Abstract
Purpose
To test the hypothesis that oxidative stress in the outer retina (OR = distance from external limiting membrane to the retinal pigment epithelium–choroid boundary) can be detected by using antioxidants (AOs) to correct an impaired light-evoked response as measured by optical coherence tomography (OCT).
Methods
C57BL/6J mice were maintained in the dark for ∼20 hours and studied by OCT before and after 1 hour of light exposure. OR thickness in dark or light was measured, and the light-dark difference (i.e., the photoresponse) was calculated. Subgroups of mice were given either saline or d-cis-diltiazem (an inducer of transient and nondamaging OR oxidative stress) ± methylene blue (24 hours before examination) and α-lipoic acid (1 hour before examination); one group was kept only in the dark and given only AOs.
Results
In uninjected or saline-injected control mice, the OR showed a similar and reproducible light-induced expansion; dark-adapted mice given AOs did not increase dark-adapted OR thickness. The d-cis-diltiazem–treated mice had no photoresponse (P > 0.05). The d-cis-diltiazem–treated mice given AOs corrected (P < 0.05) the suppressed OR photoresponse, indicating the presence of oxidative stress.
Conclusions
QUEnch-assiSTed (QUEST) OCT reproduced results from previous gold standard assays, showing that oxidative stress impairs the OR photoresponse and that d-cis-diltiazem produces OR oxidative stress. We envision future applications of QUEST OCT in a range of oxidative stress–based retinopathies.
Keywords: optical coherence tomography, dark, light, free radicals, reactive oxygen species
Oxidative stress in the outer retina (OR = distance from external limiting membrane to the retinal pigment epithelium–choroid boundary) is a condition of unregulated and continuous production of free radicals (e.g., superoxide and hydroxyl radicals). Experimental studies implicate OR oxidative stress/damage, as measured ex vivo, as pathogenic in many incurable blinding diseases, such as age-related macular degeneration, diabetic retinopathy, and retinitis pigmentosa, as well as in visual performance declines during “healthy” aging.1–7 However, it is not possible to measure OR oxidative stress in patients and so therapies that appear promising in preclinical studies have not been adequately tested clinically. At present, physicians must make educated guesses regarding antioxidant (AO) dose, timing, drug combinations, and whether the selected treatment strategy indeed reduces oxidative stress in the target tissue. Also, clinical studies often rely on a one-antioxidant-solves-all approach that may be too simple or started too late to be effective in changing disease outcomes. Not surprisingly, many clinical trials find unclear medical benefits from “guesstimated” AO dosing, leaving the role of oxidative stress in aging and disease largely uncertain. Poor outcomes in AO clinical trials are insufficient evidence for ruling out a pathogenic role of oxidative stress in patients.
We have been addressing this problem with the development of various QUEnch-assiSTed (QUEST) MRI protocols that involve AO correction (i.e., a “quench”) of retinal function or excessive free radical production.8 Agreement between QUEST magnetic resonance imaging (MRI) approaches and gold standard methods has underscored their usefulness as noninvasive indices of OR oxidative stress.1,8–12 Recently, we have found that d-cis-diltiazem, an Food and Drug Administration (FDA)-approved drug, induces a temporary and nondamaging production of excessive reactive oxygen species in the OR of C57BL/6J mice in vivo as measured by QUEST MRI and by a gold standard ex vivo assay.9,13,14
We, and others, have found in various mice strains that when light stimulation causes a significant increase in hydration of the OR (measured by proton density MRI), the distance (i.e., expansion) between external limiting membrane (ELM) and retinal pigment epithelium (RPE) increases as measured in vivo by optical coherence tomography (OCT), and water mobility in this region increases as measured by diffusion MRI in agreement with previous microelectrode studies of an impermanent probe.1,15–26 Furthermore, in 2- to 3-month-old diabetic mice (i.e., before vascular histopathology), light-stimulated OR expansion as measured by MRI is impaired but could be restored by AO, supporting the conclusions of OR oxidative stress early in the course of diabetes.1,10–12 However, it has remained unclear if the light-stimulated expansion of the OR measured by OCT is similarly affected by oxidative stress.
In this study, we applied a QUEST strategy with ultrahigh-resolution OCT imaging to test the hypothesis that correcting oxidative stress–suppressed light-evoked expansion of OR with AOs is a novel way to encode oxidative stress information into the OCT image.
