Abstract
Polycystic ovary syndrome (PCOS) is a common female condition typified by reproductive, hyperandrogenic, and metabolic features. Polycystic ovary syndrome is a genetic condition, exacerbated by obesity. There is a close link between obesity and PCOS based on epidemiological data, and more recently corroborated through genetic studies. There are many mechanisms mediating the effects of weight-gain and obesity on the development of PCOS. The metabolic effects of insulin resistance and steroidogenic and reproductive effects of hyperinsulinaemia are important mechanisms. Adipokine production by subcutaneous and visceral fat appears to play a part in metabolic function. However, given the complexity of PCOS pathogenesis, it is important also to consider possible effects of PCOS on further weight-gain, or at least on hampering attempts at weight-loss and maintenance through lifestyle changes. Possible mediators of these effects include changes in energy expenditure, mental ill health, or physical inactivity. In this brief review, we discuss the main mechanisms that underlie the association between obesity and PCOS, from divergent perspectives of weight-gain contributing to development of PCOS and vice versa. We also consider novel management options for women with obesity and PCOS.
Keywords: Polycystic ovary syndrome, obesity, metabolism
Introduction
For billions of years, the eukaryotic cell and, more recently, its multi-cellular manifestations have evolved to mitigate against nutrient scarcity. This, combined with oxygen free radicals and hypothermia, represents major threats to species survival. In response to these threats, our complex physiology has adapted through diverse mechanisms. Provision of a ready supply of nutrients through storage of energy in the liver and adipose tissue in times of plenty and use of alternate fuel sources such as ketone bodies when food is scarce mitigate against starvation. Efficient and timely eradication of oxygen free radicals, generated through mitochondrial oxidative respiration through enzymes such as superoxide dismutase, mitigates against the harmful effects of oxygen free radicals. Maintenance of a constant body temperature, through shivering activity and activation of brown adipose tissue in response to cold exposure, mitigates against hypothermia.
For the last 50 years, Homo sapiens has been navigating a white water ride. Although environmental turbulence is usually necessary for evolutionary change, what is unusual about our current ‘white water ride’ is the assumption of its cause being antipodal to a more familiar threat of nutrient scarcity: that of nutrient abundance. (Abundance of a substance essential for life is not altogether unprecedented: oxygen abundance during the Cambrian era may have precipitated the Cambrian explosion of multi-cellular life.) Of course, this perspective is almost certainly a gross over-simplification. The development of the global obesity epidemic over the last half century is likely to be multi-factorial and complex, and go far beyond simple food abundance. However, whatever its actual cause(s), obesity accounts for a huge component of global ill health and is associated with at least 50 obesity-related comorbidities.1-3 Global obesity also confers a substantial socio-economic burden. Expenditure on obesity and its numerous sequelae accounts for a substantial proportion of healthcare costs globally.
Most obesity-related comorbidities associate with cardiometabolic dysfunction. This includes development of conditions like type 2 diabetes mellitus (T2D), hypertension, and other features of the metabolic syndrome. Obesity-related malignancies such as endometrial carcinoma also associate with underlying cardiometabolic dysfunction, insulin resistance, and compensatory hyperinsulinaemia.4
Polycystic ovary syndrome is an important example of a metabolic disorder, associated with insulin resistance, the manifestations of which include cardiometabolic risk, and the effects of which are greatly amplified by obesity.2,5,6 Accordingly, PCOS associates with heightened risk for the development of T2D,7 impaired glucose tolerance,8 dyslipidaemia, non-alcoholic fatty liver disease,9 and obstructive sleep apnoea (OSA).10 Polycystic ovary syndrome has a prevalence of between 6% and 10% in reproductive-age women.11-14 Although PCOS can manifest at any stage of reproductive life, it often develops during adolescence.2 Polycystic ovary syndrome is typified by both reproductive and hyperandrogenic features that include oligo-amenorrhoea, impaired fertility, hirsutism, acne, and androgenic alopecia. Polycystic ovary syndrome often also presents with hyperandrogenaemia.2
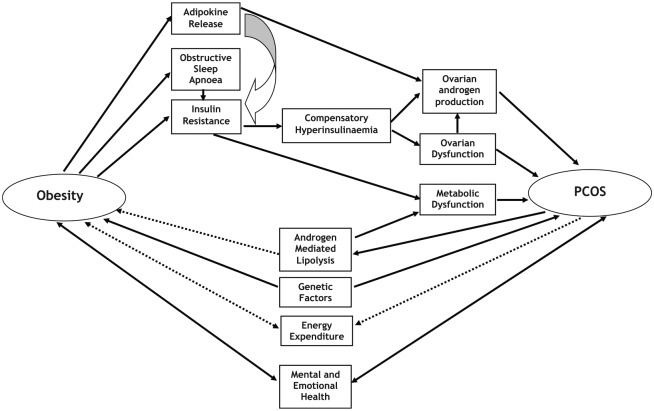
The frequent co-occurrence of obesity with PCOS, the inherent complexity of each condition, and the association of each with cardiometabolic dysfunction and insulin resistance can make discernment of pathogenic pathways challenging. Polycystic ovary syndrome is an obesity-related condition. As such, weight-gain and obesity contribute towards the development of PCOS. However, there are also mechanisms whereby the development of PCOS can contribute towards further weight-gain and hamper efforts to establish effective weight-loss. In this brief review, we explore the mechanisms whereby weight-gain and obesity contribute towards development of PCOS, and vice versa (summarized in Figure 1). We also explore novel strategies for sustained weight-loss in the management of women with PCOS, and in its prevention.
Figure 1.
Summary of mechanisms linking obesity with PCOS. PCOS indicates polycystic ovary syndrome.
Obesity as a Risk Factor for the Development of PCOS
In women who are genetically predisposed to development of PCOS, weight-gain and obesity often result in its clinical and biochemical manifestation. Accordingly, there are close links between obesity and PCOS. The majority of women with PCOS (38%-88%) are either overweight or obese.2,15,16 Data from the Northern Finland Birth Cohort (NFBC) 1966 show a significant association between body mass index (BMI) and features of PCOS at all ages.17 Furthermore, modest weight-loss (around 5%) often results in clinically meaningful improvements in the reproductive, hyperandrogenic, and metabolic features of PCOS.18,19 Outlined below are factors that mediate the effects of weight-gain and obesity on the pathogenesis of PCOS.
Insulin resistance and fat distribution in the development of PCOS
The majority of women with PCOS (50%-90%) are insulin resistant.20-22 There is poor understanding of the origin of insulin resistance in PCOS and the mechanisms implicated. It is likely that testosterone and the CAG repeat number within the androgen receptor contribute towards insulin resistance in PCOS.23 In one study in PCOS, there was demonstration of a post-receptor defect specific to the phosphatidylinositol 3-kinase (PI3-kinase) pathway. This pathway is responsible for mediating the metabolic effects of insulin (including glucose disposal into skeletal muscle).2 Whatever the mechanisms, insulin resistance underlies association of PCOS with dysmetabolic features. Insulin also mediates steroidogenic and cell-growth effects, however, through a separate post-receptor pathway, the mitogen-activated protein (MAP) kinase pathway, which remains intact.2,24 Compensatory hyperinsulinaemia, secondary to insulin resistance in PCOS, has stimulatory effects on the intact MAP kinase (and the impaired PI3-kinase) pathway to enhance steroidogenesis.25 Therefore, although PCOS is a condition that associates with insulin resistance, it is important to appreciate that this only accounts for one side of the coin. An equally important aspect of PCOS pathogenesis relates to the pleiotropic steroidogenic effects (including hyperandrogenaemia and reproductive dysfunction) of excessive insulin, enabled through an intact MAP kinase post-receptor insulin pathway.
