Abstract
Experimental techniques for identification of essential genes (EGs) in prokaryotes are usually expensive, time-consuming and sometimes unrealistic. Emerging in silico methods provide alternative methods for EG prediction, but often possess limitations including heavy computational requirements and lack of biological explanation. Here we propose a new computational algorithm for EG prediction in prokaryotes with an online database (ePath) for quick access to the EG prediction results of over 4,000 prokaryotes (https://www.pubapps.vcu.edu/epath/). In ePath, gene essentiality is linked to biological functions annotated by KEGG Ortholog (KO). Two new scoring systems, namely, E_score and P_score, are proposed for each KO as the EG evaluation criteria. E_score represents appearance and essentiality of a given KO in existing experimental results of gene essentiality, while P_score denotes gene essentiality based on the principle that a gene is essential if it plays a role in genetic information processing, cell envelope maintenance or energy production. The new EG prediction algorithm shows prediction accuracy ranging from 75% to 91% based on validation from five new experimental studies on EG identification. Our overall goal with ePath is to provide a comprehensive and reliable reference for gene essentiality annotation, facilitating the study of those prokaryotes without experimentally derived gene essentiality information.
Subject terms: Genetic markers, Bacterial genes
Introduction
Essential genes (EGs) are defined as those genes that are critical for the survival of an organism1,2. Identification and prediction of EGs are therefore of great importance for understanding cellular functions3, developing drugs against emerging pathogens and antibiotic-resistant pathogens4,5, and exploring evolutionary divergence6 as well as the origin of life7.
However, experimental identification of EGs in prokaryotes is costly and time-consuming8. Thus far, sufficient information on gene essentiality is only available for limited prokaryotic strains with genome-wide experimental data9,10 and the number is slowly increasing11–13. Many prokaryotic species are uncultivable, are too dangerous to handle, or have no genetic system available, making the experimental approach for EG identification unrealistic. Furthermore, available experimental results are derived from different methods in different instances and are more reliable for model organisms such as Escherichia coli and Bacillus subtilis. Generating these outcomes for other organisms is not a simple task.
In silico EG prediction emerges as a potential alternative method, which may greatly reduce cost in terms of both time and expense14. Computational methods for EG prediction are rapidly being developed, such as those using biological features of genes15,16, flux balance analysis of metabolic networks using constraint-based modelling17 and homolog and evolutionary distance18 combined with machine learning algorithms (e.g. support vector machine and artificial neural network (ANN))18–20. However, existing computational methods for EG prediction have several limitations. Predictions using metabolic models are constrained by the availability of the models corresponding to the organism of interest21. Moreover, these predictions are only available for those genes involved in metabolic pathways, whereas other genes such as those involved in genetic information processing and some cell envelope maintenance genes are excluded. In addition, computational methods using machine learning algorithms for EG prediction require existing gene essentiality information derived from laboratory experiments18 and extensive computational resources. Although they may show relatively high predictive power within their training sets, the general application of these tools remains largely uncertain outside their data domain. Moreover, these purely data-driven methods tend to establish quantitatively algorithms for EG prediction as a ‘black box’ (such as ANN), so that biological explanations underlying these methods are unclear. Overall, new methods for EG prediction with sound biological mechanisms and fast procedures, as well as databases for easy access to the prediction results, are highly desired for both genetic research and biological application.
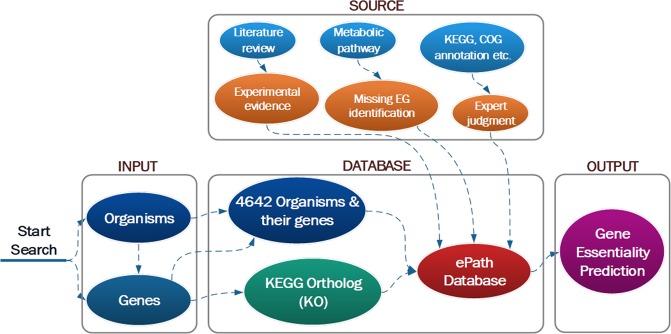
We report here the development of the ePath database (Fig. 1) for EG annotation and prediction in prokaryotic genomes, covering the complete genomes of over 4,000 prokaryotic strains available in NCBI. We have proposed two criteria for EG prediction:
Genes serving the same molecular function but without any paralogs (isozyme or alternative pathway) should be consistently considered as either essential or non-essential.
A non-essential gene should be categorized as ‘genes playing essential functions but with paralogs (isozyme or alternative pathway) in the corresponding genome’, if there are EGs linked to both nodes of the corresponding edge in a KEGG pathway.
Figure 1.
Conceptual diagram of the ePath online database and search engine.
Accordingly, in ePath, the essentiality of genes is annotated based on two pieces of information. The first piece is the gene function annotation obtained from various databases. For a single gene, we retrieve the corresponding KEGG Ortholog (KO), and link the KO to a group of annotations including KEGG KO annotation, KEGG pathway/Module/Reaction annotation, Gene Ontology (GO), and Clusters of Orthologous Groups (COGs). We subsequently score the essentiality of this gene (‘P_score’ hereafter) based on the principle that a gene should be essential if it performs one of the following functions: genetic information processing, cell envelope maintenance or energy production8. The second piece is gene essentiality based on data provided from existing genome-wide experimental results. We have collected data from 31 strains listed in Database of Essential Genes (DEG)9 and linked all the experimental EGs to KO when possible, and summarized the essentiality frequency of these KOs in the 31 strains. Furthermore, we identify all the genes in the 31 strains if their projections on the KEGG metabolic pathway map (ko01100) have experimentally-verified EG neighbors on both sides of the edges, and consider these genes as ‘gap’ EGs that are missing in experiments (the ‘remapping’ algorithm). This is based on our previous finding using single gene-knockout technology that genes playing essential functions become non-essential when isozymes (paralogs or alternative pathway) exist in the genome8. For each specific gene, we thus develop an essentiality scoring criteria based on the essentiality of its orthologue in the 31 strains (E_score hereafter). Finally, the essentiality of this gene is annotated based on the E_score and P_score. The predictions are subsequently validated by five recent experimental studies that are not included in our training dataset. Overall, with the ePath database, we aim to provide a comprehensive and reliable reference for gene essentiality annotation with an easily accessible online database and searching tool, in order to facilitate studies for organisms lacking gene essentiality information. The ePath database is freely available at: https://www.pubapps.vcu.edu/epath/.