Materials and Methods
All animals were treated in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals, the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research, and Institutional Animal and Care Use Committee authorization. Animals were housed and maintained in 14 hour:10 hour light-dark cycle laboratory lighting, unless otherwise noted.
Groups
Two- to 3-month-old C57BL/6J (Jackson Labs, Bar Harbor, ME, USA) mice were studied as outlined in Figure 1. After overnight dark adaptation, animals were injected under dim-red light with either d-cis-diltiazem dissolved in saline (n = 9, bolus subcutaneous, 30 mg/kg; D-2521, >99% purity, Sigma-Aldrich Corp., St. Louis, MO, USA) or saline three times (i.e., instead of d-cis-diltiazem and the two AO injections, n = 4) as a control group; uninjected mice were also used as a control group (n = 6). On each eye, OCT imaging was first performed in darkness ∼1 hour after the d-cis-diltiazem or saline injection.9 After imaging in dark, mice were exposed to room light (∼500 lux) for ∼1 hour, and second OCT images were captured for each eye under room light. The d-cis-diltiazem–treated mice were given AOs (n = 5): 1 mg/kg methylene blue (MB, intraperitoneal (i.p.), dissolved in saline) before overnight dark adaptation (∼20 hours before d-cis-diltiazem, Fig. 1) and 50 mg/kg α-lipoic acid (ALA, i.p., dissolved in saline and pH adjusted to ∼7.4) after the first OCT imaging (i.e., ∼1 hour before the second OCT examination in light, Fig. 1). To test effects of AOs on dark-adapted OR thickness, a subgroup of animal (n = 4) received only MB and ALA, but no d-cis-diltiazem, and were kept in dark throughout the imaging examination.
Figure 1.
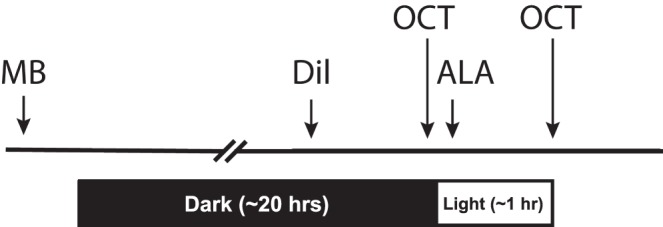
Timeline of drug administration and dark versus light exposure.
Ultrahigh-Resolution OCT
Light exposure elicits an increase in OR thickness as measured by OCT in mice and humans; the procedure has been previously published.22–24,26 Briefly, after anesthetizing mice with ketamine (100 mg/kg) and xylazine (6 mg/kg), retina OCT images were captured with Envisu UHR2200 (Bioptigen, Durham, NC, USA), with OCT beam bandwidth of 160 nm, and theoretic axial resolution of 1.6 μm in tissue. The mouse eye was positioned with the optic nerve head (ONH) in the center of the OCT scan. Full-field (50° fixed field view, corresponding to 1.4 mm × 1.4 mm for a typical mouse eye) volume scans (at 1000 A-scan × 100 B-scan × 5) and a vertical B-scan (averaged 40 times) were collected. Mice used in this study were of similar age, so between-mice eye size differences were small. Vertical B-scan images were studied from our previous results showing that d-cis-diltiazem produces oxidative stress in superior and inferior retina,9 and OR thickness was measured at location ∼450 μm superior (“12-o'clock” position) and ∼450 μm inferior (“6-o'clock” position) to the center of the ONH, by using vendor-provided Reader program (Bioptigen) and an in-house MATLAB program. OR length was measured from external limiting membrane (ELM) to the RPE-choroid boundary.