Compensatory hyperinsulinaemia in PCOS has multiple effects on peripheral tissues, including co-gonadotrophic effects within ovarian theca cells (characterized by synergism with luteinising hormone [LH] through activation of CYP17 [P450c17α], a key enzyme in androgen biosynthesis within the ovary).26-28 It is also well established that ovulatory dysfunction in PCOS results from the adverse effects of hyperinsulinaemia on preantral follicular development.29,30 Other effects of insulin in PCOS that likely contribute towards the development of hyperandrogenaemia in this condition include enhancement of LH pulse amplitude in pituitary tissue (shown in rodent models)21,31 and stimulation of adrenal P450c17α activity.32 Furthermore, insulin may also suppress production of sex hormone-binding globulin (SHBG) within the liver,33,34 thereby further enhancing androgenicity through increased levels of free (biologically available) testosterone.
Weight-gain and obesity worsen insulin resistance and features of the metabolic syndrome.35 Weight-gain and obesity in women with PCOS also promote worsening insulin resistance,2 and both metabolic dysfunction (mediated through further impairment of the PI3-kinase post-receptor insulin pathway) and the characteristic reproductive and hyperandrogenic features of this condition. Therefore, the effects of weight-gain on insulin resistance and hyperinsulinaemia, and the dysmetabolic and steroidogenic implications of the impaired PI3-kinase and intact MAP kinase post-receptor insulin pathways, respectively, form a central component of PCOS pathogenesis and underlie the association of weight-gain and obesity with PCOS. This explanation also provides a rationale for the benefits of successful weight-loss in obese and overweight women with PCOS, through improved insulin sensitivity and serum insulin levels and favourable impact on metabolic health, reproductive function (including restoration of ovulation, menstrual cyclicity, and fertility), and hyperandrogenic features.2,19,36
Regarding pathogenesis of insulin resistance inherent to PCOS, it is important to consider a possible role for visceral fat preponderance as a contributor, given the known effects of visceral fat on metabolic dysfunction through adipokine and fatty acid release.37-39 Unfortunately, some of the earlier imaging studies in PCOS used techniques such as lipometer40 and ultrasound,41 which are limited by operator-dependence.42 Dual-energy X-ray absorptiometry (DEXA) has also been used,43,44 although this technique does not allow for discernment between visceral and subcutaneous abdominal fat depots.42 Another limitation of some imaging studies in PCOS includes lack of an appropriately BMI-matched control group for comparison.42
Our own group employed MR (with highly resolved images and clear delineation of fat depots) in 22 obese BMI- and fat mass-matched pairs of PCOS cases and controls to determine cross-sectional areas of fat depots (including visceral fat) at anatomically pre-defined sites.45 We demonstrated that fat depots, including visceral fat, are equivalent between women with PCOS and control women,45 despite significant differences in insulin resistant between the two groups. Subsequent MR-based studies in women with PCOS versus control women show similar findings.46,47 Based on these more recent imaging studies using a gold standard approach of MR-based methodology, it seems that women with PCOS manifest global adiposity, rather than a preponderance of visceral fat.45 However, visceral fat may still be important in PCOS as a contributor towards insulin resistance. There is a positive correlation between total body fat mass and visceral fat mass in women regardless of PCOS status.42,45,48 Changes in body weight in women with PCOS would therefore influence visceral fat content and metabolic risk. The role of ectopic fat as a mediator of metabolic risk in PCOS remains incompletely explored. It is likely, however, that non-alcoholic fatty liver disease (NAFLD), which is common in obese women with PCOS, contributes towards the metabolic dysfunction associated with PCOS.49
Obstructive sleep apnoea in the development of PCOS
Obstructive sleep apnoea (OSA) presents with recurrent episodes of complete or partial obstruction of the upper airway leading to intermittent hypoxia and chronic sleep loss.50 Obstructive sleep apnoea is more common in men51 and in patients with obesity.52
Patients with OSA have increased risk of insulin resistance, which is present even when adjusted for BMI.53 In this sense, OSA is similar to PCOS.20-22 Association of OSA with insulin resistance and metabolic derangement is complex, but changes in levels of cortisol, catecholamines, and adipokines are likely to be contributory.54
Obstructive sleep apnoea is commoner in women with PCOS than in BMI- and age-adjusted control women.55 A recent large longitudinal study showed that women with PCOS have a 2.26 higher risk of developing OSA compared with women without PCOS, irrespective of BMI or age.56 Polycystic ovary syndrome may increase the risk of OSA through effects on the sex hormones progesterone and testosterone. In one study, progesterone reduced airway resistance by enhancing activity of the upper airway dilator muscle,57 thereby conferring protection from OSA in women. Furthermore, testosterone influences apnoeic threshold, with increased levels of testosterone resulting in breathing instability during sleep.58 Anovulation in PCOS typically associates with lowered levels of progesterone.2 This combined with increased levels of testosterone provides one sex hormone-related explanation for association of PCOS with OSA. The effects of testosterone on apnoeic threshold58 may also provide one explanation (in addition to increased truncal fat around the neck) for the increased risk of developing OSA in men. Interestingly, visceral fat content (rather than total body fat) correlates with the risk for development of OSA.59 As visceral fat content in women (regardless of PCOS status) increases commensurately with total fat content,45 this may contribute towards heightened risk of OSA in women with PCOS who gain weight.
There are similarities between the metabolic derangements that typify OSA and PCOS.60 However, women with PCOS who also develop OSA have a metabolic double-whammy, with enhanced insulin resistance and glucose intolerance, and greater risk of development of T2D and metabolic syndrome compared with women with PCOS without OSA, adjusted for BMI, ethnicity, and age.60 Given the greater risk of development of OSA in women with PCOS, it is important that healthcare professionals are aware and institute regular OSA screening for women with PCOS. Treatment of OSA with continuous positive airway pressure (CPAP) therapy is effective at alleviating insulin resistance in women with PCOS and can at least partially reverse the associated metabolic derangements.61 Bariatric surgery is an excellent treatment option for OSA (resolution rate 85%).62
Steroidogenesis and adipokines in the development of PCOS
As alluded to earlier, insulin resistance and the associated hyperinsulinaemia is a driver for enhanced steroidogenesis in women with PCOS. Enhanced steroidogenesis (through co-gonadotrophic effects within the ovary and direct effects within the adrenal2) results in hyperandrogenaemia and hyperandrogenic features including hirsutism that typify women with this condition. Weight-gain and obesity in women with PCOS, through its effects on insulin resistance, thereby drive enhanced steroidogenesis and hyperandrogenism. This provides an explanation for the close association between body weight and severity of the hyperandrogenic features of PCOS.
