Abstract
Traffic-related air pollution has been linked to higher risks of infertility and miscarriage. We evaluated whether folate intake modified the relationship between air pollution and livebirth among women using assisted reproductive technology (ART). Our study included 304 women (513 cycles) presenting to a fertility center in Boston, Massachusetts (2005–2015). Diet and supplements were assessed by food frequency questionnaire. Spatiotemporal models estimated residence-based daily nitrogen dioxide (NO2), ozone, fine particulate, and black carbon concentrations in the 3 months before ART. We used generalized linear mixed models with interaction terms to evaluate whether the associations between air pollutants and livebirth were modified by folate intake, adjusting for age, body mass index, race, smoking, education, infertility diagnosis, and ART cycle year. Supplemental folate intake significantly modified the association of NO2 exposure and livebirth (P = 0.01). Among women with supplemental folate intakes of <800 μg/day, the odds of livebirth were 24% (95% confidence interval: 2, 42) lower for every 20-parts-per-billion increase in NO2 exposure. There was no association among women with intakes of ≥800 μg/day. There was no effect modification of folate on the associations between other air pollutants and livebirth. High supplemental folate intake might protect against the adverse reproductive consequences of traffic-related air pollution.
Keywords: air pollution, fecundity, fertility, folate, infertility, pregnancy loss, traffic
There is increasing evidence that exposure to traffic-related air pollution (TRAP) is related to lower fecundity and fertility among women attempting to conceive spontaneously and through assisted reproductive technology (ART) (1, 2). In previous studies, women residing closer to major roadways were shown to have a higher risk of infertility (3), longer time to pregnancy (4), higher risk of spontaneous abortion (5), and reduced likelihood of livebirth following ART (6). Moreover, higher exposure to nitrogen dioxide (NO2), a marker of TRAP (7, 8), was linked to lower census-tract level livebirth rates (9), decreased fecundability (10), and lower probability of implantation (11), pregnancy (12), and livebirth (13) following ART.
In addition, there is growing acceptance that nutrients, such as folic acid, might be related to enhanced reproductive success among women (14) and could reduce the toxicity of environmental pollutants (15). A high intake of folic acid has been related to lower risk of infertility (16), shorter time to pregnancy (17), lower risk of pregnancy loss (18), and better outcomes of ART (19–21). Furthermore, a high folic acid intake has been shown to attenuate the associations of certain reproductive toxicants such as bisphenols and phthalates (22, 23), pesticides (24, 25), and arsenic (26) on various health outcomes. Emerging evidence also suggests that folate intake, as measured by dietary folate equivalents (as well as other methyl donors), might mute the association between TRAP and risk of birth defects (27) and that folic acid intake (from diet and supplements) could attenuate the association between TRAP on risk of autism (28).
Although the specific underlying biological pathways through which TRAP exposure and folic acid intake could affect female fertility are unknown, it is hypothesized that both are involved in the induction of epigenetic changes, including alterations in DNA methylation (29–31), as well as the generation of oxidative stress and inflammation (32, 33). Therefore, we hypothesized that folate, in particular folic acid from supplements (due to the higher bioavailability of folate in this form), might attenuate the adverse reproductive consequences of air pollution exposure on likelihood of livebirth following ART.
METHODS
Study population
Study subjects were recruited into the Environment and Reproductive Health (EARTH) Study starting in November 2004 from patients presenting at the Massachusetts General Hospital Fertility Center. All women aged 18–46 years were eligible, and approximately 60% of those contacted participated. Upon enrollment, all participants provided their residential address. Women were followed through their infertility treatment until discontinuation or livebirth. Women received financial incentives for participation: $100 for the entry visit, $50 for baseline take-home questionnaires, and $50 per cycle visit.
Of the 696 completed ART cycles contributed between April 2005 (when the first ART cycle was initiated) and March 2017 (when our master data set was updated), we excluded 60 cycles ending after December 31, 2015 (due to the temporal constraints of the air pollution models); 3 cycles contributed by women residing outside of the United States; and 3 cycles missing data on the air pollutants. From the remaining 630 ART cycles, we excluded 127 cycles from women who did not complete a dietary assessment (introduced in 2007), bringing our final sample size to 513 ART cycles from 304 women. For the analysis of black carbon (BC), we excluded 14 cycles (7 women) who resided in Michigan, Maine, or northern New Hampshire, which were outside the range of this model. Work on the main effects of these air pollutants on ART outcomes has been published elsewhere (34). The EARTH Study was approved by the Human Studies Institutional Review Boards of Massachusetts General Hospital and the Harvard T.H. Chan School of Public Health. All study participants signed an informed consent after the study procedures were explained by trained study staff.
Air pollution exposures
The residential address of each woman was geocoded using ArcGIS and linked to validated spatiotemporal models for each air pollutant. Daily concentrations of particulate matter with an aerodynamic diameter equal to or less than 2.5 μm (PM2.5) and NO2 were both modeled using satellite remote sensing data in combination with land-use terms (35, 36). Daily ozone (O3) models additionally included chemical transport models, O3 vertical profiles, meteorological variables, and other atmospheric compounds (37). Daily BC exposure was estimated at the address using ambient BC measurements from over 300 monitors in Massachusetts, Rhode Island, and southern New Hampshire (38). For each pollutant, we averaged daily estimated concentrations from 3 months prior to the start of the ART cycle to represent a women’s average air pollution exposure. This time window also roughly corresponds to the proposed window of follicular development (39). Distance to major roadways (meters) was determined using the ESRI StreetPro 2007 data layer. We selected US Census feature class codes to include A1, A2, or A3 road segments.
Dietary assessment
Dietary folate intake was assessed using a validated food frequency questionnaire (40). Participants were asked to report how often they consumed 131 food items during the previous year. Multivitamin and supplement users were asked to specify the brand, the dose, and frequency of use. Nutrient intakes were estimated by summing the nutrient contribution of all foods and supplements. Nutrient contents were obtained from the US Department of Agriculture, with additional information from manufacturers (41). Folate intake with this questionnaire has been validated against dietary records (r = 0.77) (42) and against red blood cell (r = 0.51) (43) and plasma (r = 0.54) (44) folate concentrations. We dichotomized total and supplemental folate intake by taking into consideration the distribution of intake in our population as well as clinically relevant cutoffs such as 400 μg/day (the recommended daily amount (RDA)), 800 μg/day (2 times the RDA), and 1,000 μg/day (the tolerable upper intake level). The cutoff closest to the 75th percentile of intake in our population was used for dichotomization.
