021/acsnano.6b02564. [DOI] [PubMed] [Google Scholar]
- Kneipp K., Wang Y., Kneipp H., Perelman L.T., Itzkan I., Dasari R.R., Feld M.S. Single molecule detection using surface-enhanced Raman scattering (SERS) Phys. Rev. Lett. 1997;78:1667–1670. [Google Scholar]
- Kneipp J., Kneippa H., Kneippac K. SERS—a single-molecule and nanoscale tool for bioanalytics. Chem. Soc. Rev. 2008;37:1052–1060. doi: 10.1039/b708459p. [DOI] [PubMed] [Google Scholar]
- Li J.F., Huang Y.F., Ding Y., Yang Z.L., Li S.B., Zhou X.S., Fan F.R., Zhang W., Zhou Z.Y., Wu D.Y. Shell-isolated nanoparticle-enhanced Raman spectroscopy. Nature. 2010;464:392–395. doi: 10.1038/nature08907. [DOI] [PubMed] [Google Scholar]
- Li W., Zamani R., Gil P.R., Pelaz B., Ibanez M., Cadavid D., Shavel A., Alvarez-Puebla R.A., Parak J.W., Arbiol J. CuTe nanocrystals: shape and size control, plasmonic properties, and use as SERS probes and photothermal agents. J. Am. Chem. Soc. 2013;135:7098–7101. doi: 10.1021/ja401428e. [DOI] [PubMed] [Google Scholar]
- Li J.M., Li J.Y., Yang Y., Qin D. Bifunctional Ag@Pd-Ag nanocubes for highly sensitive monitoring of catalytic reactions by surface-enhanced Raman spectroscopy. J. Am. Chem. Soc. 2015;137:7039–7042. doi: 10.1021/jacs.5b03528. [DOI] [PubMed] [Google Scholar]
- Li T., Chen K., Chen Z.F., Cong C.X., Qiu C.Y., Chen J.J., Wang X.M., Chen H.J., Yu T., Xie W.J. 1T’ transition metal telluride atomic Layers for plasmon-free SERS at femtomolar levels. J. Am. Chem. Soc. 2018;140:8696–8704. doi: 10.1021/jacs.8b02972. [DOI] [PubMed] [Google Scholar]
- Li A.R., Lin J., Huang Z.N., Wang X.T., Guo L. Surface-enhanced Raman spectroscopy on amorphous semiconducting rhodium sulfide microbowl substrates. iScience. 2018;10:1–10. doi: 10.1016/j.isci.2018.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z.L., Ganapathy S., Xu Y.L., Zhou Z., Sarilar M., Wagemaker M. Mechanistic insight into the electrochemical performance of Zn/VO2 batteries with an aqueous ZnSO4 electrolyte. Adv. Energy Mater. 2019;9:1900237. [Google Scholar]
- Lin X.M., Cui Y., Xu Y.H., Ren B., Tian Z.Q. Surface-enhanced Raman spectroscopy: substrate-related issues. Anal. Bioanal. Chem. 2009;394:1729–1745. doi: 10.1007/s00216-009-2761-5. [DOI] [PubMed] [Google Scholar]
- Lin J., Shang Y., Li X.X., Yu J., Wang X.T., Guo L. Ultrasensitive SERS detection by defect engineering on single Cu2O superstructure particle. Adv. Mater. 2017;29:1604797. doi: 10.1002/adma.201604797. [DOI] [PubMed] [Google Scholar]
- Lin J., Hao W., Shang Y., Wang X.T., Qiu D.L., Ma G.S., Chen C., Li S.Z., Guo L. Direct experimental observation of facet-dependent SERS of Cu2O polyhedra. Small. 2018;14:1703274. doi: 10.1002/smll.201703274. [DOI] [PubMed] [Google Scholar]
- Lombardi J.R., Birke R.L., Lu T.H., Xu J. Charge-transfer theory of surface enhanced Raman spectroscopy: Herzberg–Teller contributions. J. Chem. Phys. 1986;84:4174–4180. [Google Scholar]
- Manthiram K., Alivisatos A.P. Tunable localized surface plasmon resonances in tungsten oxide nanocrystals. J. Am. Chem. Soc. 2012;134:3995–3998. doi: 10.1021/ja211363w. [DOI] [PubMed] [Google Scholar]
- Morrison V.R., Chatelain R.P., Tiwari K.L., Hendaoui A., Bruhacs A., Chaker M., Siwick B.G. A photoinduced metal-like phase of monoclinic VO2 revealed by ultrafast electron diffraction. Science. 2014;346:445–448. doi: 10.1126/science.1253779. [DOI] [PubMed] [Google Scholar]
- Moskovits M. Surface roughness and the enhanced intensity of Raman scattering by molecules adsorbed on metals. J. Chem. Phys. 1978;69:4159–4161. [Google Scholar]
- Muehlethaler C., Considine C.R., Menon V., Lin W.C., Lee Y.H., Lombardi G.Y. Ultrahigh Raman enhancement on monolayer MoS2. ACS Photon. 2016;3:1164–1169. [Google Scholar]
- Mulvihill M., Tao A., Benjauthrit K., Arnold J., Yang P.D. Surface enhanced Raman spectroscopy for trace arsenic detection in contaminated water. Angew. Chem. Int. Ed. 2008;120:6556–6560. doi: 10.1002/anie.200800776. [DOI] [PubMed] [Google Scholar]