Our own group has demonstrated the association of PCOS with enhanced 5-alpha reductase activity in the largest study to date on urinary steroid profiles in women with PCOS (n = 178) versus BMI-matched control women (n = 100).63 Furthermore, 5-alpha reductase activity correlated positively with increasing adiposity in both groups.63 The main effects of enhanced 5-alpha reductase activity on androgenicity in PCOS are two-fold: (1) enhanced conversion of testosterone into 5-dihydroxytestosterone (a potent androgen) and (2) enhanced conversion of cortisol into its breakdown products. As cortisol is broken down, negative feedback effect at the level of the pituitary diminishes, resulting in enhancement of hypothalamo-pituitary adrenal (HPA) axis activity and adrenal (including androgen) steroidogenesis.63
Weight-gain and obesity may also contribute to the clinical and biochemical expression of PCOS through effects on adipokine release.42 In a large meta-analysis of more than 3400 subjects, it was demonstrated that serum adiponectin levels are lower in women with PCOS compared with BMI-adjusted control women, possibly contributing towards insulin resistance in PCOS.64 One of the steroidogenic effects of adiponectin is inhibition of androgen production from ovarian theca cells.65 It seems likely that enhancement of ovarian androgen production in women with PCOS is influenced, at least in part by suppressed levels of adiponectin.42 Consistent with this hypothesis is an observation of inverse correlation between levels of adiponectin and testosterone (and ovarian volume) in pubertal girls (n = 56) with type 1 diabetes mellitus.66 The role of high molecular weight adiponectin in PCOS should be a focus for future research.67
In addition to adiponectin, visfatin (implicated in metabolism, inflammation, and insulin resistance42,68) may also contribute towards metabolic dysfunction in PCOS,42 serum levels of visfatin being greater in women with PCOS than in control women.68-71 Retinol-binding protein 4 may also play a role in mediating effects of weight-gain and obesity on the development of PCOS.67,72 However, another adipokine, adipocyte fatty acid-binding protein, while correlated with markers of obesity, does not appear to associate with metabolic or hyperandrogenic features of PCOS.73
Genetic factors in the development of PCOS
Genetic factors contribute towards the development of both PCOS74-76 and obesity.77 A recently reported large-scale genome-wide meta-analysis of PCOS showed evidence for shared genetic architecture between metabolic traits, including a causal link between obesity and PCOS.78 Given the epidemiological link between both conditions, it is important to consider a possible role for genetic variants.
FTO (fat mass and obesity-associated gene) was the first gene identified from a genome-wide association study (GWAS) to have a robust effect on susceptibility for the development of common polygenic obesity.79,80 Variants within FTO influence fat mass (per-allele difference in BMI 0.36 kg·m−2).79 In a UK-based study on 463 UK PCOS cases and more than 1300 UK female controls, our own group demonstrated a significant association between a variant within FTO (rs9939609 single nucleotide polymorphism) and PCOS status (odds ratio [OR] per minor allele copy 1.30), attenuated by adjustment for BMI between cases and controls.81 These data showed for the first time a genetic corroboration for a mechanistic link between PCOS and obesity.81 Subsequent studies in diverse populations have confirmed association between variants in FTO and PCOS status.82-84 Based on the data outlined here, it seems likely that genetic factors that include FTO variants contribute towards development of PCOS through effects on increased fat mass and weight-gain. Such genetic effects on fat mass are likely to provide an explanation for at least some of the heritability of PCOS.
Polycystic Ovary Syndrome as a Risk Factor for the Development of Obesity
Much epidemiological data confirm a close association between obesity and PCOS.2,17 As outlined earlier, much evidence confirms a clear effect of weight-gain on development of PCOS and weight-loss on its alleviation, mediated, for example, through effects on insulin sensitivity.2 Furthermore, data from NFBC 1966 show association between early adiposity rebound during childhood and diagnosis of PCOS and obesity in adulthood.85 However, PCOS is a complex condition, and it is likely that its relationship with obesity is also complex. It is important to consider possible mechanisms whereby PCOS may contribute towards further weight-gain or hamper successful attempts at weight-loss and maintenance of body weight through lifestyle means in women with this condition, outlined in this section.
Energy expenditure as a contributor to weight-gain in PCOS
Changes in energy expenditure over prolonged periods could influence body weight. It is important to consider, therefore, whether PCOS is associated with changes in any aspect of metabolism. Robinson and colleagues86 performed continuous indirect calorimetry in 14 women with PCOS and 14 controls in a cross-sectional design. Compared with controls, postprandial thermogenesis was significantly lower in women with PCOS, and the difference between groups was more marked for obese women with PCOS (a difference of 41.1 kJ). Furthermore, the level of insulin resistance correlated with the reduction in postprandial thermogenesis in the PCOS group.86 Interestingly, resting energy expenditure was similar between groups in this study.86 More recently, measurement of resting metabolic rate using a SenseWear Armband in 109 women with PCOS versus 31 control women87 showed that resting metabolic rate was equivalent between groups.87
There is a lack of clear data in the literature to enable any meaningful conclusions regarding potential effects of PCOS on energy expenditure. Furthermore, even if PCOS is truly associated with diminished energy expenditure (either postprandial and/or resting components), this would not necessarily result in weight-gain over time: central control of the components of metabolism and interlinks between appetite, gut peptides, and nutrient supply is highly complex and incompletely understood. Postprandial thermogenesis accounts for a relatively small proportion of overall metabolism. It seems unlikely that modest changes in postprandial thermogenesis would contribute towards clinically meaningful weight-gain in PCOS. Without long-term and more complete data, and a better understanding of the complexity of body weight regulation, the data outlined above remain interesting observations. Potential implications for body weight regulation, however, are uncertain.
Androgen-mediated lipolysis as a contributor towards weight-gain in PCOS
Polycystic ovary syndrome may associate with abnormalities in lipolytic functioning of adipocytes.2 In one study, catecholamine-induced lipolysis within isolated visceral adipocytes was increased two-fold in non-obese women with PCOS compared with BMI-matched control women,88 possibly mediated by changes in function of the post-receptor protein kinase A – hormone-sensitive lipase complex.2,88 Furthermore, in normal men and women, testosterone facilitates release of non-esterified fatty acids from visceral adipocytes in vivo.89 Most studies of androgen effects on the adipocyte have shown a stimulatory effect on lipolysis, and impaired adipocyte differentiation, insulin signalling, and generation of adipokines.90 However, there is tight control of exposure of adipocytes to androgens through key isoenzymes. Within abdominal subcutaneous adipose tissue, androgen inactivation usually predominates.90
The effects of androgens on lipolysis and adipocyte function in women with PCOS, the site-specificity, and possible mediating effects of fat mass are incompletely understood, and worthy of further research focus. It remains possible that androgen-mediated enhanced visceral lipolysis contributes towards metabolic dysfunction in PCOS. However, based on current data, it is premature to hypothesize a role for changes in subcutaneous adipocyte lipolysis as a factor that contributes towards weight-gain in some women with PCOS.
Mental health problems as a contributor to weight-gain in PCOS
Implementation of effective lifestyle strategies to achieve and maintain weight-loss and mitigate against ongoing weight-gain usually requires focus, drive, and commitment. Any mental or emotional health problem can potentially hinder such attempts at lifestyle implementations (including dietary changes and engagement in physical activity). There are complex interactions between obesity and mental health problems that often become established during childhood.91 It is likely that interlinks between obesity and mental health problems (including poor emotional health and self-esteem) are complex and multi-directional.
In obese women with PCOS, there are also often features like hirsutism, menstrual irregularities, and fertility problems that amalgamate with the already complex interlinks between obesity and mental and emotional functioning. Unsurprisingly, women with PCOS are susceptible to mental health problems. In one cross-sectional and self-reported study on 177 women with PCOS and 109 healthy controls, scores for anxiety, depression, and negative body image were significantly higher in the women with PCOS.92 Body image and self-worth were predictors of anxiety and depression (regardless of PCOS status), and time taken to diagnose PCOS was associated with poor psychological functioning.92 In another cross-sectional study on 50 patients with PCOS and 41 healthy controls, women with PCOS had significantly higher depression and anxiety scores compared with the controls.93 Interestingly, however, temperament and character were equivalent between PCOS and control groups.93 In a further study that focused on adolescent girls aged 13 to 18 years and diagnosed with PCOS, there was very little perceived self-control (including menstrual irregularities and threat of infertility). Perception of poor self-control was a predictor for depression.94
Data from the NFBC 1966 show that at ages 31 and 46 years, compared with controls, women with PCOS have increased anxiety and/or depression symptoms.95 It is reasonable to hypothesize that depression and anxiety (and perceived diminishment in self-control) at different ages would all potentially hinder attempts at lifestyle implementation and successful weight-loss. This includes engagement in physical activity (as outlined in the next section), but would also extend to other aspects of healthy living including diet and sleep sufficiency for example. The data outlined stem from cross-sectional (association) studies. Therefore, inference of causality is not possible. It is likely though that the clinical manifestation of PCOS (with frequent co-occurrence of obesity) and mental/emotional problems have complex and multi-directional interlinks and vary between individuals. While pre-existent mental health problems may contribute towards weight-gain and the manifestation of PCOS in those who are genetically predisposed,77 it seems likely also that features of PCOS, including the perceived lack of self-control especially at the vulnerable age of adolescence,94 would predispose to new development or worsening of pre-existing mental and emotional problems. A vicious cycle may ensue in which worsening features of PCOS beget heightened anxiety, depression, body image, and perceived lack of self-control that in turn hinders attempts at implementing lifestyle measures to facilitate weight-loss. Therefore, in at least some women, PCOS may lead to further weight-gain (or at least dampen attempts at successful weight-loss), mediated indirectly through associated mental and emotional problems.