Outcome assessment
For fresh ART cycles, patients underwent luteal-phase gonadotropin-releasing hormone agonist protocol, follicular-phase gonadotropin-releasing-hormone-agonist/flare protocol, or gonadotropin-releasing-hormone-antagonist protocol, as clinically indicated. During stimulation, patients were monitored for serum estradiol, follicle size, and endometrial thickness. Human chorionic gonadotropin was administered approximately 36 hours before scheduled oocyte retrieval to induce oocyte maturation. Following retrieval, oocytes were fertilized using conventional insemination or intracytoplasmic sperm injection. For cryo-thaw or donor-egg recipient cycles, patients underwent endometrial preparation protocols. Following embryo transfer, all clinical outcomes were assessed identically for fresh, cryo-thaw, and donor-egg recipient cycles. Successful implantation was defined as a serum β-human chorionic gonadotropin level >6 mIU/mL (approximately 17 days after egg retrieval), clinical pregnancy as an intrauterine pregnancy confirmed on the 6-week ultrasound, and livebirth as the birth of a neonate of ≥24 weeks’ gestation.
Covariate assessment
At enrollment, height and weight were measured to calculate body mass index (BMI; weight (kg)/height(m)2). Participants completed a take-home questionnaire regarding lifestyle factors, reproductive health, and medical history. Census-tract level, median family income in the past 12 months (in 2011 inflation-adjusted dollars) was calculated from the American Community Survey 2007–2010. The alternate Healthy Eating Index 2010 (aHEI-2010) was calculated as a measure of a healthy diet (45). Clinical information including infertility diagnosis (diminished ovarian reserve, endometriosis, male factor, ovulatory, tubal, uterine, and unexplained) and protocol type was abstracted from electronic medical records.
Statistical analysis
Descriptive statistics were calculated comparing the bottom and top quartiles of estimated NO2 exposure stratified by supplemental folate intake (<800 μg/day vs. ≥800 μg/day). To test whether the associations between air pollutants and livebirth were modified by folate intake, multivariate mixed-effects logistic regression models with a subject-specific random effect and a product of the continuous air pollutant and dichotomized folate intake were used, because they can account for within-person correlations in outcomes (46). Each air pollutant was modeled as a continuous exposure, and effect sizes are reported in interquartile range (IQR) increments. Distance to major roadways was modeled per 250-m increment. Additional models were fitted, including a product of the continuous air pollutant and continuous folate intake. An interaction term was considered suggestive when the P value was <0.10. Results are presented as the multiplicative change in odds of livebirth (95% confidence interval) per IQR increase in the air pollutant or predicted probabilities of livebirth at the average value of continuous covariates and the most common value of categorical covariates (47).
We also evaluated evidence of additive interaction by placing women into one of 4 categories based on air pollution exposure (< or ≥ median) and folate intake. Generalized estimating equations with binomial distribution and log link function were used to assess the combined effect of air pollution and folate intake on the risk of livebirth. The relative excess risk due to interaction was calculated as RR11−R10−R01 + 1 and confidence bounds were estimated using approximate variance estimators (48).
Intermediate ART endpoints were investigated only if an interaction was detected between folate, the pollutant, and livebirth to minimize multiple testing and type 1 error. We used generalized linear mixed models with random intercepts and normal (for peak estradiol and endometrial thickness), Poisson (for oocyte counts), or binomial (for implantation, clinical pregnancy, and pregnancy loss) distribution. Pregnancy loss, including therapeutic abortions (all of which were performed due to life-threatening abnormalities), was examined only among cycles with successful implantation (n = 290 cycles). Confounding was evaluated using prior knowledge and descriptive statistics from our cohort through the use of directed acyclic graphs (49). Variables retained in the final multivariable models were maternal age (continuous), race (white, other), BMI (continuous), smoking status (ever, never), educational level (less than graduate degree, graduate degree), initial infertility diagnosis (female, male, unexplained), and year of ART cycle (continuous). Multivariable models were also fitted with further adjustment for the other pollutants and without adjustment for infertility diagnosis (due to concerns that it might be a downstream consequence of exposure). Several sensitivity analyses were conducted, including restricting our analyses to noncurrent smokers (n = 501 cycles, 98%), autologous fresh ART cycles (n = 427 cycles, 83%), cycles with embryo transfer (n = 468, 91%), cycles with prospective diet (n = 495, 96%), first in-study ART cycles (n = 304 cycles, 59%), first ART cycles at Massachusetts General Hospital (n = 235, 46%), and nulligravid women (n = 300, 58%).
RESULTS
Among the 304 women in our analysis, the mean age was 35.2 (standard deviation, 4.0) years and BMI was 24.3 (standard deviation, 4.4). The majority of women were white (83%), had a college education or higher (91%), had never smoked (72%), and had unexplained infertility at baseline (38%). During the study period, the median estimated air pollution concentrations in the 3 months prior to ART were 22.9 (10th–90th percentile, 5.3–47.1) parts per billion for NO2, 34.2 (10th–90th percentile, 19.9–50.2) parts per billion for O3, 8.5 (10th–90th percentile, 6.8–10.9) μg/m3 for PM2.5, and 0.49 (10th–90th percentile, 0.36–0.76) μg/m3 for BC. The median distance to A1–A3 roadways was 107.8 (10th–90th percentile, 3.7–414.1) m. The correlations between pollutants were modest (Web Table 1, available at https://academic.oup.com/aje), with the highest magnitude observed for NO2 and BC (r = 0.39). Ozone had weak inverse correlations with NO2 (r = −0.13) and BC (r = −0.22).
Women in this cohort were high consumers of total and supplemental folate, with median intakes of 1,100 (range, 187–2,675) μg/day and 571 (range, 0–2,400) μg/day, respectively. Only 16% of our women consumed <400 μg/day and 20% of women consumed ≥1,000 μg/day of supplemental folate, which precluded a meaningful evaluation of these cutoffs. A total of 468 (91%) of the initiated cycles had at least 1 embryo transferred, with 290 (57%) resulting in implantation, 257 (50%) in clinical pregnancy, and 206 (40%) in livebirth (Web Figure 1). Women contributed an average of 1.7 ART cycles (range, 1–6).
Across both strata of supplemental folate intake, women in the highest quartile of estimated NO2 exposure had lower BMIs (Table 1). Among women with high intake of supplemental folate, those with the highest estimated exposure to NO2 were more likely to be diagnosed with female factor infertility; however, no other baseline characteristics were significantly related to NO2 exposure.
