ABSTRACT
Background
Whether changes in fruit and vegetable intake can modify the effect of genetic susceptibility to obesity on long-term changes in BMI and body weight are uncertain.
Objective
We analyzed the interactions of changes in total and specific fruit and vegetable intake with genetic susceptibility to obesity in relation to changes in BMI and body weight.
Methods
We calculated a genetic risk score on the basis of 77 BMI-associated loci to determine the genetic susceptibility to obesity, and examined the interactions of changes in total and specific fruit and vegetable intake with the genetic risk score on changes in BMI and body weight within five 4-y intervals over 20 y of follow-up in 8943 women from the Nurses’ Health Study (NHS) and 5308 men from the Health Professionals Follow-Up Study (HPFS).
Results
In the combined cohorts, repeated 4-y BMI change per 10-risk allele increment was 0.09 kg/m2 among participants with the greatest decrease in total fruit and vegetable intake and −0.02 among those with the greatest increase in intake (P-interaction <0.001; corresponding weight change: 0.20 kg compared with −0.06 kg). The magnitude of decrease in BMI associated with increasing fruit and vegetable intake was more prominent among participants with high genetic risk than those with low risk. Reproducible interactions were observed for fruits and vegetables separately (both P-interaction <0.001). Based on similar nutritional content, the interaction effect was greatest for berries, citrus fruits, and green leafy vegetables, and the interaction pattern persisted regardless of the different fiber content or glycemic load of fruits and vegetables.
Conclusions
Genetically associated increased BMI and body weight could be mitigated by increasing fruit and vegetable intake, and the beneficial effect of improving fruit and vegetable intake on weight management was more pronounced in individuals with greater genetic susceptibility to obesity.
Keywords: fruits, vegetables, genetic susceptibility, weight gain, gene–diet interaction
Introduction
Obesity has become a major health challenge in the United States and worldwide (1), with the obesity epidemic mainly attributed to dramatic changes in diet and lifestyle patterns, in concert with genetic susceptibility (2). Emerging evidence from observational studies and randomized controlled trials suggests that particular dietary intake may modify genetically associated adiposity (3, 4). In our recent study, we showed that increased adherence to healthy dietary patterns could substantially attenuate the effect of genetic predisposition to long-term weight gain (5). It is noticeable, although very preliminary, that among individual components of dietary patterns, changes in fruit and vegetable intake exhibited the most evident interactions with genetic predisposition in relation to weight gain (5).
Fruits and vegetables are essential components of a healthy diet. However, the majority of Americans have consumed less fruits and vegetables than the recommended levels over the past few decades (6, 7), and consumption has even decreased in several subpopulations (8), in parallel with the rising obesity epidemic. Analyses in prospective cohorts such as the Nurses’ Health Study (NHS) and the Health Professionals Follow-Up Study (HPFS) have shown that increasing fruit and vegetable intake over time was associated with less weight gain (9, 10). However, data from meta-analyses of randomized controlled trials on the relation of fruit and vegetable intake with weight change are not entirely consistent (11, 12). We hypothesized that the interactions between the variations in human genome and dietary intake may partly account for these heterogeneous results.
It is currently unclear if the interaction pattern of changes in fruit and vegetable intake and genetic predisposition to obesity on weight gain exists, independent of the overall dietary quality. Furthermore, robust evidence has suggested that on the basis of varied nutritional components, fiber content, and glycemic loads, particular fruits and vegetables have different effects on weight management (10). Nevertheless, whether, and to what extent, specific fruits and vegetables modify genetically associated weight gain is unknown. Therefore, in this study, we examined the interaction between changes in total and specific fruit and vegetable intake and the genetic susceptibility to obesity on long-term changes in BMI and body weight in US men and women. We analyzed longitudinal changes in BMI and body weight as outcomes to minimize potential reverse causations, examined total fruits and vegetables and subgroups by nutritional components, fiber content, and glycemic load, and assessed interaction robustness by controlling baseline and concurrent changes of lifestyle factors and dietary quality by comparing the results from 2 independent cohorts—the NHS and HPFS.
Methods
Study design and population
The NHS is a cohort of 121,701 female registered nurses aged 30–55 y at enrollment in 1976 (13), and the HPFS is a cohort of 51,529 male health professionals aged 40–75 y at enrollment in 1986 (14). Participants were followed every 2 y using validated questionnaires to collect information on demographics, lifestyle, and medical history. Detailed information of dietary and lifestyle factors was available from 1986. Blood samples were collected from 32,826 women in the NHS between 1989 and 1990 and from 18,225 men in the HPFS between 1993 and 1995. In this study, we included 8943 women and 5308 men of European ancestry, with complete baseline information and available genotype data based on genome-wide association studies (15–19), and were free from diabetes, cancer, or cardiovascular disease at baseline in 1986 (Supplemental Figure 1). This study was approved by the Institutional Review Boards of Brigham and Women's Hospital and Harvard TH Chan School of Public Health, and all participants provided written informed consents.
Assessment of fruit and vegetable intake
Dietary information was measured with a validated 131-item semiquantitative FFQ, administered in 1986 and every 4 y thereafter (20). Participants were asked to report the frequencies of 16 fruit items and 28 vegetable items consumed during the previous year in 9 responses ranging from “never, or less than once per month” to “6 or more times per day”. A standard unit or portion size was specified for each fruit or vegetable item, and the response to each item was converted into average daily intake. The Pearson correlation coefficients comparing diet assessed by the FFQ with multiple 7-d diet records ranged from 0.24 to 0.76 for individual fruits and 0.13 to 0.53 for individual vegetables (21). Changes in intake of fruits and vegetables were calculated as the differences between the end and the beginning of each 4-y period, with positive differences representing increased intake and negative differences decreased intake.