Physical inactivity as a contributor to weight-gain in PCOS
Physical inactivity is an important contributor to most chronic diseases.96 The benefits of physical activity for the populace are legion and include interesting cross talk between muscle and brain.97 As in obesity management in general, maintenance of physical activity and exercise forms a key aspect of lifestyle advice for women with PCOS.2 Benefits include promotion of weight-loss2; fasting insulin levels and waist circumference98; free androgen index99; and blood pressure, lipid profile, glucose levels, and reduction in fat mass.100 It is important to consider whether PCOS may contribute towards weight-gain and obesity (or at least resistance to effective weight-loss), through effects of the condition on ability to engage in physical activity, mediated by possible PCOS-related emotional and physical factors. It is important to highlight that the factors discussed below may also apply to the general obese populace regardless of PCOS status, given the complex association between obesity and emotional and physical functioning.101,102
Emotional factors implicated in physical inactivity in PCOS
Despite a lack of well-controlled studies reported in the literature on physical activity in PCOS,103 one study reported by Banting and colleagues104 compared self-reported measures of physical activity and depression and anxiety scores in women with and without PCOS. Compared with controls, women with PCOS experienced more barriers to physical activity including emotional factors like lack of confidence and fear of injury, and physical limitations.104 Encouragingly, however, physically active women (regardless of PCOS status) experienced less severe depression than that in their inactive counterparts.104 Other studies support the benefits of physical activity on mental health in PCOS. These include beneficial effects of brisk-walking over 6 months on body image distress score,105 and beneficial effects of enhanced exercise as part of lifestyle modification on improvements on self-esteem, depression, and anxiety.106
Physical factors implicated in physical inactivity in PCOS
There is some controversy in the literature regarding physical cardiorespiratory fitness in women with PCOS. One study demonstrated that VO2 max is impaired in young overweight women with PCOS,107 whereas another study showed equivalence of physical fitness between women with PCOS and age- and BMI-matched controls.108 Differences in insulin sensitivity between subjects in each study may have contributed towards some of the discrepancies regarding physical fitness in PCOS between studies.103 No apparent differences exist between women with PCOS and healthy controls regarding muscle strength108 and levels of free-living physical activity.109
To summarize this section, evidence to support a direct effect of PCOS on physical ability to engage in physical activity (including muscle strength and fitness) appears scant. However, there is clear evidence to support the notion that PCOS may have a negative impact on engagement in physical activity, mediated indirectly through emotional barriers. Although long-term prospective studies are not available, it seems reasonable to hypothesize that such disengagement in physical activity would eventually contribute towards weight-gain and hamper efforts to lose weight. Given the huge benefits of physical activity (including improvements in mental and emotional health), it is important to manage any emotional barriers to its effective execution proactively, sensitively, and in a timely manner.
Novel Management Strategies for Obesity and PCOS
Although many therapies exist for management of PCOS, most of these target manifestations of PCOS rather than underlying causal mechanisms. Effective weight-loss implementation remains the most effective and promising management strategy for women with PCOS. However, this presents a problem: weight-loss maintenance through lifestyle implementation is challenging and has a high failure rate.110 While bariatric surgery represents an excellent alternative strategy to lifestyle implementation for effective and long-term weight-loss,111 surgical management strategies for obesity associated with PCOS will never be scalable to a population level. Our current therapeutic armamentarium for obesity in the United Kingdom is diminutive. Novel management strategies for PCOS in the context of obesity should therefore focus on effective means of maintaining weight-loss over the longer term on a population level.
Appetite enhancement is a key driver of weight-regain following weight-loss, and one that persists for at least a year.112 It is logical to develop future therapies that suppress appetite enhancement following initial weight-loss. Data on appetite suppressant effects of key gut peptides are promising and promote certain gut peptides such as peptide tyrosine tyrosine (PYY) as potential future therapies to complement lifestyle strategies for weight-maintenance following initial weight-loss.113 However, these gut peptide therapies are likely to be expensive and will likely require injectable administration. In addition to appetite suppression, therapies to improve hyperlipidaemia may impact beneficially on hyperandrogenaemia in PCOS.114
An alternate novel strategy for weight-loss, one that is entirely scalable on a population level and which everyone can implement, is the application of mindfulness to lifestyle strategies. Our own group demonstrated significant improvements in dietary-induced weight-loss and healthy eating-related behaviours among obese patients attending group sessions in the context of a UK-based tier 3 obesity service.115 It is reasonable to hypothesize application of mindfulness techniques to other aspects of lifestyle change including physical activity to facilitate successful adoption of lifestyle change in obese women with PCOS. We envision that self-taught mindfulness techniques, administered through appropriate and wide-reaching infrastructure and adopted by the populace at scale, could represent a novel and effective strategy to promote maintenance of body weight following initial weight-loss in obese women with PCOS. In this way, mindfulness approaches could complement changes in diet and physical activity in obese women with PCOS. This should be a focus of future research.
Although not a novel strategy, it is worth considering the benefits of physical exercise in PCOS (independent of any weight-loss) in more detail. Aerobic exercise can improve reproductive function in women with PCOS, including normalization of menstrual cyclicity98,99 and ovulation rates.99 In fact, exercise (possibly through improvements in insulin sensitivity) appears to result in improvements in menstrual cyclicity and/or ovulation in around 50% of women with PCOS.103 It is worth highlighting, though, that excessive exercise (>60 minutes per day) can increase risk of anovulation, whereas exercise duration of 30 to 60 minutes per day reduces the risk of anovulatory infertility.116 Therefore, promotion of moderate (and avoidance of excessive) exercise are important health messages for women with PCOS.
Conclusions and Future Directions
It is widely accepted based on current evidence that, weight-gain and obesity are important risk factors for the clinical and biochemical manifestations of PCOS in those women who are genetically predisposed.117 The multiple mechanisms mediating this process are complex. However, the association between obesity and PCOS is more complex than a simple cause-and-effect process and likely includes complicated interlinks between multiple factors. As outlined, it seems likely that in at least some obese women with PCOS, development of PCOS through indirect mechanisms (including depression and perceived lack of self-control and increased distress) may hamper ongoing attempts at lifestyle change and therefore effective weight-loss. Indeed, such factors may even promote further weight-gain, thereby setting in play a vicious circle that can be difficult to vanquish.