Results
Comparison of predicted and experimental EGs and the missing EGs
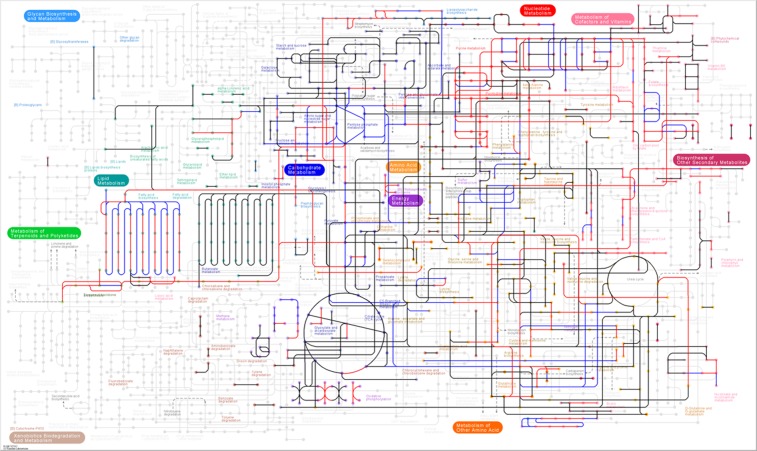
We selected 31 strains in the DEG database with corresponding EGs identified experimentally (Table 1). These EGs were linked to KO numbers (see Table 2 for basic information). The E_score of every KO number was based on the knowledge of essential genes in these 31 strains. Predicted EGs were compared to the experimentally defined EGs and the missing EGs identified by the ‘remapping’ algorithm were highlighted. Among the 31 strains, 27 were found to possess missing EGs via the ‘remapping’ algorithm. The number of missing EGs ranged from 224 to 839 with an average value of approximately 350. Using E. coli K12 as an example, we found that 3,139 genes in the genome were assigned with KO numbers. Experimentally, 296 genes were identified as essential22, among which 286 EGs were labeled on the KEGG pathway (eco01100) with KO numbers (Fig. 2). Our ‘remapping’ analysis showed another 469 genes were potentially essential that could have been missing in the experimental investigation.
Table 1.
List of the 31 strains collected from the database of essential genes (DEG) used for training data and 5 strains collected from the literature used for validation.
| No | Organism | KEGG abbreviation | EG number | Condition | Reference |
|---|---|---|---|---|---|
| Training datasets | |||||
| 1 | Acinetobacter baumannii ATCC 17978 | acb | 458 | Rich medium | 35 |
| 2 | Acinetobacter baylyi ADP1 | aci | 499 | Rich medium | 36 |
| 3 | Agrobacterium fabrum str. C58 | atu | 361 | Rich medium | 37 |
| 4 | Bacillus subtilis 168 | bsu | 271 | Rich medium | 1 |
| 5 | Bacteroides fragilis 638 R | bfg | 547 | Rich medium. | 38 |
| 6 | Brevundimonas subvibrioides ATCC 15264 | bsb | 412 | Rich medium | 37 |
| 7 | Burkholderia pseudomallei K96243 | bps | 505 | Rich medium | 39 |
| 8 | Burkholderia thailandensis E264 | bte | 406 | Rich medium. | 40 |
| 9 | Campylobacter jejuni NCTC 11168 = ATCC 700819 | cje | 228 | Rich medium | 41 |
| 10 | Caulobacter crescentus | ccr | 480 | Rich medium | 42 |
| 11 | Escherichia coli MG1655 II | eco | 296 | Rich medium | 22 |
| 12 | Francisella novicida U112 | ftn | 392 | Rich medium | 43 |
| 13 | Haemophilus influenzae Rd KW20 | hin | 642 | Rich medium | 44 |
| 14 | Helicobacter pylori 26695 | hpy | 323 | Rich medium | 45 |
| 15 | Mycobacterium tuberculosis H37Rv III | mtu | 687 | Rich medium | 46 |
| 16 | Mycoplasma genitalium G37 | mge | 381 | Rich medium | 7 |
| 17 | Mycoplasma pulmonis UAB CTIP | mpu | 310 | Rich medium | 47 |
| 18 | Porphyromonas gingivalis ATCC 33277 | pgn | 463 | Rich medium | 48 |
| 19 | Pseudomonas aeruginosa PAO1 | pae | 336 | Rich medium | 49 |
| 20 | CGA009 | rpa | 522 | Rich medium | 50 |
| 21 | Salmonella enterica serovar Typhi Ty2 | stt | 358 | Rich medium | 51 |
| 22 | Salmonella enterica serovar Typhimurium SL1344 | sey | 353 | Rich medium | 51 |
| 23 | Salmonella typhimurium LT2 | stm | 230 | Rich medium | 52 |
| 24 | Shewanella oneidensis MR-1 | son | 403 | Rich medium | 53 |
| 25 | Sphingomonas wittichii RW1 | swi | 535 | Rich medium | 54 |
| 26 | Staphylococcus aureus NCTC 8325 | sao | 351 | Rich medium | 55 |
| 27 | Streptococcus agalactiae A909 | sak | 317 | Rich medium | 56 |
| 28 | Streptococcus pyogenes NZ131 | soz | 241 | Todd-Hewitt medium | 57 |
| 29 | Streptococcus sanguinis SK36 | ssa | 218 | Rich medium | 8 |
| 30 | Synechococcus elongatus PCC 7942 | syf | 682 | Rich medium | 58 |
| 31 | Vibrio cholerae N16961 | vch | 779 | Rich medium | 59 |
| Validation datasets | |||||
| 1 | Campylobacter jejuni NCTC 11168 | cje | 166 | Rich medium | 12 |
| 2 | Mycobacterium tuberculosis H37Rv | mtu | 461 | Rich medium | 11 |
| 3 | Burkholderia cenocepacia H111 | bceo | 398 | Rich medium | 60 |
| 4 | Herbaspirillum seropedicae SmR1 | hse | 397 | Rich medium | 61 |
| 5 | Bacillus subtilis 168 | bsu | 257 | Rich medium | 13 |
Table 2.
Information for EGs collected from the database of essential genes (DEG).
| Item | Value | Note |
|---|---|---|
| DEG strains included | 31 | — |
| Total genes | 134,525 | — |
| Essential genes | 16,308 | — |
| Essential genes with KO available | 13,370 | 81.98%; 2,311 KOs without duplication |
| Non-essential genes | 118,217 | — |
| Non-essential genes with KO available | 64,107 | 54.23%; 6,290 KOs without duplication |
| Total KO without duplication | 6,839 | 31.10% of total 21,987 KOs in KEGG |
| Additional KO linked by #R | 312 | 7,151 appear in at least one of the 31 strains |
| Total KO in database | 21,987 | — |
Figure 2.