Table 1.
Demographic and Reproductive Characteristics at Baseline According to Quartiles of Estimated Nitrogen Dioxide Exposure in the 3 Months Prior to Assisted Reproductive Technology Use and Intake of Supplemental Folate Among 304 Women in the Environment and Reproductive Health Study, Massachusetts, 2005–2015
| Characteristic | Women Who Consumed <800 μg/day of Supplemental Folate (n = 173) | Women Who Consumed ≥800 μg/day of Supplemental Folate (n = 131) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NO2 Quartile 1 (n = 44) | NO2 Quartile 4 (n = 47) | NO2 Quartile 1 (n = 32) | NO2 Quartile 4 (n = 29) | |||||||||
| Mean (SD) | No. | % | Mean (SD) | No. | % | Mean (SD) | No. | % | Mean (SD) | No. | % | |
| Estimated NO2 exposure, ppb | 8.8 (3.1) | 56.9 (37.2) | 8.3 (4.4) | 58.4 (57.0) | ||||||||
| Demographic characteristics | ||||||||||||
| Age, years | 35.1 (4.0) | 35.0 (4.1) | 35.1 (4.5) | 35.3 (4.4) | ||||||||
| White race | 37 | 84.1 | 40 | 85.1 | 29 | 90.6 | 24 | 82.8 | ||||
| BMIa | 25.5 (5.2) | 23.9 (4.1) | 25.2 (4.3) | 23.6 (3.8) | ||||||||
| Smoking status | ||||||||||||
| Never | 32 | 72.7 | 32 | 68.1 | 23 | 71.9 | 21 | 72.4 | ||||
| Former | 9 | 20.5 | 14 | 29.8 | 9 | 28.1 | 7 | 24.1 | ||||
| Current | 3 | 6.8 | 1 | 2.1 | 0 | 0.0 | 1 | 3.5 | ||||
| Educational level | ||||||||||||
| High school or less | 5 | 11.4 | 4 | 8.5 | 3 | 9.4 | 1 | 3.5 | ||||
| College | 13 | 29.0 | 13 | 27.7 | 16 | 50.0 | 5 | 17.2 | ||||
| Graduate school | 26 | 59.1 | 30 | 63.8 | 13 | 40.6 | 23 | 79.3 | ||||
| Distance to A1–A3 roadway, m | 279.7 (337.3) | 129.4 (105.1) | 517.3 (627.5) | 78.5 (75.9) | ||||||||
| Census tract median income, $ | 105,327 (31,418) | 95,474 (45,496) | 99,071 (31,182) | 101,610 (42,076) | ||||||||
| Dietary characteristics | ||||||||||||
| Total calories, kcal/day | 1,877.0 (625.8) | 1,834.3 (609.0) | 1,807.7 (586.8) | 1,705.7 (648.0) | ||||||||
| Total folate, μg/day | 797.5 (291.0) | 808.9 (308.3) | 1,387.5 (337.7) | 1,408.1 (294.5) | ||||||||
| Food folate, μg/day | 459.1 (202.7) | 483.6 (168.4) | 422.9 (211.7) | 454.2 (225.3) | ||||||||
| Supplemental folate, μg/day | 338.4 (194.2) | 325.3 (218.8) | 964.5 (305.7) | 953.9 (210.4) | ||||||||
| Alcohol intake, g/day | 9.0 (14.3) | 12.3 (13.3) | 7.7 (9.3) | 7.4 (5.6) | ||||||||
| aHEI-2010 score | 62.9 (11.0) | 68.0 (10.1) | 62.9 (12.5) | 69.0 (9.8) | ||||||||
| Baseline reproductive characteristics | ||||||||||||
| Duration of pregnancy attempt, months | ||||||||||||
| ≤12 | 8 | 18.2 | 20 | 42.6 | 11 | 34.4 | 6 | 20.7 | ||||
| 13–24 | 25 | 56.8 | 21 | 44.7 | 14 | 43.8 | 15 | 51.7 | ||||
| ≥24 | 11 | 25.0 | 6 | 12.8 | 7 | 21.9 | 8 | 27.6 | ||||
| Previous IVF cycle at MGH | 13 | 29.6 | 5 | 10.6 | 10 | 31.3 | 5 | 17.2 | ||||
| Nulligravid | 27 | 61.4 | 31 | 66.0 | 18 | 56.3 | 17 | 58.6 | ||||
| Nulliparous | 38 | 86.4 | 42 | 89.4 | 24 | 75.0 | 26 | 89.7 | ||||
| Infertility diagnosis | ||||||||||||
| Male factor | 13 | 29.6 | 17 | 36.2 | 7 | 21.9 | 7 | 24.1 | ||||
| Female | 15 | 34.1 | 13 | 27.7 | 9 | 28.1 | 12 | 41.4 | ||||
| Unexplained | 16 | 36.4 | 17 | 36.2 | 16 | 50.0 | 10 | 34.5 | ||||
| Protocol | ||||||||||||
| Antagonist or flare | 9 | 20.5 | 11 | 23.4 | 9 | 28.1 | 2 | 6.9 | ||||
| Luteal phase agonist | 29 | 65.9 | 33 | 70.2 | 20 | 62.5 | 24 | 82.8 | ||||
| Egg-donor or cryo-thaw cycle | 6 | 13.6 | 3 | 6.4 | 3 | 9.4 | 3 | 10.3 | ||||
| Number of ART cycles contributed to analysis | 1.8 (1.0) | 1.9 (1.1) | 1.4 (0.8) | 1.6 (1.0) | ||||||||
Abbreviations: aHEI-2010, alternate Health Eating Index 2010; ART, assisted reproductive technology; BMI, body mass index; IVF, in vitro fertilization; MGH, Massachusetts General Hospital; NO2, nitrogen dioxide; ppb, parts per billion; SD, standard deviation.
a BMI was calculated as weight (kg)/height (m)2.
