Abstract
STUDY QUESTION
What is the association of female and male partner marijuana smoking with infertility treatment outcomes with ART?
SUMMARY ANSWER
Women who were marijuana smokers at enrollment had a significantly higher adjusted probability of pregnancy loss during infertility treatment with ART whereas, unexpectedly, there was a suggestion of more favorable treatment outcomes in couples where the man was a marijuana smoker at enrollment.
WHAT IS KNOWN ALREADY
Data on the relation of female and male partner marijuana use with outcomes of infertility treatment is scarce despite increased use and legalization worldwide.
STUDY DESIGN, SIZE, DURATION
We followed 421 women who underwent 730 ART cycles while participating in a prospective cohort (the Environment and Reproductive Health Study) at a fertility center between 2004 and 2017. Among them, 200 women (368 cycles) were part of a couple in which their male partner also enrolled in the study.
PARTICIPANTS/MATERIALS, SETTING, METHODS
Participants self-reported marijuana smoking at baseline. Clinical endpoints were abstracted from electronic medical records. We used generalized linear mixed models with empirical standard errors to evaluate the association of baseline marijuana smoking with ART outcomes adjusting for participants’ age, race, BMI, tobacco smoking, coffee and alcohol consumption, and cocaine use. We estimated the adjusted probability of implantation, clinical pregnancy, and live birth per ART cycle, as well as the probability of pregnancy loss among those with a positive B-hCG.
MAIN RESULTS AND THE ROLE OF CHANCE
The 44% of the women and 61% of the men had ever smoked marijuana; 3% and 12% were marijuana smokers at enrollment, respectively. Among 317 women (395 cycles) with a positive B-hCG, those who were marijuana smokers at enrollment (N = 9, cycles = 16) had more than double the adjusted probability of pregnancy loss than those who were past marijuana smokers or had never smoked marijuana (N = 308, 379 cycles) (54% vs 26%; P = 0.0003). This estimate was based on sparse data. However, couples in which the male partner was a marijuana smoker at enrollment (N = 23, 41 cycles) had a significantly higher adjusted probability of live birth than couples in which the male partner was a past marijuana smoker or had never smoked marijuana (N= 177, 327 cycles) (48% vs 29%; P = 0.04), independently of the women’s marijuana smoking status. Treatment outcomes of past marijuana smokers, male and female, did not differ significantly from those who had never smoked marijuana.
LIMITATIONS, REASONS FOR CAUTION
Marijuana smoking was self-reported with possible exposure misclassification. Chance findings cannot be excluded due to the small number of exposed cases. The results may not be generalizable to couples from the general population.
WIDER IMPLICATIONS OF THE FINDINGS
Even though marijuana smoking has not been found in past studies to impact the ability to become pregnant among pregnancy planners in the general population, it may increase the risk of pregnancy loss among couples undergoing infertility treatment. Marijuana smoking by females and males may have opposing effects on outcomes of infertility treatment with ART.
STUDY FUNDING/COMPETING INTEREST(S)
The project was financed by grants R01ES009718, P30ES000002, and K99ES026648 from the National Institute of Environmental Health Sciences (NIEHS). None of the authors has any conflicts of interest to declare.
Keywords: assisted reproductive technologies, couple, infertility, pregnancy loss, live birth, marijuana
Introduction
One in six couples trying to conceive experience infertility (Louis et al., 2013; Thoma, et al., 2013) and many seek treatment with ART. In the last decade, multiple studies linked fertility to environmental factors and lifestyle choices including exposure to environmental chemicals (Dodge et al., 2015; Minguez-Alarcon et al., 2016), air pollution (Gaskins et al., 2018; Nassan et al., 2018a), diet (Gaskins and Chavarro, 2018; Nassan et al., 2018b), tobacco smoking (Budani et al., 2018), and drug use (Joesoef et al., 1990; Joesoef et al., 1993; du Plessis et al., 2015; Samplaski et al., 2015).
Marijuana is the most widely used illicit drug in the world (UNODC, 2017). In 2016, more than 24 million Americans reported using marijuana (SAMHSA, 2016). Prevalent marijuana use by women and men of reproductive age is of concern given data scarcity on its potential reproductive effects. To date, only three (Klonoff-Cohen et al., 2006; Kasman et al., 2018; Wise et al., 2018) studies have evaluated the relation of marijuana smoking in both partners on fertility. Two studies among pregnancy planners found no evidence that either partner’s marijuana use was related to time to pregnancy (Kasman et al., 2018; Wise et al., 2018). Another study (Klonoff-Cohen et al., 2006) among couples undergoing ART, reported that marijuana smoking from both partners was related to lower oocyte yield and fertilization rate but unrelated to pregnancy or live birth rates. These last findings, however, may not be applicable to current practice as more than half of participants underwent gamete or zygote intrafallopian transfer (GIFT/ZIFT) cycles, which currently represent <1% of ART (CDC, 2015). The dearth of literature on the effects on reproduction has been acknowledged by the American College of Obstetricians and Gynecologists (ACOG) (ACOG, 2017). To further study this question using more recent data, we evaluated the relation of marijuana smoking with outcomes of infertility treatment among couples attending the Massachusetts General Hospital (MGH) fertility center. We hypothesized that marijuana smoking in couples would be unrelated to outcomes of infertility treatment.
Materials and Methods
Study design
The Environment and Reproductive Health (EARTH) Study is an ongoing prospective cohort started in 2004 aimed at identifying environmental and lifestyle determinants to fertility among couples presenting to the MGH fertility center, in Boston, Massachusetts (Messerlian et al., 2018). Couples who met the eligibility criteria (18–45 years for women; 18–55 years without vasectomy for men) were invited to participate. Approximately 65% of women and 45% of men approached by study staff enrolled in the study (Messerlian et al., 2018). Joint participation as a couple is encouraged but not required. Of the 850 women who enrolled between 2004 and 2017, 476 women had completed at least one treatment cycle with ART as of December 2017. Of those, 421 (88%) women had answered recreational drug use questions at study enrollment and subsequently underwent 730 ART cycles. There were no statistically significant differences in age, race, education, smoking status, and ART protocol compared between participants who provided data on recreational drug use and those who did not (data not shown). Among these women, 200 (48%) had a non-azoospermic male partner who also enrolled in the study and answered drug use questions. Those 200 couples completed 368 ART cycles between 2005 and 2017 (Supplementary Fig. S1). The Institutional Review Boards of the Harvard T.H. Chan School of Public Health and MGH approved the study. Every participant provided written informed consent.
Assessment of marijuana smoking and covariates
Participants self-reported marijuana smoking at enrollment. Ever marijuana smokers (>2 joints/cigarettes or equivalent amount of marijuana in their lifetime) were also asked to report the average number of joints/cigarettes they smoked per week, age at which they started to smoke marijuana, if they ever quit, last time they smoked marijuana, and the lifetime duration of marijuana smoking. The questionnaire had parallel questions about cocaine use. Participants also self-reported demographic information, data on other lifestyle factors, and medical history.