Metabolic pathway diagram of E. coli (eco01100 in KEGG pathway database34) with the gene essentiality information. The edges in red represent the EGs identified by experiment22. The edges in blue represent the missing EGs identified by the ‘remapping’ algorithm in this study. The edges in black represent the non-EGs. The original metabolic pathway map from KEGG34 is used with KEGG copyright permission number 190185.
We propose that the ‘gap genes’ identified by the ‘remapping’ algorithm from earlier experimental studies (Table 1) are non-EGs but playing essential functions, which could be largely attributed to the existence of isozymes, paralogs or alternative pathways in the genome. In this case, single gene-knockout technology cannot distinguish these ‘gap genes’ from the real EGs. In our previous work, we selected three pairs of paralogous or isozyme genes, SSA_0791/SSA_1494, SSA_0578/SSA_2195, and SSA_0352/SSA_1188, in Streptococcus sanguinis SK36. Indeed, double gene deletion mutants could not be constructed for these gene pairs, which supported our hypothesis8. Our ‘remapping’ algorithm therefore provides a new method to collect a comprehensive essential functions/reactions pool for EG prediction in prokaryotes. Overall, our approach in ePath is the annotation of ‘essential functions’ rather than ‘essential genes’, which is an important distinction for gene classification in prokaryotes23.
KO essentiality scoring
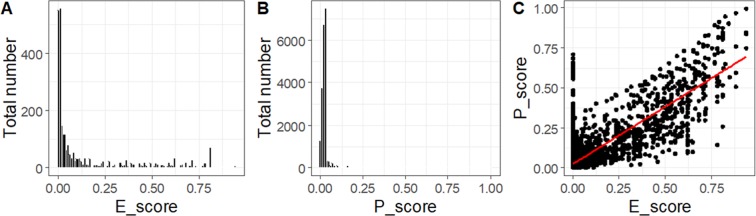
To expand the prediction for strains without experimental data, we attempted to calculate E_score and P_score for all 21,987 KOs. Note that 6,839 KOs (31.1%) appeared in at least one of the 31 strains, while the other KOs do not appear in any of the 31 strains. With 312 additional KOs that are linked using the reaction number in KEGG (#R), there are 7,151 KOs in total that can be assigned an E_score. Therefore, E_score was only available for 32.5% of the total KOs. For P_score, on the other hand, all 21,987 KOs were assigned. Analysis shows that distributions of E_score and P_score are both skewed to the left near zero (Fig. 3). E_scores range from 0 to 0.938, with an average value of 0.018 and standard deviation of 0.096. P_scores range from 0 to 0.997, with an average value of 0.037 and standard deviation of 0.084. We define the threshold for E_score as 0.6 and P_score as 0.03, which are both in the upper 90th percentile of all the data. We note a significant positive correlation between E_score and P_score (R2 = 0.67, p < 0.001; Fig. 3), suggesting that these two criteria are closely related for EG prediction. As both E_score and P_score are readily available for KOs, essentiality of genes among the 4,642 strains can be evaluated as long as they have been assigned one KO number.
Figure 3.
Frequency score distribution (A) E_score (only values higher than 0 are shown; N = 2546); (B) P_score (only values higher than 0 are shown; N = 21667); (C) correlation between P_score and E_score. The solid red line represents the best linear fit to the data with R2 = 0.67 (p < 0.001).
ePath: the online EG database and search engine
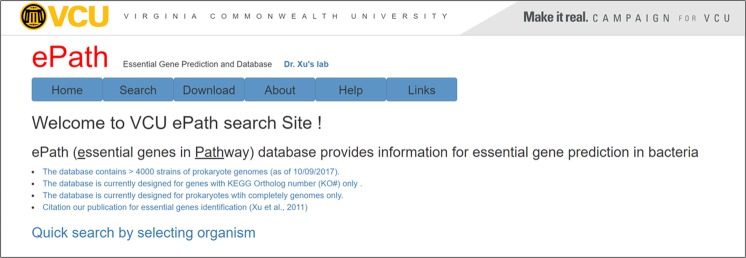
Serving as an online EG database and search engine, the ePath website provides access to the information described above (Fig. 4). End users can access the data easily and freely. Different searching strategies are possible, including (1) search by organism name (e.g. Escherichia coli K-12 MG1655), (2) search by organism then by gene locus (e.g. Escherichia coli K-12 MG1655 then eco:b0002), (3) search by organism then by KO# (e.g. Escherichia coli K-12 MG1655 then K02313), and (4) search by organism then by gene name (Escherichia coli K-12 MG1655 then purL). The outcomes include Organism, KEGG abbreviation, Gene_Locus, KO number (KO_Nbr), Gene_Name, Gene_Function, E_Score and P_Score. For convenience, search results can be downloaded as a ‘.csv’ document. The entire database in ePath is also available for downloading and reanalysis by end users.
Figure 4.
Interface of ePath website. The ePath searchable online database for essential genes for 4,000 + strains of prokaryote genomes.
Validation using new data
For validation, we collected five new datasets recently published for experimentally-derived EG identification, which did not include any of the 31 strains used for the training set (Table 1). The genes in these five datasets were used to query the ePath database for predictions (i.e. among the 4,642 strains). We compared the experimental results provided in each individual study with the EG prediction scores (E_score and P_score) from ePath, focusing on those genes with available KO numbers. Five criteria were applied for assessment of performance: (1) sensitivity; (2) specificity; (3) precision; (4) accuracy; and (5) F-measure (Table 3). Results showed that the proportion of essential genes that have been correctly identified ranged from 46% to 83% (sensitivity). Our method displayed better performance in predicting non-EGs (specificity) than EGs (precision), which ranged from 77% to 92% and 28% to 60%, respectively. The proportion of overall samples that were correctly identified (accuracy) ranged from 75% to 91%. The F-measure parameter indicates that harmonic mean of precision and sensitivity ranged from 34% to 70%. Our prediction performance was comparable to other approaches using either machine learning24 or evolutionary information18. Overall, the validation results indicate that our predictions are reliable and can serve as critical information for EG identification.
Table 3.