As healthcare professionals, we are all likely to encounter women with PCOS given its prevalence and close association with obesity. Given the future projection of the obesity epidemic, it is likely that the overall prevalence of PCOS will continue to increase. Furthermore, PCOS often becomes manifest during adolescence, a vulnerable time of life. It is incumbent upon all healthcare professionals to recognize the plight of women and girls with PCOS, and the complexity of its pathogenesis. We need to manage these patients with sensitivity and empathy, while also pursuing a proactive approach, particularly regarding presence and emergence of mental and emotional health problems. This is particularly important given the stigmatization of mental ill health and obesity within our society. We need to adopt a holistic approach to weight management in PCOS: one that addresses not just lifestyle change but any potential mental and emotional barriers to its effective implementation. Only then we can hope to convert the typified vicious circle of weight-gain and worsened features of PCOS into a liberated virtuous cycle of effective weight-loss and eventual freedom from the shackles of PCOS.
Footnotes
Funding:The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of conflicting interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: All authors contributed substantively to the preparation of this manuscript.
ORCID iDs: Thomas M Barber  https://orcid.org/0000-0003-0689-9195
https://orcid.org/0000-0003-0689-9195
Petra Hanson  https://orcid.org/0000-0002-6845-1049
https://orcid.org/0000-0002-6845-1049
References
- 1. Guh DP, Zhang W, Bansback N, Amarsi Z, Birmingham CL, Anis AH. The incidence of co-morbidities related to obesity and overweight: a systematic review and meta-analysis. BMC Public Health. 2009;9:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Barber TM, McCarthy MI, Wass JA, Franks S. Obesity and polycystic ovary syndrome. Clin Endocrinol (Oxf). 2006;65:137-145. [DOI] [PubMed] [Google Scholar]
- 3. Saboor Aftab SA, Kumar S, Barber TM. The role of obesity and type 2 diabetes mellitus in the development of male obesity-associated secondary hypogonadism. Clin Endocrinol (Oxf). 2013;78:330-337. [DOI] [PubMed] [Google Scholar]
- 4. Passarello K, Kurian S, Villanueva V. Endometrial cancer: an overview of pathophysiology, management, and care. Semin Oncol Nurs. 2019;35:157-165. [DOI] [PubMed] [Google Scholar]
- 5. Teede HJ, Joham AE, Paul E, et al. Longitudinal weight gain in women identified with polycystic ovary syndrome: results of an observational study in young women. Obesity (Silver Spring). 2013;21:1526-1532. [DOI] [PubMed] [Google Scholar]
- 6. Wild S, Pierpoint T, McKeigue P, Jacobs H. Cardiovascular disease in women with polycystic ovary syndrome at long-term follow-up: a retrospective cohort study. Clin Endocrinol (Oxf). 2000;52:595-600. [DOI] [PubMed] [Google Scholar]
- 7. Barber TM, McCarthy MI, Franks S, Wass JA. Metabolic syndrome in polycystic ovary syndrome. Endokrynol Pol. 2007;58:34-41. [PubMed] [Google Scholar]
- 8. Mohlig M, Floter A, Spranger J, et al. Predicting impaired glucose metabolism in women with polycystic ovary syndrome by decision tree modelling. Diabetologia. 2006;49:2572-2579. [DOI] [PubMed] [Google Scholar]
- 9. Ramezani-Binabaj M, Motalebi M, Karimi-Sari H, Rezaee-Zavareh MS, Alavian SM. Are women with polycystic ovarian syndrome at a high risk of non-alcoholic Fatty liver disease; a meta-analysis. Hepat Mon. 2014;14:e23235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ehrmann DA. Metabolic dysfunction in PCOS: relationship to obstructive sleep apnea. Steroids. 2012;77:290-294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Azziz R, Woods KS, Reyna R, Key TJ, Knochenhauer ES, Yildiz BO. The prevalence and features of the polycystic ovary syndrome in an unselected population. J Clin Endocrinol Metab. 2004;89:2745-2749. [DOI] [PubMed] [Google Scholar]
- 12. Sanchon R, Gambineri A, Alpanes M, Martinez-Garcia MA, Pasquali R, Escobar-Morreale HF. Prevalence of functional disorders of androgen excess in unselected premenopausal women: a study in blood donors. Hum Reprod. 2012;27:1209-1216. [DOI] [PubMed] [Google Scholar]
- 13. Yildiz BO, Bozdag G, Yapici Z, Esinler I, Yarali H. Prevalence, phenotype and cardiometabolic risk of polycystic ovary syndrome under different diagnostic criteria. Hum Reprod. 2012;27:3067-3073. [DOI] [PubMed] [Google Scholar]
- 14. Asuncion M, Calvo RM, San Millan JL, Sancho J, Avila S, Escobar-Morreale HF. A prospective study of the prevalence of the polycystic ovary syndrome in unselected Caucasian women from Spain. J Clin Endocrinol Metab. 2000;85:2434-2438. [DOI] [PubMed] [Google Scholar]
- 15. Legro RS. The genetics of obesity. Lessons for polycystic ovary syndrome. Ann N Y Acad Sci. 2000;900:193-202. [DOI] [PubMed] [Google Scholar]
- 16. Balen AH, Conway GS, Kaltsas G, et al. Polycystic ovary syndrome: the spectrum of the disorder in 1741 patients. Hum Reprod. 1995;10:2107-2111. [DOI] [PubMed] [Google Scholar]
- 17. Ollila MM, Piltonen T, Puukka K, et al. Weight gain and dyslipidemia in early adulthood associate with polycystic ovary syndrome: prospective cohort study. J Clin Endocrinol Metab. 2016;101:739-747. [DOI] [PubMed] [Google Scholar]
- 18. Kiddy DS, Hamilton-Fairley D, Bush A, et al. Improvement in endocrine and ovarian function during dietary treatment of obese women with polycystic ovary syndrome. Clin Endocrinol (Oxf). 1992;36:105-111. [DOI] [PubMed] [Google Scholar]
- 19. Holte J, Bergh T, Berne C, Wide L, Lithell H. Restored insulin sensitivity but persistently increased early insulin secretion after weight loss in obese women with polycystic ovary syndrome. J Clin Endocrinol Metab. 1995;80:2586-2593. [DOI] [PubMed] [Google Scholar]
- 20. Dunaif A, Segal KR, Futterweit W, Dobrjansky A. Profound peripheral insulin resistance, independent of obesity, in polycystic ovary syndrome. Diabetes. 1989;38:1165-1174. [DOI] [PubMed] [Google Scholar]
- 21. Dunaif A. Insulin resistance and the polycystic ovary syndrome: mechanism and implications for pathogenesis. Endocr Rev. 1997;18:774-800. [DOI] [PubMed] [Google Scholar]
- 22. Venkatesan AM, Dunaif A, Corbould A. Insulin resistance in polycystic ovary syndrome: progress and paradoxes. Recent Prog Horm Res. 2001;56:295-308. [DOI] [PubMed] [Google Scholar]
- 23. Mohlig M, Jurgens A, Spranger J, et al. The androgen receptor CAG repeat modifies the impact of testosterone on insulin resistance in women with polycystic ovary syndrome. Eur J Endocrinol. 2006;155:127-130. [DOI] [PubMed] [Google Scholar]
- 24. Cusi K, Maezono K, Osman A, et al. Insulin resistance differentially affects the PI 3-kinase- and MAP kinase-mediated signaling in human muscle. J Clin Invest. 2000;105:311-320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rice S, Christoforidis N, Gadd C, et al. Impaired insulin-dependent glucose metabolism in granulosa-lutein cells from anovulatory women with polycystic ovaries. Hum Reprod. 2005;20:373-381. [DOI] [PubMed] [Google Scholar]