Assessment of ART outcomes
Trained study staff abstracted the clinical information from the participants’ electronic medical records and measured their height and weight at baseline to calculate BMI. Details of participants’ clinical management are described elsewhere (Chavarro et al., 2012). Briefly, women underwent a cycle of oral contraceptives for 2–5 weeks to suppress ovulation before their ART cycles, unless contraindicated. On Day 3 of induced menses, women began controlled ovarian stimulation using one of three protocols as clinically indicated: (i) luteal-phase GnRH agonist, (ii) follicular-phase GnRH-agonist/flare, or (iii) follicular-phase GnRH-antagonist. Clinical staff monitored women during stimulation for serum estradiol (E2), follicle size and counts, and endometrial thickness until 2 days before oocyte retrieval. hCG was administered 35 h before the scheduled oocyte retrieval procedure to induce ovulation. Embryologists classified oocytes as germinal vesicle, metaphase I, metaphase II (MII), or degenerated. Following retrieval, oocytes underwent conventional insemination or ICSI for fertilization, as clinically indicated. Embryologists determined fertilization proportions 17–20 h after insemination as the number of oocytes with two pro-nuclei divided by the number of mature (MII) oocytes inseminated or injected. Women undergoing cryopreservation-thaw or oocyte donor cycles underwent endometrial preparation prior to transfer. Following embryo transfer, clinical staff assessed clinical outcomes (i.e. implantation, clinical pregnancy, and live birth) identically for fresh, cryo-thaw, and donor-egg recipient cycles. We defined implantation as a serum B-hCG concentration >6m IU/ml, measured ~17 days after oocyte retrieval, clinical pregnancy as the presence of intrauterine gestational sac(s) on transvaginal ultrasonography at 6 weeks gestation, and live birth as the birth of a neonate on or after 24 weeks of gestation. The denominator for the clinical outcomes was the total number of initiated ART cycles. We defined pregnancy loss as positive B-hCG test without a live birth.
Statistical analysis
Participants were categorized according to their baseline marijuana smoking in three different ways: never versus ever marijuana smokers; never, past, or current (at enrollment); and non-current versus current marijuana smokers. We used the Chi-square test and Fisher exact test when appropriate for discrete variables and Kruskal–Wallis test for continuous variables to assess differences in demographic and lifestyle characteristics across marijuana smoking categories. We used multivariable generalized linear mixed models to evaluate the associations between baseline marijuana smoking and ART outcomes, with random intercepts to account for multiple treatment cycles in the same women and empirical (robust) standard errors. We used binomial distribution with logit link function for clinical outcomes (implantation, clinical pregnancy, live birth, and pregnancy loss) and fertilization proportion, normal distribution with identity link for E2 and endometrial thickness, and Poisson distribution and log link function for total and mature oocyte yields. We accounted for the over-dispersion in the Poisson models by including a scale parameter. We presented the results as population marginal means adjusted for the covariates at their average levels for continuous variables and weighted average level of categorical variable in the model (Searle et al., 1980). We considered the covariates in the model based on the literature and based on the differences between groups in the baseline characteristics. We fitted age-adjusted and multivariable models that included age (years, continuous), BMI (kg/m2, continuous), race (Caucasian or not), tobacco smoking history (ever vs never), coffee intake (≥5 cup/week vs not), alcohol intake (≥1 day/week), and cocaine use (ever vs never).
We first examined the association between women’s marijuana smoking and the clinical outcomes not accounting for men’s marijuana smoking or men’s covariates among the full cohort of 421 eligible women. Then we considered both partners’ marijuana smoking among the 200 enrolled couples by co-adjusting for each partner’s marijuana smoking and covariates (as above) of both partners. We further explored the intensity of marijuana smoking, measured in joint-years among all participants and age of start of marijuana smoking among ever marijuana smokers. We calculated joint-years by multiplying the average number of joints of marijuana smoked per day by the number of years the person had smoked. Finally, we cross-classified the couples according to their joint-marijuana smoking as a couple, accounting for both partner’s covariates.
We conducted several sensitivity analyses. Specifically, we restricted analyses to the first ART cycle per couple to account for the variable number of cycles per couple, conducted analyses with further adjustment for stimulation protocol, sexually transmitted diseases, education history, and without using the empirical standard errors. All analyses were conducted using the SAS 9.4 (SAS Institute Inc., Cary NC, USA).
Results
Analyses of women’s marijuana smoking were based on 421 women who underwent 730 ART cycles (average = 1.7; range: 1–7 cycles/woman). Analyses of men’s marijuana smoking and couple co-exposure were based on a subset of 200 couples who underwent 368 ART cycles (average = 1.8; range of 1–7 cycles/couple) (Supplementary Fig. S1). Participants were mostly Caucasian, had college degrees or higher, and had never smoked tobacco (Table I). Mean (SD) age and BMI of women and men was 35.4 (4.0) and 36.6 (5.0) years, and 24.2 (4.3) and 27.2 (4.6) kg/m2, respectively. Overall, 44% of the women and 61% of the men had ever smoked marijuana, including 12 (3%) women (25 cycles) and 23 (12%) men (41 cycles) who were marijuana smokers at enrollment. Marijuana smoking was positively correlated within couples; 65 couples (33%) had both partners and 60 couples (30%) had neither partners ever smoked marijuana. Marijuana smokers were also more likely to be tobacco smokers and to consume more alcohol and coffee. All but two participants (one woman and one man) who used cocaine had also smoked marijuana (Table I). Women who enrolled as part of a couple had similar characteristics to women who joined alone (Supplementary Table SI).
Table I.
Demographic and cycle characteristics by marijuana smoking status among women and men participating in the EARTH study.