Definitions of fruits and vegetables are presented in Supplemental Tables 1 and 2. Based on similar nutritional content, we grouped fruits into categories of berries, citrus fruits, apples and pears, and melon, and vegetables into categories of green leafy vegetables, cruciferous vegetables, legumes, and tomatoes. Because fiber content and glycemic load may differentiate the effect of fruits and vegetables on weight change, we categorized fruits and vegetables into high or low fiber according to median fiber content per serving of fruits and vegetables, and into high or low glycemic load with cutoffs of 6.5 for fruits and 0.7 for vegetables (10).
Assessment of BMI and body weight
Height and body weight were assessed by questionnaire administered at enrollment, and weight was requested on each follow-up questionnaire. Questionnaire-reported and technician-measured weights were highly correlated (r = 0.97 in the NHS and HPFS) in a validation subsample (22). BMI was calculated as weight in kilograms divided by the square of height in meters. Changes in BMI and weight were evaluated every 4 y as the differences between the end and the beginning of each 4-y period, with positive differences representing weight gain and negative differences representing weight loss.
Genotyping and calculation of genetic risk score
Seventy-seven single-nucleotide polymorphisms (SNPs) that represent all 77 genetic loci associated with BMI in people of European descent were selected (Supplemental Table 3) (23). Details of SNP genotyping and imputation have been described previously (15–19). All SNPs were genotyped or had a high imputation quality score (r2 ≥ 0.8) as assessed by MACH software (version 1.0.16).
The genetic risk score was calculated based on the 77 SNPs using a weighted method (4). Each SNP was scored as 0, 1, or 2 according to the number of BMI increasing alleles and was weighted by its relative effect size (β coefficient) on BMI derived from the previous genome-wide association study (23). The genetic risk score ranged from 0 to 154, with each point representing 1 risk allele and higher scores indicating a higher genetic susceptibility to obesity.
Assessment of covariates
Information on demographics, lifestyle, and medical history was derived from the biennial questionnaires. Total energy intake was derived from the FFQs, and diet quality was assessed by the Alternative Healthy Eating Index (AHEI), with a higher score indicating a better diet quality (24). The reproducibility and validity of physical activity have been described previously (25), and the level of physical activity was evaluated by metabolic equivalent hours per week.
Statistical analysis
In the NHS and HPFS, data were analyzed within five 4-y intervals over a follow-up period of 20 y, that is, 5 measures during the period from 1986 to 2006. The primary outcome was change in BMI, and the secondary outcome was change in body weight. Multivariable generalized linear models with repeated-measures analyses were used to examine the main associations of the genetic risk score and changes in fruit and vegetable intake with changes in BMI, 4-y changes in BMI per increment of 10 risk alleles stratified by quartiles of changes in fruit and vegetable intake, and mean values of changes in BMI according to joint categories of the genetic risk score and changes in fruit and vegetable intake. Genetic risk was classified into 3 subgroups as low, intermediate, and high according to thirds of the genetic risk score. Interactions between the genetic risk score and changes in fruit and vegetable intake were examined by including the respective interaction terms (for example, genetic risk score × changes in fruit and vegetable intake) in the models. Specific fruits and vegetables were analyzed by nutritional components, fiber content, and glycemic load. We tested whether the interaction patterns were independent of the overall dietary quality by including baseline and concurrent changes of the AHEI score in multivariable adjustments, and by replicating the analyses within subgroups of the AHEI score. Main associations and interactions on change in body weight were also examined. Missing data for main exposure and outcome variables, such as dietary factors, BMI, and body weight were carried forward only once and after that the follow-up was censored. Missing data for covariates during any follow-up period were coded as a missing indicator category for categorical variables or with carried forward values for continuous variables. In sensitivity analyses, given that old age or smoking may influence weight change, we replicated main analyses among participants younger than 65 y and in nonsmokers throughout the follow-up period. Results from the 2 cohorts were pooled by means of inverse-variance-weighted fixed-effects meta-analysis. All reported P values are nominal and 2-sided. Statistical analyses were performed using SAS software, version 9.4 (SAS Institute).
Results
Baseline characteristics and the first 4-y diet and lifestyle changes
Within the first 4-y period (1986–1990), compared to participants with a decreased intake of total fruits and vegetables (quartile 1 to 3), participants with increased intake (quartile 4) appeared to have less increases in BMI and body weight and greater increases in physical activity, total energy intake, and AHEI score (Table 1). The mean (SD) value of the genetic risk score was 69.5 (5.5) in the NHS and 69.3 (5.6) in the HPFS. The genetic risk score was positively correlated with BMI and showed normal distributions across the 2 cohorts (Supplemental Figure 2).
TABLE 1.