Results for EG prediction validation.
| Criterion | Calculation | cje | mtu | bceo | hse | bsu |
|---|---|---|---|---|---|---|
| sensitivity | TP/(TP + FN) | 0.65 | 0.49 | 0.46 | 0.49 | 0.83 |
| specificity | TN/(TN + FP) | 0.77 | 0.87 | 0.89 | 0.87 | 0.92 |
| precision | TP/(TP + FP) | 0.31 | 0.60 | 0.28 | 0.32 | 0.60 |
| accuracy | (TP + TN)/(TP + TN + FP + FN) | 0.75 | 0.76 | 0.85 | 0.83 | 0.91 |
| F-measure | 2 × sensitivity × precision/(sensitivity + precision) | 0.42 | 0.54 | 0.34 | 0.39 | 0.70 |
Note: (1) species abbreviations refer to Table 1. (2) TP: numbers of true positive; FN: numbers of false negative; TN: numbers of true negative; FP: numbers of false positive.
Discussion
Genome-wide experimental efforts can be expensive and time-consuming, which has resulted in an increase in predictive methodologies. We present a quick and efficient tool to identify putative essential genes in prokaryotic species lacking genome-wide experimental data. The new tool (ePath) covers the completed genomes of over 4,000 prokaryotic strains, which is broader than approaches using metabolic models. ePath provides information to drive the study of essential genes. For example, (1) to understand important knockout genes due to paralogs8, (2) for elucidating gene functions of hypothetical genes (unpublished data), (3) For many organisms, experiments with Tn-seq and other whole-genome mutagenesis are difficult and time consuming. Their EG results are often difficult to assess. ePath predictions can provide independent information to evaluate these experimental datasets and assess the success of the mutagenesis methods used, and (4) to identify antibacterial targets for drug development25,26.
Comparison with existing EG databases
Existing databases for EG information and annotations are generally for the currently available experimental outcomes. For example, the database of essential genes (DEG)9 contains collected and updated published experimental data concerning essential genes in different genomes. The Online GEne Essentiality database (OGEE)10, on the other hand, makes a step forward by collecting not only experimental outcomes but also gene features, so that there are possibilities to explore the distinction of EGs from non-EGs. Both the DEG and OGEE databases include comprehensive experimental essential gene data. Other studies attempt to either project experimentally identified EGs to functional roles in metabolic pathways27, or linking features of prokaryotic genes (e.g. genomic islands) to the possibility of a gene to be essential25. These studies significantly improve our ability and confidence in EG prediction in prokaryotes, but have not provided EG predictions of unknown organisms and their evaluations in these organisms.
The ePath database distinguishes itself from the other essential gene prediction resources by the following three distinct criteria: (1) end users of ePath may have access to EG annotations for over 4,000 prokaryotes with complete genome annotation available. This number is significantly higher than any other resource, potentially leading to more users and applications. (2) ePath demonstrates prediction accuracy ranging from 75%–91% based on validation. Comparatively, this performance is equivalent to other methods with similar objectives. However, in ePath, all prediction results are readily available so there is no requirement for further computation. The bias and uncertainty found in complicated machine learning are not present, making ePath more stable and comparable in EG predictions; and (3) ePath predicts EGs based on both sound principles from biological knowledge and existing experimental outcomes. The prediction algorithm in ePath is simple, facilitating its generalization to the whole prokaryote domain.
Limitations and future perspectives
One of the limitations of ePath is that the EG predictions are only available for those genes with KO numbers available, which means that genes without functional annotation by KEGG (in most cases ‘hypothetical protein’) cannot be assessed. For example, there are 819 out of 2270 genes in Streptococcus sanguinis SK36 that have been annotated with KO. ePath can therefore provide predictions of essentiality to these 819 genes but not to the others. Despite the fact that ePath is limited by KO annotation, this limitation is continuously decreasing in importance, as the KEGG database performs updates on a regular basis, so that the number of genes with KO numbers available is rapidly increasing. We obtained KO numbers for the genomes of the 4,642 strains directly from the KEGG-KO database (as of October, 2017), and ePath will be updated following updates of the KEGG database in the future on a regular basis. Moreover, it appears that only a minor fraction of these ‘hypothetical genes’ without KO annotations are essential. For example, only 3 out of the 218 EGs in Streptococcus sanguinis SK36 are ‘hypothetical’8 and all of them are functionally related with the three basic categories (unpublished data). Meanwhile, KEGG also helpfully provides an online tool (BlastKOALA) for automatic KO assignment28. In theory, we can run BlastKOALA for all the 4,642 strains with available complete genome sequence data, which however would be computationally overwhelming. Therefore, we propose that the KEGG-KO database should be applied with caution for any of the annotated strains in KEGG. We suggest that for genes of interest to researchers but without KEGG annotations, it is possible to assign KOs using BlastKOALA. E_score and P_score could be obtained for the KOs using the KO essentiality by annotation table of the ePath website. With the accumulation of experimental data for essential genes in different organisms, more missed KO will be assigned with more accurate E_scores (and derived P_scores).
Another limitation that could be resolved in future studies is the determination of thresholds for the new E_score and P_score. To ascertain thresholds for newly proposed indicators, a large training dataset is usually required, which is difficult for EG studies. Although we observed good prediction performance with the E_score and P_score with validation data, determination of the thresholds for these two scores are based on quantitative decisions that require further evaluation. Given the small but increasing number of experimental EG studies, refinement of the scoring system for EG prediction in the near future is promising.
P_score is based on the assumption that one gene is more likely to be essential when it performs the functions among the three categories: cell envelope, energy production, and processing of genetic information, which, however, could be blurred as more refined functions may be found23. Nevertheless, P_score may nevertheless provide hints for gene essentiality. Therefore, we advocate that when ePath is used, E_score should be more weighted, while the P_score should be considered as supplementary information for EG prediction, especially when E_score is not available.
Finally, knowledge of paralogous genes would be beneficial for use of ePath. Notably, all EGs from DEG are identified using single gene knock-outs. Because of the existence of paralogous genes in prokaryotic species (isozymes or alternative pathways)8, even if a function is essential, the deletion of one paralogous gene may not lead to prokaryotic death (due to alternative gene functional compensation). In another case, if an essential compound is supplied in the growth condition (e.g. essential amino acid), the genes for related biosynthetic processes would not be essential under the experimental condition8. To resolve the issue, a remapping process is proposed in the present study to obtain a comprehensive essential function pool for the prediction of EGs in various species under different environmental conditions. However, this strategy brings some problems. In the case of a very high E_score indicating essentiality of a gene, a false positive prediction could be given due to the existence of paralogous genes or essential compounds, which results in low accuracy of EG predictions. End users could partially avoid these problems based on their knowledge of paralogous genes, isozymes and alternative pathways in the target species or the nutritional composition of their chosen growth medium.