| Marijuana smoking in women (N = 421) | Marijuana smoking in men (N = 200) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Baseline characteristics | All women | Never | Past | At enrollment | P-value1 | All men | Never | Past | At enrollment | P-value1 |
| Participants, N (%) | 421 | 238 (57) | 171 (41) | 12 (3) | 200 | 80 (40) | 97 (49) | 23 (12) | ||
| Cycles, n (%) | 730 | 405 (55) | 300 (41) | 25 (3) | 368 | 152 (41) | 175 (48) | 41 (11) | ||
| Age, years | 35.4 (4.0) | 35.6 (4.1) | 35.1 (3.8) | 36.8 (1.9) | 0.12 | 36.6 (5.0) | 37.1 (5.2) | 36.5 (4.9) | 34.9 (4.2) | 0.21 |
| Caucasian, N (%) | 350 (83) | 185 (78) | 154 (90) | 11 (92) | 0.003 | 180 (90) | 68 (85) | 93 (96) | 19 (83) | 0.01 |
| BMI, kg/m2 | 24.2 (4.3) | 24.3 (4.6) | 23.9 (3.8) | 25.9 (5.0) | 0.38 | 27.2 (4.6) | 26.8 (4.0) | 27.4 (5.4) | 27.7 (3.2) | 0.34 |
| Tobacco smoking, N (%) | <0.0001 | <0.0001 | ||||||||
| Never | 305 (72) | 201 (84) | 98 (57) | 6 (50) | 133 (67) | 65 (81) | 58 (60) | 10 (43) | ||
| Former | 106 (25) | 35 (15) | 68 (40) | 3 (25) | 56 (28) | 12 (15) | 36 (37) | 8 (35) | ||
| At enrollment | 10 (2) | 2 (1) | 5 (3) | 3 (25) | 11 (6) | 3 (4) | 3 (3) | 5 (22) | ||
| College degree or higher, N (%) | 392 (93) | 221 (93) | 163 (95) | 8 (67) | 0.006 | 168 (84) | 69 (86) | 82 (85) | 17 (74) | 0.36 |
| Cocaine use, N (%) | 47 (11) | 1 (0.4) | 42 (25) | 4 (33) | <0.0001 | 39 (20) | 1 (1) | 28 (29) | 10 (43) | <0.0001 |
| Coffee intake ≥5 cup/week, N (%) | 198 (47) | 97 (41) | 94 (55) | 7 (58) | 0.01 | 139 (70) | 52 (65) | 72 (74) | 15 (65) | 0.37 |
| Alcohol intake ≥1 day/week, N (%) | 242 (57) | 112 (47) | 119 (70) | 11 (92) | <0.0001 | 145 (73) | 45 (56) | 78 (80) | 22 (96) | <0.0001 |
| Moderate to vigorous physical activity, hours/week2 | 4.0 (6.0) | 3.6 (4.0) | 4.5 (8.2) | 4.0 (3.4) | 0.55 | 6.2 (8.0) | 7.0 (9.6) | 4.9 (4.8) | 8.4 (11.1) | 0.58 |
| History of Sexually transmitted diseases, N (%)3 | 135 (32) | 64 (27) | 66 (39) | 5 (42) | 0.03 | 17 (9) | 6 (8) | 7 (7) | 4 (17) | 0.28 |
| Initial infertility diagnosis/ couple, N (%) | ||||||||||
| Male factor4 | 128 (30) | 72 (30) | 55 (32) | 1 (8) | 0.26 | 65 (33) | 29 (36) | 27 (28) | 9 (39) | 0.42 |
| Female factor5 | 127 (30) | 76 (32) | 45 (26) | 6 (50) | 59 (30) | 24 (30) | 27 (28) | 8 (35) | ||
| Unexplained | 166 (39) | 90 (38) | 71 (42) | 5 (42) | 76 (38) | 27 (34) | 43 (44) | 6 (26) | ||
| Stimulation protocol, N (%) | 0.99 | 0.34 | ||||||||
| Luteal phase agonist6 | 292 (69) | 163 (68) | 120 (70) | 9 (75) | 145 (73) | 64 (80) | 64 (66) | 17 (74) | ||
| Flare7 | 51 (12) | 29 (12) | 21 (12) | 1 (8) | 20 (10) | 8 (10) | 10 (10) | 2 (9) | ||
| Antagonist | 48 (11) | 28 (12) | 19 (11) | 1 (8) | 21 (11) | 4 (5) | 14 (14) | 3 (13) | ||
| Egg donor or cryo-thaw cycle | 30 (7) | 18 (8) | 11 (6) | 1 (8) | 14 (7) | 4 (5) | 9 (9) | 1 (4) | ||
| Day 3 FSH concentrations, mIU/l | 7.4 (2.8) | 7.4 (2.4) | 7.6 (3.2) | 6.6 (1.4) | 0.38 | 7.2 (2.5) | 6.9 (2.1) | 7.2 (2.8) | 7.7 (2.5) | 0.38 |
| Average marijuana joints smoked/week8 | 0 | 0.9 (1.1) | 0.7 (0.3) | 0.65 | 0 | 1.3 (1.8) | 1.9 (2.4) | 0.09 | ||
| Duration of marijuana-smoking, years8 | 0 | 10.5 (5.6) | 15.9 (.)9 | 0.30 | 0 | 5.8 (6.3) | 13.2 (5.3) | 0.009 | ||
| Marijuana joint-year8 | 0 | 1.3 (1.5) | 1.1 (.)9 | 0.55 | 0 | 1.6 (3.7) | 6.8 (8.8) | 0.009 | ||
| Age of marijuana-smoking start, years8 | NA | 17.8 (3.7) | 23.3 (7.3) | 0.02 | NA | 17.7 (2.8) | 17.5 (4.1) | 0.48 | ||
| Partner’s age, years10 | 36.5 (5.0) | 36.8 (5.5) | 36.2 (4.3) | 36.9 (2.5) | 0.92 | 34.9 (3.8) | 34.5 (3.8) | 35.5 (3.7) | 34.2 (3.7) | 0.13 |
| Partner’s marijuana smoking at enrollment, N (%)10 | 23 (12) | 12 (10) | 9 (11) | 2 (50) | 0.0001 | 4 (2) | 0 | 2 (2) | 2 (9) | <0.0001 |
N (%) is presented for categorical/binary variables and mean (SD) is presented for continuous variables.
1From Chi-square (or Fisher’s exact test when appropriate) for discrete variables and Kruskal–Wallis for continuous variables.
2Includes weight and aerobic exercise and sports.
3Syphilis, gonorrhea, mycoplasma/ureaplasma, chlamydia, trichomonas, herpes, human papilloma virus, lymphogranuloma, group-B strep, or other STDs.
4Male factor was defined as having one (or more) of the semen analysis parameters below the lower reference limits of the WHO on two different semen samples collected at least 4 weeks apart.
5Female factor was defined as diminished ovarian reserve (DOR), endometriosis, ovulatory, or tubal factors as defined by the Society of Assisted Reproductive Technology (SART) diagnosis of infertility (SART, 2017).
6Luteal-phase GnRH-agonist protocol.
7Follicular-phase GnRH-agonist/flare protocol.
8The numbers presented for the entire cohort are restricted to ever marijuana smokers and comparing past versus marijuana smokers at enrollment.
9Only one woman was in this category with non-missing information; therefore, SD could not be estimated.
10For women, those values only represent women who were part of participating couples. Abbreviations: EARTH, the Environment and Reproductive Health Study; ART; BMI; FSH, follicle stimulating hormone; STDs, sexually transmitted diseases; mins, minutes.