Characteristics of participants in the NHS and HPFS, according to change in intake of total fruits and vegetables1
| Change in intake of total fruits and vegetables | |||||
|---|---|---|---|---|---|
| Characteristic | Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | P value2 |
| NHS | |||||
| Participants, n | 2235 | 2237 | 2235 | 2236 | |
| Age, y | 54.7 ± 6.5 | 53.9 ± 6.6 | 53.7 ± 6.7 | 54.5 ± 6.6 | 0.17 |
| Initial BMI, kg/m2 | 25.7 ± 4.8 | 25.5 ± 4.9 | 25.4 ± 4.7 | 25.7 ± 5.0 | 0.53 |
| BMI change, kg/m2 | 0.6 ± 2.1 | 0.5 ± 2.0 | 0.4 ± 2.1 | 0.1 ± 2.2 | <0.001 |
| Initial weight, kg | 68.7 ± 13.5 | 68.2 ± 14.1 | 67.8 ± 13.2 | 68.5 ± 14.1 | 0.22 |
| Weight change, kg | 1.4 ± 4.9 | 1.1 ± 4.5 | 1.0 ± 4.8 | 0.3 ± 5.2 | <0.001 |
| Initial physical activity, MET-h/wk | 16.6 ± 19.4 | 13.7 ± 17.7 | 12.7 ± 19.5 | 13.3 ± 18.1 | <0.001 |
| Change in physical activity, MET-h/wk | 0.3 ± 16.7 | 1.4 ± 15.1 | 1.5 ± 15.2 | 2.6 ± 16.0 | <0.001 |
| Initial alcohol intake, g/d | 7.0 ± 11.4 | 6.9 ± 11.2 | 6.4 ± 11.2 | 5.7 ± 10.1 | <0.001 |
| Change in alcohol intake, g/d | −1.4 ± 6.5 | −1.1 ± 6.9 | −1.0 ± 6.1 | −0.9 ± 6.4 | 0.01 |
| Current smoker, % | 14.1 | 17.4 | 18.8 | 18.9 | <0.001 |
| Remained current smoker, % | 10.6 | 13.2 | 14.1 | 12.8 | 0.004 |
| Total energy intake, kcal/d | 1978 ± 522 | 1807 ± 496 | 1715 ± 493 | 1638 ± 501 | <0.001 |
| Change in total energy intake, kcal/d | −212 ± 433 | −68 ± 390 | 22 ± 376 | 199 ± 428 | <0.001 |
| AHEI score | 56.9 ± 10.4 | 51.8 ± 10.8 | 48.3 ± 10.3 | 49.5 ± 10.8 | <0.001 |
| Change in AHEI score | −2.0 ± 8.4 | 0.3 ± 8.2 | 2.6 ± 7.5 | 5.7 ± 8.4 | <0.001 |
| Initial fruit intake, servings/d | 2.6 ± 1.6 | 1.7 ± 1.0 | 1.4 ± 0.9 | 1.3 ± 1.0 | <0.001 |
| Change in fruit intake, servings/d | −1.2 ± 1.4 | −0.4 ± 0.7 | −0.1 ± 0.6 | 0.6 ± 1.2 | <0.001 |
| Initial vegetable intake, servings/d | 6.2 ± 2.6 | 3.9 ± 1.5 | 3.1 ± 1.4 | 2.9 ± 1.5 | <0.001 |
| Change in vegetable intake, servings/d | −3.9 ± 2.6 | −1.4 ± 0.8 | −0.5 ± 0.7 | 1.0 ± 1.9 | <0.001 |
| Genetic risk score | 69.5 ± 5.5 | 69.4 ± 5.5 | 69.4 ± 5.4 | 69.5 ± 5.6 | 0.74 |
| HPFS | |||||
| Participants, n | 1327 | 1327 | 1327 | 1327 | |
| Age, y | 55.6 ± 8.8 | 54.5 ± 8.5 | 54.5 ± 8.5 | 56.0 ± 8.6 | 0.14 |
| Initial BMI, kg/m2 | 25.7 ± 3.3 | 25.8 ± 3.3 | 25.8 ± 3.2 | 25.9 ± 3.4 | 0.23 |
| BMI change, kg/m2 | 0.3 ± 1.3 | 0.2 ± 1.3 | 0.3 ± 1.2 | 0.0 ± 1.4 | <0.001 |
| Initial weight, kg | 81.6 ± 11.9 | 81.7 ± 12.0 | 81.6 ± 11.7 | 82.1 ± 12.3 | 0.61 |
| Weight change, kg | 0.8 ± 3.8 | 0.8 ± 3.6 | 0.8 ± 3.7 | 0.1 ± 4.1 | <0.001 |
| Initial physical activity, MET-h/wk | 22.8 ± 27.3 | 18.0 ± 22.8 | 19.2 ± 26.6 | 19.3 ± 22.3 | 0.003 |
| Change in physical activity, MET-h/wk | 15.7 ± 35.5 | 16.6 ± 31.1 | 13.8 ± 29.1 | 20.9 ± 35.1 | 0.001 |
| Initial alcohol intake, g/d | 12.5 ± 16.0 | 12.4 ± 15.1 | 13.2 ± 16.6 | 12.4 ± 17.3 | 0.96 |
| Change in alcohol intake, g/d | −1.5 ± 9.9 | −1.2 ± 8.9 | −1.1 ± 8.0 | −1.4 ± 9.6 | 0.67 |
| Current smoker, % | 8.5 | 10.1 | 9.6 | 6.7 | 0.01 |
| Remained current smoker, % | 6.1 | 7.1 | 7.0 | 4.3 | 0.009 |
| Total energy intake, kcal/d | 2197 ± 651 | 1996 ± 579 | 1975 ± 603 | 1979 ± 608 | <0.001 |
| Change in total energy intake, kcal/d | −319 ± 513 | −124 ± 432 | −22 ± 391 | 144 ± 502 | <0.001 |
| AHEI score | 56.4 ± 11.6 | 51.2 ± 11.6 | 50.5 ± 11.1 | 52.0 ± 11.7 | <0.001 |
| Change in AHEI score | −2.5 ± 8.4 | −0.1 ± 7.9 | 1.6 ± 6.9 | 4.9 ± 8.3 | <0.001 |
| Initial fruit intake, servings/d | 2.2 ± 1.5 | 1.4 ± 1.0 | 1.3 ± 1.0 | 1.4 ± 1.1 | <0.001 |
| Change in fruit intake, servings/d | −0.7 ± 1.1 | −0.1 ± 0.6 | 0.2 ± 0.5 | 0.9 ± 1.2 | <0.001 |
| Initial vegetable intake, servings/d | 4.8 ± 2.4 | 3.1 ± 1.5 | 2.9 ± 1.6 | 3.0 ± 1.7 | <0.001 |
| Change in vegetable intake, servings/d | −1.8 ± 1.6 | −0.4 ± 0.6 | 0.2 ± 0.5 | 1.5 ± 1.8 | <0.001 |
| Genetic risk score | 69.2 ± 5.5 | 69.3 ± 5.7 | 69.3 ± 5.6 | 69.5 ± 5.5 | 0.33 |
Plus-minus values are means ± SDs for variables at baseline (1986) and the first 4-y changes (1986–1990) in the NHS and HPFS. AHEI, Alternative Healthy Eating Index; HPFS, Health Professionals Follow-Up Study; MET, metabolic equivalent; NHS, Nurses’ Health Study.
P values are for the trend across the quartiles of change in total fruit and vegetable intake.