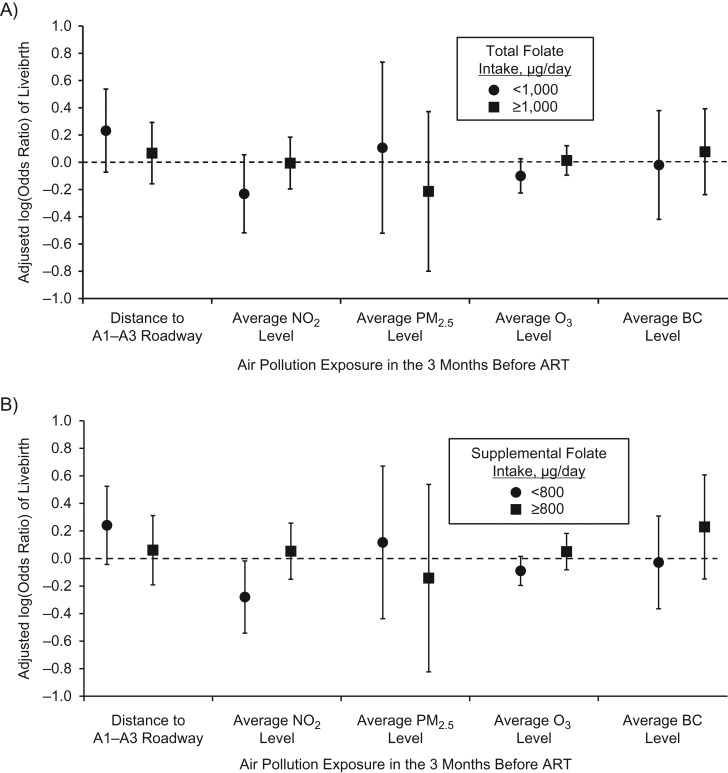
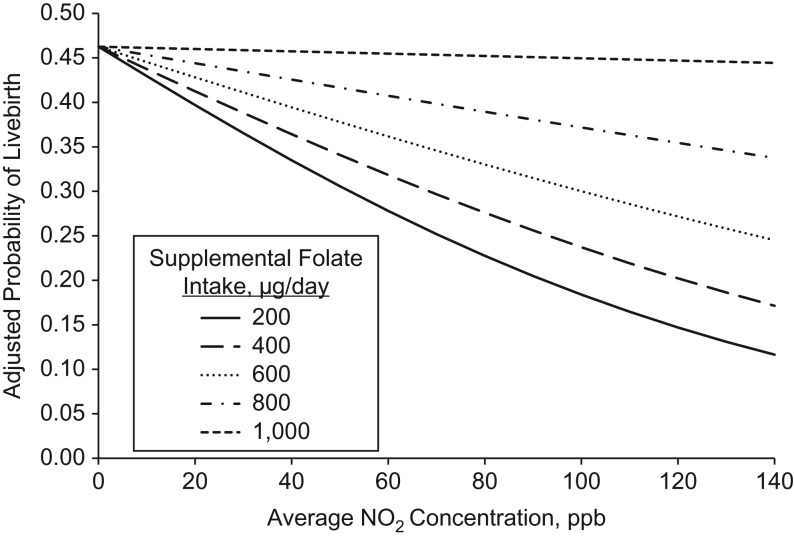
Intake of total folate statistically significantly modified the association between estimated exposure to NO2 in the 3 months prior to ART and livebirth (P = 0.02) (Figure 1). This association was driven primarily by intake of supplemental folate (P = 0.01) as opposed to food folate (P = 0.24). Among women with supplemental folate intakes of <800 μg/day, every 20-parts-per-billion increase in estimated NO2 was associated with 24% (95% confidence interval: 2, 42) lower odds of livebirth. There was no association between estimated NO2 exposure and livebirth among women with intakes of ≥800 μg/day. The effect modification of the NO2 and livebirth association by supplemental folate persisted when supplemental folate was modeled as a continuous variable (Figure 2). The inverse association between estimated NO2 and probability of livebirth was strongest among women with the lowest intake of supplemental folate (e.g., 200 μg/day) and became increasingly weak as intakes increased up until 1,000 μg/day at which point the association became essentially null. Folate intake did not modify the associations between estimated O3, PM2.5, BC, or distance to A1–A3 roadways and livebirth (Figure 1).
Figure 1.
Effect modification of total and supplemental folate intake on the relationship between the distance to A1–A3 roadways and estimated nitrogen dioxide (NO2), ozone (O3), fine particulate matter (PM2.5), and black carbon (BC) concentrations in the 3 months prior to using assisted reproductive technology (ART) and the odds of livebirth in the Environment and Reproductive Health Study, Massachusetts, 2005–2015. A) Total folate; B) supplemental folate. All air pollutants are modeled per interquartile-range increase (20 parts per billion for NO2, 3 μg/m3 for PM2.5, 15 parts per billion for O3, and 0.2 μg/m3 for BC), and distance to major roadway was modeled per 250-m increase. Analyses were performed using multivariable generalized linear mixed models with random intercepts (binomial distribution and logit link function), adjusting for age, body mass index, race, smoking status, educational level, initial infertility diagnosis, and year of cycle start. There were 134 women (239 cycles) who consumed <1,000 μg/day of total folate and 170 (274 cycles) who consumed ≥1,000 μg/day of total folate. There were 173 women (303 cycles) who consumed <800 μg/day of supplemental folate and 131 (210 cycles) who consumed ≥800 μg/day of supplemental folate. For total folate, the P values for interaction were 0.63 for distance to major roadways, 0.02 for NO2, 0.90 for PM2.5, 0.14 for O3, and 0.67 for BC. For supplemental folate, the P values for interaction were 0.92 for distance to major roadways, 0.01 for NO2, 0.78 for PM2.5, 0.13 for O3, and 0.33 for BC.
Figure 2.
Effect modification of supplemental folate intake (modeled continuously) on the association between estimated nitrogen dioxide (NO2) concentrations in the 3 months prior to using assisted reproductive technology and adjusted probability of livebirth in the Environment and Reproductive Health Study, Massachusetts, 2005–2015. Analyses were performed using generalized linear mixed models with random intercepts (binomial distribution and logit link function). Data are presented as predicted marginal probabilities of livebirth at the mean age (35.6 years), body mass index (24.3, calculated as weight (kg)/height (m)2), and year (2010.9); the most frequent category of race (white), smoking status (never smoker), educational level (graduate degree), and infertility diagnosis (unexplained); and varying levels of supplemental folate intake. The values of 200, 400, 600, 800, and 1,000 μg/day correspond to the 10th, 42nd, 51st, 77th, and 91st percentiles of intake in our population. ppb, parts per billion.
When evaluating the models for additive interaction, we found that women with higher estimated exposure to NO2, PM2.5, O3, and BC and low supplemental folate intake had the lowest risk of livebirth compared with women with lower exposure to those pollutants and high supplemental folate intake (Web Table 2). However, only for NO2 did the effect estimate suggest a departure from expected interaction (relative excess risk due to interaction = −0.36, 95% confidence interval: −0.82, 0.09).