- 26. Nestler JE, Strauss JF., 3rd Insulin as an effector of human ovarian and adrenal steroid metabolism. Endocrinol Metab Clin North Am. 1991;20:807-823. [PubMed] [Google Scholar]
- 27. Franks SMH, White D, Willis D. Mechanisms of An ovulation in Polycystic Ovary Syndrome. Filicori MF, ed. Amsterdam: Elsevier; 1996:183-186. [Google Scholar]
- 28. White D, Leigh A, Wilson C, Donaldson A, Franks S. Gonadotrophin and gonadal steroid response to a single dose of a long-acting agonist of gonadotrophin-releasing hormone in ovulatory and anovulatory women with polycystic ovary syndrome. Clin Endocrinol (Oxf). 1995;42:475-481. [DOI] [PubMed] [Google Scholar]
- 29. Robinson S, Kiddy D, Gelding SV, et al. The relationship of insulin insensitivity to menstrual pattern in women with hyperandrogenism and polycystic ovaries. Clin Endocrinol (Oxf). 1993;39:351-355. [DOI] [PubMed] [Google Scholar]
- 30. Willis DS, Watson H, Mason HD, Galea R, Brincat M, Franks S. Premature response to luteinizing hormone of granulosa cells from anovulatory women with polycystic ovary syndrome: relevance to mechanism of anovulation. J Clin Endocrinol Metab. 1998;83:3984-3991. [DOI] [PubMed] [Google Scholar]
- 31. Adashi EY, Hsueh AJ, Yen SS. Insulin enhancement of luteinizing hormone and follicle-stimulating hormone release by cultured pituitary cells. Endocrinology. 1981;108:1441-1449. [DOI] [PubMed] [Google Scholar]
- 32. Morin-Papunen LC, Vauhkonen I, Koivunen RM, Ruokonen A, Tapanainen JS. Insulin sensitivity, insulin secretion, and metabolic and hormonal parameters in healthy women and women with polycystic ovarian syndrome. Hum Reprod. 2000;15:1266-1274. [DOI] [PubMed] [Google Scholar]
- 33. Nestler JE, Powers LP, Matt DW, et al. A direct effect of hyperinsulinemia on serum sex hormone-binding globulin levels in obese women with the polycystic ovary syndrome. J Clin Endocrinol Metab. 1991;72:83-89. [DOI] [PubMed] [Google Scholar]
- 34. Yki-Jarvinen H, Makimattila S, Utriainen T, Rutanen EM. Portal insulin concentrations rather than insulin sensitivity regulate serum sex hormone-binding globulin and insulin-like growth factor binding protein 1 in vivo. J Clin Endocrinol Metab. 1995;80:3227-3232. [DOI] [PubMed] [Google Scholar]
- 35. Reaven GM. The metabolic syndrome: requiescat in pace. Clin Chem. 2005;51:931-938. [DOI] [PubMed] [Google Scholar]
- 36. Azziz R, Ehrmann D, Legro RS, et al. Troglitazone improves ovulation and hirsutism in the polycystic ovary syndrome: a multicenter, double blind, placebo-controlled trial. J Clin Endocrinol Metab. 2001;86:1626-1632. [DOI] [PubMed] [Google Scholar]
- 37. Banerji MA, Faridi N, Atluri R, Chaiken RL, Lebovitz HE. Body composition, visceral fat, leptin, and insulin resistance in Asian Indian men. J Clin Endocrinol Metab. 1999;84:137-144. [DOI] [PubMed] [Google Scholar]
- 38. Marks SJ, Moore NR, Clark ML, Strauss BJ, Hockaday TD. Reduction of visceral adipose tissue and improvement of metabolic indices: effect of dexfenfluramine in NIDDM. Obes Res. 1996;4:1-7. [DOI] [PubMed] [Google Scholar]
- 39. Zhuang XF, Zhao MM, Weng CL, Sun NL. Adipocytokines: a bridge connecting obesity and insulin resistance. Med Hypotheses. 2009;73:981-985. [DOI] [PubMed] [Google Scholar]
- 40. Horejsi R, Moller R, Rackl S, et al. Android subcutaneous adipose tissue topography in lean and obese women suffering from PCOS: comparison with type 2 diabetic women. Am J Phys Anthropol. 2004;124:275-281. [DOI] [PubMed] [Google Scholar]
- 41. Yildirim B, Sabir N, Kaleli B. Relation of intra-abdominal fat distribution to metabolic disorders in nonobese patients with polycystic ovary syndrome. Fertil Steril. 2003;79:1358-1364. [DOI] [PubMed] [Google Scholar]
- 42. Barber TM, Franks S. Adipocyte biology in polycystic ovary syndrome. Mol Cell Endocrinol. 2013;373:68-76. [DOI] [PubMed] [Google Scholar]
- 43. Kirchengast S, Huber J. Body composition characteristics and body fat distribution in lean women with polycystic ovary syndrome. Hum Reprod. 2001;16:1255-1260. [DOI] [PubMed] [Google Scholar]
- 44. Puder JJ, Varga S, Kraenzlin M, De Geyter C, Keller U, Muller B. Central fat excess in polycystic ovary syndrome: relation to low-grade inflammation and insulin resistance. J Clin Endocrinol Metab. 2005;90:6014-6021. [DOI] [PubMed] [Google Scholar]
- 45. Barber TM, Golding SJ, Alvey C, et al. Global adiposity rather than abnormal regional fat distribution characterises women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2008;93:999-1004. [DOI] [PubMed] [Google Scholar]
- 46. Manneras-Holm L, Leonhardt H, Kullberg J, et al. Adipose tissue has aberrant morphology and function in PCOS: enlarged adipocytes and low serum adiponectin, but not circulating sex steroids, are strongly associated with insulin resistance. J Clin Endocrinol Metab. 2011;96:E304-E311. [DOI] [PubMed] [Google Scholar]
- 47. Dolfing JG, Stassen CM, van Haard PM, Wolffenbuttel BH, Schweitzer DH. Comparison of MRI-assessed body fat content between lean women with polycystic ovary syndrome (PCOS) and matched controls: less visceral fat with PCOS. Hum Reprod. 2011;26:1495-1500. [DOI] [PubMed] [Google Scholar]
- 48. Carmina E, Bucchieri S, Esposito A, et al. Abdominal fat quantity and distribution in women with polycystic ovary syndrome and extent of its relation to insulin resistance. J Clin Endocrinol Metab. 2007;92:2500-2505. [DOI] [PubMed] [Google Scholar]
- 49. Macut D, Bjekic-Macut J, Livadas S, et al. Nonalcoholic fatty liver disease in patients with polycystic ovary syndrome. Curr Pharm Des. 2018;24:4593-4597. [DOI] [PubMed] [Google Scholar]
- 50. Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328:1230-1235. [DOI] [PubMed] [Google Scholar]
- 51. Young T, Evans L, Finn L, Palta M. Estimation of the clinically diagnosed proportion of sleep apnea syndrome in middle-aged men and women. Sleep. 1997;20:705-706. [DOI] [PubMed] [Google Scholar]
- 52. Romero-Corral A, Caples SM, Lopez-Jimenez F, Somers VK. Interactions between obesity and obstructive sleep apnea: implications for treatment. Chest. 2010;137:711-719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Tassone F, Lanfranco F, Gianotti L, et al. Obstructive sleep apnoea syndrome impairs insulin sensitivity independently of anthropometric variables. Clin Endocrinol (Oxf). 2003;59:374-379. [DOI] [PubMed] [Google Scholar]
- 54. Nitsche K, Ehrmann DA. Obstructive sleep apnea and metabolic dysfunction in polycystic ovary syndrome. Best Pract Res Clin Endocrinol Metab. 2010;24:717-730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Fogel RB, Malhotra A, Pillar G, Pittman SD, Dunaif A, White DP. Increased prevalence of obstructive sleep apnea syndrome in obese women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2001;86:1175-1180. [DOI] [PubMed] [Google Scholar]