Genetic association with BMI according to changes in fruit and vegetable intake
During 20 y of follow-up, changes in BMI every 4 y were 0.03 kg/m2 (SE: 0.01; P = 0.008) per 10-risk allele increment and −0.03 (SE: 0.004; P <0.001) per increased daily serving of fruits and vegetables in the combined cohorts (Supplemental Table 4). We found consistent interactions between the genetic risk score and changes in total fruit and vegetable intake on BMI change in the NHS (P-interaction = 0.009) and HPFS (P-interaction <0.001) (Table 2). In the combined cohorts, changes in BMI per 10-risk allele increment were 0.09 (SE: 0.02), 0.02 (SE: 0.02), 0.02 (SE: 0.02), and −0.02 (SE: 0.02) [corresponding weight change: 0.20 kg (SE: 0.06), 0.04 kg (SE: 0.06), 0.05 kg (SE: 0.06), and −0.06 kg (SE: 0.06); Supplemental Table 5] across quartiles of changes in total fruit and vegetable intake (P-interaction <0.001). Similar interaction patterns between the genetic risk score and changes in separate intake of fruits and vegetables on changes in BMI and body weight were also observed (Table 2, Supplemental Table 5).
TABLE 2.
Change in BMI per 10-risk allele increment of the genetic risk score, according to quartiles of changes in fruits and vegetables intake1
| Change in fruit and vegetable intake | |||||
|---|---|---|---|---|---|
| Analysis | Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | P-interaction |
| Total fruits and vegetables | |||||
| NHS | |||||
| Baseline participants, n | 2235 | 2237 | 2235 | 2236 | — |
| Change in BMI, kg/m2 | 0.07 ± 0.04 | 0.03 ± 0.03 | −0.01 ± 0.03 | −0.02 ± 0.03 | 0.009 |
| HPFS | |||||
| Baseline participants, n | 1327 | 1327 | 1327 | 1327 | — |
| Change in BMI, kg/m2 | 0.11 ± 0.03 | 0.01 ± 0.03 | 0.05 ± 0.03 | −0.02 ± 0.04 | <0.001 |
| Pooled results2 | |||||
| Baseline participants, n | 3562 | 3564 | 3562 | 3563 | — |
| Change in BMI, kg/m2 | 0.09 ± 0.02 | 0.02 ± 0.02 | 0.02 ± 0.02 | −0.02 ± 0.02 | <0.001 |
| Fruits | |||||
| NHS | |||||
| Baseline participants, n | 2232 | 2239 | 2236 | 2236 | — |
| Change in BMI, kg/m2 | 0.09 ± 0.03 | −0.04 ± 0.04 | 0.02 ± 0.03 | 0.01 ± 0.04 | 0.001 |
| HPFS | |||||
| Baseline participants, n | 1326 | 1328 | 1331 | 1323 | — |
| Change in BMI, kg/m2 | 0.06 ± 0.03 | 0.05 ± 0.03 | 0.01 ± 0.03 | 0.03 ± 0.03 | 0.01 |
| Pooled results2 | |||||
| Baseline participants, n | 3558 | 3567 | 3567 | 3559 | — |
| Change in BMI, kg/m2 | 0.08 ± 0.02 | 0.01 ± 0.02 | 0.01 ± 0.02 | 0.02 ± 0.03 | <0.001 |
| Vegetables | |||||
| NHS | |||||
| Baseline participants, n | 2235 | 2236 | 2237 | 2235 | — |
| Change in BMI, kg/m2 | 0.08 ± 0.04 | 0.01 ± 0.03 | −0.01 ± 0.03 | −0.01 ± 0.03 | 0.02 |
| HPFS | |||||
| Baseline participants, n | 1326 | 1329 | 1327 | 1326 | — |
| Change in BMI, kg/m2 | 0.09 ± 0.03 | 0.04 ± 0.03 | 0.01 ± 0.03 | −0.01 ± 0.03 | <0.001 |
| Pooled results2 | |||||
| Baseline participants, n | 3561 | 3565 | 3564 | 3561 | — |
| Change in BMI, kg/m2 | 0.08 ± 0.03 | 0.02 ± 0.02 | 0.01 ± 0.02 | −0.01 ± 0.02 | <0.001 |
Plus-minus values are β coefficients ± SEs for changes in BMI (kg/m2) per 10-risk allele increment of the genetic risk score. Data were derived from repeated-measurements analyses for women in the NHS (five 4-y intervals from 1986 to 2006) and men in the HPFS (five 4-y intervals from 1986 to 2006). Results were adjusted for age, genotyping source, baseline variables at the beginning of each 4-y period: BMI (quintiles), fruit and vegetable intake (quintiles), physical activity (quintiles), alcohol intake (0, 0.1–4.9, 5–9.9, 10–14.9, ≥15 g/d), smoking status (never, former, current), total energy intake (quintiles), and AHEI score (quintiles), and concurrent 4-y changes in lifestyle factors: physical activity (quintiles), alcohol intake (quintiles), smoking status (never to never, never to current, past to past, past to current, current to past, current to current), total energy intake (quintiles), and AHEI score (quintiles). AHEI, Alternative Healthy Eating Index; HPFS, Health Professionals Follow-Up Study; NHS, Nurses’ Health Study.
Results for the 2 cohorts were pooled by means of inverse-variance-weighted fixed-effects meta-analysis (all P for heterogeneity >0.05).
Changes in BMI according to joint categories of the genetic risk score and changes in fruit and vegetable intake
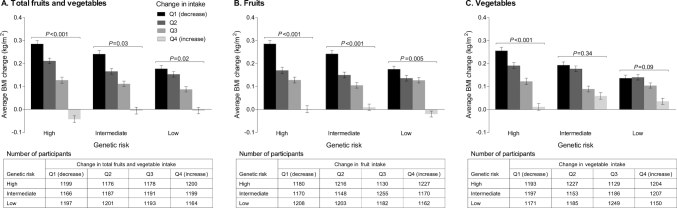
In the combined cohorts, participants who had high genetic risk and the greatest decrease in fruit and vegetable intake exhibited the greatest increases in BMI every 4 y over 20 y of follow-up (Figure 1). The magnitude of decrease in BMI associated with increasing fruit and vegetable intake was more prominent among participants with high genetic risk than those with low genetic risk. In particular, among participants who had high genetic risk but with the greatest increment in fruit and vegetable intake, the average BMI decreased or there was basically no gain. Changes in body weight according to joint categories of genetic risk and changes in fruits and vegetables are shown in Supplemental Figure 3.