- Nethravathi C., Rajamathi C.R., Rajamathi M., Gautam U.K., Wang X., Golberg D., Bando Y. N-doped graphene−VO2 (B) nanosheet-built 3D flower hybrid for lithium ion battery. ACS Appl. Mater. Interfaces. 2013;5:2708–2714. doi: 10.1021/am400202v. [DOI] [PubMed] [Google Scholar]
- Nie S., Emory S.R. Probing single molecules and single nanoparticles by surface-enhanced Raman scattering. Science. 1997;275:1102–1106. doi: 10.1126/science.275.5303.1102. [DOI] [PubMed] [Google Scholar]
- Palonpon A.F., Ando J., Yamakoshi H., Dodo K., Sodeoka M., Kawata S., Fujita K. Raman and SERS microscopy for molecular imaging of live cells. Nat. Protoc. 2013;8:677–692. doi: 10.1038/nprot.2013.030. [DOI] [PubMed] [Google Scholar]
- Park W.H., Kim Z.H. Charge transfer enhancement in the SERS of a single molecule. Nano Lett. 2010;10:4040–4048. doi: 10.1021/nl102026p. [DOI] [PubMed] [Google Scholar]
- Peksa V., Jahn M., Stolcova L., Schulz V., Proska J., Prochazka M., Weber K., Cialla-May D., Poppet J. Quantitative SERS analysis of azorubine (E 122) in sweet drinks. Anal. Chem. 2015;87:2840–2844. doi: 10.1021/ac504254k. [DOI] [PubMed] [Google Scholar]
- Phan-Quang G.C., Lee H.K., Phang Y.Y., Ling X.Y. Plasmonic colloidosomes as three-dimensional SERS platforms with enhanced surface area for multiphase sub-microliter toxin sensing. Angew. Chem. Int. Ed. 2015;54:9691–9695. doi: 10.1002/anie.201504027. [DOI] [PubMed] [Google Scholar]
- Qazilbash M.M., Brehm M., Chae B.G., Ho P.C., Andreev G.O., Kim B.J., Yun S.J., Balatsky A.V., Maple M.B., Keilmann F. Mott transition in VO2 revealed by infrared spectroscopy and nano-imaging. Science. 2007;318:1750–1753. doi: 10.1126/science.1150124. [DOI] [PubMed] [Google Scholar]
- Qi D.Y., Lu L.G., Wang L.Z., Zhang J.L. Improved SERS sensitivity on plasmon-free TiO2 photonic microarray by enhancing light-matter coupling. J. Am. Chem. Soc. 2014;136:9886–9889. doi: 10.1021/ja5052632. [DOI] [PubMed] [Google Scholar]
- Qian X.M., Peng X.H., Ansari1 D.O., Yin-Goen Q., Chen G.Z., Shin D.M., Yang L., Young A.N., Wang M.D., Nie S. In vivo tumor targeting and spectroscopic detection with surface-enhanced Raman nanoparticle tags. Nat. Biotechnol. 2008;26:83–90. doi: 10.1038/nbt1377. [DOI] [PubMed] [Google Scholar]
- Qiu B.C., Xing M.Y., Yi Q.Y., Zhang J.L. Chiral carbonaceous nanotubes modified with titania nanocrystals: plasmon-free and recyclable SERS sensitivity. Angew. Chem. Int. Ed. 2015;54:10643–10647. doi: 10.1002/anie.201505319. [DOI] [PubMed] [Google Scholar]
- Quagliano L.G. Observation of molecules adsorbed on III-V semiconductor quantum dots by surface-enhanced Raman scattering. J. Am. Chem. Soc. 2004;126:7393–7398. doi: 10.1021/ja031640f. [DOI] [PubMed] [Google Scholar]
- Schlucker S. Surface-enhanced Raman spectroscopy: concepts and chemical applications. Angew. Chem. Int. Ed. 2014;53:4756–4795. doi: 10.1002/anie.201205748. [DOI] [PubMed] [Google Scholar]
- Strelcov E., Lilach Y., Kolmakov A. Gas sensor based on metal−insulator transition in VO2 nanowire thermistor. Nano Lett. 2009;9:2322–2326. doi: 10.1021/nl900676n. [DOI] [PubMed] [Google Scholar]
- Sun H.Z., Cong S., Zheng Z.H., Wang Z., Chen Z.G., Zhao Z.G. Metal–organic frameworks as surface enhanced Raman scattering substrates with high tailorability. J. Am. Chem. Soc. 2019;141:870–878. doi: 10.1021/jacs.8b09414. [DOI] [PubMed] [Google Scholar]
- Taylor R.W., Coulston R.J., Biedermann F., Mahajan S., Baumberg J.J., Scherman O.A. In situ SERS monitoring of photochemistry within a nanojunction reactor. Nano Lett. 2013;13:5985–5990. doi: 10.1021/nl403164c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X.T., Shi W.S., She G.W., Ma L.X. Using Si and Ge nanostructures as substrates for surface-enhanced Raman scattering based on photoinduced charge transfer mechanism. J. Am. Chem. Soc. 2011;133:16518–16523. doi: 10.1021/ja2057874. [DOI] [PubMed] [Google Scholar]
