Tick-borne diseases have increased in prevalence in the United States and abroad. The reasons for these increases are multifactorial, but climate change is likely to be a major factor. One of the main features of the increase is the geographic expansion of tick vectors, notably Amblyomma americanum, which has brought new pathogens to new areas. The clinical spectrum of tick-borne diseases can range from asymptomatic to fatal infections, with a disproportionate incidence in children and the elderly. In addition, new pathogens that are cotransmitted by Ixodes scapularis have been discovered and have led to difficult diagnoses and to disease severity. Of these, Borrelia burgdorferi, the agent of Lyme disease, continues to be the most frequently transmitted pathogen. However, Babesia microti, Borrelia miyamotoi (another spirochete), Anaplasma phagocytophilum, and Powassan virus are frequent cotransmitted agents. Polymicrobial infection has important consequences for the diagnosis and management of tick-borne diseases.
KEYWORDS: Amblyomma, Anaplasma, Babesia, Borrelia burgdorferi, Ehrlichia, Ixodes, Lyme disease, Powassan, Rickettsia
ABSTRACT
Tick-borne diseases have doubled in the last 12 years, and their geographic distribution has spread as well. The clinical spectrum of tick-borne diseases can range from asymptomatic to fatal infections, with a disproportionate incidence in children and the elderly. In the last few years, new agents have been discovered, and genetic changes have helped in the spread of pathogens and ticks. Polymicrobial infections, mostly in Ixodes scapularis, can complicate diagnostics and augment disease severity. Amblyomma americanum ticks have expanded their range, resulting in a dynamic and complex situation, possibly fueled by climate change. To document these changes, using molecular biology strategies for pathogen detection, an assessment of 12 microbes (9 pathogens and 3 symbionts) in three species of ticks was done in Suffolk County, New York. At least one agent was detected in 63% of I. scapularis ticks. Borrelia burgdorferi was the most prevalent pathogen (57% in adults; 27% in nymphs), followed by Babesia microti (14% in adults; 15% in nymphs), Anaplasma phagocytophilum (14% in adults; 2% in nymphs), Borrelia miyamotoi (3% in adults), and Powassan virus (2% in adults). Polymicrobial infections were detected in 22% of I. scapularis ticks, with coinfections of B. burgdorferi and B. microti (9%) and of B. burgdorferi and A. phagocytophilum (7%). Three Ehrlichia species were detected in 4% of A. americanum ticks. The rickettsiae constituted the largest prokaryotic biomass of all the ticks tested and included Rickettsia amblyommatis, Rickettsia buchneri, and Rickettsia montanensis. The high rates of polymicrobial infection in ticks present an opportunity to study the biological interrelationships of pathogens and their vectors.
INTRODUCTION
Polymicrobial infections are known for a variety of disorders, such as urinary tract infections, sexually transmitted diseases, periodontal disease, otitis media, and opportunistic infections associated with hospital procedures, to name just a few. In these settings, coexisting microbes can generate synergy or interference of the infectious process. However, regardless of the outcome of a specific polymicrobial infection, the interrelationships among the microbes are likely to have an impact on the course of the infection. Unlike some of the examples above where polymicrobial infections affect only the patient, the various pathogens and symbionts of ticks interact in both the tick itself and in the patient with consequences that have not been totally foreseen (1).
Tick-borne diseases have become a worldwide threat to public health. In the United States, cases increased from 22,000 in 2004 to >48,000 in 2016 (2). Tick-borne diseases range from subclinical to fatal infections, with a disproportionate incidence in children and the elderly. Moreover, some can also be transmitted by blood transfusions and cause severe disease in patients with underlying disorders. New agents have also been discovered (3, 4). Notably, formerly geographically confined tick species have expanded their range, resulting in a dynamic and complex situation, possibly fueled by climate change. Polymicrobial infections represent another aspect of tick-borne diseases that can complicate diagnosis and augment disease severity. Since the discovery of Borrelia burgdorferi in 1982 (5) and human babesiosis caused by the hemoprotozoan Babesia microti (6, 7) in Long Island and Nantucket in the late 1970s, more cotransmitted infections have been recognized. Two early serosurveys disclosed a link between babesiosis and erythema migrans and double infections (8, 9). Since then, Ixodes scapularis has been implicated as the vector of five human pathogens in the northeast United States. Polymicrobial infections occur in both North America and Europe (10).
Over the past 3 decades, there has been a steady increase in the number of newly discovered tick-borne agents. In addition to B. burgdorferi and B. microti, Anaplasma phagocytophilum (11–15), which was originally classified as a granulocytic Ehrlichia species (16), a relapsing fever-like Borrelia species, Borrelia miyamotoi (3), and the deer tick virus, a variant of Powassan virus (POWV) (4), are also transmitted by I. scapularis. POWV was first isolated from a patient with encephalitis (17), and over 100 cases have been reported in the United States (18). The capacity of I. scapularis to harbor such a diverse pathogen microbiome increases the risk of polymicrobial infections from a single tick bite. Almost 25 years ago, Telford et al. warned that cotransmission of pathogens will have a unique impact on public health in sites of endemicity (19).
Pathogens transmitted by Amblyomma americanum and Dermacentor variabilis contribute to the broad spectrum of tick-borne diseases. A. americanum, the lone star tick, has historically been found in the southern United States, but it has substantially expanded its range. Anecdotally, it appears that this aggressive species has become the most abundant tick in Long Island, New York, and in other regions previously outside its range (20, 21), and it has done so in a very short time (22, 23). A collateral effect of the expansion of A. americanum could be the displacement of I. scapularis and D. variabilis through competitive interactions that are not understood. Significant shifts in disease prevalence in the future could be due to shifts in the vector populations, and systematic tick-pathogen surveys may answer this question. A. americanum is a vector of Ehrlichia chaffeensis and Ehrlichia ewingii (24–27), both capable of causing severe disease in patients who are elderly or immunodeficient (28). A. americanum has been linked to a Lyme disease-like syndrome called southern tick-associated rash illness (STARI). Borrelia lonestari, a relapsing fever-like species found in A. americanum (29), has been associated with STARI, but its role has not been corroborated. Most recently, A. americanum has been implicated in meat allergy syndrome, an intriguing condition that may be due to this tick directly without the intervention of a microbe (30). Haemaphysalis longicornis, a newly discovered exotic tick species introduced in the United States (31), is an important vector of human and animal disease agents in its original geographic range (32, 33).
Of note is the increasing awareness of the rickettsial biome in the three vector ticks. The American dog tick, D. variabilis, is a vector of Rickettsia rickettsii, the agent of Rocky Mountain spotted fever (RMSF). The number of reported cases of spotted fever rickettsioses (SFR) in the United States has increased from 1,713 in 2004 to 4,269 in 2016 (2). However, tick surveillance studies have rarely reported R. rickettsii in D. variabilis (34, 35). This suggests that other species of Rickettsia contribute to the rise in incidence of SFR.
Rickettsia buchneri, an ovarian symbiont, (36), is the most abundant prokaryote in I. scapularis (37–39). Rickettsia amblyommatis (40) is pervasively associated with A. americanum (41). Rickettsia montanensis in D. variabilis has been implicated circumstantially in an RMSF-like infection (42). These three rickettsial species phylogenetically belong to the spotted fever group but are not agents of disease. However, these rickettsiae, by virtue of their abundance, may have a critical role in pathogen-vector interactions.
The presence of known pathogens as well as probable pathogens and symbionts in ticks requires a polymicrobial approach to the clinical aspects of tick-borne infections. Concurrent polymicrobial infections in humans can have a synergistic effect and result in a more severe course of illness. In addition, the clinical course of one tick-borne disease could be influenced simply by exposure to another microbe. For example, A. phagocytophilum and Ehrlichia spp. target cells of the innate immune system, and it is possible that even a self-limited transient exposure may influence development of diseases caused by the other pathogens. Excellent reviews have considered the pathogenesis of these organisms (43–46). Of equal importance as the role of polymicrobial infections in clinical disease, the combination of pathogens and symbionts no doubt constitutes a species-specific tick microbiome. There is evidence for detrimental, beneficial, or neutral effects among the interactions of the prokaryotes with each other and with the host tick. These effects can influence access to nutrients, which in turn influence overall fitness of the organisms. For these reasons, and to verify the changing conditions of tick-borne diseases, we performed a polymicrobial assessment of the three species of ticks associated with human disease, including one that is a recent invader. Our results reveal a complex pattern of tick infections with new and emergent pathogens on a background of rapidly shifting tick populations that justify a polymicrobial approach to the study of tick-borne diseases.
RESULTS
Ticks were collected in the spring and fall of 2018 from multiple locations throughout Suffolk, a suburban county that occupies the central and eastern part of Long Island, NY (https://gisportal.suffolkcountyny.gov/gis/home/). All locations were clustered into northern sites and southern sites (Fig. 1). In the spring, we collected both adults and nymphs of A. americanum (676) and I. scapularis (198) and adults of D. variabilis (296). Fall collections were comprised of 480 I. scapularis adults. In total, we examined 1,633 individual ticks and 17 pools of 170 A. americanum nymphs (10 nymphs per pool). I. scapularis ticks were screened for the presence of B. burgdorferi, B. miyamotoi, A. phagocytophilum, B. microti, POWV, and Rickettsia spp. We did not screen I. scapularis ticks for other ehrlichial agents, because they were not detected in a previous study in our area and their geographic range appears to be limited to the Upper Midwest (39). A. americanum ticks were screened for B. lonestari, Ehrlichia spp., and Rickettsia spp., as well as for the five agents tested in I. scapularis. D. variabilis ticks were screened for Rickettsia spp. only.
FIG 1.
Map of Suffolk County, New York, showing locations of tick collection in the northern and southern regions.
Pathogen burden of I. scapularis.
In the northeastern United States, I. scapularis is implicated in the transmission of five agents, including B. burgdorferi, A. phagocytophilum, B. microti, B. miyamotoi, and POWV. All five agents and R. buchneri were detected in our study. A total of 430 of 678 (63%) I. scapularis ticks were positive for at least one human pathogen. B. burgdorferi was the most prevalent pathogen in both adults and nymphs (57% and 27%, respectively), followed by B. microti (14% and 15%, respectively) and A. phagocytophilum (14% and 2%, respectively). B. miyamotoi and POWV were detected only in adult ticks and at lower prevalences (3% and 2%, respectively) (Table 1; Fig. 2). We performed additional assays to characterize the strains of A. phagocytophilum and POWV. There are two strains of A. phagocytophilum that differ by 2 bp from their 16S rRNA sequences. One variant (AP variant 1–nonpathogenic) does not infect humans and is carried by deer (47, 48). The HA strain that is implicated in human disease was present in 67 out of 90 (75%) A. phagocytophilum-positive ticks; 19 were found to be of the nonpathogenic strain (21%), and 4 could not be identified further. All POWV strains were part of lineage II, which is associated exclusively with transmission by I. scapularis. In addition to these five agents, we found that of the 187 ticks tested, 87% were positive for Rickettsia. Of sequences of PCR products from a 20% subset of the Rickettsia-positive ticks, all were identified as R. buchneri, an ovarian symbiont. Overall, there were no differences in regional infection rates, with the exceptions of B. microti (P = 0.02) and POWV (P = 0.01), which were more prevalent in northern Suffolk County (Table 1; Fig. 2). There were no statistically significant differences in infection rates between spring and fall collections in all the pathogens tested for I. scapularis (Table 2). With the exceptions of R. buchneri in I. scapularis ticks and R. amblyommatis in lone star ticks, adult ticks had significantly higher infection rates with B. burgdorferi and A. phagocytophilum than nymphs (P = 0.00003 and P = 0.004, respectively) (Tables 1, 2, and 5; Fig. 2). This is a very well-known feature of tick-borne diseases.
TABLE 1.