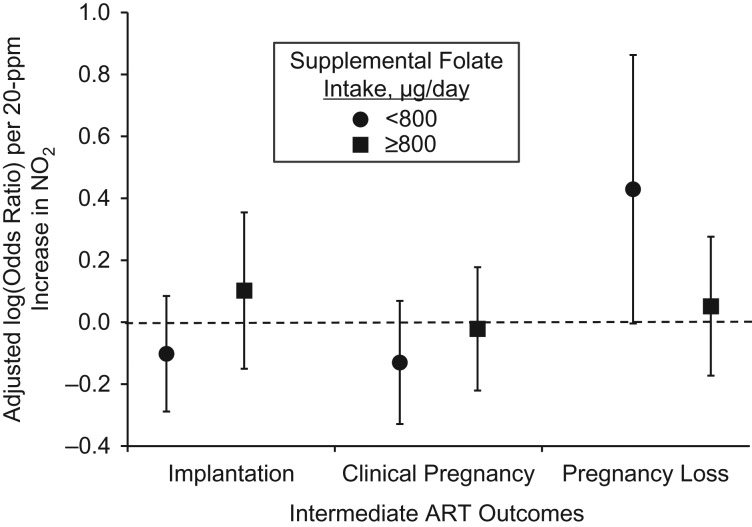
There was no evidence of effect modification of supplemental folate on the associations between estimated NO2 and estradiol levels, endometrial thickness, or total or mature oocyte yield (Web Table 3). We observed a weak interaction between supplemental folate, estimated NO2, and odds of implantation and clinical pregnancy, but the effect estimate of estimated NO2 on these outcomes, while stronger among women with supplemental folate intakes <800 μg/day, was not significant (Figure 3). Supplemental folate intake did modify the association between estimated NO2 and pregnancy loss (P = 0.06). The odds ratio for pregnancy loss was 1.54 (95% confidence interval: 1.00, 2.37) per 20-parts-per-billion increase in estimated NO2 among women with intakes <800 μg/day. There was no association among women with intakes of ≥800 μg/day.
Figure 3.
Effect modification of supplemental folate intake on the relationship between estimated nitrogen dioxide (NO2) concentrations in the 3 months prior to using assisted reproductive technology (ART) and odds of implantation, clinical pregnancy, and pregnancy loss in the Environment and Reproductive Health Study, Massachusetts, 2005–2015. Analyses were performed using multivariable generalized linear mixed models with random intercepts (binomial distribution and logit link function), adjusting for age, body mass index, race, smoking status, educational level, initial infertility diagnosis, and year of cycle start. The P values for interaction were 0.22 for implantation, 0.35 for clinical pregnancy, and 0.06 for pregnancy loss. There were 290 implantations and 257 clinical pregnancies out of 513 ART cycles. For the analysis of pregnancy loss, only cycles with a positive implantation were included (n = 290 cycles, 84 pregnancy losses). The circles and squares are the adjusted odds ratio for the outcome per interquartile-range increase in NO2 concentrations among women with supplemental folate intake of, respectively, less than or at least 800 μg/day. The 95% confidence intervals are represented by the vertical bars.
The effect modification of folate on the relationship between estimated NO2 and livebirth remained similar when analyses were restricted to cycles from noncurrent smokers, fresh autologous cycles, cycles with embryo transfer, cycles with prospective diet, first in-study cycles, first cycles at Massachusetts General Hospital, and nulligravid women (Web Table 4). Results were also similar after coadjustment for the other pollutants (Web Table 5) and without adjustment for infertility diagnosis (data not shown).
DISCUSSION
In our prospective cohort study of women undergoing ART, intake of supplemental folate modified the relationship of estimated NO2 exposure with probability of livebirth. Specifically, there was a lower likelihood of livebirth with increasing estimated exposure to NO2 3 months prior to ART among women consuming <800 μg/day of supplemental folate; however, estimated NO2 exposure was unrelated to livebirth among women with higher intake. The negative association of estimated NO2 on livebirth among women with low supplemental folate intake was driven primarily by a higher risk of pregnancy loss with increasing NO2 exposure. We found no evidence of an interaction with total or supplemental folate intake on the relationships between estimated O3, PM2.5, BC, or distance to A1–A3 roadways and livebirth following ART.
While this is, to our knowledge, the first time that an interaction between NO2 and supplemental folate has been investigated in a study focused on fertility, previous studies have observed a similar interaction of TRAP, and specifically NO2 exposure, with folate intake during pregnancy on risk of birth defects (27) and autism (28). In the National Birth Defects Prevention Study, a greater than additive interaction was observed for methyl donor intakes of methionine, choline, vitamin B6, and folate with NO2 exposure and odds of perimembranous ventricular septal defect (27). In the Childhood Autism Risk from Genetics and the Environment study, women with low folic acid intake (≤800 μg) who were exposed to higher levels of air pollution (e.g., near-roadway pollution, NO2, PM10, and PM2.5) during the first trimester had a higher risk of having a baby with autism compared with women with high folic acid intake who were exposed to lower air pollution levels (28).
Although the consistency of our findings with previous studies suggests that they might represent a true biological interaction, it was not possible to discern the underlying biological mechanisms. There are two leading hypotheses, however, based on mechanistic studies in humans. The first is that higher short- and long-term exposure to ambient TRAP decreases DNA methylation in peripheral blood leukocytes as well as the placenta (31, 50–52), and this might adversely affect early embryo development and pregnancy maintenance (29). Because dietary folate is a key source of the one carbon group used to methylate DNA (53), adequate folate intake could protect against DNA hypomethylation. A recent intervention study further supports this hypothesis, showing that, among heathy adults receiving a B-vitamin supplement, the association between a 2-hour controlled exposure to PM2.5 and DNA methylation was attenuated (54). The second is that higher exposure to TRAP, including NO2, promotes the generation of oxidative stress and inflammation (32), which has been implicated in a full spectrum of reproductive functions (55). Due to folic acid’s antioxidant properties (33), this could also explain the attenuated association we observed between NO2 and livebirth in women with high supplemental folate intake.
Similar to previous studies, our data suggest that supplemental folate intake is preferable to food folate in the context of counteracting the negative reproductive consequences of NO2 exposure. This could be because natural food folate has a lower proportion of folate that is absorbed and available for metabolic reactions and/or storage compared with folic acid, due to food folate’s limited release from the food matrix, its destruction within the gastrointestinal tract, and/or incomplete hydrolysis of glutamates (56). Second, even in the context of a fortified food supply, it is difficult to consume high levels of folate from diet alone. Therefore, the stronger associations we observed with supplemental folate could be driven by wider intakes and greater absorption, which combined allow for more extreme comparisons.