- Wang X.J., Wang C., Cheng L., Lee S.T., Liu Z. Noble metal coated single-walled carbon nanotubes for applications in surface enhanced Raman scattering imaging and photothermal therapy. J. Am. Chem. Soc. 2012;134:7414–7422. doi: 10.1021/ja300140c. [DOI] [PubMed] [Google Scholar]
- Wang X.T., Shi W.X., Jin Z., Huang W.F., Lin J., Ma G.S., Li S.Z., Guo L. Remarkable SERS activity observed from amorphous ZnO nanocages. Angew. Chem. Int. Ed. 2017;56:9851–9855. doi: 10.1002/anie.201705187. [DOI] [PubMed] [Google Scholar]
- Wang X.T., Shi W.X., Wang S.X., Zhao H.W., Lin J., Yang Z., Chen M., Guoet L. Two-dimensional amorphous TiO2 nanosheets enabling high-efficiency photoinduced charge transfer for excellent SERS activity. J. Am. Chem. Soc. 2019;141:5856–5862. doi: 10.1021/jacs.9b00029. [DOI] [PubMed] [Google Scholar]
- Whittaker L., Jaye C., Fu Z., Fischer D.A., Banerjee S. Depressed phase transition in solution-grown VO2 nanostructures. J. Am. Chem. Soc. 2009;131:8884–8894. doi: 10.1021/ja902054w. [DOI] [PubMed] [Google Scholar]
- Willets K.A. Super-resolution imaging of SERS hot spots. Chem. Soc. Rev. 2014;43:3854–3864. doi: 10.1039/c3cs60334b. [DOI] [PubMed] [Google Scholar]
- Xi G.C., Ouyang S.X., Li P., Ye J.H., Ma Q., Su N., Bai H., Wang C. Ultrathin W18O49 nanowires with diameters below 1 nm: synthesis, near-infrared absorption, photoluminescence, and photochemical reduction of carbon dioxide. Angew. Chem. Int. Ed. 2012;51:2395–2399. doi: 10.1002/anie.201107681. [DOI] [PubMed] [Google Scholar]
- Xi G.C., Ye J.H., Ma Q., Su N., Bai H., Wang C. In situ growth of metal particles on 3D urchin-like WO3 nanostructures. J. Am. Chem. Soc. 2012;134:6508–6511. doi: 10.1021/ja211638e. [DOI] [PubMed] [Google Scholar]
- Xie L.M., Ling X., Fang Y., Zhang J., Liu Z.F. Graphene as a substrate to suppress fluorescence in resonance Raman spectroscopy. J. Am. Chem. Soc. 2009;131:9890–9891. doi: 10.1021/ja9037593. [DOI] [PubMed] [Google Scholar]
- Yilmaz M., Babur E., Ozdemir M., Gieseking R.L., Dede Y., Tamer U., Schatz G.C., Facchetti A., Usta H., Demirel G. Nanostructured organic semiconductor films for molecular detection with surface-enhanced Raman spectroscopy. Nat. Mater. 2017;16:918–924. doi: 10.1038/nmat4957. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Qian J., Wang D., Wang Y.L., He S.L. Multifunctional gold nanorods with ultrahigh stability and tunability for in vivo fluorescence imaging, SERS detection, and photodynamic therapy. Angew. Chem. Int. Ed. 2013;52:1148–1151. doi: 10.1002/anie.201207909. [DOI] [PubMed] [Google Scholar]
- Zhang X.Y., Zheng Y.H., Liu X., Lu W., Dai J.Y., Lei D.Y., MacFarlane D.R. Hierarchical porous plasmonic metamaterials for reproducible ultrasensitive surface-enhanced Raman spectroscopy. Adv. Mater. 2015;27:1090–1096. doi: 10.1002/adma.201404107. [DOI] [PubMed] [Google Scholar]
- Zhang Q.Q., Li X.S., Ma Q., Zhang Q., Bai H., Yi W.C., Liu J.Y., Han J., Xi G.C. A metallic molybdenum dioxide with high stability for surface enhanced Raman spectroscopy. Nat. Commun. 2017;8:14903. doi: 10.1038/ncomms14903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Z.H., Cong S., Gong W.B., Xuan J.N., Li G.H., Lu W.B., Geng F.X., Zhao Z.G. Semiconductor SERS enhancement enabled by oxygen incorporation. Nat. Commun. 2017;8:1993. doi: 10.1038/s41467-017-02166-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu C.H., Meng G.W., Zheng P., Huang Q., Li Z.B., Hu X.Y., Wang X.J., Huang Z.L., Li F.D., Wu N.Q. A hierarchically ordered array of silver-nanorod bundles for surface-enhanced Raman scattering detection of phenolic pollutants. Adv. Mater. 2016;28:4871–4876. doi: 10.1002/adma.201506251. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.