Prevalence of five pathogens and R. buchneri in I. scapularis ticks by seasona
| Agentb | No. of positive ticks (% [95% CI]) |
||
|---|---|---|---|
| Spring (n = 198 [140 A/58 N]) | Fall (n = 480) |
Total (n = 678 [620 A/58 N]) |
|
| Borrelia burgdorferi | |||
| A | 85 (61 [52–69]) | 266 (55 [51–60]) | 351 (57 [53–60]) |
| N | 16 (27 [17–41]) | 16 (27 [17–41]) | |
| Borrelia miyamotoi | |||
| A | 4 (3 [1–8]) | 16 (3 [2–5]) | 20 (3 [2–5]) |
| N | 0 (0 [0–8]) | 0 (0 [0–8]) | |
| Anaplasma phagocytophilum | |||
| A | 19 (14 [9–21]) | 70 (15 [12–18]) | 89 (14 [12–17]) |
| N | 1 (2 [0.1–10]) | 1 (2 [0.1–10]) | |
| Babesia microti | |||
| A | 27 (19 [13–27]) | 61 (13 [10–16]) | 88 (14 [12–17]) |
| N | 9 (15 [8–28]) | 9 (15 [8–28]) | |
| POWV | |||
| A | 5 (3 [1–8]) | 6 (1 [0.5–3]) | 11 (2 [1–3]) |
| N | 0 (0 [0–8]) | 0 (0 [0–8]) | |
| Rickettsia buchneric | |||
| A | 26 (81 [63–92]) | 64 (89 [79–95]) | 90 (86 [78–92]) |
| N | 21 (91 [70–98]) | 21 (91 [70–98]) | |
Total prevalences for both spring and fall seasons are included (excluding R. buchneri). Values are means and 95% confidence intervals (CI).
A, adults; N, nymphs.
Thirty-two adults and 23 nymphs were tested for the spring season; 72 adult ticks were tested for the fall season.
FIG 2.
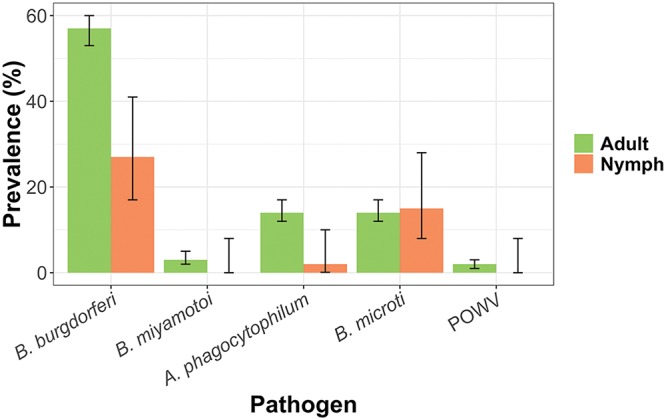
Prevalence of five pathogens in I. scapularis adults and nymphs.
TABLE 2.
Prevalence of five pathogens and R. buchneri in I. scapularis ticks by geographical region of Suffolk Countya
| Agentb | No. of positive ticks (% [95% CI]) |
|||
|---|---|---|---|---|
| Spring |
Fall |
|||
| North (n = 125 [80 A/45 N]) | South (n = 73 [60 A/13 N]) | North (n = 240) | South (n = 240) | |
| Borrelia burgdorferi | ||||
| A | 58 (72 [61–82]) | 27 (45 [32–58]) | 139 (58 [51–64]) | 127 (53 [46–59]) |
| N | 12 (27 [15–42]) | 4 (31 [10–61]) | ||
| Borrelia miyamotoi | ||||
| A | 3 (4 [1–11]) | 1 (2 [0.1–10]) | 9 (4 [2–7]) | 7 (3 [1–6]) |
| N | 0 (0 [0–10]) | 0 (0 [0–28]) | ||
| Anaplasma phagocytophilum | ||||
| A | 11 (14 [7–24]) | 8 (13 [6–25]) | 35 (15 [10–20]) | 35 (15 [10–20]) |
| N | 1 (2 [0.1–13]) | 0 (0 [0–28]) | ||
| Babesia microti | ||||
| A | 19 (24 [15–35]) | 8 (13 [6–25]) | 35 (15 [10–20]) | 26 (11 [7–16]) |
| N | 9 (20 [10–35]) | 0 (0 [0–28]) | ||
| POWV | ||||
| A | 5 (6 [2–15]) | 0 (0 [0–7]) | 5 (2 [1–5]) | 1 (0.4 [0–3]) |
| N | 0 (0 [0–10]) | 0 (0 [0–28]) | ||
| Rickettsia buchneric | ||||
| A | 18 (78 [56–92]) | 8 (89 [51–99]) | 29 (91 [74–97]) | 35 (87 [72–95]) |
| N | 21 (91 [70–98]) | |||
Values are means and 95% confidence intervals (CI).
A, adults; N, nymphs.
Thirty-two adults and 23 nymphs were tested for the spring season; 72 adult ticks were tested for the fall season.
TABLE 5.
Agents detected in A. americanum and D. variabilis ticksa
| Tick species and agentb | No. of positive ticks (% [95% CI]) |
||
|---|---|---|---|
| Spring |
Total | ||
| North | South | ||
| Amblyomma americanum | n = 352 (128 A/224 N) | n = 324 (194 A/130 N) | n = 676 (322 A/354 N) |
| Borrelia lonestaric | |||
| A | 1 (1 [0.05–6]) | 5 (3 [1–8]) | 6 (2 [1–5]) |
| N | 1 (0.5 [0.2–3]) | 1 (1 [0.5–6]) | 2 (0.6 [0.1–3]) |
| Ehrlichia chaffeensis | |||
| A | 3 (2 [0.6–7]) | 3 (1 [0.4–5]) | 6 (2 [0.7–4]) |
| N | 1 (0.4 [0.2–3]) | 1 (0.8 [0.4–5]) | 2 (0.6 [0.1–2]) |
| Ehrlichia ewingii | |||
| A | 8 (6 [3–12]) | 4 (2 [0.7–5]) | 12 (4 [2–6]) |
| N | 2 (1 [0.1–3]) | 0 (0 [0–3]) | 2 (0.6 [0.1–2]) |
| E. ruminantium-like species | |||
| A | 2 (2 [0.3–6]) | 3 (1 [0.4–5]) | 5 (1 [0.6–4]) |
| N | 0 (0 [0–2]) | 0 (0 [0–3]) | 0 (0 [0–1]) |
| Total Ehrlichia spp. | |||
| A | 13 (10 [6–17]) | 10 (5 [3–9]) | 23 (7 [5–11]) |
| N | 3 (1 [0.3–4]) | 1 (0.8 [0.4–5]) | 4 (1 [0.4–3]) |
| Rickettsia amblyommatis | |||
| A | 79 (62 [53–70]) | 119 (61 [54–68]) | 198 (61 [56–67]) |
| N | 111 (49 [43–56]) | 85 (65 [56–73]) | 196 (55 [50–60]) |
| Dermacentor variabilis | n = 124 | n = 172 | n = 296 |
| Rickettsia montanensis | 6 (5 [2–11]) | 2 (1 [0.2–5]) | 8 (3 [1–5]) |
Values are means and 95% confidence intervals (CIs).
A, adult; N, nymph.
A total of 548 ticks were tested from the northern (102 adults, 210 nymphs) and southern (140 adults, 96 nymphs) regions of Suffolk County.
A total of 22% (147 of 678) of I. scapularis ticks were infected with more than one pathogen. Dual infections were detected in 126 (19%) of the ticks. Triple infections were observed in 21 (3%) of the ticks. A single adult tick collected in the fall was coinfected with four pathogens (Table 3). The highest coinfection prevalence was found with B. burgdorferi and B. microti, with 9% of the total ticks analyzed. Ticks coinfected with B. burgdorferi and A. phagocytophilum accounted for 7% of the total ticks. Triple infections with these three pathogens were more prevalent in both seasons than dual infections with B. burgdorferi-B. miyamotoi, B. burgdorferi-POWV, B. miyamotoi-A. phagocyotophilum, and B. miyamotoi-B. microti (Table 3).
TABLE 3.
Polymicrobial infections detected in I. scapularis ticks
| Polymicrobial infections | No. (%) of coinfected I. scapularis ticks |
||
|---|---|---|---|
| Spring (n = 198) | Fall (n = 480) | Total (n = 678) | |
| Two pathogens | |||
| B. burgdorferi-B. miyamotoi | 2 (1) | 6 (1) | 8 (1) |
| B. burgdorferi-A. phagocytophilum | 11 (6) | 39 (8) | 50 (7) |
| B. burgdorferi-B. microti | 20 (10) | 41 (8) | 61 (9) |
| B. burgdorferi-POWV | 2 (1) | 1 (0.2) | 3 (0.4) |
| B. miyamotoi-A. phagocytophilum | 3 (0.6) | 3 (0.4) | |
| B. miyamotoi-B. microti | 1 (0.2) | 1 (0.1) | |
| Three pathogens | |||
| B. burgdorferi-B. miyamotoi-A. phagocytophilum | 2 (0.4) | 2 (0.3) | |
| B. burgdorferi-A. phagocytophilum-B. microti | 5 (2) | 2 (0.4) | 7 (1) |
| B. burgdorferi-B. microti-POWV | 2 (1) | 1 (0.2) | 3 (0.4) |
| A. phagocytophilum-B. microti-POWV | 1 (0.2) | 1 (0.1) | |
| Four pathogens | |||
| B. burgdorferi-B. miyamotoi-A. phagocytophilum-B. microti | 1 (0.2) | 1 (0.1) | |
Coinfection of A. phagocytophilum and B. microti with B. burgdorferi in host-seeking I. scapularis ticks has been reported in several regions of the United States (Table 4). The 54%, 14%, and 13% prevalences of B. burgdorferi, B. microti, and A. phagocytophilum, respectively, in this study are consistent with the prevalence rates found in New York State (39, 49, 50) but are higher than those of other regions in the Northeast (Table 4). The insularity of Long Island could explain and be a determinant of the high global prevalence rate of infected I. scapularis ticks found in this study, since rodent population densities inhabiting this region could be affected by the island syndrome (51) that favors increases in rodent populations, which, in turn, could lead to greater opportunities for juvenile stages of I. scapularis to find a rodent host and therefore get infected by one or more pathogens.
TABLE 4.
