Abstract
Psychotic experiences may be understood as altered information processing due to aberrant neural computations. A prominent example of such neural computations is the computation of prediction errors (PEs), which signal the difference between expected and experienced events. Among other areas showing PE coding, hippocampal-prefrontal-striatal neurocircuits play a prominent role in information processing. Dysregulation of dopaminergic signaling, often secondary to psychosocial stress, is thought to interfere with the processing of biologically important events (such as reward prediction errors) and result in the aberrant attribution of salience to irrelevant sensory stimuli and internal representations. Bayesian hierarchical predictive coding offers a promising framework for the identification of dysfunctional neurocomputational processes and the development of a mechanistic understanding of psychotic experience. According to this framework, mismatches between prior beliefs encoded at higher levels of the cortical hierarchy and lower-level (sensory) information can also be thought of as PEs, with important consequences for belief updating. Low levels of precision in the representation of prior beliefs relative to sensory data, as well as dysfunctional interactions between prior beliefs and sensory data in an ever-changing environment, have been suggested as a general mechanism underlying psychotic experiences. Translating the promise of the Bayesian hierarchical predictive coding into patient benefit will come from integrating this framework with existing knowledge of the etiology and pathophysiology of psychosis, especially regarding hippocampal-prefrontal-striatal network function and neural mechanisms of information processing and belief updating.
Keywords: schizophrenia, computational modeling, dopamine, reward, prediction error, delusions, hallucinations
Introduction
Biological accounts of schizophrenia and related psychotic states have long focused on dopamine dysfunction, based on the effects of antipsychotic medication and neuroimaging studies showing increased striatal dopamine synthesis capacity and release in unmedicated psychotic patients.1–4 Decisive steps toward computational accounts of dopamine function were taken when Schultz and coworkers5 showed that phasic dopamine release reflects the valence and magnitude of mismatches between expected and obtained reward outcomes (called “reward prediction errors,” or RPEs), and when Robinson and Berridge6 postulated that phasic dopamine release reflects the attribution of incentive salience to sensory stimuli and internal representations (for further early theories on altered learning and inference in schizophrenia, see supplementary section 1). We and others7–9 proposed that, in schizophrenia, delusion formation is promoted by dysregulated, and therefore noisy, firing of dopaminergic neurons, thus imbuing otherwise irrelevant stimuli with meaning and motivating subjects to focus their attention on these cues—a phenomenon termed “aberrant salience attribution.” Increased dopamine tone may “drown” the encoding of actually relevant stimuli, such as primary reinforcers and conditioned cues associated with rewards.10
Dopaminergic prediction error (PE) signaling and its postulated cognitive correlate of salience attribution can be grounded in a more general computational framework of how individuals make inferences about their environment and thus shape their subjective model of the world.11 According to the theory of Bayesian inference, beliefs are continuously updated by testing prior beliefs against novel incoming information, resulting in posterior beliefs. It has been proposed that the brain uses this mechanism of belief updating to infer the hidden states of the world from incomplete and noisy sensory data.12 In a Bayesian sense, the term belief is not exclusively used for higher-level cognition but also refers to a probability distribution over some unknown environmental or internal state and may or may not be consciously accessible.13 The mean of this distribution denotes the most expected state, whereas its variance denotes the uncertainty of the belief (and the inverse variance its precision). Critically, if a belief is held with high uncertainty (ie, low precision), mismatching sensory information will be very effective in updating the belief. In contrast, if a belief has low uncertainty (ie, high precision), mismatching information can be ignored and will have little effect on belief updating.13
The “predictive coding” framework has been suggested as one biologically plausible algorithmic implementation of Bayesian inference in the brain.14,15 This framework describes a hierarchical organization of beliefs, where mismatches between prior beliefs and incoming signals result in PEs, which serve as learning signals for the updating of posterior beliefs and are propagated upwards in the hierarchy from low to high levels.16 The updating of lower-level beliefs by PEs is weighted by higher-level beliefs regarding the certainty attributed to a lower-level belief. The predictive coding hierarchy is thought to reflect the hierarchical structure of information in the world,17 whereby different sources of uncertainty are computed at different levels of the hierarchy: low-level uncertainty results from limited or noisy sensory information, whereas high-level uncertainty results from unpredictable state changes over time, ie, environmental volatility.13,18 This hierarchical structure in uncertainty computation is supposedly represented in the sensory cortices for lower-level processing and in associative cortices for higher-level processing.18,19
In schizophrenia, an altered balance between the encoding of prior beliefs and sensory information processing may result in maladaptive PE signaling, not only in dopamine-dependent subcortical circuits, but also in cortical brain circuits.16,20,21 In a Bayesian framework,12 this impairment can be formalized as a relative imprecision in the signaling of prior beliefs vis-a-vis input signals emanating from low-level sensory cortices, leading to a kind of aberrant PE signaling, apart from dopamine-dependent subcortical RPE signaling. Such erratic PE signaling could impair the ability to distinguish between relevant and irrelevant sensory information, resulting in maladaptive inferences and belief-updating. Maladaptive inferences may arise from aberrant encoding of the precision of prior beliefs at different levels of the predictive coding hierarchy. However, such accounts need to be integrated with existing knowledge regarding hippocampal-prefrontal-striatal network dysfunction in schizophrenia.22,23 In this review, we focus on Bayesian predictive coding accounts of psychotic experience and discuss the neural circuits related to altered information processing in psychosis.
Computational Models of Reinforcement Learning Alterations in Psychosis
Computational models of reinforcement learning (RL) have been frequently applied to data from individuals performing tasks reliant on the ability to process and learn from feedback, and to decide based on representations of value.11,24 While the in-the-moment experience of rewarding stimuli appears to be largely intact in the majority of schizophrenia patients, there is solid evidence for deficits in value-based decision making and altered neuronal signals during aspects of reward-based learning.25–27 In animal studies, RPEs have been shown to evoke phasic dopamine bursts that act as teaching signals during associative learning about rewards,28–30 as well as salient nonrewarding events.31 Importantly, alterations in the (largely dopaminergic) signaling of RPEs have been proposed to underlie both the increased tendency to attribute aberrant salience to irrelevant stimuli (thought to contribute to positive symptoms7,8,10) and the reduced ability to adaptively learn about reward value and attribute incentive salience to biologically important stimuli and events (thought to contribute to motivational impairments32,33).