Of note, while most previous studies on TRAP and fertility have consistently found an adverse link, the majority of women in these studies also likely had supplemental folate intakes well below 800 μg/day, where we observed an adverse association between NO2 and livebirth. For example, the expected percentage of women taking folic acid before conception is <25% among pregnancy cohorts in Europe (57–59) and <33% in the United States (60). Even among highly motivated women planning pregnancy, such as those enrolled in the Longitudinal Investigation of Fertility and the Environment Study, only 63% were taking a multivitamin, 22% were taking a prenatal vitamin, and 4% were taking a folic acid supplement (61). Given that the standard amount of folic acid in multivitamins is 400 μg/day—up to 600–800 μg/day in prenatal vitamins—even with higher rates of supplementation (such as in the EARTH Study where 92% of women took either a prenatal or folic acid supplement), few women would be expected to have supplemental folate intakes >800 μg/day.
Our study has several limitations. For the air pollutants, we used residence-based ambient air pollution exposures as a proxy of personal exposure. Furthermore, we lacked information on the women’s work addresses or their time-activity patterns, which might have improved exposure assessment. We also did not update a woman’s address over the follow-up period. However, the spatiotemporal models of air pollution that we used are validated and specific to the woman’s home address, which is important for pollutants with small-scale spatial variation such as NO2 (62). Given that most women in our study resided at the same address as their male partner, it is also difficult to rule out the possibility that male exposure to TRAP could partially or fully explain the associations. Dietary assessment by food frequency questionnaire is also subject to measurement error; however, we used a questionnaire known to relate well to biomarker levels (43, 63). Due to the prospective nature of our study, measurement error of both air pollution and folate would most likely be nondifferential with respect to the outcomes and result in an attenuation of the observed associations. Our analysis included only women undergoing ART at a single academic medical center in Massachusetts. While this benefitted our analysis by limiting confounding across regions and treatment centers, the air pollution concentrations were generally low and thus it is unclear how generalizable our results are to women undergoing ART worldwide. Due to our relatively small sample size, it is possible that small but clinically meaningful interactions could have been missed. While residual confounding could still be explaining associations, unlike many other pregnancy cohorts, supplemental folate intake was not correlated with measures of socioeconomic status or healthy diet in this population. Strengths of our study include its prospective design and complete follow-up of participants, the comprehensive adjustment for confounding variables, and a wide range of folate intake.
In conclusion, we found that intake of supplemental folate of ≥800 μg/day appeared to attenuate the adverse association between estimated NO2 exposure and probability of livebirth following ART. Given that women have little control over their daily air pollution exposures, our findings provide potentially valuable guidance as to an individual-level dietary change that could ameliorate some of the negative reproductive consequences of TRAP exposure. In conjunction with the broader literature, which has observed interactions between folate and environmental exposures (22–26), such as TRAP (27, 28), on reproductive outcomes, our findings provide further support for research investigating interactions between nutrients and environmental exposures on reproductive outcomes.
Supplementary Material
ACKNOWLEDGMENTS
Author affiliations: Department of Nutrition, Harvard T.H. Chan School of Public Health, Boston, Massachusetts (Audrey J. Gaskins, Jorge E. Chavarro); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, Massachusetts (Audrey J. Gaskins, Jorge E. Chavarro, Joel Schwartz, Francine Laden); Department of Epidemiology, Rollins School of Public Health at Emory University, Atlanta, Georgia (Audrey J. Gaskins); Department of Environmental Health, Harvard T.H. Chan School of Public Health, Boston, Massachusetts (Lidia Mínguez-Alarcón, Kelvin C. Fong, Yara Abu Awad, Qian Di, Jennifer B. Ford, Brent A. Coull, Joel Schwartz, Russ Hauser, Francine Laden); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, Massachusetts (Jorge E. Chavarro, Brent A. Coull, Joel Schwartz, Russ Hauser, Francine Laden); Department of Biostatistics, Harvard T.H. Chan School of Public Health, Boston, Massachusetts (Brent A. Coull); Department of Environmental Geography, Ben Gurion University of the Negev, Beersheva, Israel (Itai Kloog); and Vincent Obstetrics and Gynecology, Massachusetts General Hospital and Harvard Medical School, Boston, Massachusetts (Jill Attaman, Russ Hauser).
This study was supported by the National Institute of Environmental Health Sciences (grants ES009718, ES022955, ES000002, and K99ES026648) and the National Institute of Diabetes and Digestive and Kidney Diseases (grant P30DK046200). This publication was made possible by US Environmental Protection Agency (grants RD-834798 and RD-83587201).
This work was presented at the annual meeting of the American Society for Reproductive Medicine, October 6–10, 2018, in Denver, Colorado.
The contents of this publication are solely the responsibility of the grantee and do not necessarily represent the official views of the US Environmental Protection Agency. Further, US Environmental Protection Agency does not endorse the purchase of any commercial products or services mentioned in the publication. The funders had no role in the design and conduct of the study; collection; management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Conflict of interest: none declared.