Prevalence of the most common single infections and pathogen coinfections reported in questing I. scapularis ticks in the United States from 2003 to 2017
| Location | Yr(s) | No. of ticks | Stagea | Single infections (%)b
|
Double infections (%) |
Triple infections (%) of Bb-Apc-Bm |
Reference | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Bb | Apc | Bm | Bb-Apc | Bb-Bm | ||||||
| NJ | 2003–2004 | 147 | A | 50 | 6 | 3 | 115 | |||
| NY | 2003–2006 | 3,300 | N | 14 | 6 | 3 | 0.5 | 1 | 116 | |
| 7,914 | A | 46 | 12 | 2 | 6 | 1 | 0.4 | 116 | ||
| ME | 2003 | 100 | A | 58 | 16 | 7 | 9 | 11d | 117 | |
| IN | 2004 | 100 | A | 72 | 5 | 4 | 4d | 117 | ||
| PA | 2005 | 94 | A | 52 | 1 | 1 | 1d | 117 | ||
| WI | 2006 | 100 | A | 35 | 14 | 8 | 4d | 117 | ||
| MI | 2006 | 119 | A | 50 | 4 | 2 | 118 | |||
| NJ | 2004–2007 | 478 | N | 10 | 4 | 3 | 119 | |||
| 610 | A | 45 | 8 | 6 | ||||||
| IA | 2007–2009 | 156 | N | 17 | 29 | 6 | 120 | |||
| NY | 2008 | 132 | A | 62 | 22 | 26 | 16 | 22 | 7 | 49 |
| CT | 2008 | 154 | A | 65 | 17 | 16 | 16 | 12 | 3 | 49 |
| WI | 2009–2013 | 748 | N | 29 | 5 | 3 | 121 | |||
| NY | 2011 | 323e | N | 67 | 34 | 19 | 50 | |||
| 922e | A | 60 | 23 | 4 | ||||||
| 466 | A | 55 | 18 | 3 | 10 | 1 | 1 | |||
| NY | 2011–2012 | 4,368 | N | 19 | 5 | 6 | 2 | 7 | 1 | 122 |
| CT | 2011–2012 | 514 | N | 13 | 3 | 6 | 1 | 2 | 123 | |
| MD | 2011–2012 | 124 | N | 19 | 1 | 123 | ||||
| NY | 2011–2012 | 207 | N | 23 | 5 | 11 | 6 | 123 | ||
| PA | 2013 | 1,363 | A | 47 | 3 | 3 | 1 | 2 | 124 | |
| MD | 2014–2015 | 168 | N | 21 | 1 | 125 | ||||
| NY | 2014–2015 | 299 | N | 17 | 9 | 3 | 3 | 4 | 125 | |
| PA | 2014–2015 | 114 | N | 22 | 3 | 2 | 125 | |||
| VA | 2014–2015 | 472 | N | 12 | 1 | 1 | 125 | |||
| DC | 2014–2015 | 253 | N | 23 | 4 | 125 | ||||
| ME | 2015 | 154 | N | 18 | 3 | 4 | 3 | 125 | ||
| MN | 2015 | 1,240 | N | 25 | 6 | 5 | 2 | 2 | 1 | 126 |
| WI | 2015 | 112 | A | 41 | 11 | 9 | 1 | 1 | 1 | 127 |
| PA | 2015–2017 | 1,721 | N | 25 | 1 | 3 | 1 | 1 | 128 | |
| NY | 2016–2017 | 197 | A | 56 | 11 | 8 | 4 | 0.5 | 2 | 39 |
N, nymphs; A, adults.
Bb, Borrelia burgdorferi; Ap, Anaplasma phagocytophilum; Bm, Babesia microti.
Ap human variant.
Coinfection of B. burgdorferi and B. microti plus Babesia odocoilei combined.
Tested in pools of single individuals to a maximum of 10.
Pathogen burden of A. americanum.
The aggressive species A. americanum is implicated in the transmission of the causative agents of ehrlichiosis (Table 5). We detected Ehrlichia spp. in 23 (7%) of adult A. americanum ticks. E. ewingii was the predominant species in our ticks (12 ticks; 4%), followed by E. chaffeensis (6 ticks; 2%), and an Ehrlichia ruminantium-like species (5 ticks; 1%). We also detected Ehrlichia in 4 out 354 individual A. americanum nymphs. Two nymphs were positive for E. ewingii and 2 for E. chaffeensis. All pools of A. americanum nymphs were negative for Ehrlichia.
B. lonestari was present in 8 ticks (1%). We did not detect B. burgdorferi in A. americanum, reasserting that this tick species does not play a role in its transmission. Early reports of the Lyme disease agent in this tick may have been mistaken with B. lonestari (52). None of the A. americanum ticks had detectable B. microti, B. miyamotoi, A. phagocytophilum, or POWV.
We detected a single Rickettsia species in A. americanum, identified as R. amblyommatis. More than half of A. americanum (58%) ticks were positive for R. amblyommatis.
Impact of tick-borne blood infections.
Three pathogens detected in this study infect blood cells. A. phagocytophilum infects neutrophils, B. microti infects erythrocytes, and the Ehrlichia spp. infect monocytes. All three pathogens can also be transmitted by transfusion of blood and blood products (53–55).
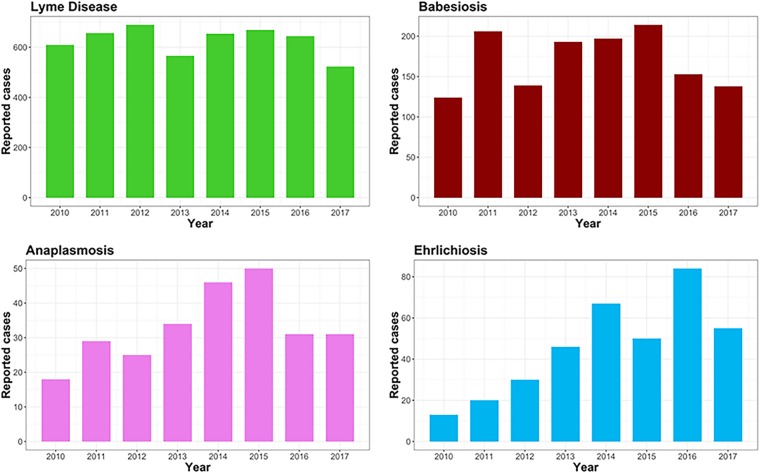
The ratio of Lyme disease cases to babesiosis cases has been approximately 4 to 1 for the last 8 years, higher than in other areas where I. scapularis is endemic (Fig. 3; Table 4). This ratio also holds for the ratio of B. burgdorferi to B. microti in ticks in this study, i.e., 3.8 to 1 (Tables 1 and 2). Conversely, during the same 8-year period, the ratio of Lyme disease cases to cases of anaplasmosis is approximately 15 to 1, but our data showed a 4-to-1 ratio of B. burgdorferi to A. phagocytophilum. This disparity between the reported cases of anaplasmosis and the tick infection rates is not readily explainable but could be due to the role that the nonpathogenic strain might have in exposure. Alternatively, a larger number of anaplasmosis cases may not be severe enough to warrant medical attention.
FIG 3.
Number of cases of anaplasmosis, babesiosis, ehrlichiosis, and Lyme disease from 2010 to 2017 in Suffolk County, New York. Numbers were derived from https://www.health.ny.gov/statistics/diseases/communicable/.
There are increasing numbers of reported cases of ehrlichiosis in Suffolk County (Fig. 3). Although the infection rate of Ehrlichia spp. in A. americanum nymphs and adults was less than 5% (Table 5), the growing populations of these ticks as well as their aggressive nature create additional anxiety over this emerging infection (Fig. 3).
The three tick-borne blood infections have several features in common. The frequency of reported cases of all three is highest in the elderly (anaplasmosis susceptibility appears to be less bound by age) and in males. Importantly, people at risk for clinical infection and for increased disease severity have compromised immune systems due to a number of causes, which can include cancer therapy, immunosuppressive drugs to treat autoimmune disorders, splenectomy, preexisting liver disease, and endogenous disorders of the immune system, among others. In communities with both high tick infestations and the presence of a sizable elderly population, the features of the three blood infections are important contributors to their high morbidity and mortality (https://www.cdc.gov/anaplasmosis/stats/index.html; https://www.cdc.gov/parasites/babesiosis/disease.html; https://www.cdc.gov/ehrlichiosis/stats/index.html).
The genus Rickettsia constitutes the largest biomass in the three tick species.
R. montanensis (2.7%) was the only agent detected in D. variabilis (Table 5). The cases of RMSF have decreased markedly in Suffolk County since the 1970s, with the numbers of cases ranging from 2 to 14 per year in the last decade (https://www.health.ny.gov/statistics/diseases/communicable/). R. amblyommatis was present in more than half of the A. americanum ticks sampled in this study (Table 5). R. buchneri is an ovarian endosymbiont with an overwhelming presence in I. scapularis (Table 1; Fig. 2) and has not been associated with human disease. However, its presence could be a factor in the associations with pathogens found simultaneously in this tick.
DISCUSSION
The polymicrobial infection approach to tick-borne diseases is rooted in the discovery of new or emergent pathogens, such as the ones detected in this study, changes in or discovery of new pathogen genotypes, such as is the case for POWV and A. phagocytophilum, and expansion of tick ranges as well as of their pathogens, as represented by A. americanum. The changes in tick populations and their pathogens may be the result of climate change, with unpredictable short- or long-term consequences. However, factors other than climate change, such as anthropogenic changes and host availability, might have great impact.
Ixodes scapularis microbiome.
I. scapularis is a public health threat (56). B. burgdorferi infects more than half of the adult I. scapularis ticks tested, and this accounts for the high number of Lyme disease cases in our area (Fig. 3). Of note is that nearly one-quarter of the I. scapularis ticks tested had polymicrobial infections, and this justifies the modification of the clinical approach to tick-borne diseases to cover all infection possibilities. At a more fundamental level, the polymicrobial infections open new possibilities for research into the microbial relationships with and within the tick.
Babesia microti causes clinical disease in elderly, immunosuppressed, and splenectomized patients (57, 58) and also in blood transfusion recipients (59). Asymptomatic babesiosis exists in individuals that do not have the above risk factors, representing a group of seropositives, and these represent a major risk as blood transfusion donors. Genomic tools have added new perspectives on the expansion of B. microti (60). There are Northeast and Midwest lineages segregating into local populations of the parasite (61, 62), suggesting dynamic adaptation changes promoting expansion.
Anaplasmosis is variable in terms of severity. The two variants of A. phagocytophilum also have ecological implications, in that the AP-HA variant is acquired from mice, whereas the reservoir of the nonpathogenic variant is deer. That these variants were detected in our study is representative of the advances at the molecular biology level that have a bearing on epidemiology.
POWV is the causative agent of a life-threatening encephalitis (63). There have not been any reported cases of POWV in our area, but its low-level presence (lineage II) in I. scapularis could result in transmission to humans. Lineage II virus circulates in an I. scapularis–white-footed mouse cycle, and it is precisely in this cycle that this virus can become a part of the polymicrobial group of pathogens (63–69).
Amblyomma americanum expansion.
Since the first confirmed records (22), and thereafter (23, 70), the presence of A. americanum has increased markedly, and it is likely the most abundant tick species in our environment. However, the lack of systematic tick surveillance in the intervening years since the first records were made makes this difficult to confirm. The expansion of A. americanum into the North Atlantic states has been mapped and documented (20, 21, 52, 71). Sonenshine connected the northward expansion of A. americanum to climate change (72). This expansion has occurred in a short time. An increase of nearly 60% in A. americanum numbers has been reported in Connecticut in the last 20 years (73), as well as in New Jersey (74). Studies in our laboratory showed that genetic changes occur in the tick population during range expansion (75).
There are serious consequences to the range expansion of A. americanum. Notably, even in the absence of transmitted pathogens, this species is an aggressive and tenacious tick. All three stages bite humans, and the larvae, in particular, can infest in large numbers, leading to an uncomfortable dermatitis. There are some subtle effects as well. Large populations of A. americanum in a community can lead to an exaggerated perception of the risk of Lyme disease, as most people cannot differentiate between I. scapularis and lone star ticks (76).
The cases of ehrlichiosis have increased steadily in our area in recent years as a function of the increases in the populations of A. americanum (Fig. 3). Our results indicate that there are three Ehrlichia species in Suffolk County. E. ewingii is the most frequent Ehrlichia species present in our ticks, and therefore, it could be the main causative agent of human ehrlichiosis in this area (49) (Table 5). E. chaffeensis is clinically indistinguishable from E. ewingii, and species identification is not possible by serology, so it may be difficult to determine the infecting species (77). An E. ruminantium-like species is present in A. americanum in our area, although its disease-causing potential is unclear (78). Transfusion-acquired E. ewingii has been documented (79), making this organism a serious threat to recipients who are at greater risk of a severe infection (80), and infection with E. ewingii has been documented in patients that were not immunosuppressed. Pediatric ehrlichiosis with an increased case fatality rate has been documented as well (81).
B. lonestari has been detected at low levels, but STARI has not been documented in our area as it has elsewhere in areas where A. americanum is abundant (82–84). B. lonestari has been linked as the causative agent of STARI from a single patient with erythema migrans (85). However, to date, this has been the only case reported.
Rickettsial biome.
The rickettsiae constitute the largest prokaryotic biomass in two of the three species of ticks detected here. The sheer abundance of these rickettsiae can be expected to have a major role in the vector-pathogen relationship and to influence the pathogen-patient relationship after transmission.