Materials and Methods
EG annotation based on information of KO
As the first step, we collected all the KOs in the KEGG database (http://www.kegg.jp/kegg/) and their annotations from the database for gene annotation. There are 21,987 KOs ranged from “K00001” to “K21987” in KEGG (as of October, 2017). For each KO, we collected its annotation from the KO database (http://www.kegg.jp/kegg-bin/get_htext). These KO annotations are molecular-level functions determined from experimental evidence of functionally characterized sequence data. They are positioned as nodes in networks and are defined in the context of KEGG molecular networks (KEGG pathway maps, BRITE hierarchies and KEGG modules)29,30. Among all 21,987 KOs, 665 do not have KO annotations. These KOs were therefore marked as “0” for further analysis. In addition, we collected information for the KOs including gene names and descriptions given by RefSeq31 or GenBank32, as well as the corresponding KEGG pathway (#ko), KEGG module (#M) and KEGG Reaction (#R) from KEGG Brite Database (http://www.kegg.jp/kegg/brite.html)33. In particular, each #ko is composed of three layers of annotation, e.g., for #ko00010, the pathway annotation is “Metabolism −>Carbohydrate metabolism −>Glycolysis/Gluconeogenesis”. Note that for one certain KO, there can be more than one (or none) corresponding #ko, #M or #R. We assigned COGs and GOs number to each KO according to the “binary relationships” provided by KEGG Brite Database, which also could be more than one or none. For COGs, their categories and annotations were collected from NCBI (ftp://ftp.ncbi.nih.gov/pub/COG/COG2014/static/lists/listCOGs.html). Furthermore, GO annotations were collected from the Gene Ontology Consortium (http://geneontology.org/page/download-annotations), in particular the “UniProt [multispecies], no IEA annotations”. Overall, we have collected multiple functional annotations for each of the 21,987 KOs. We therefore have established the first database for this study, which is presented in detail on the ePath online database.
EG annotation based on existing experimental discovery
We collected a group of strains (Table 1) from the database of essential genes (DEG: http://www.essentialgene.org/)9, for which EGs have been identified and validated using experimental approaches. The selected 31 strains used as the training set include 134,525 genes in total, in which 16,308 genes were identified as EG using experimental methods, mostly in rich media (Table 2). For all 134,525 genes, we assigned KOs to each by implementing the BlastKOALA tool (http://www.kegg.jp/kegg/tool/annotate_sequence.html), which determines the most appropriate KO for one gene based on a modified version of the KOALA algorithm after the BLAST search against a non-redundant dataset of pan-genome sequences generated from the KEGG GENES database28. If one gene is annotated with more than one KO, we selected the best match provided by BlastKOALA. As the input to the BlastKOALA, the genome sequences for the 31 strains were collected from NCBI together with the ‘gene_id’, which is also provided by the DEG. This variable therefore serves as the linkage between the outcomes from BlastKOALA and the DEG. All 31 strains have been included in KEGG so that the GENES family/genus abbreviation (Table 1) was pre-assigned after uploading the genome sequence in BlastKOALA. Among the 16,308 EGs, 13,370 were successfully linked to one KO (2,311 KOs without duplication), while for the rest of the 118,217 non-EGs, 64,107 had a KO available (6,290 KOs without duplication). Therefore, 77,477 KOs were obtained in total, belonging to 6,839 KOs after the removal of duplicates. The details of the gene information above are presented in the ePath online database. We also elaborated to match each of the 6,839 KOs with other KOs if they share the same #R, as we hope to add additional information to understand KO function. This procedure resulted in an additional 312 KOs. These 7,151 KOs were further analyzed in the following sections. The details of the gene information above are presented in the ePath online database.
Remapping: a new algorithm for identifying missing EGs
We used KEGG pathways to identify those potential EGs that could have been overlooked in experiments with a new algorithm called ‘remapping’. As the first step, we collected the ‘Locus-tag’ for all the EGs from DEG, which is critical for subsequent analysis with KEGG pathways labeled by the ‘Locus-tag’ along the edges of the pathway map. Due to a recent update of NCBI, the ‘Locus-tags’ for many strains have been changed. We therefore obtained the ‘Locus-tag’ for all the EGs in the 31 strains from their original publications (Table 1). We assigned the ‘Locus-tag’ for all the 16,308 EGs (except for 102 missing).
Next, we focused on the metabolic pathways in the category of “Global and overview” maps in KEGG, i.e., the ‘ko01100’ pathway. We downloaded this pathway file in “.xml” format from KEGG for all the 31 strains, using the python package ‘requests’. The pathway document contains the information for all the chemical reactions of the metabolism with the corresponding functional genes in the organism according to state-of-the-art knowledge in literature29. The nodes serve as chemical compounds either as substrate or products, while all the edges act as the KO group(s) that produce the enzymes for the reaction. For any given strain, the edges are labeled by the corresponding genes’ ‘Locus-tag’.
For each strain, we further parsed the “.xml” file for the ‘ko01100’ pathway using the python package ‘xml.etree.ElementTree’. By collecting the attributions with ‘entry’ and type ==’gene’, we obtained the gene ‘Locus-tag’ list and the corresponding reaction ID list within the pathway. In addition, the ‘reaction’ attribution provides the reaction information including the compound id for the substrate and product. Based on the table for the linkage between #R and #K, we further determined all the corresponding KO for each reaction in the pathway. Overall, we summarized all the information from the pathway’s.xml file. We established a table for each strain accordingly, in which each row represents a biochemical reaction and the columns were as follows: substrate(s) (#C), product(s) (#C), reactions (#R), KOs (#K), and gene ‘Locus-tag’. Furthermore, for each reaction, we queried the corresponding genes to the EGs database above based on ‘Locus-tag’. We scored the reaction with 5 points if at least one gene was essential, and scored the reaction with 1 point if all the genes were non-essential. We therefore built a chemical reaction matrix (S) for all the compounds in each strain, serving as the linkage matrix for one pathway. The element Sij (i = 1, 2, …, n; j = 1, 2, …, n) represents the existence and essentiality of the reaction between two compounds, where i and j are the location of the element representing the chemical’s index, and n is the total number of chemicals. For essential genes, Sij = 5; for non-essential genes, Sij = 1; and for reactions that do not exist, Sij = 0. With the Sij available for one strain, we then went through the KEGG pathways and identified the missing EGs that were not experimentally determined. We used the Depth-first search (DFS) algorithm combined with our criteria for traversing the pathway map represented by the matrix S. We started the search from each row in the matrix representing one edge in the map. The algorithm for DFS can be described as follows:
The search will go across the next edge either if the edge represents one EG, or if there is at least one EG linked to the node on the other end of the edge.
The search will stop if the edge represents one non-EG and there is no EG linked to the node on the other end of the edge.