Statistical Analysis
Data are presented as mean ± standard error of the mean (SEM). We used linear mixed models to compare OR thickness as measured by OCT among five groups: noninjected control, thrice-saline-injected control, AO, d-cis-diltiazem (DIL), and DIL+AO. The OCT measurements were taken over two regions (inferior/superior) for both eyes and at two periods (either dark/light or dark/dark) for each mouse. The dark AO group had no light exposure for both periods, while the animals in the remaining groups were exposed to light in the second period. Our modeling initially only considered the period effect, and not the light effect. The initial model included the fixed effects of period, group, eye, region, and all interactions, as well as a random intercept for mice. We did evaluate random coefficients for period, eye, and region, but both the Akaike information criterion and the Bayesian information criterion indicated that these random coefficients did not improve the model fit. As such, our final model only included the random intercept. The model fixed effects were evaluated by using the likelihood ratio test. We evaluated higher-order interactions, removing any interactions that were not significant. The final model included the two-way interactions period*group, eye*group, and region*group. The period*group interaction was the effect of interest, since this interaction described the differential response between the two periods between groups. No interactions that included region and eye with period and group were significant, indicating that region and eye did not affect the period difference between groups. Still, both region and eye had effects on mean OCT. We compared the difference in the two periods among groups, using all pairwise comparisons of groups. We also compared all groups pairwise both in the dark and in the light (for groups measured in the light). We used Tukey's honestly significant difference (HSD) to adjust for multiple comparisons. All analyses were initially conducted in SAS 9.4 (SAS Institute Inc., Cary, NC, USA), using Proc Mixed to fit the mixed model. The pairwise comparisons were conducted by using an “Estimate” statement in Proc Mixed and the resulting standard error and degrees of freedom as calculated by using the Kenward-Roger method. Since region and eye did not affect the period difference between groups, we averaged the region and eye effects (i.e., inferior/superior were averaged, and right/left eyes were averaged) in the estimate statements. The estimates, standard errors, and degrees of freedom were used to determine adjusted P values and confidence intervals (CIs), using the ptukey() and qtukey() functions in R version 3.5.1.
We also evaluated reliability/repeatability of the multiple measurements on individual mice by using the intraclass correlation (ICC). We used the final model from above to estimate the ICC as 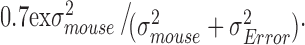 We used Proc NLMIXED to fit the model and calculate the standard error and 95% CI, using the delta method.
We used Proc NLMIXED to fit the model and calculate the standard error and 95% CI, using the delta method.
Results
Overall Repeatability Within Subjects
The ICC estimated from our data was 0.347 (95% CI: 0.186, 0.509), which differed from 0 and reflects a significant correlation within subjects. As such, OR thickness showed repeatability within subjects.
OR Thickness and Photoresponse in Controls
Shown in Figure 2 are examples of OCT images obtained under darkness and after 1-hour exposure to light for a mouse eye at the inferior retinal region. The OR photoresponse in control mice can be appreciated upon visual inspection of the OCT data. Quantitative analysis showed that light-exposed uninjected or thrice-saline-injected controls had OR thicknesses of 57.7 ± 0.7 μm (mean ± SEM) and 59.7 ± 0.8 μm, respectively (Fig. 3); these were not different from each other (P > 0.05). In the dark, these controls had OR thicknesses of 54.2 ± 0.7 μm and 54.5 ± 0.8 μm, respectively; no statistically significant difference was found between these values (P > 0.05). OR thickness in dark-only mice given only AOs was 54.8 ± 0.8 μm and was not different (P > 0.05) from that in dark-adapted control mice.
Figure 2.
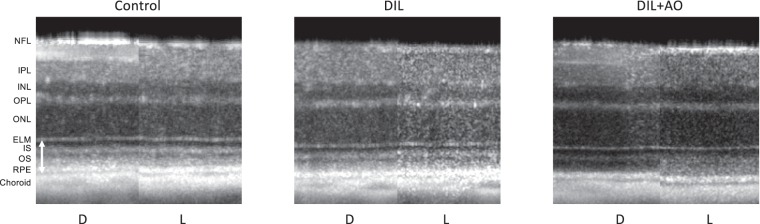
Representative OCT images of inferior retina in dark (D) and light (L) for an uninjected control mouse (control), a d-cis-diltiazem (DIL)–injected mouse, and a d-cis-diltiazem + MB/ALA (DIL+AO) mouse; images from thrice-saline-injected controls and dark only + AO mice appear similar to controls and DIL images, respectively (data not shown); similar results are seen in superior retina (data not shown). Layer assignments: nerve fiber layer (NFL), inner plexiform layer (IPL), inner nuclear layer (INL), outer plexiform layer (OPL), ELM; rod inner segment layer (IS); rod outer segment layer (OS); RPE, and anterior choroid boundary (choroid).37 Vertical double arrowhead bar between the ELM and retina-choroid border indicates the OR region measured.