There was overwhelming infection of R. amblyommatis (40) in our A. americanum populations. This rickettsia may be more than a symbiont, as cases of rickettsiosis may have been caused by R. amblyommatis (86). RMSF-like illness may be associated with the expanding range of A. americanum (21). This is further supported by the lack of detection of R. rickettsii in tick surveillance studies, including the work presented here. However, that there is a high prevalence of R. amblyommatis does not necessarily support its role in human disease. Whether a symbiont, a pathogen under certain conditions, or an outright pathogen, R. amblyommatis has introduced a confounding factor in the diagnoses of febrile tick-borne infections, as positive serologies may be misinterpreted.
The rickettsiae present in the ovaries of I. scapularis belong to the spotted fever group and are closely related to R. montanensis (36, 87, 88). The most abundant prokaryotic DNA sequences found in I. scapularis were from R. buchneri (37–39). R. buchneri underwent the introduction of transposons with genomic deletions and mutations that resulted in the loss of pathogenicity (89). R. buchneri contains multiple mobile genetic elements that endow this species with a plastic and repetitive genome that is thought to account for its symbiotic lifestyle (90). Its overwhelming presence in this study suggests that this organism plays an undisputed role in the homeostasis of this tick.
R. montanensis was the only agent detected in D. variabilis ticks, with no detection of R. rickettsii. This tick was associated with an outbreak of RMSF in our area in the late 1970s (91). Given our findings in this study, we support the suggestion that other rickettsial species could confound a diagnosis of RMSF (92, 93), although the prevalence of this symbiotic organism in D. variabilis seems to have remained stable for many years (92).
Polymicrobial infections may be synergistic in enhancing the severity of human illnesses, and more-deleterious interactions could be discovered if there was greater emphasis on a pluralistic approach to tick-borne diseases. There is an extensive literature on the topic of coinfections, and we cite the most recent for American patients (94) and for Europe (95). However, despite the high levels of tick coinfections, there is at least one therapeutic feature that could mitigate the impact of polymicrobial infections in patients. Two of the most common coinfecting organisms, B. burgdorferi and A. phagocytophilum, are responsive to doxycycline, so both could be treated simultaneously. Polymicrobial infections can lead to treatment problems as well. The possibility of side effects has resulted in doxycycline not being prescribed to young children, an overrepresented group in Lyme disease. B. microti requires antiparasitic drugs that do not work against B. burgdorferi and A. phagocytophilum. Likewise, beta-lactam antibiotics can be active against B. burgdorferi but not A. phagocytophilum, and it is in these cases where the complexity of polymicrobial infections requires further treatment evaluation.
Nonetheless, the relationships of pathogens and symbionts in the tick have biological significance but appear to be complex. Pathogen burden could be important in maintaining the balance of organisms within the tick, where deviations may alter the mechanics of transmission. On one hand, there is evidence for the possibility of limited interactions among the several coinfecting pathogens in I. scapularis. These pathogens occupy different anatomical niches in the tick, as some are extracellular in the lumen of the midgut (Borrelia) and others are obligate intracellular organisms (Anaplasma, Babesia, and POWV), so it is possible that direct contact among them may be limited. There is a hierarchy of transmission of pathogens in relation to the duration of blood feeding. POWV can be transmitted within 15 min of tick attachment, and both A. phagocytophilum and B. miyamotoi can be transmitted within the first 24 h of attachment. Transmission of B. burgdorferi increases with the length of attachment (96–98), and B. microti needs to change its morphology while in the salivary glands of the tick, requiring a longer period of time for transmission. This pattern of staggered transmission may further segregate the pathogens within the vector and actually favor acquisition of the organisms that are transmitted quickly, as people may remove ticks in time to abort infections with the slower pathogens.
Notwithstanding, there is increasing evidence for far-reaching interactions among symbionts and pathogens with each other and, collectively, with the ticks. In some instances, the interaction can be to the benefit of the pathogen. A. phagocytophilum induces the production of the antifreeze glycoprotein (IAFGP), which in turn makes infection easier (99). Relationships between the symbiont-pathogen and the tick may result in a neutral status quo; for example, the vector competence of A. americanum for R. rickettsii was not significantly affected by R. amblyommatis (100). In other instances, tick responses can be harmful to pathogens. Hemocytes of I. scapularis ingest B. burgdorferi (101), and this tick regulates infection with A. phagocytophilum through the production of antimicrobial peptides, and actin phosphorylation for survival, as well as proteases to increase vector fitness (102–104). R. montanensis (an organism of unproven pathogen status) induces ticks to produce defensins (105) and protease inhibitors that limit colonization by this Rickettsia species (106). Anaplasma, Borrelia, Ehrlichia, and Rickettsia spp. do not have interbacterial effector and immunity genes whose products regulate interactions among bacteria utilizing the same niche (38). The lack of these genes can lead to a system of shared tolerance for each other that would be clearly to their advantage for survival in the vector. It appears that we have only begun to appreciate the interactions of the microbes with each other and with the tick. The best approach to study the interactions among the prokaryotes in the tick is to consider the polymicrobial nature of these fascinating biological systems (107–109).
MATERIALS AND METHODS
Tick collection and study areas.
Two active tick-borne pathogen surveillance programs were conducted during the spring season peak (from May to July) and the fall season peak (from October to November) throughout Suffolk County, New York, in 2018. Questing ticks were collected from vegetation along trails by flagging a 1-m2 cotton flannel fabric attached on both ends to a wooden pole between 10:00 and 14:00 h during sunny days. The area of study was divided into two geographical regions to compare potential differences in infection rates between the northern and the southern regions of the county (Fig. 1). Ticks were collected for a minimum of 60 min per site in order to collect a representative number of each species to estimate the prevalence of pathogens in each geographical region.
All collected ticks were identified morphologically to species, life stage, and sex by use of a dissecting microscope and the appropriate taxonomic keys and stored at –80°C until further processing (110–112).
TNA extraction from ticks.
Total nucleic acids (TNA) were extracted using the NucliSENS easyMAG platform (bioMérieux, Durham, NC). Ticks were grouped by location and day of collection. To remove environmental contaminants, pools of 10 ticks were washed with 3% hydrogen peroxide, followed by three washes with 1 ml of 1× phosphate-buffered saline (PBS). The ticks were then placed individually in a sterile 1.5-ml centrifuge tube and homogenized using a 21-gauge, 1.5-in needle in 50 μl of 1× PBS. The entire volume was then added to NucliSENS easyMAG lysis buffer (bioMérieux, Durham, NC). TNA were extracted according to the manufacturer’s protocol, eluted in 40 μl, and stored at –80°C.
Pathogen detection by real-time PCR.
We utilized a pathogen detection strategy in which we tested each tick TNA by quantitative PCR (qPCR) using two approaches, consisting of either a single-agent qPCR using DNA as a template or a multiplex one-step reverse transcription PCR (RT-qPCR), using both cDNA and DNA as a template (113).
All I. scapularis and A. americanum samples were screened for the presence of B. burgdorferi, B. miyamotoi/B. lonestari, B. microti, A. phagocytophilum, and POWV with the multiplex RT-qPCR (Table 6). Our rationale for testing A. americanum was to evaluate the role of this tick as a potential vector of these five agents. For positive controls of the multiplex assay, we employed quantified plasmid standards at 10, 100, and 1,000 copies (113). All remaining tests consisted of single-agent qPCR assays. For detection of Rickettsia, we developed a qPCR assay designed to detect a fragment within the ompB gene of the most common spotted fever group Rickettsia species. This assay was used to screen all TNA from A. americanum and D. variabilis ticks and from 127 I. scapularis ticks. For positive controls, we used TNA from an I. scapularis adult previously shown to be infected with R. buchneri. To detect Ehrlichia spp., we employed an Ehrlichia-specific assay targeting a portion of the 16S rRNA gene. This assay was used to test all A. americanum ticks (Table 6). For positive controls, we used DNA from a lysate of E. chaffeensis-infected DH82 cells.
TABLE 6.
Primer and probe sequences for RT-PCRa
| Pathogen | Gene target | Primer pair | Probe | 5′ dye | 3′ quencher |
|---|---|---|---|---|---|
| Borrelia burgdorferi | ospA | Fwd: CCTTCAAGTACTCCAGATCCATTG | CAACAGTAGACAAGCTTGA | 6-FAM | MGB |
| Rev: AACAAAGACGGCAAGTACGATC | |||||
| Borrelia miyamotoi/B. lonestari | flaB | Fwd: AGCACAAGCTTCATGGACATTGA | TGTGGGTGCAAATCAGGATGAAGCA | HEX | BHQ-1 |
| Rev: GAGCTGCTTGAGCACCTTCTC | |||||
| Babesia microti | cox1 | Fwd: CATCATGCCAGGCCTGTTTG | TACTACCCATACTGGTCGGTGCTCC | Quasar 705 | BHQ-2 |
| Rev: GAAGAAACCACAAGAGCAAATGC | |||||
| Anaplasma phagocytophilum | 16S rRNA | Fwd: GGCATGTAGGCGGTTCGGT | GCCAGGGCTTAACCCTGGAGCT | Cy5 | BHQ-2 |
| Rev: CACTAGGAATTCCGCTATCCTCTCC | |||||
| Powassan virus | 3′UTR | Fwd: GTGATGTGGCAGCGCACC | CCTACTGCGGCAGCACACACAGTG | Texas Red | BHQ-2 |
| Rev: CTGCGTCGGGAGCGACCA | |||||
| Ehrlichia spp. | 16S rRNA | Fwd: CGTAAAGGGCACGTAGGTGGACTA | TCGAAAGAGGATAGCGGA | VIC | MGB |
| Rev: CACCTCAGTGTCAGTATCGAACCA | |||||
| Rickettsia spp. | ompB | Fwd: AACAAGCTGCTGGGCACCATAT | AGAGAATGAGAAACCGTTAACGT | FAM | MGB |
| Rev: CGGTGCTGCTATCGGTATCACT |
UTR, untranslated region; 6FAM, 6-carboxyfluorescein; HEX, 6-carboxy-2,4,4,5,7,7-hexachlorofluorescein; BHQ, black hole quencher.
All qPCRs were performed on a Bio-Rad C1000 Touch system with a CFX96 optical module (Bio-Rad, Hercules, CA) using the RNA UltraSense one-step quantitative RT-PCR system (Invitrogen, Carlsbad, CA). The final reaction mixture contained 5 μl of template and 20 μl of master mixture. The master mixture contained 0.2 μM each forward primer, 0.3 μM each reverse primer, 0.1 μM each probe, 1.25 μl of RNA UltraSense enzyme mix, and 5 μl RNA UltraSense 5× reaction mix. The reverse transcription step was performed at 55°C for 15 min, followed by incubation at 95°C for 10 min. The RT step was omitted for the Ehrlichia and Rickettsia qPCR assays. The PCR consisted of 40 cycles at 95°C for 15 s and 60°C for 30 s.
Qualitative PCR.
We developed qualitative PCR assays to further characterize the species detected by qPCR. For Rickettsia, we employed an assay that amplified 380 nucleotides (nt) within ompB. For Anaplasma, we designed an assay within gltA to differentiate between the pathogenic agent (AP-HA) and nonpathogenic variants (AP-variant 1). For Ehrlichia, we first employed an assay targeting the 16S rRNA gene. Any samples found to be positive for the E. ruminantium variant were tested with a nested PCR targeting the gltA gene.
For characterization of B. burgdorferi, B. miyamotoi, B. lonestari, and POWV, we used our previously established assays (49, 114).
All qualitative PCRs were performed using AmpliTaq Gold 360 master mix (Applied Biosystems, Foster City, CA) in a 25 μl-reaction mixture with 2 μl of template, 0.2 μM forward primer, and 0.3 μM reverse primer and 12.5 μl of the polymerase. Primers and reaction conditions used for each pathogen are described in Table 7. A nontemplate control was included in each assay. PCR products were resolved through 2% agarose gel in Tris-borate-EDTA (TBE) buffer and sequenced by dideoxy sequencing. All sequences were analyzed using Geneious 10.0.9 software.