Several groups have used functional magnetic resonance imaging (fMRI) in conjunction with computational modeling in a RL framework, point to a disruption in midbrain RPE signaling in acutely psychotic individuals,34–38 which has also been observed in conjunction with the encoding of informative errors in the ventral striatum during reversal learning.39 Given the evidence for dopaminergic dysfunction in psychosis,2,3,40 a link between alterations in dopamine function and RPE signaling in psychotic illness is very plausible. Indeed, one study in healthy volunteers found an inverse association between dopamine synthesis capacity and the strength of striatal RPE signals.41 Additional fMRI studies have demonstrated that individuals with schizophrenia (especially those with more severe negative symptoms and/or deficits in intellectual function)42–44 exhibit attenuations in ventral striatal activation during reward anticipation, relative to healthy controls. Such alterations in neuronal learning signals can help to explain previously observed RL deficits in schizophrenia patients.45–47
Recent Bayesian accounts of learning and inference provide a computational account of dopamine that goes beyond signaling the magnitude and valence of RPEs, postulating that dopamine plays an important role in belief updating by encoding the precision of PEs.48–51 In short, the effects of PEs on learning are weighted by the belief about the current precision of the learning signal.52,53 This probabilistic belief is thought to reflect prior assumptions about informational uncertainty and is, in turn, modulated by higher-level beliefs regarding the volatility of the environment, encoded at higher levels of neural computation including the prefrontal cortex (PFC) and hippocampus (figure 1).54 A computational framework that models this hierarchical structure of belief updating is the Hierarchical Gaussian Filter13 (figure 2). This approach extends RL accounts and provides a broader framework that goes beyond learning of beliefs about reward-related value expectation. Using this framework, a recent model-based fMRI study of associative audiovisual learning found that high-level beliefs regarding the strength of dynamic cue-target contingencies relied on hippocampus and orbitofrontal cortex, whereas the representations of low-level conditional target probabilities was associated with early visual cortex activity.19
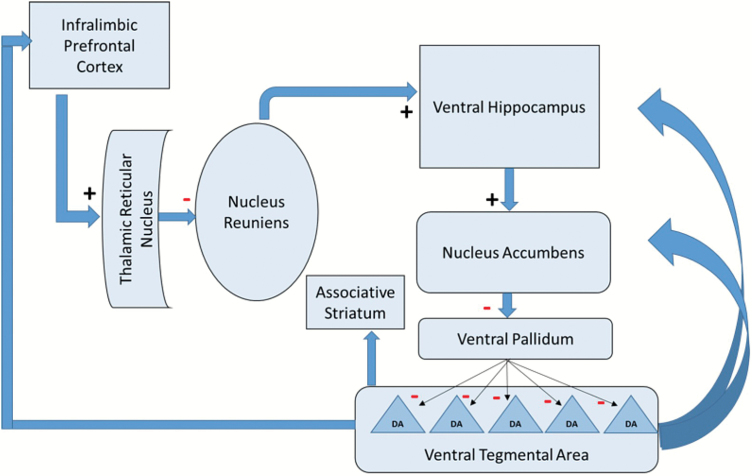
Fig. 1.
Circuit model of frontal and hippocampal control of dopamine neuron firing. The ventral hippocampus exerts potent control over dopamine neurons firing spontaneously. The number firing determines the amplitude, and hence salience, of the signal. Dopamine neurons are normally inhibited by the ventral pallidum, which in turn is inhibited by the nucleus accumbens. When the ventral hippocampus is activated, it activates the nucleus accumbens, which in turn inhibits the ventral pallidum and releases dopamine neurons from inhibition, allowing them to initiate firing.55 The ventral hippocampus is potently regulated by the PFC via the thalamus. The (infralimbic) PFC normally holds the ventral hippocampus in a less-active state. However, when infralimbic PFC activity is decreased, the primary effect is deactivation of the reticular nucleus of the thalamus, which in turn disinhibits the thalamic nucleus reuniens. This increases the tonic excitatory drive of the nucleus reuniens on the ventral hippocampus, disinhibiting dopamine firing, which impacts cognitive control via the associative striatum.56 The human analogue of the infralimbic cortex is the subgenual cingulate area 25. The arrows from the VTA to cortical and subcortical regions denote modulatory dopaminergic projections.
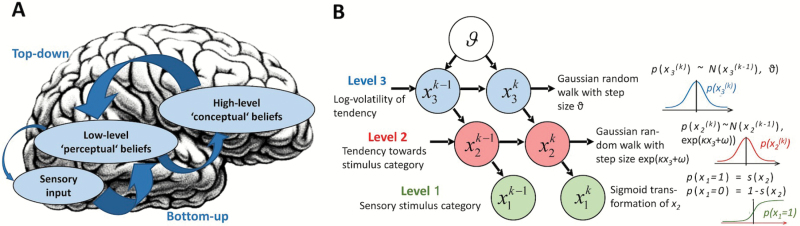
Fig. 2.
(A) Schematic model of altered hierarchical inference in the visual system. Sensory input represents processing in early visual cortex. Low-level “sensory” beliefs are encoded at the next higher hierarchical level, eg, mid- or high-level visual areas, and high-level “conceptual” beliefs at the highest cortical levels, eg, PFC. Arrows represent top-down signaling of prior beliefs and bottom-up signaling of prediction errors (PEs), with arrow thickness representing their respective precisions. The putative decrease in precision of low-level beliefs may lead to increased weighting of the sensory input, thus enhancing PEs, potentially compensated by increased precision of conceptual high-level beliefs.57 Brain image courtesy of Flickr/IsaacMao. (B) Schematic representation of the Hierarchical Gaussian Filter (adapted from Mathys et al13). Levels represent hidden environmental states at time k. They depend on their immediately preceding values and on the parameters κ (coupling of levels 2 and 3), ω (step size at level 2), and ϑ (learning speed about environmental volatility). The probability at each level is determined by the variables and parameters at the level above. The levels relate to each other by determining the step size of a random walk. Note that the levels of this model are not equivalent to those in (A). However, reduced learning speed about environmental volatility might contribute to a stronger top-down influence of high-level beliefs.