Figure 3.
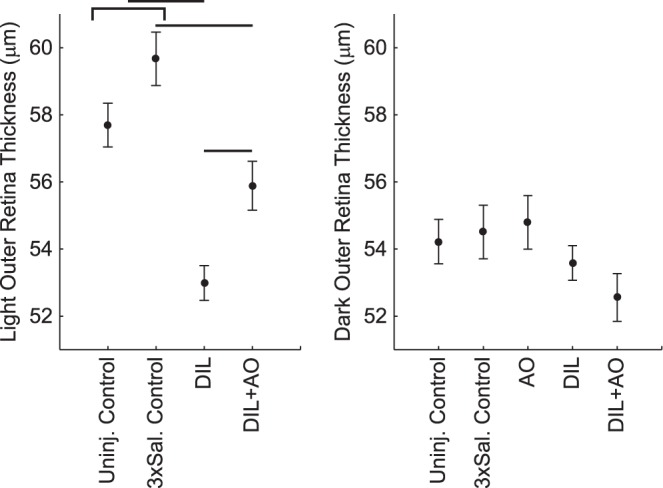
OR thicknesses in light (left) and dark (right) for the uninjected controls (Uninj. Control), thrice-saline-injected control (3xSal. control), d-cis-diltiazem (DIL), and d-cis-diltiazem + MB/ALA (DIL+AO) groups. There was no significant difference between control groups exposed to light, and both control groups (indicated by the bracket) were different from the DIL group (horizontal line). MB/ALA increased light OR thickness to uninjected control values but not to thrice-saline-injected control values. OR thickness in dark-adapted mice was unremarkable. Data presented as mean ± SEM.
In uninjected or thrice-saline-injected controls, 1-hour light exposure produced an elongation in OR thickness of 3.5 ± 0.5 μm or 5.2 ± 0.6 μm, respectively (Fig. 3). Both of these OR photoresponses were different (P < 0.0001) from 0 (but not from each other [P = 0.2]) (Fig. 3). Mice that were kept in the dark and given AOs did not change (P = 0.64) OR thickness (−0.3 ± 0.6 μm) from 0 (Fig. 4).
Figure 4.
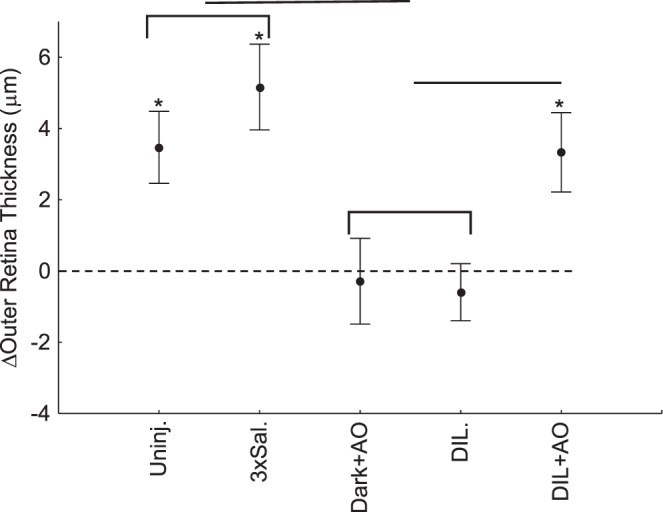
Post-pre change (Δ) in OR thickness for dark/light studies (i.e., the OR photoresponse) or dark/dark study for the uninjected controls (Uninj.), thrice-saline-injected control (3xSal.), dark-only controls given only MB/ALA (dark+AO), d-cis-diltiazem (DIL), and d-cis-diltiazem + MB/ALA (DIL+AO) groups. Both dark/light-exposed groups had a photoresponse different from 0 (asterisk), and both control groups' exposure to light (indicated by the bracket) was different from that of the dark+AO and DIL groups (horizontal line). MB/ALA restored the OR photoresponse to control values. Data presented as mean ± 95% confidence intervals.