- 56. Balachandran K, Sumilo D, O’Reilly MW, et al. Increased risk of obstructive sleep apnoea in women with polycystic ovary syndrome: a population-based cohort study [published online ahead of print February 1, 2019]. Eur J Endocrinol. doi: 10.1530/EJE-18-0693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Popovic RM, White DP. Upper airway muscle activity in normal women: influence of hormonal status. J Appl Physiol (1985). 1998;84:1055-1062. [DOI] [PubMed] [Google Scholar]
- 58. Zhou XS, Rowley JA, Demirovic F, Diamond MP, Badr MS. Effect of testosterone on the apneic threshold in women during NREM sleep. J Appl Physiol (1985). 2003;94:101-107. [DOI] [PubMed] [Google Scholar]
- 59. Shinohara E, Kihara S, Yamashita S, et al. Visceral fat accumulation as an important risk factor for obstructive sleep apnoea syndrome in obese subjects. J Intern Med. 1997;241:11-18. [DOI] [PubMed] [Google Scholar]
- 60. Tasali E, Van Cauter E, Hoffman L, Ehrmann DA. Impact of obstructive sleep apnea on insulin resistance and glucose tolerance in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2008;93:3878-3884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Tasali E, Chapotot F, Leproult R, Whitmore H, Ehrmann DA. Treatment of obstructive sleep apnea improves cardiometabolic function in young obese women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2011;96:365-374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Ashrafian H, le Roux CW, Rowland SP, et al. Metabolic surgery and obstructive sleep apnoea: the protective effects of bariatric procedures. Thorax. 2012;67:442-449. [DOI] [PubMed] [Google Scholar]
- 63. Vassiliadi DA, Barber TM, Hughes BA, et al. Increased 5α-reductase activity and adrenocortical drive in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2009;94:3558-3566. [DOI] [PubMed] [Google Scholar]
- 64. Toulis KA, Goulis DG, Farmakiotis D, et al. Adiponectin levels in women with polycystic ovary syndrome: a systematic review and a meta-analysis. Hum Reprod Update. 2009;15:297-307. [DOI] [PubMed] [Google Scholar]
- 65. Lagaly DV, Aad PY, Grado-Ahuir JA, Hulsey LB, Spicer LJ. Role of adiponectin in regulating ovarian theca and granulosa cell function. Mol Cell Endocrinol. 2008;284:38-45. [DOI] [PubMed] [Google Scholar]
- 66. Iniguez G, Torrealba IM, Avila A, Cassorla F, Codner E. Adiponectin serum levels and their relationships to androgen concentrations and ovarian volume during puberty in girls with type 1 diabetes mellitus. Horm Res. 2008;70:112-117. [DOI] [PubMed] [Google Scholar]
- 67. Barber TM, Hazell M, Christodoulides C, et al. Serum levels of retinol-binding protein 4 and adiponectin in women with polycystic ovary syndrome: associations with visceral fat but no evidence for fat mass-independent effects on pathogenesis in this condition. J Clin Endocrinol Metab. 2008;93:2859-2865. [DOI] [PubMed] [Google Scholar]
- 68. Yildiz BO, Bozdag G, Otegen U, et al. Visfatin and retinol-binding protein 4 concentrations in lean, glucose-tolerant women with PCOS. Reprod Biomed Online. 2010;20:150-155. [DOI] [PubMed] [Google Scholar]
- 69. Cekmez F, Cekmez Y, Pirgon O, et al. Evaluation of new adipocytokines and insulin resistance in adolescents with polycystic ovary syndrome. Eur Cytokine Netw. 2011;22:32-37. [DOI] [PubMed] [Google Scholar]
- 70. Dikmen E, Tarkun I, Canturk Z, Cetinarslan B. Plasma visfatin level in women with polycystic ovary syndrome. Gynecol Endocrinol. 2011;27:475-479. [DOI] [PubMed] [Google Scholar]
- 71. Jongwutiwes T, Lertvikool S, Leelaphiwat S, Rattanasiri S, Jultanmas R, Weerakiet S. Serum visfatin in Asian women with polycystic ovary syndrome. Gynecol Endocrinol. 2009;25:536-542. [DOI] [PubMed] [Google Scholar]
- 72. Mohlig M, Weickert MO, Ghadamgahi E, et al. Retinol-binding protein 4 is associated with insulin resistance, but appears unsuited for metabolic screening in women with polycystic ovary syndrome. Eur J Endocrinol. 2008;158:517-523. [DOI] [PubMed] [Google Scholar]
- 73. Mohlig M, Weickert MO, Ghadamgadai E, et al. Adipocyte fatty acid-binding protein is associated with markers of obesity, but is an unlikely link between obesity, insulin resistance, and hyperandrogenism in polycystic ovary syndrome women. Eur J Endocrinol. 2007;157:195-200. [DOI] [PubMed] [Google Scholar]
- 74. Walley AJ, Blakemore AI, Froguel P. Genetics of obesity and the prediction of risk for health. Hum Mol Genet. 2006;15 Spec No 2:R124-R130. [DOI] [PubMed] [Google Scholar]
- 75. Vink JM, Sadrzadeh S, Lambalk CB, Boomsma DI. Heritability of polycystic ovary syndrome in a Dutch twin-family study. J Clin Endocrinol Metab. 2006;91:2100-2104. [DOI] [PubMed] [Google Scholar]
- 76. Franks S. Polycystic ovary syndrome. N Engl J Med. 1995;333:853-861. [DOI] [PubMed] [Google Scholar]
- 77. Barber TM, Franks S. Genetics of polycystic ovary syndrome. Front Horm Res. 2013;40:28-39. [DOI] [PubMed] [Google Scholar]
- 78. Day F, Karaderi T, Jones MR, et al. Large-scale genome-wide meta-analysis of polycystic ovary syndrome suggests shared genetic architecture for different diagnosis criteria. Plos Genet. 2018;14:e1007813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Frayling TM, Timpson NJ, Weedon MN, et al. A common variant in the FTO gene is associated with body mass index and predisposes to childhood and adult obesity. Science. 2007;316:889-894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Dina C, Meyre D, Gallina S, et al. Variation in FTO contributes to childhood obesity and severe adult obesity. Nat Genet. 2007;39:724-726. [DOI] [PubMed] [Google Scholar]
- 81. Barber TM, Bennett AJ, Groves CJ, et al. Association of variants in the fat mass and obesity associated (FTO) gene with polycystic ovary syndrome. Diabetologia. 2008;51:1153-1158. [DOI] [PubMed] [Google Scholar]
- 82. Li T, Wu K, You L, et al. Common variant rs9939609 in gene FTO confers risk to polycystic ovary syndrome. PLoS ONE 2013;8:e66250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Ewens KG, Jones MR, Ankener W, et al. FTO and MC4R gene variants are associated with obesity in polycystic ovary syndrome. PLoS ONE. 2011;6:e16390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Yan Q, Hong J, Gu W, et al. Association of the common rs9939609 variant of FTO gene with polycystic ovary syndrome in Chinese women. Endocrine. 2009;36:377-382. [DOI] [PubMed] [Google Scholar]
- 85. Koivuaho E, Laru J, Ojaniemi M, et al. Age at adiposity rebound in childhood is associated with PCOS diagnosis and obesity in adulthood-longitudinal analysis of BMI data from birth to age 46 in cases of PCOS. Int J Obes (Lond). 2019;43:1370-1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Robinson S, Chan SP, Spacey S, Anyaoku V, Johnston DG, Franks S. Postprandial thermogenesis is reduced in polycystic ovary syndrome and is associated with increased insulin resistance. Clin Endocrinol (Oxf). 1992;36:537-543. [DOI] [PubMed] [Google Scholar]