The idea that dopaminergic transmission contributes to the signaling of the precision of learning signals also suggests a mechanism by which dopamine may influence higher-level decision making. Specifically, representations of uncertainty have been shown to drive exploratory behavior.58–60 It is therefore noteworthy that dopamine synthesis capacity in the striatum has been found to be associated with the extent to which participants used a goal-directed learning strategy, in the context of a complex cognitive task,61 and that deficits in goal-directed exploration have been found to contribute to impairments in learning and motivation in schizophrenia patients.25,47
Further evidence points to roles for glutamatergic neurotransmission (eg, via NMDA receptors) and GABAergic inhibition in encoding the precision of learning signals in hippocampal and cortical networks (figure 1). While the signaling of precision-weighted PEs has not been investigated in schizophrenia patients thus far, the administration of the NMDA receptor antagonist ketamine, to healthy controls, was found to be associated with both a reduced ability to use confidence (precision) estimates to regulate RL parameters and altered fronto-parietal activity.62
Finally, dopamine has also been implicated in hippocampus-dependent novelty detection and novelty seeking behavior in both humans and rodents.54,63–65 Of course, merely because a stimulus is “novel” does not necessarily mean that it would be involved in belief updating. Rather, it has been proposed that a stimulus must be both novel and salient to impact belief, and that prefrontal-hippocampal dynamics thought to underlie novelty detection interact with dopaminergic salience signals16,54 to bring about relevant memory incorporation.54
Regarding neurobiological correlates of the symptoms of psychotic illness, positive symptoms are thought to be mediated by dopamine hyper-responsivity, with cognitive symptoms arising via frontal cortical dysfunction, and negative symptoms via impaired function of fronto-striatal circuits, cingulate cortex, and the amygdalae.66,67 While decades of research have revealed that dopamine can have different actions depending on its projection site,54,68,69 one central aspect of dopamine function seems to be the signaling of salience or, in Bayesian terms, of the precision during information processing.
Sensory Information Processing and Its Association With the Formation and Maintenance of Delusions
Insufficient precision in the encoding of low-level prior beliefs relative to sensory input could occur either due to the faulty acquisition of prior beliefs, or due to a reduced ability to appropriately use prior beliefs in perceptual inference. The acquisition of prior beliefs could be faulty if sensory information is misleading, if the detection and/or encoding of relevant sensory information are perturbed (or excessively stochastic),54 or if the detection and/or computation of higher-level PEs are perturbed or insufficiently precise.16,21 A reduced ability to appropriately use prior beliefs in perceptual inference could occur if high-level beliefs include false assumptions regarding the volatility of a novel environment.
These considerations are consistent with the view that both genetic and psychosocial factors contribute to the development of psychosis. While it is still necessary to differentiate among the various forms of stress, a mechanistic understanding of stress effects on neural circuits is emerging, indicating, eg, that stress can elevate microglial activity in hippocampus70 and increase striatal dopamine release.71 Experiences of both acute and early-life stress have been shown to engender and exacerbate psychotic symptoms—possibly via elevated striatal dopamine synthesis72—especially in those with elevated genetic liability for, or social vulnerability to, psychotic illness.73–75 As yet, no studies of individuals at elevated genetic or environmental risk for psychosis have examined the precision in encoding of prior beliefs, or influence of priors on decision making. Regardless of whether they are driven by psychosocial or primary neurobiological factors, maladaptive inferences related to sensory and high-level processing may ultimately implicate the same circuits involved in the comparison of prior beliefs and new sensory information. Such neural circuits include temporo-limbic brain regions involved in executive control and stress reactivity.23,54
Importantly, different levels of neural processing may be differentially involved in false inferences underlying psychotic experience; Schmack and coworkers76 observed that delusional ideation in both healthy volunteers and schizophrenia patients is associated with reduced perceptual stability during the viewing of ambiguous visual stimuli that represent informational uncertainty,76,77 which points to a weaker influence of perceptual priors built up at previous encounters with the same visual information. This observation is in line with the well-known finding of reduced susceptibility to some visual illusions in schizophrenia, pointing to reduced precision of prior beliefs at low hierarchical levels.21,78 Interestingly, the finding of a decreased influence of such lower-level perceptual beliefs was accompanied by an increased influence of higher-level cognitive beliefs on sensory processing. Belief-related connectivity between regions encoding high-level beliefs in the orbitofrontal cortex and visual brain areas encoding visual motion perception was increased in delusion-prone individuals and schizophrenia patients.76,79 Accordingly, reduced precision of perceptual beliefs encoded at low levels (eg, in sensory cortices) may be compensated by increased precision of more abstract conceptual beliefs encoded in higher-level brain circuits (figure 2).79 In this case, such a disambiguating top-down signal may reflect a belief in low volatility of the environment, as previously suggested.16 In the context of social interactions, an individual may falsely categorize ambivalent social interactions as threatening if informational uncertainty regarding social cues is wrongly disambiguated by delusion-congruent top-down signals (thereby contributing to the stability of delusions).
At this point, our understanding of the role of dopaminergic modulation of (and by) fronto-striatal circuits comes back into view.22,80 In the following section, we review a series of studies suggesting that aberrations in glutamatergic and dopaminergic signaling emerge from dysfunctional interactions among temporo-limbic, prefrontal, and striatal brain areas, and consider how these empirical findings relate to the theoretical framework of Bayesian predictive coding.
Reverberating Circuits and Temporal Limbic-Cortical Dysfunction in Psychosis
Despite genetic associations between dopamine receptor variants and schizophrenia risk,81 even the most ardent proponents of the dopamine hypothesis of schizophrenia would not suggest that schizophrenia involves a primary deficit in dopamine dysfunction akin to that characterizing Parkinson’s disease. Rather, it has been proposed that fronto-striatal circuits may be disrupted in schizophrenia, thus putting a focus on dopamine–glutamate interactions1,22,23 (see supplementary section 2.1).