TABLE 7.
Primer sequences and PCR conditions for qualitative assay
| Pathogen | Gene target | Primer pair | Product length (nt) | PCR conditions |
|---|---|---|---|---|
| Borrelia burgdorferi | ospA | Fwd: GCGTTTCAGTAGATTTGCCT | 676 | 95°C for 10 min; 40 cycles of 95°C for 30 s, 57°C for 30 s, and 72°C for 60 s |
| Rev: TTGGTGCCATTTGAGTCGTA | ||||
|
Borrelia miyamotoi/ B. lonestari |
flaB | Fwd: GGGATTATMAATCATAATACRTCAGC | 967 (B. miyamotoi)/ 949 (B. lonestari) |
95°C for 10 min; 40 cycles of 95°C for 30 s, 57°C for 30 s, and 72°C for 70 s |
| Rev: TTGCTTGTGCAATCATAGCCATTGC | ||||
| Babesia microti | 18S rRNA | Fwd: GGGACTTTGCGTTCATAAAACGC | 171 | 95°C for 10 min; 40 cycles of 95°C for 30 s, 60°C for 30 s, and 72°C for 45 s |
| Rev: GCAATAATCTATCCCCATCACGAT | ||||
|
Anaplasma phagocytophilum |
gltA | Primary reaction | 668 | 95°C for 10 min; 40 cycles of 95°C for 30 s, 62°C for 30 s, and 72°C for 60 s (same conditions for primary and secondary reactions) |
| Fwd: ACCGGAACCCCCATAGCTCT | ||||
| Rev: GCAAGTCGCATTGATCCGCT | ||||
| Secondary reaction | 589 | |||
| Fwd: TCGACATTTGGGTACAACTTGCG | ||||
| Rev: CAATTGCGAAAGTACCCGGCA | ||||
| Powassan virus | Envelope (E) glycoprotein |
Fwd: GGCAACTGCATCTCTATRAATCC | 395 | 95°C for 10 min; 40 cycles of 95°C for 30 s, 58°C for 30 s, and 72°C for 45 s |
| Rev: CCTCATGCAGTGAAAATGGATATCTT | ||||
| Ehrlichia spp. | 16S rRNA | Fwd: ATGCGTAGGAATCTACCTAGTAGTA | 460 | |
| Rev: GCCTTGGTATTTCACTTTTAACTTACT | ||||
| Ehrlichia spp. | gltA | Primary reaction | 777 | 95°C for 10 min; 40 cycles of 95°C for 30 s, 56°C (primary)/58°C (secondary) for 30 s, and 72°C for 60 s |
| Fwd: CCAGGATTTATGTCTACTGCTGC | ||||
| Rev: GCATACYCTATGACCAAAMCCCAT | ||||
| Secondary reaction | 627 | |||
| Fwd: GCGCGGATTACRTTTATTGATGG | ||||
| Rev: ATTGGCHCCACCATGAGCTG | ||||
| Rickettsia spp. | ompB | Fwd: GGTACTGCCGAGTTACGTTTAG | 380 | 95°C for 10 min; 40 cycles of 95°C for 30 s, 57°C for 30 s, and 72°C for 45 s |
| Rev: CTCGCATCAACAACRCCTG |
Statistical analysis.
A Fisher’s exact test was performed for each agent to compare seasonal and geographical infection rates with the five pathogens. A two-tailed P value of <0.05 was considered statistically significant. Statistical analyses were performed using the software package R (R-Development Core Team; www.r-project.org). The mean and 95% confidence intervals of pathogen prevalence were calculated using the proportion test in R.
ACKNOWLEDGMENTS
DNA from cell-cultured E. chaffeensis was used as a positive control for PCR and was provided by J. Stephen Dumler at the Walter Reed National Military Medical Center. We thank the New York State Office of Parks, Recreation and Historic Preservation and the U.S. National Park Service for permission to collect ticks. The assistance of Erik Kopping, Peter T. Benziger, and Indra Jayatilaka is gratefully acknowledged.
This study was funded by a grant from the National Institutes of Health (AI-027044-29 to J.L.B.). Support was also provided by the Island Outreach Foundation, Blue Point, NY, to the Stony Brook Renaissance School of Medicine. Support from the Steven & Alexandra Cohen Foundation (CU18-2692) was provided to R.T.
Footnotes
Citation Sanchez-Vicente S, Tagliafierro T, Coleman JL, Benach JL, Tokarz R. 2019. Polymicrobial nature of tick-borne diseases. mBio 10:e02055-19. https://doi.org/10.1128/mBio.02055-19.
Contributor Information
Liise-anne Pirofski, Albert Einstein College of Medicine.
Abdu Azad, University of Maryland School of Medicine.
Erol Fikrig, Yale School of Medicine.
Ulrike Munderloh, University of Minnesota.
Sam R. Telford, III, Tufts University School of Veterinary Medicine.
REFERENCES
- 1.Telford SRT, Goethert HK. 2008. Emerging and emergent tick-borne infections, p 344–376. In Bowman AS, Nuttall PA (ed), Ticks: biology, disease and control. Cambridge University Press, Cambridge, United Kingdom. [Google Scholar]
- 2.Rosenberg R, Lindsey NP, Fischer M, Gregory CJ, Hinckley AF, Mead PS, Paz-Bailey G, Waterman SH, Drexler NA, Kersh GJ, Hooks H, Partridge SK, Visser SN, Beard CB, Petersen LR. 2018. Vital signs: trends in reported vectorborne disease cases–United States and Territories, 2004–2016. MMWR Morb Mortal Wkly Rep 67:496–501. doi: 10.15585/mmwr.mm6717e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fukunaga M, Takahashi Y, Tsuruta Y, Matsushita O, Ralph D, McClelland M, Nakao M. 1995. Genetic and phenotypic analysis of Borrelia miyamotoi sp. nov., isolated from the ixodid tick Ixodes persulcatus, the vector for Lyme disease in Japan. Int J Syst Bacteriol 45:804–810. doi: 10.1099/00207713-45-4-804. [DOI] [PubMed] [Google Scholar]
- 4.Telford SR, Armstrong PM, Katavolos P, Foppa I, Garcia AS, Wilson ML, Spielman A, Spielman A. 1997. A new tick-borne encephalitis-like virus infecting New England deer ticks, Ixodes dammini. Emerg Infect Dis 3:165–170. doi: 10.3201/eid0302.970209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burgdorfer W, Barbour AG, Hayes SF, Benach JL, Grunwaldt E, Davis JP. 1982. Lyme disease–a tick-borne spirochetosis? Science 216:1317–1319. doi: 10.1126/science.7043737. [DOI] [PubMed] [Google Scholar]
- 6.Grunwaldt E. 1977. Babesiosis on Shelter Island. N Y State J Med 77:1320–1321. [PubMed] [Google Scholar]
- 7.Ruebush TK, Juranek DD, Chisholm ES, Snow PC, Healy GR, Sulzer AJ. 1977. Human babesiosis on Nantucket Island. N Engl J Med 297:825–827. doi: 10.1056/NEJM197710132971511. [DOI] [PubMed] [Google Scholar]
- 8.Filstein MR, Benach JL, White DJ, Brody BA, Goldman WD, Bakal CW, Schwartz RS. 1980. Serosurvey for human babesiosis in New York. J Infect Dis 141:518–521. doi: 10.1093/infdis/141.4.518. [DOI] [PubMed] [Google Scholar]
- 9.Benach JL, Habicht GS. 1981. Clinical characteristics of human babesiosis. J Infect Dis 144:481. doi: 10.1093/infdis/144.5.481. [DOI] [PubMed] [Google Scholar]
- 10.Swanson SJ, Neitzel D, Reed KD, Belongia EA. 2006. Coinfections acquired from ixodes ticks. Clin Microbiol Rev 19:708–727. doi: 10.1128/CMR.00011-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen SM, Dumler JS, Bakken JS, Walker DH. 1994. Identification of a granulocytotropic Ehrlichia species as the etiologic agent of human disease. J Clin Microbiol 32:589–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bakken JS, Dumler JS, Chen S-M, Eckman MR, Van Etta LL, Walker DH. 1994. Human granulocytic ehrlichiosis in the Upper Midwest United States. JAMA 272:212. doi: 10.1001/jama.1994.03520030054028. [DOI] [PubMed] [Google Scholar]
- 13.Moss WJ, Dumler JS. 2003. Simultaneous infection with Borrelia burgdorferi and human granulocytic ehrlichiosis. Pediatr Infect Dis J 22:91–92. doi: 10.1097/00006454-200301000-00023. [DOI] [PubMed] [Google Scholar]
- 14.Aguero-Rosenfeld ME, Donnarumma L, Zentmaier L, Jacob J, Frey M, Noto R, Carbonaro CA, Wormser GP. 2002. Seroprevalence of antibodies that react with Anaplasma phagocytophila, the agent of human granulocytic ehrlichiosis, in different populations in Westchester County, New York. J Clin Microbiol 40:2612–2615. doi: 10.1128/jcm.40.7.2612-2615.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Steere AC, McHugh G, Suarez C, Hoitt J, Damle N, Sikand VK. 2003. Prospective study of coinfection in patients with erythema migrans. Clin Infect Dis 36:1078–1081. doi: 10.1086/368187. [DOI] [PubMed] [Google Scholar]
- 16.Dumler JS, Barbet AF, Bekker C, Dasch GA, Palmer GH, Ray SC, Rikihisa Y, Rurangirwa FR. 2001. Reorganization of genera in the families Rickettsiaceae and Anaplasmataceae in the order Rickettsiales: unification of some species of Ehrlichia with Anaplasma, Cowdria with Ehrlichia and Ehrlichia with Neorickettsia, descriptions of six new species combinations and designation of Ehrlichia equi and “HGE agent” as subjective synonyms of Ehrlichia phagocytophila. Int J Syst Evol Microbiol 51:2145–2165. doi: 10.1099/00207713-51-6-2145. [DOI] [PubMed] [Google Scholar]
- 17.McLean DM, Donohue WL. 1959. Powassan virus: isolation of virus from a fatal case of encephalitis. Can Med Assoc J 80:708–711. [PMC free article] [PubMed] [Google Scholar]
- 18.Piantadosi A, Rubin DB, McQuillen DP, Hsu L, Lederer PA, Ashbaugh CD, Duffalo C, Duncan R, Thon J, Bhattacharyya S, Basgoz N, Feske SK, Lyons JL. 2016. Emerging cases of Powassan virus encephalitis in New England: clinical presentation, imaging, and review of the literature. Clin Infect Dis 62:707–713. doi: 10.1093/cid/civ1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Telford SR, Dawson JE, Katavolos P, Warner CK, Kolbert CP, Persing DH, Persing DH. 1996. Perpetuation of the agent of human granulocytic ehrlichiosis in a deer tick-rodent cycle. Proc Natl Acad Sci U S A 93:6209–6214. doi: 10.1073/pnas.93.12.6209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Springer YP, Eisen L, Beati L, James AM, Eisen RJ. 2014. Spatial distribution of counties in the continental United States with records of occurrence of Amblyomma americanum (Ixodida: Ixodidae). J Med Entomol 51:342–351. doi: 10.1603/me13115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dahlgren FS, Paddock CD, Springer YP, Eisen RJ, Behravesh CB. 2016. Expanding range of Amblyomma americanum and simultaneous changes in the epidemiology of spotted fever group rickettsiosis in the United States. Am J Trop Med Hyg 94:35–42. doi: 10.4269/ajtmh.15-0580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Means RG, White DJ. 1997. New distribution records of Amblyomma americanum (L.) (Acari: Ixodidae) in New York State. J Vector Ecol 22:133–145. [PubMed] [Google Scholar]
- 23.Mixson TR, Ginsberg HS, Campbell SR, Sumner JW, Paddock CD. 2004. Detection of Ehrlichia chaffeensis in adult and nymphal Amblyomma americanum (Acari: Ixodidae) ticks from Long Island, New York. J Med Entomol 41:1104–1110. doi: 10.1603/0022-2585-41.6.1104. [DOI] [PubMed] [Google Scholar]
- 24.Maupin GO, Anderson BE, Piesman JF, Johnson BJB, Sims KG, Happ CM, Olson JG, Childs JE. 1993. Amblyomma americanum: a potential vector of human ehrlichiosis. Am J Trop Med Hyg 49:239–244. doi: 10.4269/ajtmh.1993.49.239. [DOI] [PubMed] [Google Scholar]