Abbreviations
- ART
assisted reproductive technology
- BC
black carbon
- BMI
body mass index
- EARTH
Environment and Reproductive Health
- IQR
interquartile range
- PM2.5
particulate matter with an aerodynamic diameter equal to or less than 2.5 μm
- NO2
nitrogen dioxide
- O3
ozone
- TRAP
traffic-related air pollution
REFERENCES
- 1. Checa Vizcaíno MA, González-Comadran M, Jacquemin B. Outdoor air pollution and human infertility: a systematic review. Fertil Steril. 2016;106(4):897–904.e1. [DOI] [PubMed] [Google Scholar]
- 2. Carré J, Gatimel N, Moreau J, et al. . Does air pollution play a role in infertility? A systematic review. Environ Health. 2017;16:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mahalingaiah S, Hart JE, Laden F, et al. . Adult air pollution exposure and risk of infertility in the Nurses’ Health Study II. Hum Reprod. 2016;31(3):638–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mendola P, Sundaram R, Louis GMB, et al. . Proximity to major roadways and prospectively-measured time-to-pregnancy and infertility. Sci Total Environ. 2017;576:172–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Green RS, Malig B, Windham GC, et al. . Residential exposure to traffic and spontaneous abortion. Environ Health Perspect. 2009;117(12):1939–1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gaskins AJ, Hart JE, Mínguez-Alarcón L, et al. . Residential proximity to major roadways and traffic in relation to outcomes of in vitro fertilization. Environ Int. 2018;115:239–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Clougherty JE, Wright RJ, Baxter LK, et al. . Land use regression modeling of intra-urban residential variability in multiple traffic-related air pollutants. Environ Health. 2008;7:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hochadel M, Heinrich J, Gehring U, et al. . Predicting long-term average concentrations of traffic-related air pollutants using GIS-based information. Atmos Environ. 2006;40(3):542–553. [Google Scholar]
- 9. Nieuwenhuijsen MJ, Basagaña X, Dadvand P, et al. . Air pollution and human fertility rates. Environ Int. 2014;70:9–14. [DOI] [PubMed] [Google Scholar]
- 10. Slama R, Bottagisi S, Solansky I, et al. . Short-term impact of atmospheric pollution on fecundability. Epidemiology. 2013;24(6):871–879. [DOI] [PubMed] [Google Scholar]
- 11. Carré J, Gatimel N, Moreau J, et al. . Influence of air quality on the results of in vitro fertilization attempts: a retrospective study. Eur J Obstet Gynecol Reprod Biol. 2017;210:116–122. [DOI] [PubMed] [Google Scholar]
- 12. Choe SA, Jun YB, Lee WS, et al. . Association between ambient air pollution and pregnancy rate in women who underwent IVF. Hum Reprod. 2018;33(6):1071–1078. [DOI] [PubMed] [Google Scholar]
- 13. Legro RS, Sauer MV, Mottla GL, et al. . Effect of air quality on assisted human reproduction. Hum Reprod. 2010;25(5):1317–1324. [DOI] [PubMed] [Google Scholar]
- 14. Gaskins AJ, Chavarro JE. Diet and fertility: a review. Am J Obstet Gynecol. 2018;218(4):379–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hoffman JB, Hennig B. Protective influence of healthful nutrition on mechanisms of environmental pollutant toxicity and disease risks. Ann N Y Acad Sci. 2017;1398(1):99–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chavarro JE, Rich-Edwards JW, Rosner BA, et al. . Use of multivitamins, intake of B vitamins, and risk of ovulatory infertility. Fertil Steril. 2008;89(3):668–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cueto HT, Riis AH, Hatch EE, et al. . Folic acid supplementation and fecundability: a Danish prospective cohort study. Eur J Clin Nutr. 2016;70:66–71. [DOI] [PubMed] [Google Scholar]
- 18. Gaskins AJ, Rich-Edwards JW, Hauser R, et al. . Maternal prepregnancy folate intake and risk of spontaneous abortion and stillbirth. Obstet Gynecol. 2014;124(1):23–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Boxmeer JC, Macklon NS, Lindemans J, et al. . IVF outcomes are associated with biomarkers of the homocysteine pathway in monofollicular fluid. Hum Reprod. 2009;24(5):1059–1066. [DOI] [PubMed] [Google Scholar]
- 20. Szymański W, Kazdepka-Ziemińska A. [Effect of homocysteine concentration in follicular fluid on a degree of oocyte maturity]. Ginekol Pol. 2003;74(10):1392–1396. [PubMed] [Google Scholar]
- 21. Gaskins AJ, Afeiche MC, Wright DL, et al. . Dietary folate and reproductive success among women undergoing assisted reproduction. Obstet Gynecol. 2014;124(4):801–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mínguez-Alarcón L, Gaskins AJ, Chiu YH, et al. . Dietary folate intake and modification of the association of urinary bisphenol A concentrations with in vitro fertilization outcomes among women from a fertility clinic. Reprod Toxicol. 2016;65:104–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Philips EM, Kahn LG, Jaddoe VWV, et al. . First trimester urinary bisphenol and phthalate concentrations and time to pregnancy: a population-based cohort analysis. J Clin Endocrinol Metab. 2018;103(9):3540–3547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ouyang F, Longnecker MP, Venners SA, et al. . Preconception serum 1,1,1-trichloro-2,2,bis(p-chlorophenyl)ethane and B-vitamin status: independent and joint effects on women’s reproductive outcomes. Am J Clin Nutr. 2014;100(6):1470–1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Schmidt RJ, Kogan V, Shelton JF, et al. . Combined prenatal pesticide exposure and folic acid intake in relation to autism spectrum disorder. Environ Health Perspect. 2017;125(9):097007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gamble MV, Liu X, Ahsan H, et al. . Folate and arsenic metabolism: a double-blind, placebo-controlled folic acid-supplementation trial in Bangladesh. Am J Clin Nutr. 2006;84(5):1093–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Stingone JA, Luben TJ, Carmichael SL, et al. . Maternal exposure to nitrogen dioxide, intake of methyl nutrients, and congenital heart defects in offspring. Am J Epidemiol. 2017;186(6):719–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Goodrich AJ, Volk HE, Tancredi DJ, et al. . Joint effects of prenatal air pollutant exposure and maternal folic acid supplementation on risk of autism spectrum disorder. Autism Res. 2018;11(1):69–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Messerschmidt DM, Knowles BB, Solter D. DNA methylation dynamics during epigenetic reprogramming in the germline and preimplantation embryos. Genes Dev. 2014;28(8):812–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Alfano R, Herceg Z, Nawrot TS, et al. . The impact of air pollution on our epigenome: how far is the evidence? (A systematic review). Curr Environ Health Rep. 2018;5(4):544–578. [DOI] [PubMed] [Google Scholar]
- 31. Baccarelli A, Wright RO, Bollati V, et al. . Rapid DNA methylation changes after exposure to traffic particles. Am J Respir Crit Care Med. 2009;179(7):572–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lodovici M, Bigagli E. Oxidative stress and air pollution exposure. J Toxicol. 2011;2011:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Joshi R, Adhikari S, Patro BS, et al. . Free radical scavenging behavior of folic acid: evidence for possible antioxidant activity. Free Radic Biol Med. 2001;30(12):1390–1399. [DOI] [PubMed] [Google Scholar]
- 34.Gaskins AJ, Fong KC, Abu Awad Y, et al. Time-varying exposure to air pollution and outcomes of in vitro fertilization among couples from a fertility clinic. Environ Health Perspect. 2019;127(7):77002. [DOI] [PMC free article] [PubMed]
- 35. Lee HJ, Koutrakis P. Daily ambient NO2 concentration predictions using satellite ozone monitoring instrument NO2 data and land use regression. Environ Sci Technol. 2014;48(4):2305–2311. [DOI] [PubMed] [Google Scholar]
- 36. Kloog I, Chudnovsky AA, Just AC, et al. . A new hybrid spatio-temporal model for estimating daily multi-year PM2.5 concentrations across Northeastern USA using high resolution aerosol optical depth data. Atmos Environ. 2014;95:581–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Di Q, Rowland S, Koutrakis P, et al. . A hybrid model for spatially and temporally resolved ozone exposures in the continental United States. J Air Waste Manag Assoc. 2017;67(1):39–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Abu Awad Y, Koutrakis P, Coull BA, et al. . A spatio-temporal prediction model based on support vector machine regression: ambient black carbon in three New England States. Environ Res. 2017;159:427–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Broekmans FJ, de Ziegler D, Howles CM, et al. . The antral follicle count: practical recommendations for better standardization. Fertil Steril. 2010;94(3):1044–1051. [DOI] [PubMed] [Google Scholar]
- 40. Yuan C, Spiegelman D, Rimm EB, et al. . Validity of a dietary questionnaire assessed by comparison with multiple weighed dietary records or 24-hour recalls. Am J Epidemiol. 2017;185(7):570–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. US Department of Agriculture, Agricultural Research Service USDA National Nutrient Database for Standard Reference, Release 25. 2012. http://www.ars.usda.gov/ba/bhnrc/ndl. Accessed July 26, 2018.