FIGURE 1.
(A–C) Pooled, multivariable-adjusted BMI change every 4 y, according to joint categories of the genetic risk score and changes in fruit and vegetable intake. The analysis included 8943 participants from the NHS and 5308 participants from the HPFS. Histograms (bars) are means (SEs) for changes in BMI (kg/m2) within joint categories of the genetic risk score and quartiles of changes in fruit and vegetable intake. P values are for the trend across the quartiles of changes in fruit and vegetable intake. Data were derived from repeated-measurements analyses for women in the NHS (five 4-y intervals from 1986 to 2006) and men in the HPFS (five 4-y intervals from 1986 to 2006). Results were adjusted for the same set of variables as denoted in Table 2; results for the 2 cohorts were pooled by means of inverse-variance-weighted fixed-effects meta-analysis. HPFS, Health Professionals Follow-Up Study; NHS, Nurses’ Health Study; Q, quartile.
Modifying effects of changes in specific fruit and vegetable intake on genetic association with BMI change
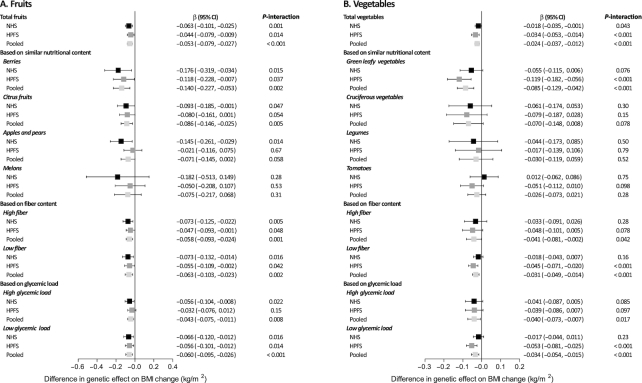
In the combined data of the 2 cohorts, each increased daily serving of fruits attenuated 0.053 (95% CI: −0.079, −0.027) in BMI (corresponding weight loss: −0.13 kg) per 10-risk allele increment (P-interaction <0.001) (Figure 2). For specific fruits based on similar nutritional content, increasing intake of berries (β: −0.14; 95% CI: −0.227, −0.053; P-interaction = 0.002) and citrus fruits (β: −0.086; 95% CI: −0.146, −0.025; P-interaction = 0.005) showed interactions with the genetic risk score on BMI change. Interaction effects were also seen for specific fruits regardless of fiber content or glycemic load (all P- interaction ≤0.008).
FIGURE 2.
(A, B) Differences in genetic association with BMI change modified by each increased daily serving of total and specific fruits and vegetables. The analysis included 8943 participants from the NHS and 5308 participants from the HPFS. Plots (bars) are β coefficients (95% CIs) for differences in genetic association (each additional 10-risk allele) with BMI change (kg/m2) modified by each increased daily serving of fruits and vegetables. Data were derived from repeated-measurements analyses for women in the NHS (five 4-y intervals from 1986 to 2006) and men in the HPFS (five 4-y intervals from 1986 to 2006). Results were adjusted for the same set of variables as denoted in Table 2; results for the 2 cohorts were pooled by means of inverse-variance-weighted fixed-effects meta-analysis. HPFS, Health Professionals Follow-Up Study; NHS, Nurses’ Health Study.
Each increased daily serving of vegetables attenuated 0.024 (95% CI: −0.037, −0.012) in BMI (corresponding weight loss: −0.05 kg) per 10-risk allele increment (P-interaction <0.001) (Figure 2). For specific vegetables, similar interaction patterns were found for green leafy vegetables (β: −0.085; 95% CI: −0.129, −0.042; P-interaction <0.001) and also for vegetables regardless of fiber content or glycemic load (all P-interaction ≤0.04). Similar interaction patterns were also observed for weight change (Supplemental Figure 4).
Interactions between the genetic risk score and changes in fruit and vegetable intake on BMI change according to AHEI score
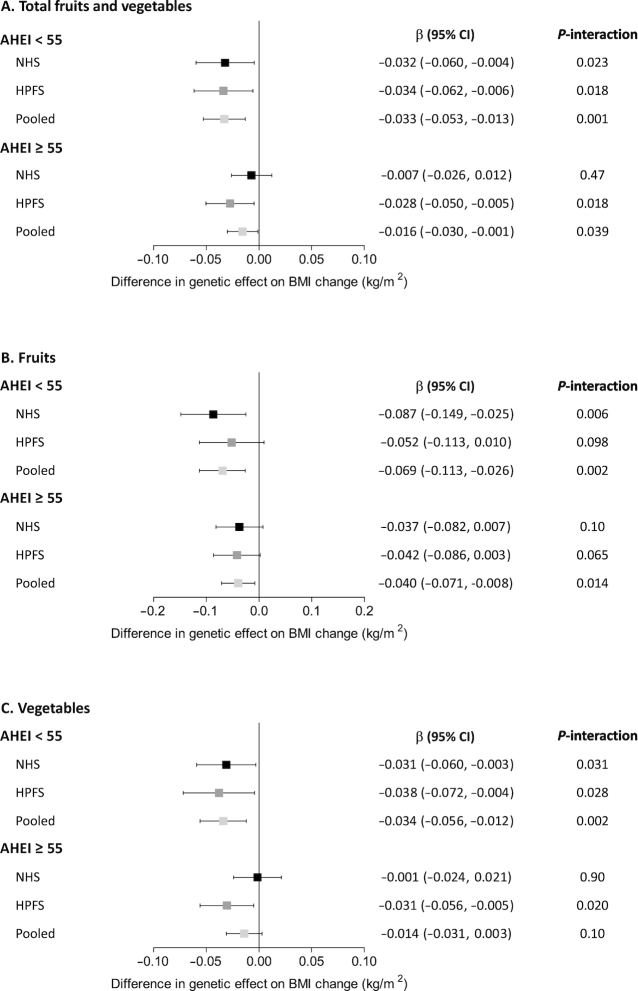
We then stratified participants by the median value of AHEI score, which represents an overall dietary quality. In general, the interaction pattern between the genetic risk score and changes in fruit and vegetable intake on BMI change was consistent within subgroups of low or high AHEI score, but the interaction effect was stronger among participants with an AHEI <55 than those with an AHEI ≥55 (Figure 3).