- 25.Buller RS, Arens M, Hmiel SP, Paddock CD, Sumner JW, Rikihisa Y, Unver A, Gaudreault-Keener M, Manian FA, Liddell AM, Schmulewitz N, Storch GA. 1999. Ehrlichia ewingii, a newly recognized agent of human ehrlichiosis. N Engl J Med 341:148–155. doi: 10.1056/NEJM199907153410303. [DOI] [PubMed] [Google Scholar]
- 26.Ewing SA, Roberson WR, Buckner RG, Hayat CS. 1971. A new strain of Ehrlichia canis. J Am Vet Med Assoc 159:1771–1774. [PubMed] [Google Scholar]
- 27.Anderson BE, Greene CE, Jones DC, Dawson JE. 1992. Ehrlichia ewingii sp. nov., the ETiologic agent of canine granulocytic ehrlichiosis. Int J Syst Bacteriol 42:299–302. doi: 10.1099/00207713-42-2-299. [DOI] [PubMed] [Google Scholar]
- 28.Walker DH, Ismail N, Olano JP, McBride JW, Yu X-J, Feng H-M. 2004. Ehrlichia chaffeensis: a prevalent, life-threatening, emerging pathogen. Trans Am Clin Climatol Assoc 115:375–384. [PMC free article] [PubMed] [Google Scholar]
- 29.Barbour AG, Maupin GO, Teltow GJ, Carter CJ, Piesman J. 1996. Identification of an uncultivable Borrelia species in the hard tick Amblyomma americanum: possible agent of a Lyme disease-like illness. J Infect Dis 173:403–409. doi: 10.1093/infdis/173.2.403. [DOI] [PubMed] [Google Scholar]
- 30.Commins SP, James HR, Kelly LA, Pochan SL, Workman LJ, Perzanowski MS, Kocan KM, Fahy JV, Nganga LW, Ronmark E, Cooper PJ, Platts-Mills T. 2011. The relevance of tick bites to the production of IgE antibodies to the mammalian oligosaccharide galactose-α-1,3-galactose. J Allergy Clin Immunol 127:1286-93.e6. doi: 10.1016/j.jaci.2011.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rainey T, Occi JL, Robbins RG, Egizi A. 2018. Discovery of Haemaphysalis longicornis (Ixodida: Ixodidae) parasitizing a sheep in New Jersey, United States. J Med Entomol 55:757–759. doi: 10.1093/jme/tjy006. [DOI] [PubMed] [Google Scholar]
- 32.Uchida T, Yan Y, Kitaoka S. 1995. Detection of Rickettsia japonica in Haemaphysalis longicornis ticks by restriction fragment length polymorphism of PCR product. J Clin Microbiol Microbiol 33:824–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Luo L-M, Zhao L, Wen H-L, Zhang Z-T, Liu J-W, Fang L-Z, Xue Z-F, Ma D-Q, Zhang X-S, Ding S-J, Lei X-Y, Yu X. 2015. Haemaphysalis longicornis ticks as reservoir and vector of severe fever with thrombocytopenia syndrome virus in China. Emerg Infect Dis 21:1770–1776. doi: 10.3201/eid2110.150126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hecht JA, Allerdice MEJ, Dykstra EA, Mastel L, Eisen RJ, Johnson TL, Gaff HD, Varela-Stokes AS, Goddard J, Pagac BB, Paddock CD, Karpathy SE. 3 April 2019. Multistate survey of American dog ticks (Dermacentor variabilis) for rickettsia species. Vector-Borne Zoonotic Dis doi: 10.1089/vbz.2018.2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Trout Fryxell RT, Hendricks BM, Pompo K, Mays SE, Paulsen DJ, Operario DJ, Houston AE. 2017. Investigating the adult ixodid tick populations and their associated Anaplasma, Ehrlichia, and Rickettsia bacteria at a Rocky Mountain spotted fever hotspot in Western Tennessee. Vector-Borne Zoonotic Dis 17:527–538. doi: 10.1089/vbz.2016.2091. [DOI] [PubMed] [Google Scholar]
- 36.Kurtti TJ, Felsheim RF, Burkhardt NY, Oliver JD, Heu CC, Munderloh UG. 2015. Rickettsia buchneri sp. nov., a rickettsial endosymbiont of the blacklegged tick Ixodes scapularis. Int J Syst Evol Microbiol 65:965–970. doi: 10.1099/ijs.0.000047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moreno CX, Moy F, Daniels TJ, Godfrey HP, Cabello FC. 2006. Molecular analysis of microbial communities identified in different developmental stages of Ixodes scapularis ticks from Westchester and Dutchess Counties, New York. Environ Microbiol 8:761–772. doi: 10.1111/j.1462-2920.2005.00955.x. [DOI] [PubMed] [Google Scholar]
- 38.Ross BD, Hayes B, Radey MC, Lee X, Josek T, Bjork J, Neitzel D, Paskewitz S, Chou S, Mougous JD. 2018. Ixodes scapularis does not harbor a stable midgut microbiome. ISME J 12:2596–2607. doi: 10.1038/s41396-018-0161-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tokarz R, Tagliafierro T, Sameroff S, Cucura DM, Oleynik A, Che X, Jain K, Lipkin WI. 2019. Microbiome analysis of Ixodes scapularis ticks from New York and Connecticut. Ticks Tick Borne Dis 10:894–900. doi: 10.1016/j.ttbdis.2019.04.011. [DOI] [PubMed] [Google Scholar]
- 40.Karpathy SE, Slater KS, Goldsmith CS, Nicholson WL, Paddock CD. 2016. Rickettsia amblyommatis sp. nov., a spotted fever group Rickettsia associated with multiple species of Amblyomma ticks in North, Central and South America. Int J Syst Evol Microbiol 66:5236–5243. doi: 10.1099/ijsem.0.001502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ponnusamy L, Gonzalez A, Van Treuren W, Weiss S, Parobek CM, Juliano JJ, Knight R, Roe RM, Apperson CS, Meshnick SR. 2014. Diversity of Rickettsiales in the microbiome of the lone star tick, Amblyomma americanum. Appl Environ Microbiol 80:354–359. doi: 10.1128/AEM.02987-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McQuiston JH, Zemtsova G, Perniciaro J, Hutson M, Singleton J, Nicholson WL, Levin ML. 2012. Afebrile spotted fever group rickettsia infection after a bite from a Dermacentor variabilis tick infected with Rickettsia montanensis. Vector Borne Zoonotic Dis 12:1059–1061. doi: 10.1089/vbz.2012.1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Carlyon JA, Fikrig E. 2006. Mechanisms of evasion of neutrophil killing by Anaplasma phagocytophilum. Curr Opin Hematol 13:28–33. doi: 10.1097/01.moh.0000190109.00532.56. [DOI] [PubMed] [Google Scholar]
- 44.Rikihisa Y. 2011. Mechanisms of obligatory intracellular infection with Anaplasma phagocytophilum. Clin Microbiol Rev 24:469–489. doi: 10.1128/CMR.00064-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rikihisa Y. 2015. Molecular pathogenesis of Ehrlichia chaffeensis infection. Annu Rev Microbiol 69:283–304. doi: 10.1146/annurev-micro-091014-104411. [DOI] [PubMed] [Google Scholar]
- 46.Ismail N, McBride JW. 2017. Tick-borne emerging infections: ehrlichiosis and anaplasmosis. Clin Lab Med 37:317–340. doi: 10.1016/j.cll.2017.01.006. [DOI] [PubMed] [Google Scholar]
- 47.Massung RF, Priestley RA, Miller NJ, Mather TN, Levin ML. 2003. Inability of a variant strain of Anaplasma phagocytophilum to infect mice. J Infect Dis 188:1757–1763. doi: 10.1086/379725. [DOI] [PubMed] [Google Scholar]
- 48.Massung RF, Courtney JW, Hiratzka SL, Pitzer VE, Smith G, Dryden RL. 2005. Anaplasma phagocytophilum in white-tailed deer. Emerg Infect Dis 11:1604–1606. doi: 10.3201/eid1110.041329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tokarz R, Jain K, Bennett A, Briese T, Lipkin WI. 2010. Assessment of polymicrobial infections in ticks in New York state. Vector Borne Zoonotic Dis 10:217–221. doi: 10.1089/vbz.2009.0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Aliota MT, Dupuis AP, Wilczek MP, Peters RJ, Ostfeld RS, Kramer LD, Kramer LD. 2014. The prevalence of zoonotic tick-borne pathogens in Ixodes scapularis collected in the Hudson Valley, New York State. Vector Borne Zoonotic Dis 14:245–250. doi: 10.1089/vbz.2013.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Adler GH, Levins R. 1994. The island syndrome in rodent populations. Q Rev Biol 69:473–490. doi: 10.1086/418744. [DOI] [PubMed] [Google Scholar]
- 52.Stromdahl EY, Nadolny RM, Hickling GJ, Hamer SA, Ogden NH, Casal C, Heck GA, Gibbons JA, Cremeans TF, Pilgard MA. 2018. Amblyomma americanum (Acari: Ixodidae) ticks are not vectors of the Lyme disease agent, Borrelia burgdorferi (Spirocheatales: Spirochaetaceae): a review of the evidence. J Med Entomol 55:501–514. doi: 10.1093/jme/tjx250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rosner F, Zarrabi MH, Benach JL, Habicht GS. 1984. Babesiosis in splenectomized adults. Review of 22 reported cases. Am J Med 76:696–701. doi: 10.1016/0002-9343(84)90298-5. [DOI] [PubMed] [Google Scholar]
- 54.Mah A, Viola GM, Ariza Heredia E, Rezvani K, Kebriaei P, Bhatti MM, Han X, Shpall EJ, Mulanovich VE. 2018. Graft loss attributed to possible transfusion-transmitted ehrlichiosis following cord blood stem cell transplant. Transpl Infect Dis 20:e12899. doi: 10.1111/tid.12899. [DOI] [PubMed] [Google Scholar]
- 55.Goel R, Westblade LF, Kessler DA, Sfeir M, Slavinski S, Backenson B, Gebhardt L, Kane K, Laurence J, Scherr D, Bussel J, Dumler JS, Cushing MM, Vasovic LV. 2018. Death from transfusion-transmitted anaplasmosis, New York, USA, 2017. Emerg Infect Dis 24:1548–1550. doi: 10.3201/eid2408.172048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Eisen RJ, Eisen L. 2018. The blacklegged tick, Ixodes scapularis: an increasing public health concern. Trends Parasitol 34:295–309. doi: 10.1016/j.pt.2017.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dammin GJ, Spielman A, Benach JL, Piesman J. 1981. The rising incidence of clinical Babesia microti infection. Hum Pathol 12:398–400. doi: 10.1016/s0046-8177(81)80020-2. [DOI] [PubMed] [Google Scholar]
- 58.Gombert ME, Goldstein EJ, Benach JL, Tenenbaum MJ, Grunwaldt E, Kaplan MH, Eveland LK. 1982. Human babesiosis. Clinical and therapeutic considerations. JAMA 248:3005–3007. doi: 10.1001/jama.1982.03330220049035. [DOI] [PubMed] [Google Scholar]
- 59.Grabowski EF, Giardina PJV, Goldberg D, Masur H, Read SE, Hirsch RL, Benach JL. 1982. Babesiosis transmitted by a transfusion of frozen-thawed blood. Ann Intern Med 96:466–467. doi: 10.7326/0003-4819-96-4-446. [DOI] [PubMed] [Google Scholar]