- 87. Romualdi D, Versace V, Tagliaferri V, et al. The resting metabolic rate in women with polycystic ovary syndrome and its relation to the hormonal milieu, insulin metabolism, and body fat distribution: a cohort study. J Endocrinol Invest. 2019;42:1089-1097. [DOI] [PubMed] [Google Scholar]
- 88. Ek I, Arner P, Ryden M, et al. A unique defect in the regulation of visceral fat cell lipolysis in the polycystic ovary syndrome as an early link to insulin resistance. Diabetes. 2002;51:484-492. [DOI] [PubMed] [Google Scholar]
- 89. Xu X, De Pergola G, Bjorntorp P. The effects of androgens on the regulation of lipolysis in adipose precursor cells. Endocrinology. 1990;126:1229-1234. [DOI] [PubMed] [Google Scholar]
- 90. O’Reilly MW, House PJ, Tomlinson JW. Understanding androgen action in adipose tissue. J Steroid Biochem Mol Biol. 2014;143:277-284. [DOI] [PubMed] [Google Scholar]
- 91. Small L, Aplasca A. Child obesity and mental health: a complex interaction. Child Adolesc Psychiatr Clin N Am. 2016;25:269-282. [DOI] [PubMed] [Google Scholar]
- 92. Deeks AA, Gibson-Helm ME, Paul E, Teede HJ. Is having polycystic ovary syndrome a predictor of poor psychological function including anxiety and depression. Hum Reprod. 2011;26:1399-1407. [DOI] [PubMed] [Google Scholar]
- 93. Ozturk A, Kucur SK, Seven A, et al. Temperament and character differences of patients with polycystic ovary syndrome. J Gynecol Obstet Hum Reprod. 2019;48:255-259. [DOI] [PubMed] [Google Scholar]
- 94. Hopkins CS, Kimble LP, Hodges HF, Koci AF, Mills BB. A mixed-methods study of coping and depression in adolescent girls with polycystic ovary syndrome. J Am Assoc Nurse Pract. 2019;31:189-197. [DOI] [PubMed] [Google Scholar]
- 95. Karjula S, Morin-Papunen L, Auvinen J, et al. Psychological Distress Is More Prevalent in Fertile Age and Premenopausal Women With PCOS Symptoms: 15-Year Follow-Up. J Clin Endocrinol Metab. 2017;102:1861-1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Booth FW, Roberts CK, Laye MJ. Lack of exercise is a major cause of chronic diseases. Compr Physiol. 2012;2:1143-1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Pedersen BK. Physical activity and muscle-brain crosstalk. Nat Rev Endocrinol. 2019;15:383-392. [DOI] [PubMed] [Google Scholar]
- 98. Vigorito C, Giallauria F, Palomba S, et al. Beneficial effects of a three-month structured exercise training program on cardiopulmonary functional capacity in young women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2007;92:1379-1384. [DOI] [PubMed] [Google Scholar]
- 99. Palomba S, Giallauria F, Falbo A, et al. Structured exercise training programme versus hypocaloric hyperproteic diet in obese polycystic ovary syndrome patients with anovulatory infertility: a 24-week pilot study. Hum Reprod. 2008;23:642-650. [DOI] [PubMed] [Google Scholar]
- 100. Thomson RL, Buckley JD, Noakes M, Clifton PM, Norman RJ, Brinkworth GD. The effect of a hypocaloric diet with and without exercise training on body composition, cardiometabolic risk profile, and reproductive function in overweight and obese women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2008;93:3373-3380. [DOI] [PubMed] [Google Scholar]
- 101. Fernandes J, Ferreira-Santos F, Miller K, Torres S. Emotional processing in obesity: a systematic review and exploratory meta-analysis. Obes Rev. 2018;19:111-120. [DOI] [PubMed] [Google Scholar]
- 102. Fontaine KR, Barofsky I. Obesity and health-related quality of life. Obes Rev. 2001;2:173-182. [DOI] [PubMed] [Google Scholar]
- 103. Thomson RL, Buckley JD, Brinkworth GD. Exercise for the treatment and management of overweight women with polycystic ovary syndrome: a review of the literature. Obes Rev. 2011;12:e202-e210. [DOI] [PubMed] [Google Scholar]
- 104. Banting LK, Gibson-Helm M, Polman R, Teede HJ, Stepto NK. Physical activity and mental health in women with polycystic ovary syndrome. BMC Womens Health. 2014;14:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Liao LM, Nesic J, Chadwick PM, Brooke-Wavell K, Prelevic GM. Exercise and body image distress in overweight and obese women with polycystic ovary syndrome: a pilot investigation. Gynecol Endocrinol. 2008;24:555-561. [DOI] [PubMed] [Google Scholar]
- 106. Clark AM, Thornley B, Tomlinson L, Galletley C, Norman RJ. Weight loss in obese infertile women results in improvement in reproductive outcome for all forms of fertility treatment. Hum Reprod. 1998;13:1502-1505. [DOI] [PubMed] [Google Scholar]
- 107. Orio F, Jr, Giallauria F, Palomba S, et al. Cardiopulmonary impairment in young women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2006;91:2967-2971. [DOI] [PubMed] [Google Scholar]
- 108. Thomson RL, Buckley JD, Moran LJ, et al. Comparison of aerobic exercise capacity and muscle strength in overweight women with and without polycystic ovary syndrome. BJOG. 2009;116:1242-1250. [DOI] [PubMed] [Google Scholar]
- 109. Wright CE, Zborowski JV, Talbott EO, McHugh-Pemu K, Youk A. Dietary intake, physical activity, and obesity in women with polycystic ovary syndrome. Int J Obes Relat Metab Disord. 2004;28:1026-1032. [DOI] [PubMed] [Google Scholar]
- 110. Mann T, Tomiyama AJ, Westling E, Lew AM, Samuels B, Chatman J. Medicare’s search for effective obesity treatments: diets are not the answer. Am Psychol. 2007;62:220-233. [DOI] [PubMed] [Google Scholar]
- 111. Saboor Aftab SA, Halder L, Piya MK, et al. Predictors of weight loss at 1 year after laparoscopic adjustable gastric banding and the role of presurgical quality of life. Obes Surg. 2014;24:885-890. [DOI] [PubMed] [Google Scholar]
- 112. Sumithran P, Prendergast LA, Delbridge E, et al. Long-term persistence of hormonal adaptations to weight loss. N Engl J Med. 2011;365:1597-1604. [DOI] [PubMed] [Google Scholar]
- 113. Zac-Varghese S, De Silva A, Bloom SR. Translational studies on PYY as a novel target in obesity. Curr Opin Pharmacol. 2011;11:582-585. [DOI] [PubMed] [Google Scholar]
- 114. Mai K, Bobbert T, Reinecke F, et al. Intravenous lipid and heparin infusion-induced elevation in free fatty acids and triglycerides modifies circulating androgen levels in women: a randomized, controlled trial. J Clin Endocrinol Metab. 2008;93:3900-3906. [DOI] [PubMed] [Google Scholar]
- 115. Hanson P, Shuttlewood E, Halder L, et al. Application of Mindfulness in a Tier 3 Obesity Service Improves Eating Behavior and Facilitates Successful Weight Loss. J Clin Endocrinol Metab. 2019;104:793-800. [DOI] [PubMed] [Google Scholar]
- 116. Hakimi O, Cameron LC. Effect of exercise on ovulation: a systematic review. Sports Med. 2017;47:1555-1567. [DOI] [PubMed] [Google Scholar]
- 117. Barber TM, Franks S. Genetic basis of polycystic ovary syndrome. Expert Rev Endocrinol Metab. 2010;5:549-561. [DOI] [PubMed] [Google Scholar]