There were no statistically significant differences in the adjusted probabilities of implantation, clinical pregnancy, or live birth according to women’s baseline marijuana smoking status (Table II). However, and despite the small number of women who were marijuana smokers at enrollment and had a positive B-hCG (9 women, 16 cycles), marijuana smokers at enrollment had more than double the adjusted proportion of pregnancy loss than women who were past or never marijuana smokers (54% vs 26%; P = 0.0003, Fig. 1). Results were similar after adjustment for joint-years of marijuana smoking. Neither joint-years of marijuana smoking nor age at start of marijuana smoking were statistically significantly related to treatment outcomes (Supplementary Table SII). Results were also similar in the subgroup of women who enrolled as a couple after additional adjustment for male partner’s marijuana smoking status and covariates (Supplementary Table SIII).
Table II.
Marginal covariate-adjusted probabilities of clinical ART outcomes associated with women’s marijuana smoking status in the EARTH study (N = 421 women, 730 cycles).
| Implantation | Clinical pregnancy | Live birth | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total women (N) | Events, n/total cycles, n | Implantation, per 100 cycles initiated | Events, n/total cycles, n | Clinical pregnancy, per 100 cycles initiated | Events, n/total cycles, n | Live birth, per 100 cycles initiated | ||||
| Women’s marijuana smoking | Age adjusted1 | MV adjusted2 | Age adjusted1 | MV adjusted2 | Age adjusted1 | MV adjusted2 | ||||
| Never | 238 | 222/405 | 55.3 (50.2, 60.3) | 54.1 (48.6, 59.4) | 197/405 | 48.7 (43.9, 53.7) | 48.1 (43.0, 53.3) | 156/405 | 38.6 (33.9, 43.6) | 38.3 (33.4, 43.5) |
| Ever | 183 | 173/325 | 53.5 (47.8, 59.0) | 55.1 (48.9, 61.1) | 149/325 | 45.4 (40.1, 50.8) | 46.2 (40.6, 52.0) | 129/325 | 39.4 (34.1, 45.0) | 39.5 (33.7, 45.6) |
| Never | 238 | 222/405 | 55.3 (50.1, 60.3) | 54.0 (48.6, 59.3) | 197/405 | 48.7 (43.9, 53.6) | 48.1 (43.0, 53.2) | 156/405 | 38.7 (34.0, 43.6) | 38.4 (33.4, 43.6) |
| Past | 171 | 157/300 | 52.4 (46.7, 58.1) | 54.0 (47.8, 60.1) | 136/300 | 44.7 (39.2, 50.4) | 45.5 (39.7, 51.5) | 122/300 | 40.3 (34.6, 46.3) | 40.3 (34.2, 46.7) |
| At enrollment | 12 | 16/25 | 65.0 (43.6, 81.7) | 67.9 (45.9, 84.1) | 13/25 | 53.4 (37.2, 68.9) | 54.7 (37.4, 71.0) | 7/25 | 29.6 (18.3, 44.1) | 30.6 (18.3, 46.5) |
| Not at enrollment | 409 | 379/705 | 54.1 (50.3, 57.8) | 54.0 (50.2, 57.7) | 333/705 | 47.1 (43.4, 50.8) | 47.0 (43.3, 50.7) | 278/705 | 39.3 (35.7, 43.1) | 39.1 (35.5, 42.9) |
| At enrollment | 12 | 16/25 | 65.0 (43.5, 81.7) | 67.9 (46.0, 84.0) | 13/25 | 53.4 (37.2, 69.0) | 55.1 (37.6, 71.5) | 7/25 | 29.6 (18.4, 44.0) | 30.3 (18.1, 46.1) |
Abbreviations: EARTH, the Environment and Reproductive Health Study; ART; MV, multivariable, n; number of cycles, N; number of women.
1Data is presented as covariate-adjusted marginal probabilities with 95% confidence intervals adjusted for women’s age.
2Data is presented as covariate-adjusted marginal probabilities with 95% confidence intervals adjusted for women’s age, BMI, race, tobacco smoking status, coffee intake, alcohol intake, and cocaine use.
All outcomes were analyzed using generalized linear mixed models with random intercepts, binary distribution, and logit link function.
The marginal covariate-adjusted probabilities were used to present the results adjusted for the covariates at their average levels for continuous variables and weighted average level of categorical variable in the model.
Figure 1.
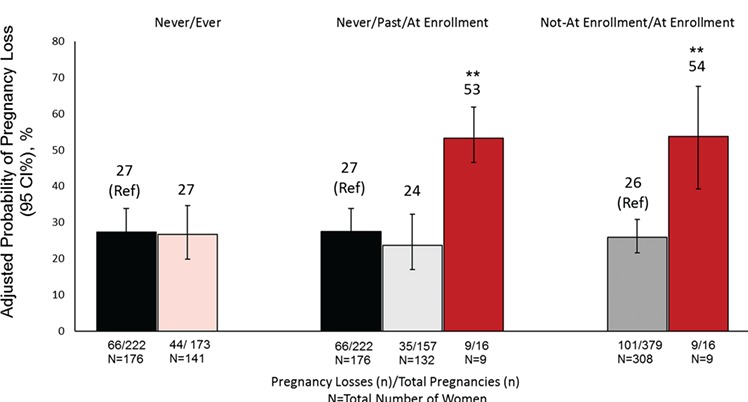
Adjusted probability (95% CI) of pregnancy loss associated with women’s marijuana smoking among 317 women who had 395 pregnancies in the EARTH study. Abbreviations: EARTH, the Environment and Reproductive Health Study; N, number of women; n, number of ART cycles. 1Defined as a positive B-hCG that did not result in live birth. 2Data is presented as predicted adjusted probabilities with 95% confidence intervals adjusted for women’s age, BMI, race, tobacco smoking status, coffee intake, alcohol intake, and cocaine use. Numbers shown below columns represent numbers of pregnancy losses/total number of pregnancies and total number of women (N) across marijuana smoking categories. Analysis was done using generalized linear mixed models with random intercepts, binary distribution and logit link function, and empirical standard error. The marginal covariate-adjusted probabilities were used to present the results adjusted for the covariates at their average levels for continuous variables and weighted average level of categorical variable in the model. **P-value < 0.005 compared to never marijuana smokers.
Couples in which the man was a marijuana smoker at enrollment had higher probabilities of implantation, clinical pregnancy, and live birth after adjusting for women’s marijuana smoking status and other potential confounders (Table III). Couples in which the male partner was a marijuana smoker at enrollment had a significantly higher probability of live birth than couples in which the male partner was a never or past marijuana smoker (48% vs 29%; P = 0.04), independently of women’s marijuana smoking status. Treatment outcomes of couples in which the man had never smoked marijuana closely mirrored those of couples in which the man was a past marijuana smoker (Table III). Intensity of marijuana smoking was statistically significantly unrelated to treatment outcomes (Supplementary Table SIV) and adjustment for intensity of use did not substantially change the results for marijuana use status.
Table III.