Preclinical studies suggest that interactions of temporo-limbic brain areas with PFC control regions secondarily disinhibit subcortical dopamine release, potentially in a “compensatory effort” to increase the signal-to-noise ratio when confronted with noisy information processing.10,82–84 Furthermore, a series of studies85,86 of temporo-limbic-prefrontal interactions has focused on the balance of excitatory glutamatergic and inhibitory GABAergic neurotransmission. Complementary fMRI and magnetic resonance spectroscopy (MRS) studies86–88 suggest that abnormal GABAergic transmission can lead to elevated hippocampal and frontocortical glutamate concentrations as well as hippocampal hyperactivity in schizophrenia patients. The most recent meta-analysis of glutamate MRS studies89 reported elevations in glutamatergic metabolites in the medial frontal cortex in individuals at high risk for schizophrenia, and in the medial temporal lobes and basal ganglia of schizophrenia patients (but see supplementary section 2.2; Schür et al90). In preclinical models, and postmortem studies, of schizophrenia, a consistent loss of parvalbumin (PV) GABAergic interneurons is found in schizophrenia, which are located at the cell body of the pyramidal neurons and have greater influence on overall activity.91 Compared with the total GABAergic interneuron population, the number of PV interneurons is small, so that measuring overall GABA concentrations will not give a precise picture of excitability.
Findings of systematic relationships between glutamatergic concentrations in brain regions of interest and PET measures of dopamine synthesis92 suggest that perturbations of excitatory glutamatergic neurotransmission contribute to dopamine system abnormalities observed in psychosis patients, in accordance with preclinical studies showing that activation of hippocampal pyramidal neurons drive dopamine neuron activity states.93,94 Within a Bayesian framework, alterations in glutamatergic neurotransmission as well as the balance between excitatory glutamatergic and inhibitory GABAergic signal transduction in the temporo-limbic and PFC may be just specific correlates of a more widespread alteration in the precision of information processing. In this context, volatility may be underestimated at high hierarchical levels (see supplementary material; Jardri et al95).
We argue that genetic- and stress-dependent vulnerability factors can contribute to psychosis by shifting the balance between the respective precisions of prior beliefs and sensory evidence. In people with these risk factors, slight alterations in the balance between excitatory and inhibitory neurotransmission may result in overall nonselective information processing and reduced precision of prior beliefs, thus increasing the relative effects of sensory inputs, which in turn results in a shift of posteriors toward the sensory evidence and enhanced precision-weighted PEs. As a result, irrelevant information could have a greater tendency to be perceived as overly salient, leading to an impaired distinction between relevant and irrelevant stimuli. This could be tested by examining relevant neurocognitive and computational measures in individuals at elevated risk for schizophrenia. These processes can strongly involve brain areas associated with novelty detection including the hippocampus,23,54 which is critical in regulating dopamine neuronal activity.94 Furthermore, all of the regions implicated in the expression of the symptoms of psychosis (eg, fronto-striatal circuits, cingulate cortex, and the amygdalae) receive innervation from the limbic hippocampus.96 Thus, dysfunctional information processing in the limbic hippocampus has far-reaching consequences, necessarily disrupting related circuits in the cerebral cortex and influencing volatility estimates at the highest levels.79,97,98
Stress-dependent and/or chaotic dopamine firing can lead to noisy signaling of common primary and secondary reinforcers by augmenting otherwise irrelevant signals: in the frontal cortex and ventral striatum, dysregulated dopamine release may blunt signals elicited by the surprising manifestation of cues associated with positive reinforcers and thus contribute to motivational deficits99 (see supplementary section 2.3). On the other hand, when phasic dopamine release is associated with stressful or chaotically coinciding stimuli, salience can be attributed to these otherwise random co-occurrences, thus contributing to delusional perceptions and delusion formation.26,100 Bayesian posteriors may be further shifted toward sensory input by circular inference, thus amplifying certain information aspects at the expense of others (supplementary section 3).
Summary and Consequences for Future Research
In sum, these findings suggest that, beyond simple effects of sensory priors on the processing of sensory input, there are complex interactions in psychotic illness between low-level information processing in sensory cortices and information processing at higher levels of the predictive coding hierarchy. We argue that such interactions of higher-level with lower-level beliefs might be a general and important mechanism underlying psychotic experiences in a volatile environment.
The framework of Bayesian inference offers a promising approach for developing computational models that account for psychotic experiences by capturing examples of nonselective information processing and failed attempts to deal with a lack of precision. Mathematical models, particularly when constrained by biological plausibility, offer the possibility of identifying neurocomputational steps that the individual has to take when solving a certain task (eg, the computation of PEs101) and the neural signals associate these steps. Importantly, the idea of nonselective information processing within a Bayesian framework, as a consequence of disrupted temporo-limbic and prefrontal-striatal interactions, might account for a wide range of psychotic experiences, including self-disorders related to the initiation and performance of action as well as the self-attribution of intentions and thoughts.102–104 Beyond RL, Bayesian approaches can be applied to a range of cognitive and perceptual processes, such as visual information processing,78,105,106 reliant on frontal-occipital connectivity.76 To further develop a mechanistic understanding of the emergence of the symptoms of psychotic illness, 4 steps are recommended.
First, future research should place greater emphasis on auditory information processing. Even though auditory hallucinations clearly occur outside of psychotic illness, complex auditory hallucinations figure prominently in psychotic illness,107–109 and substantial evidence points to predictive coding alterations in relation to auditory processing in schizophrenia.98,110
Secondly, we strongly recommend employing multimodal imaging approaches, including the combination of MRS assessment of excitatory and inhibitory neurotransmitters and their metabolites with PET studies of monoaminergic neurotransmission and fMRI, EEG, or magnetoencephalographic measures of neural signals corresponding to the magnitude, valence, and precision of predictions and violations thereof. This would allow us to assess relationships among molecular measures of neurochemical concentration and function and measures of neural circuit function.