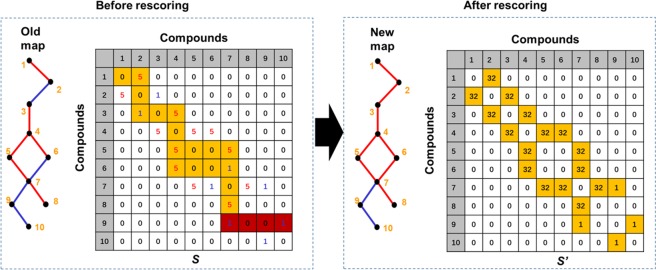
After DFS, we would be able to identify multiple ‘essential sections’ inside the pathway, in which all the missing EGs would be included and identified. Then, we rescored the starting edge by summing all the scores of the edges linked to the starting edge identified by the DFS search, and we obtained the updated matrix (S’). Note that in the KEGG pathways, one gene may appear in multiple edges in one map. If one non-essential gene is identified as a missing EG at one location, all the other edges where this gene appears will be labeled as essential, as will all the other genes in those edges. This situation could produce an infinite loop. Therefore, we only ran the search once for the sake of simplicity. We provided an illustrative example for the algorithm with 10 chemicals (genes) in Fig. 5.
Figure 5.
Link missing essential genes in pathway. An illustrative example of the ‘remapping’ algorithm processed on the KEGG pathway map with 10 hypothetical compounds. The left panel represents the map and matrix (S) before the rescoring. In the old map (left panel), the blue edges are non-essential genes, while the red ones are essential genes. The elements in the matrix (S) show the existence and essentiality of the reaction between the two corresponding compounds. The colored elements highlight how the DFS algorithm searches for the linked edges for the first edge. The yellow boxes are the linked edges and the red boxes are the discarded edges. The right panel shows the new map and the updated matrix (S’). Note that in the new map, edges (2–3) and (6–7) are considered as the missing EGs and are labeled red. The S’ provides the final score for each edge, which serves as the basis for EG determination.
EG scoring system
Two dimensions of scoring for KO essentiality are developed based on the data collected. First, an experimental score (E_score) is assigned for each of 7,151 KOs. E_score is based on the appearance and essentiality of each KO among the 31 stains. We propose a formula for calculating E_scorei for gene i (Eq. 1) in the range of 0–1, where a higher value suggests a higher potential for essentiality.
| 1 |
where EGi,e represents the number of strains that have a particular KO that is essential according to experimental outcomes; EGi,m represents the number of strains that have a particular KO that is missing essential according to ‘remapping’; nonEGi represents the number of strains that have a particularKO that is not essential. The first term on the right of Eq. 1 represents the probability of the KO as EG among the strains in which it appears. The second term on the right of Eq. 1, on the other hand, indicates the probability of the KO to appear among strains as another aspect of the gene’s essentiality.
Second, a prediction score (P_score) was assigned for each KO. The P_score originates from the E_score and was determined by expert judgment on the essentiality of the KO based on comprehensive annotations for prokaryotes. For a KO without an E_score, at first two additional scores, i.e. P_score_KEGG and P_score_COG, were determined based on the KEGG or COG annotation and calculated from the E_score of the KOs with the same pathway annotation. These two scores were manually assigned within a range of 0–1. For example, K15792 has no E-score, but its KEGG pathway annotation is “Lysine biosynthesis//Peptidoglycan biosynthesis//”. There is one KO (K01928) with E_score 0.59 for the same KEGG pathway annotation. As a result, P_score_KEGG for K15792 is 0.59. Its COG annotation is “UDP-N-acetylmuramyl tripeptide synthase//UDP-N-acetylmuramyl pentapeptide synthase//”. However, no KO with the same COGs and with available E_score is found. Therefore, P_score_COG for K15792 is 0. As another example, K10781 has no E-score, but its KEGG pathway annotation is “Fatty acid metabolism//Fatty acid biosynthesis//”. There are 8 other KOs with the same annotation (K01716, K18473, K00645, K02371, K00648, K10780, K00667, and K00668) with an average E_score of 0.57. Therefore, P_score_KEGG of K10781 is 0.57. There is no COG annotation. There are a total of 1292 empty cells for COG annotations with average E_score 0.04. As a result, P_score_COG of K10781 is 0.04. The rationale originates from the earlier findings8 that KOs belonging to the following three functional categories are essential: (1) gene information processing, (2) energy production, and (3) cell envelope. We manually examined all KO annotations with E_score and found that the KOs with higher E_scores were highly related with the three functional categories above. To expand the prediction for KOs without E_scores, P_score is assigned as the average of standardized P_score_KEGG and P_score_COG for each KO. Note that we cannot assign P_score for the KOs having neither KEGG pathways nor COG annotations. Overall, a P_score may range from 0 to 1, with 0 representing no potential for essentiality, while 1 denotes certain essentiality based on expert judgment.
Essential gene prediction for 4,642 strains
We collected all the prokaryotes in KEGG Organisms database (in total 4,642, as of October, 2017). For the genomes of each collected strain, we assigned a KO number based on the existing information in KEGG (KEGG-KO database). For all the 4,642 strains, KO annotations for genes in each genome were collected by parsing the ‘.keg’ file via python, which serves as the basis for further EG annotation based on both E_ score and P_score. All EG prediction results for the 4,642 strains are provided in the ePath online database.
Acknowledgements
We are grateful to previous laboratory members who contributed to the development of this annotation, Jordan L Niermeyer (initial ePath) and Nihar U Sheth (bioinformatic consultant). This work was supported by National Institutes of Health grants R01DE023078 and R01DE018138 (P. Xu). We thank Dr. Todd Kitten for editing manuscript and the anonymous reviewers who provided constructive comments and suggestions to improve the manuscript. The funders had no role in study design, data collections and interpretation, or the decision to submit the work for publication.