Marginal adjusted probabilities of clinical ART outcomes associated with men’s marijuana smoking status in the EARTH study (N = 200 couples, 368 cycles).
| Implantation | Clinical pregnancy | Live birth | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total men (N) | Events, n/total cycles, n | Implantation, per 100 cycles initiated | Events, n/total cycles, n | Clinical pregnancy, per 100 cycles initiated | Events, n/total cycles, n | Live birth, per 100 cycles initiated | ||||
| Men’s marijuana smoking | Age adjusted1 | MV adjusted2 | Age adjusted1 | MV adjusted2 | Age adjusted1 | MV adjusted2 | ||||
| Never | 80 | 81/152 | 54.2 (45.5, 62.7) | 56.3 (46.7, 65.5) | 67/152 | 43.1 (34.8, 51.8) | 44.4 (35.5, 53.7) | 57/152 | 37.2 (28.4, 46.9) | 38.7 (29.4, 49) |
| Ever | 120 | 122/216 | 57.0 (49.7, 64.1) | 56.8 (48.8, 64.5) | 110/216 | 51.7 (44.7, 58.6) | 51.3 (43.9, 58.5) | 89/216 | 42.8 (36.2, 49.6) | 41.8 (34.5, 49.5) |
| Never | 80 | 81/152 | 59.2 (39.2, 76.5) | 57.4 (37.5, 75.2) | 67/152 | 43.6 (30.1, 58.0) | 42.2 (28.9, 56.6) | 57/152 | 33.4 (22.1, 47.1) | 31.2 (20.0, 45.3) |
| Past | 97 | 92/175 | 58.3 (38.5, 75.7) | 55.4 (36.6, 72.8) | 85/175 | 49.8 (36.2, 63.5) | 47.6 (35.4, 60.0) | 67/175 | 35.8 (24.9, 48.5) | 32.9 (23.0, 44.6) |
| At enrollment | 23 | 30/41 | 75.5 (54.7, 88.7) | 77.2 (57.7, 89.3) | 25/41 | 61.0 (44.0, 75.7) | 61.6 (45.5, 75.4) | 22/41 | 52.6 (37.2, 67.6) | 52.1 (37.1, 66.8)* |
| Not at enrollment | 177 | 173/327 | 61.3 (33.8, 83.1) | 56.9 (31.0, 79.5) | 152/327 | 47.6 (30.2, 65.6) | 45.1 (30.0, 61.3) | 124/327 | 32.6 (19.8, 48.7) | 29.2 (18.0, 43.5) |
| At enrollment | 23 | 30/41 | 77.4 (51.8, 91.6) | 77.9 (53.5, 91.5)* | 25/41 | 61.5 (42.1, 77.9) | 60.1 (42.6, 75.4) | 22/41 | 50.1 (33.9, 66.4) | 47.6 (32.4, 63.3)* |
Abbreviations: EARTH, the Environment and Reproductive Health Study; ART; MV, multivariable, n; number of cycles, N; number of men.
1Data is presented as adjusted marginal probabilities with 95% confidence intervals adjusted for women’s marijuana smoking and both men’s and women’s age.
2Data is presented as adjusted marginal probabilities with 95% confidence intervals adjusted for women’s marijuana smoking, both men’s and women’s age, BMI, race, tobacco smoking status, coffee intake, alcohol intake, and cocaine use for both partners. Analysis was done using generalized linear mixed models with random intercepts, binary distribution and logit link function, and empirical standard error. The marginal adjusted probabilities were used to present the results adjusted for the covariates at their average levels for continuous variables and weighted average level of categorical variable in the model. *P < 0.05.
Finally, when couples were jointly stratified according to both partners’ marijuana smoking status, the highest adjusted probabilities of live birth were observed in couples where the woman was not a marijuana smoker at enrollment and the man was a marijuana smoker at enrollment which was significantly higher compared to couples where neither partner was a marijuana smoker at enrollment (P = 0.04) and compared to couples where both partners were marijuana smokers at enrollment (P = 0.01) (Fig. 2). However, estimates for couples with a woman who was a marijuana smoker at enrollment were based on very limited data (four couples, nine cycles).
Figure 2.
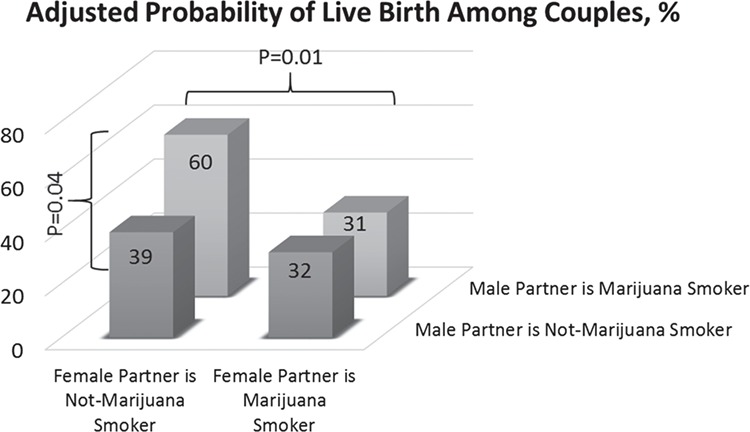
Adjusted probability of clinical ART outcomes associated with joint male and female partners’ marijuana smoking at enrollment among 200 couples (368 cycles) in the EARTH study. Abbreviations: EARTH, the Environment and Reproductive Health Study. Couples with not at enrollment marijuana smoker woman and not at enrollment marijuana smoker man were 175 couples and 324 cycles. Couples with not at enrollment marijuana smoker woman and marijuana smoker at enrollment man were 21 couples and 35 cycles. Couples with marijuana smoker woman at enrollment and not at enrollment marijuana smoker man were 2 couples and 3 cycles. Couples with marijuana smoker woman at enrollment and marijuana smoker man at enrollment were 2 couples and 6 cycles. Data is presented as covariate-adjusted marginal means adjusted for both men’s and women’s age, BMI, race, tobacco smoking status, coffee intake, alcohol intake, and cocaine use. Analysis was done using generalized linear mixed models with random intercepts, binary distribution and logit link function, and empirical standard error. The marginal covariate-adjusted probabilities were used to present the results adjusted for the covariates at their average levels for continuous variables and weighted average level of categorical variable in the model. *Indicates <0.05 compared to the couples with not at enrollment marijuana smoker woman and marijuana smoker man at enrollment.
Marijuana smoking in men or women was not significantly associated with ovarian response to stimulation or fertilization rate (data not shown). All results were consistent when we restricted analyses to the first treatment cycle for each couple and after further adjustment for treatment protocol, history of sexually transmitted diseases, education history, and without empirical standard errors (data not shown).
Discussion
In this prospective study of couples undergoing infertility treatment based at a fertility center, women’s marijuana smoking at enrollment was significantly associated with higher risk of pregnancy loss, although very few women were marijuana smokers at enrollment. On the other hand, men’s marijuana smoking at enrollment was significantly associated with higher probability of live birth independent of women’s marijuana smoking. Intensity of marijuana in men or women was not associated with these outcomes. Moreover, past and never marijuana smokers had similar success rates. While the results should be interpreted with caution given the low frequency of marijuana smoking at enrollment among women, they suggest that marijuana smoking among women may be related to worse infertility treatment outcomes. They also highlight the importance of simultaneously considering lifestyle factors of both partners when evaluating risk factors for couple-based outcomes.