- 42. Rimm EB, Giovannucci EL, Stampfer MJ, et al. . Reproducibility and validity of an expanded self-administered semiquantitative food frequency questionnaire among male health professionals. Am J Epidemiol. 1992;135(10):1114–1126; discussion 1127–1136. [DOI] [PubMed] [Google Scholar]
- 43. Giovannucci E, Stampfer MJ, Colditz GA, et al. . Multivitamin use, folate, and colon cancer in women in the Nurses’ Health Study. Ann Intern Med. 1998;129(7):517–524. [DOI] [PubMed] [Google Scholar]
- 44. Yuan C, Spiegelman D, Rimm EB, et al. . Relative validity of nutrient intakes assessed by questionnaire, 24-hour recalls, and diet records as compared with urinary recovery and plasma concentration biomarkers: findings for women. Am J Epidemiol. 2018;187(5):1051–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Chiuve SE, Fung TT, Rimm EB, et al. . Alternative dietary indices both strongly predict risk of chronic disease. J Nutr. 2012;142(6):1009–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Fitzmaurice GM, Laird NM, Ware JH. Chapter 14: missing data and dropout Applied Longitudinal Analysis. Hoboken, NJ: John Wiley and Sons, Inc.; 2004. [Google Scholar]
- 47. Searle SR, Speed FM, Milliken GA. Population marginal means in the linear model: an alternative to least square means. Am Stat. 1980;34(4):216–221. [Google Scholar]
- 48. Hosmer DW, Lemeshow S. Confidence interval estimation of interaction. Epidemiology. 1992;3(5):452–456. [DOI] [PubMed] [Google Scholar]
- 49. Weng HY, Hsueh YH, Messam LL, et al. . Methods of covariate selection: directed acyclic graphs and the change-in-estimate procedure. Am J Epidemiol. 2009;169(10):1182–1190. [DOI] [PubMed] [Google Scholar]
- 50. Bellavia A, Urch B, Speck M, et al. . DNA hypomethylation, ambient particulate matter, and increased blood pressure: findings from controlled human exposure experiments. J Am Heart Assoc. 2013;2(3):e000212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Plusquin M, Guida F, Polidoro S, et al. . DNA methylation and exposure to ambient air pollution in two prospective cohorts. Environ Int. 2017;108:127–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Cai J, Zhao Y, Liu P, et al. . Exposure to particulate air pollution during early pregnancy is associated with placental DNA methylation. Sci Total Environ. 2017;607–608:1103–1108. [DOI] [PubMed] [Google Scholar]
- 53. Crider KS, Yang TP, Berry RJ, et al. . Folate and DNA methylation: a review of molecular mechanisms and the evidence for folate’s role. Adv Nutr. 2012;3(1):21–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Zhong J, Karlsson O, Wang G, et al. . B vitamins attenuate the epigenetic effects of ambient fine particles in a pilot human intervention trial. Proc Natl Acad Sci U S A. 2017;114(13):3503–3508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Agarwal A, Aponte-Mellado A, Premkumar BJ, et al. . The effects of oxidative stress on female reproduction: a review. Reprod Biol Endocrinol. 2012;10:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. McNulty H, Pentieva K. Folate bioavailability. Proc Nutr Soc. 2004;63(4):529–536. [DOI] [PubMed] [Google Scholar]
- 57. Tort J, Lelong N, Prunet C, et al. . Maternal and health care determinants of preconceptional use of folic acid supplementation in France: results from the 2010 National Perinatal Survey. BJOG. 2013;120(13):1661–1667. [DOI] [PubMed] [Google Scholar]
- 58. Nilsen RM, Leoncini E, Gastaldi P, et al. . Prevalence and determinants of preconception folic acid use: an Italian multicenter survey. Ital J Pediatr. 2016;42:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Nilsen RM, Vollset SE, Gjessing HK, et al. . Patterns and predictors of folic acid supplement use among pregnant women: the Norwegian Mother and Child Cohort Study. Am J Clin Nutr. 2006;84(5):1134–1141. [DOI] [PubMed] [Google Scholar]
- 60. Khodr ZG, Lupo PJ, Agopian AJ, et al. . Preconceptional folic acid-containing supplement use in the National Birth Defects Prevention Study. Birth Defects Res A Clin Mol Teratol. 2014;100(6):472–482. [DOI] [PubMed] [Google Scholar]
- 61. Palmsten K, Flores KF, Chambers CD, et al. . Most frequently reported prescription medications and supplements in couples planning pregnancy: the LIFE Study. Reprod Sci. 2018;25(1):94–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Monn C. Exposure assessment of air pollutants: a review on spatial heterogeneity and indoor/outdoor/personal exposure to suspended particulate matter, nitrogen dioxide and ozone. Atmos Environ. 2001;35(1):1–32. [Google Scholar]
- 63. Jacques PF, Sulsky SI, Sadowski JA, et al. . Comparison of micronutrient intake measured by a dietary questionnaire and biochemical indicators of micronutrient status. Am J Clin Nutr. 1993;57(2):182–189. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.