Thirdly, Bayesian models of decision making should make increasing use of more formalized approaches to dynamic interactions between brain areas, such as dynamic causal modeling and laminar neuroimaging methods. Dynamic causal modeling has already helped to identify subgroups of schizophrenia patients based on the severity of cognitive impairments and negative symptoms,111,112 while laminar neuroimaging methods may provide more precise assays of top-down and bottom-up information flow.113,114
Finally, more studies should attempt to integrate computational neuropsychiatry methods investigating the pathophysiology of psychosis with advances in identification of genetic and environmental risk factors for schizophrenia to better understand how, why, and when psychopathology emerges.115 In the larger picture, computational modeling—specifically Bayesian models of predictive coding—offer the possibility to link various, thus far largely separate, strains of research focused on different aspects of psychotic experience, such as aberrant salience attribution in delusion formation, the tenacity of delusions, and the development of impaired self-ascription of thoughts and actions.
Funding
This work was supported by the German Research Foundation (SCHL 1969/1-2/3-1 and 4-1 to F.S.) and the US National Institutes of Health (5R01MH094460 to J.A.W. and MH057440 to A.A.G.).
Supplementary Material
References
- 1. Carlsson A, Waters N, Carlsson ML. Neurotransmitter interactions in schizophrenia—therapeutic implications. Biol Psychiatry. 1999;46:1388–1395. [DOI] [PubMed] [Google Scholar]
- 2. Laruelle M, Abi-Dargham A, van Dyck CH, et al. Single photon emission computerized tomography imaging of amphetamine-induced dopamine release in drug-free schizophrenic subjects. Proc Natl Acad Sci USA. 1996;93:9235–9240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Howes OD, Kambeitz J, Kim E, et al. The nature of dopamine dysfunction in schizophrenia and what this means for treatment. Arch Gen Psychiatry. 2012;69:776–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jauhar S, Nour MM, Veronese M, et al. A test of the transdiagnostic dopamine hypothesis of psychosis using positron emission tomographic imaging in bipolar affective disorder and schizophrenia. JAMA Psychiatry. 2017;74:1206–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Schultz W, Dayan P, Montague PR. A neural substrate of prediction and reward. Science. 1997;275:1593–1599. [DOI] [PubMed] [Google Scholar]
- 6. Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev. 1993;18:247–291. [DOI] [PubMed] [Google Scholar]
- 7. Heinz A. Dopaminergic dysfunction in alcoholism and schizophrenia—psychopathological and behavioral correlates. Eur Psychiatry. 2002;17:9–16. [DOI] [PubMed] [Google Scholar]
- 8. Kapur S. Psychosis as a state of aberrant salience: a framework linking biology, phenomenology, and pharmacology in schizophrenia. Am J Psychiatry. 2003;160:13–23. [DOI] [PubMed] [Google Scholar]
- 9. Miller R. Schizophrenic psychology, associative learning and the role of forebrain dopamine. Med Hypotheses. 1976;2:203–211. [DOI] [PubMed] [Google Scholar]
- 10. Heinz A, Schlagenhauf F. Dopaminergic dysfunction in schizophrenia: salience attribution revisited. Schizophr Bull. 2010;36:472–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Maia TV, Frank MJ. An integrative perspective on the role of dopamine in schizophrenia. Biol Psychiatry. 2017;81:52–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Friston KJ, Stephan KE, Montague R, Dolan RJ. Computational psychiatry: the brain as a phantastic organ. Lancet Psychiatry. 2014;1:148–158. [DOI] [PubMed] [Google Scholar]
- 13. Mathys CD, Lomakina EI, Daunizeau J, et al. Uncertainty in perception and the Hierarchical Gaussian Filter. Front Hum Neurosci. 2014;8:825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Aitchison L, Lengyel M. With or without you: predictive coding and Bayesian inference in the brain. Curr Opin Neurobiol. 2017;46:219–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Valton V, Romaniuk L, Douglas Steele J, Lawrie S, Seriès P. Comprehensive review: computational modelling of schizophrenia. Neurosci Biobehav Rev. 2017;83:631–646. [DOI] [PubMed] [Google Scholar]
- 16. Adams RA, Huys QJ, Roiser JP. Computational psychiatry: towards a mathematically informed understanding of mental illness. J Neurol Neurosurg Psychiatry. 2016;87:53–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Friston K. A theory of cortical responses. Philos Trans R Soc Lond B Biol Sci. 2005;360:815–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Payzan-LeNestour E, Bossaerts P. Risk, unexpected uncertainty, and estimation uncertainty: Bayesian learning in unstable settings. PLoS Comput Biol. 2011;7:e1001048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Weilnhammer VA, Stuke H, Sterzer P, Schmack K. The neural correlates of hierarchical predictions for perceptual decisions. J Neurosci. 2018;38:5008–5021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fletcher PC, Frith CD. Perceiving is believing: a Bayesian approach to explaining the positive symptoms of schizophrenia. Nat Rev Neurosci. 2009;10:48–58. [DOI] [PubMed] [Google Scholar]
- 21. Adams RA, Stephan KE, Brown HR, Frith CD, Friston KJ. The computational anatomy of psychosis. Front Psychiatry. 2013;4:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Weinberger DR. Implications of normal brain development for the pathogenesis of schizophrenia. Arch Gen Psychiatry. 1987;44:660–669. [DOI] [PubMed] [Google Scholar]
- 23. Meyer-Lindenberg A. From maps to mechanisms through neuroimaging of schizophrenia. Nature. 2010;468:194–202. [DOI] [PubMed] [Google Scholar]
- 24. Deserno L, Boehme R, Heinz A, Schlagenhauf F. Reinforcement learning and dopamine in schizophrenia: dimensions of symptoms or specific features of a disease group?Front Psychiatry. 2013;4:172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Strauss GP, Cohen AS. A transdiagnostic review of negative symptom phenomenology and etiology. Schizophr Bull. 2017;43:712–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pankow A, Friedel E, Sterzer P, et al. Altered amygdala activation in schizophrenia patients during emotion processing. Schizophr Res. 2013;150:101–106. [DOI] [PubMed] [Google Scholar]