We did not observe statistically significant differences in the adjusted probability of biochemical or clinical pregnancy according to women’s marijuana smoking status. This finding is in agreement with the three previous studies (Klonoff-Cohen et al., 2006; Kasman et al., 2018; Wise et al., 2018) that have addressed this question. Klonoff-Cohen et al. (2006) examined the association between marijuana use and outcomes of infertility treatment with ART among 221 couples enrolled in California between 1993 and 1997. Similar to our findings, this study (Klonoff-Cohen et al., 2006) found no significant association of women’s marijuana smoking with clinical pregnancy and live birth; however, estimates for non-statistically significant associations were not reported in this study making it difficult to make a full comparison between studies including a comparison of the magnitude of associations. Our findings are also consistent with the two studies among pregnancy planners attempting conception without medical assistance (Kasman et al., 2018; Wise et al., 2018) that reported no significant association between women’s marijuana use and time to pregnancy. However, these three studies (Klonoff-Cohen et al., 2006; Kasman et al., 2018; Wise et al., 2018) did not assess pregnancy loss. Data on marijuana use and pregnancy loss is equally scarce. A meta-analysis (Conner et al., 2016) summarizing the results of two previous studies (Wilcox et al., 1990; Kline et al., 1991) evaluating the association of maternal marijuana use and spontaneous abortion concluded that maternal use of marijuana was not associated with spontaneous abortion. It should be noted that most of the pregnancy losses in the meta-analyses were clinical losses, whereas 49 (45%) of the 110 losses in our study were losses of biochemical pregnancies. Hence, results may not be directly comparable. However, these results are supported by experimental studies. In female rodent and primate models, Delta 9 tetrahydrocannabinol (THC)—marijuana’s active component—was associated with reduced gonadotropin concentrations (by suppressing of LH pulsatile secretion) (Chakravarty et al., 1975; Besch et al., 1977; Dalterio et al., 1983). In addition, in female monkeys, administration of marijuana in early pregnancy led to pregnancy loss that was associated with a rapid decline in chorionic gonadotropin and a subsequent fall in progesterone concentrations to non-detectable levels (Asch and Smith, 1986). Furthermore, endocannabinoid disruption led to high nitric oxide (NO) production, as an inflammation and sepsis marker and a free radical, that was associated with septic abortion in female animals (Vercelli et al., 2009; Aisemberg et al., 2010). Given the increased use and legalization of marijuana in the United States and the scarcity of data regarding the reproductive effects of marijuana smoking, additional studies that include a greater proportion of marijuana smokers at enrollment are warranted.
We found that couples where the male partner was a marijuana smoker at enrollment had a higher adjusted probability of live birth. These findings stand in contrast not only to our hypothesis but to the results of the three previous studies (Klonoff-Cohen et al., 2006; Kasman et al., 2018; Wise et al., 2018) and to findings of a rodent model that also finds no effect of chronic exposure of male mice to THC on outcomes of IVF (Lopez-Cardona et al., 2018). These apparent discrepancies should be carefully examined. As previously mentioned, Klonoff-Cohen et al. (2006) did not report relationships that were not statistically significant, including the association between male partner marijuana smoking and adjusted probability of clinical pregnancy or live birth. Therefore, it is not possible to determine whether the nominal differences between the two studies are due to true differences related to study population characteristics (e.g. more frequent marijuana use and high frequency of GIFT/ZIFT cycles in the Klonoff-Cohen study), approaches to data analysis (e.g. co-adjustment of marijuana smoking status of both partners and consideration of other lifestyle factors including use of other drugs in this study but not in Klonoff-Cohen’s), or are due to differences in statistical power resulting from differences in sample size. While unexpected, positive health effects of marijuana have been reported. Of greatest relevance, we have reported that men in this study who had ever smoked marijuana had significantly higher sperm concentration (62.7 (95% CI: 56.0, 70.3) million/ml) than men who had never smoked marijuana (45.4 (95% CI: 38.6, 53.3) million/ml) after adjusting for potential confounders (P = 0.0003). There were no significant differences in sperm concentration between at enrollment (59.5 (95% CI: 47.3, 74.8) million/ml) and past marijuana smokers (63.5 (95% CI: 56.1, 72.0) million/ml; P = 0.60) (Nassan et al., 2019). Others also reported non-deleterious relations with other health outcomes. For example, marijuana use has been previously related to improved pulmonary function (Pletcher et al., 2012; Papatheodorou et al., 2016), lower fasting insulin concentrations, improved insulin resistance, smaller waist circumference, and lower diabetes prevalence (Rajavashisth et al., 2012; Penner et al., 2013). Nevertheless, given the preponderance of evidence, our findings may be better interpreted as lack of evidence for a deleterious effect rather than as evidence of a positive effect of male partner marijuana smoking on outcomes of infertility treatment. Furthermore, we have previously observed in this same study population that men’s exposure to certain environmental chemicals (Dodge et al., 2015; Carignan et al., 2018) and nutritional factors (e.g. meat intake) (Xia et al., 2015) can influence outcomes of ART (independent of their female partner); therefore, it is not implausible that marijuana smoking among men could impact these same endpoints.
Although we adjusted for tobacco smoking as an ever versus never variable, the study population consisted mostly of women who never smoked (72%) and men who never smoked (67%). Most of those who reported smoking were past smokers with only 10 (2%) women out of 421 and 11 (6%) men out of 200 men were current smokers at baseline. In addition, only 3 (25%) out of the 12 women who were marijuana smokers at enrollment were also tobacco smokers at enrollment. Similarly, for men, only 5 men (22%) out of the 23 men who were marijuana smokers at enrollment were tobacco smokers at enrollment. Of note, we have previously reported the association between tobacco smoking and outcomes of ART in this population (Vanegas et al., 2017). In our previous report, we found that female partner tobacco smoking was associated to a higher rate of failure during ART, but most of the failures were cycle cancellations prior to oocyte retrieval (e.g. cancellation due to poor response) and to a lesser extent chemical losses. Moreover, we did not observe clear relations of male partner smoking with ART outcomes. The low frequency of smoking and of concurrent use of tobacco and marijuana smoking, along with the divergent pattern of association for tobacco and marijuana smoking in the same study is further evidence that the results for marijuana smoking reported here are unlikely to be explained by residual confounding due to tobacco smoking.