OR Thickness and Photoresponse After d-cis-Diltiazem
In a d-cis-diltiazem–treated mouse, light OR thickness appeared similar to that in the dark (Fig. 2), suggesting an impaired OR photoresponse. Light-exposed d-cis-diltiazem–injected mice had an OR thickness of 53.0 ± 0.5 μm, which was different (P < 0.0001) from both uninjected and thrice-saline–injected control values (Figs. 2, 3). In dark-adapted d-cis-diltiazem–treated mice, OR thickness was 53.6 ± 0.5 μm, which was not different from the other dark OR thicknesses.
d-cis-Diltiazem prevented the light-induced extension of OR thickness (Fig. 4). Averaged light-induced OR thickness change was −0.6 ± 0.4 μm, which was not different (P = 0.14) from 0 and was smaller (P < 0.0001) than those observed in control animals (Fig. 4).
OR Thickness and Photoresponse After d-cis-Diltiazem and AOs
Treatment with AOs visibly restored the photoresponse in an inferior image of a d-cis-diltiazem + AO–injected mouse (Fig. 2). In light-exposed mice given both d-cis-diltiazem and AOs, the OR thickness was 55.9 ± 0.7 μm, which is not different (P = 0.4) from that in uninjected controls but was different (P = 0.01) from thrice-saline-injected control values (Figs. 2, 3). In dark-adapted d-cis-diltiazem and AO–treated mice, OR thickness was 52.6 ± 0.7 μm, which was not different from the other dark OR thicknesses.
d-cis-Diltiazem mice treated with AOs showed a light-evoked OR expansion of 3.3 ± 0.6 μm, which is different (P < 0.0001) from 0 but not from that in uninjected (P = 1.0) and thrice-saline-injected control mice (P = 0.2; Fig. 4).
Discussion
In this study, we demonstrated for the first time that OR oxidative stress can be detected with OCT. MB is an alternative electron transporter that effectively suppresses generation of superoxide from a variety of sources, and ALA is a potent free radical neutralizer.27,28 MB and ALA are both FDA approved and have been shown to be effective when given either individually or in combination in the d-cis-diltiazem model or in disease models with OR oxidative stress.1,2,9,11,29,30 Having established feasibility of QUEST OCT with MB and ALA, we anticipate that future studies will investigate other AO approaches and how their dosing and timing can be optimized in animal models and in humans.
The present results further support earlier observations that a robust aspect of OR physiology (i.e., its light-evoked expansion) is measurable by imaging the OR microstructure photoresponse with OCT.1,15,22,23 Unlike OCT images of fully (>5 hours) light-adapted mouse retina that exhibit a prominent hyporeflective band between photoreceptor tip and RPE bands, at only 1 hour of light exposure the hyporeflective band is often visually undetectable on OCT images (Fig. 2); the hyporeflective band is very likely linked to fluid accumulation in the subretinal space. Although local changes in refractive index could, in theory, alter the optical path in the retina and induce apparent changes in OR thickness, native laminar structure corresponds well with OCT images for mouse retina (unpublished observation), suggesting that local variations in refractive index are relatively small; similar light-evoked OR changes were measured with OCT, diffusion MRI, and hydration-sensitive MRI measurements and support this conclusion.21 In addition, the data herein highlight that oxidative stress impairs the normal OR photoresponse and can be corrected by acute AO treatment (QUEST protocol) as a noninvasive way to detect OR oxidative stress.1 In our previous study, we have found that diabetes impaired the OR photoresponse owing to oxidative stress as measured by QUEST diffusion MRI.1,11 Also, we have found that OR oxidative stress is a feature of the rd10 mouse model of retinitis pigmentosa, and it has recently been reported that this model shows an impaired OR photoresponse.2,24 These data suggest that the impaired OCT photoresponse in the rd10 mouse is due to oxidative stress. Together, these considerations raise the strong possibility that QUEST OCT will be useful for mapping oxidative stress and treatment efficacy in various OR diseases.