FIGURE 3.
(A–C) Interactions between the genetic risk score and changes in fruit and vegetable intake on BMI change according to AHEI score. The analysis included 8854 participants who had an AHEI <55 (5668 from the NHS and 3186 from the HPFS) and 5397 participants who had an AHEI ≥55 (3275 from the NHS and 2122 from the HPFS). Plots (bars) are β coefficients (95% CIs) for differences in genetic association (each additional 10-risk allele) with BMI change (kg/m2) modified by each increased daily serving of fruits and vegetables. Data were derived from repeated-measurements analyses for women in the NHS (five 4-y intervals from 1986 to 2006) and men in the HPFS (five 4-y intervals from 1986 to 2006). Results were adjusted for age, genotyping source, baseline variables at the beginning of each 4-y period: BMI (quintiles), fruit and vegetable intake (quintiles), physical activity (quintiles), alcohol intake (0, 0.1–4.9, 5–9.9, 10–14.9, ≥15 g/d), smoking status (never, former, current), and total energy intake (quintiles), and concurrent 4-y changes in lifestyle factors: physical activity (quintiles), alcohol intake (quintiles), smoking status (never to never, never to current, past to past, past to current, current to past, current to current), and total energy intake (quintiles); results for the 2 cohorts were pooled by means of inverse-variance-weighted fixed-effects meta-analysis. AHEI, Alternative Healthy Eating Index; HPFS, Health Professionals Follow-Up Study; NHS, Nurses’ Health Study.
Sensitivity analyses
In smaller samples of participants younger than 65 y and nonsmokers throughout the follow-up period, we observed similar but weaker interactions of the genetic risk score with changes in fruit and vegetable intake on BMI change (Supplemental Table 6).
Discussion
In 2 independent, prospective cohorts of US men and women, we found consistent interactions of changes in total and separate intake of fruits and vegetables with genetic susceptibility to obesity on longitudinal changes in BMI and body weight. Increasing intake of fruits and vegetables remarkably attenuated the genetically associated increases in BMI and body weight, and the beneficial effect of improving fruit and vegetable intake on changes in BMI and body weight was more prominent in individuals with high genetic risk. The interaction effect was independent of an overall dietary quality, and was stronger for fruits compared with vegetables; for specific fruits and vegetables, the interaction effect was greatest for berries, citrus fruits, and green leafy vegetables, and the interaction pattern persisted regardless of different fiber content or glycemic load of fruits and vegetables.
Fruits and vegetables are the primary dietary components highlighted by the American Heart Association 2020 Strategic Impact Goals and the US Dietary Guidelines (26–28). However, intake of fruits and vegetables have long been inadequate among most Americans, and even decreased in older populations (6–8). Although the majority of epidemiological investigations suggest an inverse association between fruit and vegetable intake and weight gain, unanimous agreement has not yet been reached (9–12). Data from the NHS and HPFS indicate that increased fruit and vegetable intake is associated with less weight gain over time (9, 10). Additionally, a meta-analysis of 8 randomized controlled trials including 1026 participants with a mean intervention period of 14.7 wk reported that increased fruit and vegetable intake, in the absence of specific advice to decrease the intake of other foods, appeared to have a role in weight maintenance or loss (11). However, another meta-analysis of 7 randomized controlled trials including 1149 participants with ≥8 wk intervention period suggests that increasing fruit and vegetable intake has no discernible effect on weight loss (12). In our study, increasing fruit and vegetable intake was associated with less weight gain, and the magnitude and significance of such associations varied across different levels of genetic risk, which lend support to the hypothesis that the heterogeneous associations between change in fruit and vegetable intake and weight change from observational studies may be partly explained by the modifying effect of genetic predisposition.
Our findings also indicate that increased intake of total and separate fruits and vegetables could attenuate the genetic associations with long-term weight gain. For specific fruits and vegetables, on the basis of similar nutritional content, the beneficial effect was strongest for berries, citrus fruits, and green leafy vegetables, and for specific fruits and vegetables on the basis of fiber content and glycemic load, such a beneficial effect was quite consistent. In addition, when stratified by an overall dietary quality, the interaction patterns were stronger among participants with an AHEI <55 than those with an AHEI ≥55, emphasizing the importance of improving fruit and vegetable intake to prevent genetically associated weight gain among individuals with a relatively low dietary quality. In this study, the magnitude of genetic associations with repeated 4-y changes in BMI and body weight were generally modest. However, given that the changes in BMI and body weight are essentially cumulative throughout life, the long-term effect size of genetic predisposition on adiposity would be substantial. In reality it is difficult to achieve substantial weight loss over a long period, even in the context of weight-loss trials (29, 30). Therefore, compared with weight gain, modest weight loss or simply maintaining weight from adulthood onward, may have an important public health implication. Taken together, these results provide supportive evidence that promoting fruit and vegetable intake could counteract genetically predisposed weight gain.