The limitations of the study must be considered when interpreting the results. First, residual confounding cannot be ruled out since marijuana use may be correlated with other lifestyle factors that we did not measure, including use of other drugs and other risk-seeking behaviors. However, we controlled for many potential confounders, including both partners’ smoking, coffee and alcohol consumption, and cocaine use. Second, there may be misclassification in self-reported marijuana use especially given the legal status (illegal during most of the study), social stigma, and potential effects on care delivery in this particular group. However, self-report of marijuana is highly correlated with cannabinoid levels in blood and urine (Fried, 1980; Greenland et al., 1982). Another limitation is that few women reported being marijuana smokers at enrollment, which limited the statistical power. Therefore, we cannot exclude the possibility that our results are a chance finding. Moreover, we assessed marijuana smoking at enrollment only. However, the percentage of the women who reported marijuana smoking at baseline in our study was similar to the percentage reported previously for women who reported marijuana smoking month before, week before, and day before the IVF procedure (Klonoff-Cohen et al., 2006) and close to rates of marijuana use among pregnant women in the general population (Brown et al., 2017). This potential exposure misclassification is expected to lead to non-differential misclassification relative to study outcomes and result in attenuation of associations. In addition, we did not have information about forms of marijuana use other than smoking. Lastly, generalizability to couples trying to conceive without medical assistance may be limited as the most important findings relate to an outcome that would not normally be observed outside the setting of ART. However, early pregnancy losses, while often time are unrecognized in spontaneous conceptions, do occur and would likely be identified as prolonged time to pregnancy. Strengths of our study include its prospective design with multiple cycles per couple and complete follow-up of all treatment cycles. We also had information on both partners’ use of marijuana and related lifestyle factors that permitted simultaneously co-adjustment for a wide range of potential confounders for both partners.
In conclusion, we found that marijuana smoking at enrollment among women in couples undergoing infertility treatment was associated with a higher probability of pregnancy loss. However, marijuana smoking at enrollment in men was associated with higher live birth rates. Importantly, success rates for couples with female and male past marijuana smokers were comparable to those of never marijuana smokers. Given the scarcity of data on the reproductive effects of marijuana smoking, despite its increased use and legalization, additional research to clarify the role of marijuana use on human reproduction and on the offspring’s health is urgently needed.
Supplementary Material
Acknowledgments
The authors gratefully acknowledge the contributions of all members of the EARTH study team, specifically research nurses, Jennifer B. Ford and Myra G. Keller, senior research staff Ramace Dadd, and the physicians and staff at the MGH fertility center. A special thank you is due to all of the study participants.
Authors’ roles
All the authors of this manuscript have made substantial contributions to the conception or design of the work, or the acquisition, analysis or interpretation of data for the work, and have contributed drafting the work or revising it critically for important intellectual content, and have approved the final version to be published, and have agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Funding
National Institute of Environmental Health Sciences (NIEHS) (NIH grants: R01-ES009718, P30ES000002, K99ES026648).
Conflict of Interest
The authors report no conflict of interest.
References
- The American College of Obstetricians and Gynecologists (ACOG) Committee Opinion. Marijuana Use During Pregnancy and Lactation. 2017, (19 March 2019, date last accessed).
- Aisemberg J, Vercelli C, Wolfson M, Salazar AI, Osycka-Salut C, Billi S, Ribeiro ML, Farina M, Franchi AM. Inflammatory agents involved in septic miscarriage. Neuroimmunomodulation 2010;17:150–152. [DOI] [PubMed] [Google Scholar]
- Asch RH, Smith CG. Effects of delta 9-THC, the principal psychoactive component of marijuana, during pregnancy in the rhesus monkey. J Reprod Med 1986;31:1071–1081. [PubMed] [Google Scholar]
- Besch NF, Smith CG, Besch PK, Kaufman RH. The effect of marihuana (delta-9-tetrahydrocannabinol) on the secretion of luteinizing hormone in the ovariectomized rhesus monkey. Am J Obstet Gynecol 1977;128:635–642. [DOI] [PubMed] [Google Scholar]
- Brown QL, Sarvet AL, Shmulewitz D, Martins SS, Wall MM, Hasin DS. Trends in marijuana use among pregnant and nonpregnant reproductive-aged women, 2002–2014. JAMA 2017;317:207–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budani MC, Fensore S, Di Marzio M, Tiboni GM. Cigarette smoking impairs clinical outcomes of assisted reproductive technologies: a meta-analysis of the literature. Reprod Toxicol 2018;80:49–59. [DOI] [PubMed] [Google Scholar]
- Carignan CC, Minguez-Alarcon L, Williams PL, Meeker JD, Stapleton HM, Butt CM, Toth TL, Ford JB, Hauser R. Paternal urinary concentrations of organophosphate flame retardant metabolites, fertility measures, and pregnancy outcomes among couples undergoing in vitro fertilization. Environ Int 2018;111:232–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC. Assisted Reproductive Technology Fertility Clinic Success Rates Report Centers for Disease Control and Prevention, American Society for Reproductive Medicine In: Society for Assisted Reproductive Technology, 2015, (28 August 2018, date last accessed).
- Chakravarty I, Sheth AR, Ghosh JJ. Effect of acute Δ9-tetrahydrocannabinol treatment on serum luteinizing hormone and prolactin levels in adult female rats. Fertil Steril 1975;26:947–948. [DOI] [PubMed] [Google Scholar]
- Chavarro JE, Ehrlich S, Colaci DS, Wright DL, Toth TL, Petrozza JC, Hauser R. Body mass index and short-term weight change in relation to treatment outcomes in women undergoing assisted reproduction. Fertil Steril 2012;98:109–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conner SN, Bedell V, Lipsey K, Macones GA, Cahill AG, Tuuli MG. Maternal marijuana use and adverse neonatal outcomes: a systematic review and meta-analysis. Obstet Gynecol 2016;128:713–723. [DOI] [PubMed] [Google Scholar]
- Dalterio SL, Mayfield DL, Bartke A. Effects of delta 9-THC on plasma hormone levels in female mice. Subst Alcohol Actions Misuse 1983;4:339–345. [PubMed] [Google Scholar]
- Dodge LE, Williams PL, Williams MA, Missmer SA, Souter I, Calafat AM, Hauser R. Associations between paternal urinary phthalate metabolite concentrations and reproductive outcomes among couples seeking fertility treatment. Reprod Toxicol 2015;58:184–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plessis SS, Agarwal A, Syriac A. Marijuana, phytocannabinoids, the endocannabinoid system, and male fertility. J Assist Reprod Genet 2015;32:1575–1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried PA. Marihuana use by pregnant women: neurobehavioral effects in neonates. Drug Alcohol Depend 1980;6:415–424. [DOI] [PubMed] [Google Scholar]