AO defenses in the retina are typically measured in preclinical studies following a provocation with a strong oxidizing agent, such as sodium iodate or paraquat, drugs that also produce substantial neurodegeneration.29,31–34 Needless to say, these drugs will not be useful clinically for evaluating antioxidant defenses. In contrast, our previous and present results support the use of the FDA-approved calcium channel blocker and cardioprotectant d-cis-diltiazem for producing a temporary OR oxidative stress in healthy dark-adapted retina without neurotoxicity.9 For example, B6 mice, which experience greater photoreceptor oxidative stress and histopathology than 129S6/Ev mice following low-dose sodium iodate, also show a greater d-cis-diltiazem OR oxidative stress than 129S6/Ev mice, as measured by QUEST MRI.9,29,34,35 One potential mechanism by which d-cis-diltiazem may generate oxidative stress is via outer retinal cytochrome P450; more work is need to test this hypothesis.9 Nonetheless, the present data raise the possibility that the combination of QUEST OCT and d-cis-diltiazem will be a useful approach to study AO defenses in the prodromal stage of aging or diseased retina.
Previously we have shown that 1 hour post d-cis-diltiazem treatment in dark-adapted B6 mice, OR oxidative stress occurs in inferior retina as measured by QUEST MRI.9 However, in this study, we found evidence that both inferior and superior OR had oxidative stress (data in Figs. 3, 4; see Analysis section of Materials and Methods for justification). One possible explanation for the somewhat different results is that OCT has higher spatial resolution and thus is better able to detect subtle microstructural changes than the lower-resolution MRI. On the other hand, QUEST MRI interrogates all retinal layers for oxidative stress, whereas QUEST OCT is limited to the outer segment and RPE layers.
In summary, the main finding here was a demonstration for the first time of a new functionality for OCT: the ability to measure localized oxidative stress in vivo. The present study carefully built on our previous findings that light-evoked expansion of the OR is an essential physiology measurable by OCT, and that light-evoked expansion of the OR is sensitive to oxidative stress as measured by MRI.1,36 However, MRI is not as widely available as OCT, and the OCT photoresponse has not previously been shown to be a useful index for measuring oxidative stress. The presents results are important because they address a longstanding problem, namely, that conventional assays are unable to noninvasively measure OR oxidative stress in vivo and potentially in patients to personalize antioxidant treatment options. We also reported that combining QUEST OCT with an acute administration of d-cis-diltiazem is a unique way to noninvasively evaluate OR AO defenses. Finally, we found that the OR thickness in the light alone can be used to detect OR oxidative stress, an observation that may help facilitate translation of QUEST OCT into a clinical setting. QUEST OCT appears to be a promising new clinically relevant tool for early diagnosis and individualized AO treatment in sight-threatening diseases with an OR oxidative stress etiology.
Acknowledgments
Supported by the National Eye Institute (RO1 EY026584, RO1 AG058171 to BAB; Intramural Research Programs EY000503 and EY000530 to HQ), NEI Core Grant P30 EY04068, and an unrestricted grant from Research to Prevent Blindness (Kresge Eye Institute).
Disclosure: B.A. Berkowitz, None; R.H. Podolsky, None; K.M. Lins-Childers, None; Y. Li, None; H. Qian, None
References
- 1.Berkowitz BA, Grady EM, Khetarpal N, Patel A, Roberts R. Oxidative stress and light-evoked responses of the posterior segment in a mouse model of diabetic retinopathy. Invest Ophthalmol Vis Sci. 2015;56:606–615. doi: 10.1167/iovs.14-15687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berkowitz BA, Podolsky RH, Berri AM, et al. Dark rearing does not prevent rod oxidative stress in vivo in Pde6brd10 mice. Invest Ophthalmol Vis Sci. 2018;59:1659–1665. doi: 10.1167/iovs.17-22734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Biswal MR, Justis BD, Han P, et al. Daily zeaxanthin supplementation prevents atrophy of the retinal pigment epithelium (RPE) in a mouse model of mitochondrial oxidative stress. PLoS One. 2018;13:e0203816. doi: 10.1371/journal.pone.0203816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bonilha VL, Bell BA, Rayborn ME, et al. Absence of DJ-1 causes age-related retinal abnormalities in association with increased oxidative stress. Free Radic Biol Med. 2017;104:226–237. doi: 10.1016/j.freeradbiomed.2017.