The biologic effects of fruits and vegetables on energy balance and adiposity function could partly account for the interactions of genetic predisposition with changes in fruit and vegetable intake on weight gain, and the different magnitudes of the interactions observed for specific fruits and vegetables might be due to their heterogeneous compositions and different consumption patterns (31). Furthermore, a number of BMI-associated genes are highly expressed in the brain and hypothalamus and play essential roles through the central nervous system in appetite control, food preference, and energy homeostasis (23), and these genes could be particularly involved in affecting weight changes related to the observed interactions. Nevertheless, functional experiments are needed to clarify the underlying mechanisms that account for gene–diet interactions.
Our findings were cross validated from 2 independent, prospective cohorts of US men and women, and the reliability of our findings was improved by several sensitivity analyses. Importantly, the repeated and well-validated dietary measures allowed us to assess changes in dietary intake rather than baseline intake, and the analyses of repeated diet changes with weight gain are more relevant to the physiologic time courses of weight change and may capture more signals of the biological relation between dietary intake and weight gain (9). Moreover, we evaluated diet changes and weight changes in discrete periods, and this type of method has been proved to reveal robust and biologically plausible relations between diet and long-term weight gain, compared with other methods evaluating diet and weight relation in baseline diet or lagged changes (32). Several limitations should also be considered when interpreting the findings. First, although we have controlled several lifestyle and dietary factors, some unmeasured or unknown confounders may exist. Second, it is possible that individuals who gained weight might increase intake of healthier foods to lose weight, therefore the results could be underestimated due to such reverse causation. Third, the data on weight and height are self-reported in the NHS and HPFS. However, a previous study has validated that these self-reported measures were highly reliable in the 2 cohorts (22). Fourth, our analyses were restricted to US health professionals of European descent, and replications in other populations are needed to confirm the generalization of our findings.
In conclusion, this prospective study in 2 large cohorts provides reproducible evidence that improving the intake of fruits and vegetables could substantially attenuate the genetic association with long-term weight gain, highlighting the importance of promoting fruit and vegetable intake to prevent weight gain, particularly for individuals genetically predisposed to obesity.
Supplementary Material
Acknowledgments
The authors’ responsibilities were as follows—TW and LQ: conceived and designed the study; EBR, JEM, FBH, WCW, and LQ: acquired the data; TW: performed analyses and drafted the manuscript; YZ, TH, and WM: provided statistical assistance; TW and LQ: had full access to all of the data in the study and took responsibility for the integrity of the data and accuracy of the data analysis; and all authors: contributed to the interpretation of the results and critical revision of the manuscript for important intellectual content, and read and approved the final manuscript. The authors declared no competing interests.
Notes
The study was supported by grants from the National Heart, Lung, and Blood Institute (HL071981, HL034594, HL35464), the National Institute of Diabetes and Digestive and Kidney Diseases (DK115679, DK091718, DK100383, DK078616), and the Fogarty International Center (TW010790). TW was supported by the Shanghai Pujiang Program (18PJ1409600), the Excellent Young Medical Talents Training Program from the Shanghai Municipal Commission of Health and Family Planning (2018YQ05), and the Shanghai Municipal Education Commission–Gaofeng Clinical Medicine Grant Support (20171901) and Natural Science Research Program (19XJ11004) from Shanghai Jiao Tong University School of Medicine. LQ was supported by NIGMS P20GM109036. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Supplemental Figures 1–4 and Supplemental Tables 1–6 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/ajcn/.
Abbreviations used: AHEI, Alternative Healthy Eating Index; HPFS, Health Professionals Follow-Up Study; NHS, Nurses’ Health Study; SNP, single-nucleotide polymorphism.
References
- 1. Ng M, Fleming T, Robinson M, Thomson B, Graetz N, Margono C, Mullany EC, Biryukov S, Abbafati C, Abera SF et al.. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384:766–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Qi L, Cho YA. Gene-environment interaction and obesity. Nutr Rev. 2008;66:684–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Qi Q, Chu AY, Kang JH, Jensen MK, Curhan GC, Pasquale LR, Ridker PM, Hunter DJ, Willett WC, Rimm EB et al.. Sugar-sweetened beverages and genetic risk of obesity. N Engl J Med. 2012;367:1387–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wang T, Xu M, Bi Y, Ning G. Interplay between diet and genetic susceptibility in obesity and related traits. Front Med. 2018;12:601–7. [DOI] [PubMed] [Google Scholar]
- 5. Wang T, Heianza Y, Sun D, Huang T, Ma W, Rimm EB, Manson JE, Hu FB, Willett WC, Qi L. Improving adherence to healthy dietary patterns, genetic risk, and long term weight gain: gene-diet interaction analysis in two prospective cohort studies. BMJ. 2018;360:j5644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Moore LV, Thompson FE. Adults meeting fruit and vegetable intake recommendations-United States, 2013. MMWR Morb Mortal Wkly Rep. 2015;64:709–13. [PMC free article] [PubMed] [Google Scholar]
- 7. Rehm CD, Peñalvo JL, Afshin A, Mozaffarian D. Dietary intake among US adults, 1999–2012. JAMA. 2016;315:2542–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ford ES, Li C, Zhao G, Pearson WS, Tsai J, Greenlund KJ. Trends in low-risk lifestyle factors among adults in the United States: findings from the Behavioral Risk Factor Surveillance System 1996–2007. Prev Med. 2010;51:403–7. [DOI] [PubMed] [Google Scholar]