- Gaskins AJ, Chavarro JE. Diet and fertility: a review. Am J Obstet Gynecol 2018;218:379–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaskins AJ, Hart JE, Minguez-Alarcon L, Chavarro JE, Laden F, Coull BA, Ford JB, Souter I, Hauser R. Residential proximity to major roadways and traffic in relation to outcomes of in vitro fertilization. Environ Int 2018;115:239–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenland S, Staisch KJ, Brown N, Gross SJ. The effects of marijuana use during pregnancy. I. A preliminary epidemiologic study. Am J Obstet Gynecol 1982;143:408–413. [DOI] [PubMed] [Google Scholar]
- Joesoef MR, Beral V, Aral SO, Rolfs RT, Cramer DW. Fertility and use of cigarettes, alcohol, marijuana, and cocaine. Ann Epidemiol 1993;3:592–594. [DOI] [PubMed] [Google Scholar]
- Joesoef MR, Beral V, Rolfs RT, Aral SO, Cramer DW. Are caffeinated beverages risk factors for delayed conception? Lancet 1990;335:136–137. [DOI] [PubMed] [Google Scholar]
- Kasman AM, Thoma ME, McLain AC, Eisenberg ML. Association between use of marijuana and time to pregnancy in men and women: findings from the National Survey of Family Growth. Fertil Steril 2018;109:866–871. [DOI] [PubMed] [Google Scholar]
- Kline J, Hutzler M, Levin B, Stein Z, Susser M, Warburton D. Marijuana and spontaneous abortion of known karyotype. Paediatr Perinat Epidemiol 1991;5:320–332. [DOI] [PubMed] [Google Scholar]
- Klonoff-Cohen HS, Natarajan L, Victoria Chen R. A prospective study of the effects of female and male marijuana use on in vitro fertilization (IVF) and gamete intrafallopian transfer (GIFT) outcomes. Am J Obstet Gynecol 2006;194:369–376. [DOI] [PubMed] [Google Scholar]
- Lopez-Cardona AP, Ibarra-Lecue I, Laguna-Barraza R, Perez-Cerezales S, Uriguen L, Agirregoitia N, Gutierrez-Adan A, Agirregoitia E. Effect of chronic THC administration in the reproductive organs of male mice, spermatozoa and in vitro fertilization. Biochem Pharmacol 2018;157:294–303. [DOI] [PubMed] [Google Scholar]
- Louis JF, Thoma ME, Sørensen DN, McLain AC, King RB, Sundaram R, Keiding N, Buck Louis GM. The prevalence of couple infertility in the United States from a male perspective: evidence from a nationally representative sample. Andrology 2013;1:741–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messerlian C, Williams PL, Ford JB, Chavarro JE, Mínguez-Alarcón L, Dadd R, Braun JM, Gaskins AJ, Meeker JD, James-Todd T et al. The environment and reproductive health (EARTH) study: a prospective preconception cohort. Hum Reprod Open 2018;2018:hoy001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minguez-Alarcon L, Hauser R, Gaskins AJ. Effects of bisphenol A on male and couple reproductive health: a review. Fertil Steril 2016;106:864–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nassan FL, Arvizu M, Minguez-Alarcon L, Williams PL, Attaman J, Petrozza J, Hauser R, Chavarro J. Marijuana smoking and markers of testicular function among men from a fertility centre. Hum Reprod 2019;34:715–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nassan FL, Chavarro JE, Minguez-Alarcon L, Williams PL, Tanrikut C, Ford JB, Dadd R, Perry MJ, Hauser R, Gaskins AJ. Residential distance to major roadways and semen quality, sperm DNA integrity, chromosomal disomy, and serum reproductive hormones among men attending a fertility clinic. Int J Hyg Environ Health 2018a;221:830–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nassan FL, Chavarro JE, Tanrikut C. Diet and men’s fertility: does diet affect sperm quality? Fertil Steril 2018b;110:570–577. [DOI] [PubMed] [Google Scholar]
- Papatheodorou SI, Buettner H, Rice MB, Mittleman MA. Recent marijuana use and associations with exhaled nitric oxide and pulmonary function in adults in the United States. Chest 2016;149:1428–1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penner EA, Buettner H, Mittleman MA. The impact of marijuana use on glucose, insulin, and insulin resistance among US adults. Am J Med 2013;126:583–589. [DOI] [PubMed] [Google Scholar]
- Pletcher MJ, Vittinghoff E, Kalhan R et al. Association between marijuana exposure and pulmonary function over 20 years. JAMA 2012;307:173–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajavashisth TB, Shaheen M, Norris KC, Pan D, Sinha SK, Ortega J, Friedman TC. Decreased prevalence of diabetes in marijuana users: cross-sectional data from the National Health and Nutrition Examination Survey (NHANES) III. BMJ Open 2012;2:e000494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration (SAMHSA) Results from the 2016 National Survey on Drug Use and Health: Detailed Tables. 2016. Center for Behavioral Health Statistics and Quality, National Survey on Drug Use and Health, (26 July 2018, date last accessed).
- Samplaski MK, Bachir BG, Lo KC, Grober ED, Lau S, Jarvi KA. Cocaine use in the infertile male population: a marker for conditions resulting in subfertility. Curr Urol 2015;8:38–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SART Preliminary Sart Clinic Summary Report: SART (Societry for Assisted Reproductive Technologies). 2017. https://www.sartcorsonline.com/rptCSR_PublicMultYear.aspx?ClinicPKID=2337
- Searle SR, Speed FM, Milliken GA. Population marginal means in the linear model: an alternative to least squares means. Am Stat 1980;34:216–221. [Google Scholar]
- Thoma ME, McLain AC, Louis JF, King RB, Trumble AC, Sundaram R, Buck Louis GM. Prevalence of infertility in the United States as estimated by the current duration approach and a traditional constructed approach. Fertil Steril 2013;99:1324–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- United Nations Office on Drugs and Crime (UNODC). World Drug Report 2017 —United Nations Office on Drugs and Crime. 2017(8 May 2018, date last accessed).
- Vanegas JC, Chavarro JE, Williams PL, Ford JB, Toth TL, Hauser R, Gaskins AJ. Discrete survival model analysis of a couple’s smoking pattern and outcomes of assisted reproduction. Fertil Res Pract 2017;3:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vercelli CA, Aisemberg J, Billi S, Wolfson ML, Franchi AM. Endocannabinoid system and nitric oxide are involved in the deleterious effects of lipopolysaccharide on murine decidua. Placenta 2009;30:579–584. [DOI] [PubMed] [Google Scholar]
- Wilcox AJ, Weinberg CR, Baird DD. Risk factors for early pregnancy loss. Epidemiology 1990;1:382–385. [DOI] [PubMed] [Google Scholar]
- Wise LA, Wesselink AK, Hatch EE, Rothman KJ, Mikkelsen EM, Sorensen HT, Mahalingaiah S. Marijuana use and fecundability in a North American preconception cohort study. J Epidemiol Community Health 2018;72:208–215. [DOI] [PubMed] [Google Scholar]
- Xia W, Chiu YH, Williams PL, Gaskins AJ, Toth TL, Tanrikut C, Hauser R, Chavarro JE. Men’s meat intake and treatment outcomes among couples undergoing assisted reproduction. Fertil Steril 2015;104:972–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