- 27. Cohen AS, Minor KS. Emotional experience in patients with schizophrenia revisited: meta-analysis of laboratory studies. Schizophr Bull. 2010;36:143–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Schultz W. Updating dopamine reward signals. Curr Opin Neurobiol. 2013;23:229–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Steinberg EE, Keiflin R, Boivin JR, Witten IB, Deisseroth K, Janak PH. A causal link between prediction errors, dopamine neurons and learning. Nat Neurosci. 2013;16:966–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Eshel N, Tian J, Bukwich M, Uchida N. Dopamine neurons share common response function for reward prediction error. Nat Neurosci. 2016;19:479–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sharpe MJ, Chang CY, Liu MA, et al. Dopamine transients are sufficient and necessary for acquisition of model-based associations. Nat Neurosci. 2017;20:735–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Waltz JA, Gold JM. Motivational deficits in schizophrenia and the representation of expected value. Curr Top Behav Neurosci. 2016;27:375–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. McClure SM, Daw ND, Montague PR. A computational substrate for incentive salience. Trends Neurosci. 2003;26:423–428. [DOI] [PubMed] [Google Scholar]
- 34. Murray GK, Corlett PR, Clark L, et al. Substantia nigra/ventral tegmental reward prediction error disruption in psychosis. Mol Psychiatry. 2008;13:239, 267–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Corlett PR, Murray GK, Honey GD, et al. Disrupted prediction-error signal in psychosis: evidence for an associative account of delusions. Brain. 2007;130:2387–2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Schlagenhauf F, Sterzer P, Schmack K, et al. Reward feedback alterations in unmedicated schizophrenia patients: relevance for delusions. Biol Psychiatry. 2009;65:1032–1039. [DOI] [PubMed] [Google Scholar]
- 37. Romaniuk L, Honey GD, King JR, et al. Midbrain activation during Pavlovian conditioning and delusional symptoms in schizophrenia. Arch Gen Psychiatry. 2010;67:1246–1254. [DOI] [PubMed] [Google Scholar]
- 38. Ermakova AO, Knolle F, Justicia A, et al. Abnormal reward prediction-error signalling in antipsychotic naive individuals with first-episode psychosis or clinical risk for psychosis. Neuropsychopharmacology. 2018;43:1691–1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Schlagenhauf F, Huys QJ, Deserno L, et al. Striatal dysfunction during reversal learning in unmedicated schizophrenia patients. Neuroimage. 2014;89:171–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Howes OD, McCutcheon R, Owen MJ, Murray RM. The role of genes, stress, and dopamine in the development of schizophrenia. Biol Psychiatry. 2017;81:9–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Schlagenhauf F, Rapp MA, Huys QJ, et al. Ventral striatal prediction error signaling is associated with dopamine synthesis capacity and fluid intelligence. Hum Brain Mapp. 2013;34:1490–1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Reddy LF, Waltz JA, Green MF, Wynn JK, Horan WP. Probabilistic reversal learning in schizophrenia: stability of deficits and potential causal mechanisms. Schizophr Bull. 2016;42:942–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Culbreth AJ, Westbrook A, Xu Z, Barch DM, Waltz JA. Intact ventral striatal prediction error signaling in medicated schizophrenia patients. Biol Psychiatry Cogn Neurosci Neuroimaging. 2016;1:474–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Dowd EC, Frank MJ, Collins A, Gold JM, Barch DM. Probabilistic reinforcement learning in patients with schizophrenia: relationships to anhedonia and avolition. Biol Psychiatry Cogn Neurosci Neuroimaging. 2016;1:460–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Waltz JA, Gold JM. Probabilistic reversal learning impairments in schizophrenia: further evidence of orbitofrontal dysfunction. Schizophr Res. 2007;93:296–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Murray GK, Cheng F, Clark L, et al. Reinforcement and reversal learning in first-episode psychosis. Schizophr Bull. 2008;34:848–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Waltz JA. The neural underpinnings of cognitive flexibility and their disruption in psychotic illness. Neuroscience. 2017;345:203–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Schwartenbeck P, FitzGerald TH, Mathys C, et al. Optimal inference with suboptimal models: addiction and active Bayesian inference. Med Hypotheses. 2015;84:109–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Friston K, Schwartenbeck P, FitzGerald T, Moutoussis M, Behrens T, Dolan RJ. The anatomy of choice: dopamine and decision-making. Philos Trans R Soc Lond B Biol Sci. 2014;369: 20130481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Iglesias S, Tomiello S, Schneebeli M, Stephan KE. Models of neuromodulation for computational psychiatry. Wiley Interdiscip Rev Cogn Sci. 2016. [DOI] [PubMed] [Google Scholar]
- 51. Haarsma J, Fletcher PC, Ziauddeen H, Spencer TJ, Diederen KMJ, Murray GK. Precision weighting of cortical unsigned prediction errors is mediated by dopamine and benefits learning. bioRxiv. 2018. [Google Scholar]
- 52. Mathys C, Daunizeau J, Friston KJ, Stephan KE. A Bayesian foundation for individual learning under uncertainty. Front Hum Neurosci. 2011;5:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Iglesias S, Mathys C, Brodersen KH, et al. Hierarchical prediction errors in midbrain and basal forebrain during sensory learning. Neuron. 2013;80:519–530. [DOI] [PubMed] [Google Scholar]
- 54. Lisman JE, Grace AA. The hippocampal-VTA loop: controlling the entry of information into long-term memory. Neuron. 2005;46:703–713. [DOI] [PubMed] [Google Scholar]
- 55. Floresco SB, West AR, Ash B, Moore H, Grace AA. Afferent modulation of dopamine neuron firing differentially regulates tonic and phasic dopamine transmission. Nat Neurosci. 2003;6:968–973. [DOI] [PubMed] [Google Scholar]