- 9. Mozaffarian D, Hao T, Rimm EB, Willett WC, Hu FB. Changes in diet and lifestyle and long-term weight gain in women and men. N Engl J Med. 2011;364:2392–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bertoia ML, Mukamal KJ, Cahill LE, Hou T, Ludwig DS, Mozaffarian D, Willett WC, Hu FB, Rimm EB. Changes in intake of fruits and vegetables and weight change in United States men and women followed for up to 24 years: analysis from three prospective cohort studies. PLoS Med. 2015;12:e1001878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mytton OT, Nnoaham K, Eyles H, Scarborough P, Ni Mhurchu C. Systematic review and meta-analysis of the effect of increased vegetable and fruit consumption on body weight and energy intake. BMC Public Health. 2014;14:886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kaiser KA, Brown AW, Bohan Brown MM, Shikany JM, Mattes RD, Allison DB. Increased fruit and vegetable intake has no discernible effect on weight loss: a systematic review and meta-analysis. Am J Clin Nutr. 2014;100:567–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Willett WC, Stampfer MJ, Colditz GA, Rosner BA, Hennekens CH, Speizer FE. Dietary fat and the risk of breast cancer. N Engl J Med. 1987;316:22–28. [DOI] [PubMed] [Google Scholar]
- 14. Rimm EB, Giovannucci EL, Willett WC, Colditz GA, Ascherio A, Rosner B, Stampfer MJ. Prospective study of alcohol consumption and risk of coronary disease in men. Lancet. 1991;338:464–8. [DOI] [PubMed] [Google Scholar]
- 15. Hunter DJ, Kraft P, Jacobs KB, Cox DG, Yeager M, Hankinson SE, Wacholder S, Wang Z, Welch R, Hutchinson A et al.. A genome-wide association study identifies alleles in FGFR2 associated with risk of sporadic postmenopausal breast cancer. Nat Genet. 2007;39:870–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Qi L, Cornelis MC, Kraft P, Stanya KJ, Linda Kao WH, Pankow JS, Dupuis J, Florez JC, Fox CS, Paré G et al.. Genetic variants at 2q24 are associated with susceptibility to type 2 diabetes. Hum Mol Genet. 2010;19:2706–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cornelis MC, Monda KL, Yu K, Paynter N, Azzato EM, Bennett SN, Berndt SI, Boerwinkle E, Chanock S, Chatterjee N et al.. Genome-wide meta-analysis identifies regions on 7p21 (AHR) and 15q24 (CYP1A2) as determinants of habitual caffeine consumption. PLoS Genet. 2011;7:e1002033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wiggs JL, Kang JH, Yaspan BL, Mirel DB, Laurie C, Crenshaw A, Brodeur W, Gogarten S, Olson LM, Abdrabou W et al.. Common variants near CAV1 and CAV2 are associated with primary open-angle glaucoma in Caucasians from the USA. Hum Mol Genet. 2011;20:4707–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jensen MK, Pers TH, Dworzynski P, Girman CJ, Brunak S, Rimm EB. Protein interaction-based genome-wide analysis of incident coronary heart disease. Circ Cardiovasc Genet. 2011;4:549–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rimm EB, Giovannucci EL, Stampfer MJ, Colditz GA, Litin LB, Willett WC. Reproducibility and validity of an expanded self-administered semiquantitative food frequency questionnaire among male health professionals. Am J Epidemiol. 1992;135:1114–26. [DOI] [PubMed] [Google Scholar]
- 21. Feskanich D, Rimm EB, Giovannucci EL, Colditz GA, Stampfer MJ, Litin LB, Willett WC. Reproducibility and validity of food intake measurements from a semiquantitative food frequency questionnaire. J Am Diet Assoc. 1993;93:790–6. [DOI] [PubMed] [Google Scholar]
- 22. Rimm EB, Stampfer MJ, Colditz GA, Chute CG, Litin LB, Willett WC. Validity of self-reported waist and hip circumferences in men and women. Epidemiology. 1990;1:466–73. [DOI] [PubMed] [Google Scholar]
- 23. Locke AE, Kahali B, Berndt SI, Justice AE, Pers TH, Day FR, Powell C, Vedantam S, Buchkovich ML, Yang J et al.. Genetic studies of body mass index yield new insights for obesity biology. Nature. 2015;518:197–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. McCullough ML, Feskanich D, Stampfer MJ, Giovannucci EL, Rimm EB, Hu FB, Spiegelman D, Hunter DJ, Colditz GA, Willett WC. Diet quality and major chronic disease risk in men and women: moving toward improved dietary guidance. Am J Clin Nutr. 2002;76:1261–71. [DOI] [PubMed] [Google Scholar]
- 25. Wolf AM, Hunter DJ, Colditz GA, Manson JE, Stampfer MJ, Corsano KA, Rosner B, Kriska A, Willett WC. Reproducibility and validity of a self-administered physical activity questionnaire. Int J Epidemiol. 1994;23:991–9. [DOI] [PubMed] [Google Scholar]
- 26. Mozaffarian D. Dietary and policy priorities for cardiovascular disease, diabetes, and obesity: a comprehensive review. Circulation. 2016;133:187–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lloyd-Jones DM, Hong Y, Labarthe D, Mozaffarian D, Appel LJ, Van Horn L, Greenlund K, Daniels S, Nichol G, Tomaselli GF et al.. Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association's strategic Impact Goal through 2020 and beyond. Circulation. 2010;121:586–613. [DOI] [PubMed] [Google Scholar]
- 28. US Department of Health and Human Services and US Department of Agriculture. 2015–2020 Dietary Guidelines for Americans. 8th ed Available from: http://health.gov/dietaryguidelines/2015/guidelines/. Accessed on 01 March, 2019. [Google Scholar]
- 29. Shai I, Schwarzfuchs D, Henkin Y, Shahar DR, Witkow S, Greenberg I, Golan R, Fraser D, Bolotin A, Vardi H et al.. Weight loss with a low-carbohydrate, Mediterranean, or low-fat diet. N Engl J Med. 2008;359:229–41. [DOI] [PubMed] [Google Scholar]
- 30. Sacks FM, Bray GA, Carey VJ, Smith SR, Ryan DH, Anton SD, McManus K, Champagne CM, Bishop LM, Laranjo N et al.. Comparison of weight-loss diets with different compositions of fat, protein, and carbohydrates. N Engl J Med. 2009;360:859–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Slavin JL, Lloyd B.. Health benefits of fruits and vegetables. Adv Nutr. 2012;3:506–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Smith JD, Hou T, Hu FB, Rimm EB, Spiegelman D, Willett WC, Mozaffarian D. A comparison of different methods for evaluating diet, physical activity, and long-term weight gain in 3 prospective cohort studies. J Nutr. 2015;145:2527–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.