- 60.Goethert HK, Telford SR. 2014. Not “out of Nantucket”: Babesia microti in southern New England comprises at least two major populations. Parasit Vectors 7:546. doi: 10.1186/s13071-014-0546-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lemieux JE, Tran AD, Freimark L, Schaffner SF, Goethert H, Andersen KG, Bazner S, Li A, McGrath G, Sloan L, Vannier E, Milner D, Pritt B, Rosenberg E, Telford S, Bailey JA, Sabeti PC. 2016. A global map of genetic diversity in Babesia microti reveals strong population structure and identifies variants associated with clinical relapse. Nat Microbiol 1:16079. doi: 10.1038/nmicrobiol.2016.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Goethert HK, Molloy P, Berardi V, Weeks K, Telford SR. 2018. Zoonotic Babesia microti in the northeastern U.S.: evidence for the expansion of a specific parasite lineage. PLoS One 13:e0193837. doi: 10.1371/journal.pone.0193837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cavanaugh CE, Muscat PL, Telford SR, Goethert H, Pendlebury W, Elias SP, Robich R, Welch M, Lubelczyk CB, Smith RP. 2017. Fatal deer tick virus infection in Maine. Clin Infect Dis 65:1043–1046. doi: 10.1093/cid/cix435. [DOI] [PubMed] [Google Scholar]
- 64.Tavakoli NP, Wang H, Dupuis M, Hull R, Ebel GD, Gilmore EJ, Faust PL. 2009. Fatal case of deer tick virus encephalitis. N Engl J Med 360:2099–2107. doi: 10.1056/NEJMoa0806326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Solomon IH, Spera KM, Ryan SL, Helgager J, Andrici J, Zaki SR, Vaitkevicius H, Leon KE, Wilson MR, DeRisi JL, Koo S, Smirnakis SM, De Girolami U. 2018. Fatal Powassan encephalitis (deer tick virus, lineage II) in a patient with fever and orchitis receiving rituximab. JAMA Neurol 75:746–750. doi: 10.1001/jamaneurol.2018.0132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hermance ME, Thangamani S. 2017. Powassan virus: an emerging arbovirus of public health concern in North America. Vector Borne Zoonotic Dis 17:453–462. doi: 10.1089/vbz.2017.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Frost HM, Schotthoefer AM, Thomm AM, Dupuis AP, Kehl SC, Kramer LD, Fritsche TR, Harrington YA, Knox KK. 2017. Serologic evidence of Powassan virus infection in patients with suspected Lyme disease. Emerg Infect Dis 23:1384–1388. doi: 10.3201/eid2308.161971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Krow-Lucal ER, Lindsey NP, Fischer M, Hills SL. 2018. Powassan virus disease in the United States, 2006–2016. Vector Borne Zoonotic Dis 18:286–290. doi: 10.1089/vbz.2017.2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Garcia-Monco JC, Benach JL. 2019. Lyme neuroborreliosis: clinical outcomes, controversy, pathogenesis, and polymicrobial infections. Ann Neurol 85:21–31. doi: 10.1002/ana.25389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mixson TR, Campbell SR, Gill JS, Ginsberg HS, Reichard MV, Schulze TL, Dasch GA. 2006. Prevalence of Ehrlichia, Borrelia, and rickettsial agents in Amblyomma americanum (Acari: Ixodidae) collected from nine states. J Med Entomol 43:1261–1268. doi: 10.1603/0022-2585(2006)43[1261:POEBAR]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 71.Childs JE, Paddock CD. 2003. The ascendancy of Amblyomma americanum as a vector of pathogens affecting humans in the United States. Annu Rev Entomol 48:307–337. doi: 10.1146/annurev.ento.48.091801.112728. [DOI] [PubMed] [Google Scholar]
- 72.Sonenshine D. 2018. Range expansion of tick disease vectors in North America: implications for spread of tick-borne disease. Int J Environ Res Public Health 15:478. doi: 10.3390/ijerph15030478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Stafford KC, Molaei G, Little EAH, Paddock CD, Karpathy SE, Labonte AM. 2018. Distribution and establishment of the lone star tick in Connecticut and implications for range expansion and public health. J Med Entomol 55:1561–1568. doi: 10.1093/jme/tjy115. [DOI] [PubMed] [Google Scholar]
- 74.Jordan RA, Egizi A. 2019. The growing importance of lone star ticks in a Lyme disease endemic county: passive tick surveillance in Monmouth County, NJ, 2006–2016. PLoS One 14:e0211778. doi: 10.1371/journal.pone.0211778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Monzón JD, Atkinson EG, Henn BM, Benach JL. 2016. Population and evolutionary genomics of Amblyomma americanum, an expanding arthropod disease vector. Genome Biol Evol 8:1351–1360. doi: 10.1093/gbe/evw080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Armstrong PM, Brunet LR, Spielman A, Telford SR. 2001. Risk of Lyme disease: perceptions of residents of a Lone Star tick-infested community. Bull World Health Organ 79:916–925. [PMC free article] [PubMed] [Google Scholar]
- 77.Beall MJ, Alleman AR, Breitschwerdt EB, Cohn LA, Couto CG, Dryden MW, Guptill LC, Iazbik C, Kania SA, Lathan P, Little SE, Roy A, Sayler KA, Stillman BA, Welles EG, Wolfson W, Yabsley MJ. 2012. Seroprevalence of Ehrlichia canis, Ehrlichia chaffeensis and Ehrlichia ewingii in dogs in North America. Parasit Vectors 5:29. doi: 10.1186/1756-3305-5-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Reeves WK, Loftis AD, Nicholson WL, Czarkowski AG. 2008. The first report of human illness associated with the Panola Mountain Ehrlichia species: a case report. J Med Case Rep 2:139. doi: 10.1186/1752-1947-2-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Regan J, Matthias J, Green-Murphy A, Stanek D, Bertholf M, Pritt BS, Sloan LM, Kelly AJ, Singleton J, McQuiston JH, Hocevar SN, Whittle JP. 2013. A confirmed Ehrlichia ewingii infection likely acquired through platelet transfusion. Clin Infect Dis 56:e105–e107. doi: 10.1093/cid/cit177. [DOI] [PubMed] [Google Scholar]
- 80.Esbenshade A, Esbenshade J, Domm J, Williams J, Frangoul H. 2010. Severe ehrlichia infection in pediatric oncology and stem cell transplant patients. Pediatr Blood Cancer 54:776–778. doi: 10.1002/pbc.22392. [DOI] [PubMed] [Google Scholar]
- 81.Nichols Heitman K, Dahlgren FS, Drexler NA, Massung RF, Behravesh CB. 2016. Increasing Incidence of ehrlichiosis in the United States: a summary of national surveillance of Ehrlichia chaffeensis and Ehrlichia ewingii infections in the United States, 2008–2012. Am J Trop Med Hyg 94:52–60. doi: 10.4269/ajtmh.15-0540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Varela AS, Luttrell MP, Howerth EW, Moore VA, Davidson WR, Stallknecht DE, Little SE. 2004. First culture isolation of Borrelia lonestari, putative agent of southern tick-associated rash illness. J Clin Microbiol 42:1163–1169. doi: 10.1128/jcm.42.3.1163-1169.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hudman DA, Sargentini NJ. 2016. Detection of Borrelia, Ehrlichia, and Rickettsia spp. in ticks in northeast Missouri. Ticks Tick Borne Dis 7:915–921. doi: 10.1016/j.ttbdis.2016.04.010. [DOI] [PubMed] [Google Scholar]
- 84.Schulze TL, Jordan RA, White JC, Roegner VE, Healy SP. 2011. Geographical distribution and prevalence of selected Borrelia, Ehrlichia, and Rickettsia infections in Amblyomma americanum (Acari: Ixodidae) in New Jersey. J Am Mosq Control Assoc 27:236–244. doi: 10.2987/11-6111.1. [DOI] [PubMed] [Google Scholar]
- 85.James AM, Liveris D, Wormser GP, Schwartz I, Montecalvo MA, Johnson B. 2001. Borrelia lonestari infection after a bite by an Amblyomma americanum tick. J Infect Dis 183:1810–1814. doi: 10.1086/320721. [DOI] [PubMed] [Google Scholar]
- 86.Apperson CS, Engber B, Nicholson WL, Mead DG, Engel J, Yabsley MJ, Dail K, Johnson J, Watson DW. 2008. Tick-borne diseases in North Carolina: is “Rickettsia amblyommii” a possible cause of rickettsiosis reported as Rocky Mountain spotted fever? Vector-Borne Zoonotic Dis 8:597–606. doi: 10.1089/vbz.2007.0271. [DOI] [PubMed] [Google Scholar]
- 87.Weller SJ, Baldridge GD, Munderloh UG, Noda H, Simser J, Kurtti TJ. 1998. Phylogenetic placement of rickettsiae from the ticks Amblyomma americanum and Ixodes scapularis. J Clin Microbiol 36:1305–1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Alowaysi M, Chen J, Stark S, Teague K, LaCourse M, Proctor J, Vigil K, Corrigan J, Harding A, Li J, Kurtti T, Zhong J. 2019. Isolation and characterization of a rickettsia from the ovary of a Western black-legged tick, Ixodes pacificus. Ticks Tick Borne Dis 10:918–923. doi: 10.1016/j.ttbdis.2019.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Felsheim RF, Kurtti TJ, Munderloh UG. 2009. Genome sequence of the endosymbiont Rickettsia peacockii and comparison with virulent Rickettsia rickettsii: identification of virulence factors. PLoS One 4:e8361. doi: 10.1371/journal.pone.0008361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gillespie JJ, Joardar V, Williams KP, Driscoll T, Hostetler JB, Nordberg E, Shukla M, Walenz B, Hill CA, Nene VM, Azad AF, Sobral BW, Caler E. 2012. A Rickettsia genome overrun by mobile genetic elements provides insight into the acquisition of genes characteristic of an obligate intracellular lifestyle. J Bacteriol 194:376–394. doi: 10.1128/JB.06244-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Benach JL, White DJ, Burgdorfer W, Keelan T, Guirgis S, Altieri RH. 1977. Changing patterns in the incidence of Rocky Mountain spotted fever on Long Island (1971–1976). Am J Epidemiol 106:380–387. doi: 10.1093/oxfordjournals.aje.a112479. [DOI] [PubMed] [Google Scholar]
- 92.Stromdahl EY, Jiang J, Vince M, Richards AL. 2011. Infrequency of Rickettsia rickettsii in Dermacentor variabilis removed from humans, with comments on the role of other human-biting ticks associated with spotted fever group Rickettsiae in the United States. Vector Borne Zoonotic Dis 11:969–977. doi: 10.1089/vbz.2010.0099. [DOI] [PubMed] [Google Scholar]
- 93.Gaines DN, Operario DJ, Stroup S, Stromdahl E, Wright C, Gaff H, Broyhill J, Smith J, Norris DE, Henning T, Lucas A, Houpt E. 2014. Ehrlichia and spotted fever group Rickettsiae surveillance in Amblyomma americanum in Virginia through use of a novel six-plex real-time PCR assay. Vector Borne Zoonotic Dis 14:307–316. doi: 10.1089/vbz.2013.1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wormser GP, McKenna D, Scavarda C, Cooper D, El Khoury MY, Nowakowski J, Sudhindra P, Ladenheim A, Wang G, Karmen CL, Demarest V, Dupuis AP, Wong SJ. 2019. Co-infections in persons with early Lyme disease, New York, USA. Emerg Infect Dis 25:748–752. doi: 10.3201/eid2504.181509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Garg K, Meriläinen L, Franz O, Pirttinen H, Quevedo-Diaz M, Croucher S, Gilbert L. 2018. Evaluating polymicrobial immune responses in patients suffering from tick-borne diseases. Sci Rep 8:15932. doi: 10.1038/s41598-018-34393-9. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 96.Piesman J, Mather TN, Sinsky RJ, Spielman A. 1987. Duration of tick attachment and Borrelia burgdorferi transmission. J Clin Microbiol 25:557–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Katavolos P, Armstrong PM, Dawson JE, Telford SR. 1998. Duration of tick attachment required for transmission of granulocytic ehrlichiosis. J Infect Dis 177:1422–1425. doi: 10.1086/517829. [DOI] [PubMed] [Google Scholar]