- 56. Zimmerman EC, Grace AA. The nucleus reuniens of the midline thalamus gates prefrontal-hippocampal modulation of ventral tegmental area dopamine neuron activity. J Neurosci. 2016;36:8977–8984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Jardri R, Hugdahl K, Hughes M, et al. Are hallucinations due to an imbalance between excitatory and inhibitory influences on the brain?Schizophr Bull. 2016;42:1124–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Behrens TE, Woolrich MW, Walton ME, Rushworth MF. Learning the value of information in an uncertain world. Nat Neurosci. 2007;10:1214–1221. [DOI] [PubMed] [Google Scholar]
- 59. Krugel LK, Biele G, Mohr PN, Li SC, Heekeren HR. Genetic variation in dopaminergic neuromodulation influences the ability to rapidly and flexibly adapt decisions. Proc Natl Acad Sci USA. 2009;106:17951–17956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Friston KJ, Shiner T, FitzGerald T, et al. Dopamine, affordance and active inference. PLoS Comput Biol. 2012;8:e1002327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Deserno L, Huys QJ, Boehme R, et al. Ventral striatal dopamine reflects behavioral and neural signatures of model-based control during sequential decision making. Proc Natl Acad Sci USA. 2015;112:1595–1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Vinckier F, Gaillard R, Palminteri S, et al. Confidence and psychosis: a neuro-computational account of contingency learning disruption by NMDA blockade. Mol Psychiatry. 2016;21:946–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Salamone JD, Correa M, Farrar A, Mingote SM. Effort-related functions of nucleus accumbens dopamine and associated forebrain circuits. Psychopharmacology (Berl). 2007;191:461–482. [DOI] [PubMed] [Google Scholar]
- 64. Wittmann BC, Daw ND, Seymour B, Dolan RJ. Striatal activity underlies novelty-based choice in humans. Neuron. 2008;58:967–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Tobler PN, O’Doherty JP, Dolan RJ, Schultz W. Reward value coding distinct from risk attitude-related uncertainty coding in human reward systems. J Neurophysiol. 2007;97:1621–1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. O’Donnell P, Grace AA. Dysfunctions in multiple interrelated systems as the neurobiological bases of schizophrenic symptom clusters. Schizophr Bull. 1998;24:267–283. [DOI] [PubMed] [Google Scholar]
- 67. O’Donnell P, Grace AA. Synaptic interactions among excitatory afferents to nucleus accumbens neurons: hippocampal gating of prefrontal cortical input. J Neurosci. 1995;15:3622–3639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Morales M, Margolis EB. Ventral tegmental area: cellular heterogeneity, connectivity and behaviour. Nat Rev Neurosci. 2017;18:73–85. [DOI] [PubMed] [Google Scholar]
- 69. Schultz W. Dopamine reward prediction-error signalling: a two-component response. Nat Rev Neurosci. 2016;17:183–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Calcia MA, Bonsall DR, Bloomfield PS, Selvaraj S, Barichello T, Howes OD. Stress and neuroinflammation: a systematic review of the effects of stress on microglia and the implications for mental illness. Psychopharmacology (Berl). 2016;233:1637–1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Vaessen T, Hernaus D, Myin-Germeys I, van Amelsvoort T. The dopaminergic response to acute stress in health and psychopathology: a systematic review. Neurosci Biobehav Rev. 2015;56:241–251. [DOI] [PubMed] [Google Scholar]
- 72. Egerton A, Howes OD, Houle S, et al. Elevated striatal dopamine function in immigrants and their children: a risk mechanism for psychosis. Schizophr Bull. 2017;43:293–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Myin-Germeys I, van Os J. Stress-reactivity in psychosis: evidence for an affective pathway to psychosis. Clin Psychol Rev. 2007;27:409–424. [DOI] [PubMed] [Google Scholar]
- 74. Veling W. Ethnic minority position and risk for psychotic disorders. Curr Opin Psychiatry. 2013;26:166–171. [DOI] [PubMed] [Google Scholar]
- 75. Cantor-Graae E, Selten JP. Schizophrenia and migration: a meta-analysis and review. Am J Psychiatry. 2005;162:12–24. [DOI] [PubMed] [Google Scholar]
- 76. Schmack K, Gòmez-Carrillo de Castro A, Rothkirch M, et al. Delusions and the role of beliefs in perceptual inference. J Neurosci. 2013;33:13701–13712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Schmack K, Schnack A, Priller J, Sterzer P. Perceptual instability in schizophrenia: probing predictive coding accounts of delusions with ambiguous stimuli. Schizophr Res Cogn. 2015;2:72–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Notredame CE, Pins D, Deneve S, Jardri R. What visual illusions teach us about schizophrenia. Front Integr Neurosci. 2014;8:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Schmack K, Rothkirch M, Priller J, Sterzer P. Enhanced predictive signalling in schizophrenia. Hum Brain Mapp. 2017;38:1767–1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci. 1986;9:357–381. [DOI] [PubMed] [Google Scholar]
- 81. Ripke S, O’Dushlaine C, Chambert K, et al. ; Multicenter Genetic Studies of Schizophrenia Consortium; Psychosis Endophenotypes International Consortium; Wellcome Trust Case Control Consortium 2 Genome-wide association analysis identifies 13 new risk loci for schizophrenia. Nat Genet. 2013;45:1150–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Winterer G, Ziller M, Dorn H, et al. Schizophrenia: reduced signal-to-noise ratio and impaired phase-locking during information processing. Clin Neurophysiol. 2000;111:837–849. [DOI] [PubMed] [Google Scholar]
- 83. Winterer G, Coppola R, Goldberg TE, et al. Prefrontal broadband noise, working memory, and genetic risk for schizophrenia. Am J Psychiatry. 2004;161:490–500. [DOI] [PubMed] [Google Scholar]