Author Contributions
P.X. conceived the study. X.K. and B.Z. carried out data collection, data analysis and wrote the manuscript. V.S., X.G. and F.E.E.-R. contributed to the discussion. H.D., X.K. and P.X. contributed to the website development. All authors reviewed the manuscript before submission.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kobayashi K, et al. Essential Bacillus subtilis genes. Proceedings of the National Academy of Sciences. 2003;100:4678–4683. doi: 10.1073/pnas.0730515100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rancati G, Moffat J, Typas A, Pavelka N. Emerging and evolving concepts in gene essentiality. Nature Reviews Genetics. 2018;19:34–49. doi: 10.1038/nrg.2017.74. [DOI] [PubMed] [Google Scholar]
- 3.Koonin EV. Comparative genomics, minimal gene-sets and the last universal common ancestor. Nature Reviews Microbiology. 2003;1:127–136. doi: 10.1038/nrmicro751. [DOI] [PubMed] [Google Scholar]
- 4.Juhas M, Eberl L, Glass JI. Essence of life: essential genes of minimal genomes. Trends in cell biology. 2011;21:562–568. doi: 10.1016/j.tcb.2011.07.005. [DOI] [PubMed] [Google Scholar]
- 5.Haselbeck R, et al. Comprehensive essential gene identification as a platform for novel antiinfective drug discovery. Current pharmaceutical design. 2002;8:1155–1172. doi: 10.2174/1381612023394818. [DOI] [PubMed] [Google Scholar]
- 6.Koonin EV, Aravind L, Kondrashov AS. The impact of comparative genomics on our understanding of evolution. Cell. 2000;101:573–576. doi: 10.1016/S0092-8674(00)80867-3. [DOI] [PubMed] [Google Scholar]
- 7.Glass JI, et al. Essential genes of a minimal bacterium. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:425–430. doi: 10.1073/pnas.0510013103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xu P, et al. Genome-wide essential gene identification in Streptococcus sanguinis. Scientific reports. 2011;1:125. doi: 10.1038/srep00125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Luo H, Lin Y, Gao F, Zhang C, Zhang R. DEG 10, an update of the database of essential genes that includes both protein-coding genes and noncoding genomic elements. Nucleic acids research. 2013;42:D574–D580. doi: 10.1093/nar/gkt1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen W-H, Minguez P, Lercher MJ, Bork P. OGEE: an online gene essentiality database. Nucleic acids research. 2011;40:D901–D906. doi: 10.1093/nar/gkr986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DeJesus MA, et al. Comprehensive essentiality analysis of the Mycobacterium tuberculosis genome via saturating transposon mutagenesis. MBio. 2017;8:e02133–02116. doi: 10.1128/mBio.02133-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mandal RK, Jiang T, Kwon YM. Essential genome of Campylobacter jejuni. BMC genomics. 2017;18:616. doi: 10.1186/s12864-017-4032-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koo B, et al. Construction and analysis of two genome-scale deletion libraries for Bacillus subtilis. Cell systems. 2017;4:291–305. e297. doi: 10.1016/j.cels.2016.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mobegi FM, Zomer A, de Jonge MI, van Hijum SA. Advances and perspectives in computational prediction of microbial gene essentiality. Briefings in functional genomics. 2017;16:70–79. doi: 10.1093/bfgp/elv063. [DOI] [PubMed] [Google Scholar]
- 15.Deng J, et al. Investigating the predictability of essential genes across distantly related organisms using an integrative approach. Nucleic acids research. 2010;39:795–807. doi: 10.1093/nar/gkq784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ning L, et al. Predicting bacterial essential genes using only sequence composition information. Genetics and molecular research: GMR. 2014;13:4564–4572. doi: 10.4238/2014.June.17.8. [DOI] [PubMed] [Google Scholar]
- 17.Edwards JS, Palsson BO. Metabolic flux balance analysis and the in silico analysis of Escherichia coli K-12 gene deletions. BMC bioinformatics. 2000;1:1. doi: 10.1186/1471-2105-1-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wei W, Ning L-W, Ye Y-N, Guo F-B. Geptop: a gene essentiality prediction tool for sequenced bacterial genomes based on orthology and phylogeny. PloS one. 2013;8:e72343. doi: 10.1371/journal.pone.0072343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guo, F., Ye, Y., Ning, L. & Wei, W. Three computational tools for predicting bacterial essential genes, in Gene Essentiality. Springer, pp 205–217 (2015). [DOI] [PubMed]
- 20.Nandi S, Subramanian A, Sarkar RR. An integrative machine learning strategy for improved prediction of essential genes in Escherichia coli metabolism using flux-coupled features. Molecular BioSystems. 2017;13:1584–1596. doi: 10.1039/C7MB00234C. [DOI] [PubMed] [Google Scholar]
- 21.Henry CS, et al. High-throughput generation, optimization and analysis of genome-scale metabolic models. Nature biotechnology. 2010;28:977–982. doi: 10.1038/nbt.1672. [DOI] [PubMed] [Google Scholar]
- 22.Baba T, et al. Construction of Escherichia coli K‐12 in‐frame, single‐gene knockout mutants: the Keio collection. Molecular systems biology. 2006;2:2006.0008. doi: 10.1038/msb4100050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xavier JC, Patil KR, Rocha I. Metabolic models and gene essentiality data reveal essential and conserved metabolism in prokaryotes. PLoS computational biology. 2018;14:e1006556. doi: 10.1371/journal.pcbi.1006556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hua H, et al. An Approach for Predicting Essential Genes Using Multiple Homology Mapping and Machine Learning Algorithms. BioMed research international. 2016;2016:e7639397. doi: 10.1155/2016/7639397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stone VN, Xu P. Targeted antimicrobial therapy in the microbiome era. Molecular oral microbiology. 2017;32:446–454. doi: 10.1111/omi.12190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stone VN, et al. Identification of small-molecule inhibitors against meso-2, 6-diaminopimelate dehydrogenase from Porphyromonas gingivalis. PloS one. 2015;10:e0141126. doi: 10.1371/journal.pone.0141126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gerdes S, et al. Essential genes on metabolic maps. Current opinion in biotechnology. 2006;17:448–456. doi: 10.1016/j.copbio.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 28.Kanehisa M, Sato Y, Morishima K. BlastKOALA and GhostKOALA: KEGG tools for functional characterization of genome and metagenome sequences. Journal of molecular biology. 2016;428:726–731. doi: 10.1016/j.jmb.2015.11.006. [DOI] [PubMed] [Google Scholar]