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hollyfield JG, Bonilha VL, Rayborn ME, et al. Oxidative damage-induced inflammation initiates age-related macular degeneration. Nat. Med. 2008;14:194–198. doi: 10.1038/nm1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Handa JT. How does the macula protect itself from oxidative stress? Mol Aspects Med. 2012;33:418–435. doi: 10.1016/j.mam.2012.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beatty S, Koh HH, Phil M, Henson D, Boulton M. The role of oxidative stress in the pathogenesis of age-related macular degeneration. Surv Ophthalmol. 2000;45:115–134. doi: 10.1016/s0039-6257(00)00140-5. [DOI] [PubMed] [Google Scholar]
- 8.Berkowitz BA, Bissig D, Roberts R. MRI of rod cell compartment-specific function in disease and treatment in vivo. Prog Retin Eye Res. 2016;51:90–106. doi: 10.1016/j.preteyeres.2015.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berkowitz BA, Podolsky RH, Farrell B, et al. D-cis-diltiazem can produce oxidative stress in healthy depolarized rods in vivo. Invest Ophthalmol Vis Sci. 2018;59:2999–3010. doi: 10.1167/iovs.18-23829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Du Y, Veenstra A, Palczewski K, Kern TS. Photoreceptor cells are major contributors to diabetes-induced oxidative stress and local inflammation in the retina. Proc Natl Acad Sci U S A. 2013;110:16586–16591. doi: 10.1073/pnas.1314575110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berkowitz BA, Bredell BX, Davis C, Samardzija M, Grimm C, Roberts R. Measuring in vivo free radical production by the outer retina. Invest Ophthalmol Vis Sci. 2015;56:7931–7938. doi: 10.1167/iovs.15-18420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Berkowitz BA, Kern TS, Bissig D, et al. Systemic retinaldehyde treatment corrects retinal oxidative stress, rod dysfunction, and impaired visual performance in diabetic micesystemic retinaldehyde treatment in diabetic mice. Invest Ophthalmol Vis Sci. 2015;56:6294–6303. doi: 10.1167/iovs.15-16990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morakinyo A, Iranloye B, Adegoke O. Calcium antagonists modulate oxidative stress and acrosomal reaction in rat spermatozoa. Arch Med Sci. 2011;7:613–618. doi: 10.5114/aoms.2011.24130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee JH, Kim H, Kim DH, Gye MC. Effects of calcium channel blockers on the spermatogenesis and gene expression in peripubertal mouse testis. Arch Androl. 2006;52:311–318. doi: 10.1080/01485010600664024. [DOI] [PubMed] [Google Scholar]
- 15.Bissig D, Berkowitz BA. Light-dependent changes in outer retinal water diffusion in rats in vivo. Mol Vis. 2012;18:2561–2577. [PMC free article] [PubMed] [Google Scholar]
- 16.Huang B, Karwoski CJ. Light-evoked expansion of subretinal space volume in the retina of the frog. J Neurosci. 1992;12:4243–4252. doi: 10.1523/JNEUROSCI.12-11-04243.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Govardovskii VI, Li JD, Dmitriev AV, Steinberg RH. Mathematical model of TMA+ diffusion and prediction of light-dependent subretinal hydration in chick retina. Invest Ophthalmol Vis Sci. 1994;35:2712–2724. [PubMed] [Google Scholar]
- 18.Li JD, Govardovskii VI, Steinberg RH. Light-dependent hydration of the space surrounding photoreceptors in the cat retina. Vis Neurosci. 1994;11:743–752. doi: 10.1017/s0952523800003047. [DOI] [PubMed] [Google Scholar]
- 19.Wolfensberger TJ, Dmitriev AV, Govardovskii VI. Inhibition of membrane-bound carbonic anhydrase decreases subretinal pH and volume. Doc Ophthalmol. 1999;97:261–271. doi: 10.1023/a:1002496223131. [DOI] [PubMed] [Google Scholar]