- 84. Abi-Dargham A, Mawlawi O, Lombardo I, et al. Prefrontal dopamine D1 receptors and working memory in schizophrenia. J Neurosci. 2002;22:3708–3719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Gonzalez-Burgos G, Lewis DA. NMDA receptor hypofunction, parvalbumin-positive neurons, and cortical gamma oscillations in schizophrenia. Schizophr Bull. 2012;38:950–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Heckers S, Konradi C. GABAergic mechanisms of hippocampal hyperactivity in schizophrenia. Schizophr Res. 2015;167:4–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Olbrich HM, Valerius G, Rüsch N, et al. Frontolimbic glutamate alterations in first episode schizophrenia: evidence from a magnetic resonance spectroscopy study. World J Biol Psychiatry. 2008;9:59–63. [DOI] [PubMed] [Google Scholar]
- 88. van Elst LT, Valerius G, Büchert M, et al. Increased prefrontal and hippocampal glutamate concentration in schizophrenia: evidence from a magnetic resonance spectroscopy study. Biol Psychiatry. 2005;58:724–730. [DOI] [PubMed] [Google Scholar]
- 89. Merritt K, Egerton A, Kempton MJ, Taylor MJ, McGuire PK. Nature of glutamate alterations in schizophrenia: a meta-analysis of proton magnetic resonance spectroscopy studies. JAMA Psychiatry. 2016;73:665–674. [DOI] [PubMed] [Google Scholar]
- 90. Schür RR, Draisma LW, Wijnen JP, et al. Brain GABA levels across psychiatric disorders: a systematic literature review and meta-analysis of (1) H-MRS studies. Hum Brain Mapp. 2016;37:3337–3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Modinos G, Simsek F, Azis M, et al. Prefrontal GABA levels, hippocampal resting perfusion and the risk of psychosis. Neuropsychopharmacology. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Gleich T, Deserno L, Lorenz RC, et al. Prefrontal and striatal glutamate differently relate to striatal dopamine: potential regulatory mechanisms of striatal presynaptic dopamine function?J Neurosci. 2015;35:9615–9621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Lodge DJ, Grace AA. The hippocampus modulates dopamine neuron responsivity by regulating the intensity of phasic neuron activation. Neuropsychopharmacology. 2006;31:1356–1361. [DOI] [PubMed] [Google Scholar]
- 94. Grace AA, Floresco SB, Goto Y, Lodge DJ. Regulation of firing of dopaminergic neurons and control of goal-directed behaviors. Trends Neurosci. 2007;30:220–227. [DOI] [PubMed] [Google Scholar]
- 95. Jardri R, Duverne S, Litvinova AS, Denève S. Experimental evidence for circular inference in schizophrenia. Nat Commun. 2017;8:14218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Grace AA, Gomes FV. The circuitry of dopamine system regulation and its disruption in schizophrenia: insights into treatment and prevention. Schizophr Bull. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Sekutowicz M, Schmack K, Steimke R, et al. Striatal activation as a neural link between cognitive and perceptual flexibility. Neuroimage. 2016;141:393–398. [DOI] [PubMed] [Google Scholar]
- 98. Powers AR, Mathys C, Corlett PR. Pavlovian conditioning-induced hallucinations result from overweighting of perceptual priors. Science. 2017;357:596–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Juckel G, Schlagenhauf F, Koslowski M, et al. Dysfunction of ventral striatal reward prediction in schizophrenia. Neuroimage. 2006;29:409–416. [DOI] [PubMed] [Google Scholar]
- 100. Kienast T, Hariri AR, Schlagenhauf F, et al. Dopamine in amygdala gates limbic processing of aversive stimuli in humans. Nat Neurosci. 2008;11:1381–1382. [DOI] [PubMed] [Google Scholar]
- 101. Montague PR, Hyman SE, Cohen JD. Computational roles for dopamine in behavioural control. Nature. 2004;431:760–767. [DOI] [PubMed] [Google Scholar]
- 102. Frith CD. The Cognitive Neuropsychology of Schizophrenia. Hillsdale, NJ: Lawrence Erlbaum Associates, Inc; 1992. [Google Scholar]
- 103. Sterzer P, Mishara AL, Voss M, Heinz A. Thought insertion as a self-disturbance: an integration of predictive coding and phenomenological approaches. Front Hum Neurosci. 2016;10:502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Voss M, Moore J, Hauser M, Gallinat J, Heinz A, Haggard P. Altered awareness of action in schizophrenia: a specific deficit in predicting action consequences. Brain. 2010;133:3104–3112. [DOI] [PubMed] [Google Scholar]
- 105. Seymour K, Stein T, Sanders LL, Guggenmos M, Theophil I, Sterzer P. Altered contextual modulation of primary visual cortex responses in schizophrenia. Neuropsychopharmacology. 2013;38:2607–2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Anderson EJ, Tibber MS, Schwarzkopf DS, et al. Visual population receptive fields in people with schizophrenia have reduced inhibitory surrounds. J Neurosci. 2017;37:1546–1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Schneider K. Psychischer Befund und Psychiatrische Diagnose. Leipzig: Georg Thieme; 1942. [Google Scholar]
- 108. Heinz A, Voss M, Lawrie SM, et al. Shall we really say goodbye to first rank symptoms?Eur Psychiatry. 2016;37:8–13. [DOI] [PubMed] [Google Scholar]
- 109. Waters F, Blom JD, Jardri R, Hugdahl K, Sommer IEC. Auditory hallucinations, not necessarily a hallmark of psychotic disorder. Psychol Med. 2018;48:529–536. [DOI] [PubMed] [Google Scholar]
- 110. Horga G, Schatz KC, Abi-Dargham A, Peterson BS. Deficits in predictive coding underlie hallucinations in schizophrenia. J Neurosci. 2014;34:8072–8082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Deserno L, Sterzer P, Wüstenberg T, Heinz A, Schlagenhauf F. Reduced prefrontal-parietal effective connectivity and working memory deficits in schizophrenia. J Neurosci. 2012;32:12–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Brodersen KH, Deserno L, Schlagenhauf F, et al. Dissecting psychiatric spectrum disorders by generative embedding. Neuroimage Clin. 2014;4:98–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Lawrence SJD, Formisano E, Muckli L, de Lange FP. Laminar fMRI: applications for cognitive neuroscience. Neuroimage. 2017. [DOI] [PubMed] [Google Scholar]
- 114. Stephan KE, Petzschner FH, Kasper L, et al. Laminar fMRI and computational theories of brain function. Neuroimage. 2017. [DOI] [PubMed] [Google Scholar]
- 115. van Os J, Kenis G, Rutten BP. The environment and schizophrenia. Nature. 2010;468:203–212. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.