- 29.Kanehisa M, et al. Data, information, knowledge and principle: back to metabolism in KEGG. Nucleic acids research. 2013;42:D199–D205. doi: 10.1093/nar/gkt1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kanehisa M, Goto S, Kawashima S, Okuno Y, Hattori M. The KEGG resource for deciphering the genome. Nucleic acids research. 2004;32:D277–D280. doi: 10.1093/nar/gkh063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pruitt KD, Tatusova T, Maglott DR. NCBI reference sequences (RefSeq): a curated non-redundant sequence database of genomes, transcripts and proteins. Nucleic acids research. 2006;35:D61–D65. doi: 10.1093/nar/gkl842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Benson DA, et al. GenBank. Nucleic acids research. 2012;41:D36–D42. doi: 10.1093/nar/gks1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kanehisa M, et al. From genomics to chemical genomics: new developments in KEGG. Nucleic acids research. 2006;34:D354–D357. doi: 10.1093/nar/gkj102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kanehisa M, Goto S. KEGG: kyoto encyclopedia of genes and genomes. Nucleic acids research. 2000;28:27–30. doi: 10.1093/nar/28.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang N, Ozer EA, Mandel MJ, Hauser AR. Genome-wide identification of Acinetobacter baumannii genes necessary for persistence in the lung. MBio. 2014;5:e01163–01114. doi: 10.1128/mBio.01163-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.De Berardinis V, et al. A complete collection of single‐gene deletion mutants of Acinetobacter baylyi ADP1. Molecular systems biology. 2008;4:174. doi: 10.1038/msb.2008.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Curtis PD, Brun YV. Identification of essential alphaproteobacterial genes reveals operational variability in conserved developmental and cell cycle systems. Molecular microbiology. 2014;93:713–735. doi: 10.1111/mmi.12686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Veeranagouda Y, Husain F, Tenorio EL, Wexler HM. Identification of genes required for the survival of B. fragilis using massive parallel sequencing of a saturated transposon mutant library. BMC genomics. 2014;15:429. doi: 10.1186/1471-2164-15-429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moule MG, et al. Genome-wide saturation mutagenesis of Burkholderia pseudomallei K96243 predicts essential genes and novel targets for antimicrobial development. MBio. 2014;5:e00926–00913. doi: 10.1128/mBio.00926-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Baugh L, et al. Combining functional and structural genomics to sample the essential Burkholderia structome. PloS one. 2013;8:e53851. doi: 10.1371/journal.pone.0053851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Metris A, Reuter M, Gaskin DJ, Baranyi J, van Vliet AH. In vivo and in silico determination of essential genes of Campylobacter jejuni. BMC genomics. 2011;12:535. doi: 10.1186/1471-2164-12-535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Christen B, et al. The essential genome of a bacterium. Molecular systems biology. 2011;7:528. doi: 10.1038/msb.2011.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gallagher LA, et al. A comprehensive transposon mutant library of Francisella novicida, a bioweapon surrogate. Proceedings of the National Academy of Sciences. 2007;104:1009–1014. doi: 10.1073/pnas.0606713104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Akerley BJ, et al. A genome-scale analysis for identification of genes required for growth or survival of Haemophilus influenzae. Proceedings of the National Academy of Sciences. 2002;99:966–971. doi: 10.1073/pnas.012602299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Salama NR, Shepherd B, Falkow S. Global transposon mutagenesis and essential gene analysis of Helicobacter pylori. Journal of bacteriology. 2004;186:7926–7935. doi: 10.1128/JB.186.23.7926-7935.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang YJ, et al. Global assessment of genomic regions required for growth in Mycobacterium tuberculosis. PLoS pathogens. 2012;8:e1002946. doi: 10.1371/journal.ppat.1002946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.French CT, et al. Large‐scale transposon mutagenesis of Mycoplasma pulmonis. Molecular microbiology. 2008;69:67–76. doi: 10.1111/j.1365-2958.2008.06262.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Klein BA, et al. Identification of essential genes of the periodontal pathogen Porphyromonas gingivalis. BMC genomics. 2012;13:578. doi: 10.1186/1471-2164-13-578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Turner KH, Wessel AK, Palmer GC, Murray JL, Whiteley M. Essential genome of Pseudomonas aeruginosa in cystic fibrosis sputum. Proceedings of the National Academy of Sciences. 2015;112:4110–4115. doi: 10.1073/pnas.1419677112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pechter KB, Gallagher L, Pyles H, Manoil CS, Harwood CS. Essential genome of the metabolically versatile alphaproteobacterium Rhodopseudomonas palustris. Journal of bacteriology. 2016;198:867–876. doi: 10.1128/JB.00771-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Barquist L, et al. A comparison of dense transposon insertion libraries in the Salmonella serovars Typhi and Typhimurium. Nucleic acids research. 2013;41:4549–4564. doi: 10.1093/nar/gkt148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Knuth K, Niesalla H, Hueck CJ, Fuchs TM. Large‐scale identification of essential Salmonella genes by trapping lethal insertions. Molecular microbiology. 2004;51:1729–1744. doi: 10.1046/j.1365-2958.2003.03944.x. [DOI] [PubMed] [Google Scholar]
- 53.Deutschbauer A, et al. Evidence-based annotation of gene function in Shewanella oneidensis MR-1 using genome-wide fitness profiling across 121 conditions. PLoS genetics. 2011;7:e1002385. doi: 10.1371/journal.pgen.1002385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Roggo C, et al. Genome‐wide transposon insertion scanning of environmental survival functions in the polycyclic aromatic hydrocarbon degrading bacterium S phingomonas wittichii RW 1. Environmental microbiology. 2013;15:2681–2695. doi: 10.1111/1462-2920.12125. [DOI] [PubMed] [Google Scholar]
- 55.Chaudhuri RR, et al. Comprehensive identification of essential Staphylococcus aureus genes using Transposon-Mediated Differential Hybridisation (TMDH) BMC genomics. 2009;10:291. doi: 10.1186/1471-2164-10-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hooven TA, et al. The essential genome of Streptococcus agalactiae. BMC genomics. 2016;17:406. doi: 10.1186/s12864-016-2741-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Le Breton Y, et al. Essential genes in the core genome of the human pathogen Streptococcus pyogenes. Scientific reports. 2015;5:9838. doi: 10.1038/srep09838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rubin BE, et al. The essential gene set of a photosynthetic organism. Proceedings of the National Academy of Sciences. 2015;112:E6634–E6643. doi: 10.1073/pnas.1519220112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cameron DE, Urbach JM, Mekalanos JJ. A defined transposon mutant library and its use in identifying motility genes in Vibrio cholerae. Proceedings of the National Academy of Sciences. 2008;105:8736–8741. doi: 10.1073/pnas.0803281105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Higgins S, et al. The essential genome of Burkholderia cenocepacia H111. Journal of bacteriology. 2017;199:e00260–00217. doi: 10.1128/JB.00260-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rosconi F, de Vries SP, Baig A, Fabiano E, Grant AJ. Essential Genes for In Vitro Growth of the Endophyte Herbaspirillum seropedicae SmR1 as Revealed by Transposon Insertion Site Sequencing. Applied and environmental microbiology. 2016;82:6664–6671. doi: 10.1128/AEM.02281-16. [DOI] [PMC free article] [PubMed] [Google Scholar]