- 98.Eisen L. 2018. Pathogen transmission in relation to duration of attachment by Ixodes scapularis ticks. Ticks Tick Borne Dis 9:535–542. doi: 10.1016/j.ttbdis.2018.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Abraham NM, Liu L, Jutras BL, Yadav AK, Narasimhan S, Gopalakrishnan V, Ansari JM, Jefferson KK, Cava F, Jacobs-Wagner C, Fikrig E. 2017. Pathogen-mediated manipulation of arthropod microbiota to promote infection. Proc Natl Acad Sci U S A 114:E781–E790. doi: 10.1073/pnas.1613422114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Levin ML, Schumacher LBM, Snellgrove A. 2018. Effects of Rickettsia amblyommatis infection on the vector competence of Amblyomma americanum ticks for Rickettsia rickettsii. Vector Borne Zoonotic Dis 18:579–587. doi: 10.1089/vbz.2018.2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Coleman JL, Gebbia JA, Piesman J, Degen JL, Bugge TH, Benach JL. 1997. Plasminogen is required for efficient dissemination of B. burgdorferi in ticks and for enhancement of spirochetemia in mice. Cell 89:1111–1119. doi: 10.1016/s0092-8674(00)80298-6. [DOI] [PubMed] [Google Scholar]
- 102.Liu L, Dai J, Zhao YO, Narasimhan S, Yang Y, Zhang L, Fikrig E. 2012. Ixodes scapularis JAK-STAT pathway regulates tick antimicrobial peptides, thereby controlling the agent of human granulocytic anaplasmosis. J Infect Dis 206:1233–1241. doi: 10.1093/infdis/jis484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sultana H, Neelakanta G, Kantor FS, Malawista SE, Fish D, Montgomery RR, Fikrig E. 2010. Anaplasma phagocytophilum induces actin phosphorylation to selectively regulate gene transcription in Ixodes scapularis ticks. J Exp Med 207:1727–1743. doi: 10.1084/jem.20100276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Villar M, López V, Ayllón N, Cabezas-Cruz A, López JA, Vázquez J, Alberdi P, de la Fuente J. 2016. The intracellular bacterium Anaplasma phagocytophilum selectively manipulates the levels of vertebrate host proteins in the tick vector Ixodes scapularis. Parasit Vectors 9:467. doi: 10.1186/s13071-016-1747-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ceraul SM, Dreher-Lesnick SM, Gillespie JJ, Rahman MS, Azad AF. 2007. New tick defensin isoform and antimicrobial gene expression in response to Rickettsia montanensis challenge. Infect Immun 75:1973–1983. doi: 10.1128/IAI.01815-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ceraul SM, Chung A, Sears KT, Popov VL, Beier-Sexton M, Rahman MS, Azad AF. 2011. A Kunitz protease inhibitor from Dermacentor variabilis, a vector for spotted fever group rickettsiae, limits Rickettsia montanensis invasion. Infect Immun 79:321–329. doi: 10.1128/IAI.00362-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ginsberg HS. 2008. Potential effects of mixed infections in ticks on transmission dynamics of pathogens: comparative analysis of published records. Exp Appl Acarol 46:29–41. doi: 10.1007/s10493-008-9175-5. [DOI] [PubMed] [Google Scholar]
- 108.Sonenshine DE, Macaluso KR. 2017. Microbial invasion vs. tick immune regulation. Front Cell Infect Microbiol 7:390. doi: 10.3389/fcimb.2017.00390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Shaw DK, Tate AT, Schneider DS, Levashina EA, Kagan JC, Pal U, Fikrig E, Pedra J. 2018. Vector immunity and evolutionary ecology: the harmonious dissonance. Trends Immunol 39:862–873. doi: 10.1016/j.it.2018.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Keirans JE, Durden LA. 1998. Illustrated key to nymphs of the tick genus Amblyomma (Acari: Ixodidae) found in the United States. J Med Entomol 35:489–495. doi: 10.1093/jmedent/35.4.489. [DOI] [PubMed] [Google Scholar]
- 111.Hoskins JD. 1991. Ixodid and argasid ticks. Keys to their identification. Vet Clin North Am Small Anim Pract 21:185–197. doi: 10.1016/S0195-5616(91)50018-8. [DOI] [PubMed] [Google Scholar]
- 112.Dubie TR, Grantham R, Coburn L, Noden BH. 2017. Pictorial key for identification of immature stages of common ixodid ticks found in pastures in Oklahoma. Southwest Entomol 42:1–14. doi: 10.3958/059.042.0101. [DOI] [Google Scholar]
- 113.Tokarz R, Tagliafierro T, Cucura DM, Rochlin I, Sameroff S, Lipkin WI. 2017. Detection of Anaplasma phagocytophilum, Babesia microti, Borrelia burgdorferi, Borrelia miyamotoi, and Powassan Virus in ticks by a multiplex real-time reverse transcription-PCR assay. mSphere 2:e00151-17. doi: 10.1128/mSphere.00151-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Tokarz R, Kapoor V, Samuel JE, Bouyer DH, Briese T, Lipkin WI. 2009. Detection of tick-borne pathogens by MassTag polymerase chain reaction. Vector Borne Zoonotic Dis 9:147–152. doi: 10.1089/vbz.2008.0088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Schulze TL, Jordan RA, Schulze CJ, Mixson T, Papero M. 2005. Relative encounter frequencies and prevalence of selected Borrelia, Ehrlichia, and Anaplasma infections in Amblyomma americanum and Ixodes scapularis (Acari: Ixodidae) ticks from central New Jersey. J Med Entomol 42:450–456. doi: 10.1093/jmedent/42.3.450. [DOI] [PubMed] [Google Scholar]
- 116.Prusinski MA, Kokas JE, Hukey KT, Kogut SJ, Lee J, Backenson PB. 2014. Prevalence of Borrelia burgdorferi (Spirochaetales: Spirochaetaceae), Anaplasma phagocytophilum (Rickettsiales: Anaplasmataceae), and Babesia microti (Piroplasmida: Babesiidae) in Ixodes scapularis (Acari: Ixodidae) collected from recreational lands in the Hudson Valley region, New York State. J Med Entomol 51:226–236. doi: 10.1603/ME13101. [DOI] [PubMed] [Google Scholar]
- 117.Steiner FE, Pinger RR, Vann CN, Grindle N, Civitello D, Clay K, Fuqua C. 2008. Infection and co-infection rates of Anaplasma phagocytophilum variants, Babesia spp., Borrelia burgdorferi, and the rickettsial endosymbiont in Ixodes scapularis (Acari: Ixodidae) from sites in Indiana, Maine, Pennsylvania, and Wisconsin. J Med Entomol 45:289–297. doi: 10.1093/jmedent/45.2.289. [DOI] [PubMed] [Google Scholar]
- 118.Hamer SA, Roy PL, Hickling GJ, Walker ED, Foster ES, Barber CC, Tsao JI. 2007. Zoonotic pathogens in Ixodes scapularis, Michigan. Emerg Infect Dis 13:1131–1133. doi: 10.3201/eid1307.070046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Schulze TL, Jordan RA, Healy SP, Roegner VE. 2013. Detection of Babesia microti and Borrelia burgdorferi in host-seeking Ixodes scapularis (Acari: Ixodidae) in Monmouth County, New Jersey. J Med Entomol 50:379–383. doi: 10.1603/me12088. [DOI] [PubMed] [Google Scholar]
- 120.Oliver JD, Bennett SW, Beati L, Bartholomay LC. 2017. Range expansion and increasing Borrelia burgdorferi infection of the tick Ixodes scapularis (Acari: Ixodidae) in Iowa, 1990–2013. J Med Entomol 54:1727–1734. doi: 10.1093/jme/tjx121. [DOI] [PubMed] [Google Scholar]
- 121.Lee X, Coyle DR, Johnson DKH, Murphy MW, McGeehin MA, Murphy RJ, Raffa KF, Paskewitz SM. 2014. Prevalence of Borrelia burgdorferi and Anaplasma phagocytophilum in Ixodes scapularis (Acari: Ixodidae) nymphs collected in managed red pine forests in Wisconsin. J Med Entomol 51:694–701. doi: 10.1603/ME13140. [DOI] [PubMed] [Google Scholar]
- 122.Hersh MH, Ostfeld RS, McHenry DJ, Tibbetts M, Brunner JL, Killilea ME, LoGiudice K, Schmidt KA, Keesing F. 2014. Co-infection of blacklegged ticks with Babesia microti and Borrelia burgdorferi is higher than expected and acquired from small mammal hosts. PLoS One 9:e99348. doi: 10.1371/journal.pone.0099348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Feldman KA, Connally NP, Hojgaard A, Jones EH, White JL, Hinckley AF. 2015. Abundance and infection rates of Ixodes scapularis nymphs collected from residential properties in Lyme disease-endemic areas of Connecticut, Maryland, and New York. J Vector Ecol 40:198–201. doi: 10.1111/jvec.12153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Hutchinson ML, Strohecker MD, Simmons TW, Kyle AD, Helwig MW. 2015. Prevalence rates of Borrelia burgdorferi (Spirochaetales: Spirochaetaceae), Anaplasma phagocytophilum (Rickettsiales: Anaplasmataceae), and Babesia microti (Piroplasmida: Babesiidae) in host-seeking Ixodes scapularis (Acari: Ixodidae) from Pennsylvania. J Med Entomol 52:693–698. doi: 10.1093/jme/tjv037. [DOI] [PubMed] [Google Scholar]
- 125.Johnson TL, Graham CB, Boegler KA, Cherry CC, Maes SE, Pilgard MA, Hojgaard A, Buttke DE, Eisen RJ. 2017. Prevalence and diversity of tick-borne pathogens in nymphal Ixodes scapularis (Acari: Ixodidae) in eastern National Parks. J Med Entomol 54:742. doi: 10.1093/jme/tjw213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Johnson TL, Graham CB, Maes SE, Hojgaard A, Fleshman A, Boegler KA, Delory MJ, Slater KS, Karpathy SE, Bjork JK, Neitzel DF, Schiffman EK, Eisen RJ. 2018. Prevalence and distribution of seven human pathogens in host-seeking Ixodes scapularis (Acari: Ixodidae) nymphs in Minnesota, USA. Ticks Tick Borne Dis 9:1499–1507. doi: 10.1016/j.ttbdis.2018.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Cross ST, Kapuscinski ML, Perino J, Maertens BL, Weger-Lucarelli J, Ebel GD, Stenglein MD. 2018. Co-infection patterns in individual Ixodes scapularis ticks reveal associations between viral, eukaryotic and bacterial microorganisms. Viruses 10:388. doi: 10.3390/v10070388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Edwards MJ, Russell JC, Davidson EN, Yanushefski TJ, Fleischman BL, Heist RO, Leep-Lazar JG, Stuppi SL, Esposito RA, Suppan LM. 2019. A 4-yr survey of the range of ticks and tick-borne pathogens in the Lehigh Valley region of eastern Pennsylvania. J Med Entomol 56:1122–1134. doi: 10.1093/jme/tjz043. [DOI] [PMC free article] [PubMed] [Google Scholar]