- 20.Adijanto J, Banzon T, Jalickee S, Wang NS, Miller SS. CO2-induced ion and fluid transport in human retinal pigment epithelium. J Gen Physiol. 2009;133:603–622. doi: 10.1085/jgp.200810169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Berkowitz BA, Podolsky RH, Qian H, et al. Mitochondrial respiration in outer retina contributes to light-evoked increase in hydration in vivo. Invest Ophthalmol Vis Sci. 2018;59:5957–5964. doi: 10.1167/iovs.18-25682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li Y, Fariss RN, Qian JW, Cohen ED, Qian H. Light-induced thickening of photoreceptor outer segment layer detected by ultra-high resolution OCT imaging. Invest Ophthalmol Vis Sci. 2016;57:OCT105–OCT111. doi: 10.1167/iovs.15-18539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang P, Zawadzki RJ, Goswami M, et al. In vivo optophysiology reveals that G-protein activation triggers osmotic swelling and increased light scattering of rod photoreceptors. Proc Natl Acad Sci U S A. 2017;114:E2937–E2946. doi: 10.1073/pnas.1620572114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li Y, Zhang Y, Chen S, Vernon G, Wong WT, Qian H. Light-dependent OCT structure changes in photoreceptor degenerative rd 10 mouse retina. Invest Ophthalmol Vis Sci. 2018;59:1084–1094. doi: 10.1167/iovs.17-23011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Azimipour M, Migacz JV, Zawadzki RJ, Werner JS, Jonnal RS. Functional retinal imaging using adaptive optics swept-source OCT at 1.6MHz. Optica. 2019;6:300–303. doi: 10.1364/OPTICA.6.000300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lu CD, Lee B, Schottenhamml J, Maier A, Pugh EN, Fujimoto JG. Photoreceptor layer thickness changes during dark adaptation observed with ultrahigh-resolution optical coherence tomography. Invest Ophthalmol Vis Sci. 2017;58:4632–4643. doi: 10.1167/iovs.17-22171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oz M, Lorke DE, Hasan M, Petroianu GA. Cellular and molecular actions of Methylene Blue in the nervous system. Med Res Rev. 2011;31:93–117. doi: 10.1002/med.20177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Packer L. Antioxidant properties of lipoic acid and its therapeutic effects in prevention of diabetes complications and cataracts. Ann N Y Acad Sci. 1994;738:257–264. doi: 10.1111/j.1749-6632.1994.tb21811.x. [DOI] [PubMed] [Google Scholar]
- 29.Berkowitz BA, Podolsky RH, Lenning J, et al. Sodium iodate produces a strain-dependent retinal oxidative stress response measured in vivo using QUEST MRI. Invest Ophthalmol Vis Sci. 2017;58:3286–3293. doi: 10.1167/iovs.17-21850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Berkowitz BA, Lewin AS, Biswal MR, Bredell BX, Davis C, Roberts R. MRI of retinal free radical production with laminar resolution in vivo. Invest Ophthalmol Vis Sci. 2016;57:577–585. doi: 10.1167/iovs.15-18972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Patel AK, Davis A, Rodriguez ME, Agron S, Hackam AS. Protective effects of a grape-supplemented diet in a mouse model of retinal degeneration. Nutrition. 2016;32:384–390. doi: 10.1016/j.nut.2015.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lederman M, Hagbi-Levi S, Grunin M, et al. Degeneration modulates retinal response to transient exogenous oxidative injury. PLoS One. 2014;9:e87751. doi: 10.1371/journal.pone.0087751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen H, Liu B, Lukas TJ, Suyeoka G, Wu G, Neufeld AH. Changes in iron regulatory proteins in the aged rodent neural retina. Neurobiol Aging. 2009;30:1865–1876. doi: 10.1016/j.neurobiolaging.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cingolani C, Rogers B, Lu L, Kachi S, Shen J, Campochiaro PA. Retinal degeneration from oxidative damage. Free Radic Biol Med. 2006;40:660–669. doi: 10.1016/j.freeradbiomed.2005.09.032. [DOI] [PubMed] [Google Scholar]
- 35.Witola WH, Kim CY, Zhang X. Inherent oxidative stress in the Lewis rat is associated with resistance to toxoplasmosis. Infect Immun. 2017;85:e00289–17. doi: 10.1128/IAI.00289-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Berkowitz BA. Oxidative stress measured in vivo without an exogenous contrast agent using QUEST MRI. J Magn Reson. 2018;291:94–100. doi: 10.1016/j.jmr.2018.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Berger A, Cavallero S, Dominguez E, et al. Spectral-domain optical coherence tomography of the rodent eye: highlighting layers of the outer retina using signal averaging and comparison with histology. PLoS One. 2014;9:e96494. doi: 10.1371/journal.pone.0096494. [DOI] [PMC free article] [PubMed] [Google Scholar]